Abstract
Study Objectives
Hypnotic medications can adversely affect behavior during unanticipated awakenings during the night. Animals treated with the hypocretin (Hcrt) receptor antagonist almorexant (ALM) have less acute cognitive impairment compared to the GABAA receptor modulator zolpidem (ZOL). This study aimed to determine whether ALM produces less acute cognitive impairment than ZOL in human subjects.
Methods
Healthy, young adult, unmedicated male and female subjects participated in a controlled trial of a single dose of ALM 100 mg (N = 48), ALM 200 mg (N = 53), ZOL 10 mg (N = 49), and placebo (PBO, N = 52).
Results
ZOL and both doses of ALM produced similar levels of subjective sleepiness and impaired the ability of subjects to remain awake in a dark, low-stimulus setting relative to PBO. For most cognitive measures, performance under ZOL was significantly worse than ALM or PBO. For tasks involving verbal memory or visual-motor coordination, ZOL impaired performance, whereas the two doses of ALM were no different than PBO. For tasks involving higher-order executive function, ZOL produced impairment in processing speed and inhibitory control, whereas the two doses of ALM were no different than PBO. Performance decrements for ALM were less than ZOL but greater than PBO for some reaction time measures.
Conclusions
The data provide support for the hypothesis that Hcrt receptor antagonists produce less functional impairment than a benzodiazepine receptor agonist (BzRA). These observations are particularly relevant to patients treated with sedative-hypnotics who are at elevated risk for falls and other untoward events during the intended hours for sleep.
Keywords: hypnotics and sedatives, cognitive dysfunction, psychomotor performance, zolpidem, almorexant, humans
Statement of Significance.
Animals treated with the hypocretin (Hcrt) receptor antagonist almorexant (ALM) have less acute cognitive impairment compared to the GABAA receptor modulator zolpidem (ZOL). In this randomized clinical trial that included 202 adults, cognitive performance measured before and after a single dose was significantly worse for ZOL versus the Hcrt receptor antagonist ALM or placebo (PBO). Performance decrement for ALM was less than ZOL but greater than PBO on some low-response-demand tasks. The results from this trial, together with the mechanistic studies in rodents, support the hypothesis that benzodiazepine receptor agonists (BzRAs) cause a general inhibition of neural activity, whereas Hcrt specifically disfacilitates wake-promoting systems but is permissive for those systems to be recruited in the setting of a task demand.
Introduction
Concerns regarding neurocognitive effects of hypnotic medications during unanticipated awakenings during sleep have led to a Food and Drug Administration (FDA) warning for all hypnotic drugs. These concerns are particularly relevant to elderly patients who disproportionally receive hypnotics and are at risk for middle-of-the-night awakenings [1]. At doses for sleep, use of benzodiazepine receptor agonists (BzRAs) is associated with a higher rate of nighttime falls and associated fractures [2–9]. Since 4.1% of adults in the United States take prescription sleep medications each month [1], there is high potential for adverse events to occur from arousals during periods of peak drug exposure.
All available FDA-approved BzRAs induce restorative sleep. However, at peak concentration, they exert substantial performance impairment in multiple neurocognitive domains. Studies have shown psychomotor impairment within the 6-hour window after ingesting zolpidem (ZOL) [10–14] or zoplicone [15]. The US Air Force has established a strict policy on the use of hypnotics by aviators, specifying the amount of aviator downtime following ingestion. For example, 10 hours is the mandated downtime following ingestion of ZOL, a BzRA with a Tmax of 1.6 hours and a t1/2 of 2–2.6 hours [16]. Hypnotic agents that do not impair cognition at peak hypnotic effect could reduce morbidity associated with unanticipated awakening. The melatonin agonist ramelteon has a desirable cognitive safety profile but limited hypnotic utility for sleep duration and sleep efficiency [17].
The hypocretin (Hcrt)/orexin neuropeptide system regulates wake and arousal [18, 19]. Disruption of the Hcrt sleep/wakefulness regulatory network results in narcolepsy in both animals and humans [20, 21]. The two Hcrt/orexin receptor subtypes (OX1R and OX2R) are found in many brain regions, with high receptor expression levels in areas associated with arousal state regulation, particularly the histaminergic, serotonergic, noradrenergic, and cholinergic wake-promoting systems, but lower expression levels occur in the cerebral cortex [22]. Since the Hcrt peptides are excitatory throughout the brain, Hcrt antagonists block such excitatory effects (disfacilitation) rather than producing a generalized inhibition as do other hypnotic agents.
After publication of the proof-of-principle study in 2007 and subsequent clinical trials [23–28], the first dual hypocretin/orexin receptor antagonist (DORA) was approved by the FDA for use in insomnia in 2014. In the past decade, there has been a rapid development of DORA compounds for treatment of insomnia, alcohol and drug addiction, and chronic pain [29–32]. Animals treated with the Hcrt receptor antagonist almorexant (ALM) had less acute cognitive impairment compared to animals treated with the GABAA receptor modulator ZOL [33–35]. Rats treated with ALM were indistinguishable from vehicle-treated rats for performance on tests of memory under normal conditions or after 6 hours of sleep deprivation [33]. Similarly, rats treated with ALM had intact learning and memory [34] and showed immediately reversibility of hypnotic effects without impairment on motor performance [35]. In contrast, BzRAs such as ZOL affect GABAA receptors, which have widespread distribution in the central nervous system (CNS), particularly in the cerebral cortex, and cause general inhibition of neural activity [36–38].
We conducted an integrated translational study of the effect of an Hcrt antagonist (ALM) as compared to a standard hypnotic (ZOL) and placebo (PBO) on neurocognitive performance at peak concentration post-dosing. The animal studies were conducted to define the neural circuitry that underlies the activity of these compounds, and the effects of these compounds on biomarkers associated with normal sleep [33, 39–41]. Based on preclinical results, we undertook the present study to determine whether ALM is superior to ZOL regarding neurocognitive side effects in humans as well. The hypothesis was that Hcrt antagonists would produce fewer functional impairments than BzRAs because the latter cause a general inhibition of neural activity, whereas Hcrt specifically disfacilitates wake-promoting systems.
