Abstract
Over the past 20 years, the regulatory approval of several novel agents to treat multiple myeloma (MM) has prolonged median patient survival from 3 to 8-10 years. Increased understanding of MM biology has led to advances in diagnosis, prognosis, and response assessment, and has informed the development of targeted and immune agents. Here we provide an overview of the recent progress in MM, and highlight the most promising research areas to further improve patient outcome in the future.
Introduction
Remarkable progress in our understanding of the pathobiology of myeloma (MM) has transformed the treatment paradigm and patient outcome. Preclinical studies have guided the discovery of more effective targeted therapies and informed clinical management. However, constitutive and ongoing genetic complexity and instability, coupled with the tumor promoting, immunosuppressive bone marrow (BM) microenvironment, remain an obstacle to cure. An estimated 32,270 new MM cases and 12,830 deaths in 2020 in the USA,1 coupled with a worldwide 126% increase in MM cases from 1990 to 2016,2 highlight the urgent need for novel therapies.
Definition of disease and precursor stages
Multiple myeloma is characterized by malignant plasma cells (PC) in the BM associated in most cases with monoclonal protein in serum and or urine; PC can also be detected in extramedullary sites and/or peripheral blood during progression of disease.3,4 Examination for MM-defining events allows for the discrimination between MM and its precursor stages, namely monoclonal gammopathy of undetermined significance (MGUS) and smoldering MM (SMM).5 Specifically, diagnosis of MM requires 10% or more PC in the BM plus one or more signs of end-organ damage including hypercalcemia, renal dysfunction, anemia, or bone disease (CRAB criteria).4 Even without CRAB features, patients who manifest MM-defining events including clonal BM PC >60%, serum : ratio >100 fold, and/or more than one bone focal lesion on magnetic resonance imaging (MRI) or positron emission tomography (PET)/computed tomography (CT) scan are also treated, as their risk of progression to symptomatic disease is approximately 80% at 2 years.5,6 Clinical manifestations of MM result from excessive production of monoclonal immunoglobulin protein by malignant PC in blood and/or urine, infiltration of BM by neoplastic clone, and aberrant cytokine secretion.4 MGUS patients are monitored for progression off all therapy, as their risk of progression overall is 1% yearly. The standard of practice is also to follow SMM patients expectantly off treatment, 7,8 as the risk of progression is 10% per year in the first 5 years, dropping to 3% per year thereafter. Recently, the new “20-20-20” Mayo Clinic criteria have identified a high-risk (HR)-SMM subgroup (patients with two or more features including: BM PC infiltration >20%, monoclonal protein >20g/L and FLC ratio >20) with a median time to progression of 29 months.9 The QuiRedex study showed that lenalidomide+dexamethasone treatment prolonged time to progression and overall survival (OS) in HR-SMM.10 More recently lenalidomide alone has been shown to delay progressions of HR-SMM;11 however, there was a high rate of treatment discontinuation and secondary cancers in the lenalidomide cohort.11 Ongoing clinical trials are also evaluating alternative treatment strategies to delay progression of HR-SMM.12Most recently, next-generation sequencing (NGS) analysis of MGUSSMM- MM patients has proven to be a useful tool to decipher the timing and chronology of disease initiation events.13,14 In the near future, the combination of genomic signatures and markers of disease burden will likely enable identification of those SMM patients who may benefit from early intervention, and definition of the optimal time to initiate treatment to avoid the development of clinical sequelae. Assessment of the value of early intervention must balance the benefit of delaying/preventing symptomatic MM against the risk of adverse events, and such early interventions should be of finite duration.
Prognostic factors and risk stratification
Clinical and laboratory factors including disease stage, cytogenetic abnormalities, and depth of response to therapy can impact survival of MM patients.15 Cytogenetic analysis and fluorescence in situ hybridization (FISH)- based genetic profiling should be routinely performed to evaluate disease biological behavior and prognosis.16 Among the poor prognostic markers, del(17p) and t(4;14) are the most informative;17,18 concomitant secondary cytogenetic abnormalities may impact prognosis.4 The International Staging System (ISS), based on albumin and β2-microglobulin levels, is most widely used,19 and has been revised (R-ISS) to incorporate lactate dehydrogenase (LDH) and HR-FISH abnormalities.20 Given the genomic complexity of MM, more sophisticated techniques including gene expression profiling, mutational status, and copy number abnormalities have been used, alone or in combination with FISH-based approaches, to more deeply characterize disease biology and prognosis. For example, newly diagnosed MM (NDMM) patients carrying HR del(17p) may be further stratified using subclonal analysis.21 Targeted sequencing has been used as an alternative to whole exon sequencing to specifically analyze fractions of the genome and provide more accurate risk stratification.22 Although not widely incorporated into clinical practice, these approaches will help to define future personalized treatment strategies in MM.
Assessment of response: minimal residual disease
The high rate of complete response (CR) observed with the introduction of novel agents has led to the need for metrics capable of detecting even deeper responses to be developed. Response criteria are based on assessment of monoclonal protein in serum and urine, as well as BM evaluation. However, these parameters alone are not sensitive enough to detect low levels of residual tumor cells in the BM.23 More recently, both retrospective meta-analyses and prospective clinical trials have demonstrated the values of measuring minimal residual disease (MRD) within the BM using next-generation flow (NGF) or NGS, and at extramedullary sites using imaging such as PET/CT.24,25 The International Myeloma Working Group (IMWG) updated response criteria now include MRD status defined by absence of BM PC by NSG or NGF with a minimum sensitivity of 1 in 105 nucleated cells in patients with CR, providing guidelines that can be uniformly interpreted and applied in the context of clinical trials.26 MRD should be evaluated over the course of the disease, informing disease biology and treatment.26 For example, the DFCI/IFM clinical trial comparing lenalidomide-bortezomib- dexamethasone followed by early versus late autologous stem cell transplant (ASCT) showed that MRD negativity at the level of 10-6 was associated with prolonged progression-free survival (PFS) and OS.27,28Moreover, those patients with MRD-BM who were also imaging (PET/CT scan) MRD negativity had the best outcome.27,28 Whether MRD-negativity should represent the goal of therapy for all patients with NDMM or relapsed/refractory (RRMM), or whether treatment decisions should be predicated on MRD status, is still the focus of ongoing clinical trials.
Biologically-based treatments
High-dose chemotherapy plus ASCT remains the standard of care for NDMM patients of physiologic age 70 years or younger who have adequate cardiac, pulmonary, hepatic and renal function.4 Patients who are ineligible for transplant receive induction regimens dependent upon their frailty status.4 In both groups, the integration of scientifically- informed combinations of novel agents including immunomodulatory drugs (IMiD), proteasome inhibitor (PI), dexamethasone, and more recently monoclonal antibodies (mAbs), has transformed the treatment paradigm and patient outcome. However, genomic and clonal evolution in the tumor-promoting BM milieu underlies relapse of disease in most patients, and novel therapies are urgently needed.
Direct targeting of multiple myeloma cell dependencies
- Multiple myeloma “lineage” dependencies
Tumor cells may crucially rely on survival mechanisms that are imprinted during lineage development, namely lineage-dependency.29 For example, the clinical success of first-in-class PI bortezomib in MM has validated the heightened dependency of MM cells on the protein quality control pathway as a therapeutic target.30-32 The ubiquitin- proteasome system (UPS) is the primary mechanism for maintaining protein homeostasis.33 In normal PC, high protein turnover due to immunoglobulin production requires intact proteasome function, and this dependency is even higher in MM PC with aberrant protein turnover which further increases proteasome load. PI can overwhelm the imbalance between proteasome degradative capacity and proteasome load,34-36 leading to endoplasmic reticulum stress due to accumulation of misfolded and unfolded proteins, activation of the unfolded protein response, and cell death. Since proteins involved in cell proliferation and apoptosis, cell-cycle, DNA repair, and metabolism are substrates of the proteasome, PI inhibitor bortezomib has broad effects.37,38 It triggers both intrinsic and extrinsic MM cell apoptosis and MM cell cycle arrest, and modifies bone turnover and osteoclast activity in the BM.38 Bortezomib inhibits the NFκB pathway by blocking degradation of its inhibitor, IκB.33,38 Importantly, NFκB is a major oncogenic pathway in MM, which mediates MM survival and DNA repair, promotes interactions of MM cells-BM accessory cells via the transcription of adhesion molecules, as well as modulating transcription of cytokines (such as IL-6, VEGF, IGF-1), which in turn mediate MM growth and drug resistance, and confer immunosuppression in the BM.33,38
Over the disease course, MM cells acquire resistance to bortezomib via genetic and non-genetic mechanisms.33 Extensive preclinical research has delineated mechanisms
of PI resistance and informed strategies to overcome resistance. Second-generation PI have been generated to overcome bortezomib resistance. The irreversible covalent epoxyketone PI carfilzomib, either alone or in combination with lenalidomide, has been approved to treat RRMM.39 Ixazomib, the first oral boronic acid-based PI, has been approved, alone and in combination with lenalidomide, to treat RRMM;40 this all-oral regimen showed low toxicity profile and improved patient quality of life. The pan-PI marizomib, which penetrates the central nervous system (CNS), also demonstrates anti-MM activity in the setting of bortezomib resistance.41 Given the multitude of available PI, the side effect profiles and identification of biomarkers of PI resistance/sensitivity will determine their optimal and rational use.
