Abstract
Acrylamide has been studied for its carcinogenicity in experimental animals, causing tumors at several organ sites, and has been considered probably carcinogenic to humans as well. Given the small number of epidemiological studies that have been conducted, it is still uncertain whether the consumption of acrylamide is associated with liver cancer. Therefore, we investigated a study to determine the possible relationship between acrylamide intake and the risk of developing liver cancer in the Japanese population. A total of 85,305 participants, from the Japan Public Health Center-based Prospective Study, who provided a validated food-frequency questionnaire were enrolled between 1995 and 1998. During a median of 16.0 years follow-up, 744 new liver cancer cases were identified. Compared to the lowest tertile of acrylamide consumption (<4.8 µg/day), the multivariate hazard ratio (HR) for the highest tertile (≥7.6 µg/day) was 0.79 (95% confidence interval [CI] = 0.65–0.95) for liver cancer using multivariable model 1, adjusted for smoking status, body mass index (BMI), physical activity, medical history, and alcohol consumption; whereas the inverse relationship disappeared after additionally adjusting for coffee consumption in multivariable model 2 with HR of 1.08 (95% CI = 0.87–1.34) for the highest tertile. The effect of dietary acrylamide intake on the risk of liver cancer was not observed in the Japanese population.
Keywords: acrylamide, liver cancer, diet, cohort
1. Introduction
Acrylamide is considered to be a potent neurotoxin agent and has been hypothesized to promote cancer risk in humans and animals, which particularly occurs via occupational exposure [1,2,3]. In 1994, the International Agency for Research on Cancer classified acrylamide as a probable human carcinogen (Group 2A) [4]. In 2002, a report from Sweden which indicated that acrylamide could be detected in carbohydrate-rich foods because of high temperature cooking over 120 degree Celsius, was aware of the concern of the harmful effects of acrylamide [5]. The formation of acrylamide depends on different cooking methods, which leads to a wide variability of acrylamide levels in the same food [6].
In western countries, although some epidemiological studies have been investigated into the effect of acrylamide consumption on the risk of cancers, the results from studies into breast cancer, endometrial cancer, rectal cancer, and lung cancer were inconsistent [7]. Currently, there are few epidemiological studies being conducted to determine the relationship between acrylamide intake from daily diet and the risk of human cancers in Asian countries. One of the reasons for this is that an appropriate method of estimating acrylamide intake has not been well-established yet. Furthermore, to our knowledge, the relationship between acrylamide consumption and liver cancer risk has not yet been investigated anywhere in the world.
The underlying mechanism of promoting the effect of acrylamide on cancers in human beings is still obscure. Although sufficient evidence from both animal experiments and epidemiological studies in humans is lacking, it has been reported that one of the pathways for acrylamide metabolism involves the process of acrylamide being converted to glycidamide, a genotoxic and mutagenic substance. This conversion occurs in the liver and is mediated by cytochrome P450 2E1 (CYP2E1) [8,9,10]. In consequence, CYP2E1 has been found to catalyze the bioactivation of some protoxins and procarcinogens, including N-nitrosodimethylamine. Exposure to N-nitrosodimethylamine has been known to develop tumors in the lung, kidney, and liver in animals [11,12]. Therefore, it is important to demonstrate the effect of acrylamide on the human liver. We conducted this study to investigate whether increasing the dietary intake of acrylamide is related to the risk of developing liver cancer in the Japanese population. To the best of our knowledge, our study is the first epidemiological study focusing on this issue.
2. Materials and Methods
2.1. Study Design and Population
The Japan Public Health Center-based Prospective Study (JPHC Study) is a prospective cohort study, established with the primary aim of establishing evidence to benefit health maintenance and improvement, including cancer prevention. Between 1990 and 1993 140,420 middle-aged Japanese adults were recruited as the target population in the JPHC Study. A self-completion questionnaire survey on lifestyle habits, including dietary habits, was conducted among the study population. Further, they were requested to provide blood samples and the results of prior local or workplace health check-ups. All cohort participants were followed up to obtain data regarding the incidence of death, migration, cancer, and cardiovascular disease. The five-year follow-up survey consisted of a second questionnaire survey among the cohort participants, being held between 1995 and 1998. We included all responders of the five-year follow-up survey, and who fulfilled the following criteria: age between 45 and 74 years old, Japanese nationality, and not registered at any age-biased designated areas [13]. A total of 93,835 men and women were enrolled into the present cohort. All subjects gave their informed consent for inclusion before they participated in the study. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Institutional Review Board of the National Cancer Center, Japan (Ethical Approval Code: 2001-021), as well as Osaka University (Ethical Approval Code: 14020-9) and Azabu University (Ethical Approval Code: 2457).
