Abstract
Introduction
Inflammasomes are central to atherosclerotic vascular dysfunction with regulatory effects on inflammation, immune modulation, and lipid metabolism. The NLRP3 inflammasome is a critical catalyst for atherogenesis thus highlighting its importance in understanding the pathophysiology of atherosclerosis and for the identification of novel therapeutic targets and biomarkers for the treatment of cardiovascular disease.
Areas covered
This review includes an overview of macrophage lipid metabolism and the role of NLRP3 inflammasome activity in cardiovascular inflammation and atherosclerosis. We highlight key activators, signal transducers and major regulatory components that are being considered as putative therapeutic targets for inhibition of NLRP3-mediated cardiovascular inflammation and atherosclerosis.
Expert opinion
NLRP3 inflammasome activity lies at the nexus between inflammation and cholesterol metabolism; it offers unique opportunities for understanding atherosclerotic pathophysiology and identifying novel modes of treatment. As such, a host of NLRP3 signaling cascade components have been identified as putative targets for drug development. We catalog these current discoveries in therapeutic targeting of the NLRP3 inflammasome and, utilizing the CANTOS trial as the translational (bench-to-bedside) archetype, we examine the complexities, challenges, and ultimate goals facing the field of atherosclerosis research.
Keywords: NLRP3 inflammasome, cardiovascular disease, atherosclerosis, inflammation, macrophage, Interleukin-1b
1. Introduction
Atherosclerosis has long been recognized as the driving force behind cardiovascular disease (CVD), which remains the leading cause of death in the United States [1]. Furthermore, despite increased use of cardio-preventive medications (i.e., potent lipid-lowering drugs and anti-platelet agents), the once high rate of decline in CVD mortality has now slowed [2,3]. This alarming deceleration, in combination with high disease burden and associated cost, has highlighted the need for novel therapeutics aimed at reversing atherogenesis. Atherosclerosis is the result of lipid accumulation and chronic inflammation within the arterial vasculature [4]. These two processes are linked via the NLRP3 inflammasome, which is predominantly expressed in macrophages and lipid-laden foam cells, neutrophils, as well as endothelial cells (ECs) residing within areas of vascular inflammation and atherosclerotic plaque formation [5,6]. NLRP3 inflammasome activation has been proposed to be a critical catalyst for atherogenesis facilitating the production and maturation of pro-inflammatory cytokines, such as interleukin‐1β (IL-1β) and interleukin‐18 (IL-18), both of which are major contributors to atherosclerosis [4,7,8].
The Canakinumab Anti-Inflammatory Thrombosis Outcome Study (CANTOS) was the first large-scale randomized controlled trial to show that targeting the inflammatory component of atherosclerosis with an anti-interleukin-1 beta (IL-1β) monoclonal antibody resulted in improved cardiovascular outcomes in patients with stable post-myocardial infarction [7,9]. Despite limitations if clinical efficaciousness, these results were posited as a breakthrough in present day vascular biology through affirmation of the “inflammatory hypothesis” of atherosclerosis and advancement of the precision-medicine approach to therapy [10]. Moreover, the results of CANTOS has substantiated the potential importance of the NLRP3 inflammasome, providing further momentum to current research efforts aimed at preclinical targeting of cardiovascular inflammation and atherosclerosis.
2. The NLRP3 inflammasome 2-step activation model
Inflammasomes are a diverse set of protein complexes grouped according to structural sensor components, enzymatic specificity, and associated upstream-downstream activation pathways [5]. The nucleotide‐binding oligomerization domain, leucine‐rich repeat–containing receptor (NLR) family pyrin domain‐containing 3 (NLRP3) inflammasome is a multi-protein complex comprised of the NLRP3 sensor, ASC adaptor, and 3) pro‐caspase‐1 that serves as a key mediator of vascular inflammation and atherogenesis [5,6]. It should be noted that NLRP3 is but one member within the NLR family of inflammasomes, which also include NLRP1, NLRP6 and NLRC4 [5,11]. Activation of the NLRP3 inflammasome requires two input signals, namely a priming signal and an activation signal (reviewed in Figure 1). The priming signal is mediated through classic inflammatory pathways, such as the mediated by IL-1 receptor (IL-1R), tumor necrosis factor α receptor (TNFR), and toll-like receptor (TLR), which activate transcription factor nuclear factor-kappa B (NF-κB) to prime the transcription, translation, and expression of inflammatory cytokines IL-1β and IL-18 precursors, along with other inflammasome components, including caspase-1 (constitutively expressed) and NLRP3. The NLRP3 serves as a sensor for the activation signal and is unique among NLR family members in its ability to recognize endogenous damage-associated molecular patterns (DAMPs), such as extracellular ATP, crystalline particulates (cholesterol crystals, uric acid crystals, silica), and ion (K+) efflux in addition to pathogen--associated molecular patterns (PAMPs) [12]. These secondary triggers promote intracellular events, such as mitochondrial ROS formation and lysosomal destabilization, which propagate inflammatory signaling and prompt structural assembly of the NLRP3 inflammasome complex (NLRP3-ASC-caspase-1). Next, proteolytic cleavage of activates caspase-1 that in turn selectively processes the inactive forms of pro-IL-1β and pro-IL-18 and facilitates their release [13–15] via cleavage of gasdermin D (GSDMD), whose pore-forming N-terminal fragment (GSDMD-N) increases plasma membrane permeability to enable pyroptotic cell death (pyroptosis) [16,17].
Figure 1. Activation of the NLRP3 inflammasome.
The NLRP3 inflammasome requires a two-step process to produce the pro-inflammatory cytokines IL-1β and IL-18. The first step (called the priming signal- depicted on the left) involves receptor-mediated activation of the transcription factor NF-κB which increases transcripts of pro-IL-1β and pro-IL-18 along with components of the inflammasome machinery including caspase-1 and NLRP3. The second step (called the activation signal- depicted on the right) involves various triggers for the assembly of the inflammasome complex including ATP, potassium efflux, and crystalline particles such as cholesterol crystals, mitochondrial ROS, and lysosomal destabilization. Oligomerization of the inflammasome components NLRP3 and ASC leads to recruitment and activation of Caspase-1 which in turn, 1) cleaves the precursor forms of IL-1β and IL-18 to their active and releasable forms, and 2) cleaves GSDMD to its active membrane pore-forming fragment GSDMD-N facilitating pyroptosis.
Abbreviations: NLRP3 (nucleotide‐binding domain, leucine‐rich repeat–containing receptor family, pyrin domain‐containing 3), IL-1β and IL-18 (interleukin-1β and −18), ASC (Apoptosis-associated speck-like protein containing a CARD), ATP (adenosine triphosphate), ROS (reactive oxygen species), GSDMD (Gasdermin D), GSDMD (Gasdermin N)
3. Macrophage cholesterol handling: the convergence of lipid metabolism and inflammasome activation
Macrophages are key regulators of lipid metabolism and their handling of excess lipids (particularly cholesterol) is critical to inflammasome activation in atherosclerosis. We will first broadly overview lipid handling in macrophages to provide context for its links to the NLRP3 inflammasome (reviewed in Figure 2).
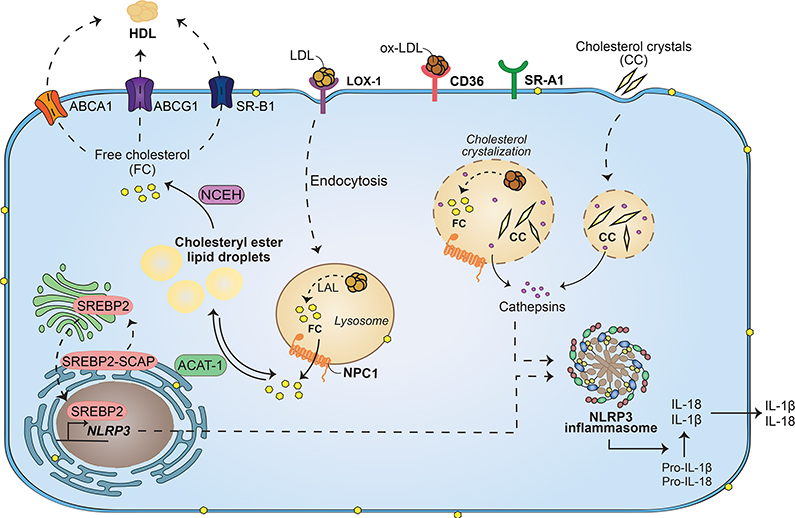
Figure 2. Macrophage lipid metabolism and links to NLRP3 inflammasome activation.
Aside from the uptake of LDL via LDL-R, macrophages take up modified lipids such as oxLDL via scavenger receptors including CD36, SR-A1, and LOX-1. The normal fate of cholesteryl esters in these lipoproteins transits through the lysosome where hydrolysis via LAL liberates free cholesterol. Lysosomal NPC1 shuttles free cholesterol out of the lysosome for re-esterification by ACAT-1 and subsequent storage in lipid droplets. Neutral lipid hydrolysis by cytoplasmic hydrolases can then re-liberate this cholesterol for efflux out of macrophages by ABCA1, ABCG1, or SR-B1 to HDL, thereby constituting the initial events in reverse cholesterol transport. Depending on intracellular cholesterol levels, endogenous cholesterol biosynthetic mechanisms involving SREBP2 can also be initiated. Each of the steps in cholesterol handling by macrophages can interact with or stimulate the NLRP3 inflammasome. Excessive oxidized lipid uptake and inefficient hydrolysis/shuttling in lysosomes can favor in situ formation of cholesterol crystals in lysosomes. Extracellular uptake of cholesterol crystals contributes to this overload. Both events compromise lysosomal membranes leading to inflammasome activation. Activation of SREBP2 can transcriptionally upregulate components of the inflammasome including NLRP3 providing increased machinery for IL-1β and IL-18 generation.
Abbreviations: LDL (low-density lipoprotein), LDL-R (low-density lipoprotein receptor), CD36 (cluster differentiation 36), SR-A1 (class A1 scavenger receptor), and LOX-1 (lipoxygenase-1), NPC1 (Niemann-Pick disease, type C1), ACAT-1 (acetyl-coenzyme A acetyltransferase 1), ABCA (ATP Binding Cassette Subfamily A Member 1), ABCG1 (ATP Binding Cassette Subfamily G Member 1), SR-B1 (class B1 scavenger receptor), HDL (high-density lipoprotein), SREBP2 (sterol response element binding protein 2)
Macrophages can take up LDL, VLDL and oxidized lipoproteins via lipoprotein receptor- and scavenger receptor-mediated pathways, as well as through micropinocytosis and phagocytosis [18]. Lipids are digested in the lysosome, generating free cholesterol and free fatty acids. Free cholesterol can undergo re-esterification in the endoplasmic reticulum (ER) to form cholesterol fatty acid esters that get stored in lipid droplets generating foam cells [19]. Alternatively, free cholesterol can be effluxed from the cell at the plasma membrane via ATP-binding cassette subfamily A member 1 (ABCA1) and subfamily G member 1 (ABCG1) transport proteins [20,21]. Build-up of intracellular cholesterol leads to activation of various transcription factors that upregulate the expression of ABCA1 and ABCG1 to efflux free cholesterol to lipid poor apolipoproteins and eventual incorporation into mature HDL [22,23]. Efflux of free cholesterol can also occur via diffusion (either passive or facilitated)[19,24]. Under normal conditions, macrophages have the capacity to maintain balanced cholesterol metabolism (uptake, processing, and efflux) that prevents formation of foam cells.
However, in the presence of excess lipids, processes such as cholesterol efflux and re-esterification become impaired, resulting in accumulation of free cholesterol within various lipid membrane structures, such as the ER, lipid rafts, and lysosomes [25–27]. Retention of free cholesterol gives rise to a complex integrated stress response causing structural-functional disruptions in ER (e.g., perturbation of lipid bilayer, altered calcium release, oxidative stress, and protein misfolding)[28]. Ultimately, excess lipid accumulation contribute to macrophage apoptosis and deposition of cellular contents within atherosclerotic plaque lending towards further plaque formation and progression [29]. Lipoprotein retention also activates endothelial cell expression of various adhesion molecules (selectin, VCAM-1) and chemoattractants (MCP-1) that recruit monocytes into the sub-endothelial space. Monocytes further differentiate into macrophages that are capable of mediating the internalization and metabolism of cholesterol in its various forms (VLDL, ApoE remnants, and oxidized LDL). Macrophages express various types of receptors that facilitate the uptake of cholesterol. Native LDL is recognized by the LDL receptor (LDLR), whereas other receptors such as CD36 (scavenger receptor A, SRA), lectin-like oxidized low-density lipoprotein receptor-1 (LOX-1), and toll-like receptor-4 (TLR-4) facilitate the uptake of modified (oxidized or glycated) forms of cholesterol that is generated as a result of the increased oxidative stress [30–32]. Oxidized LDL (oxLDL) induces macrophage pro-inflammatory gene expression, including IL-1β, and IL-6 [33]. Scavenger receptors not only function in lipid uptake, but serve as pattern recognition receptors (PRRs), mediating macrophage proliferation and stress response. Most notably, CD36, TLR-4, and LOX-1 have all been implicated in inflammasome activation [34,35]. Resident macrophages within an atherosclerotic plaque, acquire lipoproteins from the surrounding environment that overwhelm normal lipid metabolism, particularly lysosomal trafficking and cholesterol efflux, resulting in increased ER stress, induction of inflammatory signaling (e.g., TLR and NFκB activation), and macrophage cell death by apoptosis [36–38]. All of these processes result in cholesterol deposition and formation of cholesterol crystals and other triggers that activate the NLRP3 inflammasome [39]. Below we explore a number of key triggers and possible mechanisms by which occur.
4. Triggers of NLRP3 inflammasome activation
The NLRP3 is a pattern recognition receptor (PRR) that serves as a key mediator of immune-mediated inflammatory signaling as part of the inflammasome complex. As mentioned, owing to its capacity to recognize a vast array of danger signal associated molecular patterns (DAMPs), as well as exogenous PAMPs related to infection, the NLRP3 serves as a global sensor of cellular stress and damage [11,40]. A number of key activation triggers have been associated with atherosclerosis as outlined below.
4.1. Cholesterol crystals
Cholesterol crystals are common components within atherosclerotic plaques that activate the NLRP3 inflammasome [41]. Cholesterol crystallization is the result of accumulation of free (un-esterified) cholesterol, which has very low solubility in aqueous environments. Crystalline cholesterol has been found in all stages of atherogenesis with established roles in plaque disruption, vascular injury, and macrophage-mediated inflammatory cytokine induction [42].
