Abstract
Peritonitis caused by Staphylococcus aureus is of major importance in peritoneal dialysis (PD) patients due to its great virulence profile and biofilm formation ability. Bacteriophages are a potential tool to treat peritonitis resulting from biofilm-associated infections. We screened S. aureus colonization in 71 PD patients from the nasal cavity, groin, and PD exit-site regions and analyzed clinical outcomes in these patients. We performed biofilm-formation testing of different strains and compared the isolates of one patient to detect phenotypic differences in S. aureus. Phage cocktails were used to detect S. aureus in vitro susceptibility. An adaptation procedure was performed in cases of bacterial resistance. Around 30% of PD patients (n = 21) were found to be S. aureus carriers; from these, a total of 34 S. aureus strains were isolated, of which 61.8% (n = 21) produced a strong biofilm. Phenotypic differences in strain biofilm production were detected in eight patients out of ten. All strains were sensitive to commonly used antibiotics. Broadly positive phage lytic activity (100%) was observed in six cocktails out of seven, and bacterial resistance towards phages was overcome using adaptation. Overall phages showed a promising in vitro effect in biofilm-forming S. aureus strains.
Keywords: S. aureus, peritoneal dialysis, phage adaptation, biofilm, phage therapy, phenotypic trait
1. Introduction
Staphylococcus aureus is a common causative agent of bacterial peritonitis in patients undergoing peritoneal dialysis (PD), representing 6–14% of cases [1,2,3], as well as being the most common causative agent of exit-site infection [4,5]. S. aureus peritonitis is associated with higher rates of relapse, repeated infection, and catheter removal in addition to increased mortality [3,6]. This can be explained by the existence of particular strains that have high expression of virulence factors such as Panton–Valentine leukocidin, enterotoxins that can act as superantigens, hemolysins, phenol-soluble modulins, and the quorum-sensing accessory gene regulator (agr) system that plays an important role in biofilm formation and virulence factor expression [7,8]. S. aureus colonizes human organisms in various sites such as the nasal cavity, inguinal region, and the PD catheter exit site [5]; therefore, treatment of culture-positive nasal carriers with topical mupirocin and the application of antibiotics to the catheter exit-site are recommended to reduce the risk of peritonitis [9]. Nevertheless, bacterial biofilm formation is of great importance as S. aureus can produce multilayered biofilms that are mainly made of polysaccharides [10]. These structures evade both the innate and adaptive immune systems of the host, leading to altered polymorphonuclear neutrophil killing and weakened phagocytosis, ineffectiveness of toll-like-receptor activation [8,11], and activation of the T-helper 1 (Th1) and Th17 immune response instead of the Th2 response that prevents S. aureus biofilm-associated infection [12]. The antimicrobial effect within the biofilm is insufficient due to lowered bacterial metabolic activity and reduced penetration that can be observed even with such bactericidal antimicrobials as oxacillin and vancomycin [13,14]. In a Brazilian study, biofilm formation was detected in 89% of S. aureus strains that were isolated from patients with PD-associated peritonitis [15]. Different anatomical niches of the xhuman organism can be colonized by phenotypically and genotypically polyclonal S. aureus strains; even the nasal cavity can be colonized with different S. aureus strains serving as a source for horizontal gene transfer with the ability for diverse antimicrobial susceptibility and virulence factor expression [16,17]. The quorum-sensing accessory gene regulator (agr) system plays a role in biofilm formation, and low agr activity is associated with increased biofilm production [18]; however, their expression activity is linked to environmental conditions [19]. Detection of strain polyclonality revealed differences in agr gene types [17], and the S. aureus biofilm-formation capacity varies between isolates and according to agr type [20]. Bacterial isolation sites among clinical strains impact biofilm production in S. aureus infections [21].
Bacteriophages or phages are natural viruses that infect bacterial cells and replicate in them, causing bacterial cell lysis. Eventually, this property can be used to eradicate bacterial pathogens. Phages are very diverse due to differences in their size, genetic structure, and morphology. Moreover, the host range of individual phage may differ from strain-specific to the extent of infecting bacteria that derive from distinctive genera [22]. Bacterial host specificity of phages is regulated via host cell surface receptors and, hence, bacteria that do not expose a specific receptor for a particular bacteriophage cannot be infected [23]. Phages replicate at the site of infection and produce substances such as endolysins that act on bacterial cell wall peptidoglycan, polysaccharide depolymerases that degrade carbohydrates, and lipopolysaccharides within bacterial biofilm. This leads to the destruction of biofilm and the bacteria embedded within regardless of their various metabolic activities. Phage-produced enzymes also mainly act on single bacterial species [24,25]. Such properties make bacteriophages and their produced particles an attractive tool for S. aureus eradication and the treatment of peritonitis caused by both antibiotic-resistant and -sensitive strains [26,27,28].
In this study, we detected S. aureus colonization in PD patients in the nasal cavity, groin, and PD exit-site region. We analyzed the impact of S. aureus colonization on peritonitis, death, kidney transplantation and PD catheter removal rates. Commercially available phage cocktails that may be potentially potent in future clinical trials were used to detect S. aureus in vitro susceptibility. The adaptation procedure was performed in the case of bacterial resistance towards phages. We performed biofilm-formation testing of different strains and compared the isolates of one patient to detect S. aureus phenotypic differences.
2. Materials and Methods
We included all peritoneal dialysis patients in Pauls Stradins Clinical University Hospital Peritoneal Dialysis Department, Latvia, between November 2016 and January 2017. S. aureus isolation and in vitro testing of antimicrobial and phage susceptibility, phage adaptation and strain biofilm formation testing were performed. Underlying diseases were noted for each patient and clinical outcomes as peritonitis, death, removal of PD catheter, and transplantation were analyzed over two-year period after S. aureus screening.
2.1. S. aureus Isolation and Microbiological Investigation
Swabs were obtained from nasal, inguinal, and PD catheter sites. Material was transported with universal transport media (AMIES) at room temperature within 2 h. Microbiological analysis was performed after overnight swab culturing on trypticase soy broth, mannitol salt agar, and Baird Parker agar with egg yolk tellurite. Gram staining and microscopy, coagulase detection, catalase test, and latex agglutination (Oxoid) were performed. For the final identification of S. aureus, a VITEK-2 (bioMerieux) system was used. Disk-diffusion antimicrobial susceptibility testing was performed according to EUCAST version 7.1, 2017.
2.2. Biofilm Growth Using Microtiter Plate Assay
Bacterial strains were isolated on trypticase soy agar (TSA) by streak method with incubation at 37 °C for 16–18 h. Two to three colonies were taken and resuspended in trypticase soy broth (TSB) supplemented with an additional 1% glucose for incubation at 37 °C for 16–18 h to receive a concentration 1–3 × 109 CFU/mL, bacterial concentration was detected using densitometer (DEN-1, Riga, Latvia) occasionally to reaffirm bacterial concentration we would perform bacterial titration. Inoculum bacteria grown in broth were diluted 1:100 in TSB supplemented with 1% glucose to receive a final concentration 1–3 × 107 CFU/mL. Then, 150 µL of diluted suspension was transferred with multichannel pipette in sterile 96-well plate (96-well TC plate, Suspension, F, Sarstedt, Germany) for biofilm cultivation. Each plate contained 11 strains and the negative control, with 8 wells per strain to provide reliable analysis of the experiment, which was triplicated. The inoculated plate was cultivated aerobically at 37 °C for 24 h. After incubation, the content of the plate was carefully removed by decanting in disinfectant. Each well was rinsed three times with 250 µL 0.9% saline to remove planktonic bacteria. After washing, the remaining bacteria attached were heat-fixed for 60 min at 60 °C. Staining was performed adding 150 µL of 0.1% crystal violet per well over 15 min; then, color was removed by decanting, and each well was washed three times with 250 µL distilled water. To detach formed cells from the wells and quantify the biofilm production degree, 150 µL of 96% ethanol was added to each well and left for 30 min (see Figure S1). Afterwards, the optical density (OD) of wells was measured with a microplate spectrophotometer (TECAN INFINITE F50, Männedorf, Switzerland) at 570 nm wavelength. Data were recovered from the TECAN interface and transferred to Microsoft Excel 10.
