Abstract
With recent prospective clinical trials that used paediatric regimens with multiple doses of pegylated form of asparaginase (PEG asparaginase) in adults reporting significantly improved survival compared with historical data with regimens that used less asparaginase, PEG asparaginase is increasingly being used in the treatment of adult acute lymphoblastic leukaemia (ALL). However, administering asparaginase still comes with its challenges, especially in adult patients. Therefore, it is important to understand how to manage its toxicities properly. An expert group met in November 2019 in London to discuss recent data of paediatric as well as adult studies using paediatric regimens with regard to the best management of several key toxicities that can occur in adults treated with asparaginase including hepatotoxicity, pancreatitis, hypertriglyceridaemia, thrombosis and hypersensitivity. Several recommendations were made for each one of these toxicities, with the goal of safe administration of the drug and to educate clinicians when the drug can be continued despite side effects.
Keywords: asparaginase, acute lymphoblastic leukaemia, toxicities, hypersensitivity, therapeutic drug monitoring, pancreatitis, thrombosis
Video Abstract.
Introduction
Despite its use in the treatment of acute lymphoblastic leukaemia (ALL) for more than four decades, administering asparaginase still comes with its challenges, especially in adult patients.
There are several reasons for this. The drug has a unique toxicity profile, different from any other chemotherapy drug. Adult oncologists are less familiar with asparaginase and its side effects, as the drug is predominantly used in ALL which is rare in adults. The toxicities can be serious; however, early discontinuation or dosing changes of the drug are often not necessary and could result in losing the improved reported outcome from multiple doses of asparaginase.1 Several randomised trials in children have shown that patients who had received prolonged post remission asparaginase had a significantly better survival rate than patients without post remission asparaginase.2 3 Other paediatric trials have shown that longer duration of asparaginase administration resulted in more favourable outcomes than shorter duration.4
Currently, the long-acting pegylated form of asparaginase (PEG asparaginase) is used more often than the short-acting native Escherichia coli derived form. Due to their success in the treatment of childhood ALL, paediatric or paediatric-inspired regimens with multiple doses of PEG asparaginase are increasingly being used in the treatment of newly diagnosed adult ALL. Prospective clinical trials using paediatric or paediatric-inspired regimens have shown a higher cure rate among adults compared with historic regimens with much less asparaginase.5–11 The results are especially good in those who achieve an early minimal residual disease negative status.5 11
Of note, asparaginase is also particularly important in improving the outcome of childhood T-cell ALL.12 In adults, 25% of patients with ALL have the T-cell subtype and their outcome with regimens with no or few asparaginase doses (ie, the hyper-CVAD chemotherapy) is particularly poor.13 14 A recent study by the Nordic Society of Pediatric Hematology and Oncology (NOPHO) ALL Group confirmed the importance of asparaginase in T-ALL and the outcome in patients aged 18–45 years was excellent.15
Taken together, as PEG asparaginase is increasingly being used in the treatment of adult ALL including T-ALL, it is important to understand how to manage its toxicities in adults properly.16
An expert group met in November 2019 in London to discuss recent data of paediatric as well as adult studies using paediatric regimens with regard to the best management of several key toxicities that can occur in adults treated with asparaginase including hepatotoxicity, pancreatitis, hypertriglyceridaemia, thrombosis and hypersensitivity.
Hepatotoxicity
Hepatotoxicity is the most common adverse effect of PEG asparaginase therapy in adults, manifesting as hyperbilirubinaemia and/or transaminitis. High-grade (grade 3 or 4) hyperbilirubinaemia has been reported in 25%–40% of adults treated with paediatric-inspired regimens5 9 17–19 and transaminitis in >50%.5 9 20 The risk of asparaginase-induced high-grade hyperbilirubinaemia increases with age, higher doses and obesity.17 20 21 In a univariate analysis performed by the German Multicenter Study Group for Adult ALL (GMALL), age >45 years and dose above 1000 IU/m2 correlated with high-grade hyperbilirubinaemia.17 The Dana Farber Cancer Institute studied patients aged 18–50 years who initially used PEG asparaginase of 2500 IU/m2/dose every 2 weeks which resulted in excessive hepatotoxicity. After the protocol was amended and the dose was reduced to 2000 IU/m2/dose every 3 weeks, the rate of hyperbilirubinaemia was significantly lower.6
Doctors who use paediatric-inspired regimens that include multiple doses of PEG asparaginase have several management dilemmas when faced with hyperbilirubinaemia, and it is important to be familiar with its characteristics.18
First, the morbidity of asparaginase-related hepatotoxicity is generally low, transitory and without hepatic failure.9–18 22 23 The aetiology of asparaginase-related hepatotoxicity remains uncertain. Hepatic failure was reported in only 1% of patients in the adult Cancer and Leukaemia Group B (CALGB) 10403 study.22 The clinical impact of the high rate of asparaginase-related hepatotoxicity could be related to its long duration. High-grade hepatotoxicity may last for a month from onset to recovery and may cause delay of subsequent chemotherapy cycles.18
Second, high-grade hyperbilirubinaemia mostly occurs after the first asparaginase dose during induction and less often in subsequent cycles,5 6 18 24 that is, perhaps at a time when patients are likely to have leukaemic infiltration of the liver.
Third, when patients did not discontinue asparaginase, nor underwent dose reduction (due to hepatotoxicity), and then were re-challenged with the drug, high-grade hepatotoxicity generally did not recur.5 6 18 24
Administering multiple successive asparaginase doses to adults despite prior hepatotoxicity is important since dose omission and/or lower total asparaginase dosing may jeopardise outcomes.1–3
Asparaginase, a key drug in all paediatric regimens, is not myelosuppressive. In fact, paediatric regimens typically use more non-myelosuppressive drugs such as vincristine and steroids; myelosuppressive drugs (such as daunorubicin) are minimised and are mostly used in induction. During induction it is important to synchronise the timing of the myelosuppressive drug with that of PEG asparaginase. One approach is to give daunorubicin only during the first 3 days of induction and to administer PEG asparaginase later, on days 16–17.9 23 24 By that time mid-induction (days 16–17), the severe neutropenia from daunorubicin myelosuppression sufficiently recovers, and PEG asparaginase-related high-grade hyperbilirubinaemia would not occur while the patients have a high risk for neutropenic sepsis.18 Two studies from the University of Southern California9 23 support this synchronisation approach, since the induction death among 76 patients was only 1%. In contrast, the UKALL14 study did not use this synchronisation strategy in a slightly older patient population19; PEG asparaginase was initially given on days 4 and 18 and daunorubicin weekly for 4 weeks throughout induction. The induction death rate among the first 91 patients was high (18%) due to neutropenic sepsis which occurred during asparaginase-related high-grade hepatotoxicity. The protocol was amended: PEG asparaginase was given only on day 18 and the dose of daunorubicin was reduced, resulting in a significantly lower (2.5%) induction death in the subsequent 244 patients.
