Abstract
Patient-centred care by a coordinated primary care team may be more effective than standard care in chronic disease management. We synthesised evidence to determine whether patient-centred medical home (PCMH)-based care models are more effective than standard general practitioner (GP) care in improving biomedical, hospital, and economic outcomes. MEDLINE, CINAHL, Embase, Cochrane Library, and Scopus were searched to identify randomised (RCTs) and non-randomised controlled trials that evaluated two or more principles of PCMH among primary care patients with chronic diseases. Study selection, data extraction, quality assessment using Joanna Briggs Institute (JBI) appraisal tools, and grading of evidence using Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach were conducted independently. A quantitative synthesis, where possible, was pooled using random effects models and the effect size estimates of standardised mean differences (SMDs) and odds ratios (ORs) with 95% confidence intervals were reported. Of the 13,820 citations, we identified 78 eligible RCTs and 7 quasi trials which included 60,617 patients. The findings suggested that PCMH-based care was associated with significant improvements in depression episodes (SMD −0.24; 95% CI −0.35, −0.14; I2 = 76%) and increased odds of remission (OR 1.79; 95% CI 1.46, 2.21; I2 = 0%). There were significant improvements in the health-related quality of life (SMD 0.10; 95% CI 0.04, 0.15; I2 = 51%), self-management outcomes (SMD 0.24; 95% CI 0.03, 0.44; I2 = 83%), and hospital admissions (OR 0.83; 95% CI 0.70, 0.98; I2 = 0%). In terms of biomedical outcomes, with exception to total cholesterol, PCMH-based care led to significant improvements in blood pressure, glycated haemoglobin, and low-density lipoprotein cholesterol outcomes. The incremental cost of PCMH care was identified to be small and significantly higher than standard care (SMD 0.17; 95% CI 0.08, 0.26; I2 = 82%). The quality of individual studies ranged from “fair” to “good” by meeting at least 60% of items on the quality appraisal checklist. Additionally, moderate to high heterogeneity across studies in outcomes resulted in downgrading the included studies as moderate or low grade of evidence. PCMH-based care has been found to be superior to standard GP care in chronic disease management. Results of the review have important implications that may inform patient, practice, and policy-level changes.
Keywords: patient-centred medical home, enhanced primary care, chronic disease management, collaborative care, meta-analysis
1. Introduction
Chronic diseases have contributed to increased mortality and morbidity worldwide with the disease burden accelerating across both developed and developing nations [1,2]. The Global Burden of Diseases (GBD) Study in 2017 reported that chronic diseases accounted for 41% of increased disability and 73% of all deaths [1,2]. Moreover, with increasing life expectancy and ageing population, the global prevalence of multiple chronic conditions or multimorbidity is also on the rise, further exacerbating complications in quality and delivery of care [3,4]. As a result, patients with one or more chronic diseases often experience poor mental and physical functioning with increased psychological distress affecting their overall health-related quality of life (HRQoL) [5,6]. In addition to negative health outcomes, chronic diseases also contribute to significant economic ramifications to both patients and health care system in the form of increased health care utilisation and costs of care [7,8].
The long-term nature of chronic diseases and complexities of care require health care systems, worldwide, to revisit guidelines on effective chronic disease management [7]. The health and economic repercussions of chronic diseases are partly connected to the fragmented design and delivery of health care systems to focus on “single disease framework” as opposed to a “whole-person approach” [9]. However, there has been an increasing advocacy towards shift from a reactive health care system to one that is proactive, enabling an integrated systems approach towards chronic disease management [10]. In view of this, the World Health Organisation (WHO) and other leading organisations have acknowledged the importance of primary care as an ideal setting to facilitate patient-centred care, which could result in better patient outcomes [11,12]. There is a large body of evidence suggesting that coordinated team-based approaches in primary care are effective in chronic disease management [13,14].
The patient-centred medical home (PCMH) model is one of the chronic care models (CCM) that has reportedly shown to provide a multidimensional solution to effectively managing chronic illness and multimorbidity in primary care [15]. This enhanced primary care model typically consists of a general practitioner (GP)-led care, as part of a multidisciplinary team (MDT) that aims to provide patient-centred care that is also comprehensive and coordinated, with emphasis on self-management and patient education [12]. There is a growing body of literature, particularly in United States and several parts of United Kingdom and other European countries, reporting the effectiveness of PCMH care models in improving biomedical [16,17], HRQoL [18,19], hospital [20,21], and economic outcomes [22] compared to standard GP care.
A comprehensive systematic review and meta-analysis of PCMH care published in 2013 [23] reported improvements in patient experiences and some reduction in health utilisation among patients with multimorbidity. However, the effect of PCMH models on patients with single-disease care management was not reviewed. Whilst the review focuses on clinical quality and processes of care, there was insufficient evidence to estimate biomedical outcomes and quality of life. In addition, the review also included patients from non-primary care settings such as tertiary care hospitals, thereby limiting understanding of the true effectiveness of PCMH model in primary care settings. The current review was warranted as there has been increased advocacy for PCMH-based care models resulting in a number of new studies evaluating PCMH models being published since 2013 [18,19,20,21].
A systematic review and meta-analysis was conducted to assess the effectiveness of PCMH-based models of care when compared to standard GP care in improving biomedical, hospital, and economic outcomes of primary care patients with one or more chronic diseases. The findings of this review may help inform guidelines and practices.
2. Methods
This review conformed to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [24]. The systematic review protocol (CRD42018085378), registered in the International Prospective Register of Systematic Reviews (PROSPERO) database, has been published elsewhere [25].
2.1. Search Strategy
We conducted literature searches on electronic databases including MEDLINE, CINAHL, Embase, Cochrane library, and Scopus from inception until 31 March 2020. The search strategy and syntaxes were developed in collaboration with an experienced university librarian. The syntax explored a broad range of terms used in definitions of PCMH, collaborative care, chronic care models, RCTs, and Quasi trials (full electronic search strings are listed in Table A1). We supplemented electronic searches by hand-searching bibliographies of several key systematic reviews [23,26,27,28] and retrieved studies to identify any relevant articles missed by the search strategy. Endnote (Version X9, Thompson Reuters, New York, NY, USA) software was used for reference management.
2.2. Eligibility Criteria and Study Selection
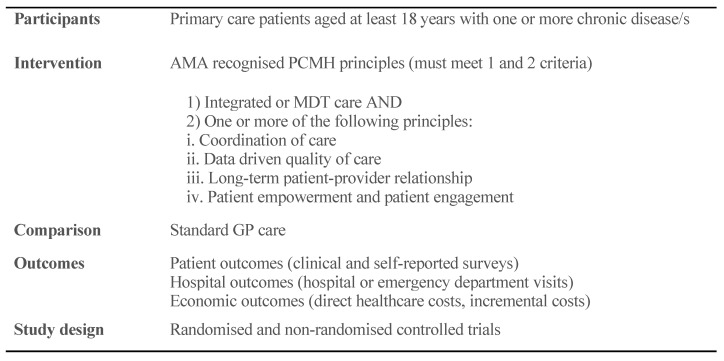
A detailed inclusion and exclusion criteria along with explanation of core PCMH principles is reported elsewhere [25]. A summary of Population, Interventions, Comparators, Outcomes, and Study designs (PICOS) framework is presented in Figure 1. Two reviewers (JRJ and KP) independently screened the titles and abstracts of all articles for eligibility. Following the title and abstract screening, a full text screening was conducted on articles which passed the title and abstract screening by two reviewers (JRJ and HJ) independently. Discrepancies were resolved and clarified through discussion.
Figure 1.
Summary of Population, Interventions, Comparators, Outcomes, and Study designs (PICOS) components. Outcomes included but not limited to patient, hospital, and economic outcomes.
2.3. Data Extraction
Data extraction of included articles was carried out independently by two reviewers (JRJ and HJ) using Excel spreadsheet (Microsoft Excel, Microsoft Corporation). Data extracted from included articles included key characteristics: first author and publication year; country of origin; sample size, age, and gender distribution; chronic disease profile; baseline characteristics reported as mean (SD) or proportions; PCMH components implemented; duration of follow-up; and outcomes. Whilst data extraction was performed using a customised spreadsheet, the Centre for Reviews and Dissemination’s (CRD) guidance for undertaking reviews in health care was followed [29]. Authors of studies with missing data were contacted by email up to two times; however, no response was received.
2.4. Quality Assessment and Risk of Bias
Two reviewers (JRJ and HJ) independently evaluated the methodological validity of included articles using relevant Joanna Briggs Institute (JBI) critical appraisal checklists (RCTs, quasi trials, and economic evaluations) [30,31]. Quality of studies were rated as good (≥8), fair (6–7), or poor (≤5) based on the summary scores. We also used risk of bias in non-randomised studies of interventions (ROBINS-I) tool to supplement JBI appraisal for non-randomised trials [32]. Additionally, the quality of evidence across included studies reporting similar outcomes was determined by applying the Grading of Recommendations Assessment, Development and Evaluation (GRADE) criteria [33]. The overall GRADE quality of evidence from the tables takes into account three factors which include (i) the average quality across the studies for each particular outcome, (ii) the level of heterogeneity between the studies, and (iii) the total number of studies reporting a particular outcome.
2.5. Outcomes
Outcomes identified from the studies include changes in mean differences or proportion of patients achieving recommended levels in
-
(1).
Biomedical outcomes—blood pressure (BP); glycated haemoglobin (HbA1c); low density lipoprotein cholesterol (LDL-C); high density lipoprotein cholesterol (HDL-C); and serum total cholesterol.
-
(2).
Self-reported health assessments (using validated questionnaires)—depression; HRQoL (overall, mental and physical functioning components); and self-management.
-
(3).
Health utilisation outcomes—hospital admissions; emergency department visits; and medications use.
-
(4).
Economic outcomes—incremental cost-effectiveness ratio (ICER) which is defined as the difference in total cost of an intervention (compared to standard care) divided by the difference in health outcome measure [22].
2.6. Data Analysis
Data of included studies were pooled together using the inverse-variance method of random-effects meta-analysis [34]. Standardised mean differences (SMD) for continuous data and odds ratios (ORs) for dichotomous data, with 95% confidence intervals (CI), were calculated and graphically presented as forest plots. Statistical heterogeneity was calculated using I2 and Cochran’s Q statistics. Subgroup analyses were considered for outcomes with substantial heterogeneity (I2 ≥ 85%). Publication bias for outcomes with at least 6 studies was assessed using funnel plots and Egger’s test of asymmetry [35]. All analyses were conducted using RevMan version 5.3 (The Nordic Cochrane Centre, Copenhagen, Denmark) and R version 4.0 software (R Foundation for Statistical Computing, Vienna, Austria).
3. Results
3.1. Literature Search
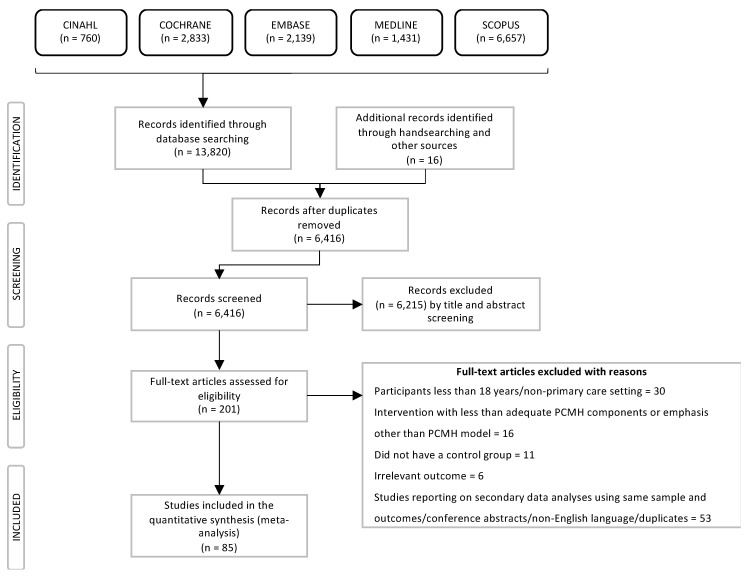
The electronic database search resulted in 13,820 citations and an additional 16 citations from hand searching key systematic reviews. After exclusion of duplicate records, 6416 articles were screened by titles and abstracts with 201 articles determined to be eligible for full-text assessment. Of these, 85 studies met the eligibility criteria and were included in our systematic review. Flowchart of the selection process from initial identification to inclusion is shown in Figure 2. Main reasons for exclusion included patients treated in non-primary care settings, not meeting minimum PCMH components or focused on intervention other than PCMH model, lack of control group, and other reasons (list of excluded articles; see Table A2).
Figure 2.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Flowchart.
3.2. Descriptive Data Synthesis
The characteristics of included studies are presented in the Appendix A Table A3 and Table A4. Of the 85 studies included in the review, 78 studies were RCTs [13,14,16,18,19,20,22,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106] and 7 studies were of non-RCTs, including quasi trials [17,21,107,108] or cohort studies with a control group [109,110,111]. The 85 studies enrolled a total of 60,617 patients with sample sizes ranging from 40 to 8366. Whilst 79 studies had sufficient data for quantitative data synthesis, 6 studies [81,85,95,97,103,107] did not have usable data and therefore, the findings were narratively summarised.
The common inclusion criteria for all 85 studies was primary care patients with diagnosis of one or more chronic conditions, whereas the predominant reason for exclusion was patients with cognitive impairment and terminal illness. In terms of the chronic disease profile of the participants in the included articles, 46% of articles were based on participants with single chronic condition whereas 54% reported on one or more conditions. The most prevalent conditions were mental illness (59%), type 2 diabetes (33%), cardiovascular diseases (CVD) including hypertension (20%), musculoskeletal disorders (6%), and chronic obstructive pulmonary disease (COPD) (6%) (Table A3 and Table A4).
More than half the studies (52%) were conducted in the United States. The mean age of patients ranged between 30 and 83 years. In terms of gender distribution, most of the studies had slightly more women than men, except for studies conducted in Veterans Affairs (VA) primary care settings [16,50,52,53,56]. The duration of follow-up varied from 3 to 48 months. Out of 85 articles included for review, in addition to MDT care, 95% of studies reported coordinated care, patient engagement and education, and self-management; 20% reported continuity of care and long-term patient provider relationship; and only 9% of studies included data driven quality of care (Table A3 and Table A4).
3.3. Quality Assessment and Risk of Bias
Quality assessment and risk of bias for individual studies are reported in the Appendix A Table A5, Table A6, Table A7, Table A8. The overall quality of studies ranged from “fair” to “good” by meeting at least 60% of items on the checklist. Two studies [62,104] were rated as poor due to general lack of information on randomisation, unclear methodology, and clarity of results. Given the nature of PCMH-based intervention, most trials employed a cluster randomisation method where a group of patients were seen by the same GP or same general practice providing PCMH care. Thereby, blinding of patients or GPs was not applicable and, as a result, items related to blinding were not necessarily graded down. However, only 32 studies reported blinding of outcome assessment whilst other studies were graded down in quality. The quality of evidence across included studies assessed using GRADE approach is presented in Table 1.
Table 1.
Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) assessment of randomised controlled trials reporting effectiveness of patient-centred medical home (PCMH) vs. standard general practitioner (GP) care on outcomes of interest.
| Outcomes | No of Studies | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | GRADE Quality of Evidence þ |
|---|---|---|---|---|---|---|---|
| Depression | 31 | Serious | Serious | Not serious | Not serious | Undetected | Moderate ‡ |
| Quality of Life | 21 | Serious | Not serious | Not serious | Not serious | Undetected | Moderate ‡ |
| Blood pressure | 13 | Serious | Not serious | Not serious | Not serious | Undetected | Moderate ‡ |
| Glycated Hemoglobin | 9 | Serious | Serious | Not serious | Not serious | Undetected | Low ঠ|
| LDL Cholesterol | 4 | Serious | Serious | Not serious | Not serious | Undetected | Low ঠ|
| HDL Cholesterol | 1 | Serious | - | Not serious | Not serious | Undetected | Low †‡^ |
| Total Cholesterol | 2 | Serious | - | Not serious | Not serious | Undetected | Low ‡^ |
| Hospital admissions | 5 | Serious | Not serious | Not serious | Not serious | Undetected | Moderate ‡ |
| Self-management (PACIC scores) | 3 | Serious | Serious | Not serious | Not serious | Undetected | Low ঠ|
| Cost-effectiveness | 19 | Serious | Serious | Not serious | Not serious | Undetected | Low ঠ|
þ High quality: Further research is very unlikely to change our confidence in the estimate of effect; Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate; Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate; Very low quality: We are very uncertain about the estimate; LDL—Low Density Lipoprotein; HDL—High Density Lipoprotein; PACIC—Patient Assessment of Care for Chronic Conditions; ‡ Most studies did not blind participants or personnel as it was not practical. Therefore, we did not downgrade for these risks/uncertainties. However, studies not reporting blinding of outcome assessment were downgraded in quality; ¶ Significant level of heterogeneity within results (I2 between 80–90%); ^ Single study—Inconsistency not applicable; † Because of the nature of the quasi-experimental designs risk of bias is unavoidable.
3.4. Depression Outcomes
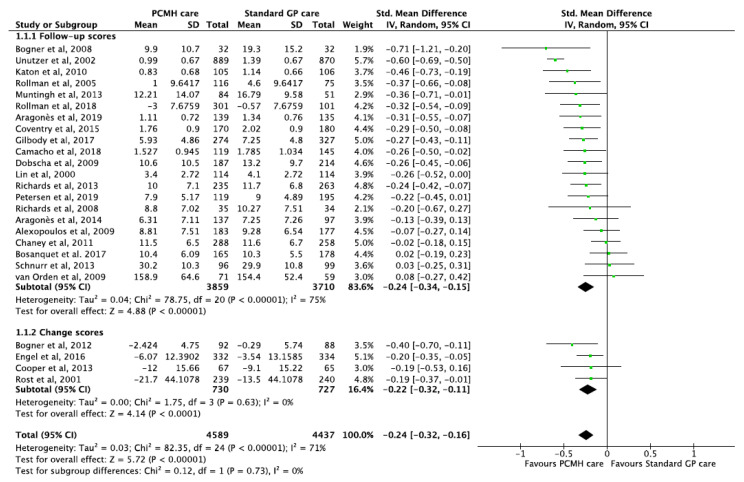
Meta-analysis of thirty-one studies [13,14,18,19,36,38,40,42,43,46,50,51,53,55,57,63,67,68,70,76,78,83,84,86,87,88,91,93,100,102,109] of patients with minor or major depression episodes after PCMH-based care reported significant improvement in depression scores compared to patients with standard primary care. With the exceptions of three studies [46,91,102], twenty-two studies reporting changes in mean differences (continuous data) of depression scores showed significant reduction with a pooled SMD of −0.24 (95% CI −0.35, −0.14; p-value < 0.001) (Figure 3).
Figure 3.
Forest plots of depression outcomes between the PCMH care and Standard GP care.
Six studies reported that PCMH care was associated with significantly increased odds of remission of depression with pooled OR 1.79 (95% CI 1.46, 2.21; p-value < 0.001) (Figure 3). Additionally, one other study [85] reported significant improvements among patients with anxiety and mood disorders with an effect size of 0.30 (95% CI 0.05, 0.55; p-value = 0.02) compared to standard care. Given most studies consistently reported improvements, the GRADE of evidence was classified as moderate quality (Table 1).
