Abstract
This manuscript presents quantitative findings on the actual effectiveness of terminal complement component 5 (C5) inhibitors and complement component 1 (C1) esterase inhibitors through their formal and common “off-label” (compassionate) indications. The results emanated from pairwise and network meta-analyses to present evidence until September 2019. Clinical trials (CT) and real-life non-randomized studies of the effects of interventions (NRSI) are consistent on the benefits of C5 inhibitors and of the absence of effects of C1 esterase inhibitors (n = 7484): Mathematically, eculizumab (surface under the cumulative ranking area (SUCRA) >0.6) and ravulizumab (SUCRA ≥ 0.7) were similar in terms of their protective effect on hemolysis in paroxysmal nocturnal hemoglobinuria (PNH), thrombotic microangiopathy (TMA) in atypical hemolytic uremic syndrome (aHUS), and acute kidney injury (AKI) in aHUS, in comparison to pre-/off-treatment state and/or placebo (SUCRA < 0.01), and eculizumab was efficacious on thrombotic events in PNH (odds ratio (OR)/95% confidence interval (95% CI) in CT and real-life NRSI, 0.07/0.03 to 0.19, 0.24/0.17 to 0.33) and chronic kidney disease (CKD) occurrence/progression in PNH (0.31/0.10 to 0.97, 0.66/0.44 to 0.98). In addition, meta-analysis on clinical trials shows that eculizumab mitigates a refractory generalized myasthenia gravis (rgMG) crisis (0.29/0.13 to 0.61) and prevents new acute antibody-mediated rejection (AMR) episodes in kidney transplant recipients (0.25/0.13 to 0.49). The update of findings from this meta-analysis will be useful to promote a better use of complement inhibitors, and to achieve personalization of treatments with this class of drugs.
Keywords: complement inactivating agents, meta-analysis as topic, biological products
1. Introduction
The complement system that functions to protect the host against infection, principally by opsonizing and lysing pathogens, can cause tissue damage and inflammation in the cases of dysregulation that occurs commonly due to rare defects genetically originated [1]. In this sense, a spectrum of diseases and patients benefit from pharmacological inhibition of various complement components. The inhibition of terminal complement component 5 (C5) is approved by the Food and Drug Administration (FDA)- and/or the European Medicine Agency (EMA) for the treatment of paroxysmal nocturnal hemoglobinuria (PNH), atypical hemolytic uremic syndrome (aHUS), and more recently to treat refractory generalized myasthenia gravis (rgMG). The two currently available C5 inhibitors, eculizumab and ravulizumab, are also used in the field of solid organ transplantation to treat acute antibody-mediated rejection (aAMR) and delayed graft function (DGF). Eculizumab and ravulizumab are used regardless of their well-known chemical differences, i.e., the four aminoacid substitutions for pH-dependent binding to increase the natural Fc receptor recycling of ravulizumab [2], but without knowledge of more advantages other than a longer half-life of ravulizumab as compared to eculizumab [2].
In addition to C5 inhibitors, various complement component 1 (C1) esterase inhibitors (e.g., Berinert®, Cinryze®, Haegarda®, Ruconest®) that are used in the treatment of hereditary angioedema (HAE) have been proposed as a possible alternative to eculizumab in the treatment/prevention of aAMR and DGF [3]. C1 esterase inhibitors act upstream in the complement classical pathway but also in the contact pathway and in decreasing the liberation of bradykinin [4].
Hypothesis and Study Objective
Targeting the complement, particularly C5, has gained a renewed interest in the last 10 years, often in an off-label manner, for severe conditions lacking any curative treatment [5,6,7]. Indeed, to date, the number of “off-label” or compassionate indications for these drugs can exceed the “officially” approved indications, and this difference might even increase in the future [8].
Importantly, as seen in other areas [9], the actual effectiveness of complement inhibition and differences between each product (or dosing scheme) can only be correctly evaluated if both clinical trials and observational studies assessing ‘real-life’ patients suffering from diseases associated with complement activation are evaluated. Innovative designs should be conceived to carry out systematic reviews to encompass all available evidence both on clinical trials and real-life studies, and network meta-analysis by using standardized tools should be performed to calculate the individual effects of each drug [10].
This report presents separate parallel one-stage pairwise and network meta-analyses on clinical trials and real-life non-randomized studies of the effects of interventions (NRSI) to address the effectiveness of complement inhibition in the treatment of PNH, aHUS, and rgMG (approved indications), and in the treatment/prevention of aAMR and DGF after solid organ transplantation (“off-label” indications).
2. Materials and Methods
Mathematical and non-mathematical results presented here respond to the review question on how efficacious are C5 inhibitors and C1 esterase inhibitors in the treatment of PNH, aHUS, and rgMG, and in the treatment/prevention of aAMR and DGF (Table 1). Literature search, study screening and selection, and data extraction are reported in details in our registered protocol in the International prospective register of systematic reviews PROSPERO, created on 21 October 2019, and updated on 3 February 2020 (CRD42019130690, https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42019130690). For the interest of readers, search strategy formulation is available online (Panel S1).
Table 1.
Study eligibility.
| Criteria | |
|---|---|
| Participants | Adult and pediatric individuals affected by or at higher risk of developing PNH attacks, aHUS, rgMG, aAMR episodes, or DGF. |
| Interventions | Commercial C5 inhibitors (e.g., eculizumab, ravulizumab) and C1-inhibitors (e.g., Berinert®, Cinryze®, Haegarda®, Ruconest®). |
| Comparisons | Placebo, pre-/off-treatment state, historical cohorts that did not receive the interventions, and any other therapeutic strategy (e.g., SOC) including active drugs when it was considered as comparators in the eligible studies. |
| Type of study | RCT including their extension follow-up studies/post-hoc analyses, in addition to historically controlled interventional studies and other non-randomized (single arm) clinical trials. |
| Real-life NRSI (e.g., registry studies and other real-world data studies). |
Abbreviations: aHUS, atypical hemolytic uremic syndrome; aAMR, acute antibody-mediated rejection; DGF, delayed graft function; NRSI, non-randomized studies of the effects of interventions; PNH, paroxysmal nocturnal hemoglobinuria; RCT, randomized controlled trial; rgMG, refractory generalized myasthenia gravis; SOC, standard of care.
Statistical analysis was carried out on aggregate data, after assessing risk of bias in both clinical trials [11] and real-life NRSI [12]. Pooled ORs and 95% CIs for treatment failure outcomes in PNH (hemolysis, thrombosis, CKD apparition/progression), aHUS (TMA, AKI), rgMG, and aAMR, prevention failure in AMR, and DGF were obtained via pairwise meta-analysis (Mantel–Haenszel random-effect method), after verifying heterogeneity (χ2, I2) and the presence of reporting bias (visual inspection of funnel plots and calculation of Egger’s test, if necessary), using Review Manager software (RevMan) version 5.3 (Cochrane Collaboration) and META-analysis package FOr R (METAFOR) version 2.4 (R project). Pooled ORs and 95% CrIs for the same outcomes evaluated at the pairwise level were calculated via Bayesian network meta-analysis (Markov chain Monte Carlo simulation on vague priors random-effect method for ‘bad’ outcomes and zero values correction), with calculation of the surface under the cumulative ranking area (SUCRA) corresponding to drugs/schemes described in the included studies, after verifying convergence (Brooks–Gelman–Rubin method) and inconsistency, using NetMetaXL software (Canadian Agency for Drugs and Technologies in Health and Cornerstone Research Group) [13].
Skewed and non-quantitative data were presented descriptively following the recommendations of the Centre for Reviews and Dissemination (University of York) [14]. As previously made in other meta-analysis from our team [10], a multidisciplinary supervision mechanism for the contextualization of findings from this summary was planned, with specialists in nephrology (D.M.-G. and D.T.), hematology (M.A.), immunology (J.V.), epidemiology (F.J.d.P. and F.L.-S.), and translational pharmacology (F.J.A.).
3. Results
3.1. Systematic Narrative Synthesis
The findings presented here are reported in accordance to the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) recommendations [15], and strictly adhere to the PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of healthcare interventions [16].
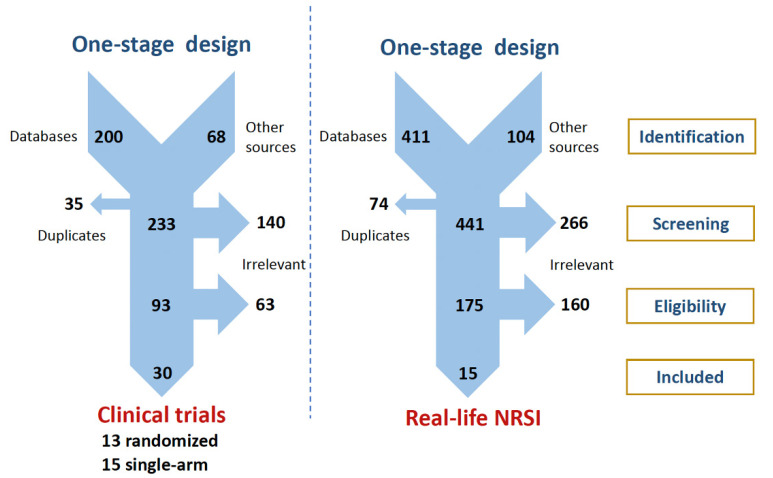
Clinical trials and real-life NRSI were searched separately in a parallel one-stage selection procedure, as depicted in Figure 1 (see Materials and Methods for further details). After excluding clearly irrelevant studies (i.e., pre-clinical studies and clinical trials with no evaluation of the eligible outcomes, other observational studies that did not meet the conditions to be considered as real-life NRSI, such as case series or case reports), 28 pharmaceutical industry-sponsored clinical trials corresponding to phases 1 to 3 evaluation of various complement inhibitors, and 15 real-life NRSI reflecting uses of these medicines in real-world settings were found to be eligible: these studies assess outcomes in PNH (No. of clinical trials/real-life NRSI: 7/7), aHUS (7/8), rgMG (3/0), aAMR (6/0), and DGF (5/0), and included a total population of 7484 participants [17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91].
Figure 1.
PRISMA flowcharts presenting our parallel one-stage systematic review selection process for retrieving complement inhibition evidence on clinical trials and real-life NRSI. NRSI, non-randomized studies of the effects of interventions; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Demographics and clinical details of study participants and the characteristics of the included studies are available for readers online (Table S1). Results from our meta-analytic calculations involved 93.3% of the total number of study participants and cover the inhibition of C5 protein (n = 3045), the inhibition of C1 esterase (n = 105), and the comparisons for these interventions (n = 4334) (Table 2).