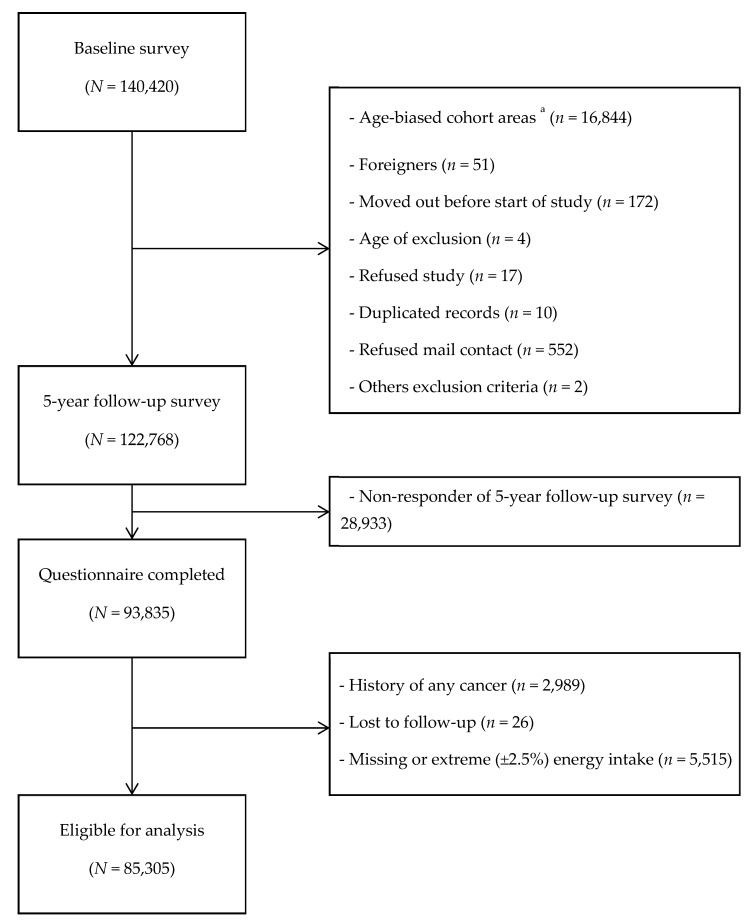
Of the initial 93,835 participants, we excluded 2989 participants who had been previously diagnosed with any cancers by the beginning of follow-up. In addition, 26 participants who were lost to follow-up were excluded from the analysis. Furthermore, we excluded 5515 participants who did not provide complete dietary data, as a result of which the total energy intake could not be calculated, and who were identified as the extreme low- and high-energy reporters. After excluding these ineligible participants, 85,305 participants were analyzed in the present study. (Figure 1)
Figure 1.
Flow diagram of eligibility for analysis. a Participants only at the age of 40 yrs. And 50 yrs. old received baseline questionnaire in these areas.
2.2. Acrylamide Intake Assessment
In the JPHC Study, the detailed information on lifestyle habits, history of health status, basic social factors, and daily dietary habits were obtained through a comprehensive questionnaire, including a food frequency questionnaire (FFQ). Participants were asked to report the general average consumption frequency of 138 food and beverage items, of standard portion size, in the last year [14]. The details of response choices for frequency, standard portion size, and relative portion sizes have been described elsewhere [15,16,17]. The validity and reproducibility study of the FFQ has been conducted by taking the dietary intakes from 28-day weighed dietary records (DRs) as a reference in a subcohort of the JPHC Study [18,19,20]. Moreover, the daily nutrients intake was calculated in reference to the Standard Tables of Food Composition in Japan (5th revised and enlarged edition) [21].
The acrylamide intake assessment from daily diet was estimated by using the measured values of acrylamide content in the following common Japanese food and beverage items as follows: baked fish paste, bread, rice cakes, Japanese-style confectionary, cakes, biscuits and cookies, chocolate, peanuts, fried tofu, miso, beer, green tea, oolong tea, black tea, coffee, and soup [22,23,24,25,26,27,28]. Furthermore, we estimated the acrylamide content by considering the different cooking methods involved. The intake of acrylamide from heated starchy vegetables (potato and sweet potato), vegetables (onion, bean sprouts, sweet pepper, squash, cabbage, snap beans, and broccoli), toast, boiled or stir-fried rice, and fried batter was calculated by multiplying the amount of raw food by the proportion of heated food (as calculated from the DRs) and the concentration of acrylamide in each heated food. The validity and reproducibility of the acrylamide intake assessment were evaluated, and showed Spearman’s correlation coefficients of 0.34–0.47 reported in detail elsewhere [29].
2.3. Statistical Analysis
Participants contributed person-years from the five-year follow-up survey until the date of diagnosis of liver cancer, death (from any cause), moving out of study areas, or 31 December 2013, whichever occurred first. Acrylamide intake was included in the multivariable-adjusted models as a categorical variable as well as a continuous variable (per 10 µg/day), to investigate the dose-response relationship. For acrylamide intake to be modeled as a categorical variable, participants were divided into tertiles according to their energy-adjusted intakes of acrylamide, which was computed using the residual method. The groups with the highest and lowest consumption were indicated as T3 and T1, respectively. Patient characteristics at the five-year follow-up survey were compared between groups, using the Kruskal−Wallis test or Chi-square test, whichever was appropriate. A Cox proportional hazards regression model was used to estimate hazard ratios (HRs) and their corresponding 95% confidence intervals (CIs), with T1 as the reference group.
In order to assess potential confounding, besides sex, 5-year age group, and public health center area, the following variables were tested, based on the previous studies: smoking status (never, former, current, or missing), body mass index (14 to <19, 19 to <21, 21 to <23, 23 to <25, 25 to <27, 27 to <30, and 30 to 40 kg/m2, or missing), physical activity (quartiles of metabolic equivalents, or missing), alcohol consumption (nondrinker, <150 or ≥150 g/week, or missing), and self-reported hepatitis and diabetes mellitus (reported at the baseline survey and five-year follow-up survey) (no or yes). The hazard ratio of acrylamide intake in the multivariable-adjusted model 1 was modified using these variables. Since the previous JPHC Study, coffee consumption has been related with a lower risk of liver cancer [30]; therefore, coffee consumption (g/day; nondrinker or quintiles) was added to the above variables in the multivariable-adjusted model 2. We additionally implemented analysis stratified by coffee consumption (nondrinker and coffee drinker).
Smoking is considered as one of major sources of acrylamide exposure, and smokers have been found to have a high-level of acrylamide-hemoglobin adducts (which is a marker of the internal dose of acrylamide) that are, on an average, three to four times higher than that seen in nonsmokers [31]. Consequently, to preclude confounding through smoking status, stratification analysis was performed according to never and ever smokers.
We also used multivariable-adjusted Cox proportional hazards regression to perform sensitivity analysis by excluding participants diagnosed with liver cancer within three years of the five-year follow-up survey (n = 69), and to perform another sensitivity analysis by excluding participants who reported a history of hepatitis (n = 1762). Furthermore, we conducted the analysis by gender to confirm whether there were any gender differences. All p-values were two-tailed. P-values less than 0.05 were considered statistically significant. We performed all the statistical analyses by using Stata/MP 14.1 (StataCorp, College Station, TX, USA).
