Abstract
Recent studies have shown that lactate coupled water flux is the underlying mechanism of the corneal endothelial pump, which is highly dependent on the presence of bicarbonate. In this study we test the hypothesis that the increased intracellular pH (pHi) caused by bicarbonate stimulates glycolytic activity and the production of lactate by endothelial cells. Primary cultures of bovine corneal endothelial cells (BCEC) were incubated in bicarbonate-free (BF) ringer, a high [HEPES] ringer, and bicarbonate-rich (BR) ringer all at pH 7.5. Lactate production and glucose consumption were greatest in BR>HEPES >BF. Similarly, pHi was greatest in BR>HEPES>BF. Increasing pHi with NH4Cl also increased lactate production in BF or BR, indicating that the increased lactate production in BR is not due to HCO3− itself. Glucose transport capacity, as measured by 2-N-(7-Nitrobenz-2-oxa-1,3-diazol-4-yl)Amino-2-Deoxyglucose (2-NBDG) uptake was unaffected by the three incubation conditions. Using Laconic, a FRET sensor for lactate, we found that intracellular [lactate] increased immediately and transiently when cells were switched from BF to BR perfusion indicating increased lactate production with subsequent matching of efflux. Moreover, induction of acute lactate influx by perfusion pulses of 10 mM lactate increased intracellular [lactate] significantly faster in BF than in BR, consistent with higher lactate production and efflux in BR. In summary, our results indicate that glycolytic flux and lactate production increase in BR due to increased pHi, consistent with the well-known pH sensitivity of phosphofructokinase, the rate limiting enzyme in glycolysis.
Keywords: Corneal endothelium, bicarbonate, intracellular pH, lactate production, glycolysis
The corneal endothelium is responsible for maintaining the transparency of the cornea by controlling stromal hydration. Steady-state hydration is achieved by the ion-linked fluid transport properties of the endothelium that offset stromal swelling pressure driven water influx. Primary active transport (Na+-K+ ATPase), located on the lateral membrane of endothelial cells, provides Na+ gradients that are required to drive basolateral Na+:2HCO3− cotransport and Na+/H+ exchange, the two major membrane transporters that control intracellular pH (pHi) in corneal endothelium (Bonanno and Giasson, 1992a, c; Diecke et al., 2004; Li et al., 2005). Regulation of pHi is strongly influenced by the robust buffering power of bicarbonate, which is supported by several carbonic anhydrases located on basolateral and apical membranes and within the cytosol (Cui et al., 2002; Fischbarg and Lim, 1974; Kuang et al., 1990; Terashima et al., 1996). These processes help drive lactate:H+ cotransport uptake on the basolateral membrane and efflux apically to the anterior chamber. Inhibiting the Na+-K+ ATPase (Anderson and Fischbarg, 1978; Li et al., 2016; Li et al., 2020; Riley et al., 1994), removing bicarbonate or inhibiting carbonic anhydrase activity (Fischbarg and Lim, 1974; Li et al., 2016; Li et al., 2020; Riley et al., 1995), inhibiting the Na+:2HCO3− cotransporter (Bonanno and Giasson, 1992c; Nguyen and Bonanno, 2011) or Na+/H+ exchanger (Liebovitch and Fischbarg, 1982; Nguyen and Bonanno, 2011), lowering extracellular buffering capacity (Li et al., 2016), shRNA knock down of monocarboxylate cotransport expression (MCTs) (Li et al., 2014), or pharmacologic inhibition of MCT cotransport (Li et al., 2016) all cause relative decreases in endothelial lactate flux with concomitant corneal edema. These findings led to the hypothesis that the corneal endothelial pump maintains hydration by linking water efflux to lactate efflux. The model (see Figure 1) (Li et al., 2016) predicts that endothelial dysfunction will reduce lactate efflux leading to increased corneal [lactate] and corneal edema, which was confirmed in rabbits (Li et al., 2016; Li et al., 2014) and in the Slc4a11 knock out mouse model of CHED (Congenital Hereditary Endothelial Dystrophy) (Li et al., 2020).
Figure 1.
