Key Points
Myeloid/lymphoid neoplasms with eosinophilia are driven by aberrant tyrosine kinases in pluripotent cells and display variable phenotypes.
FGFR-driven hematolymphoid neoplasms are targetable by TKI inhibitors such as ponatinib; studies of specific FGFR inhibitors are ongoing.
Introduction
We report the first case of ETV6-FGFR2 rearrangement with features of a myeloid/lymphoid neoplasm with eosinophilia presenting with extramedullary transformation to mixed phenotype (T-myeloid/lymphoid) acute leukemia in a 41-year-old woman.
Case description
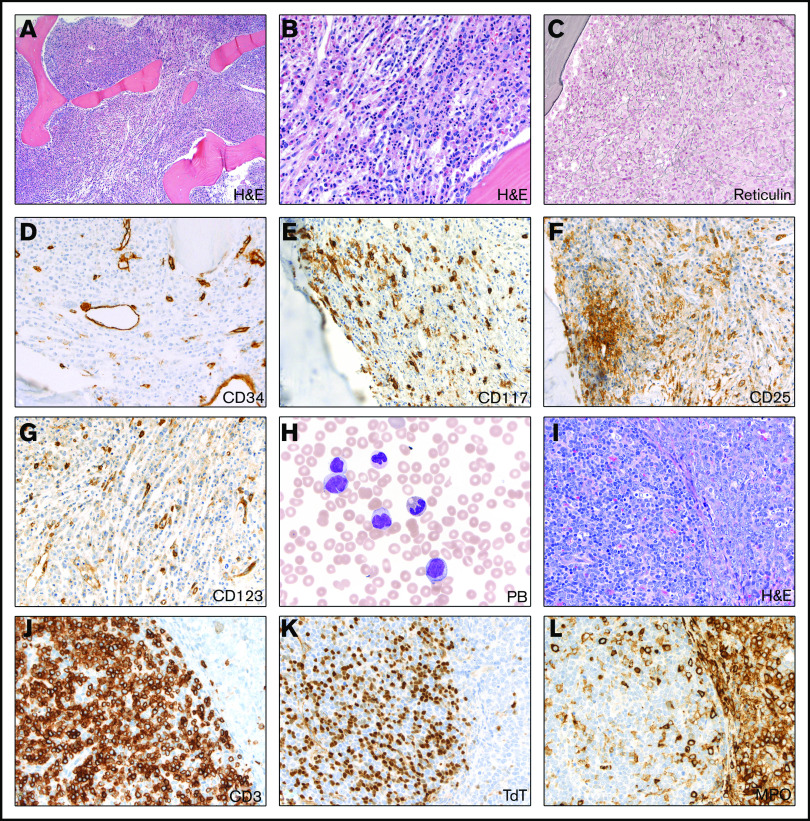
The patient presented with significant weight loss, drenching nights sweats, and diffuse bone pain over many weeks. Diffuse lymphadenopathy was seen on physical examination. Laboratory studies revealed leukocytosis (white blood cell count [WBC], 64.2 × 103/μL), anemia (hemoglobin, 9.2 g/dL), and thrombocytopenia (platelets 29 × 103/μL); diffuse lymphadenopathy was noted on whole body imaging. The WBC differential showed 6% blasts, 14% myelocytes, 7% metamyelocytes, 48% neutrophils with dyspoietic features, 8% monocytes with abnormal or immature forms, and 15% lymphocytes. Bone marrow biopsy was hypercellular and consistent with myeloproliferative or myelodysplastic neoplasms with fibrosis and eosinophilia but showed no increase in blasts by CD34 immunohistochemistry (Figure 1A-D). Aspirates were paucicellular. Of note, the bone marrow showed peritrabecular clusters of spindle cells with CD117, CD123, CD25, and focal mast cell tryptase expression suggestive of abnormal mast cells (Figure 1E-G).
Figure 1.
