Abstract
Background:
Several ongoing phase 2 trials are evaluating new neoadjuvant therapy regimens in patients with muscle-invasive bladder cancer (MIBC). The 1-yr recurrence-free survival (RFS) after radical cystectomy (RC), with or without perioperative chemotherapy, can be used to model statistical assumptions and interpret outcomes from these studies.
Objective:
To provide a benchmark for predicting 1-yr RFS in patients with cT2–4N0 MIBC.
Design, setting, and participants:
We identified 950 patients with clinical stage T2–4N0 MIBC undergoing RC at 27 centers between 1990 and 2016. We assessed 1-yr RFS rates for patients managed with no perioperative chemotherapy, neoadjuvant chemotherapy (NAC), adjuvant chemotherapy (AC), or NAC followed by AC. Cox regression analyses tested for 1-yr postsurgical RFS predictors. A Cox-based nomogram was developed to estimate 1-yr RFS and its accuracy was assessed in terms of Harrell’s c-index, a calibration plot, and decision curve analysis. We report 1-yr RFS rates across the nomogram tertiles.
Results and limitations:
The 1-yr RFS rates were 67.9% (95% confidence interval [CI] 64–72) after no perioperative chemotherapy, 76.9% (95% CI 72–83%) after NAC, 77.8% (95% CI 71–85%) after AC, and 57% (95% CI 37–87) after NAC + AC. On multivariable analysis, positive surgical margins (p = 0.002), pT stage (p < 0.0001), and pN stage (p<.0001) were significantly associated with RFS, while NAC was not (p = 0.6). The model including all these factors yielded a c-index of 0.76 (95% CI 0.72–0.79), good calibration, and a high net benefit. The 1-yr RFS rates across nomogram tertiles were 90.5% (95% CI 87–94%), 73.4% (95% CI 68–79%), and 51.1% (95% CI 45–58%), respectively. The results lack external validation.
Conclusions:
Benchmark 1-yr RFS estimates for phase 2 design of new neoadjuvant trials are proposed and can be used for statistical assumptions, pending external validation.
Patient summary:
Our prognostic model predicting 1-yr survival free from recurrence of bladder cancer after radical cystectomy, with or without standard chemotherapy, could provide an improvement to the quality of phase 2 clinical trial designs and interpretation of their results.
Keywords: Urothelial carcinoma, Bladder cancer, Perioperative chemotherapy, Nomogram, Relapse-free survival
1. Introduction
Over the last three decades, perioperative cisplatin-based chemotherapy, added to radical cystectomy (RC), represented the recommended therapeutic option for patients with muscle-invasive urothelial bladder cancer (MIBC) owing to the possibility of better relapse-free survival (RFS) and overall survival (OS) compared to surgery alone [1–5]. Nevertheless, patients treated with platinum-based neoadjuvant therapies and RC exhibit suboptimal disease control. The small benefit perceived by the urologic oncology community for standard chemotherapy has been responsible for low rates of chemotherapy administration worldwide [6–8]. Meanwhile, the proven efficacy of immune checkpoint inhibitors in patients with locally advanced or metastatic urothelial carcinoma [9] has supported the evaluation of these drugs in the perioperative setting, alone or in combination with platinum-based chemotherapy. To date, several ongoing phase 2 trials are testing neoadjuvant immunotherapy agents in MIBC patients who are candidates for RC. The same situation applies to the use of targeted agents in patients with molecularly selected tumors, in particular for agents targeting the FGFR pathway.
Results from these studies are largely pending. However, there are several uncertainties surrounding the activity of neoadjuvant therapy with checkpoint inhibitors. Primarily, identification of novel agent activity may be impaired (or inflated) by the use of pathologic response as the primary endpoint for such trials. In fact, the proportion of patients who might achieve a complete response after transurethral resection of the bladder (TURB) alone may vary substantially, and this issue has remained widely unaddressed in the literature. Despite the availability of multiple postcystectomy nomograms to predict OS in patients according to pathological findings, very limited information is available regarding RFS prediction in the short term (ie, 1 yr). Formulating such data with a specific focus on patients with cT2–4N0M0 disease may help in the design of single-arm phase 2 trials with novel agents and in comparison of findings across studies [10–12]. If available, a prognostic model predicting 1-yr RFS after RC, with or without standard chemotherapy, could aid in improving the quality of these study designs and interpretation of the results. To address this issue, we analyzed a large multi-institutional patient population.
2. Patients and methods
2.1. Study design
The study was approved by the institutional review boards of the 27 participating institutions. The analysis population included the cystectomy database of the Urological Research Institute (URI) of San Raffaele Hospital in Milan (n = 1067), and the Retrospective International Study of Invasive/Advanced Cancer of the Urothelium (RISC) database (n = 3024). This contemporary database includes data gathered between 1990 and 2016 from hospitals in the USA, Europe, Israel, and Canada. Criteria for patient selection included: pure or predominant urothelial carcinoma (UC) histology, cT2–4N0M0 stage, and radical cystectomy treatment. For patients who received chemotherapy, administration of at least two cycles of any chemotherapy course in either the neoadjuvant or adjuvant setting was required. Administration of any new drug, either alone or combined with chemotherapy, was an exclusion criterion. Concomitant or sequential delivery of radiotherapy was also an exclusion criterion. The study flow chart, with patient numbers and reasons for patient exclusions, is provided in Supplementary Figure 1.
