Abstract
Purpose
Although ovarian tissue transportation has been validated for up to 24 h, there is no standard protocol to date. We aimed to elucidate how existing media currently used for ovarian tissue transportation affect ovarian tissue metabolism and cell viability.
Methods
Cow ovarian fragments were immersed in 0.9% NaCl solution, IVF medium, Leibovitz 15 medium (L-15), or PBS for 1, 4, or 24 h at 4 °C. Media were analyzed for pH, lactate dehydrogenase (LDH) activity, and glucose, pyruvate, and lactate concentrations, while apoptosis was assessed by TUNEL assays in fixed fragments. Viability rates were assessed by flow cytometry (FACS).
Results
There were lower pH levels in NaCl at all time points compared with other media. LDH activity increased with time and was lowest in NaCl at 1 and 4 h. There was no significant difference in glucose levels, but a significant pyruvate decrease in L-15 and a significant lactate increase in all media. TUNEL showed apoptosis rates ranging from 0 to 5%. FACS showed a mean of 4% necrotic cells and 15–19% apoptotic cells after 1 h of incubation, but less than 1% necrotic cells and 2–6% apoptotic cells after 24 h in all media.
Conclusion
Our results indicate marked metabolic activity in ovarian tissue at 4 °C and suggest that cells use internal sources of energy, which may influence transplantation outcomes. This highlights the importance of better understanding whole tissue dynamics to develop a standard protocol for ovarian tissue transportation.
Electronic supplementary material
The online version of this article (10.1007/s10815-020-01935-y) contains supplementary material, which is available to authorized users.
Keywords: Metabolism, Assisted reproduction, Fertility preservation, Ovarian tissue transportation, Ovarian tissue cryopreservation
Introduction
Ovarian tissue cryopreservation and transplantation is a widely applied approach for fertility preservation in cancer patients [1]. More than 130 live births have been reported to date with use of this technique, yielding pregnancy rates of 30–60% and live birth rates of around 40% [2, 3].
While much emphasis is placed on improving ovarian tissue cryopreservation and grafting procedures and understanding events occurring before and after revascularization of grafted tissue [4–7], the importance of ovarian tissue transportation is progressively coming to the fore. In the early days of cryopreservation/transplantation, ovarian biopsies were usually collected and cryopreserved in the same hospital. In recent years, however, due to strict European directives on tissue banking and high costs involved in maintaining an ovarian tissue cryobank, only large hospitals can have such facilities. The concept of “the woman stays, the tissue moves,” first established by the Danish network for transport of ovarian tissue for up to 4 h [8], is becoming increasingly accepted, steadily extending the duration of ovarian tissue transit. In order to reach larger numbers of patients, the FertiPROTEKT network has now validated ovarian tissue transportation for up 24 h [9, 10].
Although pregnancies and live births have been reported after such a long period of transportation [10], it remains an empirical procedure and there is no standard protocol [9, 11]. Only three studies have evaluated the effect of different media on human ovarian tissue transport [12–14], but none of the studied media is used in clinical practice for ovarian tissue transportation [10, 15–17]. Moreover, we must bear in mind that the ovary is a highly complex organ with a diversity of cells and structures of vital importance to ensuring the survival and development of follicles [18], and available knowledge on ovarian tissue metabolism refers mainly to follicles [19], which are composed of two of the six different cell populations present in the ovary [20]. Transportation of this tissue is undoubtedly a challenging prospect. We therefore aimed to elucidate how existing media currently used for ovarian tissue transportation affect the metabolism of whole ovarian tissue during short- and long-term cold ischemia. Our results suggest that considerable metabolic activity was preserved in ovarian tissue samples at low temperatures.
Material and methods
Experimental design
To assess the effect of ovarian tissue transport at 4 °C, we used cow ovaries as a translational model for humans because of their physiological similarities [21], availability, and size, which allowed us to perform all media comparisons in the same animals. A total of 30 ovaries were obtained from 15 adult cows (Belgian Blue, 3–6 years of age) euthanized in a local slaughterhouse (Abattoir of Anderlecht, Brussels, Belgium). The ovaries were used in two different set of experiments. In the first study, we aimed to demonstrate metabolic activity in ovarian tissue transported at 4 °C. For this, ovarian fragments were incubated in three different media and, after 1, 4, or 24 h, both the media and tissue were analyzed. Based on results obtained from these first experiments, we carried out a second study to correlate metabolic activity and cell viability during ovarian tissue transportation. Modifications were made to transport media in order to avoid bias and store the tissue in similar conditions to those in clinical practice. Ovarian tissue fragments were incubated in one of the three media for 1 or 24 h at 4 °C, and media and isolated ovarian cells were then analyzed.
Experiment 1
Immediately after retrieval in the slaughterhouse, adipose tissue and ligaments were removed and the ovaries were cut into 10 fragments of 1 × 1.5 × 0.5 cm. One piece was immediately fixed in 4% paraformaldehyde (Alfa Aesar, ref. J61899, Karlsruhe, Germany), and the others were randomly immersed in 15-mL Falcon tubes containing 10 mL of one of the following solutions, with one fragment per tube (online resource 1: media composition) at 4 °C:
Universal IVF medium (Origio, Malov, Denmark, ref. #10310060A).
Leibovitz 15 medium (L-15) + GlutaMAX™ (Gibco, Bleiswijk, the Netherlands, ref. #31415-029) supplemented with 5% antibiotic-antimycotic (anti-anti, Gibco, Bleiswijk, the Netherlands, ref. #15240-062).
0.9% sodium chloride (NaCl) solution (Merck, Darmstadt, Germany, ref. #1.06404.5000) supplemented with 5% anti-anti.
After 1, 4, or 24 h, the fragments were fixed in 4% paraformaldehyde and media were aliquoted for further analysis. A few aliquots of 1 mL were snap-frozen in liquid nitrogen to assess glucose, lactate, and pyruvate concentrations and lactate dehydrogenase (LDH) activity, and the remaining media were kept on ice for pH analysis.
Media analyses
Levels of pH were measured in each medium (n = 9 animals) using a pH meter (Consort™ C3010 multi-parameter analyzer). For glucose, pyruvate, and lactate, media were first thawed on ice, and then substrates and products of metabolism were evaluated in 24-h samples of each medium (n = 6 animals) using specific enzymatic assays on a CMA600 microdialysis analyzer (CMA Microdialysis, Kista, Sweden), as previously reported [22]. LDH quantification (n = 9 animals) was performed in each medium thawed on ice using an LDH assay kit (Abcam, ref. #ab102526) and read using a luminometer (2030 Multilabel Reader, Victor™ X4, PerkinElmer), according to the manufacturer’s instructions.