Initial (pretreatment) bone marrow and lymph node evaluation. (A-B) Hypercellular bone marrow showing myeloid proliferation with focal increase in eosinophils but no increase in blasts (hematoxylin and eosin [H&E] stain; 10× and 40× objective lenses, respectively). (C-D) Reticulin stain showing moderate increase in fibrosis (40× objective lens) (C) with no increase in CD34+ blasts (40× objective lens) (D). (E-G) CD117 immunostain demonstrates focal paratrabecular areas of spindled mast cells (40× objective lens) (E) with aberrant expression of CD25 (F) and CD123 (G) (40× objective lens). (H) Peripheral blood (PB) smear showing leukocytosis with dysgranulopoiesis and abnormal monocytes (Wright-Giemsa stain; 100× oil objective lens). (I-L) Excisional lymph node biopsy showing 2 phenotypically and geographically distinct blast populations (H&E stain; 40× objective lens) (I), with separate T-lymphoblastic differentiation (J: CD3 and K: TDT; 40× objective lens) and myeloblastic differentiation (myeloperoxidase [MPO] stain; 40× objective lens) (L).
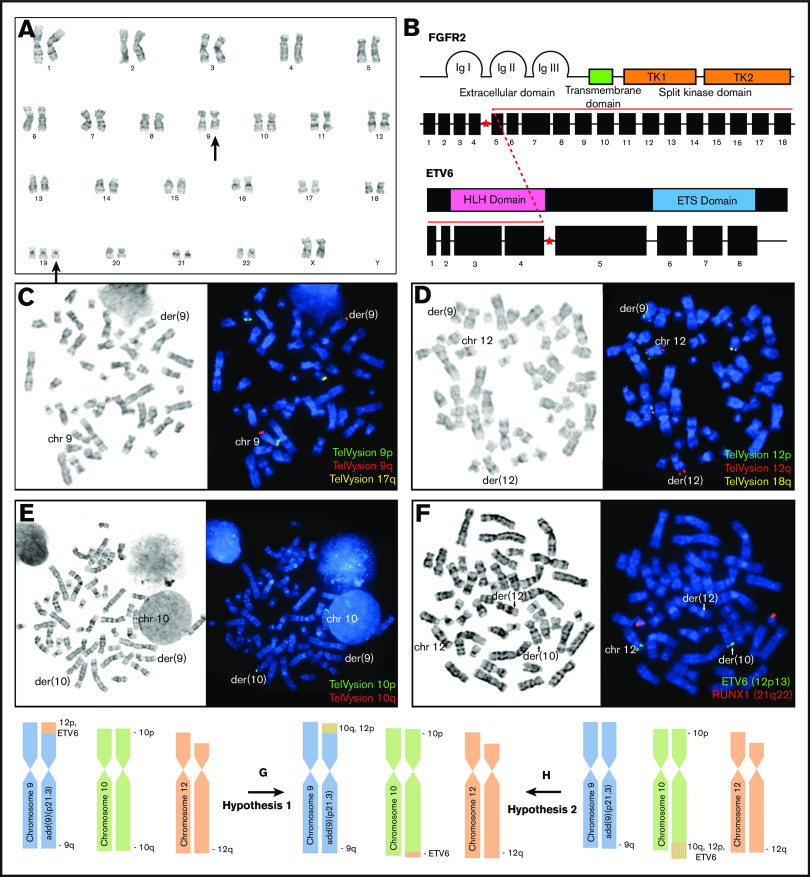
Lymph node biopsy showed an acute leukemic infiltrate with distinct T-cell and myeloid components and focal eosinophilia (Figure 1I-L). Karyotypic analysis of the lymph node showed an abnormal female karyotype with loss of material from chromosome 9p and gain of chromosome 19 (ISCN: 46,XX,add(9)(p21.3)[1]/47,idem,+19[19]) (Figure 2A). Next-generation sequencing (NGS) of the lymph node revealed RUNX1 and STAG2 mutations. BCR-ABL1 studies were negative. KIT mutational analysis was negative. No rearrangements of platelet-derived growth factor receptor A (PDGFRA), PDGFRB, or FGFR1 were detected by interphase fluorescence in situ hybridization (FISH) of the peripheral blood sample. However, given the clinical presentation and morphologic features suggestive of the myeloid/lymphoid neoplasms with eosinophilia, hybrid capture targeted RNA NGS analysis for gene fusions in FGFR1/2/3 and NTRK1/2/3 genes was performed as follows: RNA was extracted from frozen-cell aliquots using the RNeasy FFPE Kit (QIAGEN, Valencia, CA) and quantified using a Qubit fluorometric assay (Thermo Fisher Scientific, Foster City, CA), adjusted for percentage of fragments greater than 100 bp using a TapeStation system (Agilent, Santa Clara, CA). Then, 300 ng of RNA was subjected to library prep using the KAPA stranded RNA-Seq Kit with RiboErase (Kapa Biosystems), followed by quantitation using the KAPA library quantification kit. Pooled libraries were captured using a custom-designed SeqCap EZcapture panel targeting 1213 genes (Roche NimbleGen, Pleasanton, CA), supplemented with select xGen Lockdown Probes (IDT, Coralville, IA). Amplified pooled captured libraries were sequenced on Illumina HiSeq-2500 instruments (Illumina, San Diego, CA) in rapid run mode (2 × 101 bp paired-end sequencing). Sequence data were aligned to the hg19 human reference transcriptome using a STAR aligner,1 and fusions were detected using a combination of python software (developed in-house) and STAR fusion software.