Methods
The study took place at the San Francisco Veterans Affairs Medical Center and the University of California, San Francisco (UCSF) Clinical Translational and Sciences Institute Inpatient Clinical Research Center (CRC). The study protocol and consent form were approved by the Committee on Human Research at UCSF. Subjects were enrolled between May 2011 through June 2014. Enrollment was closed when the study successfully obtained the sample size projected in the initial power analysis (see below). The study involved healthy volunteers who were considered normal sleepers per the Research Diagnostic Criteria for Normal Sleepers and who were free of medical and psychiatric disorders. After informed consent and confirmation of eligibility, subjects were asked to maintain a sleep diary and wear a wrist actigraph 24 hours/day for 7 days. Subjects were admitted to the CRC on the eighth day of the study period, 2 days prior to study drug administration. Subjects’ sleep was monitored with polysomnography (PSG) during each night on the CRC. Subjects could awaken ad lib and were allowed exposure to natural light through an outside window. Double-blind randomization to one of four groups (ALM 100 mg, ALM 200 mg, ZOL 10 mg, or PBO) took place after the second night at the CRC, following the administration of several baseline measures of neurocognitive performance. The 100 and 200 mg doses of ALM were chosen because these were the doses undergoing testing in Phase III trials at the time of study initiation. Following dosing at 15:00, subjects were accompanied by study personnel and instructed to remain awake. Neurocognitive performance, objective alertness, subjective symptoms, and adverse events were assessed several hours before and after dosing. Subjects were debriefed and discharged from the CRC on the morning of the fourth day on the unit.
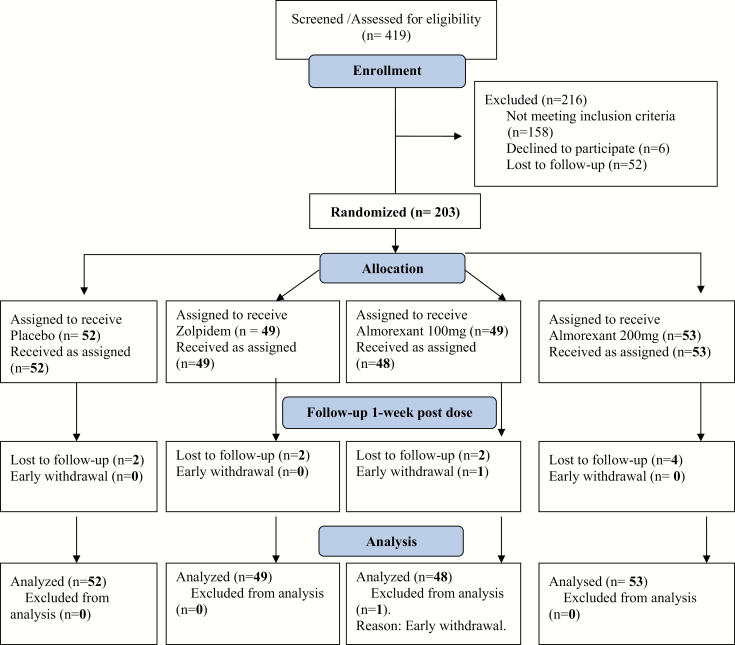
Medically healthy men and women ages 19–39 (N = 214) were recruited from web-based postings and flyers posted in community sites. See Figure 1 for Consort diagram. The inclusion criteria were as follows: (1) male and female subjects between the ages of 19 and 39 determined to be physically healthy by physical exam and laboratory assessments; (2) habitual wake time between 06:00 and 08:00 hours maintained within the past month; (3) habitual bedtime between 22:00 and 00:00 hours maintained within the past month; (4) body mass index (BMI) >18 and <28 kg/m2; (5) ability to communicate well with the Investigator and to understand the study requirements; (6) meet research criteria for healthy sleeper [42].
Figure 1.
Consort diagram for the randomized controlled trial.
Exclusion criteria were as follows: (1) diagnosis of a sleep disorder within 2 years of screening or current sleep disturbance as suggested by a global score of >5 on the Pittsburgh Sleep Quality Index (PSQI) [43]; (2) current presence of two or more risk categories on the Berlin Questionnaire for sleep apnea [44] and overnight oximetry showing 10 desaturation events per hour; (3) a current or lifetime diagnosis of any psychiatric disorder with psychotic features, major depression, bipolar disorder, panic disorder, obsessive-compulsive disorder, post-traumatic stress disorder, generalized anxiety disorder, dysthymia, or agoraphobia without panic disorder, assessed using the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR) Axis I Disorders (SCID) [45]; (4) a current diagnosis of alcohol or substance abuse or dependence or a history of alcohol or substance abuse or dependence within the past year, assessed using the SCID; (5) subjects who were pregnant, lactating, or planning to become pregnant or subjects who are not willing to use an acceptable form of birth control during the study; (6) lifetime history of brain injury (including concussions, mild traumatic brain injuries, or loss of consciousness for ≥10 minutes which resulted in the development of persistent symptoms lasting ≥1 month), stroke, brain hemorrhage, seizures (not including infantile febrile seizures), epilepsy, or brain infection caused by meningitis, encephalitis, or any other infectious agent; (7) systemic illness affecting CNS function; (8) cardiovascular disease (to include but not limited to arrhythmias, valvular heart disease, congestive heart failure, history of myocardial infarction, or family history of sudden cardiac death), hypertension, or hypercholesterolemia; (9) asthma or other reactive airway diseases; (10) any other chronic or unstable medical conditions; (11) current use of statins, ketoconazole, prescription or over-the-counter medications, or herbal supplements containing psychoactive properties or stimulants; (12) treatment with another investigational drug; (13) current daily use of any other medication; (14) consumption of grapefruit (including grapefruit juice) or treatment with moderate or strong inhibitors of cytochrome P450 3A4 (CYP3A4) within 1 week prior to randomization; (15) treatment with drugs metabolized by CYP2D6 isoenzyme with a narrow therapeutic index within 1 week prior to randomization; (16) self-reported regular nicotine use within the past 30 days involving >4 cigarettes per week or >2 cigarettes per day; (17) self-reported consumption of alcohol within the past 30 days of >14 standard drinks per week or ≥5 standard drinks on any day (men), or >7 standard drinks per week or ≥4 standard drinks on any day (women); (18) use of opioids, benzodiazepines, amphetamines, cocaine, cannabis, or any other illicit drugs within 30 days of screening by self-report or a urine toxicology screen; (19) known liver disease or abnormal liver function tests assessed at the time of screening; (20) self-reported regular caffeine use more than 200 mg per day on average within 6 months of screening; (21) habitual long sleepers (>9 hours) or short sleepers (<5 hours); (22) shift work within 1 month prior to the screening visit or planned shift work during the study; (23) subjects who have traveled >3 time zones within 1 week prior to the screening visit or any other visit; (24) subjects with extreme evening tendencies (score < 23) and extreme morning tendencies (score > 43) [46]; (25) known hypersensitivity or contraindication to any excipients of the drug formulation.