3.5. Quality of Life Outcomes
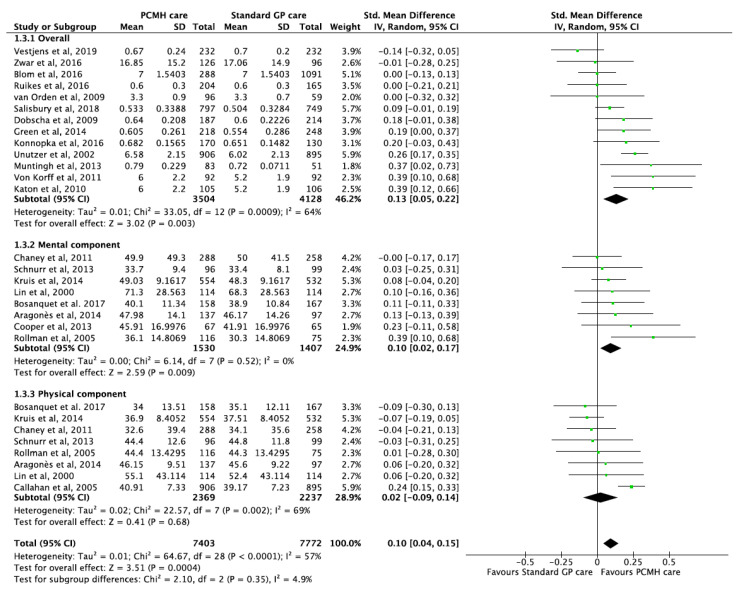
Twenty-two studies [18,19,21,22,41,46,49,50,51,53,59,68,72,76,86,89,91,100,102,105,106,108] evaluated the effectiveness of PCMH-based care on HRQoL (overall, physical component and mental component). Patients enrolled in PMCH-based care reported small but significant improvements in HRQoL compared to standard care with a pooled SMD of 0.10 (95% CI 0.04, 0.15; p-value < 0.001) (Figure 4). Additionally, one other study [85] reported significant improvements with an effect size of 0.38 (95 % CI 0.13, 0.63; p-value = 0.003). Moderate heterogeneity was observed among included studies (I2 = 57%), but test for sub-group differences were not significant. The GRADE of evidence was classified as moderate quality (Table 1).
Figure 4.
Forest plots of Quality of life (QoL) outcomes between the PCMH care and Standard GP care.
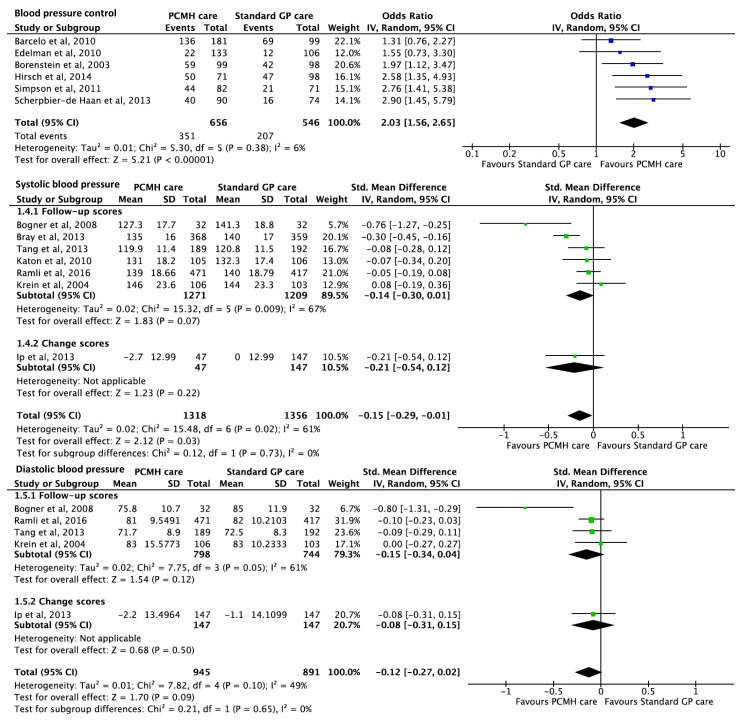
3.6. Blood Pressure Outcomes
Thirteen studies [16,17,39,42,45,61,64,68,71,82,90,94,96] reported on the effect of PCMH care on blood pressure outcomes. Six studies reported that PCMH care was associated with significantly increased odds of BP control with pooled OR 2.03 (95% CI 1.56, 2.65; p-value < 0.001) (Figure 5). Seven studies reported significant improvements in systolic blood pressure (SBP), in favour of PCMH care, with pooled estimates of SMD −0.15 (95% CI −0.29, −0.01; p-value = 0.03). Similar reduction was observed across five studies reporting on diastolic blood pressure (DBP), but the pooled estimate of SMD −0.12 (95% CI −0.27, 0.02; p-value = 0.09) failed to meet significance (Figure 5). The GRADE of evidence was classified as moderate quality (Table 1).
Figure 5.
Forest plots of blood pressure outcomes between the PCMH care and Standard GP care. BP control refers to blood pressure levels within the guideline’s recommended range.
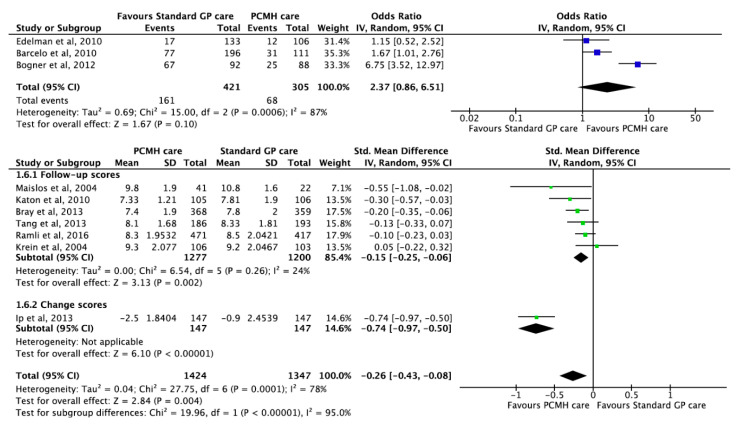
3.7. Glycated Haemoglobin Outcomes
Ten studies [16,17,39,43,64,68,71,77,82,96] reported on the effect of PCMH care on HbA1c outcomes. HbA1c levels were recorded among patients with a positive diagnosis of Type 2 diabetes. Three studies reported that PCMH care was associated with increased odds of glycaemic control with pooled OR 2.37 (95% CI 0.86, 6.51; p-value = 0.100). However, the pooled estimate was not statistically significant (Figure 6). The substantial heterogeneity of 87% in the three studies reporting ORs was due to a shorter follow-up duration of three months reported by Bogner et al. [43] compared to the other two studies which had follow-up duration of 12 to 13 months. Seven studies reported significant improvements in HbA1c, in favour of PCMH care with pooled estimates of SMD −0.26 (95% CI −0.43, −0.08; p-value = 0.004) (Figure 6). Given the substantial amount of heterogeneity, the GRADE of evidence was classified as low quality (Table 1).
Figure 6.
Forest plots of HbA1c outcomes between the PCMH care and Standard GP care. HbA1c control refers to HbA1c levels within the guideline’s recommended range.
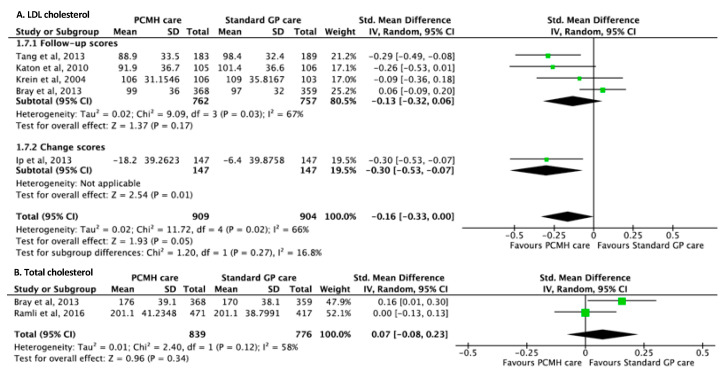
3.8. Cholesterol Outcomes
For LDL-cholesterol outcomes, five studies [17,64,68,71,96] reported significant improvements in favour of PCMH care with pooled SMD of −0.16 (95% CI −0.33, −0.00; p-value = 0.05) compared to standard GP care. Test for subgroup difference between follow-up and change scores showed no statistical significance (I2 = 16.8%, p-value = 0.27) (Figure 7A). For total cholesterol outcomes, two studies [17,82] reported a non-significant increase in total cholesterol with a pooled SMD of 0.07 (95% CI −0.08, 0.23; p-value = 0.34) (Figure 7B). The GRADE of evidence of both LDL and total cholesterol outcomes were classified as low quality given the limited number of studies (Table 1).
Figure 7.
Forest plots of (A) LDLcholesterol and (B) Total cholesterol outcomes between the PCMH care and Standard GP care.
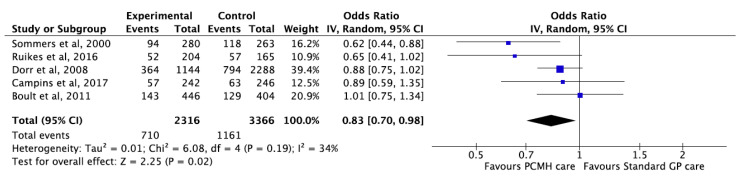
3.9. Hospital Admissions
Five studies [20,21,48,54,111] reported that PCMH care was associated with significant reduction in hospital admissions compared to standard care with pooled OR 0.83 (95% CI 0.70, 0.98; p-value = 0.02) (Figure 8). Additionally, one study [110] reported a reduction in mean hospital admission rates related to diabetic complications 12 months after PCMH based care compared to standard care. Nonetheless, the change in mean difference failed to meet statistical significance. The GRADE of evidence was classified as moderate quality (Table 1).
Figure 8.
Forest plot for hospital admissions between PMCH care and Standard GP care.
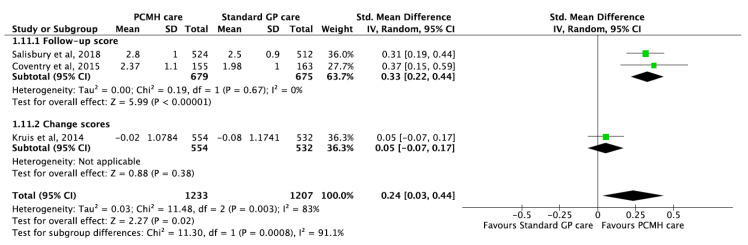
3.10. Self-Management Outcomes
Three studies [14,72,89] reported significant improvements in self-management scores in favour of PCMH care compared to standard care with pooled estimates of SMD 0.24 (95% CI 0.03, 0.44; p-value < 0.001) (Figure 9). Given the substantial amount of heterogeneity (I2 = 83%), the GRADE of evidence was classified as low quality (Table 1).
Figure 9.
Forest plots of self-management outcomes (Patient Assessment of Care for Chronic Conditions (PACIC) scores) between the PCMH care and Standard GP care.
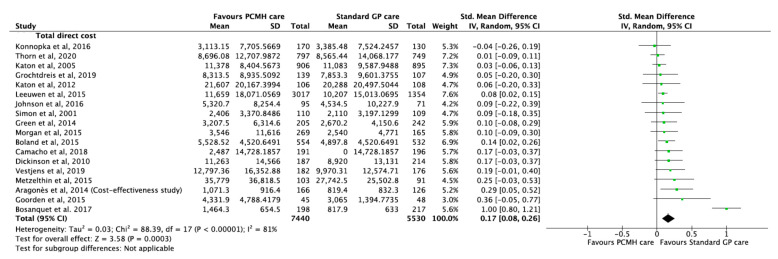
3.11. Economic Outcomes
A total of 18 studies [13,22,37,44,46,52,58,59,60,65,66,69,73,79,80,92,98,108] reported cost-effectiveness of PCMH-based models of care compared to standard care. To avoid bias in analysis, all currencies were converted to US Dollars at the time of the respective trials and cost effectiveness was measured in terms of incremental cost of intervention. The incremental cost of PCMH care was small but significantly higher than standard care with a pooled estimate of 0.17 (95% CI 0.08, 0.26; p-value < 0.001) (Figure 10). The substantial heterogeneity of 81% was due to higher costs of intervention reported by Bosanquet et al. [46]. The GRADE of evidence was classified as low quality (Table 1).
Figure 10.
Forest plots of incremental cost of intervention between the PCMH care and Standard GP care.
A summary of results from meta-analyses (where possible) and individual studies from randomised and non-randomised controlled trials are presented in Table 2.
Table 2.
Summary of findings from meta-analyses (where possible) or individual studies from randomised and non-randomised controlled trials.
| Outcome | No of Studies | No of Participants | Effect Size (95% CI) | p-Value | Q Statistic | I2 | Egger’s Test p-Value ‡ |
Citations | Figure |
|---|---|---|---|---|---|---|---|---|---|
| Randomised controlled trials | |||||||||
| Depression | 24 6 |
7255 1520 |
SMD −0.24 (−0.35, −0.14) OR 1.79 (1.46, 2.21) |
<0.001 <0.001 |
78.3 3.58 |
76% 0% |
0.275 0.608 |
[13,14,18,19,36,38,40,42,43,46,50,51,53,55,57,63,67,68,70,76,78,83,84,86,87,88,91,93,100,102,109] | Figure 3 |
| Quality of Life | 22 | 12,370 | SMD 0.12 (0.09, 0.15) | <0.001 | 57.38 | 51% | 0.556 | [18,19,21,22,41,46,49,50,51,53,59,68,72,76,86,89,91,100,102,105,106,108] | Figure 4 |
| Blood pressure | |||||||||
| BP control | 6 | 1202 | OR 2.03 (1.56, 2.65) | <0.001 | 5.30 | 6% | 0.347 | [16,39,42,45,61,64,68,71,82,90,94,96] | Figure 5 |
| Systolic BP | 6 | 1947 | SMD −0.08 (−0.17, 0.01) | 0.09 | 8.97 | 44% | 0.737 | ||
| Diastolic BP | 5 | 1836 | SMD −0.12 (−0.27, 0.02) | 0.10 | 7.82 | 49% | 0.260 | ||
| Glycated haemoglobin | [16,39,43,64,68,71,77,82,96] | Figure 6 | |||||||
| Glycaemic control | 3 | 726 | OR 2.37 (0.86, 6.51) | 0.001 | 15.00 | 87% | NA | ||
| HbA1c | 6 | 2044 | SMD −0.21 (−0.30, −0.12) | <0.001 | 27.75 | 82% | 0.405 | ||
| LDL Cholesterol | 4 | 1086 | SMD −0.25 (−0.37, −0.13) | <0.001 | 1.64 | 0% | NA | [64,68,71,96] | Figure 7A |
| Total Cholesterol | 1 | 888 | SMD 0.00 (−0.13, 0.13) | 1.00 | NA | NA | NA | [82] | Figure 7B |
| Hospital admissions | 3 | 4770 | OR 0.90 (0.80, 1.03) | 0.12 | 0.67 | 0% | NA | [20,48,54] | Figure 8 |
| Self-management (PACIC scores) | 3 | 2440 | SMD 0.24 (0.03, 0.44) | 0.02 | 11.48 | 83% | NA | [14,72,89] | Figure 9 |
| Cost-effectiveness | 17 | 12,612 | SMD 0.17 (0.07, 0.26) | 0.001 | 87.84 | 82% | 0.206 | [13,22,37,44,46,52,58,59,60,65,66,69,73,79,80,92,98] | Figure 10 |
| Non-randomised trials | |||||||||
| Depression | 1 | 314 | SMD −0.22 (−0.45, 0.01) | 0.06 | NA | NA | NA | [109] | Figure 3 |
| Quality of Life | 2 | 833 | SMD −0.08 (−0.21, 0.06) | 0.28 | 0.94 | 0% | NA | [22,108] | Figure 4 |
| Blood pressure | Figure 5 | ||||||||
| Systolic BP | 1 | 727 | SMD −0.30 (−0.45, −0.16) | <0.001 | NA | NA | NA | [17] | |
| Glycated haemoglobin | 1 | 727 | SMD −0.20 (−0.35, −0.06) | 0.006 | NA | NA | NA | [17] | Figure 6 |
| LDL Cholesterol | 1 | 727 | SMD 0.06 (−0.09, 0.20) | 0.43 | NA | NA | NA | [17] | Figure 7 |
| HDL Cholesterol | 1 | 727 | SMD 0.15 (0.00, 0.29) | 0.05 | NA | NA | NA | [17] | - |
| Total Cholesterol | 1 | 727 | SMD 0.16 (0.01, 0.30) | 0.04 | NA | NA | NA | [17] | Figure 8 |
| Hospital admissions | 2 | 912 | OR 0.63 (0.48, 0.83) | 0.001 | 0.02 | 0% | NA | [21,111] | Figure 9 |
| Cost-effectiveness | 1 | 358 | SMD 0.19 (−0.01, 0.40) | 0.07 | NA | NA | NA | [108] | Figure 10 |
NA—not applicable; SMD—Standard Mean Difference; OR—Odds ratio; ‡ Egger’s test was conducted only for outcomes with at least 6 studies. Note: The slight discrepancy in the effect sizes in this table to that reported in the manuscript and figures is because the effects sizes are classified based on their study design. I2 describes the percentage of total variation across studies that is due to heterogeneity rather than chance. A value of 0% indicates no observed heterogeneity, and larger values show increasing heterogeneity.
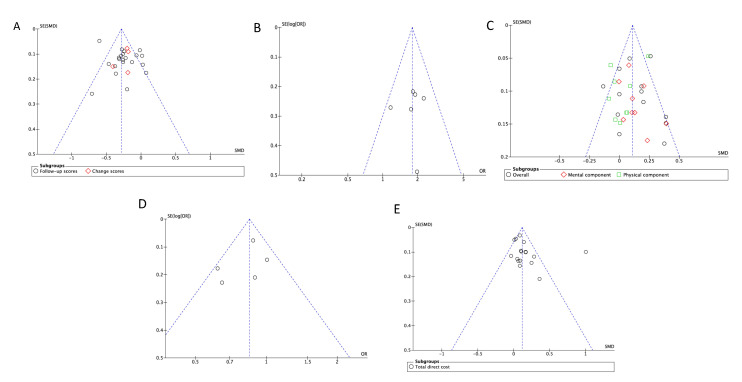
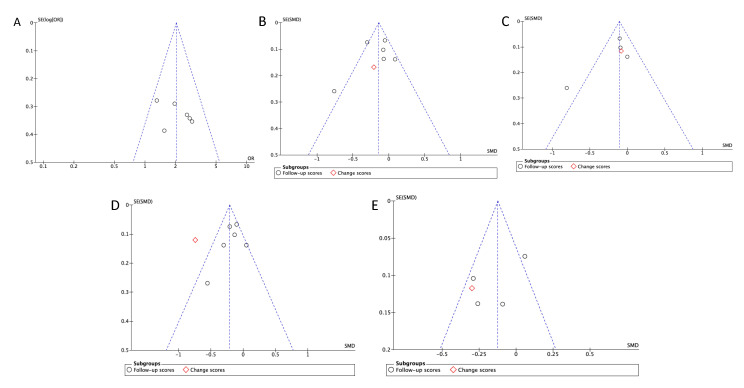
3.12. Publication Bias
Six or more articles with similar outcomes were inspected for publication bias visually by using funnel plots and statistically by determining the significance from Egger’s test of asymmetry. Visual inspection of included studies reporting similar outcomes did not indicate any obvious sign of asymmetry (Figure 11 and Figure 12). Consistent with visual findings, no evidence of publication bias was detected with Egger’s test, as all outcomes had p > 0.05, showing evidence of funnel plot symmetry (Table 2).