Table 2.
Number of participants from studies for which numerical data were available for analysis.
| Clinical Trials | Real-Life NRSI | |||||
|---|---|---|---|---|---|---|
| C5 Inhibition | C1 Inhibition | Controls | C5 Inhibition | C1 Inhibition | Controls | |
| PNH | 665 | - | 86 | 1338 | - | 2851 |
| aHUS | 137 | - | 185 | 463 | - | 627 |
| rgMG | 69 | - | 70 | - | - | - |
| aAMR | 186 | 60 | 285 | - | - | - |
| DGF | 187 | 45 | 230 | - | - | - |
| Total | 1244 | 105 | 856 | 1801 | - | 3478 |
Abbreviations: aAMR, acute antibody-mediated rejection; aHUS, atypical hemolytic uremic syndrome; DGF, delayed graft function; NRSI, non-randomized studies of the effects of interventions; PNH, paroxysmal nocturnal hemoglobinuria; rgMG, refractory generalized myasthenia gravis.
All real-life NRSI and all clinical trials but one randomized study [91] were published in peer-reviewed journals. In most cases, data of a single study were displayed in more than one published article. Oral communications and posters presented in meetings of medical societies involved in the treatment of the diseases addressed in this meta-analysis contain also critical information and can be part of the included studies [52,56,64,74,84,85].
In 15 clinical trials (number of trials in PNH/aHUS/rgMG/aAMR: 4/7/1/3, respectively), inclusion of participants into the study was not followed by random allocation of such individuals into intervention and control groups. These single-arm trials used pre- and/or off-treatment state [17,18,20,21,22,23,24,25,45,46,47,48,49,50,51,52,79] or historical cohorts [80,82,87] as comparators for the evaluation of complement inhibition effectiveness.
Randomization was performed in trials in PNH (number of placebo-/standard of care (SOC)-/active-controlled trials: 1/0/2) [19,26,27], in trials assessing eculizumab as treatment of rgMG crises (2/0/0) [77,78], as well as in trials assessing complement inhibitors, respectively, as treatment (1/0/0) or prevention of new aAMR episodes (1/1/0) in kidney recipients [81,83,84,85,86], and as prevention of DGF after kidney transplantation (5/0/0) [88,89,90,91].
Importantly, all participants in non-randomized single-arm trials and all those undergoing placebo or the intervention (complement inhibition) in randomized two-arm trials did not stop rescue treatments (e.g., plasma exchanges and/or intravenous immunoglobulin infusion for treating aHUS, aAMR, and rgMG, immunosuppressive schemes for preventing DGF), nor maintenance treatments (e.g., erythropoietin, corticosteroids and anticoagulants in PNH, maintenance immunosuppression in transplant recipients).
Real-life NRSI comprised most analyzed cases (n = 5279), i.e., approximately two-thirds, while the remaining were participants in clinical trials (n = 2205). The comparators in real-life NRSI were pre-eculizumab era individuals, i.e., patients who never underwent complement inhibition (56%), and individuals treated with complement inhibitors in their off-treatment state, i.e., patients who discontinued complement inhibitors for various reasons (33%), and patients in their pre-treatment state, i.e., before receiving complement inhibition (11%).
Furthermore, clinical trials and real-life NRSI did not assess adult and pediatric populations separately [17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76]. In one trial in aAMR [83] and in two trials in DGF [88,89], DGF prevention and aAMR prevention were not pre-specified as study outcomes, suggesting a potential high risk of alpha inflation (false discovery rate). Results from our evaluation of risk of bias in included studies are available for readers online (Table S1).
3.2. Quantitative Analysis
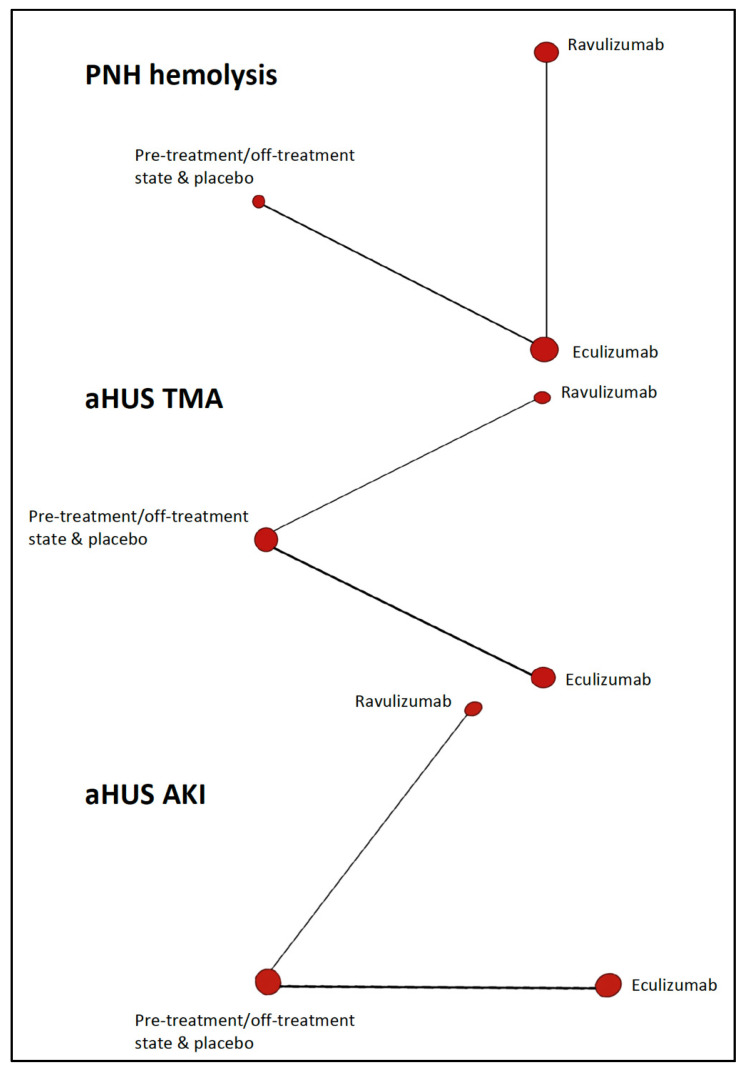
Thirteen non-randomized single-arm trials and one randomized two-arms trial provided numerical data from individuals with PNH (n = 751) and aHUS (n = 322), that were entered in multiple-treatments’ meta-analysis calculations. As depicted in Figure 2, the Bayesian network diagrams corresponding to these analyses illustrates the scarcity of available evidence.
Figure 2.
Bayesian network diagrams for the competing complement C5 inhibitors corresponding to three outcomes (clinical trials). aHUS, atypical hemolytic uremic syndrome; AKI, acute kidney injury; PNH, paroxysmal nocturnal hemoglobinuria; TMA, thrombotic microangiopathy.
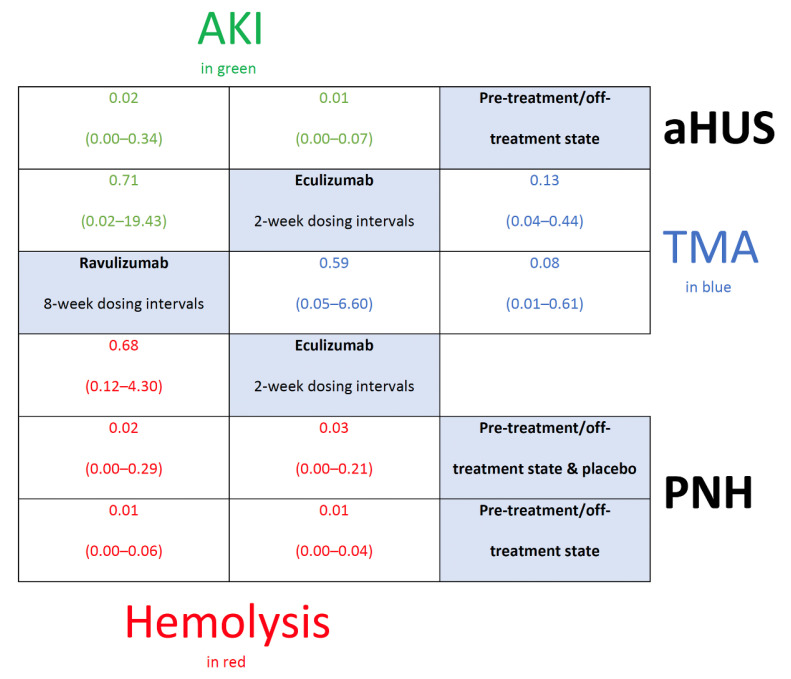
Nevertheless, as presented in Figure 3, summary estimates on, respectively, hemolysis in PNH [17,18,19,20,21,22,23,24,25,26,27], thrombotic microangiopathy (TMA) in aHUS [45,46,47,48,49,50,51,52], and acute kidney injury (AKI) in aHUS [45,46,47,48,49,50,51,52], demonstrate a significant protective effect of eculizumab (odds ratio (OR)/95% credible interval (95% CrI): 0.03/0.00 to 0.21, 0.13/0.04 to 0.44, 0.01/0.00 to 0.07) and ravulizumab (0.02/0.00 to 0.29, 0.08/0.01 to 0.61, 0.02/0.00 to 0.34) compared to pre-/off-treatment state and/or placebo (which including rescue/maintenance treatments).
Figure 3.
League table showing effect estimates of the assessed complement C5 inhibitors (eculizumab vs. ravulizumab) against the comparators on three outcomes (acute kidney injury (green), thrombotic microangiopathy (blue), and hemolysis (red)) (clinical trials). aHUS, atypical hemolytic uremic syndrome; AKI, acute kidney injury; PNH, paroxysmal nocturnal hemoglobinuria; TMA, thrombotic microangiopathy.
Importantly, with regard to hemolysis in PNH, taking into account only trials using pre-/off-treatment state as comparators (i.e., by excluding the only randomized placebo-controlled trial available), the protective effect of eculizumab (0.01/0.00 to 0.04), and ravulizumab (0.01/0.00 to 0.06) persisted.
On the basis of the surface under the cumulative ranking area (SUCRA), eculizumab (>0.6) and ravulizumab (≥0.7) were similar in terms of their effects on the above mentioned three outcomes, and a markedly difference between treat and not to treat with C5 inhibitors (<0.01) was observed (Table 3). Vague prior random-effects heterogeneity in these calculations was in part counterbalanced by the absence of inconsistency (Figure S1).
Table 3.
