Abstract
Background.
Enthusiasm for the use of metal nanoparticles in human and veterinary medicine is high. Many articles describe the effects of metal nanoparticles on microbes in vitro, and a smaller number of articles describe effects on the immune system, which is the focus of this review.
Methods.
Articles were retrieved by performing literature searches in Medline, of the National Institute of Medicine, as well as via Google Scholar.
Results.
In vitro studies show that metal nanoparticles have antimicrobial effects. Some metal nanoparticles augment innate host immune defenses, such as endogenous antimicrobial peptides, and nitric oxide. Metal nanoparticles may also function as vaccine adjuvants. Metal nanoparticles can migrate to locations distant from the site of administration, however, requiring careful monitoring for toxicity.
Conclusions.
Metal nanoparticles show a great deal of potential as immunomodulators, as well as direct antimicrobial effects. Before metal particles can be adopted as therapies, however, more studies are needed to determine how nanoparticles migrate though the body and on possible adverse effects.
Keywords: Gold, Silver, Zinc, Nitric oxide, Vaccine Adjuvants
The literature on metal nanoparticles is vast, and has increased greatly over the last several years, as shown in Figure 1 A. Of the 34,956 articles on metal nanoparticles in the biomedical literature since 2005, however, only 41 have appeared in journals considered “core clinical medical journals,” based on the Abridged Index Medicus (AIM) list, as defined by the National Library of Medicine and PubMed. To the relief of readers of this journal, as well as to the author, most of the articles citing metal nanoparticles do not have anything to do with infection or immunity.
Fig. 1.
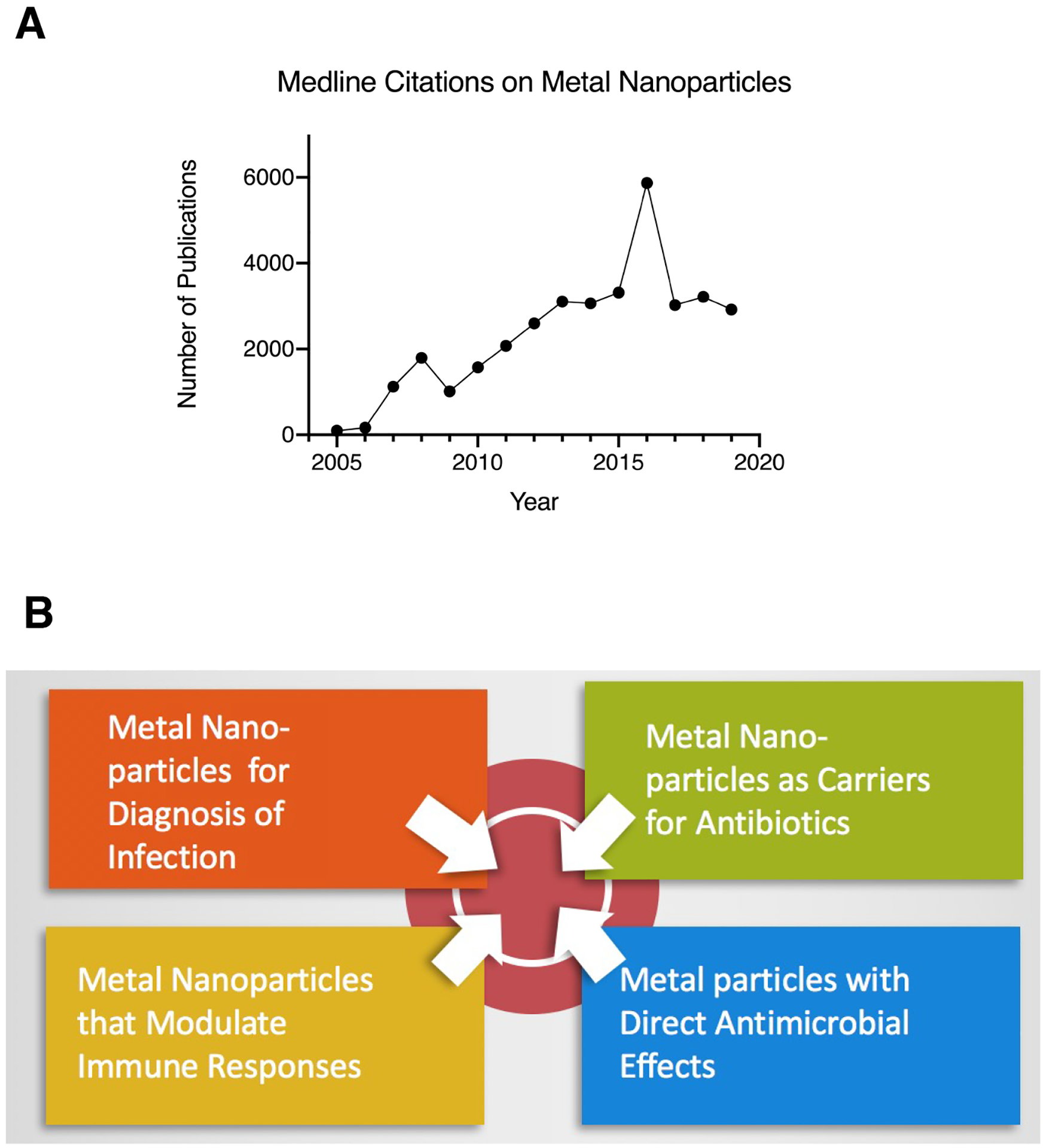
Panel A, Results from the Medline database of the National Library of Medicine (NLM) from a search for articles on “Metal Nanoparticles,” indexed yearly from 2005 to 2019. Panel B, Illustration showing the categories into which articles on metal nanoparticles effects in infection and immunity can be sorted. Articles on metal particles for in vitro diagnosis and on metal particles used as carriers for other, known antimicrobials are not discussed in this review.
Review of the literature showed that many of the articles retrieved described using metal nanoparticles in vitro in diagnostic tests, including those for infectious diseases. This is not a surprise because magnetic beads have been used for many years in immunoassays, and other diagnostic assays, because of their ability to help separate a target molecule (such as a DNA sequence or a protein) from background material in the sample. Immunogold labeling of antibodies has been used for many years in electron microscopy. Fig. 1B shows that there are at least four separate categories of articles on metal nanoparticles related to infection and immunity. This review will completely omit the articles using metal nanoparticles for in vitro diagnosis alone, and will only briefly mention articles which used nanoparticles as carriers for other known antibiotics or antimicrobials.