2.3. Biofilm Calculation
The optical density (OD) values for each strain were averaged and expressed as a number. The cut-off value (ODc) was separately calculated as three standard deviations (SDs) over the mean negative control value for each plate. To classify bacterial strains according to their capability for biofilm production, they were grouped according to Stepanovic et al. [29], respectively, biofilm nonproducer ODs ≤ ODc, weak producer ODc < ODs ≤ 2 × ODc, moderate producer 2 × ODc < ODs ≤ 4 × ODc, and strong producer ODs ≥ 4 × ODc.
2.4. Bacteriophage Preparation
Seven commercial bacteriophage stocks, i.e., cocktails were used: 6 produced by Eliava BioPreparations Ltd. (Staphylococcal, Pyo, Ses, Fersisi, Enko, and Intesti; Tbilisi, Georgiaand 1 by Microgen Ltd. (Pyobacteriophag; Perm, Perm Territory, Russia). S. aureus ATCC 4336 and ATCC 15923 were used as reference strains.
In terms of commercially produced phage stocks, main constituents, and host range of them have been disclosed before [30,31,32,33,34,35,36,37,38]. Staphylococcal bacteriophage containing Sb-1 (Myoviridae), active against S. aureus [30,31]. Pyo bacteriophage containing Sb-1 (Myoviridae) and ISP (Myoviridae) active against Staphylococcus spp., Streptococcus spp., Proteus spp., P. aeruginosa, and E. coli [32,33]. Ses active against Staphylococcus spp., Streptococcus spp., enteropathogenic E. coli [34]. Fersisi containing B1 (Myoviridae) and JA1 (Myoviridae) active against Staphylococcus spp., Streptococcus spp. [35,36]. Enko active against Salmonella spp., Shigella spp., Staphylococcus spp., enteropathogenic E. coli [34]. Intesti containing D1-D18, F1-F4 and Proteus phage (Myoviridae, Siphoviridae, Podoviridae) [37], active against Shigella spp., Salmonella spp., E.coli, Proteus spp., Staphylococcus spp., P.aeruginosa, Enterococcus spp. [32,34]. Pyobacteriophag containing fRuSau02 (Myoviridae) and SCH1 (Podoviridae) active against Staphylococcus spp., Streptococcus spp., Enterococcus spp., Proteus spp., Klebsiella spp., P. aeruginosa, and E.coli [33,36,38].
Plaque assay was used for the determination of the original titer of all 7 commercial phage cocktails that was measured in plaque-forming units per milliliter (PFU/mL). Serial tenfold dilutions of each commercial bacteriophage cocktail were made in 1.5 mL microcentrifuge tubes labeled from 10−1 to 10−5. For sequential dilutions, each microcentrifuge tube was filled with 900 µL sterile physiological saline and 100 µL bacteriophage suspension. Two to three pure colonies of S. aureus ATCC 4336 bacterial strain freshly grown on TSA plate were transferred to Luria–Bertani (LB) broth medium and incubated overnight at 37 °C. Then, 100 µL of bacterial culture suspension grown overnight and 50 µL of each previously made dilution of every bacteriophage cocktail were mixed together in 4 mL of previously molten 0.7% TSA tube, gently mixed, and poured evenly onto each corresponding TSA plate. After solidification of the top agar, plates were inverted and incubated overnight at 37 °C. Each bacteriophage commercial stock titer (PFU/mL) was calculated by using the following equation: (number of plaques)/(dilution factor × volume of diluted bacteriophage in mL). All procedures were performed in duplicate.
To attain the higher concentration of each bacteriophage, cocktail phage propagation using S. aureus ATCC 4336 was performed. After performing the plaque assay, the webbed plates of each bacteriophage cocktail were selected. Then, 12 mL of the LB broth medium was poured on each webbed plate. The flooded plates were left at room temperature on an orbital shaker (50 rpm) for 1–2 h. Subsequently, supernatant and soft overlay agar were collected for each bacteriophage cocktail in a labeled 15 mL plastic centrifuge tube. For each labeled tube, chloroform (CHCl3) with a final volume of 2–3% was added. After brief vortexing, all tubes were stored for 1–2 h at 4 °C. Tubes were centrifuged at 6000× g for 15 min at 4 °C to remove bacterial cell debris. To avoid bacterial contamination, the bacteriophage supernatant after centrifugation was filtered using a 0.20 µm filter. The final titer of the freshly acquired bacteriophage lysate was assessed via plaque assay. To equate the dilution factor among each bacteriophage cocktail, dilution with LB broth medium was accordingly performed.
2.5. Bacteriophage Adaptation
The adaptation procedure was applied to overcome bacteriophage resistance and ensure the enhanced bacteriophage lytic efficacy of bacteriophage cocktails. For bacteriophage adaptation, a protocol of a modified Appelman’s method was followed [39]. To optimize the method, strain ATCC 15923 was adapted using all 7 bacteriophage cocktails. Briefly, consecutive serial tenfold dilutions were made by adding 0.5 mL bacteriophage cocktail to 4.5 mL LB broth using bacteriophage lysate that was attained after the propagation procedure. For each tube except the first, 50 µL of the overnight bacterial host was added. The first tube, labeled as 10−1 containing only bacteriophage cocktail, was used as negative control, whereas the tube containing only bacterial suspension was the positive control. All prepared tubes were incubated for 48 h at 37 °C. Tubes were subsequently examined by measuring OD at 600 nm. The tube with the highest dilution factor but with OD similar to negative control was selected for further steps of the adaptation procedure. Prior to centrifugation, chloroform (CHCl3) with a final concentration of 2–3% was added to the selected bacteriophage and bacterial host mixture and incubated at 4 °C for 2 h. Centrifugation for 15 min at 4 °C was performed at 6000× g to pellet bacterial debris. The bacteriophage lysate from the supernatant above the solid residue was filtered through a 0.20 µm filter. The acquired lysate was subject to 3 cycles of the described adaptation procedure before performing a spot test to visually demonstrate the lytic ability of adapted bacteriophage stock.
2.6. Bacteriophage Lytic Activity Detection
To qualitatively demonstrate the lytic activity of the original and adapted bacteriophages, a spot test was applied. To make a bacterial lawn, 100 µL of overnight bacterial culture was mixed with 4.5 mL of previously molten top 0.7% TSA in a tube and gently mixed. Afterwards, it was transferred to the corresponding stiffened TSA plate and left at room temperature for approximately 20 min until complete solidification. A drop containing 10 µL of bacteriophage lysate was pipetted onto previously labeled area on the bacterial host lawn. Plates were left at room temperature to allow the drops to absorb into the agar. Plates were incubated in inverted position overnight at 37 °C. After incubation, the zones of clearing were visually examined for each plate. A positive lytic effect was recorded as confluent lysis (+++), partial lysis (++), or individual plaques (+), while a negative effect as resistant or no lysis (−) (see Figure 2). To validate the results, the spot test procedure duplicated for each phage preparation.