Targeting upstream components of the ubiquitin proteasome system (UPS) has recently emerged as a promising strategy to overcome PI resistance. Therapeutic targeting of deubiquitylating enzymes (DUB) and the 19S proteasome- associated ubiquitin receptor Rpn13 overcame PI resistance in preclinical studies.42-45 However, the first-inhuman trial of USP14/UCHL5 DUB inhibitor for RRMM has been stopped due to dose-limiting toxicity. Targeted therapies against Rpn13 have been developed for evaluation in setting of PI resistance.46 An alternative approach to overcome PI resistance is the concomitant block of the aggresome/autophagy pathway using an inhibitor of histone deacetylase 6 (HDAC6), which is recruited to maintain proteostasis balance as an adaptive response mechanism. 38
Lineage vulnerabilities in MM also include aberrant transcription factor (TF) regulatory networks controlling lineage factor IRF4.47 Although direct targeting of TF represents an attractive strategy, there are no available inhibitors for clinical application. However, we found that aberrant regulatory KDM3A-IRF4-KLF2 loop may be efficiently targeted by KDM3A inhibitor, which restores IRF4 and KLF2 promoter methylation and suppresses their transcription, thereby resulting in decreased MM cell homing to the BM and direct anti-MM toxicity.48Moreover, repression of IRF4 transcription is observed after lenalidomide treatment, which triggers cereblon (CRBN)-mediated degradation of IRF4 transcriptional activator IKZF3.49,50 From these findings, a new platform technology has been developed to trigger selective protein degradation. Specifically, degronimids, also known as proteolysis-targeting chimeras (PROTAC), are designed by conjugating the small-molecule binder of the target protein to an E3 ubiquitin ligase binding scaffold, such as the analogs of thalidomide which bind CRBN.51 This approach will allow for the therapeutic degradation of protein substrates that are otherwise challenging to target.
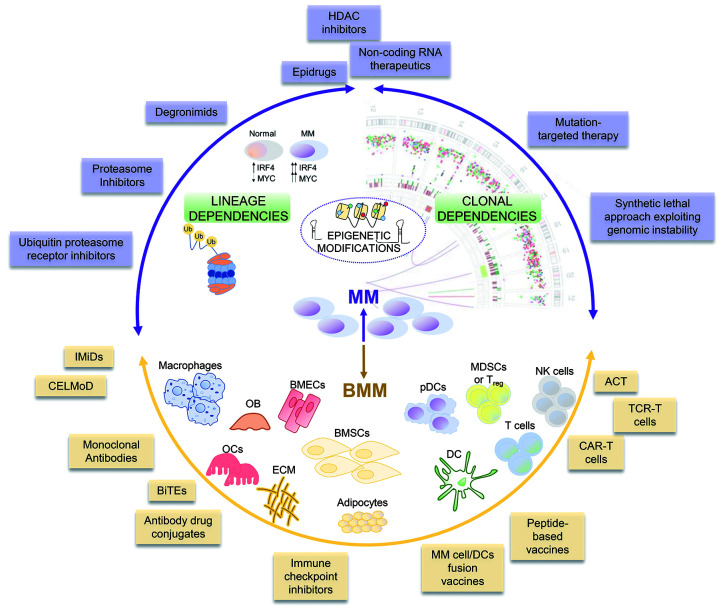
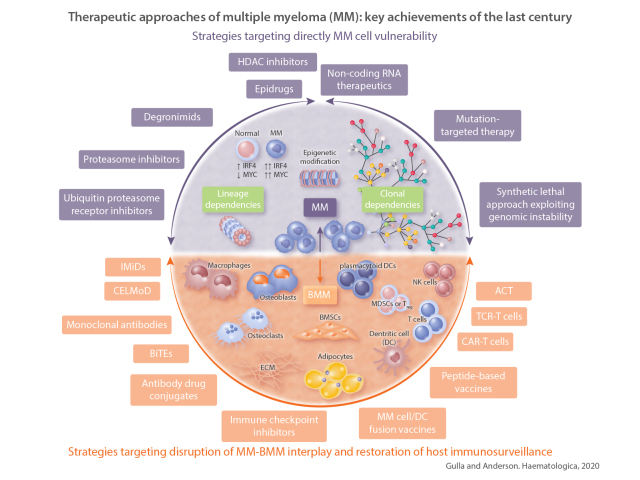
Figure 1.
Overview of the different anti-multiple myeloma (MM) strategies discussed in the review. Purple: strategies designed to directly target MM cell vulnerabilities; we can distinguish those exploiting “lineage dependencies” or “clonal dependencies”. (Center) Strategy targeting “epigenetic modifications” that may broadly affect both lineage and clonal vulnerabilities. (Bottom, yellow) Strategies aiming to disrupt MM-bone marrow microenvironment (BMM) interplay and restore host immunosurveillance. Purple double pointed arrows: MM-related approaches; yellow double pointed arrows: BMM-related approaches. These highlight the fact that one specific treatment, even in case of target therapy, may also affect multiple cellular components/interactions thus amplifying the therapeutic effects. OB: osteoblast; OC: osteoclasts; BMEC: bone marrow endothelial cells; ECM: extracellular matrix; BMSC: bone marrow stromal cells; DC: dendritic cell; pDC: plasmacytoid DC; MDSC: myeloid derived suppressor cells; Treg: regulatory T cell.
- Multiple myeloma “clonal” dependencies
Large inter-patient and intra-patient genetic heterogeneity limits the identification of universal drivers of MM. However, several oncogenic dependencies are primary events related to mutations in driver genes and primary translocations.52 Translocations or gains of MYC locus (along with dysregulation of upstream signaling pathways, such as IRF4) support an oncogenic role of MYC in MM, especially in the context of MGUS-MM transition.53 Alteration of the transcriptional program of MYC, and of its functional collaborators such as E2F1, promotes oncogenic signaling and PC survival.54,55 Frequent gene mutations in MM include RAS, either KRAS or NRAS, with subsequent activation of the MAPK pathway, BRAF, DIS3, and FAM46C.56 Their role as prognostic factors has not been completely defined, as only TP53 mutation (6-8% of patients at diagnosis) clearly confers worse patient outcome. 57 Mutation-targeted treatments in MM are often compromised by intra-clonal heterogeneity. Specifically, deep sequencing has identified a complex subclonal structure in MM with different patterns of clonal evolution impacted by BM, immune response, and therapy.58 In this complex scenario, MM cells may share common mutations, but they may also express additional subclones which compromise mutation-targeting therapies.58,59 The MyDRUG trial is enrolling patients with relapsed MM based upon genomic sequencing; patients receive a specific treatment targeting their unique tumor mutations, along with standard-of-care treatment.60 This trial will reveal whether the abnormal clone can be targeted, and provide the rationale for further derived clinical trials of targeted therapies.