Generation of cholesterol crystals can occur via a number of mechanisms, including both intracellular and extracellular sources. Macrophages phagocytose cholesterol crystals and store crystal-derived un-esterified cholesterol as cellular cholesteryl esters [39,42]. As mentioned, release of internalized cholesterol stores occurs when macrophages undergo cell death and apoptosis. A well-established apoptotic cascade involves an ER stress pathway (induced by excess free cholesterol) that promotes and activates the unfolded protein response (UPR) and its effector C/EBP-homologous protein (CHOP). In turn, CHOP increases calmodulin kinase II (CaMKII) activity, triggering death receptor signaling and mitochondrial dysfunction that ultimately results in macrophage apoptosis [43,44]. Dysfunction of lysosomal and/or intracellular cholesterol trafficking has also been linked to lipid accumulation and shown to modulate NLRP3 activation [6,35,45,46]. Free cholesterol generated by lysosomal hydrolysis can partition into the lysosomal membrane and influence lysosomal function through perturbations of proteins and/or physical properties of membranes [47]. Lysosomal acidification is a key step required for the hydrolysis of cholesterol esters from oxLDL by lysosomal acid lipase to free cholesterol, which is more readily crystallized. This catabolic event was found to be a requisite for cholesterol crystallization and NLRP3 activation [35]. Furthermore, CD36 (pattern recognition receptor) serves as a scavenger receptor for oxLDL [35]. In regards to extracellular lipid contributions, it has been shown that aggregated and fused extracellular lipoprotein particles within human atherosclerotic lesions can subsequently induce formation of cholesterol crystals, as well as extracellular lipid accumulation. These extracellular-derived lipoprotein particles (together with cholesterol crystals) can be taken up by macrophages, induce foam cell formation, as well as incite inflammasome complex activation triggering the subsequent release of IL-1β [48]. However, other non-cholesterol lipid metabolites have also been shown to induce NLRP3 inflammasome activation and IL-1β secretion in monocytes-macrophages [49–51]. For example, in vitro studies in human monocytes showed lysophosphatidylcholine (LPC) was capable of inducing foam cell formation, as well as inflammasome activation (indicated by IL-1β secretion) that was dependent upon lysosomal dysfunction, generation of ROS and potassium efflux [51]. LPC has been shown to be a key immune mediator of phagocytic recruitment to apoptotic cells [52].
4.2. Lysosomal damage
As mentioned, lysosomal damage is one mechanism by which cholesterol crystals induce NLRP3 inflammasome activation. Studies have shown that uptake and incorporation of cholesterol crystals into macrophage lysosomes can result in overloading with subsequent membrane destabilization and rupture of phagolysosomes. This process has been referred to as ‘frustrated phagocytosis’ whereby crystal-induced lysosomal overloading prevents appropriate endocytosis, cholesterol processing, and clearance. Subsequent lysosomal destabilization and rupture induces leakage of the lysosomal the enzyme cathepsin B (cysteine protease), resulting in NLRP3 inflammasome activation [41]. The exact role of cathepsin B after its release is unclear. Similarly, the involvement of other cathepsins has been debated. Some evidence has shown that chemical inhibition (i.e., CA-074-Me; selective inhibitor of cathepsin B) blocks NLRP3 activation, suggesting a specific and perhaps direct interaction [53,54]. However, studies using cathepsin B–deficient macrophages, in addition to a broad-spectrum, non-selective cathepsin inhibitor (K777), suggested functional redundancy among various cathepsins, showing that both K777- and CA-074-Me-mediated suppression of particle-induced IL-1β could be achieved in cathepsin B–deficient macrophages [55]. Interestingly, ion flux (calcium release and/or potassium efflux) has also been associated with both particulate-induced lysosomal damage- and cathepsin-mediated mechanism of NLRP3 inflammasome activation [12,39,56–58]. A unifying link among all these associated observations may reside with ATP purinergic signaling, which has been described as common pathway involved in particulate- or crystal-mediated NLRP3 inflammasome activation, and can modulate intracellular Ca2+ and K+ [59]. However, while seemingly important, the exact role for cathepsins in NLRP3 inflammasome activity is unclear and further investigations are needed.
4.3. Reactive oxygen species (ROS)
Evidence also indicates that NLRP3 inflammasome activation is dependent upon ROS generation. Certain NLRP3 activators, such as ATP and crystalline particulates (i.e., asbestos, silica, and urate), are triggers of short-lived ROS and oxidative stress [60]. Similarly, cholesterol crystals produce ROS which induces a cytokine response dependent upon complement activation, wherein C5a and TNF together play a role in controlling inflammasome caspase-1 activation [61]. Furthermore, cholesterol crystals induce the up-regulation of complement receptor-3 (CR3) and phagocytosis of cholesterol crystals could be reduced by more than 60% in a complement-dependent fashion using compstatin (C3 inhibitor), eculizumab (anti-C5 monoclonal Ab), or the C5a receptor antagonist, all suggesting complement receptor-3 (CR3) may be a key surface protein mediating phagocytosis of cholesterol crystals [61]. As outlined in the section below, ROS formation is largely related to mitochondrial dysfunction.
4.5. ATP, purinergic receptors, and ion flux
Adenosine triphosphate (ATP) is a well-known and potent activator of the NLRP3 inflammasome pathway leading to IL-1β processing and secretion in plaque-resident macrophages [62–66]. ATP released from dead or dying cells within the necrotic core of an atherosclerotic plaque binds to purinergic receptors, such as the P2X7 receptor (P2X7R), which is a ligand-gated ion channel abundantly expressed on macrophages that subsequently leads to ion flux, mitochondrial ROS formation, and mitochondrial DNA release [64–66]. ATP serves as an endogenous danger signal capable of triggering mitochondrial dysfunction (i.e., ROS and loss of mitochondrial membrane potential) and apoptosis, resulting in release of oxidized mitochondrial DNA (mtDNA) into the cytosol that binds and activates the NLRP3 inflammasome [67]. Overexpression of Bcl-2 (anti-apoptotic protein) inhibits mitochondrial dysfunction and subsequent NLRP3 inflammasome activation, presumably by abrogating mitochondrial-mediated apoptosis. Given that both mitochondrial dysfunction and apoptosis are inextricably linked to ROS, intracellular potassium, and lysosomal degradation, Shimada and colleagues present mitochondria as a central sensor for NLRP3 activity [67]. ATP and P2X7R signaling in atherosclerosis has been demonstrated in P2X7R-deficient mice, whereby lack of this receptor results in the inability to elicit activation of caspase-1, diminished NLRP3 activity, reduced plaque size, and overall mitigation of atherosclerosis in these mice [68].
High concentrations of extracellular ATP leads to sustained P2X7R stimulation that triggers non-selective cationic pore formation and disrupts normal cellular ion homeostasis through Ca2+ influx and K+ efflux [69]. In addition to its intrinsic pore-forming capacity, P2X7R activation upregulates Pannexin-1 hemi-channels, which facilitates additional release of both ATP and IL-1β that further propagates intra- and inter-cellular purinergic signaling and pro-inflammatory cascades, serving as an amplification loop [70]. Baron and colleagues propose that newly released extracellular ATP as well as its hydrolyzed derivatives (ADP, AMP, adenosine) are all capable of amplifying second signal NLRP3 inflammasome activation concurrently via multiple membrane receptor signaling pathways, including a) stimulation of ATP-gated P2X7 receptors, b) activation of other purinergic receptors, including P2Y2 or P2Y1, both of which can couple to the Gi-PLC-β-IP3 pathway to modulate cellular Ca2+ and/or K+ flux, c) activation of adenosine receptors (A2A, A2B, and A3) coupled to Gs and cAMP production, and lastly d) reuptake of extracellular adenosine via equilibrative nucleotide transporters (ENTs), which may be specifically targeted with various ENT inhibitors (MBMPR and/or 5-Iodotubercidin) [71]. ATP purinergic signaling is a common pathway involved in inflammasome activation, especially activation mediated by particulate or crystal triggers (e.g., cholesterol) [59,71,72]. However, the mechanisms by which ATP-mediated ion flux activates NLRP3 inflammasome still remains an active area of investigation. Moreover, it should be noted that while purinergic signaling may be a common pathway for particulate-mediated inflammasome activation, it is not solely dependent upon the P2X7 (or other specific) purinergic receptor, as ATP-mediated IL-1β production has been demonstrated to occur in the absence of various P2X and P2Y receptors [73].
Depletion of intracellular K+ (K+ efflux) has long been observed as a common trigger for NLRP3 inflammasome activation, particularly in association with ATP and purinergic signaling [12,63–65]. K+ efflux in and of itself has been shown to be capable of NLRP3 activation [12,65]. While alternative, ion (K+) efflux-independent NLRP3 activation mechanisms have been proposed, they seemingly involve non-canonical signaling and activation pathways [74–76].
The role of increased intracellular Ca2+ is thought to facilitate mitochondrial damage by overloading the mitochondrial matrix, increase oxidative stress (enhance NADH dehydrogenase activity) and ROS formation that, ultimately, causes loss of membrane potential and release of mtDNA into the cytosol [77]. P2RX7, along with other plasma membrane channels like TRPM2 and TRPM7 (transient receptor potential cation channel subfamily M member 2 and 7) have been linked to Ca2+ influx and NLRP3 inflammasome activation [78]. Ca2+ influx has also been associated with endoplasmic reticulum (ER) stress and apoptosis [79,80]. A number of studies have shown that inhibition of Ca2+ influx, at both the level of the ER and extracellular membrane-mediated entry, attenuated IL-1β production, suggesting it to be a requisite process for NLRP3 inflammasome activation that may be contingent upon ER stress-related apoptosis [28,77,81]. However, others have shown that NLRP3 inflammasome activation can occur independently from Ca2+ mobilization, suggesting more of a modulatory role [57]. Stimulation with an allosteric agonist to the calcium-sensing receptor (CaSR), a G-protein coupled receptor (GPCR) that triggered by extracellular Ca2+ has also been shown to activate the NLRP3 through both Gαq-mediated Ca2+ increase and with Giα-mediated inhibition of cAMP [82]. Peng et al demonstrated in oxLDL-stimulated macrophages, P2X7R activation triggers NLRP3 inflammasome activity through regulatory effects on protein kinase R (PKR)[83], which is recognized as a central mediator of various immune- and inflammatory-mediated processes, including autophagy and ER stress‐induced apoptosis [84,85]. PKR has been shown to be integral for ATP-induced inflammasome activation in both human and murine macrophages, whereby genetic deletion (PKR−/− knockout) or pharmacological blockade (2-aminopurine, 2-AP) markedly suppressed NLRP3 inflammasome activity, as measured by caspase-1 activation and IL-1β processing-release [86]. PKR phosphorylation may be influenced by ion flux.
4.4. Neutrophil extracellular traps (NETs) and Pyroptosis
Cholesterol crystals have also been shown to activate neutrophils, triggering the release of neutrophil extracellular traps (NETs), a process referred to as NETosis [87]. NETosis is a specialized form of programmed cell death in neutrophils that was originally described in the defense against pathogens and involves the release of intracellular granule contents (e.g., chromatin, DNA, elastase, myeloperoxidase) to form large extracellular webs that trap and inactivate pathogens [88,89]. Interactions between neutrophils and other non-pathogenic cells (macrophages, platelets) and substances (cholesterol crystals, P-selectin glycoprotein) have been shown to facilitate NET formation, suggesting a possible interplay among contributing processes (inflammation-immune modulation, lipid accumulation, thrombosis-coagulation) of atherosclerosis and atherothrombosis [90]. It has been proposed that NETosis further triggers the activation of the NLRP3 inflammasome in lesional macrophages, although the mechanism is not fully understood [89].
Warnatsch and colleagues have demonstrated that NETs help drive atherosclerosis by modulating cytokine production and NET-mediated priming of macrophages by promoting a self-amplifying IL-1/IL-17 cascade thought to sustain chronic inflammation [87]. Pyroptosis is a novel form of inflammatory programmed cell death in macrophages shown to be an inflammasome-dependent process. In response to canonical inflammasome activators and caspase-1-mediated cleavage, the N-terminal fragment of gasdermin D (GSDMD) is released to the plasma membrane where it oligomerizes with inner leaflet lipids to form pores, allowing the release of mature IL-1β and triggering pyroptosis [91]. In addition to cellular swelling and lysis, which are protective mechanisms to prevent intracellular pathogen replication, pyroptosis also facilitates the extracellular release of the inflammatory cytokine IL-1β [91]. This ability of macrophages to respond to pathogen- and/or damage-associated molecular patterns (PAMPs and DAMPs) is also replicated in other immune cells, specifically neutrophils. Pyroptosis in neutrophils requires inflammasome activation, caspase-1 activation of gasdermin D (GSDMD) with pore formation, and results in secretion of IL-1β and a host of other immunomodulatory cytokines, including IL-10, IL-13, IL-8, myeloperoxidase (MPO), cathepsin G and other granzymes [92,93]. Pyroptosis and neutrophil extracellular trap formation (NETosis) are effectively the same process that results in the secretion of IL-1β, one of the most potent pro-inflammatory cytokines known [94]. Although subtle, yet potentially important distinctions likely exist, NETosis has been implicated in atherothrombosis, notably in plaque erosion and thrombosis [95,96], a process that may be increasingly important in acute coronary syndromes [97]. The manner in which these two cell types (macrophages and neutrophils) functionally interrelate in the pathogenesis of atherosclerosis is an area of keen interest.
4.6. Hypoxia
Hypoxia has long been recognized as a key mediator of metabolic and cellular stress that has recently been implicated as a potential inflammasome activator in atherosclerotic macrophages [98–100]. Hypoxia has been associated with pro-inflammatory stimulation, as well as accelerated atherosclerotic plaque progression and lesion complexity [101–105]. Additional studies have shown that hypoxia perturbs macrophage lipid metabolism, promoting foam cell formation [106]. Specific association to inflammasome activation has been limited. Some have shown hypoxia-mediated activation of the nuclear factor-κB signaling cascade, which may drive IL-1β transcription [107,108]. Some studies have implicated the involvement of hypoxia-inducible factor-1α (HIF-1α)[109–111], which itself has been linked to mitochondrial reactive oxygen species formation [112,113]. Both mitochondrial ROS and hypoxia-inducible factor-1α (HIF-1α) have been linked to inflammasome activation and induction of IL-1β transcription [99,109,114]. Folco and colleagues report that selective (reduced) autophagy occurs under conditions of hypoxia that, in the presence of additional NLRP3 triggers (e.g., cholesterol crystals), serves as a transcriptional amplifier that increases NLRP3 activation and subsequent IL-1β production [98]. Further details regarding the effect of hypoxia in atherosclerotic macrophages can be found elsewhere [100]. It should be noted that HIF-1α has been shown to be an important and direct mediator of IL-1β expression through the promotion of glycolysis and inflammation in activated macrophages and not via direct activation on NLRP3 [99,115].
5. Role of the NLRP3 inflammasome in atherosclerosis
The presumptive role of NLRP3 inflammasome in atherosclerosis stems from its role as an upstream activator of IL-1β [7,8,116]. Homozygous Nlrp3-deficient (Nlrp3−/−) mice are viable and fertile, suggesting its major function as a pathophysiological mediator. NLRP3 deficiency within an atherogenic background (Ldlr−/− knockout) has been shown to abrogate atherogenesis via inflammasome-mediated pathways signaling through Il-1 [6]. Furthermore, the same has been shown for knockouts of NLRP3 inflammasome components, such as caspase-1 (caspase-1−/−) within either Ldlr- or ApoE-deficient atherogenic mouse models [6,56,117,118]. Dual ApoE− and Caspase-1−deficient mice were found to have markedly less atherosclerotic development-progression (i.e., 35%−45% reduction in plaque area of ascending aorta) in response to either high-fat Western or regular chow diets [117]. Usui and colleagues demonstrated similar reductions in plaque complexity (reduced macrophage and vascular smooth muscle cell content) in ApoE− and Caspase-1−deficient mice [56]. This work has established the supposition that the NLRP3 inflammasome plays a central role in atherogenic signaling and inhibition (either direct or indirect) reduces the development and progression of atherosclerosis. Additional studies of 8-oxoguanine glycosylase (OGG1), which is a key DNA glycosylase whose function is to support mitochondrial function via clearance of oxidative mtDNA damage [119] have also lent insight. Dual Ogg1- and Ldlr-deficient mice exhibited increased oxidized mtDNA-mediated inflammasome activation (increased serum IL-1β levels) resulting in enhanced atherosclerotic lesion size. Concurrent NLRP3 and OGG1 deficiency (Nlrp3−/−, Ogg1−/−) mitigated this Ogg1-mediated effect, resulting in reduced aortic sinus atherosclerotic lesion area [120]. However, NLRP3 deficiency alone did not show significant differences in atherosclerotic lesion size compared to wild-type mice [120].
