ABSTRACT
Virulence of the human fungal pathogen Candida albicans depends on the switch from budding to filamentous growth. Deletion of the Arf GTPase Arl1 results in hyphae that are shorter as well as reduced virulence. How Arl1 is regulated during hyphal growth, a process characteristic of filamentous fungi, yet absent in S. cerevisiae, is unknown. Here, we investigated the importance of the Rab6 homolog, Ypt6, in Arl1-dependent hyphal growth and determined that YPT6 overexpression specifically rescued the hyphal growth defect of an arl1 mutant, but not the converse. Furthermore, we show that deletion of ARL1 results in an alteration of the distribution of the Rab8 homolog, Sec4, in hyphal cells and that this defect is restored upon YPT6 overexpression.
KEYWORDS: Arf, hyphal growth, secretion, Rab, yeast morphogenesis
Introduction
Cell shape changes are critical for a range of biological processes, such as neuronal development, and cell shape abnormalities are characteristic of cancer cells. Morphological changes are also crucial for the virulence of a range of plant and human fungal pathogens. Candida albicans is a major fungal pathogen of humans that accounts for ∼10% of hospital-acquired bloodstream infections, with mortality rate exceeding 30%. The success of C. albicans as a pathogen is associated with its ability to switch between different morphological states.1–3 These dramatic cell shape changes require cytoskeleton reorganization and sustained membrane traffic, resulting, in particular, in the secretion of hydrolytic enzymes, critical for pathogenicity.4–6 During filamentous growth, C. albicans secretory vesicles are clustered at a structure called the Spitzenkörper at the tip of the filament,7 the Golgi apparatus redistributes to the apex region8,9 and endocytosis sites form a collar below the hyphal tip.8,10 Trafficking to the plasma membrane is mediated by vesicular transport regulated by small GTPases of the Arf (ADP-ribosylation factor) and Rab (Ras-related in brain) families.11–16
C. albicans has 5 Arf/Arl homologs compared to 26 in Human. C. albicans is a diploid yeast, thought to have diverged from Saccharomyces cerevisiae approximately 800 millions years ago.17 We recently showed that of these 5 Arf/Arl proteins, only Arf2 is essential for viability and antifungal drug sensitivity.18 While both Arf2 and Arl1 are required for hyphal growth and virulence, Arl1 is additionally critical for restricting hyphal growth to a single site, likely via regulation of protein secretion. Arl1 is involved in multiple cellular processes both in mammalian and yeast cells.19 How is Arl1 regulated during hyphal growth, a process characteristic of filamentous fungi, is at present unknown. Several studies have shed light on the crosstalk between Arf and Rab proteins; in budding yeast, Arl1 was shown to genetically interact with the Human Rab6 homolog, Ypt6.20–22 Recently, a role of Arl1 and Ypt6 in S. cerevisiae autophagy was also reported.23–25 Here, we investigated the importance of Ypt6 in C. albicans Arl1-dependent filamentous growth.
Results and discussion
We have shown that an arl1/arl1 deletion mutant was dramatically reduced in invasive growth in response to fetal calf serum (FCS) or the carbon source-poor Spider medium.18 Fig. 1A and 1B show that the arl1/arl1 defect was partially rescued by over-expression of YPT6, on both media. To examine if GTP-GDP cycling of Ypt6 was critical for such a rescue, we generated constitutive active and constitutive negative mutants of Ypt6. Fig. 1C shows that, compared to over-expression of wild-type Ypt6, over-expression of the constitutive negative form of Ypt6, Ypt6[T25N], did not rescue the defect while over-expression of the constitutive active form, Ypt6[Q71L], rescued the defect to an intermediate level, suggesting that GTP-GDP cycling of Ypt6 is indeed critical. Alternatively, it is possible that the reduced efficiency of Ypt6[Q71L], compared to Ypt6, to restore invasive growth is due to sub-optimal activation by a GEF.26 We next investigated the importance of YPT6 for invasive growth and Fig. 1D shows that YPT6 is also critical, as a ypt6/ypt6 deletion mutant was dramatically reduced in invasive growth. This defect in invasive growth of the ypt6/ypt6 mutant was complemented by over-expression of YPT6, but not over-expression of ARL1. The deletion and over-expressing mutants were verified by RT-PCR (Fig. 1F and 2G).
Figure 1.
