Abstract
The blood–brain barrier (BBB) plays a crucial role in maintaining the specialized microenvironment of the central nervous system (CNS). In aging, the stability of the BBB declines and the permeability increases. The list of CNS pathologies involving BBB dysfunction is growing. The opening of the BBB and subsequent infiltration of serum components to the brain can lead to a host of processes resulting in progressive synaptic, neuronal dysfunction, and detrimental neuroinflammatory changes. Such processes have been implicated in different diseases, including vascular dementia, stroke, Alzheimer’s disease (AD), Parkinson’s disease, multiple sclerosis, amyotrophic lateral sclerosis, hypoxia, ischemia, and diabetes mellitus. The BBB damage is also observed in tauopathies that lack amyloid-β overproduction, suggesting a role for tau in BBB damage. Tauopathies represent a heterogeneous group of around 20 different neurodegenerative diseases characterized by abnormal deposition of the MAPT in cells of the nervous system. Neuropathology of tauopathies is defined as intracellular accumulation of neurofibrillary tangles (NFTs) consisting of aggregated hyper- and abnormal phosphorylation of tau protein and neuroinflammation. Disruption of the BBB found in tauopathies is driven by chronic neuroinflammation. Production of pro-inflammatory signaling molecules such as cytokines, chemokines, and adhesion molecules by glial cells, neurons, and endothelial cells determine the integrity of the BBB and migration of immune cells into the brain. The inflammatory processes promote structural changes in capillaries such as fragmentation, thickening, atrophy of pericytes, accumulation of laminin in the basement membrane, and increased permeability of blood vessels to plasma proteins. Here, we summarize the knowledge about the role of tau protein in BBB structural and functional changes.
Keywords: neurovascular unit, blood-brain barrier, tauopathy, tau protein, neuroinflammation
Introduction
Tau proteins are the most frequent microtubule-associated proteins in the brain and are characterized as intrinsically disordered proteins. They are abundant in the neurons of the central nervous system (CNS) and have roles primarily in maintaining the stability of microtubules in axons. They are also less expressed in brain-resident immune cells: astrocytes and oligodendrocytes (Williams, 2006). Tau proteins are important for cell signaling, synaptic plasticity, and regulation of genomic stability (Guo et al., 2017). The adult human brain expresses at least six isoforms of tau protein, which are derived from a mictorubule-associated protein tau (MAPT) tau gene as a result of alternative splicing of its messenger RNA (Goedert et al., 1989; Hanes et al., 2009; Zhang et al., 2009). The tau isoforms range in size from 352 to 441 amino acid residues and differed on the presence of 0, 1, or 2 sequence inserts in the amino-terminus of the protein and inclusion or exclusion of the second of four microtubule-binding potential repeat domains coded by exon 10 (Hanes et al., 2009; Grinberg et al., 2013). In humans, tau is subject to many post-translational modifications, including hyperphosphorylation, truncation, nitration, glycation, glycosylation, ubiquitination, polyaminations, and self-aggregation into insoluble paired helical filaments (Mena et al., 1996; Mohandas et al., 2009; Novak, 2012; Beharry et al., 2014).
Tauopathies represent a heterogeneous group of around 20 neurodegenerative diseases characterized by abnormal deposition of the MAPT in neurons and glial cells (Zilka et al., 2009; Ferrer et al., 2014). Histopathologically, the tauopathies are characterized by the presence of intracellular insoluble inclusions of abnormally modified protein tau into neurofibrillary or gliofibrillary tangles. Tangles are formed by hyperphosphorylated tau protein, causing the tau to dissociate from microtubules and form insoluble inclusions. Tauopathies are classified by the predominance of tau isoforms found in cytoplasmic inclusions of tau protein: those with inclusions predominantly composed of tau with 3-repeat (3R-tauopathies), those with predominantly 4-repeat (4R-tauopathies), or an equal ratio of 3R:4R tau. The most common tauopathies are Alzheimer’s disease, frontotemporal dementia with parkinsonism liked to chromosome 17 (FTDP-17), progressive supranuclear palsy (PSP), corticobasal degeneration (CBD), and Pick’s disease (PiD). There are also rarer tauopathies including argyrophilic grain disease (AGD), postencephalitic parkinsonism (PEP), parkinsonism dementia complex of Guam (PDCG), tangle-dominant dementia, and a new category of tauopathy known as the globular glial tauopathies (GGTs). According to their tau pathology, we can divide tauopathies into three main groups (Table 1).
Table 1.
The first subgroup of tauopathies is represented by 3R/4R tauopathies, such as Alzheimer’s disease (AD), frontotemporal dementia, and parkinsonism linked to chromosome 17, parkinsonism dementia complex of Guam (Lytico-bodig disease), chronic traumatic encephalopathy, postencephalitic parkinsonism, atypical parkinsonism of Guadeloupe, primary age-related tauopathy, or diffuse neurofilament tangles with calcification.
| Disease | Predominant isoform | Blood–brain barrier changes | Amyloid-β deposition | References |
|---|---|---|---|---|
| Alzheimer’s disease | 3R + 4R |
|
Present | Higuchi et al. (1987), Scheibel et al. (1987), Miyakawa et al. (1988), Stewart et al. (1992), Buee et al. (1997), Zlokovic (2005), Abbott et al. (2006), Desai et al. (2007), Pérez et al. (2007), Liu et al. (2008), Zlokovic (2011), Østergaard et al. (2013), Cai et al. (2018), and Yamazaki et al. (2019) |
| Frontotemporal dementia and parkinsonism linked to chromosome 17 | 3R, 4R/3R + 4R |
|
Absent | De Reuck et al. (2012) and De Reuck et al. (2013) |
| Progressive supranuclear palsy | 4R |
|
Absent | Bartels et al. (2008) |
| Corticobasal degeneration | 4R |
|
Absent | Koga et al. (2019) |
| Chronic traumatic encephalopathy | 3R + 4R |
|
Present in approximately half of the cases | McKee et al. (2009) and Farrell et al. (2019) |
| Parkinson–dementia complex of Guam (Lytico-bodig disease) | 3R + 4R |
|
Present in some cases | Buee et al. (1994), Buee et al. (1997), and Majerova et al. (2018) |
| Pick disease | 3R |
|
Absent | Buee et al. (1994), Komori (1999), and Buee et al. (1997) |
| Argyrophilic grain disease | 4R | - | Absent | - |
| Globular glial tauopathies | 4R |
|
Absent | Zlokovic (2008), Bartels et al. (2009), and Haydon (2001) |
| Aging-related tau astrogliopathy | 4R |
|
Absent | Kovacs et al. (2016) |
| Primary age-related tauopathy | 3R + 4R | - | Absent | - |
| Postencephalitic parkinsonism | 3R + 4R | - | Very rare | - |
| Atypical parkinsonism of Guadeloupe | 3R + 4R | - | Absent | - |
| Diffuse neurofibrillary tangles with calcification | 3R + 4R | - | Absent | - |
Three-repeat tauopathies include Pick’s disease and some forms of frontotemporal dementia and parkinsonism linked to chromosome 17 and to the four-repeat tauopathies belong progressive supranuclear palsy, corticobasal degeneration, argyrophilic grain disease, globular glial tauopathies, or aging-related tau astrogliopathy.
3R tauopathies
4R tauopathies
3R/4R tauopathies
However, tau neuropathology is rarely isolated, and it is associated with the deposition of at least one other amyloidogenic protein, such as α-synuclein or huntingtin in most tauopathies. Based on this, we can assume, that tau may have important pathological roles in these disorders with multiple pathologies (Jensen et al., 1999; Hashiguchi et al., 2000).
AD, representing around 70% of all dementia cases, is the best-described tauopathy (Avila et al., 2004). Brain pathology in AD is characterized by the presence of extracellular amyloid-β plaques and intracellular (NFTs; Glenner and Wong, 1984; Grundke-Iqbal et al., 1986). For more than 20 years, the research has been focused on “amyloid-β cascade hypothesis” (Armstrong, 2013), according to which, the amyloid-β is the central pathological feature of AD. This theory assumed that the elimination of amyloid-β could be therapeutic in AD patients. This theory was mainly supported by the discovery of a familial form of AD, which is connected with an APP gene mutation (Korczyn, 2008). The majority of Alzheimer’s patients do not have a mutation causing an increase in APP; therefore, other processes, such as the impaired elimination of amyloid-β, can cause the accumulation of amyloid-β (Preston et al., 2003; Bell and Zlokovic, 2009). On the other hand, the “tau hypothesis” is based on a strong correlation between the clinical symptoms of AD and neurofibrillary pathology. Under pathological conditions, highly soluble tau protein undergoes different post-translational with the latter formation of insoluble paired helical filaments (Mena et al., 1996; Mohandas et al., 2009). These processes lead to the neuronal death and subsequent release of pathologically modified tau proteins into the extracellular environment, activating microglia and inducing the spread of tau pathology by a prion-like mechanism (Maccioni et al., 2010; Kovac et al., 2011). These facts clearly demonstrate that unlike amyloid-β senile plaques, tau pathology correlates with the progress of AD, and therefore, it is considered by many to be the main cause of neurodegeneration (Kovacech et al., 2009).
Neurovascular Changes in Tauopathies
CNS is considered to be one of the most delicate systems in the human body. This fact explains the necessity to maintain the extracellular environment of the CNS highly regulated (Hawkins and Davis, 2005). Three main barrier layers at the interface between blood and tissue protect the CNS: (i) the choroid plexus epithelium located between the blood and the ventricular cerebrospinal fluid; (ii) the arachnoid epithelium situated between the blood and the subarachnoid cerebrospinal fluid; and (iii) the vascular blood-brain barrier (BBB), which mediates the communication between the periphery and the CNS (Abbott, 2005).
