Abstract
IMPORTANCE
The rabies virus causes a fatal encephalitis and can be transmitted through tissue or organ transplantation. In February 2013, a kidney recipient with no reported exposures to potentially rabid animals died from rabies 18 months after transplantation.
OBJECTIVES
To investigate whether organ transplantation was the source of rabies virus exposure in the kidney recipient, and to evaluate for and prevent rabies in other transplant recipients from the same donor.
DESIGN
Organ donor and all transplant recipient medical records were reviewed. Laboratory tests to detect rabies virus–specific binding antibodies, rabies virus neutralizing antibodies, and rabies virus antigens were conducted on available specimens, including serum, cerebrospinal fluid, and tissues from the donor and the recipients. Viral ribonucleic acid was extracted from tissues and amplified for nucleoprotein gene sequencing for phylogenetic comparisons.
MAIN OUTCOMES AND MEASURES
Determination of whether the donor died from undiagnosed rabies and whether other organ recipients developed rabies.
RESULTS
In retrospect, the donor’s clinical presentation (which began with vomiting and upper extremity paresthesias and progressed to fever, seizures, dysphagia, autonomic dysfunction, and brain death) was consistent with rabies. Rabies virus antigen was detected in archived autopsy brain tissue collected from the donor. The rabies viruses infecting the donor and the deceased kidney recipient were consistent with the raccoon rabies virus variant and were more than 99.9% identical across the entire N gene (1349/1350 nucleotides), thus confirming organ transplantation as the route of transmission. The 3 other organ recipients remained asymptomatic, with rabies virus neutralizing antibodies detected in their serum after completion of postexposure prophylaxis (range, 0.3–40.8 IU/mL).
CONCLUSIONS AND RELEVANCE
Unlike the 2 previous clusters of rabies virus transmission through solid organ transplantation, there was a long incubation period in the recipient who developed rabies, and survival of 3 other recipients without pretransplant rabies vaccination. Rabies should be considered in patients with acute progressive encephalitis of unexplained etiology, especially for potential organ donors. A standard evaluation of potential donors who meet screening criteria for infectious encephalitis should be considered, and risks and benefits for recipients of organs from these donors should be evaluated.
Rabies is a fatal, acute progressive encephalitis caused by neurotropic zoonotic viruses belonging to the genus Lyssavirus.1 Unique rabies virus variants, distinguishable by molecular typing methods, are associated with specific animal reservoirs. Globally, an estimated 55 000 persons die of rabies every year, with most transmission attributable to dog bites.2 Approximately 2 human rabies deaths are reported in the United States every year, and during 2000 through 2010, all but 2 domestically acquired cases were associated with bats.3–5 Despite raccoons being the most frequently reported rabid animal in the United States, only 1 human rabies case associated with the raccoon rabies virus variant has been reported.3,5
Rabies virus transmission has occurred through tissue and solid organ transplantation.6–8 In the 2 previously recognized clusters of rabies virus transmission through organ transplantation, which were attributed to a bat and a canine rabies virus variant, all recipients except 1 who was previously vaccinated had rabies symptom onset within 6 weeks of transplantation and died.6,7 These observations suggest a high infectivity rate and an incubation period of approximately 6 weeks in unvaccinated immunosuppressed recipients of solid organs from donors with rabies.
In February 2013, a patient died of rabies 18 months after receiving a deceased-donor kidney transplant. The Centers for Disease Control and Prevention (CDC) and state and local health departments conducted an investigation to determine whether rabies virus was transmitted through transplantation and to prevent rabies in other recipients and contacts.
Case Report (Deceased Kidney Recipient)
In February 2013, a man who received a deceased-donor kidney transplant in September 2011 presented to an emergency department (ED) complaining of right hip pain with radiation to the lower extremity (Figure 1). He was diagnosed with sciatica and discharged but was admitted 4 days later with fever, diaphoresis, nausea, right lower extremity weakness, and right lower abdominal pain near the site of his transplanted kidney. His symptoms progressed to bilateral lower extremity weakness with ascending paresthesias. He developed encephalopathy, excessive salivation, and hemodynamic instability and died 22 days after admission.
Figure 1.
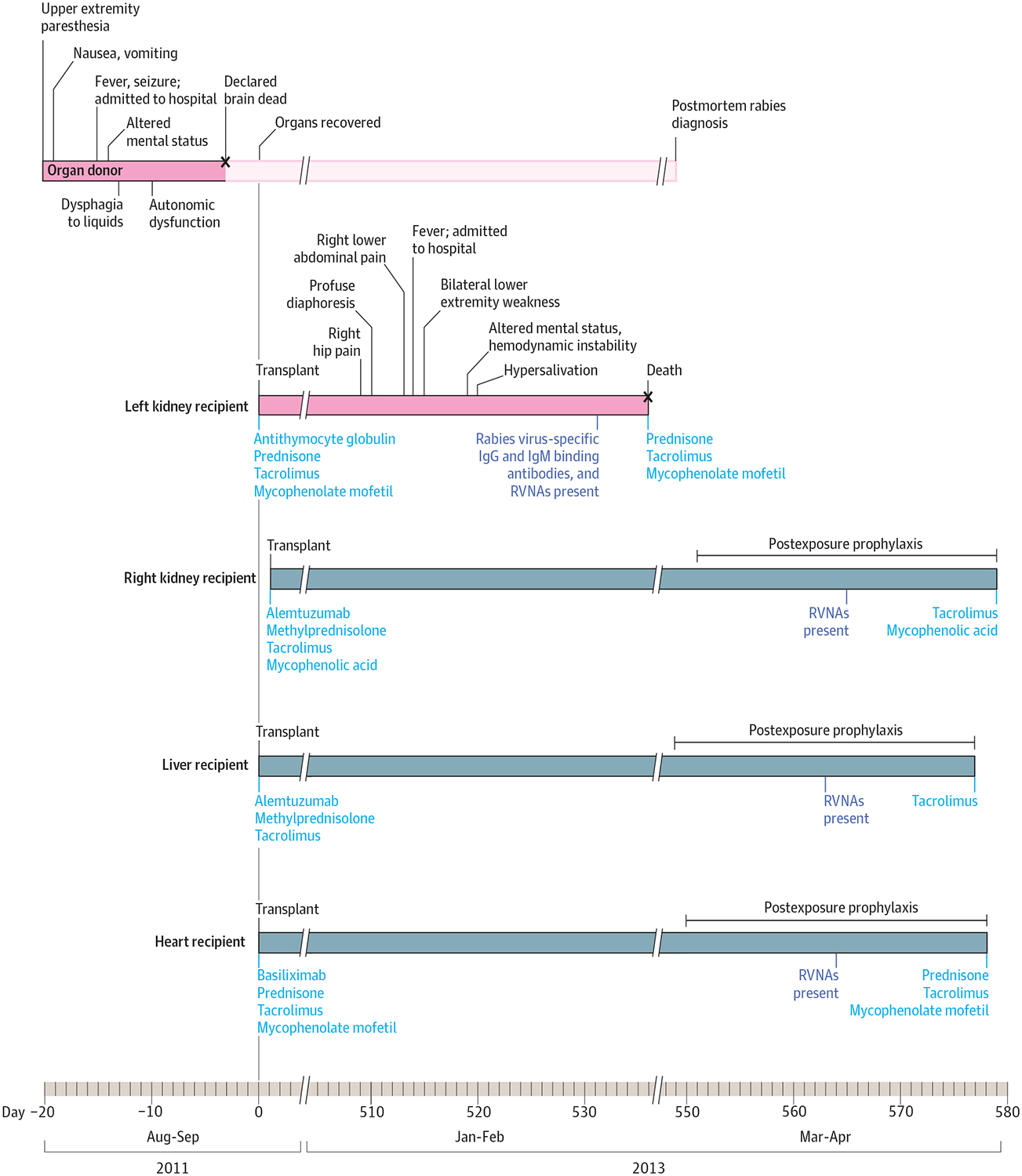
Clinical Course of the Transplant Recipients Who Received Solid Organs From a Donor With Rabies
RVNAs indicates rabies virus neutralizing antibodies.