3. Results
The distribution of characteristics for participants at 5-year study survey are shown in Table 1. After adjusting for total energy intake, the mean intake (±SD) of acrylamide by our study population was 6.9 ± 3.8 µg per day. The major food sources of acrylamide in Japan were coffee (27.4%), green tea (21.6%), potato (11.0%), vegetables (10.8%), and biscuits (10.6%), which were different from western countries [27,30,31]. Participants who consumed more acrylamide were, on an average, younger, had lower intake of alcohol, and less likely to have diabetes or hepatitis.
Table 1.
Characteristics of participants (n = 85,305) according to tertile of energy-adjusted acrylamide intake at five-year-study survey.
| Characteristics | Tertile of Energy-Adjusted Acrylamide Intake | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tertile 1 | Tertile 2 | Tertile 3 | p-Value c | ||||||||||||
| Number of Participants | 28,435 | 28,435 | 28,435 | ||||||||||||
| Men, % | 57.7 | 44.6 | 38.4 | ||||||||||||
| Dietary variables | |||||||||||||||
| Acrylamide intake | |||||||||||||||
| Range, μg/d | 0.04 | − | 4.81 | 4.82 | − | 7.63 | 7.64 | − | 67.11 | ||||||
| Mean and SD, a μg /d | 3.4 | ± | 1.0 | 6.1 | ± | 0.8 | 11.1 | ± | 3.5 | <0.001 | |||||
| Mean and SD, a μg·kg body weight−1·d−1 | 0.06 | ± | 0.05 | 0.11 | ± | 0.09 | 0.21 | ± | 0.23 | <0.001 | |||||
| Coffee, a g/d | 42.0 | ± | 57.8 | 107.1 | ± | 105.4 | 276.5 | ± | 283.8 | <0.001 | |||||
| Green tea, a g/d | 313.9 | ± | 337.9 | 510.7 | ± | 423.1 | 764.4 | ± | 680.8 | <0.001 | |||||
| Alcohol intake, a g/d | 155.1 | ± | 241.7 | 92.2 | ± | 179.2 | 58.5 | ± | 134.2 | <0.001 | |||||
| Biscuits, a g/d | 0.8 | ± | 1.2 | 2.0 | ± | 2.5 | 5.0 | ± | 7.9 | <0.001 | |||||
| Potato, a g/d | 10.2 | ± | 9.6 | 17.8 | ± | 14.8 | 21.5 | ± | 24.0 | <0.001 | |||||
| Vegetables, a g/d | 180.4 | ± | 119.8 | 220.9 | ± | 128.4 | 231.1 | ± | 143.3 | <0.001 | |||||
| Fruit, a g/d | 178.9 | ± | 161.4 | 219.8 | ± | 163.0 | 221.1 | ± | 168.5 | <0.001 | |||||
| Meat, a g/d | 58.1 | ± | 43.5 | 57.1 | ± | 36.6 | 56.2 | ± | 35.6 | <0.001 | |||||
| Fish, a g/d | 85.9 | ± | 56.1 | 88.4 | ± | 48.9 | 83.7 | ± | 47.9 | <0.001 | |||||
| Total energy intake, a kcal/d | 1997.1 | ± | 641.5 | 2019.3 | ± | 610.4 | 1971.3 | ± | 610.1 | <0.001 | |||||
| Nondietary variables | |||||||||||||||
| Age at 5-year follow-up study, a y | 57.8 | ± | 7.6 | 57.1 | ± | 7.9 | 55.9 | ± | 8.0 | <0.001 | |||||
| Body mass index, a b kg/m2 | 23.7 | ± | 3.1 | 23.6 | ± | 3.0 | 23.4 | ± | 3.0 | <0.001 | |||||
| Smoking status, % | |||||||||||||||
| Never | 57.9 | 64.8 | 64.2 | <0.001 | |||||||||||
| Former | 10.6 | 8.3 | 6.8 | ||||||||||||
| Current | 25.1 | 21.1 | 23.3 | ||||||||||||
| Missing | 6.4 | 5.8 | 5.7 | ||||||||||||
| Number of cigarettes/d, a b only for current | 20.5 | ± | 14.3 | 21.2 | ± | 11.9 | 22.7 | ± | 11.4 | <0.001 | |||||
| Physical activity (METs) a | 33.2 | ± | 6.4 | 33.2 | ± | 6.2 | 33.1 | ± | 6.1 | <0.001 | |||||
| Diabetes, % yes | 8.3 | 6.6 | 5.4 | <0.001 | |||||||||||
| Hepatitis, % yes | 2.4 | 2.1 | 1.7 | <0.001 | |||||||||||
a Mean ± s.d. b Number of participants missing the following: body mass index: 2163; number of cigarettes per day for current smokers: 378; physical activity: 15,446. c Kruskal−Wallis test for continuous variables and Chi-square test for categorical variables.
During a total of 1,267,791 person-years of follow-up (14.9 years on average), 744 cases of liver cancer were ascertained among the 85,305 eligible participants. Table 2 shows that the higher energy-adjusted acrylamide intake was associated with a significantly lower risk of liver cancer in the age- and area-adjusted model and the multivariable model 1. Subjects with a high acrylamide intake (T3) had a 21% lower liver cancer risk than those who had a low acrylamide intake (T1) (HR = 0.79; 95% CI = 0.65–0.95; P trend = 0.01) in model 1. Results were similar in sensitivity analyses. However, the statistically significant decrease disappeared after additional adjustment for coffee consumption (HR = 1.08; 95% CI = 0.87–1.34). Exclusion of cases where liver cancer was diagnosed during the first three years of follow-up did not change the results in model 2. In addition, the negative result found in model 2 did not change after considering the information on hepatitis B and C virus infection. Besides, exclusion of participants who reported a history of hepatitis did not change the results in multivariable model 3.
Table 2.
