Abstract
A metagenomic study was performed on 498 female and 40 male Aedes albopictus mosquitos collected in August and September 2019 in Ticino, a region in southern Switzerland, to address the question regarding the risk of the local transmission of zoonotic viruses. A total of 13 viruses from seven different virus families and several unclassified viral taxa were identified. Reads of insect-specific flaviviruses were present in all pools, and a complete genome of aedes flavivirus was assembled and phylogenetically analysed. The most abundant virus was Wenzhou sobemo-like virus, assembled from 1.3 × 105 to 3.6 × 106 reads in each pool. In a pool of male mosquitos, a complete genome of aedes Iflavi-like virus was detected and phylogenetically analysed. Most importantly, genomes of human pathogenic viruses were not found. This is the first study to determine the virome of Ae. albopictus from Switzerland and forms a baseline for future longitudinal investigations concerning the potential role of Ae. albopictus as a vector of clinically relevant viruses.
Keywords: metagenomic, Aedes albopictus, virome
1. Introduction
The Asian tiger mosquito Aedes albopictus plays an important role as a vector of arboviruses, many of which can severely affect human health, including dengue virus (DENV), Zika virus (ZIKV), yellow fever virus (YFV), and chikungunya virus (CHIKV) [1]. Changing environmental factors and progressing human development can support the establishment of invasive arthropod species, affect the vector competence of endemic species, and enhance viral virulence, thereby potentially contributing to the spread of emerging viral diseases. Ae. albopictus, originally endemic in South-East Asia, has spread over the past approx. 40 years throughout large parts of the Americas, Africa, Australia, and Southern Europe [2]. In Switzerland, Ae. albopictus was spotted for the first time in 2003 in canton Ticino, where since 2007 it has been firmly established; occasionally it is also found in northern parts of Switzerland [3,4,5,6,7]. To date, no autochthonous cases of DENV or CHIKV infections have occurred in Switzerland, while in neighbouring countries, i.e., Italy and France, several cases have been reported. The first transmission of CHIKV by Ae. albopictus outside of a tropical area was reported in 2007 in Italy [8]. Several cases of autochthonous DENV and CHIKV virus infections have been linked to Ae. albopictus as a vector in France as well [9,10]. Hence, Ae. albopictus appears to retain its competence to harbour and transmit zoonotic viruses outside of the tropical regions.
In addition to zoonotic viruses, mosquitos are known to host many different insect-specific viruses (ISVs) which persistently infect mosquitos, but not vertebrates [11], although many ISVs are closely related to human pathogens [12]. Interestingly, coinfection with some ISVs may modulate or even supress replication of specific zoonotic viruses, such as West Nile virus (WNV), in mosquito cells and therefore inhibit their transmission [13,14,15,16]. In addition, coinfection with specific bacteria such as Wolbachia sp. may limit the ability of mosquitos to transmit specific zoonotic viruses [17].
As Ae. albopictus is endemic in Ticino and progressively invades other regions of Switzerland, and because the presence of the vector facilitates the possibility that zoonotic viruses may be locally transmitted to humans, we addressed the question as to whether these mosquitos indeed harbor such viruses. Specifically, using next-generation sequencing and metagenomic analyses, we determined the full, unbiased virus population diversity of Ae. albopictus collected in five municipalities of Lugano in Ticino. A similar study has not previously been performed in Switzerland.
2. Materials and Methods
2.1. Sample Collection
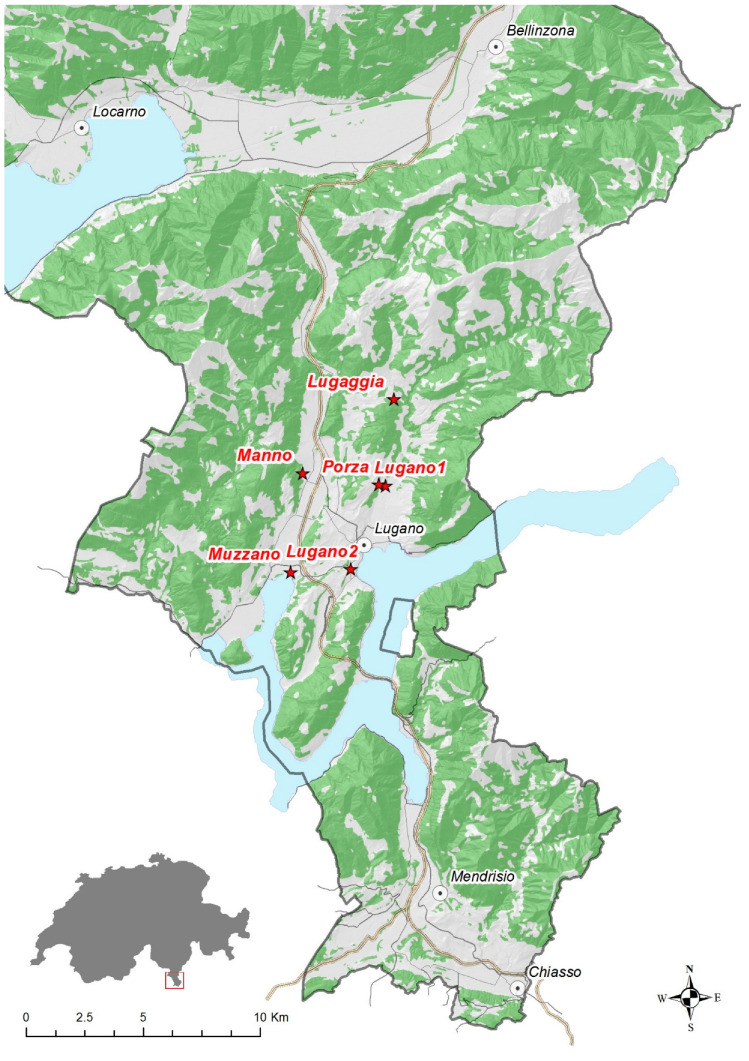
Adult mosquitos were captured in urban and public areas, using electric manual aspirators and entomological nets in August and September 2019 in five municipalities of the Lugano area (Lugaggia, Manno, Porza, Muzzano, and Lugano), canton Ticino, Switzerland (Figure 1). Mosquitos were euthanised by exposure to dry ice [18] and identified to the species level using morphological keys [19,20,21,22,23]. In total, 538 adults (males n = 40; females n = 498) were collected, divided into 15 pools according to collection date, place, and gender (5–59 mosquitos per pool; see Table 1), and stored at −20 °C until further use.
Figure 1.
