Abstract
Introduction
Plants have always been a significant source of natural active components with biological properties. Celery seed oil (extracted from Apium graveolens) has several potential applications, but its therapeutic uses in the form of nanoemulsion formulation need to be investigated further in order to meet the demand in cancer treatment, and to alleviate the prevailing crisis arising from increased antimicrobial resistance.
Methods
The therapeutic potential of celery seed oil was investigated through the formulation and testing of a nanoemulsion developed with Tween 80 (a non-ionic surfactant) and the utilization of an ultrasonication technique. Anticancer and apoptotic properties of the formulation were evaluated through MTT and Annexin V-FITC assays. The clonogenic assay aided in the identification of the antiproliferative properties of the formulation on oral squamous cell carcinoma. The antimicrobial study was supported by agar well diffusion assay, membrane integrity test and scanning electron microscopy.
Results
Experiments identified relevant parameters, including optimal surfactant concentration and emulsification time. GC-MS analysis identified various components in the celery oil, but not their biological activities. A sonication time of 20 min resulted in a droplet diameter of 23.4 ± 1.80 nm. The IC50 concentration of the optimal nanoemulsion formulation against SAS cells was 1.4 µL/mL. At this concentration, cell proliferation was significantly reduced through inhibition of the anchorage-independent cell growth by disrupting colony formation and inducing cell death (apoptosis) of cancer cells. The nanoemulsion was also treated with a microbial suspension of S. aureus, and displayed antibacterial properties through lipid membrane fusion, causing cytoplasmic leakage as verified through agar well diffusion and membrane permeability assays. Scanning electron microscopy revealed complete distortion of the bacterial pathogen.
Conclusion
The results in this study present celery as a possible constituent for cancer therapeutics and as a candidate for aggressive, yet safe cancer treatment. The celery-based nanoemulsion has the potential to act as a key alternative to standard antibiotic therapy.
Keywords: celery, nanoemulsion, ultrasonication, SAS cell line, antibacterial activity
Introduction
It is observed that conventional cancer therapies such as surgery, chemo and radiotherapy have not shown much success in the management of solid tumours. This is due to the multiple side effects associated with these modes of treatment, coupled with the growing concern over multi-drug resistances. The development of new effective therapeutic strategies which, along with high efficacy, come with reduced levels of toxicity as well as lower risk of adverse side effects is the need of the hour.1 The side effects associated with synthetic drugs, or even chemotherapy, can be mitigated by essential oils (EOs)/phytochemicals, according to earlier studies.2,3 The lipophilic nature of EOs enables them to easily penetrate the cell membrane.4,5
Smoking and drinking have increasingly become a part of the lifestyle followed by today’s population. In light of this, oral squamous cell carcinoma has turned into a fatal disease on a global scale.6 Largely, developing countries are affected by this type of cancer, and around 500,000 cases of oral and pharyngeal cancer are diagnosed annually on a global scale.7 This raises a serious concern, and renders it very important to pay immediate attention towards developing a compound to hinder the growth of such a cancer. Historically, the treatment of oral squamous cell carcinoma (OSCC) has been through conventional methods such as surgery and radiation, with or without chemotherapy, all of which lead to significant post-treatment morbidity.8 The stage in which the patient is suffering from OSCC, ranging from preliminary to advanced, is of significant importance in its treatment.9 In the preliminary stage, either surgery or radiotherapy or both are used, whereas a combination of chemotherapy, surgery and radiotherapy is preferred for the subsequent stages.10
Through a span of 15 years from 2000 to 2015, the level of antibiotic consumption has increased by approximately 65% worldwide, and by two-fold in most low- and middle-income countries.11 As a consequence of this increased and unbefitting intake of antibiotics, resistance to antibiotics– commonly known as antimicrobial resistance– began taking root, and currently constitutes a large threat to the effectiveness of treatment routes via antibiotics. Examples include treatment of newborns suffering from sepsis12 or of infections caused during surgery, cancer treatment, etc.13,14 This brings therapeutics to a critical juncture where there is an urgent need to circumvent antimicrobial resistance by various methods such as development of new antibiotics, along with efforts to develop new alternatives such as immunomodulatory peptides,15 vaccination,16 anti-virulence inventions,17 antibody therapeutics,18 phage treatment,19,20 and antibiotic-based potentiators;21 these have steadily gained traction over the years.
A nanoemulsion, as the name suggests, is an emulsion with its particle dimension in the nanometer scale. Currently, to improve the delivery of therapeutic agents, nanoemulsions are in wide-spread investigation as drug carriers.22 The method of ultrasonic emulsification has emerged as a very efficient technique where viscous resistance is overcome by applying a strong shear force using high-energy ultrasound23,24 to reduce droplet size. Translucent nanoemulsions have been successfully produced using high-power ultrasound in laboratory scale.25 The reduced particle size of a nanoemulsion aids in increasing drug retention time and bioavailability, and thereby prevents loss of drug.26
Ultrasonication is the most attractive high-energy method to prepare nanoemulsions. Ultrasonic homogenization has proven to be cost-effective as well as efficient owing to its ability to generate small droplet sizes with minimum consummation of energy. The underlying principle of ultrasonication revolves around coupling of waves that have been generated by an acoustic field of frequency in the 20–100 kHz range with a liquid. These waves create localized microturbulence, causing the dispersed phase (oil) to become unstable and, as a consequence, create cavitation in the continuous phase.27 Voids are created during the rarefaction cycle of the waves, and keep growing until they collapse during the compression cycle causing high pressure and high shear which induce extreme heating and cooling rates, as well as severe turbulence through liquid jets which break the dispersed phase into nano-sized droplets.28 This study therefore employed ultrasonication to prepare the nanoemulsion.
Cancer treatments have become more advanced, and have significantly brought down the associated death rate.29 Anticancer therapy mostly involves targeting multiple signalling pathways. However, at a certain stage, patients with aggressive cancer have poor prognosis and become resistant to chemotherapeutic drugs.30 Recent oncological research experiments including animal models, pre-clinical and clinical trials have proven that many natural compounds, mostly phytochemicals derived from plant extracts, have been effective in cancer treatment and chemoprevention.31–35
In the past few years, there has been an increasing interest in research on the anticancer properties of spice-based nanoemulsions owing to their favourable characteristics such as higher bioavailability and stability. A nanoemulsion formulation using two spices, Drimys angustifolia and D. brasiliensis Miers, was developed to reduce the cell viability of U-138 MG (human glioblastoma) and T24 (human bladder carcinoma) cell lines.36 Another study documented that eugenol-loaded nanoemulsions demonstrate apoptosis of colon (HTB37) and liver (HB8065) cancer cell lines.37 Significant progress has been made in the investigation of curcuminoid nanoemulsions with studies conducted on enhancing their oral bioavailability38 and on demonstrating apoptosis of A549 (adenocarcinoma) and H460 (cellosaurus cell line).39
This study has investigated the anticancer and antibacterial activity of celery nanoemulsion. Celery (Apium Graveolens) is a traditional medicinal plant which has enormous health benefits.40 Celery has the antioxidant property, meaning that it can remove the free radical due to the presence of p-coumaric acid, tannin, luteonin, apigenin, saponin, and caffeic acid in its composition.41 For the treatment of spleen, liver diseases, jaundice, rheumatism, gout and inflammation, people have traditionally used celery seeds. Celery seed oil inhibits liver tumor by cell proliferation inhibition, apoptosis upregulation, and downregulation of inflammatory markers.42 Although studies have been conducted to substantiate the therapeutic properties of celery nanoemulsion for treating chronic osteoarthritic diseases,43 for antihypertensive activity44 and for its anti-nociceptive effects,45 no in-vitro studies have been conducted on the anticancer properties of celery nanoemulsions. This study, therefore, focuses on the anticancer and antibacterial effects of celery oil-based nanoemulsion.
Methods
Materials
Celery seed oil (Apium graveolens) of Indian source was obtained from Cyrus Enterprises, India. Steam distillation process was employed in extracting oil as per the manufacturer’s datasheet. Polyethylene glycol sorbitan monooleate (commonly known as Tween 80), which has been used as a surfactant in the study, was procured from Merck, India. For this study, the Annexin V-FITC Early Apoptosis Detection Kit was procured from CST, Inc., while the MTT assay kit was procured from Hi Media Laboratories, India. Double-distilled water from milli-Q systemTM was used in all experiments conducted for this study.
GC-MS
For investigating the active compounds present inside the celery seed oil and their composition, GC-MS analysis has been carried out. The celery essential oil was injected into the capillary column (30 m x 0.25 μm ID) of the Hewlett Packard gas chromatograph (6890 Series) at an injector temperature of 280°C, and split ratio of 1:50. The carrier gas (99.999% He) flowed in the column at a linear velocity of 44.2 cm/sec. The column oven temperature was set to an initial value of 45°C for 1 min and programmed to then rise to 250°C at 5°C/min. It was subsequently maintained at 250°C for 20 min.
Formulation of Nanoemulsion
The nanoemulsion of celery oil was formed using the oil, water and surfactant. The non-ionic surfactant used in this study– Tween 80– exhibits high solubility in essential oils and coalescence with the essential oil droplets, which boosts the stability of the system. Preparation of the nanoemulsions in this study was a twofold process, where the initial step was formation of emulsions by mixing the three key components, namely – oil, water and surfactant, at varying oil: surfactant ratios – 1:1 (CEL – A), 1:2 (CEL – B), and 1:3 (CEL – C). They were mixed using a magnetic stirrer rotating at a speed of 500 rpm for 10 min (Table 1). These emulsions were then made into their respective nanoemulsions using an ultrasonicator, a Probe Sonicator Advanced Model from PCI Analytics Private Limited. Each concentration was checked before sonication (0 min) and after subjecting to sonication times of 5, 10, 15 and 20 min, respectively.