Tissue analyses
Fixed ovarian fragments were dehydrated, embedded in paraffin, and cut into 5-μm-thick sections. To evaluate the apoptotic response, one sample of each medium was chosen from three different animals based on the highest concentrations of lactate measured (totaling nine samples). Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assays were performed using the In Situ Cell Death Detection Kit, TMR Red (Roche, ref. 12 156 792 910, Mannheim, Germany), as described by Vanacker et al. [23] Paraffin sections were deparaffinized with paraffin-clearing solvent (X-Solv, Yvsolab NV/SA, Belgium) and rehydrated in propanol. DNAse was used to damage DNA in positive controls, and negative control sections were incubated with a label without enzyme solution. Slides were further stained with 4′,6′-diamidino-2-phenylindole (DAPI) and scanned with a Mirax digital slide scanner. Areas of TUNEL-positive cells and DAPI-stained cells were automatically analyzed using Visiopharm® software, and the percentage of TUNEL-positive/TUNEL-negative areas was calculated.
Experiment 2
To confirm cell viability after transportation, ovaries from 4 cows were obtained from a local slaughterhouse. Fragments of 1 × 1 × 0.5 cm were incubated in 20 mL of either phosphate-buffered saline without calcium chloride and magnesium chloride (PBS, Gibco, ref. 10010-023, Bleiswijk, the Netherlands) and IVF-medium or L-15 medium for 1 or 24 h at 4 °C. PBS was used in experiment 2 to avoid bias in the results due to pH alterations, as NaCl in experiment 1 was not a buffered solution.
Media analyses
Media were analyzed for pH, glucose, pyruvate, and lactate concentrations as previously described.
Viability assessment
Immediately upon arrival in the laboratory (1 h of transportation), to serve as a control group for each medium, one fragment of 5 × 5 mm was immersed in 5 mL McCoy 5A medium (Gibco, ref. 16600082) containing 100 μL Liberase™ DH (Merck, ref. 5401054001, Bornem, Belgium) and disrupted in the gentleMacs™ Dissociator (Miltenyi Biotec, Germany) for 1 h at 37 °C (program 37C_h_TDK_1). After digestion, the ovarian cell suspension was passed through a 100-μm filter and rinsed in PBS, before being centrifuged for 5 min at 1500 rpm. Supernatant was removed, and cells were stained with FITC Annexin V/Dead Cell Apoptosis Kit (Thermo Fisher, ref. V13242) according to the manufacturer’s instructions. Flow cytometry analysis was carried out with BD FACSCantoII (BD Biosciences) equipped with FACSDiva v8.0 software and analyzed by FlowJo software (BD Biosciences). At least 10,000 ovarian cells were analyzed. The same procedure was performed after 24 h of transportation.
Statistical analyses
Statistical analyses were carried out using IBM SPSS Statistics 22 software. Data were expressed as means ± SD. Continuous variables were evaluated for normal distribution using the Shapiro-Wilk test. Possible differences were investigated by one-way ANOVA followed by Tukey’s post hoc test (normal distribution) or the Kruskal-Wallis test followed by the Mann Whitney U test (not normal distribution). P < 0.05 was considered statistically significant.
Results
Experiment 1
pH
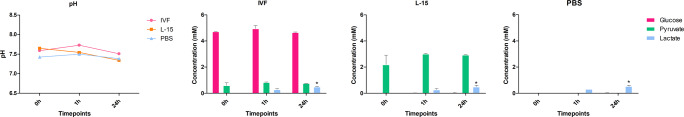
First, we measured pH as an indicator of medium acidification [24–26] to obtain pH values (n = 9) in the different media over time (Fig. 1). We found a significantly lower pH in NaCl at all time points compared with IVF medium and L-15. While the pH increased with time in NaCl, it slightly but significantly decreased in L-15 after 24 h. It was not significantly altered in IVF medium.
Fig. 1.
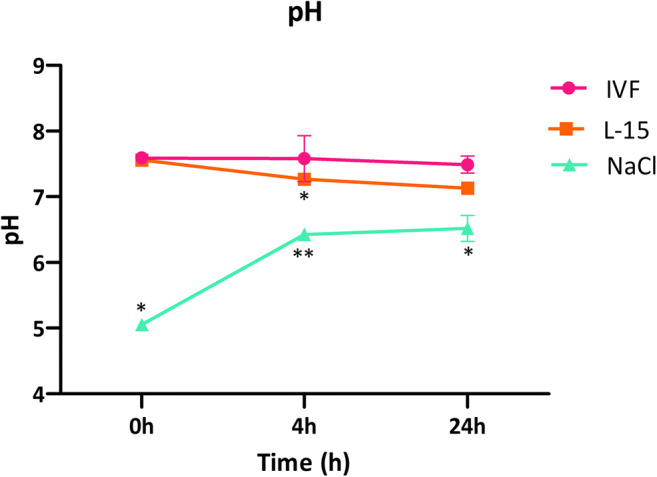
pH variation over time in different media used for ovarian tissue transportation. Ovarian fragments of 1 × 1.5 × 0.5 cm were stored at 4 °C in 10 mL of IVF medium, L-15, or NaCl for the indicated times, at which pH values were measured using a pH meter (n = 9). Asterisk indicates p < 0.05 at the same time points
Glucose, pyruvate, and lactate
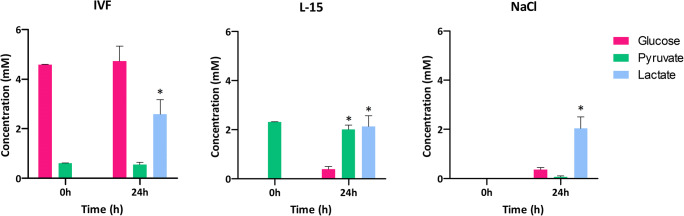
We measured glucose, pyruvate, and lactate concentrations in media after 24 h (n = 6) as markers of metabolic activity for the longest period investigated (Fig. 2). Glucose levels were stable in IVF medium over 24 h of incubation. Pyruvate values were stable in all media except L-15, where a small but significant decrease was noted at 24 h compared with 0 h. There was a significant release of lactate from ovarian samples in all media, with no significant difference between media.
Fig. 2.
Glucose, pyruvate, and lactate concentrations in different media used for ovarian tissue transportation. Ovarian fragments of 1 × 1.5 × 0.5 cm were stored at 4 °C in IVF medium, L-15, or NaCl for the indicated times, at which glucose, pyruvate, and lactate concentrations were determined in incubation media using a CMA600 enzymatic analyzer (n = 6). Asterisk indicates p < 0.05 for a given metabolite between 0 and 24 h
LDH activity
LDH release is a marker of tissue damage, as only necrotic cells can release the enzyme [27]. In our experiments, LDH activity (n = 9) increased with time in all media (Fig. 3). Comparatively, however, it was significantly lower in NaCl than in IVF medium and L-15 at 1 h and 4 h of incubation. There was no difference in LDH activity at the 24-h time point between the three media.
Fig. 3.