(Fig. 1.)
I. Design of Metal Nanoparticles.
As with other nanoparticles, formulating a metal as a tiny particle greatly increases the surface area to volume ratio, and this can drastically change the biological effects of the metal, just as can be seen with nonmetal nanoparticles. Metal nanoparticles have come to attention of biomedical researchers because the technology for creating metal nanoparticles has developed over many years for use in other fields, such as electronics and industrial manufacturing. Metal nanoparticles can be generated by use of chemical reducing agents from the soluble metal salts, such as silver nitrate, for example. Nanoparticles can also be created by “sputtering,” electrospray, and aerosol techniques (Singh et al., 2013), and by incorporating ultrasound (sonochemistry). Coprecipitation and use of microemulsions are other methods used to create metal oxide nanoparticles.
Noble metals have featured prominently in the search for metal nanoparticles with biological effects, especially silver and gold nanoparticles. Because of their resistance to degradation, however gold, silver and platinum nanoparticles should be considered as non-biodegradable.
Other metals which have been investigated as nanoparticles, include those composed of copper, iron, and zinc. In addition, semi-metals such as gallium and bismuth have been incorporated into nanoparticles as well (Hernandez-Delgadillo et al., 2013; Vega-Jimenez et al., 2017). Iron and zinc may decompose into the ionic forms of those elements in acidic cellular compartments, and therefore might be considered partially biodegradable. In addition to pure metal, metal oxides feature prominently in the field of nanoparticles, such as iron oxide NPs, zinc oxide (ZnO) NPs, titanium oxide (TiO2) NPs, and others. Iron oxide can be in the form of Fe2O3 (ferric iron, Fe III) or Fe3O4 (Fe II/III). The latter is magnetic, which means it can be used to separate a target from background in vitro or in vivo. Fe3O4 nanoparticles can also be injected into a target tissue (such as cancerous tumor) and then heated by application of a high frequency alternating magnetic field, known as magnetic hyperthermia.
II. Metals as Carriers for Other Antimicrobial Agents
As shown in Figure 1B, top right, many articles in the literature described the use of metal nanoparticles as carriers for other antibiotics or antimicrobials. Examples of this include gold-chlorhexidine , silver-aztreonam nanoparticles (Habash et al., 2014), silver-tobramycin nanoparticles (Habash et al., 2017), and silver particles combined with cationic peptides (Ruden et al., 2009). In most of these cases, the primary antimicrobial activity was attributed to the antibiotic or antimicrobial drug that was conjugated to the particle, with the metal particle providing an additive or synergistic effect. Because of the non-biodegradability of these nanoparticles, and the lack of clear-cut advantages over other nanoparticles, it is difficult to envision where these metal nanoparticles carriers would find a niche for actual therapy.
One exception is the article by Coates et al. showing that, by incorporating super-paramagnetic nanoparticles into an inhaled aerosol, nebulized droplets of medication could be directed preferentially to one lung, rather than the other, by the use of a magnet placed over the chest (Coates, 2008).
For the sake of perspective, there are many other types of nanoparticles other than those of metal. Examples include carbon nanoparticles, which can come in various sizes and shapes, including the icosahedral shapes of buckminsterfullerene or “Buckyballs” (which resemble soccer balls). Biodegradable nanoparticles include poly-lactic- co-glycolic acid (PLGA), hydroxyapatite, calcium phosphate, chitosan, chitosan-mannitol, and tannic acid hydrogels. Chitosan is the N-deacetylated derivative of chitin, a polysaccharide found in the exoskeletons of arthropods and crustaceans, and in fungi. The biodegradability of some of the organic nanoparticles should be considered a strong advantage from the point of view of safety, compared to gold and silver particles, which are not biodegradable in the human body.
II. Metal Nanoparticles with Direct Antimicrobial Effects.
As shown in Fig. 1B, bottom right, the next category of metal nanoparticles are those with direct antimicrobial effects.
The antimicrobial effects of metals have been known for centuries, as reviewed recently by Chernousova et al. (Chernousova and Epple, 2013). Some of the earliest metal antimicrobials were mercury -containing compounds, and lead is also a potent biocide, active against bacteria, fungi, plant, and animal cells. Because of their toxicity, however, nano-metals composed of mercury and lead have mostly been avoided (Bellinger, 2016).
Table I shows examples of some direct antimicrobial effects of metal nano-particles. As shown in the Table, metal nanoparticles have been shown in have antimicrobial activity in vitro against a wide range of organisms, including Gram-positive and Gram-negative bacteria, mycobacteria, fungi, and few viruses.
Table I.
Selected Examples of Direct Antimicrobial Effects of Metal Nanoparticles.
| Type of Metal Nano-Particle | Type of Microbe Tested | Comments | Reference |
|---|---|---|---|
| Bacteria | |||
| Silver | Staphylococcus aureus | In vitro | (Iwalokun et al., 2019) |
| Silver | Pseudomonas aeruginosa | In vitro | (Liao et al., 2019) |
| Silver | Acinetobacter baumannii | In vitro | (Singh et al., 2018) |
| Platinum | Aeromonas hydrophila | In vitro, zebrafish | (Ahmed et al., 2018) |
| Chitosan†-Silver Nanoparticle | Bacterial burn wound pathogens: MRSA, Pseudomonas, others | Marked synergy in vitro; effective when applied topically on infected burn wounds in vivo | (Huang et al., 2011) |
| Zinc Oxide | E. coli and Klebsiella pneumoniae | In vitro | (Ansari et al., 2012) |
| Zinc Oxide | Campylobacter jejuni | in vitro | (Xie et al., 2011) |
| Fe3O4, Iron oxide | Bacillus subtilis Staphylococcus aureus E. coli Pseudomonas aeruginosa |
In vitro. | (Arakha et al., 2015; Mohan and Mala, 2019); (Wikipedia_contributors, 14 October 2019) |
| Copper | Experimentally Infected wounds | Topical, in vivo | (Babushkina et al., 2017) |
| Silver | Mycobacterium tuberculosis | In vitro | (Selim et al., 2018) |
| Fungi | |||
| Silver | Candida albicans | Tested in combination with fluconazole; Aspergillus & Cryptococcus tested but were mostly unaffected by silver. | (Singh et al., 2013) |
| Viruses | |||
| Silver- Tannic acid hydrogel | Herpes simplex virus | For Topical use | (Szymanska et al., 2018) ; (Orlowski et al., 2014) |
, chitosan is the N-deacetylated derivative of chitin; chitin is a polysaccharide found in the exoskeleton of insects and crustaceans, and also in fungi.