BBB strictly controls the exchange of cells and molecules between blood and CNS. Previously, the BBB has been characterized as a layer of endothelial cells forming the vessel/capillary wall. Recently, the BBB is a component of the neurovascular unit (NVU: Abbott et al., 2006). The NVU represents a highly dynamic system, where the proper functioning of the brain depends on the functional interactions of the endothelial cells, neurons, pericytes, mast cells, and glial cells. Moreover, the NVU can be expanded to include circulating immune cells and peripheral tissue cells, which are connected to it by humoral secretions and thus can influence the physical, biochemical, and immune processes of the CNS barriers (Kousik et al., 2012; Wong et al., 2013).
The BBB is formed by endothelial cells firmly attached by tight junctions that restrict the paracellular pathway (Stamatovic et al., 2008). Intercellular junctions play crucial roles in tissue integrity and also in vascular permeability (Wallez and Huber, 2008; Zlokovic, 2008; Tietz and Engelhardt, 2015). Endothelial cells are closely surrounded by pericytes and enclosed by the basement membrane. Pericytes occupy the perivascular space between the capillary wall and astrocytic endfeet, except in the large vessels where smooth muscle cells replace them. Astrocytic endfeet surround the endothelial cells of the BBB and provide biochemical support (Figure 1A). The BBB phenotype has developed mainly under the influence of astrocytes and pericytes and is generally characterized by more complex tight junctions than in other endothelial cells and a number of specific transport and enzyme systems that are responsible for the transport of molecules across the endothelial cells (Abbott, 2002; Bell et al., 2010). The physical breakdown of the BBB is caused by disruption of cell-to-cell junctions between endothelial cells, rising bulk-flow fluid transcytosis, and/or enzymatic degradation of the capillary basement membrane (Zlokovic, 2011).
Figure 1.
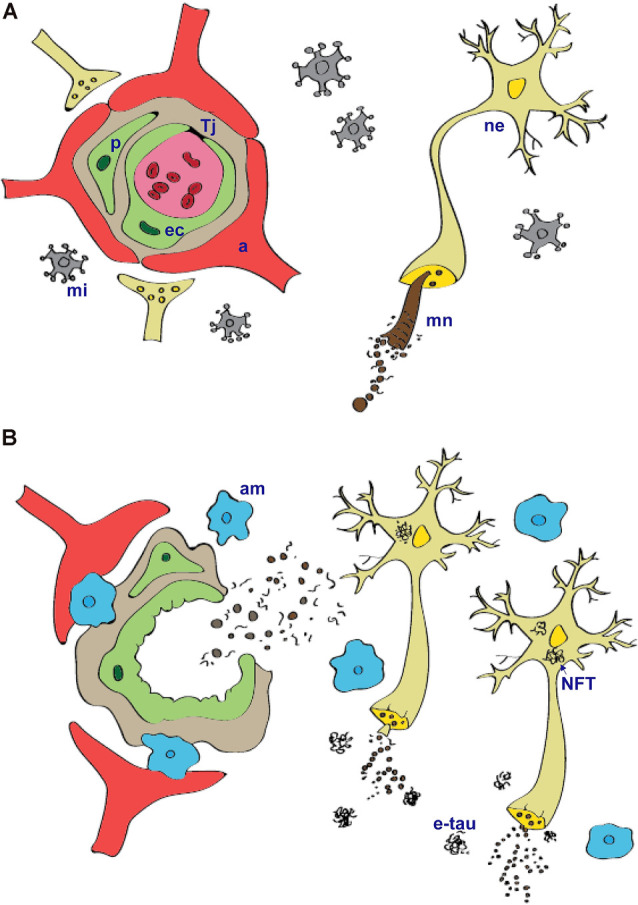
Tau protein physiology (A) and pathology (B) within the neurovascular unit. Microtubule-associated protein tau is important for assembly and stabilization of microtubular network (mn) in the neurons (ne). In tauopathies, abnormally modified tau protein that is hyperphosporylated and truncated form insoluble intracellular aggregates—neurofibrillary tangles. Microtubular network in axons disintegrate, and this leads to impairment in communication between neurons. Injured neurons and extracellular tau aggregates (e-tau) signal to the microglia (mi) that become activated. Activated microglia (am) produce pro-inflammatory cytokines and chemokines that can cause structural and functional changes of the blood–brain barrier. Prolonged local neuroinflammation stimulate transmigration of peripheral blood monocyte-derived macrophages and release of plasma components into extravascular space. a, astrocytes; Tj, tight junctions; ne, neurons; ec, endothelial cells; p, pericytes; mi, microglia; mn, microtubular network; am, activated microglia; NFT, neurofibrillary tangles; etau, extracellular tau aggregates.
Paracellular and transcellular pathways are the most common ways for the transport of molecules across the BBB. During the paracellular pathway, molecules are transported between the endothelial cells through the junctional complex. The paracellular pathway is described as a passive transport based on the movement of hydrophilic molecules across the barrier, depending on their electrochemical, hydrostatic, and osmotic gradient (Fu and Wright, 2018). The transport is controlled by tight junctions regulated by various signaling cascades. The principal integral membrane tight junction proteins are occludin, claudin-1, claudin-5, and members of the JAMs family. These proteins interact with other tight junctions proteins, such as ZO-1, ZO-2, and cingulin in the cytoplasm. Subsequently, they are connected to actin filaments (Di Liegro and Savettieri, 2005).
Transcellular transport provides the movement of nutrients, ions, or particles across the BBB depending or not depending on energy. During the transcellular transport, molecules are transported across the luminal and abluminal membrane of the capillary endothelium by different mechanisms including receptor-mediated transcytosis, efflux transport systems, endocytosis of positively charged molecules, and carrier-mediated transport (Fu and Wright, 2018).
The list of CNS pathologies involving BBB dysfunction is rapidly expanding. BBB disruption is associated with numerous pathological conditions that affect the CNS such as ischemia, infections, epilepsy, tumors, and neuroinflammatory diseases including tauopathies. AD is the only tauopathy with described mechanisms of how NVU changes contribute to its pathology. Few reports characterize the general morphology of vessels in PiD, PDCG, FTDP-17, traumatic brain injury/chronic traumatic encephalopathy (CTE), and vascular PSP. In case of PSP, GGTs, and aging-related tau astrogliopathy, a few reports describing functional changes of NVU exists. For other tauopathies, there are no reports dealing with vascular or NVU changes (Michalicova et al., 2017).
The Role of Tau in the Regulation of BBB Integrity in Physiological and Pathological Conditions
The “healthy” BBB is crucial for proper neuronal function and “healthy” neurons are important for maintaining local milieu of NVU (Rhea et al., 2019). Neurons are very closely associated with brain capillaries. It was estimated that almost every neuron has its own capillary (Zlokovic, 2008). Neurons are important for maintaining the tight junctions (Savettieri et al., 2000), metabolism, regulation of blood flow, and permeability (Zvi et al., 1997). Thus, pathological processes in neurons inevitably affect the NVU.
That the neuropathological hallmarks such as intra- and extracellular protein aggregates are associated with chronic neuroinflammation has long been known. Chronic neuroinflammation affects the BBB by increasing vascular permeability, promoting structural changes in brain capillaries such as fragmentation, thickening, atrophy of pericytes, accumulation of laminin in the basement membrane, increasing permeability to small molecules and plasma proteins, enhancing the migration of immune cells, altering transport systems, or influencing the role of BBB as signaling interface (De Vries et al., 2012; Erickson and Banks, 2013b; Persidsky et al., 2016; Figure 1B). The inflammatory mediators play an important role in regulating blood-to-brain cell transmigration, perpetuating inflammation, and thus exacerbating the disease pathology. Structural and functional changes of BBB lead to progressive synaptic and neuronal dysfunction (Di Liegro and Savettieri, 2005; Zlokovic, 2008; Abbott et al., 2010).
Previously, it has been shown that tangle formation correlated with neuroinflammation in AD (Dipatre and Gelman, 1997; Overmyer et al., 1999; Sheffield et al., 2000; Laurent et al., 2018) and in non-AD human tauopathies such as tangle-predominant dementia, Guam parkinsonism dementia, PSP, and CBD (Imamura et al., 2001; Ishizawa and Dickson, 2001). Moreover, neuroinflammation linked to tau deposition has been well documented in mice transgenic for human mutant tau protein (Bellucci et al., 2004; Yoshiyama et al., 2007) or in transgenic rats expressing misfolded truncated tau protein derived from AD (Zilka et al., 2006; Stozicka et al., 2010).
We showed that in contrast to amyloid-β peptides, truncated tau is not directly toxic to brain endothelial cells. The effect of tau is however mediated through the activation of glial cells (Kovac et al., 2009). Moreover, tau-induced activation of glial cells increased expression of endothelial adhesion molecules and increased transport of leukocytes across BBB (Majerova et al., 2019).
Together, these findings suggest that tau protein has an important role in regulation of microenvironment within the NVU during the physiological and pathological conditions.
Alzheimer’s Disease
AD is a globally widespread chronic disease affecting around 25 million people worldwide. AD is characterized by cerebrovascular and neuronal dysfunctions leading to a progressive decrease in cognitive functions (Bell and Zlokovic, 2009; Dalvi, 2012). On the neuropathological level, AD is defined by the presence of extracellular amyloid plaques composed of amyloid-β peptide aggregates and NFTs formed by hyperphosphorylated, truncated, and aggregated tau protein, neural loss, loss of synapses, neuroinflammation, and oxidative stress (Cai et al., 2018). The neurofibrillary pathology in AD contains insoluble inclusions of 3R and 4R tau isoforms (Siddiqua and Margittai, 2010).