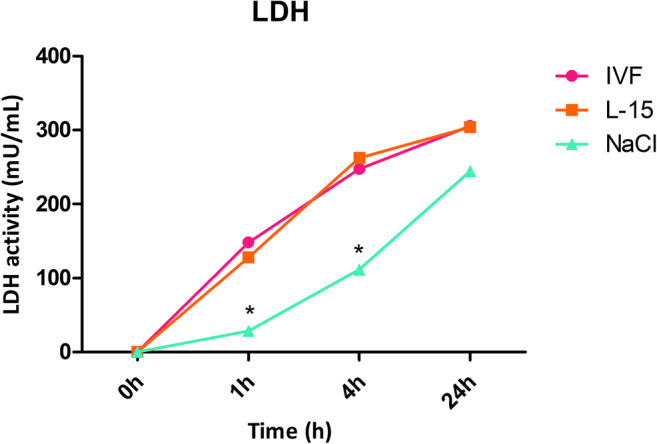
LDH release from ovarian samples occurs at early time points in all storage media. Ovarian fragments of 1 × 1.5 × 0.5 cm were stored at 4 °C in IVF medium, L-15, or NaCl for the indicated times, at which LDH activity was calculated in incubation media (n = 9). Asterisk indicates p < 0.05 at the same time points
Tissue analyses
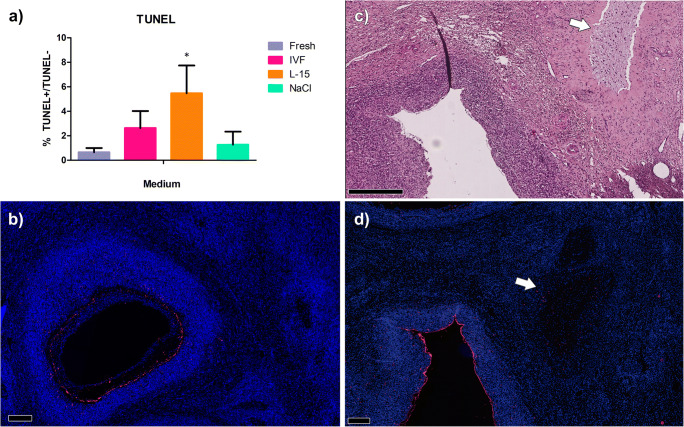
As LDH release increased during storage, indicating necrosis, we finally evaluated apoptosis as a second major form of cell death. TUNEL-positive areas (n = 3) were 0.65 ± 0.35% in fresh tissue, 2.63 ± 1.39% after 24 h of storage in IVF medium, 5.47 ± 2.27% in L-15, and 1.27 ± 1.06% in NaCl (Fig. 4a). L-15 was the only medium displaying significantly higher apoptosis rates than fresh tissue. Upon closer inspection, apoptotic cells were mainly located in antral follicles (Fig. 4b), corpus albicans, and fibrotic tissue (Fig. 4c, d). No oocyte nuclei were observed on TUNEL slides.
Fig. 4.
Apoptosis was observed mainly in granulosa cells of antral follicles and fibrotic tissue after 24 h of storage in different media. Ovarian samples were stored at 4 °C in IVF medium, L-15, or NaCl solution for the designated times, after which apoptosis was evaluated using TUNEL assays (a). Asterisk indicates significantly different results (p < 0.05). Representative pictures show ovarian follicles in fresh tissue (b) and after 24 h of incubation in L-15 medium (c, d). Red = dead cells. Blue = DAPI. White arrows point to fibrotic tissue. a, b, d Scale bar = 200 μm. c Scale bar = 500 μm
Experiment 2
Media analyses
No significant differences were observed in pH between media at 0, 1, and 24 h. There was no consumption of glucose or pyruvate in IVF and L-15 media after 24 h of incubation (p > 0.05), but there was significant lactate release in all media (p < 0.05) (Fig. 5).
Fig. 5.
pH and glucose, lactate, and pyruvate concentrations after ovarian tissue transportation in different media. Ovarian fragments of 1 × 1.5 × 0.5 cm were stored at 4 °C in 20 mL of IVF medium, L-15, or PBS for the indicated times, at which pH values were measured using a pH meter, and glucose, pyruvate, and lactate concentrations were determined in incubation media using a CMA600 enzymatic analyzer (n = 4). Asterisk indicates p < 0.05 for a given metabolite between 0, 1, and 24 h
Viability assessment
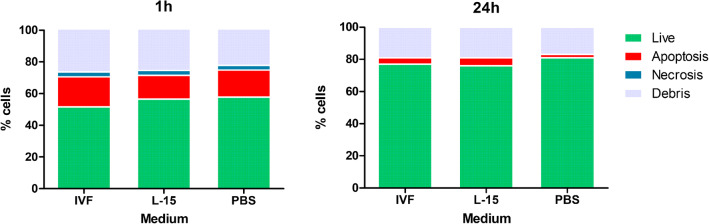
FACS showed a mean of 4% necrotic ovarian cells in tissue samples incubated in all media and 15–19% apoptotic ovarian cells after 1 h of incubation, but less than 1% necrotic ovarian cells and 2–6% apoptotic ovarian cells after 24 h in all media (Fig. 6). No significant differences were observed for necrosis or apoptosis in different media or time points (p > 0.05).
Fig. 6.
Cell viability assessed by flow cytometry after ovarian tissue transportation in different media. Ovarian fragments of 1 × 1.5 × 0.5 cm were stored at 4 °C in 20 mL of IVF medium, L-15, or PBS for the indicated times. A piece of tissue was dissociated and cell viability was assessed with annexin V/propidium iodide with flow cytometry. Live cells are shown in green, apoptotic cells in red, necrotic cells in blue, and debris in gray
Discussion
To our knowledge, this is the first report to evaluate metabolic substrates in different media currently used for ovarian tissue transportation. Our results shed new light on the impact of different media on ovarian tissue during storage. We observed high lactate release in all tested media after 24 h of storage at 4 °C, but no consumption of glucose and only low consumption of pyruvate. These results point to considerable metabolic activity in ovarian tissue during transportation.
This study evolved as a consequence of data obtained from the first set of experiments. Believing that storing ovarian tissue at 4 °C would not have a great impact on cell metabolism, in the first experiment, we evaluated only the longest period of transport. After analyzing our results, we decided to conduct a second series of experiments with an added control (1 h), in order to demonstrate the impact of 24 h of transport. Media used in the present study were chosen based on ovarian tissue transportation reports. IVF medium [15] and L-15 [16] are used in clinical practice in humans, while NaCl and PBS act as controls and have been used in ovarian transportation studies in different animal species [25, 26, 28–47].