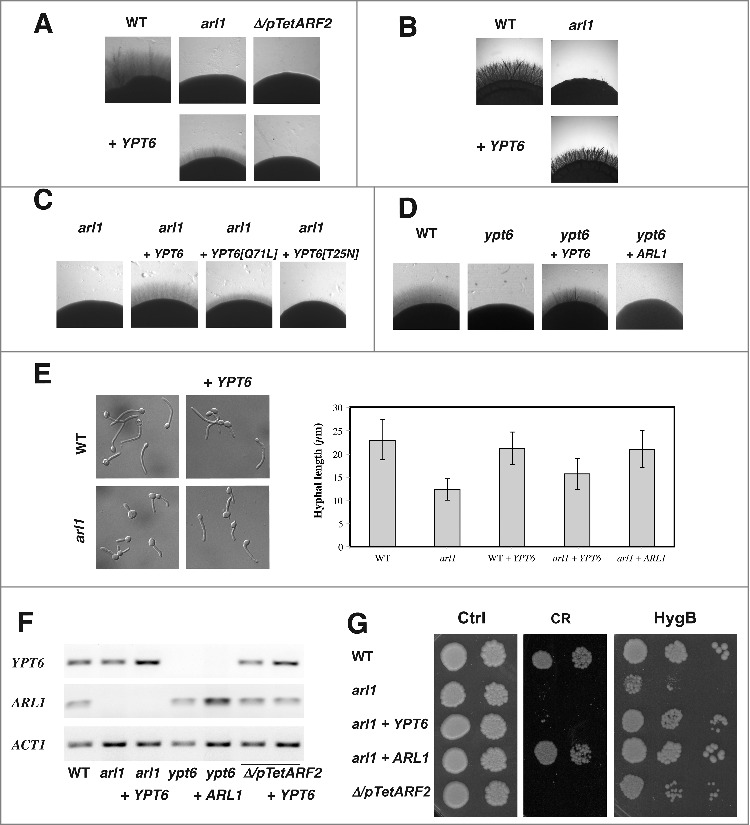
Overexpression of YPT6 rescues the hyphal invasive growth defect in an arl1 deletion mutant. (A-D) Overexpression of YPT6 specifically rescues invasive growth in arl1/arl1 cells. Indicated strains were grown on agar-containing YEPD with 50% FCS (A, C, D) or on Spider media (B) and images were taken after 5–6 days. Similar results were observed in 2 independent experiments. (C) Rescue of invasive growth in arl1/arl1 cells depends on Ypt6 activity. Indicated strains were grown on agar-containing YEPD with FCS as in A. (D) Overexpression of ARL1 does not rescue invasive growth in ypt6/ypt6 cells. Indicated strains were grown on agar-containing YEPD with FCS as in A. (E) Overexpression of YPT6 partially rescues arl1/arl1 hyphal length defect. Cells from the indicated strains were incubated with FCS for 90 min and the graph shows the average hyphal length (mean of 200–400 cells each strain, from 3 experiments); error bars indicate SD. Student t test was used to calculate the p values: arl1 + ARL1 vs arl1: 0.0028 and arl1 + YPT6 vs arl1: 0.0042. (F) YPT6 and ARL1 transcripts in the overexpression mutants. mRNA and cDNA were prepared from the indicated strains and the transcripts were quantified by RT-PCR; actin (ACT1) was used for normalization. (G) Overexpression of YPT6 does not rescue cell wall defects in arl1/arl1 cells. Serial dilutions of the indicated strains were spotted on YEPD media (Ctrl) containing 400 μg/ml Congo red (CR) or 1 mg/ml hygromycin B (HygB). Images were taken after 2 days.
Figure 2.