Figure 2.
Initial molecular and cytogenetic evaluation. (A) Karyogram of G-banded chromosomes reveals an aberrant add(9)(p21.3) in all 20 metaphases and trisomy 19 in 19 of 20 metaphases analyzed (46,XX,add(9)(p21.3)[1]/47,idem,+19[19]). (B) Next-generation RNA sequencing identified an aberrant transcript corresponding to a fusion between exon 4 of ETV6 (upstream) and exon 5 of FGFR2 (downstream). (C-E) FISH with Vysis TelVysion subtelomeric probes post G-banding reveals subtelomeric loss of 9p from the add(9)(p21.3) chromosome (C), abnormal localization of 1 copy of 12p to the short arm of the add(9)(p21.3) chromosome (D), and abnormal localization of 1 copy of 10q to the short arm of the add(9)(p21.3) chromosome (E). (F) FISH with Vysis LSI ETV6(TEL)/RUNX1(AML1) probe set reveals abnormal localization of 1 copy of ETV6 to the long arm of chromosome 10. These findings are favored to represent the sum of the 2 rearrangement events among chromosomes 9, 10, and 12, resulting in the add(9)(p21.3) aberrancy as well as cryptic aberrancies of chromosomes 10 and 12, including abnormal localization of the ETV6 FISH probe to the long arm of chromosome 10. (G-H) The order of these rearrangement events cannot be determined, but 2 speculative hypotheses are proposed. Ig, immunoglobulin.
A novel ETV6-FGFR2 fusion between exon 4 of ETV6 (nm_001987) and exon 5 of FGFR2 (nm_022970) was identified (Figure 2B) and seems to involve their canonical splicing sites. The split depth of coverage is 1088 reads (supplemental Material). An ETV6/RUNX1 extra-signal, dual color DNA probe set was hybridized to G-banded metaphase chromosomes which demonstrated an ETV6 (12p13.2, green) signal localizing to the distal portion of the long arm of chromosome 10 (der(10)) (Figure 2F). Additional FISH analysis using Vysis TelVysion (Downers Grove, IL) subtelomeric probes demonstrated multiple karyotypically cryptic rearrangements resulting in localization of segments of 10q and 12p to the short arm of the derivative chromosome 9 with loss of 9p (Figure 2C-H). Immunohistochemical staining for FGFR2 directed at the tyrosine kinase II domain (ab58201, Abcam, Cambridge, United Kingdom) demonstrated abnormal cytoplasmic FGFR2 protein overexpression in comparison with normal lymphoid tissue (supplemental Material).