A Medical History was obtained in all participants. Laboratory tests included a serum chemistry panel, liver function tests, thyroid functions, complete blood count, urine toxicology screen, and pregnancy test (if appropriate). In addition, the following assessments were utilized in all subjects: Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders (SCID) [45], Alcohol Use Disorders Identification Test (AUDIT) [47], and Smith Morningness Scale [46]. Additional details are in Supplementary Methods.
Subjects were randomly assigned in a 1:1:1:1 ratio to: ALM 100 mg, ALM 200 mg, ZOL 10 mg, or PBO. Subjects were stratified by gender and randomized in blocks of N = 8 within each stratum in lists generated by the study statistician (TJM). Study drug was provided to a nurse on the CRC by a research pharmacist. The nurse and all study personnel remained blinded until enrollment for the study was completed and all measures scored.
Sociodemographic factors were collected at baseline (Table 1). Subjects underwent a series of tests intended to assess their sleepiness (Stanford Sleepiness Scale [SSS], Modified Maintenance of Wakefulness Test [MWT]), attention (Psychomotor Vigilance Task [PVT], Continuous Performance Task [CPT] II), visual-motor coordination (Grooved Pegboard Test), verbal memory (Rey Verbal Auditory Learning Test), working memory (Digit Span), short- and long-term lexical memory (Paired Associate Learning Test), and executive function (Stroop Color-Word Test, Tower Test from Delis-Kaplan Executive Function System). In contrast to the other tests, the Stroop Color-Word, Grooved Pegboard, Digit Span, and Tower Tests were only administered once after dosing. Additional details are in Supplementary Methods.
Table 1.
Characteristics of participants by treatment group
| ALM 100 mg | ALM 200 mg | ZOL 10 mg | PBO | |
|---|---|---|---|---|
| N | 48 | 53 | 49 | 52 |
| Age, years (range) | 26 (20–38) | 26 (19–38) | 27 (20–38) | 26 (19–38) |
| % Female | 63 | 66 | 61 | 62 |
| Education, years (SD) | 16.2 (1.6) | 16.0 (2.1) | 16.0 (1.7) | (1.5) |
| Total sleep time, min (SD) | 418 (52) | 421 (44) | 418 (69) | 417 (52) |
| Sleep efficiency, % (SD) | 91.3 (7.9) | 91.0 (7.3) | 89.3 (13.7) | 91.3 (8.6) |
Enrollment was estimated to include 216 subjects to obtain 200 evaluable subjects. An equal number of subjects were to be randomly assigned to each group. Sample size was chosen to provide power = 0.80, with alpha = 0.05, to detect an effect size (Cohen’s f) of approximately 0.29. The effect of ZOL 10 mg versus PBO on the cognitive performance was estimated to range from f = 0.34 to f = 0.80, based on prior reports [10–14]. Given the hypothesis that both doses of ALM would be associated with less impairment than ZOL 10 mg, we anticipated that a range of effect sizes might be found with ALM. If ALM was no different than PBO, the study would be slightly overpowered to demonstrate its superiority to ZOL. However, if ALM produced impairment of cognition that was intermediate between ZOL 10 mg and PBO, the study would be appropriately powered.
We hypothesized that subjects receiving ZOL 10 mg would show greater impairment in neurocognitive performance compared to subjects receiving PBO, ALM 100 mg, or ALM 200 mg. This hypothesis was tested by comparing groups on post-medication performance tests using pre-medication test scores as covariates. When multiple administrations of a performance test occurred, mixed effects models were used, with hypotheses tested by the pre-medication-adjusted mean differences in post-medication scores among the four groups. Linear mixed models were used for all outcome measures except for the MWT, where a mixed effects tobit model was used to accommodate the fact that the maximal sleep latency was 20 minutes, and for number of lapses on the PVT, where a mixed effects Poisson model was used to accommodate the distribution of count data.
When a test was administered only once pre- and post-medication, the linear mixed model reduced to a one-way ANCOVA comparing mean scores on the four groups, with the pre-medication test score serving as the covariate. The Hommel step-up procedure [48] was used to adjust p-values for the six possible between-arm comparisons of each outcome measure averaged over all post-dosing time points. The Hommel procedure was also used to adjust p-values for between-arm comparisons conducted at individual time points, e.g. when there were four post-dosing time points, p-values were corrected for 24 comparisons (6 comparisons across 4 time points).
Two-tailed significance tests were conducted at the p = 0.05 level using adjusted p-values. Primary analyses were intent-to-treat analyses based on all participants randomized, regardless of dropout or missing data status. The exact form of each mixed model, for example, the correlational structure of repeated measures and whether heterogeneous group variances are included, was made based on best fit according to the Bayesian Information Criterion (BIC) before hypothesis testing was conducted. Model residuals were checked for normality (or Poisson distribution for counts of PVT lapses), heterogeneity of variance among arms, and potential outliers. Potential outliers at the subject level were also identified by computing Cook’s distance for each of the group effects. No influential outliers were observed. Violations of the normality assumption were apparent in the originally proposed linear mixed model for sleep latency in the MWT task. This was attributed to the ceiling of sleep latency scores at 20 minutes (for subjects who did not fall asleep during the allotted 20-minute observation period), and this was accommodated by adopting a tobit model with right censoring at 20 minutes in place of the standard linear model. Model checks and modifications were made prior to conducting any significance tests.
Results
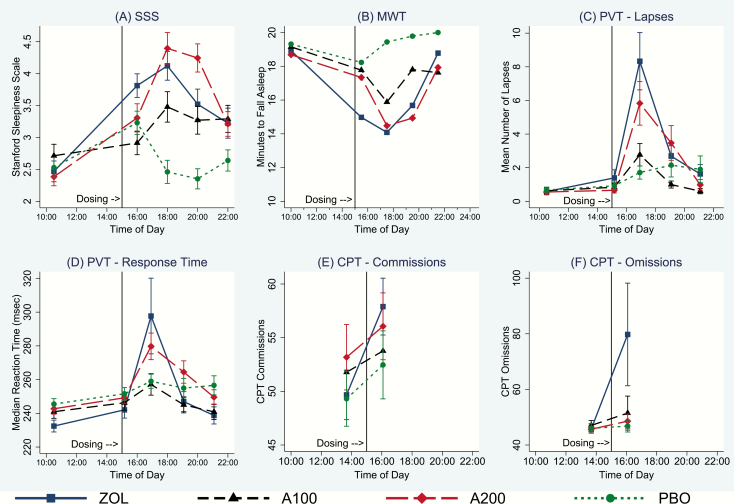
ZOL and the 200 mg dose of ALM increased subjective sleepiness as measured by the SSS relative to PBO, although the ALM 100-mg dose produced marginally less sleepiness than ZOL (Figure 2A; Supplementary Table S1). ZOL and both doses of ALM impaired the ability of subjects to remain awake in a dark, low-stimulus setting relative to PBO in the MWT (Figure 2B; Supplementary Table S2). The latency to sleep on the MWT did not differ across treatment with any of the three active drugs.
