Abstract
Influenza virus neuraminidase (NA) contains a universally conserved epitope (NAe, NA222–230). However, no studies have reported vaccines targeting this NA conserved epitope and inducing antibodies recognizing NAe. The extracellular domain of M2 (M2e) is considered as an attractive target for a universal influenza vaccine. We generated recombinant influenza H1N1 viruses expressing conserved epitopes in hemagglutinin (HA) molecules: NAe (NAe-HA) or M2e (M2e-HA) within the HA head domain. Inactivated recombinant NAe-HA and M2e-HA viruses were more effective in inducing IgG antibodies specific for an inserted conserved epitope than live recombinant virus. Recombinant inactivated M2e-HA virus vaccination induced cross protection against H3N2 virus with less weight loss compared to NAe-HA and was more effective in inducing humoral and cellular M2e immune responses. This study provides insight into developing recombinant influenza virus vaccines compatible with current platforms to induce antibody responses to conserved poorly immunogenic epitopes.
Keywords: Recombinant chimeric influenza virus, Cross protection, Neuraminidase epitope, M2 extracellular domain epitope, heterosubtypic immunity
Introduction
Influenza virus, belonging to the Orthomyxoviridae family, contains negative-sense single-stranded RNA genomes composed of 8 segments facilitating the emergence of diverse reassorted strains in nature. Influenza virus causes respiratory diseases in humans, resulting in 300,000–500,000 deaths worldwide (Iuliano et al., 2018; Krammer et al., 2018). Annual vaccination with inactivated influenza vaccines is routinely recommended in 6 months old ages and older populations, and live-attenuated influenza virus (LAIV) vaccine for 2 to 49 years old populations. Influenza A virus exists in different serotypes with a wide range out of 18 subtypes (H1-H18) of hemagglutinin (HA) and 11 subtypes (N1-N11) of neuraminidase (NA) (Tong et al., 2012; Tong et al., 2013). Although current vaccination based on HA immunity has been proven to be effective in providing strain-specific protection, cross protective influenza vaccines remain to be developed.
Previous studies demonstrated universal vaccines targeting to conserved domains or epitopes. An influenza A virus M2 ion channel protein extracellular domain (M2e) has been extensively studied to develop M2e-based universal vaccines in different platforms including protein carrier conjugates with experimental adjuvants (De Filette et al., 2008; Jegerlehner et al., 2004; Neirynck et al., 1999), virus-like particles (Kim et al., 2013; Song et al., 2011; Wang et al., 2012), and vectored vaccines (Hashemi et al., 2012; Hessel et al., 2014; Zhou et al., 2010). Immunity to NA, the second major influenza virus surface protein, can provide an independent protective correlate (Couch et al., 2013; Memoli et al., 2016; Monto et al., 2015; Murphy et al., 1972). NA contains an epitope (NA amino acid residues 222–230, NA222–230, termed NAe), which is universally 100% conserved across influenza A and B viruses (Gravel et al., 2010). This NA222–230 sequence was identified to be located close to the NA enzymatic active site (Doyle et al., 2013a; Doyle et al., 2013b; Doyle et al., 2013c). The monoclonal antibody against NA222–230 (HCA-2 mAb) was used to quantitatively determine NA contents in different influenza vaccine lots (Gravel et al., 2010). Point mutations within the NA222–230 sequence were fatal to the virus (Doyle et al., 2013b), suggesting a potential universal vaccine target, which has not been tested yet.
HA is composed of the highly variable antigenic head domain and relatively conserved stalk domain (Neu et al., 2016; Steel et al., 2010). Using reverse genetics techniques, recombinant influenza viruses expressing chimeric HAs with the same stalk but with different subtype HA heads were generated (Chen et al., 2016). As a universal vaccination strategy, sequential immunizations with different head and stalk chimeric HA virus vaccines were reported to boost anti-stalk antibody responses (Choi et al., 2019; Ermler et al., 2017; Krammer et al., 2014; Liu et al., 2019). Recombinant influenza H1N1 virus A/Puerto Rico/8/1934 (A/PR8), a mouse-adapted pathogenic wild type (WT) strain was used to express chimeric HA with tandem M2e epitopes inserted in the N-terminus HA (4xM2e-HA), conferring extra cross protection (Kim et al., 2017a). However, a pathogenic nature of WT A/PR8 virus in mice would not represent attenuated influenza virus vaccines. A chimeric HA with a single M2e epitope in the head domain antigenic site Ca was tested in inactivated recombinant WT A/PR8 viruses as a cross protective virus vaccine platform (Sun et al., 2019).
In this study to test whether antibody responses to universally conserved NAe (NA222–230) epitope enhance cross protection in comparison with immune responses to M2e, we generated chimeric HA conjugates with M2e (M2e-HA) or NAe (NAe-HA) in the HA head domain. Replication competent recombinant A/PR8 viruses expressing these chimeric HA molecules (M2e-HA, NAe-HA) were rescued by reverse genetics techniques using an attenuated A/PR8 backbone. Immune responses to M2e and NAe epitopes and cross protection were determined in mice after intramuscular (IM) immunization with inactivated chimeric A/PR8 viruses or intranasal (IN) infection with live chimeric A/PR8 viruses. Inactivated chimeric virus vaccine platforms effectively raised antibody responses to M2e and NAe and conferred cross protection. This study provides insight into developing recombinant influenza virus vaccines compatible with current platforms to induce antibody responses to conserved poorly immunogenic epitopes.
Materials and Methods
Viruses, protein, and antibodies
Influenza A viruses A/Philippines/2/1982 (A/Phil, H3N2), A/Puerto Rico/8/34 (A/PR8, H1N1), and rgH5N1 were propagated in embryonated chicken eggs (Hy-line North America, Mansfield, GA). The rgH5N1 virus is a reassortant containing H5 HA with the polybasic cleavage site deleted and N1 NA derived from A/Vietnam/1203/2004 (H5N1) and the backbone genes from A/PR8 virus (H1N1) (Song et al., 2011). The rgH9N2 virus (BEI resources) is a low pathogenic reassortant containing HA and NA from A/chicken/Hong Kong/1997 (H9N2) and 6 internal genes derived from A/PR8. A/PR8 (H1N1) and rgH5N1 viruses were inactivated by treating formalin (1:4000, v/v) as previously described (Kim et al., 2019a; Ko et al., 2018). A monoclonal antibody HCA-2 against NA222–230 was kindly provided by Dr. Xuguang Li (University of Ottawa, Canada). Influenza A virus M2e monoclonal antibody (mAb) 14C2 was purchased (Abcam, Cambridge, MA).
Generation and characterization of recombinant chimeric influenza viruses
Chimeric HA genes encoding conserved 9 amino acid (aa), ILRTQESES (NA222–230, NAe-HA) and 10 aa, human M2e7–16, VETPIRNEWG (M2e-HA) were constructed (Fig. 1) by following a similar cloning strategy as described previously (Lee et al., 2016). First, we constructed an intermediate plasmid A/PR8-mHA in pHW2000 where PstI and HindIII restriction enzyme sites were introduced at nucleotide positions 489 and 563 of the HA gene by site-directed mutagenesis using the Quick Change Multi Site-Directed Mutagenesis Kit (Agilent Technologies, Boblingen, Germany). Next, two recombinant plasmids of chimeric NAe-HA and M2e-HA were generated by inserting a gene fragment encoding the NA222–230 or M2e7–16 epitope into the HA head antigenic site between residues 171–172 in the PR8-mHA plasmid using PstI and HindIII restriction enzyme cloning sites (Fig. 1A).
Figure 1. Generation of replication competent recombinant A/PR8 viruses containing chimeric HA constructs with a conserved epitope inserted into the head domain.
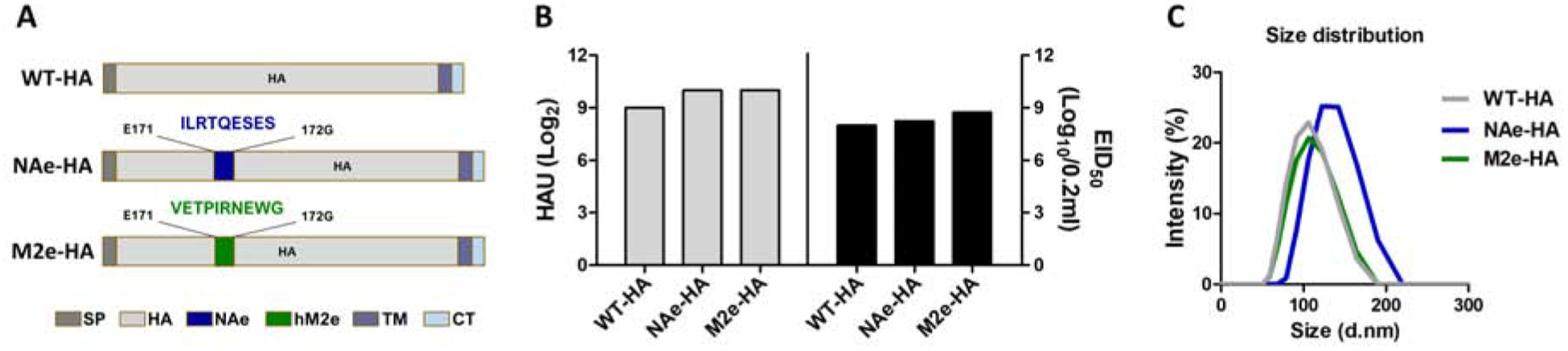
Recombinant A/PR8 influenza viruses containing chimeric NAe-HA and M2e-HA were generated via the reverse genetics system using the attenuated pHW2000-based eight-plasmid system. (A) Chimeric HA constructs with a conserved epitope inserted into the head domain. NA222–230 (NAe-HA) or human M2e7–16 (M2e-HA) were inserted between positions 171 and 177 as indicated by the color boxes, respectively. (B) In vitro growth of recombinant A/PR8 influenza viruses containing chimeric HA. The 293T cell culture supernatants at 48h from co-transfected with eight pHW2000 plasmids including the chimeric HA constructs and attenuated PB1 and PB2 genes were inoculated into embryonated chicken eggs. Hemagglutination activity units (HAU, left panel) and the egg infective dose titers (right panel) in allantoic fluids containing rescued virus were determined using the chicken red blood cells and an egg infection assay. EID50; 50% egg infective dose. (C) Size distribution of influenza PR8 viruses containing chimeric HA molecules.
