Abstract
We present the synthesis of 17 macrocyclic compounds having the structure of so-called unclosed cryptands, acting as anion receptors. These compounds possess amide functions playing the role of hydrogen-bond-donating systems. We have synthesized the presented compounds both by standard methods (using batch conditions) and by static combinatorial chemistry methods, using tetrabutylammonium dihydrogen phosphate as a template, promoting the lariat arm postfunctionalization reaction.
Introduction
Combinatorial chemistry is a technology that allows for the parallel synthesis of a large number of chemical compounds, reaching hundreds of thousands or even millions, in a single chemical process. It is usually used to search for new drugs in the pharmaceutical industry,1 although it is increasingly being used in creating new semiconductors,2 catalysts,3 and polymers.4 Parallel synthesis and analysis of the resulting library allow costs to be minimized and significantly shorten the time of studies. Subsequently, potentially useful compounds are synthesized and analyzed by classical methods, which allow compounds that exhibit false positive properties to be eliminated, for example, as components showing synergic effects. Combinatorial chemistry is equally successfully used in the synthesis of small compounds with low-molecular weight5 and macromolecules, for example, peptides.6 Although combinatorial chemistry has been used in the industry since the 1990s,7 its roots can be traced back to the 1960s, when Merrifield began researching the synthesis of peptides using solid-phase methods.8 Merrifield’s discovery became a milestone in the development of peptide synthesis, and his solid-phase synthesis method was developed in the second half of the 20th century and is used in the synthesis of DNA and RNA fragments,9 as well as in the synthesis of low-molecular-weight organic compounds.10
The increase in the number of components in libraries is strongly correlated with the requirements for analysis methods. One of the possible problems involves cross effects occurring in large sets, which may lead to the inhibition of interactions of the tested template with a given library. At the end of the 1990s, combinatorial chemistry branched into two parts: classical or static combinatorial chemistry and dynamic combinatorial chemistry, presented by Sanders,11 Lehn,12 and others.
Static combinatorial chemistry uses a number of various irreversible reactions that the chosen substrates can undergo. Therefore, it is not possible to recover substrates from the product library, once generated. An additional requirement for the resulting static combinatorial library is its representativeness, which requires that the library should contain all elements that can be obtained based on the substrates used.13
Results and Discussion
The use of combinatorial chemistry methods has allowed significant progress to be made in the synthesis of demanding supramolecular systems. In our previous works,14 we presented efficient synthesis of unclosed cryptands (UCs) by postfunctionalization of the lariat arm after yield-limiting macrocyclization. Therefore, in this work, we decided to apply our well-established knowledge in the design and synthesis of supramolecular ion receptors while harnessing the advantages of static combinatorial chemistry. As a result, we obtained a representative library of 17 macrocyclic compounds having the character of anion receptors. We obtained UCs, macrocyclic polyamides, differing in substituents in the lariat arm, both by the methods of static combinatorial chemistry (while examining the effect of the presence of the template on the composition of the resulting libraries) as well as by a classical synthetic approach. These two processes carried out in parallel allowed us to perform a full spectroscopic analysis of the receptors obtained and to examine their complexing properties.
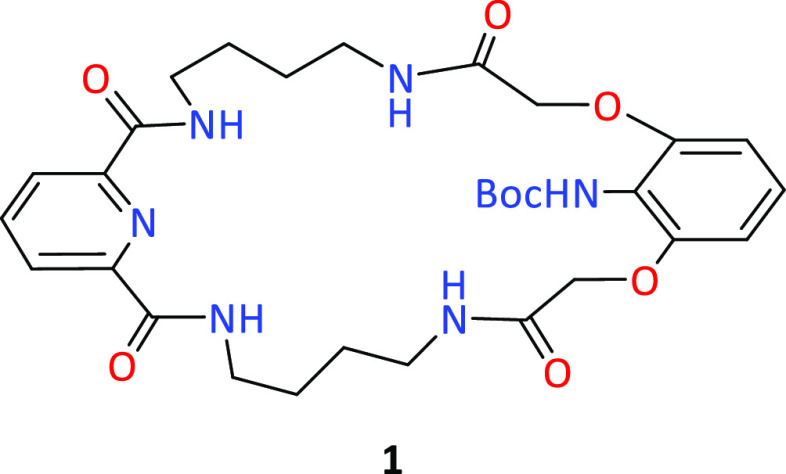
We began our research with the preparation of a macrocyclic precursor, for which we used the ICHOPAN II synthesis method, which consists of obtaining polyamide macrorings by a double-amidation reaction using α,ω-diamines and methyl esters of α,ω-dicarboxylic acids.15 This method makes it possible to obtain the expected products, as shown in Figure 1, with relatively high yields.
Figure 1.
Structure of the macrocyclic polyamide.
The use of selected substrates, which have functions properly protected during the macrocyclization reaction, makes it possible to modify the complexing properties of the receptors received during the irreversible amidation reaction. We have recently used this type of approach in the synthesis of chiral amide phase-transfer catalysts that selectively affect the asymmetric synthesis of amino acid derivatives.16 This is the basic requirement for obtaining static libraries, whose composition is kinetically controlled and does not change after completion of the reaction, including during the testing stage.