Cerebrospinal fluid (CSF) analysis performed 5 days before death revealed a pleocytosis (white blood cells, 622/μL). Tests for human immunodeficiency virus (HIV), human T-cell lymphotropic virus types 1 and 2, cytomegalovirus, JC virus, BK virus, enteroviruses, varicella-zostervirus, herpes simplex virus, West Nile virus, and Cryptococcus had negative results. Epstein-Barr virus nucleic acid was detected in serum. Magnetic resonance imaging (MRI) revealed diffuse signal abnormality throughout the brain and spinal cord. Rabies virus–specific binding IgG (≥1:128 dilution) and IgM (1:8 dilution) antibodies and rabies virus neutralizing antibodies (RVNAs) (0.4 IU/mL) were present in serum collected 5 days before death. The recipient was not previously vaccinated for rabies, and family members denied exposures to potentially rabid animals. Because of his history, kidney transplantation was considered as a possible but unlikely source of transmission.
Methods
This activity was reviewed according to CDC National Center for Emerging and Zoonotic Infectious Diseases institutional procedures. It was deemed to not constitute human subjects research and was therefore not subject to institutional review board requirements.
Clinical and Epidemiologic Review
To determine whether the deceased kidney recipient acquired rabies virus infection through transplantation and to identify other potentially infected recipients from the same donor, medical records of the donor and recipients were reviewed. Interviews with family members of the deceased kidney recipient and donor were conducted.
Laboratory Specimen Collection and Testing
Laboratory testing was conducted at CDC. Antemortem rabies testing of the deceased kidney recipient was performed on serum, CSF, nuchal skin biopsy, and saliva specimens. After the kidney recipient’s death, additional tests were conducted on urine and transplanted kidney biopsy specimens collected during hospitalization and on tissues collected during autopsy. Donor specimens were obtained from storage, including serum and oral cavity biopsy tissue collected during unrelated dental surgery and tissues collected during autopsy. Specimens from the other recipients were also tested.
Serum and CSF were analyzed for rabies virus–specific binding IgG and IgM antibodies using the indirect fluorescent antibody test and for RVNAs using the rapid fluorescent focus inhibition test.9,10 The direct fluorescent antibody test was used to detect rabies virus antigen in nuchal skin biopsy.9,10 Tissue specimens were examined using hematoxylin-eosin stains or immunohistochemical (IHC) stains with mouse or rabbit hyperimmune rabies virus antiserums.5,7,11,12 Ribonucleic acid was extracted and amplified from saliva, urine, and tissues by heminested reverse transcriptase–polymerase chain reaction (RT-PCR) targeting the rabies virus nucleoprotein (N) gene.10 Polymerase chain reaction products of the expected molecular weight were sequenced. Phylogenetic analysis was performed (Molecular Evolutionary Genetics Analysis version 5.0; Biodesign Institute) by comparing these sequences to those available in GenBank and to those of rabies viruses circulating within raccoon populations in the eastern United States that were newly sequenced for this investigation.10,13
Results
Organ Donor
In August 2011, a previously healthy man presented to a primary care clinic after returning from a fishing trip with nausea, vomiting, and upper extremity paresthesias on each of 4 consecutive days. He was transported to an ED where he was febrile and had peripheral leukocytosis (white blood cells, 24 800/μL), hyponatremia (sodium, 128 mEq/L), and hypokalemia (potassium, 2.0 mEq/L). Shortly after arrival to the ED, he had a seizure and was admitted. While hospitalized, he had dysphagia to liquids and altered mental status requiring intubation. He experienced autonomic dysfunction with hypothermia and hemodynamic instability. The patient was declared brain dead 17 days after symptom onset with a presumed diagnosis of ciguatera poisoning.
Cerebrospinal fluid analysis performed during the initial ED evaluation revealed a pleocytosis (white blood cells, 9/μL). Tests for HIV, cytomegalovirus, varicella-zoster virus, herpes simplex virus, and Cryptococcus had negative results, but Epstein-Barr virus–specific serum IgG antibodies were detected. No abnormalities were noted on brain MRI. Organ donor eligibility screening was conducted; the questionnaire administered to family members included an item assessing exposure to potentially rabid animals or receipt of rabies postexposure prophylaxis (PEP) due to suspected exposure within the previous 6 months. No increased risk for infectious disease transmission was identified, and kidneys, heart, and liver were transplanted into 4 recipients. No vessels or tissues were transplanted.