It should be noted that opposing studies have suggested that NLRP3 inflammasome deficiency has no effect on the development-progression of atherosclerosis [121]. Using dual inflammasome-component knockouts (i.e., Nlrp3−/−, Asc−/− or caspase-1−/−) within atherogenic ApoE−/− mice did not have any significant effect on atherosclerotic plaque size (total surface area), complexity (macrophage content), or stability (smooth muscle actin) is unaltered in NLRP3 inflammasome-deficient ApoE−/− mice [121]. Potential explanations for these conflicting results have been postulated to be related to differences in atherogenic background (ApoE−/− versus Ldlr−/−), which may yield differing levels of hyperlipidemia and/or differing levels of IL-1α versus IL-1β signaling in these two models [122]. Further studies are needed to parse out differences in NLRP3 inflammasome signaling in different atherogenic murine models.
Lentivirus-mediated NLRP3 gene silencing in ApoE-deficient atherosclerotic mouse model lowered both lipid and macrophage content of aortic plaques compared to controls [123]. Increased plaque stability was also reported, as evidenced by increased collagen content and thickness within the fibrous cap, as well as decreased mRNA expression of matrix metalloproteinase-2 (MMP-2) and matrix metalloproteinase-9 (MMP-9), which destabilize plaques through collagen breakdown [123]. Another recent study using Lentiviral-silencing of NLRP3 demonstrated inhibition of homocysteine-mediated inflammasome signaling and atherosclerosis within ApoE-deficient mice [124].
6. Regulation of the NLRP3 inflammasome in atherosclerosis
Other means of achieving negative regulation of NLRP3 inflammasome will be important for understanding atherosclerosis as well as identifying potential therapeutic targets.
6.1. High-density lipoprotein (HDL)
High‐density lipoprotein (HDL) exerts many different and antagonistic effects on cholesterol crystal (CC)-induced inflammasome activation. While HDL binds directly to CCs, this interaction did not dissipate CC formation, nor did it prevent phagocytosis or inhibit cellular uptake [125,126]. Niyonzima and colleagues demonstrated that HDL blocks complement deposition to the surface of CC and this resulted in the suppression of markers of activation in monocytes [126]. HDL has specifically been shown to inhibit complement C3b deposition on the surface of CCs [61,126]. High‐density lipoprotein (HDL) suppresses inflammasome activation in response to a wide range of activators, including ATP and nigericin (microbial toxin derived from Streptomyces), and stabilizes lysosomal integrity in response to treatment with CCs. Loss of lysosomal integrity activates lysosomal cathepsins capable of generating secondary messengers that trigger NLRP3 with resultant activation of caspase‐1 and pro‐IL‐1β cleavage [127]. Decreased transcription of NLRP3 and IL-1β genes in monocyte‐derived macrophages has been observed following treatment with lipopolysaccharide (LPS) and HDL [125,128]. Some have shown this to be mediated through activation of ATF3.
6.2. Autophagy
Autophagy has been identified as an important regulatory process in atherosclerosis, as well as inflammasome activity. Autophagy stimulates cholesterol efflux and is induced in lipid-laden macrophages as a means of reducing excess intracellular lipid burden [27]. Lipid droplets are encapsulated by autophagosomes, which then fuse with lysosomes containing lysosomal acid lipase (LAL) that hydrolyzes cholesterol esters into free cholesterol available for efflux [129,130]. Autophagy induction in Wip1-deficient macrophages prevented accumulation of lipid droplets and suppressed conversion into foam cells [131]. Mice deficient in macrophage autophagy (ATG5−/−) have been show to develop accelerated atherosclerosis accompanied by enhanced activation of NLRP3 inflammasome [132]. Similarly, given that mitochondrial-mediated ROS overproduction serves as a key inflammasome trigger, the clearance of damaged mitochondria via organelle-specific autophagy (mitophagy) has been described as a means of inhibiting NLRP3 inflammasome activation [133]. A recent study has also highlighted the importance of mitophagy, showing suppression of mitophagy (via mTORC1 activation) to be associated with increased cardiovascular risk, although the role of NLRP3 is not clear [134]. Additionally, several mechanisms have been identified for direct capture of assembled NLRP3 inflammasomes coupled with targeted degradation in autophagosomes [135,136]. Such selective autophagy is triggered by inflammatory signaling cascades and serves as a crucial molecular brake to limit inflammation. Similarly, the concept of ‘inflammasomophagy’ (inflammasome-specific autophagy) has been described via two distinct mechanisms: 1) precision autophagy, which involves direct recognition of multiple inflammasome components by TRIM20 that in turn recruits autophagosome machinery (e.g., Beclin, ULK1, and ATG8) and 2) selective autophagy, which involves ASC subunit poly-ubiquitination and p62-mediated interaction with the autophagosome [13,137–139]. Additionally, over-expression of transcription factor EB (TFEB), a key transcriptional regulator of autophagy and lysosome function, in macrophages inhibits cholesterol crystal-induced NLRP3 inflammasome activation and attenuates the progression of atherosclerosis by promoting lysosomal biogenesis [45].
6.3. Endogenous Modulators-Effectors
NEK7 (NIMA Related Kinase 7) is a serine-threonine protein kinase important in mitosis and cell cycle progression. A number of studies have demonstrated NEK7 to be a requisite NLRP3-specific inflammasome activator [140–142]. While the exact mechanism of NEK7-NLRP3 interaction remains unclear, binding apparently occurred via the NEK7 catalytic domain, although not exclusively, and was contingent upon potassium efflux [141]. Reduced caspase-1 activation and IL-1β secretion was observed in NEK7-deficient cells and NEK7-deficient mice showed diminished IL-1β response to NLRP3 activators (i.e., LPS and nigericin) [140].
Caspase recruitment domain (CARD)-only proteins (COPs) and Pyrin domain (PYD)-only proteins (POPs) are endogenous analogues of caspase-1 and ASC proteins identified as regulators of NLRP3 inflammasome activity [143,144]. It was initially hypothesized that these regulatory proteins functioned as negative or inhibitory regulators to prevent excessive or persistent inflammasome activation. For example, POP1 was shown to impede ASC oligomerization with NLRP3, thereby, inhibiting inflammasome activation [145]. However, positive regulators have also been identified, such as CARD16, which promotes caspase-1 association and inflammasome activation, and subsequent IL-1β release [146]. While the function of these proteins remains controversial, they may provide critical insights into NLRP3 modulation and therapeutic target development.
7. Current approaches to therapeutic targeting of the NLRP3 inflammasome
Several years of research at the preclinical stage and recent clinical trial data have raised excitement in targeting the NLRP3 and IL-1β signaling cascade in cardiovascular therapeutics. We discuss the relevant observations below (Table 1).
Table 1.
Pharmacological targeting of the NLRP3 inflammasome
| Agent | Target | Preclinical Data | Clinical Data |
|---|---|---|---|
| MCC950 | NLRP3 | Reduced atherosclerosis in ApoE-deficient mice, reduced macrophage VCAM-1 & ICAM-1 [151] | N/A |
| CY-90 | NLRP3 | Improved glycemic control and insulin sensitivity in diabetic mice [153] | N/A |
| BOT-4-one | NLRP3 | In vitro studies; reduced ATPase activity of recombinant NLRP3 protein and increased level of NLRP3 ubiquitination (macrophages)[154] | N/A |
| BAY 11–7082 | NLRP3 and IκB kinase | In vitro studies; reverses autophagy-regulated atherosclerosis and VSMC phenotype switching mediated via NF-κB signaling [155,156] | N/A |
| Canakinumab (Ilaris) | IL-1β | N/A | Reduced fibrinogen, IL-6, and hsCRP [166] CANTOS: reduction in MI, stroke, and related death [9,169] |
| Anakinra (Kineret) | IL-1Ra | N/A | MRC-ILA Heart Study; reduced CRP, no effect on MACE [177] VCU-ART, VCU-ART2; no effect on recurrent ischemic CV events [179,180] VCU-ART3; reduced IL-6, hsCRP, and post-MI HF; improved LV remodeling [178] |
| GSK1070806 | IL-18 | Levels correlate with carotid intima thickness and IL-6 and CRP in humans [181–183] | N/A |
| Tocilizumab (Actemra), Sarilumab (Kevzara) | IL-6R | N/A | ENTRACTE; non-inferior to Etanercept for non-fatal MI, stroke, or CV-related death; reduced CRP raised LDL [189] SARIL-RA-MOBILITY; reduced hsCRP [188] unclear CV benefit |
| VX-740 (Pralnacasan) and VX-765 | Caspase-1 | VX-765 plus P2Y12 antagonist (ticagrelor or cangrelor) at reperfusion; decreased infarction and preserved ventricular function in rats [160] | N/A |
| MLN1202, Plozalizumab | CCR2 | 3-fold reduction in mean aortic lesion area [190–192] | Reduced hsCRP levels [193] Phase II study atherosclerosis withdrawn |
| Arglabin | farnesyl transferase inhibitor | lowers plasma total cholesterol and triglycerides, reduced IL-1β, IL-18, and IL-1α in vitro and in vivo [210] | N/A |
| Methotrexate | Dihydrofolate reductase inhibitor | CIRT; no reduction in IL-1β, IL-6, hsCRP, or MACE [194] | |
| Colchicine | Β-tubulin polymerization inhibitor | Reduced CRP; synergism with atorvastatin [202] Reduced levels IL-1β, IL-18, caspase-1 human ACS [206] |
Reduced levels IL-1β, IL-18, and IL-6 [204,205] LoDoCo; reduction in ACS [203] COLCOT; reduction CV events acute post-MI [207] |
| JTX92 | LOX-1 | LOX-1 deficiency decreased atherosclerosis, decreased NLRP3 triggers and activation [208,209] | N/A |
| Trehalose | TFEB activator | reduces IL-1β and atherosclerosis in macrophage ATG5- and SQSTM1-dependent manner [213] | N/A |
Abbreviations: nucleotide‐binding oligomerization domain, leucine‐rich repeat–containing receptor (NLR) family pyrin domain‐containing 3 (NLRP3), C-C Motif Chemokine Receptor 2 (CCR-2), lipoxygenase-1 (LOX-1), Transcription Factor EB (TFEB), vascular cell adhesion molecule 1 (VCAM-1), intercellular adhesion molecule 1 (ICAM-1), autophagy related 5 (ATG5), sequestosome 1 (SQSTM1), high-sensitivity C-reactive protein (hsCRP), major adverse cardiovascular events (MACE), Cardiovascular Inflammation Reduction Trial (CIRT), Colchicine Cardiovascular Outcomes Trial (COLCOT), Canakinumab Antiinflammatory Thrombosis Outcome Study (CANTOS), Investigation of the effect of Interleukin-1 receptor antagonist (IL-1Ra) on markers of inflammation in non-ST elevation acute coronary syndromes (The MRC-ILA-HEART Study), Virginia Commonwealth University Anakinra Remodeling Trial (VCU-ART), A Study of Etanercept in Comparison to Tocilizumab in Rheumatoid Arthritis and Cardiovascular Disease Risk Factors (ENTRACTE), Evaluation of Sarilumab (SAR153191/REGN88) on Top of Methotrexate in Rheumatoid Arthritis Patients (SARIL-RA-MOBILITY)
7.1. Direct NLRP3 inhibitors
MCC950 is a direct, small-molecule inhibitor of NLRP3 that directly interacts with the Walker B motif within the NACHT domain, blocking ATP hydrolysis required for NLRP3 activation and inflammasome formation [147,148]. Given its mechanism of action, MCC950 maintains high specificity and reportedly inhibits NLRP3 activation by all stimuli. Interestingly, murine studies of cryopyrin-associated periodic syndromes (CAPS)-related NLRP3 mutants (Nlrp3L351P knock-in mice and ex vivo-stimulated mutant macrophages) were resistant to inhibitory properties of MCC950, whereas wild-type NLRP3 triggered with LPS demonstrated reduced IL-1β and IL-18 [149]. MCC950 was also shown to be similarly efficacious and neuroprotective against dopaminergic degeneration in α-synuclein-mediated NLRP3 activation mouse model of Parkinson’s disease (PD)[150]. In relation to cardiovascular disease and atherosclerosis, studies in ApoE-deficient mice have shown that MCC950 markedly reduced atherosclerotic lesion size, reduced the number of intra-lesional macrophage content and expression of vascular cell adhesion molecule 1 (VCAM-1) and intercellular adhesion molecule 1 (ICAM-1)[151]. Other direct NLRP3 inhibitors have been investigated. CY-09 is an analog of the cystic fibrosis transmembrane conductance regulator (CFTR) channel inhibitor (CFTR-172) [152]. In relation to NLRP3, its mechanism of action mimics that of MCC950 by directly binding the Walker A motif within the NACHT domain, blocking ATP hydrolysis required for NLRP3 activation and inflammasome formation [153]. Similarly, it does not affect other inflammasome sensors (NLRP1, NLRC4, RIG-1, or NOD2)[153]. Although no direct investigation regarding atherosclerosis, CY-90 was shown to have beneficial effects on NLRP3-mediated blood glucose control and insulin resistance in diabetic mice [153]. Other direct NLRP3 inhibitors exist, including BOT-4-one (covalent alkylation of NLRP3)[154] and BAY 11–7082 (IκB kinase inhibitor and covalent inhibitor of NLRP3), the latter of which inhibits NLRP3 inflammasome activity in macrophages via alteration of the ubiquitin system and may play a role in autophagy-regulated atherosclerosis and VSMC phenotype switching mediated via NF-κB signaling [155,156]. Interestingly, there is some evidence showing that dietary intake (specifically high-fat, high-calorie Western diet) is capable of inducing systemic inflammation and epigenetic re-programming in myeloid progenitors in murine models (Ldlr−/−) that is NLRP3-dependent (absent in Nlrp3−/−, Ldlr−/− mice) [157–159]. Moreover, while dietary modification can reverse systemic inflammation, a sustained priming of bone marrow immune cells remains, suggesting that the NLRP3 inflammasome may be involved in sensing-mediating both acute and chronic inflammation related to Western diet, which would further support direct, NLRP3 inhibition as a potential preventative and therapeutic approach in atherosclerotic cardiovascular disease [157].
7.2. Caspase-1 inhibitors
Both VX-740 (Pralnacasan) and VX-765 are covalent inhibitors at cysteine residues within the catalytic domain of caspase-1, resulting in reduced cleavage and activation of precursor forms of IL-1β and IL-18. In relation to atherosclerotic cardiovascular disease, studies using VX-765 plus P2Y12 antagonist decreased infarction and preserved ventricular function when reperfusion in rats [160]. A number of other caspase-1 inhibitors have been tested in osteoarthritis, epilepsy and psoriasis with some reaching phase II clinical trials, but were not further developed due to concerns relating to hepatotoxicity [161,162].