Figure 2.
Effect of study medications on sleepiness, ability to maintain wake, psychomotor vigilance, sustained attention, and inhibitory control. PVT, Psychomotor Vigilance Task; A100, almorexant 100 mg; A200, almorexant 200 mg. Red vertical line indicates time of dosing at 15:00. Error bars represent 95% confidence intervals, uncorrected for multiple comparisons.
The number of PVT trials with more than one 500-ms lapse increased significantly in the ZOL condition relative to ALM 100 mg and PBO (Figure 2C; Supplementary Table S3). The ALM 200-mg dose produced more lapses than the ALM 100-mg dose across all time points, and significantly more lapses than PBO at 115 minutes post-dosing (16:55). Despite the increased subjective sleepiness (Figure 2A) and impaired ability to stay awake (Figure 2B), the 100 mg dose of ALM did not produce more lapses than PBO at any time point. Median reaction time (RT) on the PVT increased in the ZOL condition relative to ALM 100 mg and PBO. Median RTs in the ALM 200 dose were slower than in ALM 100 overall, and slower than PBO at 1:55 post-dosing (Figure 2D; Supplementary Table S4).
Errors of commission on the CPT (measured when a response occurs in the absence of a target and is an index of inhibitory control) increased between the pre-dose test at 13:30 and the post-dose test at 16:00 for all conditions (Figure 2E), suggesting a time-of-day effect. However, the increase in commission errors was significant only for the ZOL condition relative to both doses of ALM and PBO (Supplementary Table S5). The increased number of commission errors for both doses of ALM were not significantly different from PBO. Errors of omission (failure to respond to target) similarly were significant in the ZOL condition relative to both doses of ALM and PBO (Figure 2F; Supplementary Table S5). Omission errors for both doses of ALM were not significantly different from PBO.
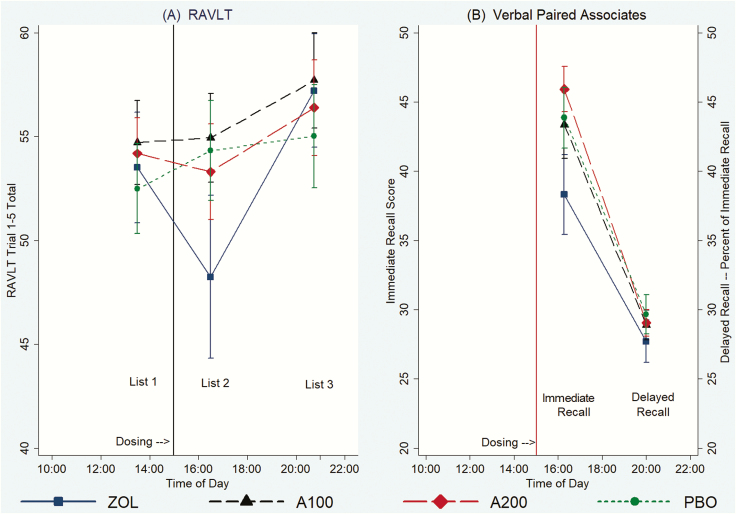
Verbal memory
Total scores on the Rey Auditory Verbal Learning Test (RAVLT), which measures the ability to learn new verbal information, were no different across conditions pre-dosing (13:30) and at 5 hours (20:10) after dosing (Figure 3A; Supplementary Table S6). However, when subjects were tested 87 minutes post-dosing at 16:27, new verbal learning was impaired by ZOL relative to both doses of ALM and PBO. Verbal learning was not significantly affected by either dose of ALM relative to PBO. Delayed recall for the word list presented at 13:30 was tested 60 minutes after dosing (16:00); there were no differences in recall of words encoded prior to taking study medication.
Figure 3.
(A) Effect of study medications on verbal memory in the RAVLT. (B) Effect of study medications on verbal memory in the Verbal Paired Associates Test. A100, almorexant 100 mg; A200, almorexant 200 mg. Vertical line indicates time of dosing at 15:00. Error bars represent 95% confidence intervals, uncorrected for multiple comparisons.
Verbal memory, as indexed by the paired associated immediate recall measured 80 minutes after dosing (16:20), showed a similar pattern as new verbal learning on the RAVLT conducted near the same time (16:27) (Figure 3B; Supplementary Table S7). New verbal learning was impaired by ZOL when measured 80 minutes post-dosing (16:20) relative to both doses of ALM and PBO. Verbal learning was not significantly affected by either dose of ALM relative to PBO. Delayed recall, tested 5 hours post-dosing (20:00) for material learned while on drug, was no different across conditions (Figure 3B; Supplementary Table S7).
Executive function
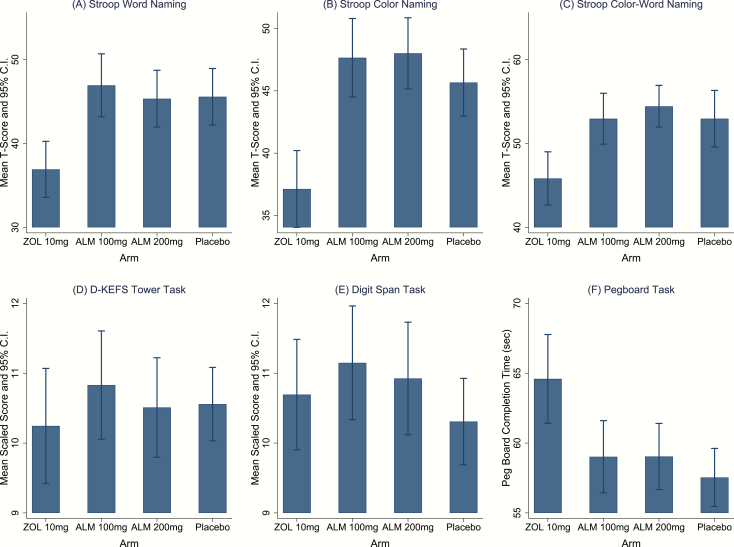
Stroop Word and Color scores (both measures of processing speed) were worse under ZOL compared to either dose of ALM or PBO (Figure 4A and B; Supplementary Table S8). Differences between either ALM group and PBO for were not significant for either measure. Stroop Color-Word scores (measure of inhibitory control and cognitive flexibility) were significantly worse under ZOL compared to either dose of ALM or PBO (Figure 4C; Supplementary Table S8). Differences between either ALM group and PBO were not significant. There were no group differences in performance on the Towers or Digit Span tasks, assessed 125 and 145 minutes, respectively, after dosing (Figure 4D and E; Supplementary Tables S9 and S10).
