Abstract
Purpose of Review
COVID-19 (coronavirus viral disease 2019), due to the novel SARS-CoV-2, may present with different types of cutaneous manifestations of varying pathophysiology. During the ongoing pandemic, publications reporting dermatologic findings in COVID-19 continue to emerge.
Recent Findings
Cutaneous vasculopathy and microthrombus-related changes including acral and sacral lesions, retiform purpura, livedo reticularis, and cutaneous vasculitis are notable findings in adult patients. Other exanthems include urticaria or angioedema, morbilliform/maculopapular exanthems, erythema multiforme, and vesicular eruptions. Increased recognition of these findings, especially those consistent with cutaneous microthrombi or vasculitis, is of particular importance. Additionally, occupational dermatologic disease related to extended personal protective equipment (PPE) use, such as skin damage and irritant or allergic contact dermatitis (ACD), represents another emerging problem amidst the pandemic.
Summary
In this review, we highlight the various cutaneous manifestations associated with COVID-19 in adult patients and occupational dermatitis in health care workers (HCWs) caring for this patient population.
Keywords: COVID-19, SARS-CoV-2, Cutaneous manifestations, Dermatologic, Rash, Contact dermatitis
Introduction
The clinical manifestations of COVID-19 viral disease, due to the novel SARS-CoV-2, range from mild flu-like symptoms to critical illness with acute respiratory distress syndrome and cytokine storm with decreased adaptive immune response, portending high morbidity and mortality. Early reports of COVID-19 described any cutaneous manifestations in only 0.2% of 1099 confirmed cases in China [1]. However, as the pandemic evolved, increased reporting on associated dermatologic conditions in adult patients with COVID-19 emerged worldwide with an incidence ranging from approximately 5–20% [2–5]. Recalcati et al. observed cutaneous manifestations in 20.4% of 88 Italian patients afflicted with COVID-19 [2]. Galván Casas and colleagues prospectively classified cutaneous manifestations in 375 Spanish patients with suspected or confirmed COVID-19 disease (1.9% mortality rate) over the course of 2 weeks [3••]. Hedou et al. reported a 5% incidence of cutaneous manifestations associated with COVID-19 among 103 non-fatal cases in France [5]. Major cutaneous manifestations in adult patients include (1) urticaria, (2) maculopapular exanthem, (3) papulovesicular exanthem, (4) chilblain-like acral lesions, (5) livedo reticularis or racemosa, (6) purpuric vasculitis. Nearly all cutaneous findings can emerge during the prodromal, active, or convalescent phases of COVID-19 disease [6].
SARS-CoV-2 can purportedly induce cutaneous manifestations via direct viral binding or secondarily through various allergic-immunologic mediated mechanisms [7]. Binding of SARS-CoV-2 to the angiotensin-converting enzyme 2 (ACE2) receptor facilitates viral entry into epithelial cells, primarily in the upper respiratory mucosa. The ACE2 receptor is also expressed in the cutaneous/subcutaneous and vascular tissues and thus may contribute to dermatologic findings in SARS-CoV-2 infection [8]. COVID-19 dermatologic manifestations can be classified into (1) viral exanthems as an immune response to viral nucleotides or (2) systemic immunologic consequences of SARS-CoV-2 such as vasculopathy or micro-thrombotic skin lesions [9]. Viral exanthems encompass urticaria/angioedema, maculopapular or morbilliform rashes, vesicular eruptions, and erythema multiforme. Vasculitic-type lesions include chilblain-like acral lesions, sacral ulcerations, purpuric lesions, and vasculitis, ischemic, or necrotic lesions. Evidence suggests that cytokine release, coagulation pathway derangement, and complement-mediated microvascular injury play a role in the pathology of this latter group [10, 11]. Medication-induced hypersensitivity exanthems and petechiae in the setting of acquired thrombocytopenia represent other cutaneous findings. Table 1 summarizes the classification of dermatologic manifestations observed in adults with COVID-19.
Table 1.
Classification of cutaneous manifestations in adult patients with COVID-19
| Viral exanthems | Pathogenesis: Allergic-immunologic host response to viral nucleotides | Cutaneous lesions due to vasculopathy or micro-thrombi | Pathogenesis: Cytokine release, coagulation pathway derangement, complement-mediated microvascular injury, and/or microthrombi |
| Urticaria/angioedema |
• Presents before or with other COVID-19 manifestations •Consider drug hypersensitivity reaction |
Chilblain-like acral lesions |
• Likely presents later in disease course • May present in asymptomatic cases • Mostly self-limited but some may be acro-ischemic lesions • More prevalent in younger patients |
| Maculopapular/morbilliform |
•Most common cutaneous manifestation •Tend to present later in disease course •May or may not be pruritic •Consider drug hypersensitivity reaction •May be associated with peripheral eosinophilia |
Sacral lesions |
•Consider other patient risk factors for sacral decubitus ulcer •May require wound care including local debridement |
| Vesicular eruption |
•Can present before other COVID-19 symptoms •May represent more specific viral exanthema Consider herpes zoster reactivation or AGEP in differential diagnosis |
Cutaneous small vessel vasculitis | •Consider differential diagnosis of hypersensitivity vasculitis or urticarial vasculitis |
| Erythema multiforme |
•Likely viral etiology •Possible medication-induced •May have purpuric or atypical features |
Petechiae or purpura |
•Associated thrombocytopenia or ITP •Evolution of maculopapular exanthem or purpuric vasculitis •Retiform purpura represents a more severe finding associated with increased mortality |
| Pityriasis rosea | •May represent reactivation of human herpesvirus | Livedo reticularis/racemosa |
•Potential for systemic thrombo-embolic events •Associated with more severe disease and greater mortality |
New literature on COVID-19 cutaneous manifestations is continuously available. It is likely that dermatologic findings are either under-recognized or under-reported, especially in subclinical disease. The American Academy of Dermatology (AAD) COVID-19 Registry was created in an effort to gather information on COVID-19 cases and better define accompanying cutaneous lesions [12]. Preliminary results on 171 confirmed cases have been published and with increasing data the medical community can expect a greater understanding on the incidence and course of exanthems associated with SARS-CoV-2 infection [13]. In this review, we summarize reports and reviews on the varied cutaneous manifestations observed in adult patients with COVID-19 as well as the dermatologic conditions in HCWs pertaining to extended PPE use and scrupulous hand hygiene practices during the pandemic. Interspersed throughout this manuscript, we briefly describe cases from our own institutional experience. Table 2 summarizes select publications of cutaneous manifestations that had accompanying histologic examination, notated throughout the text.
Table 2.
Histopathological examination findings in cutaneous findings in adults with COVID-19
| Cutaneous manifestation | Histologic examination (other notes) | References |
|---|---|---|
| Maculopapular exanthema | Slight spongiosis, basal cell vacuolation; mild perivascular lymphocytic infiltrate (negative RT-PCR SARS-CoV-2 on whole-skin biopsy) | Ahouach et al. [14] |
| Superficial perivascular inflammation with eosinophils compatible with drug reaction | Rosell-Díaz et al. [15] | |
| Lichenoid pattern with eosinophils compatible with drug reaction | Rosell-Díaz et al. [15] | |
| Mild superficial perivascular lymphocytic infiltrate; spongiosis | Reymundo et al. [16] | |
| Erythema multiforme | Epidermal spongiosis; dilated vessels in dermis filled with neutrophils, extravasation of red blood cells; lymphocytic perivascular and interstitial infiltrate | Jimenez- Cauhe et al. [17•] |
| Vacuolar-type interface dermatitis with occasional necrotic keratinocytes | Rodríguez-Jiménez et al. [18] | |
| Vesicular eruption | Prominent non-ballooning acantholysis leading to the constitution of an intraepidermal unilocular vesicle, suprabasal location | Mahé et al. [19] |
| Basketweave hyperkeratosis; slightly atrophic epidermis; vacuolar degeneration of the basal layer with multinucleate, hyperchromatic keratinocytes and dyskeratotic cells; absence of inflammatory infiltrate | Marzano et al. [20••] | |
| Pityriasis rosea | Spongiosis with focal parakeratosis in the epidermis and a few rounded spongiotic vesicles containing aggregates of lymphocytes and Langerhans cells; moderate lymphohistiocytic infiltrate present in the superficial dermis; papillary dermal edema | Sanchez et al. [21] |
| Acral lesion | Diffuse dense lymphoid infiltrate of superficial and deep dermis with a perivascular pattern and signs of endothelial activation | Recalcati et al. [22] |
| Lichenoid dermatitis with perivascular mononuclear infiltrate and vascular microthrombi | de Masson et al. [23•] | |
| Lymphocytic perivascular and peri-eccrine infiltration; no vascular occlusion; no intravascular thrombi | Saenz Aguirre et al. [24•] | |
| Superficial and deep perivascular and perisudoral infiltrate of lymphocytes and histiocytes; slightly lichenoid; partial fibrinoid necrosis in deep dermal arteriole | Mahieu et al. [25] | |
| Superficial and deep lichenoid, perivascular, and peri-eccrine infiltrate of lymphocytes, with occasional plasma cells; vacuolar alteration along the basal layer of the epidermis; scattered necrotic keratinocytes; no intraluminal fibrin thrombi | Kolivras et al. [26•] | |
| Sacral ulcer | Fibrin thrombi in numerous blood vessels, consistent with a thrombotic vasculopathy | Young et al. [27] |
| Vasculitis | Leukocytoclastic vasculitis with extravasation of red blood cells; basal epidermal necrosis; dermal perivascular neutrophilic infiltration and fibrin deposition | Mayor-Ibarguren et al. [28] |
| Small vessel damage with fibrinoid necrosis of vessel wall; neutrophilic infiltration; leukocytoclasia; extravasated erythrocytes; granular deposition of C3 | Dominguez-Santas et al. [29] | |
| Spongiosis, focal vacuolar degeneration of base keratinocytes and focal lymphocytic exocytosis; slight inflammatory lymphomorphonuclear infiltrate of superficial dermis; occasional aspects of vessel wall damage (suspected drug-induced urticarial vasculitis) | Skroza et al. [30] | |
