Abstract
Females have more robust immune responses than males, and viral infections are more severe for males. Hormones and genetic sex, namely the X chromosome, influence sex differences with immune responses. Here, we review recent findings underlying sexual dimorphism of disease susceptibility for two prevalent viral infections, influenza and SARS-CoV-2, which exhibit male-biased disease severity. Viral infections are proposed to be an initiating event for autoimmunity, which exhibits a female bias. We also review recent work elucidating the epigenetic and genetic contribution of X-Chromosome Inactivation maintenance, and X-linked gene expression, for the autoimmune disorder Systemic Lupus Erythematosus, and highlight the complex considerations required for identifying underlying hormonal and genetic contributions responsible for sex differences in immune responses.
Current Opinion in Physiology 2021, 19:62–72
This review comes from a themed issue on Inflammation
Edited by Pilar Alcaide and Michael Schnoor
Available online 13th October 2020
For complete overview of the section, please refer the article collection - Inflammation
https://doi.org/10.1016/j.cophys.2020.09.015
2468-8673/© 2020 Elsevier Ltd. All rights reserved.
Introduction
Women have more robust immune responses following infection from a variety of pathogens, leading to decreased mortality. Yet this propensity for stronger immune responses may contribute towards increased incidence of autoimmune disease in women. Sexual dimorphism with immune responses originates from genetic and hormonal differences with the immune system, which influence how immune responses to pathogens. Innate immune cells, including neutrophils, natural killer (NK) cells, and macrophages, respond to pathogens by lysing infected cells, phagocytosing infectious particles, and releasing soluble signals such as inflammatory cytokines [1, 2, 3, 4]. B and T cells, the major players of the adaptive immune system, become activated in a targeted manner against invading pathogens. B cells produce and secrete specific antibodies that neutralize viral particles and facilitate clearance of the pathogen [1,5]. Upon antigen encounter, some virus-specific B cells enter germinal centers of secondary lymphoid organs to somatically mutate the antigen binding domain and class-switch the constant domains of their immunoglobulin genes [1,5]. This increases the affinity of viral-specific antibodies for the viral antigen and alters effector capabilities of the antibody [1,5]. T cells, either CD8+ or CD4+, can directly lyse infected cells and secrete pro-inflammatory cytokines [1,6]. Two specialized subsets of CD4+ T cells are T follicular helper cells (Tfh), which facilitate B cell affinity maturation, and T regulatory cells (Tregs) suppress aberrant immune responses in the periphery [1,7,8]. The adaptive immune system generates long-lived memory cells that can respond quickly and robustly in the event of a secondary challenge by the same pathogen [1,6,9].
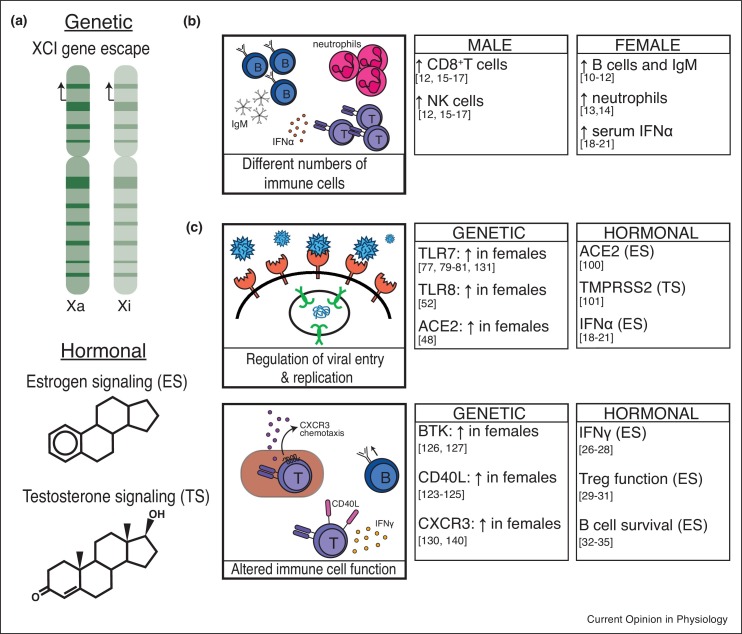
There is sexual dimorphism with immune responses in healthy individuals, with observed differences in numbers of immune cell populations and serum cytokine concentrations (Figure 1 ). Women have higher numbers of B cells and elevated serum levels of non-class switched antibodies [10, 11, 12]. Woman and female rats also have elevated neutrophil counts [13,14] as well as a higher proportion of CD4+ T cells [12,15, 16, 17]. In contrast, men have more CD8+ T cells and NK cells [12,15, 16, 17]. There are also sex differences with serum cytokine production, in particular Type I Interferons (IFNα) as well as IL-10 [18, 19, 20, 21, 22]. These baseline differences contribute to sex biases when the immune system is activated in the presence of a pathogen.
Figure 1.
Genetic and hormonal contributions to sexually biased immune responses. (a) Possible factors leading to sexual dimorphic gene expression. (b) Differential immune cell populations and soluble mediators in males compared to females. Legend: B cells (dark blue), neutrophils (pink), T cells (purple), IFNα (pink dots). (c) Examples of alterations in gene expression driven by genetic or hormonal factors leading to sex-biased immune responses. Legend: Virus (blue), viral receptor (salmon), endosomal TLR7 and TLR8 (green), viral RNA (blue strands), B cells (dark blue), T cells (purple), CD40L (pink, on T cell), CXCR3 (purple dots), IFNγ (orange dots).
Genetics and hormones contribute to sex differences with immune responses. Sex hormones such as estrogen and testosterone are produced at different ratios, and receptor signaling events regulate a number of important immunity-related genes. Sex hormone signaling can also alter the susceptibility of non-immune cells to viral infection, as well as drive both innate and adaptive immune cell activation and function [23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35] (Figure 1). Estrogen receptor signaling is associated with elevated production of innate pro-inflammatory cytokines, particularly antiviral IFNs [19,21,23]. Elevated estrogen levels during pregnancy and estrus cycles dampen pro-inflammatory responses, demonstrating the complex interactions sex hormones have with immune responses [23]. The genetic basis for sex differences resides with the sex chromosomes, and the X chromosome is enriched for important regulatory immune-related genes [36,37]. Female mammals have two X chromosomes and use X-chromosome inactivation (XCI), a hallmark example of epigenetic gene regulation, to equalize X-linked gene expression between the sexes. XCI is established early in female embryonic development and maintained through each cell division through adulthood by various epigenetic modifications, including Xist RNA, repressive histone modifications (H3K27me3, H2AK119-ubiquitin, H4K20me1), the histone variant macroH2a, and DNA methylation, which are enriched across the inactive X (Xi) [38, 39, 40, 41, 42, 43]. The Xi is often found near the nucleolus and at the nuclear periphery, which is enriched for heterochromatin [44]. Some genes escape XCI and are expressed from both X chromosomes, leading to altered expression levels between the sexes [45, 46, 47, 48, 49, 50, 51, 52]. Here, we will review recent advances investigating sex differences with viral responses to influenza and coronaviruses, and genetic as well as epigenetic contributions to female-biased autoimmunity.
Sex differences with viral infections and vaccinations: influenza and coronaviruses
Sex differences with viral infections and clinical outcomes have been observed for a variety of viruses. Male children under age four had significantly more occurrences of measles, viral meningitis, and hepatitis infections [53]. Male sex in adults was also a significant predictor of severe disease outcome with several viruses, including from hepatitis A, hepatitis B, Epstein-Barr virus, and West Nile virus [54, 55, 56, 57, 58]. Men also had higher levels of HIV RNA in their blood, although when accounting for viral load, women were more likely to develop AIDS [59]. Some viruses display a bias towards more severe disease in female mammals, which might be indicative of heightened antiviral immune responses also causing aberrant immunopathology [58]. Successful viral vaccination strategies rely on the adaptive immune system generating a memory response to inactivated viral particles or viral subunits, and sex biases have also been reported. Women mounted stronger class-switched antibody profiles for vaccinations against yellow fever, measles mumps and rubella, hepatitis A, hepatitis B, herpes, smallpox, and influenza [60, 61, 62, 63, 64, 65]. Women also displayed increased local inflammation around the site of vaccination, which may reflect sexual dimorphism with innate immune activation [60,62,65].
Immune responses to viruses exhibit sexual dimorphism, including in viral cell entry, recognition of viral motifs, and immune cell activation (Figure 1). In this section, we highlight recent advances in understanding the underlying sex differences in response to two viral infections that impose great disease burdens worldwide: influenza and SARS-CoV-2. The current mechanistic explanations for sex-biased responses to these infections involve both hormonal and genetic factors as well as interactions between the immune system and other biological processes, serving to highlight the complexities inherent in identifying the underlying causes of sex-biased disease parameters.