Table 1.
Formulations Used for the Optimization of Celery Oil-Based Nanoemulsion
| Formulation Code | Oil:Surfactant (v/v) | Sonication Time | Celery Oil:Tween 80:Water (v/v) |
|---|---|---|---|
| CEL-A0 | 1:1 | 0 min | 6:6:88 |
| CEL-A1 | 1:1 | 5 min | 6:6:88 |
| CEL-A2 | 1:1 | 10 min | 6:6:88 |
| CEL-A3 | 1:1 | 15 min | 6:6:88 |
| CEL-A4 | 1:1 | 20 min | 6:6:88 |
| CEL-B0 | 1:2 | 0 min | 6:12:82 |
| CEL-B1 | 1:2 | 5 min | 6:12:82 |
| CEL-B2 | 1:2 | 10 min | 6:12:82 |
| CEL-B3 | 1:2 | 15 min | 6:12:82 |
| CEL-B4 | 1:2 | 20 min | 6:12:82 |
| CEL-C0 | 1:3 | 0 min | 6:18:76 |
| CEL-C1 | 1:3 | 5 min | 6:18:76 |
| CEL-C2 | 1:3 | 10 min | 6:18:76 |
| CEL-C3 | 1:3 | 15 min | 6:18:76 |
| CEL-C4 | 1:3 | 20 min | 6:18:76 |
The transducer of the sonicator produces mechanical vibrations in the liquid system, and these induce an oscillating acoustic pressure owing to the collapse of bubbles.46 This acoustic pressure (P) which forms microbubbles resulting in local conditions that break the liquid system into nanosized droplets may be expressed as:47
P = Pmax sin (2πft), where
Pmax is the electric power given as input to the probe sonicator, and f is frequency of the waveform. It is evident that acoustic pressure caused by cavitation has high and low amplitudes owing to its sinusoidal nature, and this induces instantaneous and extreme heating and rapid cooling in the system leading to amplified disruption of the liquid system into nanodroplets.
Characterization of Nanoemulsion
The following characteristics of the nanoemulsions were studied: droplet size, absorbance, polydispersity index, viscosity, pH and stability.
Droplet size was measured through Dynamic Light Scattering whereby a correlation is made between the extent of dispersion of nanoparticles and the intensity of scattered light. Tracking the droplet size helps in characterizing the homogeneity, stability and bioavailability of the solution.48 Droplet size has a direct impact on the interfacial area available for the nanoemulsion, which, in turn, affects the permeability of the active compounds through membranes of vessels and tissue, thereby dictating their bioavailability. A smaller droplet size also aids in suppressing coalescence and precipitation of droplets, thereby minimizing breakdown of the nanoemulsion through well-known phenomena such as sedimentation and creaming through Ostwald ripening.49 Polydispersity index, which is also measured through DLS, describes the extent of homogeneity, which increases with enhanced surfactant concentration. A high surfactant concentration implies that there is sufficient amount of it to be absorbed onto all the oil droplets to form a pliable film, which prevents the adhesion or cohesion of the oil droplets, thereby ensuring homogeneity across all oil droplets in the nanoemulsion.50 The DLS measurements were done in triplicate with mean ± S.D. calculated.
Studying the absorbance of the nanoemulsion at 600 nm helps in physically characterizing the system for turbidity, and quantitatively confirms visually clear solutions for different oil-to-surfactant ratios. The measurements were carried out in triplicate with mean ± S.D. calculated.
Viscosity and pH are other parameters that help in the physicochemical characterization of nanoemulsions used in this study. Viscosity offers critical insights into the rheological properties of the nanoemulsion, and further helps estimate the rate of drug release and its efficacy. Viscosity also aids in understanding micelle formation in the nanoemulsion, as well in distinguishing between their formulation types. This helps in understanding inversion between phases during formulation.51 pH indicates the stability of the nanoemulsion, since any fluctuations in its value will be due to chemical reactions.52 Both these measurements were done in triplicate with mean ± S.D. calculated.
Physical stability is characterized for different formulations by assessing the extent of phase separation post-centrifugation, while stability over long periods of time is characterized only for formulations found to be stable after centrifugation; this is done by subjecting them to recurring heating-cooling and freeze-thaw cycles between 25°C and 4°C. Characterizing physicochemical stability for different formulations helps in choosing the most suitable one to be taken forward in the study, thereby optimizing both surfactant utilization and physicochemical stability.
Instruments used in the physicochemical characterization of the nanoemulsion are listed in Table 2.
Table 2.
Instruments Used for Physiochemical Characterization of Nanoemulsion
| Parameter | Instrumentation |
|---|---|
| Absorbance | V-630 Series, Jasco Analytical Instruments, Asia |
| Particle Size and Polydispersity Index | Horiba, Nanopartica SZ-100 series |
| Viscosity | Brookfield Viscometer with Brookfield Rheocalc Software [DV-II+ Pro EXTRA, LV-II, UL Adapter |
| pH | Digital pH meter [EUTECH Instruments, Oakton, Singapore] |
| Centrifugation | REMI International, India |
Anticancer Activity
After the formulation of celery essential oil nanoemulsion, its anticancer effect against SAS cell line was determined by MTT assay, clonogenic assay and Annexin V-FITC assay. Oral squamous cell carcinoma (SAS) cell line of human origin was gifted by Prof. D. Karunagaran, IIT Madras, who procured it from the Japanese Collection of Research Bioresources Cell Bank, Japan. The oral squamous carcinoma cells were cultured in Roswell Park Memorial Institute 1640 medium (RPMI 1640 media) supplemented with 10% Fetal Bovine Serum (FBS) at 37°C, 5% CO2 and Penicillin (100 units/mL)/Streptomycin (100 µg/mL) (Gibco, Grand Island, NY, USA).
MTT
In this assay, the SAS cells were treated with various concentrations of celery oil and celery nanoemulsion separately in an ascending order after the cells were seeded in 96-well plates. After a period of 48 h, 10 µL of 5 mg/mL MTT was added in every well and allowed to incubate for 3 h. Formazan crystals of purple colour would form in viable cells, followed by aspiration of media and thereafter by the solubilization of crystals with 100 µL DMSO. The colour developed was read using Bio-Rad 680 colorimeter at 570 nm.
Clonogenic Assay
In the colony formation assay, SAS cells were cultured in 6-well plates (hundred cells/well), and after 24 h, interacted with celery nanoemulsion at IC50 concentration of 1.4 µL/mL. This is followed by incubation at 37°C, 5% CO2 for 14 days till the optimal clones are obtained. Later, cells in the plates were methanol-fixed and stained with crystal violet. The colony counts were calculated using ImageJ software.
Flow Cytometry Assay
The SAS cells were cultured and treated with celery nanoemulsion at IC50 concentration 1.4 µL/mL. After 48 h, cells were detached by 0.25% trypsin, washed two times with phosphate-buffered saline, and interacted with Annexin V-FITC conjugate and PI (Propidium Iodide) for 10 min at laboratory temperature as per manufacturer’s instructions. The suspension was then analyzed using BD FACSVERSE to assess the ability of the suspension to induce apoptosis.
Antibacterial Activity
The antibacterial effects of the formulation against Staphylococcus aureus (ATCC 29213) were determined. S. aureus was sustained at 4°C in agar slants as stock. A few colonies were segregated from this culture, and inoculated into MHB (Mueller-Hinton Broth). A further subculture was made to obtain pure and viable culture. This was then diluted with the required medium to attain an O.D. of 1.5 x 107–108 CFU/mL.
Well Diffusion
One hundred microliters of the Staphylococcus aureus culture was swabbed over the surface of agar and a hole of 6–8 mm diameter was punched using a cork-borer. After this, 20–100 µL of the celery oil-based formulation was added. After incubating the agar plate under suitable conditions, the phytochemical compound was observed to diffuse and inhibit the microbial growth. The zone of inhibition was measured as diameter after an incubation period of 24 h.53 The experiment was carried out in triplicate with mean ± S.D. calculated.
Membrane Permeability Assay
The bacterial culture was incubated overnight and resuspended by diluting in Phosphate-buffered saline (PBS).54 0.5 mL of the microbial suspension with 9.5 mL of the optimized celery oil-based formulation acted as a test sample. The suspension was then diluted to 9.5 mL of PBS, and this acted as a negative control. 0.5 mL of the bacterial suspension diluted with 9.5 mL of sodium benzoate (500 mg/L) was used as a positive control. All the formulations were incubated for 1 h and centrifuged at 6000 g for 10 min to help in the excretion of cytoplasmic substance from the microbial cells. The supernatant’s absorbance was then calculated using UV–Visible Spectrophotometry at 260 nm. The experiment was carried out in triplicate with mean ± S.D. calculated.
Scanning Electron Microscopic Analysis
Scanning electron microscope (SEM) is a versatile instrument for material characterization.55 An image is formed utilising a focused electron beam of fairly low energy. This technique enables an understanding of the structural and morphological variations in the cell membrane of the pathogen due to cell damage.56 In this analysis, S. aureus was centrifuged at 5000 rpm for 15 min during the course of the night and was washed with sterile PBS (pH 7.4) twice. A 1% v/v of the desired concentration of the inoculum (1 x 108 CFU/mL) was interacted with 10-times dilution of celery nanoemulsion for 120 min after which it was subsequently washed two times with PBS and adhered to a metal stub. Finally, the sample for analysis was gold sputter-coated and examined under HR-SEM (FEI Quanta FEG 200).
Statistical Analysis
All experiments in this study were done in triplicate. GraphPad Prism 5.0, proprietary software of GraphPad Software, Inc., San Diego, CA was used to plot concentration correlation curves and to differentiate data points between the test and control group. T-test was used in calculating the P-value of two groups compared. Values of P≤0.05 were considered to be statistically significant.