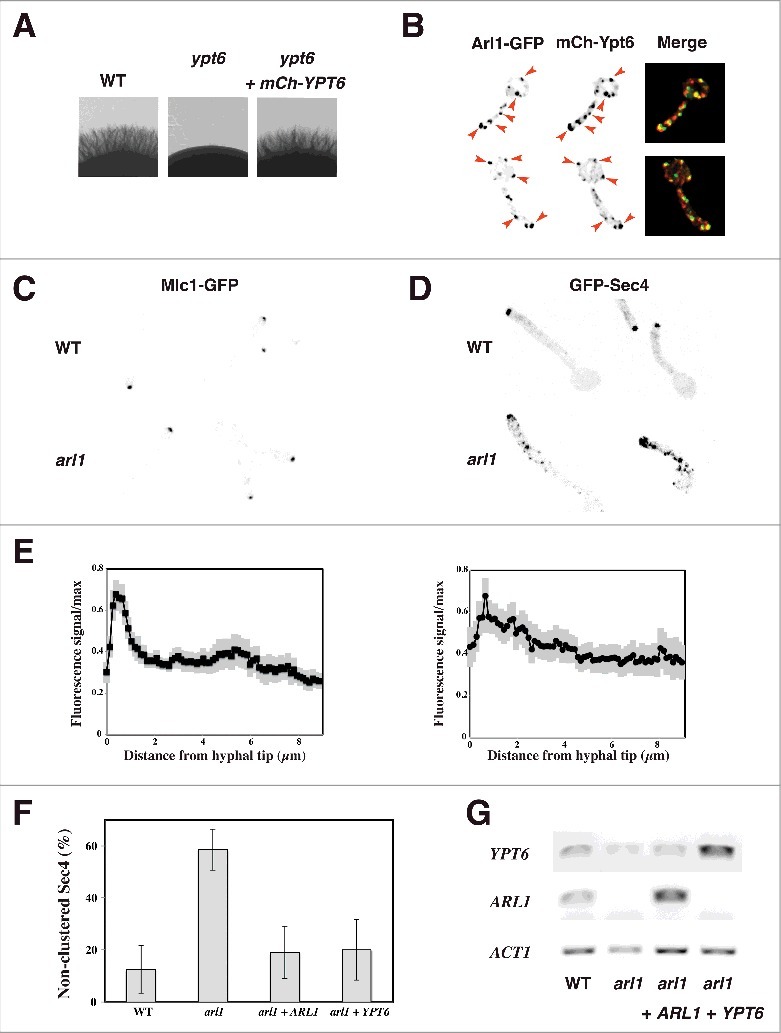
Overexpression of YPT6 rescues the altered Sec4 distribution in the arl1 deletion mutant. (A-B) Arl1 and Ypt6 partially co-localize in hyphae. The mCh-Ypt6 fusion is functional (A) Cells from the indicated strains were incubated on Spider media and images were taken after 5 days. Similar results were observed in 2 independent experiments. Maximum projections of 21 deconvolved z-sections of representative cells expressing Arl1-GFP together with mCh-Ypt6 after 90 min FCS-induced hyphal growth; GFP and mCherry signals were acquired simultaneously. (B) (C-E) Sec4, but not Mlc1, distribution is altered in arl1/arl1 cells. WT and arl1/arl1 cells, expressing Mlc1-GFP (C) or GFP-Sec4 (D), were incubated with FCS for 45 and 90 min, respectively. Representative images are shown. Cell fluorescence concentration profiles were analyzed with the HyphalPolarity program,47 to quantify the GFP-Sec4 signal concentration along the major axis of hyphal cells, from sum projection images generated with Image J (E). (F) Overexpression of YPT6 restores Sec4 clustering in arl1/arl1 cells. The graph shows averages of 3 independent experiments (n = 10–30 cells each) for each indicated strain, with p values: arl1 vs WT: 0.0004, arl1 + ARL1 vs arl1: 0.0028 and arl1 + YPT6 vs arl1: 0.0042; no statistically significant difference was observed between the values for WT, arl1 + ARL1 and arl1 + YPT6. (G) YPT6 and ARL1 transcripts in strains expressing GFP-Sec4. Transcripts were determined as in Fig. 1F.
Previously, we also observed that the arl1/arl1 mutant had reduced hyphal growth in response to FCS in liquid media, with filaments ∼2-fold shorter compared to that of the wild-type.18 Fig. 1E shows that arl1/arl1 cells over-expressing YPT6 have an average hyphal length of 16 ± 3 μm (n = 400 cells), which is intermediate compared to 12 ± 2 μm (n = 250 cells) and 21 ± 4 μm (n = 160 cells) for the arl1/arl1 and the arl1/arl1 over-expressing ARL1 cells, respectively. Over-expression of YPT6 in WT cells did not result in an increase of hyphal length (21 ± 4 μm, n = 300 cells, compared to 23 ± 4 μm, n = 200 cells, for the WT cells).
Furthermore, the Δ/pTetARF2 mutant, and to a lesser extent arl1/arl1 mutant, had reduced growth on the cell wall perturbant congo red (CR) a defect complemented by the addition of the respective gene.18 Fig. 1G shows that the arl1/arl1 defects on this cell wall perturbant is not rescued by the over-expression of YPT6. In C. albicans, cell wall integrity mutants often exhibit a growth defect on hygromycin B (HygB). In S. cerevisiae, the hypersensitivity of an arl1 deletion mutant to hygromycin B was not rescued by YPT6 overexpression.21 Fig. 1G shows, intriguingly, that the growth defect on hygromycin B was stronger for the arl1/arl1 than the Δ/pTetARF2 mutant and, in contrast to growth on CR, the growth defect of the arl1/arl1 mutant was rescued by over-expression of YPT6.