7.1. Interleukin-1 beta (IL-1β) and the CANTOS Trial
IL-1β is a macrophage-produced pro-inflammatory cytokine that promotes atherogenesis via its actions on various cell types and tissues, including vascular smooth muscle cells (VSMCs), vascular endothelial cells (ECs) and macrophages. IL-1β acquires its biological activity via the NLRP3 inflammasome and subsequent proteolytic processing by caspase-1. Cells within an atheroma produce and secrete IL-1β in response to inflammatory stimuli. In turn, IL-1β stimulates proliferation of VSMCs and other pro-inflammatory mediators, such as intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1) and MCP-1 (CCL-2). IL-1β has also been shown to induce its own gene expression (i.e., auto-induction) and, thus, mediates an inflammatory-amplification loop central to the progression of atherogenesis. Furthermore, some evidence suggests differential contributions from IL-1β versus IL-1α in atherosclerotic lipid accumulation and inflammation that occurs in a temporal-specific manner. In an early atherosclerosis progression model (ApoE-deficient mice, Western diet, no pre-established atherosclerosis), treatment with monoclonal antibodies against IL-1α or IL-1α and IL-1β together resulted in decreased atherosclerotic lesion area and aortic root vascular remodeling (IL-1β inhibition had no effect on remodeling) [163]. In an advanced atherosclerosis model (ApoE-deficient mice, Western diet, established atherosclerosis), selective inhibition of IL-1β reverted the intravascular inflammatory state, as evidenced by an increase IL-10 (anti-inflammatory cytokine) by monocytes and a decrease in atheroma size in the aortic root. By contrast, IL-1α inhibition had no such effect, suggesting temporal differences in atherogenesis contributions via IL-1α (early) versus IL-1β (late), which was postulated to promote inflammation in advanced atherosclerosis [163].
Elucidation of the NLRP3 inflammasome pathway in both the development and progression of atherosclerosis has reclassified atherosclerotic cardiovascular disease as a chronic inflammatory disorder and broadened the scope for new therapeutic strategies. Understanding the contributions of inflammatory and immune mechanisms in atherogenesis may transcend traditional therapeutic approaches beyond lipid-lowering drugs, anti-platelet agents, and intravascular interventions. This is best evidenced by the therapeutic targeting of IL-1β, as a downstream product of NLRP3 activation. A plethora of evidence existed to support the therapeutic targeting of IL-1β in the treatment of atherosclerotic cardiovascular disease. IL-1β is a soluble, secreted mediator that exerts pro-inflammatory, pro-atherogenic and pro-thrombotic effects on various cells in the vasculature, including macrophages-monocytes, endothelial cells (ECs), and vascular smooth muscle cells (VSMCs). In fact, hypotheses of IL-1β as a central mediator of atherogenesis dates back nearly four decades [7,164,165]. Initial promise for the safety and efficacy of targeted IL-1β inhibition came by way of some smaller clinical trials, including a phase II study of diabetic patients, whereby the human interleukin-1 beta (IL-1β) monoclonal antibody, Canakinumab (Ilaris), dose-dependently reduced plasma levels of the pro-inflammatory mediators fibrinogen, IL-6, and hsCRP [166]. Canakinumab demonstrated only modest and statistically non-significant effects on hemoglobin A1c, glucose, and insulin levels. However, median reductions at 4 months in CRP were marked (36.4%, 53.0%, 64.6%, and 58.7%) and dose-dependent (for the 5-, 15-, 50-, and 150-mg), respectively, when compared with placebo (4.7%) (all P values ≤0.02) [166]. Similar results were observed for IL-6 and fibrinogen and were noticeable in both women and men [166]. Interestingly, no effects were observed for lipids (i.e., LDL, HDL, and non-HDL cholesterol). The anti-inflammatory benefits of Canakinumab had been demonstrated in certain forms of juvenile and gouty arthritis also showing mediation by IL-1β [167,168].
The CANTOS study was touted as the first large-scale, randomized clinical trial to transform theory into reality (bench to bedside), showing that targeted neutralization of IL-1β reduced recurrent cardiovascular events in statin-treated patients with stable, prior myocardial infarction [9]. Study participants were shown to have residual underlying inflammation with elevated hsCRP (>2.0 mg/L, approximate population median) with a 15% reduction in the primary end point of major adverse cardiovascular events (MACE). However, no significant change in MACE was observed with low-dose (50 mg) Canakinumab. In addition to IL-1β, CANTOS highlighted modulation of IL-6 signaling and reduced cardiovascular event rates with a 32% reduction in MACE and a 48% reduction in all-cause mortality in those achieving on-treatment IL-6 levels below 1.65 ng/L (study median value)[169]. Interestingly, no significant benefit for any endpoints was seen in those with on-treatment IL-6 levels at or above 1.65 ng/L with initiation of Canakinumab. It is important to note that response to Canakinumab related to presumed residual inflammatory risk as indicated by hsCRP reduction. Therefore, if patients are divided at the median of hsCRP response, the patients that fall in the upper half of hsCRP reduction – the responders – had a 27% reduction in cardiovascular events compared to only 5% for the lower half [169]. Fuster and colleagues have shown that mutations in human hematopoietic stem cells (HSC) can markedly enhance IL-1β production by macrophages, which in turn augments progression of atherosclerosis [170]. The theory is that patients harboring such pro-inflammatory and pro-atherogenic clonal hematopoiesis may respond differently and/or require more aggressive therapeutic targeting with anti-inflammatory medications [10]. However, some animal studies have highlighted differential effects of IL-1β inhibition, in terms of prevention versus intervention. IL-1β inhibition (using IL-1β antibody) in ApoE-deficient mice at the start of atherogenic (Western diet) feeding was beneficial and reduced the development of atherosclerotic plaque formation [171]. However, others have shown that IL-1β inhibition was also beneficial in maintaining late stage atherosclerotic plaque stability and outward remodeling via increased macrophage and decreased smooth muscle cell content within the fibrous cap of lesions [172]. The combined implication of these results remains to be seen and whether treatment with IL-1β inhibitors is beneficial at various stages of atherosclerosis. Thus, while the results from CANTOS elude to the significance of the NLRP3 inflammasome pathway as a pathophysiological contributor to atherosclerosis by targeting IL-1β (as a theoretical LDL-cholesterol-independent inflammatory effector), further research is needed to parse out the differential effects of IL-1β activity, as well as that of direct targeting of NLRP3 or other inflammasome-related mediators. Below we highlight some of this research.
7.2. Interleukin-1α (IL-1α) receptor antagonist (IL-1Ra)
The role of the NLRP3 inflammasome in atherosclerosis, as well as the contributions of specific IL-1 isoforms remains controversial. Studies using dual ApoE- and inflammasome-deficient (ApoE−/− crossed with NLRP3, Caspase-1, or ASC knock-out) mice found no differences in atherosclerotic plaque development, progression, or stability among dual knockouts versus controls [121]. While activity of the NLRP3 inflammasome has been proposed to be requisite for IL-1β secretion, studies have also shown that IL-1α secretion can be induced via inflammasome-independent, calcium flux that was not affected by caspase-1 deficiency, K+ efflux or ROS formation [173]. Furthermore, while much attention has been placed on IL-1β as a key mediator of inflammation and atherosclerosis, Freigang et al. have demonstrated IL-1α to be a major, inflammasome-independent driver of vascular inflammation and atherosclerosis, in response to unsaturated fatty acids, demonstrating mice deficient in bone marrow-derived IL-1α had fewer atherosclerotic lesions than controls [174]. This effect was substantial, although less robust in comparison with IL-1β [174,175].
Other IL-1 modulators have also shown anti-atherogenic and protective efficacy in cardiovascular disease. Anakinra (Kineret) is a recombinant, non-glycosylated human interleukin-1 receptor antagonist (IL-1Ra) used in the treatment of rheumatoid arthritis (RA) that decreases signaling via both IL-1α and IL-1β [176]. Several studies in post-MI patients have shown IL-1Ra antagonism to facilitate reductions in both hsCRP and IL-6, improve left ventricular remodeling, as well as reduced incidence of heart failure [177–180]. A drawback to IL-1Ra therapy is its lack of specificity on IL-1 isoforms, meaning it antagonizes the activity of both IL-1α and IL-1β, which may lessen efficacy and augment infection risk.
7.3. Interleukin-18 (IL-18)
Interleukin 18 (IL-18) is also a potent pro-inflammatory and pro-atherogenic cytokine that becomes activated and released by the inflammasome pathway along with and analogous to IL-1β [181,182]. As such, targeted inhibition of this cytokine is postulated to warrant similar consideration for the treatment of targeting NLRP3 inflammasome-mediated atherosclerosis and simultaneous inhibition of both IL-1β and IL-18 has been proposed, although it is felt that adverse effects would likely outweigh benefits [183]. Serum levels of IL-18 in humans with atherosclerosis correlate with carotid intima thickness and other biomarkers of inflammation (IL-6 and CRP) [182,184].
7.4. Interleukin-6 (IL-6)
Given the findings with the aforementioned Canakinumab studies, IL-6 seems to have a central role within the inflammasome cascade and atherosclerosis. Targeted inhibition of IL-6 has been postulated to have a significant impact on cardiovascular disease. This has been evidenced by IL-6 receptor gene (IL-6R) variants leading to reduced IL-6 signaling. The IL-6R (Asp358Ala) SNP (rs2228145; rs4129267) has been associated with decreased risk of cardiovascular events (per allele odds ratio 0.95; 95% CI, 0.93–0.97) [185], reduced atherosclerotic disease burden and diminished ischemic heart disease (odds ratio 0.95; 95% CI, 0.94–0.97) [186]. Interestingly, this SNP was associated with increased CRP levels in both Asian and European populations [187]. Tocilizumab (Actemra) is a humanized monoclonal antibody against IL-6 receptor (IL-6R) was shown to be non-inferior to Etanercept (Enbrel) for primary outcome of non-fatal MI, non-fatal stroke, or cardiovascular-related death in the ENTRACTE trial (www.clinicaltrials.gov/NCT01331837). The SARIL-RA-MOBILITY trial used another anti-IL-6R antibody, Sarilumab (Kevzara), to demonstrate a reduction in hsCRP levels (by more than 90% relative to baseline), however, increase in both LDL and HDL cholesterol were observed at higher dosages (>100 mg every 2 weeks) as well as transaminase elevation, and opportunistic infections. Effect on cardiovascular events remains unclear [188]. Thus, the potential for reduction in cardiovascular outcomes may be diminished by these adverse effects, particularly increased LDL-C [189].
7.5. Monocyte chemoattractant protein-1 (MCP-1)
Monocyte chemoattractant protein-1 (MCP-1) is a potent chemokine that is highly expressed in oxidized lipids and atherosclerotic plaques that is critical for monocyte-macrophage migration and infiltration. MCP-1 signals via the CC chemokine receptor type 2 (CCR2) as part of the inflammatory response and atherogenesis. In transgenic mouse models, it has long been known that MCP-1 deficiency confers protection from macrophage recruitment and atherosclerotic lesion formation [190,191]. Similarly, absence of CCR2 reduces atherosclerotic burden (3-fold reduction in mean aortic lesion area) [192] and an anti-CCR2 neutralizing monoclonal antibody (MLN1202) has been shown to reduce hsCRP levels (median change −24.2% vs. 2.5%, p = 0.009) [193].
7.6. Methotrexate and Colchicine
An interesting corollary and contrast to the CANTOS trial is the Cardiovascular Inflammation Reduction Trial (CIRT) [194], which was a randomized, double-blinded, placebo-controlled clinical trial using low-dose Methotrexate as a generalized anti-inflammatory agent in an attempt to reduce cardiovascular events, based upon cardiovascular event rates in other studies using systemic anti-inflammatory agents and disease-modifying anti-rheumatic drugs (DMARDs) [195,196]. Results from CIRT showed that low-dose methotrexate (15–20 mg weekly) had no significant effect in terms of reducing IL-1β, IL-6, hsCRP, or major adverse CV events (MACE) compared with placebo in a patient population with established CAD and either type 2 diabetes mellitus (T2DM) or metabolic syndrome or both [194]. However, the seemingly neutral results from CIRT can be related to its lack of pathway-specific targeting of inflammation (e.g. via the IL-1β to IL-6 to CRP cascade) which appears to be critical to reductions in cardiovascular event rates [197]. The anti-inflammatory efficacy of methotrexate in rheumatoid arthritis is thought to adenosine-mediated [198,199]. Similarly, Canakinumab has shown little additional benefit in combination with methotrexate in treating rheumatoid arthritis [200]. On the heels of trials such as CANTOS and CIRT, the search is on for therapies targeting inflammation, particularly via the NLRP3 inflammasome pathway. Given that both uric acid and cholesterol crystals are activators of the NLRP3 inflammasome, colchicine became a candidate to antagonize this process and reduce release of IL-1β [201]. Colchicine is an anti-gout medication that disrupts cytoskeletal functions by inhibiting β-tubulin polymerization into microtubules, preventing activation and degranulation of inflammatory mediators by neutrophils. Small studies in both animals and humans have shown that colchicine could lower plasma CRP levels both independently and synergistically with atorvastatin therapy (4.03 mg/L vs. 5.35 mg/L, p < 0.05) [202,203]. Moreover, reductions in IL-1β, IL-18, and IL-6 sampled at the coronary sinus in ACS patients undergoing coronary angiography were seen with colchicine [204,205]. In ACS patients treated with short-term colchicine (given as 1 mg followed by 0.5 mg one hour later), monocyte sampling with ex vivo analysis after ATP stimulation revealed a significant reduction in IL-1β levels (versus pre-treatment levels, p<0.05)[206]. Similar effects were observed for caspase-1 levels (decreased by 30.2%, p<0.05) and pro-caspase-1 mRNA levels (decreased by 57.7%, p<0.05) in treated versus untreated control patients. Of note, short-term colchicine treatment had no effect on IL-1β levels in peripheral venous blood samples, implying the absence of second activation signal (i.e., no ATP stimulation) [206]. The 2013 Low-Dose Colchicine (LoDoCo) trial showed a significant reduction in ACS (4.6% vs. 13.6%) and 67% reduction in the composite endpoint (acute coronary syndrome, cardiac arrest, ischemic stroke) in patients treated with colchicine (0.5 mg daily) or standard secondary prevention, at a median follow-up of 3 years [204]. Results from the 2019 Colchicine Cardiovascular Outcomes Trial (COLCOT) also showed a significantly lower risk of ischemic cardiovascular events (composite of deaths from cardiovascular causes, resuscitated cardiac arrest, myocardial infarction, stroke, or urgent hospitalization for angina leading to coronary revascularization) in patients treated with colchicine 0.5 mg daily compared to placebo (5.5% vs 7.1%; HR 0.77; 95% CI 0.61–0.96). However, median follow-up was 22.6 months and decreases in individual components of the primary end point were not statistically significant [207]. Interestingly, other anti-gout therapies, such as allopurinol and Febuxostat have not been shown to modulate cardiovascular inflammatory risk independent of hyperuricemia.
7.7. Lectin-like oxLDL receptor-1 (LOX-1)
Lectin-like oxLDL receptor-1 (LOX-1) is the cognate receptor for oxLDL in macrophages that contributes to lipid accumulation and foam cell transformation in atherosclerosis. Several studies have shown that in vivo deletion of LOX-1 in LDLR−/− knockout mice decreased atherosclerosis fed with a high-fat diet, whereas in vitro LOX-1 silencing in macrophages mitigated mitochondrial DNA damage, ROS formation, and NLRP3 activation [34,208,209].