Hepatic steatosis can be seen post-PEG asparaginase, often in patients with high-grade hyperbilirubinaemia. In one small study, all eight patients with high-grade hyperbilirubinaemia had hepatic steatosis. However, no evidence of liver failure was reported in patients with hepatic steatosis.18 25–28 Hepatic steatosis can be present before the first dose of PEG asparaginase but the practice of performing liver ultrasound is debatable and not widely applied. Others perform liver ultrasound and detected steatosis leads to a lower dose of PEG asparaginase.17 This is because steatosis was reported to be associated with a significant threefold bilirubin increase which delays treatment of asparaginase and the other chemotherapy regimen.17
Single case observations and small case-series reported that L-carnitine can result in rapid amelioration of PEG asparaginase-induced hyperbilirubinaemia.29 A prospective clinical trial (NCT03564678) is testing levocarnitine’s role.
Recommendation from the roundtable regarding the management of hepatoxicity
The recommended dose of PEG asparaginase is not finalised, although in adults it is not greater than 2000 IU/m2. Several studies use 2000 IU/m2 6 9 17 18 24 while others use 1000 IU/m2 with good results.17 30 The rarity of severe clinical liver disease and the reversibility of hepatotoxicity during induction therapy suggest continuation of PEG asparaginase therapy without dose modification when hyperbilirubinaemia and transaminitis occur. However, some investigators argue that after high-grade hyperbilirubinaemia its long duration can delay the next cycle of chemotherapy and thus recommend dose reduction in the subsequent dose to 1000 IU/m2 or 500 IU/m2 and try thereafter to give full doses. In patients with high risk of severe clinical liver toxicity including morbid obesity, that is, a body mass index (BMI) >30–35, age >45–50 years and a history of liver disease, the GMALL group reduces all doses of PEG asparaginase to 1000 or even 500 IU/m2.
The risk can be further reduced by administration of myelosuppressive drugs (such as daunorubicin) early in induction therapy and PEG asparaginase 2 weeks later.
Pancreatitis
Another common asparaginase-related adverse event is clinical pancreatitis which in adults should always lead to discontinuation of any form of asparaginase. It should be distinguished from chemical pancreatitis (high amylase and lipase without clinical symptoms of pancreatitis) in which asparaginase should be continued.
Although the pathophysiology remains elusive, clinical asparaginase-associated pancreatitis (AAP) occurs with an incidence of 2%–18% in adult patients with ALL treated with paediatric-inspired asparaginase regimens.5 9 10 16 18 Short-term acute complications of AAP include systemic inflammatory response syndrome (SIRS), hyperglycaemic, development of pseudocysts and pancreatic necrosis, while long-term complications include insulin-dependent diabetes and chronic pancreatitis, although the later weans off within 5–6 years after cessation of antileukaemic therapy.31 Several studies have reported that older age and higher cumulative asparaginase dose and duration32 correlate with higher incidences of pancreatitis, but these as well as host genome variants33 are too weak predictors to be applied for asparaginase treatment stratification.
Doctors should be aware and anticipate this side effect, as pancreatitis may easily be misinterpreted as other abdominal complications or sepsis. It is important to educate the patient to be aware of pancreatitis symptoms and alert their treating physicians at the onset of these symptoms. Diagnostic tests should include imaging as well as both lipase and amylase.31 Immediate supportive treatment includes no oral intake, intravenous hydration, antibiotics and analgesic medication, although it remains unknown if this prevents progression to SIRS, pseudocyst and haemorrhagic pancreatitis.
It is a general understanding that there is a higher risk of recurrent pancreatitis with re-exposure to asparaginase following an initial diagnosis of pancreatitis. Almost all re-exposures were reported in children with reoccurrence rates of 44%–63%.31 34 35 In childhood ALL, a study by Wolthers et al 31 reported that risk of second AAP is not more severe than the first AAP and is not associated with any other clinical parameters. Since a second AAP did not involve an increased risk of complications and since the second episode occurred after a median of several additional doses of PEG asparaginase, the authors carefully argued that asparaginase re-exposure should be determined mainly by the anticipated need for asparaginase for antileukaemic efficacy.31 However, in adults the approach still is to permanently discontinue asparaginase for symptomatic pancreatitis, because of the potential severe morbidity or even mortality if asparaginase is given again.16 Pancreatitis is usually the main reason for termination of asparaginase treatment in adults. However, in asymptomatic patients with elevated amylase or lipase (so-called ‘chemical pancreatitis’), asparaginase should be continued.16
Recommendation from the roundtable regarding the management of pancreatitis
Educate the patient to report any symptoms for an early diagnosis of pancreatitis and obtain imaging on suspicion of pancreatitis.
Immediately on the diagnosis of clinical pancreatitis start treatment with no oral intake, hydration as well as antibiotic and analgesic medication.
Asparaginase should be permanently discontinued in adult patients with symptomatic pancreatitis.
Hypertriglyceridaemia
True incidences of hypertriglyceridaemia in adults treated with paediatric-inspired asparaginase regimen are not exactly known, but several prospective studies of paediatric patients and young adults with ALL reported 1%–18% incidences of grade 3–4 hypertriglyceridaemia.5 9 10 16 18 In another study, grade 3–4 hypertriglyceridaemia was reported in 50% of the patients.20 In a study conducted at Memorial Sloan Kettering Cancer Center where triglyceride (TG) levels were measured twice a week for at least 2 weeks following each PEG asparaginase dose regardless of symptoms, grade 3–4 hypertriglyceridaemia rates of 59% were observed and in a multivariate analysis, older age and higher PEG asparaginase doses were associated with higher incidences of hypertriglyceridaemia.36 Although higher-grade hypertriglyceridaemia rarely leads to any clinically significant medical complications, TG can clog laboratory devices causing interference with the measurement of routine blood biochemical tests and therefore treatment delays.
Adjustments or discontinuation of asparaginase therapy are generally not necessary. Several treatment approaches have been reported for patients with hypertriglyceridaemia. Strong evidence for any specific option is lacking, and there is currently no standard treatment for hypertriglyceridaemia. However, monitoring strategy can be adopted, of checking a baseline TG level before starting asparaginase, especially for older adults, and starting a fibrate if TG levels reach ≥1000 mg/dL to prevent high TG interfering with laboratory testing.
