Abstract
Background & aims
Angiotensin converting enzyme (ACE)-2 is a modulator of adipose tissue metabolism. However, human data of adipose ACE-2 is rarely available. Considering that, ACE-2 is believed to be the receptor responsible for cell entry of SARS-CoV-2, a better understanding of its regulation is desirable. We therefore characterized the modulation of subcutaneous adipose ACE-2 mRNA expression during weight loss and the impact of ACE-2 expression on weight loss induced short- and long-term improvements of glucose metabolism.
Methods
143 subjects (age > 18; BMI ≥ 27 kg/m2) were analyzed before and after a standardized 12-week dietary weight reduction program. Afterwards subjects were randomized to a 12-month lifestyle intervention or a control group (Maintain-Adults trial). Insulin sensitivity (IS) was estimated by HOMA-IR (as an estimate of liver IS) and ISIClamp (as an estimate of skeletal muscle IS). ACE-2 mRNA expression (ACE-2AT) was measured in subcutaneous adipose tissue before and after weight loss.
Results
ACE-2AT was not affected by obesity, but was reduced in insulin resistant subjects. Weight loss resulted in a decline of ACE-2AT (29.0 (20.0–47.9) vs. 21.0 (13.0–31.0); p = 1.6 ∗ 10−7). A smaller reduction of ACE-2 AT (ΔACE-2AT) was associated with a larger improvement of ISIClamp (p = 0.013) during weight reduction over 3 months, but not with the extend of weight loss. The degree of changes in insulin resistance were preserved until month 12 and was also predicted by the weight loss induced degree of ΔACE-2AT (p = 0.011).
Conclusions
Our data indicate that subcutaneous adipose ACE-2 expression correlates with insulin sensitivity. Weight loss induced decline of subcutaneous adipose ACE-2 expression might affect short- and long-term improvement of myocellular insulin sensitivity, which might be also relevant in the context of ACE-2 downregulation by SARS-CoV-2.
Trial registration: ClinicalTrials.gov number: NCT00850629, https://clinicaltrials.gov/ct2/show/NCT00850629, date of registration: February 25, 2009.
Keywords: Adipose tissue, Insulin sensitivity, Obesity, ACE-2, Weight loss
Highlights
-
•
Subcutaneous adipose ACE-2 mRNA expression (ACE-2AT) is reduced in insulin resistance.
-
•
Weight loss resulted in a decline of ACE-2AT.
-
•
This was associated with smaller short- and long-term improvement of insulin sensitivity.
-
•
This might be also relevant in the context of ACE-2 downregulation by SARS-CoV-2.
1. Introduction
Metabolic and cardiovascular morbidity is highly associated with an unfavorable clinical course of COVID-19 infections [1,2]. As those comorbidities are often seen in obese subjects, obesity was early thought to represent an additional risk factor. Actually, very recent reports support this assumption, as a higher BMI and especially visceral adiposity was associated with increased disease severity of COVID-19 infections [3,4] as well as worse clinical outcome in patients with COVID-19 [[5], [6], [7]]. Several parameters are discussed to be responsible for this finding including increased airway resistance, impaired respiratory mechanics and gas exchange as well as lower strength of respiratory muscles in obesity [8]. Based on recent findings, identifying angiotensin converting enzyme (ACE)-2 as the receptor responsible for cell entry of SARS-CoV-2 by binding coronavirus spike protein [9], the amount of ACE-2 expression was also discussed as a potential risk factor for higher infection rate, although reasonable doubt was also mentioned recently [10].
Apart from this recently described function, ACE-2 protein is known to be involved in regulation of local and global renin-angiotensin system. It represents a homolog of ACE, which converts angiotensin (Ang) I into Ang II. Activation of angiotensinogen II type I receptor by Ang II results in vasoconstriction, inflammation and increased sodium absorption. In contrast, ACE-2 decreases Ang II level by transforming it into Ang-(1–7). High levels of Ang-(1–7) results in improved metabolism and lower inflammation via Mas receptor (MasR) activation [11]. Thus, high ACE-2 activity exerts its beneficial effects via both reduction of Ang II and increased Ang-(1–7)/MasR signaling. Although ACE-2 is abundantly expressed in airway epithelium of the lungs, a remarkable expression was also detected in small intestine, testis, kidneys, heart, thyroid, and adipose tissue [12].
