Abstract
Expression of cytokines/chemokines is tightly regulated at the transcription level. This is crucial in the central nervous system to maintain neuroimmune homeostasis. IL-8 a chemoattractant, which recruits neutrophils, T cells, and basophils into the brain in response to inflammation and/or injury is secreted predominantly by neurons, microglia, and astrocytes. Here, we investigated the mechanism by which astrocytes regulate IL-8 expression. We demonstrate that while β-catenin negatively regulated IL-8 transcription, its canonical transcriptional partners, members of the TCF/LEF transcription factors (TCF1, TCF3, TCF4 and LEF1) and Activating transcription factor 2 (ATF2) positively regulated IL-8 transcription. We further identified a putative TCF/LEF binding site at −175nt close to the minimal transcription region on the IL-8 promoter, mutation of which caused a significant reduction in IL-8 promoter activity. Chromatin immunoprecipitation demonstrated binding of TCF1, TCF4, LEF1 and ATF2 on the IL-8 promoter suggesting that TCFs/LEF partner with ATF2 to induce IL-8 transcription. These findings demonstrate a novel role for β-catenin in suppression of IL-8 expression and for TCFs/LEF/ATF2 in inducing IL-8. These findings reveal a unique mechanism by which astrocytes tightly regulate IL-8 expression.
Keywords: Human astrocytes, β-catenin, TCFs/LEF, IL-8, ATF2, neuroinflammation
Graphical Abstract
Introduction
Chemokines constitute a large family of structurally and functionally related small proteins (8–10 kDa). In addition to their well-established role in the immune system, they are implicated in the maintenance of central nervous system (CNS) homeostasis and as mediators of neuroinflammation, playing an essential role in leukocyte infiltration into the brain. IL-8, in particular, is a proinflammatory CXC family member α type chemokine, which is a chemoattractant for neutrophils, T cells, and basophils upon injury or infection [1–3]. In the brain, elevated IL-8 levels in response to injury recruits neutrophils into the CNS where they degranulate and release chemoattractants for T lymphocytes [4–7] [8, 9]. In addition, IL-8 primes neutrophils for superoxide production along with other neurotoxic molecules such as basophils, which exacerbate neuropathology in context of a number of neurodegenerative diseases [4–7, 9, 10]. IL-8 is secreted by a number of resident brain cells including neurons, microglia, astrocytes, as well as endothelial cells [11–13]. Under physiological conditions, IL-8 level within the CNS is relatively low, estimated at 0–170 pg/mL as measured in the cerebrospinal fluid (CSF) [4]. In several neurologic diseases (e.g. Alzheimer’s Disease (AD) [5], Multiple sclerosis (MS) [6], HIV [2, 14, 15], bacterial meningitis [16–20], severe traumatic brain injuries (TBI) [4, 7]), IL-8 level in CSF is elevated, as high as 8000 pg/ml [4]. High IL-8 levels are also implicated in pain [21, 22], gliomagenesis [23], tumoral angiogenesis [23] and systemic lupus erythematous [24].
At the transcriptional level, IL-8 is regulated by a number of transcriptional factors and pathways, depending on the cell type (e.g. epithelial, endothelial, or leukocyte origin) such as AP-1, c-Jun, MAPK, C/EBP and NF-κB signaling pathways [1, 25–28]. However, regulation of IL-8 at the molecular level in the CNS and in astrocytes in particular is unknown. Given that astrocytes secrete IL-8, that astrocytes are the most abundant cell type in the brain, and that IL-8 regulation needs to be tightly controlled to prevent hyper-activation within the CNS while balancing the need for neuroimmune communication to elicit an appropriate biologic response; we evaluated here the mechanism by which IL-8 is regulated in human astrocytes. We previously found that inhibition of Wnt/β-catenin signaling in astrocytes leads to induction of a senescence associated secretory phenotype (SASP), a collection of cytokines and other secretory factors secreted under cellular senescence. IL-8 is a hallmark feature of SASP. Therefore, we probed the impact of Wnt/β-catenin integrity in astrocytes on IL-8 expression.
The Wnt/β-catenin signaling pathway is an important neuroprotective pathway and its dysregulation is linked to a number of neurodegenerative diseases, including AD, PD, neuroHIV, and psychiatric disorders such as bipolar disorder and depression [29–31]. The pathway is vital to various functions in the CNS ranging from memory consolidation in astrocytes, neurogenesis, neurotransmitter release, to induction of long-term potentiation and depolarization resulting in increased synaptic strengths [30, 32, 33]. Disruption of β-catenin signaling has profound biologic consequences on astrocyte/neuron communication [34, 35], particularly disruption of the glutamate/glutamine cycle and induction of astrocyte senescence [36, 37]. β-catenin is a transcriptional coactivator and the central mediator in the canonical Wnt/β-catenin pathway. Wnt/β-catenin signaling is initiated by 19 small secreted glycoproteins (Wnts) which bind to seven transmembrane receptors (frizzled) and a co-receptor (the low-density lipoprotein receptor related protein (LRP) 5/6). This ligand/receptor binding leads to the destabilization of a β-catenin destruction complex [Axin, Disheveled (DVL), casein kinase-1α (CK-1α), and glycogen synthase kinase-3β (GSK-3β)] and stabilization of β-catenin. Stabilized β-catenin translocates to the nucleus, where it binds to TCFs/LEF transcription factors, displacing co-repressors such as transducing-like enhancer protein (TLE) and histone deacetylases (HDACs) and recruiting additional co-activators (Pygo, Bcl9, and CBP/p300) to regulate target genes [30, 38].
The TCFs/LEF family are part of the high mobility group (HMG) major end point mediators of Wnt/β-catenin pathway, which bind to specific DNA sequences in gene promoters to regulate their cognate gene expression. TCFs/LEF are differentially expressed in cells and tissues [39–41]. TCF1 (encoded by the TCF7 gene) can act as either an activator or repressor of transcription, depending on cell and tissue type [42–44]. LEF1 (encoded by the LEF1 gene) is generally known as an activator of transcription [45, 46]. TCF3 (encoded by the TCF7L1 gene) is the most abundant and is generally known as a repressor for transcription [45, 47–50], while TCF4 (encoded by the TCF7L2 gene) is the most extensively studied member of the family because it is more ubiquitously expressed in many human adult tissues in comparison to TCF1, TCF3, and LEF1 [51]. Like TCF1, TCF4 can act as either a transcriptional activator or repressor for transcription depending on cell and tissue type [43, 44, 52, 53]. Astrocytes are unique in that they have robust Wnt signaling and express all TCFs/LEF and β-catenin [51, 52]. We reveal here that in astrocytes β-catenin and TCFs/LEF regulate IL-8 at the mRNA level and do so by engaging independent pathways for gene regulation. These findings highlight a novel pathway for IL-8 regulation in astrocytes and point to potentially the β-catenin/TCF/LEF pathway as a means by which to regulate IL-8 expression in the CNS.
