Abstract
The objective of this study is to investigate the effects of sea buckthorn oil (SBO) on proliferation, adipogenic differentiation and insulin sensitivity of 3T3-L1 cells. Results showed that SBO increased cell proliferation ability, accompanied by up-regulated proliferating cell nuclear antigen content (p < 0.05) and p38 activity (p < 0.05). SBO also promoted adipogenesis and enhanced adipogenic transcriptional factors expression. Mitochondrial biogenesis related gene expressions were elevated in SBO treated cells (p < 0.05). Of note, SBO also increased glucose uptake and glucose transporter 4 abundance (p < 0.05). Cells treated with SBO exhibited greater phosphorylated insulin receptor substrate 1 (p < 0.05), phosphorylated-Akt (p < 0.05) and phosphorylated AMP-activated protein kinase (p < 0.01) contents. When taken together, these results suggest that SBO promotes 3T3-L1 cells proliferation, adipogenesis and insulin sensitivity.
Keywords: Sea buckthorn oil, Proliferation, Adipogenesis, Insulin sensitivity, AMP-activated protein kinase
Introduction
Type 2 diabetes mellitus (T2DM) is characterized by lower insulin utilization ability in metabolic organs and tissues, causing elevated circulating blood glucose concentration and resulting side effects, including hypertension, dyslipidemia, arteriosclerosis, obesity and even cancer (Lewis et al., 2002). Insulin resistance is the main contributor to T2DM pathogenesis; high insulin sensitivity allows cells to utilize blood glucose more effectively, while lower insulin sensitivity may cause failure of insulin-sensitive tissues to respond to insulin, and eventually lead to T2DM (Zeyda and Stulnig, 2009). Adipose tissue is a highly active metabolic and endocrine organ, playing an important role in regulating whole body glucose homeostasis. Adipocyte effectively sequestrates fatty acids in the form of triglycerides, irregular functioning results in higher level of circulating fatty acids, further inducing insulin resistance (Delarue and Magnan, 2007). As a result, attempts to identify therapeutic agents which may enhance insulin sensitivity has received considerable attention. Plants possess the potential of increasing insulin sensitivity, which may provide an alternative strategy for developing future effective, safe antidiabetic drugs (Eddouks et al., 2014).
Sea buckthorn (Hippophae rhamnoides L.) is a spiny deciduous shrub, which for centuries has been widely used in traditional medicine in both Asia and Europe (Suryakumar and Gupta, 2011). All parts of sea buckthorn plant contain substantial volumes of bioactive substances, which have varied health benefits, including anti-inflammatory properties, anticancer, antioxidant, and anti-atherosclerotic effects, among others (Olas, 2018). Observed in diet-induced obesity within mice, supplementation of sea buckthorn leaves extract ameliorates adiposity and insulin resistance through suppressing lipogenesis while increasing energy expenditure (Kwon et al., 2017). Oils extracted from sea buckthorn berry are abundant in fat-soluble vitamins and plant sterols, and has been successfully used in the cosmetic industry for improving dry, flaky or rapidly aging skin in addition to skin regeneration and repair treatments (Zielińska and Nowak, 2017). Moreover, a previous study demonstrates that sea buckthorn fruit oil extract alleviates insulin resistance in both type 2 diabetes mellitus cells found within rats (Gao et al., 2017). As noted above, sea buckthorn bioactive components possess anti-obesity effects, but the underlying mechanism for this remains unclear.
It is well established that AMP-activated protein kinase (AMPK) is the central regulator of cellular and organismal metabolism. AMPK activation promotes glucose uptake, insulin sensitivity, mitochondrial biogenesis and fatty acid oxidation, while dysfunctions contribute to development of insulin resistance (Ruderman et al., 2013). Numerous pharmacological agents and natural compounds can directly or indirectly activate AMPK, and some of these agents are currently used as insulin sensitizers to improve hyperglycemia (Coughlan et al., 2014). Considering the role of adipocytes in glucose homeostasis and insulin sensitivity, we hypothesize that sea buckthorn oil (SBO) enhances insulin sensitivity in adipocyte through activation of AMPK. The objective of our present study is to explore the effects and mechanisms of SBO on 3T3-L1 pre-adipocytes proliferation, differentiation and insulin sensitivity.
Materials and methods
Cell culture and adipogenic induction
Mouse 3T3-L1 pre-adipocytes were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). Cells were grown in Dulbecco’s Modified Eagle Medium (DMEM, #SH30021.01, HyClone, Logan, UT, USA) containing 10% fetal bovine serum (FBS, #10099-141, Gibco, Grand Island, NY) and 1% penicillin–streptomycin-l-glutamine (#G0200, Solarbio, Beijing, China) in a 37 °C incubator with 5% CO2. Preadipocytes were induced to adipogenic differentiation with and without SBO (10−4 and 10−5, volume of SBO/volume of medium (V/V)) following previous described protocol (Ukita et al., 2016). The SBO (YZ111627) was purchased from Beijing Solarbio Science & Technology Co., Ltd. (Beijing, China).