Particularly in adipose tissue ACE-2/Ang-(1–7)/MasR pathway represent a crucial element involved in lipid storage and regulation of adipokine production such as resistin and leptin. Clearly, ACE-2 mediated higher Ang-(1–7) levels can directly improve myocellular insulin efficacy. However, the modulation of adipokine secretion might represent an addition factor involved in modulation of myocellular insulin sensitivity by ACE-2 via inter-organ crosstalk. Finally, ACE-2 exerts anti-obesity effects by induction of browning in white adipose tissue in (HFD)-induced obesity mice [13]. Thus, this protein seems to represent a crucial modulator of metabolic health in obesity.
Although there is currently no evidence for SARS-CoV-2 infection of AT and detected virus level in peripheral blood samples were rather low [14], the presence of ACE-2 on adipocytes as well as the fact that adipose tissue is a proven target tissue for multiple viruses [15] makes it not implausible, that adipose tissue could be also targeted by SARS-CoV-2. In this context, increased knowledge of adipose ACE-2 regulation in humans might be crucial. As current data is sparse in humans, we intend to analyze adipose ACE-2 expression in obesity and insulin resistant state. Moreover, we aimed to investigate, if ACE-2 expression can be modified by weight loss, and finally, if metabolic short- and long-term improvements are associated with weight loss induced changes of adipose ACE-2 expression in humans.
2. Materials and methods
2.1. Participants
Details of the performed weight loss-weight maintenance trial in adults (Maintain-Adult, ClinicalTrials.gov NCT00850629) were already described [16,17]. In brief, 156 overweight or obese subjects (120 female and 36 male) (BMI ≥ 27 kg/m2) underwent a 12 week multimodal weight loss. Afterwards 143 of those lost at least 8% of initial body weight and were enrolled into a 12-month randomized controlled weight maintenance intervention. 61% of these subjects had no abnormality of glucose metabolism, while 27% were characterized by either impaired fasting glucose or impaired glucose tolerance. Type 2 diabetes was detected in 12%. None of the enrolled subjects reported intake of nutraceuticals affecting insulin sensitivity. The study was performed between 2010 and 2016 at a University Center.
2.2. Study design
The major characteristics of the trial are shown in Fig. S1. After the initial weight loss period, we compared the effects of a 12-months multimodal lifestyle intervention to maintain body weight with a control group within a randomized controlled trial.
Pre-trial weight loss phase: A structured weight reduction program (caloric restriction using a very low energy diet and nutritional counseling, physical exercises and psychological advices) was used to achieve a weight loss of at least 8%. The detailed protocol was already reported previously [18] and is given in the supplement.
12 months randomized weight maintenance phase: Eligible subjects (n = 143, 112 female and 31 male) were randomized into the intervention or control group. Subjects in the control group were no longer involved in any form of counseling. A continuous counseling was performed in the intervention group for the next 12 months. This multimodal lifestyle intervention was comparable to sessions of the weight loss period. Details of the protocol and the intervention were already reported [16,18] and are described in the supplement.
Follow-up period: After 12 months all subjects (intervention and control group) underwent a free living period of 6 months without any further active intervention.
2.3. Phenotyping
A comprehensive phenotyping was performed before (T-3) and after (T0) weight loss, 12 months (T12) after randomization and after the additional follow-up of 6 months (T18). All participants received a dietary recommendation of a balanced energy intake for the 3 days preceding phenotyping. The phenotyping focused on anthropometric, hormonal and metabolic evaluation. Following a 10-hour overnight fast, all patients were investigated at the endocrine trial center of the Charité Medical School at 8.00 a.m. Waist circumference was measured three times and the means were calculated. At 9.00 a.m. fasting samples were taken. Moreover, subjects also underwent an oral glucose tolerance test and a body impedance analysis using AKERN BIA 101 (SMT medical GmbH & Co. KG, Würzburg, Germany) at T-3, T0, T12 and T18. A hyperinsulinemic-euglycemic clamp was performed at T-3, T0 and T12 and adipose tissue biopsies from subcutaneous adipose tissue were performed at T-3 and T0 as previously described [16,19]. Blood samples were centrifuged, and plasma and serum samples were frozen immediately at −80 °C.