Locations of mosquito collection. Detailed coordinates and sample descriptions are given in Table 1.
Table 1.
Summary of the mosquito samples used in this study. Male mosquitos were collected at all locations and during the whole sampling period.
| Pool Name | Sample Location | Position | Number of Mosquitos | Gender | Sampling Time Point |
|---|---|---|---|---|---|
| MO1 | Lugaggia | 46.062 N 8.969 E | 5 | female | August |
| MO2 | Manno | 46.034 N 8.918 E | 16 | female | August |
| MO3 | Lugano1 | 46.028 N 8.964 E | 42 | female | August |
| MO4 | Porza | 46.029 N 8.960 E | 21 | female | August |
| MO5 | Lugano1 | 46.028 N 8.964 E | 48 | female | September |
| MO6 | Lugano1 | 34 | female | September | |
| MO7 | Lugano1 | 35 | female | September | |
| MO8 | Lugano1 | 40 | female | September | |
| MO9 | Muzzano | 45.996 N 8.911 E | 40 | female | September |
| MO10 | Muzzano | 40 | female | September | |
| MO11 | Muzzano | 40 | female | September | |
| MO12 | Muzzano | 50 | female | September | |
| MO13 | Lugano2 | 45.997 N 8.944 E | 59 | female | September |
| MO14 | Lugano2 | 28 | female | September | |
| MO15 | Mix | 40 | male | mix |
2.2. Sample Processing and Sequencing
To each pool, 500 µL of phosphate-buffered saline (PBS) (Merck, Darmstadt, Germany) and a stainless-steel bead (5 mm, Qiagen, Hilde, Germany) were added, and samples were mechanically homogenised in a TissueLyser II (Qiagen, Hilde, Germany) at 20 Hz for 2 min. Then, homogenates were centrifuged at 16,060× g for 5 min, and the supernatants were passed through a 0.45 µm syringe filter (Puradisc, 13 mm, Whatman GE Healthcare, Chicago, IL, USA). To enrich nucleic acids that are protected by a virus capsid, 14 µL of micrococcal nuclease buffer, 1 µL of micrococcal nuclease (both New England Biolabs, Ipswich, MA, USA), and 1 µL of ribonuclease A from bovine pancreas (Merck, Darmstadt, Germany) were added to 134 µL of each filtrate from the previous step and incubated for 15 min at 45 °C and 1 h at 37 °C. Total RNA and DNA were extracted using the QIAmp Viral RNA mini kit (Qiagen, Darmstadt, Germany) without RNA carrier. β-mercaptoethanol (Bio-rad, Cressier, Switzerland) was added at a final concentration of 1% in order to inactivate nucleases. RNA was transcribed using 2.5 µM of random primer with a known 20 nucleotide (nt) tag sequence at the 5′ end (SISPA-N: GTTGGAGCTCTGCAGTCATCNNNNNN) and the RevertAid First Strand H minus cDNA Synthesis Kit (Thermo Fisher Scientific, Basel, Switzerland) following the manufacturer’s recommended protocol. Then, 1 µL of RNase H (New England Biolabs, Ipswich, MA, USA) was added to degrade remaining RNA. A premix of 0.8 µM SISPA-N primer, 10× Klenow buffer, and 0.2 mM dNTP was added to 45.5 µL of the first-strand DNA. Following denaturation at 95 °C for 1 min and cooling down on ice, the second strand was synthesized using Klenow polymerase (5 U/20 µL; Thermo Fisher Scientific, Basel, Switzerland) for 15 min at 25 °C followed by 1 h at 37 °C. An additional step of second-strand synthesis using Klenow polymerase was performed at the same conditions, followed by DNA purification using the PureLink® PCR Micro Kit (Invitrogen-ThermoFisher, Waltham, MA, USA). Then, dsDNA was amplified non-specifically by sequence-independent single primer amplification (SISPA). For this, the HotStarTaq DNA polymerase (Qiagen, Darmstadt, Germany) and the SISPA primer (GTT GGA GCT CTG CAG TCA TC) were used under the following conditions: 15 min of activation at 95 °C, 18 cycles of 30 s at 94 °C, 30 s at 58 °C and 1 min at 72 °C, followed by 10 min at 72 °C and cooling down to 4 °C. Finally, the amplified products were purified using the QIAquick PCR purification kit (Qiagen, Darmstadt, Germany). The total DNA was quantified on the Agilent 4200 TapeStation (Santa Clara, CA, USA). Sequencing libraries were made using NEBNext Ultra II DNA library prep kit and NEBNext® Multiplex Oligos for Illumina® (96 Unique Dual Index Primer Pairs) (both New England Biolabs, Ipswich, MA, USA). After quantifying at the TapeStation and pooling at equal molar concentrations, libraries were sequenced using the Illumina NovaSeq system in a single-end 1 × 100 nt run at the Functional Genomics Center Zurich (FGCZ, Zurich, Switzerland).
2.3. Quality Control, Pre-Processing, and Assembly of Metagenomics Reads
Individual metagenomes per sample and the metagenome of all samples combined were assembled and analyzed using the same pipeline. In detail, the technical quality of Illumina single-end (SE) DNA-seq reads was evaluated using FastQC version 0.11.7 (https://www.bioinformatics.babraham.ac.uk/projects/fastqc/). Raw reads were pre-processed using Trimmomatic (version 0.36) to trim off PCR primers, sequencing adaptors, and low-quality ends (average quality lower than 20 within a 4 nucleotide (nt) window) [24]. Quality-controlled reads (average quality 20 and above, read length 40 and above) were assembled using megahit (version 1.1.3) with multiple k-mers of 21, 29, 39, 59, 79, 99, and 119 [25]. To annotate contigs taxonomically, assembled contigs were compared against the NCBI nt database (ftp://ftp.ncbi.nlm.nih.gov/blast/db/) using BLASTN (version 2.6.0+) [26]. Hits were sorted by bit scores. The top hit was defined as the hit with the maximal bit score. Only hits with e value ≤ 1 × 10−5 and bit score ≥ 100 were kept for contig taxonomy annotation. The naïve best-hit method was used to obtain the specific taxa assignment per contig after manual inspection of the alignments. The LCA (lowest common ancestor) algorithm (contig mode) based on multiple BLAST hits was also applied to obtain a more accurate and general taxa assignment [27]. To quantify the abundance of contigs, quality-controlled reads were mapped back to the assembled genomes using BWA-MEM (version 0.7.17) [28]. Mapped reads were quantified using samtools idxstats (version 1.5) [29]. Unmapped reads were extracted again and aligned to an in-house database containing genomes using Bowtie 2 (parameters: -a --very-sensitive --no-mixed --no-discordant -X 1000). Mapped reads and mapped bases per viral genome were calculated using bedtools. Viral genomes with at least five mapped reads were reported using R markdown (http://rmarkdown.rstudio.com/). Additionally, de novo assembled contigs were further investigated in the metagenomic pipeline of SeqMan Ultra software (Lasergene, DNAStar, USA), viral sequences were analyzed with NCBIs ORF finder (https://www.ncbi.nlm.nih.gov/orffinder/), and phylogenetic trees were built in MEGA X [30]. MUSCLE was used for multiple sequence alignment of the amino acid (aa) sequences, and the maximum likelihood method with 1000 bootstraps replicates was employed to build up phylogenetic trees. Statistical analysis was performed in IBM SPSS software version 24 using Fisher’s exact test (p value ≤ 0.05).