Elevated lipids have been associated with risk of osteonecrosis in children,37 but similar studies are lacking for adults. Both hypertriglyceridaemia and pancreatitis occur with PEG asparaginase, however early discontinuation of asparaginase or overtreating hypertriglyceridaemia can be avoided since no correlation was found between the two toxicities.20 38–40
Recommendations from the roundtable regarding the management of hypertriglyceridaemia
Adjustments or discontinuation of asparaginase therapy in case of hypertriglyceridaemia are not necessary.
Several interventions, including fibrates, may reduce hypertriglyceridaemia, but data are lacking to support that this reduces the risk of thromboembolism (TE), osteonecrosis or pancreatitis.
Monitoring of lipids is recommended in a research setting and can be considered in clinical practice.
Thrombosis
With the recent increased use of asparaginase and intensified use of dexamethasone partly due to paediatric-inspired therapy among adults with ALL,10 TE has become a frequent and serious treatment-related toxicity challenging protocol adherence and cure rates.41 42 Most TE cases occur during asparaginase and corticosteroid therapy.43–47 Asparaginase has been associated with increased levels of procoagulant factors,48–52 while corticosteroids may inhibit fibrinolysis by increasing levels of factor VIII, von Willebrandt factor (vWF) and plasminogen activator inhibitor-1.48 53
The reported incidence of symptomatic TE in childhood ALL is about 5%, but increases with age41 42 45 49 54 55 already at the age of 10 years. When including asymptomatic cases, TE has been reported in up to 37%–73% of children.56 57 TE incidence is for adults 1.4%–2.2% at diagnosis43 58 and 5%–41% during chemotherapy.47 52 54 55 59–63
In the NOPHO ALL-2008 protocol (2008–2017) that recruited patients aged 1–45 years, the 2.5-year cumulative incidence of TE was 7.9%, being higher in patients aged 10–17 years as well as 18–45 years (15%–20%; p<0.0001). Adjusted HR (HRa) were associated with greater age (10.0–17.9 years: HRa 4.9, 95% CI 3.1 to 7.8, p<0.0001; 18.0–45.9 years: HRa 6.06, 95% CI 3.65 to 10.1, p<0.0001) and mediastinal mass at ALL diagnosis (HRa 2.1, 95% CI 1.0 to 4.3, p=0.04). Compared with younger patients, those aged 18.0–45.9 years had an increased hazard of pulmonary embolism, while those aged 10.0–17.9 years had an increased hazard of cerebral sinus venous TE. Five deaths were attributable to TE.10
Genome-wide association studies have so far not identified significant single nucleotide polymorphisms (SNPs) associated with TE in children. As an alternative, NOPHO explored the impact of SNPs that in the general population has been associated with TE. They interrogated four TEhigh risk SNPs (F5 rs6025, F11 rs2036914, FGG rs2066865, ABO rs8176719) among 1252 patients with ALL (aged 1–45 years). F11 rs2036914 was significantly associated with TE (HR 1.52, 95% CI 1.11 to 2.07), while FGG rs2066865 was borderline significantly associated (HR 1.37, 95% CI 0.99 to 1.91). A genetic risk score based on F11 rs2036914 and FGG rs2066865 was associated with TE (HR 1.45 per risk allele, 95% CI 1.15 to 1.81). Importantly, the association was significant for adolescents aged 10.0–17.9 years (HR 1.64), whereas the risk for TE was high in adults aged 18–45 years independently of their genetic risk score.64
TE may lead to truncation of asparaginase therapy, which in several studies have been associated with increased risk of relapse. Continuation of asparaginase therapy is thus generally recommended after TE with co-administration of low molecular weight heparin (LMWH).20 55 65 Although randomised studies have not been performed, for central nervous system TE, there is a lack of data to clarify whether re-exposure to asparaginase is safe. Randomised trials on TE prophylaxis are also lacking in adults, although a recent paediatric randomisation gave support to prophylactic use of LMWH during induction therapy.66
Hypofibrinogenaemia often occurs after PEG asparaginase administration but bleeding is rare.9 20 23 Cryoprecipate replacement contains prothrombotic factors including vWF and factor VIII, which may promote thrombosis. Its administration was reported to be associated with a higher rate of thrombosis.20 65 The panel therefore discourages the use of cryoprecipitate replacement in the absence of active bleeding or planned surgical intervention.
Recommendation regarding the management of thrombosis
Continuation of asparaginase therapy is recommended after TE with co-administration of LMWH.
A recent paediatric randomisation gave support to prophylactic use of LMWH but in adults prophylactic LMWH is controversial and is not routinely applied.
Hypersensitivity
One notable asparaginase adverse effect is hypersensitivity. This is because asparaginase is a foreign protein synthesised by either E. coli (native enzyme or PEG asparaginase) or Erwinia chrysanthemi (Erwinia asparaginase). Hypersensitivity can pose symptom management challenges and significant long-term clinical outcome ramifications since enzymatic inactivation of the drug can occur with hypersensitivity. Asparaginase can provoke symptomatic (ie, overt or clinical) hypersensitivity or silent hypersensitivity through asparaginase antibody formation. Silent asparaginase antibodies may complicate therapy via enzymatic pharmacokinetic and pharmacodynamic effects.37 67
The asparaginase agent and its route of delivery also appear to impact hypersensitivity risk, with the native enzyme and intramuscular delivery demonstrating higher hypersensitivity rates compared with PEG asparaginase/Erwinia and intravenous administration, respectively.68
Several confounding factors may complicate adult hypersensitivity management. First, while the constellation of symptoms that may accompany clinical hypersensitivity are well described, different grading systems exist, which may render if difficult to compare data across agents and across clinical trials. Second, considerable overlap exists between low-grade clinical hypersensitivity, an immune-mediated process, and symptomatic hyperammonaemia, a function of rapid ammonia liberation—from the hydrolytic enzymatic activity—during and shortly after asparaginase administration.69 70
Clinical hypersensitivity presents with anaphylaxis or symptoms that include dyspnoea, pruritus, oedema, rash, cough and vomiting, which often lead to discontinuation of treatment. Anaphylaxis and other severe forms of clinical hypersensitivity is an indication for asparaginase infusion interruption and parenteral medication (eg, antihistamines, glucocorticoids, acetaminophen and when necessary epinephrine). With high-grade clinical hypersensitivity and/or low enzymatic activity of asparaginase, patients should be switched to Erwinia asparaginase.71
Clinical hypersensitivity to PEG asparaginase is less common in adults than children16; 1%–23% of adult PEG asparaginase recipients experience hypersensitivity reactions of any grade and high-grade (≥3) hypersensitivity reactions are seen in 2%–10% of patients.5 9 72
Silent inactivation is particularly relevant, as the decreased asparaginase activity is asymptomatic and will stay undetected if asparaginase activity is not monitored. In the case of diagnosed silent inactivation, the standard step of management is the prompt switch to Erwinia asparaginase therapy71 73; although in some patients treated with PEG asparaginase, the inactivating antibodies may be directed towards the PEG moiety rather than asparaginase.
