Abstract
Strategies to reduce obesity have become public health priorities as the prevalence of obesity has risen in the United States and around the world. While the anti-inflammatory and hypotriglyceridemic properties of long-chain omega-3 polyunsaturated fatty acids (n-3 PUFAs) are well known, their antiobesity effects and efficacy against metabolic syndrome, especially in humans, are still under debate. In animal models, evidence consistently suggests a role for n-3 PUFAs in reducing fat mass, particularly in the retroperitoneal and epididymal regions. In humans, however, published research suggests that though n-3 PUFAs may not aid weight loss, they may attenuate further weight gain and could be useful in the diet or as a supplement to help maintain weight loss. Proposed mechanisms by which n-3 PUFAs may work to improve body composition and counteract obesity-related metabolic changes include modulating lipid metabolism; regulating adipokines, such as adiponectin and leptin; alleviating adipose tissue inflammation; promoting adipogenesis and altering epigenetic mechanisms.
Keywords: Adipocytes, Fish oil, Metabolic syndrome, Obesity, Omega-3 polyunsaturated fatty acids, Weight loss
1. Introduction
The American Medical Association recognizes obesity as a disease [1] and considers it a major public health problem. In the United States, 36.5% of adults are obese [2], while approximately 39% of the world’s adult population is overweight and more than 13% are obese [3]. Obesity increases morbidity risks for heart disease, type 2 diabetes mellitus (T2DM) and some types of cancer [4]. Metabolic changes associated with these diseases comprise metabolic syndrome (MetS), which is diagnosed when three of the following five conditions exist: abdominal obesity, elevated triglycerides (TG), reduced high-density lipoprotein (HDL) cholesterol, high blood pressure and elevated fasting blood glucose [5].
Lipids are key macronutrients in the human diet. The type and proportion of dietary fatty acids consumed impact health and whole-body physiology [6]. Research has shown that saturated fatty acids (SFAs) are detrimental to health, while monounsaturated (MUFAs) and polyunsaturated fatty acids (PUFAs) offer health benefits [7]. In the diet, fatty fish and fish oil rich in omega-3 (n-3) PUFAs, such as eicosapentaenoic acid (EPA) or docosahexaenoic acid (DHA), have demonstrated cardioprotective, anti-inflammatory and hypotriglyceridemic properties. Hence, these fatty acids may assist in the treatment and prevention of obesity comorbidities, especially by improving individual components of the metabolic syndrome [7–9]. Therefore, the effect of n-3 PUFAs on body weight and body composition is of particular interest.
In this review, we provide an update on the effects of n-3 PUFAs on obesity and MetS in both animal and human studies, highlighting potential mechanisms for n-3 PUFAs in reducing body weight, improving body composition and counteracting the adverse metabolic consequences of obesity.
2. Adipose tissue and obesity
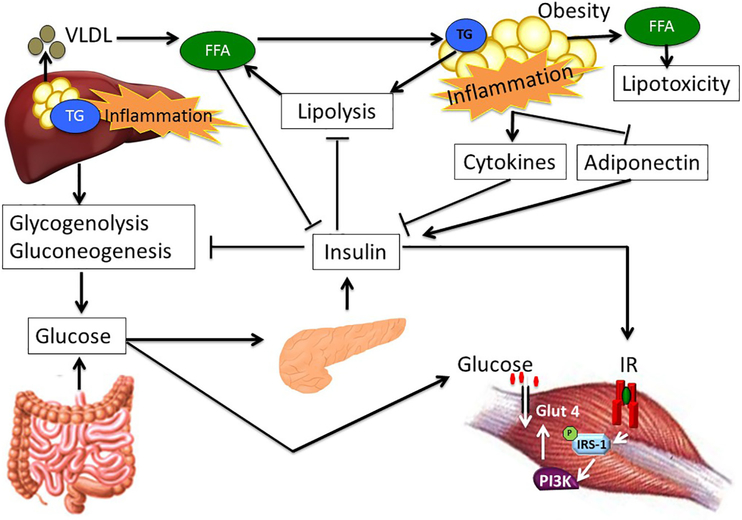
Body fat is primarily stored in adipose tissue, a connective tissue composed of adipocytes, preadipoctyes, vascular endothelial cells, fibroblasts and various types of immune cells, including adipose tissue macrophages [10]. Adipose tissue is an active endocrine organ that secretes numerous hormones, including leptin and adiponectin, and cytokines (adipokines) such as interleukin (lL)-6 [11]. Three major types of adipose tissue have been identified: white adipose tissue (WAT), brown adipose tissue (BAT) and beige (“brite”) adipose tissue. WAT is primarily responsible for energy storage in the form of TG and the release of fatty acids during periods of fasting; it is mainly located in two distinct depots, as subcutaneous adipose tissue or visceral adipose tissue [10]. The adipocytes of visceral fat surrounding internal organs are more metabolically active than those of subcutaneous adipose tissue and thus contribute to the risks of cardiovascular disease and T2DM [12]. BAT plays a key role in thermogenesis and is mainly found above the clavicle and scapula in adults [12]. Obesity leads to adipose tissue dysfunction, which is mechanistically linked to the pathogenesis of insulin resistance in the liver and in skeletal muscle (Fig. 1) and may result in MetS [13].
Fig. 1.
Adipose tissue, liver and skeletal muscle cross talk in obesity and insulin resistance. The liver maintains normoglycemia during fasting via glycogenolysis and gluconeogenesis. Following a meal, increased glucose delivery to the pancreas stimulates insulin secretion, which acts on the liver, adipose tissue and skeletal muscle. The primary action of insulin on the liver is to suppress hepatic glucose output, while insulin increases glucose uptake by the skeletal muscle and adipose tissue. Insulin additionally inhibits lipolysis in adipose tissue. In obesity, changes in adipokines produced and released from adipose tissue, such as decreased adiponectin and increased TNF-α and other inflammatory cytokines, coupled with increased free fatty acids contribute to hepatic and skeletal muscle insulin resistance.
While weight loss via lifestyle modification is the primary treatment in the management of obesity and its comorbidities, compliance is difficult. Adjunct treatments for the management of obesity include pharmaceuticals [14], surgery [15] and dietary supplements [16]. Despite these measures, however, the prevalence of obesity has continued to rise. Thus, alternative strategies to assist in weight loss and reduce body fat are necessary. Natural bioactives such as n-3 PUFAs present few side effects and so may be safer than other modalities for the treatment of obesity. This review summarizes current basic and clinical research and mechanistic insights regarding the effects of n-3 PUFAs on obesity.
3. Omega-3 fatty acids
3.1. Synthesis and metabolism
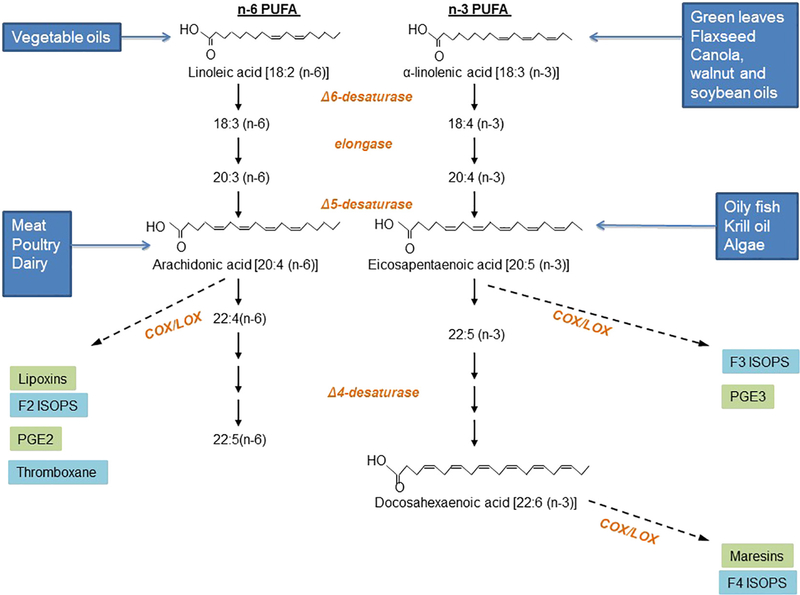
The human body can synthesize many fatty acids, but not linoleic acid (LA; omega-6; C18:2 n-6) or α-linolenic acid (ALA; C18:3 n-3), which must be consumed in the diet. ALA is the precursor for EPA (C20:5 n-3),docosapentaenoic acid (DPA; C22:5 n-3) and DHA (C22:6 n-3) in the human body (Fig. 2) [6]. Many studies have found low conversion rates of ALA to EPA and DPA, and little to no DHA synthesis [17,18]; hence, any direct benefits of these very long chain fatty acids depend on dietary intake [17]. Both dietary intake and fatty acid desaturase activity determine plasma n-3 PUFA levels [19]. A balanced n-6:n-3 fatty acid ratio (1:1 to 2:1 is optimal) is important for homeostasis and normal development throughout the lifespan. High n-6 PUFA intake in the Western diet increases the n-6:n-3 ratio to a range from 10:1 to 20:1 and may play a role in the pathogenesis of obesity and related diseases [19,20].
Fig. 2.
Metabolism of omega-6 and omega-3 polyunsaturated fatty acids. LA is an essential n-6 PUFA that is metabolized to AA and further to proinflammatory eicosanoids. ALA is an essential n-3 PUFA that is metabolized to EPA, DPA and DHA. Eicosanoids derived from the metabolism of EPA, DPA and DHA also aid in the regulation of inflammation and are considered more anti-inflammatory. ISOPS, isoprostanes; COX, cyclooxygenases; LOX, lipooxygenases.
3.2. Sources of omega-3 PUFAs
Dietary sources of n-3 PUFAs are much less abundant than n-6 PUFAs. ALA is synthesized by plants from LA and can thus be found in green leafy vegetables, seeds such as flaxseed (linseed), nuts and legumes. Vegetable oils such as sunflower, corn, perilla, canola and soybean also provide ALA but are much more abundant in LA. Fish, such as salmon, tuna, trout, mackerel, anchovy, bluefish, herring, mullet, sturgeon and sardines, are also rich sources of n-3 PUFAs, particularly EPA and DHA. Lean fish, such as cod, store lipids in their liver, and for this reason, cod liver oil is a good source of n-3 PUFAs. Fatty fish, such as salmon, mackerel, sardines and tuna, store lipids throughout their bodies and are good whole sources of n-3 PUFAs [6].
The 2015 Dietary Guidelines for Americans recommend consuming about 8 oz per week of seafood, which would provide about 250 mg/day of EPA and DHA [21]. The recommended intake for n-3 PUFAs corresponds to consuming fish twice weekly, including one serving of oily fish. Even though there is ample evidence for a role of n-3 PUFAs in modulating chronic diseases, an optimal dose has not been agreed upon, and recommendations vary based on governing body. The U.S. Food and Drug Administration has stated that levels up to 3 g/day are generally recognized as safe [22], although other authorities have reported no adverse effects at up to 5–6 g/day [23]. It has been suggested that the bioavailability of n-3 PUFAs is improved by emulsification. Emulsified n-3 PUFA is more easily exposed to pancreatic lipase and colipase, enhancing its digestion. Additionally, emulsified n-3 PUFA is easily transported into enterocytes, thus increasing fatty acid absorption [24,25].
3.3. Dietary omega-3 PUFA intake, obesity and metabolic disorders
Dietary fish intake is considerably higher in people of the circumpolar arctic regions and relatively much lower in those living in the United States, Australia, France and the United Kingdom. Fish intake closely reflects n-3 PUFA consumption, with intakes of approximately 3 to 4 g/day by Eskimos, 5 to 6 g/day by Japanese, 0.189 g/day by Australians and 0.25 g/day by Europeans and North Americans [26,27]. After it was reported that Japanese and Eskimo populations had healthier metabolic profiles associated with elevated plasma n-3 PUFA levels attributed to high fatty fish intake [28], many prospective studies began to examine whether fish or fish oil intake prevents the development of obesity.
The Health Professional Follow-up Study suggested that men with a high level of fish consumption were less likely to be overweight [29]. In contrast, the Nurses’ Health Study found that women with higher fish intake (two or more fish meals per week) had a higher risk of being overweight [30]. In China, data from the Shanghai Women’s and Men’s Health studies found similar indices of body mass among groups of varying fish intake [31]. Clearly, findings of prospective studies regarding the beneficial effects of fish intake on obesity are far from agreement. The evident discrepancies may have arisen due to differing or inadequate methods of data collection on fish intake (food frequency questionnaires), differences in cooking methods and other unaccounted for lifestyle practices (exercise, etc.) from study to study and among different study populations.
Plasma, erythrocyte and tissue n-3 PUFA concentrations are largely determined by consumption and thus may be taken to accurately reflect n-3 PUFA consumption. Plasma levels of fatty acids reflect recent intake, whereas tissue levels of fatty acids reflect long-term intake [32,33]. Erythrocyte fatty acid content (i.e., the omega-3 index) correlates with fatty acid intake and parallels tissue concentrations and thus is more reflective of long-term intake [34]. An increase in n-6: n-3 ratio [35] and overall lower serum phospholipid n-3 concentrations, particularly of DHA have been associated with obesity, specifically waist circumference measures [36] in obese adolescents [35] and obese adults [36]. Thus, prospective studies on the relationship between plasma and erythrocyte fatty acid content and the long-term risk of obesity are warranted to clarify this issue.
4. Omega-3 PUFA in animal studies
Animal studies performed to investigate the antiobesity effects of n-3 PUFA have used a variety of models, diet compositions, and n-3 PUFA compositions and doses. Such differences across experimental designs complicate the interpretation of their results into cohesive and conclusive findings (Table 1).
Table 1.