Having compounds comprehensively documented by analytical and spectral data, it was possible to start constructing static combinatorial libraries and tracking their composition under the influence of experimental variables. We conducted the analysis of the obtained libraries using high-performance liquid chromatography (HPLC) techniques, which proved to be an excellent tool for analyzing combinatorial mixtures. We then referred the obtained results to the complexing properties of the presented macrocyclic receptors, as determined using the 1H NMR titration techniques.
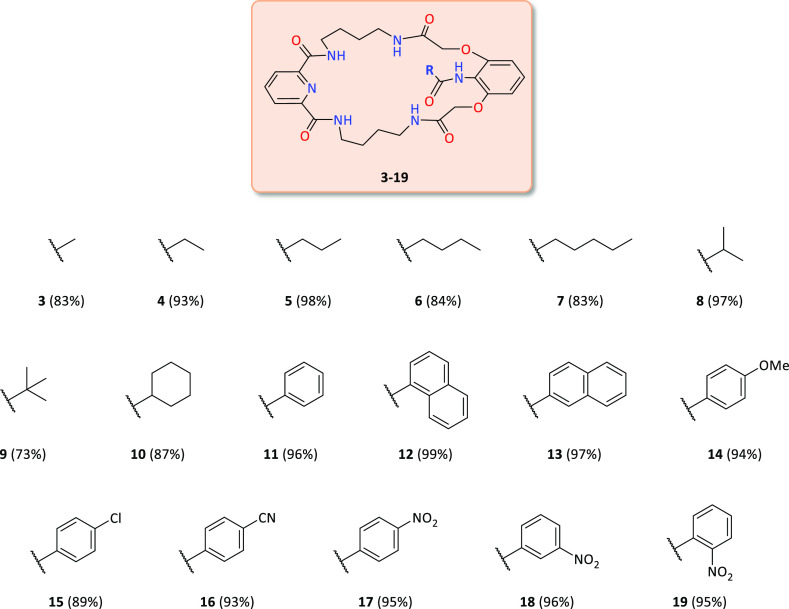
The synthesis of macrocyclic precursor 1 was presented in our previous work.14a By postfunctionalization of precursor 1, carried out under one-pot conditions (deprotection of the amine function of 1 and subsequent reactions with one of the 17 acid chlorides shown in Scheme 1), we obtained 17 derivatives with the amide function in the lariat arm. These reactions occurred with excellent yields (Figure 2).
Scheme 1. Synthesis of the Library of Macrocyclic Receptors 3–19.
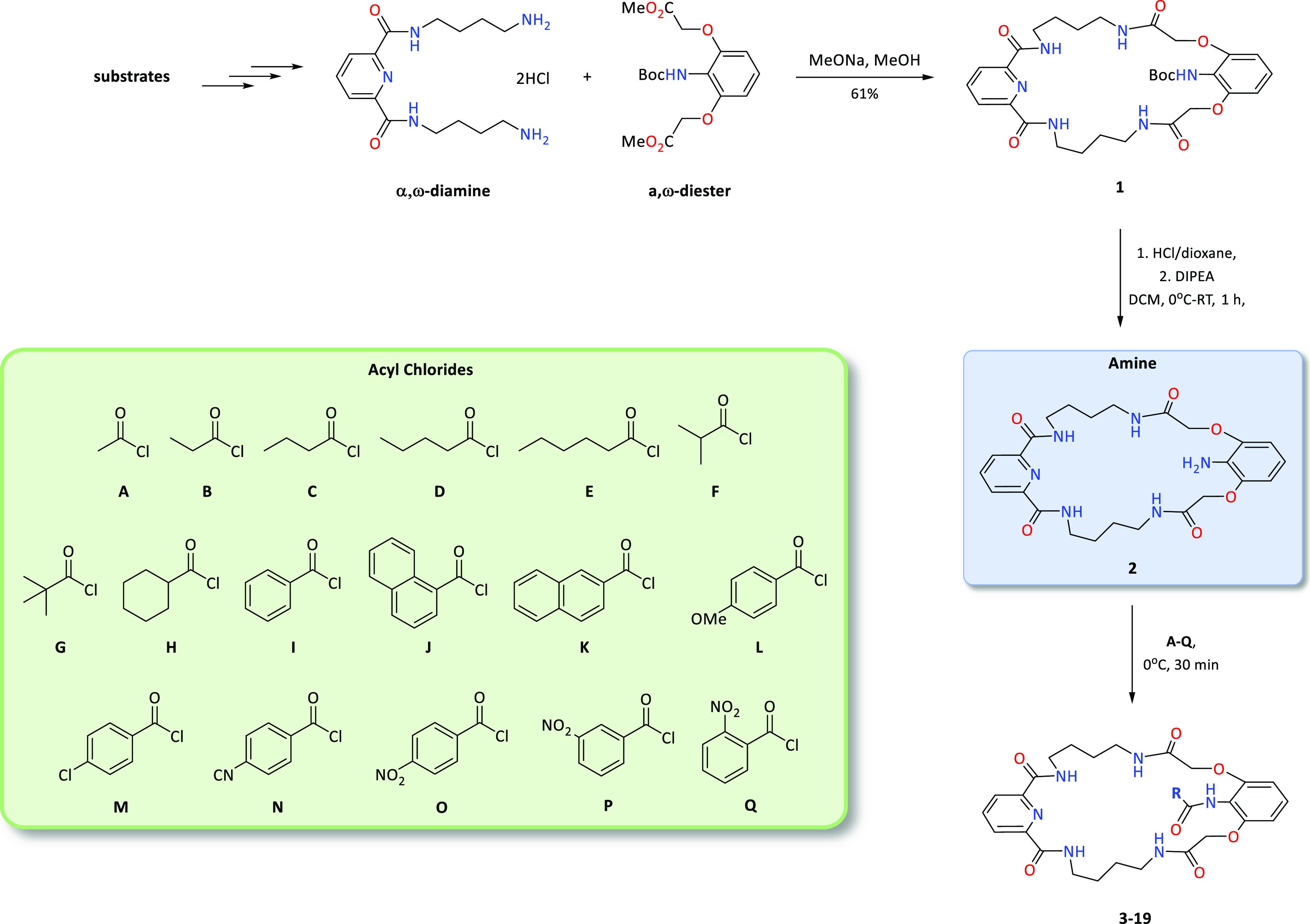
Figure 2.