Results
Identification of Celery Essential Oil Constituents by GC-MS
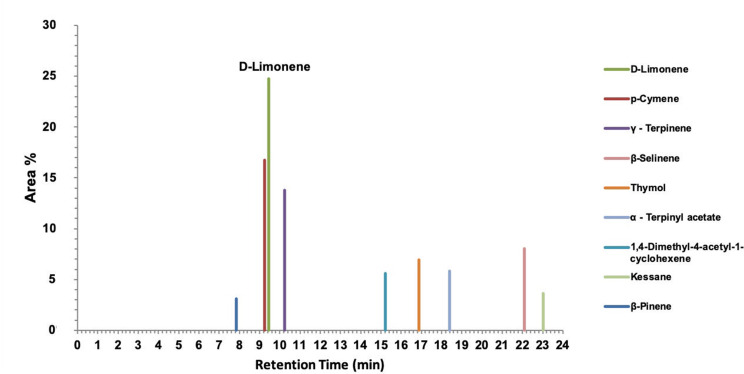
Celery oil has been tested through Gas Chromatography-Mass Spectroscopy. Figure 1 depicts the area % and also the time of retention of the active components. GC-MS reported D-limonene as the component with the highest peak area % of 24.73. The different compounds identified by GC-MS include p-cymene (16.73), γ – Terpinene (13.78), β-Selinene (8.05), thymol (6.93), α - Terpinyl acetate (5.81), 1,4-Dimethyl-4-acetyl-1-cyclohexene (5.59), kessane (3.64), and β-Pinene (3.08), as depicted in the chromatogram.
Figure 1.
Gas Chromatography-Mass Spectroscopy of celery seed (Apium graveolens) essential oil of Indian origin.
Nanoemulsion Characterization
The characterization studies were conducted for all preparations to find the optimal formulation. Samples prepared with various oil-to-surfactant ratios, prior to and after sonication of 5, 10, 15 and 20 min, were taken for characterization. Figure 2 shows the visual appearances of these formulations. With an increase in both surfactant concentration and sonication time, there was a noticeable colour change in the emulsions from milky to a clear formulation.
Figure 2.
Visual inspection of all celery oil-based formulations with varying oil-to surfactant ratios before and after sonication time of 5, 10, 15 and 20 min, showing colour change from milky to transparent.
Both surfactant concentration and sonication time are important factors that dictate the stability of the nanoemulsion. As elucidated in Characterization of Nanoemulsion, droplet size dictates the stability of the nanoemulsion. In this context, the consequences of sonication time and the concentration of surfactant on the size of droplets were examined. As further elucidated in Characterization of Nanoemulsion, the effect of the extent of homogeneity on the stability of the formulation was characterized by assessing the polydispersity index for all the formulations.
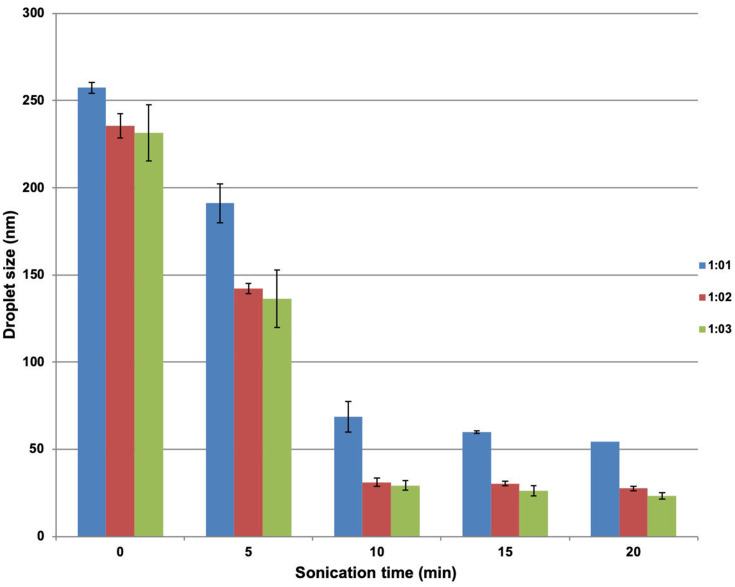
A reduced droplet size was observed as the concentration of surfactant increased—for example, for a sonication period of 20 min, droplet diameters of 54.5 ± 0.72 nm, 27.46 ± 1.28 nm and 23.4 ± 1.8 nm were observed for oil: surfactant ratios of 1:1, 1:2 and 1:3, respectively. In parallel, a reduced droplet size was also noticed with the increase in sonication time—for example, for the formulation where oil: surfactant ratio was 1:3, droplet diameters 29.2 ± 2.26 nm, 26.3 ± 2.91 nm and 23.4 ± 1.8 nm were observed for sonication times of 10 min, 15 min and 20 min, respectively. These trends in droplet size are reproduced in Figure 3. An increase in homogeneity was observed along with the decrease in polydispersity index with increase in surfactant concentration—for example, for a sonication time for 20 min, polydispersity indices of 0.346 ± 0.03, 0.299 ± 0.02 and 0.275 ± 0.01 were observed for oil: surfactant ratios of 1:1, 1:2 and 1:3, respectively.
Figure 3.
Variation in mean droplet size of celery oil-based formulations with oil-to surfactant ratio and sonication time.
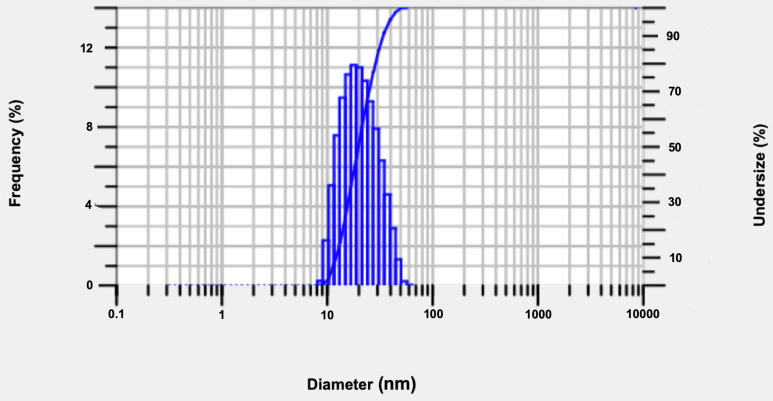
The celery oil-based nanoemulsion formulation CEL-C4, with an oil-to-surfactant ratio of 1:3, demonstrated the minimum droplet size diameter and produced more uniform and stable droplets after sonication for 20 min (Figure 4). Thus, CEL-C4 was identified as the optimal formulation for further application studies.
Figure 4.
Droplet size distribution of optimized celery nanoemulsion (CEL-C4) obtained using dynamic light scattering technique.
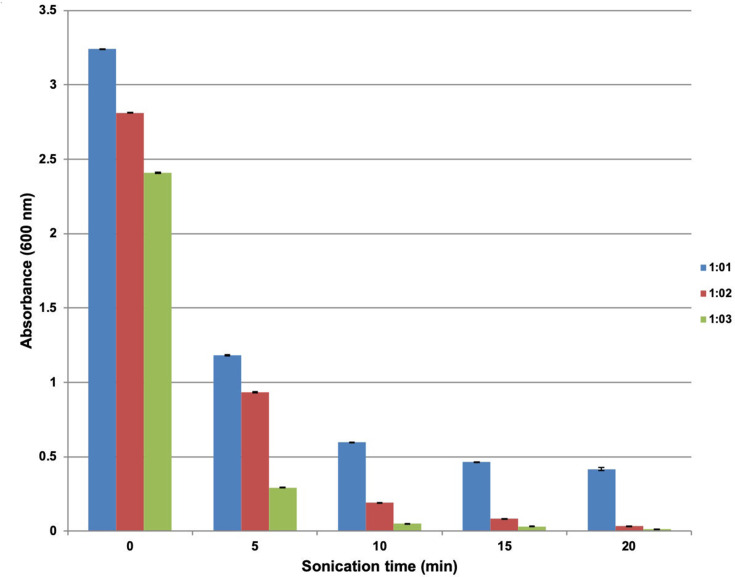
Absorbance was observed to decrease with an increased surfactant concentration both before and after sonication (Figure 5).
Figure 5.
UV-Spectroscopic measurements (600 nm) of celery oil-based formulations with oil-to-surfactant ratios of 1:1, 1:2 and 1:3 before and after sonication for various times.
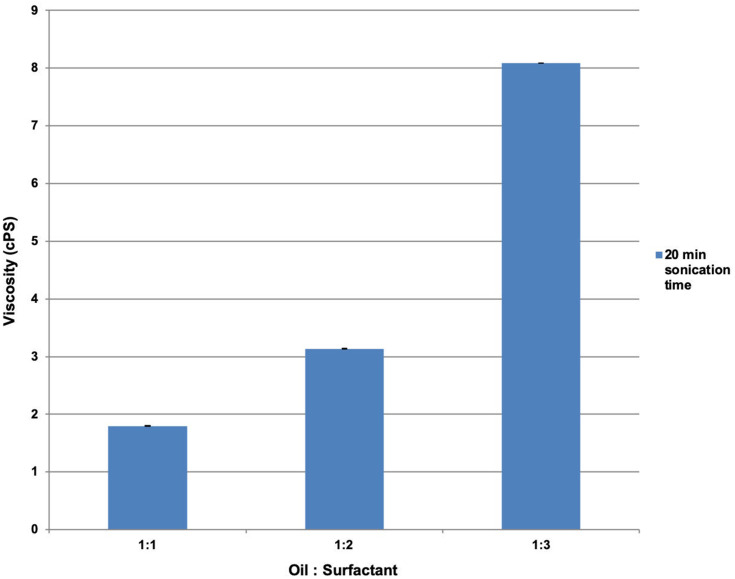
The increment in viscosity is directly proportional to that in surfactant concentration. The increase in viscosity from CEL-A to CEL-C after a 20 min sonication period is shown in Figure 6.