SUCRA-based ranking of C5 inhibitors evaluated (clinical trials).
| Drug Intervention † C5 Inhibitors | SUCRA ‡ Outcomes: A/B/C § |
|---|---|
| Eculizumab | 0.637/0.642/0.797 |
| Ravulizumab | 0.860/0.850/0.700 |
| Pre-treatment/off-treatment states or placebo | 0.002/0.007/0.003 |
§ Hemolysis (A) in PNH, and TMA (B) and AKI (C) in aHUS, were the outcomes assessed into network level. † The two commercial C5 inhibitors analyzed were ranked according to probabilities for being the best, the second best, the third best, and so on , following Markov chain Monte Carlo methods. ‡ SUCRA for each C5 inhibitor out of the competing C5 inhibitors requires calculation of the vector of the cumulative probabilities to be among the best drug, . Abbreviations: aHUS, atypical hemolytic uremic syndrome; AKI, acute kidney injury; PNH, paroxysmal nocturnal hemoglobinuria; SUCRA, surface under the cumulative ranking area; TMA, thrombotic microangiopathy.
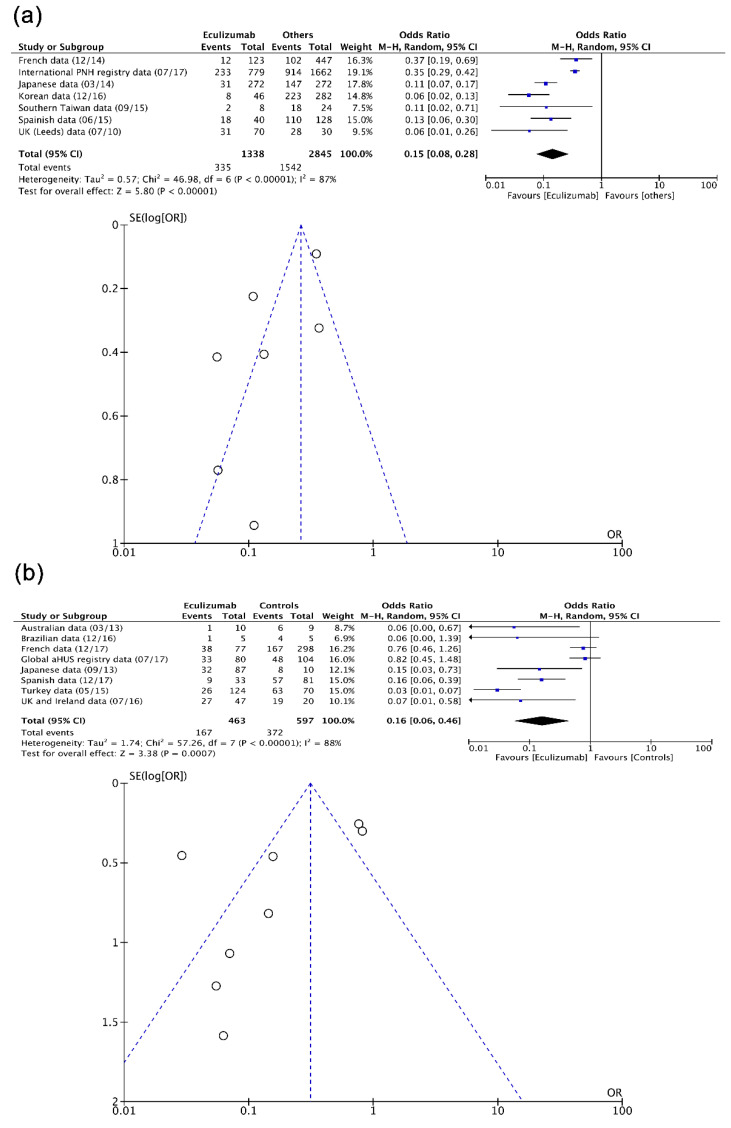
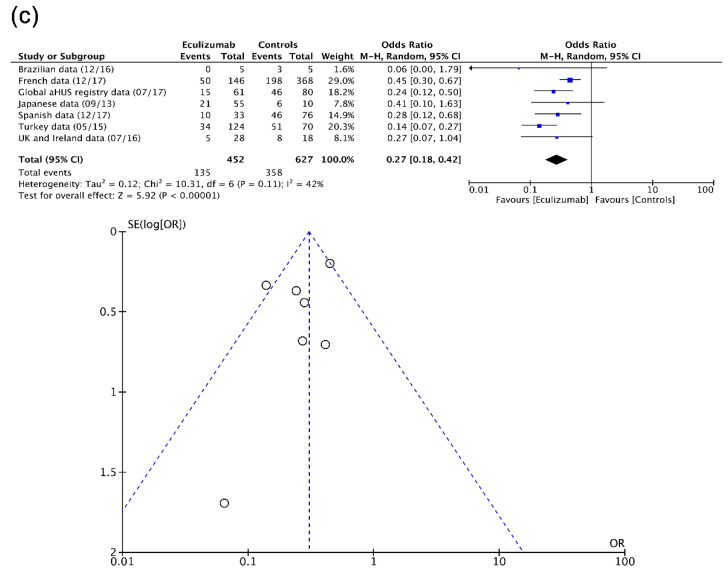
Fifteen real-life NRSI including individuals with PNH (n = 4189) and aHUS (n = 1090) were assessed mathematically into pairwise level. As observed in Figure 4, a protective effect from eculizumab was evident in hemolysis in PNH [28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44], TMA in aHUS [53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76], and AKI in aHUS [53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76] according to summary estimates obtained (OR/95% confidence interval (95% CI): 0.15/0.08 to 0.28, 0.16/0.06 to 0.46, 0.27/0.18 to 0.42). Considerable heterogeneity (I2 >80%) and funnel plot asymmetry affected effect estimates, particularly those corresponding to hemolysis in PNH (Egger’s test (t)/degrees of freedom (df)/p: −2.8186, 17, 0.0372) and TMA in aHUS (−2.3591, 13, 0.0414).
Figure 4.
Forest and funnel plots showing effect estimates of eculizumab (real-life NRSI) in (a) hemolysis in PNH, (b) TMA in aHUS, and (c) AKI in aHUS. aHUS, atypical hemolytic uremic syndrome; AKI, acute kidney injury; CI, confidence interval; M–H, Mantel–Haenszel test; NRSI, non-randomized studies of the effects of interventions; PNH, paroxysmal nocturnal hemoglobinuria; SE, standard error; TMA, thrombotic microangiopathy.
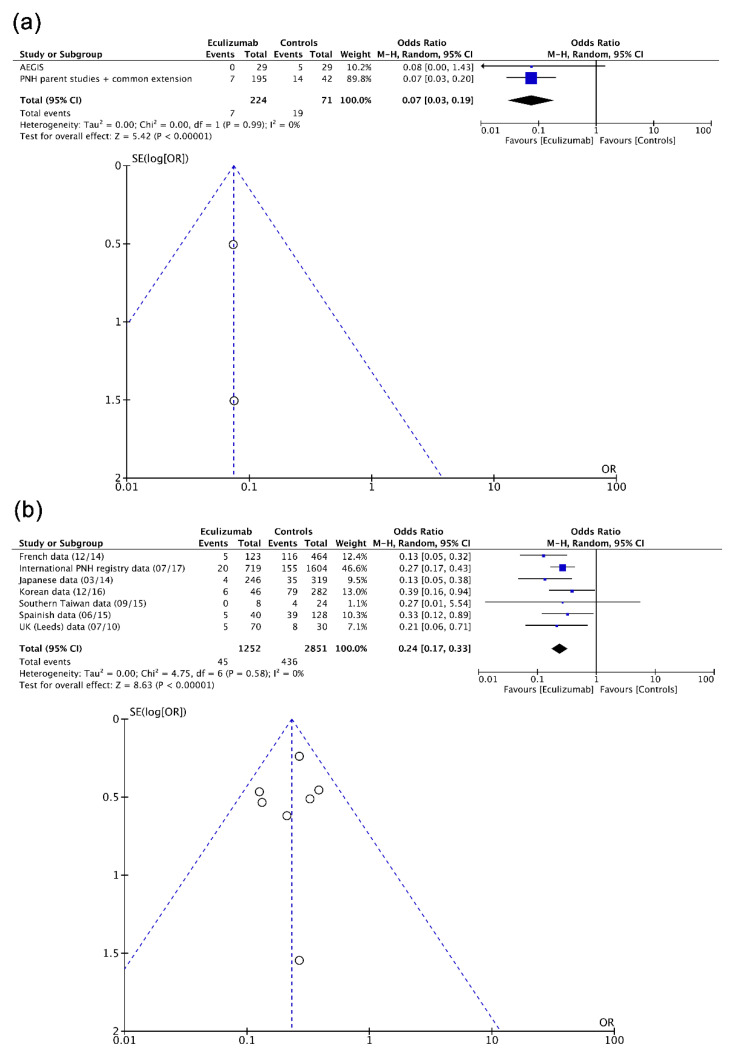
As shown in Figure 5, eculizumab had a positive effect, respectively, in clinical trials and real-life NRSI, on thrombotic events in PNH (0.07/0.03 to 0.19, 0.24/0.17 to 0.33) and chronic kidney disease (CKD) occurrence/progression in PNH (0.31/0.10 to 0.97, 0.66/0.44 to 0.98). No (or non-important) heterogeneity and almost no (or weak) funnel plot asymmetry affected these calculations.
Figure 5.
Forest and funnel plots showing effect estimates of eculizumab for (a) clinical trials and (b) real-life NRSI in thrombotic events in PNH, and for (c) clinical trials and (d) real-life NRSI in CKD apparition/progression in PNH. CI, confidence interval; CKD, chronic kidney disease; M–H, Mantel–Haenszel test; NRSI, non-randomized studies of the effects of interventions; PNH, paroxysmal nocturnal hemoglobinuria; SE, standard error.