Sex differences with influenza infections and vaccines
Seasonal influenza infections result in 3–5 million severe cases and 290 000–650 000 deaths per year worldwide [66] and while many reports do not disaggregate by sex, there is a clear sex bias in influenza susceptibility that changes with age. Young men before puberty exhibit more severe disease compared to age-matched women, which suggests genetic origins for observed sex differences [67,68]. In addition, older men are more likely to be hospitalized versus older women [69]. In contrast, adult pre-menopausal women have increased likelihood of severe illness [67] and increased lung pathology. The picture of sex bias during influenza infection becomes more complicated when examining responses to pandemic influenza outbreaks [70]. For example, in the 2009 H1N1 pandemic, pre-menopausal women had higher rates of hospitalization and also higher risk of death [71, 72, 73, 74] ; in contrast, during the 1917–1918 epidemic there was increased mortality for men [75]. Both viral-mediated and immunopathologic lung damage can occur during influenza infection, resulting in the development of Acute Respiratory Distress Syndrome (ARDS) and hypoxemic respiratory failure [76]. Therefore, the increased susceptibility to influenza for women (post-puberty and pre-menopausal) and male-predominance of disease severity particularly with increased age could arise from genetic differences between the sexes in addition to hormonal changes during aging.
Influenza infections in mice also exhibit sex differences. Female mice challenged with the H1N1 strain of influenza produced higher levels of neutralizing and class-switched antibodies compared to male mice [77••]. Higher antibody titers were also observed in female mice following vaccination with inactivated virus, which correlated with higher numbers of germinal center B cells, CD8+ and CD4+ T cells in lymph nodes [77••]. Female immunized mice also had higher transcription of Toll-like receptor 7 (Tlr7) and reduced DNA methylation at the Tlr7 promoter in B cells [77••]. Tlr7 is X-linked and plays a role in inducing class-switch recombination in B cells [78], thus female-specific elevations with Tlr7 could explain the higher levels of class-switched antibodies that provide protection from influenza infections (Figure 1 ). We and others have reported Tlr7 escape from XCI in human and mouse B cells [79, 80, 81], yet additional work is required to establish whether biallelic expression of Tlr7 provides increased protection from influenza.
Sex hormones also contribute to observed sex differences with immune responses to influenza. Estrogen treatment reduced influenza A replication in human nasal epithelial cells derived from female, but not male, donors, suggesting that estrogen receptor signaling directly affects influenza virus replication [82]. Testosterone also impacts immune responses. Using a machine learning approach, a cluster of lipid metabolism genes regulated by testosterone were identified which correlated with male-specific poor vaccine-induced antibody production [65]. Men with elevated testosterone levels also had the lowest level of antibody production [65]. Yet studies in mice found that testosterone reduced lung inflammation and improved survival following influenza infection, and androgen replacement treatment did not impact the susceptibility of aged mice [83, 84, 85, 86]. Additional work is necessary to elucidate the molecular details and species-specificity of sex hormones affecting viral-induced injury and lung repair following influenza infection.
Sex differences with coronavirus and resulting respiratory disease
The current COVID-19 global pandemic is caused by a coronavirus (SARS-CoV-2) and men are more susceptible to infection, severe disease, and mortality [87, 88, 89]. Previous outbreaks of the coronaviruses SARS-CoV and MERS-CoV, which resulted in SARS and MERS, also exhibited a male bias for severe disease [90, 91, 92]. While epidemiological data from the COVID-19 pandemic is complicated by uneven reporting of sex-disaggregated data and country-by-country testing criterion, a recent analysis from 38 countries identified a male bias for COVID-19 fatalities in 37 countries [89]. For the 12 countries for which the data was available, further breakdown by both sex and age revealed a significant male bias in case fatality for every age above 30 years [89]. Peripheral blood mononuclear cells (PBMCs) from patients in the United States presenting with mild to moderate COVID-19 symptoms exhibited sexual dimorphism with immune populations, where male patients had more non-classical monocytes and female patients had more robust activated T cell profiles [93•]. In addition, when analyzing immune determinants that correlated with progression to severe disease, men had reduced T cell signatures characteristic of severe disease, and women had elevated viral antigen-specific class-switched antibodies which is predictive of disease protection [93•].
Mouse modeling suggests that estrogen may play a protective role in SARS-CoV-2 infection and COVID-19 disease. Utilizing a mouse-adapted strain of SARS-CoV, the Perlman lab demonstrated that male mice were more susceptible to infection, with significantly increased lung damage and mortality [94••]. In addition, male mice had elevated levels of pro-inflammatory cytokines as well as inflammatory macrophage/monocyte infiltration as compared to female mice [94••]. Importantly, while gonadectomies or treatment with a nonsteroidal anti-androgen had no effect on viral pathogenesis in male mice, gonadectomies and estrogen-receptor antagonist treatment significantly increased viral susceptibility in female mice [94••]. Whether estrogen treatment impacts SARS-CoV infection or disease in male mice remains an open question. While this study demonstrated that estrogen signaling is an important factor in the sex differential susceptibility to SARS-CoV infection, the applicability to SARS-CoV-2 infection is unknown.
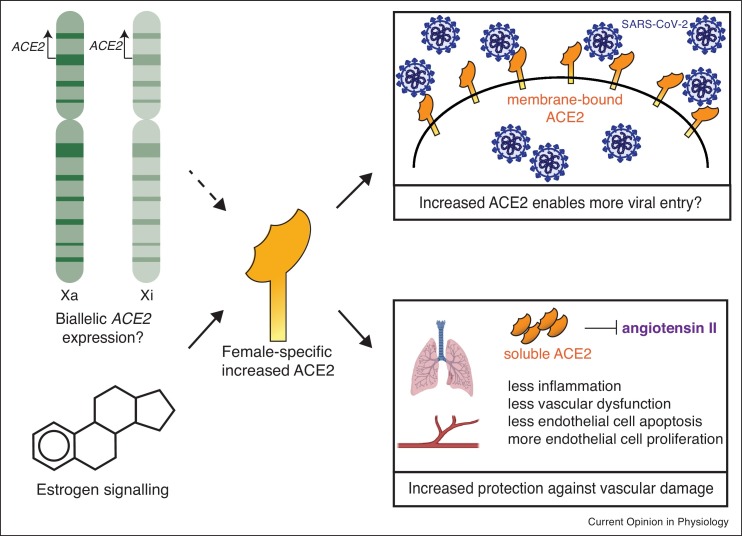
An intriguing aspect to the pathogenesis of SARS-CoV and SARS-CoV-2 is that cell tropism relies on the cellular receptor Angiotensin Converting Enzyme 2 (ACE2) and the serine protease TMPRSS2 [95,96,97•,98,99], and expression of both genes exhibits sex differences [100,101]. Both ACE2 and TMPRSS2 contain hormone response elements in their promoter regions, and ACE2 expression is increased by estrogen in human airway epithelial cells [100]. Estrogen-mediated regulation of ACE2 alone does not account for the male-bias with COVID-19 disease and mortality, as the male bias is observed among older adults, including post-menopausal women who may/may not be on hormone replacement therapy [89]. The ACE2 gene is X-linked, thus women have the potential to express more ACE2 protein if this gene escapes XCI in specific cell types, as ACE2 escapes XCI in human fibroblasts [48]. Female-specific increased expression of ACE2 could facilitate greater SARS-CoV-2 infection (Figure 2 ), which would likely result in higher cases or greater severity of disease, which is not observed clinically [89]. Recent observations suggest that women express higher levels of ACE2 in a variety of tissues, and that ACE2 expression may decrease with age specifically in human male tissues [102]. ACE2 negatively regulates angiotensin II, which promotes lung injury by increasing vascular dysfunction and inflammation [103]. Moreover, angiotensin II can directly induce endothelial apoptosis [104,105] and inhibit endothelial cell proliferation, worsening lung injury [106] and providing an explanatory context for the vascular dysfunction observed in COVID-19 [107]. Thus, elevated ACE2 levels are actually protective, and reduce susceptibility for severe lung injury as well as other vascular-related injuries resulting from SARS-CoV-2 infection [96,103] (Figure 2). An intriguing alternative explanation for elevated ACE2 expression that may facilitate protection is that ACE2 is an IFN-stimulated gene [108], thus ACE2 upregulation may increase viral entry into cells already in a heightened antiviral state [109]. Further examination of the genetic and hormonal contributions that result in differences with ACE2 and TMPRSS2 expression in the context of SARS-CoV-2 infection and COVID-19 disease is necessary.
Figure 2.
Female-biased increased expression of ACE2 could have different outcomes that alter susceptibility to SARS-CoV-2 and COVID-19 disease severity. Female-specific increases in ACE2 expression due to genetic and hormonal factors might lead to more viral entry but also leads more active ACE2 and subsequent protection from vascular dysfunction. Legend: ACE2 (orange), SARS-CoV-2 virions (blue).
