Abstract
Obesity is an increasingly costly and widespread epidemic, effecting 1 in 10 adults worldwide. It has been causally linked with both the metabolic syndrome and insulin resistance, both of which are associated with increased chronic inflammation. The exact mechanisms through which inflammation may contribute to both MetS and IR are numerous and their details are still largely unknown. Recently, micro-RNAs (miRNAs) have emerged as potential interventional targets due to their potential preventive roles in the pathogenesis of several diseases, including MetS and obesity. The purpose of this review paper is to discuss some of the known roles of miRNAs as mediators of inflammation-associated obesity and IR and how omega-3 polyunsaturated fatty acids may be used as a nutritional intervention for these disorders.
INTRODUCTION
Current estimates claim that over 78 million US adults are obese in the USA,1 and obesity is now recognized as a disease of epidemic proportions.2 It is a very expensive disease as the total medical costs for obese patients is estimated to be over US$147 billion annually in the USA alone.3 Obesity increases the risk of other comorbidities such as hypertension, type II diabetes (T2D), coronary heart disease, stroke, and some cancers.4 It is causally linked with metabolic syndrome (MetS), characterized by at least three of the following: hypertension, hyperglycemia, abdominal obesity, elevated plasma triglyceride, and reduced plasma high-density lipoproteins (HDL).5 Inflammation and disruption of adipose tissue function are underlying causes of MetS and obesity.6,7
Adipose tissue secretes adipokines, bioactive peptides and lipids that modulate cardiovascular function, insulin sensitivity, inflammation, and adipose tissue function.8,9 In lean individuals, adipose tissue secretes high levels of anti-inflammatory mediators such as interleukin-10 (IL-10). On the contrary, in individuals with obesity, adipose tissue secretes high levels of proinflammatory adipokines such as IL-1β, monocyte chemoattractant protein 1 (MCP-1), and tumor necrosis factor (TNF-α)9 as well as IL-6, whose role in the obesity-associated inflammation is still debatable. This preponderance of proinflammatory versus anti-inflammatory adipokines is a hallmark of obesity-associated low-grade inflammation which leads to macrophage infiltration. Adipose tissue macrophages (ATM) fall into two broad categories: classically activated proinflammatory M1 and alternatively activated anti-inflammatory M2 subtypes. M1 macrophages secrete proinflammatory cytokines such as IL-1β, IL-6, and TNF-α, while M2 macrophages are characterized by the production of different cytokines which includes both proinflammatory and anti-inflammatory mediators including IL-10, IL-12, tumor growth factor-β (TGF-β), and TNF-α.9,10
In healthy ‘lean’ adipose tissue, the anti-inflammatory M2 phenotype is typically dominant; however, in obesity, the proinflammatory M1 macrophages become the dominating phenotype in adipose tissue. The mechanisms of this switch have been extensively reviewed.10,11 Briefly, due to leakage of the intestinal barrier, whose permeability is pathologically increased in obesity, ATMs are continuously stimulated by bacterial lipopolysaccharides (LPS). Bacterial toxin binds macrophage-associated toll-like receptor 4 (TLR-4) and stimulates proinflammatory nuclear factor-κappa B (NF-κB) pathway.Further, saturated fatty acids (SFA) and sterols, whose blood levels are elevated in obesity, make their independent contribution to the proinflammatory polarization of adipose macrophages and T cells. A major role in the monocyte recruitment to adipose tissue belongs to the MCP-1 produced by adipocytes. MCP-1 is increased in both genetically modified obese ob/ob mice and under diet-induced obese conditions (high fat diet (HFD) in wild type (WT) animals. Moreover, a chronic MCP-1 increase in mice results in insulin resistance (IR) (discussed below), which was experimentally improved by pharmacological inhibition of MCP-1 signaling.12,13
INSULIN AND INSULIN SIGNALING
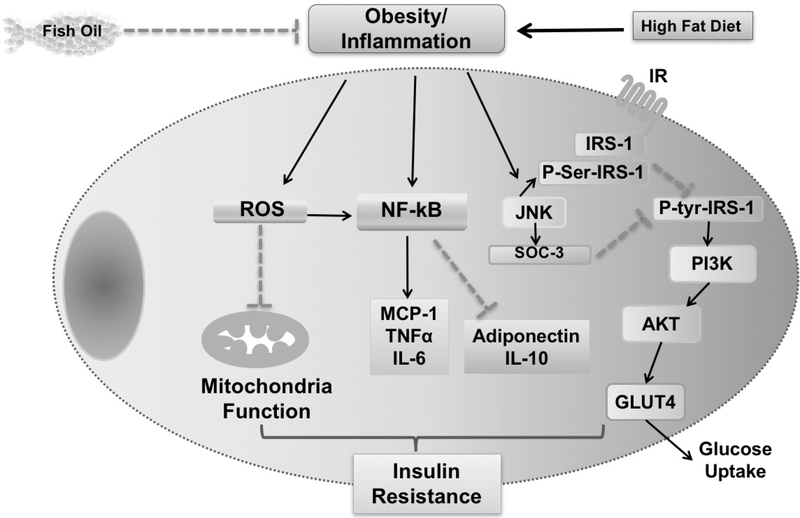
A principal regulator of carbohydrate metabolism in mammals is insulin, a hormone secreted by the pancreatic β-cells in response to increased blood glucose. In adipose tissue, insulin also inhibits lipolysis, thereby reducing free fatty acid (FFA) release from adipocytes and decreasing blood FFA. Binding of insulin to its tetrameric receptor expressed on the cell membrane results in the cascade of intracellular signaling events within the insulin-sensitive tissues (adipose tissue, muscle, liver).14–16 Briefly, the receptor conformation changes once insulin binds, leading to the autophosphorylation of specific tyrosine residues. This tyrosine kinase activation is associated with the cytoplasmic moiety of the receptor. The activated kinase then phosphorylates tyrosine residues on insulin receptor substrates (IRS): G-proteins, kinases, phosphatases, and other signal transductors.17 It launches a cascade of several downstream effects, including the activation of the phosphatidylinositol 3-kinase-protein kinase B (PI3K)-AKT. The PI3K-AKT pathway is known for its essential role in mediating insulin-stimulated glucose uptake and gluconeogenesis suppression. The effects of PI3K-AKT are opposed by the c-Jun N-terminal kinase (JNK), serine kinase as it acts as a negative regulator of insulin signaling and mediates the proinflammatory intracellular signaling pathways.18 Thus, under conditions of inflammation associated with obesity, the molecular signals coming from the inflamed environment interfere the insulin signaling and promote a reduction in the cell insulin sensitivity at a molecular level.19 Furthermore, other known mechanisms include phosphorylation of serine instead of tyrosine in the IRS blocks the insulin signaling pathway (figure 1).