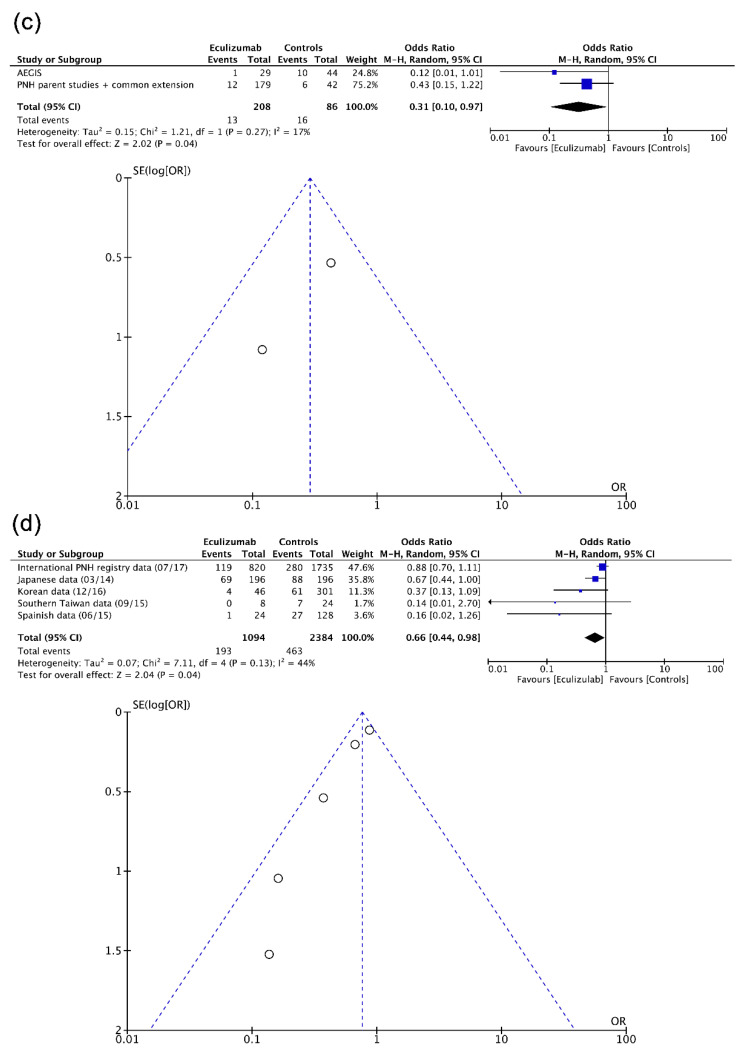
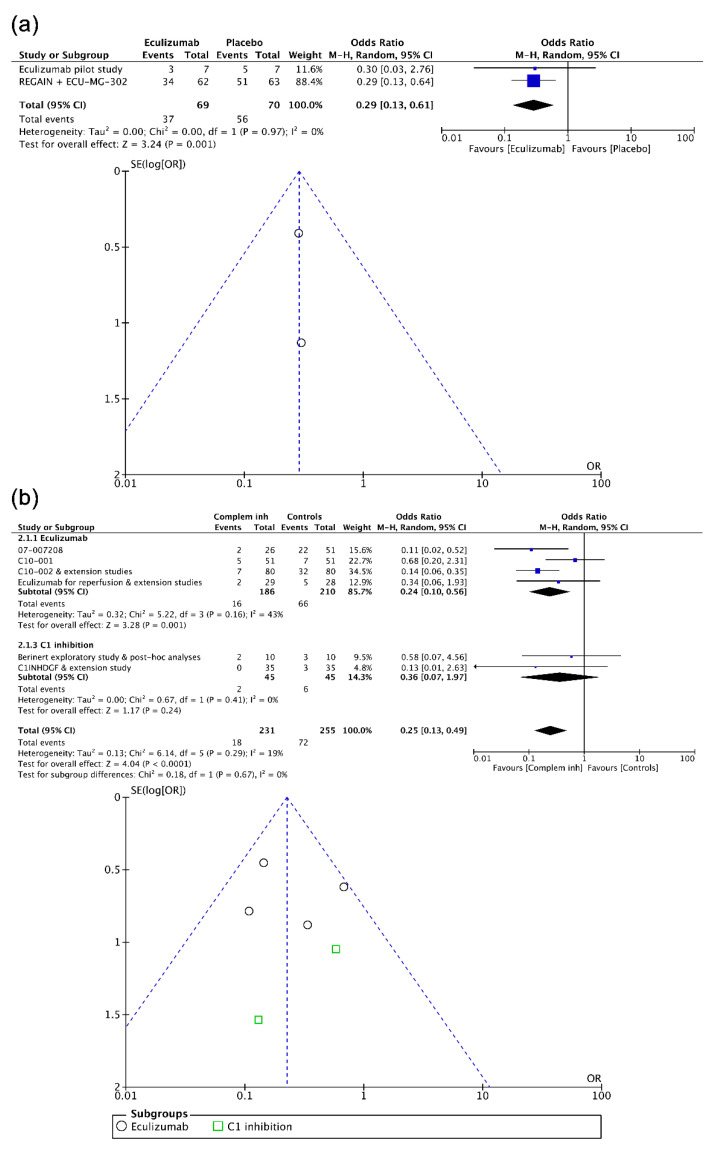
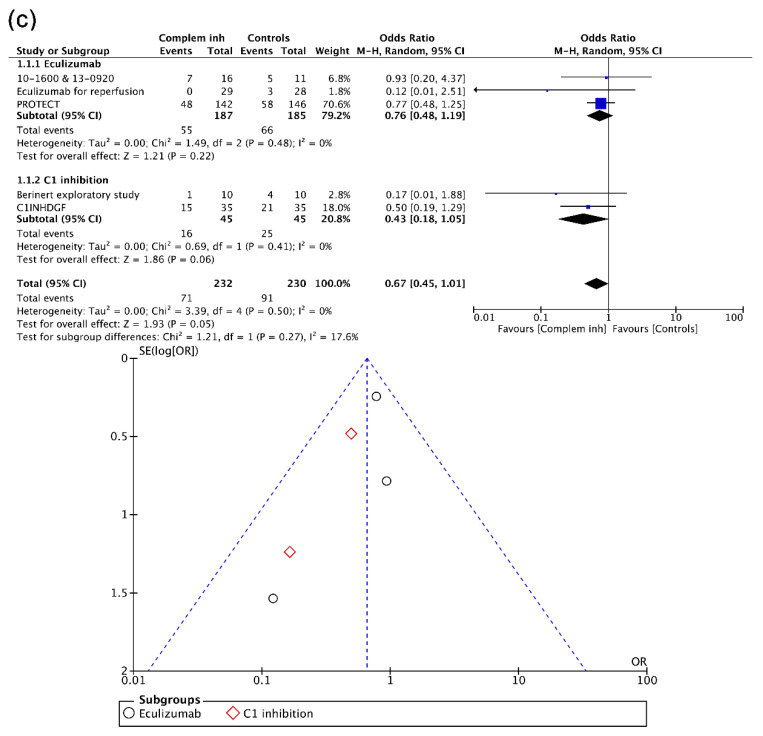
Again at the pairwise level, when combining data from, respectively, the two randomized trials in rgMG and the extension follow-up study of one of them [76,77,78], and the four trials in aAMR prevention [82,86,87,88], eculizumab had a positive effect (0.29/0.13 to 0.61, 0.25/0.13 to 0.49). As shown in Figure 6, although the overall effect of complement inhibition regarding the prevention of new aAMR episodes in kidney recipients was protective, the absence of an effect from the inhibition of C1 esterase allows for seeing an effect only from eculizumab (0.24/0.10 to 0.56) [83,84,85,89], suggesting the efficacy of targeting C5. Contrarily, no effect from complement inhibitors on the prevention of DGF was observed [83,88,89,90,91]. Heterogeneity (43%) affected summary estimates in subgroup analysis of eculizumab to prevent aAMR, and funnel plot asymmetry was particularly noted in the analysis on prevention of DGF. No effect from the inhibition of C1 esterase for treating aAMR [80,81] was found (data not shown).
Figure 6.
Forest and funnel plots showing effect estimates of complement inhibition (clinical trials) corresponding to (a) the treatment of rgMG crises, (b) the prevention of new acute AMR episodes, and (c) the prevention of DGF after kidney transplantation. AMR, antibody-mediated prevention; CI, confidence interval; M–H, Mantel–Haenszel test; PBO, placebo; rgMG, refractory generalized myasthenia gravis; SE, standard error.
4. Discussion
4.1. Important Messages
This manuscript presented gathers and evidence on the effectiveness of complement inhibition. Formal and common “off-label” (compassionate) indications of these medicines are covered. Our results include a total of 7484 participants and confirmed that C5 inhibitors are effective (i) to treat PNH, aHUS, and rgMG crises, and (ii) to prevent aAMR episodes. Clinical trials and real-life NRSI are consistent on the beneficial effect of C5 inhibition. The two available C5 inhibitors eculizumab and ravulizumab are similar regarding their effect. The evidence on the inhibition of C1 esterase is still scarce, and data from our analysis showed no effects.
Complement inhibition has become in the last decade a new therapeutic option for a number of rare diseases, most of them leading to death, and that constitute a hard societal burden all over the world [5,6,7]. In some of these indications (such as PNH), it is important to emphasize that C5 inhibition provides only symptomatic relief. Over the past decade, there has been an impressive increase in our comprehension of the role of complement in diverse physiological and pathophysiological states. Together with the discovery of complement inhibitors, assessing both clinical trials and real-life studies, it may allow an optimal use of these drugs. This is the first summary on the benefits of complement inhibitors in various conditions, after verifying that mathematical dichotomous data on various indications for these drugs were available, and with the intention to go beyond clinical trial evidence.
The effect of complement inhibition on several key outcomes is reported. This effect persists in RCT and also in real-life NRSI, despite the fact that the quality of the included individual studies greatly differs, and the existence of statistical heterogeneity and reporting bias in many studies. In particular, with regard to differences between participants in different latitudes leading to heterogeneity, the genetic background should be considered, among other factors.
Moreover, separate summary effect estimates for eculizumab and ravulizumab are presented only for PNH and aHUS. In these two disorders, eculizumab was found to be similar to ravulizumab in terms of their protective effect. Evidence shows thus that eculizumab and ravulizumab play an important role in the management of PNH and aHUS. In our opinion, decisions to use one or the other C5 inhibitor should be based on other factors such as costs, insurance coverage, availability, local expertise, etc. In addition, in these and other indications, long-term use of complement inhibitors remains to be evaluated.
Finally, very few publications investigating the effect of C1 esterase inhibitors were included, and no effects on various pre-defined outcomes were found.
4.2. Findings in Context
The most recent summaries on complement inhibition have not addressed the impact of these drugs from a pharmacoepidemiological perspective, that is, for the moment, there are no comprehensive assessments on the benefits of using these medications through their multiple indications [92,93]. However, the evidence body is still small enough to cover mathematically all complement activation diseases.
Our pharmacometrical assessement of complement inhibition clearly showed a significant benefit of treating patients (compared to placebo or historical cohort or standard-of-care), and this is the main finding of our research. Furthermore, we found no difference between eculizumab and ravulizumab.
As demonstrated in a previous work performed by our team, staged systematic review processes and network meta-analysis assessments lead to a more exhaustive evaluation of evidence [10], particularly if the diseases/conditions studied are rare. Updating regularly systematic reviews and meta-analyses is also another very important aspect to take into account [94]. In this sense, the findings presented here should not be interpreted as definitive or categorical: results from a new 26-week, single arm, open-label, phase 3 study of ravulizumab in aHUS, called the 312 study (NCT03131219), will be available soon [95].