2.4. Laboratory analyses
Standard laboratory analyses are described in the supplements. Leptin was analyzed using commercial ELISA (R&D Systems, Abingdon, UK) (inter-assay CV 3.5–5.4%, intra-assay CV 3.0–3.3%). Tissue samples were analyzed by RNA sequencing using the HiSeq2000 system (TruSeq SBS Kit-Hs 200 cycles, Illumina San Diego, US) (details see supplement). Aldosterone and renin were measured by radioimmunoassay (Siemens, Germany and CIS bio GmbH, Berlin, Germany).
2.5. Statistics and calculations
Insulin sensitivity was assessed by dividing the average glucose infusion rate (GIR, mg glucose/min) during the steady state of the hyperinsulinemic-euglycemic clamp by the body weight (M-value). The insulin sensitivity index (ISIClamp), which reflects predominantly skeletal muscle insulin sensitivity, was calculated as ratio of M-value to the serum insulin concentration (I, mU/l) in this period of the clamp. HOMA-IR was calculated to assess whole body insulin sensitivity [20].
Weight loss induced changes (T-3 to T0) of specific parameters (body mass index (BMI), fat mass (FM), waist circumference (WC), HOMA-IR, FFA, ISIClamp, leptin per fat mass (Leptin/FM), adipose leptin (LeptinAT) and ACE-2 mRNA expression (ACE-2AT)) were expressed as percentage of baseline values at T-3 (ΔBMI, ΔFM, ΔWC, ΔHOMA-IR, ΔFFA, ΔISIClamp, ΔLeptin/FM, ΔLeptinAT and ΔACE-2AT). Changes of BMI, HOMA-IR and ISIClamp between T-3 and T12 were expressed as percentage of baseline values at T-3 (ΔBMIT3T12, ΔHOMA-IRT3T12 and ΔISIClampT3T12).
Statistical procedures were performed using SPSS version 25.0 (SPSS Inc., Chicago, IL, USA) and SAS software, version 9.4 (SAS Institute). The here reported data reflects secondary analyses and are based on per protocol analysis including data of all available participants at the corresponding time point. Comparisons were made via paired Student's t-test for normally distributed data and Wilcoxon test for skewed data. Correlations between variables were investigated by Pearson's correlation coefficient for normally distributed data or Spearman's rank correlation coefficient for skewed data. Data were adjusted for age and gender as described in the Results section. Results were considered significant, if the two-sided α was below 0.05. Data were presented as median and limits of the interquartile range (IQR: 25th – 75th percentile) and plotted as raw values unless stated otherwise.
Multivariate linear regression models were performed to analyze the impact of weight loss induced changes of adipose ACE-2 mRNA expression at T-3 on weight loss induced relative changes of BMI (ΔBMI), HOMA-IR (ΔHOMA-IR) and ΔISIClamp (ΔISIClamp). These models included age, gender and concomitant decline of BMI (except the model focusing on ΔBMI) as potential confounders. Comparable regression models were performed to analyze the impact of baseline ACE-2 mRNA expression on long-term improvement of BMI, HOMA-IR and ISIClamp. As the hyperinsulinemic-euglycemic clamp was only performed at T-3, T0 and T12, we have chosen these time-points for calculation. Given the effect of the 12 months intervention on BMI [16] at T12, the models regarding long-term improvement were also adjusted for treatment group and concomitant BMI changes. Finally, the additive predictive effect of both ΔACE-2AT on the dependent variables was assessed by likelihood ratio test comparing the models including or excluding ΔACE-2AT.
2.6. Study approval
The study protocol was approved by the Institutional Review Board of the Charité Medical School and all subjects gave written informed consent. The trial was registered at ClinicalTrials.gov (NCT00850629).
3. Results
In general, 143 middle aged, obese subjects were included in the performed multimodal weight loss intervention. More than 50% were moderate or severe obese (Table 1 ). ACE-2 mRNA expression could be detected in subcutaneous adipose tissue (29.0 (20.0–47.0)) but not in skeletal muscle. Subcutaneous adipose tissue ACE-2 mRNA expression (ACE-2AT) did not differ between men (28.0 (17.0–48.5)) and women (29.5 (20.8–48.0)) (p = 0.806) and were not related to age (p = 0.108), estimates of obesity (BMI (p = 0.625), waist circumference (p = 0.492)), renin (p = 0.714) and aldosterone concentration (p = 0.275). However, a lower ACE-2AT was seen in insulin resistant subjects (HOMA-IR: r = −0.228, p = 0.049; ISIClamp: r = 0.212, p = 0.070). Accordingly analysis of ACE-2AT reveals lowest levels in type 2 diabetes (20.5 (15.0–55.0) compared to prediabetes (35.0 (19.3–55.0)) and healthy obese (28.0 (22.0–47.0)). However, given the limited sample size of these subgroups, the difference was not significant. ACE-2AT was also related to estimates of adipose tissue leptin production (Leptin/FM: r = 0.356; p = 0.003 and LeptinAT: r = 0.262; p = 0.023).
