Key Points
Question
Is there an association between maternal labor epidural analgesia given for vaginal delivery and risk of autism spectrum disorders in children?
Findings
In this multiethnic population-based clinical birth cohort that included 147 895 children, autism spectrum disorders were diagnosed in 1.9% of the children delivered vaginally with epidural analgesia vs 1.3% of the children delivered vaginally without the exposure, a 37% relative increase in risk that was significant after adjusting for potential confounders.
Meaning
This study suggests that exposure to epidural analgesia for vaginal delivery may be associated with increased risk of autism in children; further research is warranted to confirm the study findings and understand the potential mechanisms.
Abstract
Importance
Although the safety of labor epidural analgesia (LEA) for neonates has been well documented, the long-term health effects of LEA on offspring remain to be investigated.
Objective
To assess the association between maternal LEA exposure and risk of autism spectrum disorders (ASDs) in offspring.
Design, Setting, and Participants
Data for this retrospective longitudinal birth cohort study were derived from electronic medical records from a population-based clinical birth cohort. A total of 147 895 singleton children delivered vaginally between January 1, 2008, and December 31, 2015, in a single integrated health care system were included. Children were followed up from the age of 1 year until the first date of the following occurrences: clinical diagnosis of ASD, last date of health plan enrollment, death, or the study end date of December 31, 2018.
Exposures
Use and duration of LEA.
Main Outcomes and Measures
The main outcome was clinical diagnosis of ASD. Cox proportional hazards regression analysis was used to estimate the hazard ratio (HR) of ASD associated with LEA exposure.
Results
Among the cohort of 147 895 singleton children (74 425 boys [50.3%]; mean [SD] gestational age at delivery, 38.9 [1.5] weeks), 109 719 (74.2%) were exposed to maternal LEA. Fever during labor was observed in 13 055 mothers (11.9%) in the LEA group and 510 of 38 176 mothers (1.3%) in the non-LEA group. Autism spectrum disorders were diagnosed in 2039 children (1.9%) in the LEA group and 485 children (1.3%) in the non-LEA group. After adjusting for potential confounders, including birth year, medical center, maternal age at delivery, parity, race/ethnicity, educational level, household income, history of comorbidity, diabetes during pregnancy, smoking during pregnancy, preeclampsia or eclampsia, prepregnancy body mass index, gestational weight gain, gestational age at delivery, and birth weight, the HR associated with LEA vs non-LEA exposure was 1.37 (95% CI, 1.23-1.53). Relative to the unexposed group, the adjusted HR associated with LEA exposure of less than 4 hours was 1.33 (95% CI, 1.17-1.53), with LEA exposure of 4 to 8 hours was 1.35 (95% CI, 1.20-1.53), and with LEA exposure of more than 8 hours was 1.46 (95% CI, 1.27-1.69). Within the LEA group, there was a significant trend of ASD risk associated with increasing duration of LEA exposure after adjusting for covariates (HR for linear trend, 1.05 [95% CI, 1.01-1.09] per 4 hours). Adding fever to the model did not change the HR estimate associated with LEA exposure (adjusted HR for LEA vs non-LEA, 1.37 [95% CI, 1.22-1.53]).
Conclusions and Relevance
This study suggests that maternal LEA may be associated with increased ASD risk in children. The risk appears to not be directly associated with epidural-related maternal fever.
This cohort study assesses the association between maternal exposure to labor epidural analgesia and risk of autism spectrum disorders in offspring
Introduction
Labor epidural analgesia (LEA) is the most commonly administered neuraxial anesthesia for labor pain.1 In the United States, more than 70% of women receive some form of a neuraxial procedure during labor. Although the effectiveness of neuraxial anesthesia for labor pain management and the safety of neuraxial anesthesia for the fetus and newborns during the perinatal period have been well documented, the long-term effects of neuraxial anesthesia on the offspring are largely unknown.2,3,4 Limited toxicology and animal studies have shown that standard clinical doses of local anesthetics (LAs) can produce neurotoxic effects and alter normal behavioral development in rhesus monkeys.5 Recent observational studies with humans found that general anesthesia for cesarean deliveries was associated with an approximately 50% increased risk for children to develop autism spectrum disorders (ASDs) compared with vaginal deliveries.6,7 A recent meta-analysis comprising 61 studies with 20 million deliveries concluded that birth by cesarean delivery was significantly associated with the risk of ASD.8 However, these studies did not address the potential risk associated with the common use of neuraxial anesthesia for routine vaginal delivery. Given that LEA is currently the criterion standard for labor pain management for routine vaginal delivery, it is critical to assess whether maternal LEA exposure has any long-term association with outcomes in offspring.
The purpose of this study was to assess whether LEA exposure for routine vaginal delivery was associated with ASD risk in offspring. Because LEA can induce fever during delivery, we also assessed the role that maternal fever plays in the association between LEA exposure and risk of ASD in offspring.9,10 Autism spectrum disorder is a neurodevelopmental disorder diagnosed relatively early in life that carries various lifelong disabilities.11,12,13 The increasing prevalence of ASD is not fully explained by improvement in ascertainment.14 Genetic and environmental factors both in early life and prenatally are thought to play important roles in the development of ASD.15,16 In this study, we controlled for various important potential confounders while assessing the risk of ASD associated with LEA exposure. Data were derived from a large birth cohort from an integrated health care system with standard clinical practices and comprehensive electronic medical records. Part of the cohort has been used in previous studies of the association between maternal diabetes and risk of ASD in offspring.17,18,19
Methods
Study Population
This retrospective longitudinal cohort study included singleton children born by vaginal delivery at 28 to 44 weeks’ gestation in Kaiser Permanente Southern California (KPSC) hospitals between January 1, 2008, and December 31, 2015. Kaiser Permanente Southern California is an integrated health care delivery system in which the continuum of prenatal, perinatal, and postnatal care for both mother and baby are standardized. All medical care data, including anesthesia records, have been captured in a systemwide integrated electronic medical record (EMR) data system. Per KPSC guidelines, a brief screening checklist (a modified version of the Checklist for Autism in Toddlers20) is administered to all children between the ages of 18 and 24 months to screen for developmental delays, including ASD. A clinical diagnosis of ASD is based on pediatric developmental specialist evaluations. Methods to obtain the demographic characteristics, covariates, and ASD diagnoses in children have been described in previously published studies on the association between maternal diabetes during pregnancy and risk of ASD in children, which broadly represent the Southern California population.17,18,19 The KPSC Institutional Review Board approved this study and waived individual participant consent; as it was a data-only study, institutional safeguards to maintain risk were well detailed, including deidentification of patient information, the research involved minimal risk to participants, and the waiver would not adversely affect the rights and welfare of participants.