4.3. Study Limitations
There are some limitations that should be mentioned. Overall, the entire body of evidence is small, even if our summary included real-life studies supporting clinical trials [9]. Analyses performed have attempted to present findings as free as possible from heterogeneity and reporting bias, the common limitations in meta-analysis [96,97]. Such limitations may constitute a discouraging finding, particularly into calculations with real-life NRSI. Nevertheless, although calculations with clinical trials were less affected by heterogeneity, clinical trials were mostly non-randomized.
Furthermore, non-negligible limits by addressing several and different diseases/conditions is to lose nuance in the discussion of actual benefits from complementary inhibition across such affections. For instance, in the setting of transplantation, the various existing types of antibody-mediated rejection should be taken into account: C5 inhibitors do not have a proven effect on chronic AMR [98], which may be interpreted as inconsistent regarding the effect on aAMR supporting our findings. The fact that evidence is available only on kidney transplantation should also be considered, as effectiveness of complement inhibition may vary in recipients of other organs.
4.4. Future Research
Head-to-head comparison between eculizumab and ravulizumab only exists for PNH and aHUS. The effect of eculizumab and ravulizumab in other complement-mediated disorders should be investigated in adequately designed RCTs, and their safety profile carefully compared. In addition, future studies should investigate the effect of complement inhibition in other diseases such as neuromyelitis optica or Guillain–Barre disease, for instance. Finally, the effect of C1 esterase inhibitors should be explored further, as the data currently available remain scarce and the potential role of C1 esterase inhibitors cannot be assessed properly at this stage.
4.5. Conclusions and Regulatory Considerations
Clinical trials and real-life studies support a beneficial effect of C5 inhibitors in the treatment of PNH, aHUS and rgMG crises, and the prevention of new aAMR episodes in kidney recipients. Available evidence that certainly involve C5 inhibitors made it clear that it is better to treat (SUCRA > 0.6) than not to treat (SUCRA < 0.01), and there is no apparent difference between eculizumab and ravulizumab.
Stronger evidence on beneficial effects in PNH, aHUS and rgMG, as well as in acute antibody-mediated rejection of kidney transplants, is currently available for C5 inhibitors, as compared to C1 esterase inhibitors. Updating this evidence (at regular intervals) is thus important, particularly to promote a better use of complement inhibitors, and to achieve personalization of treatments with this class of drugs.
Acknowledgments
The authors thank Beatriz Muñoz (Clinical Epidemiology Support Office, Sanidad de Castilla y León, Zamora, Spain) and Begoña Valdés (Pharmacological Big Data Laboratory, University of Valladolid, Valladolid, Spain) who helped coordinate the data retrieval and Eduardo Gutiérrez who helped in images performing.
Supplementary Materials
The following are available online at https://www.mdpi.com/2227-9059/8/9/355/s1: Table S1: The included studies after searches with respect to (a) their relevant information from study participants grouped by diseases, (b) the risk of bias in clinical trials, and (c) the risk of bias in real-life NRSI; Figure S1: Forest and inconsistency plot for (a) hemolysis in PNH, (b) TMA in aHUS, and (c) AKI in aHUS; Panel S1: Full search strategy and search results.
Author Contributions
Conceptualization, F.H.-G., H.C., J.V., M.P., and Y.D.M.; methodology, F.H.-G.; software, C.B.-G., C.O.-S., and F.H.-G.; validation, C.O.-S., F.H.-G., F.J.Á., and M.P.; formal analysis, C.B.-G., C.O.-S., and F.H.-G.; investigation, C.B.-G., C.O.-S., D.M.-G., D.T., F.C., F.J.d.P., F.H.-G., F.J.Á., F.L.-S., G.G., H.C., J.V., M.A., M.P., and Y.D.M.; resources, F.H.-G. and M.P.; data curation, C.B.-G., F.H.-G., H.C., J.V., and Y.D.M.; writing—original draft preparation, C.B.-G., F.H.-G., and M.P.; writing—review and editing, C.B.-G., C.O.-S., F.C., F.H.-G., H.C., J.V., M.P., and Y.D.M.; visualization, F.H.-G. and M.P.; supervision, F.H.-G. and M.P.; project administration, F.H.-G. and M.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Ricklin D., Hajishengallis G., Yang K., Lambris J.D. Complement: A key system for immune surveillance and homeostasis. Nat. Immunol. 2010;11:785–797. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sheridan D., Yu Z.-X., Zhang Y., Patel R., Sun F., Lasaro M.A., Bouchard K., Andrien B., Marozsan A., Wang Y., et al. Design and preclinical characterization of ALXN1210: A novel anti-C5 antibody with extended duration of action. PLoS ONE. 2018;13:e0195909. doi: 10.1371/journal.pone.0195909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berger M., Lefaucheur C., Jordan S.C. Update on C1 Esterase Inhibitor in Human Solid Organ Transplantation. Transplant. 2019;103:1763–1775. doi: 10.1097/TP.0000000000002717. [DOI] [PubMed] [Google Scholar]
- 4.Muller Y.D., Harr T., Dayer E., Seebach J.D. C1 esterase inhibitor concentrates and attenuated androgens. Lancet. 2018;391:1355–1356. doi: 10.1016/S0140-6736(18)30583-X. [DOI] [PubMed] [Google Scholar]
- 5.Ricklin D., Mastellos D.C., Reis E.S., Lambris J.D. The renaissance of complement therapeutics. Nat. Rev. Nephrol. 2018;14:26–47. doi: 10.1038/nrneph.2017.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ricklin D., Reis E.S., Lambris J.D. Complement in disease: A defence system turning offensive. Nat. Rev. Nephrol. 2016;12:383–401. doi: 10.1038/nrneph.2016.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reis E.S., Mastellos D.C., Yancopoulou D., Risitano A.M., Ricklin D., Lambris J.D. Applying complement therapeutics to rare diseases. Clin. Immunol. 2015;161:225–240. doi: 10.1016/j.clim.2015.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ricklin D., Barratt-Due A., Mollnes T.E. Complement in clinical medicine: Clinical trials, case reports and therapy monitoring. Mol. Immunol. 2017;89:10–21. doi: 10.1016/j.molimm.2017.05.013. [DOI] [PubMed] [Google Scholar]
- 9.Oneil M.E., Berkman N.D., Hartling L., Chang S.M., Anderson J., Motu’Apuaka M., Guise J.-M., McDonagh M.S. Observational evidence and strength of evidence domains: Case examples. Syst. Rev. 2014;3:35. doi: 10.1186/2046-4053-3-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herrera-Gómez F., Chimeno M.M., García D.M., Lizaraso-Soto F., Maurtua-Briseño-Meiggs Á., Grande-Villoria J., Bustamante-Munguira J., Alamartine E., Vilardell M., Sangrador C.O., et al. Cholesterol-Lowering Treatment in Chronic Kidney Disease: Multistage Pairwise and Network Meta-Analyses. Sci. Rep. 2019;9:1–11. doi: 10.1038/s41598-019-45431-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Higgins J.P.T., Green S., editors. Cochrane Handbook for Systematic Reviews of Interventions—Version 5.1.0. The Cochrane Collaboration; London, UK: 2011. [Google Scholar]
- 12.Sterne J.A.C., Hernán M.A., Reeves B.C., Savović J., Berkman N.D., Viswanathan M., Henry D., Altman D.G., Ansari M.T., Boutron I., et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown S., Hutton B., Clifford T.J., Coyle D., Grima D.T., Wells G.A., Cameron C. A Microsoft-Excel-based tool for running and critically appraising network meta-analyses—An overview and application of NetMetaXL. Syst. Rev. 2014;3:110. doi: 10.1186/2046-4053-3-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Systematic Reviews: Centre for Reviews and Dissemination’s (CRD) Guidance for Undertaking Reviews in Health Care. University of York; York, UK: 2008. [Google Scholar]
- 15.Shamseer L., Moher D., Clarke M., Ghersi D., Liberati A., Petticrew M., Shekelle P., Stewart L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. BMJ. 2015;350:g7647. doi: 10.1136/bmj.g7647. [DOI] [PubMed] [Google Scholar]
- 16.Hutton B., Salanti G., Caldwell D.M., Chaimani A., Schmid C.H., Cameron C., Ioannidis J.P., Straus S., Thorlund K., Jansen J.P., et al. The PRISMA Extension Statement for Reporting of Systematic Reviews Incorporating Network Meta-analyses of Health Care Interventions: Checklist and Explanations. Ann. Intern. Med. 2015;162:777–784. doi: 10.7326/M14-2385. [DOI] [PubMed] [Google Scholar]
- 17.Hillmen P., Hall C., Marsh J.C., Elebute M., Bombara M.P., Petro B.E., Cullen M.J., Richards S.J., Rollins S.A., Mojcik C.F., et al. Effect of Eculizumab on Hemolysis and Transfusion Requirements in Patients with Paroxysmal Nocturnal Hemoglobinuria. N. Engl. J. Med. 2004;350:552–559. doi: 10.1056/NEJMoa031688. [DOI] [PubMed] [Google Scholar]
- 18.Hill A., Hillmen P., Richards S.J., Elebute D., Marsh J.C., Chan J., Mojcik C.F., Rother R.P. Sustained response and long-term safety of eculizumab in paroxysmal nocturnal hemoglobinuria. Blood. 2005;106:2559–2565. doi: 10.1182/blood-2005-02-0564. [DOI] [PubMed] [Google Scholar]
