Abstract
Duodenal hyperpermeability and low-grade inflammation in functional dyspepsia is potentially related to duodenal acid exposure. We aimed to evaluate in healthy volunteers the involvement of mast cell activation on the duodenogastric reflex and epithelial integrity during duodenal acidification. This study consisted of 2 parts: (1) Duodenal infusion of acid or saline during thirty minutes in a randomized, double-blind cross-over manner with measurement of intragastric pressure (IGP) using high resolution manometry and collection of duodenal biopsies to measure epithelial barrier function and the expression of cell-to-cell adhesion proteins. Mast cells and eosinophils were counted and activation and degranulation status were assessed. (2) Oral treatment with placebo or mast cell stabilizer disodiumcromoglycate (DSCG) prior to duodenal perfusion with acid, followed by the procedures described above. Compared with saline, acidification resulted in lower IGP (P < 0.01), increased duodenal permeability (P < 0.01) and lower protein expression of claudin-3 (P < 0.001). Protein expression of tryptase (P < 0.001) was increased after acid perfusion. Nevertheless, an ultrastructural examination did not reveal degranulation of mast cells. DSCG did not modify the drop in IGP and barrier dysfunction induced by acid. Duodenal acidification activates an inhibitory duodenogastric motor reflex and, impairs epithelial integrity in healthy volunteers. However, these acid mediated effects occur independently from mast cell activation.
Subject terms: Small intestine, Tight junctions, Translational research, Functional dyspepsia
Introduction
Functional gastrointestinal disorders (FGID) represent the most frequently diagnosed class of disorders in gastroenterology clinical practice1. Functional dyspepsia (FD) is one of the most common FGID occurring in up to 20% of the population and defined by the Rome IV criteria as the presence of dyspeptic symptoms in the absence of underlying organic, systemic or metabolic disease likely to explain the symptoms2. However, the pathophysiology of FD is incompletely elucidated, resulting in a paucity of effective treatment options.
Initial research focused on functional alterations of the stomach as a possible cause of dyspeptic symptoms, such as impaired accommodation, delayed emptying and hypersensitivity to distension3. More recent reports, however, point towards the duodenum as a central integrator in the pathophysiology of FD. One of the most consistent duodenal alterations is mucosal low-grade immune activation, mainly characterized by mast cell and eosinophil infiltration and activation4,5. The mechanism underlying low-grade inflammation in FD remains to be identified, but in a previous study, we demonstrated increased duodenal permeability, which was associated with low-grade inflammation5 and mast cell and eosinophil activation/degranulation4. We hypothesized that impaired barrier function allows increased transepithelial passage of luminal substances triggering an immune response, which in turn can activate sensory neurons causing symptoms or alter duodenogastric reflex pathways6. Mast cell activation has also been suggested to be involved in physiological conditions such as fat absorption7 and postprandial disruption of the migrating motor complex by cholecystokinin8.
Several potential players have been identified in the pathogenesis of the impaired barrier function in FD, including psychological stress, an altered bile acid pool and microbiota and acid exposure. Indeed, it has been shown that patients with FD display hypersensitivity to duodenal perfusion with acid and lipids3,9 and an increased acid exposure of the duodenum has been demonstrated in patients with FD10,11. Moreover, exogenous duodenal acid perfusion affects gastric sensorimotor function through duodenogastric reflex pathways in healthy volunteers, resulting in delayed gastric emptying, impaired gastric accommodation and hypersensitivity to gastric distension12–16. However, whether an increased duodenal acid exposure can also explain the observed increased duodenal permeability and low-grade inflammation in humans with FD, has not been studied.
Hence, the aim of the present study was to evaluate the effect of duodenal acid perfusion on barrier function and mast cell activation in healthy humans. Furthermore, we evaluated whether mast cell activation is required for activation of the duodenogastric reflex and acid-induced impairment of mucosal integrity by pretreatment with a mast cell stabilizer. Finally, we performed an ex vivo acid exposure study in wild type and mast cell deficient mice.