Library of macrocyclic receptors 3–19.
The presented compounds contain various substituents in the lariat arm and comprise both aliphatic and aromatic units. Within the second group, compounds with electron-donating and electron-withdrawing substituents can be distinguished.
Having 17 expected macrocyclic receptors, we proceeded to concurrent synthesis of the presented compounds by combinatorial chemistry using the library of acid chlorides.
In this study, we used three-substrate libraries, whose components were as follows: macrocyclic precursor 2 (in every case) and pairs of changing acid chlorides. The libraries were constructed in such a way that all substrates were used equimolarly, so each of them contained one equivalent of compound 2 and one equivalent of both acid chlorides. In such systems, the ratio of the contents of the two resulting macrocyclic products was tested, as well as the total conversion determined on the residual content of compound 2 in the reaction mixture. Then, we compared the composition of the generated libraries, in both libraries without the addition of a template and in the presence of dihydrogen phosphate.
First, we generated static three-substrate libraries in non-templated systems. Reactions were carried out in anhydrous dichloromethane (DCM) at a concentration of c = 1 mM for each of the substrates for 1 h (Scheme 2). As reagents differentiating emerging substituents in the lariat arm, we used mixtures of appropriate acid chlorides. The reaction mixture thus obtained was then analyzed using HPLC. The composition of the mixtures was calculated based on the obtained chromatograms. Overlapping signals in the HPLC spectra of some macrocyclic products effectively prevented the determination of the library composition. Therefore, in the following Figures 3–6, these libraries are omitted.
Scheme 2. Composition of Libraries in Non-templated Systems.
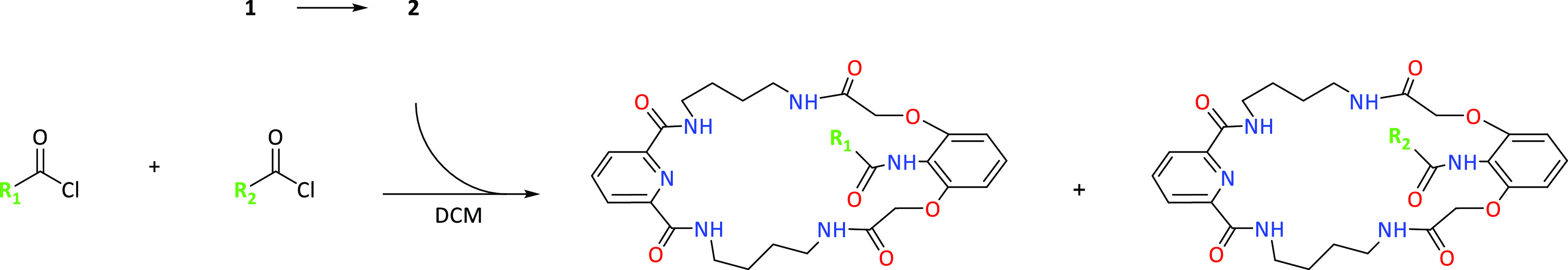
Figure 3.
Macrocyclic product 3 content in combinatorial mixtures in untemplated systems (green) and with the use of TBA-H2PO4 as a template (blue).
Figure 6.
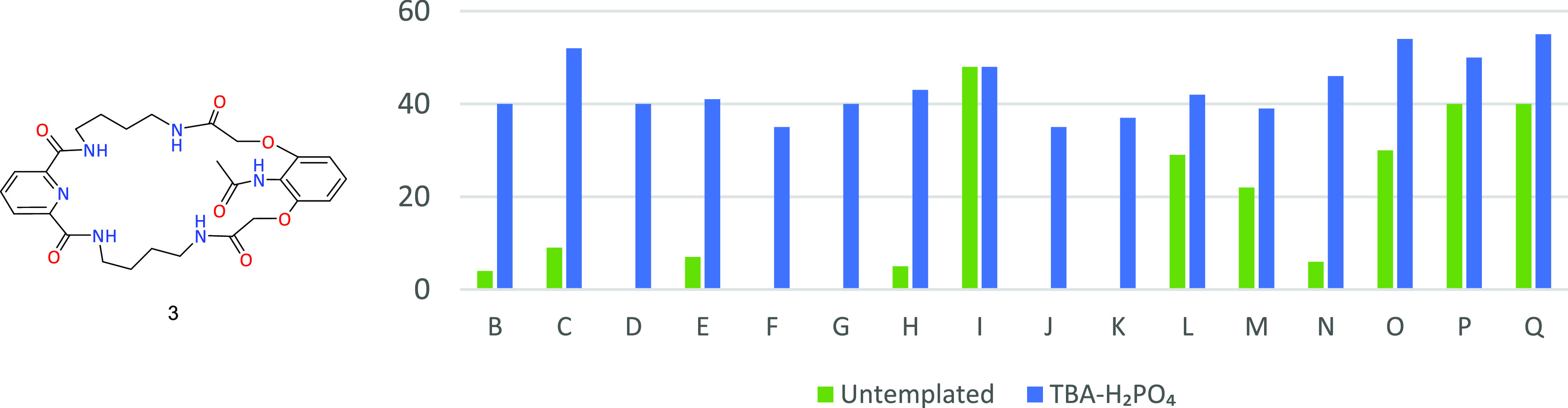
Macrocyclic product 17 content in combinatorial mixtures in untemplated systems (green) and with the use of TBA-H2PO4 as a template (blue).
