Graphical abstract
Keywords: Hesperidin, Dyslipidemia, Hepatic damage, Cadmium and toxicity
Highlights
-
•
Exposure of rats to CdCl2 led to hepatic dysfunction and dyslipidemia.
-
•
CdCl2 caused abnormal rapid weight reduction in rats.
-
•
Hesperidin abrogated dyslipidemia in rats exposed to CdCl2.
-
•
Hesperidin had hepatoprotective effect in rats against CdCl2 toxicity.
Abstract
The ever-increasing human population with attendant industrialization poses serious global health challenge. Cadmium (Cd) with other heavy metals contribute greatly to environmental pollutions and humans are daily exposed to them, leading to diverse ailments. We explored whether Hesperidin (HSP) could protect against hepatic damage and dyslipidemia in Wistar rats exposed to Cd. Forty wistar rats were randomly assigned into five groups (n = 8). Group 1 received 2 mL/kg body weight of normal saline; Group 2 received 100 mg/kg body weight of HSP while Group 3 received 5 mg/kg body weight of Cadmium Chloride (CdCl2) for 28 days. Group 4 received 100 mg/kg body weight of HSP and after 90 min, CdCl2 (5 mg/kg) body weight was administered for 28 days. Group 5 received 50 mg/kg body weight of HSP and after 90 min, CdCl2 (5 mg/kg) body weight was administered for 28 days. The serum lipid profiles, hepatic dysfunction and oxidative stress markers were determined using standard methods. Cd toxicity in rats prominently elevated serum activities of AST, ALT, ALP and levels of total bilirubin, direct bilirubin, cholesterol, LDL-C and malondialdehyde with decreased levels of HDL-C, triglycerides, superoxide dismutase, catalase, glutathione and body weights. The pre-treatment of HSP before Cd intoxication prevented the dysregulated activities of liver enzymes and levels of lipid profiles, enzymatic and non-enzymatic antioxidants and other biomarkers investigated, thus suggesting anti-hyperlipidemic and hepato-protective potentials. HSP may have great potentials for development of therapeutics that could enhance the management of dyslipidemia and liver disorders associated with heavy metal exposure.
1. Introduction
Cadmium (Cd) is a ubiquitous and non-biodegradable pollutant representing a great concern to human health. The metal is naturally distributed, but industrial development has dramatically increased their concentrations in the environment [1,2]. Increased emissions of the metal in the environment and its non-biodegradability have increased the risk of human exposure. The main routes of Cd exposure are by ingestion and inhalation due to their presence in food, air, dairy products and tobacco leaves [[3], [4], [5], [6]]. Efforts have been made to understand the exact mechanisms of toxicity of Cd and available body of knowledge suggests oxidative stress as one of the critical mechanism of toxicity of this metal, albeit the metal is a Fenton’s metal [[6], [7], [8]]. Other possible mechanisms of toxicity include binding to oxygen, nitrogen, and sulphur ligands, which may affect numerous enzymes and proteins; interaction with bio-elements; inhibition of apoptosis and changes in DNA structure and the inhibition of damaged DNA repair, which may lead to aberrant gene expression [2,9,10]. Additionally, Cd has been shown to exert toxicity in the hippocampus of the brain [11], prostate, pancreas, endocrine system and the heart causing memory defects, cardiovascular dysfunctions, immune and reproductive system damages [[12], [13], [14], [15]]. Regardless of oral, pulmonary or parenteral exposure, the liver and the kidney are by far the primary organs that take up the greatest amounts of Cd during its exposure [[16], [17], [18]].
Hesperidin (HSP) is a bioflavonoid (flavonoids have diphenol structure with the molecular formula C28H34O15) and found mainly in vegetables, herbs, fruits and legumes. Nearly 5000 kinds of flavonoids have been identified to be abundantly present in citrus fruits [19,20]. Hesperidin derives its name from the word “hesperidium”, the kind of fruit produced by citrus trees [21]. Hesperidin is believed to be a secondary metabolite playing a role in plant defense against fungal and bacterial invasions [22]. The sweet oranges (Citrus sinensis) and tangelos are known to contain very high amount of hesperidin and are the major sources of hesperidin extraction albeit recent study have shown that it can be synthesized [19,23]. Hesperidin is therefore a classical dietary polyphenol, which has been shown to exert varieties of pharmacological actions. Some of which include anti-inflammatory [24], anti-cancer, anti-diabetic, antioxidant, free radical scavenging and antiulcer effects [[25], [26], [27], [28]]. It also inhibits selected cytochrome P450 enzymes resulting in drug interactions. Hesperidin is also known to increase the strength of the capillaries (blood vessels) and regulate their permeability [20]. More interestingly, the pharmacokinetics of hesperidin is well known and previous studies have investigated its safety and bioavailability in the human plasma after removal of rutinoside moiety attached to the compound in citrus juice by rhamnosidase activity and gut microbiota composition [29,30]. For instance, Manach et al. [30] reported that after 3 h of consumption of citrus juice, plasma concentrations of Hesperetin-7-glucoside gotten from hesperidin peaks between 5 h–7 h and returns to baseline in 24 h indicating improved bioavailability and safety. However, the use of gold nanoparticles for more effective delivery and bioavailability of Hesperidin have been developed recently [31,32].
Indeed, the efficacy of many varieties of pure phyto-compounds such as hesperidin has been reported with identified wide spectrum of pharmacological properties including anti-inflammatory, anti-allergic, anti-tumour and anti-oxidant characteristics [33]. Recently, the potentials of dietary polyphenols such as hesperidin in protecting organs against oxidative stress-mediated toxicity came into limelight and has been reviewed elsewhere [34]. However, there is still gap in scientific knowledge concerning the effects of hesperidin on dysregulation of lipid profiles and hepatic dysfunctions in rats exposed to heavy metals toxicity. More so, the roles of pure natural bioactive compounds such as hesperidin in ameliorating dyslipidemia and hepatic damages triggered by environmental contaminants have not been investigated adequately. More recently, Elhelaly et al. [35] revealed that hesperidin administration provided protective effects on liver, kidney and the brain against oxidative damages due to exposure of rats to acrylamide, which is a heat-induced toxin formed during thermal processing of many commonly consumed foods. It is an imperative therefore, that hesperidin is currently an emerging new therapeutic agent from natural products, which could be used in modulating physiological dysregulations due to various disease pathologies in the body [36]. However, more studies would be needed to provide sufficient scientific evidence of these potentials; we therefore investigated whether hesperidin could ameliorate possible hepatic damage and dyslipidemia in male wistar rats due to their exposure to Cd toxicity.