| Retiform purpura | Pauci-inflammatory thrombogenic vasculopathy involving capillaries, venules, and/or arterioles or small arteries; dermal arterial thrombosis; deposits of complement C5b-9 (also with features of acral livedo racemosa) | Droesch et al. [31] |
| Multiple thrombi occluding small vessels of the superficial and mid dermis; deposition of IgM, C3, fibrinogen, and C9 (retiform purpura with progressive thrombocytopenia) | Bosch-Amate et al. [32] | |
| Thrombogenic vasculopathy; extensive necrosis of epidermis and adnexal structures; interstitial and perivascular neutrophilia with leukocytoclasia; extensive deposition of C5b-9 in microvasculature | Magro et al. [6] | |
| Livedo reticularis or racemosa | Perivascular lymphocytic inflammation; increased superficial dermal mucin; necrotic keratinocytes consistent with viral exanthem | Khalil et al. [33] |
| Nest of Langerhans cells in the epidermis; microthrombi admixed with nuclear and eosinophilic debris in superficial and deep dermis | Giannotti et al. [34] | |
| Perivascular lymphocytic infiltrate in superficial dermis along with deeper-seated small thrombi within venules of deep dermis; vascular deposits of C5b-9 and C4d | Magro et al. [35•] | |
| SDRIFE | Subcorneal pustules and superficial infiltrates of lymphocytes and eosinophils | Chicharro et al. , [36] |
| AGEP | Subcorneal pustule with mild focal acanthosis and spongiosis, neutrophilic exocytosis, sparse keratinocyte necrosis, and a perivascular lymphocytic infiltrate with rare neutrophils and eosinophils | Robustelli Test et al. [37] |
| Spongiform subcorneal and intracorneal pustules; some keratinocyte necrosis; dermal inflammatory infiltrate of neutrophils with perivascular accentuation | Delaleu et al. [38] |
Cutaneous Manifestations in Adult Patients with COVID-19
Viral Exanthems
Urticaria and Angioedema
Urticaria represents a histamine-mediated reaction due to cutaneous mast cell degranulation, characterized by circumscribed wheals with surrounding erythema, either localized, scattered, or generalized in distribution. Histamine-mediated angioedema may accompany urticaria or occur in isolation, representing deeper dermal edema. Viral infections account for a major etiology of acute urticaria and/or angioedema. In the series by Galván Casas et al., 19% of patients had urticarial eruptions that lasted for about 1 week [3••]. Similarly, an extensive review of 997 patients from 9 different countries analyzed by Jia et al. found that approximately 22% of patients had urticarial eruptions [4••]. Urticaria with or without angioedema in the setting of confirmed or highly suspected COVID-19 infection has been observed in several reports and case series [2, 5, 39–47]. Similar to other viral infections, urticarial rash may precede or occur simultaneously with COVID-19 systemic manifestations (i.e., fever, cough) and last for several days [5, 39, 43, 44, 47, 48]. Larger studies suggest that urticaria is associated with more severe COVID-19 disease, though case reports anecdotally recount otherwise [3••]. Furthermore, acute urticaria may occur in asymptomatic or subclinical SARS-CoV-2 infection.
At our institution, we observed two cases of histaminergic-angioedema in the setting of confirmed SARS-CoV-2 infection. Both middle-aged adult patients had mild-to-moderate COVID-19 respiratory disease: one presented with profound lip and eyelid angioedema while the other experienced an episode of generalized facial angioedema with flushing and pruritus. There was a concern for azithromycin-related reaction in the latter which was subsequently disproven. These cutaneous-limited reactions rapidly responded to anti-histamine treatment.
Urticaria or angioedema in the setting of viral infection may be attributed to direct mast cell degranulation. Increased levels of cytokine IL-6 stimulate mast cells, resulting in activation and subsequent degranulation, leading to urticaria and/or angioedema [7, 49]. Another proposed mechanism includes the deposition of antigen-antibody complexes with complement activation and subsequent mast cell degranulation [49]. Immediate hypersensitivity due to medication must be considered in the differential diagnosis of acute urticaria/angioedema, especially if a temporal association between drug administration and urticaria/angioedema onset is apparent [41]. Non-sedating anti-histamines represent the preferred treatment for isolated acute urticaria/angioedema not part of a systemic anaphylactic reaction.
Maculopapular Exanthems
Maculopapular or morbilliform appearing exanthems can emerge during viral infections (i.e., EBV, HHV-6, HIV-1) in the presence or absence of medications, secondary to an immunologic response to viral antigens, and are mostly benign with a self-limited course. Maculopapular exanthems are characterized by erythematous, blanching scattered to confluent lesions that can be distributed throughout the body, with prominence for the chest, back, abdomen, and extremities. Maculopapular exanthem seems to be the most common cutaneous manifestation associated with COVID-19, occurring in 22–47% of cases [3, 4, 13]. Reports associated with SARS-CoV-2 infection have varied clinical descriptions such as development at disease onset or as a later manifestation with negative repeat SARS-CoV-2 RT-PCR, suggesting a potential drug-induced etiology [2, 3, 5, 14–16, 50–55]. These exanthems may be pruritic or non-pruritic. Reymundo and colleagues reported on 7 adult patients without recent medication use who primarily developed truncal exanthems later on in the course of SARS-CoV-2 infection [16]. Histologic findings are varied (Table 2). Galván Casas et al. noted that maculopapular exanthems were associated with more severe COVID-19 disease and a 2% mortality rate [3••]. Topical corticosteroids and liberal moisturization for skincare represent mainstays of treatment. Severe erythroderma or extensive body surface area involvement may require systemic steroid therapy.
Medications may be implicated in maculopapular exanthems, sometimes associated with peripheral eosinophilia. A sub-analysis of the cohort studied by Galván Casas et al. noted that 78% of maculopapular rashes had concomitant drug intake [56]. A case series by Rosell-Díaz et al. reported on 12 patients mainly with improvement in COVID-19 disease and in recent receipt of medications (i.e., lopinavir/ritonavir, hydroxychloroquine, remdesivir) who developed pruritic, maculopapular exanthems associated with peripheral eosinophilia [15]. Other reports note associated administration of hydroxychloroquine, azithromycin, beta-lactam antibiotics, and anti-viral medications [56]. Our allergy/immunology service evaluated critically ill patients with COVID-19 maculopapular exanthems with possible drug culprits including meropenem, hydroxychloroquine, and tocilizumab. Drug reactions remain a leading differential diagnosis of maculopapular rash. Thus, a comprehensive medication administration record review must be part of the evaluation. Severe cutaneous adverse reactions including DiHS/DRESS (drug-induced hypersensitivity syndrome/drug reaction with eosinophilia and systemic symptoms) syndrome represents a significant diagnostic consideration.
Erythema Multiforme
Erythema multiforme is characterized by macules, papules, and classic target lesions with a predominance to occur on the distal extremities. Mucosal involvement and systemic symptoms such as fever and arthralgias may be accompanying features. Associated with viral infections in the majority of cases, erythema multiforme has been observed in the setting of COVID-19 disease [17, 57]. Medications represent another etiology of erythema multiforme. The sub-analysis by Català et al. noted that erythema multiforme-like eruptions occurred in 9.7% (n = 17/176) of cases with a mean duration of 9.7 days [56]. Jimenez-Cauhe et al. described 4 adult women who developed erythema multiforme 16–24 days after the onset of COVID-19 symptoms [17•]. Typical target lesions and erythematous papules progressing to erythematous-violaceous patches with central pseudo-vesicles were noted; some had associated palatal macules and petechiae (Table 2). A report of erythema multiforme major with mucosal involvement also exists [58]. Furthermore, there is an overlap of erythema multiforme-like lesions with non-evanescent urticarial eruptions and atypical palmar plaques [18, 59].
Vesicular Eruptions
Vesicular rashes usually manifest as small, fluid-filled lesions on an erythematous base. In the cohort analyzed by Galván Casas and colleagues, vesicular eruptions with distinct monomorphic lesions primarily on the trunk or limbs were observed in 9% of cases [3••]. Most lesions were pruritic, appeared before the onset of other COVID-19 symptoms in 15% of cases, and lasted for a mean of about 10 days; some had hemorrhagic features. A case series on 22 patients with varicella-like papular-vesicular lesions and confirmed SARS-CoV-2 infection is insightful [20••]. The majority were middle-aged adult men with non-pruritic to mildly pruritic scattered lesions distributed on the trunk that presented a median of 3 days after onset of systemic COVID-19 symptoms; the mortality rate was 13.6% (n = 3/22) [20••]. Lesions resolved in about 8 days without scarring. Table 2 lists the histologic findings. The authors advised that vesicular eruption may be a specific COVID-19 cutaneous finding. A prospective observational study by Fernandez-Nieto et al. described the findings in 24 patients with vesicular eruptions; 10 required hospitalization and there were no fatalities [60••]. Two morphological patterns were noted: (1) diffuse polymorphic or (2) localized monomorphic lesions [60••]. The systematic review by Jia and colleagues found that 10% (n = 101/997) had vesicular rash [4••]. Other publications also reported pruritic vesicular eruption [2, 19, 54]. An important differential diagnosis in vesicular rash includes medication-induced acute generalized exanthematous pustulosis (AGEP). Additionally, a perceived increase in herpes zoster infection was documented among Spanish dermatologists and vesicular herpetic-like lesions were noted in patients afflicted with SARS-CoV-2 infection in Spain and Italy [3, 61]. It is unclear whether or not these lesions are due to Herpesviridae viral reactivation.