In the next step, we generated similar libraries that this time contained one equivalent of dihydrogen phosphate. Analysis of the combinatorial libraries obtained indicates that the resulting macrocyclic products do not form in a statistical manner. The composition of the reaction mixture is influenced by the structural (geometric) factors of the substrates used, as well as their electronic properties, which influenced the composition of static libraries (proportions of macrocyclic products differing in a lariat arm).
Considering the amide systems formed with the participation of acetyl chloride (A) as a fixed element in competition with a series of 16 different acid chlorides B–Q, we observed that the addition of dihydrogen phosphate in the form of a tetrabutylammonium (TBA) salt used as a template has a strong effect on the distribution of products in the reaction mixture, with a predominance of receptor 3, containing an acetyl group in the lariat arm (Figure 3).
Considering intra-aliphatic competition of acid chlorides B–H, we observed a very strong increase in the content of macrocyclic compound 3 in all cases. The addition of a template promotes the formation of a product with the geometrically smallest substituent in the lariat arm. A similar relationship occurs in the case of aliphatic–aromatic competition with the use of large aromatic 1-naphthyl (J) and 2-naphthyl (K) chlorides, as well as with 4-cyanobenzoyl chloride (N) and isomers of nitrobenzoyl chlorides (O, P, Q), containing a strong electron-withdrawing group.
An analogous analysis of competitive reactions involving butyryl chloride (C) as a fixed element is presented in Figure 4.
Figure 4.
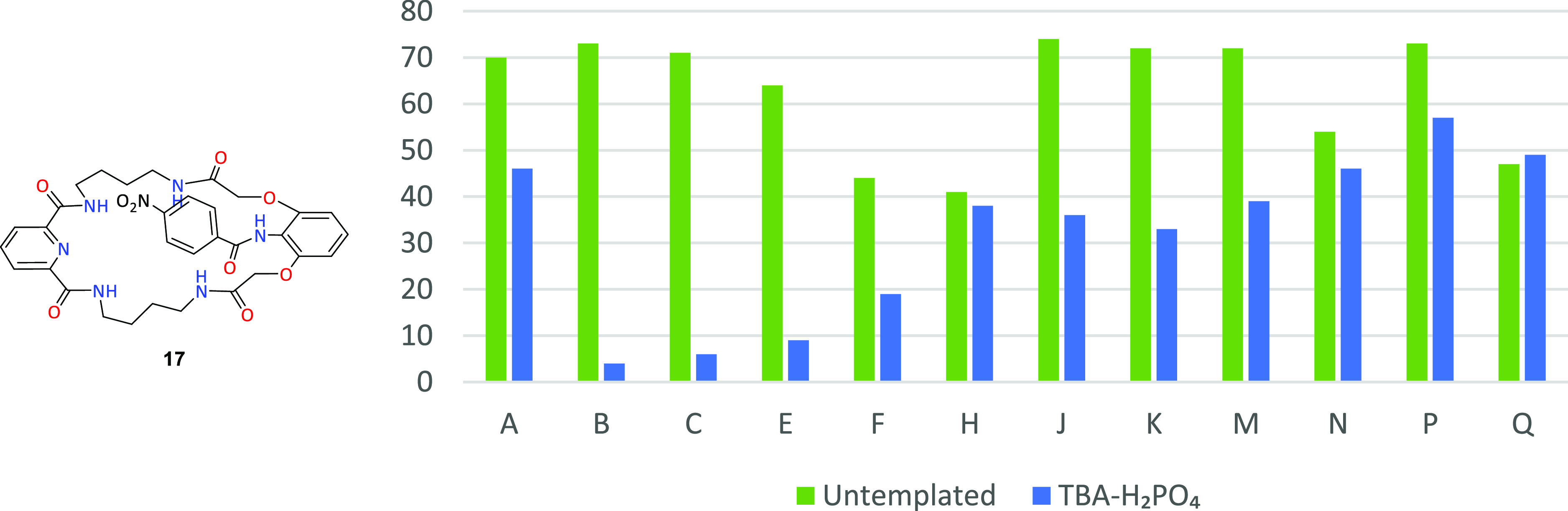
Macrocyclic product 5 content in combinatorial mixtures in untemplated systems (green) and with the use of TBA-H2PO4 as a template (blue).