2. Materials and methods
2.1. Chemicals and reagents
Cadmium Chloride (CdCl2) and Hesperidin were purchased from Sigma-Aldrich (USA). Serum liver function and lipid profile parameters kits were purchased from Randox Laboratories Ltd (UK). All other reagents and chemicals were of standard analytical grade.
2.2. Experimental animals
Forty male Wistar rats (8 weeks old) of an average weight of 135 ± 5 g were obtained from the animal farm of Anatomy Department, Ebonyi State University, Abakaliki. The rats were kept in stainless steel rat cages in a well-ventilated animal house of Biochemistry Department, Ebonyi State University Abakaliki. They were acclimatized for 7 days under good laboratory conditions (12 h light/dark cycle; room temperature: 25−30 °C). They were allowed access to standard rodent chow (Vital feed®, Grand Cereals Ltd, Jos, Nigeria) and water ad libitum. The procedures for experimental studies were performed in consistent with the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 8023, revised in 1978). The Department of Biochemistry, Ebonyi State University Institutional Ethical Review Committee, approved the study with approval number EBSU/BCH/ET/20/001.
2.2.1. Experimental design
At the end of acclimatization period, the animals were randomly assigned into 5 experimental groups (n = 8). The cadmium chloride was administered orally at the dose of 5 mg/kg body weight, which was 1/5 of the oral median lethal dose values in rats previously obtained by other studies [15,37]. Hesperidin was administered at 100 mg/kg and 50 mg/kg as used in previous studies [28,38,39]. The details of the study design are shown below.
Group 1 (Normal control): Rats received normal saline (2 mL/kg body weight) for 28 days.
Group 2 (Hesperidin control): Rats received hesperidin dissolved in saline (100 mg/kg body weight) orally for 28 days.
Group 3 (CdCl2 control): Rats received CdCl2 dissolved in normal saline (5 mg/kg body weight) orally for 28 days.
Group 4 (Hesperidin + CdCl2): Rats were pre-administered with hesperidin (100 mg/kg BW) 90 min before CdCl2 (5 mg/kg BW) intoxication for 28 days.
Group 5 (Hesperidin + CdCl2): Rats were pre-administered with hesperidin (50 mg/kg BW) 90 min before CdCl2 (5 mg/kg BW) intoxication for 28 days.
After initial body weights, the body weight of the rats were taken at every 7 days intervals. After 28 days of treatment, rats were fasted overnight and were euthanized through rapid exposure to overdose of CO2. The blood was collected through cardiac puncture into plain sample bottles. The blood was centrifuged (3000 g for 10 min) and serum separated for analyses of lipid profiles and liver function parameters.
2.3. Biochemical analyses
2.3.1. Determination of lipid profile
Total cholesterol was done by the method of Allain and Roschlaw [40]. Briefly, 10 μL of distilled water, 10 μL of standard solution and 10 μL of sample (serum) were added to the blank, standard and sample test tubes respectively. The solutions were mixed and incubated at 37 °C for 5 min. The absorbance at 500 nm was determined using a spectrophotometer. Concentration of cholesterol in the sample (mg/dL) = x concentration of standard.
High Density Lipoprotein cholesterol (HDL-C) concentration was determined by centrifugation method as described by Allain and Roschlaw [40]. Briefly, the precipitating reagent was diluted in the ratio of 4:1 with redistilled water (80 mL of reagent was diluted with 20 mL of redistilled water). The solutions were mixed thoroughly, incubated at room temperature for 10 min and then centrifuged for 10 min at 4000 × g. Then, 100 μL of the supernatant was pipetted into the sample test tube. Furthermore, 100 μL of distilled water was added into the reagent blank test tube, and 10 μL of standard solution was added into the standard test tube. Afterwards, the solutions were mixed thoroughly, incubated at 25 °C for 5 min. The absorbance at 500 nm was determined using a spectrophotometer. Calculation: Concentration of HDL cholesterol in the supernatant (mg/dL) = x concentration of standard.
For Triacylglyceride determination, 10 μL of sample serum was added to the sample test tube and 10 μL of standard solution was added to the standard test tube. A third test tube was marked reagent test tube. After that, 1000 μL of reagent (R1) were added to each of the test tubes. The solutions were mixed and incubated for 5 min at 37 °C. The absorbance was read at 500 nm using a spectrophotometer. TG concentration = x concentration of standard.
LDL– cholesterol concentrations of the samples were determined by equation method of Friedewald et al. [41]. Briefly, after the determination of total cholesterol, HDL cholesterol and triglycerides, the values obtained were used to calculate the concentration of LDL as shown:
| LDL Cholesterol (mg/dL) = Total cholesterol – (trigycerides + HDL). |
2.3.2. Determination of liver function markers
2.3.2.1. ALP determination
Alkaline phosphatase activity was determined using commercial kit manual (Randox, UK). A sample of 0.05 mL of was pipetted into a test tube, and then 3.0 mL of reagent (a mixture of diethanolamine buffer, MgCl2 and a substrate, p-nitrophenylphosphate) was added and mixed. The initial absorbance was taken and a timer was started simultaneously. The absorbance was read after 1, 2 and 3 min at the wavelength of 450 nm. Calculation [42]:
| ALP (U/L) = 2790 x change in absorbance. |
2.3.2.2. Bilirubin determination
Bilirubin was determined using the method of Jendrassik and Grof [43], briefly the sample (200 μL), R1 (1000 μL), R2 (50 μL) and R3 (1000 μL) were mixed and left for 10 min at 25 °C. Then R4 (1000 μL) was added, mixed and left for 30 min at 25 °C. Absorbance of the sample was read at 578 nm against the sample blank (ATB). R1- sulphanilic acid; R2- Nitrite; R3-Caffeine; R4- Tartrate. Calculation: Total bilirubin (mg/dL) = 10.8 x ATB (578 nm).
2.3.2.3. AST determination
The method of Reitman and Frankel [44] was used to assay for the activities of alanine aminotransferase and aspartate aminotransferase. Briefly, Aspartate aminotransaminase (AST) activity was determined using commercial kit manual (Randox, UK). Sample of 0.5 mL was mixed with 0.5 mL of Reagent 1 (Buffer: phosphate buffer, l-aspartate and α-oxoglutarate) and incubated for exactly 30 min at room temperature. Then 0.1 mL of reagent (2,4-dinitrophenylhydrazine) was added, mixed and allowed to stand for 20 min at a temperature of 20–25 °C. Furthermore, 5 mL of NaOH was then added, mixed and read after 5 min at a wavelength of 546 nm. The AST activity was obtained from the table in the manual [44].