2.7. Data Analysis
IBM SPSS Statistics version 26 and Microsoft Excel 10 were used for data analysis. Graphical analysis was done using GraphPad Prism software version 8.1.0.
2.8. Ethical Statement
The study protocol was approved by the Ethics Committee of Riga Stradins University (document no. 32/28.01.2016). Written informed consent was obtained from all subjects before the study.
3. Results
Seventy-one patients were screened for S. aureus carriage, but seventy patients were included in the study, as one patient with PD catheter had not initiated dialysis; 51% (n = 36) were male and 49% (n = 34) were female, with an average age 59.96 years (SD15.9). For detailed patient data see Table S1.
Causes for end-stage renal disease vary in the study group: glomerulonephritis (GN; 40%, n = 28), diabetic nephropathy (14.3%, n = 10), chronic interstitial nephritis (20%, n = 14), autosomal dominant polycystic kidney disease (ADPKD; 10%, n = 7), hypertensive nephropathy (12.9%, n = 9); and unknown (2.9%, n = 2).
S. aureus carriers were 30% (n = 21; see Table 1); 71.4% of carriers were male (n = 15) and 28.6% female (n = 6). Of the carrier group patients, 28.6% had diabetes mellitus (n = 6), compared to only 10.2% in the noncarrier group (n = 5). Prior to and during the study, none of the patients used topical antimicrobial for S. aureus decolonization. The mean length of patient participation in the study in the noncarrier group was 16.61 months, and 13.95 months in the carrier group.
Table 1.
S. aureus carrier characteristics. Note: PD, peritoneal dialysis; ADPKD, autosomal dominant polycystic kidney disease.
| N | Age | Sex | Cause of End-Stage Renal Disease | Carriage Site (Biofilm Production Group) | Outcome in Two Years | ||
|---|---|---|---|---|---|---|---|
| Nose | Groin | PD Catheter | |||||
| 1 | 55 | ♂ | Diabetic nephropathy | 1N strong | 1 episode of S. aureus/Pseudomonas spp. Peritonitis, transplantation | ||
| 2 | 74 | ♀ | Glomerulonephritis | 3N strong | Death | ||
| 3 | 76 | ♂ | Hypertensive nephropathy | 6N moderate | 6G strong | 3 episodes of peritonitis (2 Streptococcus spp., 1 culture-negative) | |
| 4 | 83 | ♂ | Chronic interstitial nephritis | 8N strong | 8G strong | Death | |
| 5 | 68 | ♀ | Diabetic nephropathy | 12N moderate | 12PD strong | Death | |
| 6 | 36 | ♂ | Chronic interstitial nephritis | 14N moderate | Transplantation | ||
| 7 | 64 | ♂ | Chronic interstitial nephritis | 15N strong | 15G strong | Uneventful PD | |
| 8 | 42 | ♀ | Chronic interstitial nephritis | 17N strong | Transplantation | ||
| 9 | 60 | ♂ | ADPKD | 19N moderate | Transplantation | ||
| 10 | 34 | ♂ | Glomerulonephritis | 25N strong | 25G strong | 25PD strong | Transplantation |
| 11 | 77 | ♀ | ADPKD | 26PD strong | Death | ||
| 12 | 68 | ♂ | Glomerulonephritis | 27N strong | 27G strong | Uneventful PD | |
| 13 | 69 | ♂ | Glomerulonephritis | 35N strong | 35G strong | 1 episode of culture-negative peritonitis | |
| 14 | 68 | ♂ | Diabetic nephropathy | 36N moderate | Transplantation | ||
| 15 | 69 | ♂ | Glomerulonephritis | 47G strong | Uneventful PD | ||
| 16 | 58 | ♂ | Hypertensive nephropathy | 48N strong | Death | ||
| 17 | 65 | ♂ | Diabetic nephropathy | 58N strong | Death | ||
| 18 | 81 | ♂ | Chronic interstitial nephritis | 59N weak | 59G weak | Death | |
| 19 | 26 | ♀ | Glomerulonephritis | 67N weak | 67G moderate | 67PD moderate | 2 episodes of peritonitis (1 Streptococcus spp., 1 culture-negative) |
| 20 | 47 | ♂ | Glomerulonephritis | 70N strong | Transplantation | ||
| 21 | 49 | ♂ | Diabetic nephropathy | 71N moderate | 71G moderate | 71PD moderate | Death |
| TOTAL n (%) | 18 (53%) | 11 (32%) | 5 (15%) | ||||
| S. aureus Strain Count | |||||||
| Biofilm Production n (%) | Strong | 10 (56%) | 8 (73%) | 3 (60%) | |||
| Moderate | 6 (33%) | 2 (18%) | 2 (40%) | ||||
| Weak | 2 (11%) | 1 (9%) | |||||
In total, 34 S. aureus strains were obtained from 213 patient samples, all sensitive to commonly used antibiotics (cefoxitin, ciprofloxacin, trimethoprim/sulfamethoxazole, clindamycin, gentamycin, tetracycline, rifampicin), two strains were resistant to erythromycin. None of the isolated strains were methicillin-resistant S. aureus (MRSA) (see Table S2). No statistically significant correlation was noted between S. aureus carriage and comorbidities such as diabetes mellitus (p = 0.05), chronic heart failure, viral hepatitis, gout, and chronic obstructive pulmonary disease.
Death as an outcome was detected in 31.1% (n = 8) of carriers and 16.3% (n = 8) in the noncarrier group. Data showed the statistical tendency that risk of death in the carrier group was 2.33 times greater than that in the noncarrier group (see Table 2).
Table 2.
Two-year outcome of screened patients.
| Clinical Outcomes | S. aureus Carriers | S. aureus | Total | RR | CI 95% |
|---|---|---|---|---|---|
| Noncarriers | |||||
| Number of patients | 30.0% | 70.0% | 100% (n = 70) | ||
| (n = 21) | (n = 49) | ||||
| Death | 31.1% | 16.3% | 22.9% (n = 16) | 2.33 | 1.01–5.38 |
| (n = 8) | (n = 8) | ||||
| Transplantation | 28.6% | 18.4% | 21.4% (n = 15) | 1.56 | 0.63–3.81 |
| (n = 6) | (n = 9) | ||||
| Removal of PD catheter | 0% | 20.4% | 14.3% (n = 10) | ||
| (n = 0) | (n = 10) | ||||
| Peritonitis | 19.1% | 34.7% | 30.0% (n = 21) | 0.55 | 0.21–1.44 |
| (n = 4) | (n = 17) |
We noted 32 cases of peritonitis during our study (78.1%, n = 25 in noncarrier group; 21.9%, n = 7 in carrier group). Causative agents in the carrier group were mixed culture of methicillin-sensitive S. aureus (MSSA)/Pseudomonas spp. (14.3%, n = 1), Streptococcus spp. (42.9%, n = 3), and culture-negative (42.9%, n = 3). In the noncarrier group, causative agents were Streptococcus spp. (36%, n = 9), culture-negative (28%, n = 7), MSSA (8%, n = 2), mixed culture of Aerococcus spp./Pseudomonas spp., Bacillus spp., Candida spp., Enterococcus spp., Aeromonas spp., methicillin-sensitive coagulase-negative Staphylococcus and methicillin-resistant coagulase-negative Staphylococcus, each 4%, n = 1.