Interplay between viral infection and initiation of autoimmune disease
The causes for autoimmune disease are unclear, yet studies suggest that viral infection is strongly correlated with the onset of autoimmunity [110]. Indeed, many different types of viruses have been linked to the development of autoimmunity in humans, including Epstein-Barr Virus, Human Cytomegalovirus, and Human T-Lymphotropic Virus 1 [110, 111, 112, 113]. Lymphocytic Choriomeningitis Virus (LCMV) accelerates onset of disease in the classic spontaneous mouse model of lupus, NZB/W F1 mice, which exhibits a female-bias [114]. Viral infection is proposed to drive autoimmunity onset through a variety of mechanisms, where viral-derived T cell-stimulatory molecules (which are structurally similar to self-peptides) result in inflammation and activation of self-reactive T and B cells [110]. Viral infections can often result in abnormal activation of self-reactive T cells, which is a hallmark of autoimmune diseases [115]. Therefore, hormonal and genetic factors that result in more robust female T and B cell activation during viral infections may contribute towards the female-bias with autoimmune disease, and additional research is necessary to elucidate the mechanisms underlying this hypothesis.
Sex differences in autoimmune diseases
About 25 autoimmune disorders have a strong female bias. Sjögren’s syndrome, Grave’s disease, and Hashimodo’s thyroidosis exhibit >90% incidence among women, while Systemic Lupus Erythematosus (SLE), antiphospholipid antibody syndrome, and systemic scleroderma patients are 70–85% female [116,117]. Indeed, autoimmune disease is one of the leading causes of death for women in the United States [116].
One genetic factor that increases the risk for developing SLE or SS is the number of X chromosomes, as 46,XX females and 47,XXY individuals with Klinefelter syndrome display heightened incidence as compared to 46,XY men [116, 117, 118]. Individuals with one X chromosome (46,XY males and 45,X Turner syndrome patients) have the lowest risk for developing SLE and SS, while people with more than two X chromosomes (47,XXX Trisomy X syndrome patients) have the highest risk [119, 120, 121]. While XCI results in dosage compensation for X-linked genes between males and females, some genes regularly escape silencing [45, 46, 47, 48, 49, 50, 51]. It is conceivable that additional immune-linked gene escape can contribute to autoimmune onset and severity, as overexpression of some immunity-related X-linked genes have been observed in SLE patients [122]. Mouse studies have shown that overexpression of CD40LG or BTK results in autoantibody production and immune complex-mediated glomerulonephritis [123, 124, 125, 126, 127], and transgenic overexpression of Tlr7 in mice can result in various symptoms of autoimmunity, including anti-nucleic acid antibodies, spontaneous lymphocyte activation, and glomerulonephritis [128,129]. Biallelic expression of Tlr7, ranging from 10 to 40% of cells, has also been recently observed in human B cells from healthy women, female SLE patients, as well as men with Kleinfelter syndrome [79,130]. Intriguingly, female lupus-prone NZB/W F1 mice also exhibit biallelic expression of Tlr7 that did not change with disease progression [131••]. A polymorphism in the X-linked gene CXorf21, an adaptor for endosomal TLRs including TLR7, is strongly associated with SLE in European populations [132, 133, 134]. Whether CXorf21 escapes XCI is an intriguing open question. These observations suggest that abnormal expression of multiple X-linked genes likely contributes to SLE disease severity.
Lupus disease also affects the localization of epigenetic modifications to the Xi, which may account for X-linked gene expression changes. Circulating T and B cells from both mice and humans display non-canonical features of XCI, as the Xi lacks Xist RNA localization and enrichment of heterochromatic modifications, although XIST/Xist is expressed at normal levels [80,135••,136]. In vitro stimulation results in accumulation of both XIST RNA and heterochromatic marks on the Xi [80,136]. We recently found that in vitro activated T cells from human SLE patients and both T and B cells from late-stage disease NZB/W F1 mice have dispersed Xist RNA localization patterns [131••,135••]. In addition, T cells from SLE patients had altered expression of X-linked genes, including overexpression of genes involved in metabolism, cell cycle, and proliferation [135••]. Furthermore, B cells from NZB/W F1 mice progressively lost H3K27me3 enrichment on the Xi with increased disease development [131••], suggesting that abnormal XCI is a consequence of lupus disease, and not causal. These findings suggest that perturbed XCI may be responsible for abnormal X-linked gene expression in SLE, and additional work is necessary to determine whether other autoimmune diseases also exhibit perturbed XCI maintenance.
Conclusion
Increasing appreciation of sex as a biological variable will likely to expand our understanding of the underlying genetic and hormonal contributions to observed sex biases for prevalence and disease severity during viral infection and autoimmunity. Here, we highlighted two examples of viral pathogens, influenza and SARS-CoV-2, where clinical observations and mouse models indicate that both X-linked gene expression and hormones contribute to the predominant male bias. The contribution of XCI escape in specific cell types important for flu and SARS-CoV-2 infection are unknown at present, yet will likely reveal important players that contribute to observed sex differences. There are other examples of pathogens, such as Leishmania and Treponema pallidum, which exhibit sexual dimorphism with infections, cytokine production, and resulting pathologies [137, 138, 139]. Yet the genetic contribution of XCI maintenance and XCI escape for these sex differences with pathogen-induced diseases remains unclear. One recent report demonstrated that during Leishmania infection, the chemokine receptor Cxcr3 escapes XCI in T cells [140]. T cells with biallelic Cxcr3 expression displayed heightened functionality in mice, suggesting that XCI escape could be a significant factor for female-bias with immune responses. While individuals with multiple X chromosomes have higher risk for autoimmune diseases, not all XX individuals will develop an autoimmune disease. Viral infections are correlated with the onset of autoimmunity in women, and more research is necessary to unravel the genetic and hormonal contributions that initiate the pathway for the loss of self-tolerance. Understanding the sex-specific mechanisms of immune responses to pathogens will reveal more effective treatment strategies for pathogen-induced diseases.
Conflict of interest statement
Nothing declared.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
CRediT authorship contribution statement
Katherine S Forsyth: Writing - original draft. Montserrat C Anguera: Writing - review & editing, Conceptualization.
Acknowledgements
We would like to thank all the members of the Anguera lab for helpful discussions regarding the article structure and feedback on figure design.