2.3.2.4. ALT determination
For alanine aminotransferase, 0.1 mL of sample was pipetted into a test tube and 0.5 mL of solution R1 (buffer containing phosphate buffer, l-alanine and α-oxoglutarate) was added, mixed and incubated for 30 min at 37 °C. Then, 0.5 mL of solution R2 (2,4-dinitrophenylhydrazine) was added and mixed and then allowed to stand for 20 min at 20–25 °C. Finally, 5.0 mL of NaOH was added, mixed and after 5 min the absorbance was read at wavelength of 546 nm [44].
2.3.2.5. Albumin determination
For albumin determination, three test tubes were prepared and designed as reagent blank, standard and sample test tubes. Then 0.01 mL of distilled water, standard and serum were added to the reagent blank, standard and sample test tubes respectively and 3.0 mL of BCG reagent was further added to the three test tubes. The tubes were mixed properly and incubated for 5 min at 20−25 °C. The absorbance of the sample and that of the standard were read against the reagent blank at the wavelength 630 nm. Calculations =
2.3.3. Determination of oxidative stress markers
2.3.3.1. MDA determination
One milliliter of the serum sample was introduced into two milliliter of (1:1: 1) TCA-TBA-HCl reagent (thiobarbituric acid 0.37 %, 0.24 N HCl and fifteen percent TCA) trichloro acetic acid-thiobabituricacid –hydrochloric acid reagent and allowed to boil at 100 °C for fifteen minutes and left to cool. A centrifuge was used to remove the flocculent materials at three thousand rotations per minute for ten minutes. The upper layer was decanted and the absorbance taken at five hundred and thirty -two nanometer against the blank with a spectrophotometer. The calculation of MDA carried out by employing molar extinction coefficient for MDA-TBA-complex of 1.56 × 105 M−1CM−1.
| Calculation of MDA: ΔA/min x VT/Ʃ x VS |
Where
ΔA = changes in absorbance
VT = totality of the volume
VS = volume of the sample and Ʃ = molar extinction
2.3.3.2. Superoxide dismutase (SOD) activity determination
The sample volume of 0.2 mL was introduced 2.5 mL of 0.05 phosphate buffer, pH 7.8 and 0.3 mL of newly prepared adrenaline solution to the reaction mixture followed by quick mixing by inversion in the cuvette. The increase in absorbance was taken every 30 s for 3 min at 480 nm against blank. Blank contained 0.3 mL of adrenaline and 2.5 mL buffer.
2.3.3.3. Catalase (CAT) determination
The sample volume of 4.0 mL of hydrogen peroxide (H2O2) solution was added to 5.0 mL of phosphate buffer and 1.0 mL of the sample was mixed at room temperature. Afterwards, 1.0 mL portion of the reaction mixture was withheld and added to 2.0 mL dichromate/acetic acid reagent at one minute interval and the steady absorbance reading taken at 570 nm.
2.3.3.4. Reduced glutathione determination (GSH) determination
The sample volume of 1.0 mL was added 4.0 % sulfo-salicyclic acid and the mixture centrifuged at 3000 rpm for 15 min at 2°C. The samples were introduced to 4.5 mL of Ellman reagent and absorbance was measured at 412 nm. The blank were prepared by addition of 0.5 mL of 4 % sulfo-salicyclic acid to 4.5 mL of Ellman reagent while absorbance was measured at 412 nm.
2.4. Statistical analysis
Results were expressed as Means ± standard deviation (SD). Graph Pad Prism 5.0 (CA, USA) was used in the data analysis. Data were analyzed using a one-way analysis of variance (ANOVA), followed by Tukey’s multiple comparison post hoc test for comparison. Values were generally considered statistically significant at p < 0.05.
3. Results
3.1. Effect of HSP on serum lipid profile in male Wistar rats exposed to Cd
Fig. 1 (A–D) shows the effect of HSP on serum lipid profile in rats exposed to Cd. It was observed that Cd significantly increased the serum levels of LDL-C, cholesterol and decreased the levels of HDL-C and triglycerides. Interestingly, pre-administration of HSP significantly normalized these distorted levels of LDL-C, HDL-C, cholesterol and triglycerides compared to Cd-treated group. HSP effect was also comparable to normal rats in all the markers investigated.
Fig. 1.
(AD)—: Effect of HSP on (A) Serum LDL-C, (B) HDL-C, (C) Cholesterol, (D) Triglyceride levels in male wistar rats exposed to Cd respectively. Data are shown as mean ± SD (n = 8). Mean of groups with different alphabet are significantly different while those with the same alphabet are not significantly different. Beginning from the normal group, the alphabet were assigned according to the significant level of each group in that order.
3.2. Effect of Hesperidin on serum liver function markers in male Wistar rats exposed to cadmium chloride toxicity
Fig. 2 (A–F) shows the effect of HSP on liver function markers in male wistar rats exposed to Cd. Our study revealed that Cd exposure for 28 days markedly increased the serum levels of AST, ALT, ALP, total bilirubin, direct bilirubin respectively and significantly decreased the serum level of albumin. Conversely, pre-administration of HSP considerably reversed the levels of these liver function markers in a dose-dependent manner.
Fig. 2.
(A–F): Effect of HSP on (A) Serum Total Bilirubin, (B) Direct Bilirubin, (C) ALT, (D) AST, (E) ALP, (F) Albumin in male wistar rats exposed to Cd respectively. Data are shown as mean ± SD (n = 8). Mean of groups with different alphabet are significantly different while those with the same alphabet are not significantly different. Beginning from the normal group, the alphabet were assigned according to the significant level of each group in that order.
3.3. Effect of Hesperidin on antioxidant enzymes and markers of oxidative stress in male Wistar rats exposed to cadmium chloride toxicity
Fig. 3 (A–D) shows the effect of HSP on antioxidant enzymes and other non-enzymatic markers of oxidative stress in male wistar rats exposed to cadmium chloride toxicity. Our result revealed that exposure of rats to cadmium for 28 days led to significant decrease in the activities of superoxide dismutase (SOD) and catalase (CAT) while there was a decrease in the level of glutathione (GSH). Cadmium also caused a significant elevation of malondialdehyde (MDA) in the exposed animals. Interestingly, HSP pre-administration normalized the deleterious effects of this exposure and further had comparable effect with the normal group in most of the markers (GSH, MDA and CAT) investigated.
Fig. 3.