Lactate dehydrogenase (LDH) assay
A cytotoxic assay was performed using a Cytotoxicity LDH Assay Kit (CK28, Dojindo Co., Ltd., Kumamoto, Japan) following the provided manual. The 3T3-L1 pre-adipocytes were cultured in 96-well plates in a growth medium overnight. Following this, mediums containing different contents of SBO (0, 10−7, 10−6, 10−5 and 10−4, V/V) were changed, and cells were cultured for 24 h in a 37 °C incubator with 5% CO2. Lysis buffer (#LH736 CK17, Dojindo Co., Ltd., Kumamoto, Japan) (20 μL) was then added and a plate was incubated for 30 min. Upon completion, cell suspension was removed to a new 96-well plate and mixed with 100 μL of working solution. Upon incubation for 30 min in the dark at 25 °C, 50 μL of stop solution was added, and absorbance at 490 nm was measured using microplate reader (Synergy H1, Winooski, VT, USA). Cell toxicity was calculated based on the formula provided by the manufacturer.
CCK8 assay
Cell proliferation was evaluated using a Cell Counting Kit-8 (CCK8, Solarbio Science & Technology Co., Ltd., Beijing, China) in accordance with the manufacturer’s instructions. Briefly, cells seeded at 1 × 103 cells per well in a 96-well plate were cultured in a 37 °C overnight. Upon the cells adhering to the bottom, mediums containing different concentrations of SBO (0, 10−7, 10−6, 10−5 and 10−4, V/V) replaced the culture for further 24 h. Upon completion, 10 μL of CCK8 solution was added to a cultured medium, cells were cultured for 2 h prior to testing. The absorbance was measured at 450 nm using a microplate reader (Synergy H1, Winooski, VT, USA).
EdU staining
The EdU assay was performed using Cell-Light EdU Apollo567 In Vitro Kit (C10310-1, RiboBio, Guangzhou, China). Cells were seeded at 4 × 103 per in a 96-wells plate and cultured in a medium which may or may not contain SBO (0, 10−5 and 10−4, V/V) for 24 h. After this, EdU reagent (50 μM) was added and cells were further incubated for 2 h. After removing the medium cells were washed twice with phosphate-buffered saline (PBS, # PYG14190, Boster Biological Technology Co., Ltd., Wuhan, China), and fixed with 4% paraformaldehyde at room temperature for 30 min, and permeabilized with 0.5% Triton X-100 (#V900502, Sigma-Aldrich, St. Louis, MO, USA) for a period of 10 min. Cells were then washed with PBS, cultured with 1 × Apollo dyeing reaction solution for 30 min in the dark at 25 °C, and stained using Hoechst 33342 (#S0116, RiboBio, Guangzhou, China). Pictures were captured using a DMi8 fluorescence microscope (Leica, Wetzlar, Germany).
Oil Red O staining
The 3T3-L1 pre-adipocytes were induced to adipogenesis with (10−5 and 10−4, V/V) or without SBO for a 6-day period, lipid droplets in adipocytes were stained with an Oil red O solution (Sigma Chemical Co., St. Louis, MO, USA). Differentiated cells were rinsed 3 times with PBS and fixed with 10% formaldehyde for 10 min. The fixed adipocytes were washed with 60% isopropyl alcohol for 15 s, and then rinsed 3 times with PBS and stained with 0.2% (w/v) Oil-red-O solution for 1 h. Following this, plates were rinsed twice with 60% isopropanol and washed 3 times with PBS. Images were acquired at a 100 × magnification with a DMi8 microscope (Leica, Wetzlar, Germany).
Quantitative Real-time PCR (qRT-PCR)
The qRT-PCR was performed using the established lab protocol (Li et al., 2020). Total RNA was extracted used Trizol reagent (Sigma, Saint Louis, MO, US), and cDNA was synthesized using a reverse transcription kit (TAKARA Co, Ltd, Dalian, China). The qRT-PCR was performed using a SYBR Green qRT-PCR kit (TAKARA Co, Ltd Dalian, China) and CFX RT-PCR detection system (BioRad, Hercules, CA). The method was employed for relative target genes expression analysis. The primer sequences were shown in Table 1.
Table 1.