In an attempt to generate recombinant live attenuated influenza virus (LAIV) backbone genes, we introduced mutations into polymerase PB1 and PB2 genes known to attribute attenuation phenotypes similar to those of cold-adapted A/Ann Arbor/6/60 influenza virus master strain (Jin et al., 2003; Jin et al., 2004). Four mutations in the PB1 (K391E, E581G, A661T) and PB2 (N265S) polymerase encoding genes were introduced into the pHW2000 PB1 and PB2 plasmids respectively by using the Quick Change Multi Site-Directed Mutagenesis Kit. To rescue attenuated recombinant viruses A/PR8 NAe-HA, A/PR8 M2e-HA, we applied reverse genetics using the pHW2000-based eight plasmid system (kindly provided by R.G. Webster) as described (Hoffmann et al., 2000). Briefly, 293T cells were transfected with eight pHW2000 plasmids including PB1 (K391E, E581G, A661T) and PB2 (N265S) mutant genes and chimeric NAe-HA or M2e-HA. At 2 days after transfection, 293T cultures with rescued viruses were inoculated into 10-day old embryonated chicken eggs at 33°C. The recombinant viruses rescued were confirmed in allantoic fluids by hemagglutination activity assays after 3 days incubation at 33°C. The 50% egg infectious dose (EID50) titers were determined in the embryonated chicken eggs. The average sizes of chimeric influenza viruses were determined by dynamic light scattering (DLS) with a Malvern Zetasizer Nano ZS (Malvern Instruments, Westborough, MA). The rescued recombinant attenuated A/PR8 NAe-HA and A/PR8 M2e-HA viruses were inactivated by treating formalin (1:4000, v/v) as described (Kim et al., 2019a; Ko et al., 2018). Expression of NAe and M2e in HA chimeric molecules and incorporation into recombinant attenuated A/PR8 viruses were confirmed by western blot and ELISA using HCA-2 mAbs and 14C2 mAbs and polyclonal antisera against A/PR8 virus.
Immunization and challenge of mice
The attenuation and immunogenic properties of live attenuated recombinant A/PR8 NAe-HA and M2e-HA viruses were initially determined in BALB/c mice after intranasal (IN) inoculation with a range of different doses (2×104 EID50, 2×105 EID50, 1×106 EID50). To test an inactivated virus vaccine platform, 6 to 8 weeks old female BALB/c mice (n=10 mice per group) were intramuscular (IM) immunized with 10 μg of inactivated A/PR8 influenza viruses containing NAe-HA, M2e-HA, and WT-HA 3 times at 0, 4, 7 weeks. Blood samples were collected at 3 weeks after immunization. Immunized mice were challenged with a lethal dose of 8× LD50 A/Phil (H3N2) at 4 weeks after second boost. Body weight changes and survival rates were monitored daily for 14 days. All animal experimental procedures in this study were performed by the guidelines of the approved Institutional Animal Care and Use Committee (IACUC) protocol (A18001).
Determination of antibody responses and the levels of cytokines
Antibody responses specific for influenza virus or peptide antigens (NAe, M2e) were determined by enzyme-linked immunosorbent assay (ELISA) in immune sera, in bronchoalveolar lavage fluids (BALF) and lung lysates harvested on 6 days post infection (dpi). The samples including serially diluted immune sera, BALF, and lung lysates were applied to a 96 well plate coated with 2μg/ml of NAe, M2e peptides, inactivated A/PR8 or rgH5N1 (A/Vietnam). The levels of IgG and isotypes were determined by using horseradish peroxidase (HRP)-conjugated anti-mouse IgG, IgG1, IgG2a, IgA (SouthernBiotech, Birmingham, AL) and tetramethylbenzidine (eBioscience, San Diego, CA) as previously described (Kim et al., 2019a). The levels of IL-6 cytokine in BALF and lung lysates were measured using cytokine ELISA kit (eBioscience, San Diego, CA) according to the manufacturer’s instructions.
Lung virus titration
Lung viral titers were determined in embryonated chicken eggs. Lung homogenates were 10-fold serially diluted and inoculated into 10 days old embryonated chicken eggs at 37 °C for 3 days. The allantoic fluids from eggs were collected and hemagglutination activity titers measured using chicken red blood cells (RBC) (Lampire Biological Laboratories, Pipersville, PA). Virus titers as EID50/ml were evaluated as described (Kim et al., 2019a).
Hemagglutination inhibition (HAI) assay
Immune sera were treated with receptor destroying enzymes (RDE, Sigma Aldrich, St. Louis, MO) at 1:4 ratio (serum: RDE) and then incubated for 16 hours at 37 °C. The RDE-treated sera were inactivated at 56 °C for 30 min then serially 2-fold diluted and incubated with 4 hemagglutination unit of A/PR8, rgH5N1, and A/Phil for 30 min, then admixed with 0.5% chicken RBC. Both chicken and turkey erythrocytes are known to be appropriate for HA and HAI assays with seasonal influenza strains (Thontiravong et al., 2016; Trombetta et al., 2018). The highest serum dilutions interfering with the red spot formation were determined for HAI titers.
Cytokine ELISpot assay
T cell responses in lung and splenocyte were determined at day 6 post infection by an enzyme-linked immunospot (ELISpot) assay as described (Kim et al., 2019a). Briefly, lung cells (2×105 cells per well) or spleen cells (5×105 cells per well) were cultured on the multi-screen 96 well plates (Millipore, Billerica, MA) coated with cytokine specific capture antibody in the presence of NAe or M2e peptides (5 μg/ml), inactivated A/PR8 (4 μg/ml) as an antigenic stimulators for 48 hours. The spots of IFN-γ secreting cells were developed with biotinylated anti-mouse IFN-γ and alkaline phosphatase-labeled streptavidin (BD Pharmingen) and visualized with 3,3’-diaminobenzidine (DAB) substrates, then the spots were counted using an ELISpot reader (BioSys, Miami, FL).
Intracellular cytokine staining analysis by flow cytometry
Bronchoalveolar lavage (BAL) fluids (BALF) were obtained by infusing 1 ml of phosphate-buffered saline (PBS) into the trachea using a 25-gauge catheter (Exelint International Co., Los Angeles, CA) to collect non-adherent cells in the airways. The lung tissues were homogenized and spun on 44/67% Percoll gradients (GE Healthcare Bio-Sciences, Pittsburgh, PA) at 2,800 rpm for 15 min. The layers containing lymphocytes were harvested and washed with cold PBS. For intracellular cytokine staining, BAL and lung cells were in vitro stimulated with the synthetic NAe or M2e peptides (5 μg/ml), with inactivated A/PR8 virus (4 μg/ml) in the presence of Golgi-stop (BD Biosciences, San Jose, CA) for 5 hours at 37 °C incubator. The cells were stained with surface markers (CD45 and CD4), then the cells secreting cytokines (IFN-γ and TNF-α) were fixed and permeabilized using a Cytofix/Cytoperm kit according to the manufacturer’s instructions (BD Biosciences). All samples were analyzed on a Becton-Bickinson LSR-II/Fortessa flow cytometer (BD, San Diego, CA) and analyzed using the Flowjo software (FlowJo V10, Tree Star, Inc., Ashland, OR).
Microneutralization assay
Microneutralization assay was performed by a modified method as described (Laurie et al., 2015). Sera collected from immunized mice were pooled per group and heat inactivated at 56 °C for 30 min. Two-folds serially diluted sera were mixed with 100× TCID50 of influenza A viruses (A/PR8, A/Phil, rgH5N1) at room temperature for 40 min. This mixture was added to Madin Darby Canine Kidney cells and then incubated at 37 °C. After three days culture in DMEM with 1 μg/ml TPCK-Trypsin, microneutralizing antibody titers were determined by a hemagglutination activity assay using 0.5% chicken RBC.
Statistical analysis
All results are presented as the mean ± the standard errors of the mean (SEM). The statistical significance for all experiments was performed by one- or two-way analysis of variance (ANOVA). Prism software (GraphPad Software, Inc., San Diego, CA) was used for all data analysis. The comparison used to generate a P value is indicated by horizontal lines (*; p<0.05, **; p<0.01, ***; p<0.001).
Results
Generation of attenuated recombinant influenza A viruses expressing chimeric HA conjugates carrying a conserved epitope
Current influenza vaccination induces strain specific immunity particularly for immunodominant HA globular head domains. In an approach to enhance the efficacy of cross protection, we constructed chimeric HA molecules with an insertion of a conserved linear epitope, 9 amino acid (aa) residues of NAe (NA222–230) [NAe-HA] or 10 aa residues of central M2e (M2e7–16) [M2eHA], between the E171 and G172 in the head domain antigenic site Sa of HA derived from A/PR8 virus (Fig. 1A). Recombinant A/PR8 influenza viruses containing chimeric NAe-HA, M2e-HA were generated using the reverse genetics system. Replication competent recombinant chimeric NAe-HA and M2e-HA influenza A/PR8 viruses were successfully rescued and generated as confirmed by hemagglutination activity units (HAU) and growth of infectious titers (EID50) in embryonated chicken eggs (Fig. 1B). These data suggest that chimeric recombinant NAe-HA and M2e-HA proteins retain functional activities in replicating virus. The average sizes of inactivated chimeric influenza viruses were 100 – 130nm similar (M2e-HA) to or slightly larger (NA2e-HA) than wild type (WT) A/PR8 virus as measured by Malvern Zetasizer Nano ZS through dynamic light scattering (DLS) property (Fig. 1C).
Chimeric HA with an insertion of NAe and M2e were determined in the western blot assay of recombinant influenza viruses by using monoclonal antibodies (mAbs) specific for M2e (14C2 mAb) and NAe (HCA-2 mAb) epitopes and A/PR8 virus-specific polyclonal antibodies (Fig. 2A). HCA-2 mAb or 14C2 mAb reactive bands were observed in the recombinant NAe-HA and M2e-HA A/PR8 virus respectively but not in the WT A/PR8 virus (Fig. 2A), suggesting the expression of chimeric NAe-HA and M2e-HA molecules. The antigenic properties of recombinant NAe-HA and M2e-HA A/PR8 viruses were determined by ELISA (Fig. 2B–C). The chimeric attenuated backbone NAe-HA A/PR8 virus displayed higher levels of reactivity to NAe specific HCA-2 mAb by 4 to 5 folds than M2e-HA and WT-HA A/PR8 viruses which show moderate HCA-2 reactivity to intrinsic NA proteins (Fig. 2B). The chimeric attenuated backbone M2e-HA A/PR8 virus exhibited highest reactivity to M2e specific 14C2 mAb, suggesting reactivity to chimeric M2e-HA whereas low reactivity to intrinsic M2 proteins in control viruses (Fig. 2C). Attenuated backbone NAe-HA and M2e-HA A/PR8 viruses displayed higher levels of HCA-2 and 14C2 mAb reactivities than WT backbone NAe-HA and M2e-HA A/PR8 viruses (data not shown). A/PR8 virus polyclonal immune sera were highly reactive to recombinant NAe-HA and M2e-HA A/PR8 viruses (Fig. 2D). These results showed that NAe and M2e epitopes are presented on the head domain of HA conjugate molecules in replication competent influenza viruses.
Figure 2. Expression of chimeric NAe-HA and M2e-HA proteins in recombinant attenuated A/PR8 viruses.