Pityriasis Rosea
Largely considered self-limited, pityriasis rosea manifests during human herpes virus reactivation. One report describes an erythematous and scaly annual plaque on the forearm starting 3 days after the onset of COVID-19 pneumonia, followed by generalized, pruritic papular and plaque-like lesions in a classic pityriasis rosea trailing collaret pattern [62]. A digitate papulosquamous, pityriasis rosea variant eruption was described in an elderly patient in another report [21]. It has been suggested that a robust immunologic-inflammatory response to SARS-CoV-2 can cause endogenous viral reactivation leading to pityriasis rosea [63].
Cutaneous Lesions Due to Vasculopathy or Micro-Thrombi
Based on autopsy findings, SARS-CoV-2 infection causes both macrovascular and microvascular thrombi in organ system vasculature including the cardio-pulmonary, renal, central nervous, and integumentary systems [64–66]. One proposed mechanism involves a cytokine storm reaction in which macrophage-induced inflammation leads to a pro-thrombotic state [67]. Purpuric or vasculitic skin manifestations due to SARS-CoV-2 infection include a spectrum of chilblains-like acral lesions, sacral “ulcers,” cutaneous small vessel vasculitis (CSSV), retiform purpura, livedo eruptions, and cutaneous ischemia/necrosis. The pathophysiology of these lesions is likely varied or multi-factorial including cutaneous micro-thrombi, vascular injury due to viral ACE2 receptor binding, or disseminated intravascular coagulation (DIC).
Chilblain-Like Eruptions and Acral Lesions
Acral cutaneous lesions (“COVID toes”) including chilblain-like lesions and those resembling pernio are likely interchangeable terms for similar or identical pathological findings in COVID-19. Acral lesions are mostly reported in pediatric patients with asymptomatic or mild disease, but may be found in adults with SARS-CoV-2 infection [22, 23, 68–70]. Chilblain-like lesions occur in adult patients with COVID-19 disease in 19–40% of cases [3, 4, 23]. Acral lesions present as asymmetric, self-limited, painful, erythematous to violaceous papules or plaques in an acral distribution with predominance for the feet [22, 27, 71]. Some may have associated bullous eruption or digital swelling [22, 25]. A description of 74 cases of acral lesions among pediatric and adult patients reported variable findings from erythematous to purpuric macules or papules [24•]. It is important to note that many patients were not subject to cold exposure nor had underlying disorders associated with pernio. Overall, pernio lesions are usually found in patients with less severe COVID-19 disease. The preliminary results published by the AAD found that 16% of patients with pernio lesions were hospitalized [13]. Chilblains may affect younger adults and tend to present later in the disease course, lasting for an approximate mean of 13 days [3••]. Mid to high potency topical corticosteroids can be considered in the management of acral lesions, particularly if they are pruritic or painful. Overall, acral lesions tend to resolve over the course of several weeks [25].
Although chilblain-like lesions during the COVID-19 pandemic are reportedly common, SARS-CoV-2 infection was not detected or not tested for in some cases [23–25, 70]. Some patients had cough and fever prior to the onset of acral lesions, whereas many had no COVID-19 symptoms and some had negative nasopharyngeal and rectal SARS-CoV-2 RT-PCR swabs; the authors suggested that patients had brief, asymptomatic COVID-19 infection prior to the onset of skin lesions [22]. Chilblain-like acral eruptions with asymptomatic or mild COVID-19 disease was also reported in a series of adolescents/young adults [72]. In a retrospective observational French study of patients with skin lesions encountered during an approximate 3-week pandemic period, acral lesions were observed in 51% (n = 142/277) of pediatric and adult patients, some of which had positive SARS-CoV-2 RT-PCR testing [23•]. Of all the cutaneous manifestations associated with COVID-19 disease, acral lesions may represent a comparatively more specific finding that could aid in the diagnosis of asymptomatic cases [3••].
The pathogenesis of acral chilblain-like lesions remains unclear. Several proposed mechanisms include cutaneous microthrombi, acquired coagulopathy, or CD8+ T lymphocyte endothelial cell cytotoxicity [23, 25]. Of note, SARS-CoV-2 viral particles were detected via electron microscopy in endothelial cells of lesional skin biopsies in pediatric patients, suggesting that chilblains are the result of vascular damage [73•]. An exaggerated innate immune response involving interferon cytokine signaling may be part of the pathogenesis [74•]. Histopathologic findings are noted in Table 2.
Sacral Ulcers
Sacral “ulcers” represent a peculiar finding in COVID-19 that require heightened awareness among medical providers, as they are distinct from sacral decubitus ulcers. Risk factors for the development of sacral decubitus ulcer include immobility and prolonged bed rest, incontinence, poor nutrition, diabetes, and vascular disease. Similar to other institutions, we have noted cases of sacral lesions and ulcerations in patients with critically ill, multi-organ system COVID-19 disease [26, 35]. Sacral ulcerations may present with purpuric lesions, violaceous induration, livedoid plaques, and eschars (Table 2). The pathogenesis is hypothesized to be multi-factorial including a combination of systemic coagulopathy, cutaneous ischemia, and pressure-induced deep tissue injury. Figure 1 depicts a slow healing, sacral “ulcer” in a middle-aged adult patient with a prolonged and complicated course of COVID-19 pneumonia including intubation for respiratory failure. Wound care consultation is warranted in these cases as some sacral ulcerative lesions require local debridement and removal of devitalized tissue. Specialized care must be taken to ensure that patients do not develop subsequent bacterial infection leading to sepsis.
Fig. 1.
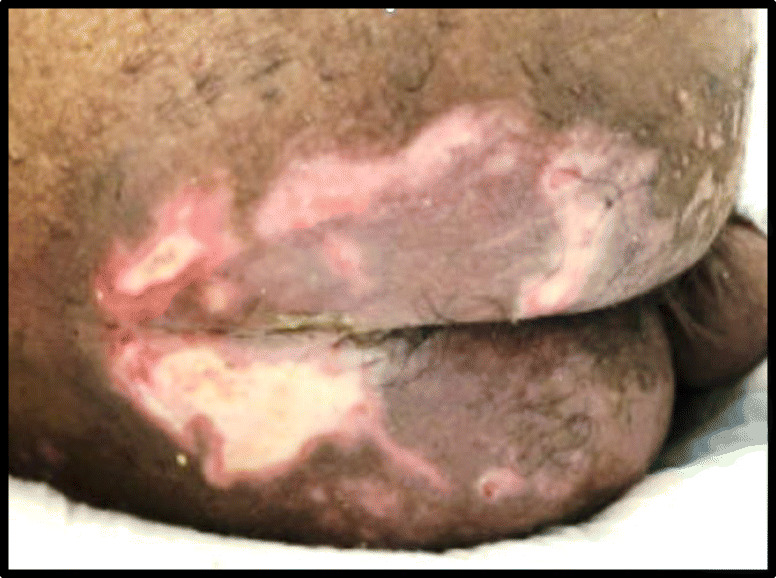
COVID-19-associated sacral “ulcer” in a patient with critical respiratory disease
Cutaneous Small Vessel Vasculitis
Cutaneous small vessel vasculitis (CSVV) is mostly mediated by immune complex deposition in the small vessels and subsequent complement-mediated inflammation and tissue destruction. CSVV is generally attributed to infection or medications, presenting as palpable purpura and/or non-blanching petechiae without extra-cutaneous organ involvement. Associated observations include urticaria, ulcerations, or hemorrhagic bullae. Several cases of CSVV associated with acute SARS-CoV-2 infection exist; findings include purpuric lesions or erythematous urticaria-like papules with central purpura or hyperpigmentation localized to the lower extremities or in a cranial-caudal distribution [28, 29, 35, 67, 75]. Vasculitic lesions may be painful and tend to appear during the latter part of active disease. Histopathological findings are listed in Table 2.
Medication-induced or hypersensitivity vasculitis is part of the differential diagnosis of CSVV. In one case, a 57-year-old woman developed a pruritic, erythematous maculopapular exanthem which progressed to painful, non-blanchable, purpuric plaques on the trunk and extremities [76]. She had received amoxicillin, ibuprofen, and metamizole; these medications were discontinued and the patient was successfully treated with topical and systemic steroids. Similarly, an adult man with COVID-19 developed a cranial-caudal, pruritic, erythematous urticarial eruption with central hyperpigmentation. Based on biopsy results and clinical features, the authors suggested a possible medication-induced urticarial vasculitis [30].