Figure 1.
Potential pathways linking obesity-induced inflammation with insulin resistance. Different pathways which were proposed to have a role in the development of insulin resistance. This includes production of reactive oxidative species (ROS), activation of the nuclear factor-κappa B (NF-κB) pathway and inactivation of insulin receptor substrate-1 (IRS-1). Solid lines indicate positive relationships and dashed lines indicate negative ones. IL, interleukin; JNK, c-Jun N-terminal kinase; MCP-1, monocyte chemoattractant protein 1; SOCS-3 suppressor of cytokine signaling; TNF, tumor necrosis factor.
MOLECULAR MECHANISMS OF IR
Obesity is associated with IR, a pathological condition and a factor in T2D and cardiovascular diseases (CVD).20 In IR, the adipocytes and muscle cells cannot produce a strong enough response to the insulin signaling and require higher concentration of insulin to mediate its effects.20 To compensate for IR, pancreatic beta-cells increase their insulin secretion to bring the blood glucose levels back to a normal range. Over time, the ability of pancreas to overproduce insulin decreases and they become exhausted, and as a result, the blood glucose levels rise. A list of described mechanisms which bridge the pathogenesis of IR and obesity includes endoplasmic reticulum (ER) stress, oxidative stress, mitochondrial dysfunction, dysregulation of lipid homeostasis (including FFA homeostasis), and hypoxia.21 Many of these mechanisms have been associated with ectopic lipid accumulation and/or obesity-induced inflammation.18
Obesity-induced inflammation and IR
Pioneer research by Hotamisligil et al demonstrated that TNF-α, an inflammatory mediator produced extensively by activated macrophages, is an IR-pathogenicity factor.22 This finding has been further confirmed by the observation that in obesity, numerous classically activated F4/80+CD11c+ M1 macrophages, phenotypically different from the IL-10 producing M2 macrophages found in ‘lean’ adipose tissue, infiltrate the adipose tissue and make their contribution to the development of obesity-associated IR.23 More recently, this finding has been further corroborated by the observation that depletion of CD11c+ cells in mice fed very HFD (60% kcal from fat) resulted in rapid and substantial recovery of insulin sensitivity.24
In a proinflammatory environment, the expression of the NF-κB transcription factor in the adipocytes of ‘obese’ adipose tissue is elevated dramatically, and it induces the expression of NF-κB-regulated inflammation-related genes (eg, TNF-α, MCP-1, IL-1β) and cell survival genes. In the canonical NF-κB activation pathway, its activation in adipocytes and immune cells may be stimulated by such obesity-associated stimuli as IL-1 and LPS of gram-negative bacteria,25 which penetrate the leaky intestinal barrier and reach the adipose tissue via systemic circulation.26 Upon binding of the respective ligand to the TNF receptor, IL-1 receptor or the toll-like receptor-4 (TLR-4), a cytoplasmic IkB kinase-beta (IKKβ) activates an NF-κB signal transduction pathway by phosphorylating the IkB proteins. Activation of IkB enables NF-κB translocation from the cytoplasm to nucleus, where it initiates transcription of target genes.25 Suppression of this IKKβ/NF-κB-dependent inflammation reduces IR and the development of T2D in both animals and humans.27–29 Therefore, the adipocytes and indigenous proinflammatory ATMs both contribute to chronic adipose tissue inflammation and promote obesity-associated IR.