References
- 1.Murphy K., Weaver C. 9th ed. Garland Science; New York: 2017. Janeway’s Immunobiology. [Google Scholar]
- 2.Amulic B., Cazalet C., Hayes G.L., Metzler K.D., Zychlinsky A. Neutrophil function: from mechanisms to disease. Annu Rev Immunol. 2012;30:459–489. doi: 10.1146/annurev-immunol-020711-074942. Epub 2012/01/10. PubMed PMID: 22224774. [DOI] [PubMed] [Google Scholar]
- 3.Freund-Brown J., Chirino L., Kambayashi T. Strategies to enhance nk cell function for the treatment of tumors and infections. Crit Rev Immunol. 2018;38:105–130. doi: 10.1615/CritRevImmunol.2018025248. Epub 2018/06/29. PubMed PMID: 29953390; PubMed Central PMCID: PMC6343663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Uribe-Querol E., Rosales C. Phagocytosis: our current understanding of a universal biological process. Front Immunol. 2020;11:1066. doi: 10.3389/fimmu.2020.01066. Epub 2020/06/26. PubMed PMID: 32582172; PubMed Central PMCID: PMC7280488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abbott R.K., Crotty S. Factors in b cell competition and immunodominance. Immunol Rev. 2020 doi: 10.1111/imr.12861. Epub 2020/06/03. PubMed PMID: 32483855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farber D.L. Form and function for t cells in health and disease. Nat Rev Immunol. 2020;20:83–84. doi: 10.1038/s41577-019-0267-8. Epub 2020/01/01. PubMed PMID: 31889162. [DOI] [PubMed] [Google Scholar]
- 7.Biram A., Davidzohn N., Shulman Z. T cell interactions with b cells during germinal center formation, a three-step model. Immunol Rev. 2019;288:37–48. doi: 10.1111/imr.12737. Epub 2019/03/16. PubMed PMID: 30874355. [DOI] [PubMed] [Google Scholar]
- 8.Sakaguchi S., Mikami N., Wing J.B., Tanaka A., Ichiyama K., Ohkura N. Regulatory t cells and human disease. Annu Rev Immunol. 2020;38:541–566. doi: 10.1146/annurev-immunol-042718-041717. Epub 2020/02/06. PubMed PMID: 32017635. [DOI] [PubMed] [Google Scholar]
- 9.Tomayko M.M., Allman D. What B cell memories are made of. Curr Opin Immunol. 2019;57:58–64. doi: 10.1016/j.coi.2019.01.003. Epub 2019/03/13. PubMed PMID: 30861463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Butterworth M., McClellan B., Allansmith M. Influence of sex in immunoglobulin levels. Nature. 1967;214:1224–1225. doi: 10.1038/2141224a0. Epub 1967/06/17. PubMed PMID: 4169229. [DOI] [PubMed] [Google Scholar]
- 11.Purtilo D.T., Sullivan J.L. Immunological bases for superior survival of females. Am J Dis Child. 1979;133:1251–1253. doi: 10.1001/archpedi.1979.02130120043008. Epub 1979/12/01. PubMed PMID: 517475. [DOI] [PubMed] [Google Scholar]
- 12.Abdullah M., Chai P.S., Chong M.Y., Tohit E.R., Ramasamy R., Pei C.P., et al. Gender effect on in vitro lymphocyte subset levels of healthy individuals. Cell Immunol. 2012;272:214–219. doi: 10.1016/j.cellimm.2011.10.009. Epub 2011/11/15. PubMed PMID: 22078320. [DOI] [PubMed] [Google Scholar]
- 13.Bain B.J. Ethnic and sex differences in the total and differential white cell count and platelet count. J Clin Pathol. 1996;49:664–666. doi: 10.1136/jcp.49.8.664. Epub 1996/08/01. PubMed PMID: 8881919; PubMed Central PMCID: PMC500612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spitzer J.A. Gender differences in some host defense mechanisms. Lupus. 1999;8:380–383. doi: 10.1177/096120339900800510. Epub 1999/08/24. PubMed PMID: 10455517. [DOI] [PubMed] [Google Scholar]
- 15.Lisse I.M., Aaby P., Whittle H., Jensen H., Engelmann M., Christensen L.B. T-lymphocyte subsets in West African children: impact of age, sex, and season. J Pediatr. 1997;130:77–85. doi: 10.1016/s0022-3476(97)70313-5. Epub 1997/01/01. PubMed PMID: 9003854. [DOI] [PubMed] [Google Scholar]
- 16.Lee B.W., Yap H.K., Chew F.T., Quah T.C., Prabhakaran K., Chan G.S., et al. Age- and sex-related changes in lymphocyte subpopulations of healthy Asian subjects: from birth to adulthood. Cytometry. 1996;26:8–15. doi: 10.1002/(SICI)1097-0320(19960315)26:1<8::AID-CYTO2>3.0.CO;2-E. Epub 1996/03/15. doi: 10.1002/(SICI)1097-0320(19960315)26:1<8::AID-CYTO2>3.0.CO;2-E. PubMed PMID: 8809475. [DOI] [PubMed] [Google Scholar]
- 17.Uppal S.S., Verma S., Dhot P.S. Normal values of cd4 and cd8 lymphocyte subsets in healthy Indian adults and the effects of sex, age, ethnicity, and smoking. Cytometry B Clin Cytom. 2003;52:32–36. doi: 10.1002/cyto.b.10011. Epub 2003/02/25. PubMed PMID: 12599179. [DOI] [PubMed] [Google Scholar]
- 18.Berghofer B., Frommer T., Haley G., Fink L., Bein G., Hackstein H. Tlr7 ligands induce higher ifn-alpha production in females. J Immunol. 2006;177:2088–2096. doi: 10.4049/jimmunol.177.4.2088. Epub 2006/08/05. PubMed PMID: 16887967. [DOI] [PubMed] [Google Scholar]
- 19.Griesbeck M., Ziegler S., Laffont S., Smith N., Chauveau L., Tomezsko P., et al. Sex differences in plasmacytoid dendritic cell levels of irf5 drive higher ifn-alpha production in women. J Immunol. 2015;195:5327–5336. doi: 10.4049/jimmunol.1501684. Epub 2015/11/01. PubMed PMID: 26519527; PubMed Central PMCID: PMC4654231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meier A., Chang J.J., Chan E.S., Pollard R.B., Sidhu H.K., Kulkarni S., et al. Sex differences in the toll-like receptor-mediated response of plasmacytoid dendritic cells to HIV-1. Nat Med. 2009;15:955–959. doi: 10.1038/nm.2004. Epub 2009/07/15. PubMed PMID: 19597505; PubMed Central PMCID: PMC2821111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seillet C., Laffont S., Tremollieres F., Rouquie N., Ribot C., Arnal J.F., et al. The tlr-mediated response of plasmacytoid dendritic cells is positively regulated by estradiol in vivo through cell-intrinsic estrogen receptor alpha signaling. Blood. 2012;119:454–464. doi: 10.1182/blood-2011-08-371831. Epub 2011/11/19. PubMed PMID: 22096248. [DOI] [PubMed] [Google Scholar]
- 22.Torcia M.G., Nencioni L., Clemente A.M., Civitelli L., Celestino I., Limongi D., et al. Sex differences in the response to viral infections: Tlr8 and tlr9 ligand stimulation induce higher il10 production in males. PLoS One. 2012;7 doi: 10.1371/journal.pone.0039853. Epub 2012/07/07. PubMed PMID: 22768144; PubMed Central PMCID: PMC3387221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kovats S. Estrogen receptors regulate innate immune cells and signaling pathways. Cell Immunol. 2015;294:63–69. doi: 10.1016/j.cellimm.2015.01.018. Epub 2015/02/07. PubMed PMID: 25682174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trigunaite A., Dimo J., Jorgensen T.N. Suppressive effects of androgens on the immune system. Cell Immunol. 2015;294:87–94. doi: 10.1016/j.cellimm.2015.02.004. Epub 2015/02/25. PubMed PMID: 25708485. [DOI] [PubMed] [Google Scholar]
- 25.Khan D., Ansar Ahmed S. The immune system is a natural target for estrogen action: opposing effects of estrogen in two prototypical autoimmune diseases. Front Immunol. 2015;6:635. doi: 10.3389/fimmu.2015.00635. Epub 2016/01/19. PubMed PMID: 26779182; PubMed Central PMCID: PMC4701921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grasso G., Muscettola M. The influence of beta-estradiol and progesterone on interferon gamma production in vitro. Int J Neurosci. 1990;51:315–317. doi: 10.3109/00207459008999730. Epub 1990/04/01. PubMed PMID: 2126259. [DOI] [PubMed] [Google Scholar]
- 27.Fox H.S., Bond B.L., Parslow T.G. Estrogen regulates the ifn-gamma promoter. J Immunol. 1991;146:4362–4367. Epub 1991/06/15. PubMed PMID: 1904081. [PubMed] [Google Scholar]
- 28.Karpuzoglu-Sahin E., Hissong B.D., Ansar Ahmed S. Interferon-gamma levels are upregulated by 17-beta-estradiol and diethylstilbestrol. J Reprod Immunol. 2001;52:113–127. doi: 10.1016/s0165-0378(01)00117-6. Epub 2001/10/16. PubMed PMID: 11600182. [DOI] [PubMed] [Google Scholar]
- 29.Tai P., Wang J., Jin H., Song X., Yan J., Kang Y., et al. Induction of regulatory t cells by physiological level estrogen. J Cell Physiol. 2008;214:456–464. doi: 10.1002/jcp.21221. Epub 2007/07/27. PubMed PMID: 17654501. [DOI] [PubMed] [Google Scholar]
- 30.Polanczyk M.J., Carson B.D., Subramanian S., Afentoulis M., Vandenbark A.A., Ziegler S.F., et al. Cutting edge: estrogen drives expansion of the cd4+cd25+ regulatory T cell compartment. J Immunol. 2004;173:2227–2230. doi: 10.4049/jimmunol.173.4.2227. Epub 2004/08/06. PubMed PMID: 15294932. [DOI] [PubMed] [Google Scholar]
- 31.Polanczyk M.J., Hopke C., Huan J., Vandenbark A.A., Offner H. Enhanced foxp3 expression and Treg cell function in pregnant and estrogen-treated mice. J Neuroimmunol. 2005;170:85–92. doi: 10.1016/j.jneuroim.2005.08.023. Epub 2005/10/29. PubMed PMID: 16253347. [DOI] [PubMed] [Google Scholar]
- 32.Verthelyi D.I., Ahmed S.A. Estrogen increases the number of plasma cells and enhances their autoantibody production in nonautoimmune c57bl/6 mice. Cell Immunol. 1998;189:125–134. doi: 10.1006/cimm.1998.1372. Epub 1998/10/29. PubMed PMID: 9790726. [DOI] [PubMed] [Google Scholar]