(A–D): Effect of HSP on (A) MDA, (B) GSH, (C) CAT, (D) SOD in Rats Exposed to Cd respectively. Data are shown as mean ± SD (n = 8). Mean of groups with different alphabet are significantly different while those with the same alphabet are not significantly different. Beginning from the normal group, the alphabet were assigned according to the significant level of each group in that order.
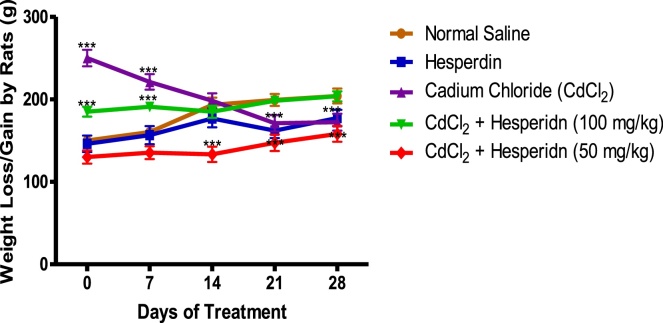
3.4. Effect of Hesperidin on body weights of rats exposed to Cd toxicity
Fig. 4 revealed that exposure of the rats to Cd caused a significant weight loss in the rats, which further decreased abruptly and stabilized on the 21st day of exposure. Interestingly, pre-administration of hesperidin (both 50 and 100 mg/kg) respectively, significantly modulated these irregularities in weight gain and loss due to Cd exposure.
Fig. 4.
Effect of HSP on body weights of Cd-induced pancreatitis in Rats. Data are shown as mean ± S.D (n = 8).
4. Discussion
Heavy metals toxicity and their consequent health challenges are currently on the increasing trend globally. We conducted an organ-function based investigation on the toxicity effects of exposure of rats to cadmium chloride and further evaluated whether pre-administration of hesperidin could ameliorate such deleterious effects. Firstly, we established that exposure of rats to CdCl2 could lead to hepatic dysfunctions using well-known liver function biochemical markers vis-à-vis Aspartate aminotransferase (AST), Alanine aminotransferase (ALT), Alkaline Phosphatase (ALP), Total bilirubin, Albumin and Direct bilirubin. Cadmium has recently been shown to induce hepatic injury leading to liver inflammation, hepatocyte death and significant alteration in biochemical markers of liver function [2,[45], [46], [47]]. We further observed that exposure of rats to Cd in this present study caused significant elevation in the serum levels of ALT, AST, ALP, total bilirubin and direct bilirubin, while albumin was significantly reduced. This significant distortion in the serum levels of these biomarkers perhaps suggests possible hepatocellular damage due to Cd toxicity in the hepatocytes. Aspartate aminotransferase (AST) and Alanine Aminotransferase (ALT) are enzymes found mainly in the liver, but also found in red blood cells, heart cells, muscle tissue and other organs, such as the pancreas and kidneys [48]. Albeit, these enzymes are constitutively present in the plasma, however, when body tissues or organs such as the liver and heart are damaged, additional AST, ALT and ALP are released into the plasma, leading to abnormal elevation in levels of these enzymes in the plasma. Increased serum levels of these enzymes in laboratory investigations, hence suggest the existence of medical problems associated to the liver [49,50].
More so, albumin is a well-known blood protein transporter and has recently been projected as a key biomarker in clinical diagnosis of early liver impairment. Its decrease in the serum has been implicated in the prognosis of chronic liver diseases such as cirrhosis of the liver, hepatitis and non-fatty acid liver disease (NAFLD) [51]. Bilirubin is an important molecule with multiple biological functions. Total bilirubin is the sum of the un-conjugated and conjugated fractions and its serum elevation has been implicated in various liver diseases in addition to pancreatic and biliary malignancy, hemolytic disorders, several inherited enzyme deficiencies, and conditions causing hepatic obstructions [52,53]. Therefore serum AST, ALT and ALP activities in addition to direct bilirubin and albumin levels are valuable aid in routine clinical diagnosis of liver diseases [49,50,54,55]. Hence, our results further validated the growing scientific evidence that Cd accumulation in the liver after exposure causes hepatic dysfunction. More interestingly, our results further revealed that pre-administration of hesperidin significantly reversed the levels and activities of these biomarkers in a concentration dependent pattern respectively. Hesperidin may have protective and healing effects on the structural integrity of the liver perhaps through opposing the oxidative damage effects of Cd on the lipid components of the hepatocyte membrane through the actions of ROS. Hesperidin has already been shown by others to have strong antioxidant potentials against oxidative damage in various tissues [25,56,57] and one of the well-known oxidative mechanism of Cd is by increase in membrane lipid peroxidation leading to elevation in MDA level [2]. We therefore deduce that the hepato-protective effect of hesperidin demonstrated in this present study against Cd toxicity may be through its scavenging action against various free radicals generated in vivo due to Cd detoxification process in the liver. Perhaps, hesperidin through its antioxidant potential already established by others [56,57], quenched free radicals in the kupffer cells and further improve the efficiency of the detoxification system of the liver against Cd toxicity.
Furthermore, our result revealed that exposure of the rats to Cd caused significant distortion in the lipid profile of the rats leading to dyslipidemia. Cadmium exposure caused a significant increase in the serum levels of LDL-C and Cholesterol while reducing HDL-C and TG. This is consistent with the reports of others in both animal models and humans [[58], [59], [60]] and perhaps suggests that Cd exposure may have high risk of triggering various cardiovascular diseases due to dyslipidemia. Dyslipidemia is one of the well-known cause of endothelial dysfunction leading to cardiovascular diseases, diabetes and other metabolic disorders [[61], [62], [63]]. Hence, elevated serum levels of LDL-C and Cholesterol with decrease in HDL-C and TG observed in our study due to exposure to Cd add to existing scientific knowledge that Cd toxicity may be systemic and perhaps elicits multi-organ damage when it accumulates in the body. This is also seen in the rapid weight loss triggered in the rats exposed to Cd. More interestingly, our result revealed that pre-administration of hesperidin significantly protected the rats from dyslipidemia due to Cd exposure and normalized the abnormal weight fluctuations in the rats. More so, hesperidin significantly reduced elevated LDL-C and Cholesterol while increasing HDL-C and TG in a slight dose dependent pattern. This was possible perhaps through the deployment of antioxidant potential of hesperidin.