Hazard ratios (95% confidence intervals) for liver cancer according to tertile of acrylamide intake.
| Quartile of Energy-Adjusted Acrylamide Intake | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 10 μg/d | Tertile 1 (Lowest) | Tertile 2 | Tertile 3 (Highest) | ||||||
| HR | (95% CI) | HR | (95% CI) | HR | (95% CI) | HR | (95% CI) | p for Trend | |
| Number of subjects | 85,305 | 28,435 | 28,435 | 28,435 | |||||
| Person-years | 1,267,791 | 417,202 | 425,177 | 425,412 | |||||
| Number of liver cancers | 744 | 311 | 248 | 185 | |||||
| Age- and area-adjusted model a | 0.96 | (0.94–0.98) | 1.00 | (Reference) | 0.88 | (0.74–1.04) | 0.73 | (0.60–0.88) | <0.01 |
| Multivariable model 1 b | 0.96 | (0.94–0.99) | 1.00 | (Reference) | 0.90 | (0.76–1.06) | 0.79 | (0.65–0.95) | 0.01 |
| Multivariable model 1 (excluding cases < 3 years) | 0.97 | (0.94–0.99) | 1.00 | (Reference) | 0.91 | (0.76–1.10) | 0.82 | (0.66–1.01) | 0.06 |
| Multivariable model 2 c | 0.99 | (0.96–1.01) | 1.00 | (Reference) | 1.00 | (0.84–1.20) | 1.08 | (0.87–1.34) | 0.51 |
| Multivariable model 2 (excluding cases < 3 years) | 0.99 | (0.96–1.01) | 1.00 | (Reference) | 0.99 | (0.81–1.21) | 1.08 | (0.85–1.37) | 0.58 |
| Multivariable model 3 d (excluding cases with history of hepatitis) | 0.99 | (0.96–1.02) | 1.00 | (Reference) | 0.95 | (0.76–1.18) | 1.12 | (0.87–1.45) | 0.47 |
| By smoking status | |||||||||
| Never smoker | |||||||||
| Number of subjects | 53,137 | 16,460 | 18,429 | 18,248 | |||||
| Person-years | 817,862 | 252,425 | 283,953 | 281,484 | |||||
| Number of liver cancers | 335 | 131 | 109 | 95 | |||||
| Multivariable model 1 | 0.98 | (0.94–1.01) | 1.00 | (Reference) | 0.86 | (0.66–1.11) | 0.93 | (0.70–1.22) | 0.54 |
| Multivariable model 2 | 0.99 | (0.95–1.02) | 1.00 | (Reference) | 0.95 | (0.72–1.25) | 1.15 | (0.85–1.56) | 0.42 |
| Ever smoker e | |||||||||
| Number of subjects | 27,083 | 10,150 | 8365 | 8568 | |||||
| Person-years | 382,550 | 141,189 | 119,153 | 122,209 | |||||
| Number of liver cancers | 352 | 147 | 128 | 77 | |||||
| Multivariable model 1 | 0.95 | (0.92–0.99) | 1.00 | (Reference) | 1.06 | (0.83–1.35) | 0.71 | (0.53–0.94) | 0.03 |
| Multivariable model 2 | 0.99 | (0.95–1.02) | 1.00 | (Reference) | 1.17 | (0.90–1.51) | 1.07 | (0.77–1.51) | 0.52 |
| By coffee consumption | |||||||||
| Nondrinker | |||||||||
| Number of subjects | 23,104 | 13,603 | 6048 | 3453 | |||||
| Acrylamide intake (mean ± SD, μg/d) | 3.0 | ±1.1 | 6.0 | ±0.8 | 10.8 | ±3.1 | |||
| Acrylamide intake (range, μg/d) | 0.0 | −4.8 | 4.8 | −7.6 | 7.6 | −67.1 | |||
| Person-years | 335,958 | 197,392 | 88,407 | 50,159 | |||||
| Number of liver cancers | 266 | 160 | 68 | 38 | |||||
| Multivariable model 1 | 1.01 | (0.96–1.06) | 1.00 | (Reference) | 1.00 | (0.74–1.34) | 1.12 | (0.77–1.62) | 0.63 |
| Drinker | |||||||||
| Number of subjects | 62,201 | 14,832 | 22,387 | 24,982 | |||||
| Acrylamide intake (mean ± SD, μg/d) | 3.7 | ±0.8 | 6.1 | ±0.8 | 11.1 | ±3.5 | |||
| Acrylamide intake (range, μg/d) | 0.4 | −4.8 | 4.8 | −7.6 | 7.6 | −62.8 | |||
| Person-years | 931,833 | 219,810 | 336,770 | 375,253 | |||||
| Number of liver cancers | 478 | 151 | 180 | 147 | |||||
| Multivariable model 1 | 0.96 | (0.93–0.98) | 1.00 | (Reference) | 0.86 | (0.69–1.07) | 0.74 | (0.58–0.94) | 0.01 |
| Multivariable model 2 | 0.98 | (0.95–1.01) | 1.00 | (Reference) | 0.97 | (0.77–1.22) | 1.05 | (0.80–1.38) | 0.73 |
Abbreviations: 95% CI = 95% confidence intervals. a Age- and area-adjusted model adjusted for gender, age (5-year age intervals) and area. b Multivariable model 1 additionally adjusted for: smoking status (never, former, current, missing), intake of alcohol (nondrinker, <150, ≥150 g/week, missing), body mass index (14−<19, 19−<21, 21−<23, 23−<25, 25−<27, 27−<30, 30–40 kg/m2, missing), physical activity (quartiles, missing), history of diabetes (yes/no), and history of hepatitis (yes/no). c Multivariable model 2 additionally adjusted for: multivariable model 1 and coffee consumption (nondrinker, quintiles). d Multivariable model 3 additionally adjusted for: smoking status (never, former, current, missing), intake of alcohol (nondrinker, <150, ≥150 g/week, missing), body mass index (14−<19, 19−<21, 21−<23, 23−<25, 25−<27, 27−<30, 30–40 kg/m2, missing), physical activity (quartiles, missing), and history of diabetes (yes/no). e Ever smoker was defined as former and current smoker.