III. Bacterial Resistance to Metal Nanoparticles.
As stated above, a large portion of the articles on metal nanoparticles reported on direct antimicrobial activity of the metal particles. This being the case, one wonders if emergence of resistance to metal nanoparticles could occur. In general, emergence of resistance to metals among bacteria appears to be less common than to other, conventional antibiotics. But resistance can emerge, as reported many years ago by McHugh et al. (Mchugh et al., 1975). They reported on an outbreak in their hospital burn unit due to a Salmonella strain resistant to silver as well as other antibiotics. Topical silver in the form of silver sulfadiazine (Silvadene™) is common in burn care due to its broad spectrum of antimicrobial activity. Resistance to zinc has been observed among strains of methicillin-resistant Staph aureus (MRSA) in swine (Hau et al., 2017). With regard to metal nanoparticles, Elbehiry et al. tested if resistance to silver or gold could emerge after prolonged exposure to increasing concentrations of nanoparticles (Elbehiry et al., 2019). They found that resistance emerged more readily to the 20 nm nanoparticles than for the 10 nm particles of both metals. For silver nanoparticles (AgNP), 10 of 20 Staphylococcal strains showed development of resistance to 20 nm particles, while for gold particles (AuNP), only 3 of 20 strains showed emergence of resistance. These findings are not reassuring for the durability of the antibacterial effects of metal nanoparticles, if such nanoparticles should come into widespread use. Resistance to metals is rarely tested for, so it is likely that resistance to metals will be missed unless it is deliberately investigated.
IV. Metal Nanoparticles that Modulate the Innate Immune Response
Studies showing effects of metal nanoparticles on the cellular immune system less frequently reported in the literature. Instead, most of the reported effects of metal NPs and metal oxide NPs are on the innate immune system, and will be discussed first.
IV.A. Metal Interactions with Nitric Oxide, an important innate host defense.
Reactive oxygen species, such as hydrogen peroxide and sodium hypochlorite, are produced by neutrophils and macrophages, and often receive top billing as host defense pathways. But reactive nitrogen species, especially nitric oxide, also play an important role against pathogenic microbes. Nitric oxide can be synthesized by 3 different versions of the heme-containing enzyme nitric oxide synthetase (NOS). All versions of NOS use the amino acid L-arginine as a reactant, along with NADPH and oxygen, to produce nitric oxide (NO). Endothelial NOS, or eNOS, generates NO to regulate vascular tone, and neuronal NOS, or nNOS, generates NO which acts as a neurotransmitter (Furchgott, 1996). The inducible form, called iNOS, is most important in antimicrobial immunity, and once fully induced, produces the largest amounts of NO (Murad, 2006). Many commonly used drugs, called nitrovasodilators, act via release of nitric oxide, including nitroglycerin, nitroprusside, and isosorbide mononitrate.
Metals, especially zinc and copper, can interact with nitric oxide host defenses against microbes. Levels of free zinc and free copper ions in prokaryotic and eukaryotic cells are kept low because of binding of these metals to sites in proteins, including metallothionein, glutathione, and others (Outten and O’Halloran, 2001). Nitric oxide can displace zinc and copper from sulfhydryl groups in these protective proteins in bacteria and increase the levels of free zinc or copper to toxic levels (Frawley et al., 2018; Pant et al., 2017; Rolim et al., 2019; St Croix et al., 2002). Fig. 2A shows that zinc oxide nanoparticles can synergize with a nitric oxide donor, S-nitroso-acetyl-penicillamine (SNAP), to kill E. coli bacteria, strain B171–8 (red arrow showing zone of inhibition). Fig 2A illustrates other results reported in the literature and emphasizes that metal nanoparticles can potentiate the effects of reactive nitrogen species (RNS), and not just reactive oxygen species (ROS) that are often emphasized (Shaikh et al., 2019).
Fig. 2.
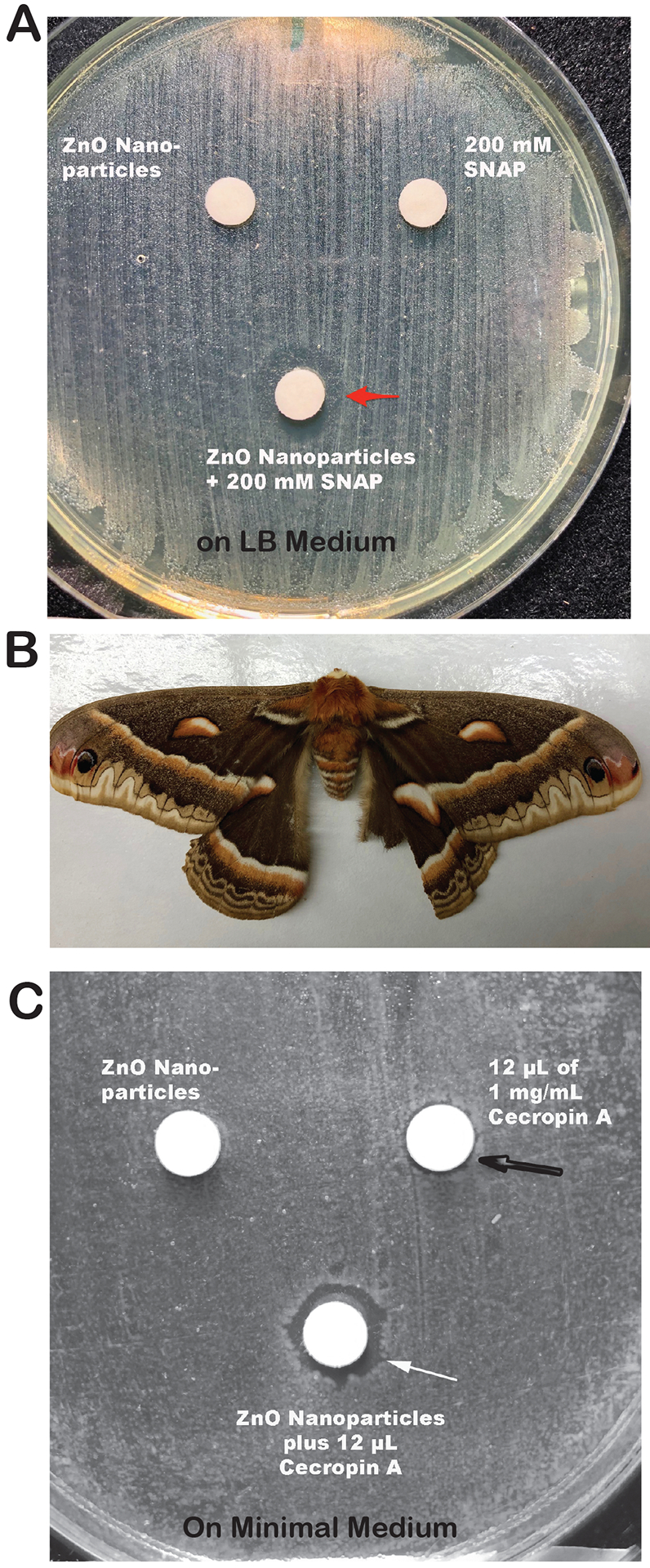
Interaction of Zinc Nanoparticles with Innate Immune Host Responses. Panel A, synergy between zinc oxide nanoparticles and the nitric oxide donor S-nitroso-acetyl-penicillamine (SNAP). Zinc oxide nanopowder was from Inframat Advanced Materials, Manchester, CT, and SNAP was from Enzo. The combination of ZnO nanoparticles and SNAP inhibited growth of E. coli strain B171–8, resulting in a zone of inhibition around the disk (red arrow).