Two basic forms of AD are known. Familial cases are predominantly early-onset (younger than 65 years), but also late-onset cases have been described (Bekris et al., 2010). This type is usually caused by specific mutations in transmembrane proteins—amyloid precursor protein (APP), presenilin 1, or presenilin 2. The latter two proteins are essential components of a protease complex involved in the generation of amyloid-β from APP (Bertram et al., 2010; Iqbal et al., 2014; Wirz et al., 2014). The late-onset (older than 65 years) sporadic form is more complex and representing more than 99% of all cases (Bertram et al., 2010). The presence of several additional risk factors was described, including traumatic brain injuries (Walter and van Echten-Deckert, 2013), diabetes mellitus, hypercholesterolemia, inflammation, environmental factors, such as diet, toxicological exposure, hormonal factors, and various aspects of lifestyle (Lahiri et al., 2007; Stozicka et al., 2010). Moreover, evidence suggests that the BBB dysfunction is one of the most common pathophysiological hallmark of AD involved in vascular risk factors (Tarasoff-Conway et al., 2016; Yamazaki and Kanekiyo, 2017; Miners et al., 2018). In AD, the functional and structural changes of BBB have been investigated for more than 30 years (Erickson et al., 2012). De La Torre reviewed more than 200 studies showing vascular involvement in AD. According to his results, he concluded that cognitive impairment and CNS pathology might be secondary to vascular changes in AD (De La Torre, 2002).
The leakage of substances from plasma into the CNS, changes in efflux and influx transporters leading to accumulation of the toxins in the CNS, and altered expression and secretion of proteins by the NVU cells are the critical pathways of vascular dysfunction connected with AD (Deane and Zlokovic, 2007; Lok et al., 2007; Neuwelt et al., 2008; Bell and Zlokovic, 2009; Bell et al., 2010; Zlokovic, 2011; Erickson and Banks, 2013a). In AD patients, changes in the regulation of cerebral blood flow caused by reduced microvascular density, an increase in endothelial pinocytosis, decrease in mitochondrial content, accumulation of collagen, and loss of tight junctions leading to the BBB breakdown and subsequent infiltration of blood-borne molecules are frequently present (Zlokovic, 2011; Østergaard et al., 2013). Thanks to the short distance between adjacent brain capillaries, a rapid exchange of substances between the CNS and blood circulation can occur. Malfunction of the nutrient and oxygen supply or impaired elimination of toxic metabolic waste can cause dysregulations of neuronal functioning. Often degeneration of brain endothelial wall in AD results in the accumulation of amyloid-β on the outer side of the basement membrane, promoting a local neuroinflammatory vascular response (Zlokovic, 2005). Failure of the amyloid-β transport from brain to the periphery is caused especially by the decreased levels of LRP-1 (low-density lipoprotein receptor-related protein 1) and increased levels of RAGE (receptor for advanced glycation end products; Cai et al., 2018). Activated NVU cells start to release pro-inflammatory cytokines and vasoactive substances. Subsequent processes, such as the decrease of cerebral blood flow and amplification of cells, contribute to cognitive impairment. Zlokovic suggests that such physiological changes of the NVU, compromised brain microcirculation, and vascular neuroinflammatory responses play an important role in the development of AD (Zlokovic, 2005). Other functional changes of brain vasculature in AD include failure of influx (glucose transporters GLUT1 and GLUT3) and efflux transport systems (P-glycoprotein), altered levels of agrin, and upregulation of aquaporin AQP4 expression (Abbott et al., 2006; Desai et al., 2007; Liu et al., 2008). Moreover, in some AD brains, structural changes of the vasculature, such as increased numbers of fragmented vessels with fewer intact branches, atrophic string vessels, different irregularities in the capillary surface, changes of vessel diameter, thickening (Zlokovic, 2005), vacuolization (Buee et al., 1997), and local disruption of the capillary basement membrane have been observed. Increased numbers of pericytes (Stewart et al., 1992), atrophy of pericytes (Miyakawa et al., 1988), swelling of astrocytic endfeet (Higuchi et al., 1987), loss of the perivascular plexus (Scheibel et al., 1987), vascular smooth muscle actin reduction, and accumulation of laminin in basement membranes have also been reported (Pérez et al., 2007). Pericytes work as gatekeepers of the BBB, and in cooperation with other NVU cells, they regulate the transport of nutrients and waste products between the interstitial fluid and peripheral blood (Thomsen et al., 2017). Multiple studies implicate the connection between pericytes, BBB dysfunction, and neurologic diseases (Machida et al., 2017), including multiple sclerosis (Zenaro et al., 2017) or AD (Winkler et al., 2014). Recent research also confirmed the association of the loss of pericytes and accumulation of fibrillar amyloid-β (Nikolakopoulou et al., 2017; Miners et al., 2018). Astrocytes are another NVU cell type that play a crucial role in the transport of amyloid-β across the BBB (Cai et al., 2017). It seems that their dysfunction can lead to the increase of RAGE activity and decrease of the LRP-1 function (Sagare et al., 2007; Askarova et al., 2011). Endothelial cells of the BBB are strongly connected by tight junctions. Yamazaki et al. (2019) have shown that the loss of cortical tight junction proteins is a common event in AD, and is correlated with synaptic degeneration. To sum up, BBB dysfunction can induce tau hyperphosphorylation, and vice versa, tau pathology can trigger the BBB damage (Ramos-Cejudo et al., 2018). Neuroinflammation and oxidative stress also contribute to the BBB damage and formation of NFTs (Ojala and Sutinen, 2017; Kumfu et al., 2018).
Cerebrovascular inflammatory changes are associated with AD pathology. iAnalysis of AD patients showed that cerebrovascular endothelium expressed increased levels of ICAM-1 and monocyte chemoattractant protein (MCP-1; Frohman et al., 1991; Grammas and Ovase, 2001). Compared to controls, AD microvessels produced significantly higher amounts of a number of inflammatory molecules such as TNF-α, transforming growth factor-β (TGF-β), nitric oxide, thrombin, cytokines such as IL-1β, IL-6, IL-8, and matrix metalloproteinases (MMPs; Grammas and Ovase, 2001). TGF-β1 as pro-inflammatory cytokine that negatively modulate vasculogenesis, angiogenesis, and vessel wall integrity. In AD patients, TGF-β1 has been associated with extracellular senile plaques and intracellular NFTs (van der Wal et al., 1993). Higher levels of TGF-β1 were found in serum and cerebrospinal fluid of demented AD patients compared to age-matched controls (Chao et al., 1994). The overexpression of TGF-β1 induced an accumulation of basement membrane proteins and led to cerebrovascular amyloidosis and vascular degeneration in Tg mice, confirming its important role in BBB changes (Wyss-Coray et al., 2000).
Frontotemporal Dementia and Parkinsonism Linked to Chromosome 17
FTDP-17 is the second most common form of dementia with 20% of all dementia cases. FTDP-17 is clinically characterized by progressive behavioral, cognitive, and motor changes, including poor impulse control, inappropriate social conduct, apathy, worse cognitive control, and limited mental flexibility. These changes often precede the extrapyramidal and corticospinal motor signs and symptoms (Sitek et al., 2014).
Numerous cases of frontotemporal dementia show dynamic neuropathological changes caused by the abnormal deposition of protein tau (Schweitzer et al., 2006). FTDP-17 is a subtype of frontotemporal dementia, and it represents a group of neurodegenerative tauopathies caused by mutations in tau and progranulin genes (Hutton et al., 1998; Poorkaj et al., 1998; Spillantini et al., 1998; Sitek et al., 2014). Different mutations in the tau gene can affect tau mRNA splicing, altering the ratio of 3R and 4R tau (Spillantini and Goedert, 2013).
There are some evidence about microvascular changes in frontotemporal dementia. De Reuck et al. (2012, 2013) performed an examination of several post-mortem brains of patients with frontotemporal dementia and found microbleeds predominantly within the areas affected by neuronal pathology, suggesting the disruption of the BBB.
PSP and Vascular PSP
PSP is a 4R tauopathy characterized by progressive deterioration of brain cells, mostly in the region of the brainstem. In addition, glial “tufted astrocytes” and neuronal tangles in gray matter and oligodendrocytic “coiled bodies” in the white matter of the neocortex are present (Irwin, 2016). The most common subtypes of PSP are Richardson’s syndrome (RS) and progressive supranuclear palsy-parkinsonism (PSP-P; Srulijes et al., 2011). Clinical features of RS include early gait instability, falls, supranuclear gaze palsy, axial rigidity, dysarthria, dysphagia, and progressive dementia. PSP-P is clinically characterized by tremor, rigid bradykinesia, levodopa responsive, late cognitive decline, and longer life expectancy. Among less common PSP syndromes belong PSP-pure akinesia with gait freezing, PSP-corticobasal syndrome, PSP-behavioral variant of frontotemporal dementia, PSP-primary lateral sclerosis, or PSP-cerebellar variant (McFarland, 2016).
The study characterizing the P-glycoprotein (P-gp) function at the BBB using [11C]-verapamil PET in PSP patients revealed increased [11C]-verapamil uptake in basal ganglia and frontal regions, suggesting decreased function of the transporter in these areas. Even if these results were not significant, they showed differences between the patients in various stages of the disease. These results are consistent with regionally decreased P-gp function with the progression of the disease (Bartels et al., 2008).
Vascular PSP is a multi-infarct disorder presenting as PSP (Josephs et al., 2002). This rare akinetic-rigid syndrome is characterized by asymmetric lower-body involvement, predominant corticospinal and pseudobulbar signs, urinary incontinence, cognitive impairment, increased frequency of stroke risk factors, and neuroimaging evidence of vascular changes in subcortical regions, especially the bilateral frontal lesions (Lanza et al., 2014). Vascular PSP differs from idiopathic PSP by a higher degree of asymmetry, lower body involvement, and evidence of corticospinal and pseudobulbar signs (Winikates and Jankovic, 1994).
CBD and Vascular Corticobasal Syndrome
CBD is a rare, progressive neurodegenerative 4R tauopathy associated with heterogeneous motor, sensory, behavioral, and cognitive symptoms. CBD pathology is characterized by circumscribed cortical atrophy with spongiosis and ballooned neurons. Tau pathology is extensively present in neurons and glial cells of the gray and white matter of the cortex, basal ganglia, diencephalon, and rostral brainstem. Abnormal tau accumulation within astrocytes forms pathognomonic astrocytic plaques. The classic clinical presentation, corticobasal syndrome, is characterized by asymmetric progressive rigidity and apraxia with limb dystonia and myoclonus (Kouri et al., 2011). The corticobasal syndrome also accompanies other diseases, including AD and PSP. Moreover, the pathology of CBD can be associated with Richardson syndrome, behavioral variant of frontotemporal dementia, primary progressive aphasia and posterior cortical syndrome (Kouri et al., 2011).
