ABSTRACT
Background
The carbon isotope ratio (CIR) is a proposed biomarker for added sugar (AS) intake in the United States; however, because the CIR is also associated with meat intake in most populations the need for specificity remains. The CIR of amino acids (AAs) has the potential to differentiate sugars from meat intakes, because essential AAs must derive from dietary protein whereas certain nonessential AAs can be synthesized from sugars.
Objectives
We tested whether serum CIR-AAs in combination with participant characteristics could meet a prespecified biomarker criterion for AS intake in the Nutrition and Physical Activity Assessment Study Feeding Study (NPAAS-FS) of the Women's Health Initiative, a population in which the whole-serum CIR was not associated with AS intake.
Methods
Postmenopausal women (n = 145) from Seattle, WA, were provided with individualized diets that approximated their habitual food intakes for 2 wk. Dietary intakes from consumed foods were characterized over the feeding period using the Nutrition Data System for Research. The CIR of 7 AAs—Ala, Gly, Val, Leu, Ile, Pro, and Phe—were measured in fasting serum collected at the end of the 2-wk feeding period, using gas chromatography–combustion isotope ratio mass spectrometry. Biomarker models were evaluated using regression R2 ≥ 0.36 as a major biomarker criterion, based on the benchmark R2 values of well-established recovery biomarkers in the NPAAS-FS.
Results
AS intake was associated with CIR-Ala (ρ = 0.32; P < 0.0001). A model of AS intake based on CIR-Ala, CIR-Gly, CIR-Ile, smoking, leisure physical activity, and body weight met the biomarker criterion (R2 = 0.37). Biomarker-estimated AS intake was not associated with meat or animal protein intake.
Conclusions
Results support serum CIR-AAs in combination with participant characteristics as potential biomarkers of AS intake in US populations, including those with low AS intake.
The Women's Health Initiative is registered at clinicaltrials.gov (NCT00000611).
Keywords: added sugar, dietary biomarker, amino acid carbon isotope ratios, controlled feeding study, Nutrition and Physical Activity Study Feeding Study (NPAAS-FS)
See corresponding commentary on page 2615.
Introduction
Consumption of added sugars (ASs) and sugar-sweetened beverages (SSBs) contributes to the etiology of multiple chronic diseases, including obesity (1, 2), diabetes (3, 4), cardiovascular disease (5–7), and some cancers (7–9). Policy initiatives and public health interventions targeting AS require accurate quantification of dietary intake, which can be hampered by random and systematic error associated with methods of self-reported dietary assessment (10–13). An objective biomarker could be used to circumvent these issues, potentially identifying patterns of systematic error in dietary data, enabling the generation of biomarker-calibrated estimates of AS/SSB intake (14). Such calibrated dietary estimates often reveal stronger diet-disease associations than were previously observed using uncalibrated dietary measures (14–18).
The carbon isotope ratio (CIR) has potential as a biomarker of AS intake because it is elevated in corn and sugar cane, which supply 70% of caloric sweeteners (ASs) and nearly all SSBs in the United States (19–21). However, due to the widespread use of corn to feed livestock in the United States (22–24), the CIR is also associated with meat and/or animal protein, often more strongly than with AS/SSB (25–27). The consistent association of the CIR with animal protein/meat intake is a major limitation of the CIR as a candidate biomarker for AS.
A potential way to improve a stable isotope biomarker of AS intake is to use the CIR of a suite of molecules that can differentiate intake of AS from intake of animal protein/meat. Amino acids (AAs) are promising candidates in this regard. The CIR of the nonessential AA (NEAA) Ala was associated with AS/SSB intake in an Alaska Native cohort (28), likely due to the synthesis of Ala from glucose via the glucose-Ala cycle (29–31). In contrast, essential AAs (EAAs) must derive from dietary protein and thus are likely to reflect the CIR of animal protein/meat. Analysis of AA CIR by gas chromatography–combustion isotope ratio mass spectrometry provides data on multiple AAs, including Ala and several EAAs (32, 33), thus potentially providing a tool to discriminate AS from animal protein/meat intakes. Despite the potential of AA CIR as a biomarker of AS based on the Alaska Native study, this approach has yet to be evaluated in other US populations, particularly those with modest or low AS intake.
This study evaluated a biomarker of AS intake based on serum AA CIR and participant characteristics in the Nutrition and Physical Activity Assessment Study Feeding Study (NPAAS-FS), an ancillary study of the Women's Health Initiative (WHI) designed to develop and evaluate dietary biomarkers in 153 postmenopausal women (34). Each participant was provided a personalized diet for 2 wk that approximated usual intake, providing dietary control while preserving typical intake distributions for the population. Our primary hypothesis was that a model of added sugar intake based on serum AA CIR and participant characteristics could meet biomarker criteria for AS intake in the NPAAS-FS study.
Methods
Study participants and design
The NPAAS-FS, an ancillary study of the WHI Extension study, was designed to evaluate dietary biomarkers in postmenopausal women within the context of their habitual diet. The NPAAS-FS was implemented from 2011 to 2013 in Seattle, WA, with biospecimens and data being investigated through the present, and included 153 WHI Extension study participants. The NPAAS-FS was approved by the Fred Hutchinson Institutional Review Board in accordance with the Declaration of Helsinki, with additional oversight from the WHI Observational Study Monitoring Board. The WHI program is registered at clinicaltrials.gov as NCT00000611. Stable isotope biomarkers of diet were evaluated in the NPAAS-FS under approved ancillary study WHI AS423, of which AA CIRs were primary outcome variables in addition to the whole serum CIR, nitrogen isotope ratio (NIR), and the sulfur isotope ratio (SIR), which were reported previously (26).
A complete description of NPAAS-FS study procedures has been previously published, including recruitment, consent, and the details of participant visits [Supplemental Figure 1 (34)]. Briefly, following written informed consent each participant completed a 4-d food record that was reviewed carefully by research dietitians and used to design an individualized 2-wk experimental diet that approximated the participant's habitual intake. All foods were prepared in the Fred Hutchinson Prevention Center Human Nutrition Laboratory (HNL), and all meal components were entered into the Nutrition Data System for Research (NDS-R; Nutrition Coordinating Center, version 2010; University of Minnesota). Participants visited the HNL 2–3 times/wk to consume a study meal on site, pick up foods for the next 2–3 d, and have body weight measured. Uneaten foods were returned to the HNL, where they were weighed and recorded. Participants were instructed to maintain their daily life and eat only study foods prepared by the HNL during the 2-wk feeding period. One exception was that alcohol was not provided as part of the study foods and beverages. Those who consumed alcohol provided their own and reported type and volume on study reporting forms. Participants completed a daily menu checklist to record consumption of all study foods and beverages (and nonstudy foods if applicable).
At the beginning and end of the 2-wk feeding period, participants provided a fasting blood specimen, and height and weight were measured by trained staff. Participants completed lifestyle questionnaires regarding smoking status, medication use, dietary supplement use, and leisure physical activity (LPA). Other variables (age, race, education) were extracted from the WHI database. Fasting blood samples were processed to serum and stored at −80°C. Serum aliquots were subsequently shipped on dry ice to the University of Alaska Fairbanks for stable isotope analysis. In this study, serum samples taken at the end of the feeding study were analyzed, because these best reflected diet provided during the study period.