Table 1.
Basal characteristics of the participants. Metabolic and anthropometric parameters of the randomized participants before weight loss. Results are presented as median and interquartile range (IQR).
| Parameter | No. of participants | Before weight loss |
|
|---|---|---|---|
| Median | (IQR) | ||
| Females [n (%)] | 143 | 112 (78) | |
| Postmenopausal females [n (%)] | 58 (51) | ||
| Age [yr] | 143 | 50.5 | (41.7–60.8) |
| BMI [kg/m2] | 143 | 35.6 | (32.9–41.0) |
| Overweight (%) | 9 | 6.3 | |
| Obesity I (%) | 54 | 37.8 | |
| Obesity II (%) | 38 | 26.6 | |
| Obesity III (%) | 42 | 29.4 | |
| Fat mass [%] | 126 | 37.4 | (32.6–40.0) |
| Waist circumference [cm] | 143 | 106.5 | (97.0–117.0) |
| Total cholesterol [mg/dl] | 143 | 200.0 | (176.0–233.0) |
| HDL-cholesterol [mg/dl] | 143 | 49.3 | (40.6–61.3) |
| LDL-cholesterol [mg/dl] | 143 | 123.1 | (103.2–146.8) |
| Triacylglycerol [mg/dl] | 143 | 126.0 | (85.0–169.0) |
| HbA1c [mmol/mol] | 143 | 37.7 | (34.4–42.1) |
| HbA1c [%] | 143 | 5.6 | (5.3–6.0) |
| HOMA-IR | 142 | 2.2 | (1.4–3.4) |
| ISIClamp [mg∙kg−1∙min−1/(mU∙l−1)] | 139 | 0.06 | (0.04–0.08) |
| Renin (ng/l) | 137 | 9.3 | (4.9–17.7) |
| Aldosterone (ng/l) | 142 | 60.8 | (32.2–93.5) |
| Aldosterone-renin-ratio | 137 | 7.7 | (3.5–11.8) |
| Leptin (ng/ml) | 138 | 40.9 | (26.2–60.0) |
| Leptin/FM (ng/(ml∙kg−1) | 122 | 1.1 | (0.8–1.5) |
| LeptinAT (counts) | 75 | 30,771 | (23,039–38,107) |
The diet-induced reduction of BMI (−4.6 (4.3–4.9) kg/m2) [17] resulted in improved insulin sensitivity (change of HOMA-IR: −37.9 (−9.6 – (−58.6)); p = 4.6*10−13 and change of ISIClamp: 47.9 (18.3–85.6) mg•kg−1•min−1/(mU•l−1); p = 9.4*10−9) and a decline of ACE-2AT in adipose tissue (29.0 (20.0–47.9) vs. 21.0 (13.0–31.0); p = 1.6 *10−7). Aldosterone and renin levels were not modified by weight loss (60.8 (32.2–92.5) vs. 68.3 (40.8–96.1); p = 0.397 and 9.3 (4.9–17.7) vs. 8.0 (5.2–14.7); p = 0.524).
Interestingly, a smaller improvement of ISIClamp was detected in subjects with a stronger decline of ACE-2AT (Fig. 1 ). Post hoc analyses revealed, that the difference of ΔISIClamp was particularly seen between the first and the third (p = 0.044) as well as the second and the third (p = 0.019) quartile of ΔACE-2AT. To adjust this relationship to potential confounders, we performed a linear regression model including age, gender and concomitant change of BMI during weight loss period. Thereby the significant effect of weight loss induced ΔACE-2AT on improvement of ISIClamp could be confirmed (Table 2 ). Moreover, the reduction of ACE-2AT was associated with weight loss induced leptin mRNA expression in adipose tissue (r = 0.390; p = 0.001). In contrast, ΔACE-2AT was not related to the decline of body weight and HOMA-IR during weight loss intervention.
Fig. 1.