Retiform Purpura
Retiform purpura is classified as a more severe cutaneous finding in COVID-19 [10, 13, 74]. Published findings from the AAD COVID-19 Registry noted that all patients with retiform purpura were hospitalized and 82% had ARDS due to SARS-CoV-2 [13••]. Painful, retiform purpura with hemorrhagic blistering and evidence of small vessel thrombi and complement activation was observed in the setting of progressive thrombocytopenia with acute SARS-CoV-2 infection [32]. Retiform purpura with concomitant acral livedo racemosa has also been noted [31]. Histopathology shows complement deposits suggesting complement pathway activation involved in coagulopathic-driven thrombi (Table 2) [31, 32]. Furthermore, Magro et al. demonstrated pauci-inflammatory vascular thrombosis with extensive complement deposits and detection of SARS-CoV-2 protein localized to endothelial cells [35, 74].
Livedo Reticularis and Racemosa
Livedo reticularis or racemosa is defined by a mottled, lace- or net-like vascular pattern of erythematous to violaceous discoloration associated with ischemia of the cutaneous capillaries. Compared with other cutaneous findings in COVID-19, livedo reticularis seems less common (2.3%) but associated with more severe disease and possibly greater mortality [3, 4, 77]. Livedo eruptions are described in multiple case reports potentially due to inflammation caused by SARS-CoV-2 binding to vascular endothelium (Table 2) [31, 33, 77, 78]. Patients may be at risk for massive systemic thromboembolic events and multi-organ involvement [34, 77].
Ischemic and Necrotic Lesions
Ischemic lesions and necrosis of the fingers and toes are reported in critically ill patients with COVID-19 [79]. Laboratory markers indicating disseminated intravascular coagulation (DIC) were observed in 4 of 7 patients with acral dry gangrene; the mortality rate was 71%. Other reports describe fatal cases of COVID-19 in patients who had a necrotic ulcer on the sole and dry gangrene of the digits, toes, and nose with clinical DIC [80, 81].
Other Cutaneous Findings in Adults with COVID-19
Petechiae and Purpura Associated with Thrombocytopenia
SARS-CoV-2 may cause thrombocytopenia by several mechanisms including bone marrow suppression, consumptive coagulopathy, immune-mediated platelet destruction, or cytokine release syndrome [82]. Petechiae and purpura associated with mild to profound levels of thrombocytopenia, including cases of immune thrombocytopenic purpura (ITP) during COVID-19 disease, have been noted [83–87]. Several therapies including immunoglobulin replacement and eltrombopag were administered. Purpura with or without associated thrombocytopenia may also represent evolution of maculopapular exanthem or a feature of purpuric vasculitis as described in the above sections [20••].
Symmetrical Drug-Related Intertriginous and Flexural Exanthema
Symmetrical drug-related intertriginous and flexural exanthema (SDRIFE) represents a cutaneous reaction to systemic drug administration, with a unique distribution and morphology. An SDRIFE-like case was reported in a woman with COVID-19 who developed an erythematous rash on the bilateral antecubital fossae, trunk, and axillary folds; she had only been in receipt of paracetamol [52]. Another case report describes an atypical SDRIFE case in a 73-year-old woman with confirmed SARS-CoV-2 infection who developed erythema on the axillae, antecubital fossae, and trunk and medial thighs [36]. Although there were no clinically evident pustules, histopathologic examination suggested AGEP and she had received azithromycin and hydroxychloroquine (Table 2).
Acute Generalized Exanthematous Pustulosis
Acute generalized exanthematous pustulosis is a rare, medication-induced, severe cutaneous adverse reaction with a mortality rate upwards of 5%. An elderly patient with COVID-19 pneumonia who had received treatment with lopinavir/ritonavir and hydroxychloroquine developed a diffuse, pruritic pustular eruption on the extremities and trunk; targetoid lesions were also present [37]. Other reports of acute generalized exanthematous pustulosis (AGEP) attributed to hydroxychloroquine in the setting of SARS-CoV-2 infection, including fatal cases, have also been described [38, 88]. Table 2 lists histopathologic findings.
Dermatologic Conditions Related to the COVID-19 Pandemic
Occupational Dermatitis
The PPE recommendations for direct encounters of patients with SARS-CoV-2 infection include a respirator facemask, eye protection (goggles or face shield), gloves, and isolation gowns. Occupational dermatitis among HCWs represents an emerging problem amidst the COVID-19 pandemic. According to data from China, 74% of HCWs (n = 280/376) reported adverse skin reactions due to PPE use and hand hygiene practices, including xerosis, desquamation, erythema, papules, and maceration [89]. Female sex, PPE usage for more than 6 h daily, and increased hand washing frequency were significant risk factors [89]. Other publications report similar findings among HCWs [90, 91]. Both irritant contact dermatitis (ICD) and allergic contact dermatitis (ACD), particularly due to frequent hand washing or alcohol-based sanitizer and extended or repeated PPE use, can occur. Pressure injuries and skin damage attributed to facemask and goggle use are other major harms incurred by HCWs.
Irritant Contact Dermatitis
ICD accounts for approximately 80% of all contact dermatitis, developing on sites exposed to irritant agents which activate the innate immune system inflammatory response causing direct skin damage. Hand hygiene represents a hallmark of SARS-CoV-2 transmission prevention. Several organizations promote the practice of frequent hand washing with surfactant-containing detergents or cleansing with alcohol-based sanitizers [92]. Increased handwashing frequency translates to increased exposure to water and detergents, which can cause local epidermal barrier dysfunction and keratinocyte impairment. Surfactants deplete protective lipids and ceramides from the skin. In turn, physical barrier disruptions increase skin permeability and susceptibility to physical or chemical irritants.
Hand dermatitis induced by frequent hand washing includes a spectrum of findings: cutaneous xerosis, dyshidrotic eczema, and ICD especially in patients with underlying atopic dermatitis (AD) [93]. The distribution may be localized to the finger webs and finger tips or involve the wrist and dorsal or ventral aspects of the hand. Repeated use of alcohol-based sanitizers can quickly lead to skin dryness and subsequent ICD. Beiu and colleagues reported on hand ICD and eczema flare due to frequent hand washing in the context of COVID-19 [93]. Prolonged and/or frequent glove wearing in the setting of compromised skin integrity can further exacerbate hand dermatitis due to an added inflammatory response. Although frequent hand washing may be unavoidable for HCWs during the pandemic, applying a skin emollient or protectant can mitigate symptoms [93]. The AAD recommends application of a petrolatum-containing moisturizer between hand washings and whenever practical, especially while not working or overnight in order to reduce trans-epidermal water loss [94].
Facemask-induced itch seems to be prevalent (19.6% of 1393 persons) according to a self-questionnaire study conducted in Poland [95]. Some patients who reported sensitive skin and AD were at significantly greater risk of pruritus; whether these patients have a tendency to develop ICD or ACD remains a subject of investigation. The application of disinfectants to face masks has been associated with retro-auricular ICD [96]. Similar to hand dermatitis, management of other ICD involves basic skin care with moisturizing creams, the use of emollients or skin protectants when feasible, and avoidance of irritant substances.
Allergic Contact Dermatitis
ACD represents a T lymphocyte–mediated, delayed-type hypersensitivity reaction of the skin due to previous sensitization to a hapten contact allergen. Acute ACD manifests as pruritic, erythematous, eczematous-appearing plaques, with papule, vesicle or bullous formation in moderate-to-severe cases. Chronic ACD tends to have scaling, fissures, or lichenification. There are thousands of contact sensitizers,; however, those likely most relevant to facemask-induced ACD include textile dyes and preservatives. Patch testing represents the gold standard in diagnosing ACD. We have evaluated two HCWs with moderate-to-severe facial ACD who exhibited positive patch test (PT) to textile dye mix. Figure 2 illustrates peri-auricular ACD related to surgical mask wear and positive PT reaction to textile dye mix at the 72-h reading. Other identified contact allergens include polyurethanes (toluene-2,4-diisocyanate, 4,4′-diaminodiphenylmethane, hexamethylene diisocyanate) and formaldehyde-releasing preservatives (formaldehyde, 2-bromo-2-nitropropane-1,3-diol) [97, 98]. Trace amounts of formaldehyde can be present in polypropylene masks as a degradation by-product [99]. A polyurethane sponge lined within a KN95 facemask was implicated in ACD involving the nasal bridge and zygomatic arches [97]. Retro-auricular ACD due to facemask ear loops composed of different materials including thermoplastic elastomer, rubber, and latex has been described [96]. Other potential allergens contained within PPE include rubber accelerators (thiuram, carbamates), other preservatives (methyldibromo glutaronitrile, quaternium-15, imidazolinylurea), paraphenylenediamine, and aluminum [100]. Upon identification of a relevant contact allergen through patch testing, the mainstay of ACD management is avoidance of that particular substance. ACD treatment may require topical and/or systemic corticosteroid therapy depending on the dermatitis severity.
Fig. 2.