Unlike the previous case, analysis of intra-aliphatic competition indicates a much less spectacular dihydrogen phosphate templating effect. Only in the case of competition with large cyclohexane carboxylic acid chloride (H) did the addition of the template result in an increase in the content of product 5 in the reaction mixture, with a simultaneous decrease in conversion from 91 to 77%. Worth mentioning against the background of other aliphatic–aromatic competitions are mixtures which include, in addition to the fixed C substrate in this series, 4-chlorobenzoyl chloride (M), containing a strongly deactivating aromatic ring substituent, as well as isomeric 4-nitro- (O) and 3-nitrobenzoyl (P) chlorides. The use of tetrabutylammonium dihydrogen phosphate (TBA-H2PO4) provided a distinct advantage of product 5 in the tested mixtures. This was associated with a drastic decrease in conversion from the quantitative level to 10–20%.
Similar dihydrogen phosphate templating properties have also been observed for the competition of 4-methoxybenzoyl chloride (L) with 16 other acid chlorides. Practically in all cases studied, the use of template significantly increased the distribution of product 14 in combinatorial mixtures (Figure 5).
Figure 5.
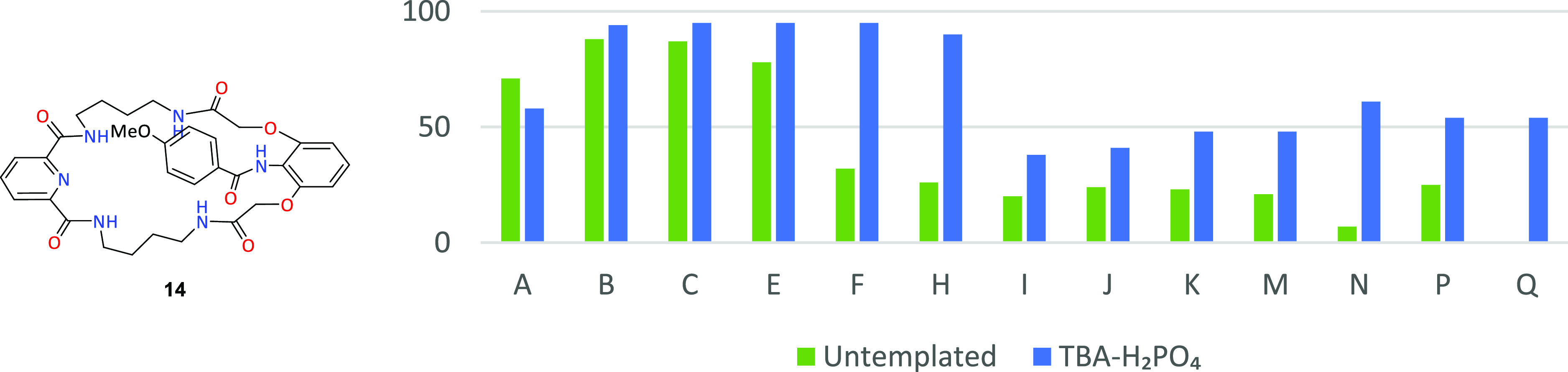
Macrocyclic product 14 content in combinatorial mixtures in untemplated systems (green) and with the use of TBA-H2PO4 as a template (blue).
In the case of aliphatic–aromatic systems, the strongest templating properties in the formation of compound 14 occur for a pair with strongly expanded substituents: isobutyl chloride (F) and cyclohexane carboxylic acid chloride (H). In addition, in templated systems, product 14 dominated in all competing aliphatic–aromatic pairs. In the case of intra-aromatic competition, in most cases, the TBA-H2PO4 templating effects are not that strong. Deviations from this principle can be seen in competition between L acid chloride and 4-cyanobenzoyl (N) and 3-nitrobenzoyl (P) chlorides. Thus, it can be concluded that the use of the TBA-H2PO4 template promotes the reaction with acid chloride containing in its structure a methoxy substituent, having donating properties. Interestingly, in this series of combinatorial reactions, we did not observe a significant reduction in conversion.
In turn, in the case of competitive processes leading to compound 17, the use of TBA-H2PO4 gives completely different results from those presented so far (Figure 6).
In this series of combinatorial reactions, we observed clear inhibitory properties of the dihydrogen phosphate anion in the formation of the macrocyclic compound 17. This process was accompanied by a strong, in most cases, decrease in conversion. The strongest inhibition of receptor 17 formation occurs in the combination of 4-nitrobenzoyl chloride (O) and aliphatic chlorides with elongated chains (B, C, and E).
Targeted recognition of phosphates is constantly being researched intensively.17 Macrocyclic receptors, such as UCs, due to their structure, are perfectly geared toward recognizing phosphates. In order to determine the affinity of the receptors for anions, we decided to use the titration technique controlled by 1H NMR spectrometry. The technique was chosen because it gives more precise information about the complexing process, unlike other methods such as microcalorimetry or spectrophotometric methods. As a model anion for research, we chose tetrahedral dihydrogen phosphate (H2PO4–) in the form of a TBA salt. As a solvent, we used the highly polar and competitive dimethyl sulfoxide (DMSO)-d6 with 0.5% water addition, and the concentration of the receptor throughout the entire complexing experiment was kept constant, thus eliminating its possible autoassociation.