Another pathway implicated in IR pathogenesis is JNK pathway which is abnormally activated in HFD-induced obese mice. Corroborating with this, selective adenovirus-induced activation of JNK pathway in mouse liver resulted in decreased insulin sensitivity, while its suppression leads to significant improvements in insulin resistance in high fat/high sucrose model of diet-induced obesity. In particular, liver gluconeogenesis was significantly reduced in these animals.30 Additionally, JNK-1 knockout mice are resistant to weight gain and IR in two different models of obesity.31
In addition to serine kinases, two members of the suppressor of cytokine signaling (SOCS) protein family members, SOCS-1 and SOCS-3, act as inhibitors of insulin signaling.32 Expression of SOCS-3 is associated with obesity in animal models of IR32 and may be induced by proinflammatory agents such as TNF-α and LPS.33,34 SOCS-1 and SOCS-3 inhibit the IRS tyrosine phosphorylation and promote IRS degradation by the proteasomes.33
In addition to the pathways mentioned above, inflammasomes are large multiprotein intracellular complexes that activate the pattern recognition receptors and induce secretion of IL-1β and IL-18 in myeloid cells resulting in obesity-associated IR. Treating LPS-stimulated bone marrow macrophages with palmitate, a saturated fatty acid, caused a dramatic increase in IL-1β and IL-18 secretion.35 Interestingly, treating cells with an unsaturated fatty acid, oleate, did not produce such effect. Moreover, apart from the insulin signaling, negatively affected by the inflammasome activation, IL-1β secretion by myeloid cells also results in inflammasome activation which can interfere with insulin sensitivity.36 In this same study, they also demonstrated that inflammasome activation depends on reactive oxygen species (ROS), another inflammation-associated group of compounds, produced extensively by the activated phagocytes.
A few studies have demonstrated that deficiency of essential components of the inflammasome (Caspase 1, NLRP3, PYCARD), suppresses the inflammation and improves IR in mouse models of diet-induced obesity.37 CD4+ T cells, which are the mature T helper lymphocytes, play an important role in overall immune response and insulin sensitivity in adipocytes.38 In ‘lean’ adipose tissue, anti-inflammatory Th2 cells are the dominant CD4+ subtype, while in diet-induced obesity, proinflammatory interferon-γ-producing Th1 cells increase their numbers and overwhelm their Th2 counterparts. Adaptive transfer of the Th2 cells into obese lymphopenic recombinase activated gene-1 deficient (Rag-1−/−) mice improved IR.38 In addition, T cell depletion with anti-CD3 antibodies in both diet-induced and genetic mouse models of obesity reduced the numbers of prevailing Th1 cells in adipose tissue and successfully reversed IR along with recovering the M2:M1 ATM ratio.38
The link between inflammation and IR in obesity was also illustrated in a study that focused on the role of eosinophils, granulocyte myeloid cells which are associated with the innate immunity and anti-inflammatory Th2 immune responses. Even though eosinophil numbers in the ‘lean’ adipose tissue are very low, they have been shown to be crucial for sustaining the M2 ATM phenotype and therefore aid in maintaining the ‘healthy’ M2:M1 ATM balance. Moreover, IR was ameliorated in hypereosinophilic HFD-fed mice, in which eosinophil numbers were elevated via the helminth infection or transgenic IL-5 expression.39
Another inflammatory mediator that has been recently implicated in the pathogenesis of several chronic diseases, including MetS, T2D and type 1 diabetes (T1D) is the lipid mediator leukotriene B4 (LTB4).40 In mice fed with HFD, the adipose tissue produces an increased level of LTB4 that recruits monocytes, skews ATM to M1 phenotype and promotes inflammation resulting in IR and hyperglycemia.40 Because activation of the LTB4 occurs through its receptor (BLT1) to induce JNK pathway, LTB4 promotes IR in adipose tissue directly through its own receptor and by enhancing the production of proinflammatory cytokines.41 In T1D mice, LTB4 is systemically produced and enhanced the myeloid differentiation primary response gene 88 (MyD88) expression in macrophages. As MyD88 is the adaptor molecule for several TLR and IL1 receptors, the LTB4 also potentiates the inflammation in T1D and is associated with increased sepsis susceptibility in T1D mice.41
Omega 3 polyunsaturated fatty acids (PUFAs), as an intervention to reduce inflammation in obesity and IR
The importance of omega-3 and omega-6 PUFAs (ω−3 and ω−6 PUFAs) for human health is related to their role as metabolic precursors to eicosanoids, a family of signaling molecules which play a crucial role in regulating the inflammatory processes in an organism. Both ω−3 and ω−6 PUFAs are essential food components because they cannot be synthesized in mammals.42 Short-chain ω−6 PUFA linoleic acid (C18:2) and short-chain ω−3 PUFA α-linolenic acid (ALA; C18:3), consumed with the diet, undergo a series of parallel and competitive desaturation, elongation and β-oxidation reactions and serve as precursors for the long-chain PUFAs: arachidonic acid (C20:4; ω−6) and eicosapentaenoic acid and docosahexaenoic acids (EPA and DHA; C20:5 and C22:6 respectively). Long-chain ω−3 PUFAs give rise further to the anti-inflammatory compounds of lipid nature, such as resolvins, protectins and maresins which also play a crucial role in tissue damage recovery.42,43 On contrast, the derivatives of ω−6 PUFAs, such as prostaglandin E2 (PGE2) and LTB4 are the mediators of inflammation.42,44 The rates of conversion from ALA to EPA are limited. As little as about 0.2%–8% of consumed ALA is converted to EPA and only 0%–4% of ALA to DHA.45,46 In this regard, the dietary interventions using EPA and/or DHA may represent a potent therapeutic tool for treating and or preventing obesity-associated inflammation and IR.47 Furthermore, dietary changes in the countries which have accepted ‘western way of life’ and more recently, in many other regions of the world, led to dramatic increase in ω−6/ω−3 diet ratio, which have been implicated in the pathogenesis of chronic inflammatory diseases.48