Genomic complexity in MM is due to genomic instability and ongoing DNA damage.61 MM cells display hyperactivation of DNA repair mechanisms which confer a survival advantage and drug resistance with increasing numbers of new mutations over time.62 These aberrant processes may reveal new vulnerabilities. For example, in MM patients, in whom ongoing DNA damage occurs concurrently with low Hippo co-transcription factor (YAP1) levels, MM cell apoptosis is prevented. Conversely, inhibition of STK4 rescues YAP1 and triggers DNA-damageinduced apoptosis, providing the framework for clinical evaluation of STK4 inhibition.63 A second example is the induction of “BRCAness” status in MM cells by bortezomib, thereby increasing their sensitivity to PARP inhibitors.37 Finally, MYC amplification in MM can induce DNA response pathway and reactive oxygen species; the former can be blocked by ATR inhibitors and the latter can be increased by bortezomib, together triggering MM cell apoptosis in a synthetic lethal mechanism.61
- Multiple myeloma epigenetic modifications
Epigenetic alterations affect regulation of gene activity and expression, without altering gene sequence. Such alterations are associated with MM onset and progression, and modulate several important biological processes.64 Among epigenetic changes, DNA methylation, histone modification, and non-coding RNA (ncRNA) deregulation are the best characterized.64 Global hypomethylation of the genome characterizes the transition from MGUS to MM, whereas pervasive genome re-methylation occurs in the transition from MM to more aggressive leukemic stage (PCL).64 Universal overexpression of histone methyltransferase MMSET is detected in patients carrying t(4;14) translocation and promotes MM cell survival by activating oncogenic MAPK pathway, increasing MYC and IRF4 transcription, and inducing chemo-resistance through enhancing DNA repair mechanisms.65,66 Therefore, development of MMSET inhibitor represents a promising therapeutic strategy for this subset of MM. We have demonstrated the oncogenic role and prognostic relevance of type II arginine methyltransferase PRMT5 in MM, whose inhibition results in MM cell killing via NFκB inhibition, thus providing the rationale for clinical trials targeting PRMT5 in MM.67
Histone deacetylases (HDAC) are generally hyperactive in MM, and HDAC inhibitors are the most investigated epigenetic drugs.65 Preclinical studies have led to clinical trials and the approval of non-selective HDAC inhibitor panobinostat in combination with bortezomib in RRMM.68 However, increased toxicity observed with panobinostat prompted the development and translation of selective HDAC6 inhibitors (ricolinostat and ACY 241), which showed promising results and lower toxicity in combination with bortezomib and dexamethasone.69,70
Over the last decade, extensive studies have also highlighted the contribution of the ncRNA compartment in MM pathogenesis and progression. Specifically, microRNAs (miRNAs) are key regulators of gene expression at the post-transcriptional level, as they can induce either translational repression or degradation of target mRNAs upon total or partial complementary binding with 3′ untranslated region (3′ UTR).71 Given the multitude of targets for a single miRNA, these molecules harbor the potential to concomitantly regulate multiple biological processes. Preclinical data have defined their oncogenic (miR-221/222,-21,-17-92 cluster) or tumor suppressive (miR-29b,-34a,-125b,-15,-16) roles in MM associated with repression or overexpression, respectively, of genes involved in essential pro-survival pathways.72-77 The role of miRNAs has been similarly investigated in the context of drug resistance, BM-PC interaction, and bone disease.78-81 Although miRNA-based therapeutics have not yet translated into US Food and Drug Administration (FDA)- approved drugs, several candidates are being tested in other diseases and will soon be evaluated in MM,82 using miRNA replacement or inhibition strategies. As they are endogenous antisense of mRNAs, their replacement is likely to induce a “natural” effect on the targets, with less off-target effects compared to siRNAs; moreover, the recent availability of in vivo delivery systems now allows for clinical trials.83 Likewise, miRNA inhibition strategies take advantage of new antisense oligonucleotide technologies, and ongoing early trials in several cancers will likely pave the way for their investigation in MM.83 Finally, long ncRNAs (lncRNA) represent major regulators of gene expression and chromatin dynamics by interacting with DNA and proteins.84 LncRNA genes outnumber protein- coding genes, with a partner of expression often restricted to specific cell types or conditions.84 With few exceptions, however, their functional role is still largely obscure in MM.85 We recently described the lncRNA landscape in MM, and their role as independent predictors of clinical outcome;86 ongoing and future studies will define their role in disease pathogenesis and as potential therapeutic targets.
Targeting the tumor-bone marrow microenvironment interface
Disrupting the interactions of MM cells with the BM represents an ideal therapeutic strategy in MM, as shown by agents such as IMiD which remain active against MM even in the BM milieu. Cellular and non-cellular components of the BM niche support MM cell proliferation, migration, survival and drug resistance, while also conferring immunosuppression, and therefore represent targets for novel therapeutics.4,87,88
- Immunomodulatory drugs
Extensive preclinical and clinical studies have led to the FDA approval of the IMiD thalidomide and its more potent derivatives lenalidomide and pomalidomide for treatment of both NDMM and RRMM.89-92 IMiD induce direct cytotoxic effects on MM cells including growth arrest and caspase-8-mediated apoptosis, associated with CRBN-dependent degradation of IKZF1/3 followed by IRF4 downregulation.49,50,93 In the BM microenvironment they abrogate MM cell adhesion to the BM, modulate cytokine and growth factor secretion, inhibit angiogenesis, and most importantly, upregulate T, NK, and NKT cells, while downregulating regulatory T cells.3,93 Mechanistically, binding to CRBN has also been implicated in mediating the immune-related effects of IMiD, as IKZF1/3 degradation in T cells increases their secretion of cytokines including IL-2.94 This mechanism is also associated with increased natural killer (NK) and NK-T-cell cytotoxicity and antibody-dependent cellular cytotoxicity (ADCC) (i.e., anti-CD38, daratumumab and -CD20, rituximab) observed after IMiD treatment.95,96 Furthermore, IMiD can also enhance NK and T-cell cytotoxicity by triggering granzyme-B via either CRBN- or ZAP-70-dependent mechanisms.95 Preclinical and clinical studies have already confirmed the strong synergism of IMiD with PI and with mAb.97-102 Recently, iberdomide, a higher affinity CRBN E3-ligase modulator (CELMoD), showed significant preclinical activity against IMiD-resistant MM cells,103 and ongoing clinical trials are examining its efficacy in RRMM resistant to lenalidomide and pomalidomide. Promising results have also been recently reported with the more potent CELMoD CC-92480, currently under investigation to treat IMiD-resistant RRMM.104
- Immune-based therapies
Loss of immune surveillance supports MM growth and resistance, and is associated with alterations in accessory and immune cells in the BM.105 Moreover, there is increasing evidence that evolving immune dysfunction is an important determinant of progression from MGUS/SMM to symptomatic MM.105 For example, functional interaction of plasmacytoid dendritic cells (DC) with MM cells promotes their survival and drug resistance,106 providing the framework for targeting this interaction in novel therapies. 107-109 Similarly, myeloid derived suppressor cells, regulatory T cells, Th17 cells, tumor-associated macrophages, mesenchymal stromal cells, and osteoclasts significantly contribute to tumor immune escape and immunocompromised clinical status.110 Immune escape is also mediated, at least in part, by increased expression of immune checkpoints, i.e., PD-1/PD-L1, in T cells and MM cells, associated with disease progression from MGUS and SMM to MM.111 Although preclinical data have suggested the therapeutic utility of PD-1/PD-L1 blockade, early clinical trials have been discouraging.111 No singleagent activity of pembrolizumab has been shown, and importantly, two randomized clinical trials evaluating pembrolizumab in combination with IMiD to treat RRMM were closed due to excessive mortality.111 Ongoing studies are characterizing the role of the other immune checkpoint or agonist proteins (i.e., LAG 3 or TIGIT and OX40, respectively) as potential therapeutic targets, alone and in combination with MM targeted and immune therapies.112 Multiple mAb targeting MM surface antigens can trigger ADCC, antibody-dependent cellular phagocytosis, complement activation, and direct effect on MM cells.113 Elotuzumab and daratumumab target SLAMF7 and CD38, respectively.113 Elotuzumab also directly activates NK cells and is FDA approved in combination with lenalidomide or pomalidomide in RRMM.99,100 Daratumumab has shown remarkable extent and frequency of response, leading to its FDA approval as a single agent or in combination with IMiD and PI in both RRMM and NDMM.97,98,114,115 The recent GRIFFIN trial compared standard lenalidomide-bortezomib-dexamethasone with or without daratumumab in transplant eligible patients, and showed deeper responses and MRD negativity rate in the daratumumab-treated patient cohort.116 Moreover, recent approval of subcutaneous formulation of daratumumab will dramatically reduce patient treatment times.117 More recently, a new CD38- directed mAb, isatuximab, has been approved in combination with pomalidomide-dexamethasone to treat RRMM;101 whether it is effective even in daratumumab refractory MM remains to be determined.
Monoclonal antibody technologies have also provided the framework for the development of Ab-drug conjugates (ADC) and bispecific T-cell engagers (BiTE). In the former, the conjugation with cytotoxic chemicals (such as auristatin) via synthetic linkers provides for direct tumor killing, and the mAb provides for selective tumor cell targeting, as well as immune-mediated cytotoxicity.118-122 Bcell maturation antigen (BCMA)-directed ADC are currently under investigation in both preclinical and clinical settings, and represent a promising approach due to the highly specific expression on BCMA on MM cells and late memory B cells, as well as the role of the BCMA/APRIL pathway in supporting MM cell survival in the BM.113,118,123 The bi-specificity of the BiTE (mainly for CD3 on T cells and several MM-associated antigens, such as BCMA and GPRC5D) allows for engagement of T cells with tumor cells, resulting in formation of cytolytic synapses and tumor lysis.124-126 Although results from early trials look promising, longer follow-up in larger studies are needed to assess the clinical benefit and potential toxicity. Importantly, BCMA ADC and BiTE have the advantage for “off the shelf” availability and universal use.