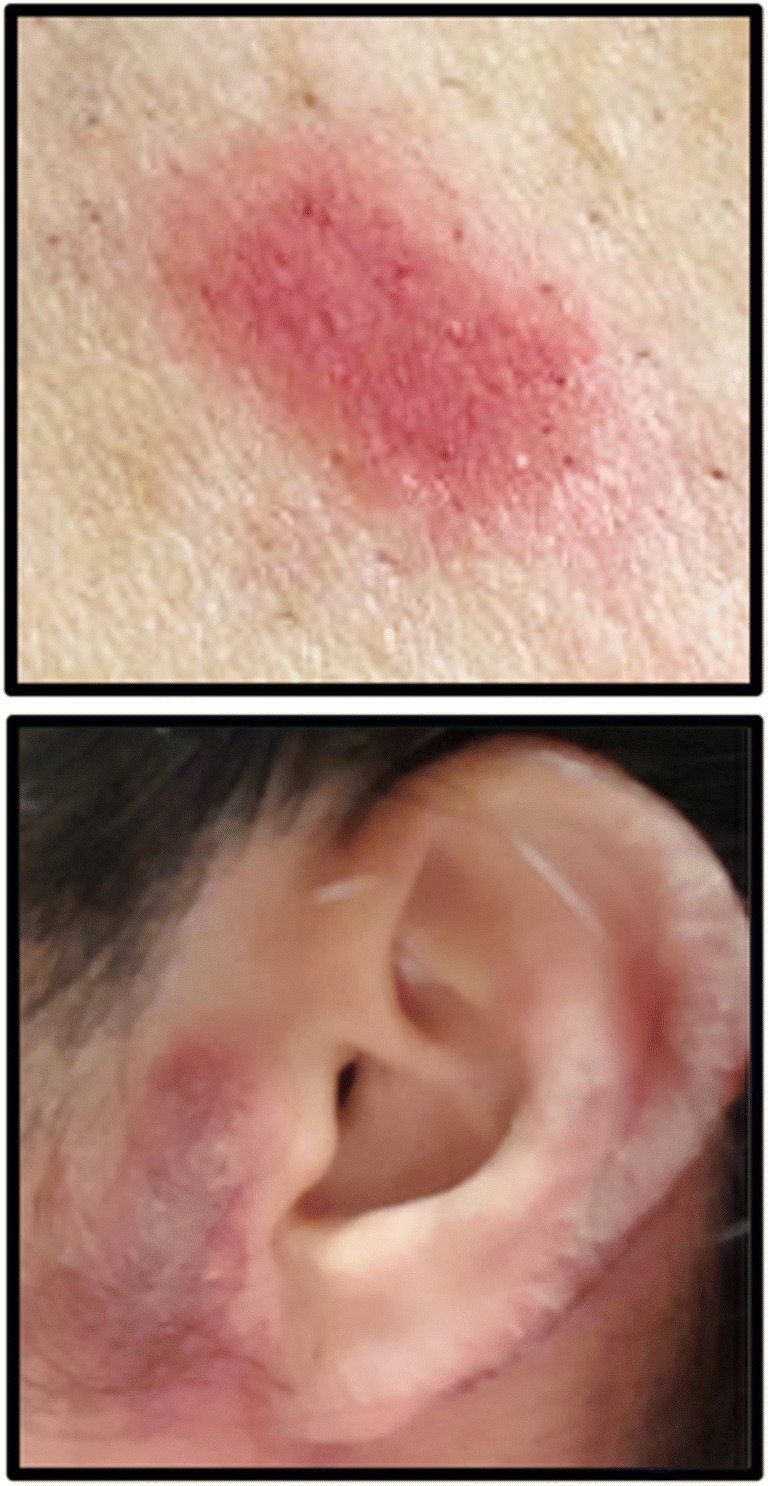
Positive patch test reaction to textile dye mix in a health care worker with severe facial allergic contact dermatitis due to surgical mask
Skin Damage
Skin damage associated with PPE use, especially N95 facemasks and goggles, among HCWs is prevalent, as high as 97% in one study (n = 526/542) [90•]. Various types of skin damage reported include skin desquamation, maceration, fissures, erosions, and ulcers on the cheeks, forehead, and nasal bridge [89, 90]. The European Task Force on Contact Dermatitis recommends the use of hydrocolloid dressings at pressure points on the face and ears to reduce friction as well as restricting prolonged duration of PPE use when possible [101]. Caution must be advised on the use of any product placed between the skin and a N95 respirator as the tight seal required for mask efficacy must not be compromised [102]. Recommendations from the AAD include applying a liquid sealant to relevant contact areas and allowing it to fully dry before N95 mask wear [94].
Conclusions
In summary, SARS-CoV-2 has been associated with several different cutaneous manifestations, likely of varying pathophysiology, some preceding COVID-19 symptomatology and others occurring during active disease or later in the course. Adult patients exhibiting COVID-19 cutaneous manifestations may demonstrate a range of illness severity. Some findings are cutaneous-limited and benign, likely due to an immunologic response to the virus itself, while vasculopathy or thrombotic-type lesions may harbor extra-cutaneous, life-threatening systemic involvement. Given the varied rashes associated with COVID-19 and their respective differential diagnoses, cutaneous manifestations related to SARS-CoV-2 infection can represent a diagnostic dilemma. A pathognomonic dermatologic finding in COVID-19 is not currently apparent. Urticaria, angioedema, maculopapular exanthems, erythema multiforme, and petechiae seem to be nonspecific while purpuric and cutaneous vasculitic findings could aid in the diagnosis and portend more severe disease. Thus, heightened awareness and timely recognition of dermatologic findings in COVID-19 are important. Additionally, HCWs may suffer deep tissue injury or dermatitis including ACD due to extended or repeated PPE use. Continued research and reporting will more precisely determine the incidence, underlying pathophysiology, potential prognostication, and optimal treatments of cutaneous manifestations in COVID-19 disease.
Abbreviations
- AAD
American Academy of Dermatology
- ACD
Allergic contact dermatitis
- ACE2
Angiotensin-converting enzyme 2
- AD
Atopic dermatitis
- AGEP
Acute generalized exanthematous pustulosis
- ARDS
Acute respiratory distress syndrome
- COVID-19
Coronarvirus viral disease 2019
- CSSV
Cutaneous small vessel vasculitis
- DIC
Disseminated intravascular coagulation
- DiHS/DRESS
Drug-induced hypersensitivity syndrome/drug reaction with eosinophilia and systemic symptoms
- HCW
Health care worker
- ICD
Irritant contact dermatitis
- ITP
Immune thrombocytopenic purpura
- PPE
Personal protective equipment
- PT
Patch test
- RT-PCR
Reverse transcriptase polymerase chain reaction
- SARS-CoV-2
Severe acute respiratory distress syndrome coronavirus 2
- SDRIFE
Symmetrical drug-related intertriginous and flexural exanthema
Compliance with Ethical Standards
Conflict of Interest
The authors declare no conflicts of interest relevant to this manuscript.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Allergic Skin Diseases
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS, China Medical Treatment Expert Group for Covid-19 Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatol Venereol. 2020;34(5):e212–e213. doi: 10.1111/jdv.16387.COMMENT. [DOI] [PubMed] [Google Scholar]
- 3.Galván Casas C, Català A, Carretero Hernández G, et al. Classification of the cutaneous manifestations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol. 2020;183(1):71–77. doi: 10.1111/bjd.19163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jia JL, Kamceva M, Rao SA, Linos E. Cutaneous manifestations of COVID-19: a preliminary review. J Am Acad Dermatol. 2020;83(2):687–690. doi: 10.1016/j.jaad.2020.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hedou M, Carsuzaa F, Chary E, Hainaut E, Cazenave-Roblot F, Masson RM. Comment on “Cutaneous manifestations in COVID-19: a first perspective” by Recalcati S. J Eur Acad Dermatol Venereol. 2020;34(7):e299–e300. doi: 10.1111/jdv.16519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rahimi H, Tehranchinia Z. A comprehensive review of cutaneous manifestations associated with COVID-19. Biomed Res Int. 2020;2020:1236520. Published 2020 Jul 5. doi:10.1155/2020/1236520 [DOI] [PMC free article] [PubMed]
- 7.Criado PR, Abdalla BMZ, de Assis IC, van Blarcum de Graaff MC, Caputo GC, Vieira IC. Are the cutaneous manifestations during or due to SARS-CoV-2 infection/COVID-19 frequent or not? Revision of possible pathophysiologic mechanisms. Inflamm Res. 2020;69(8):745–756. doi: 10.1007/s00011-020-01370-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li MY, Li L, Zhang Y, Wang XS. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect Dis Poverty. 2020;9(1):45. doi: 10.1186/s40249-020-00662-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suchonwanit P, Leerunyakul K, Kositkuljorn C. Cutaneous manifestations in COVID-19: lessons learned from current evidence. J Am Acad Dermatol. 2020;83(1):e57–e60. doi: 10.1016/j.jaad.2020.04.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suchonwanit P, Leerunyakul K, Kositkuljorn C. Diagnostic and prognostic values of cutaneous manifestations in COVID-19 [published online ahead of print, 2020 May 23] Dermatol Ther. 2020;2020:e13650. doi: 10.1111/dth.13650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xiong M, Liang X, Wei YD. Changes in blood coagulation in patients with severe coronavirus disease 2019 (COVID-19): a meta-analysis. Br J Haematol. 2020;189(6):1050–1052. doi: 10.1111/bjh.16725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.COVID-19 Dermatology Registry. American Academy of Dermatology Association. https://www.aad.org/member/practice/coronavirus/registry. Accessed August 30, 2020.
- 13.•• Freeman EE, McMahon DE, Lipoff JB, et al. The spectrum of COVID-19-associated dermatologic manifestations: an international registry of 716 patients from 31 countries [published online ahead of print, 2020 Jul 2]. J Am Acad Dermatol. 2020;S0190–9622(20)32126–5. 10.1016/j.jaad.2020.06.1016. A large data registry collection of suspected or confirmed COVID-19 cases and associated dermatologic findings, submitted by medical professionals.