Materials and Methods
Cell Culture and Reagents:
Normal Human Astrocytes (NHAs) (Lonza, Walkersville, MD) and U138MG astrocytoma cell line (ATCC; Manassas, VA) were maintained and cultured as previously described [36]. Briefly, NHAs were propagated in astrocyte basal media (ABM, Lonza) supplemented with 0.3% heat-inactivated fetal bovine serum (HI-FBS), 30 μL/mL ascorbic acid, 1 μL/mL rhEGF, 1 μL/mL GA-1000 (30 μg/mL gentamicin and 15 μg/mL amphotericin), 2.5 μL/mL insulin, and 10 μL/mL L-glutamine. Passages (1–6) were used in these experiments. U138MG cells were propagated in Dulbecco’s modified eagle’s medium (DMEM; ThermoFisher, Waltham, MA) supplemented with 10% HI-FBS serum (Sigma, St. Louis, MO) and 1% penicillin-streptomycin (ThermoFisher, Waltham, MA). Cells were maintained in a 5% CO2 humidified atmosphere at 37°C. 6-bromoindirubin-3’-oxime (BIO, lot #025M4611V), LiCl (lot #0001402019) and human recombinant TNF-α (H8916, lot #MKCF5139) were purchased from Sigma (St. Louis, MO). Small molecule inhibitors of Smads, LDN-193189 (lot #S261803) and SB525334 (lot #S147602) were purchased from Selleckchem (Houston, TX) and re-suspended in appropriate vehicle and stored per manufacturer’s instructions. A custom-made ELISA kit (HSTCMAG-28SK, Millipore Sigma, Darmstadt, Germany) was procured to measure IL-8 and TNF-α analytes. Data was acquired and analyzed on the FLEXMAP 3D machine (Luminex Corp, Northbrook, IL).
Bioinformatic search for TCF/LEF DNA binding sequences on IL-8 promoter:
The locus of IL-8 gene (CXCL8) on chromosome q4 of human genome was identified by searching on Pubmed in FASTA format. Approximately 1.5kb sequence upstream of transcriptional start site of this gene was identified as putative promoter region. Previously identified promoter elements such as C/EBP, NF-κB, and AP-1 for IL-8 [1, 23] were all found to be present in our putative promoter sequence. The Sequencher program, software version 5.4.6 (Genes Code incorporation, Ann Arbor, MI) was used to predict a TCF/LEF binding site on the IL-8 promoter using the TCF/LEF consensus sequence 5’-CAAAGA-3’.
Plasmid Construction and Site-directed Mutagenesis:
Genomic DNA was extracted from cultured NHAs using a QIAampDNA mini and blood mini kit (Qiagen, Hilden, Germany). A 1.3kb promoter region of IL-8 was amplified using the primers KR9 (F-5’-ctggcctaactggccggtacGGTGTCCTTGGATAAAGAG-3’) and KR10 (R-5’-gaggccagatcttgatatccTACCAAAGCATCAAGAATAG-3’) and inserted into KpnI and XhoI linearized pGL4.12 vector via Gibson Assembly (New England Biolabs, Ipswich, MA). The following PCR conditions were used: initial denaturation at 95 °C for 10 min; followed by 35 cycles of denaturation at 95 °C for 30 sec, annealing at 60 °C for 30 sec, and extension at 72 °C for 1.5 min and a final extension at 72 °C for 10 min using the Q5 hot start Taq polymerase. Recombinant plasmid was subjected to restriction digestion with SfiI and sequencing to confirm the presence of a single copy of insert in proper orientation. This wild type IL-8 plasmid (p576) was subjected to site-directed mutagenesis using the Q5 site-directed mutagenesis kit (New England Biolabs) with primers, (F-5’-TTTAAAGATCctgGAAAACTTTCGTCATACTC-3’ and R-5’-ATAATTTAATTTTAATATACATTTAAAATACTG-3’) to obtain the substitution plasmid at the TCF/LEF binding site (p594). This plasmid was sequenced from both directions to confirm proper substitution.
Plasmid and siRNA transfections:
ON-TARGET plus SMARTpool siRNAs specific for TCF1 (L-019735–00), TCF3 (L-014703–00), TCF4 (L-003816–00), LEF1 (L-015396–00), β-catenin (L-003482–00), C/EBP-β (L-006423–00), C/EBP-δ (L-010453–00), RelA (L-003533–00), ATF2 (L-009871–00), Zeb1 (L-006564–01), and scrambled (D-001810–10) were procured from Dharmacon. A pcDNA plasmid harboring the constitutively active β-catenin gene termed as pABC (S33Y mutation; #19286) or control pcDNA (#10792) was obtained from Addgene (Watertown, MA). Expression vector pCMV6 harboring cDNAs of each TCF/LEF family members were obtained from ABMgood (Richmond, BC, Canada). Transfections of siRNAs at 100nm were performed using Lipofectamine RNAiMax (Invitrogen, Carlsbad, CA) and plasmids at 0.25μg/24 well format with or without the indicated siRNAs were performed using Lipofectamine 3000 (Invitrogen, Carlsbad, CA).
Quantitative real-time PCR:
Total cellular RNA was isolated using the RNeasy mini kit (Qiagen, Hilden, Germany). RNA was digested with DNaseI (Sigma, St. Louis, MO) for 15 min at RT and DNaseI was inactivated at 70 °C for 15 min. cDNA was synthesized using Qscript supermix (Quanta Biosciences, Beverly, MA). Real-time PCR was performed using SSO fast SYBR green supermix (Biorad, Hercules, CA) in a 7900HT fast real-time PCR system (Applied Biosystems, Waltham, MA) using SDS v2.4 software. Reaction conditions were 95 °C for 10 min; followed by 35 cycles of denaturation at 95 °C for 15 sec, annealing and extension at 60 °C for 60 sec and a final extension at 72 °C for 10 min. Melting curve analysis was performed to ensure the amplification of a single product. Primers used are: β-catenin-F-5’-TCTTGCCCTTTGTCCCGCAAATCA-3’ and β-catenin-R-5’-TCCACAAATTGCTGTGTCCCA-3’; IL-8-F-5’-CTTGGCAGCCTTCTTGATTT-3’ and IL-8-R-5’-GGGTGGAAAGGTTTGGAGTATG-3’; MCP-1-F-5’-TCATAGCAGCCACCTTCATTC-3’ and MCP-1-R-5’-CTCTGCACTGAGATCTTCCTATTG-3’; TNF-α-F-5’-CCAGGGACCTCTCTCTAATCA-3’ and TNF-α-R-5’-TCAGCTTGAGGGTTTGCTAC-3’; TCF1-F-5’-AGGCCAAGAAGCCAACCATCAAGA-3 and TCF1-R-5’-ACTCTGCAATGACCTTGGCTCTCA-3’; TCF3-F-5’-TGCAGTGAGCGTGAAATCACCAGT-3’ and TCF3-R-5’-AATGGCTGCACTTTCCTTCAGGGT-3’; TCF4-F-5’-TCGGCAGAGAGGGATTTAGCTGATGT-3’ and TCF4-R-5’-CTTTCCCGGGATTTGTCTCGGAAACT-3’; LEF1-F-5’-AAGCATCCAGATGGAGGCCTCTACAA-3’ and LEF1-R-5’-TGATGTTCTCGGGATGGGTGGAGAAA-3’; C/EBP-β-F-5’-CGCGACAGGGCCAAGAT-3’and C/EBP-β-R-5’-GCTGCTCCACCTTCTTCTG-3’; C/EBP-δ-F-5’– CATCGACTTCAGCGCCTAC-3’ and C/EBP-δ-R-5’-GCCTTGTGATTGCTGTTGAAG-3’; RelA-F-5’-TGGGAATCCAGTGTGTGAAG-3’ and RelA-R-5’-CACAGCATTCAGGTCGTAGT-3’; ATF2-F-5’- GTCATGGTAGCGGATTGGTTAG-3’ and ATF2-R-5’-CGGAGTTTCTGTAGTGGATGTG-3’; Zeb1-F-5’-CCCAGGACAGCACAGTAAAT-3’ and Zeb1-R-5’-GATGGTGTACTACTTCTGGAACC-3’; ID1-F-5’-CGACATGAACGGCTGTTACTC-3’ and ID1-R-5’-GGTCCCTGATGTAGTCGATGA-3’; PAI-F-5’-CTGGTGAATGCCCTCTACTTC-3’ and PAIR-5’-TGCTGCCGTCTGATTTGT-3’; and GAPDH-F-5’-TGACTTCAACAGCGACACCCACT-3’ and GAPDH-R-5’-ACCACCCTGTTGCTGTAGCCAAAT-3’. Fold change in mRNA expression was calculated by relative quantification using the comparative CT method with GAPDH as the endogenous control.