Figure 6.
The effect of oil-to-surfactant ratio on viscosity as measured on a Brookfield Viscometer.
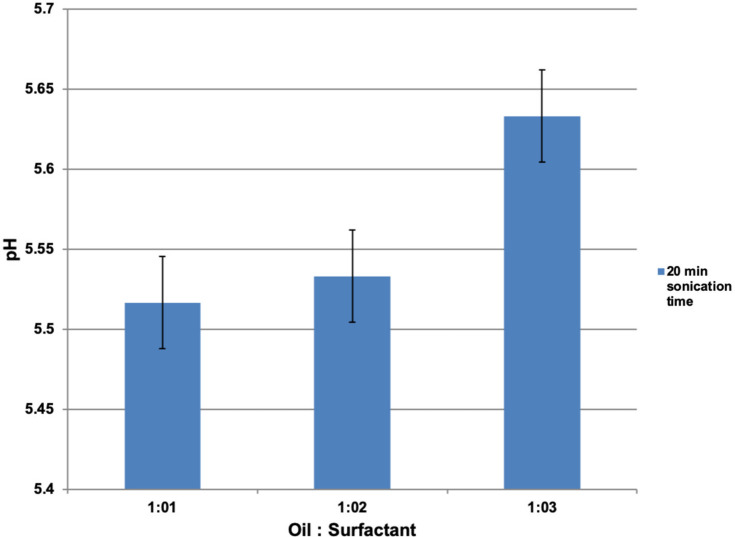
The pH values also increase with increase in surfactant concentration from CEL-A to CEL-C after a sonication period of 20 min, as shown in Figure 7.
Figure 7.
The effect of oil-to-surfactant ratio on pH as measured on a digital pH meter.
Stability Studies
All CEL-A formulations displayed phase separation within 72 h of preparation irrespective of the variation in their sonication time. Among the sonicated formulations of CEL-B and CEL-C, only CEL-B1 and CEL-C1 phase-separated in the centrifugation test, and were discarded from other tests. The remaining formulations—CEL-B2, B3, B4 and CEL-C2, C3 and C4—displayed good stability as they did not phase separate during centrifugation, while also maintaining stability through recursive heating–cooling cycles and freeze–thaw tests.
From the narrowed list of formulations, the optimal formulation was identified based on the particle size and PDI value. As established in the previous section, higher sonication times lead to greater stability (lower droplet size) of a nanoemulsion, thereby narrowing the list of optimal formulations further to CEL-B4 and C4. The previous section also contained data showing that higher surfactant concentrations lead to greater stability (lower droplet size) and superior homogeneity (lower polydispersity index), which led to the selection of CEL-C4 as the optimal formulation to be taken forward in the study.
Anticancer Activity
Cytotoxicity Assay
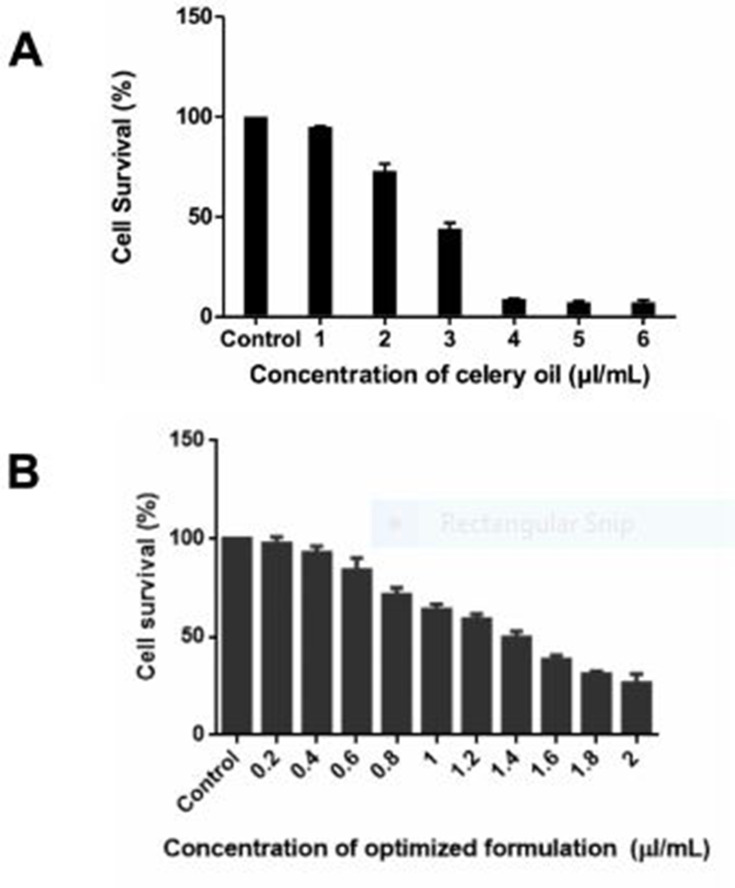
Celery oil demonstrated cytotoxicity against SAS cells at an IC50 concentration of 2.7 µL/mL (Figure 8A). DMSO (vehicle control) was used in the solubilization of celery oil. As essential oils cannot be used for any in-vivo applications due to their volatility, instability and poor aqueous solubility, celery oil was not considered for further mechanistic studies.
Figure 8.
(A and B) Cell viability measurement of SAS carcinoma cell line on treatment with (A) celery oil compared with (B) nanoemulsion, after 48 h incubation by MTT assay measured at 570 nm (*P 0.05 compared with respective control).
SAS cells resulted in concentration-dependent inhibition of cell growth after 48 h of interaction with the nanoemulsion formulated at an IC50 concentration of 1.4 µL/mL (Figure 8B). The vehicle control consisting of surfactant-water mixture does not pose cytotoxicity on cells.
Clonogenic Assay
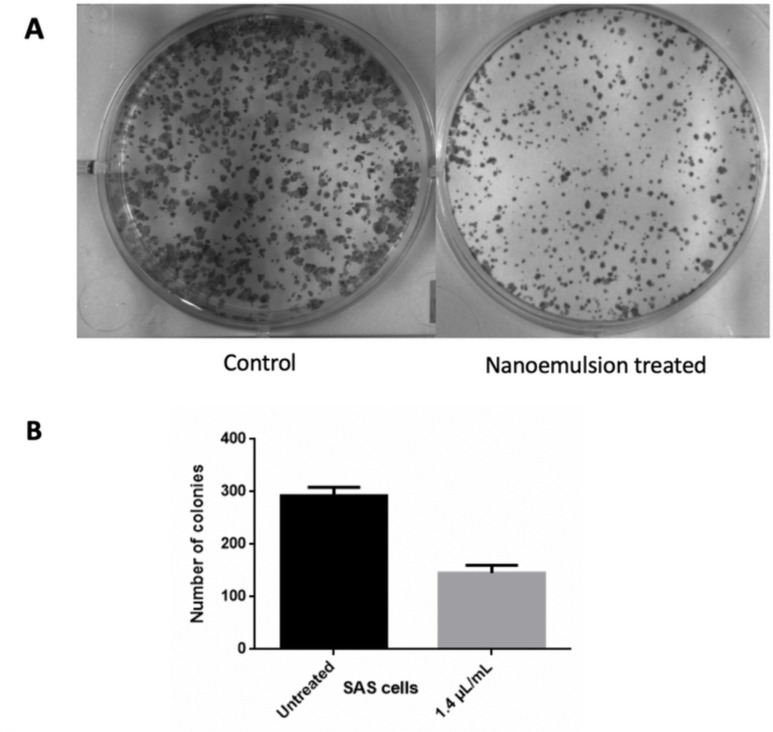
Oral squamous carcinoma cells (SAS) were treated with 1.4 µL/mL concentrated celery nanoemulsion and incubated for a period of 14 days at 37°C. Figure 9A and B depicts the decreased extent of colony formation on treatment with celery oil nanoemulsion in comparison with the untreated control cells. This indicates the antiproliferative effect of the nanoemulsion. This assay is most commonly used in investigating the endurance of radiated cancer cells.57
Figure 9.
(A and B) Clonogenic assay indicating a decline in colony formation of SAS carcinoma cell line on treatment with nanoemulsion (right) in comparison with untreated control (left) (*P ≤ 0.05 compared with respective control).
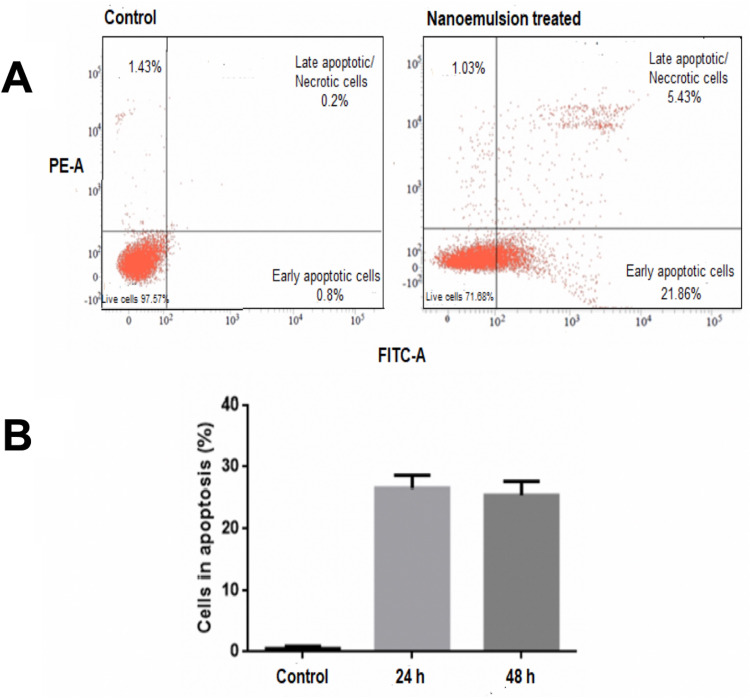
Annexin V-FITC Assay
The assay was performed in SAS cells after treatment with the optimized nanoemulsion concentration (1.4 µL/mL) and compared with the control cells (Figure 10B). The untreated control (Annexin V−/PI−) indicated viable cells, implying that they do not undergo apoptosis (Figure 10A(left)). After 48 h of treatment with nanoemulsion, a majority of the cells had undergone apoptosis (Annexin V+/PI−) or had already died (Annexin V+/PI+), as depicted in Figure 10A (right). This assay indicated 27% cell death through apoptosis mechanism.