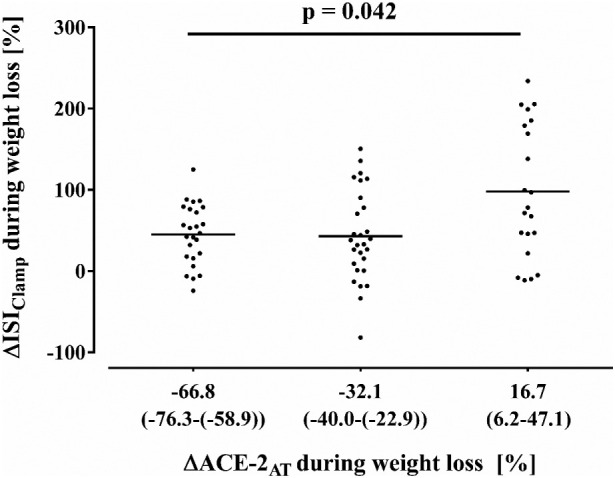
Association of weight loss induced changes of ACE-2AT with ΔISIClamp during weight loss.
Table 2.
Impact of weight loss induced changes of adipose ACE-2 mRNA expression on ΔISIClamp.
| Predictors | Coefficients | 95% CI | p value | R2 |
|---|---|---|---|---|
| ΔACE-2AT | 0.40 | 0.09–0.71 | 0.013 | 0.406 |
| Gender (reference: male) | −36.83 | −74.21–0.55 | 0.053 | |
| ΔBMI | −2.38 | −6.27–1.53 | 0.229 | |
| Age | −0.41 | −1.51–0.68 | 0.453 |
3.1. Prediction of long-term modulation of insulin sensitivity
The weight loss induced changes of insulin resistance were preserved until month 12, as reported previously (Table S1) [21]. Given the current data, we aimed to analyze the effect of weight loss induced decline of ACE-2AT on long-term improvement of these estimates of insulin sensitivity. We revealed a prediction of higher long-term improvement of ISIClampT3T12 by a smaller decline of ACE-2AT. As long-term improvement of ISIClampT3T12 might be also affected by concomitant weight loss or short-term changes of ISIClamp, (ISIClampT3T0), we adjusted this analysis to these parameters, which does not change this finding (Table 3 ). A comparable model without ΔACE-2AT explained a marginally, but significant lower variability (R2 = 0.780; p = 0.011 for comparison between models) than the full model (Table 3).
Table 3.
Impact of weight loss induced changes of adipose ACE-2 mRNA expression on long-term improvement of ISIClamp (ΔISIClampT3T12).
| Predictors | Coefficients | 95% CI | p value | R2 |
|---|---|---|---|---|
| ΔACE-2AT | 0.37 | 0.09–0.66 | 0.011 | 0.807 |
| Gender (reference: male) | 3.93 | −28.47–36.32 | 0.808 | |
| Age | −0.48 | −6.52 to −3.49 | 0.331 | |
| ΔBMIT3T12 | −5.00 | −6.27–1.53 | 2.5 ∗ 10−8 | |
| ΔISIClamp | 1.02 | 0.81–1.24 | 1.3 ∗ 10−12 | |
| Randomization | 9.14 | −17.19–35.48 | 0.489 |
Long-term changes of HOMA-IR or BMI were not predicted by ΔACE-2AT.
4. Discussion
Recent data have clearly highlighted the role of ACE-2 in COVID-19 infection, which supports more research on the role of ACE-2 in human health. Previous experimental and animal data indicate, that the ACE-2/Ang-(1–7)/MasR signaling is responsible for numerous beneficial effects of adipose ACE-2 [11]. Although we did not find an obesity dependent pattern of subcutaneous adipose ACE-2 mRNA expression at baseline in our human subjects, we identified a significant relationship of ACE-2 expression with estimates of hormonal activity in subcutaneous adipose tissue. As leptin secretion is known to be stimulated by Ang-(1–7) [22], the observed association of ACE-2AT with both adipose leptin expression as well as fat mass adjusted circulating leptin level, might be caused by increased Ang-(1–7) level due to higher adipose ACE-2 activity. Given the fact, that increased leptin signaling results in improved insulin sensitivity [23], this pathway may be also relevant regarding the inter-organ cross talk between adipose tissue and skeletal muscle. The higher ACE-2 expression found in insulin sensitive subjects of our cohort supports this assumed interaction, even if circulating Ang-(1–7) levels, generated by adipose ACE-2, might also cause direct effects on myocellular insulin sensitivity [24]. Taken together, these data support an impact of subcutaneous adipose ACE-2 on subcutaneous adipose tissue function. As ACE-2AT level did not differ regarding age and gender, which is in line with previous reports [12], the impact of adipose ACE-2 might not depend on these factors.