Chromatin Immunoprecipitation (ChIP):
ChIP was performed using the Magna Chromatin Immunoprecipitation kit (Millipore) with antibodies for β-catenin (anti-Rabbit, Sigma-Aldrich; #C2206), TCFs/LEF Family Antibody Sampler Kit (Cell Signaling, #9383T), ATF2 (Cell Signaling, #D4L2X) and Rabbit IgG control (Cell Signaling, #3900). Per immunoprecipitation, ~1–2 × 106 cells and 5μg of antibody was used. ChIP DNA was processed as manufacturer’s protocol and analyzed by qPCR as indicated above. Primers used to amplify IL-8 promoter region were, F-5’-ACCAAATTGTGGAGCTTCAGT-3’ and R-5’-CCTGAGTCATCACACTTCCTATTT-3’. Data is normalized to IgG and shown as fold change with respect to IgG.
Western blot and Luciferase assay:
For western blot, cells were lysed with RIPA buffer to extract cellular proteins and total protein content was measured by bicinchonic acid (BCA) assay (Bio-Rad, Des Plaines, IL). Ten to twenty micrograms of total cell lysate was separated by 10% SDS-PAGE, transferred onto a nitrocellulose membrane, blocked with superblock (ThermoFisher) containing 0.1% Tween 20 (T20) for 1h, incubated with primary antibody for 1h at RT for β-catenin (1:10,000; Rabbit; Sigma, #C2206) or Glyceraldehyde 3-phosphate dehydrogenase (GAPDH, Rabbit, Sigma, #G9545) overnight at 4°C in superblock-0.1% T20. Membranes were washed extensively in TBS-T20 and incubated with secondary antibody conjugated to horseradish peroxidase (HRP) (1:50,000; Cell Signaling, #7074) in superblock-0.1% T20 for 45 min at RT. Membranes were again washed extensively in TBS-T20 and developed with supersignal west femto maximum sensitivity substrate (ThermoFisher) according to the instructions. For luciferase assay, cells were harvested and lysed with passive lysis buffer by incubating at 37 °C for 10 min followed by pipetting several times. Lysate was spun at 5,000 rpm for 4 min to remove debris, and 20 μl was used to measure luciferase activity using firefly luciferase assay system (Promega, E1500, Madison, WI) in a single-injector luminometer. Total protein content was measured using BCA and relative light units were normalized to μg/ml of protein.
Statistical analysis:
Statistical analyses were performed with consultation of Rush statistical core using Prism software (GraphPad Prism, San Diego, CA). The variables were compared using either the two-tailed one sample T-test or the one-way ANOVA with the post hoc Dunnett’s on the data. p ≤0.05 was considered significant. All experiments were performed independently at least three times.
Results
β-catenin negatively regulates IL-8 transcription in human astrocytes:
We assessed mRNA expression levels of cytokines/chemokines expressed by astrocytes, namely IL-8, MCP-1, and TNF-α. qPCR analysis of cellular RNA from astrocytes seeded at 90% confluency for 24h demonstrated detectable levels of mRNAs for IL-8 and MCP-1, while TNF-α was undetected (Fig. 1a). We next assessed the role of β-catenin in regulation of these cytokines/chemokines by performing loss- and gain-of-function studies. siRNA mediated knockdown (KD) of β-catenin resulted in~ 90% loss in β-catenin protein expression (Fig. 1b), consistent with our previous studies [36, 54]. KD of β-catenin led to an induction in IL-8 mRNA and IL-8 protein at 2–2.5-fold and 1.3–1.5 fold, measured by qPCR and ELISA, respectively (Figs. 1b, c). Conversely, gain-of function studies using pharmacologic agents (BIO and LiCl) which induce β-catenin [55, 56] resulted in ≥50% reduction in IL-8 mRNA (Fig. 1d) and a ≥40% reduction in secreted IL-8 protein (Figs. 1e, f). A dose response study with LiCl indicated a significant inhibition of IL-8 mRNA at 5mM and 10mM concentrations (Suppl. Fig. 1). BIO on the other hand, had a narrow window for effects on β-catenin and doses ≥ 2μM were toxic to the cells. Loss and gain-of- function studies had no impact on MCP-1 and TNF-α (Figs. 1b, d). Finally, densitometry analysis on the western blots of GAPDH under β-catenin KD or induction using pharmacological agents indicated no significant changes in its expression demonstrating that GAPDH is a suitable control in these cells (Suppl. Figs 1b, c). Together, these studies indicate that β-catenin negatively regulates IL-8 transcription in astrocytes.
Figure 1: β-catenin negatively regulates IL-8 transcription in human astrocytes.

a) Human astrocytes were evaluated for endogenous expression of IL-8, MCP-1, TNF-α, and GAPDH mRNA by real-time PCR after 48h. b) Astrocytes were transfected with β-catenin or scrambled (scrm) siRNA and at 48hrs, β-catenin and IL-8 mRNA levels were measured by real-time PCR. Efficacy of KD of β-catenin protein is shown by Western blot within the graph. *indicates p≤0.05 in comparison to scrambled siRNA. c) NHAs were transfected with β-catenin or scrambled (scrm) siRNA and at 48hrs, IL-8 protein in the supernatant was measured by ELISA. d) Astrocytes were either treated with activators of β-catenin (0.5μM BIO or 5mM LiCl) or left untreated and at 24h, mRNA levels were measured by real-time PCR. e and f) IL-8 protein in the supernatant was measured by ELISA *indicates p≤0.05 in comparison to respective control. The data is presented as mean ± SEM (n ≥3, one sample T-Test).
TCFs/LEF positively regulate IL-8 mRNA expression in human astrocytes.