Previously, we showed that a functional Arl1-mCh fusion localizes to the late Golgi by colocalization with Sec7-GFP.18 Here, we generated a functional mCh-Ypt6 fusion, which complements the invasive growth defect of the ypt6/ypt6 mutant (Fig. 2A). In S. cerevisiae budding cells, Ypt6 was shown to localize both to the cis- and trans-Golgi, with ∼ 30% colocalization with Sec7,27 while in Aspergillus nidulans, its homolog RabC also localized to the Spitzenkörper.28 Comparison of the localization of mCh-Ypt6 to that of Arl1-GFP in C. albicans WT cells (Fig. 2B) revealed that the majority of Arl1 containing punctae also had Ypt6 signal (67 ± 8% punctae had both signals, n = 55 cells), indicating that Arl1 and Ypt6 are largely co-localized during hyphal growth. To confirm this result, we compared the localization of Ypt6 to that of Sec7 and determined a similar level of colocalization (63% ± 4% of Ypt6 containing punctae had Sec7 signal, n = 90 cells).
Our previous results suggested that the polarized growth defect in the arl1/arl1 mutant results from misregulated secretion.18 We further investigated secretion in this arl1/arl1 mutant by examining the distribution of a Spitzenkörper marker, the myosin light chain, Mlc1, and that of secretory vesicles, the small Rab G-protein Sec4.7 Fig. 2C shows that Mlc1 was clustered similarly in the arl1/arl1 and the WT hyphal cells and quantification of the fluorescent signal at the tip indicated that it was comparable in the two strains, suggesting that the Spitzenkörper is not substantially altered. In contrast, Fig. 2D shows that the distribution of Sec4 was altered in the arl1/arl1 mutant, compared to the WT. During hyphal growth, Sec4 is predominantly clustered at the tip of the hyphal cell in C. albicans, as well as in A. nidulans 7,29 which was reflected by the distribution profiles in the WT cells, shown in Fig. 2E. In comparison, the Sec4 distribution in the arl1/arl1 cells was less tip-clustered. As illustrated in Fig. 2F, there was a higher percentage of arl1/arl1 cells exhibiting non-clustered secretory vesicles, compared to the WT cells and the mutant over-expressing ARL1, 58 ± 8% compared to 12 ± 9% and 19 ± 10%, respectively. Interestingly, the WT Sec4 distribution in the arl1/arl1 mutant was restored by over-expression of YPT6, with only 20 ± 12% of cells with non-clustered secretory vesicles. Similar to that reported previously,18 the filament extension rate in the arl1/arl1 cells expressing GFP-Sec4 was slightly reduced compared to that of WT cells expressing GFP-Sec4 (12 ± 2 μm/h in arl1 compared to 15 ± 2 μm/h in WT). Overexpression of either ARL1 or YPT6 in this arl1/arl1 strain background restored the filament extension rate to that of the WT (19 ± 3 μm/h and 15 ± 2 μm/h, respectively) suggesting that the alteration in Sec4 distribution is associated with reduced filament extension rate. Interestingly, we observed that the ypt6 mutant also had a reduced filament extension rate (in preparation), similar to what was observed in A. nidulans.28
This study revealed that overexpression of the RAB6 homolog Ypt6 can rescue the hyphal growth defect and secretory vesicles clustering defect of an arl1 mutant. In HeLa cells, RAB6 regulates the movement and docking of secretory carriers to the plasma membrane30 and in specialized cell types, such as macrophages and developing neurons, this RAB6-dependent secretory pathway fulfills specific functions.31,32 In A. nidulans, deletion of the Ypt6 homolog, RabC, also resulted in impaired secretion.28 An attractive possibility is that the increased level of Ypt6 facilitates membrane traffic in the C. albicans arl1 mutant, perhaps via promoting targeting of secretory vesicles to the growth site. Alternatively, as the arl1 deletion mutant has a reduced hyphal extension rate, it is also possible that the alteration of Sec4 distribution results indirectly from reduced growth rather than from Arl1 regulation. Hence, over-expression of Ypt6 could rescue such a defect as a result of increasing the hyphal extension rate. In such a scenario, we can imagine that the Arl1 and Ypt6 genetic interaction during retrograde vesicular transport via the GARP (Golgi-associated retrograde protein) complex, observed in S. cerevisiae33–35, is critical specifically for hyphal growth, perhaps for lipid homeostasis.36 Further characterization of the arl1 and ypt6 mutants will be necessary to define the specific roles of these small GTPases during hyphal growth. C. albicans has only 9 Rab proteins, compared to nearly 70 in mammalian cells and 11 in S. cerevisiae. Indeed, homologs of S. cerevisiae Ypt10 and Ypt11 and homologs of Ypt4 (Human Rab4 homolog), present in filamentous fungi such as A. nidulans, are not present in C. albicans. Hence, C. albicans has the minimal protein trafficking machinery37 and identification of the regulators and effectors of critical Rab and Arf GTPases during transition between yeast and hyphal growth should shed light on the key elements of membrane traffic, sufficient to achieve morphological changes.