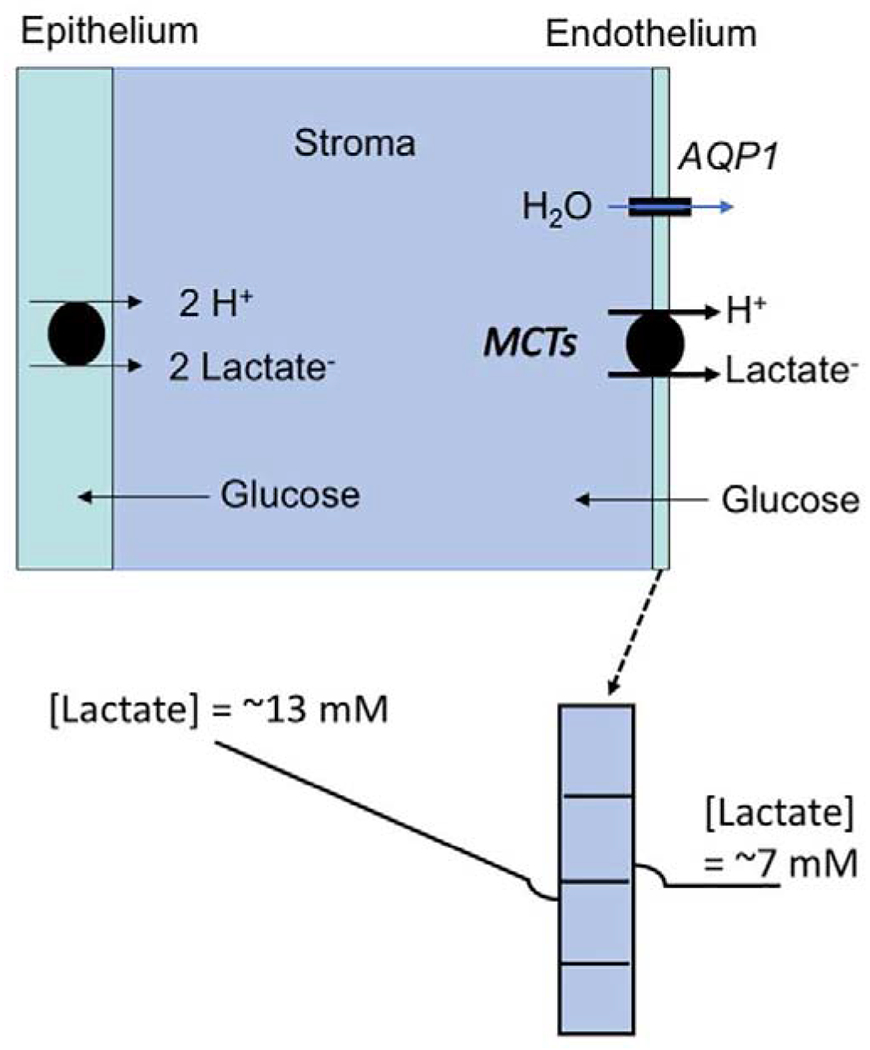
Lactate- Water Flux Schematic Model. (Top) Glucose is taken up by corneal endothelial cells from the aqueous humor and diffuses into the stroma to the epithelium. Epithelial cells and stromal keratocytes produce 2 lactate molecules for every glucose metabolized in glycolysis. (Bottom) This creates a high [lactate] in the anterior cornea (average ~13 mM (Klyce, 1981)) relative to the aqueous (~7 mM) (Bergmanson et al., 1985). Therefore, a [lactate] gradient is formed decreasing from anterior cornea to endothelium, where moncarboxylate transporters (MCT1 and 4) take up lactate:H+ by secondary active transport, creating a slight dip in [lactate] at the basolateral domain. Apical membrane MCT2 transfers lactate:H+ to the apical domain creating a slightly higher [lactate] in the apical unstirred layer. This transendothelial [lactate] gradient creates an osmotic driving force for water flux that is facilitated by aquaporin 1.
Eighty-five percent of the glucose taken up by the cornea is metabolized to lactate (Riley, 1969), indicating that the cornea is highly glycolytic. The epithelium and stromal keratocytes are responsible for most of the lactate production, consistent with a relatively lower density of mitochondria compared with endothelial cells (Hogan et al., 1971). Lactate does not diffuse anteriorly (Klyce, 1981), so it must all diffuse posteriorly across the endothelium providing substrate for the pump mechanism. Seminal studies showed that pump function is unchanged when the epithelium is removed, proving that the endothelium is responsible for the pump, not the epithelium (Dikstein and Maurice, 1972; Riley et al., 1995). Epithelial removal significantly reduced corneal lactate production by 50% (Li et al., 2020). However, the linkage between lactate efflux and water efflux was unchanged, suggesting that the endothelium can form the basolateral to apical [lactate] gradients needed for osmotic water flux as long as some lactate is present.
Confluent monolayers of primary cultures of bovine corneal endothelial cells have been shown to transport water in the absence of epithelial cells or keratocytes (Kuang et al., 2004; Narula et al., 1992). The fluid transport rate was ~50-60% of that measured in intact corneas and was eliminated by inhibition of the Na+-K+ ATPase or bicarbonate removal. These cultures also produce lactate where apical efflux is approximately twice basolateral efflux and the flux is dependent on bicarbonate, carbonic anhydrase activity, and the pHi regulatory transporters (Nguyen and Bonanno, 2011, 2012). These studies suggest that the endothelium by itself retains some intrinsic water pumping capability that is dependent on bicarbonate and linked to lactate flux. Regulation of glycolysis and therefore production of lactate is primarily via phosphofructokinase (PFK). Classic studies have shown that PFK activity increases with increasing pH (Kitajima et al., 1983; Trivedi and Danforth, 1966). In the current study, we test the hypothesis that lactate production by cultured bovine corneal endothelial cells is enhanced in bicarbonate due to the effects of pHi on glycolytic activity.
METHODS
Cell culture
Fresh bovine eyes were obtained from a local slaughterhouse and corneal endothelial cells were harvested as previously described (Bonanno and Giasson, 1992a). Primary culture was established in T-25 flasks with 5 ml Dulbecco Modified Eagle Medium (DMEM), 10% bovine calf serum, and 1% antibiotic/antimycotic (penicillin 100 U/mL, and fungizone 0.25 ug/mL), gassed with 5% CO2-95% air at 37° C and medium changed every 2-3 days. All bovine corneal endothelial cells (BCEC) used in experiments are passage P1 – P3.
Ringer solutions and chemicals.