Primer sequences for q-RT-PCR
| Name | Sequence (5′–3′) | Accession Number |
|---|---|---|
| PCNA |
GAACCTCACCAGCATGTCCA TGGGATTCCAAGTTGCTCCA |
NM_011045.2 |
| PPARγ |
ACCTCTGCTGGGGATCTGAA ATCACGGAGAGGTCCACAGA |
NM_001127330.2 |
| C/EBPα |
CCCTTGCTTTTTGCACCTCC TGCCCCCATTCTCCATGAAC |
NM_001287514.1 |
| aP2 |
GGATTTGGTCACCATCCGGT TTCCATCCCACTTCTGCACC |
NM_024406.3 |
| Glut4 |
CTAGGCATCAATGCTGTTTTCTA CGAGACCAACGTGAAGACCGTATT |
AB008453.1 |
| 18S rRNA |
GTAACCCGTTGAACCCCATT CCATCCAATCGGTAGTAGCG |
M35283.1 |
Western blotting
Western blotting was performed using the established protocol in our lab (Li et al., 2020). Briefly, cells grown on 12-well plates were harvested and lysed in an ice-cold lysis buffer. Soluble protein was separated through homogenization and centrifugation (12,000 × g for 10 min at 4 °C). Samples were then subjected to SDS-PAGE for protein separation, and isolated protein was transferred to nitrocellulose membrane. After blocking with 5% skim milk power (Sangon Biotech Co, Ltd., Shanghai, China) in PBS for 1 h, the membrane was subjected to a first antibody (4 °C, overnight), followed by a secondary antibody (room temperate, 1 h). The immunoblotting bands in the membrane were scanned and analyzed using an Odyssey Infrared Imaging System (LI-COR Biosciences, Lincoln, NE, US).
The following antibodies were used: Proliferating cell nuclear antigen (PCNA, bs-0754R), p-p38 MAPK (bs-2210R), peroxisome proliferator-activated receptor γ (PPARγ, bs-4590R), CCAAT-enhancer-binding protein α (C/EBPα, bs-1630R), Insulin receptor substrate 1 (IRS, bs-0172R), p-IRS (bs-0172R) and p-Akt (bsm-33280 M) were obtained from Biosynthesis Biotechnology Co., Ltd. (Beijing, China). p38 AMPK (#9212), glucose transporter 4 (Glut4, #2213), Akt (#9272), p-AMPKα (#2535) and AMPKα (#2532) were purchased from Cell Signaling (Danvers, MA, USA). Superoxide dismutase 1 (SOD, no. sc-8637), CAT (no. sc34281), glutathione peroxidase 4 (GPx-4, no. sc-50497) and fatty acid binding protein (aP2, sc-18661) were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, US). The goat anti-mouse secondary antibody (926-68070) and anti-rabbit secondary antibody (926-32211) were sourced from LI-COR Biosciences (LI-COR Biosciences, Lincoln, NE, US).
Glucose uptake assay
Glucose uptake was determined using 2-(N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl) amino)-2-deoxyglucose (2-NBDG, Life Technologies, Carlsbad, CA, USA). Briefly, 3T3-L1 pre-adipocytes were induced into differentiation in an adipogenic induction medium containing with (10−5 and 10−4, V/V) or without SBO for 6 days. The differentiated adipocytes were then serum-starved in DMEM for 4 h, washed with PBS for 3 times, and incubated with 100 μM 2-NBDG for 30 min at 37 °C. Upon washing twice with cold PBS, images were acquired using a DMi8 fluorescence microscope (Leica, Wetzlar, Germany).
Enzyme-linked immunosorbent assay
Cells seeded in a 6-well plate were induced into adipogenic differentiation with (10−5 and 10−4, V/V) or without SBO for 3 days. Upon washing with PBS, cells were cultured in DMEM medium (no serum) containing 10 μL of MDCFH-DA for 30 min at 37 °C. Then, cells were collected and centrifuged at 1000 × g for 5 min at 4 °C. Finally, cell pellets were suspended with PBS and used for reactive oxygen species content analysis (E004, Nanjing Jiancheng Bioengineering Institute, Nanjing, Jiangsu, China).
Statistical analysis
Data has been expressed as mean ± standard error of the mean (SEM) and normality and homogeneity of variance were determined using the Shapiro–Wilk and the BrownForsythe test using GraphPad Prism 7 software (Monrovia, CA, USA). The student’s t test (LDH assay data) or one-way analysis of variance followed by Duncan’s multiple-range test for post hoc were employed for all data analysis. A p < 0.05 was considered as significant for all data.
Results and discussion
Effect of SBO on 3T3-L1 pre-adipocytes proliferation
Cytotoxicity is one a key indicator in evaluating effects of a certain chemical compound on a given cell line. Within our study, the LDH assay was employed to determine the cytotoxicity of SBO on 3T3-L1 pre-adipocytes. As shown in Fig. 1A, the addition of SBO at specific amounts (from 10−4 to 10−7, V/V) did not induce cellular cytotoxicity, indicating SBO’s safety for further potential application. Similarly, a study of the effects of SBO on burn wounds demonstrates SOB displayed no associated toxicity or side effects in rats (Upadhyay et al., 2009).