Materials and methods
Growth conditions
Yeast extract-peptone dextrose (YEPD) was used and strains were grown at 30°C, unless indicated otherwise. Filamentous growth induction was carried out as described previously either with 50% serum38 or Spider medium.39 Congo red and Hygromycin B were from Fluka, Sigma-Aldrich, Saint Quentin Fallavier, France.
Strains and plasmids
Strains used are listed in Table S1. All strains were derived from BWP17.40 The ypt6∆/ypt6∆ strain was generated by homologous recombination. Each copy was replaced by either HIS1 or URA3, using knockout cassettes generated by amplification of pGemHIS1 and pGemURA3.40 Primers with a unique RsrII, at the 5′ end, and a unique MluI, at the 3′ end, were used to amplify the YPT6 and ARL1 ORFs, and the fragments subsequently cloned into pExpArg-pADH1RAC1,41 yielding to pExpArg-pADH1YPT6 and pExpArg-pADH1ARL1, respectively.
pExpArg-pADH1YPT6[Q71L] and pExpArg-pADH1YPT6[T25N] were generated by site-directed mutagenesis of pExpArg-pADH1YPT6. The GFP-Sec4 expressing strains and the Mlc1-GFPγ expressing strains were generated as described.42,43 pExpArg-pYPT6mChYPT6 was constructed by cloning yemCherry (yeast enhanced monomeric Cherry), amplified by PCR from pFA-yemCherry plasmid using primer pairs with unique Pac1 site, into pExpArg-pYPT6YPT6. pExpArg-pYPT6YPT6 was constructed by amplifying from gDNA YPT6 ORF with 1 kb upstream and downstream, using primer pairs with unique Xho1 and Not1 sites at the 5′ and 3′ ends; pExpArg-pARF2ARF2,18 with unique Xho1 and Not1 sites, was used to subclone the PCR amplified fragment.
All pExpArg plasmids were linearized with StuI and integrated into the RP10 locus. Two independent clones of each strain were generated and confirmed by PCR. All PCR amplified products and site-directed mutagenesis products were confirmed by sequencing (Eurofins MWG Operon, Ebersberg, Germany).
Microscopy analyses
For colony morphology analyses, plates were incubated for 3–6 days prior to imaging. For cell morphology studies, cells were imaged by differential interference contrast. For GFP-Sec4 imaging, z-stacks were acquired as described.8 For Arl1 and Ypt6 co-localization experiments, GFP and yemCherry signals were acquired simultaneously and the analyses were carried out as described.8 Images were deconvolved, using Huygens Professional software (V3.7)
General techniques
RT-PCR analyses were carried out as described.44,45 Genomic DNA from C. albicans strains was isolated as described.46
Supplementary Material
Ackowledgments
We thank J. Konopka, Y. Wang and J. Wendland for reagents and S. Bogliolo for assistance. This work was supported by the CNRS, the iBV PRISM microscopy facility and grants from the ANR (ANR-13-BSV3-0006-01 and ANR-11-LABX-0028-01) and EU FP7 (PITN-GA-2013-607963).