In addition to total energy and macronutrient intakes, we examined the intakes of specific foods that have been demonstrated to have elevated CIR in previous studies (35). These included 4 variables characterizing dietary intake of sugars [total sugars, ASs, SSBs, and “nonadded” sugars (calculated as total − ASs)], 6 variables characterizing intake of animal-derived foods (animal protein, red meat, poultry, fish/seafood, eggs, and dairy), and 1 variable characterizing corn intake (corn products). We note that ASs by definition include all sugars added to foods during preparation or processing, not only those deriving from corn or sugar cane.
The following dietary intake variables were extracted from the NPAAS-FS “consumed” NDS-R files over the period of dietary intake: total energy (kilocalories per day), carbohydrate (grams per day), fat (grams per day), protein (grams per day), animal protein (grams per day), total sugar (grams per day), and AS (grams per day). Additional constructed variables were created from the NPAAS-FS “consumed” NDS-R food components including: corn products, SSBs, red meat, fish/seafood, poultry, eggs, and dairy, with each dietary component coded as 0 or 1 for each new variable. Detailed coding inclusion/exclusion criteria are presented in Supplemental Table 1. Briefly, “red meat” included beef, pork, lamb, and liver; “fish/seafood” included fish and shellfish; “dairy” included animal milk and all milk-derived products such as cream, butter, cheese, and yogurt; and “corn products” included whole corn, popcorn, corn chips, corn tortillas, corn cereals, and corn oil. If a category was the second or third ingredient listed in a commercial food component it was coded as 0.5; for example, some breakfast cereals were coded 0.5 for “corn products” (26). Coding variables were multiplied by grams of intake, summed over each participant, and divided by the number of days of intake to generate daily intake in grams per day for each participant for each new food category. For components that included beverages (dairy and SSBs), we subtracted grams of water from grams of intake.
AA extraction and derivatization
Serum AAs (free and bound) were extracted from samples collected at the end of the 2-wk feeding period using protocols described by Walsh et al. (33). Serum (50–100 μL) was hydrolyzed with 1 mL 6 N HCl (110°C, 20 h), and hydrolysates were lipid-extracted using n-hexane/dichloromethane (6:5 v:v). An “external” standard mixture of 12 AAs with known δ13C values (0.05 mM each in 0.1 M HCl) was prepared alongside each batch of serum samples. An “internal” standard, norleucine (Nle), was added to both samples and external standards (33). Sample hydrolysates and external standards were dried at room temperature under a gentle stream of dinitrogen, resuspended in 200 μL 0.1 N HCl, and stored at −20°C. A 100-μL aliquot of each hydrolyzed sample and external standard was transferred into a GC vial with a fixed 300-μL insert, and AAs were derivatized to methoxycarbonyl methyl esters (MCMEs) as described in detail elsewhere (33), allowing them to be separated and measured in a GC system. AA-MCME derivatives were stored at −20°C.
Analysis of AA CIRs
AA-MCME CIRs were measured by using gas chromatography–combustion isotope ratio mass spectrometry (GC-CIRMS), using a Thermo Trace gas chromatograph, the GC-IsoLink combustion interface, the Conflo VI gas interface, and a Delta-V isotope ratio mass spectrometer (Thermo Fisher Scientific) at the Alaska Stable Isotope Facility at the University of Alaska Fairbanks. Each sample was injected and measured in duplicate, and the external standard was injected and measured before and after every 3 samples. The AA-MCMEs were injected in splitless mode at 250°C and separated on a DB-23 capillary column (Agilent Technologies; 30 m × 0.25 mm internal diameter; 0.25 μm film thickness) at a constant flow rate of 1.2 mL/min under the following temperature program: 50°C (hold 2 min), 120°C at 15°C/min (hold 3 min), 150°C at 6°C/min (hold 10 min), 200°C at 6°C/min (hold 5 min), 250°C at 6°C/min (hold 20 min). The separated AA-MCMEs were completely oxidized to carbon dioxide and introduced into the isotope ratio mass spectrometer via the GC-IsoLink and Conflo IV interfaces (Thermo Fisher Scientific, Inc).
The CIR-AAs were expressed as δ13C values in permil (‰) abundance of 13C relative to an international standard [Vienna PeeDee Belemnite (V-PDB); 13C/12C = 0.0112372], as follows:
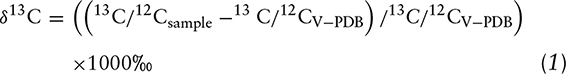
The measured δ13C value of each AA-MCME peak was normalized relative to the internal standard (Nle-MCME) in ISODAT, the Thermo analytical software package (33). To account for the nonanalyte carbon added to AA-MCMEs during derivatization, measured sample AA-MCME δ13C values were corrected to underivatized AA δ13C values using an arithmetic procedure based on the external standard, for which the relation between measured, derivatized AA-MCME δ13C values and known, underivatized AA δ13C values could be characterized for each AA (36).
In this article, we present the δ13C values of 3 NEAAs—Ala, Gly, and Pro—and 4 EAAs—Ile, Leu, Phe, and Val—based on good chromatographic separation and reproducibility as assessed using blind duplicates (10% of analyses). Analytical error was estimated as ±1.3‰ (Ala), ±2.0‰ (Gly), ±0.8‰ (Ile), ±0.8‰ (Leu), ±0.6‰ (Phe), ±0.9‰ (Pro), and ±0.9‰ (Val), respectively, by propagating measurement error of the samples and external standards as described elsewhere (37). These estimates agreed very well with the average absolute values of the difference between blind duplicates for each AA: 1.2‰, 2.1‰, 0.6‰, 0.6‰, 0.9‰, 0.8‰, and 0.7‰, respectively.
Statistical analysis
All statistical analyses were performed using JMP version 11 (SAS Institute). Because stable isotope ratios reflect diet over periods longer than 2 wk (38) we excluded from dietary analyses participants (n = 6) who were found in a previous study to have whole-serum stable isotope ratios (including the CIR, NIR, and SIR) that were not stable over the 2 wk of dietary control, suggesting that those participants were not in isotopic equilibrium with the diet provided (26). Our criterion for exclusion was a difference in CIR, NIR, or SIR between pre- and postfeeding samples that exceeded the mean difference by ≥3 IQR widths, <4% of 153 participants (26). We described the participant characteristics and the range of the AA CIRs across tertiles of AS intake by using medians (±IQR). Differences across tertiles were analyzed by either a Kruskall–Wallis or a χ 2 square test.
We examined associations between dietary intake variables and AA CIRs measured in postfeeding serum samples using Spearman rank correlation (ρ), with 95% CIs estimated using Fisher z-transformation. We used a significance level of P = 0.005, based on Bonferroni adjustment to account for the number of comparisons made with each potential biomarker [k = 11, where k refers to the number of dietary comparisons made for each biomarker (39)]. Stable isotope biomarkers were evaluated for their associations with intakes of sweeteners [total sugar (grams per day), AS (grams per day), “nonadded” sugar (total sugar − AS, grams per day), and SSBs (grams per day)], animal-derived foods [animal protein (grams per day), fish/seafood (grams per day), red meat (grams per day), poultry (grams per day), eggs (grams per day), and dairy (grams per day)], and corn products (grams per day).