Cell metabolism is intrinsically linked to acid-base balance. This balance is at risk, or lost, during severe ischemia, mainly because of lactic acid release [24, 48]. In this context, Tellado et al. [26] reported that follicular fluid pH was lower after porcine ovarian tissue storage in NaCl. This is consistent with the pH reduction we observed in L-15 medium in experiment 1 of the present study. On the other hand, the pH was altered in NaCl over time, possibly because it was the only medium that did not contain any buffer, since pH levels in PBS remained constant in experiment 2. Moreover, lactate concentrations were similar in all three media after 24 h of incubation in both experiments. Hence, the relatively acidic nature of NaCl at the beginning of the experiment did not appear to interfere with cell metabolism at 4 °C. In fact, cells can maintain their metabolism when extracellular pH levels are as low as 5.6 [24, 48]. Mild extracellular acidification may actually be beneficial to cells [49], inhibiting free radical generation and decreasing energy demands [24]. The levels of pH did not significantly alter in any media in experiment 2, possibly because the volume of media was higher and PBS contains phosphate buffer, avoiding great pH changes.
A lower pH might also affect LDH activity in NaCl [50]. Evaluating LDH activity in media is an indirect way of measuring necrosis [27], while TUNEL assays measure apoptosis. Our results showed higher apoptosis rates (5.47%) only in L-15 compared with fresh tissue (0.65%). The present study shows lower apoptosis rates than in sheep ovarian tissue stored in minimal essential medium (MEM) or Dulbecco’s modified Eagle’s medium (DMEM) for up to 24 h and cultured for 5 to 7 days [51, 52] and in mouse ovaries stored in DMEM for 24 h [53]. Our TUNEL findings and viability assessment by FACS support the hypothesis of increased lactate levels due to cell metabolism, since the percentage of apoptotic/necrotic cells was too low to affect lactate release in any media after 24 h. Experiment 1 revealed increasing LDH activity with time and similar LDH levels in all media after 24 h of storage, while experiment 2 showed less than 1% necrotic cells after 24 h. This discrepancy might be because media were not centrifuged before LDH assays. Curiously, we observed a higher percentage of viable cells after 24 h of transportation than after 1 h. This is consistent with reports by Isachenko et al. [54], who found more viable cells after storage of human ovarian tissue for 24 h at 5 °C, followed by in vitro culture (IVC) or culture in the chorioallantoic membrane of chick embryos. It may have been due to mitosis stimulation induced by the cold, as observed in neurons of rats exposed to low temperatures [55].
Glucose and lactate were quantified in porcine follicular fluid during transportation in NaCl [26] at 25 °C and showed decreased glucose and increased lactate concentrations, suggesting anaerobic glycolysis as a major metabolic event during transport. In contrast to these findings, we observed no glucose consumption in any media, low pyruvate consumption in L-15, but comparable lactate release across all media. Lactate concentrations were lower in experiment 2, undoubtedly because they were diluted in higher media volumes. Although glycine and glutamine present in L-15 might contribute to lactate release [56], there are no sources of energy in NaCl or PBS that could account for lactate production. Therefore, in our study, it is possible that ovarian cells used their internal energy sources rather than nutrients present in media to release lactate. Indeed, almost all mammalian cells, including ovarian cells, store fatty acids, which constitute sources of acetyl-CoA for lactate production [57], in lipid droplets [58, 59]. In oocytes and cumulus cells, triacylglycerol is the main lipid found in lipid droplets [60]. Palmitic, stearic, oleic, and linoleic fatty acids are most prevalent in oocytes of mammalian species, and saturated fatty acids make up the majority of lipids stored in oocytes [61]. Lipids are also an important source of energy during oocyte maturation; β-oxidation of fatty acids plays an essential role in oocyte maturation and developmental competence, as well as meiosis resumption and nuclear maturation in murine, bovine, and porcine oocytes [62, 63].
While the transport of vital organs is widely established, ovarian tissue transportation studies lag far behind [64]. Studies on organ preservation report that although cold ischemia during storage reduces cellular metabolism 12-fold, ATP and ADP are gradually depleted [65, 66]. In ovarian tissue, the pathway would then involve mitochondrial export of the tricarboxylic acid (TCA) cycle intermediate malate and its cytosolic conversion to pyruvate and then lactate. Cells might also produce energy by autophagy, consuming cell components to survive [67]. Further studies are warranted to test these hypotheses, such as inhibition of fatty acid oxidation (FAO) [68] or LDH activity [69].
In addition, while follicle metabolism has been extensively studied (reviewed by Collado-Fernandez et al. [19]), follicles are just one of the numerous cell populations residing in ovarian cortex. These different cell populations are directly or indirectly involved in follicle survival and development. For instance, stromal cells make up 83% of the ovarian population [20] and play key roles in primordial follicle activation and further development [18, 70]. Indeed, our results may have been affected by the size of fragments [71], the presence of medullary tissue, and the volume of media used. Glucose, pyruvate, and lactate levels evaluated in the present study correspond to the metabolism of all ovarian cell populations, demonstrating the importance of investigating whole tissue dynamics.
Many effects of ischemia have been studied after ovarian tissue transplantation [5, 6, 72–75]. In particular, it has been reported that ovarian tissue hypoxia lasts for 3–5 days, with partial pressure of oxygen stabilizing around 10 days after transplantation of human ovary xenografts to mice [74]. Microdialysis assessments showed that anaerobic metabolism was maintained for up to 10 days after grafting human ovarian fragments to nude mice, namely until revascularization of the tissue [6]. When cells are engaged in anaerobic metabolism, they often consume their internal energy stores, accumulate metabolic byproducts, and export protons. Reperfusion triggers another series of events, generating reactive oxygen species (ROS) that cause more damage to cells [76]. In grafted ovarian tissue, the main consequences of ischemia revascularization include limited graft life span, empty follicles upon aspiration, and poor responder hormone profiles [77]. However, it is important to point out that ischemia does not begin after transplantation, but as soon as the tissue is removed from the patient, and continues until it is cryopreserved [9, 78]. Transportation of the ovary may take up to 24 h in clinical settings [9], and little is known about this process to date.
Studies in humans have assessed transportation by analyzing ovarian tissue after IVC [12–14]. While all of them concurred that low temperatures are useful when tissue needs to be transported for long distances, and observed little alteration to follicle morphology after transportation, evaluation by IVC may lead to bias, as this system is not well established for ovarian tissue and may not maintain cell interactions and follicle growth [79]. Investigating transportation protocols by techniques that resemble the physiological environment of ovarian tissue will provide us with more reliable information on understanding how this process affects fertility. Indeed, evaluating ovarian tissue transportation through transplantation, IVF, and embryo transfer, Kamoshita et al. [53] observed lower implantation rates after 24 h of transport, even though follicle morphology was similar to fresh controls. Our data indicate that ovarian cells might be consuming their own internal energy reserve in the first 24 h of transportation, which may play a role in the cascade of events that occur in the first 10 days post-grafting. To elucidate the impact of metabolic activity observed in the present study on fertility outcomes, xenotransplantation of transported tissue is recommended.