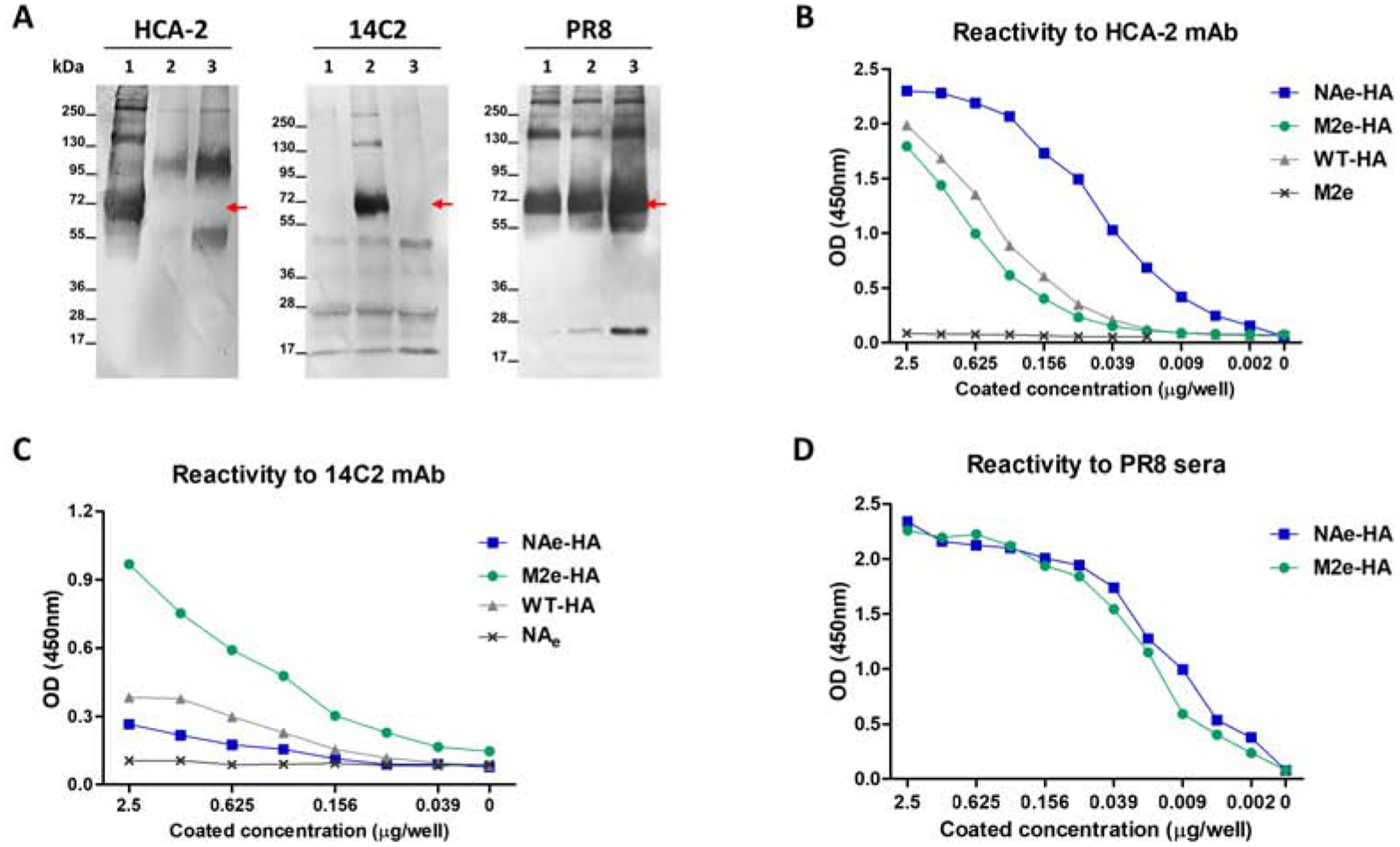
The expression of chimeric NAe-HA and M2e-HA proteins in recombinant A/PR8 viruses was determined by Western blot and ELISA. HCA-2 or 14C2 mAbs specific for NA222–230 (NAe) and M2e epitope respectively were used to probe chimeric NAe-HA and M2e-HA proteins. Mouse immune sera collected from mice infected with sublethal A/PR8 virus were used to prove A/PR8 HA proteins. (A) Western blot analysis. Lane 1: NAe-HA A/PR8 virus (10 μg), Lane 2: M2e-HA A/PR8 virus (10 μg), Lane 3: WT-HA A/PR8 virus (10 μg). Red color arrows indicate chimeric HA proteins with NAe or M2e, and WT-HA. (B-D) The reactivity of recombinant PR8 viruses to specific HCA-2 and 14C2 mAbs or polyclonal antibodies was determined by ELISA. (B) The reactivity of recombinant PR8 viruses to NA222–230 specific HCA-2 mAb. (C) The reactivity of recombinant PR8 viruses to M2e specific 14C2 mAb. (D) The reactivity of recombinant PR8 viruses to A/PR8 polyclonal anti-sera.
Vaccination with inactivated recombinant influenza virus enhances IgG2a antibody responses specific for a conserved mono epitope inserted into the HA head domain
First, we determined the pathogenic phenotypes and immunogenicity of chimeric influenza viruses since these recombinant viruses were generated using the backbone genes containing the polymerase encoding genes (PB1, PB2) with mutations conferring LAIV-like phenotypes in addition to a chimeric HA mutant gene (Supplementary Fig. S1). The mice that were intranasally (IN) inoculated with a range of infectious egg titers (2×104 and 2×105 EID50) of chimeric HA recombinant viruses did not show any sign of weight loss disease, while mice inoculated with a range of 106 EID50 were observed to display a slight weight loss (2.8%), confirming the attenuated phenotypes of recombinant virus pathogenicity in mice. In contrast, the WT A/PR8 backbone virus caused severe weight loss and lethality to the mice with a titer of 2×104 EID50 (Supplementary Fig. S1). High levels of IgG antibodies specific for A/PR8 virus antigen were induced in mice with prime inoculation of infectious egg titers (2×104 to 1×106 EID50) of chimeric HA recombinant viruses (Supplementary Fig. S2A–B). In contrast, antibody responses specific for M2e and NAe epitope antigens were induced to minimal or below the levels of detection in mice with prime inoculation of chimeric M2e-HA or NAe-HA recombinant viruses (Supplementary Fig. S2C–F). These data indicate that M2e or NAe epitope inserted into the HA head domain was not effective in generating IgG antibodies in mice after prime inoculation with live virus platforms. IN boost inoculation (1×107 EID50) resulted in further enhanced levels of IgG antibodies specific for A/PR8 and inducing low levels of IgG specific for M2e but no significant levels of IgG for NAe epitopes (data not shown).
To determine the immunogenicity of inactivated recombinant influenza virus platforms, the groups of mice were intramuscularly (IM) immunized with chimeric HA inactivated viruses (10 μg per mouse) as indicated (Fig. 3). IgG antibodies specific for A/PR8 virus were induced at significant levels after prime dose, which were further enhanced after boost dose (Fig. 3C, Supplementary Fig. S3). IgG responses specific for M2e single epitope but not for NAe was induced at detectable levels after prime dose in the M2e-HA group, suggesting that M2e epitope might be more immunogenic than NAe (Supplementary Fig. S4A–B). After boost dose, IgG antibody responses specific for NAe and M2e epitopes in the NAe-HA and the M2e-HA groups were induced respectively but not in the control WT-HA group (Supplementary Fig. S4C–D). After the 2nd boost, total IgG antibodies specific for NAe or M2e were further increased to high levels in the NAe-HA or M2e-HA immunized mouse groups respectively whereas WT-HA immunization did not (Fig. 3A–B). Interestingly, both NAe-HA or M2e-HA vaccine groups induced dominant IgG2a isotype antibodies specific for NAe or M2e peptides (Fig. 3D–E). It is interesting to find that the IgG responses for NAe and M2e epitopes were IgG2a isotype dominant, whereas IgG2a and IgG1 isotype antibodies for A/PR8 virus were induced at a similar level in all three groups with NAe-HA, M2e-HA, and WT-HA (Fig. 3F).
Figure 3. Immunization with recombinant inactivated PR8 viruses containing chimeric HA induces IgG2a dominant isotype antibody specific for NAe and M2e.
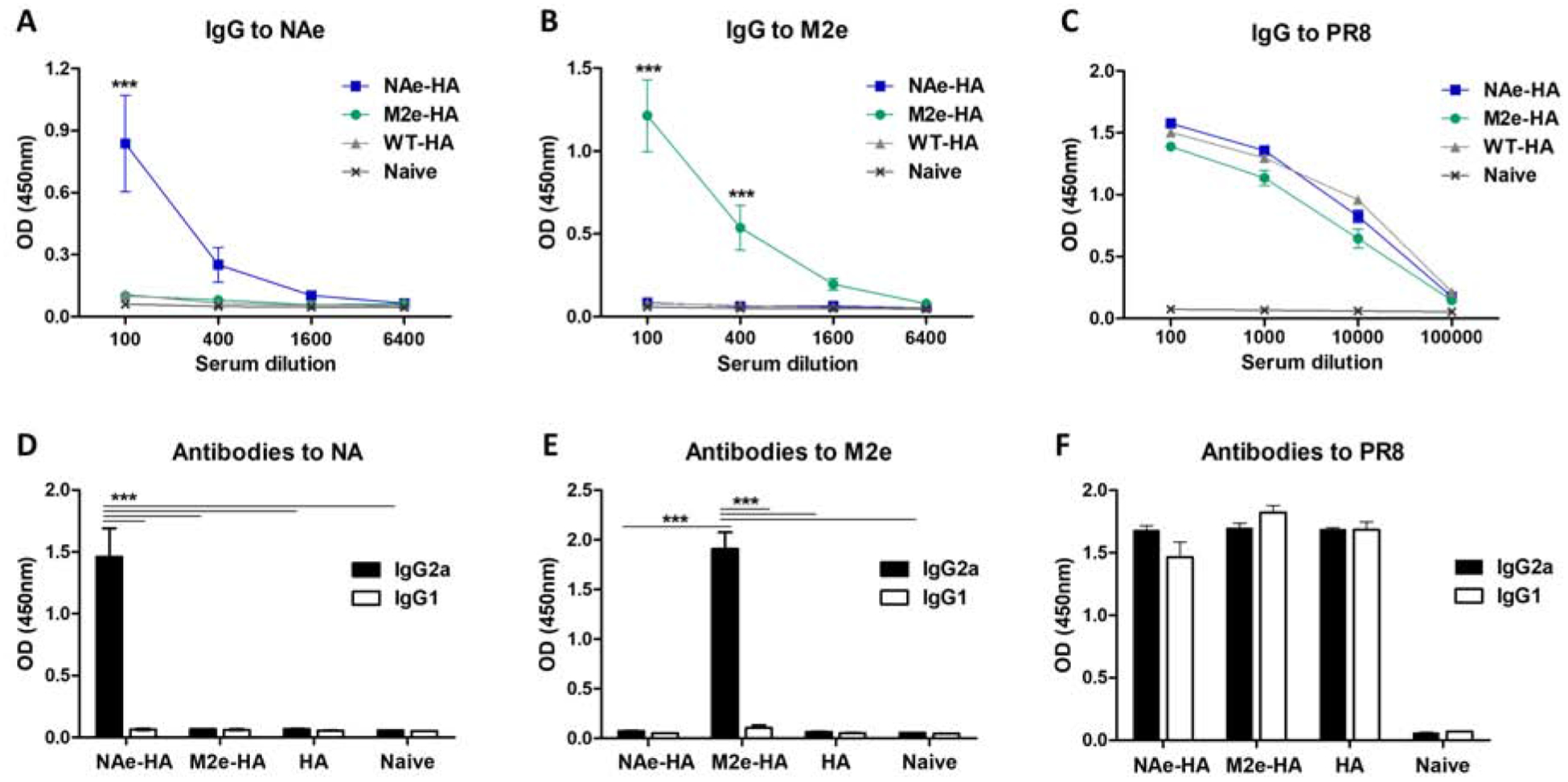
BALB/c mice (n=10) were intramuscularly (IM) immunized with recombinant A/PR8 influenza viruses containing NAe or M2e inserted into the HA head domain. Antigen-specific antibody responses in immune sera collected after second boost immunization were determined by ELISA. (A and B) Total IgG to NAe or M2e peptides. (C) Total IgG to A/PR8 virus. (D and E) IgG2a and IgG1 isotype antibodies against NAe or M2e peptides in 100-fold diluted immune sera harvested after second boost immunization. (F) IgG2a and IgG1 isotype antibodies to PR8 virus in 100-fold diluted boost immune sera. Statistical significance was determined by using two-way ANOVA. Data of optical density (OD) values are representative of individual animal out of two independent experiments. Error bars indicate the means ± SEM. ***; p<0.001.