Independent of organ procurement, an autopsy was performed, and tests for various arboviruses, enteroviruses, coronaviruses, adenoviruses, influenza viruses, parainfluenza viruses, and human metapneumovirus had negative results. The brain was soft and friable with blurring of the gray-white matter junction reflecting gross and histologic changes consistent with prolonged ventilator-induced effect. At the time of autopsy, histopathologic features to suggest a definitive diagnosis were not observed on examination of brain tissue. As part of the autopsy-related investigation, local water temperature evaluation determined the presence of ciguatoxin to be extremely unlikely. The cause of death was ultimately attributed to complications of severe gastroenteritis.
Although the organ procurement organization screening questionnaire queried exposure to potentially rabid animals in the last 6 months, subsequent interviews with family members conducted during the epidemiologic investigation revealed that the donor had significant wildlife exposure, such as hunting and trapping animals in North Carolina. Activities specific to raccoons included trapping and keeping them in captivity, using them as live bait during dog training exercises, and preparing pelts for display. Through these activities, the donor sustained at least 2 raccoon bites, 18 and 7 months prior to symptom onset, for which he did not seek medical care. The captive raccoon responsible for the latter bite was healthy up to 4 weeks after the bite. Neither raccoon was available for testing.
Other Transplant Recipients
The 3 other recipients (right kidney, heart, and liver) did not have signs or symptoms consistent with rabies or encephalitis. All received PEP with rabies immune globulin and 5 doses of rabies vaccine, and remain asymptomatic.14,15 Immunosuppressive regimens are summarized in Figure 1. The heart recipient experienced mild graft rejection. Other posttransplantation complications included BK virus–associated nephropathy in the asymptomatic kidney recipient and herpes zoster in the liver recipient.
Laboratory Findings
For the deceased kidney recipient, rabies virus–specific binding IgG and IgM antibodies and RVNAs were detected in serum collected during his illness but were not found in CSF (Table). Diffuse and extensive encephalomyelitis and rare intracytoplasmic inclusions typical of rabies were observed in central nervous system (CNS) tissues following hematoxylin-eosin staining (Figure 2). Abundant rabies virus antigens were also detected by IHC staining in examined CNS tissues but not the transplanted kidney. Rabies virus RNA was detected in saliva, nuchal skin biopsy, and postmortem CNS tissues but not the transplanted kidney.
Table.
Laboratory Findings of Recipients of Solid Organs From a Donor With Rabies in September 2011
| Patient | Specimen | Date of Collection | Rabies Virus-Specific Binding IgG and IgM Antibodies (IFA)a | Rabies Virus Neutralizing Antibodies (RFFIT)b | Rabies Virus Nucleic Acid (RT-PCR) | Rabies Virus Antigen (IHC) |
|---|---|---|---|---|---|---|
| Donor | Serum | 12/2010 | Not detected | Not detected | ||
| Serum | 5/2011 | Not detected | Not detected | |||
| Oral cavity biopsy | 7/2011 | Not detected | Not detected | |||
| Serum | 19 d ARSO | IgG ≥1:128, IgM ≥1:32 | 7.2 IU/mLc | |||
| Brain | Autopsy | Detected | Detected | |||
| Gastrointestinal tract | Autopsy | Detected | ||||
| Lungs, thyroid, spleen, pancreas | Autopsy | Not detected | ||||
| Deceased left kidney recipient | Transplanted kidney biopsy | 7 d ARSO | Not detected | |||
| Serum | 22 d ARSO | IgG ≥1:128, IgM 1:8 | 0.4 IU/mLc | |||
| CSF | 22 d ARSO | Not detected | Not detected | |||
| Saliva | 22 d ARSO | Detected | ||||
| Nuchal skin biopsy | 22 d ARSO | Detected | ||||
| Brain | Autopsy | Detected | Detected | |||
| Spinal cord | Autopsy | Detected | ||||
| Heart, lungs, thyroid, liver | Autopsy | Not detected | ||||
| Transplanted kidney | Autopsy | Not detected | Not detected | |||
| Native kidneys | Autopsy | Not detected | Not detected | |||
| Asymptomatic right kidney recipient | Thyroid, parathyroid, thymus, lymph nodes | 11/2011 | Not detected | |||
| Transplanted kidney biopsies | 12/2011, 9/2012 | Not detected | Not detected | |||
| Urine | 3/2013 | Not detected | ||||
| Serum, PEP day 0 | 3/2013 | Not detected | Not detected | |||
| Serum, PEP day 14 | 3/2013 | 0.4 IU/mLc | ||||
| Serum, PEP day 28 | 4/2013 | 0.3 IU/mLc | ||||
| Serum, PEP day 39 | 4/2013 | 0.3 IU/mLc | ||||
| Asymptomatic heart recipient | Transplanted heart biopsies | 9/2011–3/2013 | Not detected | Not detected | ||
| Serum, PEP day 0 | 3/2013 | Not detected | Not detected | |||
| Serum, PEP day 14 | 3/2013 | 0.4 IU/mLc | ||||
| Serum, PEP day 49 | 5/2013 | 0.3 IU/mLc | ||||
| Asymptomatic liver recipient | Serum, PEP day 0 | 3/2013 | Not detected | Not detected | ||
| Serum, PEP day 14 | 3/2013 | 0.5 IU/mLc | ||||
| Serum, PEP day 28 | 4/2013 | 2.2 IU/mLc | ||||
| Serum, PEP day 42 | 4/2013 | 40.8 IU/mLc |
Abbreviations: ARSO, after rabies symptom onset; CSF, cerebrospinal fluid; IFA, indirect fluorescent antibody test; IHC, immunohistochemical test; PEP, rabies postexposure prophylaxis; RFFIT, rapid fluorescent focus inhibition test; RT-PCR, reverse transcriptase–polymerase chain reaction.
Specimens were incrementally diluted (ranging from 1:1 through 1:128) to screen for rabies virus–specific binding IgG and IgM antibodies. If a positive reaction did not occur at the dilutions tested, the result is listed as “not detected.”
Specimens were incrementally diluted to determine end-point titers against a known rabies virus dose, with a 50% reduction of virus at a dilution of 1:5 considered the lower limit of detection. End-point titers were converted to IU/mL by comparison with a known dilution of US Standard Rabies Immune Globulin.
Complete rabies virus neutralization was demonstrated at a serum dilution of 1:5.