Panel B, cecropia moth, from which the antimicrobial peptides called cecropins were first isolated.
Panel C, synergy between zinc oxide nanoparticles and cecropin A. ZnO particles alone failed to produce any zone of inhibition with E. coli strain B171–8 (disk on top left). Cecropin A alone produced a very small, barely detectable zone of inhibition (black arrow), but the combination of ZnO particles and cecropin A formed a definite zone of inhibition of growth (white arrow).
IV. B. Metal Interactions with Antimicrobial Peptides
Antimicrobial peptides have been discovered in a wide variety of animals, such as the magainins in frog skin (Zasloff, 1987), and cecropins in the hemolymph of moths (Moore et al., 1996). Closely related peptides with antimicrobial effects have subsequently found in many organisms, including horseshoe crabs, other insects, and mammals. For example, cecropin P1 is expressed in Paneth cells in intestinal crypts of the human intestine (Boman et al., 1993; Lee et al., 1989). Antimicrobial peptides are also synthesized by bacteria, such as Polymyxin B, and its derivative colistin, produced by Bacillus polymyxus. In a similar manner to their interactions with nitric oxide, metals can potentiate the antimicrobial effect of cationic peptides, as shown in Figure 2B and 2C. These effects can often be observed with metal salts as well as with metal nanoparticles. Fig. 2B shows a cecropia moth, a large North American moth with vivid eye spots on the wings, and from which the antimicrobial peptide cecropin was first isolated.
As shown in Figure 2C, zinc oxide nanoparticles potentiated the inhibitory effect of cecropin A against E. coli. To observe this effect, the medium used was changed to minimal medium, because the high ionic strength of typical growth media often inhibits the actions of antimicrobial peptides (Koshlukova et al., 1999). Metal nanoparticles can also synergize with polymyxin B (Jasim et al., 2017). Metals, including those in nanoparticle formulations, can also potentiate the effects of other antimicrobial peptides such as the histatins found in saliva (Dong et al., 2003). Talukder et al. showed that zinc stimulated the release of the peptide LL-37, generated from its precursor cathelicidin, from CaCo-2 colonic cells (Talukder et al., 2011). In summary, various formulations of various metals can potentiate the effects of antimicrobial peptides, and this may be considered a bona fide immunomodulatory effect of metals on this aspect of the innate host defenses. Zinc, copper, and silver appear to be the metals with the greatest activity in this regard.
V. Effects of Nanometals as Vaccine Adjuvants.
As mentioned above, reports of metal nanoparticles on acquired immune responses are fewer than those on innate immunity. One area where metal nanoparticles have been tested is as immune adjuvants. Immunologists have known for many decades that particulate antigens usually provoke a stronger immune response in the same antigen (e.g., ovalbumin) presented in a soluble form. As explained by Gause et al., this is due to the fact that the immune system has evolved to respond to particulate threats, be they viral, bacterial, fungal, or other. As another simple example, if a soluble antigen is presented, for example by intramuscular injection, then the soluble protein may diffuse away before there is time for antigen presenting cells to take up the antigen and present them to T cells.
For many years, the only adjuvant available for use in vaccines in the United States was alum, which is itself a little bit like metal nanoparticle. Alum is aluminum sulfate salt, and is complexed with another cation such as potassium or ammonium. Alum has been used in many ways over the centuries, for example in making textiles, the tanning of leather, as well as use as a topical medication. Since the advent of vaccines to prevent infectious diseases, alum has frequently been used as an adjuvant. In some vaccines, however, no adjuvant is needed, because of the vaccine protein itself forms into a particle, for example virus- like particles (VLPs) of hepatitis B surface antigen, used in the hepatitis B vaccine. Most vaccines, however, do require the use of an adjuvant.
More recently, the scientific and immunological principles that guide production of a good adjuvant particle have been more clearly defined. One of the critical characteristics of a nanoparticle is the particle size. Since the technology for producing metal nanoparticles is fairly advanced, metal nanoparticles of various sizes can be produced and tested for biological activity. For example, Chithrani et al found that 50 nm gold particles had the greatest ability to be taken up into HeLa cells (Chithrani et al., 2006). In a similar vein, Fifis (Fifis et al., 2004) explored the optimum size of polystyrene beads used as adjuvants in inducing antitumor immunity, and found that beads of 40 nm diameter appeared to give the best response. The size of the adjuvant particle used may also be able to skew the antigen-presenting cell which presents the antigen to T cells, with larger particles preferentially being trafficked to macrophages, while smaller particles might traffic to dendritic cells (Gause et al., 2017).
Given what is known about particulate adjuvants, metal nanoparticles, not surprisingly, have been shown to be able to act as vaccine adjuvants (Dakterzada et al., 2016; Poon et al., 2017; Roy et al., 2014). Few of those reported studies, however, have compared metal nanoparticles with non-metallic adjuvants, to alum, or to lipid adjuvants such as monophosphoryl lipid A (MPL) to determine if metal nanoparticles might be superior. The wide variety of sizes, shapes, and compositions of nanoparticles used as adjuvants have been reviewed by Singha et al. (Singha et al., 2018). Those authors concluded that by clever choice of adjuvant composition and size, vaccine designers might be able to help direct an antigen to different immune cells, and also to various intracellular compartments (lysosome vs. endosome vs. cytosol), and this would influence the vigor and nature of the immune response to the antigen. For example, the choice of nanoparticle used to induce anti-tumor immunity might be different from the optimal nanoparticle intended to induce anti-microbial immunity. Those authors could not conclude, however, that metal nanoparticles were superior to non-metallic nanoparticles as vaccine adjuvants. Gregory et al. reached similar conclusions in comparing metal vs. other nanoparticles as vaccine adjuvants (Gregory et al., 2013). Those authors suggested that metal nanoparticles might be best suited to optimizing particle size, and for tracking the migration of nanoparticles to tissues, then using that data to design non-metal nanoparticles with similar activity for actual use as vaccine adjuvants (Gregory et al., 2013).