Figure 11.
Funnel plots assessing asymmetry of depression, QoL, hospital admissions, and cost outcomes between the PCMH care and Standard GP care. (A)—Depression (SMD); (B)—Depression (OR); (C)—Quality of Life (SMD); (D)—Hospital admissions (OR); (E)—Direct costs.
Figure 12.
Funnel plots assessing asymmetry of biomedical outcomes between the PCMH care and Standard GP care. (A)—Blood pressure (SMD); (B)—Systolic blood pressure (OR); (C)—Diastolic blood pressure (SMD); (D)—HbA1C (OR); (E)—LDL cholesterol.
4. Discussion
4.1. Summary of Findings
This systematic review comprehensively summarised current evidence on the effectiveness of PCMH-based models on chronic disease management among primary care patients. Compared to standard GP care, PCMH-based care led to significant improvements in depression episodes, quality of life, HbA1c, LDL cholesterol, hospital admissions, and self-management outcomes. Whilst PCMH care was significantly associated with increased odds of blood pressure control, reductions in both pooled estimates of SBP and DBP were not statistically significant. In contrast, the findings suggest that PCMH-based interventions have higher costs and was not cost-effective when compared to standard care. Additionally, the narrative synthesis of studies also corroborated with pooled estimates of the meta-analyses.
4.2. Consistency with Other Systematic Reviews
The most commonly reported PCMH principles in the included studies were patient engagement through education and self-management, and care coordination in addition to team-based care. Findings of this review, underscoring these PMCH elements in primary care, are consistent with previous systematic reviews reporting quality of care and overall patient experiences [26,112]. In terms of study outcomes, depression and HRQoL were frequently reported outcomes in the included studies. Systematic reviews focusing on depression outcomes as a result of collaborative care reported similar improvements, which were consistent with our pooled estimates of SMDs and ORs [113,114]. Similarly, our review showed small but significant improvements in the self-reported HRQoL and self-management scores, which is consistent with previous reviews [115,116]. Variabilities in the duration of intervention and baseline severity of chronic illness may explain smaller pooled estimates of HRQoL outcome.
Changes in biomedical outcomes are common measures employed in evaluating the effectiveness of chronic disease management interventions. With the exception of total cholesterol outcomes, findings of our studies were consistent with previous reviews [117,118], showing improvements in biomedical outcomes in favour of PCMH-based care compared to standard care. In terms of cost-effectiveness of PCMH-based models, some meta-analytic reviews on economic evaluations showed that PCMH care was associated with decreases in total costs compared to standard care [119,120]. However, our review supports evidence from prior reviews [115,121], suggesting that PCMH-based care was not associated with improvement in cost outcomes compared to standard care. This discordance could be due to the variability in the initial and sustained amount of costs incurred as a result of additional staffing and other infrastructure as well as the sample of patients and their comorbidity profile in the included trials [121].
4.3. Strengths and Limitations
Quality assessment for risk of bias was assessed within and across studies of similar outcomes. As aforementioned, blinding of patients and GPs was not possible due to the nature of intervention and design of trials, as reported in other systematic reviews conducted in primary care settings [114,122]. A substantial amount of heterogeneity was observed for measures of depression, HbA1c, and incremental cost of intervention, justifying the choice of random effects model. Higher heterogeneity is expected when pooling results of complex interventions, given the varying levels of intensity of different interventions, follow-up times, chronic disease profile of participants, and country’s primary care setting [115]. Nonetheless, pooled estimates are to be interpreted with caution given unexplained variation observed in outcomes with higher heterogeneity. The review did not consider unpublished data or non-English language studies given the exhaustive number of citations identified. This may have had potential impact on effect size estimates.
Whilst previous reviews and meta-analyses on collaborative care for either single specific disease or multimorbidity have been studied, this review provides a comprehensive current evidence with quantitative synthesis on the effectiveness of PCMH-based care models exclusively on primary care patients with one or more chronic diseases. Other strengths include a registered and published protocol, with a peer-reviewed search strategy, conducted on a wide range of electronic databases.
4.4. Patient, Provider, and Policy-Level Implications and Future Directions
Findings of our systematic review have important implications at patient, practice, and policy-level. The evidence may inform patients on the enhanced biomedical outcomes and quality of life resulting from improved education and self-management support. The transformational changes at practice level may enable GPs to better target and deliver care according to the level and complexity of different patients [123]. Additionally, our study findings may also impact policy and implementation guidelines given the growing advocacy towards patient-centred care. Future research should focus on evaluating sustained benefits of PCMH-based care as well as supporting holistic experiences of patients receiving patient-centred care.
5. Conclusions
Current evidence suggests that PCMH-based care showed significant improvements in depression, HRQoL, self-management, biomedical, and health utilisation outcomes compared to standard GP care. Whilst studies included for pooled estimates showed consistent trend for several outcomes, high heterogeneity in some outcomes resulted in low to moderate grade of evidence, limiting firmer conclusion from the pooled evidence. Further research is needed to evaluate the long-term cost-effectiveness of PCMH-based care after the initial higher costs incurred for intervention, which may prove to be more cost-effective than standard care.
Acknowledgments
The authors would like to express their gratitude to Katrina Chaudhary (Librarian, School of Science and Health, Western Sydney University) and Lily Collison (Librarian, School of Medicine, Western Sydney University) for their help in developing search terms and guidance during the initial search process. We are also particularly grateful to Evan Atlantis for his valuable expertise and feedback provided for this study.
Appendix A
Table A1.
Search strategy.
| No | Search Terms |
|---|---|
| 1 | PCMH.tw. |
| 2 | (patient-centred adj medical adj home *).tw. |
| 3 | (patient adj centred adj medical adj home *).tw. |
| 4 | (patient-centered adj medical adj home *).tw. |
| 5 | (patient adj centered adj medical adj home *).tw. |
| 6 | (Medical adj home *).tw. |
| 7 | (Home adj based adj care).tw. |
| 8 | (home adj based adj model).tw. |
| 9 | (Health adj home *).tw. |
| 10 | (Health adj care adj home *).tw. |
| 11 | (Health-care adj home *).tw. |
| 12 | (Patient adj centred adj care).tw. |
| 13 | (Patient-centred adj care).tw. |
| 14 | (Patient adj centered adj care).tw. |
| 15 | (Patient-centered adj care).tw. |
| 16 | (Patient adj focused adj care).tw. |
| 17 | (Patient-focused adj care).tw. |
| 18 | (Integrated adj primary adj care).tw. |
| 19 | (Integrated adj care).tw. |
| 20 | (Integrated adj health adj care).tw. |
| 21 | (Integrated adj service *).tw. |
| 22 | (Integrated adj delivery).tw. |
| 23 | (Team-based adj care).tw. |
| 24 | (multidisciplinary adj care *).tw. |
| 25 | (care adj team).tw. |
| 26 | (care adj coordination).tw. |
| 27 | (coordinated adj care).tw. |
| 28 | (coordinated adj health adj care).tw. |
| 29 | (coordinated adj primary adj care).tw. |
| 30 | (collaborative adj practice).tw. |
| 31 | (Collaborative adj care).tw. |
| 32 | (Advanced adj primary adj care).tw. |
| 33 | (enhanced adj primary adj care).tw. |
| 34 | (augmented adj care).tw. |
| 35 | (augmented adj service *).tw. |
| 36 | (guided adj care).tw. |
| 37 | (chronic adj care adj model *).tw. |
| 38 | (Patient adj aligned adj care adj team).tw. |
| 39 | (patient adj care adj team).tw. |
| 40 | 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 |
| 41 | (primary adj health adj care).tw. |
| 42 | (family adj practice *).tw. |
| 43 | (primary adj care *).tw. |
| 44 | (community adj network *).tw. |
| 45 | (health adj care adj coalitions).tw. |
| 46 | (chronic adj care *).tw. |
| 47 | (primary adj physician *).tw. |
| 48 | (primary adj care adj physician *).tw. |
| 49 | (general adj practice *).tw. |
| 50 | (general adj physician *).tw. |
| 51 | (general adj practitioner *).tw. |
| 52 | (community adj based adj provider *).tw. |
| 53 | (community adj practice).tw. |
| 54 | (community adj care).tw. |
| 55 | (preventive adj service *).tw. |
| 56 | (patient adj care).tw. |
| 57 | Adult *.tw. |
| 58 | (middle adj age *).tw. |
| 59 | geriatric.tw. |
| 60 | (geriatric adj practice).tw. |
| 61 | elder *.tw. |
| 62 | exp Chronic Disease/ |
| 63 | (Chronic adj disease *).tw. |
| 64 | (Chronic adj illness *).tw. |
| 65 | exp COMORBIDITY/ |
| 66 | comorbid *.tw. |
| 67 | multimorbid *.tw. |
| 68 | exp Diabetes Mellitus/ |
| 69 | ((Diabetes adj mellitus) or Diabet *).tw. |
| 70 | exp ASTHMA/ |
| 71 | Asthma *.tw. |
| 72 | exp ARTHRITIS/ |
| 73 | Arthritis.tw. |
| 74 | exp Back Pain/ |
| 75 | (Back adj pain).tw. |
| 76 | exp Cardiovascular Diseases/ |
| 77 | (cardiovascular adj disease *).tw. |
| 78 | (Heart adj disease *).tw. |
| 79 | exp Neoplasms/ |
| 80 | cancer *.tw. |
| 81 | (malignant adj neoplasm *).tw. |
| 82 | exp Pulmonary Disease, Chronic Obstructive/ |
| 83 | (chronic adj obstructive adj pulmonary adj disease).tw. |
| 84 | (respiratory adj disease *).tw. |
| 85 | exp Kidney Diseases/ |
| 86 | (Kidney adj disease *).tw. |
| 87 | 41 or 42 or 43 or 44 or 45 or 46 or 47 or 48 or 49 or 50 or 51 or 52 or 53 or 54 or 55 or 56 or 57 or 58 or 59 or 60 or 61 or 62 or 63 or 64 or 65 or 66 or 67 or 68 or 69 or 70 or 71 or 72 or 73 or 74 or 75 or 76 or 77 or 78 or 79 or80 or 81 or 82 or 83 or 84 or 85 or 86 |
| 88 | 40 and 87 |
| 89 | Randomized Controlled Trials as Topic/ |
| 90 | (Randomized adj controlled adj trial *).tw. |
| 91 | (Randomised adj controlled adj trial *).tw. |
| 92 | (Clinical adj Trial *).tw. |
| 93 | Random adj allocat * |
| 94 | (Clinical adj trial).pt. |
| 95 | (Controlled adj trial *).tw. |
| 96 | 89 or 90 or 91 or 92 or 93 or 94 or 95 |
| 97 | 88 and 96 |
| 98 | limit 97 to (English language and humans) |
* represents wildcard symbol that broadens a search by finding words that start with the same letters.
Table A2.
List of excluded articles from full-text screening stage with an overarching reason.
| Articles | Number of Articles | Overarching Reason for Exclusion |
|---|---|---|
| (Aguiar, 2016; Bartels, 2004; Battersby, 2013; Bekelman, 2015; Berry, 2016; Brunisholz, 2017; Casas, 2006; de Stampa, 2014; Druss, 2001; Fors, 2015; Gjerdingen, 2009; Grochtdreis, 2018; Gums, 2016; Gums, 2014; Jakobsen, 2017; Jiao, 2014; Joubert, 2008; Kane, 2016; King, 2019; Ku, 2015; Peikes, 2009; Pourat, 2019; Schillinger, 2009; Siaw, 2018; Speyer, 2016; Walker, 2014; Wolff, 2010; Yoon, 2016; Yuting, 2017; Zatzick, 2015) | 30 | Participants: Patients less than 18 years; patients recruited and treated in a non-primary care setting; patients diagnosed with a communicable disease. |
| (Adam, 2010; Anderson, 2009; Borgermans, 2009; Campbell-Sills, 2016; Counsell, 2007; Eggers, 2018; Grunfeld, 2013; Ishani, 2016; Liu, 2003; Oosterbaan, 2013; Raftery, 1996; Rinfret, 2009; Rothman, 2005; Tao, 2015; Uittenbroek, 2017; Vermunt, 2012) | 16 | Intervention: Does not meet the PCMH definition or not sufficient components of PCMH or more focus on other intervention than PCMH model. |
| (Anjara, 2019; Bauer, 2019; Callahan, 2006; Ell, 2010; Hedrick, 2003; Jaen, 2010; Kearns, 2017; Kuhmmer, 2016; Meredith, 2016; Meulepas, 2007; Moran, 2011) | 11 | Comparison: Does not have a comparison group or comparison group received some amount of intervention other than standard care. |
| (Dwight-Johnson, 2010; Gill, 2017; Griffiths, 2016; Harpole, 2005; Marsteller, 2010; Marsteller, 2013) | 6 | Irrelevant outcomes |
| (Areán, 2005; Areán, 2007; Boland, 2015; Boult, 2013; Boyd, 2010; Buist-Bouwman, 2005; Campbell-Scherer, 2018; Chan, 2011; Conn, 2005; Ell, 2012; Ell, 2011; Fann, 2009; Ford, 2019; Fortney, 2014; Gensichen, 2006; Gilbody, 2007; Goering, 2003; Goertz, 2016; Hegel, 2005; Hendricks, 2016; Hirsch, 2014; Houles, 2010; Hunkeler, 2006; Jansen, 2017; Katon, 2006; Katon, 2003; Khambaty, 2015; Kinder, 2006; Kindy, 2003; Kumar, 2005; Lewis, 2017;Lin, 2014; McCusker, 2019; McGregor, 2011; Menchetti, 2013; Mills, 2003; Pieters, 2002; Price, 2004; Romano, 2011; Ruescas-Escolano, 2014; Sepers, 2015; Slimmer, 2003; Spoorenberg, 2016; Stone, 2010; Turner, 2011; Uittenbroek, 2017; Unutzer, 2001; Unutzer, 2006; Upchurch, 2005; Vester, 2019; Wang, 2011; Williams Jr, 2004; Zulman, 2015) | 53 | Other reasons: Non-English, conference abstracts, secondary data analyses using same sample, duplicate with different title, design and early implementation experiences paper, thesis, commentary, same outcome with same sample but different follow-up times. |
Table A3.
Characteristics of randomised controlled trials reviewed.