The composition of the standard bicarbonate-free Ringer (BF) used throughout this study was (in mM) 160 Na+, 4 K+, 0.6 Mg2+, 1.4 Ca2+, 102 Cl−, 1 HPO42−, 60 gluconate, and 5 glucose. HEPES Ringer was identical except that 60 mM Na-HEPES was substituted for sodium gluconate. Bicarbonate-rich ringer solution (BR) was prepared by equimolar substitution of sodium gluconate with 28.5 mM NaHCO3−. Ringer solutions were equilibrated with air (BF & HEPES) or 5% CO2 (BR) and pH was adjusted to 7.50 with NaOH at 37°C. Lactate solutions were prepared on the morning of the experiment by equimolar substitution of Na+-gluconate by Na+-L(+) lactate. Osmolarities of all solutions were adjusted to 295-300 mOsmol by adding sucrose. Na+ L(+) lactate, nigericin, and iodoacetate were obtained from Sigma Aldrich (St. Louis, MO). The pH-sensitive fluorescent dye 2’7’-bis(2-carboxyethel)-5(6)-carboxyfluorescein (BCECF-AM) was purchased from Molecular Probes (Thermo Fisher Scientific). 2-NBDG was obtained from (Sigma).
Lactate Production & Glucose Consumption
Primary cultured BCEC were subcultured to 12 well plates at 2 x 105 cells/well and grown to confluency within 3-5 days. Cells were washed with PBS and 0.5 ml of Ringer solutions (BF, HEPES or BR) was added. BF and HEPES plates were maintained at 37°C in air, and BR plates were maintained at 37°C and connected to 5% CO2. For measuring lactate production, a 50 μl sample was taken at 30, 60, 90, 120 and 150 minutes respectively, while replenishing with a 50 μl of Ringers at the same time. In order to measure glucose consumption, the glucose concentration was reduced to 0.1 mM in all ringer solutions, and samples were taken at 1, 2, 3, 4 and 5 hours respectively. Measurements of lactate and glucose were carried out by using assay kits from BioVision (Milpitas, CA).
Oxygen Consumption
Primary bovine corneal endothelial cells were seeded at 5 x 104 cells/well (250 μl volume) in 24-well plates (Agilent 100777-004) and grown to confluence in DMEM, 10% bovine calf serum, and 1% antibiotic/antimycotic (penicillin 100 U/mL, and fungizone 0.25 ug/mL), gassed with 5% CO2-95% air at 37° C. The day of the experiments, cells were washed and incubated in BF or HEPES 60 mM ringers for 2 hours at 37 ° C in a non-CO2 incubator. Mito Stress kit for Seahorse XFe24 (Agilent #103015-100) was run using reagents prepared in BF or HEPES 60 mM: Oligomycin 1.5 μM, FCCP 0.5 μM, Rotenone 0.5 μM Antimycin A: 0.5 μM. Data analysis was performed with Wave Software and Multi-File Report Generator. At the end of the assay, cells were trypsinized and counted in a Cellometer. Oxygen consumption rates were normalized to cell number.
Glucose Transport Capacity
Bovine primary corneal endothelial cells were incubated with BR, BF or HEPES ringers containing 10 μM 2-NBDG for 30 mins, followed by dissociation with 0.25% Trypsin EDTA. Cells were washed with the respective buffers containing 5% FBS to neutralize trypsin and centrifuged at 4000g for 5 mins. Cell pellets were re-suspended in BF, BR or HEPES and filtered using CellTrics filters (Sysmex, Germany). A glucose transporter (Glut1) inhibitor, WZB117 (Tocris, Bristol, U.K) 20 μM in BR solution, was used as a negative control for 2-NBDG uptake, and unstained cells were used to gate for autofluorescence. Flow cytometry analysis was conducted on MACSQuant VYB (Miltenyi Biotech, Germany). Immediately prior to analysis, 7-ADD reagent (Bio Legend, San Diego, CA) was added to all samples to stain dead cells, which were excluded. 10,000 cells were used for each acquisition, and treatment conditions were analyzed in triplicate. Data were analyzed using Flow-Jo software (FlowJo LLC., Ashland, OR).
Measurement of Intracellular pH
Bovine corneal endothelial cells were cultured onto 25-mm round glass coverslips and loaded with the pH-sensitive fluorescent dye 2’7’-bis(2-carboxyethyl)-5(6)-carboxyfluorescein (BCECF) by incubation in BF Ringer solution containing 2 μM BCECF-AM at room temperature for 30 min. Dye-loaded cells were then kept in BF Ringer solution for at least 30 minutes before use. Coverslips were placed in a single-sided perfusion chamber (Bonanno and Giasson, 1992a). The assembled chambers were placed on a water-jacketed (37°C) brass collar held on the stage of an inverted microscope (Diaphot 200; Nikon, Tokyo, Japan) and viewed with an oil immersion objective (40x; Nikon). Hanging syringes with Ringer solution were placed in a Plexiglas warming box (37°C) and connected to an eight-way Hamilton valve used to select the desired ringer. The flow of the perfusate (approximately 0.5 mL/min) was achieved by gravity. Connections to the valve and the valve to the chamber used Phar-Med tubing (Saint-Gobain Performance Plastics, Paris, France). BCECF fluorescence was excited alternately at 495 ± 10 and 440 ± 10 nm. Fluorescence emission (520-550 nm) ratios (F495/F440) obtained at 1 Hz were calibrated against pHi by the high-K+-nigericin technique (Bonanno and Giasson, 1992b). Excitation light and data collection was controlled by PTI Felix software.