With similar symptoms to those of real allergic hypersensitivity reactions, non-allergic infusion reactions are becoming more common with the use of intravenous PEG asparaginase. These non-allergic infusion reactions often occur shortly into the infusion.69 70 A switch to Erwinia asparaginase is not necessary for these infusion symptoms that are related to hyperammonaemia.
In suspected hypersensitivity, it may be helpful to measure the activity of asparaginase through therapeutic drug monitoring (TDM).
Therapeutic drug monitoring
In addition to the identification of patients with silent inactivation, TDM of asparaginase may help distinguish between clinical hypersensitivity and non-allergic infusion reactions.74 75
TDM can also be used for adjusting asparaginase doses, but there is no sufficiently vetted algorithm to support this approach yet and this is not performed routinely outside of clinical trials.54
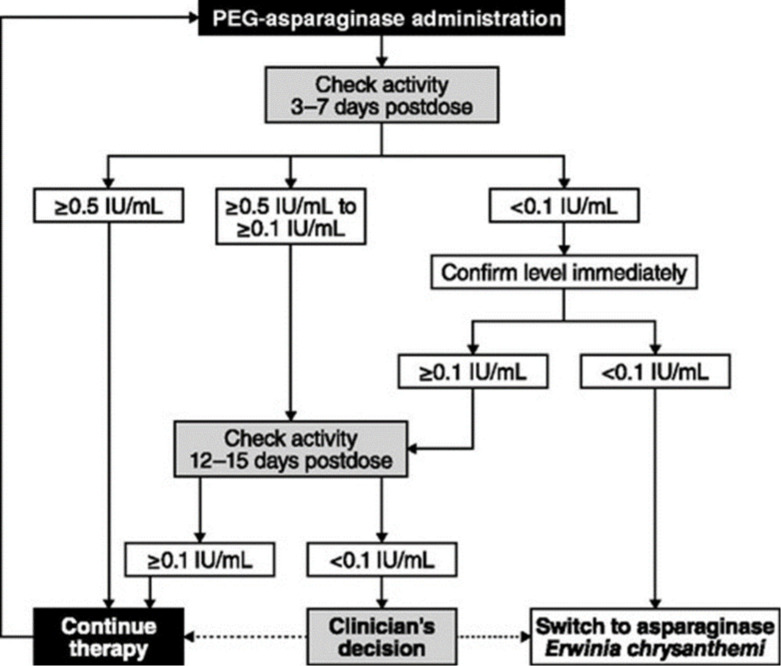
There are different ways to undertake TDM of asparaginase therapy, yet expert consensus recommends the monitoring of serum asparaginase activity (SAA), which is now most used in practice.31 To date, an SAA level ≥0.1 IU/mL appears to be a reasonable target level to ensure therapeutic benefit.74
To provide recommendations for measurement of asparaginase activity, Salzer et al 74 propose a treatment algorithm to guide the use of activity monitoring in patients receiving PEG asparaginase therapy for ALL (figure 1).
Figure 1.
Algorithm for pegylated form of asparaginase (PEG asparaginase) activity monitoring administration.74 Reproduced with permission from Taylor and Francis Group.
There is not a standardisation of TDM for adults, however adult prospective clinical studies should incorporate TDM and report on it.
Premedication
A recent single-institution retrospective study of paediatric patients with ALL analysed hypersensitivity data from two cohorts76: one group of patients who were treated prior to the institution performing asparaginase premedication and a second later cohort who received standard premedication. Premedication with histamine antagonists, acetaminophen and (in some cases) corticosteroids was associated with both a lower rate of substitutions of PEG asparaginase to Erwinia asparaginase and lower acute adverse event rates. Patients who received premedication prior to PEG asparaginase demonstrated rare silent inactivation on SAA measurements 1 week postdose. Also, a substantial financial saving was achieved in the premedication cohort, as fewer patients required Erwinia asparaginase. Interestingly, in a large US trial (CALGB 10403) using a paediatric regimen in adults aged 17–39 years, the rate of high-grade hypersensitivity was 10% of the patients. After an amendment that mandated premedication with acetaminophen and hydrocortisone before each dose of PEG asparaginase the rate decreased to 4%.5
Importantly, if premedication is administered, TDM should become mandatory in order not to miss silent inactivation, although the yield of finding inactivation would be low even if done after every dose.
Recommendations from the panel regarding the management of infusion-related reaction
Systematic and standardised TDM detects silent inactivation, distinguishes between true hypersensitivity versus other infusion reactions, and allows premedication, thus permitting more patients to benefit from asparaginase.
If hypersensitivity occurs, all subsequent doses of PEG asparaginase (including the dose that caused the reaction) should be switched to Erwinia asparaginase. Discontinuation of PEG asparaginase for hypersensitivity without replacing all subsequent scheduled PEG asparaginase doses with Erwinia asparaginase was associated with inferior disease-free survival.1 On the other hand, after a clinical or silent allergic reaction, switching and replacing all subsequent scheduled doses with Erwinia asparaginase had a similar outcome to receiving all scheduled doses of PEG asparaginase.1 71
Conclusion
Prolonged postremission asparaginase is a key component of a multiagent chemotherapy regimen for patients with newly diagnosed ALL, as confirmed in children by several randomised clinical trials. A recent study in children reported that discontinuation of asparaginase doses is often associated with inferior outcome.1 In adults, recent prospective clinical trials that used paediatric or paediatric-inspired regimens with multiple doses of PEG asparaginase have reported significantly improved survival compared with historical data with regimens that used less asparaginase. However, since asparaginase is almost exclusively used in ALL, clinicians who treat adults are less familiar with the drug and may be reluctant to use it or discontinue it prematurely. The expert panel reviewed the most common toxicities of asparaginase (ie, hepatoxicity, pancreatitis, hypertriglyceridaemia, thrombosis and hypersensitivity) and how to manage them. Several recommendations were made for each one of these toxicities, with the goal of safe administration of the drug and to educate clinicians when the drug can be continued despite side effects. With the recent studies showing clinical benefit of paediatric regimens in adults with ALL, this information will help clinicians who use such regimens in this patient population.
Table 1 summarises prevention and management of the discussed PEG asparaginase toxicities.
Table 1.