Fig. 1.
Sea buckthorn oil (SBO) promoted mouse 3T3-L1 pre-adipocytes proliferation. (A) Lactate dehydrogenase (LDH) assay was used to evaluated cellular cytotoxicity under different concentration of SBO. (B) Cell Counting Kit-8 (CCK8) assay. (C) 5-Ethynyl-2′-deoxyuridine (EdU) staining. (D) Proliferating Cell Nuclear Antigen (PCNA) mRNA expression. (E) PCNA protein content. f Both p38 MAPK and p-p38 MAPK protein abundances. (Mean ± SEM, n = 6; scar bar stands for 100 µm; Different letters indicate significant difference; **p < 0.01)
The ability to form new fat cells through adipogenesis is critical to healthy adipose tissue distribution (Vishvanath and Gupta, 2019). Adipogenesis is a tightly regulated process, which involves preadipocyte proliferation and adipocyte differentiation. It is well-established that mitotic clonal expansion or proliferation is a prerequisite for adipogenic differentiation in 3T3-L1 cells (Tang et al., 2003). The CCK8 assay suggests that 3T3-L1 proliferative ability improved when the cells were treated with 10−6, 10−5 and 10−4 SBO compared to control cells (p < 0.01, Fig. 1B). Moreover, cells which received both 10−5 and 10−4 SBO effectively increased their EdU-positive number (Fig. 1C). The data clearly indicates that SBP promoted 3T3-L1 preadipocytes proliferation. PCNA is one of the most reliable markers for evaluating cell proliferation ability. Worthy of note, a previous study shows that extracts from sea buckthorn berries increase PCNA expression in acetic acid‐induced gastric ulcers within rats (Xu et al., 2007). Compared with control cells, 10−5 and 10−4 SBO treatment increased PCNA mRNA content, with no difference observed between 10−5 and 10−4 SBO treated cells (p < 0.05, Fig. 1D). As anticipated, PCNA protein abundance showed consistent manner as mRNA expression (p < 0.05, Fig. 1E).
To order to explore underlying proliferative mechanisms, p38 MAPK protein abundance was measured. Although p38 MAPK protein abundance did not vary among groups, cells received 10−4 SBO showed increased phospous-p38 MAPK content (p < 0.05, Fig. 1F), and no difference was found between control and 10−5 SBO treated cells. It has been well established that C/EBPβ is necessary for 3T3-L1 preadipocytes proliferation, and that DNA binding activity is dependent on sequentially phosphorylation exerted by p38 MAPK and glycogen synthase kinase-3β (Guindi et al., 2018), and specific inhibitors of p38 mitogen-activated protein kinase block 3T3-L1 adipogenesis (Engelman et al., 1998). As such, SBO promoted 3T3-L1 preadipocytes proliferation might be possible through activation of p38 MAPK.
SBO enhanced 3T3-L1 pre-adipocytes differentiation and mitochondrial biogenesis
Adipogenesis is tightly regulated by several transcriptional factors, with both PPARγ and C/EBPα playing key roles. During this process, early-phase transcription factors, including C/EBPβ and C/EBPδ, activate both C/EBPα and PPARγ expressions, whose expressions in turn transcriptionally activate target genes expression which characterize adipocyte phenotype (Rosen and MacDougald, 2006). As Fig. 2A shows, cells treated with both 10−5 and 10−4 SBO showed more lipid droplets compared with that of the control group. Both C/EBPα and aP2 mRNA contents were both elevated in a dose dependent manner when cells received SBO (control < 10−5 < 10−4, p < 0.05, Fig. 2B). Compared with control cells, cells subjected to both 10−5 and 10−4 SBO exhibited greater PPARγ mRNA content, there was no difference between 10−5 and 10−4 SBO treated cells (p < 0.05, Fig. 2B). Treated cells with 10−4 SBO increased both PPARγ and aP2 protein abundance (p < 0.05, Fig. 2C), whereas no difference observed in the other two groups. Likewise, SBO treatment effectively increased PPARγ protein levels (p < 0.05, Fig. 2C). Considering the relationship between cell proliferation and adipogenic differentiation, SBO may facilitate adipogenesis through accelerating mitotic clonal expansion.
Fig. 2.