Cellular therapies represent an additional strategy to boost MM-specific immunity using either adoptive T-cell (ACT) or engineered T-cell approaches.3 Clinical experience with ACT using marrow-infiltrating lymphocytes (MIL) in MM has shown promise in achieving memory immune responses and stable disease.127 Importantly, advances in engineering technologies have allowed for both T-cell receptor (TCR) and chimeric antigen receptor (CAR) T-cell approaches.128 CAR are chimeric proteins that bring together the signaling moieties of TCR complexes and the variable domains of Ab which recognize a tumor-associated antigen.128,129 Co-stimulatory molecules have been included in the second-generation CAR-T cells to enhance T-cell activation by mimicking a physiological T-cell response.128,129 After genetic modification, a patient’s T cells expressing the chimeric protein can be expanded ex vivo, and then activate a specific T-cell response once reinfused to the patient.128,129 This allows CAR-T cells, in contrast to TCR-T cells, to recognize unprocessed tumor antigen in an MHC-independent manner.128,129 A major determinant of successful CAR-T therapy is the identification of a target uniquely and highly expressed by MM cells, thus limiting the occurrence of “off-target” effects. Among a variety of antigen targets, BCMA is the most frequently used due to its selectivity for normal plasma and MM cells. Several CAR-T products have been clinically tested in heavily pre-treated (PI-IMiD-CD38 mAb) RRMM, and have demonstrated remarkable deep (MRD- ) responses.129-132 Clinical experience has also helped to improve management of the most commonly observed toxicities of CAR-T cells, including cytokine release syndrome and neurotoxicity.128,129 To date, however, most patients have relapsed, and ongoing research is assessing mechanisms of resistance to CAR-T, utilizing combination immune approaches with CAR-T, and using CAR-T earlier in the disease course in order to achieve more durable responses.
Lastly, vaccination strategies have been developed to improve antigen-specific memory anti-MM immunity. Specifically, multi-peptide-based vaccines induce effective and durable memory peptide-specific CTL in SMM patients, providing the rationale for their clinical evaluation to delay progression from SMM to active disease. 133,134 More recently, a novel engineered heteroclitic BCMA peptide has been used to induce a BCMA-specific memory anti-MM immunity, suggesting its potential use in vaccination and/or ACT strategies to generate longlasting immunity against MM.135 As alternative vaccination strategy employs MM cell/DC fusion vaccines to generate anti-MM immunity in the post-ASCT setting, this vaccine induces anti-MM immunity and enhances depth of response.136 The most significant obstacle to successful vaccination therapy in MM is the disease- and treatment-related immune dysfunction, which may limit the immune responses in vivo. As such, a randomized trial comparing lenalidomide versus lenalidomide plus MM cell/DC fusion vaccine post-transplant is ongoing (clinicaltrials. gov identifier: NCT02728102) to determine whether combination of vaccination with IMiD may improve its efficacy. Overall, future treatment approaches will likely rely on the optimal combination of targeted and immunebased strategies to obtain a durable anti-MM response and restore the host immune-surveillance.
Future directions
Despite tremendous advances, the clinical management of MM patients remains challenging, since acquisition of resistance underlies relapse of disease in most patients. Correlative science studies on patient samples are delineating mechanisms of resistance to both targeted and immune agents in order to inform clinical strategies to overcome resistance and improve patient outcome. Development of second-generation more potent drugs of the same class has overcome both PI and IMiD resistance, as have combination therapies with agents targeting pathways mediating resistance. Identification of biomarkers of patient MM resistance/sensitivity may further inform sequential and combination therapies in the future. Importantly, agents targeting novel MM vulnerabilities are urgently needed. For example, selinexor, a selective inhibitor of nuclear export of tumor suppressor proteins, growth factors, and mRNAs of oncogenic proteins, has recently been approved in triple-class (PI-IMiD-anti-CD38 mAb) refractory MM.137
A similar scenario of resistance is now beginning to appear for immune-based approaches, with several possible explanations. Loss of targeted antigens (such as BCMA, CD38) is a common event, either due to loss with tumor evolution or to suppression in the face of immune pressure. Multi-antigen targeting may potentially overcome this obstacle, and several trials are evaluating this strategy.129 Similarly, circulating antigen in a soluble form may potentially interfere with immune-targeted approaches. For example, high levels of soluble BCMA have been detected in MM patients, and anti-BCMA CAR-T therapy is being combined with γ-secretase inhibitor to block BCMA cleavage from the MM cell surface (clinicaltrials.gov identifier: NCT03502577). Additional resistance may be intrinsic to the technology or modality, and ongoing efforts to increase CAR-T cell expansion and persistence in vivo include enriching for early memory Tcell phenotype, optimizing CAR design to avoid antigenindependent tonic signaling, and/or intensifying lymphodepletion to promote CAR-T cell persistence.129
Lastly, T-cell exhaustion and the immunosuppressive BM may contribute to both targeted- and immune-therapy resistance.128 Restoration of host anti-MM immunity represents an important unmet need in MM. Several models, such as SCID-hu and SCID-synth-hu, have been developed to recapitulate the in vivo growth on patient MM cells in the context of BM.138,139 However, understanding the role of the immune system in disease pathobiology requires the use of immunocompetent models (such as the 5T and Vk*MYC).53,140 This is critical for evaluating not only immune therapies, but also targeted MM agents, such as bortezomib, which induce immunogenic cell death in vivo.
A recent area of investigation in MM is assessing the role of gut microbiome in shaping the immune system response, including anti-tumor immunity. For example, a commensal bacterium Prevotella heparinolytica promotes progression of MM by favoring differentiation of Th17 cells in the gut, which migrate to the BM of Vk*MYC mice and activate eosinophils; targeting IL-17-eosinophil immune axis may, therefore, represent a potential treatment for HR-SMM.141 Abundance of Eubacterium limosum bacteria in the intestinal flora has been associated with relapse after allogeneic stem cell transplantation.142 Similarly, high presence of Eubacterium hallii in the intestinal microbiota correlates with achievement of MRD negativity. 143 Although still in an early stage, studies of the MM microbiome may identify future biomarkers or therapeutic agents to improve MM patient outcome.
Finally, identification of specific biomarkers predictive of therapy response within a patient’s heterogeneous MM has been a major focus of research. The first example of biomarker-driven anti-MM treatment in MM is the Bcl-2 inhibitor venetoclax, whose safety and efficacy are predicated upon occurrence of t(11;14) or presence of high levels of BCL2.144 Several trials in RRMM are showing efficacy of venetoclax, as monotherapy and in combination, restricted to this patient subset.145,146 Integration of current and future technologies may further guide disease management and allow for precision medicine. For example, encouraging results have recently been reported in a trial using a multi-omics approach integrating DNA and RNA sequencing to inform drug treatment for RRMM.147 The ongoing MyDRUG trial profiles relapsed MM, and is then examining whether targeting genomic abnormalities in combination with standard relapse MM therapy can delete the abnormal MM clone.60 This and other trials will inform the utility of precision medicine in MM, especially in the presence of concomitant genetic abnormalities.
Given the multiple available treatment options, welldesigned randomized clinical trials are necessary to assess the superior efficacy of specific regimens with head-tohead comparison. For example, interim analysis of the phase III ENDURANCE trial did not show superior PFS of carfilzomib versus bortezomib in combination with lenalidomide-dexamethasone for NDMM. Importantly, regulatory randomized trial results require real-world validation, since patient age, frailty status, and comorbidity frequently do not reflect trial patients. High-dose melphalan with ASCT remains a standard of care, and its role in the era of novel therapies is under evaluation in the IFM/DFCI 2009 DETERMINATION and FORTE clinical trials. However, the recent use of quadruplet therapies including daratumumab in the CASSIOPEIA and GRIFFIN trials shows that the addition of mAb can achieve increased extent and depth of response to induction therapy, 116,148 and whether high-dose melphalan and ASCT improves outcome of quadruplet therapy remains to be determined. Nonetheless, research continues to improve alkylating agents as well. For example, melflufen is a prodrug which is digested to melphalan by high levels of aminopeptidase in MM cells, thereby improving its therapeutic index.149,150 Ultimately, the future use of novel targeted and immune therapies, as well as the role of conventional therapies, will be defined by vulnerabilities within individual patients and/or patient subgroups.