Effects of n-3 PUFA on body weight and body composition in male animals
| Rats/mice (animal number/days on diet) | Dietary fat (% kcal from fat) | Saturated fat (SFA) (%) | n-3 PUFA (% in diet)/replaced/added | n-6/n-3 ratio | Control diet | Body weight | Fat mass/site | Ref. |
|---|---|---|---|---|---|---|---|---|
| OLETF rats (8/175) | 5 | NA | EPA Added | NA | Safflower oil | NC | −/ABD | [40] |
| Wistar rats (30/28) | 20 | NA | 0.22–5.48 EPA 0.00–5.38 DHA NA |
NA | Lard and olive oil | NC | −/RP | [41] |
| C57BL/6J mice (8/49) | 20 |
1.86 |
6.5 n-3 PUFA 0.60 EPA 5.40 DHA Replaced 44% |
1.08 | Corn oil | − | −/EPI | [42] |
| 20 | 2.12 | 5.94 n-3 PUFA 4.26 EPA 0.74 DHA Replaced 15% |
1.04 | Corn oil | − | NC | ||
| 20 | 1.4 | 12.48 n-3 PUFA 0.72 EPA 1.60 DHA Replaced 15% |
0.21 | Flaxseed oil | NC | NC | ||
| 20 | 1.14 | 14.14 n-3 PUFA 2.12 EPA 4.72 DHA Replaced 44% |
0.14 | Flaxseed oil | − | −/EPI | ||
| 35.2 | 6.79 | 5.56–12.70 n-3 PUFA 0.40–1.16 EPA 3.60–10.28 DHA Replaced 15%–44% |
1.15 | Rapeseed oil, sunflower oil | − | −/EPI | ||
| C57BL/6J mice (12/35) | 20 | 1.14 | 14.14 n-3 PUFA 2.12 EPA 4.72 DHA Replaced 44% |
0.14 | Flaxseed oil | − | −/EPI | [43] |
| 35 | 6.79 | 5.56 n-3 PUFA 0.40 EPA 3.60 DHA Replaced 15% |
1.15 | Chow fed | − | −/EPI | ||
| Wistarrats (29/35) | 62 | 0.10 EPA Added | SFA | NC | −/RP | [37] | ||
| 21-week sucrose-induced obese | 7.5 | 2.7 | 2.7 n-3 PUFA | 0.02 | Corn-canola oil | NC | NC/EPI | [59] |
| Wistar rats (10/42) | 1.5 EPA 0.98 DHA NA |
|||||||
| fa/fa and lean Zucker rats (8/63) | 10 | 2.542–2.624 | .0089–3.55 ALA 0.80 EPA 0.161 DPA 0.915 DHA NA |
0.54–58.59 | Flaxseed oil Menhaden oil or Safflower oil |
NC | NC | [44] |
| C57BL/6JOlaHsd mice (30/182) | 40 | 9.6 | 21.9 n-3 NA |
7.5 | HF | NC | NC | [45] |
| Sprague-Dawley rats (48/21) | 14 | 5.24 | 3.42 n-3 0.21 EPA 1.08 DHA NA |
0.2 | Sucrose diet | NC | −/RP and EPI | [39] |
| Wistar rats (8/270) | 8 | 1.65 | 1.66 n-3 Replaced 7% |
2.30 | Cornstarch | NC | −/RP and EPI | [46] |
| C57Bl/6J mice (10/77) | 45 | 13.5 | 7.25 EPA (6.75) Added |
HF diet | − | − | [47] | |
| C57Bl/6J mice (14/70) | 45 | NA | 3.6 EPA (also says 36g/kg) Added |
NA | HF diet | − | −/SubQ PG MES |
[48] |
| Golden Syrian hamsters (12/140) | 45 | NA | 2.0 n-3 0.9 EPA 0.5 DHA Replaced 10% |
3.75 | HF lard diet | − | NC | [154] |
| Wistar rats (NA/16–20) | 50 | NA | FO Replaced 30% |
NA | HF lard diet | NC | −/SubQ RP EPI |
[49] |
| C57BL/6J Ob/ob (NA/112) | NA | NA | Cod liver oil 80 g EPA 80 g DHA NA |
NA | Primrose oil | − | NC | [50] |
| C57BL/6J mice (7/140) | 38.1 | NA | 5% EPA ethyl ester Replaced 5% |
NA | HF diet | NC | NC/WAT | [51] |
| 25 | NA | 5% EPA ethyl ester Replaced 5% |
NA | HF-HS diet | − | −/WAT | ||
| C57BL/6J mice (8/213) | 60 | NA | 5.3 n-3 PUFA 0.74 EPA 2.4 DHA Replaced 15% |
NA | cHF diet | NC | −/EPI | [52] |
| Wistar rats (16/28) | NA | NA | NA Replaced 6% |
NA | HF-HS diet | NC | −/EPI PER |
[53] |
| C57BL/6J mice (50/49) | 35 | NA | 0.42 n-3 PUFA 0.19 DHA 0.05 EPA Replaced 15% |
NA | HF diet Corn oil |
NC | −/BAT | [54] |
| Sprague–Dawley rats (28/21) | 20 | 9.4 | 20 n-3 PUFA 2 EPA 6.4 DHA |
NA | Safflower | NC | −/EPI PER |
[61] |
| C57BL/6J mice (8/49) | 35 | NA | 0.5 EPA+DHA Replaced 10% |
NA | Corn oil | − | −/EPI | [63] |
| Goto–Kakizaki rats (15/28) | NA | NA | 0.5 g/kg EPA Gavage |
NA | CMC | NC | NC | [55] |
| KKAy mice (36/84) | 10 | NA | 12 EPA 18 DHA Added 30 % |
NA | (1) Perilla or (2) Soybean oil & (3) Lard |
NC | −/EPI | [56] |
| Fisher rats (10/42) | 20 | NA | 18 n-3 PUFA | NA | Corn oil | NA | −/EPI | [64] |
| Wistar rats (28/16) | 50 | 9 | 5% n-3 PUFA 2.62 EPA & 1.62 Replaced 10% |
0.4 | Lard | NC | −/EPI | [62] |
| Wistar rats (30/24) | 40 | 23.7 | 18–6 or 40.6 n-3 PUFA 5 or 17.9 EPA 7.7 or 14.7 DHA |
0.29 or 0.12 | Olive oil + Beef tallow |
NC | −/EPI & RP | [57] |
+, increase; −, decrease; A, addition; ABD, abdominal; CMC, carboxymethylcellulose; EPI, epididymal; FO, fish oil; HF-HS, high fat and high sugar; MES, mesenteric; NA, information not available; NC, no change; OTLEF, Otsuka Long–Evans Tokushima fatty; PG, perigonadal; PER; perirenal; RP, retroperitoneal adipose tissue; R, reversal; SubQ, subcutaneous.
4.1. Omega-3 PUFA, energy intake and obesity
Studies relating effects on body weight and energy intake with n-3 PUFA supplementation are inconsistent; while some show either decreased [37,38] or increased energy intake [39], most show unchanged energy intake with the addition of n-3 PUFA or with varying n-3 PUFA doses [40–57]. Only one study performed with female mice reported decreased energy intake with no significant effect on body weight [38].
Supplementation with n-3 PUFA prevented high-fat (HF)-diet-induced weight gain in a number of rodent studies, most of which supplemented an HF diet provided from the start, concurrent with inducing obesity [41,43,45,48,49,51]. Several studies have utilized a design that investigated the effectiveness of n-3 PUFA to reverse diet-induced weight gain and related metabolic changes by adding n-3 PUFA to the HF diet at approximately midstudy [46,47,58,59]. Our lab found that mice on an EPA reversal diet (6 weeks of HF followed by 5 weeks of HF-EPA) had body weights similar to mice fed the HF-only diet [47]. In a different study, body weight decreased significantly at the beginning of the 6-week reversal period, returning to weights similar to the low-fat-fed group for the remainder of the study (18 weeks total) [58]. Taken together, these studies suggest that antiobesity effects of N-3 PUFA in mice are predominantly seen when it is fed from the start rather than introduced after obesity is already established.
4.2. Omega-3 PUFA and insulin resistance
Obesity leads to insulin resistance, which is at least in part responsible for the pathogenesis of MetS [13]. Most weight loss interventions improve insulin resistance. Similarly, most animal studies document a beneficial effect of n-3 PUFAs on insulin sensitivity [60]. Since n-3 PUFAs induce weight loss in rodent models of obesity, it is difficult to state whether there are direct effects of n-3 PUFAs on insulin sensitivity. By contrast, we have shown weight-independent benefits of EPA on insulin sensitivity in HF-diet-induced obese C57BL/6J mice. These EPA-fed mice had significantly improved homeostatic model assessment of insulin resistance (HOMA-IR) scores when compared to HF-fed mice, despite similar body weights [47].
4.3. Animal study conclusions
Thus far, most rodent studies have shown an antiobesity effect of n-3 PUFA, while fewer studies have found no change in body weight [37,39–41,44–46,49,51–57,59,61,62]. These studies do suggest that n-3 PUFA plays a role in reducing adipose tissue mass [40], particularly in the epididymal [39,42,43,46,49,52,53,56,57,61–64] and retroperitoneal locations [37,39,41,46,49,57]. Differences in the outcomes of studies on the effects of n-3 PUFA on body weight could be due to differing animal models of obesity (genetic vs. diet-induced obesity), the content of the diet (HF vs. high sucrose), the n-3 PUFA (EPA or DHA) formulation, the form of n-3 PUFA (TG form or as ethyl ester) provided or various combinations of these factors. Differences in n-3 PUFA dosage and duration may contribute to differences in outcomes as well. Failure to assess energy expenditure also limits meaningful comparisons. The combination of calorie restriction and n-3 PUFA supplementation may be the most effective strategy for reducing weight and improving body composition [54]. Interestingly, Ruzickova et al. extrapolated findings from their animal study to humans to suggest that with a daily intake of 100 g dietary fat, 11 g of EPA/DHA would be required to limit weight gain [42]. Few animal studies have considered translation to human studies since the amounts of n-3 PUFA, as EPA, DHA or both, in animal studies far exceed amounts feasible in humans [42,45,64]. It should be noted, however, that human studies of fish oil intake among Eskimo and Japanese populations have shown beneficial effects of these fatty acids even at intake levels below 11 g/day. Since these populations consume more fish and less red meat, it is plausible that a relatively lower arachidonic acid (AA) intake leading to a decreased n-6:n-3 ratio is contributing to the beneficial effects observed.
5. Omega-3 PUFA and weight loss in humans
There are a variety of approaches to investigating the effects of n-3 PUFA on body weight, body composition and energy intake in human interventions that use different types of fish and varying levels of fish oil content, particularly EPA and DHA (Table 2). Fish and fish oil have also been used in addition to a variety of weight loss and dietary interventions of different durations with or without an exercise regimen. Participants have ranged from healthy to obese, with a variety of obesity-associated disorders, including T2DM, hyperinsulinemia and other features of MetS. The control, or type of placebo, which consists of assorted oils containing n-6 PUFA, such as sunflower, corn, soybean and paraffin oils, also varies among studies.
Table 2.
Effects of n-3 PUFA on body weight, body composition and energy intake in humans
| N (M/F) | Participants Age (years) |
n-3 PUFA (% in diet) | Other diet | Duration | Body weight | Fat mass | Fat site | Lean mass | Energy intake | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| 15 (10/5) | Healthy adults 69–73 years |
4 g Lovaza/day 1.86 g EPA 1.50 g DHA |
Corn oil NA |
8 weeks | NC | NC | NA | NC | [186] | |
| 44(15/29) | Healthy adults 62–76 years |
4 g n-3 1.86 g EPA 1.5 g DHA |
Corn oil | 6 months | NC | NC | NA | + | NA | [210] |
| 17 (8/9) | Nonobese, T2DM Obese, T2DM 41–66 years |
PUFA diet NA |
SF diet | 10 weeks 5 week crossover design |
NC | − | Abdominal SubQ | NA | NC | [71] |
| 33 (20/13) | Overweight, impaired glucose 40–61 years |
3.9 g EPA + DHA | Maize oil | 9 months | NC | NC | NA | NC | NA | [187] |
| 53 (17/36) | Decreased muscle mass 60+ years |
1.3 g n-3 660 mg EPA 440 mg DHA |
Vitamin E | 12 weeks | NC | NC | NA | NC | NA | [211] |
| 76 | Overweight, IR 9–18 years |
540 mg EPA 360 mg DHA |
Corn starch | 1 month | NC | NC | NA | NA | NA | [126] |
| 126 F | Healthy, postmenopausal 68–82 years |
1.2 g EPA + DHA | Olive oil | 6 months | NC | NA | NA | NA | NC | [70] |
| 44 (14/30) | Healthy 27–41 years |
1.6 g EPA 800 mg DHA |
Safflower oil | 6 weeks | NC | − | NA | + | NA | [66] |
| 12 F | Postmenopausal, T2DM, without hypertriglyceridemia 54–56 years |
1.8 g n-3 1.08 EPA 0.72 DHA |
Placebo Paraffin oil |
2 months | NC | − | SubQ | NA | NC | [69] |
| 47 M | Overweight 35–55 years |
230 mg EPA and 154 mg DHA | Corn oil | 8 weeks | NC | NA | NA | NA | NC | [68] |
| 66 (45/21) | T2DM 40–70 years |
2.8 g EPA + DHA | Placebo | 24 weeks | NC | NA | NA | NA | NA | [208] |
| 24 M | Viscerally Obese | 1.8 g EPA + 1.56g EPA | Placebo | 6 weeks | NC | NA | NA | NA | NA | [212] |
| 27F | Overweight/obese 23–60 years |
2.8 g DHA | Oleic acid | 12 weeks | NC | NC | NA | NC | − | [67] |
F, female; IR, insulin resistant; M, male.
With n-3 PUFA supplementation alone, studies report no change in body weight (Table 2). One study in healthy adults supplemented with fish oil diets demonstrated decreased body fat mass, basal respiratory quotient and increased basal lipid oxidation when dietary intake was controlled [65]. Another study found reduction in fat mass along with significantly increased lean mass (fat-free mass) despite no alterations in total body mass, resting metabolic rate (RMR) or respiratory exchange ratio when compared to placebo supplementation [66]. Participant-reported diet diaries indicate significant reductions in carbohydrate, fat and total caloric intake with n-3 PUFA supplementation in one study [67], but others show no change in energy intake [68–71]. Since most studies only report total caloric intake, the effect of n-3 PUFA supplementation on macronutrient and energy intake should be repeated in larger studies to conclusively determine the role of n-3 PUFA in weight loss in humans.
5.1. Omega-3 PUFA in combination with dietary interventions
Weight loss results appear more promising when n-3 PUFA supplementation is combined with calorie restriction (Table 3), but it is difficult to draw conclusions due to the variety of calorie restriction programs in different studies. Greater improvements in metabolic parameters, such as improved insulin resistance and decreased TGs, were attained with combined n-3 PUFA supplementation and calorie restriction compared to calorie restriction alone [72–74] or replacement of SFA [71]. Interestingly, results appear to be independent of the source, form or dose, i.e., different fish species (salmon, tuna, sardines, etc.) or fish oil capsules, of n-3 PUFA supplied [72,73,75]. This was confirmed by Thorsdottir et al., who compared the effects of various fish (cod or salmon) and fish oil (DHA/EPA capsules) in conjunction with 30% calorie restriction on weight loss in young, overweight adults for 8 weeks. After 4 weeks, men receiving cod, salmon or fish oil capsules lost approximately 1 kg more than those on 30% calorie restriction alone. The fish species and fish oil capsules supplied various amounts of n-3 PUFA: 0.3 g/day from cod, 3.0 g/day from salmon and 1.5 g/day from fish oil capsules, yet the n-3 PUFA dose did not influence weight loss outcomes. This suggests that variations in weight loss benefits may not depend solely on variations in n-3 PUFA dosages from study to study [76].
Table 3.
Effects of n-3 PUFA in addition to dietary intervention on body weight, body composition and energy intake in humans
| N (M/F) | Participants | Diet intervention | Dietary fat (%) | n-3 PUFA (% in diet) | Control (placebo) | Duration | Body weight | Fat mass | Lean mass | Energy intake | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| P18 (6/12) 15 (5/10) | Obese 28–53 years |
HEWLD | NA | 6 g n-3 0.42 g EPA 1.62 g DHA |
MUFA | 12 weeks | NC | NC | NA | NC | [213] |
| 24 (6/18) | Obese 25–65 years |
LCD | 25 | 1.8 g n-3 DHA:EPA5:1 |
Placebo Corn oil |
12 weeks | NC | NC | NA | NA | [73] |
| 35 F | Overweight, IR 21–69 years |
Low fat/high carb diet | 35 | 1.3 g EPA 2.9 g DHA |
2.8 g LA 1.4 g OA |
24 weeks | NC | NC | NC | NA | [120] |
| 6(5/1) | Healthy 21–25 years |
Controlled diet | 32 | Replaced 6g: 6 g FO 1.1 g EPA 0.7 g DHA |
None | 3 weeks | NC | − | NC | NC | [65] |
| 14 (8/6) | Overweight, hypertensive 51–55 years |
CR + daily fish meal | NA | 3.65 g n-3 | CR only | 16 weeks | NC | NC | NA | NC | [75] |
| 35 M | Overweight 20–40 years |
30% CR + cod | NA | 0.3 g n-3 | CR only | 8 weeks | − | NA | NA | NC | [76] |
| 42 M | Overweight 20–40 years |
30% CR + salmon | NA | 3.0 g n-3 | CR only | 8 weeks | − | NA | NA | NC | |
| 29M | Overweight 20–40 years |
30% CR + FO | NA | 1.5 g n-3 | CR only | 8 weeks | − | NA | NA | NC | |
| 34 M | Overweight 25–65 years |
Ketogenic Mediterranean |
45.8 ± 4 | Krill oil 57.5 mg EPA 32.5 mg DHA |
Ketogenic Mediterranean diet only |
4 weeks | NC | NC | NC | NA | [74] |
| 45 | MetS 37–63 years |
CR+ FO | 27.7 | 2.13 G N-3 PUFA | CR | 12 weeks | − | NC | NC | NC | [72] |
| 278 | Overweight 20–40 years |
30% CR | Lean fish or fatty fish | 0.26 g in lean fish & 2.1 g in fatty fish | CR + placebo | 8 weeks | NC | NC | NC | NC | [214] |
| 30 % CR | Fish oil | 1.3 g EPA and DHA | Placebo | NC | NC | NC | NC | ||||
| 51 F | Healthy 20–50 years |
30% CR | 30 | 1.3 g EPA | Sunflower flower CR only |
10 weeks | NC | NC | NC | NC | [118] |
CR, calorie restriction; HEWLD, healthy eating weight loss diet; LCD, low-calorie diet; MetH, metabolically healthy; OA, oleic acid.