Results
Part 1: Duodenal acid perfusion decreases intragastric pressure, increases duodenal permeability and activates duodenal mast cells
Symptoms and duodenal pH
Duodenal saline/acid perfusion was performed in 10 healthy volunteers (3 men, 7 women; age 34.6 ± 4.2 years) to evaluate the effect of duodenal acidification on the duodenogastric reflex, mucosal barrier function and immune activation. No significant dyspeptic symptoms were induced during acid perfusion (all P > 0.1; supplementary Table S1). Acid perfusion significantly decreased the mean pH in the duodenum (7.29 ± 0.17 before vs. 3.94 ± 0.34 during perfusion; P = 0.002), whereas saline perfusion did not (7.34 ± 0.23 before vs. 7.13 ± 0.20 during perfusion; P = 0.25).
Intragastric pressure (IGP)
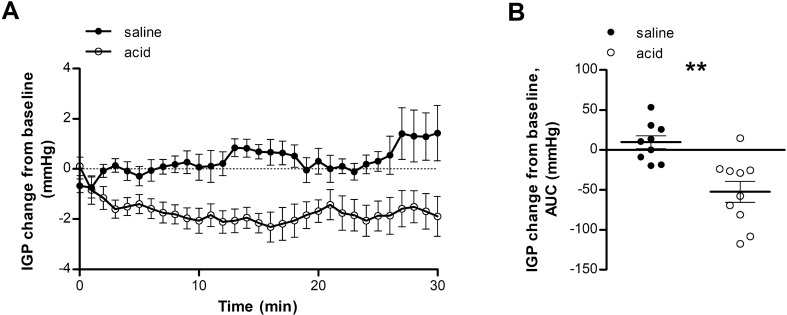
Perfusion with an acid solution resulted in activation of a duodenogastric reflex resulting in a relaxation of the proximal stomach, demonstrated by a decreased IGP compared with saline perfusion (AUC: − 52.4 ± 13.2 vs. 9.6 ± 8.1 mmHg; P = 0.003) (Fig. 1A,B).
Figure 1.
Duodenal acid perfusion relaxes the proximal stomach. IGP during saline perfusion (black dots) and acid perfusion (white dots) was evaluated using a high-resolution manometry catheter. (A) Time curve of IGP during saline or acid perfusion. (B) AUC of IGP during saline or acid perfusion. n = 9 for saline perfusion and n = 10 for acid perfusion. Data are mean ± SEM; **P < 0.01. AUC, area under the curve; IGP, intragastric pressure.
Duodenal mucosal barrier function
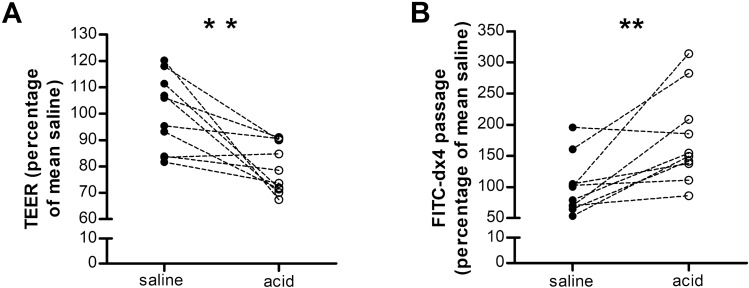
To determine mucosal integrity, duodenal biopsy samples were mounted in Ussing chambers to measure transepithelial electrical resistance (TEER) and permeability to 4 kDa dextran labeled to fluorescein isothiocyanate (FITC-dx4) after duodenal saline and acid perfusion. Compared to saline perfusion, acid perfusion resulted in a lower TEER (78.9 ± 2.9 vs. 100.0 ± 4.4%, P = 0.005) (Fig. 2A) and higher passage of FITC-dx4 (176.9 ± 23.1% vs. 100.0 ± 14.4%, P = 0.007) (Fig. 2B). These data indicate that duodenal acid perfusion impairs mucosal barrier function.
Figure 2.
Duodenal acid impairs the mucosal barrier functions. Mucosal barrier function after saline perfusion (black dots) and acid perfusion (white dots) was evaluated in Ussing chambers by measuring TEER (A) and passage of FITC-dx4 (B). n = 9 for saline perfusion and n = 10 for acid perfusion. Results are expressed relative to the mean of the control group; **P < 0.01. FITC-dx4, fluorescently labeled dextran of 4 kDa; TEER, transepithelial electrical resistance.