- 19.Hillmen P., Young N.S., Schubert J., Brodsky R.A., Socié G., Muus P., Röth A., Szer J., Elebute M.O., Nakamura R., et al. The Complement Inhibitor Eculizumab in Paroxysmal Nocturnal Hemoglobinuria. N. Engl. J. Med. 2006;355:1233–1243. doi: 10.1056/NEJMoa061648. [DOI] [PubMed] [Google Scholar]
- 20.Brodsky R.A., Young N.S., Antonioli E., Risitano A.M., Schrezenmeier H., Schubert J., Gaya A., Coyle L., De Castro C., Fu C.-L., et al. Multicenter phase 3 study of the complement inhibitor eculizumab for the treatment of patients with paroxysmal nocturnal hemoglobinuria. Blood. 2008;111:1840–1847. doi: 10.1182/blood-2007-06-094136. [DOI] [PubMed] [Google Scholar]
- 21.Hillmen P., Muus P., Roth A., Elebute M.O., Risitano A.M., Schrezenmeier H., Szer J., Browne P., Maciejewski J.P., Schubert J., et al. Long-term safety and efficacy of sustained eculizumab treatment in patients with paroxysmal nocturnal haemoglobinuria. Br. J. Haematol. 2013;162:62–73. doi: 10.1111/bjh.12347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hillmen P., Muus P., Dührsen U., Risitano A., Schubert J., Luzzatto L., Schrezenmeier H., Szer J., Brodsky R.A., Hill A., et al. Effect of the complement inhibitor eculizumab on thromboembolism in patients with paroxysmal nocturnal hemoglobinuria. Blood. 2007;110:4123–4128. doi: 10.1182/blood-2007-06-095646. [DOI] [PubMed] [Google Scholar]
- 23.Hillmen P., Elebute M., Kelly R., Urbano-Ispizua A., Hill A., Rother R.P., Khursigara G., Fu C.-L., Omine M., Browne P., et al. Long-term effect of the complement inhibitor eculizumab on kidney function in patients with paroxysmal nocturnal hemoglobinuria. Am. J. Hematol. 2010;85:553–559. doi: 10.1002/ajh.21757. [DOI] [PubMed] [Google Scholar]
- 24.Kanakura Y., Ohyashiki K., Shichishima T., Okamoto S., Ando K., Ninomiya H., Kawaguchi T., Nakao S., Nakakuma H., Nishimura J.-I., et al. Safety and efficacy of the terminal complement inhibitor eculizumab in Japanese patients with paroxysmal nocturnal hemoglobinuria: The AEGIS Clinical Trial. Int. J. Hematol. 2011;93:36–46. doi: 10.1007/s12185-010-0748-9. [DOI] [PubMed] [Google Scholar]
- 25.Kanakura Y., Ohyashiki K., Shichishima T., Okamoto S., Ando K., Ninomiya H., Kawaguchi T., Nakao S., Nakakuma H., Nishimura J.-I., et al. Long-term efficacy and safety of eculizumab in Japanese patients with PNH: AEGIS trial. Int. J. Hematol. 2013;98:406–416. doi: 10.1007/s12185-013-1404-y. [DOI] [PubMed] [Google Scholar]
- 26.Lee J.W., De Fontbrune F.S., Lee L.W.L., Pessoa V., Gualandro S., Füreder W., Ptushkin V., Rottinghaus S.T., Volles L., Shafner L., et al. Ravulizumab (ALXN1210) vs. eculizumab in adult patients with PNH naive to complement inhibitors: The 301 study. Blood. 2019;133:530–539. doi: 10.1182/blood-2018-09-876136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kulasekararaj A.G., Hill A., Rottinghaus S.T., Langemeijer S., Wells R., Gonzalez-Fernandez F.A., Gaya A., Lee J.W., Gutierrez E.O., Piatek C.I., et al. Ravulizumab (ALXN1210) vs. eculizumab in C5-inhibitor–experienced adult patients with PNH: The 302 study. Blood. 2019;133:540–549. doi: 10.1182/blood-2018-09-876805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schrezenmeier H., Muus P., Socié G., Szer J., Urbano-Ispizua A., Maciejewski J.P., Brodsky R.A., Bessler M., Kanakura Y., Rosse W., et al. Baseline characteristics and disease burden in patients in the International Paroxysmal Nocturnal Hemoglobinuria Registry. Haematologica. 2014;99:922–929. doi: 10.3324/haematol.2013.093161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Socié G., Schrezenmeier H., Muus P., Lisukov I., Röth A., Kulasekararaj A., Lee J.W., Araten D., Hill A., Brodsky R., et al. Changing prognosis in Paroxysmal Nocturnal Haemoglobinuria disease subcategories; an analysis of International PNH Registry. Intern. Med. J. 2016;46:1044–1053. doi: 10.1111/imj.13160. [DOI] [PubMed] [Google Scholar]
- 30.Urbano-Ispizua A., Muus P., Schrezenmeier H., Almeida A., Wilson A., Ware R.E. Different clinical characteristics of paroxysmal nocturnal hemoglobinuria in pediatric and adult patients. Haematologica. 2016;102:e76–e79. doi: 10.3324/haematol.2016.151852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee J.W., De Latour R.P., Brodsky R.A., Jang J.H., Hill A., Röth A., Schrezenmeier H., Wilson A., Marantz J.L., Maciejewski J.P. Effectiveness of eculizumab in patients with paroxysmal nocturnal hemoglobinuria (PNH) with or without aplastic anemia in the International PNH Registry. Am. J. Hematol. 2019;94:E37–E41. doi: 10.1002/ajh.25334. [DOI] [PubMed] [Google Scholar]
- 32.Sakurai M., Jang J.H., Chou W.-C., Kim J.S., Wilson A., Nishimura J.-I., Chiou T.-J., Kanakura Y., Lee J.W., Okamoto S. Comparative study on baseline clinical characteristics of Asian versus non-Asian patients with paroxysmal nocturnal hemoglobinuria. Int. J. Hematol. 2019;110:411–418. doi: 10.1007/s12185-019-02699-7. [DOI] [PubMed] [Google Scholar]
- 33.Ninomiya H., Obara N., Chiba S., Usuki K., Nishiwaki K., Matsumura I., Shichishima T., Okamoto S., Nishimura J.-I., Ohyashiki K., et al. Interim analysis of post-marketing surveillance of eculizumab for paroxysmal nocturnal hemoglobinuria in Japan. Int. J. Hematol. 2016;104:548–558. doi: 10.1007/s12185-016-2065-4. [DOI] [PubMed] [Google Scholar]
- 34.Choi C.W., Jang J.H., Kim J.S., Jo D.-Y., Lee J.-H., Kim S.-H., Kim Y.-K., Won J.-H., Chung J.S., Kim H., et al. Efficacy of eculizumab in paroxysmal nocturnal hemoglobinuria patients with or without aplastic anemia: Prospective study of a Korean PNH cohort. Blood Res. 2017;52:207–211. doi: 10.5045/br.2017.52.3.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jang J.H., Kim J.S., Yoon S.-S., Lee J.-H., Kim Y.-K., Jo D.-Y., Chung J.S., Sohn S.K., Lee J.W. Predictive Factors of Mortality in Population of Patients with Paroxysmal Nocturnal Hemoglobinuria (PNH): Results from a Korean PNH Registry. J. Korean Med. Sci. 2016;31:214–221. doi: 10.3346/jkms.2016.31.2.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim J.S., Jang J.H., Yoon S.-S., Lee J.-H., Kim Y.-K., Jo D.-Y., Chung J.S., Sohn S.K., Lee J.W. Distinct subgroups of paroxysmal nocturnal hemoglobinuria (PNH) with cytopenia: Results from South Korean National PNH Registry. Ann. Hematol. 2016;95:125–133. doi: 10.1007/s00277-015-2511-z. [DOI] [PubMed] [Google Scholar]
- 37.Kim J.S., Cheong J.-W., Mun Y.-C., Jang J.H., Jo D.-Y., Lee J.W., Hematology A.A.W.P.O.T.K.S.O., Hematology O.B.O.A.A.W.P.O.T.K.S.O. Clinical implication of renal dysfunction during the clinical course in patients with paroxysmal nocturnal hemoglobinuria: A longitudinal analysis. Ann. Hematol. 2019;98:2273–2281. doi: 10.1007/s00277-019-03735-6. [DOI] [PubMed] [Google Scholar]
- 38.Loschi M., Porcher R., Barraco F., Terriou L., Mohty M., De Guibert S., Mahe B., Lemal R., Dumas P., Etienne G., et al. Impact of eculizumab treatment on paroxysmal nocturnal hemoglobinuria: A treatment versus no-treatment study. Am. J. Hematol. 2016;91:366–370. doi: 10.1002/ajh.24278. [DOI] [PubMed] [Google Scholar]
- 39.De Latour R.P., Mary J.Y., Salanoubat C., Terriou L., Etienne G., Mohty M., Roth S., De Guibert S., Maury S., Cahn J.Y., et al. Paroxysmal nocturnal hemoglobinuria: Natural history of disease subcategories. Blood. 2008;112:3099–3106. doi: 10.1182/blood-2008-01-133918. [DOI] [PubMed] [Google Scholar]
- 40.Villegas A., Núñez R., Gaya A., Cuevas-Ruiz M.V., Carral A., Arrizabalaga B., Gómez-Roncero M.I., Mora A., Bravo P., Lavilla E., et al. Presence of acute and chronic renal failure in patients with paroxysmal nocturnal hemoglobinuria: Results of a retrospective analysis from the Spanish PNH Registry. Ann. Hematol. 2017;96:1727–1733. doi: 10.1007/s00277-017-3059-x. [DOI] [PubMed] [Google Scholar]
- 41.Muñoz-Linares C., Ojeda E., Forés R., Pastrana M., Cabero M., Morillo D., Bautista G., Baños I., Monteserin C., Bravo P., et al. Paroxysmal nocturnal hemoglobinuria: A single Spanish center’s experience over the last 40 yr. Eur. J. Haematol. 2014;93:309–319. doi: 10.1111/ejh.12346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rubio M.L., Morado M., Gaya A., Rosa D.A., Ojeda E., Muñoz J.A., De Mendiguren B.P., Monteagudo M.D., Durán J.M., Fisac R.M., et al. Paroxysmal nocturnal hemoglobinuria therapy with eculizumab: Spanish experience. Med. Clin. Barc. 2011;137:8–13. doi: 10.1016/j.medcli.2010.09.035. [DOI] [PubMed] [Google Scholar]
- 43.Wang H.-C., Kuo C.-Y., Liu I.-T., Chen T.-Y., Chang Y.-H., Lin S.-J., Cho S.-F., Liu Y.-C., Liu T.-C., Lin S.-F., et al. Distinct clinical characteristics of paroxysmal nocturnal hemoglobinuria in patients in Southern Taiwan: A multicenter investigation. Kaohsiung J. Med. Sci. 2017;33:405–410. doi: 10.1016/j.kjms.2017.05.011. [DOI] [PubMed] [Google Scholar]
- 44.Kelly R.J., Hill A., Arnold L.M., Brooksbank G.L., Richards S.J., Cullen M., Mitchell L.D., Cohen D.R., Gregory W.M., Hillmen P. Long-term treatment with eculizumab in paroxysmal nocturnal hemoglobinuria: Sustained efficacy and improved survival. Blood. 2011;117:6786–6792. doi: 10.1182/blood-2011-02-333997. [DOI] [PubMed] [Google Scholar]