Intracellular Lactate
Relative changes in intracellular [Lactate] were measured using the lactate sensitive FRET probe Laconic (addgene.org, Cat# 44238) [27]. This probe was transiently transfected into primary cultured bovine corneal endothelial cells (BCEC) by using Lipofectamine 3000 (Life technologies, Cat# L3000-008). In brief, 5x105 BCEC were seeded on 25 mm diameter coverslips, placed in 6-well plates. 2 μg of laconic plasmid was mixed with 10 μl of transfection reagent, and the transfection complex was brought to a volume of 0.75 ml by adding serum-free DMEM before being applied to the cells that have grown to ~60-70% confluence. After a 3-hour transfection the cells were grown in normal conditions for another 24 ~ 48 hour. The coverslip was mounted in the perfusion chamber, fluorescent cells were located and perfused with BF, HEPES, or BR. In some experiments 10 mM Na-lactate (substituted for Na-gluconate) was added. Fluorescence was excited at 440 nm and dual emission (490 and 520 nm) collected from transfected cells at 1 Hz. Excitation and data collection was controlled by PTI Felix software.
Statistical analysis
Summary data is presented as mean values ± standard deviations. Student’s t-test (paired or independent) was used to test for significance (p<0.05). Bonferroni’s multiple comparisons.
RESULTS
Lactate Production
Previous studies have shown that steady-state pHi of corneal endothelial cells is higher in bicarbonate-rich ringer (BR) than in bicarbonate-free (BF) (Bonanno and Giasson, 1992a, c). To test the effect of pHi on lactate production, primary cultures of bovine corneal endothelial cells (BCEC) were washed with PBS and incubated with BF, 60 mM HEPES buffered bicarbonate-free (HEPES), or BR ringer. Each experiment was done in triplicate and repeated with new cultures on three separate days. Figure 2A shows that lactate production was greatest in BR, then HEPES, and least in BF over 120 minutes. To measure glucose consumption rates under the same conditions, we had to lower the starting [glucose] in order to bring the [glucose] of samples within the assay range. Figure 2B shows that glucose consumption was greatest in BR, then HEPES, and least in BF, consistent with the differences in lactate production. These results suggest that glycolysis is accelerated in BCEC under conditions of higher pHi. To confirm that this effect is via glycolysis and not from tricarboxylic acid cycle (TCA) intermediates feeding back to lactate production, the same experiments were repeated in the presence of 100 μM iodoactetate, a Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) inhibitor. Figure 2C shows that iodoacetate reduced lactate production to less than 10% under all three conditions.
Figure 2.

Effect of BF, HEPES, and BR Ringer on Glycolytic Activity in BCEC. A) Lactate production, n=3 per data point, *significantly different from BF, p<0.05. B) Glucose consumption. C) % of Control Lactate production at 2 hours in the presence of 100 μM iodoacetate, n=3 per condition. D) Oxygen Consumption Rate in BF (n=5) vs HEPES (n=6), *p<0.05. E) OCR related to ATP production. F) 2-NBDG uptake in BF, HEPES, and BR, n=3 per condition. No NBDG gate control and 20 μM Glut inhibitor WZB117 control are shown.
Oxygen Consumption
The end product of glycolysis, pyruvate, can be converted to lactate or it can enter mitochondria and contribute to the TCA cycle and ultimately consume oxygen. Increased lactate production implies that the availability of pyruvate is also increased, some of which could enter mitochondria and increase oxygen consumption. To test this possibility, we measured oxygen consumption. Figure 2D shows that the basal oxygen consumption rate (OCR) is greater in HEPES then BF. (BR with CO2 equilibration was not possible within the Seahorse machine). Moreover, ATP related oxygen consumption (Figure 2E) was also higher in HEPES, consistent with greater oxidative flux in endothelial cells with greater glycolytic activity.
Glucose Transport
We next asked if facilitated glucose uptake via Glut transporters is affected differentially by BR, HEPES, and BF incubation. We used 2-NBDG, a non-metabolizable fluorescent glucose analog (Zou et al., 2005), uptake assay to test this possibility. Figure 2F shows that there was no difference in the capacity to bring glucose into the cells under the three conditions.
Intracellular pHi
Next, we measured pHi under the three conditions to confirm that BR has higher pHi than BF and to also test if HEPES incubation is intermediate. Figure 3A shows the calibration of fluorescence ratio (Ex495/440, Em 520-540) to pHi. The average steady-state pHi was 7.038 ± 0.091 in BF, 7.116 ±0.118 in HEPES, and 7.226 ±0.104 in BR. Figure 3B shows a representative trace of pHi of BCEC perfused with BF, then switched to HEPES, then BR. The average of the paired differences between HEPES & BF (+0.074 ±0.045) and BR & BF (+0.197 ±0.067) were significantly different (n=5 pairs, p<0.05) confirming the rank order of pHi as BR>HEPES>BF. NH4Cl exposure is a well-known way to increase pHi in all cells including corneal endothelium (Bonanno and Giasson, 1992a, c; Ogando et al., 2013; Roos and Boron, 1981). If increased pHi stimulates glycolysis, then increasing pHi by NH4Cl incubation should increase lactate production. BCEC were washed with PBS and then incubated in BF or BR with or without 15 mM NH4Cl all at pH 7.5, which increases steady-state pHi by ~0.13 (Bonanno and Giasson, 1992a, c; Ogando et al., 2013; Roos and Boron, 1981). Figure 3C shows that lactate production was significantly increased in both BF and BR in the presence of NH4Cl.
Figure 3.