- 33.Bernardi A.I., Andersson A., Grahnemo L., Nurkkala-Karlsson M., Ohlsson C., Carlsten H., et al. Effects of lasofoxifene and bazedoxifene on B cell development and function. Immun Inflamm Dis. 2014;2:214–225. doi: 10.1002/iid3.37. Epub 2015/04/14. PubMed PMID: 25866629; PubMed Central PMCID: PMC4386916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grimaldi C.M., Jeganathan V., Diamond B. Hormonal regulation of B cell development: 17 beta-estradiol impairs negative selection of high-affinity DNA-reactive B cells at more than one developmental checkpoint. J Immunol. 2006;176:2703–2710. doi: 10.4049/jimmunol.176.5.2703. Epub 2006/02/24. PubMed PMID: 16493025. [DOI] [PubMed] [Google Scholar]
- 35.Hill L., Jeganathan V., Chinnasamy P., Grimaldi C., Diamond B. Differential roles of estrogen receptors alpha and beta in control of B-cell maturation and selection. Mol Med. 2011;17:211–220. doi: 10.2119/molmed.2010.00172. Epub 2010/11/26. PubMed PMID: 21107497; PubMed Central PMCID: PMC3060981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ross M.T., Grafham D.V., Coffey A.J., Scherer S., McLay K., Muzny D., et al. The DNA sequence of the human x chromosome. Nature. 2005;434:325–337. doi: 10.1038/nature03440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bianchi I., Lleo A., Gershwin M.E., Invernizzi P. The X chromosome and immune associated genes. J Autoimmun. 2012;38:J187–J192. doi: 10.1016/j.jaut.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 38.Payer B., Lee J.T. X chromosome dosage compensation: how mammals keep the balance. Annu Rev Genet. 2008;42:733–772. doi: 10.1146/annurev.genet.42.110807.091711. [DOI] [PubMed] [Google Scholar]
- 39.Brockdorff N., Ashworth A., Kay G.F., Cooper P., Smith S., McCabe V.M., et al. Conservation of position and exclusive expression of mouse xist from the inactive x chromosome. Nature. 1991;351:329–331. doi: 10.1038/351329a0. [DOI] [PubMed] [Google Scholar]
- 40.Penny G.D., Kay G.F., Sheardown S.A., Rastan S., Brockdorff N. Requirement for Xist in X chromosome inactivation. Nature. 1996;379:131–137. doi: 10.1038/379131a0. [DOI] [PubMed] [Google Scholar]
- 41.Plath K., Fang J., Mlynarczyk-Evans S.K., Cao R., Worringer K.A., Wang H., et al. Role of histone h3 lysine 27 methylation in X inactivation. Science. 2003;300:131. doi: 10.1126/science.1084274. [DOI] [PubMed] [Google Scholar]
- 42.Silva J., Mak W., Zvetkova I., Appanah R., Nesterova T.B., Webster Z., et al. Establishment of histone h3 methylation on the inactive X chromosome requires transient recruitment of eed-enx1 polycomb group complexes. Dev Cell. 2003;4:481–495. doi: 10.1016/S1534-5807(03)00068-6. [DOI] [PubMed] [Google Scholar]
- 43.Galupa R., Heard E. X-chromosome inactivation: a crossroads between chromosome architecture and gene regulation. Annu Rev Genet. 2018;52:535–566. doi: 10.1146/annurev-genet-120116-024611. Epub 2018/09/27. PubMed PMID: 30256677. [DOI] [PubMed] [Google Scholar]
- 44.Zhang L.F., Huynh K.D., Lee J.T. Perinucleolar targeting of the inactive X during S phase: Evidence for a role in the maintenance of silencing. Cell. 2007;129:693–706. doi: 10.1016/j.cell.2007.03.036. Epub 2007/05/22. doi: S0092-8674(07)00452-7 [pii]10.1016/j.cell.2007.03.036. PubMed PMID: 17512404. [DOI] [PubMed] [Google Scholar]
- 45.Berletch J.B., Yang F., Disteche C.M. Escape from X inactivation in mice and humans. Genome Biol. 2010;11:213. doi: 10.1186/gb-2010-11-6-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Berletch J.B., Yang F., Xu J., Carrel L., Disteche C.M. Genes that escape from X inactivation. Hum Genet. 2011;130:237–245. doi: 10.1007/s00439-011-1011-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carrel L., Willard H.F. X-inactivation profile reveals extensive variability in X-linked gene expression in females. Nature. 2005;434:400–404. doi: 10.1038/nature03479. [DOI] [PubMed] [Google Scholar]
- 48.Balaton B.P., Cotton A.M., Brown C.J. Derivation of consensus inactivation status for X-linked genes from genome-wide studies. Biol Sex Differences. 2015;6:35. doi: 10.1186/s13293-015-0053-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Berletch J.B., Ma W., Yang F., Shendure J., Noble W.S., Disteche C.M., et al. Escape from X inactivation varies in mouse tissues. PLoS Genet. 2015;11 doi: 10.1371/journal.pgen.1005079. Epub 2015/03/19. PubMed PMID: 25785854; PubMed Central PMCID: PMC4364777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang F., Babak T., Shendure J., Disteche C.M. Global survey of escape from X inactivation by RNA-sequencing in mouse. Genome Res. 2010;20:614–622. doi: 10.1101/gr.103200.109. Epub 2010/04/07. PubMed PMID: 20363980; PubMed Central PMCID: PMC2860163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Carrel L., Brown C.J. When the lyon(ized chromosome) roars: ongoing expression from an inactive X chromosome. Philos Trans R Soc Lond B Biol Sci. 2017;372 doi: 10.1098/rstb.2016.0355. Epub 2017/09/28. PubMed PMID: 28947654; PubMed Central PMCID: PMC5627157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McDonald G., Cabal N., Vannier A., Umiker B., Yin R.H., Orjalo A.V., Jr, et al. Female bias in systemic lupus erythematosus is associated with the differential expression of X-linked toll-like receptor 8. Front Immunol. 2015;6 doi: 10.3389/fimmu.2015.00457. Epub 2015/10/07. PubMed PMID: 26441962; PubMed Central PMCID: PMC4561825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Green M.S. The male predominance in the incidence of infectious diseases in children: a postulated explanation for disparities in the literature. Int J Epidemiol. 1992;21:381–386. doi: 10.1093/ije/21.2.381. [DOI] [PubMed] [Google Scholar]
- 54.Chen C.M., Chen S.C.C., Yang H.Y., Yang S.T., Wang C.M. Hospitalization and mortality due to hepatitis a in Taiwan: a 15-year nationwide cohort study. J Viral Hepat. 2016;23:940–945. doi: 10.1111/jvh.12564. [DOI] [PubMed] [Google Scholar]
- 55.Yu M.W., Cheng S.W., Lin M.W., Yang S.Y., Liaw Y.F., Chang H.C., et al. Androgen-receptor gene cag repeats, plasma testosterone levels, and risk of hepatitis B-related hepatocellular carcinoma. J Natl Cancer Inst. 2000;92:2023–2028. doi: 10.1093/jnci/92.24.2023. Epub 2000/12/21. PubMed PMID: 11121465. [DOI] [PubMed] [Google Scholar]
- 56.Murphy G., Pfeiffer R., Camargo M.C., Rabkin C.S. Meta-analysis shows that prevalence of Epstein-Barr virus-positive gastric cancer differs based on sex and anatomic location. Gastroenterology. 2009;137:824–833. doi: 10.1053/j.gastro.2009.05.001. Epub 2009/05/19. PubMed PMID: 19445939; PubMed Central PMCID: PMC3513767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jean C.M., Honarmand S., Louie J.K., Glaser C.A. Risk factors for west Nile virus neuroinvasive disease, California, 2005. Emerg Infect Dis. 2007;13:1918–1920. doi: 10.3201/eid1312.061265. Epub 2008/02/09. PubMed PMID: 18258047; PubMed Central PMCID: PMC2876738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Klein S.L. Sex influences immune responses to viruses, and efficacy of prophylaxis and treatments for viral diseases. Bioessays. 2012;34:1050–1059. doi: 10.1002/bies.201200099. Epub 2012/09/27. PubMed PMID: 23012250; PubMed Central PMCID: PMC4120666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Griesbeck M., Altfeld M. In: Sex and Gender Differences in Infection and Treatments for Infectious Diseases. Klein S.L., Roberts C.W., editors. Springer International Publishing; Switzerland: 2015. Sex differences in the manifestations of HIV-1 infection; pp. 103–181. [Google Scholar]
- 60.Klein S.L., Jedlicka A., Pekosz A. The XS and Y of immune responses to viral vaccines. Lancet Infect Dis. 2010;10:338–349. doi: 10.1016/S1473-3099(10)70049-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Domínguez A., Plans P., Costa J., Torner N., Cardenosa N., Batalla J., et al. Seroprevalence of measles, rubella, and mumps antibodies in Catalonia, Spain: results of a cross-sectional study. Eur J Clin Microbiol Infect Dis. 2006;25:310–317. doi: 10.1007/s10096-006-0133-z. [DOI] [PubMed] [Google Scholar]
- 62.Gaucher D., Therrien R., Kettaf N., Angermann B.R., Boucher Gv, Filali-Mouhim A., et al. Yellow fever vaccine induces integrated multilineage and polyfunctional immune responses. J Exp Med. 2008;205:3119–3131. doi: 10.1084/jem.20082292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Troy J.D., Hill H.R., Ewell M.G., Frey S.E. Sex difference in immune response to vaccination: a participant-level meta-analysis of randomized trials of imvamune smallpox vaccine. Vaccine. 2015:5425–5431. doi: 10.1016/j.vaccine.2015.08.032. PubMed Central PMCID: PMC4581981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Trevisan A., Giuliani A.A.-O.X., Scapellato M.L., Anticoli S.A.-O., Carsetti R., Zaffina S., et al. Sex disparity in response to hepatitis b vaccine related to the age of vaccination. Int J Environ Res Public Health. 2020 doi: 10.3390/ijerph17010327. 1660-4601 (Electronic) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Furman D., Hejblum B.P., Simon N., Jojic V., Dekker C.L., Thiébaut R., et al. Systems analysis of sex differences reveals an immunosuppressive role for testosterone in the response to influenza vaccination. Proc Natl Acad Sci U S A. 2014;111:869. doi: 10.1073/pnas.1321060111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Organization WH . World Health Organization; 2018. Influenza (seasonal)https://www.who.int/news-room/fact-sheets/detail/influenza-(seasonal) Available from: [Google Scholar]