Because of the strong T-lymphoid component of her disease, the patient underwent induction therapy with course I of the CALGB 10403 regimen.2,3 She was found to have a mixed metabolic response on positron emission tomography scan with recovery bone marrow biopsy showing 5% to 30% cellularity and persistence of her myeloid neoplasm with 5% to 10% CD34+ blasts with myeloid phenotype but no blasts with T-cell phenotype (supplemental Material). She then underwent re-induction with a myeloid-based regimen of high-dose cytarabine and mitoxantrone.4 Bone marrow biopsy upon count recovery was variably cellular (5% to 40%) and demonstrated persistent disease with 10% to 18% CD34+ blasts with myeloid phenotype. The patient then received a course of decitabine with venetoclax; the recovery bone marrow biopsy showed 5% cellularity and 10% atypical myeloid blasts.
Subsequently, the patient underwent an allogeneic matched related donor stem cell transplantation with fludarabine and busulfan conditioning; however, her day +30 bone marrow biopsy demonstrated 60% to 70% cellularity and recurrent or persistent disease with 60% to 70% blasts with myeloid phenotype and monocytic differentiation. No blasts with a T-cell phenotype were identified. Her clinical condition quickly deteriorated after diagnosis of recurrent disease, with extramedullary involvement including malignant ascites containing myeloid blasts with monocytic differentiation. An attempt was made to obtain ponatinib for this patient. However, she elected for hospice care and died 235 days after her original diagnosis.
Results and discussion
We report here the first case of ETV6-FGFR2 rearrangement in a myeloid/lymphoid neoplasm with eosinophilia presenting with nodal acute mixed phenotype (T-lymphocytic/myeloid) transformation with an aggressive clinical course resistant to conventional therapies including allogeneic stem cell transplantation.
The FGFR family has emerged as a frequently altered set of genes across a wide range of tumors, which is likely a result of the involvement of the FGFR pathway in signal transduction pathways that regulate cell proliferation, differentiation, and migration, all of which are critical for tumorigenesis.5 FGFR2 aberrations have been reported at significantly increased rates in cholangiocarcinoma, endometrial adenocarcinoma, and gastric adenocarcinoma,6 but have not been previously described as a driver in myeloid or lymphoid malignancies. Conversely, FGFR1 abnormalities have been well documented in hematologic malignancies. Hematopoietic neoplasms associated with FGFR1 rearrangement are included in the family of myeloid and lymphoid neoplasms with eosinophilia (MLN/Eo’s), a group of myeloid/lymphoid neoplasms that stem from the formation of a fusion gene (or rarely a mutation) resulting in expression of an aberrant tyrosine kinase in a pluripotent (myeloid-lymphoid) stem cell, with varying responsiveness to tyrosine kinase inhibitors (TKIs). This is frequently a result of 1 of many fusion partners inducing receptor oligomerization and activation, which results in phenotypes that are dependent on both the partner protein and kinase domain.7
Myeloid/lymphoid neoplasms associated with FGFR1 rearrangement may present at different stages of maturation in the course of treatment in different patients or in varying stages in the same patient. Case presentations can range from myeloproliferative neoplasms (MPNs), acute myeloid leukemias (AMLs), B- or T-lymphoblastic leukemias or lymphomas, or mixed phenotype acute leukemias, but all are uniformly characterized by aggressive clinical behavior and resistance to imatinib.8 These malignancies may, however, respond to specific FGFR1/2/3 inhibitors. Fusion of upstream exon 5 of ETV6 to downstream exon 10 of FGFR3 has been described in peripheral T-cell lymphoma,9 resulting in an open reading frame for a chimeric protein consisting of the helix-loop-helix (HLH) domain of ETV6 and the tyrosine kinase domains of FGFR3. Similarly, we postulate that the translocation of ETV6 with FGFR2 may result in analogous fusion with ETV6 to allow receptor oligomerization and activation.