The raw sequence reads generated in this study are available at the NCBI sequence read archive (SRA) database under Bioproject accession PRJNA638077. Virus genomes generated have been deposited in GenBank under the accession numbers MT577804, MT577805, and MT591567.
3. Results
In total, 125 million sequencing reads were generated from 15 pools (3.520 × 106 sequencing reads per pool) and applied to reference alignment and de novo analysis to construct contigs. In each mosquito pool, between 1.9% and 36.1% of the total generated sequences were classified as viral reads (Table 2). From a total number of 30,754 contigs, identification of obtained viral contigs using the blastn algorithms resulted in detection of 167 viral contigs belonging to the viral families Flaviviridae, Rhabdoviridae, Iflaviridae, Orthomyxoviridae, Dicistroviridae, Tymoviridae, Genomoviridae, and several unclassified viral taxa. The majority of the detected viral sequences were classified as ISVs. Importantly, no human pathogenic viruses were detected.
Table 2.
Viral reads detected shown as a percentage of the total number of reads generated from each pool of mosquitos.
| Virus | MO1 | MO2 | MO3 | MO4 | MO5 | MO6 | MO7 | MO8 | MO9 | MO10 | MO11 | MO12 | MO13 | MO14 | MO15 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aedes albopictus cell fusing agent virus | 0.003% | 0.0002% | - | - | - | 0.0002% | 0.0003% | 0.0001% | 0.0006% | 0.0001% | 0.0002% | 0.0002% | - | - | - |
| Aedes flavivirus | - | 0.5% | 0.06% | 0.005% | 0.03% | 0.06% | 0.01% | 0.02% | 0.007% | 0.04% | 0.02% | 0.04% | 0.04% | 0.002% | 0.153% |
| Aphid lethal paralysis virus | - | - | - | 0.001% | - | - | - | - | - | - | - | - | - | - | - |
| Arboretum virus | - | - | - | - | - | - | - | 0.002% | - | 0.001% | - | - | - | - | - |
| Culex Iflavi-like virus 4 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 4.6% |
| Fig fleck-associated virus | - | - | - | - | - | - | - | - | - | 0.004% | 0.008% | - | - | - | - |
| Guato virus | - | 0.002% | - | 0.0004% | - | 0.0003% | 0.001% | - | - | 0.001% | 0.002% | 0.0003% | - | - | - |
| Hubei mosquito virus 2 | 1.3% | 0.058% | - | 0.42% | 0.77% | 0.19% | 0.3% | 0.2% | - | 0.037% | - | 0.2% | 1.2% | - | - |
| Kaiowa virus | - | 0.0002% | - | - | - | 0.0002% | - | - | - | - | - | - | - | - | - |
| Lake Sarah-associated circular virus-48 | - | - | - | - | - | - | - | - | - | - | - | 0.002% | - | - | - |
| Plant associated genomovirus 3 | - | - | - | - | - | - | - | - | - | - | - | - | 0.003% | - | - |
| Wenzhou sobemo-like virus 4 | 21.4% | 1.3% | 33.1% | 19.9% | 29.0% | 13.8% | 17.9% | 15.7% | 20.5% | 17.3% | 12.6% | 15.5% | 25.2% | 14.4% | 31.4% |
| Whidbey virus | - | 0.0002% | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Total % of viruses | 22.7% | 1.9% | 33.2% | 20.3% | 29.8% | 14.0% | 18.2% | 16.0% | 20.5% | 17.4% | 12.6% | 15.7% | 26.5% | 14.4% | 36.1% |
| Reads generated in million | 4.2 | 9.4 | 6.7 | 7.5 | 7.1 | 9.9 | 12.2 | 9.5 | 8.1 | 20.6 | 6.7 | 8.9 | 6.8 | 3.5 | 3.6 |
3.1. Mosquito-Associated Viruses
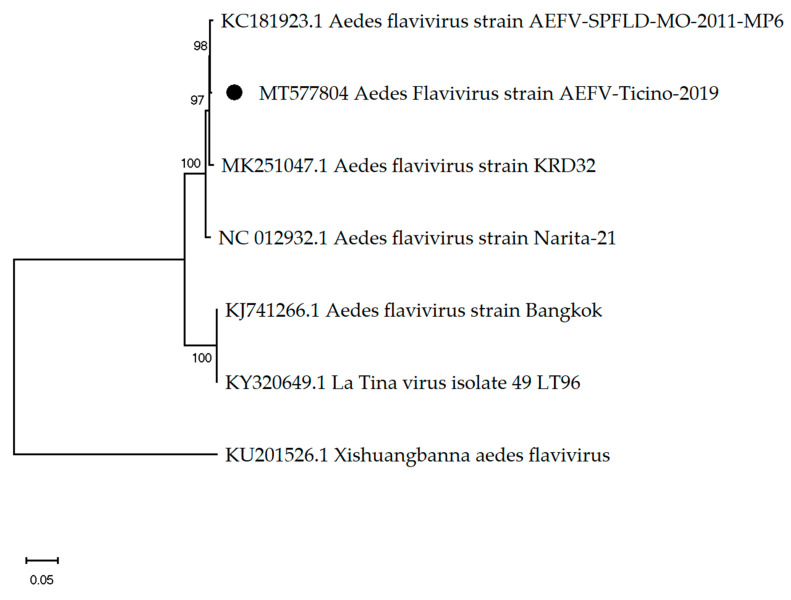
In all 15 pools, viral reads of flavivirus or flavivirus-related virus have been detected. In 14 pools, between 0.0020% and 0.5% (Table 2) of the total generated reads were assembled to Aedes flavivirus (AEFV). In the pool MO2, with 42,862 sequencing reads (0.5% of total reads), the complete AEFV of 11,038 nt in length encoding a polyprotein of 3341 amino acids in length was assembled and showed 99.62% similarity to the Aedes flavivirus strain AEFV-SPFLD-MO-2011-MP6 (GenBank accession: AGJ91136.1) (Figure 2) [31]. Additionally, in nine pools contigs with an identity above 99% to Aedes albopictus cell fusing agent virus (CFAV) have been assembled (GenBank acc: AF411835.2) [32].