Screening for ASD did not begin in KPSC until after 1 year of age; therefore, children who did not enroll as KPSC health plan members by 1 year of age were not eligible. Follow-up ended on the first date that any one of the following occurred: clinical diagnosis of ASD, last date of continuous KPSC plan membership, death of the child (any cause), or study end date of December 31, 2018.
All maternal and child data were extracted from the KPSC EMR and birth certificate records and were linked by a unique membership identifier used for all patient care delivery. All data were validated for quality through data plots and frequency tables. Potential outliers and data errors were rectified by cross-checking against historical data in the EMR. Validation of the data was established in previous reports.17,18,19,21
Exposures and Outcomes
The exposure variable was LEA administered during labor and delivery. This information was extracted from LEA procedure notes and pharmacy data stored in the EMR. The duration of LEA exposure was approximated by the duration between the LEA placement time and the delivery time. Fever was defined as a body temperature of 38 °C or higher at any time between hospital admission and time of delivery for the non-LEA group, and epidural-related maternal fever (ERMF) was defined as a body temperature of ≥38 °C or higher after LEA placement and before the time of delivery for the LEA group.
The outcome measure was the presence or absence of ASD during the follow-up period, which was identified by International Classification of Diseases, Ninth Revision codes 299.x or equivalent KPSC codes. These codes included autistic disorders, Asperger syndrome, or pervasive developmental disorder not otherwise specified and excluded childhood disintegrative disorder and Rett syndrome. Codes from at least 2 separate visits were required for an ASD diagnosis; these codes were validated with a positive predictive value of 88% and used in previous publications.17,18,19
Covariates
Covariates to control for potential confounders at the time of the epidural event were maternal social demographic characteristics (age at delivery, parity, educational level, self-reported maternal race/ethnicity, and median family household income based on census tract of residence), medical center of delivery, history of comorbidity (≥1 diagnoses of heart, lung, kidney, or liver disease or cancer), maternal obesity (prepregnancy body mass index and gestational weight gain), diabetes (preexisting type 1 or 2 diabetes or gestational diabetes), preeclampsia or eclampsia, and smoking during pregnancy, as well as child characteristics at delivery (gestational age at delivery, birth weight, sex, and presence of any birth defect).
Statistical Analysis
Maternal characteristics, obstetrical outcomes, and neonatal outcomes were compared between the LEA and non-LEA groups by use of χ2 tests for proportions and t tests for mean values. The cumulative incidence of ASD in each exposure group was estimated by use of the Kaplan-Meier method. Relative risks of ASD were estimated by hazard ratios (HRs) using Cox proportional hazards regression models in which the time variable is child’s age minus 1. To control for potential correlation owing to multiple siblings born to the same mother, robust SEs were used for statistical testing. Data analyses were also repeated by randomly sampling 1 child per family. The proportional hazard assumption was assessed by examining the log (−log) plot of the survival function vs the log of the child’s age and showed a parallel association and, thus, was not violated. Use of LEA was modeled as a binary (yes or no) variable. Duration of LEA exposure was considered as a categorical variable with the following 3 strata: less than 4 hours, 4 to 8 hours, and more than 8 hours. Birth year was included as a covariate to control for possible confounding due to changes in delivery practice and ASD screening during the study period. Medical center of delivery was included as a covariate to control for geographical variation in LEA use and ASD diagnosis.1,19,21,22 Maternal social demographic characteristics, history of comorbidity, obesity, diabetes, preeclampsia or eclampsia, and smoking during pregnancy as well as gestational age at delivery and birth weight were included as covariates to adjust for potential confounding. The child’s sex was similarly distributed between the LEA and non-LEA groups; although boys have a much higher ASD prevalence than girls, additionally adjusting for the child’s sex in the data analysis did not change the risk estimates associated with LEA use. Results are presented without the adjustment of the child’s sex.
Primary data analyses used inverse probability of treatment weighting (IPTW) to balance all potential confounders between LEA and non-LEA use as well as standard covariate adjustment. In the IPTW analysis, the propensity of receiving LEA was calculated using a logistic regression model of all covariates included in the adjusted analysis. Sensitivity analyses were conducted by excluding children with preterm birth (defined as gestational age of delivery, <37 weeks) and excluding children with any birth defect. We also reported potential confounding due to unmeasured confounders by computing the E-value.23 Data analysis was conducted using SAS Enterprise Guide, version 7.1 (SAS Institute Inc) and R, version 3.6.0 (64 bit; R Foundation for Statistical Computing). All P values were from 2-sided tests and results were deemed statistically significant at P < .05. Point estimates and 95% CIs are presented.