2.2. Study outcomes
Descriptive statistics included the frequency and proportion for categorical variables, and the median and interquartile range (IQR) for continuous variables. The statistical significance of differences in medians and proportions was determined using Kruskal-Wallis and χ2 tests, respectively. The primary endpoint of the analyses was 1-yr RFS. Recurrence after RC was defined as any radiologic evidence of pelvic recurrences or distant metastases (cases with newly diagnosed upper-tract tumors were excluded). The Kaplan-Meier method was used to estimate RFS and OS, both defined as the period of survival from RC.
2.3. Statistical analyses
The analyses consisted of five steps. First, we assessed 1-yr RFS rates in the overall population, as well as according to the use of perioperative chemotherapy: no perioperative chemotherapy (n = 545) versus neoadjuvant chemotherapy (NAC; n = 242) versus adjuvant chemotherapy (AC; n = 146) versus NAC followed by AC (n = 17). Second, univariable and multivariable Cox regression models [13] were applied to test for predictors of recurrence and predictors of death from any cause after RC. Third, to reject the hypothesis of an immortal time bias, we used a 3-mo landmark analysis and refitted the multivariable Cox regression analysis testing for death from any cause. Fourth, we developed a Cox-based nomogram for prediction of 1-yr RFS, including variables that were significantly associated with RFS after RC on multivariable analysis; we decided a priori to include NAC given its univariable significance and to more finely estimate the outcomes according to precystectomy chemotherapy administration. The performance of the model was assessed in terms of discrimination (Harrell’s c-index) and calibration (calibration plots). Furthermore, decision curve analysis (DCA) [14] was applied to assess the net benefit related to nomogram use, and validation (2000 bootstrap resamples) was internally tested. Finally, we examined 1-yr RFS rates across nomogram-derived tertiles. All statistical tests were two-sided, with the level of significance set at p < 0.05. Analyses were performed using the R software environment for statistical computing and graphics (R Foundation for Statistical Computing, Vienna, Austria).
3. Results
3.1. Study population
Between 1990 and 2016, 950 patients with cT2–4N0M0 MIBC were suitable for the study purposes (Table 1). The median age at diagnosis was 68 yr (IQR 60–74). Most patients were Caucasian (89.5%), former (36.9%) or current smokers (22.6%), and had a Charlson comorbidity index of ≥1 (59.7%). Overall, 567 patients (59.7%) harbored cT2N0 MIBC, while 223 (23.5%) had cT3–4N0 tumors. NAC was administered in 259 patients (27.3%). Cisplatin-based combination chemotherapy was the regimen most commonly administered (82.6%). Pathological examination revealed that 350 (36.8%), 294 (30.9%), and 278 patients (29.3%) harbored ≤pT2N0, pT3–4N0, and pTanyN+ MIBC, respectively. In addition, 28 patients (3%) had pT0–4NX disease. After RC, AC was administered in 163 patients (17.2%), with cisplatin-based regimens accounting for 50.9% of this group.
Table 1 –
General characteristics of the study patients
| Characteristic | Overall | No NAC or AC | NAC | AC | NAC + AC |
|---|---|---|---|---|---|
| Median age at surgery, yr (range) | 68 (60.2–74) | 71 (63–77) | 64 (57–70) | 65 (56–70) | 62 (55–68) |
| Median LNs removed, n (range) | 15 (9–22) | 15 (9–22) | 16 (10–22) | 16 (10–24) | 20.5 (14–26.5) |
| Race, n (%) | |||||
| Caucasian | 850 (89.5) | 479 (87.9) | 229 (94.6) | 128 (87.7) | 14 (82.4) |
| Hispanic or Latino | 69 (7.3) | 51 (9.4) | 1 (0.4) | 16 (11) | 1 (5.9) |
| Black | 22 (2.3) | 9 (1.7) | 9 (3.7) | 2 (1.4) | 2 (11.8) |
| Other or mixed | 9 (0.9) | 6 (1.1) | 3 (1.2) | 0 (0) | 0 (0) |
| Smoking status, n (%) | |||||
| Never smoker | 192 (20.2) | 111 (20.4) | 55 (22.7) | 22 (15.1) | 4 (23.5) |