The overall incidence of peritonitis was 0.35 episodes per patient year. In the noncarrier group, there were 0.37 episodes per patient year compared to 0.29 per patient year in carrier group.
3.1. Biofilm Growth
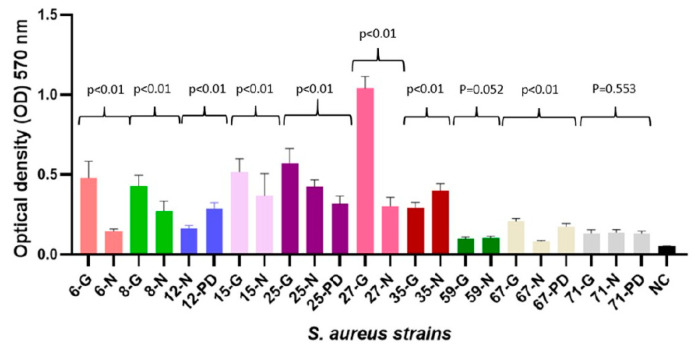
Biofilm production was observed among all isolated strains. Most commonly, strains produced strong biofilm (21, 61.8%), moderate (10, 29.4%), and weak (3, 8.8%) biofilm, all isolated strain biofilm mean optical densities are summarized in Table S2. Strong biofilm production of S. aureus strains was detected in seven out of ten individuals who did carry S. aureus in multiple isolation sites (see Table 1). The biofilm production capacity of S. aureus strains significantly varied in 8 out of 10 patients when strains were compared among different patient S. aureus isolation sites (see Figure 1).
Figure 1.
Biofilm production capability on microtiter plate of the 34 clinical isolates of S. aureus and negative control (NC). Bars represent mean values of OD (measured at wavelength of 570 nm). Differences between one suspect S. aureus strain capability in biofilm formation were analyzed using T test and Mann–Whitney U Test for two strain analyses, and one-way ANOVA and Kruskal–Wallis test for three strain analysis and are expressed as p-values.
3.2. Bacteriophage Susceptibility and Adaptation
From all seven commercial bacteriophage cocktails, the Staphylococcal Bacteriophage (Eliava) had an original titer of 104 PFU/mL, while Pyo, Enko, Intesti Bacteriophages (Eliava) and Pyobacteriophag (Microgen) had 105 PFU/mL. Only the Ses and Fersisi Bacteriophages (Eliava) demonstrated a titer of 106 PFU/mL. After bacteriophage cocktail propagation, of seven phage stocks all but one presented a titer of 109 PFU/mL, namely Pyo, Ses, Fersisi, Intesti and Pyobacteriophag. Conversely, an estimated titer of Staphylococcal Bacteriophage was 107 PFU/mL. All phage titers were equalised to 107 PFU/mL before phage lytic activity testing against S. aureus strains (see Table 3).
Table 3.
Lytic activity of seven commercial bacteriophage cocktails against 34 Staphylococcus aureus strains acquired from peritoneal dialysis patient samples.
| S. aureus Strain Code | Bacteriophages | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Biofilm Production Degree | Eliava BioPreparations Ltd. | Microgen Ltd. | |||||||
| Staphylococcal | Pyo | Ses | Fersisi | Enko | Intesti | Pyobacteriophag | |||
| 1.2 × 107 | 3.4 × 107 | 4.0 × 107 | 2.4 × 107 | 1.4 × 107 | 2.0 × 107 | 2.0 × 107 | |||
| Not Adapted | Adapted | ||||||||
| 1N | Strong | PL | PL | PL | PL | PL | PL | PL | |
| 3N | Strong | PL | PL | PL | PL | PL | PL | PL | |
| 6N | Moderate | R a | PL | PL | PL | PL | PL | PL | PL |
| 6G | Strong | R a | PL | PL | PL | PL | PL | PL | PL |
| 8N | Strong | PL | PL | PL | PL | PL | PL | PL | |
| 8G | Strong | PL | PL | PL | PL | PL | PL | PL | |
| 12N | Moderate | IP a | PL | PL | PL | PL | PL | PL | IP |
| 12PD | Strong | R a | PL | PL | PL | PL | PL | PL | PL |
| 14N | Moderate | PL | PL | PL | PL | PL | PL | PL | |
| 15N | Strong | R a | PL | PL | PL | PL | PL | PL | PL |
| 15G | Strong | R a | PL | PL | PL | PL | PL | PL | PL |
| 17N | Strong | R a | PL | IP | PL | PL | PL | PL | IP |
| 19N | Moderate | R a | PL | PL | PL | PL | PL | PL | IP |
| 25N | Strong | PL | PL | PL | PL | PL | PL | PL | |
| 25PD | Strong | PL | PL | PL | PL | PL | PL | PL | |
| 25G | Strong | PL | PL | PL | PL | PL | PL | PL | |
| 26PD | Strong | PL | PL | PL | PL | PL | PL | PL | |
| 27N | Strong | PL | PL | IP | PL | IP | PL | PL | |
| 27G | Strong | PL | CL | PL | PL | CL | PL | PL | |
| 35N | Strong | IP a | PL | PL | PL | PL | PL | PL | PL |
| 35G | Strong | PL | PL | PL | PL | PL | PL | PL | |
| 36N | Moderate | PL | PL | PL | PL | PL | PL | PL | |
| 47G | Strong | CL | PL | PL | PL | PL | PL | PL | |
| 48G | Strong | CL | PL | PL | PL | PL | PL | PL | |
| 58N | Strong | PL | PL | PL | PL | PL | PL | PL | |
| 59G | Weak | CL | PL | IP | PL | PL | PL | PL | |
| 59N | Weak | CL | CL | PL | IP | PL | PL | PL | |
| 67G | Weak | R a | PL | PL | PL | PL | PL | PL | PL |
| 67N | Moderate | R a | PL | PL | PL | PL | PL | PL | PL |
| 67PD | Moderate | PL | PL | PL | PL | PL | PL | PL | |
| 70N | Strong | PL | PL | PL | PL | PL | PL | PL | |
| 71PD | Moderate | PL | PL | PL | PL | PL | PL | PL | |
| 71G | Moderate | PL | PL | PL | PL | PL | PL | PL | |
| 71N | Moderate | PL | PL | PL | PL | PL | PL | PL | |
| Phage lytic activity | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |
| CL (+++) | 4 (12) | 0 (0) | 2 (6) | 0 (0) | 0 (0) | 1 (3) | 0 (0) | 0 (0) | |
| PL (++) | 19 (56) | 11 (100) | 31 (91) | 32 (94) | 33 (97) | 32 (94) | 34 (100) | 31 (91) | |
| IP (+) | 2 a (6) | 0 (0) | 1 (3) | 2 (6) | 1 (3) | 1 (3) | 0 (0) | 3 (9) | |
| R (−) | 9 a (26) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
a Staphylococcal Bacteriophage (Eliava) taken to adaptation procedure after showing spot test result as individual plaques (+) or no lysis (−) against 11 Staphylococcus aureus strains acquired from peritoneal dialysis patients samples: CL (+++), confluent lysis; PL (++), partial lysis; IP (+), individual plaques; R (−), resistant or no lysis.