Our study further revealed significant distortions in the activities of antioxidant enzymes investigated in the study in addition to other non-enzymatic markers of oxidative stress. Cadmium exposure markedly decreased the activities of superoxide dismutase and catalase. This is consistent with the most recent finding of others [[64], [65], [66], [67]]. Cadmium being a Fenton metal [7,8], may have bound to these enzymes, interacted with the covalent bonds of their amino acids residues and thus abruptly inhibited their activities. More so, cadmium exposure decreased the level of glutathione (GSH) in the animals. Perhaps, cadmium accumulation in the liver led to its binding with the sulfhydryls group of GSH and limited the expansive reductive actions of GSH [68]. This further weakened the antioxidant defense system of the body leading to overproduction of reactive oxygen species (ROS) [69], which subsequently enhanced lipid peroxidation evidenced with significant elevation of MDA observed in this present study. MDA is a product of lipid peroxidation due to elevation of ROS in the physiological system and particularly in tissues [2,69]. Cd is also known to increase MDA in the physiological system [2,67]. Interestingly, hesperidin in this present study ameliorated all the oxidative dysregulations of both the enzymatic and non-enzymatic antioxidants caused by Cd exposure. Hesperidin perhaps by enhancing the activities and/or synthesis of this endogenous antioxidant enzymes and non-enzymes defense system, further countered the oxidative effects of Cd toxicity in the physiological system of the animals. In general, dyslipidemia is multifactorial, however oxidative stress caused by increase in ROS generation has also been implicated in its etiology [70] and hesperidin may have scavenged these ROS generated in vivo due to Cd toxicity. It follows that the beneficial effects of hesperidin against dyslipidemia observed in this present study may further account for its recent proposed use in the treatment of cardiovascular, diabetes and other metabolic diseases [36]. Further mechanistic and computational studies however, would be needed to provide sufficient scientific evidence and this would form our next line of research plan in this area of study. Notice that administration of hesperidin alone significantly elevated serum levels of TG; this perhaps suggests that hesperidin should only be recommended for therapeutic purposes and might be discouraged for leisure intake at high concentration to avoid increase of cardiovascular risk; hesperidin may not be helpful except in disease and pathological conditions. Our data however is not enough to validate this hypothesis at this present time.
5. Conclusion
We have further validated the fact that cadmium exposure exacerbates hepatotoxicity in addition to eliciting dyslipidemia and dysregulation of antioxidant defense system in the physiological system of rat through induction of oxidative stress. Interestingly, we demonstrated for the first time that pre-administration of hesperidin was able to provide strong protective effect against these toxicity effects of cadmium. Hesperidin may hold a new horizon of hope in pharmacological development of therapies against toxicities due to exposure to heavy metals.
Research funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
P.M. Aja: Conceptualization, Investigation, Project administration, Supervision, Writing - original draft. E.U. Ekpono: Methodology, Formal analysis, Project administration. J.N. Awoke: Data curation, Software, Validation, Writing - review & editing. A.C. Famurewa: Conceptualization, Methodology, Validation, Project administration. F.I. Izekwe: Investigation, Project administration, Resources, Methodology. E.J. Okoro: Methodology, Resources, Investigation. C.F. Okorie: Methodology, Resources, Investigation. C.L. Orji: Methodology, Resources, Investigation. F. Nwite: Project administration, Investigation. B.A. Ale: Project administration, Investigation. A.F. Aku: Methodology, Resources, Investigation. I.O. Igwenyi: Project administration, Supervision, Validation, Visualization. B.U. Nwali: Validation, Supervision, Resources. O.U. Orji: Validation, Supervision, Resources. O.G. Ani: Project administration, Investigation. C.R. Ozoemena: Methodology, Resources, Investigation. G.C. Anizoba: Methodology, Resources, Investigation.
Declaration of Competing Interest
The authors declare no conflict of interest.
References
- 1.Naka K.S., Mendes L.C.S., de Queiroz T.K.L., Costa B.N.S., de Jesus I.M., Câmara M.C., Lima M.O. A comparative study of cadmium levels in blood from exposed populations in an industrial area of the Amazon, Brazil. Sci. Total Environ. 2020;698:134309. doi: 10.1016/j.scitotenv.2019.134309. [DOI] [PubMed] [Google Scholar]
- 2.Andjelkovic M., Djordjevic A.B., Antonijevic E., Antonijevic B., Stanic M., Kotur-Stevuljevic J., VSpasojevic-Kalimanovska V., Jovanovic M., Boricic N., Wallace D., Bulat Z. Toxic effect of acute cadmium and lead exposure in rat blood, liver, and kidney. Int. J. Environ. Res. Public Health. 2019;16(274):1–22. doi: 10.3390/ijerph16020274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ma C., Iwai-Shimada M., Tatsuta N., Nakai K., Isobe T., Takagi M., Nishihama Y., Nakayama S.F. Health risk assessment and source apportionment of mercury, lead, cadmium, selenium, and manganese in Japanese women: an adjunct study to the Japan environment and children’s study. Int. J. Environ. Res. Public Health. 2020;17:2231. doi: 10.3390/ijerph17072231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tchounwou P.B., Yedjou C.G., Patlolla A.K., Sutton D.J. Heavy metal toxicity and the environment. Experientia Suppl. 2014;101:133–164. doi: 10.1007/978-3-7643-8340-4_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zafarzadeh A., Bonyadi Z., Feyzi K. Health risk assessment related to cadmium in dairy products in Gorgan, Iran. Int. J. Environ. Anal. Chem. 2020 doi: 10.1080/03067319.2020.1779244. [DOI] [Google Scholar]