For complexation studies, we decided to use representative compounds 3, 5, 6, 10, 11, 14, and 17, selected from those synthesized in the course of the work. The resulting stability constants (Ka) for the complexes of these receptors with the dihydrogen phosphate anion are summarized in Table 1.
Table 1. Stability Constants Ka [M–1] of Receptor Complexes with H2PO4– in DMSO-d6 + 0.5% H2O at 298 Ka.
| entry | receptor | Ka |
|---|---|---|
| 1 | 3 | 8600 (1:1) |
| 210 (1:2) | ||
| 2 | 5 | 40 (1:1) |
| 100 (2:1) | ||
| 3 | 6 | 1520 (1:1) |
| 6 (1:2) | ||
| 4 | 10 | 1620 (1:1) |
| 20 (1:2) | ||
| 5 | 11 | 1700 (1:1) |
| 290 (2:1) | ||
| 6 | 14 | 1020 (1:1) |
| 210 (2:1) | ||
| 7 | 17 | 3770 (1:1) |
| 40 (1:2) |
Anion in the form of a TBA salt, constants determined using the HypNMR program18 for the 1:1, 1:2, or 2:1 binding model (receptor/anion).
Their analysis indicates that among the presented receptors, the H2PO4– anion is most strongly complexed by receptor 3 with the acetyl substituent in the lariat arm and by receptor 17, which contains a strong electron-withdrawing nitro group in its structure, which increases the acidity of the amide proton located in the lariat arm.
Moreover, we conducted additional titration experiments under the control of UV–vis spectroscopy for receptors 3 and 17 using DCM as solvent and H2PO4– as anionic guest. Unfortunately, in both cases, attempts to fit the 1:1 [H/G] complexation model were unsuccessful (see the Supporting Information). At the same time, both: the use of the 1:2 [H/G] model and the 2:1 [H/G] model did not allow to obtain the realistic values of the Ka association constant (e.g., for the 1:2 model [H/G], the constant K1 ∼0, and the constant K2 was very large). Furthermore, the solvation of the TBA-H2PO4 ion pair in DCM is incomplete.19
During syntheses, using combinatorial chemistry methods, we observed a very strong influence of templating factors on the composition of the reaction mixture and substrate conversion. The addition of TBA-H2PO4 repeatedly significantly changed the content of individual macrocyclic components in the created library.
The complexing properties of a number of macrocyclic receptors with various substituents in the lariat arm that we presented show a certain analogy to anion–receptor affinity with the promotion of the formation of this macrocyclic compound using the template. Despite the fact that the complexation constant should not be directly related to promoting the formation of a given macrocyclic compound, the preorganization of substrates in the lariat arm postfunctionalization under the influence of the template is a certain reflection of the subsequent complexation studies.
Experimental Procedures
General Procedure A for Obtaining Receptors 3–19
To a suspension of macrocyclic compound 1 (0.140 g, 0.23 mmol) in anhydrous DCM (3 mL) at 0 °C, 4 M HCl in dioxane (0.286 mL, 1.15 mmol) was added. Then, the mixture was stirred at room temperature for 1 h. Subsequently, the mixture was cooled to 0 °C, and then N,N-diisopropylethylamine (0.288 mL, 1.65 mmol) and the corresponding acyl chloride (0.28 mmol) were added. The mixture was stirred for a further 30 min, the solvent was evaporated under a vacuum, and the residue was purified employing column chromatography and using a DCM/methanol mixture [99:1 → 95:5, v/v] as the eluent. The obtained colorless oil was dissolved in methanol and then sonicated in water.
General Procedure B for Parallel Synthesis of Receptors 3–19
To a solution of two corresponding acyl chlorides (0.001 mmol each) and additionally in templated mixtures TBA-H2PO4 (0.340 mg, 0.001 mmol) in DCM (0.5 mL) at 0 °C, the solution of macrocyclic precursor 2 (0.513 mg, 0.001 mmol) in DCM (0.5 mmol) was added. Then, the mixture was stirred for 1 h, the solvent was evaporated under a vacuum, and the residue was filtered through a short pad of Celite using methanol. The mixture thus obtained was then analyzed using HPLC.
Acknowledgments
We acknowledge Poland’s National Science Centre (project 2016/21/B/ST5/03352) for financial support.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c04228.