- 67.Morgan R., Klein S.L. The intersection of sex and gender in the treatment of influenza. Curr Opin Virol. 2019;35:35–41. doi: 10.1016/j.coviro.2019.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jensen-Fangel S., Mohey R., Johnsen S.P., Andersen P.L., Sorensen H.T., Ostergaard L. Gender differences in hospitalization rates for respiratory tract infections in Danish youth. Scand J Infect Dis. 2004;36:31–36. doi: 10.1080/00365540310017618. PubMed PMID: 15000556. [DOI] [PubMed] [Google Scholar]
- 69.Wang C.S., Wang S.T., Chou P. Efficacy and cost-effectiveness of influenza vaccination of the elderly in a densely populated and unvaccinated community. Vaccine. 2002;20:2494–2499. doi: 10.1016/s0264-410x(02)00181-0. Epub 2002/06/12. PubMed PMID: 12057604. [DOI] [PubMed] [Google Scholar]
- 70.Vom Steeg L.G., Klein S.L. Sex and sex steroids impact influenza pathogenesis across the life course. Semin Immunopathol. 2019;41:189–194. doi: 10.1007/s00281-018-0718-5. Epub 2018/10/10. PubMed PMID: 30298431; PubMed Central PMCID: PMC6370518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jacobs J.H., Archer B.N., Baker M.G., Cowling B.J., Heffernan R.T., Mercer G., et al. Searching for sharp drops in the incidence of pandemic A/H1N1 influenza by single year of age. PLoS One. 2012;7 doi: 10.1371/journal.pone.0042328. Epub 2012/08/10. PubMed PMID: 22876316; PubMed Central PMCID: PMC3410923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Eshima N., Tokumaru O., Hara S., Bacal K., Korematsu S., Tabata M., et al. Sex- and age-related differences in morbidity rates of 2009 pandemic influenza a h1n1 virus of swine origin in japan. PLoS One. 2011;6 doi: 10.1371/journal.pone.0019409. Epub 2011/05/12. PubMed PMID: 21559366; PubMed Central PMCID: PMC3084848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kumar A., Zarychanski R., Pinto R., Cook D.J., Marshall J., Lacroix J., et al. Critically ill patients with 2009 influenza a(H1N1) infection in canada. JAMA. 2009;302:1872–1879. doi: 10.1001/jama.2009.1496. Epub 2009/10/14. PubMed PMID: 19822627. [DOI] [PubMed] [Google Scholar]
- 74.Klein S.L., Pekosz A., Passaretti C., Anker M., Olukoya P. World Health Organization; 2010. Sex, Gender and Influenza. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Noymer A., Garenne M. The 1918 influenza epidemic’s effects on sex differentials in mortality in the United States. Popul Dev Rev. 2000;26:565–581. doi: 10.1111/j.1728-4457.2000.00565.x. Epub 2000/01/01. PubMed PMID: 19530360; PubMed Central PMCID: PMC2740912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Herold S., Becker C., Ridge K.M., Budinger G.R. Influenza virus-induced lung injury: pathogenesis and implications for treatment. Eur Respir J. 2015;45:1463–1478. doi: 10.1183/09031936.00186214. Epub 2015/03/21. PubMed PMID: 25792631. [DOI] [PubMed] [Google Scholar]
- 77••.Fink A.L., Engle K., Ursin R.L., Tang W.-Y., Klein S.L. Biological sex affects vaccine efficacy and protection against influenza in mice. Proc Natl Acad Sci U S A. 2018;115:12477. doi: 10.1073/pnas.1805268115. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper provides a mechanistic explanation for elevated neutralizing antibody production in female mice infected with influenza, where female-specific increased Tlr7 expression in B cells correlated with disease protection.
- 78.Rawlings D.J., Schwartz M.A., Jackson S.W., Meyer-Bahlburg A. Integration of b cell responses through toll-like receptors and antigen receptors. Nat Rev Immunol. 2012;12:282–294. doi: 10.1038/nri3190. Epub 2012/03/17. PubMed PMID: 22421786; PubMed Central PMCID: PMC3437941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Souyris M., Cenac C., Azar P., Daviaud D., Canivet A., Grunenwald S., et al. Tlr7 escapes x chromosome inactivation in immune cells. Sci Immunol. 2018;3 doi: 10.1126/sciimmunol.aap8855. [DOI] [PubMed] [Google Scholar]
- 80.Wang J., Syrett C.M., Kramer M.C., Basu A., Atchison M.L., Anguera M.C. Unusual maintenance of X chromosome inactivation predisposes female lymphocytes for increased expression from the inactive X. Proc Natl Acad Sci U S A. 2016;113 doi: 10.1073/pnas.1520113113. E2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Syrett C.M., Sindhava V., Sierra I., Dubin A.H., Atchison M., Anguera M.C. Diversity of epigenetic features of the inactive X-chromosome in NK cells, dendritic cells, and macrophages. Front Immunol. 2019;9:3087. doi: 10.3389/fimmu.2018.03087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Peretz J., Pekosz A., Lane A.P., Klein S.L. Estrogenic compounds reduce influenza A virus replication in primary human nasal epithelial cells derived from female, but not male, donors. Am J Physiol-Lung Cell Mol Physiol. 2015;310:L415–L425. doi: 10.1152/ajplung.00398.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vom Steeg L.G., Dhakal S., Woletsadik Y.A., Park H.S., Mulka K.R., Reilly E.C., et al. Androgen receptor signaling in the lungs mitigates inflammation and improves the outcome of influenza in mice. PLoS Pathog. 2020;16 doi: 10.1371/journal.ppat.1008506. Epub 2020/07/10. PubMed PMID: 32645119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vermillion M.S., Ursin R.L., Kuok D.I.T., Vom Steeg L.G., Wohlgemuth N., Hall O.J., et al. Production of amphiregulin and recovery from influenza is greater in males than females. Biol Sex Differ. 2018;9:24. doi: 10.1186/s13293-018-0184-8. Epub 2018/07/18. PubMed PMID: 30012205; PubMed Central PMCID: PMC6048771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vom Steeg L.G., Vermillion M.S., Hall O.J., Alam O., McFarland R., Chen H., et al. Age and testosterone mediate influenza pathogenesis in male mice. Am J Physiol Lung Cell Mol Physiol. 2016;311:L1234–L1244. doi: 10.1152/ajplung.00352.2016. Epub 2016/11/07. PubMed PMID: 27815260; PubMed Central PMCID: PMC5206399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.vom Steeg L.G., Attreed S.E., Zirkin B., Klein S.L. Testosterone treatment of aged male mice improves some but not all aspects of age-associated increases in influenza severity. Cell Immunol. 2019;345 doi: 10.1016/j.cellimm.2019.103988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gebhard C., Regitz-Zagrosek V., Neuhauser H.K., Morgan R., Klein S.L. Impact of sex and gender on Covid-19 outcomes in Europe. Biol Sex Differ. 2020;11:29. doi: 10.1186/s13293-020-00304-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wenham C., Smith J., Morgan R. Covid-19: the gendered impacts of the outbreak. Lancet. 2020;395:846–848. doi: 10.1016/S0140-6736(20)30526-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Scully E.P., Haverfield J., Ursin R.L., Tannenbaum C., Klein S.L. Considering how biological sex impacts immune responses and covid-19 outcomes. Nat Rev Immunol. 2020;20:442–447. doi: 10.1038/s41577-020-0348-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Alghamdi I.G., Hussain I.I., Almalki S.S., Alghamdi M.S., Alghamdi M.M., El-Sheemy M.A. The pattern of Middle East respiratory syndrome coronavirus in Saudi Arabia: a descriptive epidemiological analysis of data from the Saudi ministry of health. Int J Gen Med. 2014:417–423. doi: 10.2147/IJGM.S67061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Karlberg J., Chong D.S.Y., Lai W.Y.Y. Do men have a higher case fatality rate of severe acute respiratory syndrome than women do? Am J Epidemiol. 2004;159:229–231. doi: 10.1093/aje/kwh056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Leong H.N., Earnest A., Lim H., Chin C., Tan C.S.H., Puhaindran M.E., et al. SARS in Singapore-predictors of disease severity. Ann Acad Med Singapore. 2006;35:326–331. [PubMed] [Google Scholar]
- 93•.Takahashi T., Wong P., Ellingson M., Lucas C., Klein J., Israelow B., et al. Sex differences in immune responses to SARS-COV-2 that underlie disease outcomes. medRxiv. 2020 doi: 10.1101/2020.06.06.20123414. 2020.06.06.20123414. [DOI] [Google Scholar]; This paper performed a comprehensive analyses of serum samples from a small cohort of COVID-19 patients, and identified sex-biased differences in immune cell populations and cytokine levels that correlated with disease severity.