Figure 2.
The phylogenetic tree of Aedes flaviviruses constructed using the maximum likelihood method and Kimura 2-parameter model. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) are shown next to the branches. The black dot indicates the genome sequenced in this study.
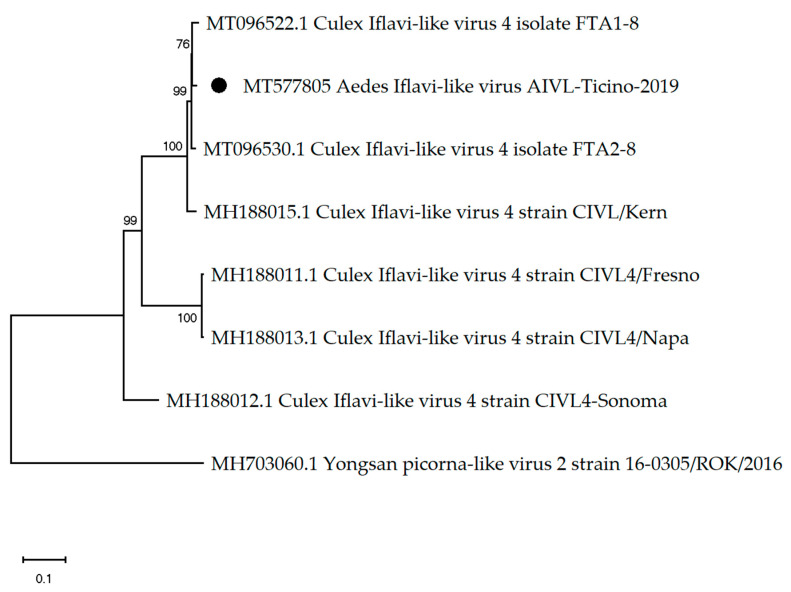
Interestingly, in pool MO15, the only pool with male mosquitos, a contig from the order Picornavirales with 9666 nt in length has been constructed using 165,192 sequencing reads (4.6% of total reads) (Table 2). Further analysis revealed a 9666 nt genome with 97.47% nt similarity to Culex Iflavi-like virus 4 isolate FTA18 (GenBank acc: MT096522.1) [33] (Figure 3), with a polyprotein starting at nt 298 and ending at nt 9639 (9342 nt and 3113 aa in length). The detection of Aedes iflavi-like virus in a pool of male mosquitos only was statistically significant (p value ≤ 0.05).
Figure 3.
The phylogenetic tree of Aedes iflavi-like virus constructed using the maximum likelihood method and Kimura 2-parameter model. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) are shown next to the branches. The black dot indicates the genome sequenced in this study.
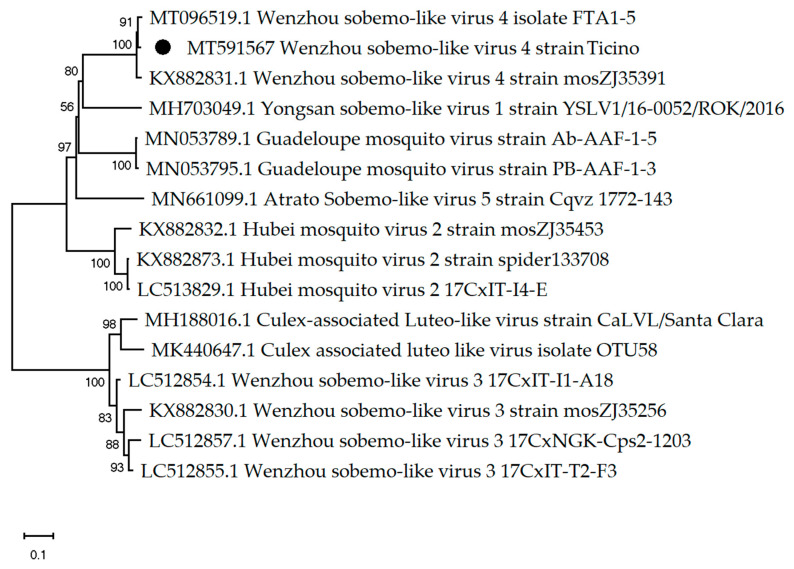
Between 1.3 × 105 and 3.6 × 106 sequenced reads (1.333–0.1% of the total reads) (Table 2) have been assembled to Wenzhou sobemo-like virus, which was the most abundant virus detected in all 15 pools. The metagenomic pipeline of SeqMan Ngen revealed in pool MO1 a genome of 2959 nt length and 98% nt identity to Wenzhou sobemo-like virus isolate FTA 15 (GenBank acc: MT096519.1) [33] (Figure 4). A hypothetical protein 1 of 1770 nt/589 aa in length starts at nt 67 and ends at nt 1836, and a hypothetical protein 2 of 1326 nt/441 aa in length starts at nt 1578 and ends at nt 2903. Additionally, in nine pools, between 0.037% and 1.3% of the total reads generated (Table 2) were assembled to Hubei mosquito virus 2 (GenBank acc: KX882764.1), which has previously been detected in mosquitos collected in China.
Figure 4.
The phylogenetic tree of Wenzhou sobemo-like virus constructed using Table 2. parameter model. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) are shown next to the branches. The black dot indicates the genome sequenced in this study.
Two contigs belonging to Rebdoviridae, particularly Arboretum virus, were detected in pools MO8 and MO10. Contigs of 1064 nt and 1235 nt in length, generated from 144 and 125 sequenced reads, respectively, showed 79.6% nt identity to novel rhabdoviruses isolated from mosquitos in Peru (GenBank acc: KC994644.1) [34]. One contig of 471 nt generated from 17 sequenced reads belonging to Orthomyoviridae has shown 75.4% nt identity to Whidbey virus strain UW1 pb1 gene (GenBank acc: KX898491.1) identified in Aedes dorsalis in Washington. Moreover, in seven pools, sequencing reads of two unclassified viruses were assembled with high nt identity to viruses detected in Brazilian mosquitos. In seven pools, 25,294 reads with 99–100% nt identity to the putative glycoprotein gene of Guato virus (GenBank acc: KT966486.1) have been assembled [35]. In two pools, 23 and 15 reads matched in 99% of the nt to a putative glycoprotein of Kaiowa virus (GenBank acc: KT966481.1 and MF344590.1) [35].