Conclusions
Over the past decades, a deeper understanding of the complex MM pathobiology has informed drug development and clinical practice, resulting in significant improvements in patient outcome. Combination approaches targeting MM cells, disrupting MM cell/BM interactions, and enhancing anti-MM immune responses, have remarkably improved response extent and frequency. Remaining obstacles to cure include constitutive and evolving genomic heterogeneity in MM cells, as well as the immunosuppressive BM milieu. In the future, integration of advanced sequencing technologies profiling both the MM cell and BM accessory/immune cells will identify novel targets and inform more potent, selective, and well tolerated targeted and immune therapies. Long-term disease- free survival and potential cure in MM will require both achieving MRD negativity and restoring host anti- MM immunity. Such patients can then be free of disease while off all therapies.
Supplementary Material
Graphical Abstract.
Funding Statement
Funding This work is supported by NIH/NCI grants SPOREP50CA100707 (KCA), R01-CA050947 (KCA), R01CA207237 (KCA), P01CA155258 (KCA) and R01- CA178264 (KCA); and the Sheldon and Miriam Medical Research Foundation (KCA). KCA is an American Cancer Society Clinical Research Professor. AG is a Fellow of The Leukemia & Lymphoma Society and a Scholar of the American Society of Hematology (ASH).
References
- 1.National Cancer Institute. Cancer stat facts: myeloma. Available from: https://seer.cancer.gov/statfacts/html/mulmy.html. Accessed 20 July 2020. [Google Scholar]
- 2.Cowan AJ, Allen C, Barac A, et al. Global burden of multiple myeloma: a systematic analysis for the Global Burden of Disease Study 2016. JAMA Oncol. 2018;4(9):1221-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson KC. Progress and paradigms in multiple myeloma. Clin Cancer Res. 2016;22(22):5419-5427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kumar SK, Rajkumar V, Kyle RA, et al. Multiple myeloma. Nat Rev Dis Primers. 2017;3:17046. [DOI] [PubMed] [Google Scholar]
- 5.Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12):e538-548. [DOI] [PubMed] [Google Scholar]
- 6.Mateos MV, Hernandez MT, Giraldo P, et al. Lenalidomide plus dexamethasone for highrisk smoldering multiple myeloma. N Engl J Med. 2013;369(5):438-447. [DOI] [PubMed] [Google Scholar]
- 7.Kyle RA, Greipp PR. Smoldering multiple myeloma. N Engl J Med. 1980;302(24):1347-1349. [DOI] [PubMed] [Google Scholar]
- 8.Kapoor P, Rajkumar SV. Smoldering multiple myeloma: to treat or not to treat. Cancer J. 2019;25(1):65-71. [DOI] [PubMed] [Google Scholar]
- 9.Lakshman A, Rajkumar SV, Buadi FK, et al. Risk stratification of smoldering multiple myeloma incorporating revised IMWG diagnostic criteria. Blood Cancer J. 2018;8(6):59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mateos MV, Hernandez MT, Giraldo P, et al. Lenalidomide plus dexamethasone versus observation in patients with high-risk smouldering multiple myeloma (QuiRedex): long-term follow-up of a randomised, controlled, phase 3 trial. Lancet Oncol. 2016;17(8):1127-1136. [DOI] [PubMed] [Google Scholar]
- 11.Lonial S, Jacobus S, Fonseca R, et al. Randomized trial of lenalidomide versus observation in smoldering multiple myeloma. J Clin Oncol. 2020;38(11):1126-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.D'Agostino M, Bertamini L, Oliva S, Boccadoro M, Gay F. Pursuing a curative approach in multiple myeloma: a review of new therapeutic strategies. Cancers (Basel). 2019;11(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rustad EH, Yellapantula V, Leongamornlert D, et al. Timing the initiation of multiple myeloma. Nat Commun. 2020;11(1):1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aktas Samur A, Minvielle S, Shammas M, et al. Deciphering the chronology of copy number alterations in multiple myeloma. Blood Cancer J. 2019;9(4):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rajkumar SV. Multiple myeloma: 2016 update on diagnosis, risk-stratification, and management. Am J Hematol. 2016;91(7): 719-734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sonneveld P, Avet-Loiseau H, Lonial S, et al. Treatment of multiple myeloma with highrisk cytogenetics: a consensus of the International Myeloma Working Group. Blood. 2016;127(24):2955-2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Avet-Loiseau H, Hulin C, Campion L, et al. Chromosomal abnormalities are major prognostic factors in elderly patients with multiple myeloma: the Intergroupe Francophone du Myelome experience. J Clin Oncol. 2013;31(22):2806-2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumar S, Fonseca R, Ketterling RP, et al. Trisomies in multiple myeloma: impact on survival in patients with high-risk cytogenetics. Blood. 2012;119(9):2100-2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greipp PR, San Miguel J, Durie BG, et al. International staging system for multiple myeloma. J Clin Oncol. 2005;23(15):3412-3420. [DOI] [PubMed] [Google Scholar]
- 20.Palumbo A, Avet-Loiseau H, Oliva S, et al. Revised International Staging System for multiple myeloma: a report from International Myeloma Working Group. J Clin Oncol. 2015;33(26):2863-2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thakurta A, Ortiz M, Blecua P, et al. High subclonal fraction of 17p deletion is associated with poor prognosis in multiple myeloma. Blood. 2019;133(11):1217-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bolli N, Biancon G, Moarii M, et al. Analysis of the genomic landscape of multiple myeloma highlights novel prognostic markers and disease subgroups. Leukemia. 2018;32(12): 2604-2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paiva B, van Dongen JJ, Orfao A. New criteria for response assessment: role of minimal residual disease in multiple myeloma. Blood. 2015;125(20):3059-3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perrot A, Lauwers-Cances V, Corre J, et al. Minimal residual disease negativity using deep sequencing is a major prognostic factor in multiple myeloma. Blood. 2018;132(23): 2456-2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Munshi NC, Avet-Loiseau H, Rawstron AC, et al. Association of minimal residual disease with superior survival outcomes in patients with multiple myeloma: a meta-analysis. JAMA Oncol. 2017;3(1):28-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumar S, Paiva B, Anderson KC, et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016;17(8):e328-e346. [DOI] [PubMed] [Google Scholar]
- 27.Moreau P, Attal M, Caillot D, et al. Prospective evaluation of magnetic resonance imaging and [(18)F]fluorodeoxyglucose positron emission tomography-computed tomography at diagnosis and before maintenance therapy in symptomatic patients with multiple myeloma included in the IFM/DFCI 2009 trial: results of the IMAJEM study. J Clin Oncol. 2017;35(25):2911-2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Attal M, Lauwers-Cances V, Hulin C, et al. Lenalidomide, bortezomib, and dexamethasone with transplantation for myeloma. N Engl J Med. 2017;376(14):1311-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garraway LA, Sellers WR. Lineage dependency and lineage-survival oncogenes in human cancer. Nat Rev Cancer. 2006;6(8): 593-602. [DOI] [PubMed] [Google Scholar]
- 30.Richardson PG, Sonneveld P, Schuster M, et al. Extended follow-up of a phase 3 trial in relapsed multiple myeloma: final time-toevent results of the APEX trial. Blood. 2007;110(10):3557-3560. [DOI] [PubMed] [Google Scholar]
- 31.Richardson PG, Sonneveld P, Schuster MW, et al. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med. 2005;352(24):2487-2498. [DOI] [PubMed] [Google Scholar]
- 32.Richardson PG, Barlogie B, Berenson J, et al. A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med. 2003;348(26):2609-2617. [DOI] [PubMed] [Google Scholar]
- 33.Gandolfi S, Laubach JP, Hideshima T, Chauhan D, Anderson KC, Richardson PG. The proteasome and proteasome inhibitors in multiple myeloma. Cancer Metastasis Rev. 2017;36(4):561-584. [DOI] [PubMed] [Google Scholar]
- 34.Cenci S, Mezghrani A, Cascio P, et al. Progressively impaired proteasomal capacity during terminal plasma cell differentiation. EMBO J. 2006;25(5):1104-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bianchi G, Oliva L, Cascio P, et al. The proteasome load versus capacity balance determines apoptotic sensitivity of multiple myeloma cells to proteasome inhibition. Blood. 2009;113(13):3040-3049. [DOI] [PubMed] [Google Scholar]