- 6.Hossein-Khannazer N., Azizi G., Eslami S., Mohammed H.A., Fayyaz F., Hosseinzadeh R., Usman A.B., Kamali A.N., Mohammadi H., Jadidi-Niaragh F., Dehghanifard E., Noorisepehr M. The effects of cadmium exposure in the induction of inflammation. Immunopharmacol. Immunotoxicol. 2020;42(1):1–8. doi: 10.1080/08923973.2019.1697284. [DOI] [PubMed] [Google Scholar]
- 7.Matović V., Buha A., Dukić-Ć osić D., Bulat Z. Insight into the oxidative stress induced by lead and/or cadmium in blood, liver and kidneys. Food Chem. Toxicol. 2015;78:130–140. doi: 10.1016/j.fct.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 8.Almenara C.C.P., Broseghini-Filho G.B., Vescovi M.V.A., Angeli J.K., Faria T.O., Stefanon I., Vassallo D.V., Padilha A.S. Chronic cadmium treatment promotes oxidative stress and endothelial damage in isolated rat aorta. PLoS One. 2013;8:1–8. doi: 10.1371/journal.pone.0068418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Júnior J.E.G.P., Moraes P.Z., Rodriguez M.D., Simões M.R., Cibin F., Pinton S., Junior F.B., Peçanha F.M., Vassallo D.V., Miguel M., Wiggers G.A. Cadmium exposure activates NADPH oxidase, renin–angiotensin system and cyclooxygenase 2 pathways in arteries, inducing hypertension and vascular damage. Toxicol. Lett. 2020;333:80–89. doi: 10.1016/j.toxlet.2020.07.027. [DOI] [PubMed] [Google Scholar]
- 10.Mouro V.G.S., Siman V.A., da Silva J. Cadmium-induced testicular toxicity in mice: subacute and subchronic route-dependent effects. Biol. Trace Elem. Res. 2020;193:466–482. doi: 10.1007/s12011-019-01731-5. [DOI] [PubMed] [Google Scholar]
- 11.El-Kott A.F., Abd-Lateif A.M., Khalifa H.S. Kaempferol protects against cadmium chloride-induced hippocampal damage and memory deficits by activation of silent information regulator 1 and inhibition of poly (ADP-Ribose) polymerase-1. Sci. Total Environ. 2020;728 doi: 10.1016/j.scitotenv.2020.138832. [DOI] [PubMed] [Google Scholar]
- 12.Kolluru V., Tyagi A., Chandrasekaran B., Damodaran C. Profiling of differentially expressed genes in cadmium-induced prostate carcinogenesis. Toxicol. Appl. Pharmacol. 2019;375:57–63. doi: 10.1016/j.taap.2019.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buha A., Matovic V., Antonijevic B., Bulat Z., Curcic M., Renieri E.A., Tsatsakis A.M., Schweitzer A., Wallace D. Overview of cadmium thyroid disrupting effects and mechanisms. Int. J. Mol. Sci. 2018;19:1501. doi: 10.3390/ijms19051501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akerstrom M., Barregard L., Lundh T., Sallsten G. The relationship between cadmium in kidney and cadmium in urine and blood in an environmentally exposed population. Toxicol. Appl. Pharmacol. 2013;268(3):286–293. doi: 10.1016/j.taap.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 15.Bashir N., Manoharan V., Miltonprabu S. Grape seed proanthocyanidins protects against cadmium induced oxidative pancreatitis in rats by attenuating oxidative stress, inflammation and apoptosis via Nrf-2/HO-1 signaling. J. Nutr. Biochem. 2016;32:128–141. doi: 10.1016/j.jnutbio.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 16.Abdeen A., Abou-Zaid O.A., Abdel-Maksoud H.A. Cadmium overload modulates piroxicam-regulated oxidative damage and apoptotic pathways. Environ. Sci. Pollut. Res. 2019;26:25167–25177. doi: 10.1007/s11356-019-05783-x. [DOI] [PubMed] [Google Scholar]
- 17.El-Sokkary G.H., Nafady A.A., Shabash E.H. Melatonin administration ameliorates cadmium-induced oxidative stress and morphological changes in the liver of rat. Ecotoxicol. Environ. Saf. 2010;73:456–463. doi: 10.1016/j.ecoenv.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 18.Abdeen A., Ghonim A., El-Shawarby R. Protective effect of cinnamon against cadmium-induced hepatorenal oxidative damage in rats. Int. J. Toxicol. 2017;5:17. doi: 10.14419/ijpt.v5i1.7119. [DOI] [Google Scholar]
- 19.Valls R.M., Pedret A., Calderón-Pérez L. Effects of hesperidin in orange juice on blood and pulse pressures in mildly hypertensive individuals: a randomized controlled trial (Citrus study) Eur. J. Nutr. 2020;2020 doi: 10.1007/s00394-020-02279-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jagetia G.C., Lalrinpuii T. Hesperidin, a Citrus bioflavonoid attnuates iron-induced biochemical oxidative stress in mouse liver. Biomed. J. Sci. Technol. Res. 2018;8(1):1–12. [Google Scholar]
- 21.Li C., Schluesener H. Health-promoting effects of the citrus flavanone hesperidin. Crit. Rev. Food Sci. Nutr. 2017;57:613–631. doi: 10.1080/10408398.2014.906382. [DOI] [PubMed] [Google Scholar]
- 22.Del-Rio J.A., Gomez P., Baidez A.G., Arcas M.C., Botia J.M. Changes in the levels of polymethoxyflavones and flavanones as part of the defense mechanism of Citrus sinensis (cv. Valencia Late) fruits against Phytophthoracitrophthora. J. Agric. Food Chem. 2004;52(7):1913–1917. doi: 10.1021/jf035038k. [DOI] [PubMed] [Google Scholar]
- 23.Binkowska I. Hesperidin: synthesis and characterization of bioflavonoid complex. SN Appl. Sci. 2020;2:445. doi: 10.1007/s42452-020-2256-8. [DOI] [Google Scholar]
- 24.Al-Rikabi R., Al-Shmgani H., Dewir Y.H., El-Hendawy S. In vivo and in vitro evaluation of the protective effects of hesperidin in lipopolysaccharide-induced inflammation and cytotoxicity of cell. Molecules. 2020;25:478. doi: 10.3390/molecules25030478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Welbat J.U., Naewla S., Pannangrong W., Sirichoat A., Aranarochana A., Wigmore P. Neuroprotective effects of hesperidin against methotrexate-induced changes in neurogenesis and oxidative stress in the adult rat. Biochem. Pharmacol. 2020;178 doi: 10.1016/j.bcp.2020.114083. [DOI] [PubMed] [Google Scholar]