3.2. Other Insect-Associated Viruses
In pool MO4, three contigs with lengths ranging from 307 to 794 nt showed 93.3%–99% nt similarity to members of the family Dicistroviridae, in particular to Aphid lethal paralysis virus isolate ALPV-CE (GenBank acc: MK704471.1), and Aphid lethal paralysis virus isolate ALPV-An (GenBank acc: JX480861.1) identified in Aphis nerii and several other insects species [36]. That was the only pool in which sequences assembled to Aphid lethal paralysis virus have been detected.
3.3. Other Viruses
Two contigs of 526 and 839 nt length showing 81% and 90% nt identity, respectively, to Lake Sarah–associated circular virus-48 were generated in pool MO12. This virus belongs to the CRESS DNA viruses identified from Lake Sarah in New Zealand (GenBank acc: KP153505.1) [37]. Viral reads assembled to a plant virus, specifically Fig fleck-associated virus from the family Tymovirdae, were detected in pools MO10 and MO11 (824 and 518 respectively) (GenBank acc: NC_015229). In pool MO13, 0.003% of the total reads have been assembled to plant associated genomovirus 3, a virus from the Genomoviridae family (GenBank acc: MH939439.1).
3.4. Wolbachia Sp. Detection
Finally, since only a fraction of the reads was found to have a viral origin (1.9% to 36.1%), we also investigated the presence of other infectious agents, Wolbachia in particular. Indeed, between 290 and 14,763 reads were assembled to Wolbachia wAlbB in each pool, thereby confirming that Ae. albopictus is naturally infected with Wolbachia strains.
4. Discussion
It is well known that arthropods constitute a major reservoir for many different viruses and therefore play an important role in virus spread and evolution [38,39]. High species diversity, worldwide distribution, and dense population support viral transmission and increase the risk of emerging and remerging viral diseases. Some mosquitos, including Ae. albopictus and Ae. Aegypti, are vectors for several medically important viruses such as ZIKAV, DENV, CHIKV, and WNV [40,41,42,43,44,45]. RNA viruses are particularly predisposed to causing new emerging diseases due to their inherently high mutation rate, which facilitates adaptation to new hosts [46,47]. Therefore, surveying the virome of invading mosquitos, in particular of species that are known vectors of human viruses, is of utmost importance in order to (i) detect potential spots of disease outbreaks and (ii) understand the role of mosquitos in virus evolution and spread.
In the current study, we used viral metagenomic sequencing to determine the virome of Ae. albopictus collected in Southern Switzerland. Importantly, genomes of medically relevant viruses were not detected. The most abundant virus genomes detected were Wenzhou sobemo-like virus and AEFV. Wenzhou sobemo-like virus has previously been found in mosquitos [48], but plants can also serve as natural host of sobemoviruses [49]. AEFV is an ISFV which replicates in mosquito cells but is unable to replicate in mammalian cells [50,51,52]. While to our knowledge this is the first report of fully sequenced AEFV in Switzerland, the virus has previously been detected in Ae. albopictus collected in Northern Italy, with a prevalence of 77.5% in 2008 and 16.8% in 2012 [53,54].
It would be interesting to investigate whether a high prevalence of ISFVs such as AEFV in Southern Switzerland (this study) and Northern Italy [53,54] correlates with a low prevalence of other medically important viruses. Indeed, cell fusing agent virus (CFAV) and Culex flavivirus, two other members of the ISFVs, are known to inhibit WNV, ZIKV, and DENV [15,55,56]. On the other hand, medically important arthropod-borne flaviviruses may have evolved from ISFVs [57,58].
Not only viruses have been shown to inhibit the replication of other viruses. Wolbachia sp. is an intracellular endosymbiont that reduces the ability of Ae. aegypti to transmit ZIKV and DENV by restricting their replication [59]. Indeed, Wolbachia-infected Ae. aegypti mosquitos have been released into the environment in Australia with the aim to supress the spread of DENV [17]. Wolbachia reads have been detected in all our mosquito pools. This was expected, as previous studies revealed a prevalence of Wolbachia in Ae. albopictus of >95% [60,61,62].
An interesting observation of this study was that Aedes iflavi-like virus genomes were found exclusively in a pool of male mosquitos. The most closely related viruses have been detected in mosquitos collected in Spain and California [33,63]. Considering that most of the studies have been performed with female mosquitos, and that the iflavi-like virus has been found in a pool of female Culex spp. in California, there is no explanation why in the present study iflavi-like virus has been detected only in a pool of male Ae. albopictus. However, the number of mosquitos collected at different locations varies greatly, and the presence of the virus may be location-specific. While it is rather unlikely that only a single male mosquito in the pool hosted the virus when considering the large number of assembled reads, this possibility cannot be excluded. The transmission of ISVs is not fully understood yet, and the transmission occurs mainly vertically from the female to progeny or on the breeding sites [33,64]. Further investigations would be necessary to determine whether this virus is sex- or location-specific.
In conclusion, this study presents a snapshot of the virome of established Swiss populations of Ae. albopictus and sets the basis for future metagenomic analyses to explore the spatial and temporal dynamics of the virus diversity in these mosquitos. This kind of study may also contribute to a better understanding of virus–virus interactions and thereby support novel strategies to prevent arbovirus diseases. The world of the ISFVs and ISVs remains largely unexplored but, if better known, may yield important insights into viral evolution and the role of these viruses in the emergence and transmission of pathogenic viruses.
Acknowledgments
We thank Anicic Nikoleta, Michela Ruinelli, and Damiana Ravasi for helping in mosquitos collecting, comments to manuscript, and helpful discussions.