- 36.Obeng EA, Carlson LM, Gutman DM, Harrington WJ, Lee KP, Boise LH. Proteasome inhibitors induce a terminal unfolded protein response in multiple myeloma cells. Blood. 2006;107(12):4907-4916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Neri P, Ren L, Gratton K, et al. Bortezomibinduced "BRCAness" sensitizes multiple myeloma cells to PARP inhibitors. Blood. 2011;118(24):6368-6379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hideshima T, Anderson KC. Biologic impact of proteasome inhibition in multiple myeloma cells-from the aspects of preclinical studies. Semin Hematol. 2012;49(3):223-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stewart AK, Rajkumar SV, Dimopoulos MA, et al. Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. N Engl J Med. 2015;372(2):142-152. [DOI] [PubMed] [Google Scholar]
- 40.Moreau P, Masszi T, Grzasko N, et al. Oral ixazomib, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;374(17):1621-1634. [DOI] [PubMed] [Google Scholar]
- 41.Richardson PG, Zimmerman TM, Hofmeister CC, et al. Phase 1 study of marizomib in relapsed or relapsed and refractory multiple myeloma: NPI-0052-101 Part 1. Blood. 2016;127(22):2693-2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Song Y, Li S, Ray A, et al. Blockade of deubiquitylating enzyme Rpn11 triggers apoptosis in multiple myeloma cells and overcomes bortezomib resistance. Oncogene. 2017;36(40):5631-5638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chauhan D, Tian Z, Nicholson B, et al. A small molecule inhibitor of ubiquitin-specific protease-7 induces apoptosis in multiple myeloma cells and overcomes bortezomib resistance. Cancer Cell. 2012;22(3):345-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tian Z, D'Arcy P, Wang X, et al. A novel small molecule inhibitor of deubiquitylating enzyme USP14 and UCHL5 induces apoptosis in multiple myeloma and overcomes bortezomib resistance. Blood. 2014;123(5): 706-716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Du T, Song Y, Ray A, Chauhan D, Anderson KC. Proteomic analysis identifies mechanism (s) of overcoming bortezomib resistance via targeting ubiquitin receptor Rpn13. Leukemia. 2020. May 18. doi: 10.1038/s41375-020-0865-2. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Song Y, Park PMC, Wu L, et al. Development and preclinical validation of a novel covalent ubiquitin receptor Rpn13 degrader in multiple myeloma. Leukemia. 2019;33(11):2685-2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shaffer AL, Emre NC, Lamy L, et al. IRF4 addiction in multiple myeloma. Nature. 2008;454(7201):226-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ohguchi H, Hideshima T, Bhasin MK, et al. The KDM3A-KLF2-IRF4 axis maintains myeloma cell survival. Nat Commun. 2016;7:10258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kronke J, Udeshi ND, Narla A, et al. Lenalidomide causes selective degradation of IKZF1 and IKZF3 in multiple myeloma cells. Science. 2014;343(6168):301-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lu G, Middleton RE, Sun H, et al. The myeloma drug lenalidomide promotes the cereblon-dependent destruction of Ikaros proteins. Science. 2014;343(6168):305-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Winter GE, Buckley DL, Paulk J, et al. Drug development. Phthalimide conjugation as a strategy for in vivo target protein degradation. Science. 2015;348(6241):1376-1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Walker BA, Mavrommatis K, Wardell CP, et al. Identification of novel mutational drivers reveals oncogene dependencies in multiple myeloma. Blood. 2018;132(6):587-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chesi M, Robbiani DF, Sebag M, et al. AIDdependent activation of a MYC transgene induces multiple myeloma in a conditional mouse model of post-germinal center malignancies. Cancer Cell. 2008;13(2):167-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jovanovic KK, Roche-Lestienne C, Ghobrial IM, Facon T, Quesnel B, Manier S. Targeting MYC in multiple myeloma. Leukemia. 2018;32(6):1295-1306. [DOI] [PubMed] [Google Scholar]
- 55.Fulciniti M, Lin CY, Samur MK, et al. Nonoverlapping control of transcriptome by promoter- and super-enhancer-associated dependencies in multiple myeloma. Cell Rep. 2018;25(13):3693-3705e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bolli N, Avet-Loiseau H, Wedge DC, et al. Heterogeneity of genomic evolution and mutational profiles in multiple myeloma. Nat Commun. 2014;5:2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Perrot A, Corre J, Avet-Loiseau H. Risk stratification and targets in multiple myeloma: from genomics to the bedside. Am Soc Clin Oncol Educ Book. 2018;38:675-680. [DOI] [PubMed] [Google Scholar]
- 58.Corre J, Cleynen A, Robiou du Pont S, et al. Multiple myeloma clonal evolution in homogeneously treated patients. Leukemia. 2018;32(12):2636-2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ledergor G, Weiner A, Zada M, et al. Single cell dissection of plasma cell heterogeneity in symptomatic and asymptomatic myeloma. Nat Med. 2018;24(12):1867-1876. [DOI] [PubMed] [Google Scholar]
- 60.Study tests targeted drugs for multiple myeloma. Cancer Discov. 2019;9(4):459. doi:10.1158/2159-8290.CD-NB2019-014. [DOI] [PubMed] [Google Scholar]
- 61.Cottini F, Hideshima T, Suzuki R, et al. Synthetic lethal approaches exploiting DNA damage in aggressive myeloma. Cancer Discov. 2015;5(9):972-987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shammas MA, Shmookler Reis RJ, Koley H, Batchu RB, Li C, Munshi NC. Dysfunctional homologous recombination mediates genomic instability and progression in myeloma. Blood. 2009;113(10):2290-2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cottini F, Hideshima T, Xu C, et al. Rescue of Hippo coactivator YAP1 triggers DNA damage-induced apoptosis in hematological cancers. Nat Med. 2014;20(6):599-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Amodio N, D'Aquila P, Passarino G, Tassone P, Bellizzi D. Epigenetic modifications in multiple myeloma: recent advances on the role of DNA and histone methylation. Expert Opin Ther Targets. 2017;21(1):91-101. [DOI] [PubMed] [Google Scholar]
- 65.Ohguchi H, Hideshima T, Anderson KC. The biological significance of histone modifiers in multiple myeloma: clinical applications. Blood Cancer J. 2018;8(9):83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mirabella F, Wu P, Wardell CP, et al. MMSET is the key molecular target in t(4;14) myeloma. Blood Cancer J. 2013;3:e114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gulla A, Hideshima T, Bianchi G, et al. Protein arginine methyltransferase 5 has prognostic relevance and is a druggable target in multiple myeloma. Leukemia. 2018;32(4):996-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.San-Miguel JF, Hungria VT, Yoon SS, et al. Overall survival of patients with relapsed multiple myeloma treated with panobinostat or placebo plus bortezomib and dexamethasone (the PANORAMA 1 trial): a randomised, placebo-controlled, phase 3 trial. Lancet Haematol. 2016;3(11):e506-e515. [DOI] [PubMed] [Google Scholar]
- 69.Hideshima T, Qi J, Paranal RM, et al. Discovery of selective small-molecule HDAC6 inhibitor for overcoming proteasome inhibitor resistance in multiple myeloma. Proc Natl Acad Sci U S A. 2016;113(46):13162-13167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vogl DT, Raje N, Jagannath S, et al. Ricolinostat, the first selective histone deacetylase 6 inhibitor, in combination with bortezomib and dexamethasone for relapsed or refractory multiple myeloma. Clin Cancer Res. 2017;23(13):3307-3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281-297. [DOI] [PubMed] [Google Scholar]
- 72.Leone E, Morelli E, Di Martino MT, et al. Targeting miR-21 inhibits in vitro and in vivo multiple myeloma cell growth. Clin Cancer Res. 2013;19(8):2096-2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Morelli E, Biamonte L, Federico C, et al. Therapeutic vulnerability of multiple myeloma to MIR17PTi, a first-in-class inhibitor of pri-miR-17-92. Blood. 2018;132(10):1050-1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Amodio N, Stamato MA, Gulla AM, et al. Therapeutic targeting of miR-29b/HDAC4 epigenetic loop in multiple myeloma. Mol Cancer Ther. 2016;15(6):1364-1375. [DOI] [PubMed] [Google Scholar]