7.8. Farnesyl transferase inhibitors
Arglabin is a plant-derived terpenoid compound that acts as a farnesyl transferase inhibitor most notably leading to the activation of RAS proto-oncogene, pivotal in human tumors. Arglabin has also shown to reduce inflammation induced by atherosclerosis through a number of unexplained mechanisms. Arglabin also decreased plasma total cholesterol and triglycerides. The inhibition of cholesterol synthesis may occur by way of blocking enzymes downstream of 3β-hydroxy-3β-methyl-glutaryl coenzyme A reductase (HMGR), such as farnesyl diphosphate farnesyl-transferase, which catalyzes the first committed step in de novo cholesterol biosynthesis [210]. As such, Arglabin be a potentially good candidate to treat hypercholesterolemia and reduce atherosclerotic plaque size in addition to its inhibition of the NLRP3 inflammasome activity. In an ApoE−/− atherosclerotic mouse model fed with a high-fat diet, Arglabin effectively inhibited the NLRP3 inflammasome activity and significantly reduced the production of pro-inflammatory cytokines IL-1β, IL-18, and IL-1α in vitro and in vivo [210]. Arglabin activated autophagy as evidenced by the increase in LC3-II protein, which is a central protein in the autophagy pathway. Thus, in an ApoE2.Ki mouse model, Arglabin seemingly has the dual distinction of reducing inflammation via NLRP3 inflammasome inhibition as well as normalizing plasma cholesterol and triglyceride levels.
7.9. TFEB and Trehalose
Transcription factor EB (TFEB) is a key transcriptional regulator of autophagy and lysosome function in macrophages (i.e., master regulator of autophagy–lysosomal biogenesis). Induction or over-expression of TFEB stimulates overall degradative capacity of cells and has been shown to specifically increase lysosomal lipid catabolism, lipolysis and cellular fatty-acid oxidation [45,211,212]. Extensive work both in vivo and in vitro has shown that macrophage-specific TFEB overexpression is atheroprotective and increases macrophage autophagy and autophagy–lysosomal biogenesis as well as increases aggrephagy and the clearance of p62-enriched protein aggregates [213]. Furthermore, TFEB overexpression decreases macrophage apoptosis and generation of the pro-inflammatory cytokine IL-1β, with a resultant decrease in atherosclerosis and plaque complexity [213]. Trehalose is a non-reducing disaccharide composed of two glucose molecules joined by an alpha-alpha (1, 1) glycosidic bond and is a diverse molecule that is capable of acting as a novel transcriptional regulator, inducing both expression and nuclear translocation of TFEB. Similar to TFEB overexpression, trehalose reduced polyubiquitinated protein aggregates, IL-1β levels, and apoptosis in cultured macrophages. Furthermore, trehalose also reduces atherosclerosis and plaque complexity in vivo and in a macrophage ATG5- and SQSTM1-dependent manner [13,213]. As such, this study has demonstrated trehalose and TFEB activation to be potent inhibitor of NLRP3 inflammasome activation. The underlying mechanism(s) hold great promise in the development of novel therapeutic agents for the treatment of atherosclerotic cardiovascular disease [13].
8. Conclusion
Inflammation has been implicated as a key contributor to the pathogenesis of a wide array of disease processes, including atherosclerotic cardiovascular disease and atherosclerosis. Increasing evidence suggests that the NLRP3 inflammasome plays a central role in the progression of inflammatory-mediated atherosclerotic cardiovascular disease, giving rise to the release of pro-inflammatory cytokines, such as IL-1β. The NLRP3 is unique among inflammasome sensors, given its ability to detect a wide array of endogenous danger signal associated molecular patterns (DAMPs), such as cholesterol crystals, which are abundant in atherogenesis and underlie macrophage-mediated dysfunction, inflammatory cytokine production, and apoptosis. Elucidation of the signaling pathways involved in NLRP3-specific inflammasome activation will allow for the development of novel and targeted therapies against the initiation and progression of atherosclerosis. As outlined in this review, the complexity and heterogeneity of upstream signals contributing to NLRP3 activation is vast. Similarly, attempts at targeted inhibition of the NLRP3 inflammasome and/or related pathways via pharmacological or genetic manipulation have yielded variable results in regards to atherosclerotic cardiovascular disease. Further investigations by way of animal and human clinical trials are required for elucidation of the specific role of NLRP3. Furthermore, we need to be mindful of functional redundancy of other NLR family (and non-NLR family) members, as well as the potential adverse effects associated with inflammasome inhibition.
9. Expert opinion
Inflammation is involved in every stage of atherosclerosis. The NLRP3 inflammasome complex, along with constituents of its upstream and downstream pathways, serve as sensor, adaptor, and effector within a crucial inflammatory cascade linked to the lipid and immune cell dysfunction often seen in atherogenesis. Backed by several years of preclinical and recent translational research, our understanding of the molecular mechanisms and signaling cascades underlying NLRP3 inflammasome activation has improved and led to the development of specific inhibitors, some of which have advanced to clinical trials for the treatment of inflammatory disorders. In the groundbreaking CANTOS trial, interleukin-1β inhibition with Canakinumab has been at the forefront of targeted anti-inflammatory therapies for the treatment of atherosclerotic cardiovascular disease. While the relative degree of cardiovascular benefits observed in CANTOS has been a topic of significant debate, the fact that salutary cardiovascular outcomes were undoubtedly observed by targeting IL-1β demonstrates a critical pathologic role of the NLRP3 inflammasome/IL-1β pathway in atherosclerosis.
Success in future IL-1β-related therapies for cardiovascular disease lies in understanding the nuanced functions of the NLRP3 inflammasome in relation to plaque biology, which inherently involves macrophages. Macrophages are key mediators of inflammation with the capacity to interact with a wide variety of damage- and/or pathogen-associated molecular patterns (DAMPs and PAMPs) resulting in NLRP3 inflammasome activation and IL‐1β production, which in turn mediate the initiation and progression of vascular inflammation and atherogenesis. The multitude of secondary signals capable of triggering inflammation, specifically NLRP3-mediated inflammation, is highly diverse. Thus, one of the challenges facing the field is elucidating the specific links between sterile inflammation and aberrant lipid metabolism. The focus must remain with inflammasome function within macrophages, which mediate atherosclerosis from inception to end, involved in all major aspects of lipid accumulation and metabolism to pro- and anti-inflammatory signal modulation. Evaluation of inflammasome activation in the context of composite upstream secondary signals and downstream effector molecules and their interrelationship to macrophage lipid metabolism is the key to understanding atherosclerosis and achieving therapeutic specificity.
Certainly much of what is known regarding NLRP3 inflammasome activation is largely focused on plaque macrophages (and to a lesser extent, neutrophils). However, one must also consider inflammasome function as a complex interplay between immune cells such as macrophages, other plaque-resident cell types such as endothelial and vascular smooth muscle cells, and even surrounding cardiac myocytes and circulating platelets. The effect of inflammation on these cell types (and vice versa), as well as their interrelationship with macrophages within the atherosclerotic milieu is likely important for an even clearer understanding of vascular dysfunction. Atherothrombosis is the major clinical endpoint characterized by combined plaque disruption and occlusive thrombus formation that leads to cardiovascular morbidity and mortality. Understanding the role of macrophage inflammasome activation on thrombus formation, platelet, vascular smooth muscle and endothelial cell dysfunction is also an area ripe for exploration.
As we have come to recognize, chronic inflammation and immune dysfunction play different, yet likely integral roles in atherogenesis and cardiovascular disease. Chronic inflammatory and autoimmune disease (e.g., rheumatoid arthritis) carries cardiovascular risk independent of traditional risk factors, such as hyperlipidemia. Thus, the former “lipid hypothesis” and “inflammatory hypothesis” are now best taken together. Successful therapeutic targeting of atherosclerosis will most likely be found at the nexus of aberrant lipid metabolism and inflammation. Identification of pathways and molecular mediators common to both processes is likely to achieve highest efficacy. For instance, aberrant cholesterol handling underlies the development of cholesterol crystals which are pathognomonic of atherosclerotic plaques. Cholesterol crystals are also one of the most important triggers of the NLRP3 inflammasome in atherosclerosis. Selective targeting of this cholesterol/inflammasome nexus brings specificity to an anti-inflammatory therapy that is inherently non-selective.
Perhaps this underlies the limitations observed with interleukin-1β inhibition with Canakinumab in the CANTOS trial, as well as other similar agents that target the inflammatory component of atherosclerotic cardiovascular disease. One could argue that inhibiting IL‐1β production may be aimed too far downstream in the NLRP3 signaling cascade. This may explain the modest degree of adverse event reduction (statin-treated individuals) in CANTOS, similar to that seen with addition of a PCSK9 inhibitor, as seen in the FOURIER trial [214]. Additionally, broad inhibition of IL‐1β production lacks specificity. Higher rates of fatal infection were associated with IL‐1β blockade. The inability to target atherosclerotic-specific macrophages clearly underlies these observations.
Beyond target identification, precision therapeutic delivery methods with the capacity to deliver the right therapy to the right tissues and cells (e.g. plaque-resident foam cells) will be another challenge to overcome. Targeted and localized therapies have been achieved with invasive drug-eluting stent delivery systems, which have obvious limitations. Lanza and colleagues have demonstrated the use of therapeutic nanoparticles targeted against surface epitopes on vascular smooth muscle cells (VSMCs) as a means of delivering anti-proliferative agents (e.g., doxorubicin or paclitaxel) to areas of vascular injury for prevention-treatment of post-angiographic restenosis [215]. Such an approach offers the benefits of increased specificity, reduced invasiveness and quantitative visualization. Similarly, Winter et al have utilized paramagnetic nanoparticle-containing contrast agent targeted against αvβ3-integrin, allowing in vivo imaging of plaque-associated angiogenesis using an in vivo animal model of atherosclerosis [216]. Studies have also shown αvβ3-integrin to be a involved in macrophage foam cell formation and NF-κB-dependent cytokine production (e.g., IL-1β, IL-8, and TNFα) [217,218]. While these methods were initially developed as enhanced molecular imaging modalities aimed at precise detection and quantification of atherosclerotic lesions, their therapeutic potential is even more intriguing.
An interesting caveat to the aforementioned nanoparticle delivery systems comes by way of studies showing nanoparticles behaving similarly to particulate matter, thus, inducing macrophage inflammatory response via NLRP3. In LPS-primed macrophages, Baron et al demonstrated that nanoparticle uptake (nano-TiO2 and nano-SiO2) triggers NLRP3 inflammasome activation and IL-1β secretion through stimulation of intracellular-to-extracellular ATP release via Pannexin hemi-channels [71]. Demento and colleagues further showed that dual-signal modified nanoparticles loaded with both LPS and ovalbumin model antigen were a) preferentially taken up by dendritic (antigen-presenting) cells, b) successful in eliciting a potent humoral-cellular immune response when administered to mice (as measured by antigen-specific IgG titers), and c) capable of stimulating IL-1β production in macrophages with no response in NLRP3- and caspase-1-deficient macrophages [219]. They additionally showed abrogated inflammasome activity with disruption of endocytosis-lysosomal function, confirming that modified nanoparticles could function similar to particulate-based triggers (e.g., alum, silica) and harness the ability to provide both the priming and activation signals required to activate the canonical NLRP3 inflammasome pathway [219].
Additionally, a recent study by Flores and colleagues also demonstrated the ability to leverage macrophage-specific processes with targeted nanotherapy, using nanoparticles loaded with a small-molecule inhibitor against a downstream component in the CD47-mediated signaling pathway, namely SHP-1 (Src homology 2 domain-containing phosphatase-1), which plays a role in atherosclerosis by suppressing phagocytosis in macrophages. It was demonstrated that these nanoparticles were preferentially taken up in atherosclerotic plaque macrophages and restoration of macrophage-specific phagocytic capacity reduced the amount of aortic plaque in atherosclerotic ApoE-deficient mice [220]. On macrophages, CD47 serves as a ligand for the signal regulatory protein-α (SIRPα) that, in turn, induces Src homology 2 domain-containing phosphatase-1 (SHP-1) to inhibit phagocytic function [221–223]. Suppression of macrophage-mediated phagocytosis (efferocytosis) via the CD47-SIRPα-SHP-1 signaling pathway allows for the propagation of cells that would otherwise be destined for phagocytic removal [224,225]. CD47-mediated anti-phagocytic signal has been established in the pathophysiology of both cancer and atherosclerosis [226,227]. Kojima et al. demonstrated increased expression of CD47 within plaques of atherosclerotic mouse models that could be selectively targeted (anti-CD47 antibody) to inhibit atherosclerotic plaque development [227].
Targeting inflammation, even in very surgical ways including highly specific NLRP3 inflammasome inhibitors, can be fraught with complexity resulting in unintended cardiovascular outcomes. Nevertheless, the great strides that have been made for preclinical targeting of the NLRP3 inflammasome in atherosclerosis are a great prelude for emerging cardiovascular therapeutics focused on inflammation and inflammatory signaling. Coupling such designer-nanoparticle delivery systems with discoveries in NLRP3-mediated signaling cascades may provide the necessary specificity to leverage the therapeutic efficacy required to target atherosclerosis at its true nexus of inflammation and lipid metabolism.
Article Highlights.
The NLRP3 (nucleotide‐binding domain, leucine‐rich repeat–containing receptor family, pyrin domain‐containing 3) inflammasome is a multimeric protein complex composed of NLRP3, ASC, and pro‐caspase‐1 that is predominately expressed on immune cells and is a key mediator of vascular inflammation and atherogenesis.
NLRP3 inflammasome activation is a critical catalyst for atherogenesis and facilitates the production and release of pro-inflammatory cytokines, such as interleukin‐1β (IL-1β).
Regulation of the NLRP3 inflammasome in atherosclerosis occurs at the nexus of cholesterol metabolism and inflammation, relying on integral cellular processes such as autophagy and lysosome function, particularly in lipid-laden macrophages.
Regulation of NLRP3 inflammasome will be important for understanding atherosclerosis as well as identifying potential therapeutic targets.
Targeting the inflammatory component of atherosclerosis via inhibitors specific to the NLRP3 inflammasome and its signaling cascade has broadened the scope for new therapeutics that may have a significant impact on cardiovascular disease.
Acknowledgments
Funding
This work was supported by NIH R01 HL125838, VA MERIT I01 BX003415, American Diabetes Association #1-18-IBS-029, and the Foundation for Barnes-Jewish Hospital.
Footnotes
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers
- [1].Benjamin EJ, Virani SS, Callaway CW, et al. Heart disease and stroke statistics - 2018 update: A report from the American Heart Association. Circulation. 2018; [DOI] [PubMed] [Google Scholar]
- [2].Sidney S, Quesenberry CP, Jaffe MG, et al. Recent trends in cardiovascular mortality in the United States and public health goals. JAMA Cardiol. 2016; [DOI] [PubMed] [Google Scholar]
- [3].Naghavi M, Abajobir AA, Abbafati C, et al. Global, regional, and national age-sex specifc mortality for 264 causes of death, 1980–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Libby P, Tabas I, Fredman G, et al. Inflammation and its resolution as determinants of acute coronary syndromes. Circ. Res. 2014; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Schroder K, Tschopp J. The Inflammasomes. Cell. 2010. [DOI] [PubMed] [Google Scholar]
- [6].Duewell P, Kono H, Rayner KJ, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;** (**= of considerable importance; key study outlining the convergence of both aberrant cholesterol handling and inflammation, with evidence that cholesterol crystals serve as endogenous danger signals, inducing phagolysosomal damage and resulting in NLRP3 activation to contribute to diet-induced atherosclerosis in mice).