Results
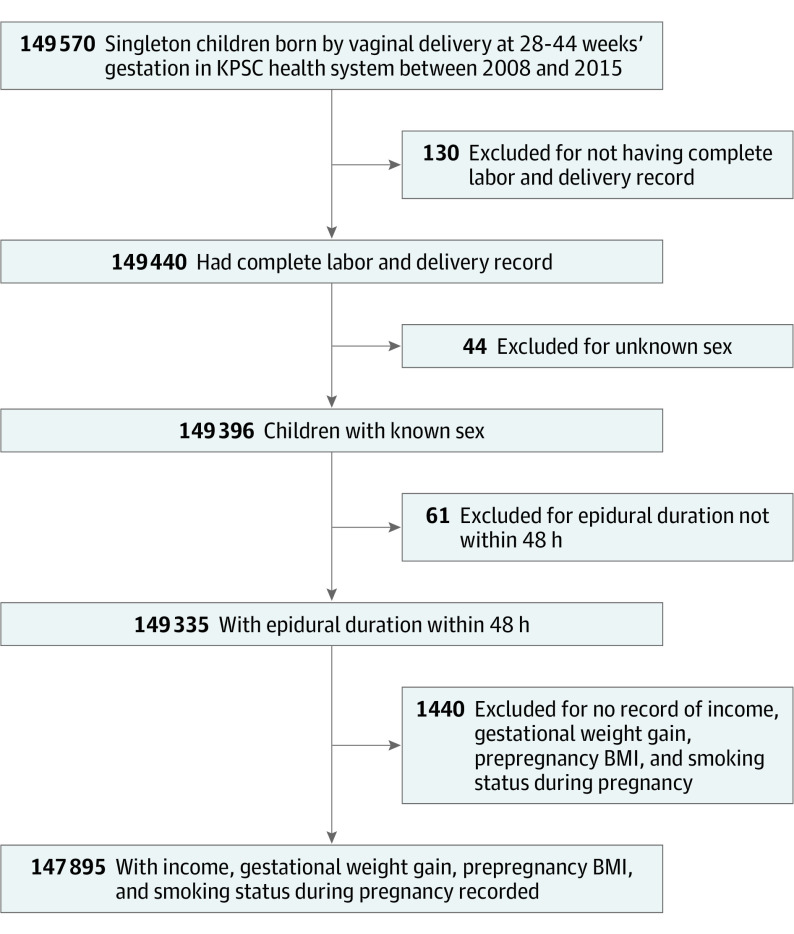
Of the 147 895 children (74 425 boys [50.3%]; mean [SD] gestational age at delivery, 38.9 [1.5] weeks) born to 119 973 unique mothers and included in the data analysis, 109 719 (74.2%) were born to women exposed to LEA. Figure 1 depicts the derivation of the study cohort. The LEA and non-LEA groups differed in all covariates (maternal age, race/ethnicity, parity, educational level, household income, diabetes status, comorbidity, smoking during pregnancy, preeclampsia or eclampsia, prepregnancy body mass index, gestational weight gain, birth weight, gestational age at delivery, and presence of any birth defect) except for sex of the child (Table 1). The LEA group had a higher fever rate than the non-LEA group (13 055 [11.9%] vs 510 [1.3%]; P < .001), where 1227 mothers [1.1%] in the LEA group had fever prior to LEA.
Figure 1. Derivation of Study Cohort.
BMI indicates body mass index; and KPSC, Kaiser Permanente Southern California.
Table 1. Characteristics of the Cohort at the Time of the Index Pregnancy.
| Characteristic | No. (%) of participants | P valuea | |
|---|---|---|---|
| Unexposed to LEA (n = 38 176) | Exposed to LEA (n = 109 719) | ||
| Maternal characteristics | |||
| Age at delivery, mean (SD), y | 30.6 (5.5) | 29.8 (5.6) | <.001 |
| Race/ethnicity | |||
| Non-Hispanic White | 8292 (21.7) | 29 149 (26.6) | <.001 |
| Non-Hispanic Black | 2749 (7.2) | 8231 (7.5) | |
| Hispanic | 21 591 (56.6) | 53 878 (49.1) | |
| Asian or Pacific Islanders | 4679 (12.3) | 15 546 (14.2) | |
| Other | 865 (2.3) | 2915 (2.7) | |
| Parity | |||
| 0 | 8821 (23.1) | 39 362 (35.9) | <.001 |
| 1 | 13 293 (34.8) | 33 891 (30.9) | |
| ≥2 | 13 349 (35) | 22 962 (20.9) | |
| Unknown | 2713 (7.1) | 13 504 (12.3) | |
| Educational level | |||
| ≤High school | 13 152 (34.5) | 31 995 (29.2) | <.001 |
| Some college | 10 988 (28.8) | 34 343 (31.3) | |
| College and postgraduate | 13 547 (35.5) | 42 108 (38.4) | |
| Unknown | 489 (1.3) | 1273 (1.2) | |
| Household income, $ | |||
| <30 000 | 1116 (2.9) | 2457 (2.2) | <.001 |
| 30 000-49 999 | 10 453 (27.4) | 25 233 (23) | |
| 50 000-69 999 | 12 787 (33.5) | 35 652 (32.5) | |
| 70 000-89 999 | 7835 (20.5) | 24 884 (22.7) | |
| ≥90 000 | 5985 (15.7) | 21 493 (19.6) | |
| History of comorbidity | 5498 (14.4) | 19 056 (17.4) | <.001 |
| Diabetes during pregnancy | |||
| Gestational diabetes | 3172 (8.3) | 8663 (7.9) | <.001 |
| Pregestational diabetes | 726 (1.9) | 2934 (2.6) | |
| Smoking during pregnancy | 188 (0.5) | 939 (0.9) | <.001 |
| Preeclampsia or eclampsia | 823 (2.2) | 3777 (3.4) | <.001 |
| Prepregnancy BMI, mean (SD) | 26.1 (5.7) | 26.4 (5.9) | <.001 |
| Gestational weight gain, mean (SD), kg | 11.7 (6.1) | 12.5 (6.3) | <.001 |
| Fever during labor | 510 (1.3) | 13 055 (11.9) | <.001 |
| Child characteristics | |||
| Birth weight, mean (SD), g | 3316.2 (495.3) | 3354.5 (475.7) | <.001 |
| Gestational age at delivery, mean (SD), wk | 38.7 (1.6) | 38.9 (1.5) | <.001 |
| Presence of any birth defect | 4447 (11.6) | 14 159 (12.9) | <.001 |
| Female | 19 031 (49.9) | 54 439 (49.6) | .43 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); LEA, labor epidural analgesia.