We regressed AS intake on multiple CIR-AAs (P = 0.05) to evaluate potential biomarkers. To evaluate whether including participant characteristics improved biomarker associations, the regression analyses were extended by considering the inclusion of age, race, BMI, body weight, current smoking status (yes or no), dietary supplement use (yes or no), LPA (Metabolic Equivalent Task hour per week (MET-h/wk) ), and alcohol consumption (grams per day). Both BMI and body weight were considered because they represent different, although related, characteristics (adiposity compared with body size). A backward-selection procedure (P = 0.10) was applied to exclude noncontributing factors from final models. Outliers were identified using Mahalanobis distance >4.0 and excluded from analyses (n = 2 for AS only). Normality of residuals was evaluated using the Shapiro–Wilk test.
We used R2 = 0.36 as the primary criterion for biomarker evaluation based on the performance of well-established recovery biomarkers in the NPAAS-FS, following other NPAAS-FS publications (26, 34, 40).
Results
The demographic, lifestyle, and dietary characteristics of the 145 participants included in this study across tertiles of AS intake are shown in Table 1. As previously reported, NPAAS-FS participants were mostly white (95%), had a median age of 75 (73, 78) y, and 60% were overweight or obese (BMI ≥25 kg/m2) (34). NPAAS-FS participants who had higher AS intakes engaged in less self-reported LPA; otherwise, participant characteristics were similar across tertiles of AS intake. The highest tertile of AS intake consumed more energy and carbohydrate relative to the lowest tertile, whereas fat and protein consumption did not differ by AS tertile. Intakes of total sugars, SSBs, and dairy increased by tertile of AS, whereas intake of “nonadded” sugars and other dietary factors did not. Measurements of AA CIRs in the study population and by tertile of AS are presented in Table 2; CIR-Ala increased by tertile of AS, whereas CIR-Gly exhibited a marginally nonsignificant decrease (P = 0.07).
TABLE 1.
NPAAS-FS participant characteristics and dietary intake in the complete study sample and by tertile of added sugar intake1
| Added sugar intake, g/d | |||||
|---|---|---|---|---|---|
| Characteristics | Complete sample | Tertile 1 (<38.7) | Tertile 2 (38.7–56.4) | Tertile 3 (>56.4) | P 2 |
| n | 145 | 48 | 49 | 48 | |
| Age, y | 75 (73, 78) | 75 (73, 78) | 76 (73, 79) | 75 (71, 77) | 0.35 |
| Race,3 white (n) | 138 | 45 | 46 | 47 | 0.56 |
| BMI, kg/m2 | 26 (24, 29) | 25 (22, 28) | 26 (24, 28) | 27 (24, 30) | 0.20 |
| Body weight, kg | 68 (61, 76) | 66 (58, 78) | 68 (60, 76) | 70 (62, 78) | 0.32 |
| Leisure physical activity, MET-h/wk | 12 (5, 24) | 13 (4, 27) | 16 (10, 25) | 8 (4, 16) | 0.007 |
| Current smokers (n) | 3 | 0 | 1 | 2 | 0.36 |
| Macronutrient intake | |||||
| Energy intake, kcal/d | 1871 (1735, 2076) | 1818 (1644, 2040) | 1858 (1758, 1985) | 1900 (1794, 2183) | 0.02 |
| Carbohydrate, g/d | 216 (188, 241) | 184 (155, 213) | 210 (190, 234) | 238 (218, 271) | <0.0001 |
| Fat, g/d | 78 (70, 89) | 76 (68, 92) | 80 (71, 87) | 79 (71, 89) | 0.90 |
| Protein, g/d | 79 (68, 90) | 79 (69, 88) | 79 (66, 90) | 76 (67, 90) | 0.83 |
| Food group intake, g/d | |||||
| Total sugar | 98 (80, 117) | 78 (64, 91) | 97 (88, 108) | 121 (103, 137) | <0.0001 |
| Added sugar | 48 (35, 63) | 29 (23, 35) | 48 (43, 52) | 69 (63, 79) | <0.0001 |
| Total sugar − added sugar | 49 (38, 61) | 49 (35, 61) | 49 (39, 61) | 49 (39, 63) | 0.97 |
| SSB | 4 (4, 10) | 4 (4, 5) | 4 (4, 6) | 9 (4, 27) | 0.0003 |
| Animal protein | 49 (41, 59) | 53 (42, 59) | 47 (39, 36) | 49 (43, 59) | 0.95 |
| Red meat | 43 (23, 64) | 48 (27, 67) | 40 (23, 66) | 42 (22, 65) | 0.79 |
| Fish/seafood | 33 (18, 49) | 36 (20, 49) | 32 (15, 55) | 30 (14, 42) | 0.40 |
| Poultry | 34 (21, 52) | 30 (18, 45) | 37 (22, 60) | 36 (21, 51) | 0.20 |
| Egg | 20 (13, 35) | 23 (16, 41) | 19 (12, 30) | 17 (11, 26) | 0.10 |
| Dairy | 58 (39, 77) | 44 (33, 67) | 55 (38, 78) | 67 (55, 81) | 0.002 |
| Corn products | 2 (0, 14) | 1 (0, 13) | 1 (0, 12) | 6 (0, 19) | 0.21 |
Data are presented as median (IQR) unless otherwise noted. MET-h, Metabolic Equivalent Task hour; NPAAS-FS, Nutrition and Physical Activity Assessment Study Feeding Study (n = 145); SSB, sugar-sweetened beverage.
Differences across tertiles of added sugar intake were assessed with either a χ2 square test (race, current smokers) or a Kruskal–Wallis test (all other variables). P < 0.05 was considered significant.
Race included 95% white and 5% nonwhite/race unknown (3 African American, 1 American Indian/Alaska Native, 1 Asian/Pacific Islander, and 2 race unknown).
TABLE 2.
NPAAS-FS whole-serum CIR and serum amino acid CIRs in the complete study sample and by tertile of added sugar intake1
| Added sugar intake, g/d | |||||
|---|---|---|---|---|---|
| Characteristics | Complete sample | Tertile 1 (<0.38.7) | Tertile 2 (38.7–56.4) | Tertile 3 (>56.4) | P 2 |
| CIR of whole serum, ‰3 | −19.9 (−20.4, −19.6) | −20.0 (−20.3, −19.6) | −20.0 (−20.5, −19.6) | −19.9 (−20.3, −19.6) | 0.60 |
| CIRs of serum NEAAs, ‰ | |||||
| Alanine | −20.8 (−21.8, −19.8) | −21.3 (−22.1, −20.4) | −20.7 (−21.8, −19.8) | −20.2 (−21.1, −19.4) | 0.0004 |
| Glycine | −9.4 (−11.2, −7.3) | −9.1 (−10.4, −6.4) | −9.3 (−11.3, −7.4) | −10.2 (−11.6, −7.9) | 0.07 |
| Proline | −17.0 (−17.7, −16.3) | −16.9 (−17.5, −16.5) | −17.0 (−17.7, −16.3) | −17.1 (−17.7, −16.3) | 0.93 |
| CIRs of serum EAAs, ‰ | |||||
| Isoleucine | −21.2 (−21.9, −20.6) | −21.1 (−21.9, −20.3) | −21.2 (−21.7, −20.5) | −21.3 (−22.1, −20.7) | 0.74 |
| Leucine | −26.2 (−26.7, −25.7) | −26.2 (−26.7, −25.5) | −26.2 (−27.0, −25.7) | −26.3 (−26.8, −25.7) | 0.57 |
| Phenylalanine | −25.5 (−26.0, −25.1) | −25.5 (−26.0, −25.1) | −25.5 (−26.0, −25.2) | −25.6 (−26.0, −25.1) | 0.85 |
| Valine | −23.9 (−24.5, −23.1) | −23.9 (−24.5, −23.0) | −23.7 (−24.6, −23.0) | −24.1 (−24.4, −23.3) | 0.76 |
Data are presented as median (IQR). CIR, carbon isotope ratio; EAA, essential amino acid; NEAA, nonessential amino acid; NPAAS-FS, Nutrition and Physical Activity Assessment Study Feeding Study (n = 145).