To define the mechanism by which β-catenin regulates IL-8 transcription, we evaluated the impact of β-catenin transcriptional partners (TCF1, TCF3, TCF4 and LEF1) on IL-8 gene transcription. Astrocytes express all four TCFs/LEF mRNAs, albeit at a lower level than the house keeping gene GAPDH [51, 52]. Efficiency of siRNA KD of individual TCFs/LEF family members is ≥65% (Fig. 2a). Individual KD of a single TCFs/LEF using these siRNA did not impact the expression of the others, as we recently demonstrated [54]. Knockdown of TCF1, TCF3, and LEF1 reduced IL-8 mRNA by >70% (Fig. 2b). Although not statistically significant, TCF4 KD showed a trending reduction (~50%) in IL-8 mRNA. Conversely, over expression of TCFs/LEF using individual cDNA plasmids led to ~2.5–3-fold induction in IL-8mRNA (Fig. 2c). These data demonstrate that TCF1, TCF3, and LEF1 positively regulate IL-8 expression in human astrocytes, pointing to a discordant effect of β-catenin and TCFs/LEF on IL-8 transcription.
Figure 2: TCFs/LEF positively regulate IL-8 expression in astrocytes.

Astrocytes were transfected with TCF1, TCF3, TCF4, LEF1, or scrambled (scrm) siRNA and at 48h a) TCF1, TCF3, TCF4, and LEF1 mRNA levels measured by real-time PCR and normalized to GAPDH. b) mRNA levels of IL-8 measured by real-time PCR and normalized to GAPDH. c) Astrocytes were transfected with TCF1, TCF3, TCF4, LEF1, or pcDNA (control) expression plasmids and at 24h, IL-8 mRNA expression was measured by real-time PCR. * indicates p≤0.05 in comparison to respective control. The data is presented as mean ± SEM (n ≥3, one-way ANOVA, post hoc Dunnett’s test).
Identification and characterization of a functional binding site of TCFs/LEF in the IL-8 promoter:
Given that TCFs/LEF are transcription factors that bind at a specific location on the promoter region of their target gene to regulate gene transcription, we assessed the interaction of TCFs/LEF on the IL-8 promoter. First, we identified a 1.3kb region upstream of IL-8 gene (transcription start site) in the human genome by Pubmed search as a putative promoter region for IL-8. Next, by bioinformatic analysis using Sequencher 5.4.6, we identified previously characterized promoter elements for IL-8 such as TATA box, binding sites for C/EBP, NF-κB and AP-1 within −150 region of transcription start site (Fig. 3a) [1]. We further found one TCF/LEF binding site on the putative IL-8 promoter at −175 position (Fig. 3a). We amplified and cloned this putative 1.3 kb promoter region into the pGL4.12 reporter vector. Transfection of this promoter reporter plasmid (p576) along with its control vector (p501) into astrocytes followed by luciferase assay showed a significantly higher luciferase activity for p576 (~8 folds) compared to p501 suggesting a strong promoter activity for the inserted 1.3kb DNA fragment (Fig. 3b). Further, stimulation of the p576 transfected cells with an IL-8 specific stimulant (TNF-α) resulted in ~1.5-fold induction (p<0.05), suggesting that this promoter insert has specificity to IL-8 gene (Fig. 3c). β-catenin KD of astrocytes transfected with p576 containing sites for TCF/LEF DNA binding induced IL-8 reporter activities by 1.8-fold further confirming that β-catenin negatively regulates IL-8 transcription (Fig. 3d). To address whether the TCFs/LEF site on the IL-8 promoter is relevant to its basal and inducible activity, we performed site-directed mutagenesis by making a CAAAGA to CCTGGA substitution at the TCFs/LEF binding site (Fig. 3e). Transfection of wild type (p576) and substituted (p594) plasmids followed by luciferase assay showed ~50% decrease in IL-8 promoter reporter activity in p594 transfected samples due to TCFs/LEF substitution (Fig. 3f). To further evaluate if loss of TCFs/LEF binding sites on the IL-8 promoter renders it non-responsive to β-catenin, we assessed the transcription activity of p594 plasmid under β-catenin KD by siRNA or β-catenin overexpression through transfection with pABC. No significant changes in the luciferase activity was noted under β-catenin KD or overexpression in comparison to their respective controls demonstrating that β-catenin requires interaction with TCFs/LEF to regulate IL-8 transcription (Suppl. Figs. 2a, b).
Figure 3: Identification and characterization of a functional TCFs/LEF binding site on the IL-8 promoter.

a) Identification of the putative TCF/LEF site located between −175 and −169 on the IL-8 promoter by bioinformatic analysis. b) An astroglioma cell line U138MG, was transfected with p501 (vector control) or IL-8 promoter reporter plasmid (p576) and at 48h luciferase assay was performed. c) U138MG cells transfected with IL-8 promoter plasmid were either unstimulated or stimulated with TNF-α and at 48h luciferase reporter assay was performed. d) NHAs were transfected with β-catenin or scrm siRNA and at 24h, the cells were transfected with p576. At 48h post transfection, luciferase reporter assay was performed. * indicates p≤0.05 in comparison to respective control. e) Wild-type IL-8 reporter plasmid (p576) was mutated by substitution on the TCF/LEF putative binding sites (p594). f) NHAs were transfected with either p576 or p594, 48h post transfection, IL-8 promoter activity was measured through luciferase reporter assay. * indicates p≤0.05 in comparison to respective control. The data is presented as mean ± SEM (n≥3, one sample T-Test).
TCFs/LEF induction of IL-8 is independent of β-catenin and depends on ATF2 transcriptional co-regulator:
Canonical β-catenin signaling involves the binding of β-catenin to TCFs/LEF family members to regulate gene expression. Because we saw a discordant role of β-catenin and TCFs/LEF on IL-8 transcription, we evaluated other transcriptional co-regulators that may be involved in TCFs/LEF induction of IL-8. We assessed the roles of Activating Transcription Factor 2 (ATF2), Caenorhabditis elegans SMA (“small” worm phenotype) and Drosophila MAD (“Mothers Against Decapentaplegic”) family of genes (SMADs), and Zinc finger E-box binding homeobox 1 (Zeb1) on IL-8 transcription. All three may partner with TCFs/LEF to regulate gene expression independent of β-catenin [57–62]. Human astrocytes express ATF2, SMADs, and Zeb1 to levels relative to GAPDH [52]. siRNA KD efficiency of ATF2 and Zeb1 in astrocytes is ≥85% (Figs. 4a, b). KD of ATF2 reduced IL-8 mRNA by >50% (Fig. 4a), while KD of Zeb1 had no effect on IL-8 mRNA (Fig. 4b). To inhibit Smads, we used several small molecule inhibitors and assessed for their efficiency of inhibition by measuring their impact on their respective target genes. SB525334 inhibits Smads 2 and 3 and inhibits its target gene Plasminogen activation inhibitor (PAI). LDN-193189 inhibits Smads 1, 5, and 8/9 and it inhibits Inhibitor of DNA Binding Protein (Id1) (Figs. 4c, d). SMAD inhibitors did not inhibit IL-8 mRNA (Figs. 4c, d). Together, these data indicate that ATF2 positively regulate IL-8 expression in astrocytes and likely does so by partnering with TCFs/LEF family members.