In conclusion, our results suggest marked metabolic activity in the ovaries at 4 °C, with ovarian cells apparently using internal sources of energy for lactate production rather than glucose or pyruvate present in some of the tested media. Since ovarian cells need their internal energy reserves for the post-grafting period prior to revascularization, this activity may have an impact on follicle and ovarian cell populations, influencing transplantation outcomes. Our findings highlight the importance of acquiring a better understanding of whole tissue dynamics in ischemia in order to develop a standard protocol that meets the needs of ovarian tissue during transport.
Electronic supplementary material
(PDF 76.9 kb)
Acknowledgments
We thank Olivier Van Kerk, Maria Dolores Gonzalez, and Sarah Storder for their technical assistance; Mira Hryniuk, B.A., for reviewing the English language of the article; Dr. Caroline Bouzin and Davide Brusa from the 2IP Imaging Platform (UCLouvain’s IREC) and the De Duve Institute for imaging resources used in this study; and Dr. Valéry Payen for valuable insights into metabolic responses.
Authors’ contributions
C.A.A. and J.M.V.V. conceived and designed the study. J.M.V.V., M.C.N.M.B., E.M., and C.A.A. acquired data. J.M.V.V., A.L.M.V., P.S., and C.A.A. analyzed and interpreted data. J.M.V.V. wrote the main manuscript text and prepared the figures. P.S., M.M.D., and C.A.A. edited the manuscript. All authors approved the final version to be published.
Funding
This work was supported by grants from Wallonia-Brussels International (WBI) (grant WBI-IN awarded to J.M.V. Vilela), and Fonds National de la Recherche Scientifique de Belgique (FNRS) (FNRS-PDR Convention T.0077.14, grant 5/4/150/5 and EOS grant 30443682 awarded to M. M. Dolmans, Télévie grant 7452917F to M.C.N.M. Blackman; C. A. Amorim is an F.R.S.-FNRS Research Associate, and P. Sonveaux, an F.R.S.-FNRS Senior Research Associate).
Data availability
Not applicable.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
Since this study was performed with animals from a slaughterhouse, it was exempt from approval from an ethics board.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cronin KA, Lake AJ, Scott S, Sherman RL, Noone A-M, Howlader N, et al. Annual report to the Nation on the Status of Cancer, part I: national cancer statistics. Cancer. 2018;124(13):2785–2800. doi: 10.1002/cncr.31551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Donnez J, Dolmans M-M. Fertility preservation in women. N Engl J Med. 2017;377(17):1657–1665. doi: 10.1056/NEJMra1614676. [DOI] [PubMed] [Google Scholar]
- 3.Shapira M, Raanani H, Chaim S, Meirow D. Challenges of fertility preservation in leukemia patients. Minerva Ginecol. 2018;70. 10.23736/S0026-4784.18.04241-7. [DOI] [PubMed]
- 4.Amorim CA, Dolmans M-M, David A, Jaeger J, Vanacker J, Camboni A, et al. Vitrification and xenografting of human ovarian tissue. Fertility and Sterility. 2012;98(5):1291–8.e2. doi: 10.1016/j.fertnstert.2012.07.1109. [DOI] [PubMed] [Google Scholar]
- 5.Van Langendonckt A, Romeu L, Ambroise J, Amorim C, Bearzatto B, Gala JL, et al. Gene expression in human ovarian tissue after xenografting. Mol Hum Reprod. 2014;20(6):514–525. doi: 10.1093/molehr/gau015. [DOI] [PubMed] [Google Scholar]
- 6.Cacciottola L, Manavella DD, Amorim CA, Donnez J, Dolmans M-M. In vivo characterization of metabolic activity and oxidative stress in grafted human ovarian tissue using microdialysis. Fertility and Sterility. 2018;110(3):534–44.e3. doi: 10.1016/j.fertnstert.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 7.Gallardo M, Paulini F, Corral A, Balcerzyk M, Lucci CM, Ambroise J, Merola M, Fernandez-maza L, Risco R, Dolmans MM, Amorim CA. Evaluation of a new freezing protocol containing 20% dimethyl sulphoxide concentration to cryopreserve human ovarian tissue. Reprod BioMed Online. 2018;37(6):653–665. doi: 10.1016/j.rbmo.2018.09.012. [DOI] [PubMed] [Google Scholar]
- 8.Jensen AK, Kristensen SG, Macklon KT, Jeppesen JV, Fedder J, Ernst E, et al. Outcomes of transplantations of cryopreserved ovarian tissue to 41 women in Denmark. Human reproduction (Oxford, England) Hum Reprod. 2015;30(12):2838–2845. doi: 10.1093/humrep/dev230. [DOI] [PubMed] [Google Scholar]
- 9.Duncan FE, Zelinski M, Gunn AH, Pahnke JE, O'Neill CL, Songsasen N, et al. Ovarian tissue transport to expand access to fertility preservation: from animals to clinical practice. Reproduction (Cambridge, England). 2016;152(6):R201–R10. 10.1530/REP-15-0598. [DOI] [PMC free article] [PubMed]
- 10.Liebenthron J, Montag M, Reinsberg J, Koster M, Isachenko V, van der Ven K, et al. Overnight ovarian tissue transportation for centralized cryobanking: a feasible option. Reprod BioMed Online. 2019;38(5):740–749. doi: 10.1016/j.rbmo.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 11.Beckmann MW, Lotz L, Toth B, Baston-Büst DM, Fehm T, Frambach T, Germeyer A, Goeckenjan M, Häberlin F, Henes M, Hirchenhain J, Hübner S, Korell M, Krüssel JS, Müller A, Reinsberg J, Schwab R, Seitz S, Sütterlin M, van der Ven H, van der Ven K, Winkler-Crepaz K, Wimberger P, von Wolff M, Liebenthron J, Dittrich R. Concept paper on the technique of cryopreservation, removal and transplantation of ovarian tissue for fertility preservation. Geburtshilfe Frauenheilkd. 2019;79(01):53–62. doi: 10.1055/a-0664-8619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klocke S, Tappehorn C, Griesinger G. Effects of supra-zero storage on human ovarian cortex prior to vitrification–warming. Reprod BioMed Online. 2014;29(2):251–258. doi: 10.1016/j.rbmo.2014.03.025. [DOI] [PubMed] [Google Scholar]
- 13.Kyoya T, Nakamura Y, Miyatani S, Miyagawa T, Tomiyama T, Kyono K. Evaluation of oxygen consumption in human vitrified and warmed pre-antral follicles after prolonged low temperatures. Reproductive Medicine and Biology. 2014;13(1):47–52. doi: 10.1007/s12522-013-0166-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Isachenko E, Isachenko V, Nawroth F, Rahimi G, Weiss JM. Effect of long-term exposure at suprazero temperatures on activity and viability of human ovarian cortex. Fertility and Sterility. 2009;91(4, Supplement):1556–1559. doi: 10.1016/j.fertnstert.2008.09.068. [DOI] [PubMed] [Google Scholar]