Similar results were observed in the stratification analysis by coffee intake. Although an increased acrylamide intake reduced the risk of liver cancer among coffee drinkers in the multivariable model 1 (HR = 0.74; 95% CI = 0.58–0.94; P trend = 0.01), the inverse association disappeared after additional adjustment in the multivariable model 2 (HR = 1.05; 95% CI = 0.80–1.38). The association between acrylamide intake and liver cancer risk was not observed in either the nondrinkers or the coffee drinkers after considering the amounts of coffee consumption. We did not observe significant differences between never or ever smokers regarding the association of acrylamide intake with the risk of liver cancer. Furthermore, no gender differences in HR were observed in the present study, shown in Table 3.
Table 3.
Hazard ratios (95% confidence intervals) for liver cancer according to tertile of acrylamide intake by gender.
| Quartile of Energy-Adjusted Acrylamide Intake | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 10 μg/d | Tertile 1 (Lowest) | Tertile 2 | Tertile 3 (Highest) | ||||||
| HR | (95% CI) | HR | (95% CI) | HR | (95% CI) | HR | (95% CI) | p for Trend | |
| Men | |||||||||
| Number of subjects | 39,996 | 16,417 | 12,669 | 10,910 | |||||
| Person-years | 569,415 | 231,895 | 181,770 | 155,751 | |||||
| Number of liver cancers | 530 | 237 | 175 | 118 | |||||
| Age- and area-adjusted model a | 0.95 | (0.93–0.98) | 1.00 | (Reference) | 0.91 | (0.74–1.10) | 0.71 | (0.57–0.89) | <0.01 |
| Multivariable model 1 b | 0.96 | (0.93–0.99) | 1.00 | (Reference) | 0.94 | (0.77–1.14) | 0.78 | (0.62–0.98) | 0.04 |
| Multivariable model 2 c | 0.99 | (0.96–1.02) | 1.00 | (Reference) | 1.05 | (0.85–1.29) | 1.15 | (0.88–1.50) | 0.31 |
| Women | |||||||||
| Number of subjects | 45,309 | 12,018 | 15,766 | 17,525 | |||||
| Person-years | 698,376 | 185,307 | 243,407 | 269,662 | |||||
| Number of liver cancers | 214 | 74 | 73 | 67 | |||||
| Age- and area-adjusted model a | 0.97 | (0.93–1.00) | 1.00 | (Reference) | 0.81 | (0.59–1.13) | 0.78 | (0.56–1.10) | 0.16 |
| Multivariable model 1 b | 0.97 | (0.94–1.01) | 1.00 | (Reference) | 0.76 | (0.55–1.06) | 0.77 | (0.54–1.08) | 0.14 |
| Multivariable model 2 c | 0.98 | (0.95–1.02) | 1.00 | (Reference) | 0.83 | (0.59–1.17) | 0.92 | (0.63–1.32) | 0.65 |
Abbreviations: 95% CI = 95% confidence intervals. a Age- and area-adjusted model adjusted for age (5-year age intervals) and area. b Multivariable model 1 additionally adjusted for: smoking status (never, former, current, missing), intake of alcohol (nondrinker, <150, ≥150 g/week), body mass index (14−<19, 19−<21, 21−<23, 23−<25, 25−<27, 27−<30, 30–40 kg/m2, missing), physical activity (quartile, missing), history of diabetes (yes/no), and history of hepatitis (yes/no). c Multivariable model 2 additionally adjusted for: multivariable model 1 and coffee consumption (nondrinker, quintiles).
4. Discussion
This large-scale population-based prospective cohort study is, to our knowledge, the first epidemiological study in humans to demonstrate the association between dietary acrylamide intake and the risk of liver cancer. We observed no significant association between dietary acrylamide intake and risk of liver cancer. Although an inverse association was observed in the multivariable model 1, this association was not observed after further adjustment in the multivariable model 2. These findings indicate that while coffee drinking may lower the risk of liver cancer, the acrylamide present in coffee contributes substantially to the total dietary acrylamide intake in Japan. Because of the dichotomic role of coffee consumption, we interpreted our results based on how coffee affects the risk of liver cancer, and by distinguishing the effect of acrylamide from the effect of coffee.
Several previous studies have suggested an inverse association between coffee consumption and liver cancer, both in Japan and in other countries [30,32,33,34,35]. Several potential mechanisms have been proposed through which coffee may lower the risk for developing liver cancer. Coffee is known to contain a variety of different biologically active chemical compounds including antioxidants and diterpenes. Antioxidants, for instance caffeine and chlorogenic acids, have been indicated for preventing oxidative DNA damage, modification effect on the apoptotic response, and reversing the cell cycle checkpoint function [36,37,38,39]. Besides, diterpenes, for instance kahweol and cafestol, have been shown to have anticarcinogenic properties, and may offer a protective effect against aflatoxin B1-induced genotoxicity [40,41,42]. These studies suggest that the ingredients in coffee may play an important role in protecting against the occurrence and development of liver cancer. Thus, the observed inverse association in the multivariable model 1, which did not adjust for coffee consumption, could not indicate the actual effect of dietary acrylamide intake. Because the inverse association disappeared after additional adjustment for coffee consumption in the multivariable model 2, coffee consumption, particularly the consumption of caffeine, chlorogenic acids, kahweol, and cafestol, should be considered as the confounding factor in this study.
To minimize the confounding effect from coffee consumption, stratified analysis was conducted by comparing HRs in coffee drinkers and nondrinkers. There was no association between acrylamide intake and liver cancer in nondrinkers. In coffee drinkers, no association was observed after adjustment for the amount of coffee consumption. These findings suggest that the association of reduced risk of liver cancer with the high intake of acrylamide was not valid.