Figure 4.
Effect of study medications on processing speed, executive function, working memory, and visual-motor coordination. CI, 95% confidence interval. Stroop T-scores are based on population norms with mean = 50 and SD = 10. D-KEFS Tower and Digit Span scaled scores are based on a population norm with mean = 10 and SD = 3. Error bars represent 95% confidence intervals, uncorrected for multiple comparisons.
Visual-motor coordination
Performance on the Pegboard task was worse under ZOL compared to either dose of ALM or PBO (Figure 4F; Supplementary Table S11).
Adverse effects
There were no differences in subjective fatigue across ZOL and both doses of ALM. ZOL was associated with higher subjective fatigue compared to PBO. ZOL was associated with higher incident dizziness, drowsiness, and nausea relative to the two doses of ALM and PBO. ZOL was also associated with greater fatigue than PBO. The 200 mg dose of ALM was associated with greater drowsiness than ALM 100 mg. There were no differences in headaches or diarrhea across the four conditions (Supplementary Table S12).
Interaction effects of sex on cognitive performance and adverse effects
We ran additional analyses to determine whether there were significant interaction effects for sex that could account for differences in performance across the four drug groups. Across all of the cognitive outcomes, there were two measures which showed an interaction effect: women performed worse than men on ZOL on the SSS and PVT lapses. For the SSS, the arm (ZOL vs. PBO) by sex interaction was borderline significant, χ 2(1, N = 203) = 3.69, p = 0.055. Excluding the final time point, ZOL versus PBO contrasts for SSS were significant in both men and women separately (for women: χ 2(1, N = 128) = 24.45, p < 0.0001; for men: χ 2(1, N = 75) = 4.03, p = 0.04). For PVT lapses, the interaction effect was significant, χ 2(1, N = 204) = 4.14, p = 0.04. During trial 3 where the effects occur, they are significant for men and women separately (women: χ 2(1, N = 128) = 17.98, p < 0.0001; for men: χ 2(1, N = 75) = 4.03, p = 0.04). Thus, despite some evidence for greater sensitivity of women to the cognitive effects of ZOL, the overall results show greater performance decrement under ZOL relative to ALM in men and that the full sample was not an artifact of sex-specific sensitivity to adverse effects.
We also ran additional analyses to determine whether there were significant interaction effects for sex that could account for differences in adverse events across the four drug groups. Women on ZOL had more reports of dizziness (63% vs. 32%, χ 2(1, N = 49) = 4.69, p = 0.030), drowsiness (97% vs. 79%, χ 2(1, N = 49) = 3.99, p = 0.046), nausea (43% vs. 16%, χ 2(1, N = 49) = 4.01, p = 0.045), and fatigue (60% vs. 32%, χ 2(1, N = 49) = 3.76, p = 0.052) relative to men. Consequently, we examined whether the higher rates of adverse events in women accounted for the greater performance decrement of subjects on ZOL relative PBO and the two doses of ALM. We created an indicator variable for whether each participant experienced one or more the adverse side effects that showed differences across the four arms (dizziness, nausea, drowsiness, or fatigue). About 70% of our sample experienced at least one of these at some time point. This indicator did not account for differences in performance across the arms; however, it is possible that this negative finding was related to the high prevalence of drowsiness across all of the arms. We then created a second indicator variable excluding drowsiness which reduced the proportion of the sample with adverse events to 42%. This narrower indicator showed some effects in a few of the outcomes; namely the SSS, PVT lapses, and Digit Span. For SSS, there was a significant effect of adverse events, z = 11.40, p < 0.001. However, there were no significant interactions between adverse events and arm or between adverse events and sex. Controlling for sex and adverse events, there were still significant contrasts between ZOL and PBO (z = 2.54, p = 0.011) and between ZOL and ALM100 (z = 3.44, p = 0.001). These contrasts were also significant when the sample was limited to those without adverse events. For PVT lapses, there was a marginal effect of adverse events, z = 1.86, p = 0.062, but no significant interactions with arm or with sex. Controlling for sex and adverse events, there were still significant contrasts between ZOL and PBO (z = 2.82, p = 0.005) and between ZOL and ALM100 (z = 2.39, p = 0.017). With the smaller subsample without adverse events, the contrast between ZOL and ALM100 was marginally significant (z = 1.81, p = 0.071), but the contrast between ZOL and PBO was no longer significant (z = 1.01, p = 0.312). For the Digit Span task, there was a marginally significant effect of adverse events, z = 1.85, p = 0.064, but no significant interactions between adverse events and study arm or sex. There were no significant contrasts between study arms, consistent with the unadjusted analyses.
Discussion
The results show that ZOL and the two doses of ALM produced similar levels of subjective sleepiness and impairment in the ability to maintain wakefulness in a dark, low-stimulus environment in which the subject’s only task was to remain awake. In a setting in which a response demand for a task of cognitive function was required, performance under ZOL was significantly worse than under ALM or PBO. ALM modestly affected sustained psychomotor vigilance and median RT to the presentation of simple targets in a dose-dependent manner. Performance with more complex tasks of verbal memory or executive function under both doses of ALM was not different than PBO. Overall, the data support the hypothesis that a Hcrt antagonist produces less functional impairment than a BzRA.
Our rationale for this interpretation is that ZOL, like all BzRAs, affect GABAA receptors which have widespread distribution in the CNS, particularly in the cerebral cortex [49, 50]. In contrast, Hcrt receptor expression is weak in the cortex and high only in brain regions associated with arousal state regulation, particularly the histaminergic, serotonergic, noradrenergic, and cholinergic wake-promoting systems [22, 51–57]. Since the Hcrt peptides are excitatory throughout the brain [19, 58–60], Hcrt antagonists work by blocking this excitation (disfacilitation). However, Hcrt antagonism does not directly inhibit monoaminergic arousal systems, which can be recruited in the setting of a task demand [40]. Rodents given ALM during the circadian active phase showed functional activation of wake systems in the hypothalamus and brainstem in contrast to animals administered ZOL [40]. Thus, these arousal systems can be recruited and activated even in the absence of Hcrt input.