- 15.Rosendahl M, Schmidt KT, Ernst E, Rasmussen PE, Loft A, Byskov AG, Andersen AN, Andersen CY. Cryopreservation of ovarian tissue for a decade in Denmark: a view of the technique. Reprod BioMed Online. 2011;22(2):162–171. doi: 10.1016/j.rbmo.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 16.Isachenko V, Dittrich R, Keck G, Isachenko E, Rahimi G, van der Ven H, Montag M, Hoffmann I, Müller A, Distler W, Beckmann M, Mallmann P. Cryopreservation of ovarian tissue: detailed description of methods for transport, Freezing and Thawing. Geburtshilfe und Frauenheilkunde. 2012;72(10):927–932. doi: 10.1055/s-0032-1327812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmidt KLT, Ernst E, Byskov AG, Nyboe Andersen A, Yding Andersen C. Survival of primordial follicles following prolonged transportation of ovarian tissue prior to cryopreservation. Hum Reprod. 2003;18(12):2654–2659. doi: 10.1093/humrep/deg500. [DOI] [PubMed] [Google Scholar]
- 18.Tagler DJ, Shea LD, Woodruff TK. Contributions of ovarian stromal cells to follicle culture. In: Donnez J, Kim SS, editors. Principles and practice of fertility preservation. Cambridge: Cambridge University Press; 2011. pp. 409–420. [Google Scholar]
- 19.Collado-Fernandez E, Picton H, Dumollard R. Metabolism throughout follicle and oocyte development in mammals. Int J Dev Biol. 2012;56:799–808. doi: 10.1387/ijdb.120140ec. [DOI] [PubMed] [Google Scholar]
- 20.Wagner M, Yoshihara M, Douagi I, Damdimopoulos A, Panula S, Petropoulos S, Lu H, Pettersson K, Palm K, Katayama S, Hovatta O, Kere J, Lanner F, Damdimopoulou P. Single-cell analysis of human ovarian cortex identifies distinct cell populations but no oogonial stem cells. Nat Commun. 2020;11(1):1147. doi: 10.1038/s41467-020-14936-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sirard MA. The ovarian follicle of cows as a model for human. In Constantinescu G, Schatten H, editors. Animal models and human reproduction; 2017. 10.1002/9781118881286.ch6.
- 22.Sonveaux P, Copetti T, De Saedeleer CJ, Vegran F, Verrax J, Kennedy KM, et al. Targeting the lactate transporter MCT1 in endothelial cells inhibits lactate-induced HIF-1 activation and tumor angiogenesis. PLoS One. 2012;7(3):e33418. doi: 10.1371/journal.pone.0033418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vanacker J, Luyckx V, Dolmans M-M, Des Rieux A, Jaeger J, Van Langendonckt A, et al. Transplantation of an alginate–matrigel matrix containing isolated ovarian cells: first step in developing a biodegradable scaffold to transplant isolated preantral follicles and ovarian cells. Biomaterials. 2012;33(26):6079–6085. doi: 10.1016/j.biomaterials.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 24.LaManna JC. Hypoxia/Ischemia and the pH Paradox. In: Ince C, Kesecioglu J, Telci L, Akpir K, editors. Oxygen transport to tissue XVII. Advances in Experimental Medicine and Biology. Boston, MA: Springer; 1996, vol 388. 10.1007/978-1-4613-0333-6_36
- 25.Wongsrikeao P, Otoi T, Karja NW, Agung B, Nii M, Nagai T. Effects of ovary storage time and temperature on DNA fragmentation and development of porcine oocytes. J Reprod Dev. 2005;51(1):87–97. doi: 10.1262/jrd.51.87. [DOI] [PubMed] [Google Scholar]
- 26.Tellado M, Alvarez G, Dalvit G, Cetica P. The conditions of ovary storage affect the quality of porcine oocytes. Advances in Reproductive Sciences. 2014;02:56–67. doi: 10.4236/arsci.2014.23007. [DOI] [Google Scholar]
- 27.Chan FK-M, Moriwaki K, De Rosa MJ. Detection of necrosis by release of lactate dehydrogenase activity. Methods in Molecular Biology (Clifton, NJ) 2013;979:65–70. doi: 10.1007/978-1-62703-290-2_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lucci CM, Kacinskis MA, Rumpf R, Báo SN. Effects of lowered temperatures and media on short-term preservation of zebu (Bos indicus) preantral ovarian follicles. Theriogenology. 2004;61(2–3):461–472. doi: 10.1016/s0093-691x(03)00226-7. [DOI] [PubMed] [Google Scholar]
- 29.Matsushita S, Tani T, Kato Y, Tsunoda Y. Effect of low-temperature bovine ovary storage on the maturation rate and developmental potential of follicular oocytes after in vitro fertilization, parthenogenetic activation, or somatic cell nucleus transfer. Anim Reprod Sci. 2004;84(3–4):293–301. doi: 10.1016/j.anireprosci.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 30.Nagao Y, Harada Y, Yamaguchi M, Igarashi A, Ooshima Y, Kato Y. Antioxidant treatment during preservation of bovine ovaries increased the development potential of embryos. Zygote. 2010;18(4):315–321. doi: 10.1017/S0967199409990384. [DOI] [PubMed] [Google Scholar]
- 31.Evecen M, Cirit Ü, Demir K, Karaman E, Hamzaoğlu Aİ, Bakırer G. Developmental competence of domestic cat oocytes from ovaries stored at various durations at 4°C temperature. Anim Reprod Sci. 2009;116(1):169–172. doi: 10.1016/j.anireprosci.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 32.Carvalho FCA, Lucci CM, Silva JRV, Andrade ER, Báo SN, Figueiredo JR. Effect of Braun-Collins and saline solutions at different temperatures and incubation times on the quality of goat preantral follicles preserved in situ. Anim Reprod Sci. 2001;66(3):195–208. doi: 10.1016/S0378-4320(01)00085-9. [DOI] [PubMed] [Google Scholar]
- 33.Santos RR, JRV S, SHF C, APR R, RNB L, Figueiredo JR. Effect of 0.9% saline solution and phosphate buffer saline at different temperatures and incubation times on the morphology of goat preantral follicles. Brazilian J Vet Res Anim Sci. 2002;39:254–259. [Google Scholar]
- 34.Lee HS, Yin XJ, Kong IK. Sensitivity of canine oocytes to low temperature. Theriogenology. 2006;66(6):1468–1470. doi: 10.1016/j.theriogenology.2006.01.051. [DOI] [PubMed] [Google Scholar]