Pegylated form of asparaginase (PEG asparaginase) toxicities prevention and management
| Toxicity | Prevention | Treatment | |
| Hepatotoxicity | High-grade hyperbilirubinaemia | Reduce dose for age >55 and BMI >35 | *See footnote |
| De-synchronisation of the administration of PEG asparaginase and myelosuppressive drugs | Try to avoid giving PEG asparaginase concomitantly with myelosupressive drugs | ||
| Liver steatosis | Liver ultrasound | †See footnote | |
| Synchronisation of PEG asparaginase and myelosuppressive drugs | |||
| Pancreatitis | Clinical | Avoid after clinical PEG asparaginase-associated pancreatitis | Early recognition and treatment Permanent discontinuation |
| ‘Chemical’ | Continue dosing | ||
| Hypertriglyceridaemia | Continue dosing | ||
| Thrombosis | Possible low molecular weight heparin (controversial) Possible antithrombin III (controversial) Minimise cryoprecipitate for hypofibrinogenaemia |
Continue dosing with co-administration of low molecular weight heparin Possible antithrombin III (needs more study) |
|
| Hypersensitivity | Premedication with hydrocortisone, antihistamine and acetaminophen before each dose | Distinguish between allergic and non-allergic infusion reaction with the use of postdosing therapeutic drug monitoring Allergic reaction—switch to Erwinia asparaginase Non-allergic reaction—no switch, continue all doses of PEG asparaginase |
*For high-grade hyperbilirubinaemia and transaminitis: some members of the panel reduce the dose of the next PEG asparaginase to 1000 or 500 and try thereafter to administer the full doses. Others continue after recovery without dose modification. We suggest that clinicians follow the approach of the protocol they use.
†There is no consensus in the literature and among the panel members about the value of ultrasound and dose reduction. However, it is not widely practised and currently the panel cannot make a specific recommendation on pre-PEG asparaginase liver ultrasound.
BMI, body mass index.
Footnotes
Contributors: All authors have contributed to and reviewed the manuscript.
Funding: The project is supported by an unrestricted educational grant from Servier.
Competing interests: DH: speaker and honoraria from Jazz Pharmaceuticals, Servier and Amgen. JHP: consulting for Amgen, Servier, Kite Pharma, Novartis, AstraZeneca, InCyte. KS: speaker honoraria from Jazz Pharmaceuticals, Servier and Amgen; educational grant from Servier. DD: speaker and consultant for Servier, Amgen and Jazz.
Patient consent for publication: Not required.
Provenance and peer review: Commissioned; externally peer reviewed.
References
- 1. Gupta S, Wang C, Raetz EA, et al. Impact of asparaginase discontinuation on outcome in childhood acute lymphoblastic leukemia: a report from the children's Oncology Group. J Clin Oncol 2020;38:1897–905. 10.1200/JCO.19.03024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sallan SE, Hitchcock-Bryan S, Gelber R, et al. Influence of intensive asparaginase in the treatment of childhood non-T-cell acute lymphoblastic leukemia. Cancer Res 1983;43:5601–7. [PubMed] [Google Scholar]
- 3. Amylon MD, Shuster J, Pullen J, et al. Intensive high-dose asparaginase consolidation improves survival for pediatric patients with T cell acute lymphoblastic leukemia and advanced stage lymphoblastic lymphoma: a pediatric Oncology Group study. Leukemia 1999;13:335–42. 10.1038/sj.leu.2401310 [DOI] [PubMed] [Google Scholar]
- 4. Douer D. Is asparaginase a critical component in the treatment of acute lymphoblastic leukemia? Best Pract Res Clin Haematol 2008;21:647–58. 10.1016/j.beha.2008.08.004 [DOI] [PubMed] [Google Scholar]
- 5. Stock W, Luger SM, Advani AS, et al. A pediatric regimen for older adolescents and young adults with acute lymphoblastic leukemia: results of CALGB 10403. Blood 2019;133:1548–59. 10.1182/blood-2018-10-881961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. DeAngelo DJ, Stevenson K, Neuberg DS, et al. A multicenter phase II study using a dose intensified Pegylated-Asparaginase pediatric regimen in adults with untreated acute lymphoblastic leukemia: a DFCI all Consortium trial. Blood 2015;126:80a 10.1182/blood.V126.23.80.80 25838348 [DOI] [Google Scholar]
- 7. Ribera J-M, Oriol A, Sanz M-A, et al. Comparison of the results of the treatment of adolescents and young adults with standard-risk acute lymphoblastic leukemia with the Programa Español de Tratamiento en Hematología pediatric-based protocol ALL-96. J Clin Oncol 2008;26:1843–9. 10.1200/JCO.2007.13.7265 [DOI] [PubMed] [Google Scholar]
- 8. Huguet F, Leguay T, Raffoux E, et al. Pediatric-inspired therapy in adults with Philadelphia chromosome-negative acute lymphoblastic leukemia: the GRAALL-2003 study. J Clin Oncol 2009;27:911–8. 10.1200/JCO.2008.18.6916 [DOI] [PubMed] [Google Scholar]
- 9. Douer D, Aldoss I, Lunning MA, et al. Pharmacokinetics-based integration of multiple doses of intravenous pegaspargase in a pediatric regimen for adults with newly diagnosed acute lymphoblastic leukemia. J Clin Oncol 2014;32:905–11. 10.1200/JCO.2013.50.2708 [DOI] [PubMed] [Google Scholar]
- 10. Toft N, Birgens H, Abrahamsson J, et al. Results of NOPHO ALL2008 treatment for patients aged 1-45 years with acute lymphoblastic leukemia. Leukemia 2018;32:606–15. 10.1038/leu.2017.265 [DOI] [PubMed] [Google Scholar]
- 11. Gökbuget N, Kneba M, Raff T, et al. Adult patients with acute lymphoblastic leukemia and molecular failure display a poor prognosis and are candidates for stem cell transplantation and targeted therapies. Blood 2012;120:1868–76. 10.1182/blood-2011-09-377713 [DOI] [PubMed] [Google Scholar]
- 12. Siegel SE, Advani A, Seibel N, et al. Treatment of young adults with Philadelphia-negative acute lymphoblastic leukemia and lymphoblastic lymphoma: Hyper-CVAD vs. pediatric-inspired regimens. Am J Hematol 2018;93:1254–66. 10.1002/ajh.25229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kozlowski P, Åström M, Ahlberg L, et al. High relapse rate of T cell acute lymphoblastic leukemia in adults treated with Hyper-CVAD chemotherapy in Sweden. Eur J Haematol 2014;92:377–81. 10.1111/ejh.12269 [DOI] [PubMed] [Google Scholar]