| Chronic Physical Conditions—Baseline Characteristics (Risk Proportion/Mean or Median and SD) | Outcomes | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Authors and Year of Publication | Country of Origin | Sample Size (N) | Mean Age/Age Groups | Gender Distribution (Female) |
Chronic Disease Profile of the Sample Population | Treatment Group | Control Group | PCMH Components | Duration of Follow-up | Depression | Quality of Life/Self-Management | Hospital Admission | Cost/Health Utility | Biomedical Outcomes |
| Alexopoulos et al., 2009 [36] | United States | Treatment = 320 Control = 279 |
Overall ≥ 60 years (mean not reported) | Overall = 71.6% | Major or minor depression according to DSM-IV criteria | HAM-D score = 18.61 (6.12) Prevalence of suicide ideation = 27.5% |
HAM-D score = 17.51 (5.82) Prevalence of suicide ideation = 18.6% |
Team based care; Co-ordinated care |
24 months | ✓ | ||||
| Aragonès et al., 2014 [18] | Spain | Treatment = 189 Control = 149 |
Overall = 47 years | Overall = 80% | Moderate or severe major depressive episode or minor depression | PHQ-9 score = 18.10 (5.20) SF12 mental health = 22.26 (9.05) SF12 physical health = 47.47 (10.98) |
PHQ-9 score = 17.66 (4.79) SF12 mental health = 22.73 (10.44) SF12 physical health = 48.23 (11.23) |
Team based care; Co-ordinated care; Patient engagement; Continuity of care. |
36 months | ✓ | ✓ | |||
| Aragonès et al., 2014 (Cost-effectiveness) [37] |
Spain | Treatment = 189 Control = 149 |
Overall = 47 years | Overall = 80% | Moderate or severe major depressive episode or minor depression | Total direct costs—776.30 (664.10) Total indirect costs—718.30 (1587.70) |
Total direct costs—593.80 (603.10) Total indirect costs—743.40 (1582.10) |
Team based care; Co-ordinated care; Patient engagement; Continuity of care. |
36 months | ✓ | ||||
| Aragonès et al., 2019 [38] | Spain | Treatment = 167 Control = 161 |
Treatment = 61.4 years Control = 59.3 years |
Treatment = 82.6% Control = 83.2% |
Major depressive episode and experiencing moderate or severe musculoskeletal pain. | HSCL-20 score; mean (SD) = 1.67 (0.80) BPI score; mean (SD) = 6.45 (1.87) |
HSCL-20 score; mean (SD) = 1.69 (0.68) BPI score; mean (SD) = 6.60 (1.77) |
MDT care, Patient engagement Coordinated care, Continuity of care |
12 months | ✓ | ||||
| Barcelo et al., 2010 [39] | Mexico | Treatment = 196 Control = 111 |
6% of <40 years; 54% of 40–59 years; and 42% of ≥60 years | NA (baseline stratified by gender) | Type 2 Diabetes | % with HbA1c (<7%) Cases: Baseline—27.6% |
% with HbA1c (<7%) Control: Baseline—20.7% |
MDT care, All other components of CCM |
13 months | ✓ | ||||
| Bjorkelund et al., 2018 [40] | Sweden | Treatment = 192 Control = 184 |
Treatment = 40.8 years Control = 41.6 years |
Treatment = 68.2% Control = 74.5% |
Mild or moderate Depression | MADRS-S Mean (SD) = 20.8 (7.2) BDI-II Mean (SD) = 23.9 (8.7) EQ5D Mean (SD) = 0.58 (0.24) |
MADRS-S Mean (SD) = 21.9 (7.1) BDI-II Mean (SD) = 25.1 (8.5) EQ5D Mean (SD) = 0.56 (0.25) |
MDT care, Patient engagement Coordinated care |
6 months | ✓ | ✓ | |||
| Blom et al., 2016 [41] | Netherlands | Treatment = 3145 Control = 4133 |
Treatment = 80.5 years Control = 81.3 years |
Treatment = 60.9% Control = 61.7% |
Depression with complex daily functioning problems | Cantri’s ladder median (range) = 7 (6–8) GARS total score median (range) = 36 (27,45) BADL subscale score median (range) = 11 (9,15) IADL subscale score median (range) = 18 (25,30) |
Cantri’s ladder median (range) = 7 (6–8) GARS total score median (range) = 37 (29,46) BADL subscale score median (range) = 11 (9,15) IADL subscale score median (range) = 20 (26,32) |
MDT care, Self-management plans, Coordinated care |
12 months | ✓ | ||||
| Bogner et al., 2008 [42] | United States | Treatment = 32 Control = 32 |
Treatment = 59.7 years Control = 57.5 years |
Treatment = 75% Control = 78.1% |
Depression and hypertension | CES-D mean score (SD) = 17.5 (13.2) SBP, mean (SD) = 146.7 (20.9) DBP, mean (SD) = 83.0 (10.7) |
CES-D mean score (SD) = 19.6 (14.2) SBP, mean (SD) = 143.1 (22.5) DBP, mean (SD) = 81.4 (11.1) |
MDT care, Patient engagement |
6 weeks | ✓ | ✓ | |||
| Bogner et al., 2012 [43] | United States | Treatment = 92 Control = 88 |
Treatment = 57.8 years Control = 57.1 years |
Treatment = 70% Control = 66% |
Type 2 Diabetes, current prescription for antidepressant. | HbA1c, mean (SD) = 7.2 (1.8) PHQ-9 score, mean (SD) = 10.6 (7.9) |
HbA1c, mean (SD) = 7.0 (1.9) PHQ-9 score, mean (SD) = 9.9 (7.2) |
MDT care, Patient engagement |
12 weeks | ✓ | ✓ | |||
| Boland et al., 2015 [44] | Netherlands | Treatment = 554 Control = 532 |
Treatment = 68.2 years Control = 68.4 years |
Treatment = 49.5% Control = 42.7% |
Chronic obstructive pulmonary disease according to GOLD (Global Initiative for COPD) guidelines. | CCQ score, mean (SD) = 1.54 (0.98) | CCQ score, mean (SD) = 1.46 (0.96) | MDT care, Self-management plans, Coordinated care |
24 months | ✓ | ||||
| Borenstein et al., 2003 [45] | United States | Treatment =98 Control = 99 |
Treatment = 62.5 years Control = 61.5 years |
Treatment = 63.2% Control = 58.5% |
Hypertension | Mean SBP = 162 Mean DBP = 92 (no SD or 95% CI reported) |
Mean SBP = 156 Mean DBP = 90 (no SD or 95% CI reported) |
MDT care Patient education |
12 months | ✓ | ||||
| Bosanquet et al. 2017 [46] | United Kingdom | Treatment = 198 Control = 217 |
Treatment = 72 years Control = 72 years |
Treatment = 59% Control = 63% |
Depression | PHQ-9 score Mean (SD) = 12.3 (5.43) | PHQ-9 score Mean (SD) = 12.0 (5.32) | MDT care, Self-management plans, Coordinated care |
18 months | ✓ | ✓ | ✓ | ||
| Boult et al., 2008 [47] | United States | Treatment = 485 Control = 419 |
Treatment = 77.2 years Control = 78.1 years |
Treatment = 54.2% Control = 55.4% |
Multimorbidity (specific conditions not reported) | PACIC aggregate score = 5.9 | PACIC aggregate score = 2.9 | MDT care, Self-management plans, Coordinated care |
6 months | ✓ | ||||
| Boult et al., 2011 [48] | United States | Treatment = 446 Control = 404 |
Treatment = 77.1 years Control = 77.8 years |
Treatment = 54.3% Control = 55.7% |
Circulatory system disorders, musculoskeletal disorders, Type 2 Diabetes, and cancers | No. of chronic diseases, mean (range) = 4.3 (1–11) | No. of chronic diseases, mean (range) = 4.3 (0–12) | MDT care, Self-management plans, Coordinated care |
6 months | ✓ | ||||
| Callahan et al., 2005 [49] | United States | Treatment = 906 Control = 895 |
Treatment = 71 years Control = 71.4 years |
Treatment = 64.1% Control = 65.6% |
Major depression and/or dysthymia | SF-12 Mean (SD) = 40.43 (7.44) IADL Mean (SD) = 0.68 (1.37) |
SF-12 Mean (SD) = 40.11 (7.40) IADL Mean (SD) = 0.61 (1.31) |
MDT care, Patient engagement Coordinated care |
12 months | ✓ | ||||
| Camacho et al., 2018 [13] | United Kingdom | Treatment = 191 Control = 196 |
Treatment = 57.9 years Control = 59.2 years |
Treatment = 41% Control = 35% |
Diabetes and/or coronary heart disease | SCL-D13 Mean (SD) = 2.364 (0.696) | SCL-D13 Mean (SD) = 2.330 (0.822) | MDT care, Patient engagement Coordinated care |
24 months | ✓ | ✓ | |||
| Campins et al., 2017 [20] | Spain | Treatment = 252 Control = 251 |
Treatment = 79.2 years Control = 78.8 years |
Treatment = 60.3% Control = 57.4% |
Patients with multimorbidity and polymedicated | Medications Mean (SD) = 10.79 (2.52) | Medications Mean (SD) = 10.91 (2.65) | MDT care, Patient engagement Coordinated care |
12 months | ✓ | ✓ | |||
| Chaney et al., 2011 [50] | United States | Treatment = 288 Control = 258 |
Treatment = 64 years Control = 64.4 years |
Treatment = 4.2% Control = 3.5% |
Subthreshold depression or dysthmia | PHQ-9 score Mean (SD) = 15.5 (4.4) SF-12 role physical score Mean (SD) = 29.2 (36.2) SF-12 role emotional score Mean (SD) = 47.1 (41.4) |
PHQ-9 score Mean (SD) = 15.7 (4.7) SF-12 role physical score Mean (SD) = 34.8 (40.7) SF-12 role emotional score Mean (SD) = 50.0 (41.8) |
MDT care, Patient engagement Coordinated care |
7 months | ✓ | ✓ | |||
| Cooper et al., 2013 [51] | United States | Treatment = 67 Control = 65 |
Treatment = 45.9 years Control = 47 years |
Treatment = 55% Control = 50% |
Major depressive disorder | CESD score, mean (SD) = 29.52 (14.48) MCS-12 score, mean (SD) = 35.97 (13.10) |
CESD score, mean (SD) = 30.17 (13.78) MCS-12 score, mean (SD) = 36.41 (12.19) |
MDT care, Patient engagement Coordinated care |
12 months | ✓ | ✓ | |||
| Coventry et al., 2015 [14] | United Kingdom | Treatment = 191 Control = 196 |
Treatment = 57.9 years Control = 59.2 years |
Treatment = 41% Control = 35% |
Diabetes and/or coronary heart disease | SCL-D-13 Mean (SD) = 2.36 (0.70) PHQ-9 Mean (SD) = 16.4 (4.2) GAD-7 Mean (SD) = 12.3 (5.1) |
SCL-D-13 Mean (SD) = 2.33 (0.82) PHQ-9 Mean (SD) = 16.5 (4.1) GAD-7 Mean (SD) = 11.9 (5.3) |
MDT care, Patient engagement Coordinated care |
4 months | ✓ | ||||
| Dickinson et al., 2010 [52] | United States | Treatment = 187 Control = 214 |
Treatment = 62.1 years Control = 61.3 years |
Treatment = 8% Control = 8% |
Musculoskeletal disorders with chronic pain | RMDQ Mean (SD) = 14.9 (4.4) Pain disability-free days 0–3 months = 31.3 (25.3) |
RMDQ Mean (SD) = 14.5 (4.4) Pain disability-free days 0–3 months = 30.0 (26.6) |
MDT care, Patient engagement Coordinated care |
12 months | ✓ | ✓ | |||
| Dobscha et al., 2009 [53] | United States | Treatment = 187Control = 214 | Treatment = 62.1 years Control = 61.3 years |
Treatment = 8% Control = 8% |
Musculoskeletal disorders with chronic pain | RMDQ Mean (SD) = 14.9 (4.4) Current pain intensity, mean (SD) = 5.3 (2.2) PHQ-9 score Mean (SD) = 8.1 (5.7) |
RMDQ Mean (SD) = 14.5 (4.4) Current pain intensity, mean (SD) = 5.1 (2.1) PHQ-9 score Mean (SD) = 8.4 (6.0) |
MDT care, Patient engagement Coordinated care |
12 months | ✓ | ✓ | ✓ | ||
| Dorr et al., 2008 [54] | United States | Treatment = 1144 Control = 2288 |
Treatment = 76.2 years Control = 76.2 years |
Treatment = 64.6% Control = 64.6% |
Circulatory system disorders, depression, and Type 2 Diabetes | Hospitalizations Mean (SD) = 257 (22.5) ED visits in previous year Mean (SD) = 407 (35.5) |
Hospitalizations Mean (SD) = 514 (22.5) ED visits in previous year = 807 (35.3) |
MDT care, Patient engagement Coordinated care, Data driven quality of care |
24 months | ✓ | ||||
| Edelman et al., 2010 [16] | United States | Treatment = 133 Control = 106 |
Treatment = 63 years Control = 60.8 years |
Treatment = 4.5% Control = 3.8% |
Diabetes and hypertension | HbA1c % Mean (SD) = 9.2 (1.3) Mean SBP (SD) mmHg = 153.7 (14.8) Mean DBP (SD) mmHg = 84.7 (12.1) |
HbA1c % Mean (SD) = 9.2 (1.5) Mean SBP (SD) mmHg = 153.7 (14.8) Mean DBP (SD) mmHg = 84.7 (12.1) |
MDT care, Patient engagement Coordinated care |
12 months | ✓ | ||||
| Engel et al., 2016 [55] | United States | Treatment = 332 Control = 334 |
Treatment = 30.9 years Control = 31.4 years |
Treatment = 80% Control = 82% |
Posttraumatic Stress Disorder and Depression | PTSD severity, mean (SD) = 29.4 (9.4) SCL-20, mean (SD) = 2.1 (0.6) |
PTSD severity, mean (SD) = 28.9 (8.9) SCL-20, mean (SD) = 2.0 (0.7) |
MDT care, Patient engagement Coordinated care, Data driven quality of care |
12 months | ✓ | ||||
| Fihn et al., 2011 [56] | United States | Treatment = 344 Control = 359 |
Treatment = 68.3 years Control = 67.2 years |
Treatment = 1.2% Control = 3.6% |
Circulatory system disorders—Angina | SAQ anginal frequency score, mean (SD) = 52.8 (17.3) | SAQ anginal frequency score, mean (SD) = 53.8 (16.5) | MDT care, Patient engagement Coordinated care |
12 months | ✓ | ||||
| Gilbody et al., 2017 [57] | United Kingdom | Treatment = 274 Control = 327 |
Treatment = 76.6 years Control = 77.4 years |
Treatment = 55.5% Control = 62.4% |
Subthreshold depression or dysthmia | PHQ-9 score, mean (SD) = 7.6 (4.32) Mean (SD) SF-12 score (physical component) = 38.5 (13.15) |
PHQ-9 score, mean (SD) = 7.6 (4.55) Mean (SD) SF-12 score (physical component) = 36.6 (13.11) |
MDT care, Self-management plans, Coordinated care |
12 months | ✓ | ✓ | |||
| Goorden et al., 2015 [58] | Netherlands | Treatment = 45 Control = 48 |
Treatment = 52 years Control = 53 years |
Treatment = 66.7% Control = 72.9% |
Major depressive disorder | Mean (SD) utility score EQ5D = 0.54 (0.25) | Mean (SD) utility score EQ5D = 0.56 (0.25) | MDT care, Patient engagement Coordinated care, Data driven quality of care |
12 months | ✓ | ✓ | |||
| Green et al., 2014 [59] | United Kingdom | Treatment = 276 Control = 305 |
Overall = 44.8 years | Overall = 71.9% | Depressive episode according to ICD-10 | Mean (SD) utility score EQ5D = 0.504 (0.288) | Mean (SD) utility score EQ5D = 0.464 (0.313) | MDT care, Self-management plans, Coordinated care |
12 months | ✓ | ✓ | |||
| Grochtdreis et al., 2019 [60] | Germany | Treatment = 139 Control = 107 |
Treatment = 71.1 years Control = 71.6 years |
Treatment = 77% Control = 79.4% |
Depressive episode, recurring depressive disorder, or dysthmia according to ICD-10 | EQ-5D-Index: mean (SD) = 0.55 (0.31) PHQ-9-Index: mean (SD) = 10.67 (4.02) Total costs: Mean (SD) = €2920 (€4425) |
EQ-5D-Index: mean (SD) = 0.55 (0.31) PHQ-9-Index: mean (SD) = 9.64 (3.62) Total costs: Mean (SD) = €4222 (€7729) |
MDT care, Patient engagement Coordinated care, Continuity of care |
12 months | ✓ | ✓ | |||
| Hirsch et al., 2014 [61] | United States | Treatment = 75 Control = 91 |
Treatment = 65.4 years Control = 69.6 years |
Treatment = 60% Control =71 % |
Diabetes and hypertension | Systolic BP (mmHg)—mean (SD) = 134.8 (17.4) Diastolic BP (mmHg)—mean (SD) = 75.1 (12.5) |
Systolic BP (mmHg)—mean (SD) = 134.4 (16.5) Diastolic BP (mmHg)—mean (SD) = 75.7 (13.4) |
MDT care, Patient engagement Coordinated care |
9 months | ✓ | ||||
| Hsu et al., 2014 [62] | Taiwan | Treatment = 789 Control = 271 |
NA | NA | Type 2 Diabetes | Mean (SD) HbA1c % = 8.4 | Mean (SD) HbA1c % = 8.6 | MDT care, Patient engagement Coordinated care |
42 months | ✓ | ||||
| Huijbregts et al., 2013 [63] | Netherlands | Treatment = 101 Control = 49 |
Treatment = 47 years Control = 52.1 years |
Treatment = 72.3% Control = 73.5% |
Major depressive disorder | Mean (SD) PHQ-9 = 15.5 (4.8) | Mean (SD) PHQ-9 = 14.8 (4.8) | MDT care, Patient engagement Coordinated care, Data driven quality of care |
12 months | ✓ | ||||
| Ip et al., 2013 [64] | United States | Treatment = 147 Control = 147 |
Treatment = 55.5years Control = 57.2 years |
Treatment = 12% Control = 12% |
Type 2 Diabetes | Mean (SD) HbA1c % = 9.5 (1.4) Mean SBP (SD) mmHg = 128.9 (16.2) Mean DBP (SD) mmHg = 73.9 (9.8) |
Mean (SD) HbA1c % = 9.3 (1.5) Mean SBP (SD) mmHg = 131 (14.8) Mean DBP (SD) mmHg = 76.6 (11.6) |
MDT care, Patient engagement Coordinated care |
12 months | ✓ | ||||
| Johnson et al., 2016 [65] | United States | Treatment = 95 Control = 71 |
Treatment = 57 years Control = 63.4 years |
Treatment = 58% Control = 40% |
Type 2 Diabetes with depressive symptoms | PHQ, mean (SD) = 14.5 (3.8) | PHQ, mean (SD) = 14.2 (3.4) | MDT care, Patient engagement Coordinated care |
12 months | ✓ | ||||
| Katon et al., 1999 [67] | United States | Treatment = 114 Control = 114 |
Treatment = 47.2 years Control = 46.7 years |
Treatment = 67.5% Control = 81.6% |
Depression or anxiety | SCL-depression mean (SD) = 1.9 (0.5) | SCL-depression mean (SD) = 1.9 (0.5) | MDT care, Patient engagement Coordinated care |
6 months | ✓ | ||||
| Katon et al., 2004 [70] | United States | Treatment = 164 Control = 165 |
Treatment = 58.6 years Control = 58.1 years |
Treatment = 65.2% Control = 64.8% |
Diabetes and depression | SCL-20 score, mean (SD) = 1.7 (0.51) | SCL-20 score, mean (SD) = 1.6 (0.45) | MDT care, Patient engagement Coordinated care |
12 months | ✓ | ||||
| Katon et al., 2005 [69] | United States | Treatment = 906 Control = 895 |
Treatment = 71 years Control = 71.4 years |
Treatment = 64% Control = 66% |
Major depression and/or dysthymia | Mean (SE) SCL-20 Depression Scores = 1.7 (0.6) | Mean (SE) SCL-20 Depression Scores = 1.7 (0.6) | MDT care, Patient engagement Coordinated care |
24 months | ✓ | ||||
| Katon et al., 2010 [68] | United States | Treatment = 106 Control = 108 |
Treatment = 57.4 years Control = 56.3 years |
Treatment = 48% Control = 56% |
Diabetes, coronary heart disease, depression, and hypertension | SCL-20 mean (SD) = 1.7 (0.6) Glycated haemoglobin % mean (SD)= 8.1 (2.0) LDL cholesterol mg/dl mean (SD)= 106.5 (35.3) Systolic blood pressure mm Hg mean (SD)= 136 (18.4) |
SCL-20 mean (SD) = 1.7 (0.6) Glycated haemoglobin % mean (SD)= 8.0 (1.9) LDL cholesterol mg/dl mean (SD)= 109.0 (36.5) Systolic blood pressure mmHg mean (SD)= 132 (17.2) |
MDT care, Patient engagement Coordinated care |
12 months | ✓ | ✓ | ✓ | ||
| Katon et al., 2012 [66] | United States | Treatment = 106 Control = 108 |
Treatment = 57.4 years Control = 56.3 years |
Treatment = 48% Control = 56% |
Diabetes and/or coronary heart disease | SCL-20 mean (SD) = 1.7 (0.6) PHQ-9 mean (SD) = 14.7 (3.8) SBP mean (SD) = 136 (18.4) HbA1c mean (SD) = 8.1 (2.0) Outpatient costs in the previous 12 months, mean (95% CI), $ = 10,026 (8312–11,741) Inpatient costs in the previous 12 months, mean (95% CI), $ = 3210 (1553–4868) |
SCL-20 mean (SD) = 1.7 (0.6) PHQ-9 mean (SD) = 13.9 (3.1) SBP mean (SD) = 132 (17.2) HbA1c mean (SD) = 8.0 (1.9) Outpatient costs in the previous 12 months, mean (95% CI), $ = 9663 (8070–11,254) Inpatient costs in the previous 12 months, mean (95% CI), $ = 2748 (1453–4043) |
MDT care, Patient engagement Coordinated care, Continuity of care |
24 months | ✓ | ||||
| Konnopka et al., 2016 [22] | Germany | Treatment = 170 Control = 130 |
Treatment = 50.8 years Control = 46.1 years |
Treatment = 75% Control = 75% |
Depression and mild somatic symptom severity | PHQ-15 score, mean (SD) = 12.6 (4.73) SF-36 PCS, mean (SD) = 43.2 (9.1) SF-36 MCS, Mean (SD) = 41.5 (10.2) |
PHQ-15 score, mean (SD) = 12.7 (4.86) SF-36 PCS, mean (SD) = 42.0 (8.9) SF-36 MCS, Mean (SD) = 40.7 (11.4) |
MDT care, Patient engagement Coordinated care |
12 months | ✓ | ✓ | |||
| Krein et al., 2004 [71] | United States | Treatment = 123 Control = 123 |