Our results are consistent with data from rats and rhesus monkeys which demonstrated that the therapeutic margin between the dose required to promote sleep versus acute cognitive impairment was much greater using the Hcrt antagonist (DORA-22) versus eszopiclone, ZOL, and diazepam [37]. At high doses, Hcrt antagonists can produce some impairment in human subjects [23, 25, 61, 62]. For example, ALM at 400 mg and 1,000 mg reduced vigilance and alertness in healthy subjects [23, 25]. These doses of ALM are higher than the range (25–200 mg) shown to improve sleep initiation, sleep maintenance, and total sleep time in a dose-dependent fashion in both young and older insomnia patients [63–65]. Although monoaminergic arousal pathways can remain functional in the presence of a Hcrt antagonist [40], at higher doses, Hcrt antagonism has measurable cognitive effects. Our data suggest that low-response-demand tasks are the most sensitive for detecting cognitive effects of ALM. However, subjects’ performance under ALM was not appreciably different than PBO on tests of active memory encoding, retrieval, or performance on higher level tests of executive function.
At present, suvorexant is the only Hcrt antagonist approved by the FDA for the treatment of insomnia [26–28, 66]. Suvorexant is effective and approved by the FDA at doses ranging from 5 to 20 mg. Performance on verbal memory and the digit symbol substitution task (a measure of processing speed and cognitive flexibility) from suvorexant at doses ≤20 mg were no different from PBO [61]. The 40-mg dose did adversely affect psychomotor function in nonelderly healthy subjects [61, 67]. Healthy elderly subjects given 15 or 30 mg of suvorexant did not show impairment in next-morning driving performance [68, 69]. Suvorexant has a half-life of approximately 12 hours [66] which, at high doses, has the potential to affect next-morning cognition [61, 67]. In contrast, ALM has a half-life of 1.4 hours and its effects on sleep EEG were absent after 6.5 hours [24]. Although development of ALM was discontinued in 2011 because of concerns about elevations in hepatic enzymes [70, 71], other Hcrt antagonists are currently in development including lemborexant [72], filorexant [73], and seltorexant [74].
Limitations
This experiment did not test performance following awakening from sleep as was recently reported by Dinges et al. [75], and the data may not generalize to how subjects with insomnia would perform under these circumstances. However, rhesus monkeys administered DORA-22 relative to eszopiclone and diazepam could arouse from sleep to salient stimuli in the environment and perform the psychomotor vigilance task without impairment [38]. It is possible that differences in sensitivity to cognitive adverse effects with Hcrt antagonists versus a BzRA may be less apparent in subjects with hyperarousal. Another significant limitation is the use of a single-dose administration; it is unknown whether the benefits of a Hcrt antagonist over a BzRA would be sustained with repeated administration. Finally, afternoon dosing may not be representative of the cognitive effects that could have been seen had the dosing been given at bedtime and the testing conducted the following morning.
In January 2013, the FDA approved new label changes for ZOL and recommended an initial dose of immediate-release ZOL of 5 mg for women and either 5 or 10 mg for men. If the 5-mg dose was ineffective, the dosage could be later increased to 10 mg if tolerated. Because the majority of our subjects were women and we were using a 10-mg dose, we conducted additional analyses to test whether there were significant interaction effects for sex that could account for differences in performance and adverse events across the four drug groups. Across all of these outcomes, there were only two measures which showed an interaction effect: women on ZOL reported more sleepiness than men on the SSS and had more lapses on the PVT. However, men on ZOL also reported more sleepiness and had more PVT lapses. Thus, despite some evidence for greater sensitivity of women to the effects of ZOL, the overall results showing greater performance decrements under ZOL relative to ALM in men and the full sample was not an artifact of sex-specific sensitivity to adverse effects.
The human component of this integrated human–animal translational study demonstrates that a Hcrt antagonist produces fewer impairments than a BzRA on neurocognitive performance tested immediately after administration. The results from this trial, together with the mechanistic studies in rodents [33, 39–41], support our hypothesis that BzRAs cause a general inhibition of neural activity, whereas Hcrt specifically disfacilitates wake-promoting systems but is permissive for those systems to be recruited in the setting of a task demand. Hcrt antagonists have the potential to reduce morbid events associated with sleep aids during the period subsequent to administration.
Supplementary Material
Funding
Supported by USAMRMC grant W81XWH-09-2-0080 and National Center for Advancing Translational Sciences, National Institutes of Health, through UCSF-CTSI grant number UL1 RR024131. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the DoD or NIH. Blinded study medications were provided by Actelion Pharmaceuticals.
Conflict of interest statement. Financial disclosure: TJM, LMR, JV, MS, TM, JH, and KS declare that they have no disclosures. TCN has received grant/research support from NIH, VA, and DoD. He received study medication from Actelion for the conduct of this study. TCN has served as scientific consultant for Jazz Pharmaceuticals and Resilience Therapeutics. AR has received grant/research support from VA and DoD and served as scientific consultant for Jazz Pharmaceuticals. AO has received grant/research support from NIH and DoD. SLB has received grant/research support from NIH, VA, and DoD. SSI has received grant/research support from VA and DoD. SRM has received research support from CHDI Foundation, F Hoffmann-LaRoche, Sunovion Pharmaceuticals, Neurocrine, Heptares, Q BioMed, Jazz, Teva, and Merck Pharmaceuticals. TSK has received grant/research support from NIH and DoD and has served as a consultant for NIH, the Japanese Society for the Promotion of Science, Teva Branded Pharmaceutical Products R&D, Inc., and Jazz Pharmaceuticals and received research support from Jazz Pharmaceuticals and Sunovion Pharmaceuticals, Inc. Nonfinancial discloure: None of the authors have any other conflicts of interest.