Figure 4: TCFs/LEF induction of IL-8 is independent of β-catenin and depends on ATF2 transcriptional co-regulator.

a) Astrocytes were transfected with siRNA for ATF2, Zeb1, or scrambled (scrm) siRNA (a and b respectively) or treated with Smads 2 and 3 inhibitor (SB525334) or Smads 1, 5, and 8/9 inhibitor (LDN-193189) (c and d respectively). Target gene as indicated was measured at 48h by real-time PCR in the siRNA KD experiments (a, b) and at 24h post small molecule inhibitor experiments (c, d). * indicates p≤0.05 in comparison to respective control. The data is presented as mean ± SEM (n≥3, one sample T-test). * indicates p≤0.05 in comparison to respective control (n≥3, one-way ANOVA, post hoc Dunnett’s).
Tethering of TCFs/LEF family members, ATF2, and β-catenin on IL-8 promoter:
We next determined whether these transcriptional factors and co-regulators are tethered on the IL-8 promoter. We performed chromatin immunoprecipitation (ChIP) assays to assess binding ability of these transcription factors and co-factors at the TCFs/LEF consensus site on the endogenous IL-8 promoter; using their respective antibodies or isotype control followed by qPCR. We show that TCF1, TCF4, LEF1, and ATF2 exhibited strong binding with statistical significance and TCF3 and β-catenin showed an appreciable binding (~5 and ~4 folds, respectively) although not significantly different when compared to IgG (Fig. 5). These studies point to TCF1, TCF4, LEF1, and ATF2 binding to IL-8 TCFs/LEF sites on the IL-8 promoter to positively regulate gene expression. To assess whether ATF2 requires interaction with the TCFs/LEF family, we performed KD of ATF2 followed by a luciferase assay of p594 in NHAs. No change in the luciferase activity of p594 with loss of ATF2 function was observed (Suppl Fig 2b).
Figure 5: Tethering of TCF/LEF, β-catenin, and ATF2 on the IL-8 promoter.

ChIP was performed using 5μg of β-catenin, TCF1, TCF3, TCF4, LEF1, ATF2 or isotype control (IgG) antibodies and DNA amplified spanning the TCF/LEF binding site (−175 to −169nt) on the IL-8 promoter. *indicates p≤0.05 in comparison to isotype control, IgG. The data is presented as mean ± SEM (n=3, one sample T-Test).
C/EBP and NF-κB pathways positively regulate IL-8 transcription:
C/EBP and NF-κB pathways are well documented to positively regulate IL-8 expression in various other cell types [1]. Given that C/EBP and NF-κB binding sites are also present on the IL-8 promoter, we assessed the roles C/EBP and NF-κB signaling in relation to IL-8 gene regulation. Human astrocytes express all NF-κB family members (RelA, RelB, NF-κB1, NF-κB2, c-Rel) and all C/EBP family members (-β, -δ, -γ, –ζ) except α and ε [52]. Knockdown of C/EBP-β, C/EBP-δ and Rel A reduced IL-8 mRNA by 60%, 80%, and 90% respectively (Fig. 6). Previously, we demonstrated that β-catenin KD inhibits C/EBP and NF-κB reporter activities by ~70% while KD of TCFs/LEF had no effect on C/EBP or NF-κB activities [54]. Together, these findings demonstrate that β-catenin independent of TCFs/LEF positively regulates C/EBP and NF-κB, which in turn are known to activate IL-8 expression.
Figure 6: C/EBP and NF-κB pathways positively regulate IL-8 transcription in astrocytes.

Astrocytes were transfected with C/EBP-β, C/EBP-δ, RelA, or scrm siRNA and at 48hrs IL-8 mRNA expression was measured by real-time PCR. * indicates p≤0.05 in comparison to respective control. The data is presented as mean ± SEM (n=3, one-way ANOVA, post hoc Dunnett’s).
Discussion
Cytokine/chemokine expression from resident brain cells is tightly regulated. A break in regulation of these molecules could lead to a cytokine/chemokine storm within the CNS that could be highly neurotoxic. Astrocytes constitute a significant population of resident brain cells and they play a key role in numerous functions within the CNS [63]. Astrocytes secrete a number of cytokines/chemokines including IL-8. Given that IL-8 is linked to a number of neurodegenerative diseases and is a chemotactic factor for neutrophils into the CNS, we evaluated the molecular mechanism by which astrocytes regulate IL-8 expression. We show that β-catenin negatively regulates IL-8 transcription while all TCFs/LEF induce it. β-catenin typically partners with one member of TCFs/LEF to regulate gene expression. In fact, we previously demonstrated that β-catenin partners with TCF4 to inhibit HIV transcription in astrocytes [51], partners with TCF1 to induce Excitatory amino acid transporter 2 and with TCF3 to induce Glutamine Synthetase [36]. Here, we report discordant effects of β-catenin and TCFs/LEF in IL-8 regulation in astrocytes, similar to that reported for IL-6 transcriptional regulation in astrocytes [54], whereby β-catenin is a negative regulator and TCF/LEF are positive regulators. Albeit, in case of IL-6 regulation, TCFs/LEF bind to ATF2 and SMADs [54] whereas, for IL-8 SMADs are not involved.
Our study also demonstrates that all TCFs/LEF exert a positive role on regulation of IL-8 gene expression. Previously, we evaluated the expression of the various TCFs/LEF in NHAs by qPCR and demonstrated that NHAs express all TCF/LEF family members and showed that siRNA for individual TCFs/LEF were highly effective and specific in that they do not cross react with each other [54]. For IL-8 transcriptional control, the uniqueness is that all of these TCF/LEFs play a role. This suggests that perhaps TCF/LEFs can compensate for each other to ensure tight regulation of promoter activity of IL-6 and IL-8 in astrocytes. To our knowledge, there are no studies to date to show involvement of more than one or all four TCFs/LEF in a given gene regulation other than IL-6 [54]. Although, a few double knockout murine models show complimentary roles of TCFs/LEF family members [42, 64]. Our work into how IL-6 and IL-8 are regulated in astrocytes demonstrates involvement of multiple TCFs/LEF on the same target gene [54]. This may underscore the importance of tight regulation of cytokines/chemokines in the CNS, as the consequence of “loose” regulation is detrimental to CNS health.