- [7].Libby P Interleukin-1 Beta as a Target for Atherosclerosis Therapy: Biological Basis of CANTOS and Beyond. J. Am. Coll. Cardiol. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Chi H, Messas E, Levine RA, et al. Interleukin-1 receptor signaling mediates atherosclerosis associated with bacterial exposure and/or a high-fat diet in a murine apolipoprotein E heterozygote model: Pharmacotherapeutic implications. Circulation. 2004; [DOI] [PubMed] [Google Scholar]
- [9].Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N. Engl. J. Med. 2017;** (**=of considerable importance; large, clinical trial targeting IL-1β as an inflammatory mediator of atherosclerosis, highlighting potential clinical-mechanistic links between inflammasome activity and atherothrombosis).
- [10].Baylis RA, Gomez D, Mallat Z, et al. The CANTOS trial one important step for clinical cardiology but a giant leap for vascular biology. Arterioscler. Thromb. Vasc. Biol. 2017; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Sharma D, Kanneganti TD. The cell biology of inflammasomes: Mechanisms of inflammasome activation and regulation. J. Cell Biol. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Muñoz-Planillo R, Kuffa P, Martínez-Colón G, et al. K+ Efflux Is the Common Trigger of NLRP3 Inflammasome Activation by Bacterial Toxins and Particulate Matter. Immunity. 2013; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Evans TD, Sergin I, Zhang X, et al. Target acquired: Selective autophagy in cardiometabolic disease. Sci. Signal. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Latz E, Xiao TS, Stutz A. Activation and regulation of the inflammasomes. Nat. Rev. Immunol. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Masters SL, Latz E, O’Neill LAJ. The inflammasome in atherosclerosis and type 2 diabetes. Sci. Transl. Med. 2011. [DOI] [PubMed] [Google Scholar]
- [16].He WT, Wan H, Hu L, et al. Gasdermin D is an executor of pyroptosis and required for interleukin-1β secretion. Cell Res. 2015; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Shi J, Zhao Y, Wang K, et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015; [DOI] [PubMed] [Google Scholar]
- [18].Tabas I, Bornfeldt KE. Macrophage Phenotype and Function in Different Stages of Atherosclerosis. Circ. Res. 2016; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Remmerie A, Scott CL. Macrophages and lipid metabolism. Cell. Immunol. 2018; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Out R, Hoekstra M, Hildebrand RB, et al. Macrophage ABCG1 deletion disrupts lipid homeostasis in alveolar macrophages and moderately influences atherosclerotic lesion development in LDL receptor-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2006; [DOI] [PubMed] [Google Scholar]
- [21].Kennedy MA, Barrera GC, Nakamura K, et al. ABCG1 has a critical role in mediating cholesterol efflux to HDL and preventing cellular lipid accumulation. Cell Metab. 2005; [DOI] [PubMed] [Google Scholar]
- [22].Hutchins PM, Heinecke JW. Cholesterol efflux capacity, macrophage reverse cholesterol transport and cardioprotective HDL. Curr. Opin. Lipidol. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Yvan-Charvet L, Wang N, Tall AR. Role of HDL, ABCA1, and ABCG1 transporters in cholesterol efflux and immune responses. Arterioscler. Thromb. Vasc. Biol. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Phillips MC. Molecular mechanisms of cellular cholesterol efflux. J. Biol. Chem. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Zhu X, Owen JS, Wilson MD, et al. Macrophage ABCA1 reduces MyD88-dependent toll-like receptor trafficking to lipid rafts by reduction of lipid raft cholesterol. J. Lipid Res. 2010; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Chowdhury SM, Zhu X, Aloor JJ, et al. Proteomic analysis of ABCA1-Null macrophages reveals a role for stomatin-like protein-2 in raft composition and toll-like receptor signaling. Mol. Cell. Proteomics. 2015; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Ouimet M, Franklin V, Mak E, et al. Autophagy regulates cholesterol efflux from macrophage foam cells via lysosomal acid lipase. Cell Metab. 2011; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Li G, Mongillo M, Chin KT, et al. Role of ERO1-α-mediated stimulation of inositol 1,4,5-triphosphate receptor activity in endoplasmic reticulum stress-induced apoptosis. J. Cell Biol. 2009; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Tabas I Macrophage apoptosis in atherosclerosis: Consequences on plaque progression and the role of endoplasmic reticulum stress. Antioxidants Redox Signal. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Podrez EA, Poliakov E, Shen Z, et al. Identification of a novel family of oxidized phospholipids that serve as ligands for the macrophage scavenger receptor CD36. J. Biol. Chem. 2002; [DOI] [PubMed] [Google Scholar]
- [31].Choi SH, Harkewicz R, Lee JH, et al. Lipoprotein accumulation in macrophages via toll-like receptor-4-dependent fluid phase uptake. Circ. Res. 2009; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kunjathoor VV, Febbraio M, Podrez EA, et al. Scavenger receptors class A-I/II and CD36 are the principal receptors responsible for the uptake of modified low density lipoprotein leading to lipid loading in macrophages. J. Biol. Chem. 2002; [DOI] [PubMed] [Google Scholar]
- [33].Van Tits LJH, Stienstra R, van Lent PL, et al. Oxidized LDL enhances pro-inflammatory responses of alternatively activated M2 macrophages: A crucial role for Krüppel-like factor 2. Atherosclerosis. 2011; [DOI] [PubMed] [Google Scholar]
- [34].Ding Z, Liu S, Wang X, et al. LOX-1, mtDNA damage, and NLRP3 inflammasome activation inmacrophages: Implications in atherogenesis. Cardiovasc. Res. 2014; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Sheedy FJ, Grebe A, Rayner KJ, et al. CD36 coordinates NLRP3 inflammasome activation by facilitating intracellular nucleation of soluble ligands into particulate ligands in sterile inflammation. Nat. Immunol. 2013; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Scull CM, Tabas I. Mechanisms of ER stress-induced apoptosis in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2011; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Tabas I, Ron D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat. Cell Biol. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Seimon TA, Nadolski MJ, Liao X, et al. Atherogenic lipids and lipoproteins trigger CD36-TLR2-dependent apoptosis in macrophages undergoing endoplasmic reticulum stress. Cell Metab. 2010; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Rajam̈aki K, Lappalainen J, Öörni K, et al. Cholesterol crystals activate the NLRP3 inflammasome in human macrophages: A novel link between cholesterol metabolism and inflammation. PLoS One. 2010; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Guo H, Callaway JB, Ting JPY. Inflammasomes: Mechanism of action, role in disease, and therapeutics. Nat. Med. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Duewell P, Kono H, Katey J. Rayner, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Grebe A, Latz E. Cholesterol crystals and inflammation. Curr. Rheumatol. Rep. 2013; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Feng B, Yaol PM, Li Y, et al. The endoplasmic reticulum is the site of cholesterol-induced cytotoxicity in macrophages. Nat. Cell Biol. 2003; [DOI] [PubMed] [Google Scholar]
- [44].Kavurma MM, Rayner KJ, Karunakaran D. The walking dead: Macrophage inflammation and death in atherosclerosis. Curr. Opin. Lipidol. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Emanuel R, Sergin I, Bhattacharya S, et al. Induction of lysosomal biogenesis in atherosclerotic macrophages can rescue lipid-induced lysosomal dysfunction and downstream sequelae. Arterioscler. Thromb. Vasc. Biol. 2014; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].de la Roche M, Hamilton C, Mortensen R, et al. Trafficking of cholesterol to the ER is required for NLRP3 inflammasome activation. J. Cell Biol. 2018; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Dubland JA, Francis GA. Lysosomal acid lipase: At the crossroads of normal and atherogenic cholesterol metabolism. Front. Cell Dev. Biol. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Lehti S, Nguyen SD, Belevich I, et al. Extracellular Lipids Accumulate in Human Carotid Arteries as Distinct Three-Dimensional Structures and Have Proinflammatory Properties. Am. J. Pathol. 2018; [DOI] [PubMed] [Google Scholar]
- [49].Yeon SH, Yang G, Lee HE, et al. Oxidized phosphatidylcholine induces the activation of NLRP3 inflammasome in macrophages. J. Leukoc. Biol. 2017; [DOI] [PubMed] [Google Scholar]
- [50].Rampanelli E, Orsó E, Ochodnicky P, et al. Metabolic injury-induced NLRP3 inflammasome activation dampens phospholipid degradation. Sci. Rep. 2017; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Corrêa R, Silva LFF, Ribeiro DJS, et al. Lysophosphatidylcholine Induces NLRP3 Inflammasome-Mediated Foam Cell Formation and Pyroptosis in Human Monocytes and Endothelial Cells. Front. Immunol. 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Lauber K, Bohn E, Kröber SM, et al. Apoptotic cells induce migration of phagocytes via caspase-3-mediated release of a lipid attraction signal. Cell. 2003; [DOI] [PubMed] [Google Scholar]
- [53].Halle A, Hornung V, Petzold GC, et al. The NALP3 inflammasome is involved in the innate immune response to amyloid-β. Nat. Immunol. 2008; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Hornung V, Bauernfeind F, Halle A, et al. Silica crystals and aluminum salts mediate NALP-3 inflammsome activation via phagosomal destabilization. Nat. Immunol. 2010; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Orlowski GM, Colbert JD, Sharma S, et al. Multiple Cathepsins Promote Pro–IL-1β Synthesis and NLRP3-Mediated IL-1β Activation. J. Immunol. 2015; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Usui F, Shirasuna K, Kimura H, et al. Critical role of caspase-1 in vascular inflammation and development of atherosclerosis in Western diet-fed apolipoprotein E-deficient mice. Biochem. Biophys. Res. Commun. 2012; [DOI] [PubMed] [Google Scholar]
- [57].Katsnelson MA, Rucker LG, Russo HM, et al. K + Efflux Agonists Induce NLRP3 Inflammasome Activation Independently of Ca 2+ Signaling. J. Immunol. 2015; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Katsnelson MA, Lozada-Soto KM, Russo HM, et al. NLRP3 inflammasome signaling is activated by low-level lysosome disruption but inhibited by extensive lysosome disruption: Roles for K+ efflux and Ca2+ influx. Am. J. Physiol. - Cell Physiol. 2016; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Riteau N, Gombault A, Baron L, et al. Purinergic signaling: A common pathway for crystals-mediated inflammasome activation. Inflamm. Res. 2011; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Dostert C, Pétrilli V, Van Bruggen R, et al. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science (80-. ). 2008; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Samstad EO, Niyonzima N, Nymo S, et al. Cholesterol Crystals Induce Complement-Dependent Inflammasome Activation and Cytokine Release. J. Immunol. 2014; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Ferrari D, Chiozzi P, Falzoni S, et al. Extracellular ATP triggers IL-1 beta release by activating the purinergic P2Z receptor of human macrophages. J. Immunol. 1997; [PubMed] [Google Scholar]
- [63].Perregaux D, Gabel CA. Interleukin-1β maturation and release in response to ATP and nigericin. Evidence that potassium depletion mediated by these agents is a necessary and common feature of their activity. J. Biol. Chem. 1994; [PubMed] [Google Scholar]
- [64].Mariathasan S, Weiss DS, Newton K, et al. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006; [DOI] [PubMed] [Google Scholar]
- [65].Pétrilli V, Papin S, Dostert C, et al. Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Differ. 2007; [DOI] [PubMed] [Google Scholar]
- [66].Kankkunen P, Välimäki E, Rintahaka J, et al. Trichothecene mycotoxins activate NLRP3 inflammasome through a P2X7 receptor and Src tyrosine kinase dependent pathway. Hum. Immunol. 2014; [DOI] [PubMed] [Google Scholar]
- [67].Shimada K, Crother TR, Karlin J, et al. Oxidized Mitochondrial DNA Activates the NLRP3 Inflammasome during Apoptosis. Immunity. 2012; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Stachon P, Heidenreich A, Merz J, et al. P2X7 Deficiency Blocks Lesional Inflammasome Activity and Ameliorates Atherosclerosis in Mice. Circulation. 2017; [DOI] [PubMed] [Google Scholar]
- [69].Coutinho-Silva R, Persechini PM. P2Z purinoceptor-associated pores induced by extracellular ATP in macrophages and J774 cells. Am. J. Physiol. - Cell Physiol. 1997; [DOI] [PubMed] [Google Scholar]
- [70].Pelegrin P, Surprenant A. Pannexin-1 mediates large pore formation and interleukin-1β release by the ATP-gated P2X7 receptor. EMBO J. 2006; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Baron L, Gombault A, Fanny M, et al. The NLRP3 inflammasome is activated by nanoparticles through ATP, ADP and adenosine. Cell Death Dis. 2015; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Paramel Varghese G, Folkersen L, Strawbridge RJ, et al. NLRP3 Inflammasome Expression and Activation in Human Atherosclerosis. J. Am. Heart Assoc. 2016; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Riteau N, Baron L, Villeret B, et al. ATP release and purinergic signaling: A common pathway for particle-mediated inflammasome activation. Cell Death Dis. 2012; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Groß CJ, Mishra R, Schneider KS, et al. K+ Efflux-Independent NLRP3 Inflammasome Activation by Small Molecules Targeting Mitochondria. Immunity. 2016; [DOI] [PubMed] [Google Scholar]
- [75].Gaidt MM, Ebert TS, Chauhan D, et al. Human Monocytes Engage an Alternative Inflammasome Pathway. Immunity. 2016; [DOI] [PubMed] [Google Scholar]