VI. Adverse Effects of Metal Nanoparticles
The toxicity of metal nanoparticles has been studied, but primarily in short-term experiments in cultured cells (Lozano et al., 2011). Of those studies, however, the results have not been very reassuring (Shakibaie et al., 2018). For example, Xu et al. found that TiO2 nanoparticles increased the ability of Staphylococcus aureus to invade HeLa cells (Xu et al., 2016). Silver nanoparticles triggered the release of neutrophil extracellular traps from neutrophils (Wang et al., 2019), and metal oxide nanoparticles showed toxicity in the zebrafish (Hou et al., 2018). Vadalasetty tested whether adding silver nanoparticles to poultry feed could protect chickens from Campylobacter jejuni (Vadalasetty et al., 2018). Although infection with Campylobacter was reduced, the nanoparticle- treated chickens also showed impaired growth compared to controls. Elbehiry et al. tested if silver or gold nanoparticles were active against Staphylococcus aureus strains causing bovine mastitis (Elbehiry et al., 2019). To their credit, they also extended their study to determine if orally administered silver or gold particles had any toxicity in rats after 30 days of administration. Lower doses of the nanoparticles seemed to be tolerated, but at 2 mg/kg orally per day the mice showed obvious and severe organ toxicity involving the brain, liver, kidney, heart, spleen, and lung. Toxicity was observed with both silver and gold particles. Their study was not able to determine if there was a dose capable of blocking S. aureus infection that was not also toxic to the rats.
An additional factor that must be taken into account in assessing toxicity of metal nanoparticles is that the nanoparticles do not necessarily remain at the site of administration (Falconer et al., 2018; Falconer and Grainger, 2018; Vila et al., 2018). Instead, particles injected intramuscularly in an animal may traffic to the regional lymph nodes. As another example, nanoparticles administered by inhalation do not remain in the lungs and airways, but may translocate to other organs, including mediastinal lymph nodes, bloodstream, and kidneys (Choi et al., 2010). This requires a careful assessment of possible action of metal nanoparticles in organs and tissues distant from the site of administration. In addition to direct toxicity, nickel nanoparticles triggered nickel allergy in mice, and this was potentiated by lipopolysaccharide (LPS)(Hirai et al., 2016). Nickel allergy is the most common type of metal allergy in humans, and is important because nickel alloys are used in buttons and snaps on clothing, in eyeglass frames, and in jewelry. Nanoparticles appear especially prone to triggering inflammatory reactions in the lung, including pulmonary fibrosis in animals such as rats (Cho et al., 2011; Lai et al., 2018; Thompson et al., 2014).
If the focus is limited to toxicity of nanoparticles in humans, the literature is more limited, but still concerning. For example, metal-on-metal prosthetic joints release tiny particles of chromium and cobalt from the alloy in the artificial joint, triggering an inflammatory phenotype (Paukkeri et al., 2016).
Putting the emerging literature on metal nanoparticles into a historical perspective may be helpful. Over the course of the past two centuries, the toxicity of many metals was not noticed until they had been in use for decades. Examples include the use of mercury salts in manufacture of wool felt for hats in the 19th century, and the use of lead additives in gasoline. Tetraethyl lead was added to gasoline beginning in the 1920’s to increase the octane of gasoline and prevent engine “knocking;” it was not removed from gasoline in the United States until the late 1970’s. Similarly, lead oxide in house paint was not banned until 1978. A more recent example is the discovery of the toxicity of gadolinium, which is used as a contrast agent for magnetic resonance imaging (MRI). Gadolinium contrast agents were first used in human is 1988, and it was not until 12 years later that a skin condition similar to scleromyxoedema was recognized in patients with kidney failure receiving hemodialysis (Cowper et al., 2000). It was not until 2006 that the link was made between this skin condition and gadolinium (Grobner, 2006; Larson et al., 2015; Swaminathan et al., 2007). The name of the condition was changed to nephrogenic systemic fibrosis after recognition of multi-organ involvement. In other words, discovery of the link between gadolinium administration and this condition took 18 years. Therefore, if there are unexpected toxicities from metal nanoparticles, these adverse effects might not be discovered right away and might require careful detective work to establish a connection. It appears that more research on potential toxicities of metal nanoparticles in sorely needed.
VII. Summary
Enthusiasm for metal nanoparticles as potential treatments for infection, or as immunomodulators, surged in recent years, with almost 6000 publications in the biomedical literature in 2016. While many different metal nanoparticles have shown promise in many different assays, in most cases the metal nanoparticles were not compared to non-metal nanoparticles of similar size, or to conventional formulations of the same metal, such as the corresponding metal salt (iron oxide or iron sulfate, for example). In addition, most of the reports have been conducted solely in vitro. As a result, possible host toxicities of metal nanoparticles could not have been detected in most of the published studies. As stated above, however, the potential for adverse effects appears real based on the few studies that were capable of detecting them. As the field of nanoparticles matures, more emphasis will need to be placed on determining safety, including tracking the migration of particles to non-intended organs, tissues, and body sites. Only with these additional studies will the possibilities of metal nanoparticles be able to converted into actual therapies for humans, animals, and possibly even in plants.
Funding:
The author has received grant support from the National Institutes of Health, NIAID, for work on zinc on the SOS response in bacteria, via grant R21 AI 145836. This work on zinc is not specifically focused on nanoparticles, however.
Footnotes
Disclosure: The author has no competing interests or conflicts of interest to declare.