- 14. Kota VK, Hathaway AR, Shah BD, et al. Poor outcomes with HyPer CVAD induction for T-cell lymphoblastic leukemia/lymphoma. Blood 2015;126:3762 10.1182/blood.V126.23.3762.3762 [DOI] [Google Scholar]
- 15. Quist-Paulsen P, Toft N, Heyman M, et al. T-Cell acute lymphoblastic leukemia in patients 1-45 years treated with the pediatric NOPHO ALL2008 protocol. Leukemia 2020;34:347–57. 10.1038/s41375-019-0598-2 [DOI] [PubMed] [Google Scholar]
- 16. Stock W, Douer D, DeAngelo DJ, et al. Prevention and management of asparaginase/pegasparaginase-associated toxicities in adults and older adolescents: recommendations of an expert panel. Leuk Lymphoma 2011;52:2237–53. 10.3109/10428194.2011.596963 [DOI] [PubMed] [Google Scholar]
- 17. Goekbuget N, Baumann A, Beck J, et al. PEG-Asparaginase intensification in adult acute lymphoblastic leukemia (ALL): significant improvement of outcome with moderate increase of liver toxicity in the German multicenter study Group for adult all (GMALL) study 07/2003. Blood 2010;116:494 10.1182/blood.V116.21.494.494 [DOI] [Google Scholar]
- 18. Burke PW, Aldoss I, Lunning MA, et al. Pegaspargase-related high-grade hepatotoxicity in a pediatric-inspired adult acute lymphoblastic leukemia regimen does not predict recurrent hepatotoxicity with subsequent doses. Leuk Res 2018;66:49–56. 10.1016/j.leukres.2017.12.013 [DOI] [PubMed] [Google Scholar]
- 19. Patel B, Kirkwood AA, Dey A, et al. Pegylated-asparaginase during induction therapy for adult acute lymphoblastic leukaemia: toxicity data from the UKALL14 trial. Leukemia 2017;31:58–64. 10.1038/leu.2016.219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Aldoss I, Douer D, Behrendt CE, et al. Toxicity profile of repeated doses of PEG-asparaginase incorporated into a pediatric-type regimen for adult acute lymphoblastic leukemia. Eur J Haematol 2016;96:375–80. 10.1111/ejh.12600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Denton CC, Rawlins YA, Oberley MJ, et al. Predictors of hepatotoxicity and pancreatitis in children and adolescents with acute lymphoblastic leukemia treated according to contemporary regimens. Pediatr Blood Cancer 2018;65:e26891 10.1002/pbc.26891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Advani AS, Sanford B, Luger S, et al. Frontline-Treatment of acute lymphoblastic leukemia (ALL) in older adolescents and young adults (AYA) using a pediatric regimen is feasible: toxicity results of the prospective US Intergroup Trial C10403 (Alliance). Blood 2013;122:3903a 10.1182/blood.V122.21.3903.3903 [DOI] [Google Scholar]
- 23. Douer D, Yampolsky H, Cohen LJ, et al. Pharmacodynamics and safety of intravenous pegaspargase during remission induction in adults aged 55 years or younger with newly diagnosed acute lymphoblastic leukemia. Blood 2007;109:2744–50. 10.1182/blood-2006-07-035006 [DOI] [PubMed] [Google Scholar]
- 24. Geyer MB, Ritchie EK, Rao AV, et al. Pediatric-Inspired chemotherapy incorporating Pegaspargase is safe and results in high rates of MRD negativity in adults ages 18-60 with Philadelphia chromosome-negative acute lymphoblastic leukemia and lymphoblastic lymphoma. Blood 2018;132:4013 10.1182/blood-2018-99-115486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bodmer M, Sulz M, Stadlmann S, et al. Fatal liver failure in an adult patient with acute lymphoblastic leukemia following treatment with L-asparaginase. Digestion 2006;74:28–32. 10.1159/000095827 [DOI] [PubMed] [Google Scholar]
- 26. Rausch CR, Marini BL, Benitez LL, et al. PEGging down risk factors for peg-asparaginase hepatotoxicity in patients with acute lymphoblastic leukemia † . Leuk Lymphoma 2018;59:617–24. 10.1080/10428194.2017.1349902 [DOI] [PubMed] [Google Scholar]
- 27. Christ TN, Stock W, Knoebel RW. Incidence of asparaginase-related hepatotoxicity, pancreatitis, and thrombotic events in adults with acute lymphoblastic leukemia treated with a pediatric-inspired regimen. J Oncol Pharm Pract 2018;24:299–308. 10.1177/1078155217701291 [DOI] [PubMed] [Google Scholar]
- 28. Kamal N, Koh C, Samala N, et al. Asparaginase-induced hepatotoxicity: rapid development of cholestasis and hepatic steatosis. Hepatol Int 2019;13:641–8. 10.1007/s12072-019-09971-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Alshiekh-Nasany R, Douer D. L-Carnitine for treatment of Pegasparaginase-Induced hepatotoxicity. Acta Haematol 2016;135:208–10. 10.1159/000442342 [DOI] [PubMed] [Google Scholar]
- 30. Albertsen BK, Grell K, Abrahamsson J, et al. Intermittent versus continuous PEG-asparaginase to reduce asparaginase-associated toxicities: a NOPHO ALL2008 randomized study. J Clin Oncol 2019;37:1638–46. 10.1200/JCO.18.01877 [DOI] [PubMed] [Google Scholar]
- 31. Wolthers BO, Frandsen TL, Baruchel A, et al. Asparaginase-associated pancreatitis in childhood acute lymphoblastic leukaemia: an observational Ponte di Legno toxicity Working Group study. Lancet Oncol 2017;18:1238–48. 10.1016/S1470-2045(17)30424-2 [DOI] [PubMed] [Google Scholar]
- 32. Liu C, Yang W, Devidas M, et al. Clinical and genetic risk factors for acute pancreatitis in patients with acute lymphoblastic leukemia. J Clin Oncol 2016;34:2133–40. 10.1200/JCO.2015.64.5812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wolthers BO, Frandsen TL, Patel CJ, et al. Trypsin-encoding PRSS1-PRSS2 variations influence the risk of asparaginase-associated pancreatitis in children with acute lymphoblastic leukemia: a Ponte di Legno toxicity working group report. Haematologica 2019;104:556–63. 10.3324/haematol.2018.199356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rank CU, Wolthers BO, Grell K, et al. Asparaginase-Associated pancreatitis in acute lymphoblastic leukemia: results from the NOPHO ALL2008 treatment of patients 1-45 years of age. J Clin Oncol 2020;38:145–54. 10.1200/JCO.19.02208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kearney SL, Dahlberg SE, Levy DE, et al. Clinical course and outcome in children with acute lymphoblastic leukemia and asparaginase-associated pancreatitis. Pediatr Blood Cancer 2009;53:162–7. 10.1002/pbc.22076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Daley RJ, Rajeeve S, Kabel CC, et al. Pegaspargase can safely be administered in adults age 40 and older with acute lymphoblastic leukemia. Blood 2019;134:3816 10.1182/blood-2019-124727 [DOI] [Google Scholar]