Copies of 1H and 13C NMR spectra of all compounds and titration experiments for receptors 3, 5, 6, 10, 11, 14, and 17 (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- For examples, see:; a Cragg G. M.; Grothaus P. G.; Newman D. J. Impact of Natural Products on Developing New Anti-Cancer Agents. Chem. Rev. 2009, 109, 3012–3043. 10.1021/cr900019j. [DOI] [PubMed] [Google Scholar]; b Horton D. A.; Bourne G. T.; Smythe M. L. The Combinatorial Synthesis of Bicyclic Privileged Structures or Privileged Substructures. Chem. Rev. 2003, 103, 893–930. 10.1021/cr020033s. [DOI] [PubMed] [Google Scholar]; c Kassel D. B. Combinatorial Chemistry and Mass Spectrometry in the 21st Century Drug Discovery Laboratory. Chem. Rev. 2001, 101, 255–268. 10.1021/cr990085q. [DOI] [PubMed] [Google Scholar]; d Santana R.; Zuluaga R.; Gañán P.; Arrasate S.; Onieva Caracuel E.; González-Díaz H. PTML Model of ChEMBL Compounds Assays for Vitamin Derivatives. ACS Comb. Sci. 2020, 22, 129–141. 10.1021/acscombsci.9b00166. [DOI] [PubMed] [Google Scholar]; Rakesh K. P.; Marichannegowda M. H.; Srivastava S.; Chen X.; Long S.; Karthik C. S.; Mallu P.; Qin H. L. Combating a Master Manipulator: Staphylococcus Aureus Immunomodulatory Molecules as Targets for Combinatorial Drug Discovery. ACS Comb. Sci. 2018, 20, 681–693. 10.1021/acscombsci.8b00088. [DOI] [PubMed] [Google Scholar]
- Fuse S.; Matsumura K.; Wakamiya A.; Masui H.; Tanaka H.; Yoshikawa S.; Takahashi T. Elucidation of the Structure-Property Relationship of p-Type Organic Semiconductors through Rapid Library Construction via a One-Pot, Suzuki-Miyaura Coupling Reaction. ACS Comb. Sci. 2014, 16, 494–499. 10.1021/co500071x. [DOI] [PubMed] [Google Scholar]
- For examples, see:; a Kingsbury J. S.; Garber S. B.; Giftos J. M.; Gray B. L.; Okamoto M. M.; Farrer R. A.; Fourkas J. T.; Hoveyda A. H. Immobilization of Olefin Metathesis Catalysts on Monolithic Sol-Gel: Practical, Efficient, and Easily Recyclable Catalysts for Organic and Combinatorial Synthesis. Angew. Chem. Int. Ed. 2001, 40, 4251–4256. . [DOI] [PubMed] [Google Scholar]; b Senkan S. M.; Ozturk S. Discovery and Optimization of Heterogeneous Catalysts by Using Combinatorial Chemistry. Angew. Chem. Int. Ed. 1999, 38, 791–795. . [DOI] [PubMed] [Google Scholar]; c Gennari C.; Piarulli U. Combinatorial Libraries of Chiral Ligands for Enantioselective Catalysis. Chem. Rev. 2003, 103, 3071–3100. 10.1021/cr020058r. [DOI] [PubMed] [Google Scholar]; d Sheng J.; Ni H.-Q.; Liu G.; Li Y.; Wang X.-S. Combinatorial Nickel-Catalyzed Monofluoroalkylation of Aryl Boronic Acids with Unactivated Fluoroalkyl Iodides. Org. Lett. 2017, 19, 4480–4483. 10.1021/acs.orglett.7b02012. [DOI] [PubMed] [Google Scholar]
- For examples, see:; a Seeberger P. H.; Haase W.-C. Solid-Phase Oligosaccharide Synthesis and Combinatorial Carbohydrate Libraries. Chem. Rev. 2000, 100, 4349–4394. 10.1021/cr9903104. [DOI] [PubMed] [Google Scholar]; b Bukhryakov K. V.; Desyatkin V. G.; O’Shea J. P.; Almahdali S. R.; Solovyeva V.; Rodionov V. O. Cooperative Catalysis with Block Copolymer Micelles: A Combinatorial Approach. ACS Comb. Sci. 2015, 17, 76–80. 10.1021/co5001713. [DOI] [PubMed] [Google Scholar]
- Burke M. D.; Berger E. M.; Schreiber S. L. A Synthesis Strategy Yielding Skeletally Diverse Small Molecules Combinatorially. J. Am. Chem. Soc. 2004, 126, 14095–14104. 10.1021/ja0457415. [DOI] [PubMed] [Google Scholar]
- For examples, see:; a Ding B.; Taotofa U.; Orsak T.; Chadwell M.; Savage P. B. Synthesis and Characterization of Peptide-Cationic Steroid Antibiotic Conjugates. Org. Lett. 2004, 6, 3433–3436. 10.1021/ol048845t. [DOI] [PubMed] [Google Scholar]; b Rathinakumar R.; Walkenhorst W. F.; Wimley W. C. Broad-Spectrum Antimicrobial Peptides by Rational Combinatorial Design and High-Throughput Screening: The Importance of Interfacial Activity. J. Am. Chem. Soc. 2009, 131, 7609–7617. 10.1021/ja8093247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasheed A.; Farhat R. Combinatorial Chemistry: A Review. Int. J. Res. Pharm. Sci. 2013, 4, 2502–2516. [Google Scholar]
- Merrifield R. B. Solid Phase Peptide Synthesis. I. The Synthesis of a Tetrapeptide. J. Am. Chem. Soc. 1963, 85, 2149–2154. 10.1021/ja00897a025. [DOI] [Google Scholar]