- 75.Di Martino MT, Leone E, Amodio N, et al. Synthetic miR-34a mimics as a novel therapeutic agent for multiple myeloma: in vitro and in vivo evidence. Clin Cancer Res. 2012;18(22):6260-6270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Morelli E, Leone E, Cantafio ME, et al. Selective targeting of IRF4 by synthetic microRNA-125b-5p mimics induces antimultiple myeloma activity in vitro and in vivo. Leukemia. 2015;29(11):2173-2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Caracciolo D, Di Martino MT, Amodio N, et al. miR-22 suppresses DNA ligase III addiction in multiple myeloma. Leukemia. 2019;33(2):487-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gulla A, Di Martino MT, Gallo Cantafio ME, et al. A 13 mer LNA-i-miR-221 inhibitor restores drug sensitivity in melphalan-refractory multiple myeloma cells. Clin Cancer Res. 2016;22(5):1222-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhao JJ, Chu ZB, Hu Y, et al. Targeting the miR-221-222/PUMA/BAK/BAX pathway abrogates dexamethasone resistance in multiple myeloma. Cancer Res. 2015;75(20): 4384-4397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Botta C, Cuce M, Pitari MR, et al. MiR-29b antagonizes the pro-inflammatory tumorpromoting activity of multiple myelomaeducated dendritic cells. Leukemia. 2018;32(4):1003-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pitari MR, Rossi M, Amodio N, et al. Inhibition of miR-21 restores RANKL/OPG ratio in multiple myeloma-derived bone marrow stromal cells and impairs the resorbing activity of mature osteoclasts. Oncotarget. 2015;6(29):27343-27358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hong DS, Kang YK, Borad M, et al. Phase 1 study of MRX34, a liposomal miR-34a mimic, in patients with advanced solid tumours. Br J Cancer. 2020;122(11):1630-1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rupaimoole R, Slack FJ. MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat Rev Drug Discov. 2017;16(3):203-222. [DOI] [PubMed] [Google Scholar]
- 84.Morelli E, Gulla A, Rocca R, et al. The noncoding RNA landscape of plasma cell dyscrasias. Cancers (Basel). 2020;12(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Amodio N, Stamato MA, Juli G, et al. Drugging the lncRNA MALAT1 via LNA gapmeR ASO inhibits gene expression of proteasome subunits and triggers anti-multiple myeloma activity. Leukemia. 2018;32(9): 1948-1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Samur MK, Minvielle S, Gulla A, et al. Long intergenic non-coding RNAs have an independent impact on survival in multiple myeloma. Leukemia. 2018;32(12):2626-2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chesi M, Mirza NN, Garbitt VM, et al. IAP antagonists induce anti-tumor immunity in multiple myeloma. Nat Med. 2016;22(12): 1411-1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fan F, Bashari MH, Morelli E, et al. The AP- 1 transcription factor JunB is essential for multiple myeloma cell proliferation and drug resistance in the bone marrow microenvironment. Leukemia. 2017;31(7): 1570-1581. [DOI] [PubMed] [Google Scholar]
- 89.Dimopoulos M, Spencer A, Attal M, et al. Lenalidomide plus dexamethasone for relapsed or refractory multiple myeloma. N Engl J Med. 2007;357(21):2123-2132. [DOI] [PubMed] [Google Scholar]
- 90.McCarthy PL, Owzar K, Hofmeister CC, et al. Lenalidomide after stem-cell transplantation for multiple myeloma. N Engl J Med. 2012;366(19):1770-1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Benboubker L, Dimopoulos MA, Dispenzieri A, et al. Lenalidomide and dexamethasone in transplant-ineligible patients with myeloma. N Engl J Med. 2014;371(10):906-917. [DOI] [PubMed] [Google Scholar]
- 92.Miguel JS, Weisel K, Moreau P, et al. Pomalidomide plus low-dose dexamethasone versus high-dose dexamethasone alone for patients with relapsed and refractory multiple myeloma (MM-003): a randomised, open-label, phase 3 trial. Lancet Oncol. 2013;14(11):1055-1066. [DOI] [PubMed] [Google Scholar]
- 93.Davies F, Baz R. Lenalidomide mode of action: linking bench and clinical findings. Blood Rev. 2010;24(Suppl 1):S13-S19. [DOI] [PubMed] [Google Scholar]
- 94.Gandhi AK, Kang J, Havens CG, et al. Immunomodulatory agents lenalidomide and pomalidomide co-stimulate T cells by inducing degradation of T cell repressors Ikaros and Aiolos via modulation of the E3 ubiquitin ligase complex CRL4(CRBN). Br J Haematol. 2014;164(6):811-821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hideshima T, Ogiya D, Liu J, et al. Immunomodulatory drugs activate NK cells via both Zap-70 and cereblon-dependent pathways. Leukemia. 2020. Apr 1. doi.org/10.1038/s41375-020-0809-x. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Luptakova K, Rosenblatt J, Glotzbecker B, et al. Lenalidomide enhances anti-myeloma cellular immunity. Cancer Immunol Immunother. 2013;62(1):39-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Facon T, Kumar S, Plesner T, et al. Daratumumab plus lenalidomide and dexamethasone for untreated myeloma. N Engl J Med. 2019;380(22):2104-2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dimopoulos MA, Oriol A, Nahi H, et al. Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375(14):1319-1331. [DOI] [PubMed] [Google Scholar]
- 99.Dimopoulos MA, Dytfeld D, Grosicki S, et al. Elotuzumab plus pomalidomide and dexamethasone for multiple myeloma. N Engl J Med. 2018;379(19):1811-1822. [DOI] [PubMed] [Google Scholar]
- 100.Lonial S, Dimopoulos M, Palumbo A, et al. Elotuzumab therapy for relapsed or refractory multiple myeloma. N Engl J Med. 2015;373(7):621-631. [DOI] [PubMed] [Google Scholar]
- 101.Attal M, Richardson PG, Rajkumar SV, et al. Isatuximab plus pomalidomide and lowdose dexamethasone versus pomalidomide and low-dose dexamethasone in patients with relapsed and refractory multiple myeloma (ICARIA-MM): a randomised, multicentre, open-label, phase 3 study. Lancet. 2019;394(10214):2096-2107. [DOI] [PubMed] [Google Scholar]
- 102.Martin T, Baz R, Benson DM, et al. A phase 1b study of isatuximab plus lenalidomide and dexamethasone for relapsed/refractory multiple myeloma. Blood. 2017;129(25): 3294-3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bjorklund CC, Kang J, Amatangelo M, et al. Iberdomide (CC-220) is a potent cereblon E3 ligase modulator with antitumor and immunostimulatory activities in lenalidomide- and pomalidomide-resistant multiple myeloma cells with dysregulated CRBN. Leukemia. 2020;34(4):1197-1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hansen JD, Correa M, Nagy MA, et al. Discovery of CRBN E3 ligase modulator CC-92480 for the treatment of relapsed and refractory multiple myeloma. J Med Chem. 2020;63(13):6648-6676. [DOI] [PubMed] [Google Scholar]
- 105.Zavidij O, Haradhvala NJ, Mouhieddine TH, et al. Ghobrial single-cell RNA sequencing reveals compromised immune microenvironment in precursor stages of multiple myeloma. Nature Cancer. 2020. Apr 27. doi.org/10.1038/s43018-020-0053-3. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chauhan D, Singh AV, Brahmandam M, et al. Functional interaction of plasmacytoid dendritic cells with multiple myeloma cells: a therapeutic target. Cancer Cell. 2009;16(4): 309-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ray A, Das DS, Song Y, et al. A novel agent SL-401 induces anti-myeloma activity by targeting plasmacytoid dendritic cells, osteoclastogenesis and cancer stem-like cells. Leukemia. 2017;31(12):2652-2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ray A, Tian Z, Das DS, et al. A novel TLR-9 agonist C792 inhibits plasmacytoid dendritic cell-induced myeloma cell growth and enhance cytotoxicity of bortezomib. Leukemia. 2014;28(8):1716-1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ray A Song Y Chauhan D Anderson KC.. Blockade of ubiquitin receptor Rpn13 in plasmacytoid dendritic cells triggers antimyeloma immunity. Blood Cancer J. 2019;9(8):64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Holthof LC, Mutis T. Challenges for immunotherapy in multiple myeloma: bone marrow microenvironment-mediated immune suppression and immune resistance. Cancers (Basel). 2020;12(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Costello C. The future of checkpoint inhibition in multiple myeloma? Lancet Haematol. 2019;6(9):e439-e440. [DOI] [PubMed] [Google Scholar]
- 112.Costa F, Das R, Kini Bailur J, Dhodapkar K, Dhodapkar MV. Checkpoint inhibition in myeloma: opportunities and challenges. Front Immunol. 2018;9:2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wudhikarn K, Wills B, Lesokhin AM. Monoclonal antibodies in multiple myeloma: current and emerging targets and mechanisms of action. Best Pract Res Clin Haematol. 2020;33(1):101143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chari A, Martinez-Lopez J, Mateos MV, et al. Daratumumab plus carfilzomib and dexamethasone in patients with relapsed or refractory multiple myeloma. Blood. 2019;134(5):421-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Spencer A, Lentzsch S, Weisel K, et al. Daratumumab plus bortezomib and dexamethasone versus bortezomib and dexamethasone in relapsed or refractory multiple myeloma: updated analysis of CASTOR. Haematologica. 2018;103(12):2079-2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Voorhees PM, Kaufman JL, Laubach JP, et al. Daratumumab, lenalidomide, bortezomib, and dexamethasone for transplant-eligible newly diagnosed multiple myeloma: GRIFFIN. Blood. 2020. Apr 23. doi: 10.1182/blood.2020005288. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mateos MV, Nahi H, Legiec W, et al. Subcutaneous versus intravenous daratumumab in patients with relapsed or refractory multiple myeloma (COLUMBA): a multicentre, open-label, non-inferiority, randomised, phase 3 trial. Lancet Haematol. 2020;7(5):e370-e380. [DOI] [PubMed] [Google Scholar]