Calculated from χ2 test for categorical variables and from analysis of variance F test for continuous variables.
Of children born to mothers in the LEA group, 32 433 (29.6%) were exposed to LEA for less than 4 hours (median, 2 hours [interquartile range, 1-3 hours]), 50 248 (45.8%) were exposed to LEA for 4 to 8 hours (median, 6 hours [interquartile range, 4-7 hours]), and 27 038 (24.6%) were exposed to LEA for more than 8 hours (median, 11 hours [interquartile range, 10-14 hours]). The ERMF rate increased with increasing duration of LEA exposure (811 of 32 433 [2.5%] for less than 4 hours LEA, 4994 of 50 248 [9.9%] for 4 to 8 hours LEA, and 7250 of 27 038 [26.8%] for more than 8 hours LEA).
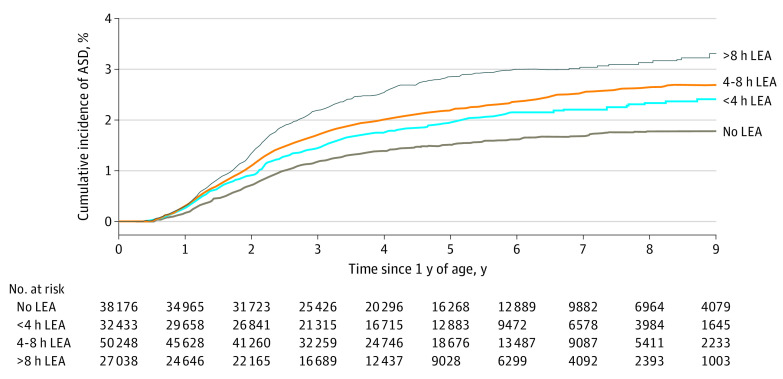
A total of 2524 children received a diagnosis of ASD during follow-up: 2039 (1.9%) in the LEA group and 485 (1.3%) in non-LEA group. A total of 527 of 32 433 children (1.6%) who had LEA exposure for less than 4 hours, 911 of 50 248 children (1.8%) who had LEA exposure for 4 to 8 hours, and 601 of 27 038 children (2.2%) who had LEA exposure for more than 8 hours received a diagnosis of ASD. Figure 2 depicts the unadjusted cumulative incidence of ASD by LEA exposure groups.
Figure 2. Unadjusted Cumulative Incidence of Autism Spectrum Disorder (ASD) by Duration of Labor Epidural Anesthesia (LEA).
In the bivariable analysis adjusted for birth year, the HR of ASD associated with LEA relative to non-LEA was 1.48 (95% CI, 1.34-1.65) (Table 2). In the IPTW analyses to balance the covariate distribution between LEA exposure and nonexposure, the risk associated with LEA was 1.38 (95% CI, 1.24-1.53). The stabilized IPTW resulted in a well-balanced covariate distribution between the LEA and non-LEA groups with standardized differences of less than 0.1 for all covariates (eTable 1 in the Supplement). Including the potential confounders as covariates in the model resulted in an HR of 1.37 (95% CI, 1.23-1.53) (Table 2). Thus, the HRs estimated from the IPTW and covariate adjustment are almost identical.
Table 2. Associations Between Labor Epidural Analgesia Use at Delivery and Risk of ASD in Offspring.
| Characteristic | No. with ASDs/total No. | Hazard ratio (95% CI) | |
|---|---|---|---|
| Bivariablea | Adjusting for covariatesb | ||
| Labor epidural analgesia | |||
| No | 485/38 176 | 1 [Reference] | 1 [Reference] |
| Yes | 2039/109 719 | 1.48 (1.34-1.65) | 1.37 (1.23-1.53) |
| Duration of labor epidural analgesia | |||
| No labor epidural analgesia | 485/38 176 | 1 [Reference] | 1 [Reference] |
| <4 h | 527/32 433 | 1.28 (1.12-1.46) | 1.33 (1.17-1.53) |
| 4-8 h | 911/50 248 | 1.46 (1.29-1.64) | 1.35 (1.20-1.53) |
| >8 h | 601/27 038 | 1.78 (1.57-2.03) | 1.46 (1.27-1.69) |
| Linear trend (per 4 h)c | 2039/109 719 | 1.11 (1.07-1.15) | 1.05 (1.01-1.09) |
Abbreviation: ASD, autism spectrum disorder.
Labor epidural analgesia was analyzed individually where only birth year was adjusted in the model.
Covariates included birth year, maternal age at delivery, parity, race/ethnicity, educational level, household income, history of comorbidity, diabetes during pregnancy, smoking during pregnancy, preeclampsia or eclampsia, prepregnancy body mass index, gestational weight gain, gestational age at delivery, birth weight, and medical center.
Linear trend is defined as the duration of labor epidural analgesia as a continuous variable within the labor epidural analgesia group.
Table 2 also presents the HRs associated with LEA exposure of less than 4 hours, 4 to 8 hours, and more than 8 hours relative to no LEA exposure, and the linear trend associated with duration of LEA within the LEA group by treating LEA duration as a continuous variable. There was no significant nonlinear association between duration of LEA and ASD risk. After adjusting for potential confounders, the HR associated with LEA exposure of less than 4 hours was 1.33 (95% CI, 1.17-1.53), with LEA exposure of 4 to 8 hours was 1.35 (95% CI, 1.20-1.53), and with LEA exposure of more than 8 hours was 1.46 (95% CI, 1.27-1.69) (Table 2). Within the LEA group, the trend of ASD risk associated with an increased duration of LEA exposure was statistically significant (adjusted HR, 1.05 [95% CI, 1.01-1.09] per 4 hours).