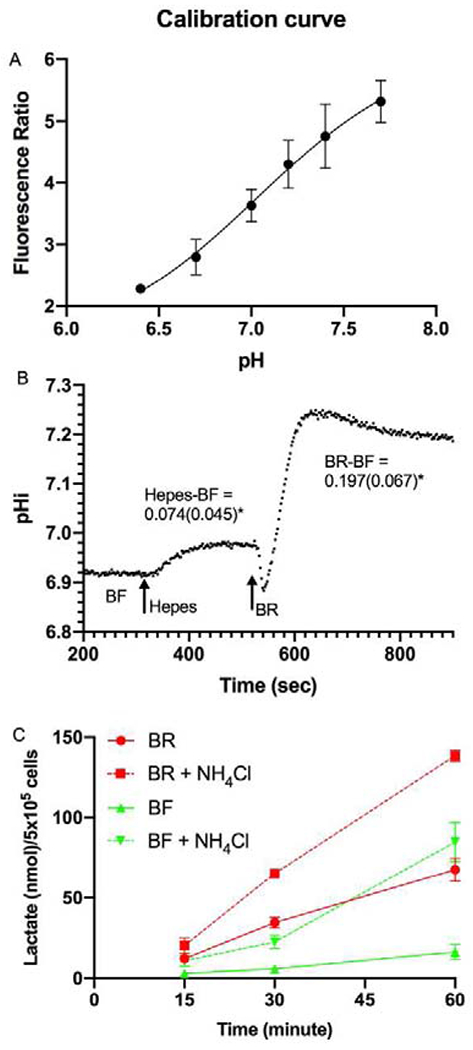
Intracellular pH. A) Standard High [K] plus Nigericin (K+/H+ exchanger) calibration of fluorescence emission ratio (Ex495/Ex440) vs pH, n=5 per data point. B) Representative trace of pHi of BCEC while perfused with BF, then HEPES, and then BR at arrows. Average (SD) of paired pHi differences (n=5) in HEPES and BR vs BF is shown. C) Lactate production in BF and BR in the presence of 15 mM NH4Cl, all at pH 7.5, n=3 per data point.
Laconic Lactate Sensor
Since Figure 3B shows that pHi changes quickly upon changing the perfusing ringer, reaching a new steady-state within two minutes, and since PFK activity would be immediately affected by this change, we examined the continuous change in intracellular lactate [Laci] using endothelial cells transiently transfected with Laconic, a lactate sensitive FRET protein. Figure 4A shows a representative trace indicating an immediate increase in intracellular lactate when perfusion was switched from BF to BR. This was followed by a decrease in [Laci] back to the same starting value. Similar changes were observed when switching from BF to HEPES, however the rate, 1.5±0.8% (ΔF/F) per min, and peak change, 2±0.1%, in HEPES were significantly smaller (n=6, p<0.05) than when switching to BR, 10±1.2% (ΔF/F) per min and 9±1.5% peak change, respectively. These results suggest that lactate production increased quickly as pHi went up. Since there is zero lactate in the perfusing ringer, the driving force for lactate efflux is infinite, so this increased rate of production is likely matched by increased efflux bringing the [Laci] back to the same resting level.
Figure 4.
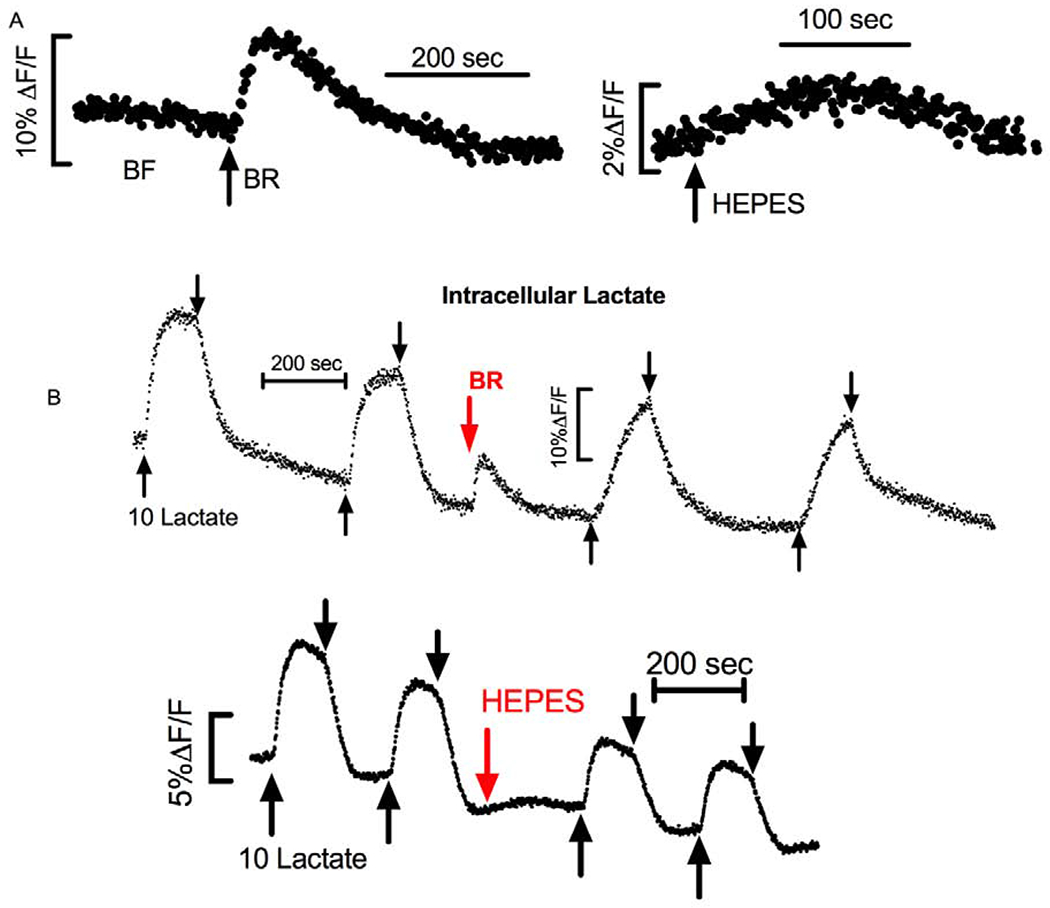
Relative Changes in Intracellular [Lactate] Measured with Laconic. A) Representative trace of Laconic transfected BCEC perfused with BF and then switched to BR at the arrow (Left) or switched to HEPES (right). B) Laconic transfected cells exposed to 2-minute pulse of 10 mM lactate (between up arrow and down arrow) in BF and then BR (top trace), and BF and then HEPES (bottom trace).