Rapid weight loss, induced by a very low calorie diet, alters adipose tissue and serum fatty acid composition [77,78]. Supplementation with n-3 PUFA during rapid weight loss increases serum n-3 PUFA concentrations [79] and may help prevent unfavorable changes in fatty acid tissue composition and essential fatty acid deficiency [77]. Some studies have utilized n-3 PUFA supplementation prior to a weight loss intervention, such as dietary restriction and/or an exercise regimen, and reported significant reductions in weight [80], while others observed no changes in body mass index (BMI) or body composition, particularly in insulin-resistant individuals [81]. Nonetheless, this type of study design should be refined and pursued further due to the relationship between tissue/plasma/erythrocyte n-3 PUFA concentrations and obesity. It will be important to verify if increasing n-3 PUFA concentrations prior to interventions would aid in weight loss and ameliorate obesity related metabolic dysfunctions.
5.2. Omega-3 PUFA and exercise
Others have explored the influence of n-3 PUFA in conjunction with exercise and with or without a dietary intervention to determine if the addition of n-3 PUFA leads to greater weight loss (Table 4). With the addition of n-3 PUFA to an exercise regimen and dietary intervention, only one study has shown a decrease in body weight [82]. However, only a few such studies have been conducted, and the dietary intervention consisted of nutritional counseling rather than a prescribed diet [83,84].
Table 4.
Effects of n-3 PUFA in addition to diet and/or exercise on body weight, body composition and energy intake in humans
| N (M/F) | Participants Age (years) |
n-3 PUFA (% in diet) | Control/diet intervention | Duration | Exercise | Body weight | Fat mass | Lean mass | Energy intake | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| 32 M | Healthy 19–34 years |
4g n-3 PUFA | No supplementation | 10 weeks | Aerobic 70%–85% HR max 1 h/3×/wk |
NC | NC | NA | NC | [86] |
| 4 g n-3 PUFA | No supplementation | 10 weeks | Maintain | NC | NC | NA | NC | |||
| 45 F | Healthy 62–66 years |
2 g n-3 0.4 g EPA 0.3 g DHA |
No supplementation | 12 weeks | Strength training 3×/wk |
NC | NA | NA | NC | [215] |
| NA | 12 weeks, 60 initial supplementation | Strength training 3×/wk |
NC | NA | NA | NC | ||||
| 50 F | Healthy 65–70 years |
Healthy diet | 24 weeks | Resistance Training 2×/wk |
NC | NA | + | NC | [88] | |
| 128 (40/88) | Overweight/obese, healthy 40–55 years |
3 g EPA + DHA 5:1 (EPA:DHA) |
Soybean and corn oil/Nutrition counseling | 24 weeks | 150 min/week at 50%–85% of VO2 max | NC | NC | NA | NC | [83] |
| 39 (6/33) | MetS 36–64 years |
3 g FO 540 mg EPA 360 mg DHA |
No supplementation/Nutrition counseling | 20 weeks | 80 min 3×/wk walking 60 min resistance training 2×/wk |
NC | NC | NA | NC | [84] |
| 29 M | Obese, IR 32–65 years |
1000 mg EPA 700 mg DHA |
Glucose/starch | 16 weeks, initial 4 week supplementation | 3–5 walking sessions/wk at 50%–65% Hrmax | NC | NA | NA | NA | [81] |
| 16 (6/11) | Overweight, hyperlipidemia, hypertensive 25–65 years |
1.9 g n-3 PUFA 360 mg EPA 1.56 g DHA |
Sunflower oil | 12 weeks | Run/ walk 3×/wk for 45 min at 75% of APHRmax | NC | − | NC | NC | [87] |
| 17 (5/11) | Overweight, hyperlipidemic, hypertensive 25–65 years | 1.9 g n-3 PUFA 360 mg EPA 1.56 g DHA |
Sunflower oil | 12 weeks | none | NC | − | NC | NC | |
| 7 (5/2) | Hyperlipidemic 27–63 years |
50 ml FO 17% EPA 12% DHA |
Corn oil | 12 weeks | Walk/jog 3×/wk for 45–50 min at 70%–85% max HR | NC | − | NA | NC | [85] |
| 7 (4/3) | Hyperlipidemic 27–63 years |
50 ml FO 17 % EPA 12% DHA |
Corn oil | None | NC | NC | NA | NC | ||
| 20 F | Severely obese 37–60 years |
2.8 g n-3 2:1 EPA: DHA |
Placebo/ VLCD | 3 weeks, inpatient | 60 min/day light to moderate |
− | NA | NA | NA | [82] |
| 50 (27/23) | Healthy 65–77 years |
3 g FO | Placebo | 18 weeks | Lower-limb resistance training 2×/wk | NC | NA | NC | NA | [89] |
APHRmax, age-predicted heart rate maximum calculated by [208−(0.7×age)]; HR, heart rate; VLCD, very-low calorie diet.
The combined effects of n-3 PUFA and exercise are currently unknown. Well-designed placebo-controlled randomized clinical trials are lacking [85], as they require healthy and lean participants [86]. Differences in the intensity and forms of exercise (i.e., aerobic or resistance training) employed prevent valid comparisons across studies. The addition of n-3 PUFA to aerobic training without dietary intervention has resulted in decreases in fat mass [87]. Furthermore, the addition of n-3 PUFA to resistance training without dietary intervention resulted in increases in lean mass [88] and improved muscle quality [89].
5.3. Limitations in human studies
Overall, findings on the effects of n-3 PUFA in humans are inconclusive. Improvements in study design and analyses could help resolve apparent inconsistencies in the effects of n-3 PUFA on weight and body composition. For example, sex, metabolic phenotype and geographic location should be taken into consideration in addition to n-3 PUFA supplementation. There is also a case for evaluating translation to real-world weight-loss diets, which are complicated by the need to control for eating behavior and physical activity. Even when participants are supplied with food, outpatient studies are difficult to translate because measurements of adherence to the recommended interventions [90] generally rely on self-reporting. Hence, studies using inpatient feeding and analysis of energy utilization should be carried out [91].
Failure to assess energy expenditure in human studies limits our understanding of the associations between n-3 PUFA status and decreased adiposity, weight loss and energy balance, especially when energy intake is unchanged. This highlights the need for tightly controlled studies, similar to that of Hall et al., to validate fuel partitioning and n-3 PUFA influence on metabolism in humans. Unfortunately, work of this nature is expensive, labor intensive and generally of short duration with small sample sizes [91].
Other limitations on current human studies of n-3 PUFA supplementation include the use of less reliable anthropometric methods [92] and failure to dose according to body weight to meet the threshold of tissue membrane n-3 PUFA phospholipid enrichment [93]. Finally, it is of utmost importance to utilize a standardized method, such as the omega-3 index, to assess n-3 PUFA status, the biological effects of n-3 PUFA and n-3 PUFA related metabolites [34]. Future human studies should employ this method to verify that n-3 PUFA consumption parallels n-3 PUFA concentrations in the body.
6. Mechanisms by which n-3 PUFA improve adiposity and metabolic disorders
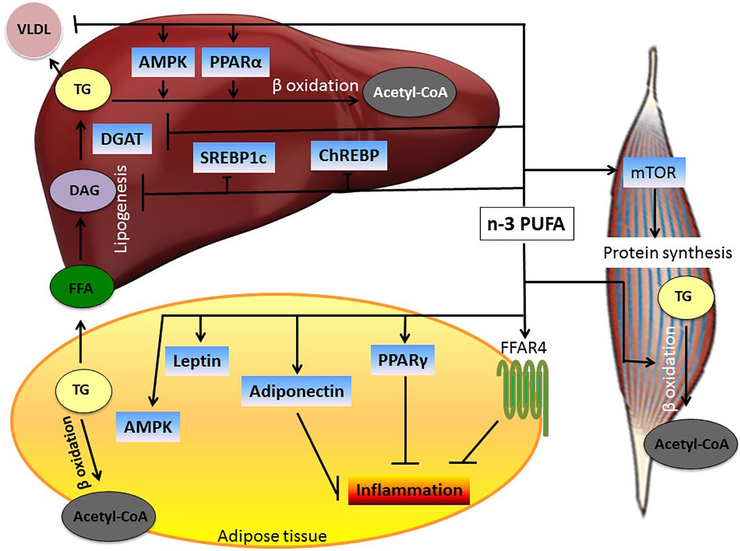
There are several proposed mechanisms by which n-3 PUFA could work in reducing body weight and improving the metabolic profile (Fig. 3). These include alterations in adipose tissue gene expression; changes in adipokine release; adipokine-mediated or adipokine-related pathways; appetite suppression; alterations in carbohydrate metabolism; increases in fat oxidation; increases in energy expenditure (possibly through thermogenesis); activating mechanisms involved in muscle anabolism; and, lastly, influence on epigenetics.
Fig. 3.
Mechanisms mediating effects of n-3 PUFA on liver, adipose tissue and skeletal muscle metabolism. Omega-3 PUFAs increase fatty acid oxidation in the liver, adipose tissue and skeletal muscle, thus limiting fat storage in these tissues. Omega-3 PUFAs also decrease the production and release of proinflammatory adipokines. In skeletal muscle, n-3 PUFAs promote protein synthesis. All mechanisms depicted here contribute to an improved metabolic profile.
6.1. Omega-3 PUFA and adipogenesis
Adipose tissue expansion in obesity occurs via adipocyte hypertrophy (enlargement of adipocytes) and hyperplasia (increase in adipocyte number due to adipogenesis). The latter is associated with smaller adipocyte size and a metabolically healthy phenotype. Both n-3 and n-6 PUFAs can bind and/or regulate transcriptional factors that control genes involved in preadipocyte differentiation. PUFAs, particularly AA and its metabolites, serve as ligands for peroxisome proliferator-activated receptors (PPAR) gamma (PPARγ) and delta (PPARδ) to induce fat cell differentiation and accelerate maturation by elevating lipoprotein lipase expression in vitro [94,95]. Elevated concentrations of n-6 and n-3 PUFA in human subcutaneous tissue correlate with reduced adipocyte size; increased SFA concentrations lead to increased fat cell size [96]. Differences in fatty acid concentrations are more strongly associated with abdominal subcutaneous than visceral adipose tissue [35].
Studies performed in clonal adipocytes (3T3-L1) also demonstrate up-regulation in PPARγ expression, adipogenesis and lipid droplet formation after the addition of n-3 PUFA [43,97]. Taken together, these studies suggest that n-3 PUFAs promote adipogenesis and a healthy expansion of adipose tissue during positive energy balance, promoting a metabolically healthy phenotype.
6.2. Adipose tissue inflammation
Chronic low-grade inflammation and changes in adipokine patterns are key factors in the pathogenesis of metabolic derangements in obesity (Fig. 1). Indeed, a relationship exists between BMI, body fat percentage and inflammatory markers [98]. Omega-3 PUFAs inhibit nuclear transcription factor kappa B, a key transcription factor in cytokine gene expression and inflammation [99]. In humans and in vitro, n-3 PUFAs also have a documented role in reducing cytokines, including 1L-1 [100,101], 1L-6 [102] and TNF-α [100,103], which are all elevated in obesity. (For an extensive review of mechanisms of n-3 PUFA and adipose tissue inflammation, see Kalupahana et al., 2011).
Omega-3 PUFAs act as agonists for different members of the free fatty acid receptor family (FFARs) present on a variety of cell types involved in both energy homeostasis and the inflammatory response. A number of saturated and unsaturated long-chain fatty acids can activate FFAR1 and FFAR4 [104,105]. Agonist stimulation that impedes the inflammatory response occurs through activation of the G-protein-independent signaling pathway through interaction with β-arrestin proteins, which may further interact with the transforming growth factor kinase protein (TAK1) and binding protein (TAB-1). Stimulation of FFAR4 or β-arrestin inhibits lipopolysaccharide (LPS)-mediated release of inflammatory cytokines, including TNF-α and 1L-6 in the macrophage-like cell line RAW264.7. In fact, decreased macrophage infiltration into adipose tissue has been shown in mice fed an n-3-PUFA-enriched diet, possibly via activation of FFAR4 (G-protein-coupled receptor 120). Since n-3 PUFAs are unable to reduce adipose tissue macrophage infiltration in FFAR4 knockout mice, this highlights the mechanistic importance of FFAR4 in mediating the anti-inflammatory effects of n-3 PUFA [106]. Furthermore, fish oil supplementation (4 g n-3 PUFA/day) in obese humans has been associated with decreased M1 macrophage presence in adipose tissue and subsequent decreases in proinflammatory markers, such as IL-8 [107]. Accordingly, monocytes differentiate preferentially into M1 macrophages when treated with human postprandial triglyceride-rich lipoproteins following a meal rich in saturated fatty acids, versus a meal high in MUFA or PUFA, after which they shift towards M2 macrophages [108].
Furthermore, n-3 PUFAs halt inflammatory processes by inhibiting activation of the NLRP3 (nucleotide-binding oligomerization domain-like receptor; NLR family, pyrin domain containing 3) inflammasome via an arrestin-FFAR4-dependent pathway [109], which triggers a caspase-dependent cascade, resulting in the release of proinflammatory cytokines [110]. The n-3 PUFA DHA acts through FFAR1 or FFAR4 to suppress caspase-1 activity via formation of a β-arrestin-2/NLRP3 or NLRP1b complex and thus decrease the release of proinflammatory cytokines [109].
Omega-3 PUFAs also influence lipid rafts, which are cholesterol- and sphingolipid-rich areas of the plasma membrane [111] that can form signaling platforms [112,113]. Incorporation of n-3 PUFAs into plasma membranes disrupts lipid rafts [114] and hence could mediate anti-inflammatory and antichemotactic n-3 PUFA properties.
6.3. Adipokine secretion
Several studies have shown that n-3 PUFAs modulate adipokine secretion. Obese individuals have high plasma leptin levels [115] suggestive of leptin resistance. Conversely, weight loss leads to parallel decreases in plasma leptin levels [116]. This weight-loss-associated decrease in leptin could contribute to hunger and a lower metabolic rate and ultimately lead to weight regain [117]. EPA supplementation attenuates the decrease in blood leptin levels that occurs during weight loss in obese women, suggesting a potentially significant role of EPA in weight loss maintenance [118]. Indeed, EPA increases the production of leptin in rodents and cultured adipocytes [37,119], suggesting a direct effect of n-3 PUFA on leptin production. However, the few studies that have assessed the role of n-3 PUFA in weight maintenance have found no significant effect on weight or blood leptin concentrations between n-3-PUFA-supplemented subjects compared to other weight loss groups [120,121]. Omega-3-PUFA-mediated effects on leptin are dependent on a number of factors, such as diet composition and energy balance, which could cause conflicting results.
Independent of body weight, both animal [37,48,122] and human [123,124] studies have found significant increases in blood levels of the insulin-sensitizing adipokine, adiponectin, following n-3 PUFA consumption. EPA appears to regulate adiponectin levels at the translational or posttranslational level rather than at the transcriptional level [123]. It has been proposed that the anti-inflammatory properties of n-3 PUFA supplementation induce an increase in adipocyte adiponectin production [123] and improve leptin sensitivity [125]. This type of interplay could have a significant influence on body weight regulation. An inverse relationship between serum adiponectin concentrations and TNF-α has also been demonstrated in ob/ob mice [123] and in overweight and insulin-resistant children following n-3 PUFA supplementation [126].