Differences across tertiles of added sugar intake were assessed with a Kruskall–Wallis test; P < 0.05 was considered significant.
CIR is expressed as δ13C values; δ13C = (Rsample/Rstandard − 1) × 1000‰, where R = 13C/12C and the standard is Vienna Pee Dee Belemnite (V-PDB).
Associations of feeding study dietary intakes with CIR-AAs are presented in Table 3. Among the NEAAs, CIR-Ala was significantly associated with intakes of AS (ρ = 0.32; P < 0.0001), dairy (ρ = 0.26; P = 0.002), and animal protein (ρ = 0.23; P = 0.0049). CIR-Gly was inversely associated with total sugar intake (ρ = −0.24; P = 0.004), and had a nonsignificant inverse association with added sugar intake (ρ = −0.22; P = 0.009) and a nonsignificant association with animal protein intake (ρ = 0.23; P = 0.006) using a Bonferroni-corrected α of 0.005. CIR-Pro was associated with animal protein intake (ρ = 0.31; P = 0.0002). The CIRs of all 4 EAAs (Val, Ile, Leu, Phe) were associated with red meat intake, with associations ranging from ρ = 0.26 to 0.33 (all P < 0.0003). Three CIR-EAAs were also associated with animal protein intake (Val, Leu, Phe), with associations ranging from ρ = 0.31 to 0.39 (all P < 0.0001). None of the CIR-EAAs were associated with total sugar or AS intake, and no CIR-AAs were associated with SSBs, poultry, or corn product intake. With the exception of CIR-Ala, associations of sugars with CIR-AAs were inverse, whereas associations of animal-based foods with CIR-AAs were positive.
TABLE 3.
Associations of serum amino acid CIRs with consumed food groups in the NPAAS-FS1
| Nonessential amino acids | Essential amino acids | ||||||
|---|---|---|---|---|---|---|---|
| Food group, g/d | Ala | Gly | Pro | Ile | Leu | Phe | Val |
| Total sugar | 0.17 (0.00, 0.32) | −0.24*(−0.39, −0.08) | −0.03 (−0.19, 0.13) | −0.16 (−0.31, 0.00) | −0.07 (−0.23, 0.10) | −0.06 (−0.22, 0.10) | −0.12 (−0.28, 0.04) |
| Added sugar | 0.32* (0.17, 0.46) | −0.22 (−0.37, −0.06) | −0.04 (−0.20, 0.13) | −0.09 (−0.25, 0.08) | −0.07 (−0.23, 0.10) | −0.06 (−0.22, 0.11) | −0.06 (−0.22, 0.11) |
| Total sugar − added sugar | −0.09 (−0.25, 0.08) | −0.11 (−0.27, 0.06) | 0.01 (−0.15, 0.18) | −0.12 (−0.28,0.04) | −0.01 (−0.17, 0.15) | −0.01 (−0.17, 0.16) | −0.11 (−0.26, 0.06) |
| SSB | 0.16 (0.00, 0.32) | −0.18 (−0.33, −0.01) | 0.03 (−0.14, 0.19) | −0.07 (−0.23, 0.09) | −0.07 (−0.23, 0.10) | −0.09 (−0.25, 0.08) | −0.12 (−0.28, 0.04) |
| Animal protein | 0.23* (0.07, 0.38) | 0.23 (0.07, 0.38) | 0.31* (0.15, 0.45) | 0.14 (−0.02, 0.30) | 0.33* (0.18, 0.47) | 0.31* (0.16, 0.45) | 0.39* (0.24, 0.52) |
| Red meat | 0.17 (0.01, 0.32) | 0.15 (−0.01, 0.31) | 0.12 (−0.05, 0.28) | 0.30* (0.14, 0.44) | 0.30* (0.14, 0.44) | 0.33* (0.17, 0.46) | 0.26* (0.10, 0.41) |
| Fish/seafood | 0.01 (−0.16, 0.17) | 0.11 (−0.06, 0.27) | 0.14 (−0.02, 0.30) | 0.06 (−0.11, 0.22) | 0.16 (0.00, 0.31) | 0.06 (−0.11, 0.22) | 0.20 (0.04, 0.35) |
| Poultry | 0.07 (−0.09, 0.23) | 0.04 (−0.12, 0.21) | 0.03 (−0.14, 0.19) | −0.07 (−0.23, −0.10) | 0.02 (−0.14, 0.19) | 0.00 (−0.16, 0.16) | 0.01 (−0.15, 0.18) |
| Eggs | 0.12 (−0.05, 0.27) | 0.11 (−0.06, 0.27) | 0.11 (−0.05, 0.27) | 0.11 (−0.05, 0.27) | 0.17 (0.00, 0.32) | 0.18 (0.01, 0.33) | 0.18 (0.02, 0.33) |
| Dairy | 0.26* (0.10, 0.40) | 0.05 (−0.12, 0.21) | 0.19 (0.03, 0.34) | −0.06 (−0.22, 0.11) | 0.08 (−0.08, 0.24) | 0.09 (−0.08, 0.25) | 0.15 (−0.01, 0.31) |
| Corn products | 0.04 (−0.12, 0.20) | −0.15 (−0.30, 0.02) | 0.04 (−0.12, 0.20) | −0.05 (−0.21, 0.11) | −0.09 (−0.25, 0.07) | −0.04 (−0.20, 0.13) | −0.11 (−0.27, 0.05) |
Data are presented as Spearman ρ, 95% CI (Fisher z-transformed). *Significant using a Bonferroni-corrected α of 0.0045 (α = 0.05 corrected for 11 comparisons). CIR, carbon isotope ratio; NPAAS-FS, Nutrition and Physical Activity Assessment Study Feeding Study (n = 145); SSB, sugar-sweetened beverage.
A biomarker model of AS intake based on CIR-AA and participant characteristics is presented in Table 4. The initial model included 3 CIR-AAs (Ala, Gly, and Ile) and achieved R2 = 0.32, which improved to R2 = 0.37 with the inclusion of body weight, LPA, and current smoking.
TABLE 4.
Biomarker model of added sugar intake based on serum amino acid CIRs and participant characteristics in the NPAAS-FS1
| Model terms | β ± SE | P | R 2 |
|---|---|---|---|
| Intercept | 134 ± 32 | <0.0001 | 0.37 |
| CIR-Ala | 8.78 ± 1.22 | <0.0001 | |
| CIR-Gly | −3.11 ± 0.58 | <0.0001 | |
| CIR-Ile | −3.19 ± 1.40 | 0.02 | |
| Smoking | 21.17 ± 9.13 | 0.02 | |
| Body weight | 0.20 ± 0.11 | 0.09 | |
| LPA | −0.16 ± 0.09 | 0.09 |
Model includes amino acid CIR and participant characteristics that remained significant on the basis of a backward-selection process (P = 0.1). CIR, carbon isotope ratio; LPA, leisure physical activity; NPAAS-FS, Nutrition and Physical Activity Assessment Study Feeding Study (n = 145).