β-catenin regulation of IL-8 promoter activity is likely context and cell type dependent. We showed here that β-catenin negatively regulates IL-8 transcription in astrocytes. However, in hepatomas and endothelial cells β-catenin positively regulates IL-8 transcription [65, 66]. Further, we showed that β-catenin induces NF-κB and C/EBP-1, two transcriptional factors reported to regulate IL-8 promoter activity in a number of cell types including immune cells, colonic epithelial cells, ovarian cancer cells, hepatocellular carcinomas, and endothelial cells [25, 65–72]. Interestingly, sequences spanning nucleotides −1 to −133 within the IL-8 promoter, which includes binding sites for NF-κB, AP-1 and C/EBP were observed to be important for transcriptional regulation of this gene [25, 26, 68]. One of the previous studies found a TCF/LEF binding site located between −177 and −186 within the IL-8 promoter [65]. By molecular approaches such as loss and gain of function, bioinformatics analysis and site directed mutagenesis of reporter plasmids, we identified a TCFs/LEF binding site on the IL-8 promoter at nt −175, we also showed a positive correlation of all four TCFs/LEFs on IL-8 transcription and a negative relationship between β-catenin and IL-8 transcription in astrocytes. IL-8 promoter is unlike that of IL-6 promoter in relation to TCF/LEF DNA binding sites. IL-8 has one TCF/LEF binding site whereas IL-6 promoter has two [54]. A complex gene regulation of IL-8 emerges where on one hand TCFs/LEF/ATF2 positively regulate IL-8 gene expression, C/EBP-β, -δ, and NF-κB also positively regulate IL-8 expression in human astrocytes, but ironically β-catenin inhibits IL-8 gene expression while inducing C/EBP-β, -δ, and NF-κB.
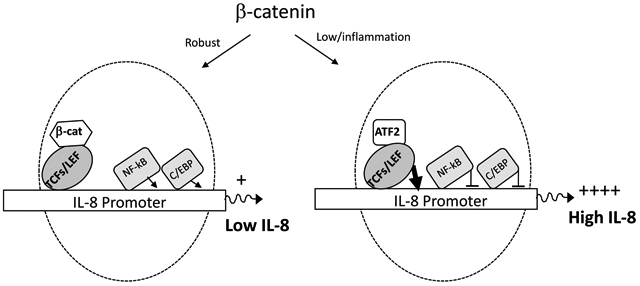
Based on published studies from our group and others demonstrating that: 1) inflammation and/or infection is associated with lower β-catenin signaling [37, 73–76] and 2) β-catenin promotes NF-κB and C/EBP promoter activity [54], a model emerges depicted in the graphical abstract whereby β-catenin may function as a master regulator of IL-8 transcription. Specifically, under homeostatic conditions with “normal/robust” levels of β-catenin, β-catenin binds to TCF/LEF to reduce IL-8 transcriptional activity while promoting IL-8 transcription through NF-κB and C/EBP mediated induction of promoter activity. The net result is a modest IL-8 expression under homeostatic condition. On the other hand, under inflammatory conditions and lower level of β-catenin, β-catenin does not interact with TCF/LEF nor mediates NFkB/C/EBP activity, TCF/LEF interact with ATF2 to induce robust IL-8 expression. Consequently, this would trigger a positive feedback loop to mediate more inflammation through recruitment of neutrophils and leukocytes. Although the co-factors interacting with TCF/LEF may be different, this model is consistent with IL-6 gene regulation in astrocytes [54]. Understanding how astrocytes differentially regulate cytokines/chemokines in the CNS can inform strategies, especially those that exploit β-catenin signaling to fine tune inflammatory responses in the CNS to promote repair, without hyper inflammation that can drive neurodegeneration/injury.
Supplementary Material
Highlights.
β-catenin negatively regulates IL-8 transcription in human astrocytes
A single TCFs/LEF binding site is present on the IL-8 promoter
TCFs/LEF along with ATF2 positively regulate IL-8 transcription in human astrocytes
Disruption of β-catenin may lead to heightened neuroinflammation by inducing IL-8 to mediate recruitment of neutrophils into the CNS.
Acknowledgements
This work was supported by NIH/NINDS R01NS060632–10 (LA) and NIH/NIGM R25 GM109421 (LA).
Abbreviations used
- NHA
normal human astrocytes
- IL-8
interleukin 8
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- C/EBP
CCAAT-enhancer-binding protein
- TCFs/LEF
T cell factor / lymphoid enhancing factor
- CNS
central nervous system
- HAND
HIV-associated neurocognitive disorder
- SASP
senescence associated secretory phenotype
- BIO
6-bromoindirubin-3’-oxime
- TF
transcription factor
- KD
knockdown
- ATF2
activating transcription factor 2
- SMDAD
Caenorhabditis elegans SMA (“small” worm phenotype) and Drosophila MAD (“Mothers Against Decapentaplegic”) family of genes
- ZEB1
zinc finger E-box binding homeobox 1
- PAI
plasminogen activation inhibitor
- EAAT2
excitatory amino acid transporter 2
- GS
glutamine synthetase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Roebuck KA, Regulation of interleukin-8 gene expression. Journal of interferon & cytokine research, 1999. 19(5): p. 429–438. [DOI] [PubMed] [Google Scholar]
- 2.Kutsch O, et al. , Induction of the chemokines interleukin-8 and IP-10 by human immunodeficiency virus type 1 tat in astrocytes. Journal of virology, 2000. 74(19): p. 9214–9221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramesh G, MacLean AG, and Philipp MT, Cytokines and chemokines at the crossroads of neuroinflammation, neurodegeneration, and neuropathic pain. Mediators of inflammation, 2013. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kossmann T, et al. , Interleukin-8 released into the cerebrospinal fluid after brain injury is associated with blood–brain barrier dysfunction and nerve growth factor production. Journal of Cerebral Blood Flow & Metabolism, 1997. 17(3): p. 280–289. [DOI] [PubMed] [Google Scholar]
- 5.Galimberti D, et al. , Intrathecal chemokine synthesis in mild cognitive impairment and Alzheimer disease. Arch Neurol, 2006. 63(4): p. 538–43. [DOI] [PubMed] [Google Scholar]
- 6.Matejčíková Z, et al. , Cerebrospinal fluid and serum levels of interleukin-8 in patients with multiple sclerosis and its correlation with Q-albumin. Multiple sclerosis and related disorders, 2017. 14: p. 12–15. [DOI] [PubMed] [Google Scholar]
- 7.Whalen MJ, et al. , Interleukin-8 is increased in cerebrospinal fluid of children with severe head injury. Crit Care Med, 2000. 28(4): p. 929–34. [DOI] [PubMed] [Google Scholar]
- 8.Taub DD, et al. , T lymphocyte recruitment by interleukin-8 (IL-8). IL-8-induced degranulation of neutrophils releases potent chemoattractants for human T lymphocytes both in vitro and in vivo. The Journal of clinical investigation, 1996. 97(8): p. 1931–1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ehrlich LC, et al. , Cytokine regulation of human microglial cell IL-8 production. The Journal of Immunology, 1998. 160(4): p. 1944–1948. [PubMed] [Google Scholar]
- 10.Metzner B, et al. , Interleukin-8 and GROα prime human neutrophils for superoxide anion production and induce up-regulation of n-formyl peptide receptors. Journal of investigative dermatology, 1995. 104(5): p. 789–791. [DOI] [PubMed] [Google Scholar]
- 11.Flynn G, et al. , Regulation of chemokine receptor expression in human microglia and astrocytes. Journal of neuroimmunology, 2003. 136(1–2): p. 84–93. [DOI] [PubMed] [Google Scholar]