References
- 1.Jacobsen ID, Wilson D, Wächtler B, Brunke S, Naglik JR, Hube B. Candida albicans dimorphism as a therapeutic target. Expert Rev Anti Infect Ther. 2012;10(1):85–93. doi: 10.1586/eri.11.152. PMID:22149617 [DOI] [PubMed] [Google Scholar]
- 2.Sudbery PE. Growth of Candida albicans hyphae. Nat Rev Microbiol. 2011;9(10):737–48. doi: 10.1038/nrmicro2636. PMID:21844880 [DOI] [PubMed] [Google Scholar]
- 3.Shapiro RS, Robbins N, Cowen LE. Regulatory circuitry governing fungal development, drug resistance, and disease. Microbiol Mol Biol Rev. 2011;75(2):213–67. doi: 10.1128/MMBR.00045-10. PMID:21646428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Naglik JR, Challacombe SJ, Hube B. Candida albicans secreted aspartyl proteinases in virulence and pathogenesis. Microbiol Mol Biol Rev. 2003;67(3):400–28, table of contents. doi: 10.1128/MMBR.67.3.400-428.2003. PMID:12966142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schaller M, Borelli C, Korting HC, Hube B. Hydrolytic enzymes as virulence factors of Candida albicans. Mycoses. 2005;48(6):365–77. doi: 10.1111/j.1439-0507.2005.01165.x. PMID:16262871 [DOI] [PubMed] [Google Scholar]
- 6.Hruskova-Heidingsfeldova O. Secreted proteins of Candida albicans. Front Biosci. 2008;13:7227–42. doi: 10.2741/3224. PMID:18508730 [DOI] [PubMed] [Google Scholar]
- 7.Jones LA, Sudbery PE. Spitzenkorper, exocyst, and polarisome components in Candida albicans hyphae show different patterns of localization and have distinct dynamic properties. Eukaryot Cell. 2010;9(10):1455–65. doi: 10.1128/EC.00109-10. PMID:20693302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghugtyal V, Garcia-Rodas R, Seminara A, Schaub S, Bassilana M, Arkowitz RA. Phosphatidylinositol-4-phosphate-dependent membrane traffic is critical for fungal filamentous growth. Proc Natl Acad Sci U S A. 2015;112(28):8644–9. doi: 10.1073/pnas.1504259112. PMID:26124136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rida PC, Nishikawa A, Won GY, Dean N. Yeast-to-hyphal transition triggers formin-dependent Golgi localization to the growing tip in Candida albicans. Mol Biol Cell. 2006;17(10):4364–78. doi: 10.1091/mbc.E06-02-0143. PMID:16855023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caballero-Lima D, Kaneva IN, Watton SP, Sudbery PE, Craven CJ. The spatial distribution of the exocyst and actin cortical patches is sufficient to organize hyphal tip growth. Eukaryot Cell. 2013;12(7):998–1008. doi: 10.1128/EC.00085-13. PMID:23666623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barr F, Lambright DG. Rab GEFs and GAPs. Curr Opin Cell Biol. 2010;22(4):461–70. doi: 10.1016/j.ceb.2010.04.007. PMID:20466531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delic M, Valli M, Graf AB, Pfeffer M, Mattanovich D, Gasser B. The secretory pathway: exploring yeast diversity. FEMS Microbiol Rev. 2013;37(6):872–914. doi: 10.1111/1574-6976.12020. PMID:23480475 [DOI] [PubMed] [Google Scholar]
- 13.Donaldson JG, Jackson CL. ARF family G proteins and their regulators: roles in membrane transport, development and disease. Nat Rev Mol Cell Biol. 2011;12(6):362–75. doi: 10.1038/nrm3117. PMID:21587297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gillingham AK, Munro S. The small G proteins of the Arf family and their regulators. Annu Rev Cell Dev Biol. 2007;23:579–611. doi: 10.1146/annurev.cellbio.23.090506.123209. PMID:17506703 [DOI] [PubMed] [Google Scholar]
- 15.Mizuno-Yamasaki E, Rivera-Molina F, Novick P. GTPase networks in membrane traffic. Annu Rev Biochem. 2012;81:637–59. doi: 10.1146/annurev-biochem-052810-093700. PMID:22463690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Segev N. Coordination of intracellular transport steps by GTPases. Semin Cell Dev Biol. 2011;22(1):33–8. doi: 10.1016/j.semcdb.2010.11.005. PMID:21130177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hedges SB. The origin and evolution of model organisms. Nat Rev Genet. 2002;3(11):838–49. doi: 10.1038/nrg929. PMID:12415314 [DOI] [PubMed] [Google Scholar]