- [76].Wolf AJ, Reyes CN, Liang W, et al. Hexokinase Is an Innate Immune Receptor for the Detection of Bacterial Peptidoglycan. Cell. 2016; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Murakami T, Ockinger J, Yu J, et al. Critical role for calcium mobilization in activation of the NLRP3 inflammasome. Proc. Natl. Acad. Sci. U. S. A. 2012; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Zhong Z, Zhai Y, Liang S, et al. TRPM2 links oxidative stress to NLRP3 inflammasome activation. Nat. Commun. 2013; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Seimon TA, Obstfeld A, Moore KJ, et al. Combinatorial pattern recognition receptor signaling alters the balance of life and death in macrophages. Proc. Natl. Acad. Sci. [Internet]. 2006;103:19794 LP–19799. Available from: http://www.pnas.org/content/103/52/19794.abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Rossol M, Pierer M, Raulien N, et al. Extracellular Ca2+ is a danger signal activating the NLRP3 inflammasome through G protein-coupled calcium sensing receptors. Nat. Commun. 2012; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Seimon TA, Obstfeld A, Moore KJ, et al. Combinatorial pattern recognition receptor signaling alters the balance of life and death in macrophages. Proc. Natl. Acad. Sci. [Internet]. 2006. [cited 2020 Jan 13];103:19794–19799. Available from: https://www.pnas.org/content/103/52/19794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Lee GS, Subramanian N, Kim AI, et al. The calcium-sensing receptor regulates the NLRP3 inflammasome through Ca2+ and cAMP. Nature. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Peng K, Liu L, Wei D, et al. P2X7R is involved in the progression of atherosclerosis by promoting NLRP3 inflammasome activation. Int. J. Mol. Med. 2015; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Lee ES, Yoon CH, Kim YS, et al. The double-strand RNA-dependent protein kinase PKR plays a significant role in a sustained ER stress-induced apoptosis. FEBS Lett. 2007; [DOI] [PubMed] [Google Scholar]
- [85].Tallóczy Z, Jiang W, Virgin IV HW, et al. Regulation of starvation- and virus-induced autophagy by the elF2α kinase signaling pathway. Proc. Natl. Acad. Sci. U. S. A. 2002; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Lu B, Nakamura T, Inouye K, et al. Novel role of PKR in inflammasome activation and HMGB1 release. Nature. 2012; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Warnatsch A, Ioannou M, Wang Q, et al. Neutrophil extracellular traps license macrophages for cytokine production in atherosclerosis. Science (80-. ). 2015; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Brinkmann V, Reichard U, Goosmann C, et al. Neutrophil Extracellular Traps Kill Bacteria. Science (80-. ). 2004; [DOI] [PubMed] [Google Scholar]
- [89].Jorch SK, Kubes P. An emerging role for neutrophil extracellular traps in noninfectious disease. Nat. Med. 2017. [DOI] [PubMed] [Google Scholar]
- [90].de Bont CM, Boelens WC, Pruijn GJM. NETosis, complement, and coagulation: a triangular relationship. Cell. Mol. Immunol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Robinson N, Ganesan R, Hegedűs C, et al. Programmed necrotic cell death of macrophages: Focus on pyroptosis, necroptosis, and parthanatos. Redox Biol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Sônego F, Castanheira FV e. S, Ferreira RG, et al. Paradoxical roles of the neutrophil in sepsis: Protective and deleterious. Front. Immunol. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Liu L, Sun B. Neutrophil pyroptosis: new perspectives on sepsis. Cell. Mol. Life Sci. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].van de Veerdonk FL, Netea MG, Dinarello CA, et al. Inflammasome activation and IL-1β and IL-18 processing during infection. Trends Immunol. 2011. [DOI] [PubMed] [Google Scholar]
- [95].Franck G, Mawson TL, Folco EJ, et al. Roles of PAD4 and netosis in experimental atherosclerosis and arterial injury implications for superfcial erosion. Circ. Res. 2018; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Quillard T, Araújo HA, Franck G, et al. TLR2 and neutrophils potentiate endothelial stress, apoptosis and detachment: Implications for superficial erosion. Eur. Heart J. 2015; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Pasterkamp G, Den Ruijter HM, Libby P. Temporal shifts in clinical presentation and underlying mechanisms of atherosclerotic disease. Nat. Rev. Cardiol. 2016. [DOI] [PubMed] [Google Scholar]
- [98].Folco EJ, Sukhova GK, Quillard T, et al. Moderate hypoxia potentiates interleukin-1â production in activated human macrophages. Circ. Res. 2014; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Tannahill GM, Curtis AM, Adamik J, et al. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature. 2013; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Sergin I, Evans TD, Bhattacharya S, et al. Hypoxia in plaque macrophages a new danger signal for interleukin-1β activation? Circ. Res. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Björnheden T, Levin M, Evaldsson M, et al. Evidence of hypoxic areas within the arterial wall in vivo. Arterioscler. Thromb. Vasc. Biol. 1999; [DOI] [PubMed] [Google Scholar]
- [102].Nakano D, Hayashi T, Tazawa N, et al. Chronic hypoxia accelerates the progression of atherosclerosis in apolipoprotein E-knockout mice. Hypertens. Res. 2005; [DOI] [PubMed] [Google Scholar]
- [103].Sluimer JC, Gasc JM, van Wanroij JL, et al. Hypoxia, Hypoxia-Inducible Transcription Factor, and Macrophages in Human Atherosclerotic Plaques Are Correlated With Intraplaque Angiogenesis. J. Am. Coll. Cardiol. 2008; [DOI] [PubMed] [Google Scholar]
- [104].Sluimer JC, Daemen MJ. Novel concepts in atherogenesis: Angiogenesis and hypoxia in atherosclerosis. J. Pathol. 2009. [DOI] [PubMed] [Google Scholar]
- [105].Jun J, Reinke C, Bedja D, et al. Effect of intermittent hypoxia on atherosclerosis in apolipoprotein E-deficient mice. Atherosclerosis. 2010; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Parathath S, Mick SL, Feig JE, et al. Hypoxia is present in murine atherosclerotic plaques and has multiple adverse effects on macrophage lipid metabolism. Circ. Res. 2011; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Ghezzi P, Dinarello CA, Bianchi M, et al. Hypoxia increases production of interleukin-1 and tumor necrosis factor by human mononuclear cells. Cytokine. 1991; [DOI] [PubMed] [Google Scholar]
- [108].Carmi Y, Voronov E, Dotan S, et al. The Role of Macrophage-Derived IL-1 in Induction and Maintenance of Angiogenesis. J. Immunol. 2009; [DOI] [PubMed] [Google Scholar]
- [109].Hultén LM, Levin M. The role of hypoxia in atherosclerosis. Curr. Opin. Lipidol. 2009. [DOI] [PubMed] [Google Scholar]
- [110].Jain T, Nikolopoulou EA, Xu Q, et al. Hypoxia inducible factor as a therapeutic target for atherosclerosis. Pharmacol. Ther. 2018. [DOI] [PubMed] [Google Scholar]
- [111].Gao L, Chen Q, Zhou X, et al. The role of hypoxia-inducible factor 1 in atherosclerosis. J. Clin. Pathol. 2012. [DOI] [PubMed] [Google Scholar]
- [112].Chandel NS, Maltepe E, Goldwasser E, et al. Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proc. Natl. Acad. Sci. U. S. A. 1998; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Chandel NS, McClintock DS, Feliciano CE, et al. Reactive oxygen species generated at mitochondrial Complex III stabilize hypoxia-inducible factor-1α during hypoxia: A mechanism of O2 sensing. J. Biol. Chem. 2000; [DOI] [PubMed] [Google Scholar]
- [114].Marsch E, Sluimer JC, Daemen MJAP. Hypoxia in atherosclerosis and inflammation. Curr. Opin. Lipidol. 2013. [DOI] [PubMed] [Google Scholar]
- [115].Mills E, O’Neill LAJ. Succinate: A metabolic signal in inflammation. Trends Cell Biol. 2014. [DOI] [PubMed] [Google Scholar]
- [116].Kirii H, Niwa T, Yamada Y, et al. Lack of interleukin-1ß decreases the severity of atherosclerosis in apoE-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2003; [DOI] [PubMed] [Google Scholar]
- [117].Gage J, Hasu M, Thabet M, et al. Caspase-1 Deficiency Decreases Atherosclerosis in Apolipoprotein E-Null Mice. Can. J. Cardiol. 2012; [DOI] [PubMed] [Google Scholar]
- [118].Hendrikx T, Jeurissen MLJ, Van Gorp PJ, et al. Bone marrow-specific caspase-1/11 deficiency inhibits atherosclerosis development in Ldlr−/− mice. FEBS J. 2015; [DOI] [PubMed] [Google Scholar]
- [119].Russo MT, De Luca G, Degan P, et al. Accumulation of the oxidative base lesion 8-hydroxyguanine in DNA of tumor-prone mice defective in both the Myh and Ogg1 DNA glycosylases. Cancer Res. 2004; [DOI] [PubMed] [Google Scholar]
- [120].Tumurkhuu G, Shimada K, Dagvadorj J, et al. Ogg1-dependent DNA repair regulates NLRP3 inflammasome and prevents atherosclerosis. Circ. Res. 2016; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Menu P, Pellegrin M, Aubert JF, et al. Atherosclerosis in ApoE-deficient mice progresses independently of the NLRP3 inflammasome. Cell Death Dis. 2011; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Kleemann R, Zadelaar S, Kooistra T. Cytokines and atherosclerosis: A comprehensive review of studies in mice. Cardiovasc. Res. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Zheng F, Xing S, Gong Z, et al. Silence of NLRP3 suppresses atherosclerosis and stabilizes plaques in apolipoprotein E-deficient mice. Mediators Inflamm. 2014; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Wang R, Wang Y, Mu N, et al. Activation of NLRP3 inflammasomes contributes to hyperhomocysteinemia-aggravated inflammation and atherosclerosis in apoE-deficient mice. Lab. Investig. 2017; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Thacker SG, Zarzour A, Chen Y, et al. High-density lipoprotein reduces inflammation from cholesterol crystals by inhibiting inflammasome activation. Immunology. 2016; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Niyonzima N, Samstad EO, Aune MH, et al. Reconstituted High-Density Lipoprotein Attenuates Cholesterol Crystal–Induced Inflammatory Responses by Reducing Complement Activation. J. Immunol. 2015; [DOI] [PubMed] [Google Scholar]
- [127].Lima H, Jacobson LS, Goldberg MF, et al. Role of lysosome rupture in controlling Nlrp3 signaling and necrotic cell death. Cell Cycle. 2013; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].De Nardo D, Labzin LI, Kono H, et al. High-density lipoprotein mediates anti-inflammatory reprogramming of macrophages via the transcriptional regulator ATF3. Nat. Immunol. 2014; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Liu K, Czaja MJ. Regulation of lipid stores and metabolism by lipophagy. Cell Death Differ. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Singh R, Kaushik S, Wang Y, et al. Autophagy regulates lipid metabolism. Nature. 2009; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Le Guezennec X, Brichkina A, Huang YF, et al. Wip1-dependent regulation of autophagy, obesity, and atherosclerosis. Cell Metab. 2012; [DOI] [PubMed] [Google Scholar]
- [132].Razani B, Feng C, Coleman T, et al. Autophagy links inflammasomes to atherosclerotic progression. Cell Metab. 2012;** (**= of considerable importance, key study linking autophagy-deficient state, atherosclerosis, and inflammasome activation, whereby a complete loss of autophagy exacerbates cholesterol crystal-mediated inflammasome hyperactivation and pro-atherogenic IL-1β response in plaque macrophages).
- [133].Zhou R, Yazdi AS, Menu P, et al. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;* (*= of importance, disruption of organelle-specific autophagy -- mitophagy -- linked to NLRP3 inflammasome activation via distinct mechanism not seen with other inflammasomes).
- [134].Zhang X, Sergin I, Evans TD, et al. High-protein diets increase cardiovascular risk by activating macrophage mTOR to suppress mitophagy. Nat. Metab. 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Shi CS, Shenderov K, Huang NN, et al. Activation of autophagy by inflammatory signals limits IL-1β production by targeting ubiquitinated inflammasomes for destruction. Nat. Immunol. 2012; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Zhong Z, Umemura A, Sanchez-Lopez E, et al. NF-κB Restricts Inflammasome Activation via Elimination of Damaged Mitochondria. Cell. 2016; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Kimura T, Mandell M, Deretic V. Precision autophagy directed by receptor regulators - emerging examples within the TRIM family. J. Cell Sci. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Kimura T, Jain A, Choi SW, et al. TRIM-directed selective autophagy regulates immune activation. Autophagy. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Kimura T, Jain A, Choi SW, et al. TRIM-mediated precision autophagy targets cytoplasmic regulators of innate immunity. J. Cell Biol. 2015; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Shi H, Wang Y, Li X, et al. NLRP3 activation and mitosis are mutually exclusive events coordinated by NEK7, a new inflammasome component. Nat. Immunol. 2016; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].He Y, Zeng MY, Yang D, et al. NEK7 is an essential mediator of NLRP3 activation downstream of potassium efflux. Nature. 2016; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Schmid-Burgk JL, Chauhan D, Schmidt T, et al. A genome-wide CRISPR screen identifies NEK7 as an essential component of NLRP3 inflammasome activation. J. Biol. Chem. 2016; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Stehlik C, Dorfleutner A. COPs and POPs: Modulators of Inflammasome Activity. J. Immunol. 2007; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Le HT, Harton JA. Pyrin- and CARD-only proteins as regulators of NLR functions. Front. Immunol. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Almeida L, Khare S, Misharin A, et al. The pyrin domain-only protein POP1 inhibits NLRP3-dependent inflammatory disease. Arthritis Rheumatol. 2015; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Karasawa T, Kawashima A, Usui F, et al. Oligomerized CARD16 promotes caspase-1 assembly and IL-1β processing. FEBS Open Bio. 2015; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Coll RC, Robertson AAB, Chae JJ, et al. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat. Med. 2015; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Coll RC, Hill JR, Day CJ, et al. MCC950 directly targets the NLRP3 ATP-hydrolysis motif for inflammasome inhibition. Nat. Chem. Biol. 2019;* (*= of considerable importance; discovery of direct, small-molecule inhibitor of the NLRP3 inflammasome and elucidation of mechanism of action relating to ATP hydrolysis as requirement for active conformation likely to play role in rational drug design).