A few studies have addressed the anti-inflammatory effects of EPA and DHA in adipose tissue (summarized in figure 1). For instance, an in vitro study showed that exposure of LPS-stimulated human adipose tissue or mature adipocytes to DHA or EPA decreased secretion of cytokines such as TNF-α, IL-6 and MCP-1.49 Spencer et al have demonstrated that supplementing the diet of human patients, suffering from IR but not a T2D, with 4 g/day ω−3-acid ethyl esters for 12 weeks reduced ATM numbers, increased vascularity and reduced MCP-1 expression in the adipose tissue.50 It was demonstrated in the same study that co-culturing human macrophages polarized from THP-1 monocytes with adipocytes in the presence of DHA or EPA resulted in decreased expression of proinflammatory cytokine genes as MCP-1 and TNF-α in both adipocytes and macrophages.50
Metabolic effects of long-chain ω−3 PUFAs are largely mediated by perixosome-proliferator-activated receptor (PPAR) transcription factors, with two receptor subtypes, PPARα and PPARγ, involved in the lipid-metabolizing effects.42,43,51 PPARγ mediates the EPA and DHA effects on adiponectin upregulation,52 while PPARα mediates EPA’s beneficial effects on hepatic insulin sensitivity, as it was shown by studies in which supplying mouse HFD with 8% fish oil resulted in the improved hepatic insulin sensitivity in WT but not in PPARα knockout animals.53 In the liver, dietary EPA also suppressed the lipogenesis and steatosis in mice fed a high-fat, high-sucrose diet.54 In addition to stimulating PPARs, ω−3 PUFAs exert their protective effects via stimulating AMP-activated protein kinase (AMPK) pathway. Similarly, in Sprague-Dawley rats fed a high glucose diet, enrichment with ω−3-rich fish oil resulted in twofold to threefold increase in hepatic AMPK phosphorylation.55 Thus promoting fatty acid oxidation and decreasing adiposity, making it an ideal bioactive food compound for treating MetS.
Micro-RNAs as mediators of inflammation-associated obesity and IR
Micro-RNAs (miRNAs) are small (21–22 nucleotides) non-coding RNAs that act as potent regulators of gene expression related to cell development, differentiation, signal transduction, and various homeostatic and pathological conditions.56–58 miRNAs can target more than one gene and promote stabilization or degradation of a mRNA by binding the complementary 3′ -UTR sequence, which repress the mRNA sequence translation.
Specific miRNAs are upregulated or downregulated in chronic diseases, such as cancer, diabetes, obesity, and CVD.59–62 White adipose tissue (WAT) is an important source of miRNAs which contributes to tissue homeostasis and inflammatory status of local as well as systemic environment. Lipohypertrophy or lipodystrophy in WAT changes regular miRNA expression.56,63 In two recently published studies by the same group of authors, miRNA-mediated effects on lipid metabolism were investigated using a genetically modified ADicerko mouse model.64,65 In these mice, the expression of Dicer, an enzyme essential for the biogenesis and regulation of miRNAs as well as small interfering RNAs (siRNAs), was specifically knocked out in adipose tissue using a Crelox gene-recombination strategy.65 The absence of Dicer in the fat tissue resulted in defective adipose miRNA processing which was associated with reduced weight gain, untypical whitening of brown adipose tissue, IR and alterations to circulating lipids.64 Manifestations of these symptoms were especially exaggerated in ADicerko animals fed HFD. The circulating miRNA levels and MetS in these mice were ameliorated by the transplantation of WAT adipose tissue. Importantly, adipose tissue-derived miRNAs, secreted in exosomes (small vesicle carriers formed in multivesicular cell compartments which are secreted by cell after fusing with the plasma membrane66) were able to regulate the expression of metabolism-related genes (fibroblast growth factor 21, FGF 21) in liver.65 These findings suggest that fat-derived miRNAs may be considered as a novel type of adipokines.67
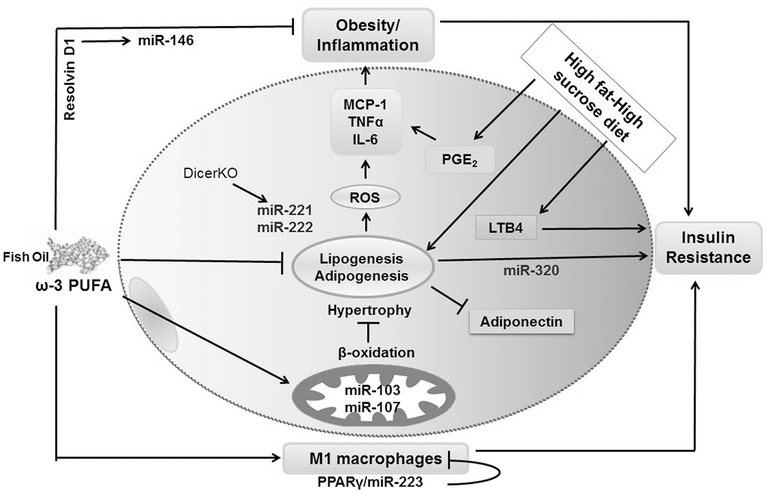
Various miRNAs have been shown to be co-expressed and/or co-regulated in obesity, affecting obesity-associated health conditions such as glucose-stimulated insulin secretion (miR-320, miR220a), adipogenesis (miR-21, miR-222, miR-146a), and insulin sensitivity (miR-103, miR107).68,69 Also, different miRNAs upregulated or downregulated in insulin-resistant adipocytes, including miR-320, have been identified by Ling et al.70 Authors demonstrated that IR in adipocytes is regulated by miR-320. It was proposed that miR-320 specifically targets the PI3K-p85 subunit. miR-320 increases Akt phosphorylation and elevates Glut4 glucose transporter protein expression levels. The paralogue miRNAs that originate from the same mother gene, miR-103 and miR-107, are both related to energy metabolism as they increase the expression of β-oxidation genes, thus stimulating lipolysis and decreasing fat deposition in adipocytes71 (figure 2).