Inactivated influenza virus vaccines containing chimeric HA with a conserved epitope improve the efficacy of cross protection
We determined whether inactivated chimeric HA influenza virus vaccination would enhance the efficacy of cross protection. Mice immunized with inactivated NAe-HA, M2e-HA, and WT-HA were intranasally infected with a lethal dose A/Philippines/82 (A/Phil, H3N2, 8×LD50) at 4 weeks after second boost immunization. Body weight changes of WT-HA immunized mice started to decrease rapidly from day 3 post challenge, displayed approximately 20% weight loss, but survived better (60%) than naïve mice showing 0% survival rates (Fig. 4A–B). Notably, chimeric M2e-HA and NAe-HA immunized groups displayed slight to moderate weight loss of approximately 9% and 13% respectively with 100% protection, and in particular, the chimeric M2e-HA group quickly recovered the weight by day 6 post infection compared to the NAe-HA vaccine group (Fig. 4A–B). Lung samples were collected at day 6 post challenge to determine the viral loads in embryonated chicken eggs (Fig. 4C). The chimeric M2e-HA and NAe-HA groups showed approximately 150-fold to 400-fold lower of lung viral titers compared to the naïve infection group and 4- to 10-folds lower lung viral titers than those in the WT-HA group (Fig. 4C).
Figure 4. Immunization with inactivated chimeric HA A/PR8 viruses confer cross protection against heterosubtypic influenza virus.
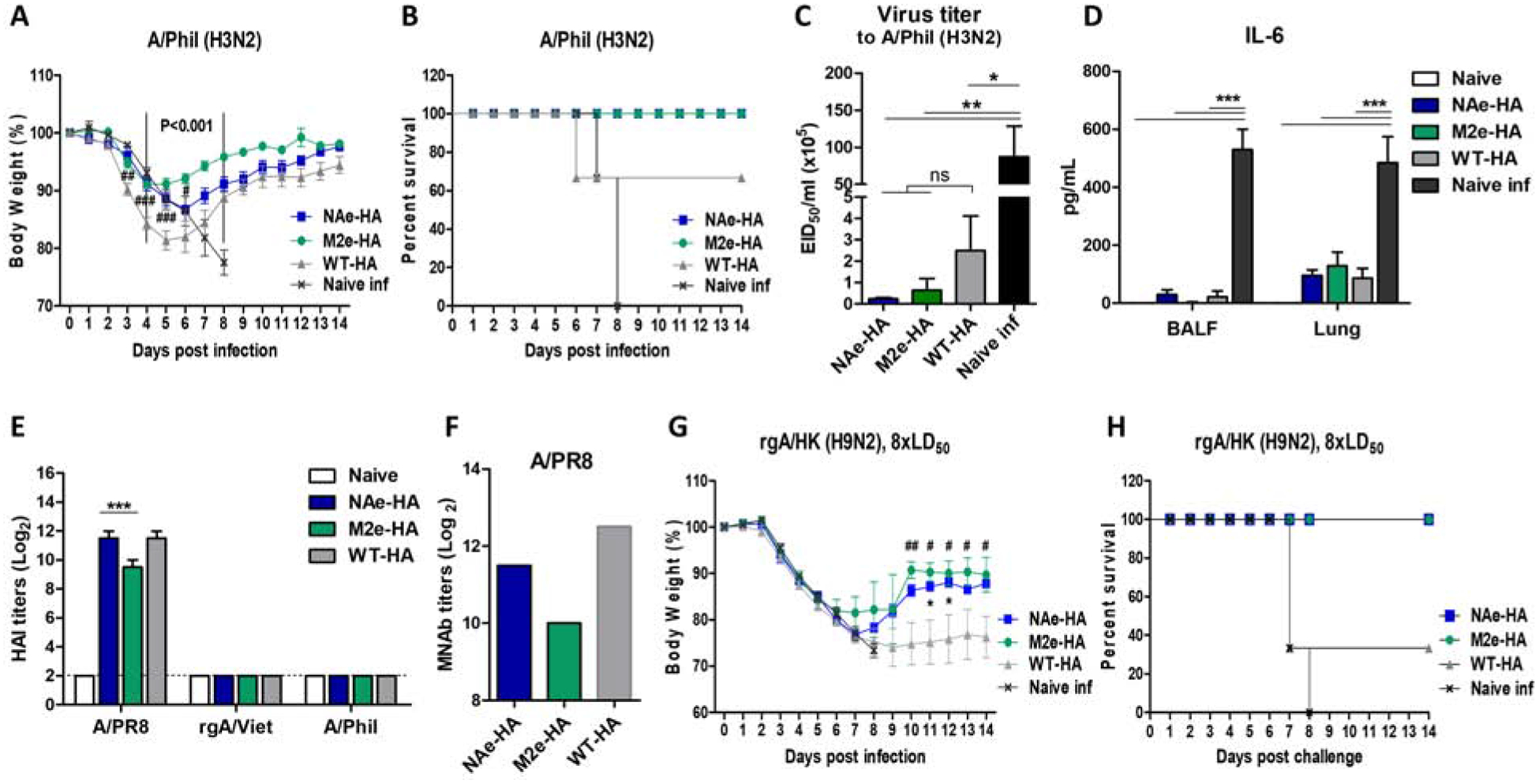
The groups of mice (n=6) immunized with chimeric HA (NAe-HA or M2e-HA) and WT-HA A/PR8 viruses were infected with A/Phil (H3N2, 8× LD50) or reassortant rgA/HK (H9N2, 8× LD50) virus and then monitored body weight changes daily for 14 days. (A) Body weight changes. P<0.001; compared between M2e-HA and WT-HA, ###; p<0.001, ##; p<0.01, #; p<0.05; compared between NAe-HA and WT-HA. (B) Survival rates. Another set of the groups of mice (n=4) was sacrificed at 6 days after infection (dpi) with the same challenge dose A/Phil (H3N2) to determine the lung viral loads and the level of IL-6 cytokine. (C) Lung viral titers at 6 dpi. Viral loads were determined by using embryonated chicken eggs and presented in EID50. (D) The level of IL-6 was determined from BALF and lung lysates by ELISA. (E) HAI titers to homologous or heterosubtypic influenza viruses. (F) Microneutralization antibody titers in pooled immune sera after second boost immunization. (G and H) Body weight changes and survival rates after challenge of mice (n=6) with rgA/HK H9N2 (8× LD50) virus. *; p<0.05 between NAe-HA and WT-HA, ##; p<0.01, #; p<0.05 between M2e-HA and WT-HA. Statistical significance was determined by using one-way ANOVA and Dunnett’s multiple comparison test. Data are representative of individual animals out of two independent experiments. Error bars indicate the means ± SEM. ***; p<0.001, **; p<0.01. *; p<0.05. ns; no significant difference between groups.
Consistent with mortality and lung viral titers, a lower level of IL-6 production was observed in BALF and lungs of chimeric HA- as well as WT-HA immunized group than those of naïve infection group which induced the highest level of IL-6 (Fig. 4D). Furthermore, we investigated hemagglutination inhibition (HAI) titers to homologous (A/PR8) or heterosubtypic influenza viruses (rgA/Vietnam/H5N1 or A/Phil) from boost immune sera of chimeric HA and WT-HA groups (Fig. 4E). Vaccination with inactivated chimeric HA and WT-HA virus induced high HAI titers against A/PR8 virus only (Fig. 4E). NAe-HA immune sera showed 2–4 folds higher HAI titers than those of M2e-HA immune sera (Fig. 4E). A similar pattern was observed in the microneutralization titers against homologous (A/PR8) virus in immune sera, displaying lower titers in the M2e-HA group (Fig. 4F). These results suggest that inactivated chimeric HA influenza virus vaccination induces HAI activity against homologous virus but not against heterosubtypic viruses, IgG antibodies specific for the inserted M2e or NAe, and improves cross protection.
We extended testing cross-protective efficacy against reverse genetic (rg) reassortant rgH9N2 virus [A/chicken/Hong Kong/1997 (H9N2)/A/PR8 reassortant]. Inactivated NAe-HA virus or M2e-HA virus vaccination induced lower body weight loss than control WT-HA virus vaccination, suggesting higher efficacy of cross protection against rgH9N2 virus (Fig. 4G–H). We further tested the protective capacity of NAe-HA virus and M2e-HA virus vaccination against reassortant rgH5N1 virus. All vaccinated mice showed 100% survival cross-protection against rgH5N1 without displaying differences among the NAe-HA, M2e-HA, and WT-HA groups probably due to the induction of stalk antibodies at similar levels within the same group 1 HA virus (data not shown).
Chimeric HA containing inactivated virus immunization induces systemic or mucosal IgG and IgA specific for NAe and M2e mono epitopes
We determined NAe and M2e mono epitope specific IgG and IgA antibody responses in BALF and lung lysates collected day 6 post challenge with heterosubtypic A/Phil (H3N2) virus (Fig. 5). The NAe-HA group showed higher levels of IgG and IgA specific for NAe peptide in their BALF and lungs compared to those in the M2e-HA and WT-HA groups (Fig. 5A, 5C). Similarly, M2e-specific mucosal antibody responses were observed at higher levels in the chimeric M2e-HA immunized group compared to NAe-HA and WT-HA-immunized groups (Fig. 5B, 5D). A similar level of IgG for A/PR8 virus was observed in all vaccinated groups (Fig. 5E). These results indicate that inactivated chimeric HA virus immunization induces IgG and IgA antibodies specific for NAe and M2e mono epitopes.