- 18.Labbaoui H, Bogliolo S, Ghugtyal V, Solis NV, Filler SG, Arkowitz RA, Bassilana M. Role of Arf GTPases in fungal morphogenesis and virulence. PLoS Pathog. 2017;13(2):e1006205. doi: 10.1371/journal.ppat.1006205. PMID:28192532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu CJ, Lee FJ. Multiple activities of Arl1 GTPase in the trans-Golgi network. J Cell Sci. 2017;130(10):1691–1699. doi: 10.1242/jcs.201319. PMID:28468990 [DOI] [PubMed] [Google Scholar]
- 20.Benjamin JJ, Poon PP, Drysdale JD, Wang X, Singer RA. Johnston GC. Dysregulated Arl1, a regulator of post-Golgi vesicle tethering, can inhibit endosomal transport and cell proliferation in yeast. Mol Biol Cell. 2011;22(13):2337–47. doi: 10.1091/mbc.E10-09-0765. PMID:21562219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maresova L, Vydareny T, Sychrova H. Comparison of the influence of small GTPases Arl1 and Ypt6 on yeast cells' tolerance to various stress factors. FEMS Yeast Res. 2012;12(3):332–40. doi: 10.1111/j.1567-1364.2011.00780.x. PMID:22188384 [DOI] [PubMed] [Google Scholar]
- 22.McDonold CM, Fromme JC. Four GTPases differentially regulate the Sec7 Arf-GEF to direct traffic at the trans-golgi network. Dev Cell. 2014;30(6):759–67. doi: 10.1016/j.devcel.2014.07.016. PMID:25220393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang IH, Chen YJ, Hsu JW, Lee FS. The Arl3 and Arl1 GTPases co-operate with Cog8 to regulate selective autophagy via Atg9 trafficking. Traffic. 2017. doi: 10.1111/tra.12498. [DOI] [PubMed] [Google Scholar]
- 24.Yang S, Rosenwald A. Small GTPase proteins in macroautophagy. Small GTPases. 2016:1–6. doi: 10.1080/21541248.2016.1246280. PMID:27763811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang S, Rosenwald AG. Autophagy in Saccharomyces cerevisiae requires the monomeric GTP-binding proteins, Arl1 and Ypt6. Autophagy. 2016;12(10):1721–1737. doi: 10.1080/15548627.2016.1196316. PMID:27462928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Langemeyer L, Nunes Bastos R, Cai Y, Itzen A, Reinisch KM, Barr FA. Diversity and plasticity in Rab GTPase nucleotide release mechanism has consequences for Rab activation and inactivation. Elife. 2014;3:e01623. doi: 10.7554/eLife.01623. PMID:24520163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kawamura S, Nagano M, Toshima JY, Toshima J. Analysis of subcellular localization and function of the yeast Rab6 homologue, Ypt6p, using a novel amino-terminal tagging strategy. Biochem Biophys Res Commun. 2014;450(1):519–25. doi: 10.1016/j.bbrc.2014.06.002. PMID:24924636 [DOI] [PubMed] [Google Scholar]
- 28.Pantazopoulou A, Penalva MA, Characterization of Aspergillus nidulans RabC/Rab6. Traffic. 2011;12(4):386–406. doi: 10.1111/j.1600-0854.2011.01164.x. PMID:21226815 [DOI] [PubMed] [Google Scholar]
- 29.Pantazopoulou A, Pinar M, Xiang X, Peñalva MA. Maturation of late Golgi cisternae into RabE(RAB11) exocytic post-Golgi carriers visualized in vivo. Mol Biol Cell. 2014;25(16):2428–43. doi: 10.1091/mbc.E14-02-0710. PMID:24943841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grigoriev I, Splinter D, Keijzer N, Wulf PS, Demmers J, Ohtsuka T, Modesti M, Maly IV, Grosveld F, Hoogenraad CC. et al., Rab6 regulates transport and targeting of exocytotic carriers. Dev Cell. 2007;13(2):305–14. doi: 10.1016/j.devcel.2007.06.010. PMID:17681140 [DOI] [PubMed] [Google Scholar]
- 31.Micaroni M, Stanley AC, Khromykh T, Venturato J, Wong CX, Lim JP, Marsh BJ, Storrie B, Gleeson PA, Stow JL. Rab6a/a' are important Golgi regulators of pro-inflammatory TNF secretion in macrophages. PLoS One. 2013;8(2):e57034. doi: 10.1371/journal.pone.0057034. PMID:23437303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schlager MA, Kapitein LC, Grigoriev I, Burzynski GM, Wulf PS, Keijzer N, de Graaff E, Fukuda M, Shepherd IT, Akhmanova A. Pericentrosomal targeting of Rab6 secretory vesicles by Bicaudal-D-related protein 1 (BICDR-1) regulates neuritogenesis. EMBO J. 2010;29(10):1637–51. doi: 10.1038/emboj.2010.51. PMID:20360680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Panic B, Whyte JR, Munro S. The ARF-like GTPases Arl1p and Arl3p act in a pathway that interacts with vesicle-tethering factors at the Golgi apparatus. Curr Biol. 2003;13(5):405–10. doi: 10.1016/S0960-9822(03)00091-5. PMID:12620189 [DOI] [PubMed] [Google Scholar]