Figure 2.
Suggested mechanisms of the anti-inflammatory effects of ω–3 polyunsaturated fatty acid (PUFA) by microRNAs (miRNA) in adipocytes. Different miRNAs are involved in adipocytes imbalance and might be targets of anti-inflammatory effects of ω−3 PUFA. ω−3 PUFAs might be regulating the expression of miR-221 and miR-222 in adipogenesis, miR-320 in insulin resistance, miR-103/miR-107 in mitochondrial respiration, miR-223 in M1 macrophages profile, and miR-146 in response to resolvin D1 effects. IL, interleukin; LTB4, leukotriene B4; MCP-1, monocyte chemoattractant protein 1; PGE2, prostaglandin E2; ROS, reactive oxidative species; TNF, tumor necrosis factor.
We have previously shown that adipose tissue angiotensiongen II (Agt) and IL-6 were significantly reduced in epididymal adipose tissue in HFD-fed mice supplemented with EPA-enriched fish oil.72 In a set of in vitro experiments, this data was corroborated by downregulation of Agt, IL-6 and MCP-1 production in mouse 3T3-L1 adipocytes treated with EPA. Gene regulation by ω−3 PUFA were exerted via reducing NF-κB activation.72 In this regard, miR-146a and miR-222, known for their associations with NF-κB and TNF-α pathways, respectively,73 look particularly promising targets for further investigations. Besides due to its well-documented proinflammatory properties, TNF-α is indirectly implicated in adipogenesis. This cytokine is a suggested molecular target for the miR-222 and is significantly upregulated in mice fed HFD (figure 2). Furthermore, miR-146a has an essential role in the LPS-hyporesponsiveness (which engages TLR-4 mediated NF-κB signaling) induced in human monocytes by prolonged bacterial toxin exposure.73
TLR-4 is a receptor for bacterial LPS, whose levels are increased in HFD-induced obesity.74 As obesity is associated with chronic TLR-4 stimulation of ATM by circulating blood toxins, a large group of miRNAs including miR-200b, miR-200c, miR-511 and miR-223, may play potential roles in TLR-4 regulation.75,76 miR-200b and miR-200c were identified as factors that modify the efficiency of TLR-4 signaling through the MyD88-dependent pathway.76 Depending on the cell cycle phase, miR-511 may positively stimulate the TLR-4 signaling or inhibit the expression of TLR-4 in myeloid-derived immune cells.75 TLR-4 binding with its ligand stimulates NF-κB activation, which can be inhibited by ω−3 PUFAs.72
Polarization of bone marrow-derived macrophages into the proinflammatory M1 or anti-inflammatory M2 phenotype is regulated by PPAR-γ/miR-223 signaling pathways, and the choice between classic and alternative activation depends on the miR-223 molecular targets (figure 2). Engaging nuclear factor of activated T-cells (Nfat5) and RAS p21 protein activator (Rasa1) genes have been shown to drive M2 polarization, and involvement of homeobox protein Pknox1 gene results in development of M1 cells.77
A significance of ω−3/ω−6 balance in human diet and the role of miRNAs in mediating the anti-inflammatory effects of ω−3 PUFAs may be illustrated lipid mediators, involved in resolution of inflammation. In a contraction phase of zymosan-induced mouse model of peritonitis, the increase of miR-146b levels in peritoneal exudates is stimulated by resolvin D1 (RvD1), an ω−3 PUFA lipid derivative with well-documented anti-inflammatory properties78 (figure 2). miR-146b interferes with NF-κB pathway, quenching the acute inflammatory responses by preventing the NF-κB nuclear translocation. This miRNA directly targets the IL-1 receptor-associated kinase 1 (IRAK1) and TNF receptor associated factor 6 (TRAF6), two key molecules, essential for NF-κB signaling.79 On the other hand, the stimulation with LTB4 decreases SOCS1 and increases MyD88 in macrophages, and these effects are mediated via induction of miR-155, miR-146b and miR-125b miRNAs.40 The miR-155 can be downregulated by the anti-inflammatory cytokine IL-10.80 As supplementation of the mouse diet with ω−3 PUFAs induces a higher IL-10 expression in insulin target tissues,81 all aforementioned miRNAs represent instrumental targets in MetS and ω−3 PUFA-devoted studies.
The experimental studies on adipose tissue miRNAs represent a potent research tool for investigating the physiology and pathophysiology of the adipose tissue. Recent discoveries have demonstrated miRNAs significance in pathogenesis of MetS and other chronic diseases, as well as miRNAs role in mediating the protective effects of ω−3 PUFAs and other bioactive food components.81 This indicates that further investigations of the regulation of miRNA-mediated metabolism have a high potential for identifying novel approaches for treatment and prevention of obesity and its associated chronic complications.