Recently, a few cases of vascular corticobasal syndrome (CBS) have been reported. Koga et al. (2019) have investigated 217 patients with an antemortem diagnosis of CBS and among them, they identified three patients with vascular CBS. Multiple infarcts in the frontal lobe and motor cortex, periventricular white matter, thalamus, and basal ganglia were observed in two patients. One patient had no cortical infarct but had multiple white matter infarcts and corticospinal tract degeneration. This autopsy study showed that, while rare, cerebrovascular pathology can underlie clinical features suggestive of CBS (Koga et al., 2019).
Chronic Traumatic Encephalopathy
In the past, CTE was referred to as dementia pugilistica. The recent studies have shown neuropathological evidence of CTE in retired American football players, professional wrestlers, professional hockey or soccer players, as well as in nonathletes. CTE may have different causes, such as falls, motor vehicle accidents, assaults, epileptic seizures, or military combat (Gavett et al., 2010). However, not all patients with repetitive brain trauma develop CTE, indicating that additional risk factors, including genetics, may play a role in the neuropathogenesis of this disease. It has also been suggested that the APOe3 allele may increase susceptibility for CTE (Gandy and Dekosky, 2012; Stern et al., 2013). In CTE, both 3R- and 4R-tau isoforms are present in neurofibrillary pathology (Woerman et al., 2016). CTE is clinically manifested by impairments in cognition, behavior, and mood, and in some cases, chronic headache and motor and cerebellar dysfunction are occasionally accompanied by dizziness and headaches (McKee et al., 2009; Stern et al., 2013). Previous microvasculature studies of several dementia pugilistica cases revealed decreased microvascular density and tortuosity with a strong correlation between the laminar distribution of NFTs and pathological microvasculature. It has been suggested that repetitive head trauma may cause vascular damage with the subsequent NFTs and neuropil neurites formation in perivascular space (McKee et al., 2009). A recent study proved the BBB disruption mainly in regions of intense perivascular tau deposition. The accumulation of tau protein was associated with loss of the tight junction protein claudin-5 and enhanced extravasation of endogenous blood components such as fibrinogen and IgG (Farrell et al., 2019).
Parkinsonism Dementia Complex of Guam (Lytico-Bodig Disease)
PDCG belongs to rare tauopathies. It is a disorder unique to the Chamorro people of Guam and the Mariana Islands. Strong familial clustering suggests the genetic origin of the disease (Hirano et al., 1961a,b; Schwab et al., 1999). Parkinsonism, dementia, or a combination of both are found as initial symptoms; however, most PDC patients show a gait disturbance with additional extrapyramidal symptoms.
Patients with PDC shows recent memory deficits, disorientation in time and place, behavioral changes, and progressive deterioration of all intellectual skills. Over time, they reach a bedridden state (Chen, 1985). Other clinical features include olfactory dysfunction and, in some individuals, oculomotor signs (Kovacs, 2015).
Neurofibrillary pathology similar to that observed in AD, but without the presence of amyloid plaques is found in most PDC cases (Lee et al., 2001).
Previous studies described brain microvascular changes in PDC. They found a decrease in vascular density, atrophic, and fragmented microvessels with a reduced number of microvascular branches mainly in the areas affected by NFTs pathology (Buee et al., 1994, 1997). Recently, we showed that neurofibrillary pathology is closely associated with cerebrovascular inflammatory changes in Guam PDC patients. The areas with significant accumulation of the tau in the NFTs correlated with upregulation of adhesion molecules, disruption of tight junctions, morphological alterations in brain microvessels such as thickening of the vessel walls, and narrowing of the vessel lumens and an increase in collagen-type IV content per vessel (Majerova et al., 2018).
Pick’s Disease
PiD is a 3R-tau predominant tauopathy characterized by the presence of “Pick bodies” comprising the aggregates of hyperphosphorylated tau and glial inclusions through the limbic and neocortical regions (Irwin, 2016). Moreover, ramified astrocytes are present (Komori, 1999). Severe neuronal and glial loss in PiD leads to frontotemporal lobe atrophy, and it is clinically manifested by the loss of verbal skills, personality changes, and progressive dementia (Hardin and Schooley, 2002; Rohn et al., 2013). Vascular changes in PiD, including thinning of microvessels, increased tortuosity, twisted vessels, and fragmentation of microvasculature. The structural changes of capillaries are comparatively severe as in AD (Buee et al., 1997). Massive disorganization of the laminar distribution of microvessels is shown mainly in atrophy-affected areas (Buee et al., 1994).
Argyrophilic Grain Disease
AGD is a highly frequent but still under-recognized neurodegenerative condition. AGD is a sporadic 4R tauopathy. In the past, AGD was reported as adult-onset dementia, but extended studies have revealed clinical features, such as changes of the personality, emotional imbalance, or memory problems. Pathologically, AGD is characterized by the presence of spindle-shaped or comma-shaped argyrophilic grains in the neuropil of the entorhinal cortex, hippocampus, and amygdala (Togo et al., 2002).
Globular Glial Tauopathies
GGTs represent a group of 4R tauopathies that are characterized neuropathologically by widespread globular glial inclusions. These tauopathies are very rare and they have a range of clinicopathological presentations. We can divide them into three main types—type I cases are typically presented with frontotemporal dementia correlating with the frontotemporal distribution of pathology, type II cases are predominately characterized by motor cortex and corticospinal tract degeneration, and type III cases can present with a combination of the frontotemporal, motor cortex, and corticospinal tract involvement. Extrapyramidal features can be present in types II and III, and in all types of globular glial tauopathies, significant degeneration of the white matter can be observed (Ahmed et al., 2013). It is known that astrocytes play a key role in maintaining the BBB via astrocytic endfeet, which are directly opposed to vascular endothelial cells (Ransom et al., 2003). Experiments on mice confirmed the mild BBB disruption after the targeted expression of human wild-type tau in murine astrocyte (Zlokovic, 2008; Bartels et al., 2009). In addition, astrocytes also modulate synaptic function by the secretion and uptake of neurotransmitters such as glutamate, the brain’s major excitatory neurotransmitter (Haydon, 2001).
Aging-Related Tau Astrogliopathy
Aging-related tau astrogliopathy is characterized by the presence of two types of tau-bearing astrocytes. The first type, thorn-shaped astrocytes, is located in the subependymal and subpial regions, perivascular spaces, and in clusters in the frontal and temporal cortices, basal forebrain, and brainstem. They were first described in association with AD and AGD. The second type is represented by granular/fuzzy astrocytes, which are mainly located in the gray matter, and they were firstly identified in a particular subgroup of patients with dementia. Both types can also be present in combination with other tauopathies (Ferrer et al., 2018). The etiology of this disease remains unclear; however, functional changes of the BBB together with metabolic encephalopathy, neurodegenerative pathologies, aging-related hypoperfusion, AD, vascular dementia, and even repeated minor trauma with possible genetic risk factors may play a role (Kovacs et al., 2016).
Clinical Challenges
As discussed in previous sections, the neurofibrillary pathology is connected with changes at the neurovascular unit. Moreover, the distribution and load of neurofibrillary pathology is correlated with clinical phenotype and severity of cognitive impairment (Nelson et al., 2012; Murray et al., 2015).
Clinical studies suggest that the NVU changes may represent an early biomarker of human cognitive dysfunction (Nation et al., 2019). The [18F]Fluoro-2-deoxy-d-glucose (FDG-PET)/CT studies suggest that in about 16% of analyzed AD cases, BBB dysfunction may be present, and this pattern is related to a worse metabolic pattern (Chiaravalloti et al., 2016). Montagne et al. (2020) performed the analysis of the BBB permeability in 245 participants using the dynamic contrast-enhanced magnetic resonance imaging. They showed increased BBB permeability in cognitively normal APOε4 carriers, compared to cognitively normal APOε3 homozygotes, both with clinical dementia rating scores of 0. This increase was independent of amyloid-β and tau levels, indicating APOε4 to be a potential therapeutic and diagnostic target in APOε4 carriers (Montagne et al., 2020).
However, major questions remain, such as how tau-induced NVU changes are presented at the clinical level and whether early biomarkers of these processes could help in more focused therapeutic intervention in the future.
Author Contributions
AM, PM, and AK wrote the manuscript. All authors read and approved the final draft of the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank to Dr. Henrieta Dudekova for her help with the preparation of graphics.
Footnotes
Funding. This work was supported by Ministry of Health of the Slovak Republic under the project registration number 2018/24-SAV-2 and by research grants VEGA 2/0088/18, VEGA 2/0147/19, VEGA 2/0150/19, APVV-16-0531, and APVV-18-0302.