Figure 2.
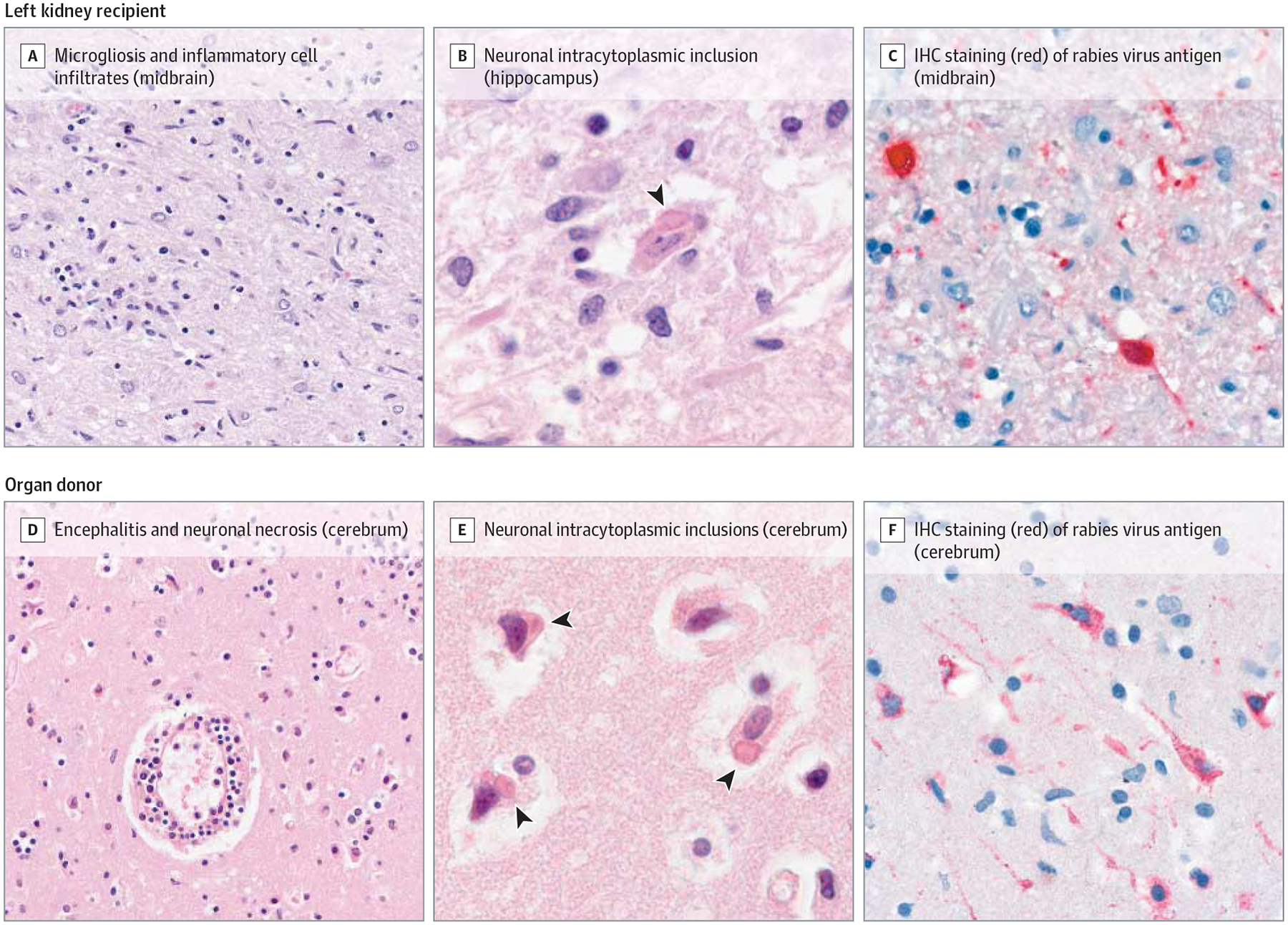
Histopathologic Features of Rabies Encephalitis in the Deceased Kidney Recipient and Organ Donor
A, Midbrain of the deceased kidney recipient showing diffuse microgliosis and mixed inflammatory cell infiltrates (hematoxylin-eosin, original magnification ×50). B, Intracytoplasmic inclusion typical of rabies in a neuron in the hippocampus of the deceased kidney recipient (hematoxylin-eosin, original magnification ×158). C, Rabies virus–infected neurons in the midbrain of the deceased kidney recipient (immunohistochemical [IHC] staining; immunoalkaline phosphatase stain, naphthol-fast red substrate [chromogen] with hematoxylin counterstain, original magnification ×100). D, Encephalitis in the donor characterized by perivascular lymphocytic cuffing, microglial proliferation, and neuronal necrosis (hematoxylin-eosin, original magnification ×50). E, Intracytoplasmic inclusions typical of rabies in cerebral neurons of the donor (hematoxylin-eosin, original magnification ×100). F, Widespread IHC staining of rabies virus antigen in cerebral neurons of the donor (immunoalkaline phosphatase stain, naphthol-fast red substrate [chromogen] with hematoxylin counterstain, original magnification ×100).
For the organ donor, rabies virus–specific binding IgG and IgM antibodies and RVNAs were retrospectively detected in serum collected during his illness (Table). Nonsuppurative encephalitis and widespread neuronal necrosis were observed in archived CNS tissues after hematoxylin-eosin staining (Figure 2). Numerous intracytoplasmic inclusions typical of rabies were evident within cortical neurons and Purkinje cells. Rabies virus antigen was detected by IHC staining in all examined brain sections and in gastric and colonic ganglia. Rabies virus RNA was detected in postmortem CNS tissues.
Sequence analysis of PCR products generated from the deceased kidney recipient and donor were more than 99.9% identical across the entire N gene (1349/1350 nucleotides) and were consistent with the raccoon rabies virus variant. Given the known variability of the N gene between different rabies virus variants (up to 20%) and between distinct raccoon rabies virus variant clades (3%), phylogenetic analysis was able to identify independent raccoon rabies foci with a geographic resolution to the county level. The deceased kidney recipient and donor rabies virus N gene sequences formed a separate clade (Figure 3) and matched each other closer than any other raccoon rabies virus variant N gene, confirming organ transplantation as the route of transmission.13
Figure 3.