- 14.Ahouach B, Harent S, Ullmer A, Martres P, Bégon E, Blum L, Tess O, Bachmeyer C. Cutaneous lesions in a patient with COVID-19: are they related? Br J Dermatol. 2020;183(2):e31. doi: 10.1111/bjd.19168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosell-Díaz AM, Mateos-Mayo A, Nieto-Benito LM, et al. Exanthema and eosinophilia in COVID-19 patients: has viral infection a role in drug induced exanthemas? [published online ahead of print, 2020 Jun 4]. J Eur Acad Dermatol Venereol. 2020. 10.1111/jdv.16709. [DOI] [PMC free article] [PubMed]
- 16.Reymundo A, Fernáldez-Bernáldez A, Reolid A, et al. Clinical and histological characterization of late appearance maculopapular eruptions in association with the coronavirus disease 2019. A case series of seven patients [published online ahead of print, 2020 Jun 4]. J Eur Acad Dermatol Venereol. 2020. 10.1111/jdv.16707. [DOI] [PMC free article] [PubMed]
- 17.• Jimenez-Cauhe J, Ortega-Quijano D, Carretero-Barrio I, et al. Erythema multiforme-like eruption in patients with COVID-19 infection: clinical and histological findings [published online ahead of print, 2020 May 9]. Clin Exp Dermatol. 2020. 10.1111/ced.14281. A small series describing the clinical characteristics of erythema-multiforme exanthems in patients with COVID-19, including histopathologic findings. [DOI] [PMC free article] [PubMed]
- 18.Rodríguez-Jiménez P, Chicharro P, de Argila D, et al. Reply to “acute urticaria with pyrexia as the first manifestations of a COVID-19 infection”: urticaria-like lesions in COVID-19 patients are not really urticaria. A case with clinicopathologic correlation. J Eur Acad Dermatol Venereol. 2020. 10.1111/jdv.16618.
- 19.Mahé A, Birckel E, Merklen C, et al. Histology of skin lesions establishes that the vesicular rash associated with COVID-19 is not ‘varicella-like’ [published online ahead of print, 2020 Jun 5]. J Eur Acad Dermatol Venereol. 2020. 10.1111/jdv.16706. [DOI] [PMC free article] [PubMed]
- 20.Marzano AV, Genovese G, Fabbrocini G, et al. Varicella-like exanthem as a specific COVID-19-associated skin manifestation: multicenter case series of 22 patients. J Am Acad Dermatol. 2020;83(1):280–285. doi: 10.1016/j.jaad.2020.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanchez A, Sohier P, Benghanem S, et al. Digitate papulosquamous eruption associated with severe acute respiratory syndrome coronavirus 2 infection [published online ahead of print, 2020 Apr 30]. JAMA Dermatol. 2020. 10.1001/jamadermatol.2020.1704. [DOI] [PubMed]
- 22.Recalcati S, Barbagallo T, Frasin LA, et al. Acral cutaneous lesions in the time of COVID-19. J Eur Acad Dermatol Venereol. 2020;34(8):e346–e347. doi: 10.1111/jdv.16533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Masson A, Bouaziz JD, Sulimovic L, et al. Chilblains is a common cutaneous finding during the COVID-19 pandemic: a retrospective nationwide study from France. J Am Acad Dermatol. 2020;83(2):667–670. doi: 10.1016/j.jaad.2020.04.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.• Saenz Aguirre A, De la Torre Gomar FJ, Rosés-Gibert P, Gimeno Castillo J, de Lagrán Alvarezr de Arcaya Martinez Z, Gonzalez-Perez R. Novel outbreak of acral lesions in times of COVID-19: a description of 74 cases from a tertiary university hospital in Spain [published online ahead of print, 2020 May 18]. Clin Exp Dermatol. 2020. 10.1111/ced.14294. A large description of acral lesions in pediatric and adult patients with mostly suspected COVID-19. [DOI] [PMC free article] [PubMed]
- 25.Mahieu R, Tillard L, Le Guillou-Guillemette H, et al. No antibody response in acral cutaneous manifestations associated with COVID-19? [published online ahead of print, 2020 Jun 2]. J Eur Acad Dermatol Venereol. 2020. 10.1111/jdv.16688. [DOI] [PMC free article] [PubMed]
- 26.• Young S, Narang J, Kumar S, et al. Large sacral/buttocks ulcerations in the setting of coagulopathy: a case series establishing the skin as a target organ of significant damage and potential morbidity in patients with severe COVID-19 [published online ahead of print, 2020 Aug 7]. Int Wound J. 2020. 10.1111/iwj.13457. A case series highlighting the findings of sacral ulcers associated with COVID-19 disease. [DOI] [PMC free article] [PubMed]
- 27.Kolivras A, Dehavay F, Delplace D, et al. Coronavirus (COVID-19) infection-induced chilblains: a case report with histopathologic findings. JAAD Case Rep. 2020;6(6):489–492. doi: 10.1016/j.jdcr.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mayor-Ibarguren A, Feito-Rodriguez M, Quintana Castanedo L, Ruiz-Bravo E, Montero Vega D, Herranz-Pinto P. Cutaneous small vessel vasculitis secondary to COVID-19 infection: a case report [published online ahead of print, 2020 May 22]. J Eur Acad Dermatol Venereol. 2020. 10.1111/jdv.16670. [DOI] [PMC free article] [PubMed]
- 29.Dominguez-Santas M, Diaz-Guimaraens B, Garcia Abellas P, Moreno-Garcia Del Real C, Burgos-Blasco P, Suarez-Valle A. Cutaneous small-vessel vasculitis associated with novel 2019 coronavirus SARS-CoV-2 infection (COVID-19) [published online ahead of print, 2020 May 26]. J Eur Acad Dermatol Venereol. 2020. 10.1111/jdv.16663. [DOI] [PMC free article] [PubMed]
- 30.Skroza N, Bernardini N, Balduzzi V, et al. A late-onset widespread skin rash in a previous COVID-19-infected patient: viral or multidrug effect? [published online ahead of print, 2020 May 18]. J Eur Acad Dermatol Venereol. 2020. 10.1111/jdv.16633. [DOI] [PMC free article] [PubMed]
- 31.Droesch C, Hoang M, DeSancho M, Lee EJ, Magro C, Harp J. Livedoid and purpuric skin eruptions associated with coagulopathy in severe COVID-19 [published online ahead of print, 2020 Aug 5] JAMA Dermatol. 2020;2020:e202800. doi: 10.1001/jamadermatol.2020.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bosch-Amate X, Giavedoni P, Podlipnik S, et al. Retiform purpura as a dermatological sign of coronavirus disease 2019 (COVID-19) coagulopathy [published online ahead of print, 2020 Jun 3]. J Eur Acad Dermatol Venereol. 2020. 10.1111/jdv.16689. [DOI] [PMC free article] [PubMed]
- 33.Khalil S, Hinds BR, Manalo IF, Vargas IM, Mallela S, Jacobs R. Livedo reticularis as a presenting sign of severe acute respiratory syndrome coronavirus 2 infection. JAAD Case Rep. 2020;6(9):871–874. doi: 10.1016/j.jdcr.2020.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gianotti R, Recalcati S, Fantini F, et al. Histopathological study of a broad spectrum of skin dermatoses in patients affected or highly suspected of infection by COVID-19 in the northern part of Italy: analysis of the many faces of the viral-induced skin diseases in previous and new reported cases. Am J Dermatopathol. 2020;42(8):564–570. doi: 10.1097/DAD.0000000000001707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of 5 cases. Transl Res. 2020;220:1–13. doi: 10.1016/j.trsl.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chicharro P, Rodríguez-Jiménez P, Muñoz-Aceituno E, De Argila D, Muñoz-Hernández P, Llamas-Velasco M. SDRIFE-like rash associated with COVID-19, clinicopathological correlation [published online ahead of print, 2020 Aug 19]. Australas J Dermatol. 2020. 10.1111/ajd.13444. [DOI] [PMC free article] [PubMed]
- 37.Robustelli Test E, Vezzoli P, Carugno A, et al. Acute generalized exanthematous pustulosis with erythema multiforme-like lesions induced by hydroxychloroquine in a woman with coronavirus disease 2019 (COVID-19) [published online ahead of print, 2020 May 9]. J Eur Acad Dermatol Venereol. 2020. 10.1111/jdv.16613. [DOI] [PMC free article] [PubMed]
- 38.Delaleu J, Deniau B, Battistella M, et al. Acute generalized exanthematous pustulosis induced by hydroxychloroquine prescribed for COVID-19. J Allergy Clin Immunol Pract. 2020;8(8):2777–2779.e1. doi: 10.1016/j.jaip.2020.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Medeiros VLS, Silva LFT. Follow-up of skin lesions during the evolution of COVID-19: a case report [published online ahead of print, 2020 May 14]. Arch Dermatol Res 2020;1–4. doi:10.1007/s00403-020-02091-0. [DOI] [PMC free article] [PubMed]
- 40.Cepeda-Valdes R, Carrion-Alvarez D, Trejo-Castro A, Hernandez-Torre M, Salas-Alanis J. Cutaneous manifestations in COVID-19: familial cluster of urticarial rash [published online ahead of print, 2020 May 14]. Clin Exp Dermatol. 2020. 10.1111/ced.14290, 10.1111/ced.14290. [DOI] [PMC free article] [PubMed]