Treatment = 61 years Control = 61 years |
Treatment = 2% Control = 5 % |
Type 2 Diabetes | Haemoglobin A1C (%) = 9.3 (1.5) LDL cholesterol (mg/dL) = 123 (37) Systolic blood pressure (mm Hg) = 145 (21) Diastolic blood pressure (mm Hg) = 86 (12) |
Haemoglobin A1C (%) = 11 (9) LDL cholesterol (mg/dL) = 9.2 (1.4) Systolic blood pressure (mm Hg) = 123 (38) Diastolic blood pressure (mm Hg) = 145 (20) |
MDT care, Patient engagement Coordinated care |
18 months | ✓ | ||||
| Kruis et al., 2014 [72] | Netherlands | Treatment = 554 Control = 532 |
Treatment = 68.2 years Control = 68.4 years |
Treatment = 50.5 % Control = 57.3% |
COPD according to GOLD (Global Initiative for COPD) guidelines. | Mean (SD) CCQ score Total = 1.5 (1.0) Mean (SD) SF-36 PCS = 38 (10.9) Mean (SD) SF-36 MCS = 48.3 (10.5) Mean (SD) PACIC score Total = 2.3 (0.9) |
Mean (SD) CCQ score Total = 1.5 (1.0) Mean (SD) SF-36 PCS = 38.6 (10.7) Mean (SD) SF-36 MCS = 48.9 (10.3) Mean (SD) PACIC score Total = 2.3 (0.9) |
MDT care, Patient engagement Coordinated care |
24 months | ✓ | ||||
| Leeuwen et al., 2015 [73] | Netherlands | Treatment = 3017 Control = 1354 |
Overall = 80.5 years | Overall = 66.5% | Multimorbidity (specific conditions not reported) with high frailty index | EQ5D, mean (SD) = 0.60 (0.28) | EQ5D, mean (SD) = 0.59 (0.29) | MDT care, Patient engagement Coordinated care |
24 months | ✓ | ||||
| Lin et al., 2000 [76] | United States | Treatment = 114 Control = 114 |
Treatment = 47.2 years Control = 46.7 years |
Treatment = 67.5 % Control = 81.6% |
Depression | Sheehan Disability Scale = 5.4 (5.0–5.8) SF-36 social functioning = 49.4 (44.6–54.2) SF-36 Role limitation due to emotional problems = 26.4 (21.1–31.7) |
Sheehan Disability Scale = 5.3 (4.9–5.7) SF-36 social functioning = 49.4 (44.6–54.2) SF-36 Role limitation due to emotional problems = 26.4 (21.1–31.7) |
MDT care, Patient engagement Coordinated care, Continuity of care |
6 months | ✓ | ||||
| Lin et al., 2006 [74] | United States | Treatment = 506 Control = 495 |
Overall = 72 years | Overall = 68.3% | Major depression and/or dysthymia | Mean (SD) arthritis pain severity = 6.1 (2.7) Mean (SD) activity interference = 5.0 (3.2) Mean (SD) HSCL score = 1.7 (0.6) |
Mean (SD) arthritis pain severity = 6.1 (2.7) Mean (SD) activity interference = 5.0 (3.2) Mean (SD) HSCL score = 1.7 (0.6) |
MDT care, Patient engagement Coordinated care |
12 months | ✓ | ||||
| Lin et al., 2012 [75] | United States | Treatment = 90 Control = 91 |
Overall = 56.8 years | Overall = 52.4% | Diabetes and/or coronary heart disease | Mean medication adherence Oral hypoglycaemic drugs = 0.83 (0.19) Antihypertensive = 0.85 (0.18) Lipid lowering = 0.82 (0.21) Antidepressant = 0.79 (0.23) |
Mean medication adherence Oral hypoglycaemic drugs = 0.83 (0.20) Antihypertensive = 0.86 (0.18) Lipid lowering = 0.85 (0.18) Antidepressant = 0.80 (0.19) |
MDT care, Patient engagement Coordinated care, Continuity of care |
12 months | ✓ | ||||
| Maislos et al., 2004 [77] | Israel | Treatment = 48 Control = 34 |
Treatment = 58 years Control = 63 years |
Treatment = 50 % Control = 65% |
Type 2 Diabetes | Mean (SD) HbA1C, % = 11.6 (1.3) | Mean (SD) HbA1C, % = 11.1 (1.1) | MDT care, Patient engagement Coordinated care |
6 months | ✓ | ||||
| Menchetti et al., 2013 [78] | Italy | Treatment = 128 Control = 99 |
Treatment = 50.1 years Control = 53.9 years |
Treatment = 78.9% Control = 72.7% |
Depression | PHQ-9, Mean (SD) = 13.7 (4.7) | PHQ-9, Mean (SD) = 12.8 (4.6) | MDT care, Patient engagement Coordinated care |
3 months | ✓ | ||||
| Metzelthin et al., 2015 [79] | Netherlands | Treatment = 103 Control = 91 |
Treatment = 77.5 years Control = 76.8 years |
Treatment = 55% Control = 60% |
Multimorbidity (specific conditions not reported) with high frailty index | GARS 18–72 = 33.1 (11.5) Mean EQ5D (SD) = 0.6 (0.2) |
GARS 18–72 = 30.6 (10.6) Mean EQ5D (SD) = 0.7 (0.2) |
MDT care, Patient engagement Coordinated care |
24 months | ✓ | ||||
| Morgan et al., 2015 [80] | United States | Treatment = 269 Control = 165 |
Treatment = 79.1 years Control = 80.3 years |
NA | Dementia | Charlson-Deyo index score Mean (SD) = 2.6 (2.4) | Charlson-Deyo index score Mean (SD) = 1.8 (1.7) | MDT care, Patient engagement Coordinated care |
30 months | ✓ | ||||
| Muntingh et al., 2013 [19] | Netherlands | Treatment = 114 Control = 66 |
Treatment = 45 years Control = 49 years |
Treatment = 73% Control = 61% |
Panic and/or general anxiety disorders | Anxiety score (BAI) mean (SD) = 24.59 (11.52) Depression score (PHQ-9) mean (SD) = 9.40 (5.62) MCS (SF-36) mean (SD) = 32.56 (11.26) PCS (SF-36) mean (SD) = 48.43 (8.73) EQ-5D score mean (SD) = 0.67 (0.17) |
Anxiety score (BAI) mean (SD) = 20.04 (11.28) Depression score (PHQ-9) mean (SD) = 8.98 (5.77) MCS (SF-36) mean (SD) = 35.74 (13.00) PCS (SF-36) mean (SD) = 47.75 (10.38) EQ-5D score mean (SD) = 0.70 (0.14) |
MDT care, Patient engagement Coordinated care |
12 months | ✓ | ✓ | |||
| Pyne et al., 2003 [81] | United States | Treatment = 115 Control = 96 |
Treatment = 40 years Control = 47 years |
Treatment = 83.5% Control = 85.4% |
Major depressive disorder | Mean mCES-D (SD) = 57.6 (18.5) Mean VAS SF-36 (SD) = 0.453 (0.127) |
Mean mCES-D (SD) = 50.8* (19.2) Mean VAS SF-36 (SD) = 0.446 (0.160) |
MDT care, Patient engagement Coordinated care |
12 months | ✓ | ||||
| Ramli et al., 2016 [82] | Malaysia | Treatment = 471 Control = 417 |
Treatment = 58 years Control = 57 years |
Treatment = 62% Control = 64% |
Type 2 Diabetes | HbA1c (%) = 8.4 (0.09) % HbA1c (≤7%) = 15.3 |
HbA1c (%) = 8.4 (0.09) % HbA1c (≤7%) = 17.0 |
MDT care, Patient engagement Coordinated care, Data driven quality of care |
12 months | ✓ | ||||
| Richards et al., 2008 [84] | United Kingdom | Treatment = 41 Control = 38 |
Treatment = 43 years Control = 43 years |
Treatment = 78% Control = 76% |
Depression | Mean (SD) PHQ-9 = 17.5 (4.9) | Mean (SD) PHQ-9 = 16.3 (4.5) | MDT care, Self-management plans, Coordinated care; Continuity of care |
3 months | ✓ | ||||
| Richards et al., 2013 [83] | United Kingdom | Treatment = 276 Control = 305 |
Treatment = 45 years Control = 44.5 years |
Treatment = 73.2% Control = 70.8% |
Depression according to ICD-10 | Mean (SD) PHQ-9 = 17.4 (5.2) Mean (SD) GAD- 7 = 12.9 (5.3) Mean (SD) SF-36 MCS = 23.2 (10.4) Mean (SD) SF-36 PCS = 44.8 (12.4) |
Mean (SD) PHQ-9 = 18.1 (5.0) Mean (SD) GAD- 7 = 13.6 (4.7) Mean (SD) SF-36 MCS = 22.3 (10.3) Mean (SD) SF-36 PCS = 44.5 (12.3) |
MDT care, Self-management plans, Coordinated care |
12 months | ✓ | ✓ | |||
| Rollman et al., 2005 [86] | United States | Treatment = 116 Control = 75 |
Treatment = 44 years Control = 45 years |
Treatment = 84% Control = 77% |
Panic and/or general anxiety disorders | Mean SIGH-A (SD) = 20.1 (6.4) Mean PDSS (SD) = 8.4 (6.0) Mean SF-12 MCS (SD) = 30.6 (8.8) Mean SF-12 PCS (SD) = 43.8 (11.8) |
Mean SIGH-A (SD) = 20.6 (6.4) Mean PDSS (SD) = 8.5 (6.1) Mean SF-12 MCS (SD) = 29.9 (10.5) Mean SF-12 PCS (SD) = 45.1 (12.1) |
MDT care, Self-management plans, Coordinated care |
12 months | ✓ | ✓ | |||
| Rollman et al., 2017 [85] | United States | Treatment = 124 Control = 126 |
Treatment = 45 years Control = 44.2 years |
Treatment = 67% Control = 68% |
Panic and/or general anxiety disorders | SF-36 MCS, mean (SD) = 27.4 (10.5) SF-36 PCS, mean (SD) = 45.6 (12.1) SIGH-A, mean (SD) = 28.4 (7.3) PDSS, mean (SD) = 12.8 (6.8) GADSS, mean (SD) = 15.9 (3.1) PHQ-9, mean (SD) = 15.2 (5.1) |
SF-36 MCS, mean (SD) = 28.7 (9.9) SF-36 PCS, mean (SD) = 45.3 (11.7) SIGH-A, mean (SD) = 28.1 (6.5) PDSS, mean (SD) = 12.4 (6.4) GADSS, mean (SD) = 15.7 (3.2) PHQ-9, mean (SD) = 15.0 (5.1) |
MDT care, Self-management plans, Coordinated care |
24 months | ✓ | ||||
| Rollman et al., 2018 [87] | United States | Treatment = 302 Control = 101 |
Treatment = 43 years Control = 42 years |
Treatment = 81% Control = 81% |
Panic and/or general anxiety disorders | SF-12 MCS, mean (SD) = 31.7 (9.4) PROMIS Depression T-score, mean (SD) = 62.0 (6.3) PHQ-9 score, mean (SD) = 13.4 (4.7) |
SF-12 MCS, mean (SD) = 31.1 (9.3) PROMIS Depression T-score, mean (SD) = 61.4 (6.4) PHQ-9 score, mean (SD) = 13.1 (4.9) |
MDT care, Self-management plans, Coordinated care |
6 months | ✓ | ||||
| Rost et al., 2001 [88] | United States | Treatment = 209 Control = 223 |
Overall = 43 years | Overall = 83.9% | Major depressive disorder | Mean mCESD = 56.9 | Mean mCESD = 57.4 | MDT care, Coordinated care |
6 months | ✓ | ||||
| Salisbury et al., 2018 [89] | United Kingdom | Treatment = 797 Control = 749 |
Treatment = 71 years Control = 70.7 years |
Treatment = 51% Control = 50% |
At least three types of chronic condition—Circulatory system disorders, musculoskeletal disorders, Type 2 Diabetes, cancers, and mental illnesses | Mean (SD) EQ-5D-5L score = 0.574 (0.282) Mean (SD) PACIC score = |
Mean (SD) EQ-5D-5L score = 0.542 (0.292) Mean (SD) PACIC score = |
MDT care, Self-management plans, Coordinated care; Continuity of care |
15 months | ✓ | ||||
| Scherpbier-de Haan et al., 2013 [90] | Netherlands | Treatment = 99 Control = 75 |
Treatment = 73.9 years Control = 72.4 years |
Treatment = 62.2% Control = 47.3% |
Depression and/or hypertension | Mean (SD) SBP = 142.7 (17.6) Mean (SD) DBP = 74.9 (9.2) |
Mean (SD) SBP = 142.5(15.1 )Mean (SD) DBP = 80.4 (8.2) |
MDT care, Self-management plans, Coordinated care |
12 months | ✓ | ||||
| Schnurr et al., 2013 [91] | United States | Treatment = 96 Control = 99 |
Treatment = 46.1 years Control = 44.4 years |
Treatment = 7% Control = 10% |
Posttraumatic Stress Disorder and Depression | PTSD Diagnostic Scale mean (SD)= 33.2 (8.3) Hopkins SCD mean (SD) = 1.98 (0.69) SF-36 Mental Component mean (SD) = 33.8 (8.8) SF-36 Physical Component mean (SD) = 42.2 (13.0) |
PTSD Diagnostic Scale mean (SD)= 34.0 (9.7) Hopkins SCD mean (SD) = 2.06 (0.78) SF-36 Mental Component mean (SD) = 32.7 (8.1) SF-36 Physical Component mean (SD) = 43.4 (12.6) |
MDT care, Self-management plans, Coordinated care |
6 months | ✓ | ✓ | |||
| Simon et al., 2001 [92] | United States | Treatment = 110 Control = 109 |
Overall = 47 years | Treatment = 67% Control = 82% |
Depression | Mean number of depression-free days was 87.7 (95% CI = 76.6–96.7) for the collaborative care group |
Mean number of depression-free days was 70.9 (95% CI = 60.8–81.3) for the usual care group |
MDT care, Self-management plans, Coordinated care |
6 months | ✓ | ||||
| Simon et al., 2004 [93] | United States | Treatment = 198 Control = 195 |
Treatment = 44.7 years Control = 44 years |
Treatment = 74% Control = 78% |
Depression | Mean (SD) SCL = 1.52 (0.58) Mean PHQ (SD) = 14.6 (5.1) |
Mean (SD) SCL = 1.55 (0.62) Mean PHQ (SD) = 15.0 (5.5) |
MDT care, Self-management plans, Coordinated care; Continuity of care |
6 months | ✓ | ||||
| Simpson et al., 2011 [94] | Canada | Treatment = 131 Control = 129 |
Treatment = 58.8 years Control = 59.4 years |
Treatment = 74% Control = 75% |
Type 2 Diabetes | Mean (SD)SBP = 130.4 (14.9) Mean (SD) DBP = 74.4 (10.0) |
Mean (SD) SBP = 128.3 (15.7) Mean (SD) DBP = 73.9 (10.8) |
MDT care, Coordinated care |
12 months | ✓ | ||||
| Smith et al., 2004 [95] | Ireland | Treatment = 96 Control = 87 |
Treatment = 64.7 years Control = 65.6 years |
Treatment = 54% Control = 57% |
Type 2 Diabetes | Mean (SD) HbA1c (%) = 6.85% (1.6) | Mean (SD) HbA1c (%) = 6.6% (1.9) | MDT care, Coordinated care |
12 months | ✓ | ||||
| Tang et al., 2013 [96] | United States | Treatment = 202 Control = 213 |
Treatment = 54 years Control = 53.5 years |
Treatment = 83% Control = 83% |
Type 2 Diabetes | Mean (SD) HbA1c (%) = 9.28 (1.74) | Mean (SD) HbA1c (%) = 9.24 (1.59) | MDT care, Self-management plans, Coordinated care; Continuity of care; Data driven quality of care |
12 months | ✓ | ||||
| Taylor et al., 2005 [97] | Canada | Treatment = 20 Control = 19 |
Treatment = 58 years Control = 67 years |
Treatment = 35% Control = 32% |
Type 2 Diabetes | HbA1c (%) = 7.69 Systolic blood pressure (mm Hg) = 134 Diastolic blood pressure (mm Hg) = 79 Cholesterol (mg/dL) = 194.1 HDL cholesterol (mg/dL) = 44.9 LDL cholesterol (mg/dL) = 116 Triglycerides (mg/dL) = 205.5 (SD or 95% CI not reported) |
HbA1c (%) = 7.69 Systolic blood pressure (mm Hg) = 129 Diastolic blood pressure (mm Hg) = 70 Cholesterol (mg/dL) = 201.01 HDL cholesterol (mg/dL) = 50.3 LDL cholesterol (mg/dL) = 119.1 Triglycerides (mg/dL) = 156.8 (SD or 95% CI not reported) |
MDT care, Self-management plans, Coordinated care |
4 months | ✓ | ||||
| Thorn et al., 2020 [98] | United Kingdom | Treatment = 797 Control = 749 |
Treatment = 71 years Control = 70.7 years |
Treatment = 51% Control = 50% |
Three or more chronic conditions from those included in the National Health Service (NHS) Quality and Outcomes Framework—Circulatory system disorders, musculoskeletal disorders, Type 2 Diabetes, cancers, and mental illnesses |
No. of long-term conditions from QOF: median (IQR) = 3.0 (3.0 to 3.0) | No. of long-term conditions from QOF: median (IQR) = 3.0 (3.0 to 3.0) | MDT care, Patient engagement Coordinated care, Continuity of care |
6 months | ✓ | ||||
| Uijen et al., 2012 [99] | Netherlands | Treatment = 64 Control = 49 |
Treatment = 64 years Control = 63 years |
Treatment = 58% Control = 75% | Chronic obstructive pulmonary disease according to ICD-10 | Self-management group GOLD stage, n (%) GOLD 1 = 13 (20.3) GOLD 2 = 42 (65.6) GOLD 3/4 = 9 (14.1) |
GOLD stage, n (%) GOLD 1 = 11 (22.4) GOLD 2 = 29 (59.2) GOLD 3/4 = 9 (18.4) |
MDT care, Self-management plans, Coordinated care; Continuity of care; |
24 months | ✓ | ||||
| Unutzer et al., 2002 [100] | United States | Treatment = 906 Control = 895 |
Treatment = 71.2 years Control = 71.4 years |
Treatment = 64% Control = 66% |
Major depression and/or dysthymia | Mean (SD) SCL-20 = 1.7 (0.6) | Mean (SD) SCL-20 = 1.7 (0.6) | MDT care, Patient engagement Coordinated care |
12 months | ✓ | ✓ | |||
| Unutzer et al., 2008 [101] | United States | Treatment = 279 Control = 272 |
Treatment = 72.6 years Control = 72.7 years |
Treatment = 70% Control = 75% |
Major depression and/or dysthymia | Depression severity score, mean (SD) = 1.7 (0.5) | Depression severity score, mean (SD) = 1.7 (0.6) | MDT care, Patient engagement Coordinated care |
48 months | ✓ | ||||
| van Orden et al., 2009 [102] | Netherlands | Treatment = 102 Control = 63 |
Treatment = 40.2 years Control = 40.4 years |
Treatment = 72% Control = 62% |
Mental disorder | SCL-90 Mean (SD) = 181.2 (58.6) WHOQOL-BREF Mean (SD) = 3.0 (0.8) |
SCL-90 Mean (SD) = 188.4 (64.2) WHOQOL-BREF Mean (SD) = 3.0 (1.0) |
MDT care, Patient engagement Coordinated care |
12 months | ✓ | ✓ | |||
| Vera et al., 2010 [103] | Puerto Rico | Treatment = 89 Control = 90 |
Treatment = 57 years Control = 53 years |
Treatment = 74% Control = 78% |
Major depression and had any of the following health conditions: diabetes, hypothyroidism, asthma, hypertension, chronic bronchitis, arthritis, heart disease, high cholesterol, or stroke. | HSCL depression Mean (SD) = 2.22 (0.54) | HSCL depression Mean (SD) = 2.34 (0.58) | MDT care, Self-management plans, Coordinated care; Continuity of care; |
6 months | ✓ | ||||
| Von Korff et al., 1998 [104] | United States | 1st trial Treatment = 41 Control = 33 2nd trialTreatment = 26 Control = 31 |
NA | NA | Depression and on anti-depressant medications | Major depression Total depression treatment costs = $1337 Minor depression Total depression treatment costs = $1298 |
Major depression Total depression treatment costs = $850 Minor depression Total depression treatment costs = $656 |
MDT care, Patient engagement Coordinated care |
12 months | ✓ | ||||
| Von Korff et al., 2011 [105] | United States | Treatment = 106 Control = 107 |
Treatment = 57.4 years Control = 56.3 years |
Treatment = 48% Control = 56% |
Diabetes, coronary heart disease, and depression | Sheehan social role disability scale = 5.6 (2.4) Global quality of life rating = 4.2 (1.9) WHODAS-2 activities of daily living = 15.8 (9.6) |
Sheehan social role disability scale = 5.1 (2.6) Global quality of life rating = 4.7 (1.8) WHODAS-2 activities of daily living = 13.8 (9.6) |
MDT care, Patient engagement Coordinated care, Continuity of care |
12 months | ✓ | ||||
| Zwar et al., 2016 [106] | Australia | Treatment = 144 Control = 110 |
Treatment = 66.5 years Control = 65.4 years |
Treatment = 61.1% Control = 58.2% |
Chronic obstructive pulmonary disease | Mean total SGRQ score (SD) = 20.0 (17.2) | Mean total SGRQ score (SD) = 18.9 (16.8) | MDT care, Patient engagement Coordinated care |
12 months | ✓ | ✓ | |||
BADL—Basic Activities of Daily Living; BAI—Beck Anxiety Inventory; BP—blood pressure; CCM—chronic care model; CCQ—Clinical COPD questionnaire; CES-D—Center for Epidemiologic Studies Depression Scale; CI—confidence interval; DBP—diastolic blood pressure; DSM-IV—Diagnostic and Statistical Manual of Mental Disorders 4th edition; EQ3D—EuroQol 3 dimensions; EQ5D—EuroQol 5 dimensions; GAD—Generalized Anxiety Disorder; GADSS—Generalized Anxiety Disorder Severity Scale; GARS—Gilliam Autism Rating Scale; GOLD—Global initiative for Chronic Obstructive Lung Disease; HAM-D—Hamilton Depression Rating Scale; HbA1c—glycated haemoglobin; HDL—high density lipoprotein; HSCL—Hopkins Symptom Checklist; IADL—Instrumental Activities of Daily Living; ICD-10—10th revision of the International Statistical Classification of Diseases and Related Health Problems; IQR—interquartile range; LDL—low density lipoprotein; MADRS-S—Montgomery and Asberg Depression Rating Scale; MCS—mental component scores; MDT—multidisciplinary team; NA—not available; PACIC- Patient Assessment of Care for Chronic Conditions; PCS—physical component scores; PDSS—Panic Disorder Severity Scale; PHQ—Patient Health Questionnaire; PROMIS—Patient-Reported Outcomes Measurement Information System; PTSD—Post-traumatic stress disorder; QOF—Quality and Outcomes Framework; RMDQ—Roland-Morris Questionnaire; SAQ—Seattle Angina Questionnaire; SBP—systolic blood pressure; SD—standard deviation; SF 12 and SF 36—short and long format of a single measures of HRQoL.