Similarly to AS intake, biomarker-estimated AS intake was associated with intakes of total sugar, SSBs, and dairy (Table 5) in addition to carbohydrate (ρ = 0.33 (0.18, 0.47), P < 0.0001). Biomarker-estimated AS intake was not associated with any other dietary factors, including animal protein, red meat, and eggs (Table 5), the factors associated with whole serum δ13C values in the NPAAS-FS. In addition, there was no association of biomarker-estimated AS intake with intake of corn products.
TABLE 5.
Associations of biomarker-estimated added sugar intake with consumed food groups in the NPAAS-FS1
| Dietary intake | ρ (95% CI) | P |
|---|---|---|
| Total sugar | 0.45 (0.31, 0.57) | <0.0001* |
| Added sugar | 0.59 (0.47, 0.68) | <0.0001* |
| Total sugar − added sugar | 0.02 (−0.14, 0.18) | 0.81 |
| SSB | 0.32 (0.17, 0.46) | <0.0001* |
| Animal protein | 0.01 (−0.15, 0.18) | 0.87 |
| Red meat | −0.05 (−0.21, 0.12) | 0.59 |
| Fish/seafood | −0.12 (−0.27, 0.05) | 0.16 |
| Poultry | 0.10 (−0.07, 0.25) | 0.25 |
| Egg | −0.04 (−0.20, 0.13) | 0.67 |
| Dairy | 0.27 (0.11, 0.41) | 0.0011* |
| Corn products | 0.13 (−0.04, 0.28) | 0.13 |
Associations are presented as Spearman's ρ, 95% CI (Fisher's z transformed). *Significant using a Bonferroni-corrected α of 0.0045 (α = 0.05 corrected for 11 comparisons). NPAAS-FS: Nutrition and Physical Activity Assessment Study Feeding Study (n = 145); SSB, sugar-sweetened beverage.
Discussion
This study examined dietary associations with the CIRs of individual serum AAs in 145 mostly white, postmenopausal women participating in the NPAAS-FS and estimated AS intake using CIR-AAs and participant characteristics. CIR-Ala was significantly positively associated with AS intake, whereas other CIR-AAs tended to have inverse associations with intakes of AS, total sugar, and SSBs. CIR-AAs tended to have positive associations with intakes of animal protein and animal-derived foods, especially red meat. A model of AS intake based on CIR-Ala, CIR-Gly, CIR-Ile, and participant characteristics met the criterion for an acceptable biomarker used by the NPAAS-FS (R2 = 0.36), which was chosen based on the performance of well-established recovery biomarkers for protein and energy in the NPAAS-FS (R2 = 0.43 and 0.53, respectively) (34). Importantly, biomarker-estimated AS intake was not associated with animal protein or red meat intake, the primary determinants of whole serum CIR in the NPAAS-FS (26). These findings suggest that CIR-AAs have promise as improved and more specific isotopic biomarkers for AS intake.
There are multiple dimensions to the validation of dietary intake biomarkers, as recently outlined by Dragsted and colleagues (41). Among these, “plausibility” requires a biological explanation for why the biomarker reflects intake of a specific food or nutrient (26, 28). Our data suggest that AA CIRs can differentiate AS intake from animal protein/red meat intake because they have differing patterns of dietary association, with the CIR of Ala reflecting primarily AS intake, whereas the CIRs of other AAs, particularly EAAs, reflect primarily animal protein/meat intake. The observation that CIR-Ala reflects dietary AS to a greater extent than dietary animal proteins suggests significant synthesis of Ala from glucose, the CIR of which reflects dietary added sugars (42). This is consistent with the activity of the glucose-Ala cycle in humans (29–31), which has been estimated to account for 40% of the blood Ala pool, and studies showing near complete Ala synthesis from sugars in some insect taxa (43, 44). The biomarker of AS intake also included CIR-Gly, which was inversely associated with AS and associated with animal protein, and CIR-Ile, which was associated with red meat intake. Finally, certain participant characteristics contributed to biomarker-estimated AS intake, including body weight, LPA, and smoking status, although we note that very few individuals in the study were current smokers (n = 3; 2%). It is plausible that body weight and LPA could alter the relation between consumed AS and CIR-AAs given their relation with energy metabolism; in particular, the metabolism of carbohydrate during exercise and mobilization of AAs for gluconeogenesis (45, 46), but further study is needed.
Another factor contributing to the validation of dietary biomarkers is “robustness,” or whether the biomarker performs similarly in the context of different diets and different study settings (41). CIR-AAs (NEAAs only) were previously evaluated as biomarkers of sugars intake in a cross-sectional study of 68 Alaska Native people, for whom diet was assessed using multiple (4) 24-h recalls (28). In the Alaska Native study, RBC CIR-Ala was significantly associated with SSBs, AS, and total sugars intake (Pearson r = 0.70, 0.59, and 0.57, respectively) (28). These stronger associations likely reflect the higher intakes of SSBs (1.4 servings/d) and AS (74 g/d) in the Alaska Native cohort relative to the NPAAS-FS, and the higher proportion of total sugars deriving from AS (83%), because naturally occurring sugars in fruit, dairy, and other foods (“nonadded” sugars) do not have elevated CIRs. Although the biomarker approach was not identical in the 2 studies, in part because the Alaska Native study did not report dietary associations of EAAs, these studies demonstrate the potential for biomarkers based on CIR-AAs to capture usual AS/SSB intake in widely different study populations and dietary contexts. The present study was the first evaluation of a CIR-AA biomarker of AS intake in a population with relatively low AS intake, for which there was no association of AS intake with the whole-sample (serum) CIR (26).
Dietary associations of the CIR-EAAs were similar to those of the whole-serum CIR in the NPAAS-FS (26): red meat intake was associated with all CIR-EAAs (Ile, Leu, Phe, Val), whereas animal protein intake was associated with the CIR of both EAAs (Val, Leu, and Phe) and NEAAs (Ala and Pro). Similar to our findings for the whole-serum CIR, poultry was not associated with any of the CIR-AAs, despite having a similar CIR to red meat in some studies (23, 24, 47). Intake of poultry was lower and less variable than that of red meat in the NPAAS-FS, which could attenuate biomarker associations. Alternatively, poultry sold in the Seattle area might have a lower CIR than red meat, as has been reported elsewhere (22, 48). Analysis of CIR-AAs by GC-CIRMS did not offer a significant advantage over whole-serum CIR for evaluating animal protein or red meat intake in the NPAAS-FS study, because association strengths were of similar magnitude (Pearson correlation coefficients = 0.3–0.4) (26). Similar to the whole-serum CIR, there was no association of CIR-AA with corn product intake, although corn product intake was very low in the NPAAS-FS.