- [149].Vande Walle L, Stowe IB, Šácha P, et al. MCC950/CRID3 potently targets the NACHT domain of wild-type NLRP3 but not disease-associated mutants for inflammasome inhibition. PLoS Biol. 2019; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Gordon R, Albornoz EA, Christie DC, et al. Inflammasome inhibition prevents -synuclein pathology and dopaminergic neurodegeneration in mice. Sci. Transl. Med. 2018; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Van Der Heijden T, Kritikou E, Venema W, et al. NLRP3 Inflammasome Inhibition by MCC950 Reduces Atherosclerotic Lesion Development in Apolipoprotein E-Deficient Mice - Brief Report. Arterioscler. Thromb. Vasc. Biol. 2017; [DOI] [PubMed] [Google Scholar]
- [152].Ma T, Thiagarajah JR, Yang H, et al. Thiazolidinone CFTR inhibitor identified by high-throughput screening blocks cholera toxin-induced intestinal fluid secretion. J. Clin. Invest. 2002; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Jiang H, He H, Chen Y, et al. Identification of a selective and direct NLRP3 inhibitor to treat inflammatory disorders. J. Exp. Med. 2017; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Shim DW, Shin WY, Yu SH, et al. BOT-4-one attenuates NLRP3 inflammasome activation: NLRP3 alkylation leading to the regulation of its ATPase activity and ubiquitination. Sci. Rep. 2017; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Wang Z, Liu B, Zhu J, et al. Nicotine-mediated autophagy of vascular smooth muscle cell accelerates atherosclerosis via nAChRs/ROS/NF-κB signaling pathway. Atherosclerosis. 2019; [DOI] [PubMed] [Google Scholar]
- [156].Strickson S, Campbell DG, Emmerich CH, et al. The anti-inflammatory drug BAY 11–7082 suppresses the MyD88-dependent signalling network by targeting the ubiquitin system. Biochem. J. 2013; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Christ A, Günther P, Lauterbach MAR, et al. Western Diet Triggers NLRP3-Dependent Innate Immune Reprogramming. Cell. 2018; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Dutta P, Courties G, Wei Y, et al. Western Diet Triggers NLRP3-Dependent Innate Immune Reprogramming. Cell. 2017; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Napier BA, Andres-Terre M, Massis LM, et al. Western diet regulates immune status and the response to LPS-driven sepsis independent of diet-associated microbiome. Proc. Natl. Acad. Sci. U. S. A. 2019; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Audia JP, Yang XM, Crockett ES, et al. Caspase-1 inhibition by VX-765 administered at reperfusion in P2Y12 receptor antagonist-treated rats provides long-term reduction in myocardial infarct size and preservation of ventricular function. Basic Res. Cardiol. 2018; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Rudolphi K, Gerwin N, Verzijl N, et al. Pralnacasan, an inhibitor of interleukin-1β converting enzyme, reduces joint damage in two murine models of osteoarthritis. Osteoarthr. Cartil. 2003; [DOI] [PubMed] [Google Scholar]
- [162].MacKenzie SH, Schipper JL, Clark AC. The potential for caspases in drug discovery. Curr. Opin. Drug Discov. Dev. 2010. [PMC free article] [PubMed] [Google Scholar]
- [163].Vromman A, Ruvkun V, Shvartz E, et al. Stage-dependent differential effects of interleukin-1 isoforms on experimental atherosclerosis. Eur. Heart J. 2019; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Libby P, Ordovas JM, Auger KR, et al. Endotoxin and tumor necrosis factor induce interleukin-1 gene expression in adult human vascular endothelial cells. Am. J. Pathol. 1986; [PMC free article] [PubMed] [Google Scholar]
- [165].Libby P, Warner SJC, Friedman GB. Interleukin 1: A mitogen for human vascular smooth muscle cells that induces the release of growth-inhibitory prostanoids. J. Clin. Invest. 1988; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Ridker PM, Howard CP, Walter V, et al. Effects of interleukin-1β inhibition with canakinumab on hemoglobin A1c, lipids, C-reactive protein, interleukin-6, and fibrinogen a phase IIb randomized, placebo-controlled trial. Circulation. 2012; [DOI] [PubMed] [Google Scholar]
- [167].Schlesinger N, Alten RE, Bardin T, et al. Canakinumab for acute gouty arthritis in patients with limited treatment options: Results from two randomised, multicentre, active-controlled, double-blind trials and their initial extensions. Ann. Rheum. Dis. 2012; [DOI] [PubMed] [Google Scholar]
- [168].Ruperto N, Brunner HI, Quartier P, et al. Two randomized trials of canakinumab in systemic juvenile idiopathic arthritis. N. Engl. J. Med. 2012; [DOI] [PubMed] [Google Scholar]
- [169].Ridker PM, Libby P, MacFadyen JG, et al. Modulation of the interleukin-6 signalling pathway and incidence rates of atherosclerotic events and all-cause mortality: Analyses from the Canakinumab Anti-Inflammatory Thrombosis Outcomes Study (CANTOS). Eur. Heart J. 2018; [DOI] [PubMed] [Google Scholar]
- [170].Fuster JJ, MacLauchlan S, Zuriaga MA, et al. Clonal hematopoiesis associated with TET2 deficiency accelerates atherosclerosis development in mice. Science (80-. ). 2017; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Bhaskar V, Yin J, Mirza AM, et al. Monoclonal antibodies targeting IL-1 beta reduce biomarkers of atherosclerosis in vitro and inhibit atherosclerotic plaque formation in Apolipoprotein E-deficient mice. Atherosclerosis. 2011; [DOI] [PubMed] [Google Scholar]
- [172].Gomez D, Baylis RA, Durgin BG, et al. Interleukin-1β has atheroprotective effects in advanced atherosclerotic lesions of mice. Nat. Med. 2018; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Groß O, Yazdi AS, Thomas CJ, et al. Inflammasome Activators Induce Interleukin-1α Secretion via Distinct Pathways with Differential Requirement for the Protease Function of Caspase-1. Immunity. 2012; [DOI] [PubMed] [Google Scholar]
- [174].Freigang S, Ampenberger F, Weiss A, et al. Fatty acid-induced mitochondrial uncoupling elicits inflammasome- independent IL-1α and sterile vascular inflammation in atherosclerosis. Nat. Immunol. 2013; [DOI] [PubMed] [Google Scholar]
- [175].Kamari Y, Werman-Venkert R, Shaish A, et al. Differential role and tissue specificity of interleukin-1α gene expression in atherogenesis and lipid metabolism. Atherosclerosis. 2007; [DOI] [PubMed] [Google Scholar]
- [176].Dinarello CA, Simon A, Van Der Meer JWM. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat. Rev. Drug Discov. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Morton AC, Rothman AMK, Greenwood JP, et al. The effect of interleukin-1 receptor antagonist therapy on markers of inflammation in non-ST elevation acute coronary syndromes: The MRC-ILA Heart Study. Eur. Heart J. 2015; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Van Tassell BW, Lipinski MJ, Appleton D, et al. Rationale and design of the Virginia Commonwealth University–Anakinra Remodeling Trial-3 (VCU-ART3): A randomized, placebo-controlled, double-blinded, multicenter study. Clin. Cardiol. 2018; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Abbate A, Kontos MC, Grizzard JD, et al. Interleukin-1 Blockade With Anakinra to Prevent Adverse Cardiac Remodeling After Acute Myocardial Infarction (Virginia Commonwealth University Anakinra Remodeling Trial [VCU-ART] Pilot Study). Am. J. Cardiol. 2010; [DOI] [PubMed] [Google Scholar]
- [180].Abbate A, Van Tassell BW, Biondi-Zoccai G, et al. Effects of interleukin-1 blockade with anakinra on adverse cardiac remodeling and heart failure after acute myocardial infarction [from the virginia commonwealth university-anakinra remodeling trial (2) (vcu-art2) pilot study]. Am. J. Cardiol. 2013; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Mallat Z, Corbaz A, Scoazec A, et al. Expression of interleukin-18 in human atherosclerotic plaques and relation to plaque instability. Circulation. 2001; [DOI] [PubMed] [Google Scholar]
- [182].Wang J, Sun C, Gerdes N, et al. Interleukin 18 function in atherosclerosis is mediated by the interleukin 18 receptor and the Na-Cl co-transporter. Nat. Med. 2015; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Gerdes N, Sukhova GK, Libby P, et al. Expression of interleukin (IL)-18 and functional IL-18 receptor on human vascular endothelial cells, smooth muscle cells, and macrophages: Implications for atherogenesis. J. Exp. Med. 2002; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Blankenberg S, Tiret L, Bickel C, et al. Interleukin-18 is a strong predictor of cardiovascular death in stable and unstable angina. Circulation. 2002; [DOI] [PubMed] [Google Scholar]
- [185].Swerdlow DI, Holmes MV, Kuchenbaecker KB, et al. The interleukin-6 receptor as a target for prevention of coronary heart disease: A mendelian randomisation analysis. Lancet. 2012; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Cai T, Zhang Y, Ho YL, et al. Association of interleukin 6 receptor variant with cardiovascular disease effects of interleukin 6 receptor blocking therapy: A phenome - Wide association study. JAMA Cardiol. 2018; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Hong EP, Kim DH, Suh JG, et al. Genetic risk assessment for cardiovascular disease with seven genes associated with plasma C-reactive protein concentrations in Asian populations. Hypertens. Res. 2014; [DOI] [PubMed] [Google Scholar]
- [188].Huizinga TWJ, Fleischmann RM, Jasson M, et al. Sarilumab, a fully human monoclonal antibody against IL-6R αin patients with rheumatoid arthritis and an inadequate response to methotrexate: Efficacy and safety results from the randomised SARIL-RA-MOBILITY part a trial. Ann. Rheum. Dis. 2014; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Kawashiri SY, Kawakami A, Yamasaki S, et al. Effects of the anti-interleukin-6 receptor antibody, tocilizumab, on serum lipid levels in patients with rheumatoid arthritis. Rheumatol. Int. 2011; [DOI] [PubMed] [Google Scholar]
- [190].Gosling J, Slaymaker S, Gu L, et al. MCP-1 deficiency reduces susceptibility to atherosclerosis in mice that overexpress human apolipoprotein B. J. Clin. Invest. 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Gu L, Okada Y, Clinton SK, et al. Absence of monocyte chemoattractant protein-1 reduces atherosclerosis in low density lipoprotein receptor-deficient mice. Mol. Cell. 1998; [DOI] [PubMed] [Google Scholar]
- [192].Dawson T C, Kuziel W A, Osahar T A, et al. Absence of CC chemokine receptor-2 reduces atherosclerosis in apolipoprotein E-deficient mice. Atherosclerosis. 1999; [DOI] [PubMed] [Google Scholar]
- [193].Gilbert J, Lekstrom-Himes J, Donaldson D, et al. Effect of CC chemokine receptor 2 CCR2 blockade on serum C-reactive protein in individuals at atherosclerotic risk and with a single nucleotide polymorphism of the monocyte chemoattractant protein-1 promoter region. Am. J. Cardiol. 2011; [DOI] [PubMed] [Google Scholar]
- [194].Everett BM, Pradhan AD, Solomon DH, et al. Rationale and design of the Cardiovascular Inflammation Reduction Trial: A test of the inflammatory hypothesis of atherothrombosis. Am. Heart J. 2013; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Ahlehoff O, Skov L, Gislason G, et al. Cardiovascular disease event rates in patients with severe psoriasis treated with systemic anti-inflammatory drugs: A Danish real-world cohort study. J. Intern. Med. 2013; [DOI] [PubMed] [Google Scholar]
- [196].Naranjo A, Sokka T, Descalzo MA, et al. Cardiovascular disease in patients with rheumatoid arthritis: Results from the QUEST-RA study. Arthritis Res. Ther. 2007; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Ridker PM, Everett BM, Pradhan A, et al. Low-dose methotrexate for the prevention of atherosclerotic events. N. Engl. J. Med. 2019; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Cronstein BN, Naime D, Ostad E. The antiinflammatory mechanism of methotrexate: Increased adenosine release at inflamed sites diminishes leukocyte accumulation in an in vivo model of inflammation. J. Clin. Invest. 1993; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Haskó G, Cronstein B. Regulation of inflammation by adenosine. Front. Immunol. 2013; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Alten R, Gomez-Reino J, Durez P, et al. Efficacy and safety of the human anti-IL-1beta monoclonal antibody canakinumab in rheumatoid arthritis: Results of a 12-week, phase II, dose-finding study. BMC Musculoskelet. Disord. 2011; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Martinon F, Pétrilli V, Mayor A, et al. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006; [DOI] [PubMed] [Google Scholar]
- [202].Huang C, Cen C, Wang C, et al. Synergistic effects of colchicine combined with atorvastatin in rats with hyperlipidemia. Lipids Health Dis. 2014; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Nidorf SM, Eikelboom JW, Budgeon CA, et al. Low-dose colchicine for secondary prevention of cardiovascular disease. J. Am. Coll. Cardiol. 2013; [DOI] [PubMed] [Google Scholar]
- [204].Nidorf M, Thompson PL. Effect of Colchicine (0.5 mg Twice Daily) on High-Sensitivity C-Reactive Protein Independent of Aspirin and Atorvastatin in Patients With Stable Coronary Artery Disease. Am. J. Cardiol. 2007; [DOI] [PubMed] [Google Scholar]
- [205].Martínez GJ, Robertson S, Barraclough J, et al. Colchicine Acutely Suppresses Local Cardiac Production of Inflammatory Cytokines in Patients With an Acute Coronary Syndrome. J. Am. Heart Assoc. 2015; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Robertson S, Martínez GJ, Payet CA, et al. Colchicine therapy in acute coronary syndrome patients acts on caspase-1 to suppress NLRP3 inflammasome monocyte activation. Clin. Sci. 2016; [DOI] [PubMed] [Google Scholar]
- [207].Tardif JC, Kouz S, Waters DD, et al. Efficacy and safety of low-dose colchicine after myocardial infarction. N. Engl. J. Med. 2019; [DOI] [PubMed] [Google Scholar]
- [208].Hu C, Dandapat A, Sun L, et al. LOX-1 deletion decreases collagen accumulation in atherosclerotic plaque in low-density lipoprotein receptor knockout mice fed a high-cholesterol diet. Cardiovasc. Res. 2008; [DOI] [PubMed] [Google Scholar]
- [209].Mehta JL, Sanada N, Hu CP, et al. Deletion of LOX-1 reduces atherogenesis in LDLR knockout mice fed high cholesterol diet. Circ. Res. 2007; [DOI] [PubMed] [Google Scholar]
- [210].Abderrazak A, Couchie D, Mahmood DFD, et al. Anti-inflammatory and antiatherogenic effects of the NLRP3 inflammasome inhibitor arglabin in ApoE2.Ki mice fed a high-fat diet. Circulation. 2015; [DOI] [PubMed] [Google Scholar]
- [211].Sardiello M, Palmieri M, Ronza A Di, et al. A gene network regulating lysosomal biogenesis and function. Science (80-. ). 2009; [DOI] [PubMed] [Google Scholar]
- [212].Settembre C, Ballabio A. Lysosomal adaptation: How the lysosome responds to external cues. Cold Spring Harb. Perspect. Biol. 2014; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Sergin I, Evans TD, Zhang X, et al. Exploiting macrophage autophagy-lysosomal biogenesis as a therapy for atherosclerosis. Nat. Commun. 2017; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [214].Sabatine MS, Giugliano RP, Keech A, et al. Rationale and design of the Further cardiovascular OUtcomes Research with PCSK9 Inhibition in subjects with Elevated Risk trial. Am. Heart J. 2016; [DOI] [PubMed] [Google Scholar]
- [215].Lanza GM, Yu X, Winter PM, et al. Targeted antiproliferative drug delivery to vascular smooth muscle cells with a magnetic resonance imaging nanoparticle contrast agent: Implications for rational therapy of restenosis. Circulation. 2002; [DOI] [PubMed] [Google Scholar]
- [216].Winter PM, Morawski AM, Caruthers SD, et al. Molecular Imaging of Angiogenesis in Early-Stage Atherosclerosis With αvβ3-Integrin-Targeted Nanoparticles. Circulation. 2003; [DOI] [PubMed] [Google Scholar]
- [217].Antonov AS, Kolodgie FD, Munn DH, et al. Regulation of macrophage foam cell formation by αVβ3 integrin: Potential role in human atherosclerosis. Am. J. Pathol. 2004; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [218].Antonov AS, Antonova GN, Munn DH, et al. αVβ3 integrin regulates macrophage inflammatory responses via PI3 kinase/Akt-dependent NF-κB activation. J. Cell. Physiol. 2011; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219].Demento SL, Eisenbarth SC, Foellmer HG, et al. Inflammasome-activating nanoparticles as modular systems for optimizing vaccine efficacy. Vaccine. 2009; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [220].Flores AM, Hosseini-Nassab N, Jarr KU, et al. Pro-efferocytic nanoparticles are specifically taken up by lesional macrophages and prevent atherosclerosis. Nat. Nanotechnol. 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [221].Henson PM, Bratton DL, Fadok VA. Apoptotic cell removal. Curr. Biol. 2001; [DOI] [PubMed] [Google Scholar]
- [222].Erwig LP, Henson PM. Clearance of apoptotic cells by phagocytes. Cell Death Differ. 2008. [DOI] [PubMed] [Google Scholar]
- [223].Lv Z, Bian Z, Shi L, et al. Loss of Cell Surface CD47 Clustering Formation and Binding Avidity to SIRPα Facilitate Apoptotic Cell Clearance by Macrophages. J. Immunol. 2015; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [224].Brown EJ, Frazier WA. Integrin-associated protein (CD47) and its ligands. Trends Cell Biol. 2001. [DOI] [PubMed] [Google Scholar]
- [225].Gresham HD, Dale BM, Potter JW, et al. Negative regulation of phagocytosis in murine macrophages by the Src kinase family member, Fgr. J. Exp. Med. 2000; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [226].Chao MP, Majeti R, Weissman IL. Programmed cell removal: A new obstacle in the road to developing cancer. Nat. Rev. Cancer. 2012. [DOI] [PubMed] [Google Scholar]
- [227].Kojima Y, Volkmer JP, McKenna K, et al. CD47-blocking antibodies restore phagocytosis and prevent atherosclerosis. Nature. 2016; [DOI] [PMC free article] [PubMed] [Google Scholar]