References
- Abbott N. J. (2002). Astrocyte-endothelial interactions and blood-brain barrier permeability. J. Anat. 200, 629–638. 10.1046/j.1469-7580.2002.00047_13.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbott N. J. (2005). Dynamics of CNS barriers: evolution, differentiation and modulation. Cell. Mol. Neurobiol. 25, 5–23. 10.1007/s10571-004-1374-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbott N. J., Patabendige A. A., Dolman D. E., Yusof S. R., Begley D. J. (2010). Structure and function of the blood-brain barrier. Neurobiol. Dis. 37, 13–25. 10.1016/j.nbd.2009.07.030 [DOI] [PubMed] [Google Scholar]
- Abbott N. J., Ronnback L., Hansson E. (2006). Astrocyte-endothelial interactions at the blood-brain barrier. Nat. Rev. Neurosci. 7, 41–53. 10.1038/nrn1824 [DOI] [PubMed] [Google Scholar]
- Ahmed Z., Bigio E. H., Budka H., Dickson D. W., Ferrer I., Ghetti B., et al. (2013). Globular glial tauopathies (GGT): consensus recommendations. Acta Neuropathol. 126, 537–544. 10.1007/s00401-013-1171-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong R. A. (2013). What causes Alzheimer’s disease? Folia Neuropathol. 51, 169–188. 10.5114/fn.2013.37702 [DOI] [PubMed] [Google Scholar]
- Askarova S., Yang X., Sheng W., Sun G. Y., Lee J. C. (2011). Role of Abeta-receptor for advanced glycation endproducts interaction in oxidative stress and cytosolic phospholipase A(2) activation in astrocytes and cerebral endothelial cells. Neuroscience 199, 375–385. 10.1016/j.neuroscience.2011.09.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila J., Lucas J. J., Perez M., Hernandez F. (2004). Role of tau protein in both physiological and pathological conditions. Physiol. Rev. 84, 361–384. 10.1152/physrev.00024.2003 [DOI] [PubMed] [Google Scholar]
- Bartels A. L., Kortekaas R., Bart J., Willemsen A. T., De Klerk O. L., De Vries J. J., et al. (2009). Blood-brain barrier P-glycoprotein function decreases in specific brain regions with aging: a possible role in progressive neurodegeneration. Neurobiol. Aging 30, 1818–1824. 10.1016/j.neurobiolaging.2008.02.002 [DOI] [PubMed] [Google Scholar]
- Bartels A. L., Willemsen A. T., Kortekaas R., De Jong B. M., De Vries R., De Klerk O., et al. (2008). Decreased blood-brain barrier P-glycoprotein function in the progression of Parkinson’s disease, PSP and MSA. J. Neural Transm. 115, 1001–1009. 10.1007/s00702-008-0030-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beharry C., Cohen L. S., Di J., Ibrahim K., Briffa-Mirabella S., Alonso Adel C. (2014). Tau-induced neurodegeneration: mechanisms and targets. Neurosci. Bull. 30, 346–358. 10.1007/s12264-013-1414-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekris L. M., Yu C. E., Bird T. D., Tsuang D. W. (2010). Genetics of Alzheimer disease. J. Geriatr. Psychiatry Neurol. 23, 213–227. 10.1177/0891988710383571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell R. D., Winkler E. A., Sagare A. P., Singh I., Larue B., Deane R., et al. (2010). Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron 68, 409–427. 10.1016/j.neuron.2010.09.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell R. D., Zlokovic B. V. (2009). Neurovascular mechanisms and blood-brain barrier disorder in Alzheimer’s disease. Acta Neuropathol. 118, 103–113. 10.1007/s00401-009-0522-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellucci A., Westwood A. J., Ingram E., Casamenti F., Goedert M., Spillantini M. G. (2004). Induction of inflammatory mediators and microglial activation in mice transgenic for mutant human P301S tau protein. Am. J. Pathol. 165, 1643–1652. 10.1016/s0002-9440(10)63421-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertram L., Lill C. M., Tanzi R. E. (2010). The genetics of Alzheimer disease: back to the future. Neuron 68, 270–281. 10.1016/j.neuron.2010.10.013 [DOI] [PubMed] [Google Scholar]
- Buee L., Hof P. R., Delacourte A. (1997). Brain microvascular changes in Alzheimer’s disease and other dementias. Ann. N Y Acad. Sci. 826, 7–24. [DOI] [PubMed] [Google Scholar]
- Buee L., Hof P. R., Bouras C., Delacourte A., Perl D. P., Morrison J. H., et al. (1994). Pathological alterations of the cerebral microvasculature in Alzheimer’s disease and related dementing disorders. Acta Neuropathol. 87, 469–480. 10.1007/s004010050112 [DOI] [PubMed] [Google Scholar]
- Cai Z., Qiao P. F., Wan C. Q., Cai M., Zhou N. K., Li Q. (2018). Role of blood-brain barrier in Alzheimer’s disease. J. Alzheimers Dis. 63, 1223–1234. 10.3233/JAD-180098 [DOI] [PubMed] [Google Scholar]
- Cai Z., Wan C. Q., Liu Z. (2017). Astrocyte and Alzheimer’s disease. J. Neurol. 264, 2068–2074. 10.1007/s00415-017-8593-x [DOI] [PubMed] [Google Scholar]
- Chao C. C., Hu S., Frey W. H., II., Ala T. A., Tourtellotte W. W., Peterson P. K. (1994). Transforming growth factor beta in Alzheimer’s disease. Clin. Diagn. Lab. Immunol. 1, 109–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K.-M. (1985). Parkinsonism-dementia,” in Handbook of Clinical Neurology, eds Vinken P. J., Bruyn G. W., Klawans H. L. (Amsterdam: Elsevier; ), 167–183. [Google Scholar]
- Chiaravalloti A., Fiorentini A., Francesco U., Martorana A., Koch G., Belli L., et al. (2016). Is Cerebral Glucose Metabolism Related to Blood-Brain Barrier Dysfunction and Intrathecal IgG Synthesis in Alzheimer Disease? Medicine 95:e4206 10.1097/MD.0000000000004206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalvi A. (2012). Alzheimer’s disease. Dis. Mon. 58, 666–677. 10.1016/j.disamonth.2012.08.008 [DOI] [PubMed] [Google Scholar]
- De La Torre J. C. (2002). Alzheimer disease as a vascular disorder: nosological evidence. Stroke 33, 1152–1162. 10.1161/01.str.0000014421.15948.67 [DOI] [PubMed] [Google Scholar]
- De Reuck J., Deramecourt V., Cordonnier C., Auger F., Durieux N., Bordet R., et al. (2012). Detection of microbleeds in post-mortem brains of patients with frontotemporal lobar degeneration: a 7.0-Tesla magnetic resonance imaging study with neuropathological correlates. Eur. J. Neurol. 19, 1355–1360. [DOI] [PubMed] [Google Scholar]
- De Reuck J., Deramecourt V., Cordonnier C., Leys D., Pasquier F., Maurage C.-A. (2013). Prevalence of cerebrovascular lesions in patients with Lewy body dementia: a neuropathological study. Clin. Neurol. Neurosurg. 115, 1094–1097. 10.1016/j.clineuro.2012.11.005 [DOI] [PubMed] [Google Scholar]
- De Vries H. E., Kooij G., Frenkel D., Georgopoulos S., Monsonego A., Janigro D. (2012). Inflammatory events at blood-brain barrier in neuroinflammatory and neurodegenerative disorders: implications for clinical disease. Epilepsia 53, 45–52. 10.1111/j.1528-1167.2012.03702.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deane R., Zlokovic B. V. (2007). Role of the blood-brain barrier in the pathogenesis of Alzheimer’s disease. Curr. Alzheimer Res. 4, 191–197. 10.2174/156720507780362245 [DOI] [PubMed] [Google Scholar]
- Desai B. S., Monahan A. J., Carvey P. M., Hendey B. (2007). Blood-brain barrier pathology in Alzheimer’s and Parkinson’s disease: implications for drug therapy. Cell Transplant. 16, 285–299. 10.3727/000000007783464731 [DOI] [PubMed] [Google Scholar]
- Di Liegro I., Savettieri G. (2005). Molecular Bases of Neurodegeneration. Trivandrum: Research Signpost. [Google Scholar]
- Dipatre P. L., Gelman B. B. (1997). Microglial cell activation in aging and Alzheimer disease: partial linkage with neurofibrillary tangle burden in the hippocampus. J. Neuropathol. Exp. Neurol. 56, 143–149. 10.1097/00005072-199702000-00004 [DOI] [PubMed] [Google Scholar]
- Erickson M. A., Banks W. A. (2013a). Blood-brain barrier dysfunction as a cause and consequence of Alzheimer’s disease. J. Cereb. Blood Flow Metab. 33, 1500–1513. 10.1038/jcbfm.2013.135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson M. A., Banks W. A. (2013b). Blood brain barrier dysfunction as a cause and consequence of Alzheimer’s disease. J. Cereb. Blood Flow Metab. 33, 1500–1513. 10.1038/jcbfm.2013.135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson M. A., Dohi K., Banks W. A. (2012). Neuroinflammation: a common pathway in CNS diseases as mediated at the blood-brain barrier. Neuroimmunomodulation 19, 121–130. 10.1159/000330247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell M., Aherne S., O’riordan S., O’keeffe E., Greene C., Campbell M. (2019). Blood-brain barrier dysfunction in a boxer with chronic traumatic encephalopathy and schizophrenia. Clin. Neuropathol. 38, 51–58. 10.5414/np301130 [DOI] [PubMed] [Google Scholar]
- Ferrer I., Garcia M. A., Gonzalez I. L., Lucena D. D., Villalonga A. R., Tech M. C., et al. (2018). Aging-related tau astrogliopathy (ARTAG): not only tau phosphorylation in astrocytes. Brain Pathol. 28, 965–985. 10.1111/bpa.12593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer I., Lopez-Gonzalez I., Carmona M., Arregui L., Dalfo E., Torrejon-Escribano B., et al. (2014). Glial and neuronal tau pathology in tauopathies: characterization of disease-specific phenotypes and tau pathology progression. J. Neuropathol. Exp. Neurol. 73, 81–97. 10.1097/nen.0000000000000030 [DOI] [PubMed] [Google Scholar]