References:
- Ahmed KBA, Raman T, and Veerappan A. 2018. Jacalin capped platinum nanoparticles confer persistent immunity against multiple Aeromonas infection in zebrafish. Scientific reports. 8:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansari MA, Khan HM, Khan AA, Sultan A, and Azam A. 2012. Synthesis and characterization of the antibacterial potential of ZnO nanoparticles against extended-spectrum beta-lactamases-producing Escherichia coli and Klebsiella pneumoniae isolated from a tertiary care hospital of North India. Applied Microbiology & Biotechnology. 94:467–477. [DOI] [PubMed] [Google Scholar]
- Arakha M, Pal S, Samantarrai D, Panigrahi TK, Mallick BC, Pramanik K, Mallick B, and Jha S. 2015. Antimicrobial activity of iron oxide nanoparticle upon modulation of nanoparticle-bacteria interface. Scientific reports. 5:14813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babushkina IV, Gladkova EV, Belova SV, and Norkin IA. 2017. Application of Preparations Containing Copper Nanoparticles for the Treatment of Experimental Septic Wounds. Bulletin of Experimental Biology & Medicine. 164:162–164. [DOI] [PubMed] [Google Scholar]
- Bellinger DC 2016. Lead contamination in Flint—an abject failure to protect public health. New England Journal of Medicine. 374:1101–1103. [DOI] [PubMed] [Google Scholar]
- Boman HG, Agerberth B, and Boman A. 1993. Mechanisms of action on Escherichia coli of cecropin P1 and PR-39, two antibacterial peptides from pig intestine. Infection and immunity. 61:2978–2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernousova S, and Epple M. 2013. Silver as antibacterial agent: ion, nanoparticle, and metal. Angewandte Chemie International Edition. 52:1636–1653. [DOI] [PubMed] [Google Scholar]
- Chithrani BD, Ghazani AA, and Chan WC. 2006. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano letters. 6:662–668. [DOI] [PubMed] [Google Scholar]
- Cho W-S, Duffin R, Howie SE, Scotton CJ, Wallace WA, MacNee W, Bradley M, Megson IL, and Donaldson K. 2011. Progressive severe lung injury by zinc oxide nanoparticles; the role of Zn 2+ dissolution inside lysosomes. Particle and fibre toxicology. 8:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi HS, Ashitate Y, Lee JH, Kim SH, Matsui A, Insin N, Bawendi MG, Semmler-Behnke M, Frangioni JV, and Tsuda A. 2010. Rapid translocation of nanoparticles from the lung airspaces to the body. Nature biotechnology. 28:1300–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coates AL 2008. Guiding aerosol deposition in the lung. New England Journal of Medicine. 358:304–305. [DOI] [PubMed] [Google Scholar]
- Cowper SE, Robin HS, Steinberg SM, Su LD, Gupta S, and LeBoit PE. 2000. Scleromyxoedema-like cutaneous diseases in renal-dialysis patients. The Lancet. 356:1000–1001. [DOI] [PubMed] [Google Scholar]
- Dakterzada F, Mohabati Mobarez A, Habibi Roudkenar M, and Mohsenifar A. 2016. Induction of humoral immune response against Pseudomonas aeruginosa flagellin(1–161) using gold nanoparticles as an adjuvant. Vaccine. 34:1472–1479. [DOI] [PubMed] [Google Scholar]
- Dong J, Vylkova S, Li X, and Edgerton M. 2003. Calcium blocks fungicidal activity of human salivary histatin 5 through disruption of binding with Candida albicans. Journal of dental research. 82:748–752. [DOI] [PubMed] [Google Scholar]
- Elbehiry A, Al-Dubaib M, Marzouk E, and Moussa I. 2019. Antibacterial effects and resistance induction of silver and gold nanoparticles against Staphylococcus aureus-induced mastitis and the potential toxicity in rats. MicrobiologyOpen. 8:e00698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falconer JL, Alt JA, and Grainger DW. 2018. Comparing ex vivo and in vitro translocation of silver nanoparticles and ions through human nasal epithelium. Biomaterials. 171:97–106. [DOI] [PubMed] [Google Scholar]
- Falconer JL, and Grainger DW. 2018. In vivo comparisons of silver nanoparticle and silver ion transport after intranasal delivery in mice. Journal of Controlled Release. 269:1–9. [DOI] [PubMed] [Google Scholar]
- Fifis T, Gamvrellis A, Crimeen-Irwin B, Pietersz GA, Li J, Mottram PL, McKenzie IF, and Plebanski M. 2004. Size-dependent immunogenicity: therapeutic and protective properties of nano-vaccines against tumors. The Journal of Immunology. 173:3148–3154. [DOI] [PubMed] [Google Scholar]
- Frawley ER, Karlinsey JE, Singhal A, Libby SJ, Doulias PT, Ischiropoulos H, and Fang FC. 2018. Nitric Oxide Disrupts Zinc Homeostasis in Salmonella enterica Serovar Typhimurium. mBio. 9:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furchgott RF 1996. The discovery of endothelium-derived relaxing factor and its importance in the identification of nitric oxide. Jama. 276:1186–1188. [PubMed] [Google Scholar]
- Gause KT, Wheatley AK, Cui J, Yan Y, Kent SJ, and Caruso F. 2017. Immunological principles guiding the rational design of particles for vaccine delivery. ACS nano. 11:54–68. [DOI] [PubMed] [Google Scholar]
- Gregory A, Williamson D, and Titball R. 2013. Vaccine delivery using nanoparticles. Frontiers in Cellular and Infection Microbiology. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grobner T 2006. Gadolinium–a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrology Dialysis Transplantation. 21:1104–1108. [DOI] [PubMed] [Google Scholar]