It has been proposed that 24-h urinary sucrose and fructose (uSF) could also be objective biomarkers of sugars intake (49–51), as could sucrose and fructose in spot urine samples (52, 53). The CIR and uSF offer different strengths and limitations as biomarkers of sugars intake; for example, uSF reflects daily intake and would be responsive to recent changes in intake, whereas blood CIR (and we assume, blood AA CIRs) reflects intake over weeks to months (27), potentially better capturing usual intake. Urine is less invasive to collect than blood but potentially more burdensome for participants, particularly when a 24-h collection is desired. A potential avenue of future investigation would be to examine how the biomarkers perform in combination, because they would be expected to have independent errors. There is no reason to expect the CIR of blood metabolites to affect the rate of sucrose and fructose excretion, or vice versa. Thus, it could be valuable to explore the efficacy of these biomarkers in combination.
Strengths of this study include the study design of the NPAAS-FS, which allowed biomarker relations to be evaluated in the context of the typical habitual diets of the study population while offering the dietary control of a controlled feeding study. Molecular stable isotope ratios as measured by GC-CIRMS are a novel type of dietary biomarker, and to our knowledge, this study is the first to evaluate a model of dietary intake based on molecular stable isotope ratios in a controlled feeding study. A limitation of this study is that the study population of postmenopausal women consumed relatively low AS and very low SSBs relative to the general US population, potentially underestimating the diet-biomarker relations in other US populations. Additionally, the sample was not diverse, being predominantly white and highly educated. Next steps will include evaluation of CIR-AAs in populations with broader intake distributions and greater demographic diversity, to examine generalizability. The time to 50% turnover of CIR in plasma is estimated to be ∼2.5 wk (38); therefore, the 2-wk period of dietary control was likely short relative to the residence time of serum CIR-Ala. This could have attenuated diet-biomarker relations, although participants who exhibited isotopic change over the feeding period were excluded as described in the Methods. The dietary database used is incomplete for certain foods, because exact ingredients and compositions are proprietary. This could affect the reliability of consumed intake estimates in the NPAAS-FS. Finally, a general limitation of all CIR-based biomarkers of AS intake is that the CIR is elevated in corn and sugar cane but not with other sources of AS, including beet sugar, honey, and maple syrup. Corn and sugar cane are “C4 plants,” and share photosynthetic adaptations that alter their CIR relative to other plants consumed as food (35). Although beet sugar, honey, and maple syrup contribute much less to total US AS intake than corn syrup and cane sugar in combination (19, 21), their consumption will cause some error in biomarker estimates.
In summary, this study demonstrated that serum CIR-AAs and participant characteristics correlated well with AS intake in a controlled feeding study of US postmenopausal women, the NPAAS-FS. The model estimated AS intake to the R2 criterion set by the performance of well-established recovery biomarkers of energy and protein intake in the NPAAS-FS, despite low levels of AS intake relative to other US populations. This was the first evaluation of a CIR-AA biomarker of AS intake in the context of a controlled feeding study and a more mainstream US diet than the Alaska Native population in which it was first proposed and reported (28). Unlike the whole-serum CIR, biomarker-estimated AS intake using CIR-AAs was not associated with red meat or animal protein intake in the NPAAS-FS (26), indicating biomarker specificity. Further evaluations are needed in US populations, including those with greater variability in AS intake and in more diverse populations. These findings highlight the potential for the CIR of serum AAs to provide an objective biomarker for AS intake in US populations, and ultimately help to clarify associations with chronic disease risk.
Supplementary Material
Acknowledgments
We gratefully acknowledge Tim Howe at the Alaska Stable Isotope Facility for laboratory assistance, and Megan Skinner Herndon at the Fred Hutchinson Cancer Research Center for data coordination.
The authors’ responsibilities were as follows—JWL, MLN, DMO'B, RLP, LFT: designed the research; JWL, MLN, DMO'B, RLP, LFT, HYY: conducted the research; DMO'B, HYY: analyzed the specimens and conducted data analyses; DMO'B, HYY: wrote the paper; CBE, JWL, YM-R, MLN, RLP, DAS, LGS, LFT, LVVH: provided critical review; DMO'B: had primary responsibility for final content; and all authors: read and approved the final manuscript.
Notes
This project was supported by the National Cancer Institute grants R01CA119171 (RLP, currently MLN and JWL) and R21CA182674 (DMO'B), National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grant 1R01DK109946 (DMO'B), and National Institute of General Medical Sciences (NIGMS) grant 2P20GM103395. The Women's Health Initiative (WHI) is supported by the National Heart, Lung, and Blood Institute, NIH, and US Department of Health and Human Services through contracts HHSN268201600046C (Fred Hutchinson Cancer Research Center), HHSN268201600001C (State University of New York, Buffalo), HHSN268201600002C (The Ohio State University), HHSN268201600003C (Stanford University), HHSN268201600004C (Wake Forest University), and HHSN271201600004C (WHI Memory Study) and grants P30 CA015704 and P30 CA023074. The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the NCI, the NIDDK, or the NIH.
Author disclosures: The authors report no conflicts of interest.
Supplemental Table 1 and Supplemental Figure 1 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn/.
Abbreviations used: AA, amino acid; AS, added sugar; CIR, carbon isotope ratio; EAA, essential amino acid; HNL, Human Nutrition Laboratory; LPA, leisure physical activity; MCME, methoxycarbonyl methyl ester; Nle, norleucine; NDS-R, Nutrition Data System for Research; NEAA, nonessential amino acid; NIR, nitrogen isotope ratio; NPAAS-FS, Nutrition and Physical Activity Assessment Study Feeding Study; SIR, sulfur isotope ratio; SSB, sugar-sweetened beverage; uSF, urinary sucrose and fructose; WHI, Women's Health Initiative.
Contributor Information
Hee Young Yun, Center for Alaska Native Health Research, Institute of Arctic Biology, Department of Biology and Wildlife, University of Alaska Fairbanks, Fairbanks, AK, USA.
Lesley F Tinker, Division of Public Health Sciences, Fred Hutchinson Cancer Research Center, Seattle, WA, USA.
Marian L Neuhouser, Division of Public Health Sciences, Fred Hutchinson Cancer Research Center, Seattle, WA, USA.
Dale A Schoeller, Department of Nutritional Sciences, University of Wisconsin, Madison, WI, USA.
Yasmin Mossavar-Rahmani, Department of Epidemiology and Population Health, Albert Einstein College of Medicine, Bronx, New York, NY, USA.
Linda G Snetselaar, Department of Epidemiology, University of Iowa, Iowa City, IA, USA.
Linda V Van Horn, Department of Preventive Medicine, Northwestern University, Chicago, IL, USA.
Charles B Eaton, Department of Family Medicine, Alpert Medical School, Department of Epidemiology, School of Public Health, Brown University, Providence, RI, USA.
Ross L Prentice, Division of Public Health Sciences, Fred Hutchinson Cancer Research Center, Seattle, WA, USA.
Johanna W Lampe, Division of Public Health Sciences, Fred Hutchinson Cancer Research Center, Seattle, WA, USA.
Diane M O'Brien, Center for Alaska Native Health Research, Institute of Arctic Biology, Department of Biology and Wildlife, University of Alaska Fairbanks, Fairbanks, AK, USA.