Acknowledgments
Funding This work was funded in part by the Presidential Cluster Hire Tier Obesity Research Cluster at Texas Tech University, SPRINT funds (TTU) FAPESP TTU to NMM and LRF, and CAPES (TR). NMM is supported in part by NIH/ NCCIH grant #R15AT00887901A1.
Footnotes
Competing interests None declared.
Provenance and peer review Commissioned; externally peer reviewed.
REFERENCES
- 1.Flegal KM, Kruszon-Moran D, Carroll MD, et al. Trends in obesity among adults in the United States, 2005 to 2014. JAMA 2016;315:2284–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ogden CL, Carroll MD, Lawman HG, et al. Trends in obesity prevalence among children and adolescents in the United States, 1988–1994 through 2013–2014. JAMA 2016;315:2292–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Finkelstein EA, Trogdon JG, Cohen JW, et al. Annual medical spending attributable to obesity: payer-and service-specific estimates. Health Aff 2009;28:w822–w831. [DOI] [PubMed] [Google Scholar]
- 4.Haslam DW, James WP. Obesity. Lancet 2005;366:1197–209. [DOI] [PubMed] [Google Scholar]
- 5.Bray GA. Medical consequences of obesity. J Clin Endocrinol Metab 2004;89:2583–9. [DOI] [PubMed] [Google Scholar]
- 6.Kalupahana NS, Moustaid-Moussa N, Claycombe KJ. Immunity as a link between obesity and insulin resistance. Mol Aspects Med 2012;33:26–34. [DOI] [PubMed] [Google Scholar]
- 7.Cottam DR, Mattar SG, Barinas-Mitchell E, et al. The chronic inflammatory hypothesis for the morbidity associated with morbid obesity: implications and effects of weight loss. Obes Surg 2004;14:589–600. [DOI] [PubMed] [Google Scholar]
- 8.Greenberg AS, Obin MS. Obesity and the role of adipose tissue in inflammation and metabolism. Am J Clin Nutr 2006;83:461S–5. [DOI] [PubMed] [Google Scholar]
- 9.Ouchi N, Parker JL, Lugus JJ, et al. Adipokines in inflammation and metabolic disease. Nat Rev Immunol 2011;11:85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chawla A, Nguyen KD, Goh YP. Macrophage-mediated inflammation in metabolic disease. Nat Rev Immunol 2011;11:738–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olefsky JM, Macrophages GCK. Inflammation, and insulin resistance. AnnRev Physiol 2010;72:219–46. [DOI] [PubMed] [Google Scholar]
- 12.Kanda H, Tateya S, Tamori Y, et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest 2006;116:1494–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tateya S, Tamori Y, Kawaguchi T, et al. An increase in the circulating concentration of monocyte chemoattractant protein-1 elicits systemic insulin resistance irrespective of adipose tissue inflammation in mice. Endocrinology 2010;151:971–9. [DOI] [PubMed] [Google Scholar]
- 14.Chang L, Chiang SH, Saltiel AR. Insulin signaling and the regulation of glucose transport. Mol Med 2004;10:65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramalingam L, Oh E, Thurmond DC . Novel roles for insulin receptor (IR) in adipocytes and skeletal muscle cells via new and unexpected substrates. Cell Mol Life Sci 2013;70:2815–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siddle K Signalling by insulin and IGF receptors: supporting acts and new players. J Mol Endocrinol 2011;47:R1–R10. [DOI] [PubMed] [Google Scholar]
- 17.Boucher J, Kleinridders A, Kahn CR. Insulin receptor signaling in normal and insulin-resistant states. Cold Spring Harb Perspect Biol 2014;6:a009191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zeyda M, Stulnig TM. Obesity, inflammation, and insulin resistance--a mini-review. Gerontology 2009;55:379–86. [DOI] [PubMed] [Google Scholar]
- 19.Manning BD. Balancing Akt with S6K: implications for both metabolic diseases and tumorigenesis. J Cell Biol 2004;167:399–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006;444:840–6. [DOI] [PubMed] [Google Scholar]
- 21.Samuel VT, Shulman GI. Mechanisms for insulin resistance: common threads and missing links. Cell 2012;148:852–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science 1993;259:87–91. [DOI] [PubMed] [Google Scholar]
- 23.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest 2007;117:175–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patsouris D, Li PP, Thapar D, et al. Ablation of CD11c-positive cells normalizes insulin sensitivity in obese insulin resistant animals. Cell Metab 2008;8:301–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lawrence T The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol 2009;1:a001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.König J, Wells J, Cani PD, et al. Human intestinal barrier function in Health and Disease. Clin Transl Gastroenterol 2016;7:e196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee BC, Lee J. Cellular and molecular players in adipose tissue inflammation in the development of obesity-induced insulin resistance. Biochim Biophys Acta 2014;1842:446–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yuan M, Konstantopoulos N, Lee J, et al. Reversal of obesityand diet-induced insulin resistance with salicylates or targeted disruption of ikkbeta. Science 2001;293:1673–7. [DOI] [PubMed] [Google Scholar]