- Habash MB, Goodyear MC, Park AJ, Surette MD, Vis EC, Harris RJ, and Khursigara CM. 2017. Potentiation of tobramycin by silver nanoparticles against Pseudomonas aeruginosa biofilms. Antimicrobial agents and chemotherapy. 61:e00415–00417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habash MB, Park AJ, Vis EC, Harris RJ, and Khursigara CM. 2014. Synergy of silver nanoparticles and aztreonam against Pseudomonas aeruginosa PAO1 biofilms. Antimicrobial Agents & Chemotherapy. 58:5818–5830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hau SJ, Frana T, Sun J, Davies PR, and Nicholson TL. 2017. Zinc Resistance within Swine-Associated Methicillin-Resistant Staphylococcus aureus Isolates in the United States Is Associated with Multilocus Sequence Type Lineage. Applied & Environmental Microbiology. 83:01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Delgadillo R, Velasco-Arias D, Martinez-Sanmiguel JJ, Diaz D, Zumeta-Dube I, Arevalo-Nino K, and Cabral-Romero C. 2013. Bismuth oxide aqueous colloidal nanoparticles inhibit Candida albicans growth and biofilm formation. International Journal of Nanomedicine. 8:1645–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai T, Yoshioka Y, Izumi N, Ichihashi K.-i., Handa T, Nishijima N, Uemura E, Sagami K.-i., Takahashi H, and Yamaguchi M. 2016. Metal nanoparticles in the presence of lipopolysaccharides trigger the onset of metal allergy in mice. Nature nanotechnology. 11:808. [DOI] [PubMed] [Google Scholar]
- Hou J, Liu H, Wang L, Duan L, Li S, and Wang X. 2018. Molecular Toxicity of Metal Oxide Nanoparticles in Danio rerio. Environmental Science & Technology. 52:7996–8004. [DOI] [PubMed] [Google Scholar]
- Huang L, Dai T, Xuan Y, Tegos GP, and Hamblin MR. 2011. Synergistic combination of chitosan acetate with nanoparticle silver as a topical antimicrobial: efficacy against bacterial burn infections. Antimicrobial Agents & Chemotherapy. 55:3432–3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwalokun BA, Akinloye O, Udoh BE, and Akinyemi KO. 2019. Efficacy of silver nanoparticles against multidrug resistant clinical Staphylococcus aureus isolates from Nigeria. Journal of Immunoassay & Immunochemistry. 40:214–236. [DOI] [PubMed] [Google Scholar]
- Jasim R, Schneider EK, Han M, Azad MA, Hussein M, Nowell C, Baker MA, Wang J, Li J, and Velkov T. 2017. A fresh shine on cystic fibrosis inhalation therapy: antimicrobial synergy of polymyxin B in combination with silver nanoparticles. Journal of biomedical nanotechnology. 13:447–457. [DOI] [PubMed] [Google Scholar]
- Koshlukova SE, Lloyd TL, Araujo MW, and Edgerton M. 1999. Salivary histatin 5 induces non-lytic release of ATP from Candida albicans leading to cell death. Journal of Biological Chemistry. 274:18872–18879. [DOI] [PubMed] [Google Scholar]
- Lai X, Zhao H, Zhang Y, Guo K, Xu Y, Chen S, and Zhang J. 2018. Intranasal delivery of copper oxide nanoparticles induces pulmonary toxicity and fibrosis in C57BL/6 mice. Scientific reports. 8:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson KN, Gagnon AL, Darling MD, Patterson JW, and Cropley TG. 2015. Nephrogenic Systemic Fibrosis Manifesting a Decade After Exposure to Gadolinium. JAMA Dermatology. 151:1117–1120. [DOI] [PubMed] [Google Scholar]
- Lee J-Y, Boman A, Sun C, Andersson M, Jörnvall H, Mutt V, and Boman HG. 1989. Antibacterial peptides from pig intestine: isolation of a mammalian cecropin. Proceedings of the National Academy of Sciences. 86:9159–9162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao S, Zhang Y, Pan X, Zhu F, Jiang C, Liu Q, Cheng Z, Dai G, Wu G, Wang L, and Chen L. 2019. Antibacterial activity and mechanism of silver nanoparticles against multidrug-resistant Pseudomonas aeruginosa. International Journal of Nanomedicine. 14:1469–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano T, Rey M, Rojas E, Moya S, Fleddermann J, Estrela-Lopis I, Donath E, Wang B, Mao Z, and Gao C. 2011. Cytotoxicity effects of metal oxide nanoparticles in human tumor cell lines In Journal of Physics: Conference Series. Vol. 304 IOP Publishing; 012046. [Google Scholar]
- Mchugh GL, Moellering R, Hopkins C, and Swartz M. 1975. Salmonella typhimurium resistant to silver nitrate, chloramphenicol, and ampicillin: A new threat in burn units? The Lancet. 305:235–240. [DOI] [PubMed] [Google Scholar]
- Mohan P, and Mala R. 2019. Comparative antibacterial activity of magnetic iron oxide nanoparticles synthesized by biological and chemical methods against poultry feed pathogens. Materials Research Express. 6:115077. [Google Scholar]
- Moore AJ, Beazley WD, Bibby MC, and Devine DA. 1996. Antimicrobial activity of cecropins. Journal of Antimicrobial Chemotherapy. 37:1077–1089. [DOI] [PubMed] [Google Scholar]
- Murad F 2006. Nitric oxide and cyclic GMP in cell signaling and drug development. New England Journal of Medicine. 355:2003–2011. [DOI] [PubMed] [Google Scholar]
- Orlowski P, Tomaszewska E, Gniadek M, Baska P, Nowakowska J, Sokolowska J, Nowak Z, Donten M, Celichowski G, Grobelny J, and Krzyzowska M. 2014. Tannic acid modified silver nanoparticles show antiviral activity in herpes simplex virus type 2 infection. PLoS ONE [Electronic Resource]. 9:e104113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Outten C, and O’Halloran T. 2001. Femtomolar sensitivity of metalloregulatory proteins controlling zinc homeostasis. Science. 292:2488–2491. [DOI] [PubMed] [Google Scholar]
- Pant J, Goudie MJ, Hopkins SP, Brisbois EJ, and Handa H. 2017. Tunable Nitric Oxide Release from S-Nitroso-N-acetylpenicillamine via Catalytic Copper Nanoparticles for Biomedical Applications. Acs Applied Materials & Interfaces. 9:15254–15264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paukkeri E-L, Korhonen R, Hämäläinen M, Pesu M, Eskelinen A, Moilanen T, and Moilanen E. 2016. The inflammatory phenotype in failed metal-on-metal hip arthroplasty correlates with blood metal concentrations. PloS one. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon WL, Alenius H, Ndika J, Fortino V, Kolhinen V, Mesceriakovas A, Wang M, Greco D, Lahde A, Jokiniemi J, Lee JC, El-Nezami H, and Karisola P. 2017. Nano-sized zinc oxide and silver, but not titanium dioxide, induce innate and adaptive immunity and antiviral response in differentiated THP-1 cells. Nanotoxicology. 11:936–951. [DOI] [PubMed] [Google Scholar]
- Rolim WR, Pieretti JC, Reno DLS, Lima BA, Nascimento MHM, Ambrosio FN, Lombello CB, Brocchi M, de Souza ACS, and Seabra AB. 2019. Antimicrobial Activity and Cytotoxicity to Tumor Cells of Nitric Oxide Donor and Silver Nanoparticles Containing PVA/PEG Films for Topical Applications. Acs Applied Materials & Interfaces. 11:6589–6604. [DOI] [PubMed] [Google Scholar]