If the rate of lactate production is increased in BR with the commensurate increased rate of efflux, then when applying a large pulse of lactate in the perfusing solution, the influx of lactate in BR will be met with greater resistance because of the greater baseline production rate and efflux. Whereas when the same lactate pulse is given in BF, where there is less baseline production and efflux, lactate influx will be easier. To test this prediction, we perfused laconic transfected cells and applied a 10 mM pulse of lactate first in BF and then in HEPES (n=6) or BR (n=10). Figure 4B shows representative traces indicating that the rate of lactate influx and peak [Laci] was greatest in BF, intermediate in HEPES, and least in BR, consistent with greater lactate production and efflux in BR. Paired differences of the initial rate and peak change between the response in BF and HEPES or between BF and BR were made and is summarized in Table 1.
Table 1.
Rate and Peak Change in Laconic Fluorescence in Response to 10 mM Lactate Pulse Relative to BF
| % of Initial Rate | % of Peak | |
|---|---|---|
| HEPES vs BF (n=6) | 75.4±15* | 59.8±25.5* |
| BR vs. BF (n=10) | 19.5±8.8* | 37.6±22.5* |
p<0.05 paired t-test
Discussion
In this study we show that lactate production by corneal endothelial cells is increased in bicarbonate-rich conditions due to the increased pHi in bicarbonate. Concomitant with increased lactate production is increased glucose consumption. Although the [glucose] used was low, the incubation conditions were identical and the rank order (BR>HEPES>BF) was consistent with lactate production. The increase in glycolytic flux was almost entirely blocked by GAPDH inhibition. An intermediate increase in lactate production and glucose consumption is observed in bicarbonate-free high HEPES ringer concomitant with an intermediate increase in pHi. Independently, increasing pHi in either BF or BR by exposure to NH4Cl increased the rate of lactate production. There are no reports that NH4Cl directly accelerates glycolytic activity, suggesting that it is the increased pHi that is activating glycolysis. The HEPES and NH4Cl results demonstrate that the increased glycolytic flux is not due to a secondary effect of CO2/HCO3−, but rather are due to increased pHi. Increasing pHi by incubation in HEPES also increased oxygen consumption, consistent with increased glycolytic flux and an increased availability of pyruvate to enter mitochondria. Our results are consistent with a recent metabolomics study (Hamuro et al., 2020) and findings that phosphofructokinase (PFK), which catalyzes the conversion of fructose-6P to Fructose 1,6-bisphosphate, is the primary rate limiting enzyme of glycolysis and is sensitive to pH, where higher pH is activating (Kitajima et al., 1983; Trivedi and Danforth, 1966).
Altered capacity for lactate efflux or glucose uptake could also play a role. Efflux is by Lactate:H+ cotransport, driven by the lactate and H+ gradients. Increased pHi in BR however, will decrease the driving force for lactate:H+ efflux. In addition, we found that glucose uptake capacity as measured by 2-NBDG uptake was unaffected by BF, HEPES, or BR. Moreover, a change in expression of monocarboxylate transporters or glucose transporters (Glut) would not be expected within the very short time frame of these incubations. Glut1 is the predominant glucose transporter in corneal endothelium (Bildin et al., 2001; Ishida et al., 1995). If plasma membrane Glut1 expression or activity were increased, then 2-NBDG accumulation would also have increased. Therefore, we conclude that the increased lactate production is not due to an increase in lactate:H+ cotransport activity or glucose transport capacity.
Real time changes in intracellular [lactate] (Laci) as measured by the FRET probe Laconic, provided additional insight to these events. [Laci] increased transiently when perfusion was switched from BF to BR (or HEPES). An increase in [Laci] could be due to increased production or decreased efflux of lactate. Since efflux is via lactate:H+ cotransport, the increase in pHi in BR (lower [H+]) could slow efflux. However, if lactate production were unaffected and efflux was decreased, then the steady-state [Laci] would remain elevated. However, as shown in Figure 4 we see that [Laci] decreased back to the starting level. Second, when a lactate pulse was introduced, [Laci] increased less and more slowly in BR relative to BF, indicating that the influx of lactate from the pulse was fighting against an increased lactate efflux in BR. We conclude that in the presence of the high buffering capacity of HEPES or BR ringer, the increased steady-state pHi activates glycolysis, most likely via PFK, increasing glycolytic flux and the production of lactate.