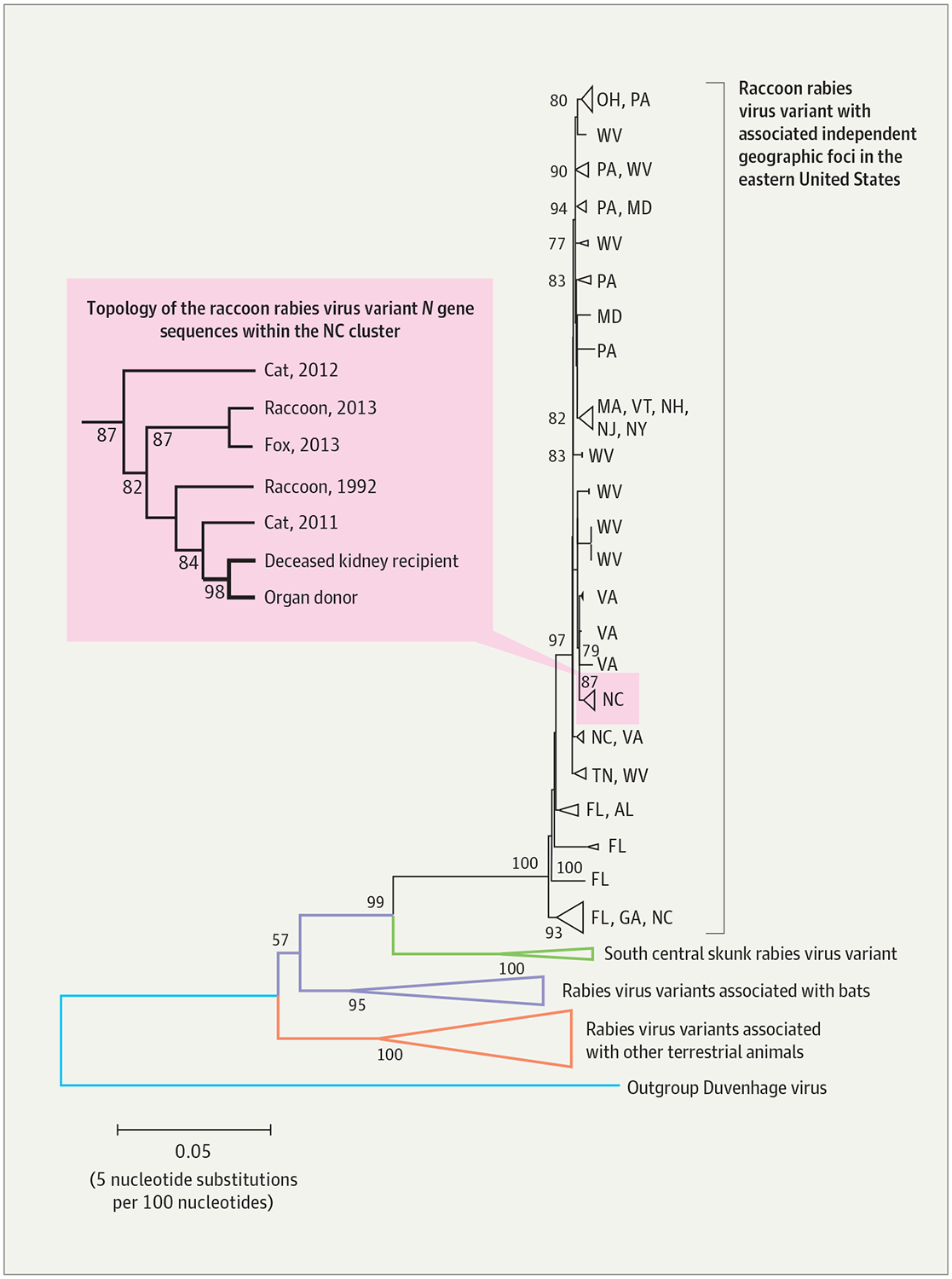
Phylogenetic Reconstruction of the Rabies Viruses Infecting the Deceased Kidney Recipient and Donor
The N gene sequences of the rabies viruses infecting the deceased kidney recipient and donor are shown in relation to N gene sequences of common rabies virus variants circulating in the United States. The phylogenetic reconstruction was generated with the neighbor-joining method under the maximum composite likelihood model to estimate nucleotide substitutions. The bootstrap method with 1000 iterations was used to assess the confidence of the branching pattern (numbers at the nodes). Branches are color coded according to reservoir host, with triangles representing collapsed branches with a common origin. The Duvenhage virus was used as an outgroup to better visualize the evolutionary relationships among rabies virus clades. Independent geographic foci associated with the raccoon rabies virus variant circulating in the eastern United States, in black, are shown along with the corresponding state (2-letter abbreviations). Enclosed in the rectangle on the left is a magnified projection showing the topology of the collapsed North Carolina cluster, which includes sequences obtained from various rabid animals, the deceased kidney recipient, and the donor.
For the 3 asymptomatic recipients, rabies virus–specific binding IgG and IgM antibodies and RVNAs were not detected in serum collected immediately prior to PEP initiation. However, titers of RVNAs suggesting an appropriate immune response were detected in all 3 recipients after PEP completion (Table).14,15 Rabies virus antigen and RNA were not detected in routine postoperative transplanted organ biopsy specimens or in specimens obtained during an unrelated post-transplant procedure.
Discussion
This is the third reported transmission event of rabies virus through solid organ transplantation and has a number of unique aspects. To our knowledge, this is the first report in which unvaccinated recipients of solid organs from a donor with rabies did not all develop disease. The symptomatic recipient’s incubation period is the longest documented in a transplant recipient who had not received prior rabies vaccination.6–8 In contrast, previous reports have noted universal transmission and fatality among unvaccinated solid organ recipients.6,7
The deceased kidney recipient and donor described here were infected by the raccoon rabies virus variant. This variant has been reported to infect only 1 other person previously.5 This transmission event provides an opportunity for enhancing rabies awareness and recognition and highlights the need for a modified approach to organ donor screening and recipient monitoring for infectious encephalitis. This investigation also underscores the importance of collaboration between clinicians, epidemiologists, and laboratory scientists. Identification of the geographic origins of the rabies viruses of the deceased kidney recipient and donor through a phylogenetic approach complemented the information obtained during epidemiologic investigations and was critical for confirming the role of transplantation in disease transmission.