Aberrations in ETV6 have been well described in both acute lymphoblastic leukemia (ALL) and AML. ETV6 was initially cloned as a fusion partner gene of PDGFRB in a t(5;12)(q33;p13) translocation, and it is a member of the erythroblast transformation–specific (ETS) family of transcription regulators,10 one of the largest families of signal-dependent transcription regulators that mediates cell proliferation, differentiation, and tumorigenesis.11 As a member of the ETS family, ETV6 is characterized by 2 functional domains, an N-terminal HLH oligomerization domain encoded by exons 3 and 4 and a C-terminal ETS DNA-binding domain encoded by exons 6 through 8.12 Repression is mediated by the HLH domain and the central portion (internal domain) of ETV6 encoded by exon 5, located between the HLH and ETS domains. When functioning appropriately, ETV6 encodes a transcription factor essential for hematopoietic regulation and embryonic development.13
ETV6 fusions are a common aberration in leukemia, and more than 30 partner genes have been characterized. Although a large number of these fusions involve aberrant activation of tyrosine kinases, fusion with transcription factors, homeobox genes, and other families have been characterized as well.14 Notably, ETV6 is one of very few genes that are recurrently found as fusion partners of tyrosine kinases (TKs) in hematologic malignancies. In ETV6/TK fusion proteins, the HLH domain of ETV6 functions as a homodimerization motif that activates the TK domain of the partner genes,15 resulting in a phenotype dependent on both ETV6 and the fusion partner and variable responses to TKIs. Fusions with PDGFRA and PDGFRB are currently recognized in the group of MLN/Eo’s and show sensitivity to imatinib therapy. In addition, several ETV6 fusions with other TK partners have been reported, including FLT3, ABL1, ABL2, JAK2, LYN, SYK, NTRK3, and FGFR3, often associated with the MLN/Eo phenotype and variable presentations, some of which showed encouraging responses to TKIs. ETV6-LYN fusion was reported in MPNs with myelofibrosis, which was insensitive to imatinib clinically but was experimentally sensitive to dasatinib.16 ETV6-ABL1 has been reported in numerous neoplasms including B-cell acute lymphoblastic leukemia (B-ALL), T-cell acute lymphoblastic leukemia (T-ALL), AML, myelodysplastic syndrome with excess blasts (MDS-EB) evolving to AML, and MPNs. Interestingly, eosinophilia seemed to be a common feature of cases associated with ETV6-ABL1 fusions. ETV6-JAK2 rearrangement is currently recognized by the World Health Organization classification as a variant translocation in myeloid/lymphoid neoplasms with PCM1-JAK2.
In addition to aberrant fusion, both somatic and germline mutations in ETV6 have been implicated in hematologic malignancies as well. Germline ETV6 mutations result in thrombocytopenia along with a predisposition for development of ALL, AML, or MDS.17 Somatic mutations in ETV6 have been noted in both myeloid and lymphoid malignancies, with the majority of mutations leading to inappropriate localization of the protein to the cytoplasm.18
Aberrant FGFR2 activation resulting from ETV6-FGFR2 fusion suggests that MLN/Eo’s associated with ETV6-FGFR2 rearrangement may respond to FGFR1/2/3 TKIs. As reviewed by Katoh,19 a number of FGFR inhibitors have been approved by the US Food and Drug Administration across multiple malignancies, and many others are currently under investigation. The FGFR1/2/3 inhibitor pemigatinib is currently being studied in the phase 2 setting in FGFR1-rearranged myeloid/lymphoid neoplasms.20 The TKI ponatinib, which has been approved by the US Food and Drug Administration for use in both chronic myeloid leukemia (CML) and BCR-ABL1-positive ALL, has demonstrated in vitro inhibition of FGFR-mediated signaling and cell growth. As a result, several clinical trials are evaluating the role of ponatinib as an FGFR inhibitor in FGFR-aberrant solid tumors.21 The 50% inhibitory concentration (IC50) for ponatinib against FGFR2 is in the range of 0.5 to 20 nM, depending on the tumor type, and the IC50 against FGFR1 in hematologic cells is 1.5 nM.22-24 Given the activity of ponatinib in both myeloid and lymphoid hematologic malignancies driven by the BCR-ABL1 fusion protein, evaluating its use in ETV6-FGFR2-driven MPN/Eo is a particularly intriguing concept. A durable response to ponatinib in combination with systemic chemotherapy in FGFR1-rearranged mixed-phenotype leukemia has been described in the literature.25 The presence of a novel FGFR2 rearrangement in a myeloid/lymphoid neoplasm with phenotypic features similar to those with FGFR1 rearrangements merits consideration for broadening the current diagnostic criteria in this category, given the potential therapeutic role of FGFR inhibition in this class of disorders.