Fatty acid-binding proteins are cytosolic proteins that bind long-chain fatty acids and promote transport to several organelles. Fatty acid-binding protein 4 (FABP4; adipocyte FABP, A-FABP; or aP2) is secreted from both macrophages and adipocytes and functions as an adipokine [127]. An elevated FABP4 serum concentration is associated with obesity, insulin resistance and hypertension [128]. Adipocytes are the predominant contributors of circulating FABP4. During lipolysis, FABP4 functions in a nonclassical secretion pathway [129]. Omega-3 PUFA dose-dependently reduced FABP4 secretion in 3T3-L1 adipocytes and reduced serum FABP4 concentrations in humans [130]. Omega-3-PUFA-mediated reductions in FABP4 could also be due in part to reduced expression of transcription factors involved in adipocyte differentiation, including PPARγ2 and C/EBPα [130]. Another possible mechanism by which FABP4 levels are lowered by n-3 PUFA is through the β-adrenergic receptor [129] since n-3 PUFAs reduce sympathetic nerve activity and thus may lower FABP4 serum level [130]. Taken together, n-3 PUFAs modulate adipokine secretion by exerting anti-inflammatory effects and promoting a metabolically healthy phenotype.
6.4. Appetite suppression
In addition to leptin, central and peripheral peptides and hormones involved in food intake and energy expenditure signaling pathways are targets for n-3-PUFA-derived endocannabinoids and thus may be implicated in the prevention and treatment of obesity. A subanalysis of the study conducted by Thorsdottir et al. reported elevated sensations of fullness in the participants who consumed higher n-3 PUFA content meals (fatty fish and fish oil) compared to those who consumed lower n-3 PUFA content meals (control and lean fish) both immediately and 2 h after consuming the meal. Feelings of hunger were consistently lower in participants who ate the meal higher in n-3 PUFA content [131]. Therefore, it is possible that increased feelings of satiety following a meal high in n-3 PUFA content could aid weight loss by reducing subsequent food intake. Appetite suppression could also be mediated through FFAR4 (GPR 120). Omega-3 PUFAs are agonists for FFAR4 [132], which elicits the secretion of cholecystokinin, a peptide hormone that is synthesized and released from the small intestine and has roles in hunger suppression [133].
6.5. Insulin resistance
Adipose tissue inflammation is at least in part responsible for obesity-associated insulin resistance. Since n-3 PUFAs alleviate adipose tissue inflammation as outlined above, reducing adipose tissue inflammation is a possible mechanism for n-3-PUFA-associated improvements in insulin sensitivity observed in animal models.
Hepatic insulin resistance in which both glucose production and lipogenesis are increased is characteristic of the metabolic dysregulation seen in obesity and T2DM. This dysregulation is attributed to reductions in proximal insulin signaling kinases, such as P13K and AKT, which hinder gluconeogenesis, as well as activation of mTORC1 and p70S6K, which control lipogenesis [134,135].
Fibroblast growth factor (FGF) 21, which is produced by the liver, adipose tissue and skeletal muscle, has been shown to reduce both hepatic glucose production and plasma glucose levels, while it also increases insulin sensitivity and adipocyte glucose uptake [136]. Circulating FGF21 levels are elevated in diet-induced obese mice [137] and obese and type 2 diabetic humans [138], suggesting obesity-related FGF21 resistance. Omega-3 PUFAs attenuate HF-diet-induced increases in FGF21 [139] with associated reductions in hyperglycemia, hypertriglyceridemia and plasma insulin levels [140,141]. This could be a potential mechanism by which n-3 PUFAs improve insulin resistance.
Omega-3 PUFA supplementation prevents insulin resistance in muscle of rats fed an HF diet [142], partly by improving glycogen synthesis [143]. Omega-3 PUFAs also decrease fat content in muscle and maintain normal PI3K activity and expression and transcription of GLUT 4 receptors in muscle and thus improve myotubule glucose uptake. Omega-3 PUFAs also promote inhibition of hepatic glucose production [142].
Hence, n-3 PUFAs may be a valuable nutritional tool for preventing or diminishing muscular and hepatic insulin resistance associated with obesity. However, n-3 PUFAs appear ineffective once T2DM is established [144].
6.6. Lipid metabolism
In both animal and human studies of n-3 PUFA supplementation, reductions in weight or fat mass were not accompanied by changes in energy intake (Tables 1–4). Omega-3 PUFAs can partition dietary fuel away from storage and toward oxidation by suppressing lipogenic genes and activating genes that encode for mitochondrial and peroxisomal fatty acid oxidation in both the liver and muscle.
Given their cardioprotective properties, n-3 PUFAs can improve endothelial function in patients with varying metabolic profiles [145], possibly through increased production of nitric oxide [146]. Furthermore, during exercise, fish oil has been shown to increase arterial dilation and blood flow to skeletal muscle [147]. Hence, improved blood flow may increase the delivery of fats to be utilized as energy in skeletal muscle, especially during exercise.
Regulation of lipid metabolism may vary by n-3 PUFA type, as well as by fat depot. For example, EPA is preferentially directed towards β-oxidation, while DHA and DPA are spared from catabolism and deposited in tissues [148]. Moreover, gene expression of fatty acid synthase [149], hormone-sensitive lipase, lipoprotein lipase and phosphoenolpyruvate carboxykinase in retroperitoneal fat is decreased with DHA and mixed EPA/DHA supplementation but not with EPA supplementation alone [41].
Portions of hepatic TG are secreted via very low density lipoprotein (VLDL), which delivers TG to peripheral tissues, such as WAT. Hepatic VLDL secretion is enhanced in obese individuals [150] possibly due to increased fatty acid delivery, elevated glucose and insulin concentrations, as well as impaired fat oxidation, which increases fatty acid esterification into TG [151]. Omega-3 PUFAs reduce lipogenesis and reduce hepatic VLDL secretion [51]. In vitro, n-3-PUFA-treated HepG2 cells have decreased hepatic VLDL secretion [152] and reduced apolipoprotein B100 production [153]. This has been validated in both DHA- and n-3-PUFA-supplemented animals [154]. Hence, through inhibition of VLDL formation, n-3 PUFAs could limit the supply of fatty acids to adipocytes and thereby limit adipocyte size and mass. In a deregulated system, n-3 PUFAs would also limit the amount of fatty acids delivered to muscle and liver. Additionally, in animal models, n-3 PUFAs modulate cholesteryl ester transfer protein mediated exchanges, resulting in increased blood HDL cholesterol and possibly apolipoprotein A-1 concentrations [155,156].
Omega-3 PUFAs alter expression and nuclear localization of both the transcription factor sterol-regulatory element-binding protein-1 (SREBP-1) and the carbohydrate response element binding protein (ChREBP), which control several lipogeneic genes, including those regulating cholesterol and fatty acid synthesis [154,157]. Nuclear translocation of ChREBP is inhibited by n-3 PUFAs and thus results in reduced expression of lipogenic and glycolytic genes, including FAS and pyruvate kinase respectively [158]. Furthermore, n-3 PUFAs suppress hepatic lipogenesis by reducing both messenger RNA (mRNA) and active protein expression of SREBP-1c, which results in reduced expression of many genes involved in lipogenesis, including FAS and acetyl-coA carboxylase [159–161]. Reduced SREBP-1c expression, via n-3 PUFA, has been attributed to inhibited transcription of nascent precursor SREBP-1c, accelerated transcript decay and reduced levels of the mature cleaved form of SREBP-1c [159,160], possibly through inhibition of proteolytic processing and reduced feed-forward activation of the Srebf1 gene. This inhibition could be due to interference with insulin signaling pathways, which promotes the proteolytic processing of SREBP-1c, potentially via an AKT-dependent mechanism [162,163].
The role of liver X receptor (LXR) is controversial. In vivo, EPA suppression of SREBP-1c promoter activity is dependent upon an intact SRE but not LXR response elements, suggesting that decreased transcription of nascent SREBP-1c with n-3 PUFA treatment results from decreased availability and thus reduced feed-forward activation [163]. In contrast, others indicate a role in the inhibition of LXRα in reduced SREBP-1c expression with n-3 PUFA, but this may be dependent upon cell types [164]. Accelerated degradation of SREBP-1c mRNA has also been proposed as a mechanism for reduced SREBP-1c expression [165]. Omega-3 PUFAs inhibit SREBP-1c cleavage processing, but the cleavage sites are unknown [166].
Activation of AMP-activated protein kinase by n-3 PUFA can also suppress SREBP-1c cleavage and nuclear translocation, perhaps via serine phosphorylation and/or by blocking activation of the insulin-responsive mechanistic target of rapamycin complex 1 (mTORC1)/S6K-signaling pathway [167]. SREBP-1c synthesis, transport and maturation are increased with insulin [162].
PUFAs, prostaglandins and leukotrienes can all act as ligands for PPARs. PPARs are transcription factors that form heterodimers with retinoid X receptors in the promoter regions of several genes involved in lipid and glucose metabolism [168,169]. For example, n-3 PUFA activation of PPARα decreases lipogenesis by suppressing FAS activity [161,170]. However, lipogenesis suppression by n-3 PUFA does not require PPARα activation [171].
PPARγ acts as a master regulator of adipogenesis and controls several genes and adipokines in lipid and glucose metabolism. Omega-3 PUFAs act as ligands for PPARγ and modulate several PPARγ target genes in mice [172] and 3T3-L1 adipocytes [97]. Omega-3 PUFAs enhance PPARγ binding to PPAR-response element in the promoter region of vascular endothelial growth factor-A, which promotes adipogenesis and alleviates hypoxia-induced adipocyte inflammation and insulin resistance [173]. It has been suggested that PPARγ plays a significant role in the ability of n-3 PUFA, specifically DHA, to stimulate M2 macrophage polarization and thereby reduce inflammation since these results are not seen in PPARγ knockdown RAW264.7 cells [174].
Omega-3 PUFAs have been shown to increase mitochondrial biogenesis and fatty acid oxidation in the liver [175,176], adipose tissue [43] and small intestine [177] of rodents, possibly through PPARα and Cox3 induction [175,178,179]. PUFA-controlled genes involved in lipid oxidation and thermogenesis include mitochondrial HMG-CoA synthase [180], peroxisomal acyl-CoA oxidase [64,181], hepatic CPT-1 [154], FABP [127] and fatty acid transporter proteins [182].
Activation of PPARα can also increase fatty acid oxidation. Increases in fatty acid oxidation by n-3 PUFA may also be mediated by AMPK, a known regulator of cellular energy metabolism. AMPK up-regulation by n-3 PUFA has been demonstrated in both adipose tissue and cultured adipocytes [55].
Taken together, n-3 PUFAs regulate lipid metabolism, favoring fatty acid oxidation and suppression of lipogenesis and leading to a favorable lipid profile and adipocyte metabolism.
6.7. Thermogenesis
Many have examined cold- and diet-induced thermogenesis mediated by mitochondrial uncoupling proteins (UCPs) in the presence of n-3 PUFA [43,48,54]. UCPs are inner mitochondrial proteins that function to transport hydrogen ions across the mitochondrial inner membrane. We have recently shown that BAT from EPA-supplemented mice expresses higher levels of thermogenic genes, such as PRDM16, peroxisome proliferator-activated receptor-gamma coactivator-1alpha and UCP1 [183].
Omega-3 PUFAs increase mitochondrial oxidative capacity in WAT [43] and skeletal muscle, possibly through UCP-3 up-regulation [48], but not in BAT or liver [43]. However, because most studies were carried out at 20°C, it is unclear whether increases in mitochondrial oxidative capacity are n-3 PUFA mediated or cold induced. Janovska et al. reported no differences in body weight but decreased epididymal fat mass after feeding an HF diet supplemented with n-3 PUFA in mice kept at 30°C, indicating that n-3 PUFA could attenuate body fat accumulation even at thermoneutrality and independent of cold-induced thermogenesis [52]. Mechanisms underlying the role of n-3 PUFA in possible induction of energy expenditure and prevention of body fat accumulation should be investigated further at various temperatures since thermogenic markers are activated even at 22°C [183].
6.8. Lean mass
The mechanism by which n-3 PUFAs have the potential to increase lean mass is not fully understood but likely involves both catabolic and anabolic pathways. Increased lean mass would result in improved body composition and possibly improved metabolism. Even though increases in RMR have been demonstrated with increases in lean mass [184], post-n-3 PUFA supplementation increases in lean mass are not always accompanied by increases in RMR [66]. Future studies are needed to examine the relationship between n-3 PUFA changes in lean mass in relation to RMR since metabolic rate significantly influences body weight.
Omega-3 PUFAs have been shown to attenuate muscle protein breakdown in isolated muscles of mice [185]. Increases in protein synthesis may be mediated by n-3 PUFA activation of the mTOR-p70s6k signaling pathway [186], a key pathway in muscle cell growth. Similarly, Clark et al. reported increased whole-body protein turnover under insulin stimulation but did not see significant increases in lean mass following 9 months of n-3 PUFA supplementation [187]. Certainly, changes in protein dynamics may not translate to increases in protein mass.
One possible mechanism of n-3 PUFA in increasing lean mass is the alteration of protein dynamics related to n-3 PUFA anti-inflammatory properties [66] since proinflammatory cytokines like TNF-α can increase protein degradation via ATP-ubiquitin-dependent pathways [188].
Another such potential mechanism relates to the ability of n-3 PUFA to lower cortisol levels [189]. Noreen et al. reported decreases in cortisol with n-3 PUFA supplementation but noted a significant correlation between cortisol level and changes in body composition [66]. Others have shown that a reduction in fat mass does not lower cortisol production [190]. Hence, n-3 PUFA supplementation may modulate cortisol levels so as to improve body composition [66]. Furthermore, proinflammatory cytokines such as 1L-6 have been shown to increase blood levels of cortisol [191], which increases protein catabolism [192]. Hence, anti-inflammatory properties of n-3 PUFA could aid in disrupting this pathway. However, increased muscle protein synthesis with n-3 PUFA supplementation is not likely mediated by changes in inflammation in a healthy population [186]. Further investigations are needed to elucidate the mechanisms by which n-3 PUFAs alter protein dynamics to increase lean mass.
6.9. Epigenetics and microRNA
Epigenetics may be an important contributor to many chronic diseases, including obesity [193,194]. Limited studies have examined n-3 PUFA and epigenetic modifications even though the expression of several genes involved in metabolic homeostasis is regulated by DNA methylation. The few that have been conducted report conflicting evidence for DNA methylation and n-3 PUFA. In a population-based study, n-3 PUFA intake was associated with DNA methylation in Alaska Yup’ik people [195]. A few studies have reported that fish oil supplementation did not alter the methylation pattern of genes [196,197], including leptin and the leptin receptor, in mouse epididymal fat [196]. It may be that n-3 PUFAs work through epigenetic mechanisms other than methylation [197]. In diet-induced obese mice, leptin expression may be regulated by n-3 PUFA via changes in methyl-CpG-binding domain protein 2 and histone modifications [198]. Since an HF diet has been shown to cause changes in the methylation of gene-specific promoter regions in the liver [199] and WAT [200], the influence of n-3 PUFAs on epigenetic modifications warrants further investigation.
MicroRNAs (miRNAs) are short noncoding RNAs that act as posttranscriptional regulators of genes by acting as sequence-specific inhibitors of mRNA. These miRNAs target transcription factors to indirectly affect entire signaling pathways. It has been documented that miRNAs act as key regulators in the pathogenesis of metabolic disease by affecting inflammation [201] and lipid metabolism [202].