- 35.Lopes CA, dos Santos RR, Celestino JJ, Melo MA, Chaves RN, Campello CC, et al. Short-term preservation of canine preantral follicles: effects of temperature, medium and time. Anim Reprod Sci. 2009;115(1–4):201–214. doi: 10.1016/j.anireprosci.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 36.Tas M, Evecen M, Ozdas OB, Cirit U, Demir K, Birler S, et al. Effect of transport and storage temperature of ovaries on in vitro maturation of bitch oocytes. Anim Reprod Sci. 2006;96(1–2):30–34. doi: 10.1016/j.anireprosci.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 37.Lucci CM, Schreier LL, Machado GM, Amorim CA, Bao SN, Dobrinsky JR. Effects of storing pig ovaries at 4 or 20 degrees C for different periods of time on the morphology and viability of pre-antral follicles. Reprod Domest Anim. 2007;42(1):76–82. doi: 10.1111/j.1439-0531.2006.00734.x. [DOI] [PubMed] [Google Scholar]
- 38.Garcia-Alvarez O, Maroto-Morales A, Berlinguer F, Fernandez-Santos MR, Esteso MC, Mermillod P, et al. Effect of storage temperature during transport of ovaries on in vitro embryo production in Iberian red deer (Cervus elaphus hispanicus) Theriogenology. 2011;75(1):65–72. doi: 10.1016/j.theriogenology.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 39.Wang YS, Zhao X, Su JM, An ZX, Xiong XR, Wang LJ, Liu J, Quan FS, Hua S, Zhang Y. Lowering storage temperature during ovary transport is beneficial to the developmental competence of bovine oocytes used for somatic cell nuclear transfer. Anim Reprod Sci. 2011;124(1–2):48–54. doi: 10.1016/j.anireprosci.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 40.Cocchia N, Corteggio A, Altamura G, Tafuri S, Rea S, Rosapane I, Sica A, Landolfi F, Ciani F. The effects of superoxide dismutase addition to the transport medium on cumulus–oocyte complex apoptosis and IVF outcome in cats (Felis catus) Reprod Biol. 2015;15(1):56–64. doi: 10.1016/j.repbio.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 41.Piras AR, Burrai GP, Ariu F, Falchi L, Zedda MT, Pau S, Gadau SD, Antuofermo E, Bebbere D, Ledda S, Bogliolo L. Structure of preantral follicles, oxidative status and developmental competence of in vitro matured oocytes after ovary storage at 4 degrees C in the domestic cat model. Reprod Biol Endocrinol. 2018;16(1):76. doi: 10.1186/s12958-018-0395-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goodarzi A, Zare Shahneh A, Kohram H, Sadeghi M, Moazenizadeh MH, Fouladi-Nashta A, Dadashpour Davachi N. Effect of melatonin supplementation in the long-term preservation of the sheep ovaries at different temperatures and subsequent in vitro embryo production. Theriogenology. 2018;106:265–270. doi: 10.1016/j.theriogenology.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 43.Evecen M, Cirit U, Demir K, Ozdas OB, Tas M, Birler S, et al. Effects of estrous cycle stage and transport temperature of ovaries on in vitro maturation of canine oocytes. Anim Reprod Sci. 2010;117(1–2):160–165. doi: 10.1016/j.anireprosci.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 44.Hanna C, Long C, Hinrichs K, Westhusin M, Kraemer D. Assessment of canine oocyte viability after transportation and storage under different conditions. Anim Reprod Sci. 2008;105(3):451–456. doi: 10.1016/j.anireprosci.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 45.Gomes RG, Andrade ER, Lisboa LA, Ciquini A, Barreiros TR, Fonseca NA, et al. Effect of holding medium, temperature and time on structural integrity of equine ovarian follicles during the non-breeding season. Theriogenology. 2012;78(4):731–736. doi: 10.1016/j.theriogenology.2012.03.019. [DOI] [PubMed] [Google Scholar]
- 46.Raffel N, Dittrich R, ORLOWSKI P, TISCHER H, SÖDER S, ERBER R, et al. Is ovarian tissue transport at supra-zero temperatures compared to body temperature optimal for follicle survival? In Vivo. 2020;34(2):533–541. doi: 10.21873/invivo.11805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lima GL, Santos EAA, Lima LF, Luz VB, Rodrigues APR, Silva AR. Short-term preservation of Pecari tajacu ovarian preantral follicles using phosphate buffered saline (PBS) or powdered coconut water (ACP(r)) media. J Arquivo Brasileiro de Medicina Veterinária e Zootecnia. 2014;66:1623–1630. doi: 10.1590/1678-7297. [DOI] [Google Scholar]
- 48.Staub F, Baethmann A, Peters J, Weigt H, Kempski O. Effects of lactacidosis on glial cell volume and viability. J Cereb Blood Flow Metab. 1990;10(6):866–876. doi: 10.1038/jcbfm.1990.143. [DOI] [PubMed] [Google Scholar]
- 49.Levine RL. Ischemia: from acidosis to oxidation. FASEB J. 1993;7(13):1242–1246. doi: 10.1096/fasebj.7.13.8405809. [DOI] [PubMed] [Google Scholar]
- 50.Mendiola P, De Costa J. The effects of pH and temperature on the kinetic properties of skeletal muscle lactate dehydrogenase from anuran amphibians. Journal of Comparative Physiology B. 1990;160(1):105–111. doi: 10.1007/bf00258769. [DOI] [Google Scholar]
- 51.Goncalves RJ, Cavalcante AY, Gouveia BB, Lins TL, Barberino RS, Menezes VG, et al. Lower apoptosis rate in ovine preantral follicles from ovaries stored in supplemented preservation media. Zygote. 2015;23(6):943–950. doi: 10.1017/s0967199414000665. [DOI] [PubMed] [Google Scholar]
- 52.Barberino R, Gonçalves R, Menezes VG, Barros V, Lins T, Gouveia B, et al. Influence of the ovarian fragmentation before storage at 4°C on the apoptosis rates and in vitro development of ovine preantral follicles. Anim Reprod. 2016;13:28–35. doi: 10.4322/1984-3143-AR772. [DOI] [Google Scholar]
- 53.Kamoshita K, Okamoto N, Nakajima M, Haino T, Sugimoto K, Okamoto A, Sugishita Y, Suzuki N. Investigation of in vitro parameters and fertility of mouse ovary after storage at an optimal temperature and duration for transportation. Hum Reprod. 2016;31(4):774–781. doi: 10.1093/humrep/dew023. [DOI] [PubMed] [Google Scholar]
- 54.Isachenko V, Orth I, Isachenko E, Mallmann P, Peters D, Schmidt T, Morgenstern B, Foth D, Hanstein B, Rahimi G. Viability of human ovarian tissue confirmed 5 years after freezing with spontaneous ice-formation by autografting and chorio-allantoic membrane culture. Cryobiology. 2013;66(3):233–238. doi: 10.1016/j.cryobiol.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 55.Fiedler J, Jara P, Luza S, Dorfman M, Grouselle D, Rage F, Lara HE, Arancibia S. Cold stress induces metabolic activation of thyrotrophin-releasing hormone-synthesising neurones in the magnocellular division of the hypothalamic paraventricular nucleus and concomitantly changes ovarian sympathetic activity parameters. J Neuroendocrinol. 2006;18(5):367–376. doi: 10.1111/j.1365-2826.2006.01427.x. [DOI] [PubMed] [Google Scholar]