- 37. Schmiegelow K, Attarbaschi A, Barzilai S, et al. Consensus definitions of 14 severe acute toxic effects for childhood lymphoblastic leukaemia treatment: a Delphi consensus. Lancet Oncol 2016;17:e231–9. 10.1016/S1470-2045(16)30035-3 [DOI] [PubMed] [Google Scholar]
- 38. Persson L, Harila-Saari A, Hed Myrberg I, et al. Hypertriglyceridemia during asparaginase treatment in children with acute lymphoblastic leukemia correlates with antithrombin activity in adolescents. Pediatr Blood Cancer 2017;64:e26559. 10.1002/pbc.26559 [DOI] [PubMed] [Google Scholar]
- 39. Raja RA, Schmiegelow K, Sørensen DN, et al. Asparaginase-associated pancreatitis is not predicted by hypertriglyceridemia or pancreatic enzyme levels in children with acute lymphoblastic leukemia. Pediatr Blood Cancer 2017;64:32–8. 10.1002/pbc.26183 [DOI] [PubMed] [Google Scholar]
- 40. Finch ER, Smith CA, Yang W, et al. Asparaginase formulation impacts hypertriglyceridemia during therapy for acute lymphoblastic leukemia. Pediatr Blood Cancer 2020;67:e28040 10.1002/pbc.28040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Toft N, Birgens H, Abrahamsson J, et al. Toxicity profile and treatment delays in NOPHO ALL2008-comparing adults and children with Philadelphia chromosome-negative acute lymphoblastic leukemia. Eur J Haematol 2016;96:160–9. 10.1111/ejh.12562 [DOI] [PubMed] [Google Scholar]
- 42. Rank CU, Toft N, Tuckuviene R, et al. Thromboembolism in acute lymphoblastic leukemia: results of NOPHO ALL2008 protocol treatment in patients aged 1 to 45 years. Blood 2018;131:2475–84. 10.1182/blood-2018-01-827949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sorà F, Sorà F, Rossi E, et al. The risk of thrombosis in patients with acute leukemia: occurrence of thrombosis at diagnosis and during treatment. J Thromb Haemost 2005;3:1985–92. 10.1111/j.1538-7836.2005.01467.x [DOI] [PubMed] [Google Scholar]
- 44. Athale UH, Chan AKC. Thrombosis in children with acute lymphoblastic leukemia: Part I. epidemiology of thrombosis in children with acute lymphoblastic leukemia. Thromb Res 2003;111:125–31. 10.1016/j.thromres.2003.10.013 [DOI] [PubMed] [Google Scholar]
- 45. Tuckuviene R, Ranta S, Albertsen BK, et al. Prospective study of thromboembolism in 1038 children with acute lymphoblastic leukemia: a Nordic Society of pediatric hematology and oncology (NOPHO) study. J Thromb Haemost 2016;14:485–94. 10.1111/jth.13236 [DOI] [PubMed] [Google Scholar]
- 46. Christ TN, Stock W, Knoebel RW. Incidence of asparaginase-related hepatotoxicity, pancreatitis, and thrombotic events in adults with acute lymphoblastic leukemia treated with a pediatric-inspired regimen. J Oncol Pharm Pract 2017;1078155217701291. [DOI] [PubMed] [Google Scholar]
- 47. DeAngelo DJ, Stevenson KE, Dahlberg SE, et al. Long-Term outcome of a pediatric-inspired regimen used for adults aged 18–50 years with newly diagnosed acute lymphoblastic leukemia. Leukemia 2015;29:526–34. 10.1038/leu.2014.229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Payne JH, Vora AJ. Thrombosis and acute lymphoblastic leukaemia. Br J Haematol 2007;138:430–45. 10.1111/j.1365-2141.2007.06677.x [DOI] [PubMed] [Google Scholar]
- 49. Truelove E, Fielding AK, Hunt BJ. The coagulopathy and thrombotic risk associated with L-asparaginase treatment in adults with acute lymphoblastic leukaemia. Leukemia 2013;27:553–9. 10.1038/leu.2012.290 [DOI] [PubMed] [Google Scholar]
- 50. Leone G, Gugliotta L, Mazzucconi MG, et al. Evidence of a hypercoagulable state in patients with acute lymphoblastic leukemia treated with low dose of E. coli L-asparaginase: a GIMEMA study. Thromb Haemost 1993;69:12–15. [PubMed] [Google Scholar]
- 51. Mitchell L, Hoogendoorn H, Giles AR, et al. Increased endogenous thrombin generation in children with acute lymphoblastic leukemia: risk of thrombotic complications in L'Asparaginase-induced antithrombin III deficiency. Blood 1994;83:386–91. 10.1182/blood.V83.2.386.386 [DOI] [PubMed] [Google Scholar]
- 52. Grace RF, DeAngelo DJ, Stevenson KE, et al. The use of prophylactic anticoagulation during induction and consolidation chemotherapy in adults with acute lymphoblastic leukemia. J Thromb Thrombolysis 2018;45:306–14. 10.1007/s11239-017-1597-7 [DOI] [PubMed] [Google Scholar]
- 53. van Zaane B, Nur E, Squizzato A, et al. Systematic review on the effect of glucocorticoid use on procoagulant, anti-coagulant and fibrinolytic factors. J Thromb Haemost 2010;8:2483–93. 10.1111/j.1538-7836.2010.04034.x [DOI] [PubMed] [Google Scholar]
- 54. Ku GH, White RH, Chew HK, et al. Venous thromboembolism in patients with acute leukemia: incidence, risk factors, and effect on survival. Blood 2009;113:3911–7. 10.1182/blood-2008-08-175745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Grace RF, Dahlberg SE, Neuberg D, et al. The frequency and management of asparaginase-related thrombosis in paediatric and adult patients with acute lymphoblastic leukaemia treated on Dana-Farber cancer Institute Consortium protocols. Br J Haematol 2011;152:452–9. 10.1111/j.1365-2141.2010.08524.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mitchell LG, Andrew M, Hanna K, et al. A prospective cohort study determining the prevalence of thrombotic events in children with acute lymphoblastic leukemia and a central venous line who are treated with L-asparaginase: results of the prophylactic antithrombin replacement in kids with acute lymphoblastic leukemia treated with asparaginase (PARKAA) study. Cancer 2003;97:508–16. 10.1002/cncr.11042 [DOI] [PubMed] [Google Scholar]