| Current smoker | 215 (22.6) | 110 (20.2) | 62 (25.6) | 39 (26.7) | 4 (23.5) |
| Former smoker | 351 (36.9) | 179 (32.8) | 108 (44.6) | 57 (39) | 7 (41.2) |
| Unknown | 192 (20.2) | 145 (26.6) | 17 (7) | 28 (19.2) | 2 (11.8) |
| CCI, n (%) | |||||
| 0 | 288 (30.3) | 133 (24.4) | 100 (41.3) | 47 (32.2) | 8 (47.1) |
| ≥1 | 567 (59.7) | 377 (69.2) | 89 (36.8) | 92 (63) | 9 (52.9) |
| Unknown | 95 (10) | 35 (6.4) | 53 (21.9) | 7 (4.8) | 0 (0) |
| Histology, n (%) | |||||
| Pure UC | 826 (86.9) | 474 (87) | 206 (85.1) | 129 (88.4) | 17 (100) |
| UC with divergent histology | 124 (13.1) | 71 (13) | 36 (14.9) | 17 (11.6) | 0 (0) |
| cT stage, n (%) | |||||
| cT2 | 567 (59.7) | 319 (58.5) | 145 (59.9) | 91 (62.3) | 12 (70.6) |
| cT3–4 | 223 (23.5) | 100 (18.3) | 91 (37.6) | 29 (19.9) | 3 (17.6) |
| Unknown | 160 (16.8) | 126 (23.1) | 6 (2.5) | 26 (17.8) | 2 (11.8) |
| pT stage, n (%) | |||||
| pT0 | 96 (10.1) | 17 (3.1) | 79 (32.6) | 0 (0) | 0 (0) |
| pT1 | 63 (6.6) | 43 (7.9) | 16 (6.6) | 3 (2.1) | 1 (5.9) |
| pT2 | 189 (19.9) | 121 (22.2) | 42 (17.4) | 21 (14.4) | 5 (29.4) |
| pT3 | 389 (40.9) | 232 (42.6) | 65 (26.9) | 84 (57.5) | 8 (47.1) |
| pT4 | 154 (16.2) | 94 (17.2) | 20 (8.3) | 37 (25.3) | 3 (17.6) |
| pTa/pTis | 59 (6.2) | 38 (7) | 20 (8.3) | 1 (0.7) | 0 (0) |
| pN stage, n (%) | |||||
| pN0 | 644 (67.8) | 379 (69.5) | 197 (81.4) | 61 (41.8) | 7 (41.2) |
| pN1 | 102 (10.7) | 50 (9.2) | 20 (8.3) | 31 (21.2) | 1 (5.9) |
| pN2 | 160 (16.8) | 89 (16.3) | 21 (8.7) | 43 (29.5) | 7 (41.2) |
| pN3 | 16 (1.7) | 7 (1.3) | 1 (0.4) | 6 (4.1) | 2 (11.8) |
| pNX | 28 (2.9) | 20 (3.7) | 3 (1.2) | 5 (3.4) | 0 (0) |
| Margin status, n (%) | |||||
| Negative | 850 (89.5) | 490 (89.9) | 216 (89.3) | 132 (90.4) | 12 (70.6) |
| Positive | 84 (8.8) | 48 (8.8) | 20 (8.3) | 12 (8.2) | 4 (23.5) |
| NAC, n (%) | |||||
| No | 691 (72.7) | 545 (100) | 0 (0) | 146 (100) | 0 (0) |
| Yes | 259 (27.3) | – | 242 (100) | – | 17 (100) |
| NAC platinum regimen, n (%) a | |||||
| Carboplatin | 22 (2.3) | – | 20 (8.3) | – | 2 (11.8) |
| Cisplatin | 214 (22.5) | – | 203 (83.9) | – | 11 (64.7) |
| Unknown | 23 (2.4) | – | 19 (7.9) | – | 4 (23.5) |
| Median NAC cycles, n (range) | 3 (2–6) | – | 3 (2–6) | – | 3 (2–4) |
| AC, n (%) | |||||
| No | 787 (82.8) | 545 (100) | 242 (100) | 0 (0) | 0 (0) |
| Yes | 163 (17.2) | 0 (0) | 0 (0) | 146 (100) | 17 (100) |
| AC platinum regimen, n (%) b | |||||
| Carboplatin | 20 (2.1) | 0 (0) | 0 (0) | 18 (12.3) | 2 (11.8) |
| Cisplatin | 83 (8.7) | 0 (0) | 0 (0) | 73 (50) | 10 (58.8) |
| Unknown | 60 (6.3) | 0 (0) | 0 (0) | 55 (37.7) | 5 (29.4) |
| Median AC cycles, n (range) | 4 (2–6) | – | – | 4 (2–6) | 4 (2–4) |
| Outcomes | – | – | |||
| RFS, n (%) | |||||
| No | 551 (58) | 313 (57.4) | 160 (66.1) | 71 (48.6) | 7 (41.2) |
| Yes | 399 (42) | 232 (42.6) | 82 (33.9) | 75 (51.4) | 10 (58.8) |
| Site of relapse, n (%) | |||||
| Distant | 334 (35.2) | 192 (35.2) | 68 (28.1) | 67 (45.9) | 7 (41.2) |
| Local | 63 (6.6) | 38 (7) | 13 (5.4) | 9 (6.2) | 3 (17.6) |
| Not specified | 3 (0.3) | 2 (0.4) | 1 (0.4) | 0 (0) | 0 (0) |
| Unknown | 550 (57.9) | 313 (57.4) | 160 (66.1) | 70 (47.9) | 7 (41.2) |
| Status at last follow-up, n (%) | |||||
| Alive | 575 (60.5) | 306 (56.1) | 176 (72.7) | 83 (56.8) | 10 (58.8) |
| Died of disease | 288 (30.3) | 177 (32.5) | 50 (20.7) | 55 (37.7) | 6 (35.3) |
| Died of other causes | 83 (8.7) | 58 (10.6) | 16 (6.6) | 8 (5.5) | 1 (5.9) |
AC = adjuvant chemotherapy; CCI = Charlson comorbidity index; CMV = cisplatin, methotrexate, vinblastine; DD = dose-dense; LN = lymph node; MVAC = methotrexate, vinblastine, doxorubicin, cisplatin; NAC = neoadjuvant chemotherapy; RFS = relapse-free survival; UC = urothelial carcinoma.
NAC regimens: CMV, n = 5; DD-MVAC, n = 36; MVAC, n = 11; gemcitabine-cisplatin, n = 162; gemcitabine-carboplatin, n = 22; unknown regimen (23)
AC regimens: DD-MVAC, n = 5; MVAC, n = 15; gemcitabine-cisplatin, n = 63; gemcitabine-carboplatin, n = 20.