Figure 10.
(A and B) Effect of celery nanoemulsion (CEL-C4), 1.4 µL/mL, against SAS carcinoma cell line with representation of apoptosis induction analysed by Annexin V-FITC assay through flow cytometry.
Antibacterial Activity
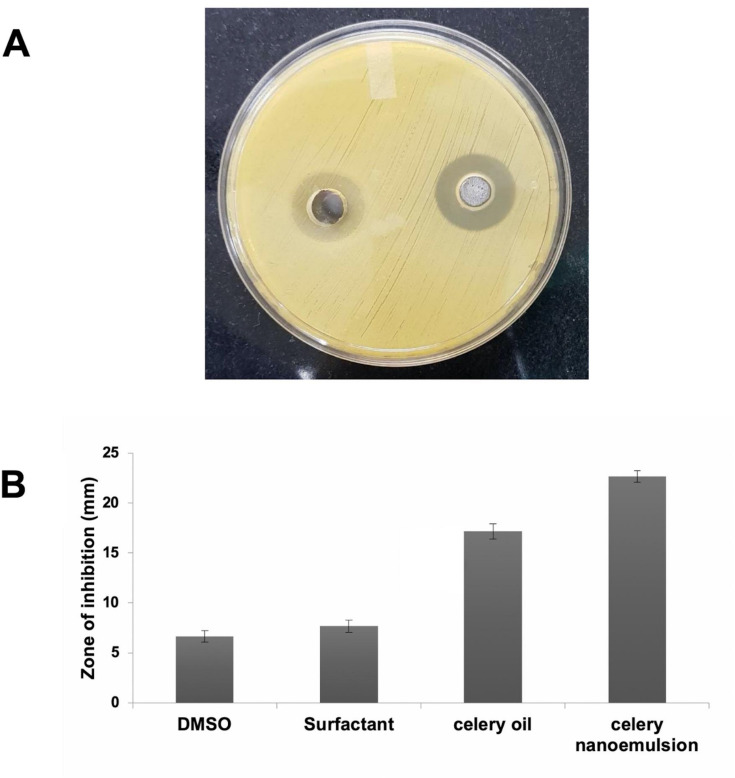
Agar Well Diffusion
This assay was performed to measure the antibacterial effects of celery nanoemulsion.58 A noticeable, clear zone of inhibition of the microbial strain on interaction with the formulation was observed for all three replicates with a mean diameter of 22.6 ± 0.57 mm, while the diameter of the zone inhibition of celery oil measured 17.1 ± 0.76 mm (Figure 11A). This indicates a higher zone of inhibition in the celery nanoemulsion as compared to its oil form. However, DMSO used in solubilization of celery oil and the surfactant-water mixture used in the formulation of celery nanoemulsion demonstrated resistance towards microbial strain interaction. The quantitative data are represented in Figure 11B. This study clearly reveals that the phytochemicals present in the celery oil, and the droplet size reduction through nanoemulsion formulation are mainly responsible for the antibacterial effect against Staphylococcus aureus.
Figure 11.
Well diffusion assay, demonstrating (A) higher zone of inhibition on treatment with celery nanoemulsion CEL-CE4 against S. aureus in comparison with celery oil (B) with quantitative representation.
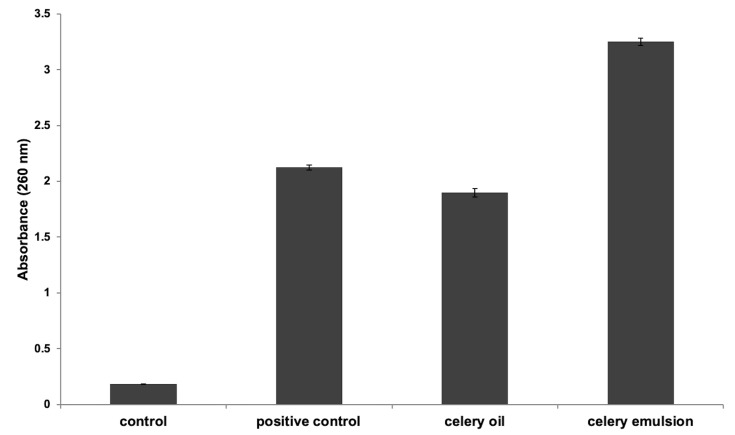
Membrane Permeability Assay
To affirm the cytoplasmic leakage from the pathogen on interaction with the formulation, membrane permeability assay was employed. UV-Visible Spectroscopy was employed to quantify the absorbance of UV and visible wavelengths in the fluid system. This aided in establishing a relationship between the absorption of light and extent of cytoplasmic leakage.59 The results depict an appreciable cytoplasmic release of the microbial suspension on nanoemulsion interaction, in contrast with oil interaction (Figure 12). The cytoplasmic leakage observed after 1 h interaction of S. aureus with celery oil and celery nanoemulsion was found to demonstrate an absorbance of 1.897 ± 0.039 and 3.251 ± 0.033, respectively, at 260 nm. Sodium benzoate, used as a positive control, showed absorbance of 2.124 ± 0.023, and evidenced a higher cytoplasmic leakage than the celery oil. However, the nanoemulsion formulation showed a greater loss of cell contents on interaction with S. aureus in comparison with the positive control and with the celery oil.
Figure 12.
Membrane permeability assay, displaying significant cytoplasmic leakage from S. aureus on treatment with CEL-C4 nanoemulsion formulation (in comparison with celery oil and positive control).
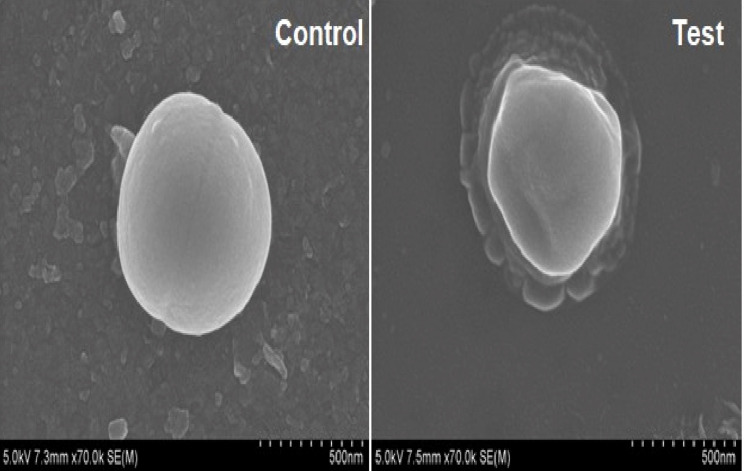
Scanning Electron Microscopy
The microscopic technique was employed to characterize the external form and structure of the materials. The untreated cell of S. aureus demonstrated an intact structure. The SEM images reveal the celery nanoemulsion-interacted cells of S. aureus as distorted with clear morphological alterations (Figure 13).
Figure 13.
Scanning electron microscopy revealing complete distortion of S. aureus on interaction with celery nanoemulsion (test) in comparison with untreated (control) cells.
Discussion
Gas chromatography–mass spectroscopy was used to identify the saturated and unsaturated hydrocarbons present in essential oil extracts.60 GC-MS indicated D–limonene as a major contributor to the biological properties exhibited by celery oil. D–limonene is reported to demonstrate fairly low toxicity with no risk to humans.61 Also, it is proven to have clinical use in dissolving cholesterol-containing gall stones, and is known for its chemopreventive effects in cancer.62 There are no proven effects attributable to the other phytochemicals present in the celery seed oil.
The nanoemulsion was prepared using celery oil, water and a bio-based Tween 80 surfactant. Based on stability and realization of uniform droplets of nanometer range, the formulation was optimized and carried forward to evaluate its anticancer and antibacterial properties.
A noticeable colour change observed during the formation of nanoemulsion could be attributed to Rayleigh scattering effect from the nanosized particles of the formulation.63 The formation was a non-spontaneous process owing to its positive Gibbs free energy. However, the surfactant has the ability to reduce the free energy of this process, thereby increasing its spontaneity and the stability of the nanoemulsion.49,64 The surfactant was also observed to have reduced the polydispersity index, which is indicative of its role in homogenizing the nanoemulsion by preventing coalescence of dispersed oil droplets.65
The small droplets produced through ultrasonication result in greater interfacial area, which increases the permeability of the active compounds through membranes of blood vessel and tissue.66 An increase in sonication time would increase the net input energy and tend to disrupt more droplets and decrease their size further. This trend was consistent with the results of this study.
An increase in sonication time (5–20 min) and surfactant concentration (1:1–1:3) led to an efficient reduction in the size of droplets and in the polydispersity index as measured through DLS. However, any increase beyond a sonication time of 20 min and oil-surfactant ratio of 1:3 was ineffective in further reducing the droplet size. CEL-C4 was therefore determined to be the optimal formulation for further characterization.