In the context of explaining the origin of the fluid transport (~60% of intact corneas) seen in cultured bovine corneal endothelial cells (Kuang et al., 2004; Narula et al., 1992), our findings support the lactate flux model for the endothelial pump. The model (Li et al., 2016; Li et al., 2020) states that lactate is taken up at the basolateral membrane of endothelial cells, where it creates a lower [lactate] in the basolateral space. Intracellular lactate is then transported to the apical surface, where it creates a slightly higher [lactate] in the apical unstirred layer. This creates a small osmotic gradient that then drives water flux from basolateral to apical. In the BCEC cultures, apical lactate efflux is greater than basolateral efflux, consistent with vectorial water flux linked to lactate. Lactate flux in cultured cells is also sensitive to the presence of bicarbonate and bicarbonate transport inhibition (Nguyen and Bonanno, 2011, 2012). Moreover, we can now add that the higher pHi in endothelial cells incubated with high buffering capacity ringers like HEPES and BR, increases lactate production as well as efflux, thereby supporting the endothelial pump.
Lastly, whether pHi is altered in endothelial dystrophies is unknown. However, in the mouse model of CHED where Slc4a11 is knocked out, cultured endothelial cells have higher pHi (Ogando et al., 2013; Zhang et al., 2017b). This is because Slc4a11 supports H+ influx driven by the membrane potential (Zhang et al., 2015). A metabolomics study indicated that glycolysis is preferred over glutaminolysis in Slc4a11 KO cells (Zhang et al., 2017a; Zhang et al., 2017b), which suggests that lactate production is increased. Further studies are needed to determine if lactate production is altered in endothelial dystrophies and how this may contribute to corneal edema.
Highlights.
Effect of Bicarbonate-free vs Bicarbonate-Rich Ringer on Corneal Endothelial Lactate Production
Increased pHi in Bicarbonate
Intracellular Lactate Measurement by Laconic
Bicarbonate Rich Ringer Accelerates Lactate Production
Acknowledgments
We would like to thank Edward Kim for expert technical assistance.
Funding Statement
This work was supported by NIH grant R01EY008834 (JAB). R.S is supported by a career starter grant from the Knights Templar Eye Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Anderson EI, Fischbarg J, 1978. Biphasic effects of insulin and ouabain on fluid transport across rabbit corneal endothelium. J Physiol 275, 377–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmanson JP, Johnsson J, Soderberg PG, Philipson BT, 1985. Lactate levels in the rabbit cornea and aqueous humor subsequent to non-gas permeable contact lens wear. Cornea 4, 173–176. [PubMed] [Google Scholar]
- Bildin VN, Iserovich P, Fischbarg J, Reinach PS, 2001. Differential expression of Na:K:2Cl cotransporter, glucose transporter 1, and aquaporin 1 in freshly isolated and cultured bovine corneal tissues. Exp Biol Med (Maywood) 226, 919–926. [DOI] [PubMed] [Google Scholar]
- Bonanno JA, Giasson C, 1992a. Intracellular pH regulation in fresh and cultured bovine corneal endothelium. I. Na/H exchange in the absence and presence of HCO3−. Invest. Ophthalmol. Vis. Sci 33, 3058–3067. [PubMed] [Google Scholar]
- Bonanno JA, Giasson C, 1992b. Intracellular pH regulation in fresh and cultured bovine corneal endothelium. I. Na+/H+ exchange in the absence and presence of HCO3. Invest Ophthalmol Vis Sci 33, 3058–3067. [PubMed] [Google Scholar]
- Bonanno JA, Giasson C, 1992c. Intracellular pH regulation in fresh and cultured bovine corneal endothelium. II. Na:HCO3 cotransport and Cl/HCO3 exchange. Invest Ophthalmol Vis Sci 33, 3068–3079. [PubMed] [Google Scholar]
- Cui W, Liu G, Liang R, 2002. Expression of carbonic anhydrase IV in rabbit corneal endothelial cells. Chin Med J (Engl) 115, 1641–1644. [PubMed] [Google Scholar]
- Diecke FP, Wen Q, Sanchez JM, Kuang K, Fischbarg J, 2004. Immunocytochemical localization of Na+-HCO3− cotransporters and carbonic anhydrase dependence of fluid transport in corneal endothelial cells. Am J Physiol Cell Physiol 286, C1434–1442. [DOI] [PubMed] [Google Scholar]
- Dikstein S, Maurice DM, 1972. The active control of corneal hydration. Isr J Med Sci 8, 1523–1528. [PubMed] [Google Scholar]
- Fischbarg J, Lim J, 1974. Role of cations, anions, and carbonic anhydrase in fluid transport across rabbit corneal endothelium. J. Physiol 241, 647–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamuro J, Numa K, Fujita T, Toda M, Ueda K, Tokuda Y, Mukai A, Nakano M, Ueno M, Kinoshita S, Sotozono C, 2020. Metabolites Interrogation in Cell Fate Decision of Cultured Human Corneal Endothelial Cells. Invest Ophthalmol Vis Sci 61, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan MJ, Alvarado JA, Weddell J, 1971. Histology of the Human Eye. W.B. Saunders, Philadelphia. [Google Scholar]