Table A4.
Characteristics of non-randomised controlled trials reviewed.
| Chronic Physical Conditions—Baseline Characteristics (Risk Proportion/Mean or Median and SD) | Outcomes | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Authors and Year of Publication | Country of origin | Sample Size (N) | Mean Age/Age Groups | Gender Distribution (Female) |
Chronic Disease Profile of the Sample Population | Treatment Group | Control GROUP | PCMH Components | Duration of Follow-up | Depression | Quality of Life/Self-Management | Hospital Admission | Cost/Health Utility | Biomedical Outcomes |
| Bray et al., 2013 [17] | United States | Treatment = 368 Control = 359 |
Treatment = 59.5 years Control = 60.6 years |
Treatment = 66% Control = 63% |
Type 2 diabetes mellitus | HbA1c, mean (SD), % = 7.9 (2.2) SBP/DBP, mean (SD), mm Hg = 138 (18)/81 (10) HDL cholesterol, mean (SD), mg/dL= 50 (13.3) Total cholesterol, mean (SD), mg/dL = 176 (39.7) |
HbA1c, mean (SD), % = 7.9 (2.2) SBP/DBP, mean (SD), mm Hg = 138 (18)/81 (10) HDL cholesterol, mean (SD), mg/dL= 50 (13.3) Total cholesterol, mean (SD), mg/dL = 176 (39.7) |
6 key elements to the intervention design: education with behavioural coaching, treatment intensification, point-of-care management, expanded roles of clinic staff to facilitate management, a team care approach, and physician leadership | 36 months | ✓ | ||||
| Kravertz et al., 2016 [107] | United States | Treatment = 350 Control = 315 |
Treatment = 72.7 years Control = 72.2 years |
NA | Hypertension | SBP = 167.7 DBP = 84 (SD or 95% CI not reported) |
NA | MDT care, Patient education Coordinated care |
4 months | ✓ | ||||
| Petersen et al., 2019 [109] | South Africa | Treatment = 137 Control = 236 |
Treatment = 42.6 years Control = 44 years |
Treatment = 83.2% Control = 80.5% |
Mental and other comorbid conditions | PHQ-9 mean (SD) = 14.5 (3.47) WHODAS mean (SD) = 37.6 (17.19) |
PHQ-9 mean (SD) = 12.8 (3.01) WHODAS mean (SD) = 40.0 (19.48) |
MDT care, Patient engagement Coordinated care |
12 months | ✓ | ||||
| Ruikes et al., 2016 [21] | Netherlands | Treatment = 287 Control = 249 |
Treatment = 83.1 years Control = 80.5 years |
Treatment = 66.9% Control = 64.3% |
Frail elderly people with multimorbidity | Katz-15 index, mean (SD) = 5.4 (2.9) | Katz-15 index, mean (SD) = 4.6 (2.7) | MDT care, Self-management plans, Coordinated care |
12 months | ✓ | ✓ | |||
| Seidu et al., 2017 [110] | United Kingdom | Treatment = 6054 Control = 2312 |
% above 65 years Treatment = 14.20 Control = 11.31 |
Treatment = 50.6% Control = 47.4% |
Type 2 diabetes mellitus | Non-elective bed days, mean (SD) = 5.62 (2.11) | Non-elective bed days, mean (SD) = 3.82 (1.62) | MDT care, Self-management plans, Coordinated care |
12 months | ✓ | ||||
| Sommers et al., 2000 [111] | United States | Treatment = 280 Control = 263 |
Treatment = 78 years Control = 77 years |
1 | Frail elderly people with multimorbidity | Hospital admissions per patient per year, mean (SD) = 0.34 (0.68) ≥1 hospital admission within 60 days % = 4.5 ≥1 ED visit % = 9.0 |
Hospital admissions per patient per year, mean (SD) = 0.39 (0.81) ≥1 hospital admission within 60 days % = 5.9 ≥1 ED visit % = 5.9 |
MDT care, Self-management plans, Coordinated care |
24 months | ✓ | ||||
| Vestjens et al., 2019 [108] | Netherlands | Treatment = 232 Control = 232 |
Treatment = 82.4 years Control = 82.4 years |
Treatment = 72.4% Control = 72.4% |
Frail elderly people with multimorbidity | EQ5D3L = 0.63 (0.26) | EQ5D3L = 0.66 (0.24) | MDT care, Patient engagement Coordinated care |
12 months | ✓ | ||||
BP—blood pressure; CI—confidence interval; DBP—diastolic blood pressure; ED—emergency department; EQ3D—EuroQol 3 dimensions; HbA1c—glycated haemoglobin; HDL—high density lipoprotein; LDL—low density lipoprotein; MDT—multidisciplinary team; NA—not available; PHQ—Patient Health Questionnaire; SBP—systolic blood pressure; SD—standard deviation; WHODAS—World Health Organization Disability Assessment Schedule.
Table A5.
Quality assessment of randomised controlled studies using Joanna Briggs Institute (JBI) critical appraisal checklist.
| Author and Year | Q 1 | Q 2 | Q 3 | Q 4 | Q 5 | Q 6 | Q 7 | Q 8 | Q 9 | Q 10 | Q 11 | Q 12 | Q 13 | Quality |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alexopoulos et al., 2009 [36] | U | U | N | NA | NA | U | Y | N | Y | Y | Y | Y | Y | Fair |
| Aragonès et al., 2014 [18] | U | U | Y | NA | NA | Y | Y | Y | Y | Y | Y | Y | Y | Good |
| Aragonès et al., 2019 [38] | Y | Y | Y | NA | NA | Y | Y | N | Y | Y | Y | Y | Y | Good |
| Barcelo et al., 2010 [39] | U | U | Y | NA | NA | U | Y | N | Y | Y | Y | U | Y | Fair |
| Bjorkelund et al., 2018 [40] | U | U | Y | NA | NA | U | Y | Y | Y | Y | Y | Y | Y | Good |
| Blom et al., 2016 [41] | U | U | Y | NA | NA | Y | Y | N | Y | Y | Y | Y | Y | Good |
| Bogner et al., 2008 [42] | U | U | Y | NA | NA | U | Y | N | Y | Y | Y | Y | Y | Fair |
| Bogner et al., 2012 [43] | Y | Y | Y | NA | NA | Y | Y | N | Y | Y | Y | Y | Y | Good |
| Borenstein et al., 2003 [45] | U | U | Y | NA | NA | U | Y | N | Y | Y | Y | U | Y | Fair |
| Bosanquet et al., 2017 [46] | Y | Y | Y | NA | NA | Y | Y | Y | Y | Y | Y | Y | Y | Good |
| Boult et al., 2008 [47] | U | U | Y | NA | NA | Y | Y | Y | Y | Y | Y | Y | Y | Good |
| Boult et al., 2011 [48] | U | U | Y | NA | NA | Y | Y | Y | Y | Y | Y | Y | Y | Good |
| Callahan et al., 2005 [49] | U | U | Y | NA | NA | U | Y | N | Y | Y | Y | Y | Y | Fair |
| Camacho et al., 2018 [13] | Y | Y | Y | NA | NA | Y | Y | N | Y | Y | Y | Y | Y | Good |
| Campins et al., 2017 [20] | Y | Y | Y | NA | NA | N | Y | N | Y | Y | Y | Y | Y | Good |
| Chaney et al., 2011 [50] | U | U | Y | NA | NA | U | Y | Y | Y | Y | Y | Y | Y | Good |
| Cooper et al., 2013 [51] | Y | Y | Y | NA | NA | N | Y | U | Y | Y | Y | Y | Y | Good |
| Coventry et al., 2015 [14] | Y | Y | Y | NA | NA | N | Y | Y | Y | Y | Y | Y | Y | Good |
| Dobscha et al., 2009 [53] | Y | Y | Y | NA | NA | Y | Y | Y | Y | Y | Y | Y | Y | Good |
| Dorr et al., 2008 [54] | U | U | Y | NA | NA | U | Y | N | U | Y | Y | Y | Y | Fair |
| Edelman et al., 2010 [16] | Y | Y | Y | NA | NA | Y | Y | U | U | Y | Y | Y | Y | Fair |
| Engel et al., 2016 [55] | Y | Y | Y | NA | NA | N | Y | N | Y | Y | Y | Y | Y | Good |
| Fihn et al., 2011 [56] | U | U | Y | NA | NA | N | Y | N | Y | Y | Y | Y | Y | Fair |
| Gilbody et al., 2017 [57] | Y | Y | Y | NA | NA | Y | Y | Y | Y | Y | Y | Y | Y | Good |
| Green et al., 2014 [59] | U | U | Y | NA | NA | N | Y | N | Y | Y | Y | Y | Y | Fair |
| Hirsch et al., 2014 [61] | Y | Y | Y | NA | NA | N | Y | N | Y | Y | Y | Y | Y | Good |
| Hsu et al., 2014 [62] | U | U | N | NA | NA | U | Y | N | U | Y | Y | Y | U | Poor |
| Huijbregts et al., 2013 [63] | Y | Y | Y | NA | NA | U | Y | Y | Y | Y | Y | Y | Y | Good |
| Ip et al., 2013 [64] | U | U | Y | NA | NA | U | Y | N | Y | Y | Y | Y | Y | Fair |
| Katon et al., 2012 [66] | U | U | Y | NA | NA | Y | Y | Y | Y | Y | Y | Y | Y | Good |
| Katon et al., 1999 [67] | Y | Y | Y | NA | NA | Y | Y | Y | Y | Y | Y | Y | Y | Good |
| Katon et al., 2010 [68] | Y | Y | Y | NA | NA | Y | Y | Y | Y | Y | Y | Y | Y | Good |
| Katon et al., 2004 [70] | Y | Y | Y | NA | NA | Y | Y | Y | Y | Y | Y | Y | Y | Good |
| Konnopka et al., 2016 [22] | U | U | Y | NA | NA | N | Y | N | Y | Y | Y | Y | Y | Fair |
| Krein et al., 2004 [71] | Y | Y | Y | NA | NA | Y | Y | Y | Y | Y | Y | Y | Y | Good |
| Kruis et al., 2014 [72] | Y | Y | Y | NA | NA | Y | Y | Y | Y | Y | Y | Y | Y | Good |
| Lin et al., 2000 [76] | Y | Y | Y | NA | NA | Y | Y | Y | Y | Y | U | Y | Y | Fair |
| Lin et al., 2006 [74] | U | U | U | NA | NA | U | Y | N | Y | Y | Y | Y | Y | Fair |
| Lin et al., 2012 [75] | Y | Y | Y | NA | NA | N | Y | N | Y | Y | Y | Y | Y | Good |
| Maislos et al., 2004 [77] | U | U | Y | NA | NA | U | Y | N | Y | Y | Y | Y | Y | Fair |
| Menchetti et al., 2013 [78] | Y | Y | Y | NA | NA | U | Y | Y | Y | Y | Y | Y | Y | Good |
| Muntingh et al., 2013 [19] | Y | Y | N | NA | NA | Y | Y | U | Y | Y | Y | Y | Y | Fair |
| Ramli et al., 2016 [82] | Y | Y | Y | NA | NA | U | Y | Y | Y | Y | Y | Y | Y | Good |
| Richards et al., 2013 [83] | Y | Y | Y | NA | NA | U | Y | Y | Y | Y | Y | Y | Y | Good |
| Richards et al., 2008 [84] | Y | Y | Y | NA | NA | Y | Y | Y | Y | Y | Y | Y | Y | Good |
| Rollman et al., 2005 [86] | Y | Y | Y | NA | NA | Y | Y | Y | Y | Y | Y | Y | Y | Good |
| Rollman et al., 2017 [85] | Y | Y | Y | NA | NA | Y | Y | Y | Y | Y | Y | Y | Y | Good |
| Rollman et al., 2018 [87] | Y | Y | Y | NA | NA | Y | Y | Y | Y | Y | Y | Y | Y | Good |
| Rost et al., 2001 [88] | N | N | Y | NA | NA | U | Y | N | Y | Y | Y | Y | Y | Fair |
| Salisbury et al., 2018 [89] | Y | Y | Y | NA | NA | Y | Y | Y | Y | Y | Y | Y | Y | Good |
| Scherpbier-de Haan et al., 2013 [90] | U | U | Y | NA | NA | N | Y | Y | Y | Y | Y | Y | Y | Good |
| Schnurr et al., 2013 [91] | Y | Y | Y | NA | NA | Y | Y | Y | Y | Y | Y | Y | Y | Good |
| Simon et al., 2004 [93] | Y | Y | Y | NA | NA | Y | Y | Y | Y | Y | Y | Y | Y | Good |
| Simpson et al., 2011 [94] | Y | Y | Y | NA | NA | Y | Y | Y | Y | Y | Y | Y | Y | Good |
| Smith et al., 2004 [95] | Y | Y | Y | NA | NA | U | Y | Y | Y | Y | Y | Y | Y | Good |
| Tang et al., 2013 [96] | Y | Y | Y | NA | NA | Y | Y | Y | Y | Y | Y | Y | Y | Good |
| Taylor et al., 2005 [97] | Y | Y | Y | NA | NA | N | Y | N | Y | Y | U | Y | Y | Good |
| Uijen et al., 2012 [99] | Y | Y | Y | NA | NA | Y | Y | Y | Y | Y | Y | Y | Y | Good |
| Unutzer et al., 2002 [100] | Y | Y | Y | NA | NA | Y | Y | Y | Y | Y | Y | Y | Y | Good |
| van Orden et al., 2009 [102] | Y | Y | Y | NA | NA | N | Y | N | Y | Y | Y | Y | Y | Good |
| Vera et al., 2010 [103] | Y | Y | Y | NA | NA | Y | Y | N | Y | Y | Y | Y | Y | Good |
| Von Korff et al., 2011 [105] | Y | Y | Y | NA | NA | N | Y | N | Y | Y | Y | Y | Y | Good |
| Zwar et al., 2016 [106] | Y | Y | Y | NA | NA | Y | Y | Y | Y | Y | Y | Y | Y | Good |
NA—Most did not blind participants or personnel as it was not practical. Therefore, we did not downgrade for these risks/uncertainties.
Table A6.
Quality assessment of non-randomised controlled studies using JBI critical appraisal checklist.
| Author and Year | Q 1 | Q 2 | Q 3 | Q 4 | Q 5 | Q 6 | Q 7 | Q 8 | Q 9 | Quality |
|---|---|---|---|---|---|---|---|---|---|---|
| Bray et al., 2013 [17] | Y | Y | Y | Y | Y | Y | Y | Y | Y | Good |
| Kravertz et al., 2016 [107] | Y | Y | Y | Y | Y | U | Y | U | U | Fair |
| Petersen et al., 2019 [109] | Y | Y | Y | Y | Y | Y | Y | Y | Y | Good |
| Ruikes et al., 2016 [21] | Y | Y | Y | Y | Y | Y | Y | Y | Y | Good |
| Seidu et al., 2017 [110] | Y | Y | Y | Y | Y | U | Y | U | U | Fair |
| Sommers et al., 2000 [111] | Y | Y | Y | Y | Y | U | Y | Y | Y | Good |
| Vestjens et al., 2019 [108] | Y | Y | Y | Y | Y | Y | Y | Y | Y | Good |
Table A7.
Quality assessment of studies on economic evaluation using JBI critical appraisal checklist.
| Author and Year | Q 1 | Q 2 | Q 3 | Q 4 | Q 5 | Q 6 | Q 7 | Q 8 | Q 9 | Q 10 | Q 11 | Quality |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aragonès et al., 2014 (Cost-effectiveness) [37] | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Good |
| Boland et al., 2015 [44] | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | Good |
| Dickinson et al., 2010 [52] | Y | Y | Y | Y | U | U | Y | Y | U | Y | U | Fair |
| Goorden et al., 2015 [58] | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | U | Good |
| Grochtdreis et al., 2019 [60] | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | Good |
| Johnson et al., 2016 [65] | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Good |
| Katon et al., 2005 [69] | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | U | Good |
| Leeuwen et al., 2015 [73] | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Good |
| Metzelthin et al., 2015 [79] | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | U | Good |
| Morgan et al., 2015 [80] | Y | Y | Y | Y | U | U | Y | N | N | Y | U | Fair |
| Pyne et al., 2003 [81] | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | U | Good |
| Simon et al., 2001 [92] | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | U | Good |
| Thorn et al., 2020 [98] | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | U | Good |
| Unutzer et al., 2008 [101] | Y | Y | Y | Y | U | U | Y | N | N | U | Y | Fair |
| Von Korff et al., 1998 [104] | Y | Y | Y | Y | U | U | U | N | N | Y | U | Poor |
Table A8.