- 26.Ahmadi A., Shadboorestan A. Oxidative stress and cancer; the role of hesperidin, a citrus natural bioflavonoid, as a cancer chemoprotective agent. Nutr. Cancer. 2016;68(1):29–39. doi: 10.1080/01635581.2015.1078822. [DOI] [PubMed] [Google Scholar]
- 27.Xiong H., Wang J., Ran Q., Lou G., Peng C., Gan Q., Hu J., Sun J., Yao R., Huang Q. Hesperidin: a therapeutic agent for obesity. Drug Des. Devel. Ther. 2019;13:3855–3866. doi: 10.2147/DDDT.S227499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hanchang W., Khamchan A., Wongmanee N., Seedadee C. Hesperidin ameliorates pancreatic β-cell dysfunction and apoptosis in streptozotocin-induced diabetic rat model. Life Sci. 2019;235(116858) doi: 10.1016/j.lfs.2019.116858. [DOI] [PubMed] [Google Scholar]
- 29.Nielsen I.L.F., Chee W.S.S., Poulsen L., Offord-Cavin E., Rasmussen S.E., Frederiksen H., Enslen M., Barron D., Horcajada M., Williamson G. Bioavailability is improved by enzymatic modification of the citrus flavonoid hesperidin in humans: a randomized, double-blind, crossover trial. J. Nutr. 2006;136(2):404–408. doi: 10.1093/jn/136.2.404. [DOI] [PubMed] [Google Scholar]
- 30.Manach C., Morand C., Gil-Izquierdo A. Bioavailability in humans of the flavanones hesperidin and narirutin after the ingestion of two doses of orange juice. Eur. J. Clin. Nutr. 2003;57:235–242. doi: 10.1038/sj.ejcn.1601547. [DOI] [PubMed] [Google Scholar]
- 31.Sulaiman G.M., Waheeb H.M., Jabir M.S. Hesperidin loaded on gold nanoparticles as a drug delivery system for a successful biocompatible, anti-cancer, anti-inflammatory and phagocytosis inducer model. Sci. Rep. 2020;10:9362. doi: 10.1038/s41598-020-66419-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saad S., Ahmad I., Kawish S.M., Khan U.A., Ahmad F.J., Ali A., Gaurav K. Improved cardioprotective effects of hesperidin solid lipid nanoparticles prepared by supercritical antisolvent technology. Colloids Surf. B Biointerfaces. 2020;187 doi: 10.1016/j.colsurfb.2019.110628. [DOI] [PubMed] [Google Scholar]
- 33.Kumara K., Khare A., Dange S. The applicability of oxidative stress Biomarkers in assembling chromium induced toxicity in the fish Labeorohita. Biomed Res. Int. 2014;2014:1–14. doi: 10.1155/2014/782493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hajialyani M., Farzaei M.H., Echeverria J., Nabavi S.M., Uriarte E., Sanchez E.S. Hesperidin as a neuroprotective agent: a review of amino acid and clinical evidence. Molecules. 2019;24(3):648. doi: 10.3390/molecules24030648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Elhelaly A.E., AlBasher G., Alfarraj S. Protective effects of hesperidin and diosmin against acrylamide-induced liver, kidney, and brain oxidative damage in rats. Environ. Sci. Pollut. Res. Int. 2019;26(34):35151–35162. doi: 10.1007/s11356-019-06660-3. [DOI] [PubMed] [Google Scholar]
- 36.Mas-Capdevila A., Teichenne J., Domenech-Coca C., Caimari A., Del Bas J.M., Escoté X., Crescenti A. Effect of hesperidin on cardiovascular disease risk factors: the role of intestinal microbiota on hesperidin bioavailability. Nutrients. 2020;12:1488. doi: 10.3390/nu12051488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Renugadevi J., MiltonPrabu S. Quercetin protects against oxidative stress- related renal dysfunction by cadmium in rats. Exp. Toxicol. Pathol. 2010;62:471–481. doi: 10.1016/j.etp.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 38.Iskender H., Dokumacioglu E., Sen T.M., Ince I., Kanbay Y., Saral S. The effect of hesperidin and quercetin on oxidative stress, NF-κB and SIRT1 levels in a STZ-induced experimental diabetes model. Biomed. Pharmacother. 2017;90:500–508. doi: 10.1016/j.biopha.2017.03.102. [DOI] [PubMed] [Google Scholar]
- 39.Kakadiya J., Mulani H., Shah N. Protective effect of hesperidin on cardiovascular complication in experimentally induced myocardial infarction in diabetes in rats. J. Basic Clin. Pharm. 2010;1:85–91. https://www.ncbi.nlm.nih.gov/pubmed/24825971 [PMC free article] [PubMed] [Google Scholar]
- 40.Allain O.P., Roschlaw A.F. 3rd edition. Oxford University Press; New York: 1979. Biochemical Analysis; pp. 80–89. [Google Scholar]
- 41.Friedewald W.T., Levy R.I., Fredrickson D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 42.Wright P.J., Leathwood A.A., Plummer D.T. Enzymes in rat urine: alkaline phosphatase. Enzymologia. 1972;42(4):317–327. [PubMed] [Google Scholar]
- 43.Jendrassik L., Grof P. Colorimetric method of determination of bilirubin. Search Results. BiochemischeZeitschrift. 1938;297(2):81–82. [Google Scholar]
- 44.Reitman S., Frankel R. Colorimetric methods for the determination of serum transaminases. Am. J. Clin. Pathol. 1957;28:56–61. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 45.Diab K.A., Ibrahim N.E., Fahmy M.A., Hassan E.M., Omara E.A. Inhibitory activity of flaxseed oil against CdCl2 induced liver and kidney damage: histopathology, genotoxicity, and gene expression study. Toxicol. Rep. 2020;7:1127–1137. doi: 10.1016/j.toxrep.2020.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cao Z., Fang Y., Lu Y. Melatonin alleviates cadmium‐induced liver injury by inhibiting the TXNIP‐NLRP3 inflammasome. J. Pineal Res. 2017;62:e12389. doi: 10.1111/jpi.12389. [DOI] [PubMed] [Google Scholar]
- 47.Sanjeev S., Bidanchi R.M., Murthy M.K. Influence of ferulic acid consumption in ameliorating the cadmium-induced liver and renal oxidative damage in rats. Environ. Sci. Pollut. Res. 2019;26:20631–20653. doi: 10.1007/s11356-019-05420-7. [DOI] [PubMed] [Google Scholar]