Author Contributions
Conceptualization, J.K., V.G., and C.F.; methodology, J.K.; software, J.K. and W.Q.; validation, J.K., W.Q. and C.F.; formal analysis, J.K.; investigation, J.K.; resources, M.T. and C.F.; data curation, J.K.; writing—original draft preparation, J.K.; writing—review and editing, J.K., E.F., W.Q., V.G., M.T., and C.F.; visualization, J.K.; supervision, C.F.; project administration, J.K.; funding acquisition, M.T. and C.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by 1. Canton of Zurich, 2. Swiss School of Public Health (SSPH+), 3. Canton Ticino.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- 1.Medlock J.M., Hansford K.M., Schaffner F., Versteirt V., Hendrickx G., Zeller H., Van Bortel W. A review of the invasive mosquitoes in Europe: Ecology, public health risks, and control options. Vector Borne Zoonotic Dis. 2012;12:435–447. doi: 10.1089/vbz.2011.0814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paupy C., Delatte H., Bagny L., Corbel V., Fontenille D. Aedes albopictus, an arbovirus vector: From the darkness to the light. Microbes Infect. 2009;11:1177–1185. doi: 10.1016/j.micinf.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 3.Wymann M.N., Flacio E., Radczuweit S., Patocchi N., Luthy P. Asian tiger mosquito (Aedes albopictus)—A threat for Switzerland? Eurosurveillance. 2008;13:3–4. doi: 10.2807/ese.13.10.08058-en. [DOI] [PubMed] [Google Scholar]
- 4.Flacio E., Engeler L., Tonolla M., Lüthy P., Patocchi N. Strategies of a thirteen year surveillance programme on Aedes albopictus (Stegomyia albopicta) in southern Switzerland. Parasites Vectors. 2015;8:208. doi: 10.1186/s13071-015-0793-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flacio E., Engeler L., Tonolla M., Müller P. Spread and establishment of Aedes albopictus in southern Switzerland between 2003 and 2014: An analysis of oviposition data and weather conditions. Parasites Vectors. 2016;9:304. doi: 10.1186/s13071-016-1577-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suter T.T., Flacio E., Feijoó Fariña B., Engeler L., Tonolla M., Regis L.N., de Melo Santos M.A., Müller P. Surveillance and Control of Aedes albopictus in the Swiss-Italian Border Region: Differences in Egg Densities between Intervention and Non-intervention Areas. PLoS Negl. Trop. Dis. 2016;10:e0004315. doi: 10.1371/journal.pntd.0004315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ravasi D., Guidi V., Flacio E., Lüthy P., Perron K., Lüdin S., Tonolla M. Investigation of temperature conditions in Swiss urban and suburban microhabitats for the overwintering suitability of diapausing Aedes albopictus eggs. Parasites Vectors. 2018;11:212. doi: 10.1186/s13071-018-2803-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sambri V., Cavrini F., Rossini G., Pierro A., Landini M.P. The 2007 epidemic outbreak of Chikungunya virus infection in the Romagna region of Italy: A new perspective for the possible diffusion of tropical diseases in temperate areas? New Microbiol. 2008;31:303–304. [PubMed] [Google Scholar]
- 9.Delisle E., Rousseau C., Broche B., Leparc-Goffart I., L’Ambert G., Cochet A., Prat C., Foulongne V., Ferre J.B., Catelinois O., et al. Chikungunya outbreak in Montpellier, France, September to October 2014. Eurosurveillance. 2015;20:21108. doi: 10.2807/1560-7917.ES2015.20.17.21108. [DOI] [PubMed] [Google Scholar]
- 10.La Ruche G., Souarès Y., Armengaud A., Peloux-Petiot F., Delaunay P., Desprès P., Lenglet A., Jourdain F., Leparc-Goffart I., Charlet F., et al. First two autochthonous dengue virus infections in metropolitan France, September 2010. Eurosurveillance. 2010;15:19676. [PubMed] [Google Scholar]
- 11.Hall R.A., Bielefeldt-Ohmann H., McLean B.J., O’Brien C.A., Colmant A.M., Piyasena T.B., Harrison J.J., Newton N.D., Barnard R.T., Prow N.A., et al. Commensal Viruses of Mosquitoes: Host Restriction, Transmission, and Interaction with Arboviral Pathogens. Evol. Bioinform. Online. 2016;12(Suppl. 2):35–44. doi: 10.4137/EBO.S40740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vasilakis N., Tesh R.B. Insect-specific viruses and their potential impact on arbovirus transmission. Curr. Opin. Virol. 2015;15:69–74. doi: 10.1016/j.coviro.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hall-Mendelin S., McLean B.J., Bielefeldt-Ohmann H., Hobson-Peters J., Hall R.A., van den Hurk A.F. The insect-specific Palm Creek virus modulates West Nile virus infection in and transmission by Australian mosquitoes. Parasites Vectors. 2016;9:414. doi: 10.1186/s13071-016-1683-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goenaga S., Kenney J.L., Duggal N.K., Delorey M., Ebel G.D., Zhang B., Levis S.C., Enria D.A., Brault A.C. Potential for Co-Infection of a Mosquito-Specific Flavivirus, Nhumirim Virus, to Block West Nile Virus Transmission in Mosquitoes. Viruses. 2015;7:5801–5812. doi: 10.3390/v7112911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bolling B.G., Olea-Popelka F.J., Eisen L., Moore C.G., Blair C.D. Transmission dynamics of an insect-specific flavivirus in a naturally infected Culex pipiens laboratory colony and effects of co-infection on vector competence for West Nile virus. Virology. 2012;427:90–97. doi: 10.1016/j.virol.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hobson-Peters J., Yam A.W., Lu J.W., Setoh Y.X., May F.J., Kurucz N., Walsh S., Prow N.A., Davis S.S., Weir R., et al. A new insect-specific flavivirus from northern Australia suppresses replication of West Nile virus and Murray Valley encephalitis virus in co-infected mosquito cells. PLoS ONE. 2013;8:e56534. doi: 10.1371/journal.pone.0056534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoffmann A.A., Montgomery B.L., Popovici J., Iturbe-Ormaetxe I., Johnson P.H., Muzzi F., Greenfield M., Durkan M., Leong Y.S., Dong Y., et al. Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature. 2011;476:454–457. doi: 10.1038/nature10356. [DOI] [PubMed] [Google Scholar]