3.2. RFS outcomes
After median follow-up of 26 mo (IQR 12–49 mo), there were 548 recurrence and 371 death events. Of these events, 288 were attributable to MIBC. Overall, the 1-yr RFS was 71.5% (95% confidence interval [CI] 69–75%). After stratification according to use of perioperative chemotherapy, 1-yr RFS rates were 67.9% (95% CI 64–72%) for no perioperative chemotherapy, 76.9% (95% CI 72–83%) for NAC, 77.8% (95% CI 71–85%) for AC, and 57% (95% CI 37–87%) for NAC + AC patients, respectively. Of note, patients with residual muscle-invasive disease after NAC, and in particular those with high-risk residual disease (pT3–4 and/or pN+) had inferior RFS compared to those without perioperative therapy or adjuvant therapy, as already reported (Table 2). Conversely, patients with downstaging to pT<2 stage after NAC had higher RFS than patients with pT<2 without NAC at 12 mo (94.2% vs 90.6%), 24 mo (88.5% vs 84.9%), and 36 mo (81.1% vs 74.9%) after RC (Supplementary Fig. 2).
Table 2 –
The 1-yr recurrence-free survival rates according to use of perioperative chemotherapy
| pT stage | Recurrence-free survival, % (95% confidence interval) | ||||
|---|---|---|---|---|---|
| Overall (n = 950) |
No NAC or AC (n = 545) |
NAC (n=242) |
AC (n=146) |
NAC + AC (n=17) |
|
| Overall | 71.5 (69–75) | 67.9 (64–72) | 76.9 (72–83) | 77.8 (71–85) | 57.0 (37–87) |
| pT0N0 (n = 91) | 97.6 (94–100) | NE. | 97.2 (94–99) | NE | NE |
| pTis/a/1N0 (n = 108) | 88.3 (82–95) | 88.8 (81–97) | 85.9 (74–99) | NE | NE |
| pT2N0 (n = 151) | 85.8 (80–92) | 87.9 (81–95) | 82.3 (70–96) | NE | NE |
| pT3–4N0 (n = 294) | 65.7 (60–72) | 61.4 (54–70) | 55.1 (42–73) | 79.2 (69–91) | NE |
| pTanyN+ (n = 278) | 54.3 (48–61) | 46.2 (38–57) | 40.3 (27–60) | 74.8 (63–86) | 45.7 (22–93) |
AC = Adjuvant chemotherapy; NAC = neoadjuvant chemotherapy; NE = not evaluable because of small numbers.
3.3. Cox regression models predicting RFS and OS
In multivariable Cox regression models, positive surgical margins (hazard ratio [HR] 1.66, 95% CI 1.21–2.30; p = 0.002), pT stage (overall p < 0.0001), and pN stage (overall p < 0.0001) were associated with high recurrence rates (Table 3). The bootstrapped (2000 samples) c-index of the model, including NAC, was 0.76 (95% CI 0.72–0.79). Of note, AC was not univariably associated with RFS (p = 0.6), whereas NAC was univariably significantly associated with RFS (HR 0.74, 95% CI 0.59–0.94; p = 0.013) but the significance was lost on multivariable analyses (p = 0.6). As noted above, NAC resulted in a detrimental HR compared to no NAC (HR 1.08, 95% CI 0.83–1.39). Sensitivity analyses were run excluding non-cisplatin regimens and the results were similar (c-index 0.76, 95% bootstrapped CI 0.73–0.78).
Table 3 –
Univariable and multivariable Cox regression models predicting recurrence after radical cystectomy in 950 MIBC patients
| Covariate | Univariable analysis | Multivariable analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Male (reference female) | 0.99 (0.77–1.27) | 0.9 | – | – |
| UC with divergent histology (reference UC) | 1.23 (0.92–1.65) | 0.2 | – | – |
| Margin status (reference negative) | 2.69 (2.01–3.60) | <0.0001 | 1.66 (1.21–2.30) | 0.002 |
| Race | ||||
| Caucasian | Reference | 0.08 | – | - |
| Hispanic or Latino | 1.15 (0.81–1.65) | – | - | |
| Black | 2.39 (1.45–3.95) | – | - | |
| Other or mixed | 1.17 (0.44–3.14) | – | - | |
| pT stage | ||||
| pT0 | Reference | <0.0001 | Reference | <0.0001 |
| pTa/is/1 | 2.26 (1.16–4.40) | 2.34 (1.16–4.74) | ||
| pT2 | 1.72 (1.17–2.53) | 2.77 (1.42–5.39) | ||
| pT3 | 3.84 (2.77–5.33) | 5.80 (3.07–11.0) | ||
| pT4 | 4.92 (3.41–7.09) | 6.08 (3.10–11.93) | ||
| pN stage | ||||
| pN0 | Reference | <0.0001 | Reference | <0.0001 |
| pN1 | 2.15 (1.61–2.88) | 1.61 (1.20–2.18) | ||
| pN2 | 2.73 (2.13–3.50) | 1.80 (1.38–2.34) | ||
| pN3 | 3.25 (1.60–6.60) | 1.87 (0.91–3.84) | ||
| pNX | 1.71 (1.00–2.94) | 1.52 (0.88–2.61) | ||
| NAC (reference no NAC) | 0.74 (0.59–0.94) | 0.0126 | 1.08 (0.83–1.39) | 0.6 |
| AC (reference AC) | 1.08 (0.85–1.37) | 0.6 | – | – |
AC = adjuvant chemotherapy; CI = confidence interval; HR = hazard ratio; NAC = neoadjuvant chemotherapy; UC = urothelial carcinoma.