References
- 1. Neuenschwander M, Ballon A, Weber KS, Norat T, Aune D, Schwingshackl L, Schlesinger S. Role of diet in type 2 diabetes incidence: umbrella review of meta-analyses of prospective observational studies. BMJ. 2019;366:l2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schlesinger S, Neuenschwander M, Schwedhelm C, Hoffmann G, Bechthold A, Boeing H, Schwingshackl L. Food groups and risk of overweight, obesity, and weight gain: a systematic review and dose-response meta-analysis of prospective studies. Adv Nutr. 2019;10:205–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Imamura F, O'Connor L, Ye Z, Mursu J, Hayashino Y, Bhupathiraju SN, Forouhi NG. Consumption of sugar sweetened beverages, artificially sweetened beverages, and fruit juice and incidence of type 2 diabetes: systematic review, meta-analysis, and estimation of population attributable fraction. Br J Sports Med. 2016;50:496–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schwingshackl L, Hoffmann G, Lampousi AM, Knuppel S, Iqbal K, Schwedhelm C, Bechthold A, Schlesinger S, Boeing H. Food groups and risk of type 2 diabetes mellitus: a systematic review and meta-analysis of prospective studies. Eur J Epidemiol. 2017;32:363–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yang Q, Zhang Z, Gregg EW, Flanders WD, Merritt R, Hu FB. Added sugar intake and cardiovascular diseases mortality among US adults. JAMA Intern Med. 2014;174:516–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bechthold A, Boeing H, Schwedhelm C, Hoffmann G, Knuppel S, Iqbal K, De Henauw S, Michels N, Devleesschauwer B, Schlesinger S et al. . Food groups and risk of coronary heart disease, stroke and heart failure: a systematic review and dose-response meta-analysis of prospective studies. Crit Rev Food Sci Nutr. 2019;59:1071–90. [DOI] [PubMed] [Google Scholar]
- 7. Malik VS, Li Y, Pan A, De Koning L, Schernhammer E, Willett WC, Hu FB. Long-term consumption of sugar-sweetened and artificially sweetened beverages and risk of mortality in US adults. Circulation. 2019;139:2113–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tasevska N, Jiao L, Cross AJ, Kipnis V, Subar AF, Hollenbeck A, Schatzkin A, Potischman N. Sugars in diet and risk of cancer in the NIH-AARP Diet and Health Study. Int J Cancer. 2012;130:159–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Qin B, Moorman PG, Alberg AJ, Barnholtz-Sloan JS, Bondy M, Cote ML, Funkhouser E, Peters ES, Schwartz AG, Terry P et al. . Dietary carbohydrate intake, glycaemic load, glycaemic index and ovarian cancer risk in African-American women. Br J Nutr. 2016;115:694–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Thompson FE, Subar AF. Dietary assessment methodology. In: Coulston AM, Boushey CJeditors. Nutrition in the prevention and treatment of disease. 2nd ed San Diego (CA): Academic Press;2008; pp. 3–39. [Google Scholar]
- 11. Dhurandhar NV, Schoeller D, Brown AW, Heymsfield SB, Thomas D, Sorensen TI, Speakman JR, Jeansonne M, Allison DB, Energy Balance Measurement Working Group . Energy balance measurement: when something is not better than nothing. Int J Obes. 2015;39:1109–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Subar AF, Freedman LS, Tooze JA, Kirkpatrick SI, Boushey C, Neuhouser ML, Thompson FE, Potischman N, Guenther PM, Tarasuk V. Addressing current criticism regarding the value of self-report dietary data. J Nutr. 2015;145:2639–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kirkpatrick SI, Collins CE, Keogh RH, Krebs-Smith SM, Neuhouser ML, Wallace A. Assessing dietary outcomes in intervention studies: pitfalls, strategies, and research needs. Nutrients. 2018;10:1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Prentice RL, Pettinger M, Tinker LF, Huang Y, Thomson CA, Johnson KC, Beasley J, Anderson G, Shikany JM, Chlebowski RT et al. . Regression calibration in nutritional epidemiology: example of fat density and total energy in relationship to postmenopausal breast cancer. Am J Epidemiol. 2013;178:1663–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tinker LF, Sarto GE, Howard BV, Huang Y, Neuhouser ML, Mossavar-Rahmani Y, Beasley JM, Margolis KL, Eaton CB, Phillips LS et al. . Biomarker-calibrated dietary energy and protein intake associations with diabetes risk among postmenopausal women from the Women's Health Initiative. Am J Clin Nutr. 2011;94:1600–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Beasley JM, LaCroix AZ, Larson JC, Huang Y, Neuhouser ML, Tinker LF, Jackson R, Snetselaar L, Johnson KC, Eaton CB et al. . Biomarker-calibrated protein intake and bone health in the Women's Health Initiative clinical trials and observational study. Am J Clin Nutr. 2014;99:934–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Prentice RL, Huang Y, Neuhouser ML, Manson JE, Mossavar-Rahmani Y, Thomas F, Tinker LF, Allison M, Johnson KC, Wassertheil-Smoller S et al. . Associations of biomarker-calibrated sodium and potassium intakes with cardiovascular disease risk among postmenopausal women. Am J Epidemiol. 2017;186:1035–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zheng C, Beresford SA, Van Horn L, Tinker LF, Thomson CA, Neuhouser ML, Di C, Manson JE, Mossavar-Rahmani Y, Seguin R. Simultaneous association of total energy consumption and activity-related energy expenditure with risks of cardiovascular disease, cancer, and diabetes among postmenopausal women. Am J Epidemiol. 2014;180:526–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Haley S. Sugar and sweeteners outlook: January 2013 [Internet]. USDA. 2013. Available from: https://www.ers.usda.gov/publications/pub-details/?pubid=39359. [Google Scholar]
- 20. Jahren AH, Bostic JN, Davy BM. The potential for a carbon stable isotope biomarker of dietary sugar intake. J Anal At Spectrom. 2014;29:795–816. [Google Scholar]
- 21. USDA Economic Research Service. Sugar and sweeteners yearbook tables. [Internet] 2019; [cited Nov 2019]. Available from: https://www.ers.usda.gov/data-products/sugar-and-sweeteners-yearbook-tables/. [Google Scholar]
- 22. Schoeller DA, Minagawa M, Slater R, Kaplan IR. Stable isotopes of carbon, nitrogen and hydrogen in the contemporary North American human food web. Ecol Food Nutr. 1986;18:159–70. [Google Scholar]