Recent studies have shown n-3 PUFA to modulate miRNA expression [201,203,204]. A diet enriched in PUFA correlated to changes in circulating miRNAs, specifically miR-106a, along with changes in other miRNAs related to lipid metabolism and adipokine secretion in healthy women [203]. In animal models, n-3 PUFAs suppress inflammation through down-regulation of miR-19b-3p, −146b-5p and −183–5p by targeting toll-like receptor, NOD-like receptor, RIG-l-like receptor, mitogen-activated protein kinase and transforming growth factor-β pathways [201]. In obese rats, DHA has been shown to counteract obesity-related increases in hepatic miR-33a and miR-122, thus improving lipid metabolism via decreased SREBP2 and FAS expression, respectively [204]. Therefore, fully characterizing n-3 PUFA modulation of miRNA involved in key pathways, such as lipid metabolism and inflammation, is warranted and could play a key role in targeting MetS and obesity-related therapies.
7. Future perspectives
Both animal and human studies have examined the beneficial effects of combining n-3 PUFA with other dietary supplements and pharmaceuticals including antidiabetic drugs, L-alanyl-L-glutamine [205], as well as α-lipoic acid [118] and krill oil [206]. Animal studies using the combination of n-3 PUFA and rosiglitazone have reported significantly greater reductions in body weight [63,143], enhanced oxidation of fatty acids [207] and counteraction of lipogenesis than with rosiglitazone therapy alone [63]. Omega-3 PUFA supplementation in addition to antidiabetic pharmaceuticals could attenuate body weight gain caused by pharmaceuticals and should be further investigated [208].
8. Conclusions
The management of obesity has shifted from a narrow focus on BMI to the wider field that includes the complications of obesity, with the goal to reduce obesity-associated comorbidities [209]. While n-3 PUFAs have not yet shown consistent benefits in terms of weight loss in humans, improvements in the metabolic profile of obese individuals have been demonstrated. Therefore, n-3 PUFAs may be an important adjunct to obesity management along with lifestyle modification and pharmacotherapy. Further study of the genetic and epigenetic molecular targets related to metabolism, appetite and energetics could aid the discovery of novel therapeutic targets for obesity-associated metabolic disorders.
Acknowledgments
Grants and funding sources: N.M.M. is in part supported by the National Institutes of Health NCCIH under award number 1 R15 AT008879-01A1. K.A.S. is a predoctoral fellow supported by the National Institute of Food and Agriculture, U.S. Department of Agriculture, AFRI ELI Predoctoral Fellowship, under award number 2017-67011-26029.
Abbreviations
- ALA
α-linolenic acid
- AA
arachidonic acid
- BMI
body mass index
- BAT
brown adipose tissue
- DHA
docosahexaenoic acid
- DPA
docosapentaenoic acid
- EPA
eicosapentaenoic acid
- FABP
fatty acid-binding protein
- FFAR
free fatty acid receptor family
- FGF
fibroblast growth factor
- HDL
high-density lipoprotein
- HF
high-fat
- IL
interleukin
- LA
linoleic acid
- MetS
metabolic syndrome
- MUFA
monounsaturated fatty acid
- PPAR
peroxisome proliferator-activated receptor
- PUFA
polyunsaturated fatty acid
- SFA
saturated fatty acid
- TG
triglycerides
- T2DM
type 2 diabetes mellitus
- UCP
uncoupling protein
- VLDL
very low density lipoprotein
- WAT
white adipose tissue
References
- [1].American Medical Association. 2013.
- [2].Ogden CL, Carroll MD, Fryar CD, Flegal KM. Prevalence of obesity among adults and youth: United States, 2011–2014. NCHS data brief, no 219. Hyattsville, MD: National Center for Health Statistics; 2015. [PubMed] [Google Scholar]
- [3].World Health Organization. Obesity and overweight. Fact sheet No 311; 2013.
- [4].Kelly T, Yang W, Chen CS, Reynolds K, He J. Global burden of obesity in 2005 and projections to 2030. Int J Obes (Lond) 2008;32:1431–7. [DOI] [PubMed] [Google Scholar]
- [5].Aguilar M, Bhuket T, Torres S, Liu B, Wong RJ. Prevalence of the metabolic syndrome in the United States, 2003–2012. JAMA 2015;313:1973–4. [DOI] [PubMed] [Google Scholar]
- [6].Ratnayake WM, Galli C. Fat and fatty acid terminology, methods of analysis and fat digestion and metabolism: a background review paper. Ann Nutr Metab 2009;55:8–43. [DOI] [PubMed] [Google Scholar]
- [7].Siriwardhana N, Kalupahana NS, Moustaid-Moussa N. Health benefits of n-3 polyunsaturated fatty acids: eicosapentaenoic acid and docosahexaenoic acid. Adv Food Nutr Res 2012;65:211–22. [DOI] [PubMed] [Google Scholar]
- [8].Kris-Etherton PM, Harris WS, Appel LJ. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation 2002;106:2747–57. [DOI] [PubMed] [Google Scholar]
- [9].Carpentier YA, Portois L, Malaisse WJ. n-3 fatty acids and the metabolic syndrome. Am J Clin Nutr 2006;83:1499s–504s. [DOI] [PubMed] [Google Scholar]
- [10].Sethi JK, Vidal-Puig AJ. Thematic review series: adipocyte biology. Adipose tissue function and plasticity orchestrate nutritional adaptation. J Lipid Res 2007;48: 1253–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kalupahana NS, Moustaid-Moussa N, Claycombe KJ. Immunity as a link between obesity and insulin resistance. Mol Asp Med 2012;33:26–34. [DOI] [PubMed] [Google Scholar]
- [12].Bjorndal B, Burri L, Staalesen V, Skorve J, Berge RK. Different adipose depots: their role in the development of metabolic syndrome and mitochondrial response to hypolipidemic agents. J Obes 2011;2011:490650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kim S, Moustaid-Moussa N. Secretory, endocrine and autocrine/paracrine function of the adipocyte. J Nutr 2000;130:3110s–5s. [DOI] [PubMed] [Google Scholar]
- [14].Patel D Pharmacotherapy for the management of obesity. Metab Clin Exp 2015; 64:1376–85. [DOI] [PubMed] [Google Scholar]
- [15].Arterburn DE, Courcoulas AP. Bariatric surgery for obesity and metabolic conditions in adults. BMJ 2014;349:g3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Raynor HA, Champagne CM. Position of the Academy of Nutrition and Dietetics: interventions for the treatment of overweight and obesity in adults. J Acad Nutr Diet 2016;116:129–47. [DOI] [PubMed] [Google Scholar]
- [17].Pawlosky RJ, Hibbeln JR, Novotny JA, Salem N Jr. Physiological compartmental analysis of alpha-linolenic acid metabolism in adult humans. J Lipid Res 2001;42: 1257–65. [PubMed] [Google Scholar]
- [18].Vermunt SH, Mensink RP, Simonis AM, Hornstra G. Effects of age and dietary n-3 fatty acids on the metabolism of [13C]-alpha-linolenic acid. Lipids 1999(34 Suppl):S127. [DOI] [PubMed] [Google Scholar]
- [19].Vessby B Dietary fat, fatty acid composition in plasma and the metabolic syndrome. Curr Opin Lipidol 2003;14:15–9. [DOI] [PubMed] [Google Scholar]
- [20].Simopoulos AP. An increase in the omega-6/omega-3 fatty acid ratio increases the risk for obesity. Nutrients 2016;8:128–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].U.S. Department of Health and Human Services, U.S. Department of Agriculture. 2015–2020 Dietary guidelines for Americans; 8th Edition; 2015. [Google Scholar]
- [22].Administration USFD. FDA announces qualified health claims for omega-3 fatty acids; 2004.
- [23].Panel on Dietetic Products NaA. Scientific opinion on the tolerable upper intake level of eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA) and docosapentaenoic acid (DPA). Eur Food Saf Authority J 2012;10:2815–82. [Google Scholar]
- [24].Garaiova I, Guschina IA, Plummer SF, Tang J, Wang D, Plummer NT. A randomised cross-over trial in healthy adults indicating improved absorption of omega-3 fatty acids by pre-emulsification. Nutr J 2007;6:4–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Raatz SK, Redmon JB, Wimmergren N, Donadio JV, Bibus DM. Enhanced absorption of n-3 fatty acids from emulsified compared with encapsulated fish oil. J Am Diet Assoc 2009;109:1076–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Blasbalg TL, Hibbeln JR, Ramsden CE, Majchrzak SF, Rawlings RR. Changes in consumption of omega-3 and omega-6 fatty acids in the United States during the 20th century. AmJ Clin Nutr 2011;93:950–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Meyer BJ. Are we consuming enough long chain omega-3 polyunsaturated fatty acids for optimal health? Prostaglandins Leukot Essent Fat Acids 2011;85: 275–80. [DOI] [PubMed] [Google Scholar]
- [28].Bang HO, Dyerberg J, Hjoorne N. The composition of food consumed by Greenland Eskimos. Acta Med Scand 1976;200:69–73. [DOI] [PubMed] [Google Scholar]
- [29].He K, Rimm EB, Merchant A, Rosner BA, Stampfer MJ, Willett WC, et al. Fish consumption and risk of stroke in men. JAMA 2002;288:3130–6. [DOI] [PubMed] [Google Scholar]
- [30].Iso H, Rexrode KM, Stampfer MJ, Manson JE, Colditz GA, Speizer FE, et al. Intake of fish and omega-3 fatty acids and risk of stroke in women. JAMA 2001;285:304–12. [DOI] [PubMed] [Google Scholar]
- [31].Takata Y, Zhang X, Li H, Gao YT, Yang G, Gao J, et al. Fish intake and risks of total and cause-specific mortality in 2 population-based cohort studies of 134,296 men and women. Am J Epidemiol 2013;178:46–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Hodge AM, Simpson JA, Gibson RA, Sinclair AJ, Makrides M, O’Dea K, et al. Plasma phospholipid fatty acid composition as a biomarker of habitual dietary fat intake in an ethnically diverse cohort. Nutr Metab Cardiovasc Dis 2007;17:415–26. [DOI] [PubMed] [Google Scholar]
- [33].Knutsen SF, Fraser GE, Beeson WL, Lindsted KD, Shavlik DJ. Comparison of adipose tissue fatty acids with dietary fatty acids as measured by 24-hour recall and food frequency questionnaire in Black and White Adventists: the Adventist Health Study. Ann Epidemiol 2003;13:119–27. [DOI] [PubMed] [Google Scholar]
- [34].Harris WS, Von Schacky C. The Omega-3 Index: a new risk factor for death from coronary heart disease? Prev Med 2004;39:212–20. [DOI] [PubMed] [Google Scholar]
- [35].Karlsson M, Marild S, Brandberg J, Lonn L, Friberg P, Strandvik B. Serum phospholipid fatty acids, adipose tissue, and metabolic markers in obese adolescents. Obesity (Silver Spring, Md) 2006;14:1931–9. [DOI] [PubMed] [Google Scholar]
- [36].Micallef M, Munro I, Phang M, Garg M. Plasma n-3 polyunsaturated fatty acids are negatively associated with obesity. Br J Nutr 2009;102:1370–4. [DOI] [PubMed] [Google Scholar]
- [37].Perez-Matute P, Perez-Echarri N, Martinez JA, Marti A, Moreno-Aliaga MJ. Eicosapentaenoic acid actions on adiposity and insulin resistance in control and high-fat-fed rats: role of apoptosis, adiponectin and tumour necrosis factor-alpha. Br J Nutr 2007;97:389–98. [DOI] [PubMed] [Google Scholar]
- [38].Borsonelo EC, Vieira L, Galduroz JC. The influence of the polyunsaturated fatty acids on body weight and anxiolytic-like behavior in female rats. Nutr Neurosci 2013;16:2–5. [DOI] [PubMed] [Google Scholar]
- [39].Peyron-Caso E, Taverna M, Guerre-Millo M, Veronese A, Pacher N, Slama G, et al. Dietary (n-3) polyunsaturated fatty acids up-regulate plasma leptin in insulin-resistant rats. J Nutr 2002;132:2235–40. [DOI] [PubMed] [Google Scholar]
- [40].Minami A, Ishimura N, Sakamoto S, Takishita E, Mawatari K, Okada K, et al. Effect of eicosapentaenoic acid ethyl ester v. oleic acid-rich safflower oil on insulin resistance in type 2 diabetic model rats with hypertriacylglycerolaemia. Br J Nutr 2002;87:157–62. [DOI] [PubMed] [Google Scholar]
- [41].Raclot T, Groscolas R, Langin D, Ferre P. Site-specific regulation of gene expression by n-3 polyunsaturated fatty acids in rat white adipose tissues. J Lipid Res 1997;38:1963–72. [PubMed] [Google Scholar]
- [42].Ruzickova J, Rossmeisl M, Prazak T, Flachs P, Sponarova J, Veck M, et al. Omega-3 PUFA of marine origin limit diet-induced obesity in mice by reducing cellularity of adipose tissue. Lipids 2004;39:1177–85. [DOI] [PubMed] [Google Scholar]
- [43].Flachs P, Horakova O, Brauner P, Rossmeisl M, Pecina P, Franssen-van Hal N, et al. Polyunsaturated fatty acids of marine origin upregulate mitochondrial biogenesis and induce beta-oxidation in white fat. Diabetologia 2005;48:2365–75. [DOI] [PubMed] [Google Scholar]
- [44].Gillam M, Noto A, Zahradka P, Taylor CG. Improved n-3 fatty acid status does not modulate insulin resistance in fa/fa Zucker rats. Prostaglandins Leukot Essent Fat Acids 2009;81:331–9. [DOI] [PubMed] [Google Scholar]
- [45].Duivenvoorde LP, van Schothorst EM, Swarts HM, Kuda O, Steenbergh E, Termeulen S, et al. A difference in fatty acid composition of isocaloric high-fat diets alters metabolic flexibility in male C57BL/6JOlaHsd mice. PLoS One 2015; 10:e0128515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Pighin D, Karabatas L, Rossi A, Chicco A, Basabe JC, Lombardo YB. Fish oil affects pancreatic fat storage, pyruvate dehydrogenase complex activity and insulin secretion in rats fed a sucrose-rich diet. J Nutr 2003;133:4095–101. [DOI] [PubMed] [Google Scholar]
- [47].Kalupahana NS, Claycombe K, Newman SJ, Stewart T, Siriwardhana N, Matthan N, et al. Eicosapentaenoic acid prevents and reverses insulin resistance in high-fat diet-induced obese mice via modulation of adipose tissue inflammation. J Nutr 2010;140:1915–22. [DOI] [PubMed] [Google Scholar]
- [48].Bertrand C, Pignalosa A, Wanecq E, Rancoule C, Batut A, Deleruyelle S, et al. Effects of dietary eicosapentaenoic acid (EPA) supplementation in high-fat fed mice on lipid metabolism and apelin/APJ system in skeletal muscle. PLoS One 2013;8:e78874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Hainault I, Carolotti M, Hajduch E, Guichard C, Lavau M. Fish oil in a high lard diet prevents obesity, hyperlipemia, and adipocyte insulin resistance in rats. Ann N Y Acad Sci 1993;683:98–101. [DOI] [PubMed] [Google Scholar]
- [50].Cunnane SC, McAdoo KR, Horrobin DF. n-3 Essential fatty acids decrease weight gain in genetically obese mice. Br J Nutr 1986;56:87–95. [DOI] [PubMed] [Google Scholar]
- [51].Sato A, Kawano H, Notsu T, Ohta M, Nakakuki M, Mizuguchi K, et al. Antiobesity effect of eicosapentaenoic acid in high-fat/high-sucrose diet-induced obesity: importance of hepatic lipogenesis. Diabetes 2010;59:2495–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Janovska P, Flachs P, Kazdova L, Kopecky J. Anti-obesity effect of n-3 polyunsaturated fatty acids in mice fed high-fat diet is independent of cold-induced thermogenesis. Physiol Res 2013;62:153–61. [DOI] [PubMed] [Google Scholar]