Apart from characterizing formulations based on their physical stability, viscosity and pH were also measured for all the formulations as they play a key role in dictating the efficacy of the nanoemulsion in drug delivery. The rise in viscosity with surfactant concentration could be attributed to the increased extent of cross-linking in the surfactant amidst which water molecules get entrapped.67–69 Characterizing a nanoemulsion based on pH and viscosity will aid in rheological studies while developing drug delivery mechanisms for an in-vivo application.
The cytotoxic potential of both celery oil and the formulation against oral squamous carcinoma cells (SAS) was evaluated by MTT assay. The mitochondrial dehydrogenases of viable cells convert the yellow-colored MTT to their corresponding purple formazan thereby indicating the existence of a direct correlation between purple formazan and metabolically active cells.70–72 On interaction of both celery oil and celery oil-based nanoemulsion against SAS cell line, it was observed that purple formazan crystals were not formed, indicating the concentration-dependant inhibition of the cancerous cells. Also, celery oil-based nanoemulsion was more effective in the inhibition of the SAS cells as compared to celery oil.
Another indicator of the antiproliferative potential of celery nanoemulsion against SAS cells verified in this study was the clonogenic assay. Here, the growth of single cell into a colony was analyzed, as cancerous cells tend to form colonies in an anchor-independent manner.73,74 The findings showed inhibited colony formation of SAS cells on treatment with the celery-based nanoemulsion formulation, substantiating the specificity of its antiproliferative properties – viz., it did not inhibit the growth of the untreated control cells.75
Annexin V-FITC76 analysed cell death pathway induced by celery nanoemulsion based on two mechanisms by which cell death occurs—apoptosis and necrosis. The apoptotic cell death is an irreversible programmed cell death, whereas necrosis is characterised by traumatic cell death caused by cellular injury.77 For differentiating between the two cell deaths, morphological changes in the cell were studied via their distinct molecular pathways, as both modes of cell death are activated by both extrinsic and intrinsic pathways. Annexin V-FITC assay was performed in flow cytometry with discrete dyes. Annexin V assay and Propidium Iodide act as an indicator of apoptosis and necrosis, respectively.78 During early apoptosis, membrane asymmetry of cell is lost, and phosphatidylserine translocates to the external membrane of cells where fluorochrome-labelled Annexin V binds to it and specifically targets and identifies apoptotic cells79 thereby acting as an indicator for apoptotic cell death. Necrotic cells are permeable for propidium iodide, which intercalates into the nuclear DNA of the necrotic cells and can be detected in red fluorescence, thereby rendering it an indicator through flow cytometry. This study confirmed that a majority of the cells had undergone apoptosis (Annexin V+/PI−), or had already died (Annexin V+/PI+) on interaction of oral squamous carcinoma cells (SAS) with celery nanoemulsion (1.4 µL/mL). These results for the first time showed anticancer activity against oral squamous carcinoma cells (SAS) via in-vitro assays using a celery-based nanoemulsion formulation. The formulation thus demonstrates potent anticancer activity, but the chemopreventive effect of the same is not yet evaluated. The chemoprevention of benzo[a]pyrene induced forestomach cancer in mouse models by the presence of phthalides such as p-mentha-2,8,-dien-1-ol, 3-n-butyl phthalide and sedanolide in the celery oil by inducing glutathione-S-transferase has been reported in literature.80
The antibacterial capability of the formulation was verified by studying the interaction between S. aureus and celery nanoemulsion. In the agar well diffusion assay, it was observed to have created a zone of inhibition within which the growth of microbial strain was inhibited by the nanoemulsion. While celery oil on its own exhibited a zone of inhibition, this study shows that the celery oil nanoemulsion exhibited a significantly larger zone of inhibition, thus verifying its enhanced efficacy. By measuring absorbance of UV light at 260 nm through the suspension, cytoplasmic leakage was observed when the microbial suspension was treated with the nanoemulsion.81 The nanoemulsion is capable of fusing with the lipid membranes of the pathogen and causing disrupted membrane integrity through destabilization leading to release of cell contents.82–84 The complete cell distortion on interaction with the nanoemulsion reveals cell membrane damage and cytoplasmic leakage, as confirmed through scanning electron microscopy. However, literature is scant on the antimicrobial properties of celery oil. The celery essential oil demonstrated antifungal effect against Malassezia furfur85 and antibacterial effect against Campylobacter jejuni.86
Conclusion
The nanoemulsion of celery oil which was used in this study was prepared through ultrasonication. This high-energy method produced acoustic pressure waves that induced cavitational conditions to disrupt the liquid system and form nanoparticles. A surfactant was used to stabilize the nanoemulsion which was then studied through centrifugation and recursive heating-cooling cycles. Formulations of varying surfactant concentrations were characterized on the basis of their physico-chemical properties in order to optimize the concentration. The optimal formulation was tested for its anti-bacterial properties through agar well diffusion assay and membrane permeability assay by treating it with S. aureus. The nanoemulsion on interaction with the pathogen caused considerable cytoplasmic leakage. This loss of cell contents through membrane disruption is possibly due to a different phenomenon or a combination of triggers (eg, the inherent antibacterial activity of celery oil). The nanoemulsion was further analysed to demonstrate its anticancer properties on oral squamous carcinoma cells (SAS), where it shows considerable antiproliferative activities by inhibiting cancer cell growth and by inducing apoptosis. Reduced droplet size with improved solubilization, coupled with the anticancer and antibacterial capabilities of celery nanoemulsion makes the formulation an attractive alternative to explore through in-vivo studies.
Acknowledgment
We thank Professor Dr. D. Karunagaran, HOD of the Department of Biotechnology, Bhupat and Jyoti Mehta School of Biosciences, Indian Institute of Technology Madras, for allowing access to the cell culture laboratory. We are thankful to CLRI, Adyar for providing access to the FACS instrumentation facility.
Funding Statement
This research was supported by Young Scientist Scheme, DST-SERB, Government of India [Grant number: YSS/2015/000163].
Disclosure
The authors report no conflicts of interest for this work.
References
- 1.Kaminski BM, Steinhilber D, Ulrich S, Ulrich S. Phytochemicals resveratrol and sulforaphane as potential agents for enhancing the anti-tumor activities of conventional cancer therapies. Curr Pharm Biotechnol. 2012;13(1):137–146. doi: 10.2174/138920112798868746 [DOI] [PubMed] [Google Scholar]
- 2.Mitoshi M, Kuriyama I, Nakayama H, et al. Effects of essential oils from herbal plants and citrus fruits on DNA polymerase inhibitory, cancer cell growth inhibitory, antiallergic, and antioxidant activities. J Agric Food Chem. 2012;60(45):11343–11350. doi: 10.1021/jf303377f [DOI] [PubMed] [Google Scholar]
- 3.Russo R, Corasaniti MT, Bagetta G, Morrone LA. Exploitation of cytotoxicity of some essential oils for translation in cancer therapy. Evid Based Complement Alternat Med. 2015;2015:1–9. doi: 10.1155/2015/397821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Swamy MK, Akhtar MS, Sinniah UR. Antimicrobial properties of plant essential oils against human pathogens and their mode of action: an updated review. Evid Based Complement Alternat Med. 2016;2016:1–21. doi: 10.1155/2016/3012462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cox SD, Mann CM, Markham JL, et al. The mode of antimicrobial action of the essential oil of melaleuca alternifolia (tea tree oil). J Appl Microbiol. 2000;88(1):170–175. doi: 10.1046/j.1365-2672.2000.00943.x [DOI] [PubMed] [Google Scholar]
- 6.Lo W-Y, Tsai M-H, Tsai Y, et al. Identification of over-expressed proteins in oral squamous cell carcinoma (OSCC) patients by clinical proteomic analysis. Clin Chim Acta. 2007;376(1–2):101–107. doi:org/10.1016/j.cca.2006.06.030 [DOI] [PubMed] [Google Scholar]