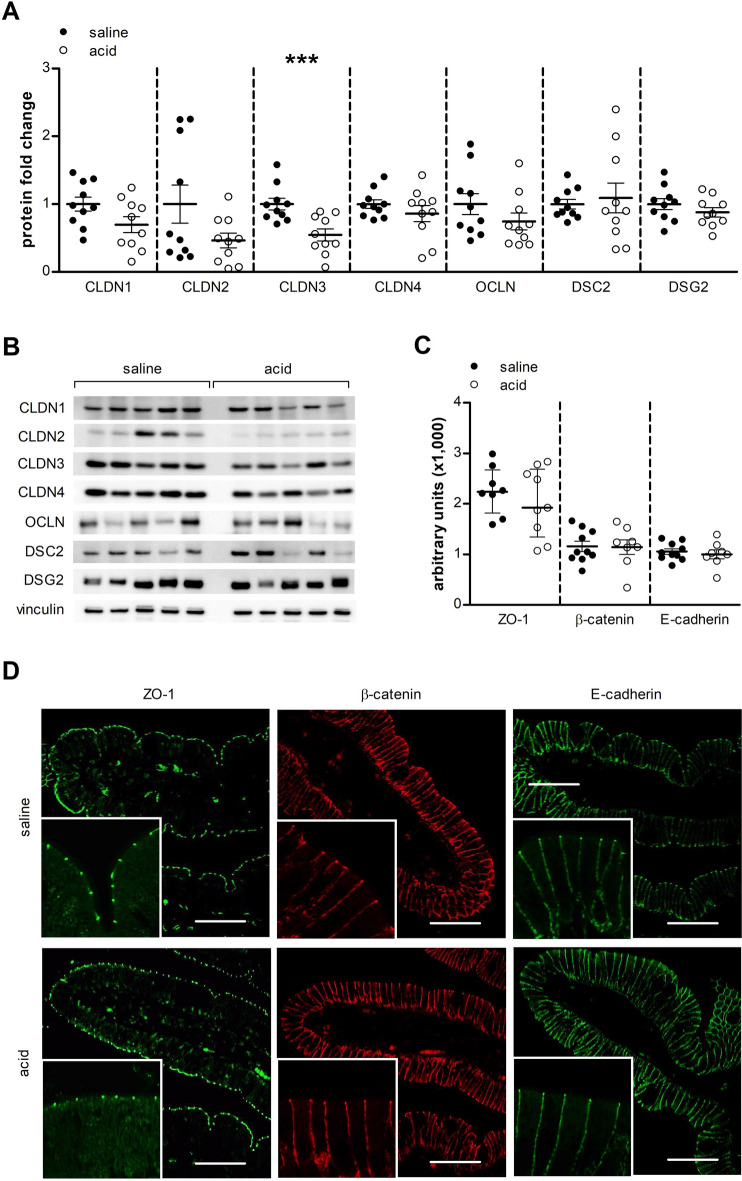
As we provided functional evidence that acid perfusion of the duodenum decreases barrier function, we investigated the expression of cell-to-cell adhesion proteins at each level of the intercellular junction after perfusion. Duodenal acid perfusion did not induce significant alterations in mRNA expression of the cell-to-cell adhesion proteins (Table 1). For the tight junction proteins, a decreased protein expression of CLDN3 (0.55-fold, P = 0.0006) was found after acid perfusion (Fig. 3A,B). The difference in protein expression of CLDN3 persisted after correction for multiple testing (P = 0.005). No difference was observed in protein expression of CLDN1 (0.70-fold, P = 0.12), CLDN2 (0.46-fold, P = 0.08), CLDN4 (0.86-fold, P = 0.37) and OCLN (0.74-fold, P = 0.26). Similarly, protein expression of the desmosomal proteins DSC2 (1.09-fold, P = 0.74) and DSG2 (0.88-fold, P = 0.13) was unaltered (Fig. 3A,B). Immunofluorescence analysis of the expression of the tight junction protein ZO-1 did not detect a difference between saline and acid perfusion (2261 ± 167 vs. 2027 ± 227 arbitrary fluorescence units; P = 0.56) (Fig. 3C,D). Duodenal acid perfusion did not affect the protein expression or localization at the adherens junction proteins β-catenin (1158 ± 102 vs. 1144 ± 148, P = 0.90) and E-cadherin (1056 ± 54 vs. 1001 ± 87, P = 0.90).