Despite these findings indicating a beneficial metabolic role of ACE-2AT, weight loss resulted in a substantial decline of ACE-2AT. Given the fact, that weight loss induced improvement of insulin sensitivity was smaller in subjects with a stronger decline of ACE-2AT, weight loss induced reduction of ACE-2AT seems not to be a primary driver of improved myocellular insulin sensitivity in obese humans. Our data rather fit to the assumption, that the decline of ACE-2 may counteract the improvement of insulin sensitivity during weight loss. Potential mechanisms might be a reduced conversion of Ang II into Ang-(1–7) and a decreased leptin production in adipocytes. Accordingly, we found a significant relationship of weight loss induced reduction of adipose ACE-2 and leptin mRNA expression. Most interestingly, this might be also relevant in long-term regulation, as improvement of insulin efficacy in skeletal muscle 12 months after weight loss was abolished by a stronger weight loss induced decline of subcutaneous adipose ACE-2 expression.
Although it could not be excluded, we would currently not interpret our findings in the context of primary lower adipose binding opportunities in insulin resistance. However, the here revealed association of reduced insulin sensitivity in case of lower ACE-2AT might be of high interest in case of infection. Although we did not investigate subjects with active COVID-19 infection, such a direct link could be relevant in regulation of blood glucose during active infection. A downregulation of ACE-2 expression on cell surface was described in the context of SARS-CoV spike protein binding [25]. Indeed, hyperglycemia was frequently observed in COVID-19 infections [26]. Vice versa poorly-controlled glucose metabolism increased the severity and mortality in diabetic patients with COVID-19 [1,27].
The interpretation of our data is limited by some factors. Most of our data are based on associations. Therefore, further experiments are clearly warranted. However, numerous experimental and animal findings published previously are in accordance to our human data and provide potential molecular explanations. Our data reflect mRNA expression. As cannot exclude that the relationship between ACE-2 at protein level/ACE-2 activity and insulin sensitivity may be different and weight loss induced changes of ACE-2 mRNA might not reflect concomitant effects on protein levels, confirmatory analyses are warranted in subsequent studies. Several behavioral, social and environmental factors are known to have substantial impact on long-term effects of dietary weight loss interventions [28,29]. Especially behavioral factors were not considered in our current analysis. Although we aimed to standardize the dietary intake and physical activity during the group sessions, we cannot exclude that these factors may have influenced our results. Given the known effect of different macronutrients on insulin sensitivity, this might have influenced our results. Moreover, recent data indicating a detrimental effect of obesity in COVID-19 also highlighted the role of visceral adipose tissue mass [5]. Weight loss induced changes of ACE-2 expression in this compartment might differ from subcutaneous adipose tissue. Therefore, our findings are not simply transferable to visceral adipose tissue.
On the other hand, numerous strengths of the current trial should be mentioned. These includes the large sample size, the long duration of the intervention, subsequent observation and the comprehensive phenotyping including detailed assessment of myocellular insulin sensitivity by hyperinsulinemic-euglycemic clamp in such a large cohort.
5. Conclusion
In summary, our data implicate, that modulation of adipose tissue ACE-2 expression may represent a mechanism linking subcutaneous adipose tissue function and myocellular insulin sensitivity. In this context, reduction of subcutaneous adipose ACE-2 expression during weight loss seems to contribute to the short- and long-term regulation of myocellular insulin sensitivity. In any case, our prospective data support that further studies elucidate the role of ACE-2 in the context of inter-organ cross talk, particularly in weight loss and possibly in the clinical course of SARS-CoV-2 infections.
Declaration of funding
This research was supported by the Deutsche Forschungsgemeinschaft (DFG KFO 218/1), the German Diabetes Society (DDG) and the German Ministry for Education and Research (BMBF) by support of the German Centre for Cardiovascular Research (DZHK; BER5.1).
CRediT authorship contribution statement
K.M., L.L., J.S. researched data and wrote the manuscript; K.M., L.S. and J.S. were responsible for data analysis; L.S., F.B. and M.B. researched data. All authors contributed to interpretation of the results. All authors critically read and edited several drafts before submission. All authors read and approved the submitted version.