Effect of SBO on adipogenic differentiation of 3T3-L1 pre-adipocytes. (A) Oil-Red-O staining after 11 days of adipogenic differentiation. (B) mRNA contents of adipogenic related genes, including proliferator-activated receptor γ (PPARγ), CCAAT/enhancer binding protein α (C/EBPα), and fatty acid binding protein 4 (aP2). (C) PPARγ, C/EBPα and aP2 protein contents. (D) mRNA expression of nuclear respiratory factor 1, mitochondrial transcription factor A and cyclooxygenase 2. (Mean ± SEM, n = 6; Different letters indicate significant difference)
As the main site for fat storage, healthy adipose tissues prevent ectopic fat accumulation in muscle or liver and enhances insulin sensitivity. Previous studies demonstrate that increased adipocyte expansion (Kim et al., 2007) or hyperplasia (Wang et al., 2017) improves insulin sensitivity due to enhanced energy storage capacity of adipose tissues. Constant across results, insufficient adipogenesis from preadipocytes limits adipocyte number and hampers the replacement of dead adipocytes, which are mainly responsible for adipose inflammation and metabolic dysfunction (Longo et al., 2019). Therefore, our data may suggest the positive clinical aspects of SBO. Additionally, white adipose tissue is important endocrine and secretory organ. Adipose tissue actively communicates by sending and receiving different types of signals to perform normal physiological functions (Wang et al., 2008). Therefore, the enhanced adipocytes differentiation by SBO might alter physiological functions, details and mechanisms require further investigation in animal models.
In the present study, we found that both 10-5 and 10-4 SBO treatments increased mRNA contents of nuclear respiratory factor 1 (NRF-1), mitochondrial transcription factor A (TFAM) and cyclooxygenase-2 (COX2) (p < 0.05, Fig. 2D), suggesting that SBO promoted mitochondria biogenesis. Mitochondria are important for energy metabolism in many tissue types, including skeletal muscle, liver and adipose tissue (Cedikova et al., 2016). The role of mitochondria in white adipocytes has long been neglected due to their low abundance (Woo et al., 2019). Given that various crucial metabolic processes, such as fatty acid oxidation, oxidative phosphorylation and ROS production take place in mitochondria, they are essential for maintaining metabolic homeostasis in white adipocytes (Boudina and Graham, 2014). Indeed, mitochondrial dysfunctions in white adipocytes induces whole body pathological consequences (Woo et al., 2019). Our data indicates that SBO not only increased the number of adipocytes but also enhances their energy and metabolic capacity. Moreover, a previous study suggests that impaired mitochondrial biogenesis in white adipose tissue is related to insulin resistance (Heinonen et al., 2015). Meanwhile, insulin sensitizer rosiglitazone induces mitochondrial biogenesis and remodeling during 3T3-L1 cell differentiation (Wilson-Fritch et al., 2003). Therefore, our results further suggested that SBO could be used as insulin sensitizer by promoting mitochondria biogenesis.
SBO stimulated glucose uptake and affected Glut4 expression
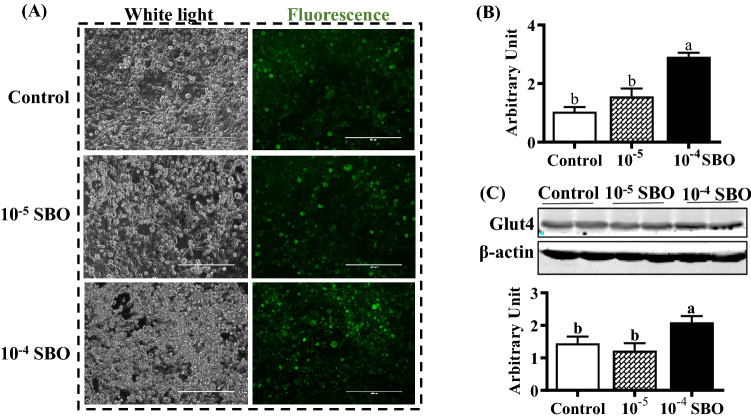
Adipocytes play a crucial role in insulin stimulated glucose uptake, relying on glucose transporter 4. As SBO may increase white adipogenic differentiation potential, we have further explored whether glucose uptake ability was altered. The 2-NBDG assay was employed to evaluate the effect of SBO on glucose uptake. As shown in Fig. 3A, fluorescence intensity in differentiated adipocytes was enhanced by SBO in a dose dependent manner (control < 10−5 < 10−4, p < 0.05). The qRT-PCR results indicated that Glut4 mRNA expression was elevated when cells were treated with 10−4 SBO (p < 0.05, Fig. 3B), and there was no difference between control and 10−5 SBO treated cells. As anticipated, the addition of 10−4 SBO increased the Glut4 protein content (p < 0.05, Fig. 3C). Similarly, a previous study undertaken identifies polyphenols from sea buckthorn stimulating glucose metabolism and increasing insulin sensitivity (Cao et al., 2019).
Fig. 3.