Since the 1970s, the raccoon rabies virus variant has spread across the eastern United States,16,17 which is concerning as raccoons increasingly inhabit urbanized areas.18 Nevertheless, recognized human infections with the raccoon rabies virus variant are rare compared with those associated with bat rabies virus variants.4,5 This may be because bites from raccoons are usually more apparent compared with smaller bites such as those from bats.19 Persons may therefore be more likely to present for medical care and receive PEP after raccoon bites than bat bites, although the lack of systematically collected data on animal bites or PEP usage makes this hypothesis difficult to verify.14,19 The donor described reportedly experienced raccoon bites but had not sought medical care and therefore did not receive PEP. Administration of PEP is efficacious against rabies, and persons who come in contact with raccoons should be evaluated for PEP.14,15 Based on his frequent contact with potentially rabid animals, the donor may have also been a candidate for rabies pre-exposure prophylaxis.14,15
Approximately one-third of domestic human rabies cases are diagnosed postmortem.9,20 More than 1000 persons die annually of unexplained encephalitis in the United States.21 Because of the rarity and lack of clinical and pathologic recognition of rabies, the disease may go undiagnosed.4,5 Diagnosis is further complicated by the variable presentation of rabies.1,4,20 Initial signs and symptoms are sometimes non-specific, and clinicians may not elicit an exposure history if they do not inquire about animal contacts and travel history.22,23 This report emphasizes the need for increased awareness among clinicians and suggests that rabies should be considered in patients with unexplained, acute progressive encephalitis.20,22 Timely diagnosis is essential for prompt initiation of experimental treatment protocols, as there are rare reports of rabies survivors.24,25 Recognition of rabies is also important to identify persons who may need PEP.14,15
The deceased kidney recipient had an unexpectedly long incubation period, and the 3 other solid organ recipients were unvaccinated but remained asymptomatic for an 18-month period between transplantation and administration of PEP, in contrast to prior reports of rabies virus transmission through transplantation.6,7 The typical rabies virus incubation period is believed to be shortened among recipients of organs or tissues from infected donors, especially when recipients are immunosuppressed.4,6,7,9 For example, the longest recorded incubation period to our knowledge in an unvaccinated recipient after a cornea transplant, a procedure that does not typically require immunosuppression, is 39 days.8 The deceased kidney recipient’s incubation period was approximately 13 times longer than this despite both immunosuppression and solid organ transplantation. It is unclear whether the donor had a similarly lengthy incubation period, as the precise exposure responsible for his infection is unknown. Notably, the incubation in the only other reported human case of raccoon rabies virus variant infection is also uncertain.5 The causes of the prolonged incubation period of the deceased kidney recipient are unclear but could include exposure to a low dose of virus or distinct characteristics of raccoon rabies virus variant pathogenesis in humans.26,27
Rabies virus–specific binding antibodies and RVNAs were not detected in the serum of the 3 asymptomatic recipients prior to PEP initiation. All subsequently developed RVNAs after PEP was initiated.28–30 The asymptomatic recipients could have been within the incubation period and might have developed clinical infection had they not received PEP. These observations raise questions about mechanisms of rabies virus spread within a host, the cells and tissues in which the virus resides during lengthy incubation periods, and how the virus evades the immune system.31 Differences in the immunosuppressive induction and maintenance regimens among the recipients could have also affected disease progression if these regimens resulted in varying degrees of immunosuppression. The effect of immunosuppressive medications, along with inoculum dose, type of transplanted organ, and host characteristics on rabies pathogenesis, is presently unclear.32,33
In addition to rabies, since 2002 there have been 11 other reported instances in the United States in which infectious encephalitis was transmitted through solid organ transplantation, including West Nile virus, lymphocytic choriomeningitis virus, and Balamuthia mandrillaris.7,34–39 Infectious encephalitis can have nonspecific clinical features easily mistaken for other disease conditions, particularly in patients with multiple comorbidities such as organ transplant recipients.40 Even if transplant-associated encephalitis is suspected, establishing the diagnosis in donors or recipients requires that appropriate specimens be collected, which may not be possible if autopsies have not been performed or archived tissue specimens are unavailable. Specimen testing for the diagnosis of rare pathogens must often be conducted by reference laboratories. Furthermore, organs recovered from a common donor are often distributed to multiple, geographically dispersed transplant centers. Hence, encephalitis that develops in one recipient may not be recognizable as transplant-associated without knowledge of the clinical status of other recipients. To facilitate the identification of transplant-associated infections, transplant centers are required to report potential donor-derived infections to the Organ Procurement and Transplantation Network.
Currently, the rarity of rabies and time required to transfer samples and perform adequate laboratory diagnostics for rabies makes universal screening of all organ donors impractical. However, given the lethality of transplant-associated encephalitis, implementation of a standardized approach for recognizing infectious encephalitis among organ donors is warranted. Prompt recognition of infectious encephalitis allows for the initiation of prophylaxis, treatment, and appropriate management of recipients. Presently, there is no standardized case definition of infectious encephalitis among organ donors, although expert opinion for recognizing CNS infections in potential organ donors is available.41 If infectious encephalitis is suspected by clinicians treating a potential organ donor, organ procurement organizations are tasked with appropriately communicating this information to transplant centers. In the present case, the questionnaire routinely administered by the organ procurement organization to family members included screening for potential rabies virus exposures but did not identify risk. Adoption of a uniform donor questionnaire that more effectively elicits risk factors may improve the screening of donors with encephalitis, but is unlikely to be sufficiently sensitive or specific compared with laboratory testing.
Efforts to develop a standardized case definition for infectious encephalitis and to identify clinical criteria that could be applied during the predonation screening process are necessary. Such organs should be used only in extreme circumstances with careful risk-benefit assessment. When criteria are met to suggest that a donor could have infectious encephalitis, a standardized approach to specimen collection, storage, and appropriate laboratory testing could improve probability of recognition. These might include performing a donor autopsy and archiving of CNS tissue and CSF in addition to the current practice of archiving serum specimens. Additionally, efforts might include identifying a panel of transplant-transmissible infections for which testing is recommended when a donor meets criteria for infectious encephalitis.