Similarly, in multivariable Cox regression models for OS, positive surgical margins (HR 1.74, 95% CI 1.24–2.45; p = 0.002), pT stage (p = 0.002), and pN stage (p < 0.0001) were the only significant predictors (Supplementary Table 1). After applying a 3-mo landmark analysis the results were comparable. The c-index of the model, including NAC and landmark analysis, was 0.70 (95% bootstrapped CI 0.74–0.78).
3.4. Nomogram prediction of 1-yr RFS and 1-yr RFS rates across nomogram-derived tertiles
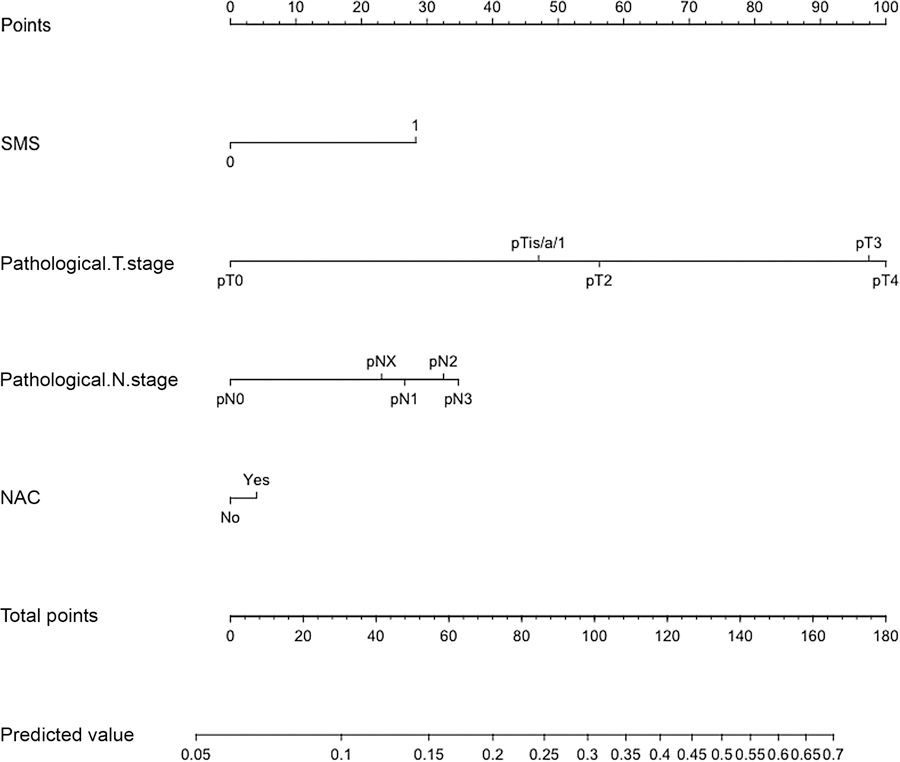
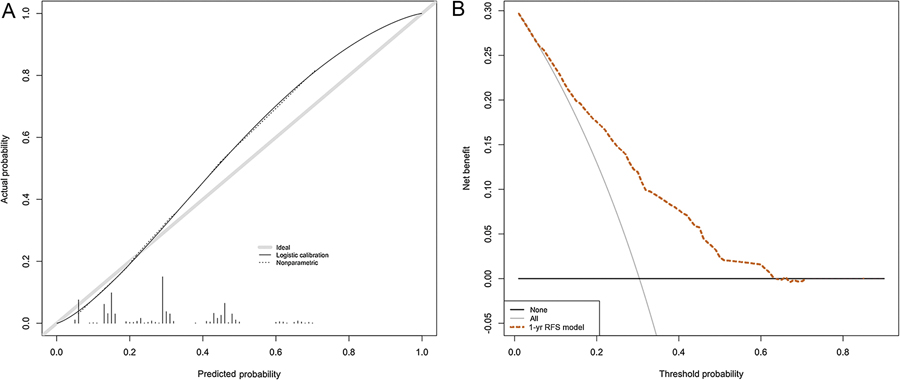
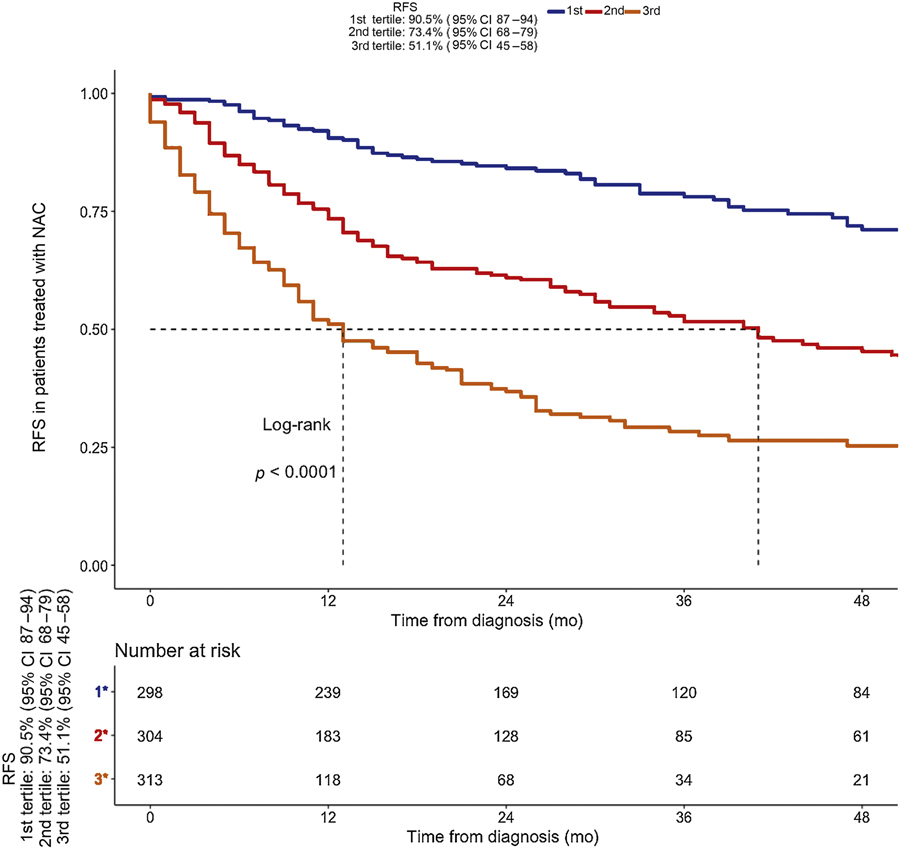
To provide 1-yr RFS estimates, we internally developed and validated a Cox-based nomogram predicting 1-yr RFS after RC (Fig. 1). The calibration plot and DCA are shown in Figure 2. According to nomogram-derived tertiles, for patients in the first, second, and third tertile the 1-yr RFS estimates were 90.5%, 73.4%, and 51.1%, respectively (Fig. 3).