To assess the role that maternal fever plays in the association between LEA and ASD, we excluded the 1227 mothers in the LEA group (1.1%) who had fever before LEA and assessed the association between fever after LEA and risk of ASD within the LEA group. Fever after LEA was not associated with ASD risk after adjusting for the same potential confounders included in the primary analysis for LEA (adjusted HR, 1.03 [95% CI, 0.89-1.20]). Thus, the risk of ASD associated with LEA exposure was not mediated by fever. Adding the presence or absence of any fever to the model for the overall cohort did not change the HR estimate associated with LEA exposure (adjusted HR, 1.37 [95% CI, 1.22-1.53]), and fever itself remained not associated with ASD (adjusted HR, 1.05 [95% CI, 0.91-1.21]).
Analyses limited to 1 child per family gave slightly higher HR estimates, but the overall conclusions remained the same as those in the full cohort analyses (eTable 2 in the Supplement). Analyses excluding children with preterm birth or children with birth defects at delivery for the full cohort also gave slightly higher HR estimates associated with LEA than the primary analyses (Table 3). In a multivariable adjusted model, LEA exposure was associated with an HR of 1.40 (95% CI, 1.25-1.57) after excluding 8805 children born at less than 37 weeks’ gestation and 1.46 (95% CI, 1.29-1.65) after excluding 18 606 children with any birth defects. The E-value for the HR of 1.37 from the full cohort was 2.08, with a lower confidence of 1.76. Thus, a minimum risk ratio of 1.76 would be required for an unmeasured confounder to be associated with both the exposure and the outcome, conditional on the measured covariates, to fully explain the observed association between LEA exposure and risk of ASD.
Table 3. Associations Between Labor Epidural Analgesia Use at Delivery and Risk of Autism Spectrum Disorders in Offspring.
| Exposure | Excluding preterm birth (<37 wk)a | Excluding children with birth defectsb | ||
|---|---|---|---|---|
| Hazard ratio (95% CI) | P value | Hazard ratio (95% CI) | P value | |
| Labor epidural analgesia | ||||
| No | 1 [Reference] | Not applicable | 1 [Reference] | Not applicable |
| Yes | 1.40 (1.25-1.57) | <.001 | 1.46 (1.29-1.65) | <.001 |
There were 8805 children excluded owing to a gestational age of less than 37 weeks. Adjustments were made for birth year, maternal age at delivery, parity, race/ethnicity, educational level, household income, history of comorbidity, diabetes during pregnancy, smoking during pregnancy, preeclampsia or eclampsia, prepregnancy body mass index, gestational weight gain, gestational age at delivery, birth weight, and medical center.
There were 18 606 children excluded owing to the presence of birth defects at birth. Adjustments were made for birth year, maternal age at delivery, parity, race/ethnicity, educational level, household income, history of comorbidity, diabetes during pregnancy, smoking during pregnancy, preeclampsia or eclampsia, prepregnancy body mass index, gestational weight gain, gestational age at delivery, birth weight, and medical center.
Discussion
In this large cohort comprised of multiethnic births, we found that maternal exposure to LEA was associated with a 37% increased risk of ASD in children after adjusting for potential confounders. Longer duration of epidural exposure was associated with greater ASD risk, in which the risk was 33% greater for LEA exposure of less than 4 hours, 35% greater for LEA exposure of 4 to 8 hours, and 46% greater for LEA exposure of more than 8 hours, compared with the unexposed group. The association with LEA exposure remained at approximately 40% after including only 1 child per family, excluding children with preterm birth or excluding children with birth defects. Despite the higher frequency of fever in the LEA group that was associated with epidural duration, the fever itself appeared to not be associated with ASD risk and did not explain the association between LEA exposure and risk of ASD.
Our findings are intriguing and bring a concern for the safety and long-term health of offspring regarding the short-term epidural use for labor pain. The current evidence on LEA safety was primarily established using the perinatal outcomes of mothers and newborns.2,4 A previous animal study has reported that labor anesthesia drugs can alter normal behavioral development in rhesus monkeys.5 Limited human studies have reported that anesthesia drug exposure for labor and delivery may be associated with ASD risk in children.3,24,25 Using a population-based case-control design, Glasson et al26 found that labor duration was not associated with ASD risk; however, the mothers of children with ASD were more likely exposed to epidural or caudal anesthesia. In a small survey study, Smallwood et al24 found that labor and delivery medications were significantly associated with elevated ASD risk, which included epidurally administered medications. To our knowledge, our study is the first large longitudinal birth cohort study that has addressed the association between regional anesthesia of LEA and ASD risk in offspring. Our findings of increased ASD risk associated with LEA are consistent with previous reports. Furthermore, we encountered a novel finding that the risk was increased with increasing duration of exposure to LEA.
Potential mechanisms showing an association between LEA and risk of ASD are largely unknown and require further studies. Although LEA can effectively block labor pain and pain-related hormonal release and changes,27,28 we speculate that its commencement may represent the beginning of a novel maternal and fetal physiology, a new homeostasis, and a dynamic biochemical equilibrium, which encompass the principles of physiology, endocrinology, immunology, pharmacology and toxicology, epigenetics, and psychology. Some mechanisms are transient, but others may be persistent and may affect major body systems.2,4,25,29 Although LEA can prolong labor,2,4,29 longer labor has not been demonstrated to be associated with an increased ASD risk.24,25,30 In this study, we found that longer duration of LEA use was associated with a higher ASD risk in the fully adjusted model, suggesting that there may be an association between anesthesia exposure and risk of ASD.