- 94••.Channappanavar R., Fett C., Mack M., Ten Eyck P.P., Meyerholz D.K., Perlman S. Sex-based differences in susceptibility to severe acute respiratory syndrome coronavirus infection. J Immunol. 2017;198:4046. doi: 10.4049/jimmunol.1601896. [DOI] [PMC free article] [PubMed] [Google Scholar]; This was the first to show sex differences with coronavirus infection, where sex hormones play a key role. This study found that male mice are more susceptible to SARS-CoV infection and that estrogen protects female mice from lung pathologies.
- 95.Li W., Moore M.J., Vasilieva N., Sui J., Wong S.K., Berne M.A., et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kuba K., Imai Y., Rao S., Gao H., Guo F., Guan B., et al. A crucial role of angiotensin converting enzyme 2 (ace2) in SARS coronavirus-induced lung injury. Nat Med. 2005;11:875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97•.Hoffmann M., Kleine-Weber H., Schroeder S., Kruger N., Herrler T., Erichsen S., et al. SARS-COV-2 cell entry depends on ace2 and tmprss2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–80 e8. doi: 10.1016/j.cell.2020.02.052. Epub 2020/03/07. PubMed PMID: 32142651; PubMed Central PMCID: PMC7102627. [DOI] [PMC free article] [PubMed] [Google Scholar]; This work found that SARS-CoV-2 viral entry requires cellular expression of ACE2 and TMPRSS2.
- 98.Iwata-Yoshikawa N., Okamura T., Shimizu Y., Hasegawa H., Takeda M., Nagata N. Tmprss2 contributes to virus spread and immunopathology in the airways of murine models after coronavirus infection. J Virol. 2019;93 doi: 10.1128/JVI.01815-18. Epub 2019/01/11. PubMed PMID: 30626688; PubMed Central PMCID: PMC6401451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Glowacka I., Bertram S., Muller M.A., Allen P., Soilleux E., Pfefferle S., et al. Evidence that tmprss2 activates the severe acute respiratory syndrome coronavirus spike protein for membrane fusion and reduces viral control by the humoral immune response. J Virol. 2011;85:4122–4134. doi: 10.1128/JVI.02232-10. Epub 2011/02/18. PubMed PMID: 21325420; PubMed Central PMCID: PMC3126222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Stelzig K.E., Canepa-Escaro F., Schiliro M., Berdnikovs S., Prakash Y.S., Chiarella S.E. Estrogen regulates the expression of SARS-COV-2 receptor ace2 in differentiated airway epithelial cells. Am J Physiol-Lung Cell Mol Physiol. 2020;318:L1280–L1281. doi: 10.1152/ajplung.00153.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lucas J.M., Heinlein C., Kim T., Hernandez S.A., Malik M.S., True L.D., et al. The androgen-regulated protease tmprss2 activates a proteolytic cascade involving components of the tumor microenvironment and promotes prostate cancer metastasis. Cancer Discov. 2014;4:1310–1325. doi: 10.1158/2159-8290.CD-13-1010. Epub 2014/08/15. PubMed PMID: 25122198; PubMed Central PMCID: PMC4409786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chen J., Jiang Q., Xia X., Liu K., Yu Z., Tao W., Gong W., Han J.J. Individual variation of the SARS-COV2 receptor ACE2 gene expression and regulation. Preprints. 2020 doi: 10.1111/acel.13168. 2020030191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Imai Y., Kuba K., Rao S., Huan Y., Guo F., Guan B., et al. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436:112–116. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dimmeler S., Rippmann V., Weiland U., Haendeler J., Zeiher A.M. Angiotensin ii induces apoptosis of human endothelial cells. Circul Res. 1997;81:970–976. doi: 10.1161/01.RES.81.6.970. [DOI] [PubMed] [Google Scholar]
- 105.Watanabe T., Barker T.A., Berk B.C. Angiotensin II and the endothelium. Hypertension. 2005;45:163–169. doi: 10.1161/01.HYP.0000153321.13792.b9. [DOI] [PubMed] [Google Scholar]
- 106.Pueyo M.E., Michel J.-B. Angiotensin ii receptors in endothelial cells. Gen Pharmacol: Vasc Syst. 1997;29:691–696. doi: 10.1016/S0306-3623(97)00021-9. [DOI] [PubMed] [Google Scholar]
- 107.Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S., et al. Endothelial cell infection and endotheliitis in Covid-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ziegler C.G.K., Allon S.J., Nyquist S.K., Mbano I.M., Miao V.N., Tzouanas C.N., et al. SARS-COV-2 receptor ace2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell. 2020;181:1016–35 e19. doi: 10.1016/j.cell.2020.04.035. Epub 2020/05/16. PubMed PMID: 32413319; PubMed Central PMCID: PMC7252096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pellegrini S., Uze G. An old cytokine against a new virus? J Interferon Cytokine Res. 2020;40:425–428. doi: 10.1089/jir.2020.0130. Epub 2020/06/26. PubMed PMID: 32584629. [DOI] [PubMed] [Google Scholar]
- 110.Hussein H.M., Rahal E.A. The role of viral infections in the development of autoimmune diseases. Crit Rev Microbiol. 2019;45:394–412. doi: 10.1080/1040841X.2019.1614904. [DOI] [PubMed] [Google Scholar]
- 111.Serafini B., Rosicarelli B., Franciotta D., Magliozzi R., Reynolds R., Cinque P., et al. Dysregulated Epstein-Barr virus infection in the multiple sclerosis brain. J Exp Med. 2007;204:2899–2912. doi: 10.1084/jem.20071030. Epub 2007/11/07. PubMed PMID: 17984305; PubMed Central PMCID: PMC2118531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Eguchi K., Matsuoka N., Ida H., Nakashima M., Sakai M., Sakito S., et al. Primary Sjogren’s syndrome with antibodies to htlv-i: clinical and laboratory features. Ann Rheum Dis. 1992;51:769–776. doi: 10.1136/ard.51.6.769. Epub 1992/06/01. PubMed PMID: 1352097; PubMed Central PMCID: PMC1004744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hiemstra H.S., Schloot N.C., van Veelen P.A., Willemen S.J., Franken K.L., van Rood J.J., et al. Cytomegalovirus in autoimmunity: T cell crossreactivity to viral antigen and autoantigen glutamic acid decarboxylase. Proc Natl Acad Sci U S A. 2001;98:3988–3991. doi: 10.1073/pnas.071050898. Epub 2001/03/29. PubMed PMID: 11274421; PubMed Central PMCID: PMC31166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gonzalez-Quintial R., Nguyen A., Kono D.H., Oldstone M.B.A., Theofilopoulos A.N., Baccala R. Lupus acceleration by a MAVS-activating RNA virus requires endosomal TLR signaling and host genetic predisposition. PLoS One. 2018;13 doi: 10.1371/journal.pone.0203118. Epub 2018/09/11. PubMed PMID: 30199535; PubMed Central PMCID: PMC6130858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Theofilopoulos A.N., Kono D.H., Baccala R. The multiple pathways to autoimmunity. Nat Immunol. 2017;18:716–724. doi: 10.1038/ni.3731. Epub 2017/06/21. PubMed PMID: 28632714; PubMed Central PMCID: PMC5791156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cooper G.S., Stroehla B.C. The epidemiology of autoimmune diseases. Autoimmune Rev. 2003;2:119–125. doi: 10.1016/s1568-9972(03)00006-5. [DOI] [PubMed] [Google Scholar]
- 117.Jacobson D.L., Gange S.J., Rose N.R., Graham N.M. Epidemiology and estimated population burden of selected autoimmune diseases in the united states. Clin Immunol Immunopathol. 1997;84:223–243. doi: 10.1006/clin.1997.4412. [DOI] [PubMed] [Google Scholar]