- 12.Murdoch C, Monk PN, and Finn A, Cxc chemokine receptor expression on human endothelial cells. Cytokine, 1999. 11(9): p. 704–712. [DOI] [PubMed] [Google Scholar]
- 13.Murphy PM, et al. , International union of pharmacology. XXII. Nomenclature for chemokine receptors. Pharmacological reviews, 2000. 52(1): p. 145–176. [PubMed] [Google Scholar]
- 14.Hofman FM, et al. , HIV-1 tat protein induces the production of interleukin-8 by human brain-derived endothelial cells. Journal of neuroimmunology, 1999. 94(1–2): p. 28–39. [DOI] [PubMed] [Google Scholar]
- 15.Kamat A, et al. , Monocyte activation markers in cerebrospinal fluid associated with impaired neurocognitive testing in advanced HIV infection. Journal of acquired immune deficiency syndromes (1999), 2012. 60(3): p. 234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spanaus K-S, et al. , CXC and CC chemokines are expressed in the cerebrospinal fluid in bacterial meningitis and mediate chemotactic activity on peripheral blood-derived polymorphonuclear and mononuclear cells in vitro. The Journal of Immunology, 1997. 158(4): p. 1956–1964. [PubMed] [Google Scholar]
- 17.Lopez-Cortes L, et al. , Interleukin-8 in cerebrospinal fluid from patients with meningitis of different etiologies: its possible role as neutrophil chemotactic factor. Journal of Infectious Diseases, 1995. 172(2): p. 581–584. [DOI] [PubMed] [Google Scholar]
- 18.Østergaard C, et al. , Interleukin-8 in cerebrospinal fluid from patients with septic and aseptic meningitis. European Journal of Clinical Microbiology & Infectious Diseases, 1996. 15(2): p. 166–169. [DOI] [PubMed] [Google Scholar]
- 19.Mastroianni CM, et al. , Cerebrospinal fluid interleukin 8 in children with purulent bacterial and tuberculous meningitis. Pediatric Infectious Disease Journal, 1994. 13(11): p. 1008–1010. [PubMed] [Google Scholar]
- 20.Van Meir E, et al. , Interleukin-8 is produced in neoplastic and infectious diseases of the human central nervous system. Cancer research, 1992. 52(16): p. 4297–4305. [PubMed] [Google Scholar]
- 21.Giron SE, et al. , Increased central nervous system interleukin-8 in a majority postlaminectomy syndrome chronic pain population. Pain Medicine, 2017. 19(5): p. 1033–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cui G. b., et al. , Elevated interleukin-8 enhances prefrontal synaptic transmission in mice with persistent inflammatory pain. Molecular pain, 2012. 8(1): p. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brat DJ, Bellail AC, and Van Meir EG, The role of interleukin-8 and its receptors in gliomagenesis and tumoral angiogenesis. Neuro-oncology, 2005. 7(2): p. 122–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang J-B, et al. , Role of IL-1β, IL-6, IL-8 and IFN-γ in pathogenesis of central nervous system neuropsychiatric systemic lupus erythematous. International journal of clinical and experimental medicine, 2015. 8(9): p. 16658. [PMC free article] [PubMed] [Google Scholar]
- 25.Hoffmann E, et al. , Multiple control of interleukin-8 gene expression. Journal of leukocyte biology, 2002. 72(5): p. 847–855. [PubMed] [Google Scholar]
- 26.Mukaida N, et al. , Molecular mechanism of interleukin-8 gene expression. Journal of leukocyte biology, 1994. 56(5): p. 554–558. [PubMed] [Google Scholar]
- 27.Matsusaka T, et al. , Transcription factors NF-IL6 and NF-kappa B synergistically activate transcription of the inflammatory cytokines, interleukin 6 and interleukin 8. Proceedings of the National Academy of Sciences, 1993. 90(21): p. 10193–10197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roux P, et al. , Activation of transcription factors NF-kappaB and NF-IL-6 by human immunodeficiency virus type 1 protein R (Vpr) induces interleukin-8 expression. J Virol, 2000. 74(10): p. 4658–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inestrosa NC, Montecinos-Oliva C, and Fuenzalida M, Wnt signaling: role in Alzheimer disease and schizophrenia. Journal of Neuroimmune Pharmacology, 2012. 7(4): p. 788–807. [DOI] [PubMed] [Google Scholar]
- 30.Al-Harthi L, Wnt/beta-catenin and its diverse physiological cell signaling pathways in neurodegenerative and neuropsychiatric disorders. J Neuroimmune Pharmacol, 2012. 7(4): p. 725–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berwick DC and Harvey K, The importance of Wnt signalling for neurodegeneration in Parkinson’s disease. 2012, Portland Press Limited. [DOI] [PubMed] [Google Scholar]
- 32.Henderson LJ and Al-Harthi L, Role of beta-catenin/TCF-4 signaling in HIV replication and pathogenesis: insights to informing novel anti-HIV molecular therapeutics. J Neuroimmune Pharmacol, 2011. 6(2): p. 247–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park M and Shen K, WNTs in synapse formation and neuronal circuitry. The EMBO journal, 2012. 31(12): p. 2697–2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Budnik V and Salinas PC, Wnt signaling during synaptic development and plasticity. Current opinion in neurobiology, 2011. 21(1): p. 151–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cerpa W, et al. , The role of Wnt signaling in neuroprotection. Drug News Perspect, 2009. 22(10): p. 579–591. [DOI] [PubMed] [Google Scholar]
- 36.Lutgen V, et al. , beta-Catenin signaling positively regulates glutamate uptake and metabolism in astrocytes. J Neuroinflammation, 2016. 13(1): p. 242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu C, et al. , HIV and drug abuse mediate astrocyte senescence in a beta-catenin-dependent manner leading to neuronal toxicity. Aging Cell, 2017. 16(5): p. 956–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Al-Harthi L, Interplay between Wnt/beta-catenin signaling and HIV: virologic and biologic consequences in the CNS. J Neuroimmune Pharmacol, 2012. 7(4): p. 731–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Genderen C, et al. , Development of several organs that require inductive epithelialmesenchymal interactions is impaired in LEF-1-deficient mice. Genes Dev, 1994. 8(22): p. 2691–703. [DOI] [PubMed] [Google Scholar]
- 40.Waterman ML, Fischer WH, and Jones KA, A thymus-specific member of the HMG protein family regulates the human T cell receptor C alpha enhancer. Genes & development, 1991. 5(4): p. 656–669. [DOI] [PubMed] [Google Scholar]
- 41.Oosterwegel M, et al. , Differential expression of the HMG box factors TCF-1 and LEF-1 during murine embryogenesis. Development, 1993. 118(2): p. 439–448. [DOI] [PubMed] [Google Scholar]
- 42.Galceran J, et al. , Wnt3a−/−-like phenotype and limb deficiency in Lef1(−/−)Tcf1(−/−) mice. Genes Dev, 1999. 13(6): p. 709–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roose J, et al. , Synergy between tumor suppressor APC and the beta-catenin-Tcf4 target Tcf1. Science, 1999. 285(5435): p. 1923–6. [DOI] [PubMed] [Google Scholar]
- 44.Tang W, et al. , A genome-wide RNAi screen for Wnt/β-catenin pathway components identifies unexpected roles for TCF transcription factors in cancer. Proceedings of the National Academy of Sciences, 2008. 105(28): p. 9697–9702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu F, et al. , Distinct roles for Xenopus Tcf/Lef genes in mediating specific responses to Wnt/β-catenin signalling in mesoderm development. Development, 2005. 132(24): p. 5375–5385. [DOI] [PubMed] [Google Scholar]