References
- 1. Chong Y, et al. Prescription Sleep Aid Use Among Adults: United States, 2005–2010. NCHS data brief, no 127. Hyattsville, MD: National Center for Health Statistics; 2013. [PubMed] [Google Scholar]
- 2. Tom SE, et al. Nonbenzodiazepine sedative hypnotics and risk of fall-related injury. Sleep. 2016;39(5):1009–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lin FY, et al. Retrospective population cohort study on hip fracture risk associated with zolpidem medication. Sleep. 2014;37(4):673–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kang DY, et al. Zolpidem use and risk of fracture in elderly insomnia patients. J Prev Med Public Health. 2012;45(4):219–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kang JH, et al. Attention-deficit/hyperactivity disorder increased the risk of injury: a population-based follow-up study. Acta Paediatr. 2013;102(6):640–643. [DOI] [PubMed] [Google Scholar]
- 6. Diem SJ, et al. ; The Osteoporotic Fractures in Men (MrOS) Study Group Use of non-benzodiazepine sedative hypnotics and risk of falls in older men. J Gerontol Geriatr Res. 2014;3(3):158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Finkle WD, et al. Risk of fractures requiring hospitalization after an initial prescription for zolpidem, alprazolam, lorazepam, or diazepam in older adults. J Am Geriatr Soc. 2011;59(10):1883–1890. [DOI] [PubMed] [Google Scholar]
- 8. Wang PS, et al. Zolpidem use and hip fractures in older people. J Am Geriatr Soc. 2001;49(12):1685–1690. [DOI] [PubMed] [Google Scholar]
- 9. Lai MM, et al. Long-term use of zolpidem increases the risk of major injury: a population-based cohort study. Mayo Clin Proc. 2014;89(5):589–594. [DOI] [PubMed] [Google Scholar]
- 10. Mattila MJ, et al. Effects of alcohol, zolpidem, and some other sedatives and hypnotics on human performance and memory. Pharmacol Biochem Behav. 1998;59(4):917–923. [DOI] [PubMed] [Google Scholar]
- 11. Verster JC, et al. Residual effects of middle-of-the-night administration of zaleplon and zolpidem on driving ability, memory functions, and psychomotor performance. J Clin Psychopharmacol. 2002;22(6):576–583. [DOI] [PubMed] [Google Scholar]
- 12. Otmani S, et al. Effects of prolonged-release melatonin, zolpidem, and their combination on psychomotor functions, memory recall, and driving skills in healthy middle aged and elderly volunteers. Hum Psychopharmacol. 2008;23(8):693–705. [DOI] [PubMed] [Google Scholar]
- 13. Zammit G, et al. Use of computerized dynamic posturography to assess balance in older adults after nighttime awakenings using zolpidem as a reference. BMC Geriatr. 2008;8:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wesensten NJ, et al. Daytime sleep and performance following a zolpidem and melatonin cocktail. Sleep. 2005;28(1):93–103. [DOI] [PubMed] [Google Scholar]
- 15. Gustavsen I, et al. Individual psychomotor impairment in relation to zopiclone and ethanol concentrations in blood–a randomized controlled double-blinded trial. Addiction. 2012;107(5):925–932. [DOI] [PubMed] [Google Scholar]
- 16. Norman JL, et al. Novel class of medications, orexin receptor antagonists, in the treatment of insomnia - critical appraisal of suvorexant. Nat Sci Sleep. 2016;8:239–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sateia MJ, et al. Efficacy and clinical safety of ramelteon: an evidence-based review. Sleep Med Rev. 2008;12(4):319–332. [DOI] [PubMed] [Google Scholar]
- 18. de Lecea L. Hypocretins and the neurobiology of sleep-wake mechanisms. Prog Brain Res. 2012;198:15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schwartz MD, et al. The neurobiology of sleep and wakefulness. Psychiatr Clin North Am. 2015;38(4):615–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hara J, et al. Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron. 2001;30(2):345–354. [DOI] [PubMed] [Google Scholar]
- 21. Thannickal TC, et al. Reduced number of hypocretin neurons in human narcolepsy. Neuron. 2000;27(3):469–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Peyron C, et al. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18(23):9996–10015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hoever P, et al. Orexin receptor antagonism, a new sleep-enabling paradigm: a proof-of-concept clinical trial. Clin Pharmacol Ther. 2012;91(6):975–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Brisbare-Roch C, et al. Promotion of sleep by targeting the orexin system in rats, dogs and humans. Nat Med. 2007;13(2):150–155. [DOI] [PubMed] [Google Scholar]
- 25. Hoever P, et al. Orexin receptor antagonism, a new sleep-promoting paradigm: an ascending single-dose study with almorexant. Clin Pharmacol Ther. 2010;87(5):593–600. [DOI] [PubMed] [Google Scholar]
- 26. Michelson D, et al. Safety and efficacy of suvorexant during 1-year treatment of insomnia with subsequent abrupt treatment discontinuation: a phase 3 randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2014;13(5):461–471. [DOI] [PubMed] [Google Scholar]
- 27. Herring WJ, et al. Orexin receptor antagonism for treatment of insomnia: a randomized clinical trial of suvorexant. Neurology. 2012;79(23):2265–2274. [DOI] [PubMed] [Google Scholar]
- 28. Herring WJ, et al. Suvorexant in patients with insomnia: results from two 3-month randomized controlled clinical trials. Biol Psychiatry. 2016;79(2):136–148. [DOI] [PubMed] [Google Scholar]
- 29. James MH, et al. Introduction to the special issue: “orexin/hypocretin receptor antagonists for the treatment of addiction and related psychiatric disease: what are the steps from here?”. Brain Res. 2020;1731:146665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Campbell EJ, et al. Suvorexant to treat alcohol use disorder and comorbid insomnia: plan for a phase II trial. Brain Res. 2020;1728:146597. [DOI] [PubMed] [Google Scholar]
- 31. Han Y, et al. Orexin receptor antagonists as emerging treatments for psychiatric disorders. Neurosci Bull. 2020;36(4):432–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Krystal AD, et al. The assessment and management of insomnia: an update. World Psychiatry. 2019;18(3):337–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Morairty SR, et al. The hypocretin/orexin antagonist almorexant promotes sleep without impairment of performance in rats. Front Neurosci. 2014;8:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dietrich H, et al. Intact learning and memory in rats following treatment with the dual orexin receptor antagonist almorexant. Psychopharmacology (Berl). 2010;212(2):145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Steiner MA, et al. Differential effects of the dual orexin receptor antagonist almorexant and the GABA(A)-α1 receptor modulator zolpidem, alone or combined with ethanol, on motor performance in the rat. Neuropsychopharmacology. 2011;36(4):848–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Möhler H, et al. A new benzodiazepine pharmacology. J Pharmacol Exp Ther. 2002;300(1):2–8. [DOI] [PubMed] [Google Scholar]
- 37. Uslaner JM, et al. Orexin receptor antagonists differ from standard sleep drugs by promoting sleep at doses that do not disrupt cognition. Sci Transl Med. 2013;5(179):179ra44. [DOI] [PubMed] [Google Scholar]