In addition, owing to their low molecular weight, all LAs given epidurally can cross the placenta and be redistributed into the maternal and fetal circulation and thereby may subject both the mother and fetus to the risk of toxic effects.31,32,33,34 The latter include abnormalities in synaptogenesis, neurogenesis, and neuronal apoptosis.35,36 These neurotoxic effects have been observed in the usual clinical concentration37 and have been reported to alter normal behavioral development in rhesus monkeys.5 Furthermore, the fetus has lower levels of serum protein binding sites, lower blood pH, more porous blood-brain barriers, and immature liver function. These factors, coupled with a larger blood supply to the fetal brain, may converge to potentially greater neurotoxic effects.38,39 Our results suggest that there is a need for further study of the neurodevelopmental effects of LAs beyond ASD.
Labor epidural analgesia may also precipitate maternal immune activation, which is a state of immune dysregulation induced by procedural trauma or by LAs.9,40,41 It can be associated with an imbalance of proinflammatory and anti-inflammatory cytokines. In animals, cytokines such as interleukin-6 (IL-6) not only can precipitate and sustain a state of maternal immune activation but also induce fetal neuroinflammation and an ASD-like phenotype.9,42 In humans, maternal IL-6 is associated with neuroinflammatory and morphologic changes in the child’s brain detected on MRI scans.43,44 In this study, we found an association between duration of LEA exposure and rate of ERMF, which was consistent with previous reports.2,4,29 However, we did not find that ERMF was associated with a risk of ASD. This result suggests that LEA-associated ASD risk may not be directly linked to ERMF and that other mechanisms may be responsible for the observation. However, the possibility of an association between maternal immune activation and ASD may still exist because maternal immune activation, a potential cause of ERMF, has many overlapping causes.9,10 Furthermore, ERMF is neither sensitive nor specific for maternal immune activation or underlying severity of cytokine abnormalities.45,46
Strengths and Limitations
This study has some strengths, including the large and multiethnic birth cohort, well-documented exposure, and outcome. An additional strength was that all data featuring continuous perinatal and pediatric care originated from a single integrated health care delivery system, in which standardized care, documentation, and screening and diagnosis of ASD were carried out systemically. We were thus able to control many confounding factors in this study. Furthermore, although this was a retrospective study, all data were captured prospectively, which we believe minimized the risk of systematic recall or ascertainment biases.
Our study has several limitations, and our findings should be interpreted with caution given the wide varieties of LEA practice and cannot be interpreted as a demonstration of a causal link between LEA exposure and subsequent development of ASD. Although the timing of LEA initiation was precisely established and the duration of exposure was reasonably approximated, in this study, the onset of the pathologic processes of ASD is unknown. Potential uncontrolled confounders may explain the association that we observed. These confounders may include factors both antecedent and subsequent to the peripartum period, such as paternal history, genetic predisposition, viral or bacterial infection, and exposure to other environmental toxins. Furthermore, the variations in the selection and total dosage of LAs, the accumulated dose, the additives such as epinephrine and opioids, and the continuous infusion rates, as well as the timing, frequency, and amount of patient-controlled bolus, may be important aspects of LEA exposure but have not been assessed.
Conclusions
The widespread use of LEA during the past few decades has significantly improved perinatal outcomes for mothers and their newborns; however, our findings raise the concern that the short duration of LEA exposure may be associated with long-term neurodevelopmental disorders in offspring. We believe that further research is warranted to confirm our study findings and to investigate the probable mechanistic association between LEA and ASD.
eTable 1. Characteristics of the Cohort at the Time of the Index Pregnancy
eTable 2. Associations Between Labor Epidural Analgesia (LEA) Use at Delivery and Risk of Autism Spectrum Disorders (ASD) in Offspring (Randomly Select One Child per Family)
References
- 1.Butwick AJ, Bentley J, Wong CA, Snowden JM, Sun E, Guo N. United States state-level variation in the use of neuraxial analgesia during labor for pregnant women. JAMA Netw Open. 2018;1(8):e186567. doi: 10.1001/jamanetworkopen.2018.6567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Birnbach DJ, Bateman BT. Obstetric anesthesia: leading the way in patient safety. Obstet Gynecol Clin North Am. 2019;46(2):329-337. doi: 10.1016/j.ogc.2019.01.015 [DOI] [PubMed] [Google Scholar]
- 3.Anim-Somuah M, Smyth RM, Cyna AM, Cuthbert A. Epidural versus non-epidural or no analgesia for pain management in labour. Cochrane Database Syst Rev. 2018;5:CD000331. doi: 10.1002/14651858.CD000331.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lim G, Facco FL, Nathan N, Waters JH, Wong CA, Eltzschig HK. A review of the impact of obstetric anesthesia on maternal and neonatal outcomes. Anesthesiology. 2018;129(1):192-215. doi: 10.1097/ALN.0000000000002182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Golub MS, Germann SL. Perinatal bupivacaine and infant behavior in rhesus monkeys. Neurotoxicol Teratol. 1998;20(1):29-41. doi: 10.1016/S0892-0362(97)00068-8 [DOI] [PubMed] [Google Scholar]