Fig. 1 –
Nomogram for prediction of disease recurrence after radical cystectomy in patients with T2–4N0M0 urothelial carcinoma of the bladder. SMS = surgical margin status; NAC = neoadjuvant chemotherapy.
Fig. 2 –
Calibration plot and decision curve analysis for the Cox-based nomogram for prediction of 1-yr recurrence-free survival (RFS). (A) Average predicted probability (nomogram-predicted 1-yr RFS) versus Kaplan-Meier estimate (observed 1-yr RFS) with 95% confidence interval for the Kaplan-Meier estimate. The grey line indicates the reference, where an ideal nomogram would lie. (B) Net benefit of a strategy involving treating all patients (grey line), no patients (black line), or according to the nomogram predictions (dotted line). The plot shows that model-based decisions are supported for a threshold probability range of approximately 10–60% at 12 mo.
Fig. 3 –
Kaplan-Meier curves of recurrence-free survival (RFS) for 950 patients with muscle-invasive bladder cancer after stratification according to nomogram-derived tertiles. NAC = neoadjuvant chemotherapy; CI = confidence interval.
4. Discussion
Cisplatin-based NAC represents the standard of care for patients with MIBC and its use is supported by European Association of Urology, National Comprehensive Cancer Network, and European Society of Medical Oncology guidelines for patients with clinical stage T2–4N0M0 MIBC (level 1 evidence) [1,15,16]. Nevertheless, a minority of patients ultimately receive chemotherapy before surgery. This is one of the reasons why several ongoing phase 2 trials are planned to evaluate immune checkpoint inhibitors or targeted agents as neoadjuvant therapy in MIBC patients before RC. Results from these studies are largely unavailable, with the exception of preliminary results from the PURE-01 study (NCT02736266) [17]. In this ongoing study, pembrolizumab is administered as single-agent therapy in a short course of three doses every 3 wk before RC. Preliminary data for 36 patients after RC revealed a pT0 rate of 38.9% (95% CI 23.1–56.5%) in the intention-to-treat population. Indeed, it is likely that results from many similar studies will be available in the near future and that chemotherapy-free regimens may represent a viable alternative. Interpreting the findings from similar noncomparative studies will be of paramount importance to drive the development of new agents versus standard chemotherapy.
Indeed, the primary endpoint used by the majority of these studies is pathologic response, represented either by pT0 or by downstaging to non–muscle-invasive residual tumor [18] primarily on the basis that pT0 response is associated with a 55% decrease in the risk of death according to a recent meta-analysis [19]. pT0 is a difficult target to use in clinical studies of MIBC. In fact, the unavoidable bias of TURB impact on pT0 results at RC is an unsolved issue for any neoadjuvant study, and this bias largely affects the results reported for standard chemotherapy. This is exemplified by the study by Brant et al [20], who found that 38% of pathologic responses could have been attributed to TURB, suggesting that TURB must also be considered in evaluating the pT0 endpoint. Moreover, it could also be argued that immune-checkpoint inhibitors will not induce high rates of pathologic complete responses. However, patients benefiting from a pT0 response may experience sustained durability compared to standard chemotherapy. As a consequence, time-based outcomes, such as 1-yr RFS, may be more useful endpoints.
Importantly, we focused on the population of patients with MIBC, clinical lymph node–negative staging, and at least predominant UC component. These patients truly represent the cleanest model for testing any new drugs given in the neoadjuvant RC setting. Data on 1-yr RFS probabilities for these patients are scarce in the literature, mostly because the prognostic models and nomograms available typically analyzed a heterogeneous population of RC patients, without selecting them according to pre-RC characteristics [12]. As a result, patients undergoing RC for non–muscle-invasive bladder cancer or presenting with enlarged lymph nodes or predominant non-urothelial carcinoma histology were included.
The clinical investigation benefits arising from the proposed model may be twofold. First, the model provides a possibility of fine tuning the design and statistical assumptions of small phase 2, open-label, single-arm studies of new neoadjuvant therapies in MIBC; in this case, use of the lower nomogram tertile (1-yr RFS of 51.1%) may be recommended as the null hypothesis for statistical assumptions. Second, the model will allow retrospective comparisons of estimated versus observed 1-yr RFS in phase 2 trials in the absence of a randomized design.