- 7.Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: globocan 2000. Int J Cancer. 2001;94:153–156. doi: 10.1002/ijc.1440 [DOI] [PubMed] [Google Scholar]
- 8.Ramineni SK, Dziubla TD, Cunningham LLJ, Puleo DA. Local delivery of imiquimod in hamsters using mucoadhesive films and their residence time in human patients. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;118(6):665–673. doi: 10.1016/j.oooo.2014.08.015 [DOI] [PubMed] [Google Scholar]
- 9.Wester A, Eyler JT, Swan JW. Topical imiquimod for the palliative treatment of recurrent oral squamous cell carcinoma. JAAD Case Rep. 2017;3(4):329–331. doi: 10.1016/j.jdcr.2017.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Loree TR, Strong EW. Significance of positive margins in oral cavity squamous carcinoma. Am J Surg. 1990;160(4):410–414. doi:org/10.1016/S0002-9610(05)80555-0 [DOI] [PubMed] [Google Scholar]
- 11.Klein EY, Van Boeckel TP, Martinez EM, et al. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc Natl Acad Sci USA. 2018;115(15):E3463 LP–E3470. doi: 10.1073/pnas.1717295115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saha SK, Schrag SJ, El Arifeen S, et al. Causes and incidence of community-acquired serious infections among young children in south Asia (ANISA): an observational cohort study. Lancet. 2018;392(10142):145–159. doi: 10.1016/S0140-6736(18)31127-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laxminarayan R, Matsoso P, Pant S, et al. Access to effective antimicrobials: a worldwide challenge. Lancet. 2016;387(10014):168–175. doi: 10.1016/S0140-6736(15)00474-2 [DOI] [PubMed] [Google Scholar]
- 14.Pieren M, Tigges M. Adjuvant strategies for potentiation of antibiotics to overcome antimicrobial resistance. Curr Opin Pharmacol. 2012;12(5):551–555. doi: 10.1016/j.coph.2012.07.005 [DOI] [PubMed] [Google Scholar]
- 15.Nijnik A, Hancock REW. Host defence peptides: antimicrobial and immunomodulatory activity and potential applications for tackling antibiotic-resistant infections. Emerg Health Threats J. 2009;2(1):7078. doi: 10.3402/ehtj.v2i0.7078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McConnell MJ, Dominguez-Herrera J, Smani Y, Lopez-Rojas R, Docobo-Perez F, Pachon J. Vaccination with outer membrane complexes elicits rapid protective immunity to multidrug-resistant acinetobacter baumannii. Infect Immun. 2011;79(1):518–526. doi: 10.1128/IAI.00741-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ni N, Li M, Wang J, Wang B. Inhibitors and antagonists of bacterial quorum sensing. Med Res Rev. 2009;29:65–124. doi: 10.1002/med.20145 [DOI] [PubMed] [Google Scholar]
- 18.Ter Meulen J. Monoclonal antibodies in infectious diseases: clinical pipeline in 2011. Infect Dis Clin North Am. 2011;25(4):789–802. doi: 10.1016/j.idc.2011.07.006 [DOI] [PubMed] [Google Scholar]
- 19.Allison KR, Brynildsen MP, Collins JJ. Heterogeneous bacterial persisters and engineering approaches to eliminate them. Curr Opin Microbiol. 2011;14(5):593–598. doi: 10.1016/j.mib.2011.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abedon ST, Thomas-Abedon C. Phage therapy pharmacology. Curr Pharm Biotechnol. 2010;11(1):28–47. doi: 10.2174/138920110790725410 [DOI] [PubMed] [Google Scholar]
- 21.Weber W, Schoenmakers R, Keller B, et al. A synthetic mammalian gene circuit reveals antituberculosis compounds. Proc Natl Acad Sci USA. 2008;105(29):9994–9998. doi: 10.1073/pnas.0800663105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shah P, Bhalodia D, Shelat P. Nanoemulsion: a pharmaceutical review. Sys Rev Pharm. 2010;1. doi: 10.4103/0975-8453.59509. [DOI] [Google Scholar]
- 23.Jaiswal M, Dudhe R, Sharma P. Nanoemulsion: an advanced mode of drug delivery system. Biotech. 2014;5. doi: 10.1007/s13205-014-0214-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abismaïl B, Canselier JP, Wilhelm AM, Delmas H, Gourdon C. Emulsification by ultrasound: drop size distribution and stability. Ultrason Sonochem. 1999;6(1–2):75–83. doi:org/10.1016/S1350-4177(98)00027-3 [DOI] [PubMed] [Google Scholar]
- 25.Peshkovsky A, Peshkovsky S, Bystryak S. Scalable high-power ultrasonic technology for the production of translucent nanoemulsions. Chem Eng Process. 2013;69:77–82. doi: 10.1016/j.cep.2013.02.010 [DOI] [Google Scholar]
- 26.Sutradhar KB, Amin ML. Nanoemulsions: increasing possibilities in drug delivery. Eur J Nanomed. 2013;5(2):97–110. doi: 10.1515/ejnm.2013.0001 [DOI] [Google Scholar]
- 27.Mawson R, Simons L, Mawson R, Simons L, Mawson R, Simons L. The use of ultrasonics for nanoemulsion preparation. Innov Food Sci Emerg Technol. 2008;9(2):170–175. doi: 10.1016/j.ifset.2007.07.005 [DOI] [Google Scholar]
- 28.Kooti W, Ali-Akbari S, Asadi-Samani M, Ghadery H, Ashtary-Larky D. A review on medicinal plant of Apium graveolens. Adv Herb Med. 2015;1:48–59. [Google Scholar]
- 29.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. doi: 10.3322/caac.21551 [DOI] [PubMed] [Google Scholar]
- 30.Faivre S, Djelloul S, Raymond E. New paradigms in anticancer therapy: targeting multiple signaling pathways with kinase inhibitors. Semin Oncol. 2006;33:407–420. doi: 10.1053/j.seminoncol.2006.04.005 [DOI] [PubMed] [Google Scholar]
- 31.Chanda S, Nagani K. In vitro and in vivo methods for anticancer activity evaluation and some indian medicinal plants possessing anticancer properties: an overview. J Pharmacogn Phytochem. 2013;2:140–152. [Google Scholar]
- 32.Jasek K, Kubatka P, Samec M, et al. DNA methylation status in cancer disease: modulations by plant-derived natural compounds and dietary interventions. Biomolecules. 2019;9(7):289. doi: 10.3390/biom9070289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kubatka P, Kello M, Kajo K, et al. Chemopreventive and therapeutic efficacy of Cinnamomum zeylanicum L. bark in experimental breast carcinoma: mechanistic in vivo and in vitro analyses. Molecules. 2020;25(6):1399. doi: 10.3390/molecules25061399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kubatka P, Uramova S, Kello M, et al. Antineoplastic effects of clove buds (Syzygium aromaticum L.) in the model of breast carcinoma. J Cell Mol Med. 2017;21(11):2837–2851. doi: 10.1111/jcmm.13197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kubatka P, Uramova S, Kello M, et al. Anticancer activities of Thymus vulgaris L. in experimental breast carcinoma in vivo and in vitro. Int J Mol Sci. 2019;20(7):1749. doi: 10.3390/ijms20071749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gomes MRF, Schuh RS, Jacques ALB, et al. Citotoxic activity evaluation of essential oils and nanoemulsions of Drimys angustifolia and D. brasiliensis on human glioblastoma (U-138 MG) and human bladder carcinoma (T24) cell lines in vitro. Rev Bras Farmacogn. 2013;23(2):259–267. doi: 10.1590/S0102-695X2012005000136 [DOI] [Google Scholar]
- 37.Majeed H, Antoniou J, Fang Z. Apoptotic effects of eugenol-loaded nanoemulsions in human colon and liver cancer cell lines. Asian Pac J Cancer Prev. 2014;15(21):9159–9164. doi: 10.1046/j.1365-2672.2000.00943.x [DOI] [PubMed] [Google Scholar]
- 38.Rachmawati H, Yee CW, Rahma A. Formulation of tablet containing curcumin nanoemulsion. Int J Pharm Pharm Sci. 2014;6(3):115–116. [Google Scholar]
- 39.Chang H-B, Chen B-H. Inhibition of lung cancer cells A549 and H460 by curcuminoid extracts and nanoemulsions prepared from curcuma longa linnaeus. Int J Nanomedicine. 2015;10:5059. doi: 10.2147/IJN.S87225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Suslick KS, Yuri Didenko MM, Fang TH, Kolbeck KJ, McNamara WB. Acoustic cavitation and its chemical consequences, philosophical transactions of the Royal Society of London. Math Phys Eng Sci. 1999;357:335–353. doi: 10.1098/rsta.1999.0330 [DOI] [Google Scholar]
- 41.Hong C, Kim H-A, Firestone GL, Bjeldanes LF. 3,3′-Diindolylmethane (DIM) induces a G1 cell cycle arrest in human breast cancer cells that is accompanied by Sp1-mediated activation of p21WAF1/CIP1 expression. Carcinogenesis. 2002;23(8):1297–1305. doi: 10.1093/carcin/23.8.1297 [DOI] [PubMed] [Google Scholar]
- 42.Saleh MM, Hashem FA, Glombitza KW. Cytotoxicity and in vitro effects on human cancer cell lines of volatiles of Apium graveolens var filicinum. Pharm Pharmacol Lett. 1998;8:97–99. [Google Scholar]
- 43.Sowbhagya HB. Chemistry, technology, and nutraceutical functions of celery (Apium graveolens L.): an overview. Crit Rev Food Sci Nutr. 2014;54(3):389–398. doi: 10.1080/10408398.2011.586740 [DOI] [PubMed] [Google Scholar]
- 44.Moodley J, Coulter I. Combination products. U.S. Patent Application 14/260,084, filed. August 21, 2014.