The evaluation results of bacteriophage lysate lytic activity obtained in the spot assay are shown in Table 3. When tested against all 34 S. aureus isolates, 6 bacteriophage stocks except Staphylococcal Bacteriophage (Eliava) revealed positive lytic results in all cases. Bacterial resistance to bacteriophage represented in the spot test was determined in 9 (26%) out of 34 Staphylococcus aureus isolates to Staphylococcal bacteriophage (Eliava).
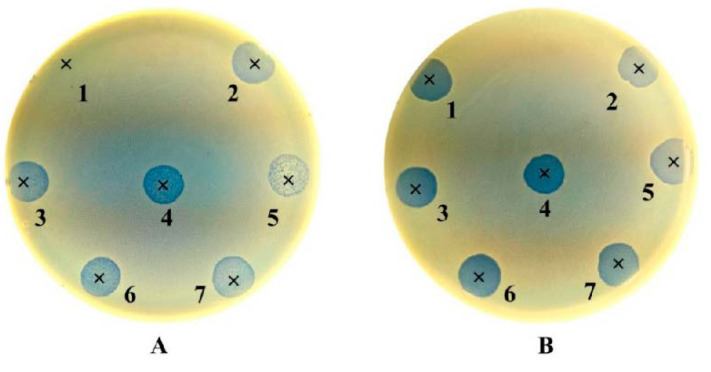
The visual confirmation results of the effect of the adaptation procedure for exposure of S. aureus ATCC 15923 to seven commercial bacteriophage cocktails is shown in Figure 2, which shows that the adaptation procedure led to both improvement in already positive lytic activity and overcoming bacterial resistance.
Figure 2.
Lytic activity of seven commercial bacteriophage cocktails against Staphylococcus aureus reference strain ATCC 15923 before and after bacteriophage adaptation. (1) Staphylococcal Bacteriophage (Eliava); (2) Pyo Bacteriophage (Eliava); (3) Ses Bacteriophage (Eliava); (4) Fersisi Bacteriophage (Eliava); (5) Enko Bacteriophage (Eliava); (6) Intesti Bacteriophage (Eliava); (7) Pyobacteriophag Bacteriophage (Microgen). (A) Lytic activity before adaptation procedure. (1) R (−), resistant or no lysis; (2–4) PL (++), partial lysis; (5) IP (+), individual plaques; (6, 7) PL (++), partial lysis. (B) Lytic activity after adaptation procedure. (1) CL (+++), confluent lysis; (2) PL (++), partial lysis; (3, 4) CL (+++), confluent lysis; (5) PL (++), partial lysis; (6, 7) CL (+++), confluent lysis.
Staphylococcal Bacteriophage (Eliava) against 11 chosen bacterial strains (9 with resistance and 2 with individual plaques) were taken for phage adaptation. In all cases, the adaptation procedure resulted in overcoming bacterial resistance; all 11 bacterial strains (100%) showed a positive lytic effect after adaptation (see Table 3).
4. Discussion
In our study, we detected the clinical relevance of S. aureus colonization in PD patients, strain antimicrobial susceptibility, biofilm characteristics, and we performed an in vitro phage susceptibility testing that showed the broad lytic activity of different commercially available bacteriophages indicating a wide phage antimicrobial effect.
The necessity of novel antimicrobial agents that can be used against S. aureus-caused infections in PD patients is of great importance, as this microorganism is one of the most prevalent causative agents of PD-associated peritonitis and exit-site infections. Due to its strong virulence profile and biofilm production capability in PD patients, it has high peritonitis relapse, PD catheter removal, and death rates. Our results suggested a 2.33 times greater relative risk of death in the S. aureus carrier group that demonstrates worse overall prognosis for S. aureus carriers, similarly to other studies. Interestingly, we did not detect higher peritonitis or S. aureus-caused peritonitis rate in the carrier group that could be explained by our relatively small study group. Nevertheless, the overall peritonitis rate was 0.35 per patient year, similar to data from other studies having larger cohorts in Northern Europe [40].
For PD patients, S. aureus colonization is one of the sources of endogenous infection; therefore, use of long-term antimicrobials is recommended by International Society for Peritoneal Dialysis guidelines, but in several countries, it is not fulfilled to avoid possible S. aureus resistance development. Our results of S. aureus isolation from the nasal cavity, groin region, and PD catheter exit-site indicated 30% (n = 21) S. aureus colonization among PD patients, which is similar but slightly greater than that in a similar study [41]. Colonization was mainly detected in several body niches, mostly in the nasal cavity (n = 18) but also in the groin region (n = 11) and around the PD catheter exit site (n = 5); however, none of the strains were MRSA.
A positive or lytic bacteriophage effect was detected in all 71 S. aureus strains using commercially available bacteriophage cocktails from Eliava (Pyo, Ses, Fersisi, Enko, Intesti) and Microgen (Pyobacteriophag), except Eliava Staphylococcal Bacteriophage, where the incidence of resistance or no lytic effect was 26% (n = 9). The most commonly observed degree of positive phage lytic effect was partial lysis, ranging 56–100% among isolates and could depend on phage concentration within the cocktail, time of incubation, and phage resistance. Our results of wide phage lytic activity of 100% for all except Eliava Staphylococcal Bacteriophage could be also associated with possible low strain genetic diversity, as in various parts of the world, we can find genetically and biologically distinct strains of the same bacterial species [42]. In cases of bacterial resistance or a weak lytic effect towards Eliava Staphylococcal Bacteriophage, an adaptation procedure or so-called host range expansion was performed. The outcome after adaptation was indicative of persistent enhancement in bactericidal action, leading to 100% lytic activity. In nine resistant strains, adaptation led to overcoming bacterial resistance; in two strains, improvement of positive lytic activity from individual plaques (+) to partial lysis (++) was achieved. The principle of phage adaptation is mainly due to spontaneous mutations and possible gene recombination between genetically diverse bacteriophages within the phage cocktail. Such changes lead to altered structural gene products, for example, encoding phage tail fiber assembly proteins that are necessary for phage attachment to the host cell or adsorption, which is the first step of phage infection. Adaptation can also reduce lysis time and increase phage burst size [43,44]. Recent findings on the Eliava Staphylococcal Bacteriophage cocktail that consist of Twort-like phage Sb-1 showed that after the adaptation process, newly formed phage clones were found in phage stock, and such a process increased phage lytic activity from 87% to 96% on globally diverse S. aureus strains. Interestingly, genetic differences between the mutant and parental Sb-1 phages were found in the phage genome hypervariable complex repeat structure, but the adsorption rate between parental and mutant phage was similar, with the conclusion that the process of host range expansion in the Sb-1 phage is still unclear [30]. Another study from Switzerland showed similar results in that after phage–bacterium adaptation of Eliava Pyo Bacteriophage with E. coli isolates from urinary tract infection, the lytic spectrum increased from 65.9% to 92.7% [45].