Figure 5. Mucosal IgG and IgA specific for NAe or M2e in mice immunized with inactivated chimeric HA A/PR8 viruses after heterosubtypic influenza virus infection.
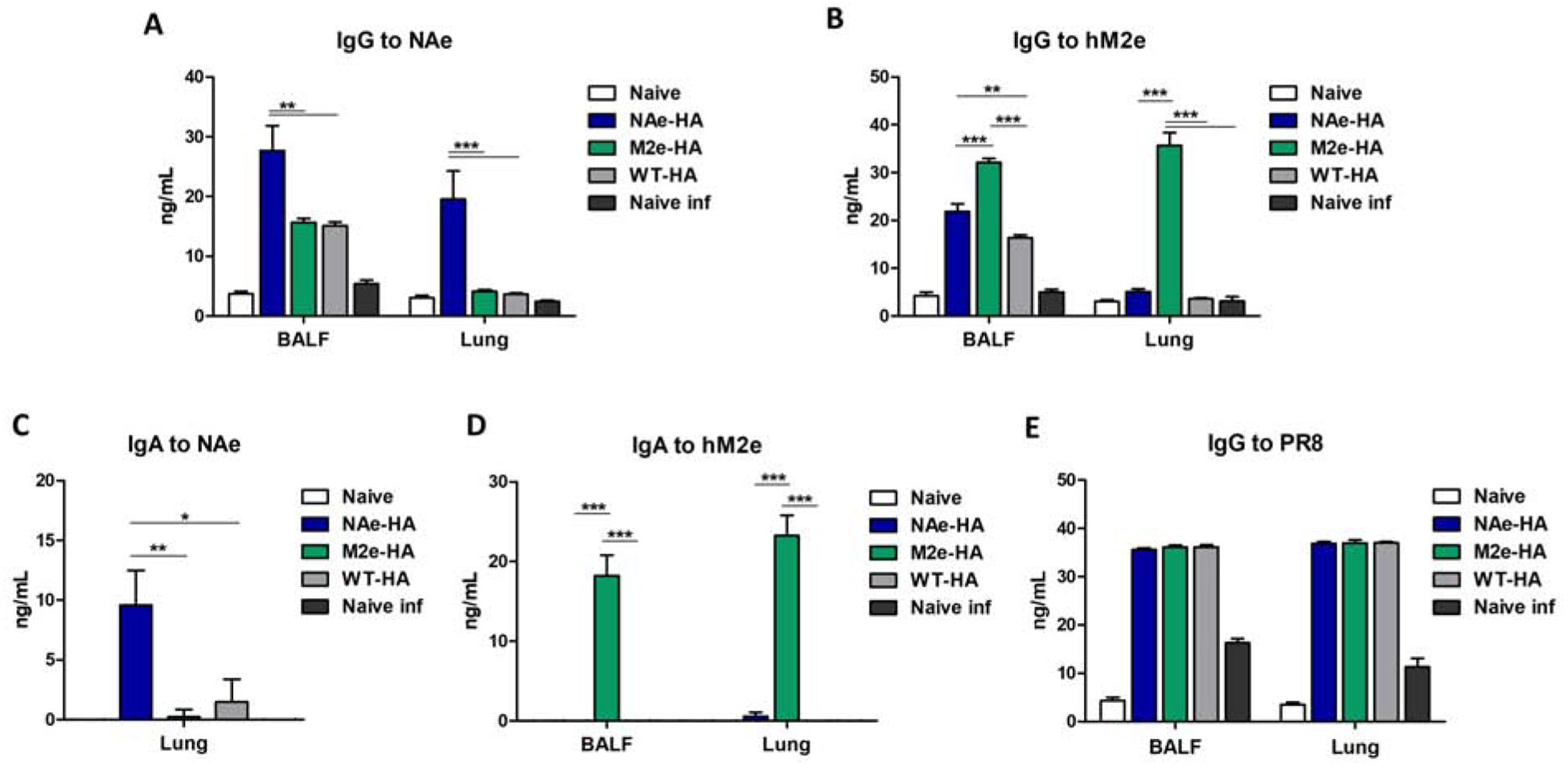
At day 6 post infection, BALF and Lung samples were collected and total IgG and IgA antibodies specific for NAe or M2e peptides were presented by ELISA. (A and B) Total IgG specific for NAe or M2e peptides. (C and D) IgA specific for NAe or M2e peptides. (E) Total IgG specific for A/PR8 virus. Statistical significance was determined by using one-way ANOVA and Tukey’s multiple comparison test. Antibody levels are presented in concentrations calculated with antibody standards and ELISA OD values. Data are representative of individual animal out of two independent experiments. Error bars indicate the means ± SEM. ***; p<0.001, **; p<0.01, *; p<0.05.
Chimeric M2e-HA inactivated virus immunization induces T cells responsive to M2e stimulation
The immune cells from the BAL, lung, and spleen tissues at day 6 post challenge were stimulated with M2e and virus antigens to analyze cellular responses secreting IFN-γ or TNF-α by an ELISpot (Fig. 6A–C) or an intracellular cytokine staining assay (Fig. 6D–E). A significant level of cellular responses secreting IFN-γ was detected in the lung of the chimeric M2e-HA group by in vitro stimulation with an M2e peptide, which is approximately 6.8-fold higher than those induced by the chimeric NAe-HA or WT-HA groups (Fig. 6A). Meanwhile, the effector cellular responses by virus antigen stimulation were observed in the chimeric NAe-HA or WT-HA immune groups at similar levels (lung, Fig. 6B) or at slightly higher levels (spleens, Fig. 6C), compared to those by the chimeric M2e-HA group (Fig. 6B–C).
Figure 6. Immunization with inactivated recombinant A/PR8 virus containing chimeric M2e-HA enhance M2-specific T cell responses.
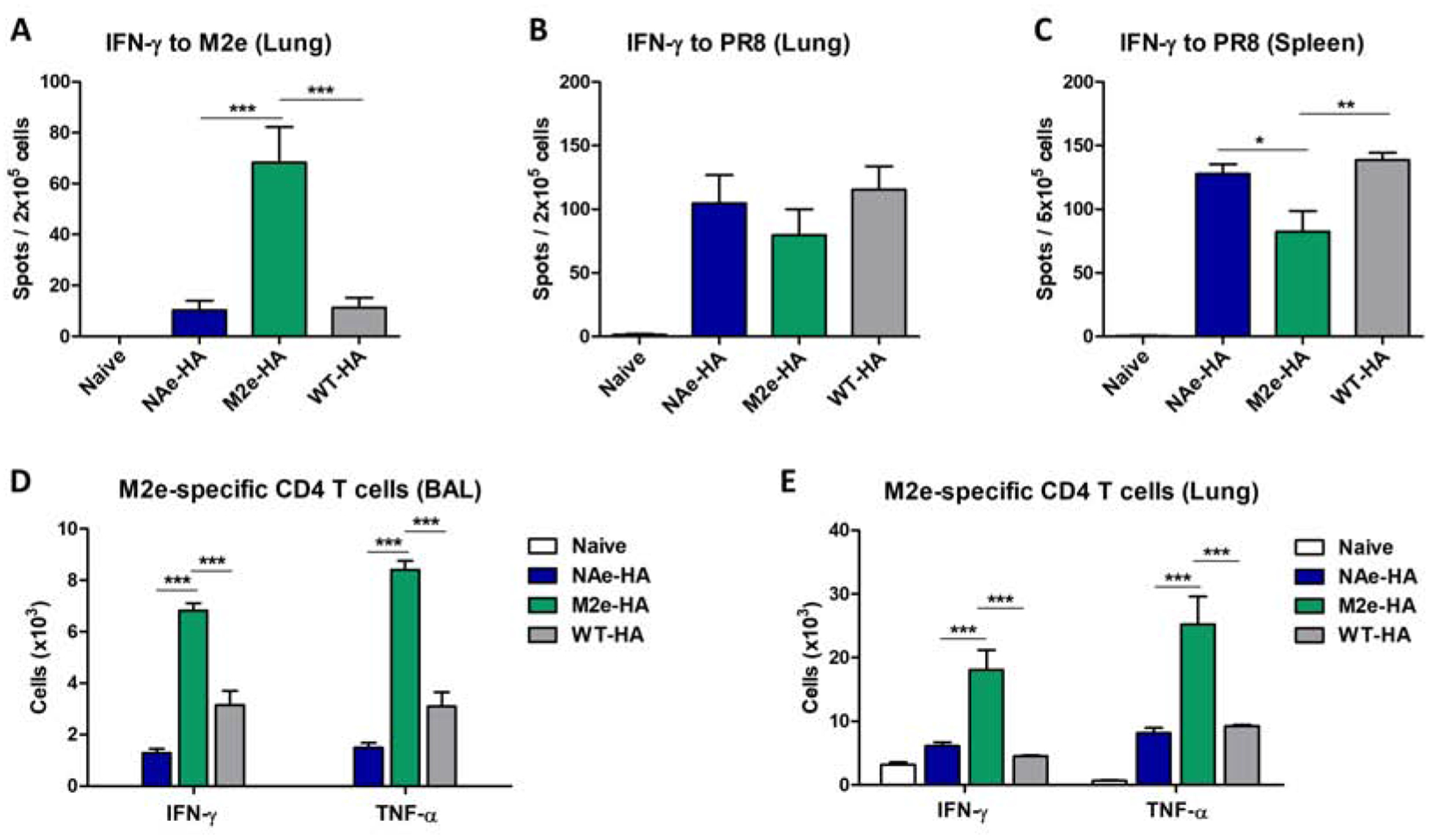
The cells were harvested from BALF, lung, and spleen tissues of immunized mice at day 6 after challenge with A/Phil/H3N2 (8× LD50). To determine IFN-γ secreting cells, lung and spleen cells were stimulated with M2e peptide or virus antigen for 3 days and then the cell spots secreting cytokine were determined by ELISpot analysis. (A) M2e-specific IFN-γ secreting cell spots in the lungs. (B and C) Virus antigen-specific IFN-γ secreting cell spots in the lung and spleen. (D and E) Antigen-specific T cell responses were analyzed by intracellular cytokine staining flow cytometry after in vitro stimulation with M2e peptide. Statistical significance was determined by using one-way ANOVA and Dunnett’s multiple comparison test. Data are representative of individual animal out of two independent experiments. Error bars indicate the means ± SEM. ***; p<0.001, **; p<0.01, *; p<0.05.
We further confirmed M2e specific cellular responses by intracellular cytokine flow cytometry assay. BAL and lung cells from chimeric M2e-HA immunization exhibited significantly higher numbers of M2e specific CD4+ T cells secreting IFN-γ or TNF-α than the chimeric NAe-HA or WT-HA groups (Fig. 6D–E). IFN-γ secreting T cells in response to NAe in vitro stimulation was not observed in the lungs of the chimeric NAe-HA immune group (Supplementary Fig S5). Thus, these results provide evidence that chimeric M2e-HA but not NAe-HA inactivated virus vaccine is effective in inducing M2e-specific effector T cell responses.