- 48.Lee K.A., Lee S.H., Lee Y.J., Baeg S.M., Shim J.H. Hesperidin induces apoptosis by inhibiting Sp1 and its regulatory protein in MSTO-211H cells. Biomol. Ther. 2012;20:273–279. doi: 10.4062/biomolther.2012.20.3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Koh C., Sakiani S., Surana P., Zhao X., Eccleston J., Kleiner D.E., Herion D., Liang T.J., Hoofnagle J.H., Chernick M., Heller T. Adult‐onset cystic fibrosis liver disease: diagnosis and characterization of an underappreciated entity. Hepatology. 2017;66:591–601. doi: 10.1002/hep.29217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cleveland E., Bandy A., VanWagner L.B. Diagnostic challenges of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Clin. Liver Dis. 2018;11(4):98–104. doi: 10.1002/cld.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sun L., Yin H., Liu M., Xu G., Zhou X., Ge P., Huayu Y., Yilei M. Impaired albumin function: a novel potential indicator for liver function damage? Ann. Med. 2019;51(7–8):333–344. doi: 10.1080/07853890.2019.1693056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chowdhury N.R., Li Y., Chowdhury J.R. In: Disorders of Bilirubin Metabolism In The Liver. Arias I.M., Alter H.J., Boyer J.L., Cohen D.E., Shafritz D.A., Thorgeirsson S.S., Wolkoff A.W., editors. 2020. [DOI] [Google Scholar]
- 53.Khan A., Lapsia S., Aslam M., Kaushik V., Reddy Y., Subar D. Serum bilirubin levels can predict pancreatic and biliary malignancies in patients with obstructive jaundice and non-conclusive cytology. Glob. j. Surg. 2018;6(1):11–15. [Google Scholar]
- 54.Mishima M., Koda M., Tsui W.M.S. Granulomatous liver diseases. In: Hashimoto E., Kwo P., Suriawinata A., Tsui W., Iwai M., editors. Diagnosis of Liver Disease. Springer; Singapore: 2019. [DOI] [Google Scholar]
- 55.Chen R.-C., Cai Y.-J., Wu J.-M., Wang X.-D., Song M., Wang Y.-Q., Zheng M.-H., Chen Y.-P., Lin Z., Shi K.Q. Usefulness of albumin‐bilirubin grade for evaluation of long‐term prognosis for hepatitis B‐related cirrhosis. J. Viral Hepat. 2017;24:238–245. doi: 10.1111/jvh.12638. [DOI] [PubMed] [Google Scholar]
- 56.Akdemir F.N.E., Gülçin I., Karagöz B., Recep S., Saleh H.A. A comparative study on the antioxidant effects of hesperidin and ellagic acid against skeletal muscle ischemia/reperfusion injury. J. Enzyme Inhib. Med. Chem. 2016;31(suppl. 4):114–118. doi: 10.1080/14756366.2016.1220378. [DOI] [PubMed] [Google Scholar]
- 57.Turk E., Kandemir F.M., Yildirim S. Protective effect of hesperidin on sodium arsenite-induced nephrotoxicity and hepatotoxicity in rats. Biol. Trace Elem. Res. 2019;189:95–108. doi: 10.1007/s12011-018-1443-6. [DOI] [PubMed] [Google Scholar]
- 58.Samarghandian S., Borji A., Farkhondeh T., Asadi-Samani M. Effect of cadmium on glucose, lipid profile and oxidative stress in streptozotocin-induced diabetic and non-diabetic rats. Toxicol. Int. 2017;24(1):9–16. [Google Scholar]
- 59.Zhou Z., Lu Y., Pi H., Gao P., Li M., Zhang L., Pei L., Mei X., Liu L., Zhao Q., Qin Q., Chen Y., Jiang Y. Cadmium exposure is associated with the prevalence of dyslipidemia. Cell. Physiol. Biochem. 2016;40:633–643. doi: 10.1159/000452576. [DOI] [PubMed] [Google Scholar]
- 60.Oyinloye B.E., Ajiboye B.O., Ojo O.A., Nwozo S.O., Kappo A.P. Cardioprotective and antioxidant influence of aqueous extracts from Sesamum indicum seeds on oxidative stress induced by cadmium in wistar rats. Pharmacogn. Mag. 2016;12(Suppl. 2):S170–S174. doi: 10.4103/0973-1296.182155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Okeahialam B.N. Dyslipidemia and cardiovascular diseases: science or fairy tale. Nig. J. Cardiol. 2020;16:1–4. http://www.nigjcardiol.org/text.asp?2019/16/1/1/269644 [Google Scholar]
- 62.Pires A., Sena C., Seiça R. Dyslipidemia and cardiovascular changes in children. Curr. Opin. Cardiol. 2016;31(1):95–100. doi: 10.1097/HCO.0000000000000249. [DOI] [PubMed] [Google Scholar]
- 63.Erem C., Hacihasanoglu A., Deger O. Prevalence of dyslipidemia and associated risk factors among Turkish adults: trabzon lipid study. Endocrine. 2008;34:36–51. doi: 10.1007/s12020-008-9100-z. [DOI] [PubMed] [Google Scholar]
- 64.Wang X., Wang T., Pan T. Senna alexandrina extract supplementation reverses hepatic oxidative, inflammatory, and apoptotic effects of cadmium chloride administration in rats. Environ. Sci. Pollut. Res. 2020;27:5981–5992. doi: 10.1007/s11356-019-07117-3. [DOI] [PubMed] [Google Scholar]
- 65.Wang J., Zhu H., Wang K., Yang Z., Liu Z. Protective effect of quercetin on rat testes against cadmium toxicity by alleviating oxidative stress and autophagy. Environ. Sci. Pollut. Res. - Int. 2020;27:25278–25286. doi: 10.1007/s11356-020-08947-2. [DOI] [PubMed] [Google Scholar]
- 66.Goodarzi Z., Karami E., Yousefi S., Dehdashti A., Bandegi A.R., Ghanbari A. Hepatoprotective effect of atorvastatin on Cadmium chloride induced hepatotoxicity in rats. Life Sci. 2020;254 doi: 10.1016/j.lfs.2020.117770. [DOI] [PubMed] [Google Scholar]
- 67.Seif M.M., Madboli A.N., Marrez D.A., Aboulthana W. Hepato-renal protective effects of egyptian purslane extract against experimental cadmium toxicity in rats with special emphasis on the functional and histopathological changes. Toxicol. Rep. 2019;6:625–631. doi: 10.1016/j.toxrep.2019.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mezynska M., Brzoska M.M. Review of polyphenol-rich products as potential protective and therapeutic factors against cadmium hepatotoxicity. J. Appl. Toxicol. 2019;39:117–145. doi: 10.1002/jat.3709. [DOI] [PubMed] [Google Scholar]
- 69.Al Omairi N.E., Al-Brakati A.Y., Kassab R.B., Lokman M.S., Elmahallawy E.K., Amin H.K., Abdel Moneim A.E. Soursop fruit extract mitigates scopolamine-induced amnesia and oxidative stress via activating cholinergic and Nrf2/HO-1 pathways. Metab. Brain Dis. 2019 doi: 10.1007/s11011-019-00407-2. [DOI] [PubMed] [Google Scholar]
- 70.Xu M., Ge C., Qin Y., Gu T., Lou D., Li Q., Hu L., Feng J., Huang P., Tan J. Prolonged PM2.5 exposure elevates risk of oxidative stress-driven nonalcoholic fatty liver disease by triggering increase of dyslipidemia. Free Radic. Biol. Med. 2019;130:542–556. doi: 10.1016/j.freeradbiomed.2018.11.016. [DOI] [PubMed] [Google Scholar]