SBO stimulated glucose uptake and promoted glucose transporter 4 (glut4) expression (A) 2-(N-(7-Nitrobenz-2-oxa-1,3-diazol-4-yl)Amino)-2-Deoxyglucose (2-NBDG) uptake assay. (B) Glut4 mRNA level in different groups. (C) Protein contents of glut4. (Mean ± SEM, n = 6; Different letters indicate significant difference)
SBO addition affected insulin receptor substrate 1 (IRS)/Akt and AMPK activity
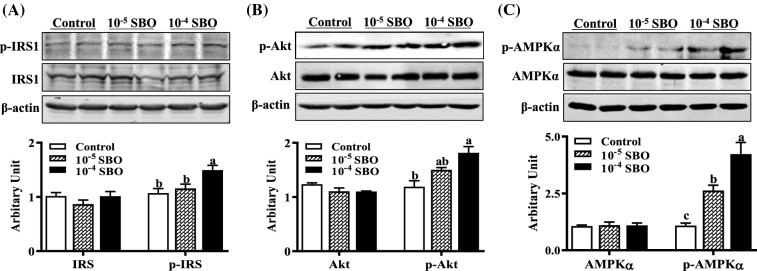
To investigate the mechanisms which stimulate glucose uptake and glut4 expression by SBO, effects of SBO on related cellular signaling pathways were evaluated. Within well-established insulin mediated PI3K/Akt pathway, the binding of insulin to its receptor activates insulin receptor tyrosine kinase, which then induces IRS1 phosphorylation causing PI3K and Akt activation and leading to GLUT4 translocation and glucose uptake. As shown in Fig. 4A, addition of 10−4 SBO increased phosphorus-IRS1 content (p < 0.05). Compared with control cells, phosphorus-Akt levels were elevated when cells were treated with 10−4 SBO (p < 0.05, Fig. 4B). This data suggests that SBO stimulated glucose uptake through activation of insulin-Akt signaling pathway, and are in line with previous reporting, demonstrating that sea buckthorn fruit oil activates PI3K/Akt signaling pathway in T2DM cells and rats (Gao et al., 2017). Additionally, PPARγ serves as the molecular target for insulin sensitizing drugs (Li and Glass, 2004), the SBO stimulated PPARγ, which may also contribute to the elevated insulin sensitivity.
Fig. 4.
SBO activated insulin receptor substrate 1 (IRS1), Akt and AMP-activated protein kinase (AMPK). (A) Both p-IRS1 and IRS1 protein abundances. (B) Both p-Akt and Akt. (C) Both AMPK and p-AMPK abundances. (Mean ± SEM, n = 6; Different letters indicate significant difference)
AMPK is an evolutionary conserved cellular energy sensor, its activation elicits insulin-sensitizing effects, making it an ideal therapeutic target for T2DM (Coughlan et al., 2014). Indeed, a wide-variety of natural extracts and chemicals from herbal medicines promote glucose uptake through activation of AMPK (Hardie, 2013). AMPK regulates glut4 transcription by directly phosphorylating histone deacetylase 5 transcriptional repressor (McGee et al., 2008), also affecting GLUT4 translocation and glucose transportation activity in 3T3-L1 adipocytes (Yamaguchi et al., 2005). Although AMPK did not vary among different groups, both 10−5 and 10−4 SBO addition effectively elevated phosphorylated AMPK content (p < 0.01, Fig. 4C), which may contribute to elevated glut4 expression and insulin sensitivity. Interestingly, AMPK activity is known to negatively correlated with adipogenesis ability, the mechanism by which SBO activates AMPK and simultaneously promotes adipogenic differentiation requires further study.
In conclusion, present results suggest that SBO serves as an insulin sensitizer in adipocytes. SBO promoted 3T3-L1 preadipocytes proliferation and adipogenic differentiation. While at the same time, SBO increased insulin sensitivity, glut4 expression and glucose uptake ability, may occur through activation of Akt and AMPK.
Acknowledgements
This work was supported by National Natural Science Foundation of China (31972559) and Modern Agro-industry Technology Research System (CARS-38).
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ting Zhang, Email: 378812259@qq.com.
Xuze Qin, Email: 437289649@qq.com.
Yuxin Cao, Email: 578584835@qq.com.
Jianxin Zhang, Email: sxndzjx@163.com.
Junxing Zhao, Email: Junxzh@sxau.edu.cn.