- 6.Chien L-N, Lin H-C, Shao Y-HJ, Chiou S-T, Chiou H-Y. Risk of autism associated with general anesthesia during cesarean delivery: a population-based birth-cohort analysis. J Autism Dev Disord. 2015;45(4):932-942. doi: 10.1007/s10803-014-2247-y [DOI] [PubMed] [Google Scholar]
- 7.Huberman Samuel M, Meiri G, Dinstein I, et al. Exposure to general anesthesia may contribute to the association between cesarean delivery and autism spectrum disorder. J Autism Dev Disord. 2019;49(8):3127-3135. doi: 10.1007/s10803-019-04034-9 [DOI] [PubMed] [Google Scholar]
- 8.Zhang T, Sidorchuk A, Sevilla-Cermeño L, et al. Association of cesarean delivery with risk of neurodevelopmental and psychiatric disorders in the offspring: a systematic review and meta-analysis. JAMA Netw Open. 2019;2(8):e1910236. doi: 10.1001/jamanetworkopen.2019.10236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sultan P, David AL, Fernando R, Ackland GL. Inflammation and epidural-related maternal fever: proposed mechanisms. Anesth Analg. 2016;122(5):1546-1553. doi: 10.1213/ANE.0000000000001195 [DOI] [PubMed] [Google Scholar]
- 10.Wohlrab P, Boehme S, Kaun C, et al. Ropivacaine activates multiple proapoptotic and inflammatory signaling pathways that might subsume to trigger epidural-related maternal fever. Anesth Analg. 2020;130(2):321-331. doi: 10.1213/ANE.0000000000004402 [DOI] [PubMed] [Google Scholar]
- 11.Treffert DA. Epidemiology of infantile autism. Arch Gen Psychiatry. 1970;22(5):431-438. doi: 10.1001/archpsyc.1970.01740290047006 [DOI] [PubMed] [Google Scholar]
- 12.Xu G, Strathearn L, Liu B, et al. Prevalence and treatment patterns of autism spectrum disorder in the United States, 2016. JAMA Pediatr. 2019;173(2):153-159. doi: 10.1001/jamapediatrics.2018.4208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dalsgaard S, Thorsteinsson E, Trabjerg BB, et al. Incidence rates and cumulative incidences of the full spectrum of diagnosed mental disorders in childhood and adolescence. JAMA Psychiatry. 2019;77(2):155-164. doi: 10.1001/jamapsychiatry.2019.3523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Christensen DL, Baio J, Van Naarden Braun K, et al. ; Developmental Disabilities Monitoring Network Surveillance Year 2010 Principal Investigators; Centers for Disease Control and Prevention (CDC) . Prevalence of autism spectrum disorder among children aged 8 years—autism and developmental disabilities monitoring network, 11 sites, United States, 2010. MMWR Surveill Summ. 2014;63(2):1-21. doi: 10.15585/mmwr.ss6802a1 [DOI] [PubMed] [Google Scholar]
- 15.Tchaconas A, Adesman A. Autism spectrum disorders: a pediatric overview and update. Curr Opin Pediatr. 2013;25(1):130-144. doi: 10.1097/MOP.0b013e32835c2b70 [DOI] [PubMed] [Google Scholar]
- 16.Blumberg SJ, Bramlett MD, Kogan MD, Schieve LA, Jones JR, Lu MC. Changes in prevalence of parent-reported autism spectrum disorder in school-aged U.S. children: 2007 to 2011-2012. Natl Health Stat Report. 2013;65(65):1-11. [PubMed] [Google Scholar]
- 17.Xiang AH, Chow T, Martinez MP, et al. Hemoglobin A1c levels during pregnancy and risk of autism spectrum disorders in offspring. JAMA. 2019. doi: 10.1001/jama.2019.8584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xiang AH, Wang X, Martinez MP, et al. Association of maternal diabetes with autism in offspring. JAMA. 2015;313(14):1425-1434. doi: 10.1001/jama.2015.2707 [DOI] [PubMed] [Google Scholar]
- 19.Xiang AH, Wang X, Martinez MP, Page K, Buchanan TA, Feldman RK. Maternal type 1 diabetes and risk of autism in offspring. JAMA. 2018;320(1):89-91. doi: 10.1001/jama.2018.7614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baron-Cohen S, Wheelwright S, Cox A, et al. Early identification of autism by the CHecklist for Autism in Toddlers (CHAT). J R Soc Med. 2000;93(10):521-525. doi: 10.1177/014107680009301007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coleman KJ, Lutsky MA, Yau V, et al. Validation of autism spectrum disorder diagnoses in large healthcare systems with electronic medical records. J Autism Dev Disord. 2015;45(7):1989-1996. doi: 10.1007/s10803-015-2358-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoffman K, Weisskopf MG, Roberts AL, et al. Geographic patterns of autism spectrum disorder among children of participants in Nurses’ Health Study II. Am J Epidemiol. 2017;186(7):834-842. doi: 10.1093/aje/kwx158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med. 2017;167(4):268-274. doi: 10.7326/M16-2607 [DOI] [PubMed] [Google Scholar]
- 24.Smallwood M, Sareen A, Baker E, Hannusch R, Kwessi E, Williams T. Increased risk of autism development in children whose mothers experienced birth complications or received labor and delivery drugs. ASN Neuro. 2016;8(4):1759091416659742. doi: 10.1177/1759091416659742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guinchat V, Thorsen P, Laurent C, Cans C, Bodeau N, Cohen D. Pre-, peri- and neonatal risk factors for autism. Acta Obstet Gynecol Scand. 2012;91(3):287-300. doi: 10.1111/j.1600-0412.2011.01325.x [DOI] [PubMed] [Google Scholar]
- 26.Glasson EJ, Bower C, Petterson B, de Klerk N, Chaney G, Hallmayer JF. Perinatal factors and the development of autism: a population study. Arch Gen Psychiatry. 2004;61(6):618-627. doi: 10.1001/archpsyc.61.6.618 [DOI] [PubMed] [Google Scholar]