- 41.Aktaş H, Hamidi AA. Urticaria in a patient with COVID-19: therapeutic and diagnostic difficulties [published online ahead of print, 2020 May 17] Dermatol Ther. 2020;2020:e13610. doi: 10.1111/dth.13610. [DOI] [PubMed] [Google Scholar]
- 42.Naziroğlu T, Sözen S, Özkan P, Şeker S, Aksu K. A case of COVID-19 pneumonia presenting with acute urticaria [published online ahead of print, 2020 May 13] Dermatol Ther. 2020;2020:e13575. doi: 10.1111/dth.13575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Henry D, Ackerman M, Sancelme E, Finon A, Esteve E. Urticarial eruption in COVID-19 infection. J Eur Acad Dermatol Venereol. 2020;34(6):e244–e245. doi: 10.1111/jdv.16472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Damme C, Berlingin E, Saussez S, Accaputo O. Acute urticaria with pyrexia as the first manifestations of a COVID-19 infection. J Eur Acad Dermatol Venereol. 2020;34(7):e300–e301. doi: 10.1111/jdv.16523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Adeliño R, Andrés-Cordón JF, De La Cruz Aracelis Martínez C. Acute urticaria with angioedema in the setting of coronavirus disease 2019. J Allergy Clin Immunol Pract. 2020;8(7):2386–2387. doi: 10.1016/j.jaip.2020.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Elhag SA, Ibrahim H, Abdelhadi S. Angioedema and urticaria in a COVID-19 patient: a case report and review of the literature [published online ahead of print, 2020 Aug 5]. JAAD Case Rep. 2020. 10.1016/j.jdcr.2020.07.042, 10.1016/j.jdcr.2020.07.042. [DOI] [PMC free article] [PubMed]
- 47.Najafzadeh M, Shahzad F, Ghaderi N, Ansari K, Jacob B, Wright A. Urticaria (angioedema) and COVID-19 infection [published online ahead of print, 2020 Jun 11]. J Eur Acad Dermatol Venereol. 2020. 10.1111/jdv.16721. [DOI] [PMC free article] [PubMed]
- 48.Hassan K. Urticaria and angioedema as a prodromal cutaneous manifestation of SARS-CoV-2 (COVID-19) infection. BMJ Case Rep. 2020;13(7):e236981. doi: 10.1136/bcr-2020-236981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kaushik A, Parsad D, Kumaran MS. Urticaria in the times of COVID-19 [published online ahead of print, 2020 Jun 12] Dermatol Ther. 2020;2020:e13817. doi: 10.1111/dth.13817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Paolino G, Canti V, Mercuri SR, Rovere Querini P, Candiani M, Pasi F. Diffuse cutaneous manifestation in a new mother with COVID-19 (SARSCov-2). Int J Dermatol. 2020;59(7):874–5. 10.1111/ijd.14919. [DOI] [PMC free article] [PubMed]
- 51.Rossi E, Lasagni C, Trakatelli M, Wertzberger Rowan S, Magnoni C. Acute maculopapular eruption in Covid-19 patient: a case report [published online ahead of print, 2020 Jun 11] Dermatol Ther. 2020;2020:e13812. doi: 10.1111/dth.13812. [DOI] [PubMed] [Google Scholar]
- 52.Mahé A, Birckel E, Krieger S, Merklen C, Bottlaender L. A distinctive skin rash associated with coronavirus disease 2019? J Eur Acad Dermatol Venereol. 2020;34(6):e246–e247. doi: 10.1111/jdv.16471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hunt M, Koziatek C. A case of COVID-19 pneumonia in a young male with full body rash as a presenting symptom. Clin Pract Cases Emerg Med. 2020;4(2):219–221. doi: 10.5811/cpcem.2020.3.47349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sachdeva M, Gianotti R, Shah M, Bradanini L, Tosi D, Veraldi S, Ziv M, Leshem E, Dodiuk-Gad RP. Cutaneous manifestations of COVID-19: report of three cases and a review of literature. J Dermatol Sci. 2020;98(2):75–81. doi: 10.1016/j.jdermsci.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Najarian DJ. Morbilliform exanthem associated with COVID-19. JAAD Case Rep. 2020;6(6):493–494. doi: 10.1016/j.jdcr.2020.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Català A, Galván-Casas C, Carretero-Hernández G, et al. Maculopapular eruptions associated to COVID-19: a subanalysis of the COVID-Piel study [published online ahead of print, 2020 Aug 10]. Dermatol Ther. 2020. 10.1111/dth.14170. [DOI] [PMC free article] [PubMed]
- 57.Reguero-Del Cura L, Gómez-Fernández C, López Obregón C, López-Sundh AE, González-López MA. Onset of erythema multiforme-like lesions in association with recurrence of symptoms of COVID-19 infection in an elderly woman [published online ahead of print, 2020 Aug 20] Dermatol Ther. 2020;2020:e14208. doi: 10.1111/dth.14208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Demirbaş A, Elmas ÖF, Atasoy M, Türsen Ü, Lotti T. A case of erythema multiforme major in a patient with COVID 19: the role of corticosteroid treatment [published online ahead of print, 2020 Jun 26] Dermatol Ther. 2020;2020:e13899. doi: 10.1111/dth.13899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Janah H, Zinebi A, Elbenaye J. Atypical erythema multiforme palmar plaques lesions due to Sars-Cov-2. J Eur Acad Dermatol Venereol. 2020;34(8):e373–e375. doi: 10.1111/jdv.16623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.•• Fernandez-Nieto D, Ortega-Quijano D, Jimenez-Cauhe J, et al. Clinical and histological characterization of vesicular COVID-19 rashes: a prospective study in a tertiary care hospital [published online ahead of print, 2020 May 8]. Clin Exp Dermatol. 2020. 10.1111/ced.14277, 10.1111/ced.14277. A prospective series of the clinical characteristics and histopathological findings of patients with COVID-19 and vesicular rash. [DOI] [PMC free article] [PubMed]
- 61.Tammaro A, Adebanjo GAR, Parisella FR, Pezzuto A, Rello J. Cutaneous manifestations in COVID-19: the experiences of Barcelona and Rome. J Eur Acad Dermatol Venereol. 2020;34(7):e306–e307. doi: 10.1111/jdv.16530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ehsani AH, Nasimi M, Bigdelo Z. Pityriasis rosea as a cutaneous manifestation of COVID-19 infection [published online ahead of print, 2020 May 2]. J Eur Acad Dermatol Venereol. 2020. 10.1111/jdv.16579. [DOI] [PMC free article] [PubMed]
- 63.Drago F, Ciccarese G, Rebora A, Muzic SI, Parodi A. SARS-CoV-2 infection: the same virus can cause different cutaneous manifestations [published online ahead of print, 2020 Jun 13]. Br J Dermatol. 2020. 10.1111/bjd.19311. [DOI] [PMC free article] [PubMed]
- 64.Rapkiewicz AV, Mai X, Carsons SE, et al. Megakaryocytes and platelet-fibrin thrombi characterize multi-organ thrombosis at autopsy in COVID-19: a case series. EClinicalMedicine. 2020;24:100434. doi: 10.1016/j.eclinm.2020.100434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jayarangaiah A, Kariyanna PT, Chen X, Jayarangaiah A, Kumar A. COVID-19-associated coagulopathy: an exacerbated immunothrombosis response. Clin Appl Thromb Hemost. 2020;26:1076029620943293. doi: 10.1177/1076029620943293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Garg S, Garg M, Prabhakar N, Malhotra P, Agarwal R. Unraveling the mystery of Covid-19 cytokine storm: from skin to organ systems [published online ahead of print, 2020 Jun 19] Dermatol Ther. 2020;2020:e13859. doi: 10.1111/dth.13859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Castelnovo L, Capelli F, Tamburello A, Faggioli PM, Mazzone A. Symmetric cutaneous vasculitis in COVID-19 pneumonia. J Eur Acad Dermatol Venereol. 2020;34(8):e362–e363. doi: 10.1111/jdv.16589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Guarneri C, Venanzi Rullo E, Gallizzi R, Ceccarelli M, Cannavò SP, Nunnari G. Diversity of clinical appearance of cutaneous manifestations in the course of COVID-19 [published online ahead of print, 2020 May 22]. J Eur Acad Dermatol Venereol. 2020. 10.1111/jdv.16669. [DOI] [PMC free article] [PubMed]
- 69.Alramthan A, Aldaraji W. Two cases of COVID-19 presenting with a clinical picture resembling chilblains: first report from the Middle East. Clin Exp Dermatol. 2020;45(6):746–748. doi: 10.1111/ced.14243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.• Bouaziz JD, Duong T, Jachiet M, et al. Vascular skin symptoms in COVID-19: a French observational study [published online ahead of print, 2020 Apr 27]. J Eur Acad Dermatol Venereol. 2020. 10.1111/jdv.16544. A small series of varying vasculitic lesions including chilblains, purpura, and livedo. [DOI] [PMC free article] [PubMed]
- 71.Ferrara G, Morgado-Carrasco D. Visual dermatology: acral eerythemato-purpuric lesions during COVID-19 pandemic. J Cutan Med Surg. 2020;24(4):409. doi: 10.1177/1203475420929919. [DOI] [PubMed] [Google Scholar]