- Ishida K, Yamashita H, Katagiri H, Oka Y, 1995. Regulation of glucose transporter 1 (GLUT1) gene expression by epidermal growth factor in bovine corneal endothelial cells. Jpn J Ophthalmol 39, 225–232. [PubMed] [Google Scholar]
- Kitajima S, Sakakibara R, Uyeda K, 1983. Significance of phosphorylation of phosphofructokinase. J Biol Chem 258, 13292–13298. [PubMed] [Google Scholar]
- Klyce S, 1981. Stromal lactate accumulation can account for corneal oedema osmotically following epithelial hypoxia in the rabbit. J Physiol (Lond) 321, 49–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang K, Xu M, Koniarek J, Fischbarg J, 1990. Effects of ambient bicarbonate, phosphate and carbonic anhydrase inhibitors on fluid transport across rabbit endothelium. Exp. Eye Res 50, 487–493. [DOI] [PubMed] [Google Scholar]
- Kuang K, Yiming M, Wen Q, Li Y, Ma L, Iserovich P, Verkman AS, Fischbarg J, 2004. Fluid transport across cultured layers of corneal endothelium from aquaporin-1 null mice. Exp Eye Res 78, 791–798. [DOI] [PubMed] [Google Scholar]
- Li J, Sun XC, Bonanno JA, 2005. Role of NBC1 in apical and basolateral HCO3− permeabilities and transendothelial HCO3− fluxes in bovine corneal endothelium. Am J Physiol Cell Physiol 288, C739–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Kim E, Bonanno JA, 2016. Fluid transport by the cornea endothelium is dependent on buffering lactic acid efflux. Am J Physiol Cell Physiol 311, C116–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Kim E, Ogando DG, Bonanno JA, 2020. Corneal Endothelial Pump Coupling to Lactic Acid Efflux in the Rabbit and Mouse. Invest Ophthalmol Vis Sci 61, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Nguyen TT, Bonanno JA, 2014. CD147 required for corneal endothelial lactate transport. Invest Ophthalmol Vis Sci 55, 4673–4681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebovitch LS, Fischbarg J, 1982. Effects of inhibitors of passive Na+ and HCO3− fluxes on electrical potential and fluid transport across rabbit corneal endothelium. Curr Eye Res 2, 183–186. [DOI] [PubMed] [Google Scholar]
- Narula P, Xu M, Kuang K, Akiyama R, Fischbarg J, 1992. Fluid transport across cultured bovine corneal endothelial cell monolayers. Am J of Physiol 262, C98-C91-93. [DOI] [PubMed] [Google Scholar]
- Nguyen TT, Bonanno JA, 2011. Bicarbonate, NBCe1, NHE, and carbonic anhydrase activity enhance lactate-H+ transport in bovine corneal endothelium. Investigative Ophthalmology & Visual Science 52, 8086–8093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TT, Bonanno JA, 2012. Lactate-H(+) transport is a significant component of the in vivo corneal endothelial pump. Investigative Ophthalmology & Visual Science 53, 2020–2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogando DG, Jalimarada SS, Zhang W, Vithana EN, Bonanno JA, 2013. SLC4A11 is an EIPA-sensitive Na(+) permeable pHi regulator. Am J Physiol Cell Physiol 305, C716–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley M, 1969. Glucose and oxygen utilization by the rabbit cornea. Experimental Eye research 8, 193–200. [DOI] [PubMed] [Google Scholar]
- Riley M, Winkler B, Czajkowski C, Peters M, 1995. The roles of bicarbonate and CO2 in transendothelial fluid movement and control of corneal thickness. Invest. Ophthalmol. Vis. Sci 36, 103–112. [PubMed] [Google Scholar]
- Riley MV, Winkler BS, Peters MI, Czajkowski CA, 1994. Relationship between fluid transport and in situ inhibition of Na(+)-K+ adenosine triphosphatase in corneal endothelium. Invest Ophthalmol Vis Sci 35, 560–567. [PubMed] [Google Scholar]
- Roos A, Boron W, 1981. Intracellular pH. Physiological Reviews 61, 297–432. [DOI] [PubMed] [Google Scholar]
- Terashima H, Suzuki K, Kato K, Sugai N, 1996. Membrane-bound carbonic anhydrase activity in the rat corneal endothelium and retina. Japanese Journal of Ophthalmology 40, 142–153. [PubMed] [Google Scholar]
- Trivedi B, Danforth W, 1966. Effect of pH on the kinetics of frog muscle phosphofructokinase. Journal of Biological Chemistry 241, 4110–4114. [PubMed] [Google Scholar]
- Zhang W, Li H, Ogando DG, Li S, Feng M, Price FW Jr., Tennessen JM, Bonanno JA, 2017a. Glutaminolysis is Essential for Energy Production and Ion Transport in Human Corneal Endothelium. EBioMedicine 16, 292–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Ogando DG, Bonanno JA, Obukhov AG, 2015. Human SLC4A11 Is a Novel NH3/H+ Co-transporter. J Biol Chem 290, 16894–16905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Ogando DG, Kim ET, Choi MJ, Li H, Tenessen JM, Bonanno JA, 2017b. Conditionally Immortal Slc4a11−/− Mouse Corneal Endothelial Cell Line Recapitulates Disrupted Glutaminolysis Seen in Slc4a11−/− Mouse Model. Invest Ophthalmol Vis Sci 58, 3723–3731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou C, Wang Y, Shen Z, 2005. 2-NBDG as a fluorescent indicator for direct glucose uptake measurement. J Biochem Biophys Methods 64, 207–215. [DOI] [PubMed] [Google Scholar]