- 118.Cho SF, Anderson KC, Tai YT. Targeting B cell maturation antigen (BCMA) in multiple myeloma: potential uses of BCMAbased immunotherapy. Front Immunol. 2018;9:1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Xing L, Lin L, Yu T, et al. A novel BCMA PBD-ADC with ATM/ATR/WEE1 inhibitors or bortezomib induce synergistic lethality in multiple myeloma. Leukemia. 2020. Feb 14. doi: 10.1038/s41375-020-0745-9. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Trudel S, Lendvai N, Popat R, et al. Targeting B-cell maturation antigen with GSK2857916 antibody-drug conjugate in relapsed or refractory multiple myeloma (BMA117159): a dose escalation and expansion phase 1 trial. Lancet Oncol. 2018;19(12):1641-1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kinneer K, Meekin J, Tiberghien AC, et al. SLC46A3 as a potential predictive biomarker for antibody-drug conjugates bearing noncleavable linked maytansinoid and pyrrolobenzodiazepine warheads. Clin Cancer Res. 2018;24(24):6570-6582. [DOI] [PubMed] [Google Scholar]
- 122.Tai YT, Mayes PA, Acharya C, et al. Novel anti-B-cell maturation antigen antibodydrug conjugate (GSK2857916) selectively induces killing of multiple myeloma. Blood. 2014;123(20):3128-3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tai YT, Acharya C, An G, et al. APRIL and BCMA promote human multiple myeloma growth and immunosuppression in the bone marrow microenvironment. Blood. 2016; 127(25):3225-3236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Frerichs KA, Broekmans MEC, Marin Soto JA, et al. Preclinical activity of JNJ-7957, a novel BCMAxCD3 bispecific antibody for the treatment of multiple myeloma, is potentiated by daratumumab. Clin Cancer Res. 2020;26(9):2203-2215. [DOI] [PubMed] [Google Scholar]
- 125.Seckinger A, Delgado JA, Moser S, et al. Target expression, generation, preclinical activity, and pharmacokinetics of the BCMA-T cell bispecific antibody EM801 for multiple myeloma treatment. Cancer Cell. 2017;31(3):396-410. [DOI] [PubMed] [Google Scholar]
- 126.Hipp S, Tai YT, Blanset D, et al. A novel BCMA/CD3 bispecific T-cell engager for the treatment of multiple myeloma induces selective lysis in vitro and in vivo. Leukemia. 2017;31(8):1743-1751. [DOI] [PubMed] [Google Scholar]
- 127.Noonan KA, Huff CA, Davis J, et al. Adoptive transfer of activated marrow-infiltrating lymphocytes induces measurable antitumor immunity in the bone marrow in multiple myeloma. Sci Transl Med. 2015;7(288):288ra78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rodriguez-Otero P, Paiva B, Engelhardt M, Prosper F, San Miguel JF. Is immunotherapy here to stay in multiple myeloma? Haematologica. 2017;102(3):423-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.D'Agostino M, Raje N. Anti-BCMA CAR Tcell therapy in multiple myeloma: can we do better? Leukemia. 2020;34(1):21-34. [DOI] [PubMed] [Google Scholar]
- 130.Yan Z, Cao J, Cheng H, et al. A combination of humanised anti-CD19 and anti-BCMA CAR T cells in patients with relapsed or refractory multiple myeloma: a single-arm, phase 2 trial. Lancet Haematol. 2019;6(10):e521-e529. [DOI] [PubMed] [Google Scholar]
- 131.Raje N, Berdeja J, Lin Y, et al. Anti-BCMA CAR T-cell therapy bb2121 in relapsed or refractory multiple myeloma. N Engl J Med. 2019;380(18):1726-1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Xu J, Chen LJ, Yang SS, et al. Exploratory trial of a biepitopic CAR T-targeting B cell maturation antigen in relapsed/refractory multiple myeloma. Proc Natl Acad Sci U S A. 2019;116(19):9543-9551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bae J, Smith R, Daley J, et al. Myeloma-specific multiple peptides able to generate cytotoxic T lymphocytes: a potential therapeutic application in multiple myeloma and other plasma cell disorders. Clin Cancer Res. 2012;18(17):4850-4860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bae J, Prabhala R, Voskertchian A, et al. A multiepitope of XBP1, CD138 and CS1 peptides induces myeloma-specific cytotoxic T lymphocytes in T cells of smoldering myeloma patients. Leukemia. 2015; 29(1):218-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bae J, Samur M, Richardson P, Munshi NC, Anderson KC. Selective targeting of multiple myeloma by B cell maturation antigen (BCMA)-specific central memory CD8(+) cytotoxic T lymphocytes: immunotherapeutic application in vaccination and adoptive immunotherapy. Leukemia. 2019; 33(9):2208-2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Rosenblatt J, Avivi I, Vasir B, et al. Vaccination with dendritic cell/tumor fusions following autologous stem cell transplant induces immunologic and clinical responses in multiple myeloma patients. Clin Cancer Res. 2013;19(13):3640-3648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chari A, Vogl DT, Gavriatopoulou M, et al. Oral selinexor-dexamethasone for tripleclass refractory multiple myeloma. N Engl J Med. 2019;381(8):727-738. [DOI] [PubMed] [Google Scholar]
- 138.Tassone P, Neri P, Carrasco DR, et al. A clinically relevant SCID-hu in vivo model of human multiple myeloma. Blood. 2005;106(2):713-716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Calimeri T, Battista E, Conforti F, et al. A unique three-dimensional SCID-polymeric scaffold (SCID-synth-hu) model for in vivo expansion of human primary multiple myeloma cells. Leukemia. 2011;25(4):707-711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Radl J, Punt YA, van den Enden-Vieveen MH, et al. The 5T mouse multiple myeloma model: absence of c-myc oncogene rearrangement in early transplant generations. Br J Cancer. 1990;61(2):276-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Calcinotto A, Brevi A, Chesi M, et al. Microbiota-driven interleukin-17-producing cells and eosinophils synergize to accelerate multiple myeloma progression. Nat Commun. 2018;9(1):4832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Peled JU, Devlin SM, Staffas A, et al. Intestinal microbiota and relapse after hematopoietic-cell transplantation. J Clin Oncol. 2017;35(15):1650-1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Pianko MJ, Devlin SM, Littmann ER, et al. Minimal residual disease negativity in multiple myeloma is associated with intestinal microbiota composition. Blood Adv. 2019;3(13):2040-2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Touzeau C, Maciag P, Amiot M, Moreau P. Targeting Bcl-2 for the treatment of multiple myeloma. Leukemia. 2018;32(9):1899-1907. [DOI] [PubMed] [Google Scholar]
- 145.Kumar S, Kaufman JL, Gasparetto C, et al. Efficacy of venetoclax as targeted therapy for relapsed/refractory t(11;14) multiple myeloma. Blood. 2017;130(22):2401-2409. [DOI] [PubMed] [Google Scholar]
- 146.Moreau P, Chanan-Khan A, Roberts AW, et al. Promising efficacy and acceptable safety of venetoclax plus bortezomib and dexamethasone in relapsed/refractory MM. Blood. 2017;130(22):2392-2400. [DOI] [PubMed] [Google Scholar]
- 147.Lagana A, Beno I, Melnekoff D, et al. Precision medicine for relapsed multiple myeloma on the basis of an integrative multiomics approach. JCO Precis Oncol. 2018;2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Moreau P, Attal M, Hulin C, et al. Bortezomib, thalidomide, and dexamethasone with or without daratumumab before and after autologous stem-cell transplantation for newly diagnosed multiple myeloma (CASSIOPEIA): a randomised, open-label, phase 3 study. Lancet. 2019;394(10192):29-38. [DOI] [PubMed] [Google Scholar]
- 149.Richardson PG, Bringhen S, Voorhees P, et al. Melflufen plus dexamethasone in relapsed and refractory multiple myeloma (O-12-M1): a multicentre, international, open-label, phase 1-2 study. Lancet Haematol. 2020;7(5):e395-e407. [DOI] [PubMed] [Google Scholar]
- 150.Schjesvold F, Robak P, Pour L, Aschan J, Sonneveld P. OCEAN: a randomized phase III study of melflufen + dexamethasone to treat relapsed refractory multiple myeloma. Future Oncol. 2020;16(11):631-641. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.