- 72.Landa N, Mendieta-Eckert M, Fonda-Pascual P, Aguirre T. Chilblain-like lesions on feet and hands during the COVID-19 pandemic. Int J Dermatol. 2020. 10.1111/ijd.14937. [DOI] [PMC free article] [PubMed]
- 73.• Colmenero I, Santonja C, Alonso-Riaño M, et al. SARS-CoV-2 endothelial infection causes COVID-19 chilblains: histopathological, immunohistochemical and ultrastructural study of seven paediatric cases [published online ahead of print, 2020 Jun 20]. Br J Dermatol. 2020. 10.1111/bjd.19327. A histopathologic study providing insight into the mechanisms of SARS-CoV-2-induced endothelial injury associated with chilblain-like skin lesions. [DOI] [PMC free article] [PubMed]
- 74.• Magro C, Mulvey JJ, Laurence J, et al. The differing pathophysiologies that underlie COVID-19 associated perniosis and thrombotic retiform purpura: a case series [published online ahead of print, 2020 Jul 22]. Br J Dermatol. 2020. 10.1111/bjd.19415. Provides insight into the histopathological mechanisms of pernio-like lesions and retiform pupura in COVID-19. [DOI] [PMC free article] [PubMed]
- 75.de Perosanz-Lobo D, Fernandez-Nieto D, Burgos-Blasco P, et al. Urticarial vasculitis in COVID-19 infection: a vasculopathy-related symptom? [published online ahead of print, 2020 Jun 8]. J Eur Acad Dermatol Venereol. 2020. 10.1111/jdv.16713. [DOI] [PMC free article] [PubMed]
- 76.Vanegas Ramirez A, Efe D, Fischer M. Drug-induced vasculitis in a patient with COVID-19. J Eur Acad Dermatol Venereol. 2020;34(8):e361–e362. doi: 10.1111/jdv.16588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chibane S, Gibeau G, Poulin F, Tessier P, Goulet M, Carrier M, Lanthier S. Hyperacute multi-organ thromboembolic storm in COVID-19: a case report [published online ahead of print, 2020 Jun 6] J Thromb Thrombolysis. 2020;2020:1–4. doi: 10.1007/s11239-020-02173-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Verheyden M, Grosber M, Gutermuth J, Velkeniers B. Relapsing symmetric livedo reticularis in a patient with COVID-19 infection [published online ahead of print, 2020 Jun 25]. J Eur Acad Dermatol Venereol. 2020. 10.1111/jdv.16773. [DOI] [PMC free article] [PubMed]
- 79.Zhang Y, Cao W, Xiao M, et al. Clinical and coagulation characteristics of 7 patients with critical COVID-2019 pneumonia and acro-ischemia. Zhonghua Xue Ye Xue Za Zhi. 2020;41:E006. doi: 10.3760/cma.j.issn.0253-2727.2020.0006. [DOI] [PubMed] [Google Scholar]
- 80.Tammaro A, Chello C, Sernicola A, et al. Necrotic acral lesions and lung failure in a fatal case of COVID-19 [published online ahead of print, 2020 Jul 16]. Aust J Dermatol. 2020. 10.1111/ajd.13400. [DOI] [PMC free article] [PubMed]
- 81.Novara E, Molinaro E, Benedetti I, Bonometti R, Lauritano EC, Boverio R. Severe acute dried gangrene in COVID-19 infection: a case report. Eur Rev Med Pharmacol Sci. 2020;24(10):5769–5771. doi: 10.26355/eurrev_202005_21369. [DOI] [PubMed] [Google Scholar]
- 82.Xu P, Zhou Q, Xu J. Mechanism of thrombocytopenia in COVID-19 patients. Ann Hematol. 2020;99(6):1205–1208. doi: 10.1007/s00277-020-04019-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lorenzo-Villalba N, Zulfiqar AA, Auburtin M, et al. Thrombocytopenia in the course of COVID-19 infection. Eur J Case Rep Intern Med. 2020;7(6):001702. doi: 10.12890/2020_001702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Murt A, Eskazan AE, Yılmaz U, Ozkan T, Ar MC. COVID-19 presenting with immune thrombocytopenia: a case report and review of the literature [published online ahead of print, 2020 Jun 4]. J Med Virol. 2020. 10.1002/jmv.26138. [DOI] [PMC free article] [PubMed]
- 85.Yang Y, Zhao J, Wu J, Teng Y, Xia X. A rare case of immune thrombocytopenic purpura, secondary to COVID-19 [published online ahead of print, 2020 May 22]. J Med Virol. 2020. 10.1002/jmv.26051. [DOI] [PMC free article] [PubMed]
- 86.Zulfiqar AA, Lorenzo-Villalba N, Hassler P, Andrès E. Immune thrombocytopenic purpura in a patient with Covid-19. N Engl J Med. 2020;382(18):e43. doi: 10.1056/NEJMc2010472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bomhof G, Mutsaers PGNJ, Leebeek FWG, Boekhorst PAW, Hofland J, Croles FN, Jansen AJG. COVID-19-associated immune thrombocytopenia. Br J Haematol. 2020;190(2):e61–e64. doi: 10.1111/bjh.16850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Litaiem N, Hajlaoui K, Karray M, Slouma M, Zeglaoui F. Acute generalized exanthematous pustulosis after COVID-19 treatment with hydroxychloroquine [published online ahead of print, 2020 May 13] Dermatol Ther. 2020;2020:e13565. doi: 10.1111/dth.13565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lin P, Zhu S, Huang Y, Li L, Tao J, Lei T, Song J, Liu D, Chen L, Shi Y, Jiang S, Liu Q, Xie J, Chen H, Duan Y, Xia Y, Zhou Y, Mei Y, Zhou X, Wu J, Fang M, Meng Z, Li H. Adverse skin reactions among healthcare workers during the coronavirus disease 2019 outbreak: a survey in Wuhan and its surrounding regions. Br J Dermatol. 2020;183(1):190–192. doi: 10.1111/bjd.19089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lan J, Song Z, Miao X, et al. Skin damage among health care workers managing coronavirus disease-2019. J Am Acad Dermatol. 2020;82(5):1215–1216. doi: 10.1016/j.jaad.2020.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zuo Y, Hua W, Luo Y, Li L. Skin reactions of N95 masks and medial masks among health-care personnel: a self-report questionnaire survey in China. Contact Dermatitis. 2020;83(2):145–147. doi: 10.1111/cod.13555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Centers for Disease Control and Prevention. Coronavirus Disease 2019 (COVID-19): Healthcare Workers: Using PPE. Updated August 19 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/using-ppe.html. Accessed September 1 2020.
- 93.Beiu C, Mihai M, Popa L, Cima L, Popescu MN. Frequent hand washing for COVID-19 prevention can cause hand dermatitis: management tips. Cureus. 2020;12(4):e7506. doi: 10.7759/cureus.7506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.American Academy of Dermatology Association (AAD). preventing and treatment occupationally induced dermatologic conditions during COVID-19. https://assets.ctfassets.net/1ny4yoiyrqia/1evNAmDqSmw6w9dhozuJGZ/303efdeff53db6e0347df52c65baf4bc/OCC_Derm_Conditions_V11_30Apr2020.pdf. Accessed September 1 2020.
- 95.Szepietowski JC, Matusiak Ł, Szepietowska M, Krajewski PK, Białynicki-Birula R. Face mask-induced itch: a self-questionnaire study of 2,315 Responders During the COVID-19 Pandemic. Acta Derm Venereol. 2020;100(10):adv00152. doi: 10.2340/00015555-3536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bothra A, Das S, Singh M, Pawar M, Maheswari A. Retroauricular dermatitis with vehement use of ear loop face masks during COVID-19 pandemic [published online ahead of print, 2020 Jun 3]. J Eur Acad Dermatol Venereol. 2020. 10.1111/jdv.16692. [DOI] [PMC free article] [PubMed]
- 97.Xie Z, Yang YX, Zhang H. Mask-induced contact dermatitis in handling COVID-19 outbreak [published online ahead of print, 2020 May 10]. Contact Dermatitis. 2020. 10.1111/cod.13599. [DOI] [PMC free article] [PubMed]
- 98.Aerts O, Dendooven E, Foubert K, Stappers S, Ulicki M, Lambert J. Surgical mask dermatitis caused by formaldehyde (releasers) during the COVID-19 pandemic. Contact Dermatitis. 2020;83(2):172–173. doi: 10.1111/cod.13626. [DOI] [PubMed] [Google Scholar]
- 99.Donovan J, Skotnicki-Grant S. Allergic contact dermatitis from formaldehyde textile resins in surgical uniforms and nonwoven textile masks. Dermatitis. 2007;18(1):40–44. doi: 10.2310/6620.2007.05003. [DOI] [PubMed] [Google Scholar]
- 100.Bhatia R, Sindhuja T, Bhatia S, et al. Iatrogenic dermatitis in times of COVID-19: a pandemic within a pandemic [published online ahead of print, 2020 Jun 4]. J Eur Acad Dermatol Venereol. 2020. 10.1111/jdv.16710. [DOI] [PMC free article] [PubMed]
- 101.Balato A, Ayala F, Bruze M, et al. European Task Force on Contact Dermatitis statement on coronavirus disease-19 (COVID-19) outbreak and the risk of adverse cutaneous reactions. J Eur Acad Dermatol Venereol. 2020;34(8):e353–e354. doi: 10.1111/jdv.16557. [DOI] [PubMed] [Google Scholar]
- 102.Sernicola A, Chello C, Cerbelli E, et al. Treatment of nasal bridge ulceration related to protective measures for the COVID-19 epidemic [published online ahead of print, 2020 May 7]. Int Wound J. 2020. 10.1111/iwj.13397. [DOI] [PMC free article] [PubMed]