- 118.Scofield R.H., Bruner G.R., Namjou B., Kimberly R.P., Ramsey-Goldman R., Petri M., et al. Klinefelter’s syndrome (47,xxy) in male systemic lupus erythematosus patients: support for the notion of a gene-dose effect from the X chromosome. Arthritis Rheum. 2008;58:2511–2517. doi: 10.1002/art.23701. Epub 2008/08/01. PubMed PMID: 18668569; PubMed Central PMCID: PMC2824898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cooney C.M., Bruner G.R., Aberle T., Namjou-Khales B., Myers L.K., Feo L., et al. 46,x,del(x)(q13) turner’s syndrome women with systemic lupus erythematosus in a pedigree multiplex for sle. Genes Immun. 2009;10:478–481. doi: 10.1038/gene.2009.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sawalha A.H., Harley J.B., Scofield R.H. Autoimmunity and Klinefelter’s syndrome: when men have two X chromosomes. J Autoimmun. 2009;33:31–34. doi: 10.1016/j.jaut.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Invernizzi P., Miozzo M., Oertelt-Prigione S., Meroni P.L., Persani L., Selmi C., et al. X monosomy in female systemic lupus erythematosus. Ann N Y Acad Sci. 2007;1110:84–91. doi: 10.1196/annals.1423.010. [DOI] [PubMed] [Google Scholar]
- 122.Syrett C.M., Anguera M.C. When the balance is broken: X-linked gene dosage from two x chromosomes and female-biased autoimmunity. J Leukoc Biol. 2019;106:919–932. doi: 10.1002/JLB.6RI0319-094R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Clegg C.H., Rulffes J.T., Haugen H.S., Hoggatt I.H., Aruffo A., Durham S.K., et al. Thymus dysfunction and chronic inflammatory disease in gp39 transgenic mice. Int Immunol. 1997;9:1111–1122. doi: 10.1093/intimm/9.8.1111. [DOI] [PubMed] [Google Scholar]
- 124.Pérez-Melgosa M., Hollenbaugh D., Wilson C.B. Cutting edge: Cd40 ligand is a limiting factor in the humoral response to T cell-dependent antigens. J Immunol. 1999;163:1123. [PubMed] [Google Scholar]
- 125.Higuchi T., Aiba Y., Nomura T., Matsuda J., Mochida K., Suzuki M., et al. Cutting edge: Ectopic expression of cd40 ligand on b cells induces lupus-like autoimmune disease. J Immunol. 2002;168:9. doi: 10.4049/jimmunol.168.1.9. [DOI] [PubMed] [Google Scholar]
- 126.Kil L.P., de Bruijn M.J.W., van Nimwegen M., Corneth O.B.J., van Hamburg J.P., Dingjan G.M., et al. Btk levels set the threshold for B-cell activation and negative selection of autoreactive b cells in mice. Blood. 2012;119:3744–3756. doi: 10.1182/blood-2011-12-397919. [DOI] [PubMed] [Google Scholar]
- 127.Corneth O.B.J., de Bruijn M.J.W., Rip J., Asmawidjaja P.S., Kil L.P., Hendriks R.W. Enhanced expression of Bruton’s tyrosine kinase in B cells drives systemic autoimmunity by disrupting t cell homeostasis. J Immunol. 2016;197:58. doi: 10.4049/jimmunol.1600208. [DOI] [PubMed] [Google Scholar]
- 128.Hwang S.-H., Lee H., Yamamoto M., Jones L.A., Dayalan J., Hopkins R., et al. B cell tlr7 expression drives anti-RNA autoantibody production and exacerbates disease in systemic lupus erythematosus–prone mice. J Immunol. 2012;189:5786. doi: 10.4049/jimmunol.1202195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Deane J.A., Pisitkun P., Barrett R.S., Feigenbaum L., Town T., Ward Jerrold M., et al. Control of toll-like receptor 7 expression is essential to restrict autoimmunity and dendritic cell proliferation. Immunity. 2007;27:801–810. doi: 10.1016/j.immuni.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wang J., Syrett C.M., Kramer M.C., Basu A., Atchison M.L., Anguera M.C. Unusual maintenance of x chromosome inactivation predisposes female lymphocytes for increased expression from the inactive x. Proc Natl Acad Sci U S A. 2016 doi: 10.1073/pnas.1520113113. Epub 2016/03/24. PubMed PMID: 27001848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131••.Syrett C.M., Sierra I., Beethem Z.T., Dubin A.H., Anguera M.C. Loss of epigenetic modifications on the inactive x chromosome and sex-biased gene expression profiles in B cells from nzb/w f1 mice with lupus-like disease. J Autoimmun. 2020;107 doi: 10.1016/j.jaut.2019.102357. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper demonstrated that B cells from female mice with lupus-like disease progressively lost repressive epigenetic marks and Xist RNA localization at the Xi. This study also observed Biallelic expression of Tlr7 (which did not change during disease progression and sex-specific gene expression differences in B cells at late stages of disease.
- 132.Heinz L.X., Lee J., Kapoor U., Kartnig F., Sedlyarov V., Papakostas K., et al. Tasl is the slc15a4-associated adaptor for irf5 activation by tlr7-9. Nature. 2020;581:316–322. doi: 10.1038/s41586-020-2282-0. Epub 2020/05/21. PubMed PMID: 32433612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bentham J., Morris D.L., Graham D.S.C., Pinder C.L., Tombleson P., Behrens T.W., et al. Genetic association analyses implicate aberrant regulation of innate and adaptive immunity genes in the pathogenesis of systemic lupus erythematosus. Nat Genet. 2015;47:1457–1464. doi: 10.1038/ng.3434. Epub 2015/10/27. PubMed PMID: 26502338; PubMed Central PMCID: PMC4668589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Odhams C.A., Roberts A.L., Vester S.K., Duarte C.S.T., Beales C.T., Clarke A.J., et al. Interferon inducible x-linked gene cxorf21 may contribute to sexual dimorphism in systemic lupus erythematosus. Nat Commun. 2019;10:2164. doi: 10.1038/s41467-019-10106-2. Epub 2019/05/17. PubMed PMID: 31092820; PubMed Central PMCID: PMC6520347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135••.Syrett C.M., Paneru B., Sandoval-Heglund D., Wang J., Banerjee S., Sindhava V., et al. Altered x-chromosome inactivation in t cells may promote sex-biased autoimmune diseases. JCI Insight. 2019;4 doi: 10.1172/jci.insight.126751. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is the first to demonstrate that altered XCI maintenance is a feature of T cells from SLE patients and mice with lupus-like disease. This study also found female-specific increased expression of X-linked immune related genes.
- 136.Syrett C.M., Sindhava V., Hodawadekar S., Myles A., Liang G., Zhang Y., et al. Loss of xist RNA from the inactive X during B cell development is restored in a dynamic yy1-dependent two-step process in activated B cells. PLoS Genet. 2017;13 doi: 10.1371/journal.pgen.1007050. e1007050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Fischer J., Jung N., Robinson N., Lehmann C. Sex differences in immune responses to infectious diseases. Infection. 2015;43:399–403. doi: 10.1007/s15010-015-0791-9. Epub 2015/05/10. PubMed PMID: 25956991. [DOI] [PubMed] [Google Scholar]
- 138.Karami M., Doudi M., Setorki M. Assessing epidemiology of cutaneous leishmaniasis in Isfahan, Iran. J Vector Borne Dis. 2013;50:30–37. Epub 2013/05/25. PubMed PMID: 23703437. [PubMed] [Google Scholar]
- 139.Satoskar A., Alexander J. Sex-determined susceptibility and differential IFN-gamma and TNF-alpha mRNA expression in dba/2 mice infected with Leishmania mexicana. Immunology. 1995;84:1–4. Epub 1995/01/01. PubMed PMID: 7890293; PubMed Central PMCID: PMC1415181. [PMC free article] [PubMed] [Google Scholar]
- 140.Oghumu S., Varikuti S., Stock J.C., Volpedo G., Saljoughian N., Terrazas C.A., et al. Cutting edge: Cxcr3 escapes x chromosome inactivation in T cells during infection: potential implications for sex differences in immune responses. J Immunol. 2019;203:789–794. doi: 10.4049/jimmunol.1800931. Epub 2019/06/30: PubMed PMID: 31253729; PubMed Central PMCID: PMC6684832. [DOI] [PMC free article] [PubMed] [Google Scholar]