Quality assessment of non-randomised controlled studies using Risk of Bias In Non-randomised Studies of Interventions (ROBINS-I) tool.
| Author and Year | Q 1 | Q 2 | Q 3 | Q 4 | Q 5 | Q 6 | Q 7 | Overall |
|---|---|---|---|---|---|---|---|---|
| Bray et al., 2013 [17] | Low | Low | Low | Low | Low | Low | Low | Good |
| Kravertz et al., 2016 [107] | Moderate | Low | Low | Low | Low | Low | Moderate | Fair |
| Petersen et al., 2019 [109] | Low | Low | Low | Low | Low | Low | Low | Good |
| Ruikes et al., 2016 [21] | Low | Low | Low | Low | Low | Low | Low | Good |
| Seidu et al., 2017 [110] | Moderate | Low | Low | Low | Low | Low | Moderate | Fair |
| Sommers et al., 2000 [111] | Low | Low | Low | Low | Low | Low | Low | Good |
| Vestjens et al., 2019 [108] | Low | Low | Low | Low | Low | Low | Low | Good |
Author Contributions
Conceptualization, J.R.J.; methodology, J.R.J. and K.A.; formal analysis, J.R.J. and K.A.; investigation, J.R.J., K.P., and H.J.; data curation, J.R.J., K.P., and H.J.; writing—original draft preparation, J.R.J.; writing—review and editing, J.R.J., W.K.T., and K.A.; and supervision, W.K.T. and K.A. All authors have read and agreed to the published version of the manuscript.
Funding
J.R.J.’s PhD scholarship was provided by Capital Markets Cooperative Research Centre (Now Rozetta Institute). The funders did not have any role in the design, methods, analysis, or preparation of this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Hay S.I., Abajobir A.A., Abate K.H., Abbafati C., Abbas K.M., Abd-Allah F., Abdulkader R.S., Abdulle A.M., Abebo T.A., Abera S.F. Global, regional, and national disability-adjusted life-years (DALYs) for 333 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1260–1344. doi: 10.1016/S0140-6736(17)32130-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Naghavi M., Abajobir A.A., Abbafati C., Abbas K.M., Abd-Allah F., Abera S.F., Aboyans V., Adetokunboh O., Afshin A., Agrawal A. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1151–1210. doi: 10.1016/S0140-6736(17)32152-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Oostrom S.H., Gijsen R., Stirbu I., Korevaar J.C., Schellevis F.G., Picavet H.S.J., Hoeymans N. Time trends in prevalence of chronic diseases and multimorbidity not only due to aging: Data from general practices and health surveys. PLoS ONE. 2016;11:e0160264. doi: 10.1371/journal.pone.0160264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Afshar S., Roderick P.J., Kowal P., Dimitrov B.D., Hill A.G. Multimorbidity and the inequalities of global ageing: A cross-sectional study of 28 countries using the World Health Surveys. BMC Public Health. 2015;15:776. doi: 10.1186/s12889-015-2008-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fortin M., Lapointe L., Hudon C., Vanasse A., Ntetu A.L., Maltais D. Multimorbidity and quality of life in primary care: A systematic review. Health Qual. Life Outcomes. 2004;2:51. doi: 10.1186/1477-7525-2-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Megari K. Quality of life in chronic disease patients. Health Psychol. Res. 2013;1:e27. doi: 10.4081/hpr.2013.932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McPhail S.M. Multimorbidity in chronic disease: Impact on health care resources and costs. Risk Manag. Healthc. Policy. 2016;9:143. doi: 10.2147/RMHP.S97248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vogeli C., Shields A.E., Lee T.A., Gibson T.B., Marder W.D., Weiss K.B., Blumenthal D. Multiple chronic conditions: Prevalence, health consequences, and implications for quality, care management, and costs. J. Gen. Intern. Med. 2007;22:391–395. doi: 10.1007/s11606-007-0322-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barnett K., Mercer S.W., Norbury M., Watt G., Wyke S., Guthrie B. Epidemiology of multimorbidity and implications for health care, research, and medical education: A cross-sectional study. Lancet. 2012;380:37–43. doi: 10.1016/S0140-6736(12)60240-2. [DOI] [PubMed] [Google Scholar]
- 10.Coleman K., Austin B.T., Brach C., Wagner E.H. Evidence on the chronic care model in the new millennium. Health Aff. 2009;28:75–85. doi: 10.1377/hlthaff.28.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organisation Multimorbidity: Technical Series on Safer Primary Care. Geneva. [(accessed on 13 April 2020)];2016 Available online: https://apps.who.int/iris/bitstream/handle/10665/252275/9789241511650-eng.pdf?sequence=1.
- 12.Australian Medical Association AMA Position Statement on the Medical Home-2015. [(accessed on 12 April 2020)]; Available online: https://ama.com.au/position-statement/ama-position-statement-medical-home.
- 13.Camacho E.M., Davies L.M., Hann M., Small N., Bower P., Chew-Graham C., Waheed W. Long-term clinical and cost-effectiveness of collaborative care (versus usual care) for people with mental–physical multimorbidity: Cluster-randomised trial. Br. J. Psychiatry. 2018;213:1–8. doi: 10.1192/bjp.2018.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coventry P., Lovell K., Dickens C., Bower P., Chew-Graham C., McElvenny D., Baguley C. Integrated primary care for patients with mental and physical multimorbidity: Cluster randomised controlled trial of collaborative care for patients with depression comorbid with diabetes or cardiovascular disease. BMJ. 2015;350:h638. doi: 10.1136/bmj.h638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maeng D.D., Graf T.R., Davis D.E., Tomcavage J., Bloom F.J., Jr. Can a patient-centered medical home lead to better patient outcomes? The quality implications of Geisinger’s ProvenHealth Navigator. Am. J. Med. Qual. 2012;27:210–216. doi: 10.1177/1062860611417421. [DOI] [PubMed] [Google Scholar]
- 16.Edelman D., Fredrickson S.K., Melnyk S.D., Coffman C.J., Jeffreys A.S., Datta S., Stein J. Medical clinics versus usual care for patients with both diabetes and hypertension: A randomized trial. Ann. Intern. Med. 2010;152:689–696. doi: 10.7326/0003-4819-152-11-201006010-00001. [DOI] [PubMed] [Google Scholar]
- 17.Bray P., Cummings D.M., Morrissey S., Thompson D., Holbert D., Wilson K., Tanenberg R. Improved outcomes in diabetes care for rural African Americans. Ann. Fam. Med. 2013;11:145–150. doi: 10.1370/afm.1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aragonès E., Caballero A., Piñol J.-L., López-Cortacans G. Persistence in the long term of the effects of a collaborative care programme for depression in primary care. J. Affect. Disord. 2014;166:36–40. doi: 10.1016/j.jad.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 19.Muntingh A., Van Der Feltz-cornelis C., Van Marwijk H., Spinhoven P., Assendelft W., De Waal M., Van Balkom A. Effectiveness of collaborative stepped care for anxiety disorders in primary care: A pragmatic cluster randomised controlled trial. Psychother. Psychosom. 2014;83:37–44. doi: 10.1159/000353682. [DOI] [PubMed] [Google Scholar]
- 20.Campins L., Serra-Prat M., Gózalo I., López D., Palomera E., Agustí C., REMEI Group Randomized controlled trial of an intervention to improve drug appropriateness in community-dwelling polymedicated elderly people. Fam. Pract. 2017;34:36–42. doi: 10.1093/fampra/cmw073. [DOI] [PubMed] [Google Scholar]
- 21.Ruikes F.G., Zuidema S.U., Akkermans R.P., Assendelft W.J., Schers H.J., Koopmans R.T. Multicomponent program to reduce functional decline in frail elderly people: A cluster controlled trial. J. Am. Board Fam. Med. 2016;29:209–217. doi: 10.3122/jabfm.2016.02.150214. [DOI] [PubMed] [Google Scholar]
- 22.Konnopka A., König H.-H., Kaufmann C., Egger N., Wild B., Szecsenyi J., Schaefert R. Cost-utility of a specific collaborative group intervention for patients with functional somatic syndromes. J. Psychosom. Res. 2016;90:43–50. doi: 10.1016/j.jpsychores.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 23.Jackson G.L., Powers B.J., Chatterjee R., Bettger J.P., Kemper A.R., Hasselblad V., Gray R. The patient-centered medical Home: A Systematic review. Ann. Intern. Med. 2013;158:169–178. doi: 10.7326/0003-4819-158-3-201302050-00579. [DOI] [PubMed] [Google Scholar]
- 24.Moher D., Shamseer L., Clarke M., Ghersi D., Liberati A., Petticrew M., Stewart L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015;4:1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.John J.R., Ghassempour S., Girosi F., Atlantis E. The effectiveness of patient-centred medical home model versus standard primary care in chronic disease management: Protocol for a systematic review and meta-analysis of randomised and non-randomised controlled trials. Syst. Rev. 2018;7:1–6. doi: 10.1186/s13643-018-0887-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rathert C., Wyrwich M.D., Boren S.A. Patient-centered care and outcomes: A systematic review of the literature. Med. Care Res. Rev. 2013;70:351–379. doi: 10.1177/1077558712465774. [DOI] [PubMed] [Google Scholar]
- 27.Hoff T., Weller W., DePuccio M. The patient-centered medical home: A review of recent research. Med. Care Res. Rev. 2012;69:619–644. doi: 10.1177/1077558712447688. [DOI] [PubMed] [Google Scholar]
- 28.Bernstein K.M., Manning D.A., Julian R.M. Multidisciplinary teams and obesity: Role of the modern patient-centered medical home. Prim. Care. 2016;43:53–59. doi: 10.1016/j.pop.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 29.Tacconelli E. Systematic reviews: CRD’s guidance for undertaking reviews in health care. Lancet Infect Dis. 2010;10:226. doi: 10.1016/S1473-3099(10)70065-7. [DOI] [Google Scholar]
- 30.Aromataris E., Munn Z. Joanna Briggs Institute Reviewer’s Manual. The Joanna Briggs Institute; Adelaide, SA, Australia: 2017. p. 299. [Google Scholar]
- 31.Gomersall J.S., Jadotte Y.T., Xue Y., Lockwood S., Riddle D., Preda A. Conducting systematic reviews of economic evaluations. Int. J. Evid. Based Healthc. 2015;13:170–178. doi: 10.1097/XEB.0000000000000063. [DOI] [PubMed] [Google Scholar]
- 32.Sterne J.A., Hernán M.A., Reeves B.C., Savović J., Berkman N.D., Viswanathan M., Carpenter J.R. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guyatt G.H., Oxman A.D., Vist G.E., Kunz R., Falck-Ytter Y., Alonso-Coello P., Schünemann H.J. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Higgins J.P., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 35.Egger M., Smith G.D., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alexopoulos G.S., Reynolds C.F., Bruce M.L., Katz I.R., Raue P.J., Mulsant B.H., PROSPECT Group Reducing suicidal ideation and depression in older primary care patients: 24-month outcomes of the PROSPECT study. Am. J. Psychiatry. 2009;166:882–890. doi: 10.1176/appi.ajp.2009.08121779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aragones E., Lopez-Cortacans G., Sanchez-Iriso E., Pinol J.L., Caballero A., Salvador-Carulla L., Cabasés J. Cost-effectiveness analysis of a collaborative care programme for depression in primary care. J. Affect. Disord. 2014;159:85–93. doi: 10.1016/j.jad.2014.01.021. [DOI] [PubMed] [Google Scholar]
- 38.Aragones E., Rambla C., Lopez-Cortacans G., Tome-Pires C., Sanchez-Rodriguez E., Caballero A., Miró J. Effectiveness of a collaborative care intervention for managing major depression and chronic musculoskeletal pain in primary care: A cluster-randomised controlled trial. J. Affect. Disord. 2019;252:221–229. doi: 10.1016/j.jad.2019.04.004. [DOI] [PubMed] [Google Scholar]
- 39.Barceló A., Cafiero E., De Boer M., Mesa A.E., Lopez M.G., Jiménez R.A., Bonfil G.M. Using collaborative learning to improve diabetes care and outcomes: The VIDA project. Prim. Care Diabetes. 2010;4:145–153. doi: 10.1016/j.pcd.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 40.Björkelund C., Svenningsson I., Hange D., Udo C., Petersson E.L., Ariai N., Wallin L. Clinical effectiveness of care managers in collaborative care for patients with depression in Swedish primary health care: A pragmatic cluster randomized controlled trial. BMC Fam. Pract. 2018;19:28. doi: 10.1186/s12875-018-0711-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blom J., Elzen W.D., Houwelingen A.H.V., Heijmans M., Stijnen T., Van Den Hout W., Gussekloo J. Effectiveness and cost-effectiveness of a proactive, goal-oriented, integrated care model in general practice for older people. A cluster randomised controlled trial: Integrated systematic care for older people-the ISCOPE study. Age Ageing. 2016;45:30–41. doi: 10.1093/ageing/afv174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bogner H.R., de Vries H.F., Bogner H.R., de Vries H.F. Integration of depression and hypertension treatment: A pilot, randomized controlled trial. Ann. Fam. Med. 2008;6:295–301. doi: 10.1370/afm.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bogner H.R., Morales K.H., de Vries H.F., Cappola A.R., Bogner H.R., Morales K.H., Cappola A.R. Integrated management of type 2 diabetes mellitus and depression treatment to improve medication adherence: A randomized controlled trial. Ann. Fam. Med. 2012;10:15–22. doi: 10.1370/afm.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boland M.R.S., Kruis A.L., Tsiachristas A., Assendelft W.J.J., Gussekloo J., Blom C.M.G., Rutten-van Mölken M.P. Cost-effectiveness of integrated COPD care: The RECODE cluster randomised trial. BMJ Open. 2015;5:e007284. doi: 10.1136/bmjopen-2014-007284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Borenstein J.E., Graber G., Saltiel E., Wallace J., Ryu S., Jackson A., Weingarten S.R. Physician-pharmacist comanagement of hypertension: A randomized, comparative trial. Pharmacotherapy. 2003;23:209–216. doi: 10.1592/phco.23.2.209.32096. [DOI] [PubMed] [Google Scholar]
- 46.Bosanquet K., Adamson J., Atherton K., Bailey D., Baxter C., Beresford-Dent J., Ekers D. Collaborative care for Screen-Positive EldeRs with major depression (CASPER plus): A multicentred randomised controlled trial of clinical effectiveness and cost-effectiveness. Health Technol. Assess. 2017;21:1–252. doi: 10.3310/hta21670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boult C., Reider L., Frey K., Left B., Boyd C.M., Wolff J.L., Scharfstein D. Early Effects of “Guided Care” on the Quality of Health Care for Multimorbid Older Persons: A Cluster-Randomized Controlled Trial. J. Gerontol. A Biol. Sci. Med. Sci. 2008;63:321–327. doi: 10.1093/gerona/63.3.321. [DOI] [PubMed] [Google Scholar]
- 48.Boult C., Reider L., Leff B., Frick K.D., Boyd C.M., Wolff J.L., Scharfstein D. The effect of guided care teams on the use of health services: Results from a cluster-randomized controlled trial. Arch. Intern. Med. 2011;171:460–466. doi: 10.1001/archinternmed.2010.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Callahan C.M., Kroenke K., Counsell S.R., Hendrie H.C., Perkins A.J., Katon W., IMPACT investigators Treatment of depression improves physical functioning in older adults. J. Am. Geriatr. Soc. 2005;53:367–373. doi: 10.1111/j.1532-5415.2005.53151.x. [DOI] [PubMed] [Google Scholar]
- 50.Chaney E.F., Rubenstein L.V., Liu C.-F., Yano E.M., Bolkan C., Lee M., Uman J. Implementing collaborative care for depression treatment in primary care: A cluster randomized evaluation of a quality improvement practice redesign. Implement Sci. 2011;6:121. doi: 10.1186/1748-5908-6-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cooper L.A., Ghods Dinoso B.K., Ford D.E., Roter D.L., Primm A.B., Larson S.M., Wang N.Y. Comparative effectiveness of standard versus patient-centered collaborative care interventions for depression among African Americans in primary care settings: The BRIDGE Study. Health Serv. Res. 2013;48:150–174. doi: 10.1111/j.1475-6773.2012.01435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dickinson K.C., Sharma R., Duckart J.P., Corson K., Gerrity M.S., Dobscha S.K. VA healthcare costs of a collaborative intervention for chronic pain in primary care. Med. Care. 2010;48:38–44. doi: 10.1097/MLR.0b013e3181bd49e2. [DOI] [PubMed] [Google Scholar]
- 53.Dobscha S.K., Corson K., Perrin N.A., Hanson G.C., Leibowitz R.Q., Doak M.N., Gerrity M.S. Collaborative care for chronic pain in primary care: A cluster randomized trial. JAMA. 2009;301:1242–1252. doi: 10.1001/jama.2009.377. [DOI] [PubMed] [Google Scholar]
- 54.Dorr D.A., Wilcox A.B., Brunker C.P., Burdon R.E., Donnelly S.M. The effect of technology-supported, multidisease care management on the mortality and hospitalization of seniors. J. Am. Geriatr. Soc. 2008;56:2195–2202. doi: 10.1111/j.1532-5415.2008.02005.x. [DOI] [PubMed] [Google Scholar]
- 55.Engel C.C., Jaycox L.H., Freed M.C., Bray R.M., Brambilla D., Zatzick D., Belsher B.E. Centrally assisted collaborative telecare for posttraumatic stress disorder and depression among military personnel attending primary care a randomized clinical trial. JAMA Intern. Med. 2016;176:948–956. doi: 10.1001/jamainternmed.2016.2402. [DOI] [PubMed] [Google Scholar]
- 56.Fihn S.D., Bucher J.B., McDonell M., Diehr P., Rumsfeld J.S., Doak M., Lee P.I. Collaborative care intervention for stable ischemic heart disease. Arch. Intern. Med. 2011;171:1471–1479. doi: 10.1001/archinternmed.2011.372. [DOI] [PubMed] [Google Scholar]
- 57.Gilbody S., Lewis H., Adamson J., Atherton K., Bailey D., Birtwistle J., Foster D. Effect of Collaborative Care vs Usual Care on Depressive Symptoms in Older Adults With Subthreshold Depression: The CASPER Randomized Clinical Trial. JAMA. 2017;317:728–737. doi: 10.1001/jama.2017.0130. [DOI] [PubMed] [Google Scholar]
- 58.Goorden M., Huijbregts K.M., van Marwijk H.W., Beekman A.T., van der Feltz-Cornelis C.M., Hakkaart-van Roijen L. Cost-utility of collaborative care for major depressive disorder in primary care in the Netherlands. J. Psychosom. Res. 2015;79:316–323. doi: 10.1016/j.jpsychores.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 59.Green C., Richards D.A., Hill J.J., Gask L., Lovell K., Chew-Graham C., Kessler D. Cost-effectiveness of collaborative care for depression in UK primary care: Economic evaluation of a randomised controlled trial (CADET) PLoS ONE. 2014;9:e104225. doi: 10.1371/journal.pone.0104225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Grochtdreis T., Brettschneider C., Bjerregaard F., Bleich C., Boczor S., Harter M., Scherer M. Cost-effectiveness analysis of collaborative treatment of late-life depression in primary care (GermanIMPACT) Eur. Psychiatry. 2019;57:10–18. doi: 10.1016/j.eurpsy.2018.12.007. [DOI] [PubMed] [Google Scholar]