Table 1.
mRNA expression of cell-to-cell adhesion proteins and eosinophil and mast cell markers.
| Gene | Saline | Acid | P value |
|---|---|---|---|
| CLDN1 | 1.06 ± 0.14 | 0.93 ± 0.13 | 0.39 |
| CLDN2 | 0.82 (0.65–2.08) | 0.93 (0.57–1.60) | 0.74 |
| CLDN3 | 1.08 (0.87–1.19) | 1.00 (0.76–1.02) | 1.00 |
| CLDN4 | 1.02 ± 0.07 | 1.15 ± 0.13 | 0.27 |
| OCLN | 1.03 ± 0.09 | 0.91 ± 0.09 | 0.29 |
| ZO1 | 1.01 ± 0.05 | 1.07 ± 0.13 | 0.81 |
| ZO2 | 1.02 ± 0.07 | 0.97 ± 0.10 | 0.84 |
| ZO3 | 1.02 ± 0.06 | 0.96 ± 0.12 | 0.83 |
| β-catenin | 1.00 ± 0.03 | 0.93 ± 0.07 | 0.53 |
| E-cadherin | 1.02 ± 0.06 | 0.89 ± 0.12 | 0.46 |
| DSC2 | 1.02 ± 0.06 | 0.95 ± 0.10 | 0.80 |
| DSG2 | 1.05 ± 0.11 | 0.85 ± 0.15 | 0.18 |
| PRG2 | 1.04 ± 0.11 | 0.93 ± 0.08 | 0.81 |
| TPSAB1 | 2.08 ± 0.58 | 1.60 ± 0.56 | 0.20 |
Real-time RT-PCR was used to evaluate the gene expression of cell-to-cell adhesion proteins (upper panel) and an eosinophil marker (PRG2) and a mast cell marker (TPSAB1) (lower panel). Data are mean ± SEM or median (IQR). CLDN, claudin; OCLN, occludin; ZO, zonula occludens; DSC2, desmocollin-2; DSG2, desmoglein-2; PRG2, eosinophil major basic protein; TPSAB1, tryptase Alpha/Beta 1.
Figure 3.
Expression of cell-to-cell adhesion proteins in the duodenal mucosa. (A) Protein expression of CLDN1-4, OCLN, DSC2 and DSG2 was evaluated by western blot (n = 10 for both groups). (B) Representative western blot of five saline perfused and five acid perfused subjects. Bands were cropped from different parts of the same gel, or from different gels. (C) Protein expression and localization of ZO-1 (n = 8 saline and n = 9 acid), β-Catenin (n = 10 saline and n = 8 acid) and E-cadherin (n = 10 saline and n = 8 acid) was assessed by immunofluorescence after intraduodenal saline (black dots) and acid (white dots). (D) Representative confocal images in mucosal biopsy specimens obtained after saline (top) and acid (bottom) perfusion. Scale bars: 50 µm. Data are mean ± SEM; ***P < 0.001. CLDN, claudin; DSC2, desmocollin-2; DSG2, desmoglein-2; OCLN, occludin; ZO-1, zonula occludens 1.