- Fu B. M., Wright N. T. (2018). Molecular, Cellular, and Tissue Engineering of the Vascular System. Cham: Springer International Publishing. [Google Scholar]
- Frohman E. M., Frohman T. C., Gupta S., de Fougerolles A., van den Noort S. (1991). Expression of intercellular adhesion molecule 1 (ICAM-1) in Alzheimer’s disease. J. Neurol. Sci. 106, 105–111. 10.1016/0022-510x(91)90202-i [DOI] [PubMed] [Google Scholar]
- Gandy S., Dekosky S. T. (2012). APOE epsilon4 status and traumatic brain injury on the gridiron or the battlefield. Sci. Transl. Med. 4:134ed134 10.1126/scitranslmed.3004274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavett B. E., Stern R. A., Cantu R. C., Nowinski C. J., Mckee A. C. (2010). Mild traumatic brain injury: a risk factor for neurodegeneration. Alzheimers Res. Ther. 2:18. 10.1186/alzrt42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenner G. G., Wong C. W. (1984). Alzheimer’s disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem. Biophys. Res. Commun. 120, 885–890. [DOI] [PubMed] [Google Scholar]
- Goedert M., Spillantini M. G., Jakes R., Rutherford D., Crowther R. A. (1989). Multiple isoforms of human microtubule-associated protein tau: sequences and localization in neurofibrillary tangles of Alzheimer’s disease. Neuron 3, 519–526. 10.1016/0896-6273(89)90210-9. [DOI] [PubMed] [Google Scholar]
- Grammas P., Ovase R. (2001). Inflammatory factors are elevated in brain microvessels in Alzheimer’s disease. Neurobiol. Aging 22, 837–842. 10.1016/s0197-4580(01)00276-7 [DOI] [PubMed] [Google Scholar]
- Grinberg L. T., Wang X., Wang C., Sohn P. D., Theofilas P., Sidhu M., et al. (2013). Argyrophilic grain disease differs from other tauopathies by lacking tau acetylation. Acta Neuropathol. 125, 581–593. 10.1007/s00401-013-1080-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundke-Iqbal I., Iqbal K., Tung Y. C., Quinlan M., Wisniewski H. M., Binder L. I. (1986). Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc. Natl. Acad. Sci. U S A 83, 4913–4917. 10.1073/pnas.83.13.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo T., Noble W., Hanger D. P. (2017). Roles of tau protein in health and disease. Acta Neuropathol. 133, 665–704. 10.1007/s00401-017-1707-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanes J., Zilka N., Bartkova M., Caletkova M., Dobrota D., Novak M. (2009). Rat tau proteome consists of six tau isoforms: implication for animal models of human tauopathies. J. Neurochem. 108, 1167–1176. 10.1111/j.1471-4159.2009.05869.x. [DOI] [PubMed] [Google Scholar]
- Hardin S., Schooley B. (2002). A story of Pick’s disease: a rare form of dementia. J. Neurosci. Nurs. 34, 117–122. 10.1097/01376517-200206000-00004. [DOI] [PubMed] [Google Scholar]
- Hashiguchi M., Sobue K., Paudel H. K. (2000). 14–3-3ζ is an effector of tau protein phosphorylation. J. Biol. Chem. 275, 25247–25254. 10.1074/jbc.M003738200 [DOI] [PubMed] [Google Scholar]
- Hawkins B. T., Davis T. P. (2005). The blood-brain barrier/neurovascular unit in health and disease. Pharmacol. Rev. 57, 173–185. 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- Haydon P. G. (2001). GLIA: listening and talking to the synapse. Nat. Rev. Neurosci. 2, 185–193. 10.1038/35058528. [DOI] [PubMed] [Google Scholar]
- Higuchi Y., Miyakawa T., Shimoji A., Katsuragi S. (1987). Ultrastructural changes of blood vessels in the cerebral cortex in Alzheimer’s disease. Jpn. J. Psychiatry Neurol. 41, 283–290. 10.1111/j.1440-1819.1987.tb00414.x [DOI] [PubMed] [Google Scholar]
- Hirano A., Kurland L. T., Krooth R. S., Lessell S. (1961a). Parkinsonism-dementia complex, an endemic disease on the island of Guam. I. Clinical features. Brain 84, 642–661. 10.1093/brain/84.4.642 [DOI] [PubMed] [Google Scholar]
- Hirano A., Malamud N., Kurland L. T. (1961b). Parkinsonism-dementia complex, an endemic disease on the island of Guam. II. Pathological features. Brain 84, 662–679. 10.1093/brain/84.4.662 [DOI] [PubMed] [Google Scholar]
- Hutton M., Lendon C. L., Rizzu P., Baker M., Froelich S., Houlden H., et al. (1998). Association of missense and 5’-splice-site mutations in tau with the inherited dementia FTDP-17. Nature 393, 702–705. [DOI] [PubMed] [Google Scholar]
- Imamura K., Sawada M., Ozaki N., Naito H., Iwata N., Ishihara R., et al. (2001). Activation mechanism of brain microglia in patients with diffuse neurofibrillary tangles with calcification: a comparison with Alzheimer disease. Alzheimer Dis. Assoc. Disord. 15, 45–50. 10.1097/00002093-200101000-00006 [DOI] [PubMed] [Google Scholar]
- Iqbal K., Liu F., Gong C. X. (2014). Alzheimer disease therapeutics: focus on the disease and not just plaques and tangles. Biochem. Pharmacol. 88, 631–639. 10.1016/j.bcp.2014.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin D. J. (2016). Tauopathies as clinicopathological entities. Parkinsonism Relat. Disord. 22, S29–S33. 10.1016/j.parkreldis.2015.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizawa K., Dickson D. W. (2001). Microglial activation parallels system degeneration in progressive supranuclear palsy and corticobasal degeneration. J. Neuropathol. Exp. Neurol. 60, 647–657. 10.1093/jnen/60.6.647 [DOI] [PubMed] [Google Scholar]
- Jensen P. H., Hager H., Nielsen M. S., Hojrup P., Gliemann J., Jakes R. (1999). α-synuclein binds to Tau and stimulates the protein kinase A-catalyzed tau phosphorylation of serine residues 262 and 356. J. Biol. Chem. 274, 25481–25489. 10.1074/jbc.274.36.25481 [DOI] [PubMed] [Google Scholar]
- Josephs K. A., Ishizawa T., Tsuboi Y., Cookson N., Dickson D. W. (2002). A clinicopathological study of vascular progressive supranuclear palsy: a multi-infarct disorder presenting as progressive supranuclear palsy. Arch. Neurol. 59, 1597–1601. 10.1001/archneur.59.10.1597 [DOI] [PubMed] [Google Scholar]
- Koga S., Roemer S. F., Kasanuki K., Dickson D. W. (2019). Cerebrovascular pathology presenting as corticobasal syndrome: an autopsy case series of vascular CBS. Parkinsonism Relat. Disord. 68, 79–84. 10.1016/j.parkreldis.2019.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komori T. (1999). Tau-positive glial inclusions in progressive supranuclear palsy, corticobasal degeneration and Pick’s disease. Brain Pathol. 9, 663–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korczyn A. D. (2008). The amyloid cascade hypothesis. Alzheimers Dement. 4, 176–178. 10.1016/j.jalz.2007.11.008 [DOI] [PubMed] [Google Scholar]
- Kouri N., Whitwell J. L., Josephs K. A., Rademakers R., Dickson D. W. (2011). Corticobasal degeneration: a pathologically distinct 4R tauopathy. Nat. Rev. Neurol. 7, 263–272. 10.1038/nrneurol.2011.43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kousik S. M., Napier T. C., Carvey P. M. (2012). The effects of psychostimulant drugs on blood brain barrier function and neuroinflammation. Front. Pharmacol. 3:121 10.3389/fphar.2012.00121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovac A., Zilkova M., Deli M. A., Zilka N., Novak M. (2009). Human truncated tau is using a different mechanism from amyloid-beta to damage the blood-brain barrier. J. Alzheimers Dis. 18, 897–906. 10.3233/jad-2009-1197 [DOI] [PubMed] [Google Scholar]
- Kovac A., Zilka N., Kazmerova Z., Cente M., Zilkova M., Novak M. (2011). Misfolded truncated protein tau induces innate immune response via MAPK pathway. J. Immunol. 187, 2732–2739. 10.4049/jimmunol.1100216 [DOI] [PubMed] [Google Scholar]
- Kovacech B., Zilka N., Novak M. (2009). New age of neuroproteomics in Alzheimer’s disease research. Cell Mol. Neurobiol. 29, 799–805. 10.1007/s10571-009-9358-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs G. G. (2015). Neuropathology of Neurodegenerative Diseases: A Practical Guide. Cambridge, UK; New York, NY: Cambridge University Press. [Google Scholar]
- Kovacs G. G., Ferrer I., Grinberg L. T., Alafuzoff I., Attems J., Budka H., et al. (2016). Aging-related tau astrogliopathy (ARTAG): harmonized evaluation strategy. Acta Neuropathol. 131, 87–102. 10.1007/s00401-015-1509-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumfu S., Charununtakorn S. T., Jaiwongkam T., Chattipakorn N., Chattipakorn S. C. (2018). Humanin exerts neuroprotection during cardiac ischemia-reperfusion injury. J. Alzheimers Dis. 61, 1343–1353. 10.3233/jad-170708 [DOI] [PubMed] [Google Scholar]
- Lahiri D. K., Maloney B., Basha M. R., Ge Y. W., Zawia N. H. (2007). How and when environmental agents and dietary factors affect the course of Alzheimer’s disease: the “LEARn” model (latent early-life associated regulation) may explain the triggering of AD. Curr. Alzheimer Res. 4, 219–228. 10.2174/156720507780362164 [DOI] [PubMed] [Google Scholar]
- Lanza G., Papotto M., Pennisi G., Bella R., Ferri R. (2014). Epileptic seizure as a precipitating factor of vascular progressive supranuclear palsy: a case report. J. Stroke Cerebrovasc. Dis. 23, E379–E381. 10.1016/j.jstrokecerebrovasdis.2013.12.043 [DOI] [PubMed] [Google Scholar]