The lack of association between acrylamide intake and liver cancer risk could be partly due to the low baseline range of acrylamide intake used in this study. The mean acrylamide intake for the reference group in this study was 3.4 µg per day, and for the highest intake category was 11.1 µg per day. In contrast, in the Netherlands cohort study, the mean acrylamide intake for the reference group was 9.5 µg per day, and for the highest intake category was 40.8 µg per day [43]. However, it is important to state that the values of acrylamide intake that were assessed by the FFQ were relative values. The determination of acrylamide exposure by using hemoglobin adducts as a biomarker of internal dose is necessary for accurate comparison.
The classification of acrylamide made by the IARC in 1994 was primarily based on evidence from animal models and mechanistic considerations [4]. In animal models, the dose of acrylamide exposure was much higher than the doses humans are exposed to through daily diet [44]. Consequently, the dose of acrylamide exposure differs between experimental animals and epidemiological studies in humans, and may help to interpret the null finding in the present study.
The present study has several strengths including a large sample size, the population-base and prospective design, and the completeness of case ascertainment through linkage to the population-based registries in Japan. However, there are also some limitations that should be discussed. First, the present study has some limitations regarding acrylamide intake assessment. The FFQ consists of a finite list of foods and beverages. Therefore, it may be a less accurate estimation of dietary intake when compared to a 24-h recall method. The correlation for acrylamide intake between the FFQ and the 28-day dietary records was relatively low [45]. Even though the use of FFQ has limitations in the assessment of dietary acrylamide exposure, it is the only feasible way of assessing dietary acrylamide intake over a long period of time in a large study population. Second, the participants were not divided into more groups based on dietary acrylamide intake because of the limited number of cases. Likewise, the associations between dietary acrylamide intake and liver cancer in never smokers and in noncoffee drinkers were based on analyses with a small number of cases. Third, in the present analysis, both acrylamide intake and information on covariates had only been measured once, while, it is highly possible that the participants may change their acrylamide consumption levels during the relatively long follow-up period. Therefore, these results should be interpreted cautiously.
5. Conclusions
In conclusion, the results of the present study indicate that there was no association between dietary intake of acrylamide and risk of liver cancer. Further studies with biomarkers of the internal dose of acrylamide are needed to investigate the carcinogenicity of acrylamide in humans.
Acknowledgments
The members of the JPHC study are listed at the following site (as of September 2019): https://epi.ncc.go.jp/en/jphc/781/7951.html. This study was supported by a grant from the Food Safety Commission, Cabinet Office, Government of Japan (Research Program for Risk Assessment Study on Food Safety, No. 1503, the principal investigator is T.S.), the National Cancer Center Research and Development Fund (since 2011, the principal investigator is S.T.), and a Grant-in-Aid for Cancer Research from the Ministry of Health, Labor and Welfare of Japan (from 1989 to 2010; principal investigator from 1997 to 2010 is S.T.).
Author Contributions
Conceptualization, all authors; methodology, all authors; software, L.Z.; validation, all authors; formal analysis, L.Z.; investigation, all authors; resources, J.I., N.S., M.I. and S.T.; data curation, L.Z.; writing—original draft preparation, L.Z.; writing—review and editing, all authors; visualization, L.Z.; supervision, T.S. and J.I.; project administration, N.S. and S.T.; funding acquisition, none. All authors have read and agreed to the published version of the manuscript.
Funding
Food Safety Commission, Cabinet Office, Government of Japan, Grant/Award Number: 1503; Grant-in-Aid for Cancer Research from the Ministry of Health, Labor and Welfare of Japan; National Cancer Center Research and Development Fund.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Friedman M. Chemistry, biochemistry, and safety of acrylamide. A review. J. Agric. Food Chem. 2003;51:4504–4526. doi: 10.1021/jf030204+. [DOI] [PubMed] [Google Scholar]
- 2.Gierisch J.M., Coeytaux R.R., Urrutia R.P., Havrilesky L.J., Moorman P.G., Lowery W.J., Dinan M., McBroom A.J., Hasselblad V., Sanders G.D., et al. Oral contraceptive use and risk of breast, cervical, colorectal, and endometrial cancers: A systematic review. Cancer Epidemiol. Prev. Biomark. 2013;22:1931–1943. doi: 10.1158/1055-9965.EPI-13-0298. [DOI] [PubMed] [Google Scholar]
- 3.Hogervorst J.G., Baars B.-J., Schouten L.J., Konings E.J., Goldbohm R.A., van den Brandt P.A. The carcinogenicity of dietary acrylamide intake: A comparative discussion of epidemiological and experimental animal research. Crit. Rev. Toxicol. 2010;40:485–512. doi: 10.3109/10408440903524254. [DOI] [PubMed] [Google Scholar]
- 4.International Agency for Research on Cancer . Some Industrial Chemicals. IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans volume 60; IARC Publication; Lyon, France: 1994. [Google Scholar]
- 5.Tareke E., Rydberg P., Karlsson P., Eriksson S., Törnqvist M. Analysis of acrylamide, a carcinogen formed in heated foodstuffs. J. Agric. Food Chem. 2002;50:4998–5006. doi: 10.1021/jf020302f. [DOI] [PubMed] [Google Scholar]