- 18.Flacio E., Bricalli-Rossi-Pedruzzi A., Bernasconi-Casati E., Patocchi N. Culicidae fauna from Canton Ticino and report of three new species for Switzerland. Mitt. Schweiz. Entomol. Gesell. 2014;87:163–182. [Google Scholar]
- 19.Becker N., Petric D., Zgomba M., Boase C., Madon M.B., Dahl C., Kaiser A. Mosquitoes and Their Control. 2nd ed. Springer; Berlin/Heidelberg, Germany: 2010. p. 577. [Google Scholar]
- 20.Romi R., Pontuale G., Sabatinelli G. Le zanzare italiane: Generalità e identificazione degli stadi preimaginali (Diptera: Culicidae) Fragm. Entomol. 1997;29:1–141. [Google Scholar]
- 21.Schaffner F., Angel G., Geoffroy B., Hervy J.-P., Rhaiem A., Brunhes J. The Mosquitoes of Europe. An Identification and Training Programme. IRD Editions; Montpellier, France: 2001. [Google Scholar]
- 22.Severini F., Toma L., Di Luca M., Romi R. Italian Mosquitoes: General Information and Identification of Adults (Diptera, Culicidae) Fragm. Entomol. 2009;41:213–372. doi: 10.4081/fe.2009.92. [DOI] [Google Scholar]
- 23.Stojanovich C.J., Scott H.G. Llustrated Key to the Adult Male Mosquitoes of America (North of Mexico) P. Stojanovich and H. G. Scott; Madison, WI, USA: 1997. [Google Scholar]
- 24.Bolger A.M., Lohse M., Usadel B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li D., Liu C.M., Luo R., Sadakane K., Lam T.W. MEGAHIT: An ultra-fast single-node solution for large and complex metagenomics assembly via succinct de Bruijn graph. Bioinformatics. 2015;31:1674–1676. doi: 10.1093/bioinformatics/btv033. [DOI] [PubMed] [Google Scholar]
- 26.Altschul S.F., Gish W., Miller W., Myers E.W., Lipman D.J. Basic local alignment search tool. J. Mol. Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 27.Huson D.H., Albrecht B., Bağcı C., Bessarab I., Górska A., Jolic D., Williams R.B.H. MEGAN-LR: New algorithms allow accurate binning and easy interactive exploration of metagenomic long reads and contigs. Biol. Direct. 2018;13:6. doi: 10.1186/s13062-018-0208-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li H., Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li H., Handsaker B., Wysoker A., Fennell T., Ruan J., Homer N., Marth G., Abecasis G., Durbin R. The Sequence Alignment/Map format and SAMtools. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumar S., Stecher G., Li M., Knyaz C., Tamura K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018;35:1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haddow A.D., Guzman H., Popov V.L., Wood T.G., Widen S.G., Haddow A.D., Tesh R.B., Weaver S.C. First isolation of Aedes flavivirus in the Western Hemisphere and evidence of vertical transmission in the mosquito Aedes (Stegomyia) albopictus (Diptera: Culicidae) Virology. 2013;440:134–139. doi: 10.1016/j.virol.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 32.Crochu S., Cook S., Attoui H., Charrel R.N., De Chesse R., Belhouchet M., Lemasson J.J., de Micco P., de Lamballerie X. Sequences of flavivirus-related RNA viruses persist in DNA form integrated in the genome of Aedes spp. mosquitoes. Pt 7J. Gen. Virol. 2004;85:1971–1980. doi: 10.1099/vir.0.79850-0. [DOI] [PubMed] [Google Scholar]
- 33.Birnberg L., Temmam S., Aranda C., Correa-Fiz F., Talavera S., Bigot T., Eloit M., Busquets N. Viromics on Honey-Baited FTA Cards as a New Tool for the Detection of Circulating Viruses in Mosquitoes. Viruses. 2020;12:274. doi: 10.3390/v12030274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vasilakis N., Castro-Llanos F., Widen S.G., Aguilar P.V., Guzman H., Guevara C., Fernandez R., Auguste A.J., Wood T.G., Popov V., et al. Arboretum and Puerto Almendras viruses: Two novel rhabdoviruses isolated from mosquitoes in Peru. Pt 4J. Gen. Virol. 2014;95:787–792. doi: 10.1099/vir.0.058685-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pauvolid-Corrêa A., Solberg O., Couto-Lima D., Nogueira R.M., Langevin S., Komar N. Novel Viruses Isolated from Mosquitoes in Pantanal, Brazil. Genome Announc. 2016;4:e01195-16. doi: 10.1128/genomeA.01195-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dombrovsky A., Luria N. The Nerium oleander aphid Aphis nerii is tolerant to a local isolate of Aphid lethal paralysis virus (ALPV) Virus Genes. 2013;46:354–361. doi: 10.1007/s11262-012-0846-2. [DOI] [PubMed] [Google Scholar]
- 37.Dayaram A., Galatowitsch M.L., Argüello-Astorga G.R., van Bysterveldt K., Kraberger S., Stainton D., Harding J.S., Roumagnac P., Martin D.P., Lefeuvre P., et al. Diverse circular replication-associated protein encoding viruses circulating in invertebrates within a lake ecosystem. Infect. Genet. Evol. 2016;39:304–316. doi: 10.1016/j.meegid.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 38.Shi M., Lin X.D., Tian J.H., Chen L.J., Chen X., Li C.X., Qin X.C., Li J., Cao J.P., Eden J.S., et al. Redefining the invertebrate RNA virosphere. Nature. 2016;540:539–543. doi: 10.1038/nature20167. [DOI] [PubMed] [Google Scholar]
- 39.Li C.X., Shi M., Tian J.H., Lin X.D., Kang Y.J., Chen L.J., Qin X.C., Xu J., Holmes E.C., Zhang Y.Z. Unprecedented genomic diversity of RNA viruses in arthropods reveals the ancestry of negative-sense RNA viruses. eLife. 2015;4:e05378. doi: 10.7554/eLife.05378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Olson J.G., Ksiazek T.G., Suhandiman, Triwibowo Zika virus, a cause of fever in Central Java, Indonesia. Trans. R. Soc. Trop. Med. Hyg. 1981;75:389–393. doi: 10.1016/0035-9203(81)90100-0. [DOI] [PubMed] [Google Scholar]
- 41.Delatte H., Paupy C., Dehecq J.S., Thiria J., Failloux A.B., Fontenille D. Aedes albopictus, vector of chikungunya and dengue viruses in Reunion Island: Biology and control. Parasite. 2008;15:3–13. doi: 10.1051/parasite/2008151003. [DOI] [PubMed] [Google Scholar]
- 42.Delatte H., Gimonneau G., Triboire A., Fontenille D. Influence of temperature on immature development, survival, longevity, fecundity, and gonotrophic cycles of Aedes albopictus, vector of chikungunya and dengue in the Indian Ocean. J. Med. Entomol. 2009;46:33–41. doi: 10.1603/033.046.0105. [DOI] [PubMed] [Google Scholar]