This case suggests that the fusion of ETV6-FGFR2 results in aberrant FGFR2 TK expression and a disease phenotype with aggressive clinical behavior that was refractory to conventional therapeutic regimens, including intensive chemotherapy and allogeneic stem cell transplantation. This may have implications for inclusion criteria of the group of myeloid/lymphoid neoplasms with eosinophilia and other specific TK gene fusions recognized by the revised 2016 World Health Organization classification.26
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank Carrie Fitzpatrick, and Kristin Petras from the Constitutional Cytogenetics Laboratory for their Vysis TelVysion probes, Jiaqi Chen and Wai-chun Hui for their technical FISH skills, and Megan Parilla with interpreting the RNA fusion results.
Footnotes
Send data sharing requests via e-mail to the corresponding author, Timothy Carll, at timothy.carll@uchospitals.edu. The RNA fusion sequence has also been provided as supplemental Material.
Authorship
Contribution: T.C. and A.P. analyzed data and helped write the paper; B.D. provided direct patient care and helped write the paper; E.H. analyzed data, provided analytical tools, and helped edit the paper; A.L. and J.S. contributed vital reagents and tools and helped edit the paper; P.W. analyzed data and helped edit the paper; O.O. provided direct patient care and helped edit the paper; and S.F. and D.A. analyzed data and helped edit the paper.
Conflict-of-interest disclosure: D.A. is a consultant for Jazz Pharmaceuticals and Monsanto. The remaining authors declare no competing financial interests.
Correspondence: Timothy Carll, Department of Pathology, University of Chicago Medicine, 5841 S Maryland Ave, Chicago, IL 60637; e-mail: timothy.carll@uchospitals.edu.
References
- 1.Dobin A, Davis CA, Schlesinger F, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29(1):15-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stock W, Luger SM, Advani AS, et al. A pediatric regimen for older adolescents and young adults with acute lymphoblastic leukemia: results of CALGB 10403. Blood. 2019;133(14):1548-1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matutes E, Pickl WF, Van’t Veer M, et al. Mixed-phenotype acute leukemia: clinical and laboratory features and outcome in 100 patients defined according to the WHO 2008 classification. Blood. 2011;117(11):3163-3171. [DOI] [PubMed] [Google Scholar]
- 4.Larson SM, Campbell NP, Huo D, et al. High dose cytarabine and mitoxantrone: an effective induction regimen for high-risk acute myeloid leukemia (AML). Leuk Lymphoma. 2012;53(3):445-450. [DOI] [PubMed] [Google Scholar]
- 5.Porta R, Borea R, Coelho A, et al. FGFR a promising druggable target in cancer: molecular biology and new drugs. Crit Rev Oncol Hematol. 2017;113:256-267. [DOI] [PubMed] [Google Scholar]
- 6.Helsten T, Elkin S, Arthur E, Tomson BN, Carter J, Kurzrock R. The FGFR landscape in cancer: analysis of 4,853 tumors by next-generation sequencing. Clin Cancer Res. 2016;22(1):259-267. [DOI] [PubMed] [Google Scholar]
- 7.Xiao S, McCarthy JG, Aster JC, Fletcher JA. ZNF198-FGFR1 transforming activity depends on a novel proline-rich ZNF198 oligomerization domain. Blood. 2000;96(2):699-704. [PubMed] [Google Scholar]
- 8.Reiter A, Gotlib J. Myeloid neoplasms with eosinophilia. Blood. 2017;129(6):704-714. [DOI] [PubMed] [Google Scholar]
- 9.Yagasaki F, Wakao D, Yokoyama Y, et al. Fusion of ETV6 to fibroblast growth factor receptor 3 in peripheral T-cell lymphoma with a t(4;12)(p16;p13) chromosomal translocation. Cancer Res. 2001;61(23):8371-8374. [PubMed] [Google Scholar]