- 56.Kaloyianni M, Freedland RA. Contribution of several amino acids and lactate to gluconeogenesis in hepatocytes isolated from rats fed various diets. The Journal of Nutrition. 1990;120(1):116–122. doi: 10.1093/jn/120.1.116. [DOI] [PubMed] [Google Scholar]
- 57.Chatham JC. Lactate -- the forgotten fuel! J Physiol. 2002;542(Pt 2):333. doi: 10.1113/jphysiol.2002.020974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Meex RCR, Schrauwen P, Hesselink MKC. Modulation of myocellular fat stores: lipid droplet dynamics in health and disease. Am J Physiol Regul Integr Comp Physiol. 2009;297(4):R913–RR24. doi: 10.1152/ajpregu.91053.2008. [DOI] [PubMed] [Google Scholar]
- 59.Jarc E, Petan T. Lipid droplets and the management of cellular stress. Yale J Biol Med. 2019;92(3):435–452. [PMC free article] [PubMed] [Google Scholar]
- 60.Homa ST, Racowsky C, McGaughey RW. Lipid analysis of immature pig oocytes. J Reprod Fertil. 1986;77(2):425–434. doi: 10.1530/jrf.0.0770425. [DOI] [PubMed] [Google Scholar]
- 61.Homa ST, Brown CA. Changes in linoleic acid during follicular development and inhibition of spontaneous breakdown of germinal vesicles in cumulus-free bovine oocytes. J Reprod Fertil. 1992;94(1):153–160. doi: 10.1530/jrf.0.0940153. [DOI] [PubMed] [Google Scholar]
- 62.Sturmey R, Reis A, Leese H, McEvoy T. Role of fatty acids in energy provision during oocyte maturation and early embryo development. Reprod Domest Anim. 2009;44(s3):50–58. doi: 10.1111/j.1439-0531.2009.01402.x. [DOI] [PubMed] [Google Scholar]
- 63.Dunning KR, Russell DL, Robker RL. Lipids and oocyte developmental competence: the role of fatty acids and β-oxidation. REPRODUCTION. 2014;148(1):R15–R27. doi: 10.1530/REP-13-0251. [DOI] [PubMed] [Google Scholar]
- 64.Rodriguez SB, Campo-Engelstein L. Conceiving wholeness: women, motherhood, and ovarian transplantation, 1902 and 2004. Perspect Biol Med. 2011;54(3):409–416. doi: 10.1353/pbm.2011.0036. [DOI] [PubMed] [Google Scholar]
- 65.Belzer FO, Southard JH. Principles of solid-organ preservation by cold storage. Transplantation. 1988;45(4):673–676. doi: 10.1097/00007890-198804000-00001. [DOI] [PubMed] [Google Scholar]
- 66.Finger EB. Organ preservation. https://emedicine.medscape.com/article/431140-overview#showall. 2019. Accessed 04/02/2020 2020.
- 67.Singh R, Cuervo AM. Autophagy in the cellular energetic balance. Cell Metab. 2011;13(5):495–504. doi: 10.1016/j.cmet.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ito K, Carracedo A, Weiss D, Arai F, Ala U, Avigan DE, Schafer ZT, Evans RM, Suda T, Lee CH, Pandolfi PP. A PML–PPAR-δ pathway for fatty acid oxidation regulates hematopoietic stem cell maintenance. Nat Med. 2012;18(9):1350–1358. doi: 10.1038/nm.2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wilkinson JH, Walter SJ. Oxamate as a differential inhibitor of lactate dehydrogenase isoenzymes. Enzyme. 1972;13:170–176. doi: 10.1159/000459658. [DOI] [PubMed] [Google Scholar]
- 70.Parrott JA, Skinner MK. Kit ligand actions on ovarian stromal cells: effects on theca cell recruitment and steroid production. Mol Reprod Dev. 2000;55(1):55–64. doi: 10.1002/(SICI)1098-2795(200001)55:1<55::AID-MRD8>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 71.Gastal GD, Alves BG, Alves KA, Souza ME, Vieira AD, Varela AS, Jr, et al. Ovarian fragment sizes affect viability and morphology of preantral follicles during storage at 4 degrees C. Reproduction. 2017;153(5):577–587. doi: 10.1530/rep-16-0621. [DOI] [PubMed] [Google Scholar]
- 72.Gallez B, Mäder K. Accurate and sensitive measurements of pO2 in vivo using low frequency EPR spectroscopy: how to confer biocompatibility to the oxygen sensors. Free Radic Biol Med. 2000;29(11):1078–1084. doi: 10.1016/S0891-5849(00)00405-6. [DOI] [PubMed] [Google Scholar]
- 73.Khan N, Williams BB, Hou H, Li H, Swartz HM. Repetitive tissue pO2 measurements by electron paramagnetic resonance oximetry: current status and future potential for experimental and clinical studies. Antioxid Redox Signal. 2007;9(8):1169–1182. doi: 10.1089/ars.2007.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Van Eyck A-S, Jordan BF, Gallez B, Heilier J-F, Van Langendonckt A, Donnez J. Electron paramagnetic resonance as a tool to evaluate human ovarian tissue reoxygenation after xenografting. Fertil Steril. 2009;92(1):374–381. doi: 10.1016/j.fertnstert.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 75.Van Eyck A-S, Bouzin C, Feron O, Romeu L, Van Langendonckt A, Donnez J, et al. Both host and graft vessels contribute to revascularization of xenografted human ovarian tissue in a murine model. Fertil Steril. 2010;93(5):1676–1685. doi: 10.1016/j.fertnstert.2009.04.048. [DOI] [PubMed] [Google Scholar]
- 76.Slegtenhorst BR, Dor FJMF, Rodriguez H, Voskuil FJ, Tullius SG. Ischemia/reperfusion injury and its consequences on immunity and inflammation. Current Transplantation Reports. 2014;1(3):147–154. doi: 10.1007/s40472-014-0017-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Camboni A, Van Langendonckt A, Pirard C, Amorim C, Demylle D, Dolmans M-M, et al. IVF outcome in patients with orthotopically transplanted ovarian tissue. Hum Reprod. 2009;24(11):2778–2787. doi: 10.1093/humrep/dep289. [DOI] [PubMed] [Google Scholar]
- 78.Jing L, Yao L, Zhao M, Peng L-P, Liu M. Organ preservation: from the past to the future. Acta Pharmacol Sin. 2018;39(5):845–857. doi: 10.1038/aps.2017.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Smitz J, Dolmans MM, Donnez J, Fortune JE, Hovatta O, Jewgenow K, Picton HM, Plancha C, Shea LD, Stouffer RL, Telfer EE, Woodruff TK, Zelinski MB. Current achievements and future research directions in ovarian tissue culture, in vitro follicle development and transplantation: implications for fertility preservation. Hum Reprod Update. 2010;16(4):395–414. doi: 10.1093/humupd/dmp056. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 76.9 kb)
Data Availability Statement
Not applicable.