- 45.Legendre C., Licht C., Muus P., Greenbaum L., Babu S., Bedrosian C., Bingham C., Cohen D., Delmas Y., Douglas K., et al. Terminal Complement Inhibitor Eculizumab in Atypical Hemolytic–Uremic Syndrome. N. Engl. J. Med. 2013;368:2169–2181. doi: 10.1056/NEJMoa1208981. [DOI] [PubMed] [Google Scholar]
- 46.Licht C., Greenbaum L.A., Muus P., Babu S., Bedrosian C.L., Cohen D.J., Delmas Y., Douglas K., Furman R.R., Gaber O.A., et al. Efficacy and safety of eculizumab in atypical hemolytic uremic syndrome from 2-year extensions of phase 2 studies. Kidney Int. 2015;87:1061–1073. doi: 10.1038/ki.2014.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Walle J.V., Delmas Y., Ardissino G., Wang J., Kincaid J.F., Haller H. Improved renal recovery in patients with atypical hemolytic uremic syndrome following rapid initiation of eculizumab treatment. J. Nephrol. 2017;30:127–134. doi: 10.1007/s40620-016-0288-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Greenbaum L.A., Fila M., Ardissino G., Al-Akash S.I., Evans J., Henning P., Lieberman K.V., Maringhini S., Pape L., Rees L., et al. Eculizumab is a safe and effective treatment in pediatric patients with atypical hemolytic uremic syndrome. Kidney Int. 2016;89:701–711. doi: 10.1016/j.kint.2015.11.026. [DOI] [PubMed] [Google Scholar]
- 49.Fakhouri F., Hourmant M., Campistol J.M., Cataland S.R., Espinosa M., Gaber A.O., Menne J., Minetti E.E., Provot F., Rondeau E., et al. Terminal Complement Inhibitor Eculizumab in Adult Patients With Atypical Hemolytic Uremic Syndrome: A Single-Arm, Open-Label Trial. Am. J. Kidney Dis. 2016;68:84–93. doi: 10.1053/j.ajkd.2015.12.034. [DOI] [PubMed] [Google Scholar]
- 50.Menne J., Delmas Y., Fakhouri F., Licht C., Lommelé Å., Minetti E.E., Provôt F., Rondeau E., Sheerin N.S., Wang J., et al. Outcomes in patients with atypical hemolytic uremic syndrome treated with eculizumab in a long-term observational study. BMC Nephrol. 2019;20:125. doi: 10.1186/s12882-019-1314-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Menne J., Delmas Y., Fakhouri F., Kincaid J.F., Licht C., Minetti E.E., Mix C., Provôt F., Rondeau É., Sheerin N.S., et al. Eculizumab prevents thrombotic microangiopathy in patients with atypical haemolytic uraemic syndrome in a long-term observational study. Clin. Kidney J. 2018;12:196–205. doi: 10.1093/ckj/sfy035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rondeau E., Scully M., Ariceta G., Barbour T.D., Cataland S.R., Heyne N., Miyakawa Y., Ortiz D., Swenson E.D., Vallee M., et al. TH-PO800: Efficacy and safety of the Long-acting C5-inhibitor ravulizumab in adult patients with atypical hemolytic uremic syndrome (aHUS); Proceedings of the American Society of Nephrology (ASN) Kidney Week 2019; Washington, DC, USA. 5–10 November 2019. [Google Scholar]
- 53.Licht C., Ardissino G., Ariceta G., Cohen D., Cole J.A., Gasteyger C., Greenbaum L.A., Johnson S., Ogawa M., Schaefer F., et al. The global aHUS registry: Methodology and initial patient characteristics. BMC Nephrol. 2015;16:207. doi: 10.1186/s12882-015-0195-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rondeau E., Cataland S.R., Al-Dakkak I., Miller B., Webb N.J., Landau D. Eculizumab Safety: Five-Year Experience From the Global Atypical Hemolytic Uremic Syndrome Registry. Kidney Int. Rep. 2019;4:1568–1576. doi: 10.1016/j.ekir.2019.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schaefer F., Ardissino G., Ariceta G., Fakhouri F., Scully M., Isbel N., Lommelé Å., Kupelian V., Gasteyger C., Greenbaum L.A., et al. Clinical and genetic predictors of atypical hemolytic uremic syndrome phenotype and outcome. Kidney Int. 2018;94:408–418. doi: 10.1016/j.kint.2018.02.029. [DOI] [PubMed] [Google Scholar]
- 56.Leanne C., Rondeau E., Ardissino G., Caby-Tosi M.-P., Al-Dakkak I., Fakhouri F., Miller B., Scully M. SP075: Pregnancy outcomes in patients enrolled in the Global aHUS Registry; Proceedings of the 56th European Renal Association—European Dialysis and Transplant Association (ERA-EDTA) Congress; Budapest, Hungary. 13–16 June 2019. [Google Scholar]
- 57.Ito S., Hidaka Y., Inoue N., Kaname S., Kato H., Matsumoto M., Miyakawa Y., Mizuno M., Okada H., Shimono A., et al. Safety and effectiveness of eculizumab for pediatric patients with atypical hemolytic-uremic syndrome in Japan: Interim analysis of post-marketing surveillance. Clin. Exp. Nephrol. 2019;23:112–121. doi: 10.1007/s10157-018-1610-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kato H., Miyakawa Y., Hidaka Y., Inoue N., Ito S., Kagami S., Kaname S., Matsumoto M., Mizuno M., Matsuda T., et al. Safety and effectiveness of eculizumab for adult patients with atypical hemolytic-uremic syndrome in Japan: Interim analysis of post-marketing surveillance. Clin. Exp. Nephrol. 2019;23:65–75. doi: 10.1007/s10157-018-1609-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ito N., Hataya H., Saida K., Amano Y., Hidaka Y., Motoyoshi Y., Ohta T., Yoshida Y., Terano C., Iwasa T., et al. Efficacy and safety of eculizumab in childhood atypical hemolytic uremic syndrome in Japan. Clin. Exp. Nephrol. 2016;20:265–272. doi: 10.1007/s10157-015-1142-y. [DOI] [PubMed] [Google Scholar]
- 60.Mallett A., Hughes P., Szer J., Tuckfield A., Van Eps C.L., Cambell S.B., Hawley C.M., Burke J., Kausman J., Hewitt I., et al. Atypical Haemolytic Uraemic Syndrome treated with the complement inhibitor Eculizumab: The experience of the Australian compassionate access cohort. Intern. Med. J. 2015;45:1054–1065. doi: 10.1111/imj.12864. [DOI] [PubMed] [Google Scholar]
- 61.De Andrade L.G.M., Contti M.M., Nga H.S., Bravin A.M., Takase H.M., Viero R.M., Da Silva T.N., Chagas K.D.N., Palma L.M.P. Long-term outcomes of the Atypical Hemolytic Uremic Syndrome after kidney transplantation treated with eculizumab as first choice. PLoS ONE. 2017;12:e0188155. doi: 10.1371/journal.pone.0188155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Besbas N., Gulhan B., Soylemezoglu O., Özçakar Z.B., Korkmaz E., Hayran M., Ozaltin F. Turkish pediatric atypical hemolytic uremic syndrome registry: Initial analysis of 146 patients. BMC Nephrol. 2017;18:6. doi: 10.1186/s12882-016-0420-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gediz F., Payzin B.K., Ecemis S., Güler N., Yilmaz A.F., Topcugil F., Berdeli A., Yılmaz A.F. Efficacy and safety of eculizumab in adult patients with atypical hemolytic uremic syndrome: A single center experience from Turkey. Transfus. Apher. Sci. 2016;55:357–362. doi: 10.1016/j.transci.2016.09.017. [DOI] [PubMed] [Google Scholar]
- 64.Yenerel M.N., Aktan M., Yildiz A., Caliskan Y., Nalcaci M. 311-II-4: The importance of Eculizumab in patients with atypical Hemolytic Uremic Syndrome: Single center experience; Proceedings of the 56th American Society of Hematology (ASH) Annual Meeting; San Francisco, CA, USA. 6–9 December 2014. [Google Scholar]
- 65.Le Clech A., Simon-Tillaux N., Provôt F., Delmas Y., Vieira-Martins P., Limou S., Halimi J.-M., Le Quintrec M., Lebourg L., Grangé S., et al. Atypical and secondary hemolytic uremic syndromes have a distinct presentation and no common genetic risk factors. Kidney Int. 2019;95:1443–1452. doi: 10.1016/j.kint.2019.01.023. [DOI] [PubMed] [Google Scholar]
- 66.Fakhouri F., Delmas Y., Provot F., Barbet C., Karras A., Makdassi R., Courivaud C., Rifard K., Servais A., Allard C., et al. Insights From the Use in Clinical Practice of Eculizumab in Adult Patients With Atypical Hemolytic Uremic Syndrome Affecting the Native Kidneys: An Analysis of 19 Cases. Am. J. Kidney Dis. 2014;63:40–48. doi: 10.1053/j.ajkd.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 67.Fremeaux-Bacchi V., Fakhouri F., Garnier A., Bienaimé F., Dragon-Durey M.-A., Ngo S., Moulin B., Servais A., Provot F., Rostaing L., et al. Genetics and Outcome of Atypical Hemolytic Uremic Syndrome: A Nationwide French Series Comparing Children and Adults. Clin. J. Am. Soc. Nephrol. 2013;8:554–562. doi: 10.2215/CJN.04760512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.De Fontbrune F.S., Galambrun C., Sirvent A., Huynh A., Faguer S., Nguyen S., Bay J.-O., Neven B., Moussi J., Simon L., et al. Use of Eculizumab in Patients With Allogeneic Stem Cell Transplant-Associated Thrombotic Microangiopathy: A study from the SFGM-TC. Transplantation. 2015;99:1953–1959. doi: 10.1097/TP.0000000000000601. [DOI] [PubMed] [Google Scholar]
- 69.Le Quintrec M., Lionet A., Kamar N., Karras A., Barbier S., Büchler M., Fakhouri F., Provost F., Fridman W.H., Thervet E., et al. Complement Mutation-AssociatedDe NovoThrombotic Microangiopathy Following Kidney Transplantation. Am. J. Transplant. 2008;8:1694–1701. doi: 10.1111/j.1600-6143.2008.02297.x. [DOI] [PubMed] [Google Scholar]
- 70.Zuber J., Le Quintrec M., Krid S., Bertoye C., Gueutin V., Lahoche A., Heyne N., Ardissino G., Chatelet V., Noël L.-H., et al. Eculizumab for atypical hemolytic uremic syndrome recurrence in renal transplantation. Am. J. Transplant. 2012;12:3337–3354. doi: 10.1111/j.1600-6143.2012.04252.x. [DOI] [PubMed] [Google Scholar]