- 6.Mottram D.S., Wedzicha B.L., Dodson A.T. Food chemistry: Acrylamide is formed in the Maillard reaction. Nature. 2002;419:448–449. doi: 10.1038/419448a. [DOI] [PubMed] [Google Scholar]
- 7.Riboldi B.P., Vinhas Á.M., Moreira J.D. Risks of dietary acrylamide exposure: A systematic review. Food Chem. 2014;157:310–322. doi: 10.1016/j.foodchem.2014.02.046. [DOI] [PubMed] [Google Scholar]
- 8.Sumner S.C., Fennell T.R., Moore T.A., Chanas B., Gonzalez F., Ghanayem B.I. Role of cytochrome P450 2E1 in the metabolism of acrylamide and acrylonitrile in mice. Chem. Res. Toxicol. 1999;12:1110–1116. doi: 10.1021/tx990040k. [DOI] [PubMed] [Google Scholar]
- 9.Ghanayem B.I., McDaniel L.P., Churchwell M.I., Twaddle N.C., Snyder R., Fennell T.R., Doerge D.R. Role of CYP2E1 in the epoxidation of acrylamide to glycidamide and formation of DNA and hemoglobin adducts. Toxicol. Sci. 2005;88:311–318. doi: 10.1093/toxsci/kfi307. [DOI] [PubMed] [Google Scholar]
- 10.Doroshyenko O., Fuhr U., Kunz D., Frank D., Kinzig M., Jetter A., Reith Y., Lazar A., Taubert D., Kirchheiner J., et al. In vivo role of cytochrome P450 2E1 and glutathione-S-transferase activity for acrylamide toxicokinetics in humans. Cancer Epidemiol. Prev. Biomark. 2009;18:433–443. doi: 10.1158/1055-9965.EPI-08-0832. [DOI] [PubMed] [Google Scholar]
- 11.Bogovski P., Bogovski S. Special report animal species in which n-nitroso compounds induce cancer. Int. J. Cancer. 1981;27:471–474. doi: 10.1002/ijc.2910270408. [DOI] [PubMed] [Google Scholar]
- 12.Klein R., Janowsky I., Schmezer P., Hermann R., Spiegelhalder B., Zeller W., Pool B. Effect of long-term inhalation of N-nitroso-dimethyl amine (NDMA) and SO2/NOx in rats. Exp. Pathol. 1989;37:273–280. doi: 10.1016/S0232-1513(89)80067-2. [DOI] [PubMed] [Google Scholar]
- 13.Tsugane S., Sawada N. The JPHC study: Design and some findings on the typical Japanese diet. Jpn. J. Clin. Oncol. 2014;44:777–782. doi: 10.1093/jjco/hyu096. [DOI] [PubMed] [Google Scholar]
- 14.Tsugane S., Kobayashi M., Sasaki S. Validity of the self-administered food frequency questionnaire used in the 5-year follow-up survey of the JPHC Study Cohort I: Comparison with dietary records for main nutrients. J. Epidemiol. 2003;13:51–56. doi: 10.2188/jea.13.1sup_51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kotemori A., Ishihara J., Zha L., Liu R., Sawada N., Iwasaki M., Sobue T., Tsugane S. Dietary acrylamide intake and risk of breast cancer: The Japan Public Health Center-based Prospective Study. Cancer Sci. 2018;109:843–853. doi: 10.1111/cas.13496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu R., Sobue T., Kitamura T., Kitamura Y., Ishihara J., Kotemori A., Zha L., Ikeda S., Sawada N., Iwasaki M. Dietary Acrylamide Intake and Risk of Esophageal, Gastric, and Colorectal Cancer: The Japan Public Health Center–Based Prospective Study. Cancer Epidemiol. Prev. Biomark. 2019;28:1461–1468. doi: 10.1158/1055-9965.EPI-18-1259. [DOI] [PubMed] [Google Scholar]
- 17.Sasaki S., Kobayashi M., Ishihara J., Tsugane S. Self-administered food frequency questionnaire used in the 5-year follow-up survey of the JPHC Study: Questionnaire structure, computation algorithms, and area-based mean intake. J. Epidemiol. 2003;13:13–22. doi: 10.2188/jea.13.1sup_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ishihara J., Inoue M., Kobayashi M., Tanaka S., Yamamoto S., Iso H., Tsugane S. Impact of the revision of a nutrient database on the validity of a self-administered food frequency questionnaire (FFQ) J. Epidemiol. 2006;16:107–116. doi: 10.2188/jea.16.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsugane S., Sasaki S., Kobayashi M., Tsubono Y., Akabane M. Validity and reproducibility of the self-administered food frequency questionnaire in the JPHC Study Cohort I: Study design, conduct and participant profiles. J. Epidemiol. 2003;13:2–12. doi: 10.2188/jea.13.1sup_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ishihara J., Sobue T., Yamamoto S., Yoshimi I., Sasaki S., Kobayashi M., Takahashi T., Iitoi Y., Akabane M., Tsugane S., et al. Validity and reproducibility of a self-administered food frequency questionnaire in the JPHC Study Cohort II: Study design, participant profile and results in comparison with Cohort I. J. Epidemiol. 2003;13:134–147. doi: 10.2188/jea.13.1sup_134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kagawa Y. Standard Tables of Food Composition in Japan Fifth Revised and Enlarged Edition. FAO; Roma, Italy: 2008. Standard tables of food composition in Japan; pp. 28–36. [Google Scholar]
- 22.Food Safety Commission of Japen . Study on Estimate of Acrylamide Intake from Food. Food Safety Commission of Japen; Tokyo, Japen: 2016. [Google Scholar]
- 23.Ministry of Agriculture, Forestry and Fisheries . Risk Profile Sheet Relating to the Food Safety for Acrylamide. Ministry of Agriculture, Forestry and Fisheries; Tokyo, Japen: 2015. [Google Scholar]
- 24.National Institute of Health Sciences Acrylamide Analysis in Food. [(accessed on 15 March 2018)]; Available online: http://www.mhlw.go.jp/topics/2002/11/tp1101-1a.html.
- 25.Mizukami Y., Kohata K., Yamaguchi Y., Hayashi N., Sawai Y., Chuda Y., Ono H., Yada H., Yoshida M. Analysis of acrylamide in green tea by gas chromatography−mass spectrometry. J. Agric. Food Chem. 2006;54:7370–7377. doi: 10.1021/jf061029a. [DOI] [PubMed] [Google Scholar]
- 26.Takatsuki S., Nemoto S., Sasaki K., Maitani T. Production of acrylamide in agricultural products by cooking. Shokuhin Eiseigaku Zasshi. J. Food Hyg. Soc. Jpn. 2004;45:44–48. doi: 10.3358/shokueishi.45.44. [DOI] [PubMed] [Google Scholar]
- 27.Food Safety Commission of Japen Information Clearing Sheet for Acrylamide. [(accessed on 4 December 2017)]; Available online: https://www.fsc.go.jp/fsciis/attachedFile/download?retrievalId=kai20111222sfc&fileId=520.