- [53].Samane S, Christon R, Dombrowski L, Turcotte S, Charrouf Z, Lavigne C, et al. Fish oil and argan oil intake differently modulate insulin resistance and glucose intolerance in a rat model of dietary-induced obesity. Metab Clin Exp 2009;58:909–19. [DOI] [PubMed] [Google Scholar]
- [54].Flachs P, Ruhl R, Hensler M, Janovska P, Zouhar P, Kus V, et al. Synergistic induction of lipid catabolism and anti-inflammatory lipids in white fat of dietary obese mice in response to calorie restriction and n-3 fatty acids. Diabetologia 2011;54:2626–38. [DOI] [PubMed] [Google Scholar]
- [55].Figueras M, Olivan M, Busquets S, Lopez-Soriano FJ, Argiles JM. Effects of eicosapentaenoic acid (EPA) treatment on insulin sensitivity in an animal model of diabetes: improvement of the inflammatory status. Obesity (Silver Spring, Md) 2011;19:362–9. [DOI] [PubMed] [Google Scholar]
- [56].Hun CS, Hasegawa K, Kawabata T, Kato M, Shimokawa T, Kagawa Y. Increased uncoupling protein2 mRNA in white adipose tissue, and decrease in leptin, visceral fat, blood glucose, and cholesterol in KK-Ay mice fed with eicosapentaenoic and docosahexaenoic acids in addition to linolenic acid. Biochem Biophys Res Commun 1999;259:85–90. [DOI] [PubMed] [Google Scholar]
- [57].Belzung F, Raclot T, Groscolas R. Fish oil n-3 fatty acids selectively limit the hypertrophy of abdominal fat depots in growing rats fed high-fat diets. Am J Physiol 1993;264:R1111-. [DOI] [PubMed] [Google Scholar]
- [58].Huang XF, Xin X, McLennan P, Storlien L. Role of fat amount and type in ameliorating diet-induced obesity: insights at the level of hypothalamic arcuate nucleus leptin receptor, neuropeptide Y and pro-opiomelanocortin mRNA expression. Diabetes Obes Metab 2004;6:35–44. [DOI] [PubMed] [Google Scholar]
- [59].Alexander-Aguilera A, Berruezo S, Hernandez-Diaz G, Angulo O, Oliart-Ros R. Dietary n-3 polyunsaturated fatty acids modify fatty acid composition in hepatic and abdominal adipose tissue of sucrose-induced obese rats. J Physiol Biochem 2011;67:595–604. [DOI] [PubMed] [Google Scholar]
- [60].Flachs P, Rossmeisl M, Kopecky J. The effect of n-3 fatty acids on glucose homeostasis and insulin sensitivity. Physiol Res 2014;63(Suppl. 1):S93–118. [DOI] [PubMed] [Google Scholar]
- [61].Takahashi Y, Ide T. Dietary n-3 fatty acids affect mRNA level of brown adipose tissue uncoupling protein 1, and white adipose tissue leptin and glucose transporter 4 in the rat. Br J Nutr 2000;84:175–84. [PubMed] [Google Scholar]
- [62].Benhizia F, Hainault I, Serougne C, Lagrange D, Hajduch E, Guichard C, et al. Effects of a fish oil-lard diet on rat plasma lipoproteins, liver FAS, and lipolytic enzymes. Am J Physiol 1994;267:E975–2. [DOI] [PubMed] [Google Scholar]
- [63].Rossmeisl M, Medrikova D, van Schothorst EM, Pavlisova J, Kuda O, Hensler M, et al. Omega-3 phospholipids from fish suppress hepatic steatosis by integrated inhibition of biosynthetic pathways in dietary obese mice. Biochim Biophys Acta 1841;2014:267–78. [DOI] [PubMed] [Google Scholar]
- [64].Baillie RA, Takada R, Nakamura M, Clarke SD. Coordinate induction of peroxisomal acyl-CoA oxidase and UCP-3 by dietary fish oil: a mechanism for decreased body fat deposition. Prostaglandins Leukot Essent Fat Acids 1999;60: 351–6. [DOI] [PubMed] [Google Scholar]
- [65].Couet C, Delarue J, Ritz P, Antoine JM, Lamisse F. Effect of dietary fish oil on body fat mass and basal fat oxidation in healthy adults. Int J Obes Relat Metab Disord 1997;21:637–43. [DOI] [PubMed] [Google Scholar]
- [66].Noreen EE, Sass MJ, Crowe ML, Pabon VA, Brandauer J, Averill LK. Effects of supplemental fish oil on resting metabolic rate, body composition, and salivary cortisol in healthy adults. J Int Soc Sports Nutr 2010;7:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Harden CJ, Dible VA, Russell JM, Garaiova I, Plummer SF, Barker ME, et al. Long-chain polyunsaturated fatty acid supplementation had no effect on body weight but reduced energy intake in overweight and obese women. Nutr Res 2014;34: 17–24. [DOI] [PubMed] [Google Scholar]
- [68].Albert BB, Derraik JG, Brennan CM, Biggs JB, Garg ML, Cameron-Smith D, et al. Supplementation with a blend of krill and salmon oil is associated with increased metabolic risk in overweight men. Am J Clin Nutr 2015;102:49–57. [DOI] [PubMed] [Google Scholar]
- [69].Kabir M, Skurnik G, Naour N, Pechtner V, Meugnier E, Rome S, et al. Treatment for 2 mo with n 3 polyunsaturated fatty acids reduces adiposity and some atherogenic factors but does not improve insulin sensitivity in women with type 2 diabetes: a randomized controlled study. Am J Clin Nutr 2007;86:1670–9. [DOI] [PubMed] [Google Scholar]
- [70].Hutchins-Wiese HL, Kleppinger A, Annis K, Liva E, Lammi-Keefe CJ, Durham HA, et al. The impact of supplemental n-3 long chain polyunsaturated fatty acids and dietary antioxidants on physical performance in postmenopausal women. J Nutr Health Aging 2013;17:76–80. [DOI] [PubMed] [Google Scholar]
- [71].Summers LK, Fielding BA, Bradshaw HA, Ilic V, Beysen C, Clark ML, et al. Substituting dietary saturated fat with polyunsaturated fat changes abdominal fat distribution and improves insulin sensitivity. Diabetologia 2002;45:369–77. [DOI] [PubMed] [Google Scholar]
- [72].Lee H-C, Cheng W-Y, Hsu Y-H, Su H-Y, B.E., Huang T-G, Lin Y-K, et al. Effects of calorie restriction with n-3 long-chain polyunsaturated fatty acids on metabolic syndrome severity in obese subjects: a randomize-controlled trial. J Funct Foods 2015;19(Part B):929–40. [Google Scholar]
- [73].Razny U, Kiec-Wilk B, Polus A, Goralska J, Malczewska-Malec M, Wnek D, et al. Effect of caloric restriction with or without n-3 polyunsaturated fatty acids on insulin sensitivity in obese subjects: a randomized placebo controlled trial. BBA Clin 2015;4:7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Paoli A, Moro T, Bosco G, Bianco A, Grimaldi KA, Camporesi E, et al. Effects ofn-3 polyunsaturated fatty acids (omega-3) supplementation on some cardiovascular risk factors with a ketogenic Mediterranean diet. Mar Drugs 2015;13:996–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Mori TA, Bao DQ, Burke V, Puddey IB, Watts GF, Beilin LJ. Dietary fish as a major component of a weight-loss diet: effect on serum lipids, glucose, and insulin metabolism in overweight hypertensive subjects. Am J Clin Nutr 1999;70:817–25. [DOI] [PubMed] [Google Scholar]
- [76].Thorsdottir I, Tomasson H, Gunnarsdottir I, Gisladottir E, Kiely M, Parra MD, et al. Randomized trial of weight-loss-diets for young adults varying in fish and fish oil content. Int J Obes (Lond) 2007;31:1560–6. [DOI] [PubMed] [Google Scholar]
- [77].Phinney SD, Tang AB, Johnson SB, Holman RT. Reduced adipose 18:3 omega 3 with weight loss by very low calorie dieting. Lipids 1990;25:798–806. [DOI] [PubMed] [Google Scholar]
- [78].Phinney SD, Davis PG, Johnson SB, Holman RT. Obesity and weight loss alter serum polyunsaturated lipids in humans. AmJ Clin Nutr 1991;53:831–8. [DOI] [PubMed] [Google Scholar]
- [79].Hlavaty P, Kunesova M, Gojova M, Tvrzicka E, Vecka M, Roubal P, et al. Change in fatty acid composition of serum lipids in obese females after short-term weight-reducing regimen with the addition of n-3 long chain polyunsaturated fatty acids in comparison to controls. Physiol Res 2008;57(Suppl. 1):S57–65. [DOI] [PubMed] [Google Scholar]
- [80].Munro IA, Garg ML. Prior supplementation with long chain omega-3 polyunsaturated fatty acids promotes weight loss in obese adults: a double-blinded randomised controlled trial. Food Funct 2013;4:650–8. [DOI] [PubMed] [Google Scholar]
- [81].Slivkoff-Clark KM, James AP, Mamo JC. The chronic effects of fish oil with exercise on postprandial lipaemia and chylomicron homeostasis in insulin resistant viscerally obese men. Nutr Metab 2012;9:9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Kunesova M, Braunerova R, Hlavaty P, Tvrzicka E, Stankova B, Skrha J, et al. The influence of n-3 polyunsaturated fatty acids and very low calorie diet during a short-term weight reducing regimen on weight loss and serum fatty acid composition in severely obese women. Physiol Res 2006;55:63–72. [DOI] [PubMed] [Google Scholar]
- [83].DeFina LF, Marcoux LG, Devers SM, Cleaver JP, Willis BL. Effects of omega-3 supplementation in combination with diet and exercise on weight loss and body composition. AmJ Clin Nutr 2011;93:455–62. [DOI] [PubMed] [Google Scholar]
- [84].de Camargo Talon L, de Oliveira EP, Moreto F, Portero-McLellana KC, Burini RC. Omega-3 fatty acids supplementation decreases metabolic syndrome prevalence after lifestyle modification program. J Funct Foods 2015;19:922–8. [Google Scholar]
- [85].Warner JG Jr, Ullrich IH, Albrink MJ, Yeater RA. Combined effects of aerobic exercise and omega-3 fatty acids in hyperlipidemic persons. Med Sci Sports Exerc 1989;21:498–505. [PubMed] [Google Scholar]
- [86].Brilla LR, Landerholm TE. Effect of fish oil supplementation and exercise on serum lipids and aerobic fitness. J Sports Med Phys Fitness 1990;30:173–80. [PubMed] [Google Scholar]
- [87].Hill AM, Buckley JD, Murphy KJ, Howe PR. Combining fish-oil supplements with regular aerobic exercise improves body composition and cardiovascular disease risk factors. Am J Clin Nutr 2007;85:1267–74. [DOI] [PubMed] [Google Scholar]
- [88].Strandberg E, Edholm P, Ponsot E, Wahlin-Larsson B, Hellmen E, Nilsson A, et al. Influence of combined resistance training and healthy diet on muscle mass in healthy elderly women: a randomized controlled trial. J Appl Physiol 2015;119: 918–25. [DOI] [PubMed] [Google Scholar]
- [89].Da Boit M, Sibson R, Sivasubramaniam S, Meakin JR, Greig CA, Aspden RM, et al. Sex differences in the effect of fish-oil supplementation on the adaptive response to resistance exercise training in older people: a randomized controlled trial. Am J Clin Nutr 2017;105:151–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Das SK, Gilhooly CH, Golden JK, Pittas AG, Fuss PJ, Cheatham RA, et al. Long-term effects of 2 energy-restricted diets differing in glycemic load on dietary adherence, body composition, and metabolism in CALERIE: a 1-y randomized controlled trial. Am J Clin Nutr 2007;85:1023–30. [DOI] [PubMed] [Google Scholar]
- [91].Hall KD, Bemis T, Brychta R, Chen KY, Courville A, Crayner EJ, et al. Calorie for calorie, dietary fat restriction results in more body fat loss than carbohydrate restriction in people with obesity. Cell Metab 2015;22:427–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Schneider HJ, Friedrich N, Klotsche J, Pieper L, Nauck M, John U, et al. The predictive value of different measures of obesity for incident cardiovascular events and mortality. J Clin Endocrinol Metab 2010;95:1777–85. [DOI] [PubMed] [Google Scholar]
- [93].Fortin M, Julien P, Couture Y, Dubreuil P, Chouinard PY, Latulippe C, et al. Regulation of glucose and protein metabolism in growing steers by long-chain n-3 fatty acids in muscle membrane phospholipids is dose-dependent. Animal 2010;4:89–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Forman BM, Tontonoz P, Chen J, Brun RP, Spiegelman BM, Evans RM. 15-Deoxy-delta 12,14-prostaglandin J2 is a ligand for the adipocyte determination factor PPAR gamma. Cell 1995;83:803–12. [DOI] [PubMed] [Google Scholar]
- [95].Mater MK, Pan D, Bergen WG, Jump DB. Arachidonic acid inhibits lipogenic gene expression in 3T3-L1 adipocytes through a prostanoid pathway. J Lipid Res 1998; 39:1327–34. [PubMed] [Google Scholar]
- [96].Garaulet M, Hernandez-Morante JJ, Lujan J, Tebar FJ, Zamora S. Relationship between fat cell size and number and fatty acid composition in adipose tissue from different fat depots in overweight/obese humans. Int J Obes (Lond) 2006; 30:899–905. [DOI] [PubMed] [Google Scholar]
- [97].Oster RT, Tishinsky JM, Yuan Z, Robinson LE. Docosahexaenoic acid increases cellular adiponectin mRNA and secreted adiponectin protein, as well as PPARgamma mRNA, in 3T3-L1 adipocytes. Appl Physiol Nutr Metab 2010;35:783–9. [DOI] [PubMed] [Google Scholar]
- [98].Yudkin JS, Stehouwer CD, Emeis JJ, Coppack SW. C-reactive protein in healthy subjects: associations with obesity, insulin resistance, and endothelial dysfunction: a potential role for cytokines originating from adipose tissue? Arterioscler Thromb Vasc Biol 1999;19:972–8. [DOI] [PubMed] [Google Scholar]
- [99].Lo CJ, Chiu KC, Fu M, Lo R, Helton S. Fish oil decreases macrophage tumor necrosis factor gene transcription by altering the NF kappa B activity. J Surg Res 1999;82:216–21. [DOI] [PubMed] [Google Scholar]
- [100].Endres S, Ghorbani R, Kelley VE, Georgilis K, Lonnemann G, van der Meer JW, et al. The effect of dietary supplementation with n-3 polyunsaturated fatty acids on the synthesis of interleukin-1 and tumor necrosis factor by mononuclear cells. N Engl J Med 1989;320:265–71. [DOI] [PubMed] [Google Scholar]
- [101].Kremer JM, Lawrence DA, Jubiz W, DiGiacomo R, Rynes R, Bartholomew LE, et al. Dietary fish oil and olive oil supplementation in patients with rheumatoid arthritis. Clinical and immunologic effects. Arthritis Rheum 1990;33:810–20. [DOI] [PubMed] [Google Scholar]
- [102].Khalfoun B, Thibault F, Watier H, Bardos P, Lebranchu Y. Docosahexaenoic and eicosapentaenoic acids inhibit in vitro human endothelial cell production of interleukin-6. Adv Exp Med Biol 1997;400b:589–97. [PubMed] [Google Scholar]
- [103].Meydani SN, Endres S, Woods MM, Goldin BR, Soo C, Morrill-Labrode A, et al. Oral (n-3) fatty acid supplementation suppresses cytokine production and lymphocyte proliferation: comparison between young and older women. J Nutr 1991;121:547–55. [DOI] [PubMed] [Google Scholar]
- [104].Hara T, Kashihara D, Ichimura A, Kimura I, Tsujimoto G, Hirasawa A. Role of free fatty acid receptors in the regulation of energy metabolism. Biochim Biophys Acta 1841;2014:1292–300. [DOI] [PubMed] [Google Scholar]