- Laurent C., Buee L., Blum D., Laurent C., Buee L., Blum D., et al. (2018). Tau and neuroinflammation: what impact for Alzheimer’s disease and tauopathies? Biomed. J. 41, 21–33. 10.1016/j.bj.2018.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee V. M., Goedert M., Trojanowski J. Q. (2001). Neurodegenerative tauopathies. Annu. Rev. Neurosci. 24, 1121–1159. 10.1146/annurev.neuro.24.1.1121 [DOI] [PubMed] [Google Scholar]
- Liu Y., Liu F., Iqbal K., Grundke-Iqbal I., Gong C. X. (2008). Decreased glucose transporters correlate to abnormal hyperphosphorylation of tau in Alzheimer disease. FEBS Lett. 582, 359–364. 10.1016/j.febslet.2007.12.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lok J., Gupta P., Guo S., Kim W. J., Whalen M. J., Van Leyen K., et al. (2007). Cell-cell signaling in the neurovascular unit. Neurochem. Res. 32, 2032–2045. 10.1007/s11064-007-9342-9 [DOI] [PubMed] [Google Scholar]
- Maccioni R. B., Farias G., Morales I., Navarrete L. (2010). The revitalized tau hypothesis on Alzheimer’s disease. Arch. Med. Res. 41, 226–231. 10.1016/j.arcmed.2010.03.007 [DOI] [PubMed] [Google Scholar]
- Machida T., Dohgu S., Takata F., Matsumoto J., Kimura I., Koga M., et al. (2017). Role of thrombin-PAR1-PKCtheta/delta axis in brain pericytes in thrombin-induced MMP-9 production and blood-brain barrier dysfunction in vitro. Neuroscience 350, 146–157. 10.1016/j.neuroscience.2017.03.026 [DOI] [PubMed] [Google Scholar]
- Majerova P., Garruto R. M., Kovac A. (2018). Cerebrovascular inflammation is associated with tau pathology in Guam parkinsonism dementia. J. Neural Transm. 125, 1013–1025. 10.1007/s00702-018-1883-3 [DOI] [PubMed] [Google Scholar]
- Majerova P., Michalicova A., Cente M., Hanes J., Vegh J., Kittel A., et al. (2019). Trafficking of immune cells across the blood-brain barrier is modulated by neurofibrillary pathology in tauopathies. PLoS One 14:e0217216. 10.1371/journal.pone.0217216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland N. R. (2016). Diagnostic approach to atypical Parkinsonian syndromes. Continuum 22, 1117–1142. 10.1212/con.0000000000000348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee A. C., Cantu R. C., Nowinski C. J., Hedley-Whyte E. T., Gavett B. E., Budson A. E., et al. (2009). Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. J. Neuropathol. Exp. Neurol. 68, 709–735. 10.1097/NEN.0b013e3181a9d503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mena R., Edwards P. C., Harrington C. R., Mukaetovaladinska E. B., Wischik C. M. (1996). Staging the pathological assembly of truncated tau protein into paired helical filaments in Alzheimer’s disease. Acta Neuropathol. 91, 633–641. 10.1016/s0197-4580(96)80510-0 [DOI] [PubMed] [Google Scholar]
- Michalicova A., Banks W. A., Legath J., Kovac A. (2017). Tauopathies—focus on changes at the neurovascular unit. Curr. Alzheimer Res. 14, 790–801. 10.2174/1567205014666170203143336 [DOI] [PubMed] [Google Scholar]
- Miners J. S., Schulz I., Love S. (2018). Differing associations between Abeta accumulation, hypoperfusion, blood-brain barrier dysfunction and loss of PDGFRB pericyte marker in the precuneus and parietal white matter in Alzheimer’s disease. J. Cereb. Blood Flow Metab. 38, 103–115. 10.1177/0271678x17690761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyakawa T., Uehara Y., Desaki J., Kimura T., Kuramoto R. (1988). Morphological changes of microvessels in the brain with Alzheimer’s disease. Jpn. J. Psychiatry Neurol. 42, 819–824. [DOI] [PubMed] [Google Scholar]
- Mohandas E., Rajmohan V., Raghunath B. (2009). Neurobiology of Alzheimer’s disease. Indian J. Psychiatry 51, 55–61. 10.4103/0019-5545.44908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagne A., Nation D. A., Sagare A. P., Barisano G., Sweeney M. D., Chakhoyan A., et al. (2020). APOE4 leads to blood-brain barrier dysfunction predicting cognitive decline. Nature 581, 71–76. 10.1038/s41586-020-2247-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray M. E., Lowe V. J., Graff-Radford N. R., Liesinger A. M., Cannon A., Przybelski S. A., et al. (2015). Clinicopathologic and 11C-Pittsburgh compound B implications of Thal amyloid phase across the Alzheimer’s disease spectrum. Brain 138, 1370–1381. 10.1093/brain/awv050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nation D. A., Sweeney M. D., Montagne A., Sagare A. P., D’orazio L. M., Pachicano M., et al. (2019). Blood-brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat. Med. 25, 270–276. 10.1038/s41591-018-0297-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson P. T., Alafuzoff I., Bigio E. H., Bouras C., Braak H., Cairns N. J., et al. (2012). Correlation of Alzheimer disease neuropathologic changes with cognitive status: a review of the literature. J. Neuropathol. Exp. Neurol. 71, 362–381. 10.1097/NEN.0b013e31825018f7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuwelt E., Abbott N. J., Abrey L., Banks W. A., Blakley B., Davis T., et al. (2008). Strategies to advance translational research into brain barriers. Lancet Neurol. 7, 84–96. 10.1016/S1474-4422(07)70326-5 [DOI] [PubMed] [Google Scholar]
- Nikolakopoulou A. M., Zhao Z., Montagne A., Zlokovic B. V. (2017). Regional early and progressive loss of brain pericytes but not vascular smooth muscle cells in adult mice with disrupted platelet-derived growth factor receptor-beta signaling. PLoS One 12:e0176225. 10.1371/journal.pone.0176225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak M. (2012). Tau truncation: the most productive post-translational modification. Alzheimers Dement. 8:424 10.1016/j.jalz.2012.05.1128 [DOI] [Google Scholar]
- Ojala J. O., Sutinen E. M. (2017). The role of interleukin-18, oxidative stress and metabolic syndrome in Alzheimer’s disease. J. Clin. Med. 6:55. 10.3390/jcm6050055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Østergaard L., Aamand R., Gutierrez-Jimenez E., Ho Y. C., Blicher J. U., Madsen S. M., et al. (2013). The capillary dysfunction hypothesis of Alzheimer’s disease. Neurobiol. Aging 34, 1018–1031. 10.1016/j.neurobiolaging.2012.09.011 [DOI] [PubMed] [Google Scholar]
- Overmyer M., Helisalmi S., Soininen H., Laakso M., Riekkinen Sr P., Alafuzoff I. (1999). Reactive microglia in aging and dementia: an immunohistochemical study of postmortem human brain tissue. Acta Neuropathol. 97, 383–392. 10.1007/s004010051002 [DOI] [PubMed] [Google Scholar]
- Pérez E., Barrachina M., Rodriguez A., Torrejon-Escribano B., Boada M., Hernandez I., et al. (2007). Aquaporin expression in the cerebral cortex is increased at early stages of Alzheimer disease. Brain Res. 1128, 164–174. 10.1016/j.brainres.2006.09.109 [DOI] [PubMed] [Google Scholar]
- Persidsky Y., Hill J., Zhang M., Dykstra H., Winfield M., Reichenbach N. L., et al. (2016). Dysfunction of brain pericytes in chronic neuroinflammation. J. Cereb. Blood Flow Metab. 36, 794–807. 10.1177/0271678X15606149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poorkaj P., Bird T. D., Wijsman E., Nemens E., Garruto R. M., Anderson L., et al. (1998). Tau is a candidate gene for chromosome 17 frontotemporal dementia. Ann. Neurol. 43, 815–825. 10.1002/ana.410430617 [DOI] [PubMed] [Google Scholar]
- Preston S. D., Steart P. V., Wilkinson A., Nicoll J. A., Weller R. O. (2003). Capillary and arterial cerebral amyloid angiopathy in Alzheimer’s disease: defining the perivascular route for the elimination of amyloid β from the human brain. Neuropathol. Appl. Neurobiol. 29, 106–117. 10.1046/j.1365-2990.2003.00424.x [DOI] [PubMed] [Google Scholar]
- Ramos-Cejudo J., Wisniewski T., Marmar C., Zetterberg H., Blennow K., De Leon M. J., et al. (2018). Traumatic brain injury and Alzheimer’s disease: the cerebrovascular link. EBioMedicine 28, 21–30. 10.1016/j.ebiom.2018.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransom B., Behar T., Nedergaard M. (2003). New roles for astrocytes (stars at last). Trends Neurosci. 26, 520–522. 10.1016/j.tins.2003.08.006 [DOI] [PubMed] [Google Scholar]
- Rhea E. M., Banks W. A., Rhea E. M., Banks W. A. (2019). Role of the blood-brain barrier in central nervous system insulin resistance. Front. Neurosci. 13:521. 10.3389/fnins.2019.00521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohn T. T., Day R. J., Catlin L. W., Brown R. J., Rajic A. J., Poon W. W. (2013). Immunolocalization of an amino-terminal fragment of apolipoprotein E in the Pick’s disease brain. PLoS One 8:e80180. 10.1371/journal.pone.0080180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagare A., Deane R., Bell R. D., Johnson B., Hamm K., Pendu R., et al. (2007). Clearance of amyloid-β by circulating lipoprotein receptors. Nat. Med. 13, 1029–1031. 10.1038/nm1635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savettieri G., Di Liegro I., Catania C., Licata L., Pitarresi G. L., D’agostino S., et al. (2000). Neurons and ECM regulate occludin localization in brain endothelial cells. Neuroreport 11, 1081–1084. 10.1097/00001756-200004070-00035 [DOI] [PubMed] [Google Scholar]
- Scheibel A. B., Duong T. H., Tomiyasu U. (1987). Denervation microangiopathy in senile dementia, Alzheimer type. Alzheimer Dis. Assoc. Disord. 1, 19–37. 10.1097/00002093-198701000-00004 [DOI] [PubMed] [Google Scholar]