Discussion
This study first time demonstrated the immunogenic comparison and protection of a conserved M2e and NAe (NA222–230) epitope which was presented on the Sa antigenic site of HA head domain of replication competent A/PR8 attenuated backbone. The technology of reverse genetics enables the generation of recombinant influenza viruses expressing foreign epitopes. We generated replication competent recombinant influenza A/PR8 viruses expressing chimeric NAe-HA and M2e-HA with a conserved epitope in the head domain. IM immunization with inactivated chimeric NAe-HA and M2e-HA viruses was more effective in inducing IgG antibody responses to a conserved NA222–230 (NAe) or M2e epitope within the HA head domain than IN inoculation with live chimeric NAe-HA or M2e-HA attenuated virus. Enhanced cross protection was observed in mice after IM immunization with inactivated chimeric NAe-HA and M2e-HA viruses compared to inactivated WT A/PR8 virus vaccination. IgG antibody recognizing NA222–230 were induced by IM vaccination with inactivated chimeric NAe-HA virus, providing less weight loss and lower lung viral replication after heterosubtypic challenge. This study supports a proof-of-concept of developing recombinant influenza virus vaccines generating antibody responses to poorly immunogenic but conserved epitopes in nature.
NA222–230 sequence was identified to be universally conserved and located near to the NA enzymatic active site (Doyle et al., 2013a; Doyle et al., 2013b; Gravel et al., 2010). Despite this universal antigenic property of an NAe epitope, neither live PR8 virus infection nor inactivated virus immunization of mice did not induce IgG antibodies recognizing the NA222–230 epitope, indicating its poor immunogenic nature. Consistent, M2e epitope was also poorly immunogenic in mice after live virus infection or inactivated virus immunization. Presenting NA222–230 or M2e7–16 epitope within the HA head domain antigenic site could be a novel approach compatible with licensed vaccine platforms inducing IgG responses to these highly conserved monomeric epitopes. An M2e epitope appears to be more immunogenic and cross protective against A/Phil H3N2 virus than NAe when compared in HA chimeric recombinant influenza virus vaccines. Both inactivated NAe-HA and M2e-HA virus vaccines provided higher efficacy of cross protection against rgH9N2 virus than control WT-HA virus vaccine. This study provides further evidence for a new approach to improve cross protective efficacy of influenza vaccination by engineering recombinant influenza viruses expressing a conserved epitope.
There was no difference between M2e-HA and NAe-HA viruses in the levels of hemagglutination activity units of virus stocks harvested from the eggs and in the capacity to replicate in the egg substrates. Nonetheless, M2e-HA virus was able to induce M2e IgG antibodies at relatively higher levels, compared to NAe-HA virus which induces NAe IgG antibodies after IM immunization. In contrast, NAe-HA virus caused slight weight loss and induced higher levels of IgG antibodies specific for A/PR8 after live virus infection. In contrast, M2e-HA virus displayed compromise in inducing HAI and microneutralization titers by 2 to 4 folds compared to chimeric NAe-HA virus vaccination. These differences between M2e-HA and NAe-HA viruses were observed in immune sera after live virus IN inoculation (Supplementary Fig. S6). It appeared that the receptor binding domain of NAe-HA molecules from NAe-HA virus might be in a more immunogenic conformation than corresponding M2e-HA molecules in M2e-HA virus. An alternative interpretation is that more immunogenic conformation of M2e in the head domain M2e-HA might be slightly interfering with B cell receptor interactions, compromising HA immunogenicity.
It is notable that IgG2a isotype dominant antibody responses to NAe and M2e were induced after IM immunization with inactivated chimeric NAe-HA and M2e-HA viruses, in contrast to the similar levels of both IgG1 and IgG2a antibodies specific for virus. M2e specific IFN-γ or TNF-α secreting cellular responses were induced in lung and BAL samples from the mice with M2e-HA chimeric virus but not with chimeric NAe-HA virus vaccination. M2e has epitopes stimulating B cells and T cells (Kim et al., 2018; Kim et al., 2019b; Kim et al., 2017b; Kolpe et al., 2017; Schepens et al., 2018) but NAe was not able to stimulate cytokine expressing cellular responses in mice after vaccination. Thus, higher levels of M2e specific antibody responses in systemic and mucosal sites and induction of T cell responses might be contributing to more effective cross protection in mice with M2e-HA chimeric virus vaccination, compared to chimeric NAe-HA virus.
In this study, BALB/c mice were IM immunized 3 times with a high dose (10 μg) of whole inactivated virus vaccines to induce significant levels of IgG responses to a single epitope, M2e or NAe. Whole inactivated virus contains multiple antigens (HA, NA, NP, M1, NS1) at enough amounts to induce cellular CD4 and CD8 T cell responses, which is consistent with a previous study using trivalent inactivated influenza vaccines (TIV) (Richards et al., 2012). The induction of cellular immune responses is likely contributing to broad protection against H3N2 (A/Phil), A/PR8 reassortant rgH9N2 viruses in mice after 3 times IM immunization with M2e-HA or NAe-HA in the absence of cross-reactive HAI titers. In contrast, a clinical study reported that inactivated virus vaccines were more effective in inducing anti-stalk antibody responses after prime dose than the live attenuated influenza vaccine (LAIV) in healthy adult individuals (Bernstein et al., 2019). In young children, LAIV was more effective in inducing T cell responses than TIV (Hoft et al., 2017). Another clinical study reported that TIV can also induce a certain level of influenza A virus-reactive T cell responses in young children but with no comparative LAIV (He et al., 2006). In previous studies, mice with prior exposure to live influenza virus acquired heterosubtypic immunity at a certain level through the induction of cross-reactive T cell and B cell responses (Guo et al., 2011; Nguyen et al., 1999; O’Neill et al., 2000). In this study, the mice that were IN inoculated with boost dose (2×10^7 EID) of live NAe-HA, M2e-HA virus, or WT A/PR8 virus were all similarly protected against H3N2 virus (data not shown), suggesting that cross protective cellular responses overwhelmed M2e (or NAe) immunity. Nonetheless, it needs to be cautious in interpreting cellular responses and protective outcomes of influenza vaccines in mouse models.
Positioning the foreign epitopes in the different sites of HA might influence the outcomes of immune responses to the epitopes inserted into HA, after live virus infection or inactivated virus immunization. In this comparison study of live and inactivated virus vaccination, it is unexpected that IN inoculation of mice with live chimeric NAe-HA and M2e-HA viruses did not effectively induce IgG antibody responses to NAe or M2e epitope inserted into the HA head domain. Induction of IgG antibody responses to M2e was reported with live virus infection of mice with recombinant WT A/PR8 virus containing chimeric HA tandem repeat 4×M2e at the N-terminus of HA (Kim et al., 2017a). IM vaccination with inactivated chimeric viruses containing foreign neutralizing epitopes inserted into the antigenic site Sa of HA derived from WT A/PR8 virus was shown to be effective in inducing protective immune responses to human respiratory syncytial virus (Lee et al., 2016). Inactivated virus platforms with M2e inserted within the HA head domain induced antibody responses to M2e and stalk domains after sequential IM vaccination with different subtypes (Sun et al., 2019). IM immunizations of mice with inactivated whole A/PR8 H1N1 virus induced significant levels of IgG antibodies specific for HA stalk domains, contributing to cross protection. Overall, these previous reports are consistent with results in this current study.
Inactivated virus vaccine platforms appear to be more effective in raising antibody responses to M2e and NAe inserted into the HA head domain than live virus inoculation. In mice, live virus inoculation generally induces stronger immunity than inactivated virus vaccine platforms. For fair comparison, we carried out additional analysis of IgG responses specific for virus, NAe, and M2e in sera of boost dose (20 folds higher dose [2×10^7 EID50] than prime dose [2×10^4 to 1×10^6 EID50] live virus inoculation to overcome prior prime immunity. Virus (A/PR8) specific IgG levels were higher in sera from prime and boost dose of live virus inoculation compared to prime and boost dose inactivated virus vaccination respectively. In contrast, IgG responses to NAe were substantially high in sera from inactivated virus vaccination after prime boost dose whereas live virus inoculation failed to induce NAe specific IgG responses even after boost dose. IgG responses to M2e were higher in sera from inactivated virus vaccination after boost dose than those in boost live virus inoculation. The 2nd boost with inactivated NAe-HA and M2e-HA virus was required to further increase the levels of IgG antibodies to NAe and M2e respectively. In summary, recombinant influenza NAe-HA and M2e-HA vaccination induces IgG responses to virus and inserted mono NAe and M2e epitopes and cellular responses contributing to cross protection. M2e might be a desirable target for incorporation into a universal vaccine due to its both B cell and T cell responses. There is a limitation in this approach, requiring multiple immunizations to induce high levels of antibody responses to an inserted single epitope.
Supplementary Material
Supplementary Figure S1. Live recombinant chimeric HA A/PR8 influenza viruses display attenuated phenotypes in mice. Six to eight weeks old female BALB/c mice (N=3 per group) were intranasally (IN) inoculated with 2×104, 2×105, 1×106 EID50 of attenuated recombinant NAe-HA, -M2e HA, -WT-HA A/PR8 viruses, or a control WT pathogenic backbone A/PR8 influenza virus. Body weigh changes were monitored daily for 14 days to assess the pathogenicity of attenuated recombinant A/PR8 viruses. (A-C) Body weight changes of mice after inoculation with attenuated recombinant NAe-HA, -M2e-HA, WT-HA A/PR8 influenza viruses. (D) Body weight changes of mice after inoculation with pathogenic backbone WT A/PR8 virus (WT HA-PR8).
Supplementary Figure S2. IgG antibody responses after prime inoculation with attenuated recombinant A/PR8 influenza viruses containing chimeric NAe-HA or -M2e-HA. BALB/c mice were IN inoculated with indicated EID50 of attenuated chimeric NAe-HA or -M2e-HA A/PR8 influenza viruses. Sera were collected at 3 weeks after prime immunization. Antibody responses specific for A/PR8 virus, NAe or M2e peptides were determined by ELISA. IgG antibody response to A/PR8 virus (A and B), NAe peptide (C and D), and M2e peptide (E and F) in prime sera.
Supplementary Figure S3. Virus specific IgG antibody responses after immunization with inactivated recombinant A/PR8 influenza viruses containing chimeric HA.
BALB/c mice were IM immunized with 10 μg of inactivated recombinant A/PR8 influenza viruses (NAe-HA, M2e-HA, WT-HA A/PR8). Sera were collected at 3 weeks after prime and boost immunization. IgG antibody responses specific for A/PR8 virus was determined by ELISA. Total IgG, IgG1, IgG2a isotype antibodies specific for A/PR8 virus in prime sera (A-C) and boost sera (D-F).