- [105].Ichimura A, Hasegawa S, Kasubuchi M, Kimura I. Free fatty acid receptors as therapeutic targets for the treatment of diabetes. Front Pharmacol 2014;5:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Oh DY, Talukdar S, Bae EJ, Imamura T, Morinaga H, Fan WQ, et al. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin sensitizing effects. Cell 2010;142:687–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Spencer M, Finlin BS, Unal R, Zhu B, Morris AJ, Shipp LR, et al. Omega-3 fatty acids reduce adipose tissue macrophages in human subjects with insulin resistance. Diabetes 2013;62:1709–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Montserrat-de la Paz S, Rodriguez D, Cardelo MP, Naranjo MC, Bermudez B, Abia R, et al. The effects of exogenous fatty acids and niacin on human monocyte-macrophage plasticity. Mol Nutr Food Res 2017;61:8–17. [DOI] [PubMed] [Google Scholar]
- [109].Yan Y, Jiang W, Spinetti T, Tardivel A, Castillo R, Bourquin C, et al. Omega-3 fatty acids prevent inflammation and metabolic disorder through inhibition of NLRP3 inflammasome activation. Immunity 2013;38:1154–63. [DOI] [PubMed] [Google Scholar]
- [110].Wen H, Gris D, Lei Y, Jha S, Zhang L, Huang MT, et al. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat Immunol 2011;12:408–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Simons K, Ikonen E. Functional rafts in cell membranes. Nature 1997;387:569–72. [DOI] [PubMed] [Google Scholar]
- [112].Kabouridis PS, Magee AI, Ley SC. S-acylation of LCK protein tyrosine kinase is essential for its signalling function in T lymphocytes. EMBO J 1997;16:4983–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Rodgers W, Crise B, Rose JK. Signals determining protein tyrosine kinase and glycosyl-phosphatidylinositol-anchored protein targeting to a glycolipid-enriched membrane fraction. Mol Cell Biol 1994;14:5384–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Kim W, Fan YY, Barhoumi R, Smith R, McMurray DN, Chapkin RS. n-3 polyunsaturated fatty acids suppress the localization and activation of signaling proteins at the immunological synapse in murine CD4(+) T cells by affecting lipid raft formation. J Immunol 2008;181:6236–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med 1996;334:292–5. [DOI] [PubMed] [Google Scholar]
- [116].Arent SM, Walker AJ, Pellegrino JK, Sanders DJ, McFadden BA, Ziegenfuss TN, et al. The combined effects of exercise, diet, and a multi-ingredient dietary supplement on body composition and adipokine changes in overweight adults. J Am Coll Nutr 2017:1–10. [DOI] [PubMed] [Google Scholar]
- [117].Hinkle W, Cordell M, Leibel R, Rosenbaum M, Hirsch J. Effects of reduced weight maintenance and leptin repletion on functional connectivity of the hypothalamus in obese humans. PLoS One 2013;8:e59114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Huerta AE, Navas-Carretero S, Prieto-Hontoria PL, Martinez JA, Moreno-Aliaga MJ. Effects of alpha-lipoic acid and eicosapentaenoic acid in overweight and obese women during weight loss. Obesity (Silver Spring) 2015;23:313–21. [DOI] [PubMed] [Google Scholar]
- [119].Perez-Matute P, Marti A, Martinez JA, Fernandez-Otero MP, Stanhope KL, Havel PJ, et al. Eicosapentaenoic fatty acid increases leptin secretion from primary cultured rat adipocytes: role of glucose metabolism. Am J Physiol Regul Integr Comp Physiol 2005;288:R1682-. [DOI] [PubMed] [Google Scholar]
- [120].Krebs JD, Browning LM, McLean NK, Rothwell JL, Mishra GD, Moore CS, et al. Additive benefits of long-chain n-3 polyunsaturated fatty acids and weight-loss in the management of cardiovascular disease risk in overweight hyperinsulinaemic women. Int J Obes (Lond) 2006;30:1535–44. [DOI] [PubMed] [Google Scholar]
- [121].Munro IA, Garg ML. Dietary supplementation with n-3 PUFA does not promote weight loss when combined with a very-low-energy diet. Br J Nutr 2012;108: 1466–74. [DOI] [PubMed] [Google Scholar]
- [122].Burghardt PR, Kemmerer ES, Buck BJ, Osetek AJ, Yan C, Koch LG, et al. Dietary n-3: n-6 fatty acid ratios differentially influence hormonal signature in a rodent model of metabolic syndrome relative to healthy controls. Nutr Metab 2010;7: 53–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Itoh M, Suganami T, Satoh N, Tanimoto-Koyama K, Yuan X, Tanaka M, et al. Increased adiponectin secretion by highly purified eicosapentaenoic acid in rodent models of obesity and human obese subjects. Arterioscler Thromb Vasc Biol 2007;27:1918–25. [DOI] [PubMed] [Google Scholar]
- [124].Gammelmark A, Madsen T, Varming K, Lundbye-Christensen S, Schmidt EB. Low-dose fish oil supplementation increases serum adiponectin without affecting inflammatory markers in overweight subjects. Nutr Res 2012;32:15–23. [DOI] [PubMed] [Google Scholar]
- [125].Zhang HH, Kumar S, Barnett AH, Eggo MC. Tumour necrosis factor-alpha exerts dual effects on human adipose leptin synthesis and release. Mol Cell Endocrinol 2000;159:79–88. [DOI] [PubMed] [Google Scholar]
- [126].Lopez-Alarcon M, Martinez-Coronado A, Velarde-Castro O, Rendon-Macias E, Fernandez J. Supplementation of n3 long-chain polyunsaturated fatty acid synergistically decreases insulin resistance with weight loss of obese prepubertal and pubertal children. Arch Med Res 2011;42:502–8. [DOI] [PubMed] [Google Scholar]
- [127].Furuhashi M, Hotamisligil GS. Fatty acid-binding proteins: role in metabolic diseases and potential as drug targets. Nat Rev Drug Discov 2008;7:489–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Ota H, Furuhashi M, Ishimura S, Koyama M, Okazaki Y, Mita T, et al. Elevation of fatty acid-binding protein 4 is predisposed by family history of hypertension and contributes to blood pressure elevation. Am J Hypertens 2012;25:1124–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Cao H, Sekiya M, Erikci M, Burak MF, Mayers JR, White A, et al. Adipocyte lipid chaperone aP2 is a secreted adipokine regulating hepatic glucose production. Cell Metab 2013;17:768–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Furuhashi M, Hiramitsu S, Mita T, Omori A, Fuseya T, Ishimura S, et al. Reduction of circulating FABP4 level by treatment with omega-3 fatty acid ethyl esters. Lipids Health Dis 2016;15:5–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Parra D, Ramel A, Bandarra N, Kiely M, Martinez JA, Thorsdottir I. A diet rich in long chain omega-3 fatty acids modulates satiety in overweight and obese volunteers during weight loss. Appetite 2008;51:676–80. [DOI] [PubMed] [Google Scholar]
- [132].Burns RN, Moniri NH. Agonism with the omega-3 fatty acids alpha-linolenic acid and docosahexaenoic acid mediates phosphorylation of both the short and long isoforms of the human GPR120 receptor. Biochem Biophys Res Commun 2010; 396:1030–5. [DOI] [PubMed] [Google Scholar]
- [133].Tanaka T, Katsuma S, Adachi T, Koshimizu TA, Hirasawa A, Tsujimoto G. Free fatty acids induce cholecystokinin secretion through GPR120. Naunyn Schmiedeberg’s Arch Pharmacol 2008;377:523–7. [DOI] [PubMed] [Google Scholar]
- [134].Laplante M, Sabatini DM. mTORC1 activates SREBP-1c and uncouples lipogenesis from gluconeogenesis. Proc Natl Acad Sci U S A 2010;107:3281 −2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Li S, Brown MS, Goldstein JL. Bifurcation of insulin signaling pathway in rat liver: mTORC1 required for stimulation of lipogenesis, but not inhibition of gluconeogenesis. Proc Natl Acad Sci U S A 2010;107:3441–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Gaich G, Chien JY, Fu H, Glass LC, Deeg MA, Holland WL, et al. The effects of LY2405319, an FGF21 analog, in obese human subjects with type 2 diabetes. Cell Metab 2013;18:333–40. [DOI] [PubMed] [Google Scholar]
- [137].Coskun T, Bina HA, Schneider MA, Dunbar JD, Hu CC, Chen Y, et al. Fibroblast growth factor 21 corrects obesity in mice. Endocrinology 2008;149:6018–27. [DOI] [PubMed] [Google Scholar]
- [138].Zhang X, Yeung DC, Karpisek M, Stejskal D, Zhou ZG, Liu F, et al. Serum FGF21 levels are increased in obesity and are independently associated with the metabolic syndrome in humans. Diabetes 2008;57:1246–53. [DOI] [PubMed] [Google Scholar]
- [139].Villarroya J, Flachs P, Redondo-Angulo I, Giralt M, Medrikova D, Villarroya F, et al. Fibroblast growth factor-21 and the beneficial effects of long-chain n-3 polyunsaturated fatty acids. Lipids 2014;49:1081–9. [DOI] [PubMed] [Google Scholar]
- [140].Nonogaki K, Yamazaki T, Murakami M, Kaji T. Ingestion of eicosapentaenoic acid in the early stage of social isolation reduces a fibroblast growth factor 21 resistant state independently of body weight in KKA(y) mice. Biochem Biophys Res Commun 2015;464:674–7. [DOI] [PubMed] [Google Scholar]
- [141].Qin Y, Zhou Y, Chen SH, Zhao XL, Ran L, Zeng XL, et al. Fish oil supplements lower serum lipids and glucose in correlation with a reduction in plasma fibroblast growth factor 21 and prostaglandin e2 in nonalcoholic fatty liver disease associated with hyperlipidemia: a randomized clinical trial. PLoS One 2015;10: e0133496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Taouis M, Dagou C, Ster C, Durand G, Pinault M, Delarue J. N-3 polyunsaturated fatty acids prevent the defect of insulin receptor signaling in muscle. Am J Physiol Endocrinol Metab 2002;282:E664–1. [DOI] [PubMed] [Google Scholar]
- [143].Kuda O, Jelenik T, Jilkova Z, Flachs P, Rossmeisl M, Hensler M, et al. n-3 fatty acids and rosiglitazone improve insulin sensitivity through additive stimulatory effects on muscle glycogen synthesis in mice fed a high-fat diet. Diabetologia 2009;52:941–51. [DOI] [PubMed] [Google Scholar]
- [144].Rivellese AA, De Natale C, Lilli S. Type of dietary fat and insulin resistance. Ann N Y Acad Sci 2002;967:329–35. [DOI] [PubMed] [Google Scholar]
- [145].Goodfellow J, Bellamy MF, Ramsey MW, Jones CJ, Lewis MJ. Dietary supplementation with marine omega-3 fatty acids improve systemic large artery endothelial function in subjects with hypercholesterolemia. J Am Coll Cardiol 2000;35:265–70. [DOI] [PubMed] [Google Scholar]
- [146].Harris WS, Rambjor GS, Windsor SL, Diederich D. n-3 fatty acids and urinary excretion of nitric oxide metabolites in humans. AmJ Clin Nutr 1997;65:459–64. [DOI] [PubMed] [Google Scholar]
- [147].Walser B, Giordano RM, Stebbins CL. Supplementation with omega-3 polyunsaturated fatty acids augments brachial artery dilation and blood flow during forearm contraction. Eur J Appl Physiol 2006;97:347–54. [DOI] [PubMed] [Google Scholar]
- [148].Ghasemifard S, Hermon K, Turchini GM, Sinclair AJ. Metabolic fate (absorption, beta-oxidation and deposition) of long-chain n-3 fatty acids is affected by sex and by the oil source (krill oil or fish oil) in the rat. Br J Nutr 2015;114:684–92. [DOI] [PubMed] [Google Scholar]
- [149].Lucero D, Miksztowicz V, Gualano G, Longo C, Landeira G, Alvarez E, et al. Nonalcoholic fatty liver disease associated with metabolic syndrome: Influence of liver fibrosis stages on characteristics of very low-density lipoproteins. Clin Chim Acta 2017;473:1–8. [DOI] [PubMed] [Google Scholar]
- [150].Simonen PP, Gylling H, Miettinen TA. Body weight modulates cholesterol metabolism in non-insulin dependent type 2 diabetics. Obes Res 2002;10:328–35. [DOI] [PubMed] [Google Scholar]
- [151].Mittendorfer B, Patterson BW, Klein S. Effect of weight loss on VLDL-triglyceride and apoB-100 kinetics in women with abdominal obesity. Am J Physiol Endocrinol Metab 2003;284:E549–6. [DOI] [PubMed] [Google Scholar]
- [152].Zheng X, Avella M, Botham KM. Comparison of the effects of dietary n-3 and n-6 polyunsaturated fatty acids on very-low-density lipoprotein secretion when delivered to hepatocytes in chylomicron remnants. Biochem J 2001;357:481–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Wu X, Shang A, Jiang H, Ginsberg HN. Demonstration of biphasic effects of docosahexaenoic acid on apolipoprotein B secretion in HepG2 cells. Arterioscler Thromb Vasc Biol 1997;17:3347–55. [DOI] [PubMed] [Google Scholar]
- [154].Kasbi Chadli F, Andre A, Prieur X, Loirand G, Meynier A, Krempf M, et al. n-3 PUFA prevent metabolic disturbances associated with obesity and improve endothelial function in golden Syrian hamsters fed with a high-fat diet. Br J Nutr 2012;107:1305–15. [DOI] [PubMed] [Google Scholar]
- [155].Kasbi Chadli F, Nazih H, Krempf M, Nguyen P, Ouguerram K. Omega 3 fatty acids promote macrophage reverse cholesterol transport in hamster fed high fat diet. PLoS One 2013;8:e61109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Xie X, Zhang T, Zhao S, Li W, Ma L, Ding M, et al. Effects of n-3 polyunsaturated fatty acids high fat diet intervention on the synthesis of hepatic high-density lipoprotein cholesterol in obesity-insulin resistance rats. Lipids Health Dis 2016; 15:81–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Kim HJ, Takahashi M, Ezaki O. Fish oil feeding decreases mature sterol regulatory element-binding protein 1 (SREBP-1) by down-regulation of SREBP-1c mRNA in mouse liver. A possible mechanism for down-regulation of lipogenic enzyme mRNAs. J Biol Chem 1999;274:25892–8. [DOI] [PubMed] [Google Scholar]
- [158].Dentin R, Benhamed F, Pegorier JP, Foufelle F, Viollet B, Vaulont S, et al. Polyunsaturated fatty acids suppress glycolytic and lipogenic genes through the inhibition of ChREBP nuclear protein translocation. J Clin Invest 2005;115:2843–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Sekiya M, Yahagi N, Matsuzaka T, Najima Y, Nakakuki M, Nagai R, et al. Polyunsaturated fatty acids ameliorate hepatic steatosis in obese mice by SREBP-1 suppression. Hepatology 2003;38:1529–39. [DOI] [PubMed] [Google Scholar]
- [160].Yahagi N, Shimano H, Hasty AH, Amemiya-Kudo M, Okazaki H, Tamura Y, et al. A crucial role of sterol regulatory element-binding protein-1 in the regulation of lipogenic gene expression by polyunsaturated fatty acids. J Biol Chem 1999;274: 35840–4. [DOI] [PubMed] [Google Scholar]
- [161].Kaur G, Sinclair AJ, Cameron-Smith D, Barr DP, Molero-Navajas JC, Konstantopoulos N. Docosapentaenoic acid (22:5n-3) down-regulates the expression of genes involved in fat synthesis in liver cells. Prostaglandins Leukot Essent Fat Acids 2011;85:155–61. [DOI] [PubMed] [Google Scholar]