Because of organ shortages and poor prognosis of patients on transplant waiting lists, the clinical decision to proceed with transplant must be made quickly. Although test results may not be available prior to transplantation, prompt notification to transplant centers of postmortem test results could improve management among recipients. Currently, recipients must provide special informed consent when an organ donor is deemed to be at increased risk for HIV, hepatitis B, or hepatitis C infection.42 In addition to a thorough risk-benefit assessment, special informed consent for receipt of an organ from a donor meeting infectious encephalitis criteria should be considered at time of organ offer. The risk of adverse events faced by recipients of organs from donors who could have infectious encephalitis is unknown. Hence, there is a need to better understand the prevalence of infectious encephalitis among donors.
In summary, rabies in the setting of solid organ transplantation can be transmitted variably and may have a long incubation period. Although recognition of rabies is challenging and solid organ transplant transmission of infectious encephalitis is rare, further education to increase awareness is needed. Concerted efforts to improve screening of donors with suspected encephalitis, to carefully consider risks and benefits of transplanting organs from these donors, and to better monitor transplant recipients for rapid recognition of infection may improve patient management and prevent further transmission.
Funding/Support:
This investigation was supported by the Centers for Disease Control and Prevention (CDC), Maryland Department of Health and Mental Hygiene, North Carolina Division of Public Health, and Florida Department of Health and funded as part of routine infectious disease outbreak investigation activities.
Role of Sponsor: The CDC, Maryland Department of Health and Mental Hygiene, North Carolina Division of Public Health and Florida Department of Health employees had a role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Glossary
- CSF
cerebrospinal fluid
- IHC
immunohistochemical
- PEP
postexposure prophylaxis
- RT-PCR
reverse transcriptase–polymerase chain reaction
- RVNAs
rabies virus neutralizing antibodies
Footnotes
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest and none were reported.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC, Department of Army/Navy/Air Force, Department of Defense, or US government.
REFERENCES
- 1.Hemachudha T, Laothamatas J, Rupprecht CE. Human rabies: a disease of complex neuropathogenetic mechanisms and diagnostic challenges. Lancet Neurol. 2002;1(2):101–109. [DOI] [PubMed] [Google Scholar]
- 2.Knobel DL, Cleaveland S, Coleman PG, et al. Re-evaluating the burden of rabies in Africa and Asia. Bull World Health Organ. 2005;83(5): 360–368. [PMC free article] [PubMed] [Google Scholar]
- 3.Blanton JD, Dyer J, McBrayer J, Rupprecht CE. Rabies surveillance in the United States during 2011. J Am Vet Med Assoc. 2012;241(6):712–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petersen B, Rupprecht C. Chapter 11: Human rabies epidemiology and diagnosis In: Tkachev S, ed. Non-Flavivirus Encephalitis. http://www.intechopen.com/books/non-flavivirus-encephalitis. Accessed July 8, 2013. [Google Scholar]
- 5.Centers for Disease Control and Prevention (CDC). First human death associated with raccoon rabies: Virginia, 2003. MMWR Morb Mortal Wkly Rep. 2003;52(45):1102–1103. [PubMed] [Google Scholar]
- 6.Maier T, Schwarting A, Mauer D, et al. Management and outcomes after multiple corneal and solid organ transplantations from a donor infected with rabies virus. Clin Infect Dis. 2010;50(8):1112–1119. [DOI] [PubMed] [Google Scholar]
- 7.Srinivasan A, Burton EC, Kuehnert MJ, et al. ; Rabies in Transplant Recipients Investigation Team. Transmission of rabies virus from an organ donor to four transplant recipients. N Engl J Med. 2005;352(11):1103–1111. [DOI] [PubMed] [Google Scholar]
- 8.Vetter JM, Frisch L, Drosten C, et al. Survival after transplantation of corneas from a rabies-infected donor. Cornea. 2011;30(2):241–244. [DOI] [PubMed] [Google Scholar]
- 9.Noah DL, Drenzek CL, Smith JS, et al. Epidemiology of human rabies in the United States, 1980 to 1996. Ann Intern Med. 1998;128(11): 922–930. [DOI] [PubMed] [Google Scholar]
- 10.Warner CK, Zaki SR, Shieh WJ, et al. Laboratory investigation of human deaths from vampire bat rabies in Peru. Am J Trop Med Hyg. 1999;60(3): 502–507. [DOI] [PubMed] [Google Scholar]
- 11.Hamir AN, Moser G, Fu ZF, Dietzschold B, Rupprecht CE. Immunohistochemical test for rabies: identification of a diagnostically superior monoclonal antibody. Vet Rec. 1995;136(12): 295–296. [DOI] [PubMed] [Google Scholar]
- 12.Hamir AN, Moser G, Wampler T, Hattel A, Dietzschold B, Rupprecht CE. Use of a single anti-nucleocapsid monoclonal antibody to detect rabies antigen in formalin-fixed, paraffin-embedded tissues. Vet Rec. 1996;138(5):114–115. [DOI] [PubMed] [Google Scholar]
- 13.Standard Nucleotide BLAST. National Library of Medicine. http://www.ncbi.nlm.nih.gov/BLAST/Blast.cgi?PROGRAM=blastn&BLAST_PROGRAMS=megaBlast&PAGE_TYPE=BlastSearch&SHOW_DEFAULTS=on&LINK_LOC=blasthome. Accessed July 8, 2013.