- 45.Atta AH, Alkofahi A. Anti-nociceptive and anti-inflammatory effects of some Jordanian medicinal plant extracts. J Ethnopharmacol. 1998;60(2):117–124. doi: 10.1016/s0378-8741(97)00137-2 [DOI] [PubMed] [Google Scholar]
- 46.Jamuna Bai A, Vittal RR. Nanoemulsions and their potential applications in food industry. Front Sustain Food Syst. 2019;3:95. doi: 10.3389/fsufs.2019.00095 [DOI] [Google Scholar]
- 47.Gharibzahedi SMT, Jafari SM. Fabrication of nanoemulsions by ultrasonication. Nanoemulsions. 2018;233–285. doi: 10.1016/b978-0-12-811838-2.00009-6 [DOI] [Google Scholar]
- 48.Zhang G, Peng Y, Cui L, Zhang L. Gold-catalyzed homogeneous oxidative cross-coupling reactions. Angew Chem Int Ed. 2009;48(17):3112–3115. doi: 10.1002/anie.200900585 [DOI] [PubMed] [Google Scholar]
- 49.Tadros T, Izquierdo P, Esquena J, Solans C. Formation and stability of nano-emulsions. Adv Colloid Interface Sci. 2004;108:303–318. doi: 10.1016/j.cis.2003.10.023 [DOI] [PubMed] [Google Scholar]
- 50.Sondari D, Tursiloadi S. The effect of surfactant on formulation and stability of nanoemulsion using extract of centella asiatica and zingiber officinale. AIP Conf Proc. 2018;2049(1):030014. doi: 10.1063/1.5082515 [DOI] [Google Scholar]
- 51.Che Marzuki NH, Wahab RA, Abdul Hamid M. An overview of nanoemulsion: concepts of development and cosmeceutical applications. Biotechnol Biotechnol Equip. 2019;33:779–797. doi: 10.1080/13102818.2019.1620124 [DOI] [Google Scholar]
- 52.Bernardi DS, Pereira TA, Maciel NR, et al. Formation and stability of oil-in-water nanoemulsions containing rice bran oil: in vitro and in vivo assessments. J Nanobiotechnology. 2011;9(1):44. doi: 10.1186/1477-3155-9-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Balouiri M, Sadiki M, Ibnsouda SK. Methods for in vitro evaluating antimicrobial activity: a review. J Pharm Anal. 2016;6(2):71–79. doi: 10.1016/J.JPHA.2015.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hou L, Shi Y, Zhai P, Le G. Inhibition of foodborne pathogens by Hf-1, a novel antibacterial peptide from the larvae of the housefly (musca domestica) in medium and orange juice. Food Control. 2007;18(11):1350–1357. doi: 10.1016/j.foodcont.2006.03.007 [DOI] [Google Scholar]
- 55.Golding CG, Lamboo LL, Beniac DR, Booth TF. The scanning electron microscope in microbiology and diagnosis of infectious disease. Sci Rep. 2016;6(1):26516. doi: 10.1038/srep26516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stokes DJ. Recent advances in electron imaging, image interpretation and applications: environmental scanning electron microscopy. Philos Trans R Soc Lond B Biol Sci. 2003;361:2771–2787. [DOI] [PubMed] [Google Scholar]
- 57.Nuryadi E, Mayang Permata TB, Komatsu S, Oike T, Nakano T. Inter-assay precision of clonogenic assays for radiosensitivity in cancer cell line A549. Oncotarget. 2018;9(17):13706–13712. doi:https://doi:10.18632/oncotarget.24448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Valgas C, de Souza SM, Smânia EFA, Smânia A. Screening methods to determine antibacterial activity of natural products. Braz J Microbiol. 2007;38(2):369–380. doi: 10.1590/S1517-83822007000200034 [DOI] [Google Scholar]
- 59.Sugumar S, Nirmala J, Ghosh V, Anjali H, Mukherjee A, Chandrasekaran N. Bio-based nanoemulsion formulation, characterization and antibacterial activity against food-borne pathogens. J Basic Microbiol. 2013;53(8):677–685. doi: 10.1002/jobm.201200060 [DOI] [PubMed] [Google Scholar]
- 60.Marriott PJ, Shellie R, Cornwell C. Gas chromatographic technologies for the analysis of essential oils. J Chromatogr A. 2001;936(1–2):1–22. doi: 10.1016/S0021-9673(01)01314-0 [DOI] [PubMed] [Google Scholar]
- 61.Kim YW, Kim MJ, Chung BY. Safety evaluation and risk assessment of d-limonene. J Toxicol Environ Health B Crit Rev. 2013;16(1):17–38. doi: 10.1080/10937404.2013.769418 [DOI] [PubMed] [Google Scholar]
- 62.Sun J. D-limonene: safety and clinical applications. Altern Med Rev. 2007;12(3):259–264. [PubMed] [Google Scholar]
- 63.Mason TG, Wilking JN, Meleson K, Chang CB, Graves SM. Nanoemulsions: formation, structure, and physical properties. J Phys Condens Matter. 2006;18:R635–R666. doi: 10.1088/0953-8984/18/41/r01 [DOI] [Google Scholar]
- 64.Hasani F, Pezeshki A, Hamishehkar H. Effect of surfactant and oil type on size droplets of betacarotene-bearing nanoemulsions. Int J Curr Microbiol Appl Sci. 2015;4:146–155. [Google Scholar]
- 65.Constantinides PP, Yiv SH. Particle size determination of phase-inverted water-in-oil microemulsions under different dilution and storage conditions. Int J Pharm. 1995;115(2):225–234. doi:org/10.1016/0378-5173(94)00272-7 [Google Scholar]
- 66.Nirmala MJ, Mukherjee A, Chandrasekaran N. Improved efficacy of fluconazole against candidiasis using bio-based microemulsion technique. Appl Biochem Biotechnol. 2013;60(4):417–429. doi: 10.1002/bab.1116 [DOI] [PubMed] [Google Scholar]
- 67.Lovelyn C, Attama A. Current state of nanoemulsions in drug delivery. J Biomater Nanobiotechnol. 2011;02(05):626–639. doi: 10.4236/jbnb.2011.225075 [DOI] [Google Scholar]
- 68.El Eini DID, Barry BW, Rhodes CT. Micellar size, shape, and hydration of long-chain polyoxyethylene nonionic surfactants. J Colloid Interf Sci. 1976;54(3):348–351. doi:org/10.1016/0021-9797(76)90314-3 [Google Scholar]
- 69.Barry BW, Eini DIE. Solubilization of hydrocortisone, dexamethasone, testosterone and progesterone by long-chain polyoxyethylene surfactants. J Pharm Pharmacol. 1976;28(3):210–218. doi: 10.1111/j.2042-7158.1976.tb04133.x [DOI] [PubMed] [Google Scholar]
- 70.Bergantini APF, Castro FA, Souza AM, Fett-Conte AC. Leucemia mielóide crônica e o sistema fas-fasL. Rev Bras Hematol Hemoter. 2005;27:120–125. [Google Scholar]
- 71.Saravanan BC, Sreekumar C, Bansal GC, Ray D, Rao JR, Mishra AK. A rapid MTT colorimetric assay to assess the proliferative index of two Indian strains of theileria annulata. Vet Parasitol. 2003;113(3–4):211–216. doi: 10.1016/s0304-4017(03)00062-1 [DOI] [PubMed] [Google Scholar]
- 72.van Meerloo J, Kaspers GJL, Cloos J. Cell sensitivity assays: the MTT assay. Methods Mol Biol. 2011;731:237–245. doi: 10.1007/978-1-61779-080-5_20 [DOI] [PubMed] [Google Scholar]
- 73.Franken NAP, Rodermond HM, Stap J, Haveman J, van Bree C. Clonogenic assay of cells in vitro. Nat Protoc. 2006;1(5):2315–2319. doi: 10.1038/nprot.2006.339 [DOI] [PubMed] [Google Scholar]
- 74.Buch K, Peters T, Nawroth T, Sanger M, Schmidberger H, Langguth P. Determination of cell survival after irradiation via clonogenic assay versus multiple MTT assay–a comparative study. Radiat Oncol. 2012;7(1):1. doi: 10.1186/1748-717X-7-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Milhomem-Paixão SSR, Fascineli ML, Muehlmann LA, et al. Carapa guianensis aublet nanoemulsions: development and assessment of cytotoxicity, genotoxicity, and hematotoxicity. J Nanomater. 2017;2017:4362046. doi: 10.1155/2017/4362046 [DOI] [Google Scholar]
- 76.Zhang G, Gurtu V, Kain SR, Yan G. Early detection of apoptosis using a fluorescent conjugate of annexin V. Biotechniques. 1997;23(3):525–531. doi: 10.2144/97233pf01 [DOI] [PubMed] [Google Scholar]
- 77.Nikoletopoulou V, Markaki M, Palikaras K, Tavernarakis N. Crosstalk between apoptosis, necrosis and autophagy. Biochim Biophys Acta. 2013;1833(12):3448–3459. doi: 10.1016/j.bbamcr.2013.06.001 [DOI] [PubMed] [Google Scholar]
- 78.Vermelho AB, da Silva Cardoso V, Ricci Junior E, Dos Santos EP, Supuran CT. Nanoemulsions of sulfonamide carbonic anhydrase inhibitors strongly inhibit the growth of trypanosoma cruzi. J Enzyme Inhib Med Chem. 2018;33(1):139–146. doi: 10.1080/14756366.2017.1405264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35(4):495–516. doi: 10.1080/01926230701320337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zheng G, Kenney PM, Zhang J, Lam LKT. Chemoprevention of benzo[a]pyrene-induced forestomach cancer in mice by natural phthalides from celery seed oil. Nutr Cancer. 1993;19(1):77–86. doi: 10.1080/01635589309514238 [DOI] [PubMed] [Google Scholar]
- 81.Zhang H, Shen Y, Weng P, Zhao G, Feng F, Zheng X. Antimicrobial activity of a food-grade fully dilutable microemulsion against Escherichia coli and Staphylococcus aureus. Int J Food Microbiol. 2009;135(3):211–215. doi: 10.1016/j.ijfoodmicro.2009.08.015 [DOI] [PubMed] [Google Scholar]
- 82.Baker JR Jr, Hamouda T, Shih A, Myc A. Non-Toxic Antimicrobial Compositions and Methods of Use. 2003. [Google Scholar]
- 83.Hwang YY, Ramalingam K, Bienek DR, Lee V, You T, Alvarez R. Antimicrobial activity of nanoemulsion in combination with cetylpyridinium chloride in multidrug-resistant Acinetobacter baumanii. Antimicrob Agents Chemother. 2013;57(8):3585. doi: 10.1128/AAC.02109-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Maiti S, Krishnan D, Barman G, Ghosh SK, Laha JK. Antimicrobial activities of silver nanoparticles synthesized from Lycopersicon esculentum extract. J Anal Sci Technol. 2014;5(1):40. doi: 10.1186/s40543-014-0040-3 [DOI] [Google Scholar]
- 85.Chee HY, Lee MH. In vitro activity of celery essential oil against malassezia furfur. Mycobiology. 2009;37(1):67–68. doi:https://doi:10.4489/MYCO.2009.37.1.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Friedman M, Henika PR, Mandrell RE. Bactericidal activities of plant essential oils and some of their isolated constituents against Campylobacter jejuni, Escherichia coli, Listeria monocytogenes, and Salmonella enterica. J Food Prot. 2002;65(10):1545–1560. doi:https://doi:10.4315/0362-028x-65.10.1545 [DOI] [PubMed] [Google Scholar]