Declaration of competing interest
We declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Acknowledgments
Acknowledgments
We thank K. Simon, B. Horchler, N. Huckauf and C. Kalischke for excellent technical assistance as well as A. Reisshauer for the support regarding to physical activity intervention. We thank Nestlé HealthCare Nutrition GmbH, Frankfurt am Main, Germany for the opportunity to purchase the Optifast 2® diet at a reduced price.
Ethics approval and consent to participate
The study protocols were approved by the Institutional Review Board of the Charité Medical School (EA1/140/12) and all subjects gave written informed consent.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.metabol.2020.154401.
Appendix A. Supplementary data
Supplementary material
References
- 1.Zhu L., She Z.G., Cheng X., Qin J.J., Zhang X.J., Cai J. Association of blood glucose control and outcomes in patients with COVID-19 and pre-existing type 2 diabetes. Cell Metab. 2020;31(6):1068–77 e3. doi: 10.1016/j.cmet.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Argenziano M.G., Bruce S.L., Slater C.L., Tiao J.R., Baldwin M.R., Barr R.G. Characterization and clinical course of 1000 patients with coronavirus disease 2019 in New York: retrospective case series. BMJ. 2020;369 doi: 10.1136/bmj.m1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kalligeros M., Shehadeh F., Mylona E.K., Benitez G., Beckwith C.G., Chan P.A. Association of obesity with disease severity among patients with COVID-19. Obesity (Silver Spring) 2020 doi: 10.1002/oby.22859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simonnet A., Chetboun M., Poissy J., Raverdy V., Noulette J., Duhamel A. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity (Silver Spring) 2020 doi: 10.1002/oby.22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Watanabe M., Caruso D., Tuccinardi D., Risi R., Zerunian M., Polici M. Visceral fat shows the strongest association with the need of intensive care in patients with COVID-19. Metabolism. 2020;111:154319. doi: 10.1016/j.metabol.2020.154319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watanabe M., Risi R., Tuccinardi D., Baquero C.J., Manfrini S., Gnessi L. Obesity and SARS-CoV-2: a population to safeguard. Diabetes Metab Res Rev. 2020 doi: 10.1002/dmrr.3325. [DOI] [PubMed] [Google Scholar]
- 7.Palaiodimos L., Kokkinidis D.G., Li W., Karamanis D., Ognibene J., Arora S. Severe obesity, increasing age and male sex are independently associated with worse in-hospital outcomes, and higher in-hospital mortality, in a cohort of patients with COVID-19 in the Bronx, New York. Metabolism. 2020;108:154262. doi: 10.1016/j.metabol.2020.154262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murugan A.T., Sharma G. Obesity and respiratory diseases. Chron Respir Dis. 2008;5(4):233–242. doi: 10.1177/1479972308096978. [DOI] [PubMed] [Google Scholar]
- 9.Hoffmann M., Kleine-Weber H., Schroeder S., Kruger N., Herrler T., Erichsen S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280. doi: 10.1016/j.cell.2020.02.052. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dalan R., Bornstein S.R., El-Armouche A., Rodionov R.N., Markov A., Wielockx B. The ACE-2 in COVID-19: foe or friend? Horm Metab Res. 2020;52(5):257–263. doi: 10.1055/a-1155-0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lelis D.F., Freitas D.F., Machado A.S., Crespo T.S., Santos S.H.S. Angiotensin-(1-7), adipokines and inflammation. Metabolism. 2019;95:36–45. doi: 10.1016/j.metabol.2019.03.006. [DOI] [PubMed] [Google Scholar]
- 12.Donoghue M., Hsieh F., Baronas E., Godbout K., Gosselin M., Stagliano N. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ Res. 2000;87(5):E1–E9. doi: 10.1161/01.res.87.5.e1. [DOI] [PubMed] [Google Scholar]
- 13.Kawabe Y, Mori J, Morimoto H, Yamaguchi M, Miyagaki S, Ota T, Tsuma Y, Fukuhara S, Nakajima H, Oudit GY,Hosoi H. ACE2 exerts anti-obesity effect via stimulating brown adipose tissue and induction of browning in white adipose tissue. Am J Physiol Endocrinol Metab 2019;317(6):E1140-E9. 10.1152/ajpendo.00311.2019. [DOI] [PubMed]
- 14.Wang W, Xu Y, Gao R, Lu R, Han K, Wu G,Tan W. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020. https://doi.org/10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed]