- [162].Yellaturu CR, Deng X, Park EA, Raghow R, Elam MB. Insulin enhances the biogenesis of nuclear sterol regulatory element-binding protein (SREBP)-1c by posttranscriptional down-regulation of Insig-2A and its dissociation from SREBP cleavage-activating protein (SCAP).SREBP-1c complex. J Biol Chem 2009;284: 31726–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Takeuchi Y, Yahagi N, Izumida Y, Nishi M, Kubota M, Teraoka Y, et al. Polyunsaturated fatty acids selectively suppress sterol regulatory element-binding protein-1 through proteolytic processing and autoloop regulatory circuit. J Biol Chem 2010;285:11681–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Pawar A, Botolin D, Mangelsdorf DJ, Jump DB. The role of liver X receptor-alpha in the fatty acid regulation of hepatic gene expression. J Biol Chem 2003;278: 40736–43. [DOI] [PubMed] [Google Scholar]
- [165].Xu J, Teran-Garcia M, Park JH, Nakamura MT, Clarke SD. Polyunsaturated fatty acids suppress hepatic sterol regulatory element-binding protein-1 expression by accelerating transcript decay. J Biol Chem 2001;276:9800–7. [DOI] [PubMed] [Google Scholar]
- [166].Nakakuki M, Kawano H, Notsu T, Imada K, Mizuguchi K, Shimano H. A novel processing system of sterol regulatory element-binding protein-1c regulated by polyunsaturated fatty acid. J Biochem 2014;155:301–13. [DOI] [PubMed] [Google Scholar]
- [167].Deng X, Dong Q, Bridges D, Raghow R, Park EA, Elam MB. Docosahexaenoic acid inhibits proteolytic processing of sterol regulatory element-binding protein-1c (SREBP-1c) via activation of AMP-activated kinase. Biochim Biophys Acta 1851; 2015:1521–9. [DOI] [PubMed] [Google Scholar]
- [168].Xu HE, Lambert MH, Montana VG, Parks DJ, Blanchard SG, Brown PJ, et al. Molecular recognition of fatty acids by peroxisome proliferator-activated receptors. Mol Cell 1999;3:397–403. [DOI] [PubMed] [Google Scholar]
- [169].Ide T, Shimano H, Yoshikawa T, Yahagi N, Amemiya-Kudo M, Matsuzaka T, et al. Cross-talk between peroxisome proliferator-activated receptor (PPAR) alpha and liver X receptor (LXR) in nutritional regulation of fatty acid metabolism. II. LXRs suppress lipid degradation gene promoters through inhibition of PPAR signaling. Mol Endocrinol 2003;17:1255–67. [DOI] [PubMed] [Google Scholar]
- [170].de Castro GS, Cardoso JF, Calder PC, Jordao AA, Vannucchi H. Fish oil decreases hepatic lipogenic genes in rats fasted and refed on a high fructose diet. Nutrients 2015;7:1644–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Ren B, Thelen AP, Peters JM, Gonzalez FJ, Jump DB. Polyunsaturated fatty acid suppression of hepatic fatty acid synthase and S14 gene expression does not require peroxisome proliferator-activated receptor alpha. J Biol Chem 1997;272: 26827–32. [DOI] [PubMed] [Google Scholar]
- [172].Neschen S, Morino K, Rossbacher JC, Pongratz RL, Cline GW, Sono S, et al. Fish oil regulates adiponectin secretion by a peroxisome proliferator-activated receptor-gamma-dependent mechanism in mice. Diabetes 2006;55:924–8. [DOI] [PubMed] [Google Scholar]
- [173].Hasan AU, Ohmori K, Konishi K, Igarashi J, Hashimoto T, Kamitori K, et al. Eicosapentaenoic acid upregulates VEGF-A through both GPR120 and PPARgamma mediated pathways in 3T3-L1 adipocytes. Mol Cell Endocrinol 2015;406: 10–8. [DOI] [PubMed] [Google Scholar]
- [174].Chang HY, Lee HN, Kim W, Surh YJ. Docosahexaenoic acid induces M2 macrophage polarization through peroxisome proliferator-activated receptor gamma activation. Life Sci 2015;120:39–47. [DOI] [PubMed] [Google Scholar]
- [175].Willumsen N, Hexeberg S, Skorve J, Lundquist M, Berge RK. Docosahexaenoic acid shows no triglyceride-lowering effects but increases the peroxisomal fatty acid oxidation in liver of rats. J Lipid Res 1993;34:13–22. [PubMed] [Google Scholar]
- [176].Rustan AC, Christiansen EN, Drevon CA. Serum lipids, hepatic glycerolipid metabolism and peroxisomal fatty acid oxidation in rats fed omega-3 and omega-6 fatty acids. Biochem J 1992;283(Pt 2):333–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].van Schothorst EM, Flachs P, Franssen-van Hal NL, Kuda O, Bunschoten A, Molthoff J, et al. Induction of lipid oxidation by polyunsaturated fatty acids of marine origin in small intestine of mice fed a high-fat diet. BMC Genomics 2009; 10:110–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Hensler M, Bardova K, Jilkova ZM, Wahli W, Meztger D, Chambon P, et al. The inhibition of fat cell proliferation by n-3 fatty acids in dietary obese mice. Lipids Health Dis 2011;10:128–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Flatmark T, Nilsson A, Kvannes J, Eikhom TS, Fukami MH, Kryvi H, et al. On the mechanism of induction of the enzyme systems for peroxisomal beta-oxidation of fatty acids in rat liver by diets rich in partially hydrogenated fish oil. Biochim Biophys Acta 1988;962:122–30. [DOI] [PubMed] [Google Scholar]
- [180].Rodriguez JC, Gil-Gomez G, Hegardt FG, Haro D. Peroxisome proliferator-activated receptor mediates induction of the mitochondrial 3-hydroxy-3-methylglutaryl-CoA synthase gene by fatty acids. J Biol Chem 1994;269: 18767–72. [PubMed] [Google Scholar]
- [181].Lee SS, Pineau T, Drago J, Lee EJ, Owens JW, Kroetz DL, et al. Targeted disruption of the alpha isoform of the peroxisome proliferator-activated receptor gene in mice results in abolishment of the pleiotropic effects of peroxisome proliferators. Mol Cell Biol 1995;15:3012–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Martin G, Schoonjans K, Lefebvre AM, Staels B, Auwerx J. Coordinate regulation of the expression of the fatty acid transport protein and acyl-CoA synthetase genes by PPARalpha and PPARgamma activators. J Biol Chem 1997;272:28210–7. [DOI] [PubMed] [Google Scholar]
- [183].Pahlavani M, Razafimanjato F, Ramalingam L, Kalupahana NS, Moussa H, Scoggin S, et al. Eicosapentaenoic acid regulates brown adipose tissue metabolism in high-fat-fed mice and in clonal brown adipocytes. J Nutr Biochem 2017;39: 101–9. [DOI] [PubMed] [Google Scholar]
- [184].Byrne HK, Wilmore JH. The effects of a 20-week exercise training program on resting metabolic rate in previously sedentary, moderately obese women. Int J Sport Nutr Exerc Metab 2001;11:15–31. [DOI] [PubMed] [Google Scholar]
- [185].Whitehouse AS, Smith HJ, Drake JL, Tisdale MJ. Mechanism of attenuation of skeletal muscle protein catabolism in cancer cachexia by eicosapentaenoic acid. Cancer Res 2001;61:3604–9. [PubMed] [Google Scholar]
- [186].Smith GI, Atherton P, Reeds DN, Mohammed BS, Rankin D, Rennie MJ, et al. Dietary omega-3 fatty acid supplementation increases the rate ofmuscle protein synthesis in older adults: a randomized controlled trial. Am J Clin Nutr 2011; 93:402–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Clark LF, Thivierge MC, Kidd CA, McGeoch SC, Abraham P, Pearson DW, et al. Fish oil supplemented for 9 months does not improve glycaemic control or insulin sensitivity in subjects with impaired glucose regulation: a parallel randomised controlled trial. BrJ Nutr 2016;115:75–86. [DOI] [PubMed] [Google Scholar]
- [188].Llovera M, Carbo N, Lopez-Soriano J, Garcia-Martinez C, Busquets S, Alvarez B, et al. Different cytokines modulate ubiquitin gene expression in rat skeletal muscle. Cancer Lett 1998;133:83–7. [DOI] [PubMed] [Google Scholar]
- [189].Delarue J, LeFoll C, Corporeau C, Lucas D. N-3 long chain polyunsaturated fatty acids: a nutritional tool to prevent insulin resistance associated to type 2 diabetes and obesity? Reprod Nutr Dev 2004;44:289–99. [DOI] [PubMed] [Google Scholar]
- [190].Purnell JQ, Kahn SE, Samuels MH, Brandon D, Loriaux DL, Brunzell JD. Enhanced cortisol production rates, free cortisol, and 11beta-HSD-1 expression correlate with visceral fat and insulin resistance in men: effect ofweight loss. Am J Physiol Endocrinol Metab 2009;296:E351-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Steensberg A, Fischer CP, Keller C, Moller K, Pedersen BK. IL-6 enhances plasma IL-1ra, IL-10, and cortisol in humans. Am J Physiol Endocrinol Metab 2003;285: E433-. [DOI] [PubMed] [Google Scholar]
- [192].Paddon-Jones D, Sheffield-Moore M, Cree MG, Hewlings SJ, Aarsland A, Wolfe RR, et al. Atrophy and impaired muscle protein synthesis during prolonged inactivity and stress. J Clin Endocrinol Metab 2006;91:4836–41. [DOI] [PubMed] [Google Scholar]
- [193].Kloting N, Berthold S, Kovacs P, Schon MR, Fasshauer M, Ruschke K, et al. MicroRNA expression in human omental and subcutaneous adipose tissue. PLoS One 2009;4:e4699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Heneghan HM, Miller N, McAnena OJ, O’Brien T, Kerin MJ. Differential miRNA expression in omental adipose tissue and in the circulation of obese patients identifies novel metabolic biomarkers. J Clin Endocrinol Metab 2011;96:E846–0. [DOI] [PubMed] [Google Scholar]
- [195].Aslibekyan S, Wiener HW, Havel PJ, Stanhope KL, O’Brien DM, Hopkins SE, et al. DNA methylation patterns are associated with n-3 fatty acid intake in Yup’ik people. J Nutr 2014;144:425–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Fan C, Liu X, Shen W, Deckelbaum RJ, Qi K. The regulation of leptin, leptin receptor and pro-opiomelanocortin expression by N-3 PUFAs in diet-induced obese mice is not related to the methylation of their promoters. Nutr Metab 2011;8:31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Amaral CL, Crisma AR, Masi LN, Martins AR, Hirabara SM, Curi R. DNA methylation changes induced by a high-fat diet and fish oil supplementation in the skeletal muscle of mice. J Nutrigenet Nutrigenomics 2014;7:314–26. [DOI] [PubMed] [Google Scholar]
- [198].Shen W, Wang C, Xia L, Fan C, Dong H, Deckelbaum RJ, et al. Epigenetic modification of the leptin promoter in diet-induced obese mice and the effects of N-3 polyunsaturated fatty acids. Sci Rep 2014;4:5282–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Cordero P, Campion J, Milagro FI, Martinez JA. Transcriptomic and epigenetic changes in early liver steatosis associated to obesity: effect of dietary methyl donor supplementation. Mol Genet Metab 2013;110:388–95. [DOI] [PubMed] [Google Scholar]
- [200].Lomba A, Martinez JA, Garcia-Diaz DF, Paternain L, Marti A, Campion J, et al. Weight gain induced by an isocaloric pair-fed high fat diet: a nutriepigenetic study on FASN and NDUFB6 gene promoters. Mol Genet Metab 2010;101:273–8. [DOI] [PubMed] [Google Scholar]
- [201].Zheng Z, Ge Y, Zhang J, Xue M, Li Q, Lin D, et al. PUFA diets alter the microRNA expression profiles in an inflammation rat model. Mol Med Rep 2015;11:4149–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Gil-Zamorano J, Martin R, Daimiel L, Richardson K, Giordano E, Nicod N, et al. Docosahexaenoic acid modulates the enterocyte Caco-2 cell expression of microRNAs involved in lipid metabolism. J Nutr 2014;144:575–85. [DOI] [PubMed] [Google Scholar]
- [203].Ortega FJ, Cardona-Alvarado MI, Mercader JM, Moreno-Navarrete JM, Moreno M, Sabater M, et al. Circulating profiling reveals the effect ofa polyunsaturated fatty acid-enriched diet on common microRNAs. J Nutr Biochem 2015;26:1095–101. [DOI] [PubMed] [Google Scholar]
- [204].Baselga-Escudero L, Arola-Arnal A, Pascual-Serrano A, Ribas-Latre A, Casanova E, Salvado MJ, et al. Chronic administration of proanthocyanidins or docosahexaenoic acid reverses the increase of miR-33a and miR-122 in dyslipidemic obese rats. PLoS One 2013;8:e69817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Wu C, Kato TS, Ji R, Zizola C, Brunjes DL, Deng Y, et al. Supplementation of l-alanyl-l-glutamine and fish oil improves body composition and quality of life in patients with chronic heart failure. Circ Heart Fail 2015;8:1077–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Schuchardt JP, Schneider I, Meyer H, Neubronner J, von Schacky C, Hahn A. Incorporation of EPA and DHA into plasma phospholipids in response to different omega-3 fatty acid formulations – a comparative bioavailability study of fish oil vs. krill oil. Lipids Health Dis 2011;10:145–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Horakova O, Medrikova D, van Schothorst EM, Bunschoten A, Flachs P, Kus V, et al. Preservation of metabolic flexibility in skeletal muscle by a combined use of n-3 PUFA and rosiglitazone in dietary obese mice. PLoS One 2012;7:e43764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Veleba J, Kopecky J Jr, Janovska P, Kuda O, Horakova O, Malinska H, et al. Combined intervention with pioglitazone and n-3 fatty acids in metformin-treated type 2 diabetic patients: improvement of lipid metabolism. Nutr Metab 2015;12:52–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Garber AJ, Abrahamson MJ, Barzilay JI, Blonde L, Bloomgarden ZT, Bush MA, et al. AACE comprehensive diabetes management algorithm 2013. Endocr Pract 2013; 19:327–36. [DOI] [PubMed] [Google Scholar]
- [210].Smith GI, Julliand S, Reeds DN, Sinacore DR, Klein S, Mittendorfer B. Fish oil-derived n-3 PUFA therapy increases muscle mass and function in healthy older adults. Am J Clin Nutr 2015;102:115–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].Krzyminska-Siemaszko R, Czepulis N, Lewandowicz M, Zasadzka E, Suwalska A, Witowski J, et al. The effect of a 12-week omega-3 supplementation on body composition, muscle strength and physical performance in elderly individuals with decreased muscle mass. Int J Environ Res Public Health 2015;12:10558–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Chan DC, Watts GF, Mori TA, Barrett PH, Redgrave TG, Beilin LJ. Randomized controlled trial of the effect of n-3 fatty acid supplementation on the metabolism of apolipoprotein B-100 and chylomicron remnants in men with visceral obesity. Am J Clin Nutr 2003;77:300–7. [DOI] [PubMed] [Google Scholar]
- [213].Munro IA, Garg ML. Dietary supplementation with long chain omega-3 polyunsaturated fatty acids and weight loss in obese adults. Obes Res Clin Pract 2013;7:e173–1. [DOI] [PubMed] [Google Scholar]
- [214].Ramel A, Parra D, Martinez JA, Kiely M, Thorsdottir I. Effects of seafood consumption and weight loss on fasting leptin and ghrelin concentrations in overweight and obese European young adults. Eur J Nutr 2009;48:107–14. [DOI] [PubMed] [Google Scholar]
- [215].Rodacki CL, Rodacki AL, Pereira G, Naliwaiko K, Coelho I, Pequito D, et al. Fish-oil supplementation enhances the effects of strength training in elderly women. Am J Clin Nutr 2012;95:428–36. [DOI] [PubMed] [Google Scholar]