- 61.Hirsch J.D., Steers N., Adler D.S., Kuo G.M., Morello C.M., Lang M., Mangione C. A randomized controlled trial of primary care based pharmacist-physician collaborative medication therapy management for hypertension. J. Gen. Intern. Med. 2013;28:S12. doi: 10.1016/j.clinthera.2014.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hsu C.-C., Tai T.-Y. Long-term glycemic control by a diabetes case-management program and the challenges of diabetes care in Taiwan. Diabetes Res. Clin. Pract. 2014;106(Suppl. 2):S328–S332. doi: 10.1016/S0168-8227(14)70738-7. [DOI] [PubMed] [Google Scholar]
- 63.Huijbregts K.M., de Jong F.J., van Marwijk H.W., Beekman A.T., Adèr H.J., Hakkaart-van Roijen L., van der Feltz-Cornelis C.M. A target-driven collaborative care model for Major Depressive Disorder is effective in primary care in the Netherlands. A randomized clinical trial from the depression initiative. J. Affect. Disord. 2013;146:328–337. doi: 10.1016/j.jad.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 64.Ip E.J., Shah B.M., Yu J., Chan J., Nguyen L.T., Bhatt D.C. Enhancing diabetes care by adding a pharmacist to the primary care team. Am. J. Health Syst. Pharm. 2013;70:877–886. doi: 10.2146/ajhp120238. [DOI] [PubMed] [Google Scholar]
- 65.Johnson J.A., Lier D.A., Soprovich A., Al Sayah F., Qiu W., Majumdar S.R. Cost-effectiveness evaluation of collaborative care for diabetes and depression in primary care. Am. J. Prev. Med. 2016;51:e13–e20. doi: 10.1016/j.amepre.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 66.Katon W., Russo J., Lin E.H.B., Schmittdiel J., Ciechanowski P., Ludman E., Von Korff M. Cost-effectiveness of a multicondition collaborative care intervention: A randomized controlled trial. Arch. Gen. Psychiatry. 2012;69:506–514. doi: 10.1001/archgenpsychiatry.2011.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Katon W., Von Korff M., Lin E., Simon G., Walker E., Unützer J., Ludman E. Stepped collaborative care for primary care patients with persistent symptoms of depression: A randomized trial. Arch. Gen. Psychiatry. 1999;56:1109–1115. doi: 10.1001/archpsyc.56.12.1109. [DOI] [PubMed] [Google Scholar]
- 68.Katon W.J., Lin E.H., Von Korff M., Ciechanowski P., Ludman E.J., Young B., McCulloch D. Collaborative care for patients with depression and chronic illnesses. N. Engl. J. Med. 2010;363:2611–2620. doi: 10.1056/NEJMoa1003955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Katon W.J., Schoenbaum M., Fan M.Y., Callahan C.M., Williams J., Hunkeler E., Unützer J. Cost-effectiveness of improving primary care treatment of late-life depression. Arch. Gen. Psychiatry. 2005;62:1313–1320. doi: 10.1001/archpsyc.62.12.1313. [DOI] [PubMed] [Google Scholar]
- 70.Katon W.J., Von Korff M., Lin E.H., Simon G., Ludman E., Russo J., Bush T. The Pathways Study: A randomized trial of collaborative care in patients with diabetes and depression. Arch. Gen. Psychiatry. 2004;61:1042–1049. doi: 10.1001/archpsyc.61.10.1042. [DOI] [PubMed] [Google Scholar]
- 71.Krein S.L., Klamerus M.L., Vijan S., Lee J.L., Fitzgerald J.T., Pawlow A., Hayward R.A. Case management for patients with poorly controlled diabetes: A randomized trial. Am. J. Med. 2004;116:732–739. doi: 10.1016/j.amjmed.2003.11.028. [DOI] [PubMed] [Google Scholar]
- 72.Kruis A.L., Boland M.R., Assendelft W.J., Gussekloo J., Tsiachristas A., Stijnen T., Bush T. Effectiveness of integrated disease management for primary care chronic obstructive pulmonary disease patients: Results of cluster randomised trial. BMJ. 2014;349:g5392. doi: 10.1136/bmj.g5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Leeuwen K.M., Bosmans J.E., Jansen A.P.D., Hoogendijk E.O., Muntinga M.E., Hout H.P.J., van Tulder M.W. Cost-Effectiveness of a Chronic Care Model for Frail Older Adults in Primary Care: Economic Evaluation Alongside a Stepped-Wedge Cluster-Randomized Trial. J. Am. Geriatr. Soc. 2015;63:2494–2504. doi: 10.1111/jgs.13834. [DOI] [PubMed] [Google Scholar]
- 74.Lin E.H., Tang L., Katon W., Hegel M.T., Sullivan M.D., Unützer J. Arthritis pain and disability: Response to collaborative depression care. Gen. Hosp. Psychiatry. 2006;28:482–486. doi: 10.1016/j.genhosppsych.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 75.Lin E.H., Von Korff M., Ciechanowski P., Peterson D., Ludman E.J., Rutter C.M., McCulloch D.K. Treatment adjustment and medication adherence for complex patients with diabetes, heart disease, and depression: A randomized controlled trial. Ann. Fam. Med. 2012;10:6–14. doi: 10.1370/afm.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lin E.H., Von Korff M., Russo J., Katon W., Simon G.E., Unützer J., Ludman E.J. Can depression treatment in primary care reduce disability? A stepped care approach. Arch. Fam. Med. 2000;9:1052–1058. doi: 10.1001/archfami.9.10.1052. [DOI] [PubMed] [Google Scholar]
- 77.Maislos M., Weisman D. Multidisciplinary approach to patients with poorly controlled type 2 diabetes mellitus: A prospective, randomized study. Acta Diabetol. 2004;41:44–48. doi: 10.1007/s00592-004-0143-1. [DOI] [PubMed] [Google Scholar]
- 78.Menchetti M., Sighinolfi C., Di Michele V., Peloso P., Nespeca C., Bandieri P.V., Gotelli S. Effectiveness of collaborative care for depression in Italy. A randomized controlled trial. Gen. Hosp. Psychiatry. 2013;35:579–586. doi: 10.1016/j.genhosppsych.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 79.Metzelthin S.F., van Rossum E., Hendriks M.R., De Witte L.P., Hobma S.O., Sipers W., Kempen G.I. Reducing disability in community-dwelling frail older people: Cost-effectiveness study alongside a cluster randomised controlled trial. Age Ageing. 2015;44:390–396. doi: 10.1093/ageing/afu200. [DOI] [PubMed] [Google Scholar]
- 80.Morgan R.O., Bass D.M., Judge K.S., Liu C.F., Wilson N., Snow A.L., Kunik M.E. A Break-Even Analysis for Dementia Care Collaboration: Partners in Dementia Care. J. Gen. Intern. Med. 2015;30:804–809. doi: 10.1007/s11606-015-3205-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pyne J.M., Rost K.M., Zhang M., Williams D.K., Smith J., Fortney J. Cost-effectiveness of a primary care depression intervention. J. Gen. Intern. Med. 2003;18:432–441. doi: 10.1046/j.1525-1497.2003.20611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ramli A.S., Selvarajah S., Daud M.H., Haniff J., Abdul-Razak S., Tg-Abu-Bakar-Sidik T.M., Shafie A.A. Effectiveness of the EMPOWER-PAR Intervention in Improving Clinical Outcomes of Type 2 Diabetes Mellitus in Primary Care: A Pragmatic Cluster Randomised Controlled Trial. BMC Fam. Pract. 2016;17:157. doi: 10.1186/s12875-016-0557-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Richards D.A., Hill J.J., Gask L., Lovell K., Chew-Graham C., Bower P., Cape J., Pilling S., Araya R., Kessler D., et al. Clinical effectiveness of collaborative care for depression in UK primary care (CADET): Cluster randomised controlled trial. BMJ. 2013;347:9. doi: 10.1136/bmj.f4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Richards D.A., Lovell K., Gilbody S., Gask L., Torgerson D., Barkham M., Fletcher J. Collaborative care for depression in UK primary care: A randomized controlled trial. Psychol. Med. 2008;38:279–287. doi: 10.1017/S0033291707001365. [DOI] [PubMed] [Google Scholar]
- 85.Rollman B., Belnap B., Mazumdar S., Abebe K., Karp J., Lenze E., Schulberg H.C. Telephone-Delivered Stepped Collaborative Care for Treating Anxiety in Primary Care: A Randomized Controlled Trial. J. Gen. Intern. Med. 2017;32:245–255. doi: 10.1007/s11606-016-3873-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rollman B.L., Belnap B.H., Mazumdar S., Houck P.R., Zhu F., Gardner W., Shear M.K. A randomized trial to improve the quality of treatment for panic and generalized anxiety disorders in primary care. Arch. Gen. Psychiatry. 2005;62:1332–1341. doi: 10.1001/archpsyc.62.12.1332. [DOI] [PubMed] [Google Scholar]
- 87.Rollman B.L., Herbeck Belnap B., Abebe K.Z., Spring M.B., Rotondi A.J., Rothenberger S.D., Karp J.F. Effectiveness of Online Collaborative Care for Treating Mood and Anxiety Disorders in Primary Care: A Randomized Clinical Trial. JAMA Psychiatry. 2018;75:56–64. doi: 10.1001/jamapsychiatry.2017.3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rost K., Nutting P., Smith J., Werner J., Duan N. Improving depression outcomes in community primary care practice: A randomized trial of the quEST intervention. Quality Enhancement by Strategic Teaming. J. Gen. Intern. Med. 2001;16:143–149. doi: 10.1111/j.1525-1497.2001.00537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Salisbury C., Mei-See M., Bower P., Guthrie B., Chaplin K., Gaunt D.M., Lee V. Management of multimorbidity using a patient-centred care model: A pragmatic cluster-randomised trial of the 3D approach. Lancet. 2018;392:41–50. doi: 10.1016/S0140-6736(18)31308-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Scherpbier-de Haan N.D., Vervoort G.M., van Weel C., Braspenning J.C., Mulder J., Wetzels J.F., de Grauw W.J. Effect of shared care on blood pressure in patients with chronic kidney disease: A cluster randomised controlled trial. Br. J. Gen. Pract. 2013;63:e798–e806. doi: 10.3399/bjgp13X675386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schnurr P.P., Friedman M.J., Oxman T.E., Dietrich A.J., Smith M.W., Shiner B., Thurston V. RESPECT-PTSD: Re-engineering systems for the primary care treatment of PTSD, a randomized controlled trial. J. Gen. Intern. Med. 2013;28:32–40. doi: 10.1007/s11606-012-2166-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Simon G.E., Katon W.J., Von Korff M., Unutzer J., Lin E.H., Walker E.A., Ludman E. Cost-effectiveness of a collaborative care program for primary care patients with persistent depression. Am. J. Psychiatry. 2001;158:1638–1644. doi: 10.1176/appi.ajp.158.10.1638. [DOI] [PubMed] [Google Scholar]
- 93.Simon G.E., Ludman E.J., Tutty S., Operaskalski B., Von Korff M., Simon G.E., Von Korff M. Telephone psychotherapy and telephone care management for primary care patients starting antidepressant treatment: A randomized controlled trial. JAMA. 2004;292:935–942. doi: 10.1001/jama.292.8.935. [DOI] [PubMed] [Google Scholar]
- 94.Simpson S.H., Majumdar S.R., Tsuyuki R.T., Lewanczuk R.Z., Spooner R., Johnson J.A. Impact of adding a pharmacist to primary care teams on blood pressure control in people with type 2 diabetes: A randomized controlled trial (ISRCTN97121854) Can. J. Diabetes. 2009;33:198–199. doi: 10.1016/S1499-2671(09)33045-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Smith S.M., Bury G., O’Leary M., Shannon W., Tynan A., Staines A., Thompson C. The North Dublin randomized controlled trial of structural diabetes shared care. Fam. Pract. 2004;21:39–45. doi: 10.1093/fampra/cmh109. [DOI] [PubMed] [Google Scholar]
- 96.Tang P.C., Overhage J.M., Chan A.S., Brown N.L., Aghighi B., Entwistle M.P., Perkins A.J. Online disease management of diabetes: Engaging and motivating patients online with enhanced resources-diabetes (EMPOWER-D), a randomized controlled trial. J. Am. Med. Inform. Assoc. 2013;20:526–534. doi: 10.1136/amiajnl-2012-001263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Taylor K.I., Oberle K.M., Crutcher R.A., Norton P.G. Promoting health in type 2 diabetes: Nurse-physician collaboration in primary care. Biol. Res. Nurs. 2005;6:207–215. doi: 10.1177/1099800404272223. [DOI] [PubMed] [Google Scholar]
- 98.Thorn J., Man M.S., Chaplin K., Bower P., Brookes S., Gaunt D., Lee V. Cost-effectiveness of a patient-centred approach to managing multimorbidity in primary care: A pragmatic cluster randomised controlled trial. BMJ Open. 2020;10:1. doi: 10.1136/bmjopen-2019-030110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Uijen A.A., Bischoff E.W., Schellevis F.G., Bor H.H., van den Bosch W.J., Schers H.J. Continuity in different care modes and its relationship to quality of life: A randomised controlled trial in patients with COPD. Br. J. Gen. Pract. 2012;62:e422–e428. doi: 10.3399/bjgp12X649115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Unutzer J., Katon W., Callahan C.M., Williams J.W., Jr., Hunkeler E., Harpole L., Areán P.A. Collaborative care management of late-life depression in the primary care setting: A randomized controlled trial. JAMA. 2002;288:2836–2845. doi: 10.1001/jama.288.22.2836. [DOI] [PubMed] [Google Scholar]
- 101.Unutzer J., Katon W.J., Fan M.Y., Schoenbaum M.C., Lin E.H.B., Della Penna R.D., Powers D. Long-term cost effects of collaborative care for late-life depression. Am. J. Manag. Care. 2008;14:95–100. [PMC free article] [PubMed] [Google Scholar]
- 102.van Orden M., Hoffman T., Haffmans J., Spinhoven P., Hoencamp E., van Orden M., Hoencamp E. Collaborative mental health care versus care as usual in a primary care setting: A randomized controlled trial. Psychiat. Serv. 2009;60:74–79. doi: 10.1176/ps.2009.60.1.74. [DOI] [PubMed] [Google Scholar]
- 103.Vera M., Perez-Pedrogo C., Huertas S.E., Reyes-Rabanillo M.L., Juarbe D., Huertas A., Chaplin W. Collaborative care for depressed patients with chronic medical conditions: A randomized trial in Puerto Rico. Psychiat. Serv. 2010;61:144–150. doi: 10.1176/ps.2010.61.2.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Von Korff M., Katon W., Bush T., Lin E.H., Simon G.E., Saunders K., Unutzer J. Treatment costs, cost offset, and cost-effectiveness of collaborative management of depression. Psychosom. Med. 1998;60:143–149. doi: 10.1097/00006842-199803000-00005. [DOI] [PubMed] [Google Scholar]
- 105.Von Korff M., Katon W.J., Lin E.H., Ciechanowski P., Peterson D., Ludman E.J., Rutter C.M. Functional outcomes of multi-condition collaborative care and successful ageing: Results of randomised trial. BMJ. 2011;343:d6612. doi: 10.1136/bmj.d6612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zwar N.A., Bunker J.M., Reddel H.K., Dennis S.M., Middleton S., van Schayck O.C., Xuan W. Early intervention for chronic obstructive pulmonary disease by practice nurse and GP teams: A cluster randomized trial. Fam. Pract. 2016;33:663–670. doi: 10.1093/fampra/cmw077. [DOI] [PubMed] [Google Scholar]
- 107.Kravetz J.D., Walsh R.F. Team-based hypertension management to improve blood pressure control. Prim. Care Community Health. 2016;7:272–275. doi: 10.1177/2150131916645580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Vestjens L., Cramm J.M., Birnie E., Nieboer A.P. Cost-effectiveness of a proactive, integrated primary care approach for community-dwelling frail older persons. Cost Eff. Resour. Allocat. 2019;17:14. doi: 10.1186/s12962-019-0181-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Petersen I., Bhana A., Fairall L.R., Selohilwe O., Kathree T., Baron E.C., Lund C. Evaluation of a collaborative care model for integrated primary care of common mental disorders comorbid with chronic conditions in South Africa. BMC Psychiat. 2019;19:107. doi: 10.1186/s12888-019-2081-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Seidu S., Bodicoat D.H., Davies M.J., Daly H., Stribling B., Farooqi A., Khunti K. Evaluating the impact of an enhanced primary care diabetes service on diabetes outcomes: A before–after study. Prim. Care Diabetes. 2017;11:171–177. doi: 10.1016/j.pcd.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 111.Sommers L.S., Marton K.I., Barbaccia J.C., Randolph J. Physician, nurse, and social worker collaboration in primary care for chronically ill seniors. Arch. Intern. Med. 2000;160:1825–1833. doi: 10.1001/archinte.160.12.1825. [DOI] [PubMed] [Google Scholar]
- 112.DePuccio M.J., Hoff T.J. Medical home interventions and quality outcomes for older adults: A systematic review. Qual. Manag. Healthc. 2013;22:327–340. doi: 10.1097/QMH.0000000000000000. [DOI] [PubMed] [Google Scholar]
- 113.Gilbody S., Bower P., Fletcher J., Richards D., Sutton A.J. Collaborative care for depression: A cumulative meta-analysis and review of longer-term outcomes. Arch. Intern. Med. 2006;166:2314–2321. doi: 10.1001/archinte.166.21.2314. [DOI] [PubMed] [Google Scholar]
- 114.Tully P.J., Baumeister H. Collaborative care for comorbid depression and coronary heart disease: A systematic review and meta-analysis of randomised controlled trials. BMJ Open. 2015;5:e009128. doi: 10.1136/bmjopen-2015-009128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Stokes J., Panagioti M., Alam R., Checkland K., Cheraghi-Sohi S., Bower P. Effectiveness of case management for ‘at risk’ patients in primary care: A systematic review and meta-analysis. PLoS ONE. 2015;10:e0132340. doi: 10.1371/journal.pone.0132340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Brady T.J., Murphy L., O’Colmain B.J., Beauchesne D., Daniels B., Greenberg M., Chervin D. A meta-analysis of health status, health behaviors, and health care utilization outcomes of the chronic disease self-management program. Prev. Chronic Dis. 2013;10:E07. doi: 10.5888/pcd10.120112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tan E.C., Stewart K., Elliott R.A., George J. Pharmacist services provided in general practice clinics: A systematic review and meta-analysis. Res. Soc. Adm. Pharm. 2014;10:608–622. doi: 10.1016/j.sapharm.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 118.Reynolds R., Dennis S., Hasan I., Slewa J., Chen W., Tian D., Hasan I. A systematic review of chronic disease management interventions in primary care. BMC Fam. Pract. 2018;19:11. doi: 10.1186/s12875-017-0692-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Milbank Memorial Fund The Impact of Primary Care Practice Transformation on Cost, Quality, and Utilization. [(accessed on 20 March 2020)];2017 Available online: https://www.pcpcc.org/sites/default/files/resources/pcmh_evidence_report_08-1-17%20FINAL.pdf.
- 120.Veet C.A., Radomski T.R., D’Avella C., Hernandez I., Wessel C., Swart E.C., Parekh N. Impact of Healthcare Delivery System Type on Clinical, Utilization, and Cost Outcomes of Patient-Centered Medical Homes: A Systematic Review. J. Gen. Intern. Med. 2020;35:1276–1284. doi: 10.1007/s11606-019-05594-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Peikes D., Zutshi A., Genevro J.L., Parchman M.L., Meyers D.S. Early evaluations of the medical home: Building on a promising start. Am. J. Manag. Care. 2012;18:105. [PubMed] [Google Scholar]
- 122.Ekers D., Murphy R., Archer J., Ebenezer C., Kemp D., Gilbody S. Nurse-delivered collaborative care for depression and long-term physical conditions: A systematic review and meta-analysis. J. Affect. Disord. 2013;149:14–22. doi: 10.1016/j.jad.2013.02.032. [DOI] [PubMed] [Google Scholar]
- 123.Primary Health Care Advisory Group Report. Better Outcomes for People with Chronic and Complex Health Conditions. [(accessed on 21 April 2020)];2015 Available online: https://www1.health.gov.au/internet/main/publishing.nsf/Content/76B2BDC12AE54540CA257F72001102B9/$File/Primary-Health-Care-Advisory-Group_Final-Report.pdf.