Our model showed high accuracy, good calibration, and a high net benefit on DCA, with a fairly wide range of decision thresholds, after internal validation. Moreover, the model will also be valuable in predicting OS with good accuracy. The incremental complexity of hypothesis testing with multiple adjustment levels and landmark analysis meant that the null hypothesis of no association between the selected variables and the primary-endpoint (1-yr RFS) was incorrectly rejected.
The availability of the proposed endpoint may avoid the critical use of pathological response (which will remain the primary goal in testing the activity of any new drug at histological and molecular levels) for translational purposes [21–24].
In addition, our results strengthen evidence of the poor prognostic impact of invasive residual disease after NAC [25]. In fact, 1-yr RFS rates after NAC were lower (<85.9% vs. 97.2%) in patients with residual disease (>pT0N0) compared with results for RC alone or RC followed by AC. On the other hand, patients with pathologic major response, represented by downstaging to pT<2 residual tumor, exhibited longer RFS post-NAC (+6.2% at 36 mo). These RFS curves will serve for comparison with RFS results obtained after a major pathologic response with new agents.
Another intriguing issue that could be evaluated using our nomogram is whether patients with residual high-risk disease after neoadjuvant immune-checkpoint inhibition have poor outcomes comparable to those reported after chemotherapy; such information could guide post-RC management of patients who received prior immune-checkpoint inhibitors.
RFS estimates for patients receiving AC are likely to be biased by unaccountable patient selection factors that typically affect retrospective studies, as AC was not significantly associated with RFS on univariable analysis.
Some limitations of the study should be acknowledged. First, despite high accuracy, good calibration, and a high net benefit on DCA, our model lacks external validation, which would have strengthened our findings. Second, a small but significant proportion of patients (n = 28, 3%) did not receive guideline-recommended lymphadenectomy at the time of RC. However, our data are in agreement with a recent publication showing that lymphadenectomy is currently avoided in approximately 18.1% of RC cases [26]. As a consequence, the decision to include those individuals stems from the intent to better reflect a real-world population, in which lymphadenectomy might be avoided for some cases because of technical problems or patient preferences. The same considerations apply to the NAC and AC regimens administered, which included non-cisplatin regimens in a minority of patients, although the sensitivity analyses that excluded non-cisplatin regimens led to similar results (comparable c-index). Third, the criteria for choosing AC instead of NAC were probably heterogeneous, being based on either clinical judgment or the policy in use at each center. Finally, an advantage of using pathologic response is the small number of patients needed to test the null hypothesis in phase 2 studies. In our nomogram, the lowest tertile still has 1-yr RFS of50%, so it might not represent a useful tool for single-center studies.
5. Conclusions
Identification of novel active agents in the neoadjuvant MIBC setting may be impaired by use of pathologic response as the primary endpoint in phase 2 trials. Use of 1-yr RFS as the endpoint for single-arm phase 2 studies and application of the proposed nomogram to set the null hypothesis and analyze outcomes may represent a useful tool to enhance the quality of results reported for new agents given preoperatively in MIBC.
Supplementary Material
To overcome the limitations of pathologic complete response as the endpoint for phase 2 trials of neoadjuvant new drugs for T2–4N0M0 muscle-invasive bladder cancer we developed a model for prediction of 1-yr recurrence-free survival. The model could help in the design of single-arm phase 2 trials of novel agents and in comparison of findings across studies.
Acknowledgments
Financial disclosures: Marco Bandini certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: This work was supported in part by National Cancer Institute Cancer Center Support grant P30 CA008748. The sponsor played a role in data collection and approval of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Witjes JA, Lebret T, Compérat EM, et al. Updated 2016 EAU guidelines on muscle-invasive and metastatic bladder cancer. Eur Urol 2017;71:462–75. 10.1016/j.eururo.2016.06.020 [DOI] [PubMed] [Google Scholar]
- 2.International Collaboration of Trialists. International phase III trial assessing neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: long-term results of the BA06 30894 trial. J Clin Oncol 2011;29:2171–7. 10.1200/JCO.2010.32.3139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zargar H, Espiritu PN, Fairey AS, et al. Multicenter assessment of neoadjuvant chemotherapy for muscle-invasive bladder cancer. Eur Urol 2015;67:241–9. 10.1016/j.eururo.2014.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Funt SA, Rosenberg JE. Systemic, perioperative management of muscle-invasive bladder cancer and future horizons. Nat Rev Clin Oncol 2017;14:221–34. 10.1038/nrclinonc.2016.188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sternberg CN, Skoneczna I, Kerst JM, et al. Immediate versus deferred chemotherapy after radical cystectomy in patients with pT3–pT4 or N+ M0 urothelial carcinoma of the bladder (EORTC 30994): an intergroup, open-label, randomised phase 3 trial. Lancet Oncol 2015;16:76–86. 10.1016/S1470-2045(14)71160-X [DOI] [PubMed] [Google Scholar]