- 43.Chang C., Ortiz K., Ansari A., Gershwin M.E. The Zika outbreak of the 21st century. J. Autoimmun. 2016;68:1–13. doi: 10.1016/j.jaut.2016.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang C.F., Hou J.N., Chen T.H., Chen W.J. Discriminable roles of Aedes aegypti and Aedes albopictus in establishment of dengue outbreaks in Taiwan. Acta Trop. 2014;130:17–23. doi: 10.1016/j.actatropica.2013.10.013. [DOI] [PubMed] [Google Scholar]
- 45.Rezza G. Dengue and chikungunya: Long-distance spread and outbreaks in naïve areas. Pathog. Glob. Health. 2014;108:349–355. doi: 10.1179/2047773214Y.0000000163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Taylor L.H., Latham S.M., Woolhouse M.E. Risk factors for human disease emergence. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2001;356:983–989. doi: 10.1098/rstb.2001.0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Woolhouse M.E., Gowtage-Sequeria S. Host range and emerging and reemerging pathogens. Emerg. Infect. Dis. 2005;11:1842–1847. doi: 10.3201/eid1112.050997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sanborn M.A., Klein T.A., Kim H.C., Fung C.K., Figueroa K.L., Yang Y., Asafo-Adjei E.A., Jarman R.G., Hang J. Metagenomic Analysis Reveals Three Novel and Prevalent Mosquito Viruses from a Single Pool of Aedes vexans nipponii Collected in the Republic of Korea. Viruses. 2019;11:222. doi: 10.3390/v11030222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sõmera M., Sarmiento C., Truve E. Overview on Sobemoviruses and a Proposal for the Creation of the Family Sobemoviridae. Viruses. 2015;7:3076–3115. doi: 10.3390/v7062761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hoshino K., Isawa H., Tsuda Y., Sawabe K., Kobayashi M. Isolation and characterization of a new insect flavivirus from Aedes albopictus and Aedes flavopictus mosquitoes in Japan. Virology. 2009;391:119–129. doi: 10.1016/j.virol.2009.06.025. [DOI] [PubMed] [Google Scholar]
- 51.Cook S., Moureau G., Kitchen A., Gould E.A., de Lamballerie X., Holmes E.C., Harbach R.E. Molecular evolution of the insect-specific flaviviruses. Pt 2J. Gen. Virol. 2012;93:223–234. doi: 10.1099/vir.0.036525-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Calzolari M., Zé-Zé L., Vázquez A., Sánchez Seco M.P., Amaro F., Dottori M. Insect-specific flaviviruses, a worldwide widespread group of viruses only detected in insects. Infect. Genet. Evol. 2016;40:381–388. doi: 10.1016/j.meegid.2015.07.032. [DOI] [PubMed] [Google Scholar]
- 53.Roiz D., Vázquez A., Rosso F., Arnoldi D., Girardi M., Cuevas L., Perez-Pastrana E., Sánchez-Seco M.P., Tenorio A., Rizzoli A. Detection of a new insect flavivirus and isolation of Aedes flavivirus in Northern Italy. Parasites Vectors. 2012;5:223. doi: 10.1186/1756-3305-5-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grisenti M., Vázquez A., Herrero L., Cuevas L., Perez-Pastrana E., Arnoldi D., Rosà R., Capelli G., Tenorio A., Sánchez-Seco M.P., et al. Wide detection of Aedes flavivirus in north-eastern Italy—A European hotspot of emerging mosquito-borne diseases. Pt 2J. Gen. Virol. 2015;96:420–430. doi: 10.1099/vir.0.069625-0. [DOI] [PubMed] [Google Scholar]
- 55.Schultz M.J., Frydman H.M., Connor J.H. Dual Insect specific virus infection limits Arbovirus replication in Aedes mosquito cells. Virology. 2018;518:406–413. doi: 10.1016/j.virol.2018.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Baidaliuk A., Miot E.F., Lequime S., Moltini-Conclois I., Delaigue F., Dabo S., Dickson L.B., Aubry F., Merkling S.H., Cao-Lormeau V.M., et al. Cell-Fusing Agent Virus Reduces Arbovirus Dissemination in Aedes aegypti Mosquitoes In Vivo. J. Virol. 2019;93:e00705-19. doi: 10.1128/JVI.00705-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cammisa-Parks H., Cisar L.A., Kane A., Stollar V. The complete nucleotide sequence of cell fusing agent (CFA): Homology between the nonstructural proteins encoded by CFA and the nonstructural proteins encoded by arthropod-borne flaviviruses. Virology. 1992;189:511–524. doi: 10.1016/0042-6822(92)90575-A. [DOI] [PubMed] [Google Scholar]
- 58.Kuno G., Chang G.J., Tsuchiya K.R., Karabatsos N., Cropp C.B. Phylogeny of the genus Flavivirus. J. Virol. 1998;72:73–83. doi: 10.1128/JVI.72.1.73-83.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chouin-Carneiro T., Ant T.H., Herd C., Louis F., Failloux A.B., Sinkins S.P. Wolbachia strain wAlbA blocks Zika virus transmission in Aedes aegypti. Med. Vet. Entomol. 2020;34:116–119. doi: 10.1111/mve.12384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ahantarig A., Trinachartvanit W., Kittayapong P. Relative Wolbachia density of field-collected Aedes albopictus mosquitoes in Thailand. J. Vector Ecol. 2008;33:173–177. doi: 10.3376/1081-1710(2008)33[173:RWDOFA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 61.Dutton T.J., Sinkins S.P. Strain-specific quantification of Wolbachia density in Aedes albopictus and effects of larval rearing conditions. Insect Mol. Biol. 2004;13:317–322. doi: 10.1111/j.0962-1075.2004.00490.x. [DOI] [PubMed] [Google Scholar]
- 62.Kittayapong P., Baisley K.J., Sharpe R.G., Baimai V., O’Neill S.L. Maternal transmission efficiency of Wolbachia superinfections in Aedes albopictus populations in Thailand. Am. J. Trop. Med. Hyg. 2002;66:103–107. doi: 10.4269/ajtmh.2002.66.103. [DOI] [PubMed] [Google Scholar]
- 63.Sadeghi M., Altan E., Deng X., Barker C.M., Fang Y., Coffey L.L., Delwart E. Virome of >12 thousand Culex mosquitoes from throughout California. Virology. 2018;523:74–88. doi: 10.1016/j.virol.2018.07.029. [DOI] [PubMed] [Google Scholar]
- 64.Saiyasombat R., Bolling B.G., Brault A.C., Bartholomay L.C., Blitvich B.J. Evidence of efficient transovarial transmission of Culex flavivirus by Culex pipiens (Diptera: Culicidae) J. Med. Entomol. 2011;48:1031–1038. doi: 10.1603/ME11043. [DOI] [PubMed] [Google Scholar]