- For examples, see:; a Stahl S.; Hultman T.; Olsson A.; Moks T.; Uhlén M. Solid Phase DNA Sequencing Using the Biotin-Avidin System. Nucleic Acids Res. 1988, 16, 3025–3038. 10.1093/nar/16.7.3025. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Lyttle M. H.; Hudson D.; Cook R. M. A New Universal Linker for Solid Phase DNA Synthesis. Nucleic Acids Res. 1996, 24, 2793–2798. 10.1093/nar/24.14.2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillier F.; Orain D.; Bradley M. Linkers and Cleavage Strategies in Solid-Phase Organic Synthesis and Combinatorial Chemistry. Chem. Rev. 2000, 100, 2091–2158. 10.1021/cr980040+. [DOI] [PubMed] [Google Scholar]
- Brady P. A.; Bonar-Law R. P.; Rowan S. J.; Suckling C. J.; Sanders J. K. M. “Living” Macrolactonisation: Thermodynamically-Controlled Cyclisation and Interconversion of Oligocholates. Chem. Commun. 1996, 319–320. 10.1039/cc9960000319. [DOI] [Google Scholar]
- Huc I.; Lehn J.-M. Virtual Combinatorial Libraries: Dynamic Generation of Molecular and Supramolecular Diversity by Self-Assembly. Proc. Natl. Acad. Sci. U.S.A. 1997, 94, 2106–2110. 10.1073/pnas.94.6.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combinatorial Chemistry; Fenniri H., Ed.; Oxford University Press: Oxford, 2000. [Google Scholar]
- For examples, see:; a Dabrowa K.; Niedbala P.; Majdecki M.; Duszewski P.; Jurczak J. A General Method for Synthesis of Unclosed Cryptands via H-Bond Templated Macrocyclization and Subsequent Mild Postfunctionalization. Org. Lett. 2015, 17, 4774–4777. 10.1021/acs.orglett.5b02324. [DOI] [PubMed] [Google Scholar]; b Jurczak J.; Sobczuk A.; Dąbrowa K.; Lindner M.; Niedbała P.; Stepniak P. Chirality of 20 - Membered Unclosed Cryptand: Macroring Distortion via Lariat Arm Modification. Chirality 2018, 30, 219–225. 10.1002/chir.22797. [DOI] [PubMed] [Google Scholar]; c Jurczak J.; Sobczuk A.; Dabrowa K.; Lindner M.; Niedbała P. An Indirect Synthetic Approach toward Conformationally Constrained 20-Membered Unclosed Cryptands via Late-Stage Installation of Intraannular Substituents. J. Org. Chem. 2018, 83, 13560–13567. 10.1021/acs.joc.8b02160. [DOI] [PubMed] [Google Scholar]; d Niedbała P.; Majdecki M.; Dąbrowa K.; Jurczak J. Selective Carboxylate Recognition Using Urea-Functionalized Unclosed Cryptands: Mild Synthesis and Complexation Studies. J. Org. Chem. 2020, 85, 5058–5064. 10.1021/acs.joc.9b03082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For examples, see:; a Jurczak J.; Kasprzyk S.; Sałański P.; Stankiewicz T. A General Method for the Synthesis of Diazacoronands. J. Chem. Soc., Chem. Commun. 1991, 956–957. 10.1039/c39910000956. [DOI] [Google Scholar]; b Jurczak J.; Stankiewicz T.; Sałański P.; Kasprzyk S.; Lipkowski P. A New Method for the Synthesis of Diazacoronands via Double-Amidation Reaction. Tetrahedron 1993, 49, 1478–1488. 10.1016/s0040-4020(01)90200-5. [DOI] [Google Scholar]
- For examples, see:; a Majdecki M.; Niedbala P.; Jurczak J. Amide-Based Cinchona Alkaloids as Phase-Transfer Catalysts: Synthesis and Potential Application. Org. Lett. 2019, 21, 8085–8090. 10.1021/acs.orglett.9b03065. [DOI] [PubMed] [Google Scholar]; b Majdecki M.; Niedbala P.; Jurczak J. Synthesis of C2 Hybrid Amide-Based PTC Catalysts and Their Comparison with Saturated Analogues. ChemistrySelect 2020, 5, 6424–6429. 10.1002/slct.202001012. [DOI] [Google Scholar]
- For examples, see:; a Katayev E. A.; Sessler J. L.; Khrustalev V. N.; Ustynyuk Y. A. Synthetic Model of the Phosphate Binding Protein: Solid-State Structure and Solution-Phase Anion Binding Properties of a Large Oligopyrrolic Macrocycle. J. Org. Chem. 2007, 72, 7244–7252. 10.1021/jo071106g. [DOI] [PubMed] [Google Scholar]; b Hargrove A. E.; Nieto S.; Zhang T.; Sessler J. L.; Anslyn E. V. Artificial Receptors for the Recognition of Phosphorylated Molecules. Chem. Rev. 2011, 111, 6603–6782. 10.1021/cr100242s. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Morozov B. S.; Namashivaya S. S. R.; Zakharko M. A.; Oshchepkov A. S.; Kataev E. A. Anthracene-Based Amido–Amine Cage Receptor for Anion Recognition under Neutral Aqueous Conditions. ChemistryOpen 2020, 9, 171–175. 10.1002/open.201900309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Barrientos D.; Rojas-Hernández A.; Gutiérrez A.; Moya-Hernández R.; Gómez-Balderas R.; Ramírez-Silva M. T. Determination of pKa Values of Tenoxicam from 1H NMR Chemical Shifts and of Oxicams from Electrophoretic Mobilities (CZE) with the Aid of Programs SQUAD and HYPNMR. Talanta 2009, 80, 754–762. 10.1016/j.talanta.2009.07.058. [DOI] [PubMed] [Google Scholar]
- Lee S.; Hua Y.; Park H.; Flood A. H. Intramolecular Hydrogen Bonds Preorganize an Aryl-triazole Receptor into a Crescent for Chloride Binding. Org. Lett. 2010, 12, 2100–2102. 10.1021/ol1005856. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.