In phage therapy, it is important that the phage is lytic, as lysogenic phages can integrate into the genome of bacteria and introduce new pathogenic properties [46,47]; for example, Shiga toxin genes responsible for E. coli-associated hemorrhagic colitis and hemolytic uremic syndrome development that are conveyed by lambdoid lysogenic bacteriophages [48]. In lysogenic phages, these properties can be transferred via horizontal gene transfer; therefore, morphologic characterization of phages and whole-genome sequencing should be performed before therapeutic use [49]. Bacterial resistance towards phages can also emerge, so the use of cocktails containing several phages even against different bacterial species is reasonable. Proper quality and safety procedures are already described by phage researchers in guidelines and articles of phage cocktail preparations [39,50]. Still, the current legal framework of phage therapy for their possible clinical use and easier clinical-study organization is a major hurdle. Already now Eliava bacteriophages have been used to treat life-threatening S. aureus caused infections with Pyo, Staphylococcal and Fersisi phage cocktails. Results from these reports show positive clinical outcome and great tolerability when used on skin and mucous membranes [51,52]. Pyo, Eliava phage cocktail and its component ISP phage [35] has been used in clinical trials. In these studies, application of bacteriophages did not elicit any side effect [53,54].
Our results revealed clinically relevant (strong and moderate) biofilm production in 91.2% (n = 31) of strains, an observation similar to another study from Brazil where S. aureus strains isolated from PD peritonitis patients produced biofilm in 88.7% (n = 55) of cases, even though a different biofilm detection method was used [15], indicating that microtiter plate assay can be used as a cost-effective screening method for biofilm detection in clinically relevant S. aureus strains. Phage lytic activity was not affected by different degrees of S. aureus biofilm production, showing that their biofilm production capability does not interfere with in vitro testing of phage on planktonic bacteria.
S. aureus isolation sites might be of major importance; although the majority of strains were isolated from the nasal cavity, two patients were only colonized in the groin and one in only the PD catheter exit-site regions. Among patients with multiple isolated S. aureus strains, their biofilm production phenotype was statistically different (see Figure 1). Based on the study of 130 S. aureus isolates, Piechota M. et al. [21] reported, that their biofilm production differed according to their isolation site, and strong biofilm producers were found mainly from tracheostomy tubes, sputum, throat, and nose. We detected strong biofilm production in 56–73% of S. aureus among all three isolation sites. The accessory gene regulator (agr) quorum-sensing system and intercellular adhesion (ica) group genes play an important role in S. aureus biofilm formation, but they are environmental factor-dependent [18,19,20,21]. Our results about biofilm production and phage susceptibility support S. aureus phenotypic variability, even within one patient; however, such phenomena did not interfere with the bacteriophage positive lytic effect that was detected in the majority of strains.
5. Conclusions
Colonization of S. aureus was associated with greater mortality rate. In vitro, bacteriophage activity showed a broad lytic spectrum against isolates. In the case of bacterial resistance, it was overcome with an adaptation procedure. Interestingly, the phenotypic differences of S. aureus isolates for phage susceptibility and biofilm production were detected, meaning that isolate virulence was variable and isolation site-dependent.
The increasing incidence of antibiotic-resistant infections and the lack of novel antimicrobials against multidrug-resistant bacteria makes phages a promising agent in treating biofilm-associated infections.
Acknowledgments
The authors would like to acknowledge and thank for the support from Mzia Kutateladze, Head of George Eliava Institute of Bacteriophage, Microbiology, and Virology, Tbilisi, Georgia, for her support and assistance to the paper.
Supplementary Materials
The following are available online at https://www.mdpi.com/2079-6382/9/9/582/s1, Table S1: Patients characteristics, Table S2: Staphylococcus aureus strain biofilm mean optical density and antimicrobial susceptibility, Figure S1: S. aureus biofilm growth using microtiter plate assay stained with crystal violet.
Author Contributions
Conceptualization, K.R.; methodology K.R. and J.K.; validation, K.R., D.R., and L.A.; formal analysis, K.R., L.A., and E.S.; investigation, K.R., D.R., L.A., and A.P. (Anna Popova); data curation, K.R., L.A., and E.S.; writing—original-draft preparation, K.R., D.R.; writing—review and editing J.K., A.P. (Anna Popova), I.P., V.K., and A.P. (Aivars Petersons); visualization, K.R., D.R., L.A., and E.S.; supervision, J.K. and A.P. (Aivars Petersons); project administration, K.R., J.K., and A.P. (Aivars Petersons) All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Whitty R., Bargman J.M., Kiss A., Dresser L., Lui P. Residual Kidney Function and Peritoneal Dialysis—Associated Peritonitis Treatment Outcomes. Clin. J. Am. Soc. Nephrol. 2017;12:2016–2022. doi: 10.2215/CJN.00630117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kitterer D., Latus J., Pöhlmann C., Alscher M.D., Kimmel M. Microbiological Surveillance of Peritoneal Dialysis Associated Peritonitis: Antimicrobial Susceptibility Profiles of a Referral Center in GERMANY over 32 Years. PLoS ONE. 2015;10:e0135969. doi: 10.1371/journal.pone.0135969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Govindarajulu S., Hawley C.M., McDonald S.P., Brown F.G., Rosman J.B., Wiggins K.J., Bannister K.M., Johnson D.W. Staphylococcus Aureus Peritonitis in Australian Peritoneal Dialysis Patients: Predictors, Treatment, and Outcomes in 503 Cases. Perit. Dial. Int. 2010;30:311–319. doi: 10.3747/pdi.2008.00258. [DOI] [PubMed] [Google Scholar]
- 4.Xu G., Tu W., Xu C. Mupirocin for Preventing Exit-site Infection and Peritonitis in Patients Undergoing Peritoneal Dialysis. Nephrol. Dial. Transplant. 2010;25:587–592. doi: 10.1093/ndt/gfp411. [DOI] [PubMed] [Google Scholar]
- 5.Simões-Silva L., Ferreira S., Santos-Araujo C., Tabaio M., Pestana M., Soares-Silva I., Sampaio-Maia B. Oral Colonization of Staphylococcus Species in a Peritoneal Dialysis Population: A Possible Reservoir for PD-Related Infections? Can. J. Infect. Dis. Med. Microbiol. 2018;2018:5789094. doi: 10.1155/2018/5789094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li P.K.-T., Chow K.M. Infectious Complications in Dialysis—Epidemiology and Outcomes. Nat. Rev. Nephrol. 2012;8:77–88. doi: 10.1038/nrneph.2011.194. [DOI] [PubMed] [Google Scholar]