- 28.FAO/WHO . Health Implications of Acrylamide in Food. FAO; Roma, Italy: 2002. [Google Scholar]
- 29.Kotemori A., Ishihara J., Nakadate M., Sawada N., Iwasaki M., Sobue T., Tsugane S. Validity of a self-administered food frequency questionnaire for the estimation of acrylamide intake in the Japanese population: The JPHC FFQ Validation Study. J. Epidemiol. 2018;28:482–487. doi: 10.2188/jea.JE20170186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Inoue M., Yoshimi I., Sobue T., Tsugane S. Influence of coffee drinking on subsequent risk of hepatocellular carcinoma: A prospective study in Japan. J. Natl. Cancer Inst. 2005;97:293–300. doi: 10.1093/jnci/dji040. [DOI] [PubMed] [Google Scholar]
- 31.Schettgen T., Rossbach B., Kütting B., Letzel S., Drexler H., Angerer J. Determination of haemoglobin adducts of acrylamide and glycidamide in smoking and non-smoking persons of the general population. Int. J. Hyg. Environ. Health. 2004;207:531–539. doi: 10.1078/1438-4639-00324. [DOI] [PubMed] [Google Scholar]
- 32.Bravi F., Bosetti C., Tavani A., Bagnardi V., Gallus S., Negri E., Franceschi S., La Vecchia C. Coffee drinking and hepatocellular carcinoma risk: A meta-analysis. Hepatology. 2007;46:430–435. doi: 10.1002/hep.21708. [DOI] [PubMed] [Google Scholar]
- 33.Larsson S.C., Wolk A. Coffee consumption and risk of liver cancer: A meta-analysis. Gastroenterology. 2007;132:1740–1745. doi: 10.1053/j.gastro.2007.03.044. [DOI] [PubMed] [Google Scholar]
- 34.Sang L.-X., Chang B., Li X.-H., Jiang M. Consumption of coffee associated with reduced risk of liver cancer: A meta-analysis. BMC Gastroenterol. 2013;13:34. doi: 10.1186/1471-230X-13-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shimazu T., Tsubono Y., Kuriyama S., Ohmori K., Koizumi Y., Nishino Y., Shibuya D., Tsuji I. Coffee consumption and the risk of primary liver cancer: Pooled analysis of two prospective studies in Japan. Int. J. Cancer. 2005;116:150–154. doi: 10.1002/ijc.20989. [DOI] [PubMed] [Google Scholar]
- 36.Asaad N.A., Zhao-Chong Z., Guan J., Thacker J., Iliakis G. Homologous recombination as a potential target for caffeine radiosensitization in mammalian cells: Reduced caffeine radiosensitization in XRCC2 and XRCC3 mutants. Oncogene. 2000;19:5788–5800. doi: 10.1038/sj.onc.1203953. [DOI] [PubMed] [Google Scholar]
- 37.Joerges C., Kuntze I., Herzinge T. Induction of a caffeine-sensitive S-phase cell cycle checkpoint by psoralen plus ultraviolet A radiation. Oncogene. 2003;22:6119–6128. doi: 10.1038/sj.onc.1206613. [DOI] [PubMed] [Google Scholar]
- 38.Saiki S., Sasazawa Y., Imamichi Y., Kawajiri S., Fujimaki T., Tanida I., Kobayashi H., Sato F., Sato S., Ishikawa K.-I., et al. Caffeine induces apoptosis by enhancement of autophagy via PI3K/Akt/mTOR/p70S6K inhibition. Autophagy. 2011;7:176–187. doi: 10.4161/auto.7.2.14074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Azam S., Hadi N., Khan N.U., Hadi S.M. Antioxidant and prooxidant properties of caffeine, theobromine and xanthine. Med Sci. Monit. 2003;9:BR325–BR330. [PubMed] [Google Scholar]
- 40.Cavin C., Holzhaeuser D., Scharf G., Constable A., Huber W., Schilter B. Cafestol and kahweol, two coffee specific diterpenes with anticarcinogenic activity. Food Chem. Toxicol. 2002;40:1155–1163. doi: 10.1016/S0278-6915(02)00029-7. [DOI] [PubMed] [Google Scholar]
- 41.Majer B., Hofer E., Cavin C., Lhoste E., Uhl M., Glatt H., Meinl W., Knasmüller S. Coffee diterpenes prevent the genotoxic effects of 2-amino-1-methyl-6-phenylimidazo [4, 5-b] pyridine (PhIP) and N-nitrosodimethylamine in a human derived liver cell line (HepG2) Food Chem. Toxicol. 2005;43:433–441. doi: 10.1016/j.fct.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 42.Cavin C., Holzhäuser D., Constable A., Huggett A.C., Schilter B. The coffee-specific diterpenes cafestol and kahweol protect against aflatoxin B1-induced genotoxicity through a dual mechanism. Carcinogenesis. 1998;19:1369–1375. doi: 10.1093/carcin/19.8.1369. [DOI] [PubMed] [Google Scholar]
- 43.Hogervorst J.G.F. Dietary Acrylamide Intake and Human Cancer Risk. Maastricht University; Maastricht, The Netherland: 2009. [Google Scholar]
- 44.Larsson S.C., Åkesson A., Bergkvist L., Wolk A. Dietary acrylamide intake and risk of colorectal cancer in a prospective cohort of men. Eur. J. Cancer. 2009;45:513–516. doi: 10.1016/j.ejca.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 45.Schatzkin A., Kipnis V., Carroll R.J., Midthune D., Subar A.F., Bingham S., Schoeller D.A., Troiano R.P., Freedman L.S. A comparison of a food frequency questionnaire with a 24-hour recall for use in an epidemiological cohort study: Results from the biomarker-based Observing Protein and Energy Nutrition (OPEN) study. Int. J. Epidemiol. 2003;32:1054–1062. doi: 10.1093/ije/dyg264. [DOI] [PubMed] [Google Scholar]