- Schwab C., Schulzer M., Steele J. C., Mcgeer P. L. (1999). On the survival time of a tangled neuron in the hippocampal CA4 region in parkinsonism dementia complex of Guam. Neurobiol. Aging 20, 57–63. 10.1016/s0197-4580(99)00005-6 [DOI] [PubMed] [Google Scholar]
- Schweitzer K., Decker E., Zhu L., Miller R. E., Mirra S. S., Spina S., et al. (2006). Aberrantly regulated proteins in frontotemporal dementia. Biochem. Biophys. Res. Commun. 348, 465–472. 10.1016/j.bbrc.2006.07.113 [DOI] [PubMed] [Google Scholar]
- Sheffield L. G., Marquis J. G., Berman N. E. (2000). Regional distribution of cortical microglia parallels that of neurofibrillary tangles in Alzheimer’s disease. Neurosci. Lett. 285, 165–168. 10.1016/s0304-3940(00)01037-5 [DOI] [PubMed] [Google Scholar]
- Siddiqua A., Margittai M. (2010). Three- and four-repeat Tau coassemble into heterogeneous filaments: an implication for Alzheimer disease. J. Biol. Chem. 285, 37920–37926. 10.1074/jbc.m110.185728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitek E. J., Narozanska E., Barczak A., Jasinska-Myga B., Harciarek M., Chodakowska-Zebrowska M., et al. (2014). Agraphia in patients with frontotemporal dementia and parkinsonism linked to chromosome 17 with P301L MAPT mutation: dysexecutive, aphasic, apraxic or spatial phenomenon? Neurocase 20, 69–86. 10.1080/13554794.2012.732087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillantini M. G., Goedert M. (2013). Tau pathology and neurodegeneration. Lancet Neurol. 12, 609–622. 10.1016/S1474-4422(13)70090-5 [DOI] [PubMed] [Google Scholar]
- Spillantini M. G., Murrell J. R., Goedert M., Farlow M. R., Klug A., Ghetti B. (1998). Mutation in the tau gene in familial multiple system tauopathy with presenile dementia. Proc. Natl. Acad. Sci. U S A 95, 7737–7741. 10.1073/pnas.95.13.7737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srulijes K., Mallien G., Bauer S., Dietzel E., Groger A., Ebersbach G., et al. (2011). In vivo comparison of Richardson’s syndrome and progressive supranuclear palsy-parkinsonism. J. Neural Transm. 118, 1191–1197. 10.1007/s00702-010-0563-8 [DOI] [PubMed] [Google Scholar]
- Stamatovic S. M., Keep R. F., Andjelkovic A. V. (2008). Brain endothelial cell-cell junctions: how to “open” the blood brain barrier. Curr. Neuropharmacol. 6, 179–192. 10.2174/157015908785777210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern R. A., Daneshvar D. H., Baugh C. M., Seichepine D. R., Montenigro P. H., Riley D. O., et al. (2013). Clinical presentation of chronic traumatic encephalopathy. Neurology 81, 1122–1129. 10.1212/WNL.0b013e3182a55f7f [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart P. A., Hayakawa K., Akers M. A., Vinters H. V. (1992). A morphometric study of the blood-brain barrier in Alzheimer’s disease. Lab Invest 67, 734–742. [PubMed] [Google Scholar]
- Stozicka Z., Zilka N., Novak P., Kovacech B., Bugos O., Novak M. (2010). Genetic background modifies neurodegeneration and neuroinflammation driven by misfolded human tau protein in rat model of tauopathy: implication for immunomodulatory approach to Alzheimer’s disease. J. Neuroinflammation 7:64. 10.1186/1742-2094-7-64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarasoff-Conway J. M., Carare R. O., Osorio R. S., Glodzik L., Butler T., Fieremans E., et al. (2016). Clearance systems in the brain–implications for Alzheimer diseaser. Nat. Rev. Neurol. 12:248. 10.1038/nrneurol.2016.36 [DOI] [PubMed] [Google Scholar]
- Thomsen M. S., Routhe L. J., Moos T. (2017). The vascular basement membrane in the healthy and pathological brain. J. Cereb. Blood Flow Metab. 37, 3300–3317. 10.1177/0271678x17722436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tietz S., Engelhardt B. (2015). Brain barriers: crosstalk between complex tight junctions and adherens junctions. J. Cell Biol. 209, 493–506. 10.1083/jcb.201412147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Togo T., Sahara N., Yen S. H., Cookson N., Ishizawa T., Hutton M., et al. (2002). Argyrophilic grain disease is a sporadic 4-repeat tauopathy. J. Neuropathol. Exp. Neurol. 61, 547–556. 10.1093/jnen/61.6.547 [DOI] [PubMed] [Google Scholar]
- van der Wal E. A., Gómez-Pinilla F., Cotman C. W. (1993). Transforming growth factor-beta 1 is in plaques in Alzheimer and Down pathologies. Neuroreport 4, 69–72. 10.1097/00001756-199301000-00018 [DOI] [PubMed] [Google Scholar]
- Wallez Y., Huber P. (2008). Endothelial adherens and tight junctions in vascular homeostasis, inflammation and angiogenesis. Biochim. Biophys. Acta 1778, 794–809. 10.1016/j.bbamem.2007.09.003 [DOI] [PubMed] [Google Scholar]
- Walter J., van Echten-Deckert G. (2013). Cross-talk of membrane lipids and Alzheimer-related proteins. Mol. Neurodegener. 8:34. 10.1186/1750-1326-8-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D. R. (2006). Tauopathies: classification and clinical update on neurodegenerative diseases associated with microtubule-associated protein tau. Intern. Med. J. 36, 652–660. 10.1111/j.1445-5994.2006.01153.x [DOI] [PubMed] [Google Scholar]
- Winikates J., Jankovic J. (1994). Vascular progressive supranuclear palsy. J. Neural Transm. Suppl. 42, 189–201. 10.1007/978-3-7091-6641-3_15 [DOI] [PubMed] [Google Scholar]
- Winkler E. A., Sagare A. P., Zlokovic B. V. (2014). The pericyte: a forgotten cell type with important implications for Alzheimer’s disease? Brain Pathol. 24, 371–386. 10.1111/bpa.12152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirz K. T., Keitel S., Swaab D. F., Verhaagen J., Bossers K. (2014). Early molecular changes in Alzheimer disease: can we catch the disease in its presymptomatic phase? J. Alzheimers Dis. 38, 719–740. 10.3233/jad-130920 [DOI] [PubMed] [Google Scholar]
- Woerman A. L., Aoyagi A., Patel S., Kazmi S. A., Lobach I., Grinberg L. T., et al. (2016). Tau prions from Alzheimer’s disease and chronic traumatic encephalopathy patients propagate in cultured cells. Proc. Natl. Acad. Sci. U S A 113, E8187–E8196. 10.1073/pnas.1616344113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong A. D., Ye M., Levy A. F., Rothstein J. D., Bergles D. E., Searson P. C. (2013). The blood-brain barrier: an engineering perspective. Front. Neuroeng. 6:7. 10.1385/1-59259-419-0:145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss-Coray T., Lin C., Sanan D. A., Mucke L., Masliah E. (2000). Chronic overproduction of transforming growth factor-beta1 by astrocytes promotes Alzheimer’s disease-like microvascular degeneration in transgenic mice. Am. J. Pathol. 156, 139–150. 10.1016/s0002-9440(10)64713-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki Y., Kanekiyo T. (2017). Blood-brain barrier dysfunction and the pathogenesis of Alzheimer’s disease. Int. J. Mol. Sci. 18:1965. 10.3390/ijms18091965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki Y., Shinohara M., Shinohara M., Yamazaki A., Murray M. E., Liesinger A. M., et al. (2019). Selective loss of cortical endothelial tight junction proteins during Alzheimer’s disease progression. Brain 142, 1077–1092. 10.1093/brain/awz011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiyama Y., Higuchi M., Zhang B., Huang S.-M., Iwata N., Saido et al. (2007). Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron 53, 337–351. 10.1016/j.neuron.2007.01.010 [DOI] [PubMed] [Google Scholar]
- Zenaro E., Piacentino G., Constantin G. (2017). The blood-brain barrier in Alzheimer’s disease. Neurobiol. Dis. 107, 41–56. 10.1016/j.nbd.2016.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Tian Q., Zhang Q., Zhou X., Liu S., Wang J. Z. (2009). Hyperphosphorylation of microtubule-associated tau protein plays dual role in neurodegeneration and neuroprotection. Pathophysiology 16, 311–316. 10.1016/j.pathophys.2009.02.003 [DOI] [PubMed] [Google Scholar]
- Zilka N., Filipcik P., Koson P., Fialova L., Skrabana R., Zilkova M., et al. (2006). Truncated tau from sporadic Alzheimers disease suffices to drive neurofibrillary degeneration in vivo. FEBS Lett. 580, 3582–3588. 10.1016/j.febslet.2006.05.029 [DOI] [PubMed] [Google Scholar]
- Zilka N., Korenova M., Novak M. (2009). Misfolded tau protein and disease modifying pathways in transgenic rodent models of human tauopathies. Acta Neuropathol. 118, 71–86. 10.1007/s00401-009-0499-y [DOI] [PubMed] [Google Scholar]
- Zlokovic B. V. (2005). Neurovascular mechanisms of Alzheimer’s neurodegeneration. Trends Neurosci. 28, 202–208. 10.1016/j.tins.2005.02.001 [DOI] [PubMed] [Google Scholar]
- Zlokovic B. V. (2008). The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron 57, 178–201. 10.1016/j.neuron.2008.01.003 [DOI] [PubMed] [Google Scholar]
- Zlokovic B. V. (2011). Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat. Rev. Neurosci. 12, 723–738. 10.1038/nrn3114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvi C., Molinatti G., Hamel E. (1997). Astroglial and vascular interactions of noradrenaline terminals in the rat cerebral cortex. J. Cereb. Blood Flow Metab. 17, 894–904. 10.1097/00004647-199708000-00008 [DOI] [PubMed] [Google Scholar]