- 34.Siniossoglou S, Pelham HR. Vps51p links the VFT complex to the SNARE Tlg1p. J Biol Chem. 2002;277(50):48318–24. doi: 10.1074/jbc.M209428200. PMID:12377769 [DOI] [PubMed] [Google Scholar]
- 35.Tong AH, Lesage G, Bader GD, Ding H, Xu H, Xin X, Young J. Berriz GF, Brost RL, Chang M. Global mapping of the yeast genetic interaction network. Science. 2004;303(5659):808–13. doi: 10.1126/science.1091317. PMID:14764870 [DOI] [PubMed] [Google Scholar]
- 36.Frohlich F, Petit C, Kory N, Christiano R, Hannibal-Bach HK, Graham M, Liu X, Ejsing CS, Farese RV, Walther TC. The GARP complex is required for cellular sphingolipid homeostasis. Elife. 2015;4. doi: 10.7554/eLife.08712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pereira-Leal JB. The Ypt/Rab family and the evolution of trafficking in fungi. Traffic. 2008;9(1):27–38. doi: 10.1111/j.1600-0854.2007.00667.x. PMID:17973655 [DOI] [PubMed] [Google Scholar]
- 38.Bassilana M, Blyth J, Arkowitz RA. Cdc24, the GDP-GTP exchange factor for Cdc42, is required for invasive hyphal growth of Candida albicans. Eukaryot Cell. 2003;2(1):9–18. doi: 10.1128/EC.2.1.9-18.2003. PMID:12582118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Calera JA, Zhao XJ, Calderone R. Defective hyphal development and avirulence caused by a deletion of the SSK1 response regulator gene in Candida albicans. Infect Immun. 2000;68(2):518–25. doi: 10.1128/IAI.68.2.518-525.2000. PMID:10639412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wilson RB, Davis D, Mitchell AP. Rapid hypothesis testing with Candida albicans through gene disruption with short homology regions. J Bacteriol, 1999;181(6):1868–74. PMID:10074081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hope H, Bogliolo S, Arkowitz RA, Bassilana M. Activation of Rac1 by the guanine nucleotide exchange factor Dck1 is required for invasive filamentous growth in the pathogen Candida albicans. Mol Biol Cell. 2008;19(9):3638–51. doi: 10.1091/mbc.E07-12-1272. PMID:18579689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li CR, Lee RT, Wang YM, Zheng XD, Wang Y. Candida albicans hyphal morphogenesis occurs in Sec3p-independent and Sec3p-dependent phases separated by septin ring formation. J Cell Sci. 2007;120(Pt 11):1898–907. doi: 10.1242/jcs.002931. PMID:17504812 [DOI] [PubMed] [Google Scholar]
- 43.Zhang C, Konopka JB. A photostable green fluorescent protein variant for analysis of protein localization in Candida albicans. Eukaryot Cell. 2010;9(1):224–6. doi: 10.1128/EC.00327-09. PMID:19915075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bassilana M, Hopkins J, Arkowitz RA. Regulation of the Cdc42/Cdc24 GTPase module during Candida albicans hyphal growth. Eukaryot Cell. 2005;4(3):588–603. doi: 10.1128/EC.4.3.588-603.2005. PMID:15755921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hope H, Schmauch C, Arkowitz RA, Bassilana M. The Candida albicans ELMO homologue functions together with Rac1 and Dck1, upstream of the MAP Kinase Cek1, in invasive filamentous growth. Mol Microbiol. 2010;76(6):1572–90. doi: 10.1111/j.1365-2958.2010.07186.x. PMID:20444104 [DOI] [PubMed] [Google Scholar]
- 46.Reuss O, Vik A, Kolter R, Morschhäuser J. The SAT1 flipper, an optimized tool for gene disruption in Candida albicans. Gene. 2004;341:119–27. doi: 10.1016/j.gene.2004.06.021. PMID:15474295 [DOI] [PubMed] [Google Scholar]
- 47.Vernay A, Schaub S, Guillas I, Bassilana M, Arkowitz RA. A steep phosphoinositide bis-phosphate gradient forms during fungal filamentous growth. J Cell Biol. 2012;198(4):711–30. doi: 10.1083/jcb.201203099. PMID:22891265 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