- 14.Manning SE, Rupprecht CE, Fishbein D, et al. ; Advisory Committee on Immunization Practices Centers for Disease Control and Prevention (CDC). Human rabies prevention: United States, 2008: recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep. 2008;57(RR-3):1–28. [PubMed] [Google Scholar]
- 15.Rupprecht CE, Briggs D, Brown CM, et al. ; Centers for Disease Control and Prevention (CDC). Use of a reduced (4-dose) vaccine schedule for postexposure prophylaxis to prevent human rabies: recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep. 2010;59(RR-2):1–9. [PubMed] [Google Scholar]
- 16.Slate D, Algeo TP, Nelson KM, et al. Oral rabies vaccination in North America: opportunities, complexities, and challenges. PLoS Negl Trop Dis. 2009;3(12):e549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sattler AC, Krogwold RA, Wittum TE, et al. Influence of oral rabies vaccine bait density on rabies seroprevalence in wild raccoons. Vaccine. 2009;27(51):7187–7193. [DOI] [PubMed] [Google Scholar]
- 18.Slate D, Rupprecht CE, Rooney JA, Donovan D, Lein DH, Chipman RB. Status of oral rabies vaccination in wild carnivores in the United States. Virus Res. 2005;111(1):68–76. [DOI] [PubMed] [Google Scholar]
- 19.Gibbons RV. Cryptogenic rabies, bats, and the question of aerosol transmission. Ann Emerg Med. 2002;39(5):528–536. [DOI] [PubMed] [Google Scholar]
- 20.Burton EC, Burns DK, Opatowsky MJ, et al. Rabies encephalomyelitis: clinical, neuroradiological, and pathological findings in 4 transplant recipients. Arch Neurol. 2005;62(6): 873–882. [DOI] [PubMed] [Google Scholar]
- 21.Khetsuriani N, Holman RC, Lamonte-Fowlkes AC, Selik RM, Anderson LJ. Trends in encephalitis-associated deaths in the United States. Epidemiol Infect. 2007;135(4):583–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention (CDC). Human rabies: Indiana and California, 2006. MMWR Morb Mortal Wkly Rep. 2007;56(15): 361–365. [PubMed] [Google Scholar]
- 23.Tunkel AR, Glaser CA, Bloch KC, et al. ; Infectious Diseases Society of America. The management of encephalitis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2008;47(3):303–327. [DOI] [PubMed] [Google Scholar]
- 24.Willoughby RE Jr, Tieves KS, Hoffman GM, et al. Survival after treatment of rabies with induction of coma. N Engl J Med. 2005;352(24):2508–2514. [DOI] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention (CDC). Presumptive abortive human rabies: Texas, 2009. MMWR Morb Mortal Wkly Rep. 2010;59(7): 185–190. [PubMed] [Google Scholar]
- 26.Niezgoda M, Briggs DJ, Shaddock J, Rupprecht CE. Viral excretion in domestic ferrets (Mustela putorius furo) inoculated with a raccoon rabies isolate. Am J Vet Res. 1998;59(12):1629–1632. [PubMed] [Google Scholar]
- 27.Liao PH, Hsu YH, Yang HH, Wang MH, Chen LK. Involvement of extraneural tissues and upregulation of inducible nitric oxide synthase after experimental infection with rabies virus in BALB/c mice and LEW/SsN rats. Pathol Int. 2012;62(9): 619–627. [DOI] [PubMed] [Google Scholar]
- 28.Cramer CH II, Shieck V, Thomas SE, Kershaw DB, Magee JC, Lopez MJ. Immune response to rabies vaccination in pediatric transplant patients. Pediatr Transplant. 2008;12(8):874–877. [DOI] [PubMed] [Google Scholar]
- 29.Rodríguez-Romo R, Morales-Buenrostro LE, Lecuona L, et al. Immune response after rabies vaccine in a kidney transplant recipient. Transpl Infect Dis. 2011;13(5):492–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eckerle I, Rosenberger KD, Zwahlen M, Junghanss T. Serologic vaccination response after solid organ transplantation: a systematic review. PLoS One. 2013;8(2):e56974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morimoto K, Patel M, Corisdeo S, et al. Characterization of a unique variant of bat rabies virus responsible for newly emerging human cases in North America. Proc Natl Acad Sci U S A. 1996;93(11):5653–5658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaplan MM, Wiktor TJ, Koprowski H. Pathogenesis of rabies in immunodeficient mice. J Immunol. 1975;114(6):1761–1765. [PubMed] [Google Scholar]
- 33.Enright JB, Franti CE, Frye FL, Behymer DE. The effects of corticosteroids on rabies in mice. Can J Microbiol. 1970;16(8):667–675. [DOI] [PubMed] [Google Scholar]
- 34.Iwamoto M, Jernigan DB, Guasch A, et al. ; West Nile Virus in Transplant Recipients Investigation Team. Transmission of West Nile virus from an organ donor to four transplant recipients. N Engl J Med. 2003;348(22):2196–2203. [DOI] [PubMed] [Google Scholar]
- 35.Centers for Disease Control and Prevention (CDC). Brief report: lymphocytic choriomeningitis virus transmitted through solid organ transplantation: Massachusetts, 2008. MMWR Morb Mortal Wkly Rep. 2008;57(29):799–801. [PubMed] [Google Scholar]
- 36.Centers for Disease Control and Prevention (CDC). Balamuthia mandrillaris transmitted through organ transplantation: Mississippi, 2009. MMWR Morb Mortal Wkly Rep. 2010;59(36):1165–1170. [PubMed] [Google Scholar]
- 37.Centers for Disease Control and Prevention (CDC). Notes from the field: transplant-transmitted Balamuthia mandrillaris: Arizona, 2010. MMWR Morb Mortal Wkly Rep. 2010;59(36):1182. [PubMed] [Google Scholar]
- 38.Fischer SA, Graham MB, Kuehnert MJ, et al. ; LCMV in Transplant Recipients Investigation Team. Transmission of lymphocytic choriomeningitis virus by organ transplantation. N Engl J Med. 2006;354(21):2235–2249. [DOI] [PubMed] [Google Scholar]
- 39.Nett RJ, Kuehnert MJ, Ison MG, Orlowski JP, Fischer M, Staples JE. Current practices and evaluation of screening solid organ donors for West Nile virus. Transpl Infect Dis. 2012;14(3):268–277. [DOI] [PubMed] [Google Scholar]
- 40.Glaser C, Bloch KC. Encephalitis: why we need to keep pushing the envelope. Clin Infect Dis. 2009;49(12):1848–1850. [DOI] [PubMed] [Google Scholar]
- 41.Guidance for recognizing central nervous system infections in potential deceased organ donors: what to consider during donor evaluation and organ offers. Organ Procurement and Transplantation Network http://optn.transplant.hrsa.gov/ContentDocuments/Guidance_DTAC_CNS_Infections_07-2012.pdf. Accessed July 8, 2013.
- 42.Seem DL, Lee I, Umscheid CA, Kuehnert MJ. PHS guideline for reducing human immunodeficiency virus, hepatitis B virus, and hepatitis C virus infection transmitted through organ transplantation. Public Health Rep. 2013;128(4):247–343. [DOI] [PMC free article] [PubMed] [Google Scholar]