References
- Boudina S, Graham TE. Mitochondrial function/dysfunction in white adipose tissue. Exp. Physiol. 2014;99:1168–1178. doi: 10.1113/expphysiol.2014.081414. [DOI] [PubMed] [Google Scholar]
- Cao H, Ou J, Chen L, Zhang Y, Szkudelski T, Delmas D, Xiao J. Dietary polyphenols and type 2 diabetes: human study and clinical trial. Crit. Rev. Food. Sci. Nutr. 2019;59:3371–3379. doi: 10.1080/10408398.2018.1492900. [DOI] [PubMed] [Google Scholar]
- Cedikova M, Kripnerová M, Dvorakova J, Pitule P, Grundmanova M, Babuska V, Kuncova J. Mitochondria in white, brown, and beige adipocytes. Stem Cells Int. 2016;2016:1–11. doi: 10.1155/2016/6067349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlan KA, Valentine RJ, Ruderman NB, Saha AK. AMPK activation: a therapeutic target for type 2 diabetes? Diabetes Metab. Syndr. Obes. 2014;7:241–253. doi: 10.2147/DMSO.S43731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delarue J, Magnan C. Free fatty acids and insulin resistance. Curr. Opin. Clin. Nutr. Metab. Care. 2007;10:142–148. doi: 10.1097/MCO.0b013e328042ba90. [DOI] [PubMed] [Google Scholar]
- Eddouks M, Bidi A, El Bouhali B, Hajji L, Zeggwagh NA. Antidiabetic plants improving insulin sensitivity. J. Pharm. Pharmacol. 2014;66:1197–1214. doi: 10.1111/jphp.12243. [DOI] [PubMed] [Google Scholar]
- Engelman JA, Lisanti MP, Scherer PE. Specific inhibitors of p38 mitogen-activated protein kinase block 3T3-L1 adipogenesis. J. Biol. Chem. 1998;273:32111–32120. doi: 10.1074/jbc.273.48.32111. [DOI] [PubMed] [Google Scholar]
- Gao S, Guo Q, Qin C, Shang R, Zhang Z. Sea buckthorn fruit oil extract alleviates insulin resistance through the PI3K/Akt signaling pathway in type 2 diabetes mellitus cells and rats. J. Agr. Food Chem. 2017;65:1328–1336. doi: 10.1021/acs.jafc.6b04682. [DOI] [PubMed] [Google Scholar]
- Guindi C, Cloutier A, Gaudreau S, Zerif E, McDonald PP, Tatsiy O, Amrani A. Role of the p38 MAPK/C/EBPβ pathway in the regulation of phenotype and IL-10 and IL-12 production by tolerogenic bone marrow-derived dendritic cells. Cells. 2018;7:256. doi: 10.3390/cells7120256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie DG. AMPK: a target for drugs and natural products with effects on both diabetes and cancer. Diabetes. 2013;62:2164–2172. doi: 10.2337/db13-0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinonen S, Buzkova J, Muniandy M, Kaksonen R, Ollikainen M, Ismail K, Vuolteenaho K. Impaired mitochondrial biogenesis in adipose tissue in acquired obesity. Diabetes. 2015;64:3135–3145. doi: 10.2337/db14-1937. [DOI] [PubMed] [Google Scholar]
- Kim JY, De Wall EV, Laplante M, Azzara A, Trujillo ME, Hofmann SM, Scherer PE. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J. Clin. Investig. 2007;117:2621–2637. doi: 10.1172/JCI31021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon EY, Lee J, Kim Y, Do A, Choi JY, Cho SJ, Choi MS. Seabuckthorn leaves extract and flavonoid glycosides extract from seabuckthorn leaves ameliorates adiposity, hepatic steatosis, insulin resistance, and inflammation in diet-induced obesity. Nutrients. 2017;9:569. doi: 10.3390/nu9060569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis GF, Carpentier A, Adeli K, Giacca A. Disordered fat storage and mobilization in the pathogenesis of insulin resistance and type 2 diabetes. Endocr. Rev. 2002;23:201–229. doi: 10.1210/edrv.23.2.0461. [DOI] [PubMed] [Google Scholar]
- Li AC, Glass CK. PPAR-and LXR-dependent pathways controlling lipid metabolism and the development of atherosclerosis. J. Lipid Res. 2004;45:2161–2173. doi: 10.1194/jlr.R400010-JLR200. [DOI] [PubMed] [Google Scholar]
- Li Q, Zhang T, Zhang R, Qin X, Zhao J. All-trans retinoic acid regulates sheep primary myoblast proliferation and differentiation in vitro. Domest. Anim. 2020;71:106394. doi: 10.1016/j.domaniend.2019.106394. [DOI] [PubMed] [Google Scholar]