- 38. Tannenbaum PL, et al. Inhibition of orexin signaling promotes sleep yet preserves salient arousability in monkeys. Sleep. 2016;39(3):603–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Vazquez-DeRose J, et al. Hypocretin/orexin antagonism enhances sleep-related adenosine and GABA neurotransmission in rat basal forebrain. Brain Struct Funct. 2016;221(2):923–940. [DOI] [PubMed] [Google Scholar]
- 40. Parks GS, et al. The dual hypocretin receptor antagonist almorexant is permissive for activation of wake-promoting systems. Neuropsychopharmacology. 2016;41(4):1144–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Schwartz MD, et al. Locus coeruleus and tuberomammillary nuclei ablations attenuate hypocretin/orexin antagonist-mediated REM sleep. eNeuro. 2016;3(2). doi: 10.1523/ENEURO.0018-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Edinger JD, et al. ; American Academy of Sleep Medicine Work Group Derivation of research diagnostic criteria for insomnia: report of an American Academy of Sleep Medicine Work Group. Sleep. 2004;27(8):1567–1596. [DOI] [PubMed] [Google Scholar]
- 43. Buysse DJ, et al. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. [DOI] [PubMed] [Google Scholar]
- 44. Netzer NC, et al. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131(7):485–491. [DOI] [PubMed] [Google Scholar]
- 45. First MB, et al. Structured Clinical Interview for DSM-IV Axis I Disorders, Research Version, Patient Edition. (SCID-I/P). New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- 46. Smith CS, et al. Evaluation of three circadian rhythm questionnaires with suggestions for an improved measure of morningness. J Appl Psychol. 1989;74(5):728–738. [DOI] [PubMed] [Google Scholar]
- 47. Saunders JB, et al. Development of the alcohol use disorders identification test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption–II. Addiction. 1993;88(6):791–804. [DOI] [PubMed] [Google Scholar]
- 48. Hommel G. A stagewise rejective multiple test procedure based on a modified Bonferroni test. Biometrika. 1988;75(2):383–386. [Google Scholar]
- 49. Connolly CN, et al. Assembly and cell surface expression of heteromeric and homomeric gamma-aminobutyric acid type A receptors. J Biol Chem. 1996;271(1):89–96. [DOI] [PubMed] [Google Scholar]
- 50. Möhler H. GABA(A) receptor diversity and pharmacology. Cell Tissue Res. 2006;326(2):505–516. [DOI] [PubMed] [Google Scholar]
- 51. Marcus JN, et al. Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol. 2001;435(1):6–25. [DOI] [PubMed] [Google Scholar]
- 52. Harrison TA, et al. Hypothalamic orexin A-immunoreactive neurons project to the rat dorsal medulla. Neurosci Lett. 1999;273(1):17–20. [DOI] [PubMed] [Google Scholar]
- 53. Nambu T, et al. Distribution of orexin neurons in the adult rat brain. Brain Res. 1999;827(1-2):243–260. [DOI] [PubMed] [Google Scholar]
- 54. Date Y, et al. Orexins, orexigenic hypothalamic peptides, interact with autonomic, neuroendocrine and neuroregulatory systems. Proc Natl Acad Sci USA. 1999;96(2):748–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Chen CT, et al. Orexin A-like immunoreactivity in the rat brain. Neurosci Lett. 1999;260(3):161–164. [DOI] [PubMed] [Google Scholar]
- 56. Horvath TL, et al. Hypocretin (orexin) activation and synaptic innervation of the locus coeruleus noradrenergic system. J Comp Neurol. 1999;415(2):145–159. [PubMed] [Google Scholar]
- 57. Hagan JJ, et al. Orexin A activates locus coeruleus cell firing and increases arousal in the rat. Proc Natl Acad Sci USA. 1999;96(19):10911–10916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sakurai T. The role of orexin in motivated behaviours. Nat Rev Neurosci. 2014;15(11):719–731. [DOI] [PubMed] [Google Scholar]
- 59. Tyree SM, et al. Hypocretin as a hub for arousal and motivation. Front Neurol. 2018;9:413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Scammell TE, et al. Neural circuitry of wakefulness and sleep. Neuron. 2017;93(4):747–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Jacobson LH, et al. Suvorexant for the treatment of insomnia. Expert Rev Clin Pharmacol. 2014;7(6):711–730. [DOI] [PubMed] [Google Scholar]
- 62. Hoch M, et al. Dual orexin receptor antagonism by almorexant does not potentiate impairing effects of alcohol in humans. Eur Neuropsychopharmacol. 2013;23(2):107–117. [DOI] [PubMed] [Google Scholar]
- 63. Black J, et al. Efficacy and safety of almorexant in adult chronic insomnia: a randomized placebo-controlled trial with an active reference. Sleep Med. 2017;36:86–94. [DOI] [PubMed] [Google Scholar]
- 64. Roth T, et al. Dual orexin receptor antagonist, almorexant, in elderly patients with primary insomnia: a randomized, controlled study. Sleep. 2017;40(2). doi: 10.1093/sleep/zsw03 [DOI] [PubMed] [Google Scholar]
- 65. Roecker AJ, et al. Orexin receptor antagonists: medicinal chemistry and therapeutic potential. Curr Top Med Chem. 2008;8(11):977–987. [DOI] [PubMed] [Google Scholar]
- 66. Sun H, et al. Effects of suvorexant, an orexin receptor antagonist, on sleep parameters as measured by polysomnography in healthy men. Sleep. 2013;36(2):259–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Vermeeren A, et al. On-the-road driving performance the morning after bedtime use of suvorexant 20 and 40 mg: a study in non-elderly healthy volunteers. Sleep. 2015;38(11):1803–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Herring WJ, et al. Suvorexant in patients with insomnia: pooled analyses of three-month data from phase-3 randomized controlled clinical trials. J Clin Sleep Med. 2016;12(9):1215–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Vermeeren A, et al. On-the-road driving performance the morning after bedtime use of suvorexant 15 and 30 mg in healthy elderly. Psychopharmacology (Berl). 2016;233(18):3341–3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Roecker AJ, et al. Orexin receptor antagonists: new therapeutic agents for the treatment of insomnia. J Med Chem. 2016;59(2):504–530. [DOI] [PubMed] [Google Scholar]
- 71. Janto K, et al. An update on dual orexin receptor antagonists and their potential role in insomnia therapeutics. J Clin Sleep Med. 2018;14(8):1399–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Murphy P, et al. Lemborexant, a dual orexin receptor antagonist (DORA) for the treatment of insomnia disorder: results from a Bayesian, adaptive, randomized, double-blind, placebo-controlled study. J Clin Sleep Med. 2017;13(11):1289–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Connor KM, et al. A phase II dose-ranging study evaluating the efficacy and safety of the orexin receptor antagonist filorexant (MK-6096) in patients with primary insomnia. Int J Neuropsychopharmacol. 2016;19(8). doi: 10.1093/ijnp/pyw022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. De Boer P, et al. A randomized phase 2 study to evaluate the orexin-2 receptor antagonist seltorexant in individuals with insomnia without psychiatric comorbidity. J Psychopharmacol. 2018;32(6):668–677. [DOI] [PubMed] [Google Scholar]
- 75. Dinges DF, et al. Effects of zolpidem and zaleplon on cognitive performance after emergent Tmax and morning awakenings: a randomized placebo-controlled trial. Sleep. 2019;42(3). doi: 10.1093/sleep/zsy258 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.