Supplementary Figure S4. NAe or M2e-specific IgG antibody responses after immunization with inactivated recombinant A/PR8 influenza viruses. Sera were collected at 3 weeks after prime and boost IM immunization of BALB/c mice with 10 μg of inactivated recombinant influenza viruses (NAe-HA, M2e-HA, WT-HA A/PR8). IgG antibody responses specific for NAe or M2e peptides were determined by ELISA. Total IgG antibody in prime sera (A and B) and boost sera (C and D). Statistical significance was determined by using two-way ANOVA test. Data are representative of individual animal out of two independent experiments. Error bars indicate the means ± SEM. ***; p<0.001, **; p<0.01.
Supplementary Figure S5. Comparison of T cell responses secreting IFN-γ upon stimulation with various antigens in lung cells from vaccinated mice. The lung cells were harvested from vaccinated mice at day 6 after challenge with A/Phil/H3N2 (8× LD50). The cells were stimulated with NAe, M2e peptide (5 μg/ml), and A/PR8 virus antigen (5 μg/ml) and then the IFN-γ positive cell spots were determined by ELISpot assay. Antigen (M2e, NAe, A/PR8)-specific IFN-γ secreting cell spots. Statistical significance was determined by using two-way ANOVA test. Data are representative of individual animal out of two independent experiments. Error bars indicate the means ± SEM. **; p<0.01.
Supplementary Figure S6. Hemagglutination inhibition titer after intranasal inoculation of live attenuated recombinant chimeric HA A/PR8 influenza viruses. HAI titers to A/PR8 influenza virus were determined after intranasal prime inoculation as described in figure 4. Statistical significance was determined by using two-way ANOVA. Error bars indicate the means ± SEM. **; p<0.01. *; p<0.05.
Acknowledgments
This work was supported by National Institutes of Health/National Institute of Allergy and Infectious Diseases (NIH/NIAID) grants AI1093772 (S.M.K), AI147042 (S.M.K), and AI152800 (S.M.K). The following reagent was obtained through BEI Resources, NIAID, NIH: A/chicken/Hong Kong/1997 (H9N2)/A/PR8 reassortant. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors do not have conflicts of interest.
References:
- Bernstein DI, Guptill J, Naficy A, Nachbagauer R, Berlanda-Scorza F, Feser J, Wilson PC, Solorzano A, Van der Wielen M, Walter EB, Albrecht RA, Buschle KN, Chen YQ, Claeys C, Dickey M, Dugan HL, Ermler ME, Freeman D, Gao M, Gast C, Guthmiller JJ, Hai R, Henry C, Lan LY, McNeal M, Palm AE, Shaw DG, Stamper CT, Sun W, Sutton V, Tepora ME, Wahid R, Wenzel H, Wohlbold TJ, Innis BL, Garcia-Sastre A, Palese P, Krammer F, 2019. Immunogenicity of chimeric haemagglutinin-based, universal influenza virus vaccine candidates: interim results of a randomised, placebo-controlled, phase 1 clinical trial. Lancet Infect Dis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CJ, Ermler ME, Tan GS, Krammer F, Palese P, Hai R, 2016. Influenza A Viruses Expressing Intra- or Intergroup Chimeric Hemagglutinins. J Virol 90, 3789–3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi A, Bouzya B, Cortes Franco KD, Stadlbauer D, Rajabhathor A, Rouxel RN, Mainil R, Van der Wielen M, Palese P, Garcia-Sastre A, Innis BL, Krammer F, Schotsaert M, Mallett CP, Nachbagauer R, 2019. Chimeric Hemagglutinin-Based Influenza Virus Vaccines Induce Protective StalkSpecific Humoral Immunity and Cellular Responses in Mice. Immunohorizons 3, 133–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couch RB, Atmar RL, Franco LM, Quarles JM, Wells J, Arden N, Nino D, Belmont JW, 2013. Antibody correlates and predictors of immunity to naturally occurring influenza in humans and the importance of antibody to the neuraminidase. J Infect Dis 207, 974–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Filette M, Martens W, Roose K, Deroo T, Vervalle F, Bentahir M, Vandekerckhove J, Fiers W, Saelens X, 2008. An influenza A vaccine based on tetrameric ectodomain of matrix protein 2. J Biol Chem 283, 11382–11387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle TM, Hashem AM, Li C, Van Domselaar G, Larocque L, Wang J, Smith D, Cyr T, Farnsworth A, He R, Hurt AC, Brown EG, Li X, 2013a. Universal anti-neuraminidase antibody inhibiting all influenza A subtypes. Antiviral research 100, 567–574. [DOI] [PubMed] [Google Scholar]
- Doyle TM, Jaentschke B, Van Domselaar G, Hashem AM, Farnsworth A, Forbes NE, Li C, Wang J, He R, Brown EG, Li X, 2013b. The universal epitope of influenza A viral neuraminidase fundamentally contributes to enzyme activity and viral replication. The Journal of biological chemistry 288, 18283–18289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle TM, Li C, Bucher DJ, Hashem AM, Van Domselaar G, Wang J, Farnsworth A, She YM, Cyr T, He R, Brown EG, Hurt AC, Li X, 2013c. A monoclonal antibody targeting a highly conserved epitope in influenza B neuraminidase provides protection against drug resistant strains. Biochemical and biophysical research communications 441, 226–229. [DOI] [PubMed] [Google Scholar]
- Ermler ME, Kirkpatrick E, Sun W, Hai R, Amanat F, Chromikova V, Palese P, Krammer F, 2017. Chimeric Hemagglutinin Constructs Induce Broad Protection against Influenza B Virus Challenge in the Mouse Model. J Virol 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravel C, Li C, Wang J, Hashem AM, Jaentschke B, Xu KW, Lorbetskie B, Gingras G, Aubin Y, Van Domselaar G, Girard M, He R, Li X, 2010. Qualitative and quantitative analyses of virtually all subtypes of influenza A and B viral neuraminidases using antibodies targeting the universally conserved sequences. Vaccine 28, 5774–5784. [DOI] [PubMed] [Google Scholar]
- Guo H, Santiago F, Lambert K, Takimoto T, Topham DJ, 2011. T cell-mediated protection against lethal 2009 pandemic H1N1 influenza virus infection in a mouse model. J Virol 85, 448–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashemi H, Pouyanfard S, Bandehpour M, Noroozbabaei Z, Kazemi B, Saelens X, Mokhtari-Azad T, 2012. Immunization with M2e-displaying T7 bacteriophage nanoparticles protects against influenza A virus challenge. PLoS One 7, e45765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He XS, Holmes TH, Zhang C, Mahmood K, Kemble GW, Lewis DB, Dekker CL, Greenberg HB, Arvin AM, 2006. Cellular immune responses in children and adults receiving inactivated or live attenuated influenza vaccines. J Virol 80, 11756–11766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessel A, Savidis-Dacho H, Coulibaly S, Portsmouth D, Kreil TR, Crowe BA, Schwendinger MG, Pilz A, Barrett PN, Falkner FG, Schafer B, 2014. MVA vectors expressing conserved influenza proteins protect mice against lethal challenge with H5N1, H9N2 and H7N1 viruses. PLoS One 9, e88340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann E, Neumann G, Kawaoka Y, Hobom G, Webster RG, 2000. A DNA transfection system for generation of influenza A virus from eight plasmids. Proceedings of the National Academy of Sciences of the United States of America 97, 6108–6113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoft DF, Lottenbach KR, Blazevic A, Turan A, Blevins TP, Pacatte TP, Yu Y, Mitchell MC, Hoft SG, Belshe RB, 2017. Comparisons of the Humoral and Cellular Immune Responses Induced by Live Attenuated Influenza Vaccine and Inactivated Influenza Vaccine in Adults. Clin Vaccine Immunol 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iuliano AD, Roguski KM, Chang HH, Muscatello DJ, Palekar R, Tempia S, Cohen C, Gran JM, Schanzer D, Cowling BJ, Wu P, Kyncl J, Ang LW, Park M, Redlberger-Fritz M, Yu H, Espenhain L, Krishnan A, Emukule G, van Asten L, Pereira da Silva S, Aungkulanon S, Buchholz U, Widdowson MA, Bresee JS, Global Seasonal Influenza-associated Mortality Collaborator, N., 2018. Estimates of global seasonal influenza-associated respiratory mortality: a modelling study. Lancet 391, 1285–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jegerlehner A, Schmitz N, Storni T, Bachmann MF, 2004. Influenza A vaccine based on the extracellular domain of M2: weak protection mediated via antibody-dependent NK cell activity. J Immunol 172, 5598–5605. [DOI] [PubMed] [Google Scholar]
- Jin H, Lu B, Zhou H, Ma C, Zhao J, Yang CF, Kemble G, Greenberg H, 2003. Multiple amino acid residues confer temperature sensitivity to human influenza virus vaccine strains (FluMist) derived from cold-adapted A/Ann Arbor/6/60. Virology 306, 18–24. [DOI] [PubMed] [Google Scholar]
- Jin H, Zhou H, Lu B, Kemble G, 2004. Imparting temperature sensitivity and attenuation in ferrets to A/Puerto Rico/8/34 influenza virus by transferring the genetic signature for temperature sensitivity from cold-adapted A/Ann Arbor/6/60. Journal of virology 78, 995–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KH, Kwon YM, Lee YT, Hwang HS, Kim MC, Ko EJ, Wang BZ, Quan FS, Kang SM, 2018. Virus-like particles presenting flagellin exhibit unique adjuvant effects on eliciting T helper type 1 humoral and cellular immune responses to poor immunogenic influenza virus M2e protein vaccine. Virology 524, 172–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KH, Lee YT, Park S, Jung YJ, Lee Y, Ko EJ, Kim YJ, Li X, Kang SM, 2019a. Neuraminidase expressing virus-like particle vaccine provides effective cross protection against influenza virus. Virology 535, 179–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MC, Kim KH, Lee JW, Lee YN, Choi HJ, Jung YJ, Kim YJ, Compans RW, Prausnitz MR, Kang SM, 2019b. Co-Delivery of M2e Virus-Like Particles with Influenza Split Vaccine to the Skin Using Microneedles Enhances the Efficacy of Cross Protection. Pharmaceutics 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MC, Lee YN, Kim YJ, Choi HJ, Kim KH, Lee YJ, Kang SM, 2017a. Immunogenicity and efficacy of replication-competent recombinant influenza virus carrying multimeric M2 extracellular domains in a chimeric hemagglutinin conjugate. Antiviral Res 148, 43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MC, Song JM, O E, Kwon YM, Lee YJ, Compans RW, Kang SM, 2013. Virus-like particles containing multiple M2 extracellular domains confer improved cross-protection against various subtypes of influenza virus. Molecular therapy: the journal of the American Society of Gene Therapy 21, 485–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YJ, Lee YT, Kim MC, Lee YN, Kim KH, Ko EJ, Song JM, Kang SM, 2017b. Cross-Protective Efficacy of Influenza Virus M2e Containing Virus-Like Particles Is Superior to Hemagglutinin Vaccines and Variable Depending on the Genetic Backgrounds of Mice. Front Immunol 8, 1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko EJ, Lee Y, Lee YT, Kim YJ, Kim KH, Kang SM, 2018. MPL and CpG combination adjuvants promote homologous and heterosubtypic cross protection of inactivated split influenza virus vaccine. Antiviral Res 156, 107–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolpe A, Schepens B, Fiers W, Saelens X, 2017. M2-based influenza vaccines: recent advances and clinical potential. Expert Rev Vaccines 16, 123–136. [DOI] [PubMed] [Google Scholar]