- 7.Kane T.L., Carothers K.E., Lee S.W. Virulence Factor Targeting of the Bacterial Pathogen Staphylococcus Aureus for Vaccine and Therapeutics. Curr. Drug Targets. 2018;19:111–127. doi: 10.2174/1389450117666161128123536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ricciardi B.F., Muthukrishnan G., Masters E., Ninomiya M., Lee C.C., Schwarz E.M. Staphylococcus Aureus Evasion of Host Immunity in the Setting of Prosthetic Joint Infection: Biofilm and Beyond. Curr. Rev. Musculoskelet Med. 2018;11:389–400. doi: 10.1007/s12178-018-9501-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Szeto C.-C., Li P.K.-T., Johnson D.W., Bernardini J., Dong J., Figueiredo A.E., Ito Y., Kazancioglu R., Moraes T., Esch S.V., et al. ISPD Catheter-Related Infection Recommendations: 2017 Update. Perit. Dial. Int. 2017;37:141–154. doi: 10.3747/pdi.2016.00120. [DOI] [PubMed] [Google Scholar]
- 10.Archer N.K., Mazaitis M.J., Costerton J.W., Leid J.G., Powers M.E., Shirtliff M.E. Staphylococcus Aureus Biofilms: Properties, Regulation, and Roles in Human Disease. Virulence. 2011;2:445–459. doi: 10.4161/viru.2.5.17724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thurlow L.R., Hanke M.L., Fritz T., Angle A., Aldrich A., Williams S.H., Engebretsen I.L., Bayles K.W., Horswill A.R., Kielian T. Staphylococcus Aureus Biofilms Prevent Macrophage Phagocytosis and Attenuate Inflammation In Vivo. J. Immunol. 2011;186:6585–6596. doi: 10.4049/jimmunol.1002794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jensen L.K., Jensen H.E., Koch J., Bjarnsholt T., Eickhardt S., Shirtliff M. Specific Antibodies to Staphylococcus Aureus Biofilm Are Present in Serum from Pigs with Osteomyelitis. In Vivo. 2015;29:555–560. [PubMed] [Google Scholar]
- 13.Singh R., Ray P., Das A., Sharma M. Penetration of Antibiotics Through Staphylococcus Aureus and Staphylococcus Epidermidis Biofilms. J. Antimicrob. Chemother. 2010;65:1955–1958. doi: 10.1093/jac/dkq257. [DOI] [PubMed] [Google Scholar]
- 14.Donlan R.M., Costerton J.W. Biofilms: Survival Mechanisms of Clinically Relevant Microorganisms. Clin. Microbiol. Rev. 2002;15:167–193. doi: 10.1128/CMR.15.2.167-193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barretti P., Moraes T.M.C., Camargo C.H., Caramori J.C.T., Mondelli A.L., Montelli A.C., Maria de Lourdes R.S. Peritoneal Dialysis-Related Peritonitis Due to Staphylococcus Aureus: A Single-Center Experience over 15 Years. PLoS ONE. 2012;7:e31780. doi: 10.1371/annotation/6ce124f8-e74f-42dc-81a6-7f85cc23fd3d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muenks C.E., Hogan P.G., Wang J.W., Eisenstein K.A., Burnham C.-A.D., Fritz S.A. Diversity of Staphylococcus Aureus Strains Colonizing Various Niches of the Human Body. J. Infect. 2016;72:698–705. doi: 10.1016/j.jinf.2016.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cespedes C., Saïd-Salim B., Miller M., Lo S.-H., Kreiswirth B.N., Gordon R.J., Vavagiakis P., Klein R.S., Lowy F.D. The Clonality of Staphylococcus Aureus Nasal Carriage. J. Infect. Dis. 2005;191:444–452. doi: 10.1086/427240. [DOI] [PubMed] [Google Scholar]
- 18.Le K.Y., Otto M. Quorum-Sensing Regulation in Staphylococci-An Overview. Front. Microbiol. 2015;6:1174. doi: 10.3389/fmicb.2015.01174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yarwood J.M., Bartels D.J., Volper E.M., Greenberg E.P. Quorum Sensing in Staphylococcus Aureus Biofilms. J. Bacteriol. 2004;186:1838–1850. doi: 10.1128/JB.186.6.1838-1850.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Y., Xu D., Shi L., Cai R., Li C., Yan H. Association Between Agr Type, Virulence Factors, Biofilm Formation and Antibiotic Resistance of Staphylococcus Aureus Isolates from Pork Production. Front. Microbiol. 2018;9:1876. doi: 10.3389/fmicb.2018.01876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Piechota M., Kot B., Frankowska-Maciejewska A., Grużewska A., Woźniak-Kosek A. Biofilm Formation by Methicillin-Resistant and Methicillin-Sensitive Staphylococcus Aureus Strains from Hospitalized Patients in Poland. Biomed Res. Int. 2018;2018 doi: 10.1155/2018/4657396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ross A., Ward S., Hyman P. More Is Better: Selecting for Broad Host Range Bacteriophages. Front. Microbiol. 2016;7:1352. doi: 10.3389/fmicb.2016.01352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kasman L.M., Porter L.D. Bacteriophages. StatPearls. [(accessed on 29 May 2020)];2019 Available online: https://www.ncbi.nlm.nih.gov/books/NBK493185/
- 24.Harada L.K., Silva E.C., Campos W.F., Del Fiol F.S., Vila M., Dąbrowska K., Krylov V.N., Balcão V.M. Biotechnological Applications of Bacteriophages: State of the Art. Microbiol. Res. 2018;212–213:38–58. doi: 10.1016/j.micres.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 25.Olsen N.M.C., Thiran E., Hasler T., Vanzieleghem T., Belibasakis G.N., Mahillon J., Loessner M.J., Schmelcher M. Synergistic Removal of Static and Dynamic Staphylococcus Aureus Biofilms by Combined Treatment with a Bacteriophage Endolysin and a Polysaccharide Depolymerase. Viruses. 2018;10:438. doi: 10.3390/v10080438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roach D.R., Donovan D.M. Antimicrobial Bacteriophage-Derived Proteins and Therapeutic Applications. Bacteriophage. 2015;5:e1062590. doi: 10.1080/21597081.2015.1062590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rashel M., Uchiyama J., Ujihara T., Uehara Y., Kuramoto S., Sugihara S., Yagyu K.-I., Muraoka A., Sugai M., Hiramatsu K., et al. Efficient Elimination of Multidrug-Resistant Staphylococcus Aureus by Cloned Lysin Derived from Bacteriophage Phi MR11. J. Infect. Dis. 2007;196:1237–1247. doi: 10.1086/521305. [DOI] [PubMed] [Google Scholar]
- 28.Chhibber S., Gupta P., Kaur S. Bacteriophage as Effective Decolonising Agent for Elimination of MRSA from Anterior Nares of BALB/c Mice. BMC Microbiol. 2014;14:212. doi: 10.1186/s12866-014-0212-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stepanović S., Vuković D., Hola V., Di Bonaventura G., Djukić S., Cirković I., Ruzicka F. Quantification of Biofilm in Microtiter Plates: Overview of Testing Conditions and Practical Recommendations for Assessment of Biofilm Production by Staphylococci. APMIS. 2007;115:891–899. doi: 10.1111/j.1600-0463.2007.apm_630.x. [DOI] [PubMed] [Google Scholar]