- 29.Kim JK, Kim YJ, Fillmore JJ, et al. Prevention of fat-induced insulin resistance by salicylate. J Clin Invest 2001;108:437–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakatani Y, Kaneto H, Kawamori D, et al. Modulation of the JNK pathway in liver affects insulin resistance status. J Biol Chem 2004;279:45803–9. [DOI] [PubMed] [Google Scholar]
- 31.Hirosumi J, Tuncman G, Chang L, et al. A central role for JNK in obesity and insulin resistance. Nature 2002;420:333–6. [DOI] [PubMed] [Google Scholar]
- 32.Ueki K, Kadowaki T, Kahn CR. Role of suppressors of cytokine signaling SOCS-1 and SOCS-3 in hepatic steatosis and the metabolic syndrome. Hepatol Res 2005;33:185–92. [DOI] [PubMed] [Google Scholar]
- 33.Ueki K, Kondo T, Kahn CR. Suppressor of cytokine signaling 1 (SOCS-1) and SOCS-3 cause insulin resistance through inhibition of tyrosine phosphorylation of insulin receptor substrate proteins by discrete mechanisms. Mol Cell Biol 2004;24:5434–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lebrun P, Van Obberghen E. SOCS proteins causing trouble in insulin action. Acta Physiol 2008;192:29–36. [DOI] [PubMed] [Google Scholar]
- 35.Wen H, Gris D, Lei Y, et al. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat Immunol 2011;12:408–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vandanmagsar B, Youm YH, Ravussin A, et al. The NLRP3 in ammasome instigates obesity-induced in ammation andinsulin resistance. NatMed 2011;17:179–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stienstra R, van Diepen JA, Tack CJ, et al. Inflammasome is a central player in the induction of obesity and insulin resistance. Proc Natl Acad Sci U S A 2011;108:15324–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Winer S, Chan Y, Paltser G, et al. Normalization of obesity-associated insulin resistance through immunotherapy. Nat Med 2009;15:921–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu D, Molofsky AB, Liang HE, et al. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science 2011;332:243–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang Z, Filgueiras LR, Wang S, et al. Leukotriene B4 enhances the generation of proinflammatory microRNAs to promote MyD88-dependent macrophage activation. J Immunol 2014;192:2349–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Filgueiras LR, Wang S, et al. Brandt SLLeukotriene B4-mediated sterile in ammation promotes susceptibilityto sepsis in a mouse model of type 1 diabetes. SciSignal 2015;8:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Flachs P, Rossmeisl M, Bryhn M, et al. Cellular and molecular effects of n-3 polyunsaturated fatty acids on adipose tissue biology and metabolism. Clin Sci 2009;116:1–16. [DOI] [PubMed] [Google Scholar]
- 43.Gonzalez-Periz A, Horrillo R, Ferre N, et al. Obesity-induced insulin resistance and hepatic steatosis are alleviated by −3 fatty acids: a role for resolvins and protectins. The FASEB Journal 2009;23:1946–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kalupahana NS, Claycombe KJ, Moustaid-Moussa N. (n-3) Fatty acids alleviate adipose tissue inflammation and insulin resistance: mechanistic insights. Adv Nutr 2011;2:304–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mozaffarian D, Wu JH, Jhy W. Omega-3 fatty acids and cardiovascular disease: effects on risk factors, molecular pathways, and clinical events. J Am Coll Cardiol 2011;58:2047–67. [DOI] [PubMed] [Google Scholar]
- 46.Burdge G Alpha-linolenic acid metabolism in men and women: nutritional and biological implications. Curr Opin Clin Nutr Metab Care 2004;7:137–44. [DOI] [PubMed] [Google Scholar]
- 47.Kalupahana NS, Claycombe K, Newman SJ, et al. Eicosapentaenoic acid prevents and reverses insulin resistance in high-fat diet-induced obese mice via modulation of adipose tissue inflammation. J Nutr 2010;140:1915–22. [DOI] [PubMed] [Google Scholar]
- 48.Patterson E, Wall R, Fitzgerald GF, et al. Health implications of high dietary omega-6 polyunsaturated fatty acids. J Nutr Metab 2012;2012:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Murumalla RK, Gunasekaran MK, Padhan JK, et al. Fatty acids do not pay the toll: effect of SFA and PUFA on human adipose tissue and mature adipocytes inflammation. Lipids Health Dis 2012;11:175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Spencer M, Finlin BS, Unal R, et al. Omega-3 fatty acids reduce adipose tissue macrophages in human subjects with insulin resistance. Diabetes 2013;62:1709–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Flachs P, Horakova O, Brauner P, et al. Polyunsaturated fatty acids of marine origin upregulate mitochondrial biogenesis and induce beta-oxidation in white fat. Diabetologia 2005;48:2365–75. [DOI] [PubMed] [Google Scholar]
- 52.Neschen S, Morino K, Rossbacher JC, et al. Fish oil regulates adiponectin secretion by a peroxisome proliferator-activated receptor-gamma-dependent mechanism in mice. Diabetes 2006;55:924–8. [DOI] [PubMed] [Google Scholar]
- 53.Neschen S, Morino K, Dong J, et al. n-3 Fatty acids preserve insulin sensitivity in vivo in a peroxisome proliferator-activated receptor-alpha-dependent manner. Diabetes 2007;56:1034–41. [DOI] [PubMed] [Google Scholar]
- 54.Sato A, Kawano H, Notsu T, et al. Antiobesity effect of eicosapentaenoic acid in high-fat/high-sucrose diet-induced obesity: importance of hepatic lipogenesis. Diabetes 2010;59:2495–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Suchankova G, Tekle M, Saha AK, et al. Dietary polyunsaturated fatty acids enhance hepatic AMP-activated protein kinase activity in rats. Biochem Biophys Res Commun 2005;326:851–8. [DOI] [PubMed] [Google Scholar]