- Longo M, Zatterale F, Naderi J, Parrillo L, Formisano P, Raciti GA, Beguinot C, Miele C. Adipose tissue dysfunction as determinant of obesity-associated metabolic complications. Int. J. Mol. Sci. 2019;20:2358. doi: 10.3390/ijms20092358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee SL, Van Denderen BJ, Howlett KF, Mollica J, Schertzer JD, Kemp BE, Hargreaves M. AMP-activated protein kinase regulates GLUT4 transcription by phosphorylating histone deacetylase 5. Diabetes. 2008;57:860–867. doi: 10.2337/db07-0843. [DOI] [PubMed] [Google Scholar]
- Olas B. The beneficial health aspects of sea buckthorn (Elaeagnus rhamnoides (L.) A. Nelson) oil. J. Ethnopharmacol. 2018;213:183–190. doi: 10.1016/j.jep.2017.11.022. [DOI] [PubMed] [Google Scholar]
- Rosen ED, MacDougald OA. Adipocyte differentiation from the inside out. Nat. Rev. Mol. Cell Biol. 2006;7:885–896. doi: 10.1038/nrm2066. [DOI] [PubMed] [Google Scholar]
- Ruderman NB, Carling D, Prentki M, Cacicedo JM. AMPK, insulin resistance, and the metabolic syndrome. J. Clin. Invest. 2013;123:2764–2772. doi: 10.1172/JCI67227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suryakumar G, Gupta A. Medicinal and therapeutic potential of Sea buckthorn (Hippophae rhamnoides L.) J. Ethnopharmacol. 2011;138:268–278. doi: 10.1016/j.jep.2011.09.024. [DOI] [PubMed] [Google Scholar]
- Tang QQ, Otto TC, Lane MD. Mitotic clonal expansion: a synchronous process required for adipogenesis. Proc Natl Acad. Sci. USA. 2003;100:44–49. doi: 10.1073/pnas.0137044100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ukita M, Yamaguchi T, Ohata N, Tamura M. Sclerostin enhances adipocyte differentiation in 3T3-L1 cells. J. Cell. Biochem. 2016;117:1419–1428. doi: 10.1002/jcb.25432. [DOI] [PubMed] [Google Scholar]
- Upadhyay NK, Kumar R, Mandotra SK, Meena RN, Siddiqui MS, Sawhney RC, Gupta A. Safety and healing efficacy of sea buckthorn (Hippophae rhamnoides L.) seed oil on burn wounds in rats. Food Chem. Toxicol. 2009;47:1146–1153. doi: 10.1016/j.fct.2009.02.002. [DOI] [PubMed] [Google Scholar]
- Vishvanath L, Gupta RK. Contribution of adipogenesis to healthy adipose tissue expansion in obesity. J. Clin. Invest. 2019;129:4022–4031. doi: 10.1172/JCI129191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Mariman E, Renes J, Keijer J. The secretory function of adipocytes in the physiology of white adipose tissue. J. Cell Physiol. 2008;216:3–13. doi: 10.1002/jcp.21386. [DOI] [PubMed] [Google Scholar]
- Wang B, Fu X, Liang X, Wang Z, Yang Q, Zou T, Du M. Maternal retinoids increase PDGFRα+ progenitor population and beige adipogenesis in progeny by stimulating vascular development. EBioMedicine. 2017;18:288–299. doi: 10.1016/j.ebiom.2017.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson-Fritch L, Burkart A, Bell G, Mendelson K, Leszyk J, Nicoloro S, Corvera S. Mitochondrial biogenesis and remodeling during adipogenesis and in response to the insulin sensitizer rosiglitazone. Mol. Cell. Biol. 2003;23:1085–1094. doi: 10.1128/MCB.23.3.1085-1094.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo CY, Jang JE, Lee SE, Koh EH, Lee KU. Mitochondrial dysfunction in adipocytes as a primary cause of adipose tissue inflammation. Diabetes Metab. 2019;43:247–256. doi: 10.4093/dmj.2018.0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Xie B, Pan S, Liu L, Wang Y, Chen C. Effects of sea buckthorn procyanidins on healing of acetic acid-induced lesions in the rat stomach. Asia. Pac. J. Clin. Nutr. 2007;16:234–238. [PubMed] [Google Scholar]
- Yamaguchi S, Katahira H, Ozawa S, Nakamichi Y, Tanaka T, Shimoyama T, Nagamatsu S. Activators of AMP-activated protein kinase enhance GLUT4 translocation and its glucose transport activity in 3T3-L1 adipocytes. Am. J. Physiol. Endocrinol. Metab. 2005;289:E643–E649. doi: 10.1152/ajpendo.00456.2004. [DOI] [PubMed] [Google Scholar]
- Zeyda M, Stulnig TM. Obesity, inflammation, and insulin resistance–a mini-review. Gerontology. 2009;55:379–386. doi: 10.1159/000212758. [DOI] [PubMed] [Google Scholar]
- Zielińska A, Nowak I. Abundance of active ingredients in sea-buckthorn oil. Lipids Health Dis. 2017;16:95. doi: 10.1186/s12944-017-0469-7. [DOI] [PMC free article] [PubMed] [Google Scholar]