- Krammer F, Margine I, Hai R, Flood A, Hirsh A, Tsvetnitsky V, Chen D, Palese P, 2014. H3 stalk-based chimeric hemagglutinin influenza virus constructs protect mice from H7N9 challenge. J Virol 88, 2340–2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krammer F, Smith GJD, Fouchier RAM, Peiris M, Kedzierska K, Doherty PC, Palese P, Shaw ML, Treanor J, Webster RG, Garcia-Sastre A, 2018. Influenza. Nat Rev Dis Primers 4, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurie KL, Engelhardt OG, Wood J, Heath A, Katz JM, Peiris M, Hoschler K, Hungnes O, Zhang W, Van Kerkhove MD, participants CLWG, 2015. International Laboratory Comparison of Influenza Microneutralization Assays for A(H1N1)pdm09, A(H3N2), and A(H5N1) Influenza Viruses by CONSISE. Clin Vaccine Immunol 22, 957–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YN, Hwang HS, Kim MC, Lee YT, Kim YJ, Lee FE, Kang SM, 2016. Protection against respiratory syncytial virus by inactivated influenza virus carrying a fusion protein neutralizing epitope in a chimeric hemagglutinin. Nanomedicine: nanotechnology, biology, and medicine 12, 759–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu WC, Nachbagauer R, Stadlbauer D, Solorzano A, Berlanda-Scorza F, Garcia-Sastre A, Palese P, Krammer F, Albrecht RA, 2019. Sequential Immunization With Live-Attenuated Chimeric Hemagglutinin-Based Vaccines Confers Heterosubtypic Immunity Against Influenza A Viruses in a Preclinical Ferret Model. Front Immunol 10, 756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memoli MJ, Shaw PA, Han A, Czajkowski L, Reed S, Athota R, Bristol T, Fargis S, Risos K, Powers JH, Davey RT Jr., Taubenberger JK, 2016. Evaluation of Antihemagglutinin and Antineuraminidase Antibodies as Correlates of Protection in an Influenza A/H1N1 Virus Healthy Human Challenge Model. MBio 7, e00417–00416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monto AS, Petrie JG, Cross RT, Johnson E, Liu M, Zhong W, Levine M, Katz JM, Ohmit SE, 2015. Antibody to Influenza Virus Neuraminidase: An Independent Correlate of Protection. The Journal of infectious diseases 212, 1191–1199. [DOI] [PubMed] [Google Scholar]
- Murphy BR, Kasel JA, Chanock RM, 1972. Association of serum anti-neuraminidase antibody with resistance to influenza in man. N Engl J Med 286, 1329–1332. [DOI] [PubMed] [Google Scholar]
- Neirynck S, Deroo T, Saelens X, Vanlandschoot P, Jou WM, Fiers W, 1999. A universal influenza A vaccine based on the extracellular domain of the M2 protein. Nat Med 5, 1157–1163. [DOI] [PubMed] [Google Scholar]
- Neu KE, Henry Dunand CJ, Wilson PC, 2016. Heads, stalks and everything else: how can antibodies eradicate influenza as a human disease? Current opinion in immunology 42, 48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen HH, Moldoveanu Z, Novak MJ, van Ginkel FW, Ban E, Kiyono H, McGhee JR, Mestecky J, 1999. Heterosubtypic immunity to lethal influenza A virus infection is associated with virus-specific CD8(+) cytotoxic T lymphocyte responses induced in mucosa-associated tissues. Virology 254, 50–60. [DOI] [PubMed] [Google Scholar]
- O’Neill E, Krauss SL, Riberdy JM, Webster RG, Woodland DL, 2000. Heterologous protection against lethal A/HongKong/156/97 (H5N1) influenza virus infection in C57BL/6 mice. The Journal of general virology 81, 2689–2696. [DOI] [PubMed] [Google Scholar]
- Richards KA, Chaves FA, Alam S, Sant AJ, 2012. Trivalent inactivated influenza vaccines induce broad immunological reactivity to both internal virion components and influenza surface proteins. Vaccine 31, 219–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepens B, De Vlieger D, Saelens X, 2018. Vaccine options for influenza: thinking small. Current opinion in immunology 53, 22–29. [DOI] [PubMed] [Google Scholar]
- Song JM, Van Rooijen N, Bozja J, Compans RW, Kang SM, 2011. Vaccination inducing broad and improved cross protection against multiple subtypes of influenza A virus. Proc Natl Acad Sci U S A 108, 757–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steel J, Lowen AC, Wang T, Yondola M, Gao Q, Haye K, Garcia-Sastre A, Palese P, 2010. Influenza virus vaccine based on the conserved hemagglutinin stalk domain. MBio 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Zheng A, Miller R, Krammer F, Palese P, 2019. An Inactivated Influenza Virus Vaccine Approach to Targeting the Conserved Hemagglutinin Stalk and M2e Domains. Vaccines (Basel) 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thontiravong A, Prakairungnamthip D, Chanvatik S, Nonthabenjawan N, Tunterak W, Tangwangvivat R, Oraveerakul K, Amonsin A, 2016. The Effect of Various Erythrocyte Species on the Detection of Avian, Swine and Canine Influenza A Viruses Isolated in Thailand. Thai J Vet Med. 46, 135–142. [Google Scholar]
- Tong S, Li Y, Rivailler P, Conrardy C, Castillo DA, Chen LM, Recuenco S, Ellison JA, Davis CT, York IA, Turmelle AS, Moran D, Rogers S, Shi M, Tao Y, Weil MR, Tang K, Rowe LA, Sammons S, Xu X, Frace M, Lindblade KA, Cox NJ, Anderson LJ, Rupprecht CE, Donis RO, 2012. A distinct lineage of influenza A virus from bats. Proceedings of the National Academy of Sciences of the United States of America 109, 4269–4274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong S, Zhu X, Li Y, Shi M, Zhang J, Bourgeois M, Yang H, Chen X, Recuenco S, Gomez J, Chen LM, Johnson A, Tao Y, Dreyfus C, Yu W, McBride R, Carney PJ, Gilbert AT, Chang J, Guo Z, Davis CT, Paulson JC, Stevens J, Rupprecht CE, Holmes EC, Wilson IA, Donis RO, 2013. New world bats harbor diverse influenza A viruses. PLoS pathogens 9, e1003657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trombetta CM, Ulivieri C, Cox RJ, Remarque EJ, Centi C, Perini D, Piccini G, Rossi S, Marchi S, Montomoli E, 2018. Impact of erythrocyte species on assays for influenza serology. J Prev Med Hyg 59, E1–E7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang BZ, Gill HS, Kang SM, Wang L, Wang YC, Vassilieva EV, Compans RW, 2012. Enhanced influenza virus-like particle vaccines containing the extracellular domain of matrix protein 2 and a toll-like receptor ligand. Clinical and vaccine immunology: CVI 19, 1119–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D, Wu TL, Lasaro MO, Latimer BP, Parzych EM, Bian A, Li Y, Li H, Erikson J, Xiang Z, Ertl HC, 2010. A universal influenza A vaccine based on adenovirus expressing matrix-2 ectodomain and nucleoprotein protects mice from lethal challenge. Mol Ther 18, 2182–2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1. Live recombinant chimeric HA A/PR8 influenza viruses display attenuated phenotypes in mice. Six to eight weeks old female BALB/c mice (N=3 per group) were intranasally (IN) inoculated with 2×104, 2×105, 1×106 EID50 of attenuated recombinant NAe-HA, -M2e HA, -WT-HA A/PR8 viruses, or a control WT pathogenic backbone A/PR8 influenza virus. Body weigh changes were monitored daily for 14 days to assess the pathogenicity of attenuated recombinant A/PR8 viruses. (A-C) Body weight changes of mice after inoculation with attenuated recombinant NAe-HA, -M2e-HA, WT-HA A/PR8 influenza viruses. (D) Body weight changes of mice after inoculation with pathogenic backbone WT A/PR8 virus (WT HA-PR8).
Supplementary Figure S2. IgG antibody responses after prime inoculation with attenuated recombinant A/PR8 influenza viruses containing chimeric NAe-HA or -M2e-HA. BALB/c mice were IN inoculated with indicated EID50 of attenuated chimeric NAe-HA or -M2e-HA A/PR8 influenza viruses. Sera were collected at 3 weeks after prime immunization. Antibody responses specific for A/PR8 virus, NAe or M2e peptides were determined by ELISA. IgG antibody response to A/PR8 virus (A and B), NAe peptide (C and D), and M2e peptide (E and F) in prime sera.
Supplementary Figure S3. Virus specific IgG antibody responses after immunization with inactivated recombinant A/PR8 influenza viruses containing chimeric HA.
BALB/c mice were IM immunized with 10 μg of inactivated recombinant A/PR8 influenza viruses (NAe-HA, M2e-HA, WT-HA A/PR8). Sera were collected at 3 weeks after prime and boost immunization. IgG antibody responses specific for A/PR8 virus was determined by ELISA. Total IgG, IgG1, IgG2a isotype antibodies specific for A/PR8 virus in prime sera (A-C) and boost sera (D-F).
Supplementary Figure S4. NAe or M2e-specific IgG antibody responses after immunization with inactivated recombinant A/PR8 influenza viruses. Sera were collected at 3 weeks after prime and boost IM immunization of BALB/c mice with 10 μg of inactivated recombinant influenza viruses (NAe-HA, M2e-HA, WT-HA A/PR8). IgG antibody responses specific for NAe or M2e peptides were determined by ELISA. Total IgG antibody in prime sera (A and B) and boost sera (C and D). Statistical significance was determined by using two-way ANOVA test. Data are representative of individual animal out of two independent experiments. Error bars indicate the means ± SEM. ***; p<0.001, **; p<0.01.
Supplementary Figure S5. Comparison of T cell responses secreting IFN-γ upon stimulation with various antigens in lung cells from vaccinated mice. The lung cells were harvested from vaccinated mice at day 6 after challenge with A/Phil/H3N2 (8× LD50). The cells were stimulated with NAe, M2e peptide (5 μg/ml), and A/PR8 virus antigen (5 μg/ml) and then the IFN-γ positive cell spots were determined by ELISpot assay. Antigen (M2e, NAe, A/PR8)-specific IFN-γ secreting cell spots. Statistical significance was determined by using two-way ANOVA test. Data are representative of individual animal out of two independent experiments. Error bars indicate the means ± SEM. **; p<0.01.
Supplementary Figure S6. Hemagglutination inhibition titer after intranasal inoculation of live attenuated recombinant chimeric HA A/PR8 influenza viruses. HAI titers to A/PR8 influenza virus were determined after intranasal prime inoculation as described in figure 4. Statistical significance was determined by using two-way ANOVA. Error bars indicate the means ± SEM. **; p<0.01. *; p<0.05.