- 10.Golub TR, Barker GF, Lovett M, Gilliland DG. Fusion of PDGF receptor β to a novel ets-like gene, tel, in chronic myelomonocytic leukemia with t(5;12) chromosomal translocation. Cell. 1994;77(2):307-316. [DOI] [PubMed] [Google Scholar]
- 11.Mavrothalassitis G, Ghysdael J. Proteins of the ETS family with transcriptional repressor activity. Oncogene. 2000;19(55):6524-6532. [DOI] [PubMed] [Google Scholar]
- 12.Fears S, Gavin M, Zhang DE, et al. Functional characterization of ETV6 and ETV6/CBFA2 in the regulation of the MCSFR proximal promoter. Proc Natl Acad Sci U S A. 1997;94(5):1949-1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hock H, Shimamura A. ETV6 in hematopoiesis and leukemia predisposition. Semin Hematol. 2017;54(2):98-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feurstein S, Godley LA. Germline ETV6 mutations and predisposition to hematological malignancies. Int J Hematol. 2017;106(2):189-195. [DOI] [PubMed] [Google Scholar]
- 15.Tomasson MH, Williams IR, Hasserjian R, et al. TEL/PDGFbetaR induces hematologic malignancies in mice that respond to a specific tyrosine kinase inhibitor. Blood. 1999;93(5):1707-1714. [PubMed] [Google Scholar]
- 16.Bhattacharyya J, Mihara K, Yasunaga S, et al. BMI-1 expression is enhanced through transcriptional and posttranscriptional regulation during the progression of chronic myeloid leukemia. Ann Hematol. 2009;88(4):333-340. [DOI] [PubMed] [Google Scholar]
- 17.De Braekeleer E, Douet-Guilbert N, Morel F, Le Bris MJ, Basinko A, De Braekeleer M. ETV6 fusion genes in hematological malignancies: a review. Leuk Res. 2012;36(8):945-961. [DOI] [PubMed] [Google Scholar]
- 18.Noetzli L, Lo RW, Lee-Sherick AB, et al. Germline mutations in ETV6 are associated with thrombocytopenia, red cell macrocytosis and predisposition to lymphoblastic leukemia. Nat Genet. 2015;47(5):535-538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katoh M. Fibroblast growth factor receptors as treatment targets in clinical oncology. Nat Rev Clin Oncol. 2019;16(2):105-122. [DOI] [PubMed] [Google Scholar]
- 20.Verstovsek S, Vannucchi AM, Rambaldi A, et al. Interim results from Fight-203, a phase 2, open-label, multicenter study evaluating the efficacy and safety of pemigatinib (INCB054828) in patients with myeloid/lymphoid neoplasms with rearrangement of fibroblast growth factor receptor 1 (FGFR1). Blood. 2018;132(suppl 1):690. [Google Scholar]
- 21.Gozgit JM, Wong MJ, Moran L, et al. Ponatinib (AP24534), a multitargeted pan-FGFR inhibitor with activity in multiple FGFR-amplified or mutated cancer models. Mol Cancer Ther. 2012;11(3):690-699. [DOI] [PubMed] [Google Scholar]
- 22.Tan FH, Putoczki TL, Stylli SS, Luwor RB. Ponatinib: a novel multi-tyrosine kinase inhibitor against human malignancies. OncoTargets Ther. 2019;12:635-645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O’Hare T, Shakespeare WC, Zhu X, et al. AP24534, a pan-BCR-ABL inhibitor for chronic myeloid leukemia, potently inhibits the T315I mutant and overcomes mutation-based resistance. Cancer Cell. 2009;16(5):401-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim DH, Kwak Y, Kim ND, Sim T. Antitumor effects and molecular mechanisms of ponatinib on endometrial cancer cells harboring activating FGFR2 mutations. Cancer Biol Ther. 2016;17(1):65-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khodadoust MS, Luo B, Medeiros BC, et al. Clinical activity of ponatinib in a patient with FGFR1-rearranged mixed-phenotype acute leukemia. Leukemia. 2016;30(4):947-950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391-2405. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.