- 6.Burger M, Mulders P, Witjes W. Use of neoadjuvant chemotherapy for muscle-invasive bladder cancer is low among major European centres: results of a feasibility questionnaire. Eur Urol 2012;61:1070–1. 10.1016/j.eururo.2012.01.039 [DOI] [PubMed] [Google Scholar]
- 7.Meeks JJ, Bellmunt J, Bochner BH, et al. A systematic review of neoadjuvant and adjuvant chemotherapy for muscle-invasive bladder cancer. Eur Urol 2012;62:523–33. 10.1016/j.eururo.2012.05.048 [DOI] [PubMed] [Google Scholar]
- 8.Reardon ZD, Patel SG, Zaid HB, et al. Trends in the use of perioperative chemotherapy for localized and locally advanced muscle-invasive bladder cancer: a sign of changing tides. Eur Urol 2015;67:165–70. 10.1016/j.eururo.2014.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Powles T, Necchi A, Rosen G, Hariharan S, Apolo AB. Anti-programmed cell death 1/ligand 1 (PD-1/PD-L1) antibodies for the treatment of urothelial carcinoma: state of the art and future development. Clin Genitourin Cancer 2018;16:117–29. 10.1016/j.clgc.2017.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karakiewicz PI, Shariat SF, Palapattu GS, et al. Nomogram for predicting disease recurrence after radical cystectomy for transitional cell carcinoma of the bladder. J Urol 2006;176:1354–61. 10.1016/j.juro.2006.06.025 [DOI] [PubMed] [Google Scholar]
- 11.Xylinas E, Cha EK, Sun M, et al. Risk stratification of pT1–3N0 patients after radical cystectomy for adjuvant chemotherapy counselling. Br J Cancer 2012;107:1826–32. 10.1038/bjc.2012.464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.International Bladder Cancer Nomogram Consortium, Bochner BH, Kattan MW, Vora KC. Postoperative nomogram predicting risk of recurrence after radical cystectomy for bladder cancer. J Clin Oncol 2006;24:3967–72. 10.1200/JCO.2005.05.3884 [DOI] [PubMed] [Google Scholar]
- 13.Gill R Understanding Cox’s regression model. Experientia Suppl 1982;41:187–99. [PubMed] [Google Scholar]
- 14.Vickers AJ, Elkin EB. Decision curve analysis: a novel method for evaluating prediction models. Med Decis Making 2006;26:565–74. 10.1177/0272989X06295361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spiess PE, Agarwal N, Bangs R, et al. Bladder cancer, version 5.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw 2017;15:1240–67. 10.6004/jnccn.2017.0156 [DOI] [PubMed] [Google Scholar]
- 16.Bellmunt J, Albiol S, Kataja V, ESMO Guidelines Working Group. Invasive bladder cancer: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol 2009;20(Suppl 4):79–80. 10.1093/annonc/mdp136 [DOI] [PubMed] [Google Scholar]
- 17.Necchi A, Briganti A, Bianchi M, et al. Preoperative pembrolizumab (pembro) before radical cystectomy (RC) for muscle-invasive urothelial bladder carcinoma (MIUC): Interim clinical and biomarker findings from the phase II PURE-01 study. Cancer Res 2018;78(13 Suppl):CT003. [Google Scholar]
- 18.Rosenblatt R, Sherif A, Rintala E, et al. Pathologic downstaging is a surrogate marker for efficacy and increased survival following neoadjuvant chemotherapy and radical cystectomy for muscle-invasive urothelial bladder cancer. Eur Urol 2012;61:1229–38. 10.1016/j.eururo.2011.12.010 [DOI] [PubMed] [Google Scholar]
- 19.Petrelli F, Coinu A, Cabiddu M, Ghilardi M, Vavassori I, Barni S. Correlation of pathologic complete response with survival after neoadjuvant chemotherapy in bladder cancer treated with cystectomy: a meta-analysis. Eur Urol 2014;65:350–7. 10.1016/j.eururo.2013.06.049 [DOI] [PubMed] [Google Scholar]
- 20.Brant A, Kates M, Chappidi MR, et al. Pathologic response in patients receiving neoadjuvant chemotherapy for muscle-invasive bladder cancer: Is therapeutic effect owing to chemotherapy or TURBT? Urol Oncol 2017;35:34.e17–25. 10.1016/j.urolonc.2016.08.005 [DOI] [PubMed] [Google Scholar]
- 21.Kassouf W, Spiess PE, Brown GA, et al. P0 stage at radical cystectomy for bladder cancer is associated with improved outcome independent of traditional clinical risk factors. Eur Urol 2007;52:769–74. 10.1016/j.eururo.2007.03.086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sonpavde G, Goldman BH, Speights VO, et al. Quality of pathologic response and surgery correlate with survival for patients with completely resected bladder cancer after neoadjuvant chemotherapy. Cancer 2009;115:4104–9. 10.1002/cncr.24466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu D, Abbosh P, Keliher D, et al. Mutational patterns in chemotherapy resistant muscle-invasive bladder cancer. Nat Commun 2017;8:2193 10.1038/s41467-017-02320-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones S, Anagnostou V, Lytle K, et al. Personalized genomic analyses for cancer mutation discovery and interpretation. Sci Transl Med 2015;7:283ra53 10.1126/scitranslmed.aaa7161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bhindi B, Frank I, Mason RJ, et al. Oncologic outcomes for patients with residual cancer at cystectomy following neoadjuvant chemotherapy: a pathologic stage-matched analysis. Eur Urol 2017;72:660–4. 10.1016/j.eururo.2017.05.016 [DOI] [PubMed] [Google Scholar]
- 26.Zaffuto E, Bandini M, Gazdovich S, et al. Contemporary rates of adherence to international guidelines for pelvic lymph node dissection in radical cystectomy: a population-based study. World J Urol. In press. 10.1007/s00345-018-2306-7 [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.