- 15.Ryan P.M., Caplice N.M. Is adipose tissue a reservoir for viral spread, immune activation, and cytokine amplification in coronavirus disease 2019? Obesity (Silver Spring) 2020 doi: 10.1002/oby.22843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mai K., Brachs M., Leupelt V., Jumpertz-von Schwartzenberg R., Maurer L., Gruters-Kieslich A. Effects of a combined dietary, exercise and behavioral intervention and sympathetic system on body weight maintenance after intended weight loss: results of a randomized controlled trial. Metabolism. 2018;83:60–67. doi: 10.1016/j.metabol.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 17.Brachs M., Wiegand S., Leupelt V., Ernert A., Kintscher U., Jumpertz von Schwarzenberg R. ANP system activity predicts variability of fat mass reduction and insulin sensitivity during weight loss. Metabolism. 2016;65(6):935–943. doi: 10.1016/j.metabol.2016.03.013. [DOI] [PubMed] [Google Scholar]
- 18.Mai K., Li L., Wiegand S., Brachs M., Leupelt V., Ernert A. An integrated understanding of the molecular mechanisms of how adipose tissue metabolism affects long-term body weight maintenance. Diabetes. 2019;68(1):57–65. doi: 10.2337/db18-0440. [DOI] [PubMed] [Google Scholar]
- 19.Mai K., Andres J., Biedasek K., Weicht J., Bobbert T., Sabath M. Free fatty acids link metabolism and regulation of the insulin-sensitizing fibroblast growth factor-21. Diabetes. 2009;58(7):1532–1538. doi: 10.2337/db08-1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matthews D.R., Hosker J.P., Rudenski A.S., Naylor B.A., Treacher D.F., Turner R.C. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 21.Li L, Spranger L, Stobäus N, Beer F, Decker A, Wernicke C, Brachs S, Brachs M, Spranger J,Mai K. Fetuin-B, a potential link of liver-adipose tissue cross talk: results of randomized weight loss- weight maintenance trial. Manuscript submitted for publication. [DOI] [PMC free article] [PubMed]
- 22.Uchiyama T., Okajima F., Mogi C., Tobo A., Tomono S., Sato K. Alamandine reduces leptin expression through the c-Src/p38 MAP kinase pathway in adipose tissue. PLoS One. 2017;12(6) doi: 10.1371/journal.pone.0178769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown R.J., Valencia A., Startzell M., Cochran E., Walter P.J., Garraffo H.M. Metreleptin-mediated improvements in insulin sensitivity are independent of food intake in humans with lipodystrophy. J Clin Invest. 2018;128(8):3504–3516. doi: 10.1172/JCI95476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dominici F.P., Burghi V., Munoz M.C., Giani J.F. Modulation of the action of insulin by angiotensin-(1-7) Clin Sci (Lond) 2014;126(9):613–630. doi: 10.1042/CS20130333. [DOI] [PubMed] [Google Scholar]
- 25.Glowacka I., Bertram S., Herzog P., Pfefferle S., Steffen I., Muench M.O. Differential downregulation of ACE2 by the spike proteins of severe acute respiratory syndrome coronavirus and human coronavirus NL63. J Virol. 2010;84(2):1198–1205. doi: 10.1128/JVI.01248-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bode B., Garrett V., Messler J., McFarland R., Crowe J., Booth R. Glycemic characteristics and clinical outcomes of COVID-19 patients hospitalized in the United States. J Diabetes Sci Technol. 2020;1932296820924469 doi: 10.1177/1932296820924469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singh A.K., Singh R. Does poor glucose control increase the severity and mortality in patients with diabetes and COVID-19? Diabetes Metab Syndr. 2020;14(5):725–727. doi: 10.1016/j.dsx.2020.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Svetkey L.P., Stevens V.J., Brantley P.J., Appel L.J., Hollis J.F., Loria C.M. Comparison of strategies for sustaining weight loss: the weight loss maintenance randomized controlled trial. JAMA. 2008;299(10):1139–1148. doi: 10.1001/jama.299.10.1139. [DOI] [PubMed] [Google Scholar]
- 29.Del C.P., Bryan D.R., Garvey W.T., Gower B.A., Hunter G.R. Dietary adherence during weight loss predicts weight regain. Obesity (Silver Spring) 2011;19(6):1177–1181. doi: 10.1038/oby.2010.298. https://doi.org/oby2010298 [pii];10.1038/oby.2010.298 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.