- Roy R, Kumar S, Verma AK, Sharma A, Chaudhari BP, Tripathi A, Das M, and Dwivedi PD. 2014. Zinc oxide nanoparticles provide an adjuvant effect to ovalbumin via a Th2 response in Balb/c mice. International Immunology. 26:159–172. [DOI] [PubMed] [Google Scholar]
- Ruden S, Hilpert K, Berditsch M, Wadhwani P, and Ulrich AS. 2009. Synergistic interaction between silver nanoparticles and membrane-permeabilizing antimicrobial peptides. Antimicrobial agents and chemotherapy. 53:3538–3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selim A, Elhaig MM, Taha SA, and Nasr EA. 2018. Antibacterial activity of silver nanoparticles against field and reference strains of Mycobacterium tuberculosis, Mycobacterium bovis and multiple-drug-resistant tuberculosis strains. Revue Scientifique et Technique. 37:823–830. [DOI] [PubMed] [Google Scholar]
- Shaikh S, Nazam N, Rizvi SMD, Ahmad K, Baig MH, Lee EJ, and Choi I. 2019. Mechanistic Insights into the Antimicrobial Actions of Metallic Nanoparticles and Their Implications for Multidrug Resistance. International Journal of Molecular Sciences. 20:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakibaie M, Forootanfar H, Ameri A, Adeli-Sardou M, Jafari M, and Rahimi HR. 2018. Cytotoxicity of biologically synthesised bismuth nanoparticles against HT-29 cell line. IET Nanobiotechnology IET. 12:653–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M, Kumar M, Kalaivani R, Manikandan S, and Kumaraguru AK. 2013. Metallic silver nanoparticle: a therapeutic agent in combination with antifungal drug against human fungal pathogen. Bioprocess & Biosystems Engineering. 36:407–415. [DOI] [PubMed] [Google Scholar]
- Singh R, Vora J, Nadhe SB, Wadhwani SA, Shedbalkar UU, and Chopade BA. 2018. Antibacterial Activities of Bacteriagenic Silver Nanoparticles Against Nosocomial Acinetobacter baumannii. Journal of Nanoscience & Nanotechnology. 18:3806–3815. [DOI] [PubMed] [Google Scholar]
- Singha S, Shao K, Ellestad KK, Yang Y, and Santamaria P. 2018. Nanoparticles for immune stimulation against infection, cancer, and autoimmunity. ACS nano. 12:10621–10635. [DOI] [PubMed] [Google Scholar]
- St Croix CM, Wasserloos KJ, Dineley KE, Reynolds IJ, Levitan ES, and Pitt BR. 2002. Nitric oxide-induced changes in intracellular zinc homeostasis are mediated by metallothionein/thionein. American Journal of Physiology - Lung Cellular & Molecular Physiology. 282:L185–192. [DOI] [PubMed] [Google Scholar]
- Swaminathan S, Horn TD, Pellowski D, Abul-Ezz S, Bornhorst JA, Viswamitra S, and Shah SV. 2007. Nephrogenic systemic fibrosis, gadolinium, and iron mobilization. New England Journal of Medicine. 357:720–722. [DOI] [PubMed] [Google Scholar]
- Szymanska E, Orlowski P, Winnicka K, Tomaszewska E, Baska P, Celichowski G, Grobelny J, Basa A, and Krzyzowska M. 2018. Multifunctional Tannic Acid/Silver Nanoparticle-Based Mucoadhesive Hydrogel for Improved Local Treatment of HSV Infection: In Vitro and In Vivo Studies. International Journal of Molecular Sciences. 19:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talukder P, Satho T, Irie K, Sharmin T, Hamady D, Nakashima Y, Kashige N, and Miake F. 2011. Trace metal zinc stimulates secretion of antimicrobial peptide LL-37 from Caco-2 cells through ERK and p38 MAP kinase. International immunopharmacology. 11:141–144. [DOI] [PubMed] [Google Scholar]
- Thompson EA, Sayers BC, Glista-Baker EE, Shipkowski KA, Taylor AJ, and Bonner JC. 2014. Innate immune responses to nanoparticle exposure in the lung. Journal of environmental immunology and toxicology. 1:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadalasetty KP, Lauridsen C, Engberg RM, Vadalasetty R, Kutwin M, Chwalibog A, and Sawosz E. 2018. Influence of silver nanoparticles on growth and health of broiler chickens after infection with Campylobacter jejuni. BMC Veterinary Research [Electronic Resource]. 14:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega-Jimenez AL, Almaguer-Flores A, Flores-Castaneda M, Camps E, Uribe-Ramirez M, Aztatzi-Aguilar OG, and De Vizcaya-Ruiz A. 2017. Bismuth subsalicylate nanoparticles with anaerobic antibacterial activity for dental applications. Nanotechnology. 28:435101. [DOI] [PubMed] [Google Scholar]
- Vila L, Garcia-Rodriguez A, Marcos R, and Hernandez A. 2018. Titanium dioxide nanoparticles translocate through differentiated Caco-2 cell monolayers, without disrupting the barrier functionality or inducing genotoxic damage. Journal of Applied Toxicology. 38:1195–1205. [DOI] [PubMed] [Google Scholar]
- Wang C, Liu X, Han Z, Zhang X, Wang J, Wang K, Yang Z, and Wei Z. 2019. Nanosilver induces the formation of neutrophil extracellular traps in mouse neutrophil granulocytes. Ecotoxicology & Environmental Safety. 183:109508. [DOI] [PubMed] [Google Scholar]
- Wikipedia_contributors. 14 October 2019. Iron oxide nanoparticle. Vol. 2019 Wikipedia, The Free Encyclopedia. [Google Scholar]
- Xie Y, He Y, Irwin PL, Jin T, and Shi X. 2011. Antibacterial activity and mechanism of action of zinc oxide nanoparticles against Campylobacter jejuni. Appl Environ Microbiol. 77:2325–2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Wei MT, Ou-Yang HD, Walker SG, Wang HZ, Gordon CR, Guterman S, Zawacki E, Applebaum E, Brink PR, Rafailovich M, and Mironava T. 2016. Exposure to TiO2 nanoparticles increases Staphylococcus aureus infection of HeLa cells. Journal of Nanobiotechnology. 14:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zasloff M 1987. Magainins, a class of antimicrobial peptides from Xenopus skin: isolation, characterization of two active forms, and partial cDNA sequence of a precursor. Proceedings of the National Academy of Sciences. 84:5449–5453. [DOI] [PMC free article] [PubMed] [Google Scholar]