- 46.Travis A, et al. , LEF-1, a gene encoding a lymphoid-specific protein with an HMG domain, regulates T-cell receptor alpha enhancer function [corrected]. Genes & development, 1991. 5(5): p. 880–894. [DOI] [PubMed] [Google Scholar]
- 47.Kim CH, et al. , Repressor activity of Headless/Tcf3 is essential for vertebrate head formation. Nature, 2000. 407(6806): p. 913–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Merrill BJ, et al. , Tcf3: a transcriptional regulator of axis induction in the early embryo. Development, 2004. 131(2): p. 263–74. [DOI] [PubMed] [Google Scholar]
- 49.Cole MF, et al. , Tcf3 is an integral component of the core regulatory circuitry of embryonic stem cells. Genes & development, 2008. 22(6): p. 746–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yi F, et al. , Opposing effects of Tcf3 and Tcf1 control Wnt stimulation of embryonic stem cell self-renewal. Nature cell biology, 2011. 13(7): p. 762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hrckulak D, et al. , TCF/LEF Transcription Factors: An Update from the Internet Resources. Cancers (Basel), 2016. 8(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Korinek V, et al. , Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat Genet, 1998. 19(4): p. 379–83. [DOI] [PubMed] [Google Scholar]
- 53.Nguyen H, et al. , Tcf3 and Tcf4 are essential for long-term homeostasis of skin epithelia. Nature genetics, 2009. 41(10): p. 1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Robinson K, et al. , β-catenin and TCFs/LEF signaling discordantly regulate IL-6 expression in astrocytes. Cell Commun Signal, 2020. 18, p. 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sato N, et al. , Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nature medicine, 2004. 10(1): p. 55. [DOI] [PubMed] [Google Scholar]
- 56.De Ferrari G, et al. , Activation of Wnt signaling rescues neurodegeneration and behavioral impairments induced by β-amyloid fibrils. Molecular psychiatry, 2003. 8(2): p. 195. [DOI] [PubMed] [Google Scholar]
- 57.Sprowl S and Waterman ML, Past Visits Present: TCF/LEFs Partner with ATFs for β-Catenin– Independent Activity. PLoS genetics, 2013. 9(8): p. e1003745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reimold AM, et al. , Decreased immediate inflammatory gene induction in activating transcription factor-2 mutant mice. International immunology, 2001. 13(2): p. 241–248. [DOI] [PubMed] [Google Scholar]
- 59.Grumolato L, et al. , β-Catenin-independent activation of TCF1/LEF1 in human hematopoietic tumor cells through interaction with ATF2 transcription factors. PLoS genetics, 2013. 9(8): p. e1003603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Labbé E, Letamendia A, and Attisano L, Association of Smads with lymphoid enhancer binding factor 1/T cell-specific factor mediates cooperative signaling by the transforming growth factor-β and Wnt pathways. Proceedings of the National Academy of Sciences, 2000. 97(15): p. 8358–8363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yanagisawa M, et al. , Signaling crosstalk underlying synergistic induction of astrocyte differentiation by BMPs and IL-6 family of cytokines. FEBS letters, 2001. 489(2–3): p. 139–143. [DOI] [PubMed] [Google Scholar]
- 62.Rosmaninho P, et al. , Zeb1 potentiates genome-wide gene transcription with Lef1 to promote glioblastoma cell invasion. The EMBO journal, 2018. 37(15). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liddelow SA and Barres BA, Reactive astrocytes: production, function, and therapeutic potential. Immunity, 2017. 46(6): p. 957–967. [DOI] [PubMed] [Google Scholar]
- 64.Gregorieff A, Grosschedl R, and Clevers H, Hindgut defects and transformation of the gastrointestinal tract in Tcf4(−/−)/Tcf1(−/−) embryos. Embo j, 2004. 23(8): p. 1825–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lévy L, et al. , Transcriptional activation of interleukin-8 by β-catenin-Tcf4. Journal of Biological Chemistry, 2002. 277(44): p. 42386–42393. [DOI] [PubMed] [Google Scholar]
- 66.Masckauchan TN, et al. , Wnt/beta-catenin signaling induces proliferation, survival and interleukin-8 in human endothelial cells. Angiogenesis, 2005. 8(1): p. 43–51. [DOI] [PubMed] [Google Scholar]
- 67.Nourbakhsh M, et al. , The NF-κB repressing factor is involved in basal repression and interleukin (IL)-1-induced activation of IL-8 transcription by binding to a conserved NF-κB-flanking sequence element. Journal of Biological Chemistry, 2001. 276(6): p. 4501–4508. [DOI] [PubMed] [Google Scholar]
- 68.Khanjani S, et al. , NFκB and AP-1 drive human myometrial IL8 expression. Mediators of inflammation, 2012. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hoffmann E, et al. , MEK1-dependent delayed expression of Fos-related antigen-1 counteracts c-Fos and p65 NF-κB-mediated interleukin-8 transcription in response to cytokines or growth factors. Journal of Biological Chemistry, 2005. 280(10): p. 9706–9718. [DOI] [PubMed] [Google Scholar]
- 70.Elliott C, et al. , Nuclear factor-kappa B is essential for up-regulation of interleukin-8 expression in human amnion and cervical epithelial cells. MHR: Basic science of reproductive medicine, 2001. 7(8): p. 787–790. [DOI] [PubMed] [Google Scholar]
- 71.Edwards MR, et al., IL-1β induces IL-8 in bronchial cells via NF-κB and NF-IL6 transcription factors and can be suppressed by glucocorticoids. Pulmonary pharmacology & therapeutics, 2005. 18(5): p. 337–345. [DOI] [PubMed] [Google Scholar]
- 72.Lakshminarayanan V, et al. , Differential regulation of interleukin-8 and intercellular adhesion molecule-1 by H2O2 and tumor necrosis factor-α in endothelial and epithelial cells. Journal of Biological Chemistry, 1997. 272(52): p. 32910–32918. [DOI] [PubMed] [Google Scholar]
- 73.Li W, et al. , IFN-gamma mediates enhancement of HIV replication in astrocytes by inducing an antagonist of the beta-catenin pathway (DKK1) in a STAT 3-dependent manner. J Immunol, 2011. 186(12): p. 6771–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Henderson LJ, et al. , Human immunodeficiency virus type 1 (HIV-1) transactivator of transcription through its intact core and cysteine-rich domains inhibits Wnt/beta-catenin signaling in astrocytes: relevance to HIV neuropathogenesis. J Neurosci, 2012. 32(46): p. 16306–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Henderson LJ, et al. , Identification of novel T cell factor 4 (TCF-4) binding sites on the HIV long terminal repeat which associate with TCF-4, beta-catenin, and SMAR1 to repress HIV transcription. J Virol, 2012. 86(17): p. 9495–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Narasipura SD, et al. , Role of beta-catenin and TCF/LEF family members in transcriptional activity of HIV in astrocytes. J Virol, 2012. 86(4): p. 1911–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