- 23. Jahren AH, Kraft RA. Carbon and nitrogen stable isotopes in fast food: signatures of corn and confinement. Proc Natl Acad Sci. 2008;105:17855–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nash SH, Bersamin A, Kristal AR, Hopkins SE, Church RS, Pasker RL, Luick BR, Mohatt GV, Boyer BB, O'Brien DM. Stable nitrogen and carbon isotope ratios indicate traditional and market food intake in an indigenous circumpolar population. J Nutr. 2012;142:84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nash SH, Kristal AR, Bersamin A, Hopkins SE, Boyer BB, O'Brien DM. Carbon and nitrogen stable isotope ratios predict intake of sweeteners in a Yup'ik study population. J Nutr. 2013;143:161–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yun HY, Lampe JW, Tinker LF, Neuhouser ML, Beresford SAA, Niles KR, Mossavar-Rahmani Y, Snetselaar LG, Van Horn L, Prentice RL et al. . Serum nitrogen and carbon stable isotope ratios meet biomarker criteria for fish and animal protein intake in a controlled feeding study of a Women's Health Initiative cohort. J Nutr. 2018;148:1931–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Votruba SB, Shaw PA, Oh EJ, Venti CA, Bonfiglio S, Krakoff J, O'Brien DM. Associations of plasma, red blood cell and hair carbon and nitrogen isotope ratios with fish, meat and SSB intake in a 12-week inpatient feeding study. Am J Clin Nutr. 2019;110:1306–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Choy K, Nash SH, Kristal AR, Hopkins SE, Boyer BB, O'Brien DM. The carbon isotope ratio of alanine in red blood cells is a new candidate biomarker of sugar-sweetened beverage intake. J Nutr. 2013;143:878–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Waterhouse C, Keilson J. The contribution of glucose to alanine metabolism in man. J Lab Clin Med. 1978;92:803–12. [PubMed] [Google Scholar]
- 30. Felig P. The glucose-alanine cycle. Metabolism. 1973;22:179–207. [DOI] [PubMed] [Google Scholar]
- 31. Perriello G, Jorde R, Nurjhan N, Stumvoll M, Dailey G, Jenssen T, Bier DM, Gerich JE. Estimation of glucose-alanine-lactate-glutamine cycles in postabsorptive humans: role of skeletal muscle. Am J Physiol. 1995;269:E443–50. [DOI] [PubMed] [Google Scholar]
- 32. Corr LT, Berstan R, Evershed RP. Optimisation of derivatisation procedures for the determination of delta C-13 values of amino acids by gas chromatography/combustion/isotope ratio mass spectrometry. Rapid Commun Mass Spectrom. 2007;21:3759–71. [DOI] [PubMed] [Google Scholar]
- 33. Walsh RG, He SN, Yarnes CT. Compound-specific delta C-13 and delta N-15 analysis of amino acids: a rapid, chloroformate-based method for ecological studies. Rapid Commun Mass Spectrom. 2014;28:96–108. [DOI] [PubMed] [Google Scholar]
- 34. Lampe JW, Huang Y, Neuhouser ML, Tinker LF, Song X, Schoeller DA, Kim S, Raftery D, Di C, Zheng C et al. . Dietary biomarker evaluation in a controlled feeding study in women from the Women's Health Initiative cohort. Am J Clin Nutr. 2017;105:466–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. O'Brien DM. Stable isotope ratios as biomarkers of diet for health research. Annu Rev Nutr. 2015;35:565–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Silfer JA, Engel MH, Macko SA, Jumeau EJ. Stable carbon isotope analysis of amino-acid enantiomers by conventional isotope ratio mass-spectrometry and combined gas-chromatography isotope ratio mass-spectrometry. Anal Chem. 1991;63:370–4. [Google Scholar]
- 37. O'Brien DM, Fogel ML, Boggs CL. Renewable and nonrenewable resources: amino acid turnover and allocation to reproduction in Lepidoptera. Proc Natl Acad SciU S A 2002;99:4413–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Votruba SB, Shaw PA, Oh EJ, Venti CA, Bonfiglio S, Krakoff J, O'Brien DM. Associations of plasma, RBCs, and hair carbon and nitrogen isotope ratios with fish, meat, and sugar-sweetened beverage intake in a 12-wk inpatient feeding study. Am J Clin Nutr. 2019;110:1306–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6:65–70. [Google Scholar]
- 40. Song X, Huang Y, Neuhouser ML, Tinker LF, Vitolins MZ, Prentice RL, Lampe JW. Dietary long-chain fatty acids and carbohydrate biomarker evaluation in a controlled feeding study in participants from the Women's Health Initiative cohort. Am J Clin Nutr. 2017;105(6):1272–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dragsted LO, Gao Q, Scalbert A, Vergeres G, Kolehmainen M, Manach C, Brennan L, Afman LA, Wishart DS, Andres Lacueva C et al. . Validation of biomarkers of food intake-critical assessment of candidate biomarkers. Genes Nutr. 2018;13:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cook CM, Alvig AL, Liu YQ, Schoeller DA. The natural 13C abundance of plasma glucose is a useful biomarker of recent dietary caloric sweetener intake. J Nutr. 2010;140:333–7. [DOI] [PubMed] [Google Scholar]
- 43. O'Brien DM, Fogel ML, Boggs CL. Renewable and nonrenewable resources: amino acid turnover and allocation to reproduction in Lepidoptera. Proc Natl Acad Sci U S A. 2002;99:4413–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. O'Brien DM, Fogel M, Boggs CL. The amino acids used in reproduction by butterflies: a comparative study of dietary sources using compound specific stable isotope analysis. Physiol Biochem Zool. 2005;78:669–777. [DOI] [PubMed] [Google Scholar]
- 45. Mul JD, Stanford KI, Hirshman MF, Goodyear LJ. Exercise and regulation of carbohydrate metabolism. Prog Mol Biol Transl Sci. 2015;135:17–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hu C, Hoene M, Plomgaard P, Hansen JS, Zhao X, Li J, Wang X, Clemmesen JO, Secher NH, Häring HU et al. . Muscle-liver substrate fluxes in exercising humans and potential effects on hepatic metabolism. J Clin Endocrinol Metab. 2020;105:1196–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Nardoto GB, Silva S, Kendall C, Ehleringer JR, Chesson LA, Ferraz ESB, Moreira MZ, Ometto JPHB, Martinelli LA. Geographical patterns of human diet derived from stable-isotope analysis of fingernails. Am J Phys Anthropol. 2006;131:137–46. [DOI] [PubMed] [Google Scholar]
- 48. Wilkinson MJ, Yai Y, O'Brien DM. Age-related variation in red blood cell stable isotope ratios (δ13C and δ15N) from two Yupik villages in Southwest Alaska: a pilot study. Int J Circumpolar Health. 2007;66:31–41. [DOI] [PubMed] [Google Scholar]
- 49. Tasevska N. Urinary sugars—a biomarker of total sugars intake. Nutrients. 2015;7:5816–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tasevska N, Runswick SA, McTaggart A, Bingham SA. Urinary sucrose and fructose as biomarkers for sugar consumption. Cancer Epidemiol Biomarkers Prev. 2005;14:1287–94. [DOI] [PubMed] [Google Scholar]
- 51. Tasevska N, Runswick SA, Welch AA, McTaggart A, Bingham SA. Urinary sugars biomarker relates better to extrinsic than to intrinsic sugars intake in a metabolic study with volunteers consuming their normal diet. Eur J Clin Nutr. 2009;63:653–9. [DOI] [PubMed] [Google Scholar]
- 52. Bingham S, Luben R, Welch A, Tasevska N, Wareham N, Khaw KT. Epidemiologic assessment of sugars consumption using biomarkers: comparisons of obese and nonobese individuals in the European Prospective Investigation of Cancer Norfolk. Cancer Epidemiol Biomarkers Prev. 2007;16:1651–4. [DOI] [PubMed] [Google Scholar]
- 53. Kuhnle GG, Tasevska N, Lentjes MA, Griffin JL, Sims MA, Richardson L, Aspinall SM, Mulligan AA, Luben RN, Khaw KT. Association between sucrose intake and risk of overweight and obesity in a prospective sub-cohort of the European Prospective Investigation into Cancer in Norfolk (EPIC-Norfolk). Public Health Nutr. 2015;18:2815–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