- 71.Servais A., Noël L.-H., Roumenina L.T., Le Quintrec M., Ngo S., Dragon-Durey M.-A., Macher M.-A., Zuber J., Karras A., Provot F., et al. Acquired and genetic complement abnormalities play a critical role in dense deposit disease and other C3 glomerulopathies. Kidney Int. 2012;82:454–464. doi: 10.1038/ki.2012.63. [DOI] [PubMed] [Google Scholar]
- 72.Cavero T., Rabasco C., López A., Román E., Avila A., Sevillano A., Huerta A., Rojas-Rivera J., Fuentes C., Blasco M., et al. Eculizumab in secondary atypical haemolytic uraemic syndrome. Nephrol. Dial. Transplant. 2017;32:466–474. doi: 10.1093/ndt/gfw453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Huerta A., Arjona E., Portoles J., Lopez P., Rabasco C., Espinosa M., Cavero T., Blasco M., Cao M., Manrique J., et al. A retrospective study of pregnancy-associated atypical hemolytic uremic syndrome. Kidney Int. 2018;93:450–459. doi: 10.1016/j.kint.2017.06.022. [DOI] [PubMed] [Google Scholar]
- 74.Cavero T., de Córdoba S.R., Praga M. 121: ¿Microangiopatía trombótica asociada a hipertensión o a hiperactividad del complemento? Implicaciones diagnósticas y terapéuticas; Proceedings of the XLVIII Congreso de la Sociedad Española de Nefrología y IX Congreso Iberoamericano de Nefrología; Madrid, Spain. 16–19 November 2018. [Google Scholar]
- 75.Sheerin N.S., Kavanagh D., Goodship T.H.J., Johnson S. A national specialized service in England for atypical haemolytic uraemic syndrome-the first year’s experience. QJM. 2016;109:27–33. doi: 10.1093/qjmed/hcv082. [DOI] [PubMed] [Google Scholar]
- 76.Brocklebank V., Johnson S., Sheerin T.P., Marks S.D., Gilbert R.D., Tyerman K.S., Kinoshita M., Awan A., Kaur A., Webb N., et al. Factor H autoantibody is associated with atypical hemolytic uremic syndrome in children in the United Kingdom and Ireland. Kidney Int. 2017;92:1261–1271. doi: 10.1016/j.kint.2017.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Howard J.F., Barohn R.J., Cutter G.R., Freimer M., Juel V.C., Mozaffar T., Mellion M.L., Benatar M., Farrugia M.E., Wang J., et al. A randomized, double-blind, placebo-controlled phase II study of eculizumab in patients with refractory generalized myasthenia gravis. Muscle Nerve. 2013;48:76–84. doi: 10.1002/mus.23839. [DOI] [PubMed] [Google Scholar]
- 78.Howard J.F., Utsugisawa K., Benatar M., Murai H., Barohn R.J., Illa I., Jacob S., Vissing J., Burns T.M., Kissel J.T., et al. Safety and efficacy of eculizumab in anti-acetylcholine receptor antibody-positive refractory generalised myasthenia gravis (REGAIN): A phase 3, randomised, double-blind, placebo-controlled, multicentre study. Lancet Neurol. 2017;16:976–986. doi: 10.1016/S1474-4422(17)30369-1. [DOI] [PubMed] [Google Scholar]
- 79.Muppidi S., Utsugisawa K., Benatar M., Murai H., Barohn R.J., Illa I., Jacob S., Vissing J., Burns T.M., Kissel J.T., et al. Long-term safety and efficacy of eculizumab in generalized myasthenia gravis. Muscle Nerve. 2019;60:14–24. doi: 10.1002/mus.26447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Viglietti D., Gosset C., Loupy A., Deville L., Verine J., Zeevi A., Glotz D., Lefaucheur C. C1 Inhibitor in Acute Antibody-Mediated Rejection Nonresponsive to Conventional Therapy in Kidney Transplant Recipients: A Pilot Study. Am. J. Transplant. 2016;16:1596–1603. doi: 10.1111/ajt.13663. [DOI] [PubMed] [Google Scholar]
- 81.Montgomery R.A., Orandi B.J., Racusen L., Jackson A.M., Garonzik-Wang J.M., Shah T., Woodle E.S., Sommerer C., Fitts D., Rockich K., et al. Plasma-Derived C1 Esterase Inhibitor for Acute Antibody Mediated Rejection Following Kidney Transplantation: Results of a Randomized, Double-Blind, Placebo-Controlled Pilot Study. Am. J. Transplant. 2016;16:3468–3478. doi: 10.1111/ajt.13871. [DOI] [PubMed] [Google Scholar]
- 82.Stegall M., Diwan T., Raghavaiah S., Cornell L.D., Burns J., Dean P.G., Cosio F.G., Gandhi M.J., Kremers W., Gloor J.M. Terminal Complement Inhibition Decreases Antibody-Mediated Rejection in Sensitized Renal Transplant Recipients. Am. J. Transplant. 2011;11:2405–2413. doi: 10.1111/j.1600-6143.2011.03757.x. [DOI] [PubMed] [Google Scholar]
- 83.Vo A.A., Zeevi A., Choi J., Cisneros K., Toyoda M., Kahwaji J., Peng A., Villicana R., Puliyanda D., Reinsmoen N., et al. A Phase I/II Placebo-Controlled Trial of C1-Inhibitor for Prevention of Antibody-Mediated Rejection in HLA Sensitized Patients. Transplantation. 2015;99:299–308. doi: 10.1097/TP.0000000000000592. [DOI] [PubMed] [Google Scholar]
- 84.Vo A.A., Choi J., Kahwaji J., Puliyanda D., Peng A., Villicana R., Jordan S. A194: Long-term analysis of a placebo-controlled trial of C1-INH for prevention of antibody-mediated rejection; Proceedings of the 2015 American Transplant Congress; Philadelphia, PA, USA. 3–6 May 2015. [Google Scholar]
- 85.Vo A.A., Choi J., Peng A., Lim K., Varanasi L., Najjar R., Huang E., Puliyanda D., Jordan S. A50: Update of a placebo-controlled trial of C1 esterase inhibitor for prevention of antibody mediated rejection (ABMR) in highly-HLA sensitized patients; Proceedings of the 2017 American Transplant Congress; Chicago, IL, USA. 29 April–3 May 2015. [Google Scholar]
- 86.Marks W.H., Mamode N., Montgomery R.A., Stegall M.D., Ratner L.E., Cornell L.D., Rowshani A.T., Colvin R.B., Dain B., Boice J.A., et al. Safety and efficacy of eculizumab in the prevention of antibody-mediated rejection in living-donor kidney transplant recipients requiring desensitization therapy: A randomized trial. Am. J. Transplant. 2019;19:2876–2888. doi: 10.1111/ajt.15364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Glotz D., Russ G., Rostaing L., Legendre C., Tufveson G., Chadban S., Grinyó J., Mamode N., Rigotti P., Couzi L., et al. Safety and efficacy of eculizumab for the prevention of antibody-mediated rejection after deceased-donor kidney transplantation in patients with preformed donor-specific antibodies. Am. J. Transplant. 2019;19:2865–2875. doi: 10.1111/ajt.15397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kaabak M.M., Babenko N., Shapiro R., Zokoyev A., Dymova O., Kim E. A prospective randomized, controlled trial of eculizumab to prevent ischemia-reperfusion injury in pediatric kidney transplantation. Pediatr. Transplant. 2018;22:e13129. doi: 10.1111/petr.13129. [DOI] [PubMed] [Google Scholar]
- 89.Jordan S.C., Choi J., Aubert O., Haas M., Loupy A., Huang E., Peng A., Kim I., Louie S., Ammerman N., et al. A phase I/II, double-blind, placebo-controlled study assessing safety and efficacy of C1 esterase inhibitor for prevention of delayed graft function in deceased donor kidney transplant recipients. Am. J. Transplant. 2018;18:2955–2964. doi: 10.1111/ajt.14767. [DOI] [PubMed] [Google Scholar]
- 90.Schröppel B., Akalin E., Baweja M., Bloom R.D., Florman S., Goldstein M., Haydel B., Hricik D.E., Kulkarni S., Levine M., et al. Peritransplant eculizumab does not prevent delayed graft function in deceased donor kidney transplant recipients: Results of two randomized controlled pilot trials. Am. J. Transplant. 2019;20:564–572. doi: 10.1111/ajt.15580. [DOI] [PubMed] [Google Scholar]
- 91.Prevention of Delayed Graft Function Using Eculizumab Therapy (PROTECT Study). ClinicalTrials.gov. [(accessed on 29 April 2020)]; Available online: https://clinicaltrials.gov/ct2/show/NCT02145182.
- 92.Kim M.S., Prasad V. The Clinical Trials Portfolio for On-label and Off-label Studies of Eculizumab. JAMA Intern. Med. 2019;180:1–3. doi: 10.1001/jamainternmed.2019.4694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Suarez M.L.G., Thongprayoon C., Mao M.A., Leeaphorn N., Bathini T., Cheungpasitporn W. Outcomes of Kidney Transplant Patients with Atypical Hemolytic Uremic Syndrome Treated with Eculizumab: A Systematic Review and Meta-Analysis. J. Clin. Med. 2019;8:919. doi: 10.3390/jcm8070919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Moher D., Tetzlaff J., Tricco A.C., Sampson M., Altman D.G. Epidemiology and Reporting Characteristics of Systematic Reviews. PLoS Med. 2007;4:e78. doi: 10.1371/journal.pmed.0040078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Study of ALXN1210 in Children and Adolescents with Atypical Hemolytic Uremic Syndrome (aHUS). ClinicalTrials.gov. [(accessed on 29 April 2020)]; Available online: https://clinicaltrials.gov/ct2/show/NCT03131219?term=NCT03131219&draw=2&rank=1.
- 96.Higgins J.P.T., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sterne J.A., Gavaghan D., Egger M. Publication and related bias in meta-analysis: Power of statistical tests and prevalence in the literature. J. Clin. Epidemiol. 2000;53:1119–1129. doi: 10.1016/S0895-4356(00)00242-0. [DOI] [PubMed] [Google Scholar]
- 98.Kulkarni S., Kirkiles-Smith N.C., Deng Y.H., Formica R.N., Moeckel G., Broecker V., Bow L., Tomlin R., Pober J.S. Eculizumab Therapy for Chronic Antibody-Mediated Injury in Kidney Transplant Recipients: A Pilot Randomized Controlled Trial. Am. J. Transplant. 2016;17:682–691. doi: 10.1111/ajt.14001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.