- 30.Sergueev K.V., Filippov A.A., Farlow J., Su W., Kvachadze L., Balarjishvili N., Kutateladze M., Nikolich M.P. Correlation of Host Range Expansion of Therapeutic Bacteriophage Sb-1 with Allele State at a Hypervariable Repeat Locus. Appl. Environ. Microbiol. 2019;85:e01209–e01219. doi: 10.1128/AEM.01209-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kvachadze L., Balarjishvili N., Meskhi T., Tevdoradze E., Skhirtladze N., Pataridze T., Adamia R., Topuria T., Kutter E., Rohde C., et al. Evaluation of Lytic Activity of Staphylococcal Bacteriophage Sb-1 Against Freshly Isolated Clinical Pathogens. Microb. Biotechnol. 2011;4:643–650. doi: 10.1111/j.1751-7915.2011.00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kutateladze M., Adamia R. Phage Therapy Experience at the Eliava Institute. Med. Mal. Infect. 2008;38:426–430. doi: 10.1016/j.medmal.2008.06.023. [DOI] [PubMed] [Google Scholar]
- 33.Azam A.H., Tanji Y. Peculiarities of Staphylococcus Aureus Phages and Their Possible Application in Phage Therapy. Appl. Microbiol. Biotechnol. 2019;103:4279–4289. doi: 10.1007/s00253-019-09810-2. [DOI] [PubMed] [Google Scholar]
- 34.Gundogdu A., Bolkvadze D., Kilic H. In Vitro Effectiveness of Commercial Bacteriophage Cocktails on Diverse Extended-Spectrum Beta-Lactamase Producing Escherichia Coli Strains. Front. Microbiol. 2016;7:1761. doi: 10.3389/fmicb.2016.01761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ajuebor J., Buttimer C., Arroyo-Moreno S., Chanishvili N., Gabriel A.M., O’Mahony J., McAuliffe O., Neve H., Franz C., Coffey A. Comparison of Staphylococcus Phage K with Close Phage Relatives Commonly Employed in Phage Therapeutics. Antibiotics. 2018;7:37. doi: 10.3390/antibiotics7020037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McCallin S., Sarker S.A., Sultana S., Oechslin F., Brüssow H. Metagenome Analysis of Russian and Georgian Pyophage Cocktails and a Placebo-Controlled Safety Trial of Single Phage Versus Phage Cocktail in Healthy Staphylococcus Aureus Carriers. Environ. Microbiol. 2018;20:3278–3293. doi: 10.1111/1462-2920.14310. [DOI] [PubMed] [Google Scholar]
- 37.Zschach H., Joensen K.G., Lindhard B., Lund O., Goderdzishvili M., Chkonia I., Jgenti G., Kvatadze N., Alavidze Z., Kutter E.M., et al. What Can We Learn from a Metagenomic Analysis of a Georgian Bacteriophage Cocktail? Viruses. 2015;7:6570–6589. doi: 10.3390/v7122958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leskinen K., Tuomala H., Wicklund A., Horsma-Heikkinen J., Kuusela P., Skurnik M., Kiljunen S. Characterization of vB_SauM-fRuSau02, a Twort-Like Bacteriophage Isolated from a Therapeutic Phage Cocktail. Viruses. 2017;9:258. doi: 10.3390/v9090258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Merabishvili M., Pirnay J.-P., De Vos D. Guidelines to Compose an Ideal Bacteriophage Cocktail. Methods Mol. Biol. 2018;1693:99–110. doi: 10.1007/978-1-4939-7395-8_9. [DOI] [PubMed] [Google Scholar]
- 40.Ljungman S., Jensen J.E., Paulsen D., Petersons A., Ots-Rosenberg M., Saha H., Struijk D.G., Wilkie M., Heimbürger O., Stegmayr B., et al. Retraining for Prevention of Peritonitis in Peritoneal Dialysis Patients: A Randomized Controlled Trial. Perit. Dial. Int. 2020;40:141–152. doi: 10.1177/0896860819887626. [DOI] [PubMed] [Google Scholar]
- 41.Grothe C., Taminato M., Belasco A., Sesso R., Barbosa D. Prophylactic Treatment of Chronic Renal Disease in Patients Undergoing Peritoneal Dialysis and Colonized by Staphylococcus Aureus: A Systematic Review and Meta-Analysis. BMC Nephrol. 2016;17 doi: 10.1186/s12882-016-0329-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grundmann H., Aanensen D.M., van den Wijngaard C.C., Spratt B.G., Harmsen D., Friedrich A.W. Geographic Distribution of Staphylococcus Aureus Causing Invasive Infections in Europe: A Molecular-Epidemiological Analysis. PLoS Med. 2010;7:e10000215. doi: 10.1371/journal.pmed.1000215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.North O.I., Sakai K., Yamashita E., Nakagawa A., Iwazaki T., Büttner C.R., Takeda S., Davidson A.R. Phage Tail Fibre Assembly Proteins Employ a Modular Structure to Drive the Correct Folding of Diverse Fibres. Nat. Microbiol. 2019;4:1645–1653. doi: 10.1038/s41564-019-0477-7. [DOI] [PubMed] [Google Scholar]
- 44.Burrowes B.H., Molineux I.J., Fralick J.A. Directed in Vitro Evolution of Therapeutic Bacteriophages: The Appelmans Protocol. Viruses. 2019;11:241. doi: 10.3390/v11030241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sybesma W., Zbinden R., Chanishvili N., Kutateladze M., Chkhotua A., Ujmajuridze A., Mehnert U., Kessler T.M. Bacteriophages as Potential Treatment for Urinary Tract Infections. Front. Microbiol. 2016;7:465. doi: 10.3389/fmicb.2016.00465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Harper D.R., Parracho H.M.R.T., Walker J., Sharp R., Hughes G., Werthén M., Lehman S., Morales S. Bacteriophages and Biofilms. Antibiotics. 2014;3:270–284. doi: 10.3390/antibiotics3030270. [DOI] [Google Scholar]
- 47.Iacovelli V., Gaziev G., Topazio L., Bove P., Vespasiani G., Finazzi Agrò E. Nosocomial Urinary Tract Infections: A Review. Urologia. 2014;81:222–227. doi: 10.5301/uro.5000092. [DOI] [PubMed] [Google Scholar]
- 48.Tozzoli R., Grande L., Michelacci V., Ranieri P., Maugliani A., Caprioli A., Morabito S. Shiga Toxin-Converting Phages and the Emergence of New Pathogenic Escherichia Coli: A World in Motion. Front. Cell. Infect. Microbiol. 2014;4:80. doi: 10.3389/fcimb.2014.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jacobsen S.M., Stickler D.J., Mobley H.L.T., Shirtliff M.E. Complicated Catheter-Associated Urinary Tract Infections Due to Escherichia Coli and Proteus Mirabilis. Clin. Microbiol. Rev. 2008;21:26–59. doi: 10.1128/CMR.00019-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pirnay J.-P., Blasdel B.G., Bretaudeau L., Buckling A., Chanishvili N., Clark J.R., Corte-Real S., Debarbieux L., Dublanchet A., De Vos D., et al. Quality and Safety Requirements for Sustainable Phage Therapy Products. Pharm. Res. 2015;32:2173–2179. doi: 10.1007/s11095-014-1617-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mulzer J., Trampuz A., Potapov E.V. Treatment of Chronic Left Ventricular Assist Device Infection with Local Application of Bacteriophages. Eur. J. Cardiothorac. Surg. 2020;57:1003–1004. doi: 10.1093/ejcts/ezz295. [DOI] [PubMed] [Google Scholar]
- 52.Zhvania P., Hoyle N.S., Nadareishvili L., Nizharadze D., Kutateladze M. Phage Therapy in a 16-Year-Old Boy with Netherton Syndrome. Front. Med. 2017;4:94. doi: 10.3389/fmed.2017.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rose T., Verbeken G., Vos D.D., Merabishvili M., Vaneechoutte M., Lavigne R., Jennes S., Zizi M., Pirnay J.-P. Experimental Phage Therapy of Burn Wound Infection: Difficult First Steps. Int. J. Burns. Trauma. 2014;4:66–73. [PMC free article] [PubMed] [Google Scholar]
- 54.Ujmajuridze A., Chanishvili N., Goderdzishvili M., Leitner L., Mehnert U., Chkhotua A., Kessler T.M., Sybesma W. Adapted Bacteriophages for Treating Urinary Tract Infections. Front. Microbiol. 2018;9:1832. doi: 10.3389/fmicb.2018.01832. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.