- 57. Farinasso L, Bertorello N, Garbarini L, et al. Risk factors of central venous lines-related thrombosis in children with acute lymphoblastic leukemia during induction therapy: a prospective study. Leukemia 2007;21:552–6. 10.1038/sj.leu.2404560 [DOI] [PubMed] [Google Scholar]
- 58. Ziegler S, Sperr WR, Knöbl P, et al. Symptomatic venous thromboembolism in acute leukemia. incidence, risk factors, and impact on prognosis. Thromb Res 2005;115:59–64. 10.1016/j.thromres.2004.07.016 [DOI] [PubMed] [Google Scholar]
- 59. Storring JM, Minden MD, Kao S, et al. Treatment of adults with BCR-ABL negative acute lymphoblastic leukaemia with a modified paediatric regimen. Br J Haematol 2009;146:76–85. 10.1111/j.1365-2141.2009.07712.x [DOI] [PubMed] [Google Scholar]
- 60. Elliott MA, Wolf RC, Hook CC, et al. Thromboembolism in adults with acute lymphoblastic leukemia during induction with L-asparaginase-containing multi-agent regimens: incidence, risk factors, and possible role of antithrombin. Leuk Lymphoma 2004;45:1545–51. 10.1080/10428190410001693588 [DOI] [PubMed] [Google Scholar]
- 61. Mohren M, Markmann I, Jentsch-Ullrich K, et al. Increased risk of venous thromboembolism in patients with acute leukaemia. Br J Cancer 2006;94:200–2. 10.1038/sj.bjc.6602945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Caruso V, Iacoviello L, Di Castelnuovo A, et al. Venous thrombotic complications in adults undergoing induction treatment for acute lymphoblastic leukemia: results from a meta-analysis. J Thromb Haemost 2007;5:621–3. 10.1111/j.1538-7836.2007.02383.x [DOI] [PubMed] [Google Scholar]
- 63. Hunault-Berger M, Chevallier P, Delain M, et al. Changes in antithrombin and fibrinogen levels during induction chemotherapy with L-asparaginase in adult patients with acute lymphoblastic leukemia or lymphoblastic lymphoma. Use of supportive coagulation therapy and clinical outcome: the CAPELAL study. Haematologica 2008;93:1488–94. 10.3324/haematol.12948 [DOI] [PubMed] [Google Scholar]
- 64. Jarvis KB, LeBlanc M, Tulstrup M, et al. Candidate single nucleotide polymorphisms and thromboembolism in acute lymphoblastic leukemia - A NOPHO ALL2008 study. Thromb Res 2019;184:92–8. 10.1016/j.thromres.2019.11.002 [DOI] [PubMed] [Google Scholar]
- 65. Orvain C, Balsat M, Tavernier E, et al. Thromboembolism prophylaxis in adult patients with acute lymphoblastic leukemia treated in the GRAALL-2005 study. Blood 2020;136:328–38. [DOI] [PubMed] [Google Scholar]
- 66. Greiner J, Schrappe M, Claviez A, et al. THROMBOTECT – a randomized study comparing low molecular weight heparin, antithrombin and unfractionated heparin for thromboprophylaxis during induction therapy of acute lymphoblastic leukemia in children and adolescents. Haematologica 2019;104:756–65. 10.3324/haematol.2018.194175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hijiya N, van der Sluis IM. Asparaginase-associated toxicity in children with acute lymphoblastic leukemia. Leuk Lymphoma 2016;57:748–57. 10.3109/10428194.2015.1101098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Burke MJ, Devidas M, Maloney K, et al. Severe pegaspargase hypersensitivity reaction rates (grade ≥3) with intravenous infusion vs. intramuscular injection: analysis of 54,280 doses administered to 16,534 patients on children’s oncology group (COG) clinical trials. Leuk Lymphoma 2018;59:1624–33. 10.1080/10428194.2017.1397658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Nussbaum V, Lubcke N, Findlay R. Hyperammonemia secondary to asparaginase: a case series. J Oncol Pharm Pract 2016;22:161–4. 10.1177/1078155214551590 [DOI] [PubMed] [Google Scholar]
- 70. Heitink-Pollé KMJ, Prinsen BHCMT, de Koning TJ, et al. High incidence of symptomatic hyperammonemia in children with acute lymphoblastic leukemia receiving PEGylated asparaginase. JIMD Rep 2013;7:103–8. 10.1007/8904_2012_156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Vrooman LM, Stevenson KE, Supko JG, et al. Postinduction dexamethasone and individualized dosing of Escherichia Coli L-asparaginase each improve outcome of children and adolescents with newly diagnosed acute lymphoblastic leukemia: results from a randomized study--Dana-Farber Cancer Institute ALL Consortium Protocol 00-01. J Clin Oncol 2013;31:1202–10. 10.1200/JCO.2012.43.2070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Rytting ME, Jabbour EJ, Jorgensen JL, et al. Final results of a single institution experience with a pediatric-based regimen, the augmented Berlin-Frankfurt-Münster, in adolescents and young adults with acute lymphoblastic leukemia, and comparison to the hyper-CVAD regimen. Am J Hematol 2016;91:819–23. 10.1002/ajh.24419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Tong WH, Pieters R, Kaspers GJL, et al. A prospective study on drug monitoring of PEGasparaginase and Erwinia asparaginase and asparaginase antibodies in pediatric acute lymphoblastic leukemia. Blood 2014;123:2026–33. 10.1182/blood-2013-10-534347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Salzer W, Bostrom B, Messinger Y, et al. Asparaginase activity levels and monitoring in patients with acute lymphoblastic leukemia. Leuk Lymphoma 2018;59:1797–806. 10.1080/10428194.2017.1386305 [DOI] [PubMed] [Google Scholar]
- 75. Bleyer A, Asselin BL, Koontz SE, et al. Clinical application of asparaginase activity levels following treatment with pegaspargase. Pediatr Blood Cancer 2015;62:1102–5. 10.1002/pbc.25299 [DOI] [PubMed] [Google Scholar]
- 76. Cooper SL, Young DJ, Bowen CJ, et al. Universal premedication and therapeutic drug monitoring for asparaginase‐based therapy prevents infusion‐associated acute adverse events and drug substitutions. Pediatr Blood Cancer 2019;66:e27797 10.1002/pbc.27797 [DOI] [PMC free article] [PubMed] [Google Scholar]