- 27.Whitburn LY, Jones LE, Davey MA, McDonald S. The nature of labour pain: an updated review of the literature. Women Birth. 2019;32(1):28-38. doi: 10.1016/j.wombi.2018.03.004 [DOI] [PubMed] [Google Scholar]
- 28.Hawkins JL. Epidural analgesia for labor and delivery. N Engl J Med. 2010;362(16):1503-1510. doi: 10.1056/NEJMct0909254 [DOI] [PubMed] [Google Scholar]
- 29.Tort S, Ciapponi A. How Does Epidural Analgesia Compare With Opioids for Pain Management During Labor? Cochrane Clinical Answers; 2018. doi: 10.1002/cca.2198 [DOI] [Google Scholar]
- 30.Gardener H, Spiegelman D, Buka SL. Perinatal and neonatal risk factors for autism: a comprehensive meta-analysis. Pediatrics. 2011;128(2):344-355. doi: 10.1542/peds.2010-1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bucklin BA, Santos A. Local Anesthetics and opioids. In: Chestnut DH, ed. Chestnut’s Obstetric Anesthesia: Principles and Practice. Elsevier; 2020: 271-312. [Google Scholar]
- 32.de Barros Duarte L, Dantas Móises EC, Cavalli RC, Lanchote VL, Duarte G, da Cunha SP. Distribution of bupivacaine enantiomers and lidocaine and its metabolite in the placental intervillous space and in the different maternal and fetal compartments in term pregnant women. J Clin Pharmacol. 2011;51(2):212-217. doi: 10.1177/0091270010365551 [DOI] [PubMed] [Google Scholar]
- 33.Mirkin BL. Perinatal pharmacology: placental transfer, fetal localization, and neonatal disposition of drugs. Anesthesiology. 1975;43(2):156-170. doi: 10.1097/00000542-197508000-00004 [DOI] [PubMed] [Google Scholar]
- 34.Ribeiro RMP, Moreira FL, Moisés ECD, et al. Lopinavir/ritonavir treatment increases the placental transfer of bupivacaine enantiomers in human immunodeficiency virus-infected pregnant women. Br J Clin Pharmacol. 2018;84(10):2415-2421. doi: 10.1111/bcp.13700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xing Y, Zhang N, Zhang W, Ren LM. Bupivacaine indirectly potentiates glutamate-induced intracellular calcium signaling in rat hippocampal neurons by impairing mitochondrial function in cocultured astrocytes. Anesthesiology. 2018;128(3):539-554. doi: 10.1097/ALN.0000000000002003 [DOI] [PubMed] [Google Scholar]
- 36.Guo Z, Liu Y, Cheng M. Resveratrol protects bupivacaine-induced neuro-apoptosis in dorsal root ganglion neurons via activation on tropomyosin receptor kinase A. Biomed Pharmacother. 2018;103:1545-1551. doi: 10.1016/j.biopha.2018.04.155 [DOI] [PubMed] [Google Scholar]
- 37.Werdehausen R, Fazeli S, Braun S, et al. Apoptosis induction by different local anaesthetics in a neuroblastoma cell line. Br J Anaesth. 2009;103(5):711-718. doi: 10.1093/bja/aep236 [DOI] [PubMed] [Google Scholar]
- 38.Souza MCO, Marques MP, Duarte G, Lanchote VL. Analysis of bupivacaine enantiomers in plasma as total and unbound concentrations using LC-MS/MS: application in a pharmacokinetic study of a parturient with placental transfer. J Pharm Biomed Anal. 2019;164:268-275. doi: 10.1016/j.jpba.2018.10.040 [DOI] [PubMed] [Google Scholar]
- 39.US Food and Drug Administration. FDA drug safety communication: FDA review results in new warnings about using general anesthetics and sedation drugs in young children and pregnant women. 2018. Accessed January 13, 2020. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-review-results-new-warnings-about-using-general-anesthetics-and
- 40.Murray KN, Edye ME, Manca M, et al. Evolution of a maternal immune activation (mIA) model in rats: early developmental effects. Brain Behav Immun. 2019;75:48-59. doi: 10.1016/j.bbi.2018.09.005 [DOI] [PubMed] [Google Scholar]
- 41.Segal S, Pancaro C, Bonney I, Marchand JE. Noninfectious fever in the near-term pregnant rat induces fetal brain inflammation: a model for the consequences of epidural-associated maternal fever. Anesth Analg. 2017;125(6):2134-2140. doi: 10.1213/ANE.0000000000002479 [DOI] [PubMed] [Google Scholar]
- 42.Saghazadeh A, Ataeinia B, Keynejad K, Abdolalizadeh A, Hirbod-Mobarakeh A, Rezaei N. A meta-analysis of pro-inflammatory cytokines in autism spectrum disorders: effects of age, gender, and latitude. J Psychiatr Res. 2019;115:90-102. doi: 10.1016/j.jpsychires.2019.05.019 [DOI] [PubMed] [Google Scholar]
- 43.Rasmussen JM, Graham AM, Entringer S, et al. Maternal interleukin-6 concentration during pregnancy is associated with variation in frontolimbic white matter and cognitive development in early life. Neuroimage. 2019;185:825-835. doi: 10.1016/j.neuroimage.2018.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guma E, Plitman E, Chakravarty MM. The role of maternal immune activation in altering the neurodevelopmental trajectories of offspring: a translational review of neuroimaging studies with implications for autism spectrum disorder and schizophrenia. Neurosci Biobehav Rev. 2019;104:141-157. doi: 10.1016/j.neubiorev.2019.06.020 [DOI] [PubMed] [Google Scholar]
- 45.Chau A, Markley JC, Juang J, Tsen LC. Cytokines in the perinatal period—part I. Int J Obstet Anesth. 2016;26:39-47. doi: 10.1016/j.ijoa.2015.12.005 [DOI] [PubMed] [Google Scholar]
- 46.Chau A, Markley JC, Juang J, Tsen LC. Cytokines in the perinatal period—part II. Int J Obstet Anesth. 2016;26:48-58. doi: 10.1016/j.ijoa.2015.12.006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Characteristics of the Cohort at the Time of the Index Pregnancy
eTable 2. Associations Between Labor Epidural Analgesia (LEA) Use at Delivery and Risk of Autism Spectrum Disorders (ASD) in Offspring (Randomly Select One Child per Family)