- 56.Arner P, Kulyté A. MicroRNA regulatory networks in human adipose tissue and obesity. Nat Rev Endocrinol 2015;11:276–88. [DOI] [PubMed] [Google Scholar]
- 57.Mukherji S, Ebert MS, Zheng GX, et al. MicroRNAs can generate thresholds in target gene expression. Nat Genet 2011;43:854–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lu J, Clark AG. Impact of microRNA regulation on variation in human gene expression. Genome Res 2012;22:1243–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dumortier O Hinault C and Van Obberghen E. MicroRNAs and metabolism crosstalk in energy homeostasis. Cell Metab 2013;18:312–24. [DOI] [PubMed] [Google Scholar]
- 60.Arner E, Mejhert N, Kulyté A, et al. Adipose tissue microRNAs as regulators of CCL2 production in human obesity. Diabetes 2012;61:1986–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Capobianco V, Nardelli C, Ferrigno M, et al. miRNA and protein expression profiles of visceral adipose tissue reveal miR-141/YWHAG and miR-520e/ RAB11A as two potential miRNA/protein target pairs associated with severe obesity. J Proteome Res 2012;11:3358–69. [DOI] [PubMed] [Google Scholar]
- 62.Caroli A, Cardillo MT, Galea R, et al. Potential therapeutic role of microRNAs in ischemic heart disease. J Cardiol 2013;61:315–20. [DOI] [PubMed] [Google Scholar]
- 63.Mori MA, Raghavan P, Thomou T, et al. Role of microRNA processing in adipose tissue in stress defense and longevity. Cell Metab 2012;16:336–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mori MA, Thomou T, Boucher J, et al. Altered miRNA processing disrupts brown/ white adipocyte determination and associates with lipodystrophy. J Clin Invest 2014;124:3339–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Thomou T, Mori MA, Dreyfuss JM, et al. Adipose-derived circulating miRNAs regulate gene expression in other tissues. Nature 2017;542:450–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhou X, Jiao Z, Ji J, et al. Characterization of mouse serum exosomal small RNA content: The origins and their roles in modulating inflammatory response. Oncotarget 2017;8:42712–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Greenhill C Non-coding RNA: exosomal microRNAs as novel adipokines. Nat Rev Genet 2017;18:212. [DOI] [PubMed] [Google Scholar]
- 68.Williams MD, Mitchell GM. MicroRNAs in insulin resistance and obesity. Exp Diabetes Res 2012;2012:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xie H, Sun L, Lodish HF. Targeting microRNAs in obesity. Expert Opin Ther Targets 2009;13:1227–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ling HY, Ou HS, Feng SD, et al. CHANGES IN microRNA (miR) profile and effects of miR-320 in insulin-resistant 3T3-L1 adipocytes. Clin Exp Pharmacol Physiol 2009;36:e32–9. [DOI] [PubMed] [Google Scholar]
- 71.Trajkovski M, Hausser J, Soutschek J, et al. MicroRNAs 103 and 107 regulate insulin sensitivity. Nature 2011;474:649–53. [DOI] [PubMed] [Google Scholar]
- 72.Siriwardhana N, Kalupahana NS, Fletcher S, et al. n-3 and n-6 polyunsaturated fatty acids differentially regulate adipose angiotensinogen and other inflammatory adipokines in part via NF-?B-dependent mechanisms. J Nutr Biochem 2012;23:1661–7. [DOI] [PubMed] [Google Scholar]
- 73.Ortega FJ, Moreno-Navarrete JM, Pardo G, et al. MiRNA expression profile of human subcutaneous adipose and during adipocyte differentiation. PLoS One 2010;5:e9022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim KA, Gu W, Lee IA, et al. High fat diet-induced gut microbiota exacerbates inflammation and obesity in mice via the TLR4 signaling pathway. PLoS One 2012;7:e47713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Olivieri F, Rippo MR, Prattichizzo F, et al. Toll like receptor signaling in “inflammaging”: microRNA as new players. Immun Ageing 2013;10:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wendlandt EB, Graff JW, Gioannini TL, et al. The role of microRNAs miR-200b and miR-200c in TLR4 signaling and NF-?B activation. Innate Immun 2012;18:846–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ying W, Tseng A, Chang RC, et al. MicroRNA-223 is a crucial mediator of PPAR?-regulated alternative macrophage activation. J Clin Invest 2015;125:4149–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Recchiuti A, Krishnamoorthy S, Fredman G, et al. MicroRNAs in resolution of acute inflammation: identification of novel resolvin D1-miRNA circuits. The FASEB Journal 2011;25:544–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ma X, Becker Buscaglia LE, Barker JR, et al. MicroRNAs in NF-kappaB signaling. J Mol Cell Biol 2011;3:159–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Quinn SR, O’Neill LA. The role of microRNAs in the control and mechanismof action of IL-10. CurrTop Microbiol Immunal 2014;380:145. [DOI] [PubMed] [Google Scholar]
- 81.Visioli F, Giordano E, Nicod NM, et al. Molecular targets of omega 3 and conjugated linoleic Fatty acids - “micromanaging” cellular response. Front Physiol 2012;3:42. [DOI] [PMC free article] [PubMed] [Google Scholar]