Low-grade inflammation
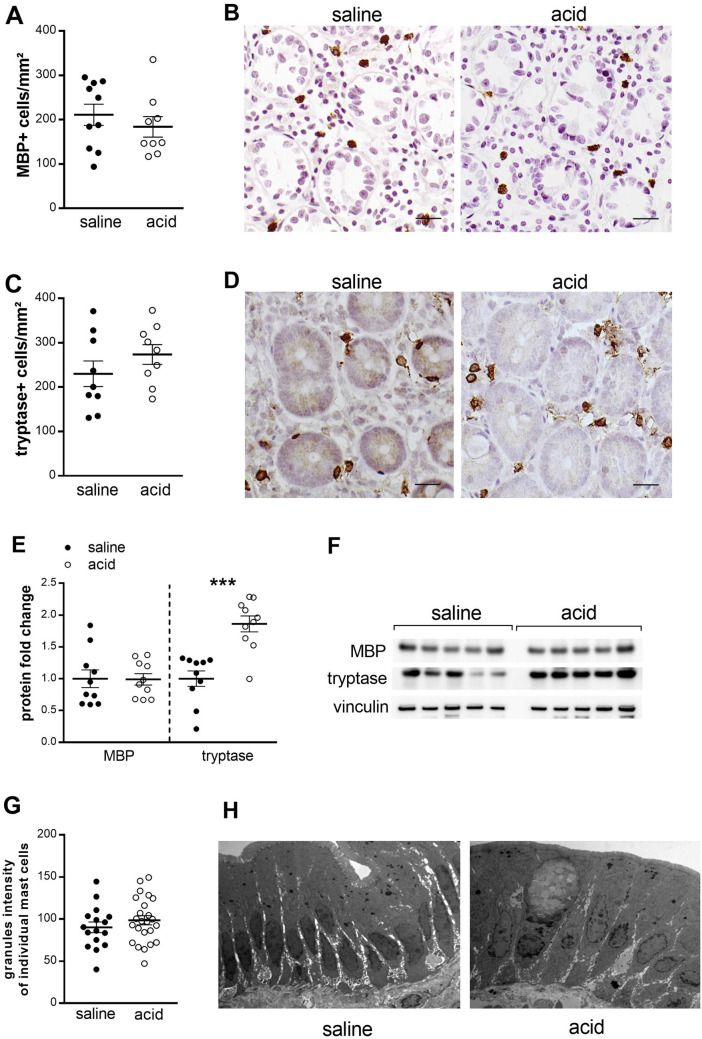
Compared to saline perfusion, no difference in eosinophil (211.0 ± 23.8 vs. 183.8 ± 23.2 MBP+ cells mm−2, P = 0.34) (Fig. 4A,B) or mast cell (230.0 ± 28.8 vs. 273.7 ± 22.1 tryptase+ cells mm−2, P = 0.34) (Fig. 4C,D) counts were detected between saline and acid perfusion. In order to exclude the possibility that degranulated mast cells may have been missed with the tryptase staining, we performed an additional quantification using an anti c-kit antibody which confirmed similar mast cell counts after saline and acid perfusion (540.0 ± 85.8 vs. 573.1 ± 60.0 c-kit+ cells mm−2, P = 0.75). No difference in mRNA expression of the eosinophil marker major basic protein (MBP) or the mast cell marker tryptase was found after acid perfusion (Table 1). However an increased expression of tryptase (1.86-fold, P = 0.0008), but not of MBP (0.99-fold, p = 0.95), was found after acid perfusion (Fig. 4E,F). These results may suggest activation of duodenal mast cells in response to acid perfusion. To explore this hypothesis more in depth, we assessed the degranulation state of mast cells (n = 16 and n = 24 respectively) by using transmission electron microscopy (TEM) in three subjects after duodenal saline and acid perfusion. The granular density of mast cells did not differ between both experimental groups (arbitrary units 90.2 ± 6.3 vs. 98.6 ± 5.4, P = 0.32, Fig. 4G). Interestingly, ultrastructural analysis of the duodenal epithelium showed that acid did not induce any damage or alteration of the epithelium, excluding a direct harmful (caustic) effect induced by acid (Fig. 4H).
Figure 4.
Duodenal acid induces tryptase expression, without changing mast cell counts or ultrastructure. Duodenal biopsy samples after saline perfusion (black dots) and after acid perfusion (white dots) were stained for eosinophils using eosinophilic MBP (n = 10 for saline and n = 9 for acid perfusion) (A) and for mast cells using tryptase (n = 9 for both groups) (C). Representative images of MBP (B) and tryptase (D) immunohistochemistry in mucosal biopsy specimens obtained after saline (left) and after acid perfusion (right). Scale bar: 20 µm. (E) Protein expression of MBP and tryptase was measured by western blot after intraduodenal saline (black dots) and acid (white dots) perfusion (n = 10 for both groups). (F) Representative western blot of five saline perfused and five acid perfused subjects. Bands were cropped from different parts of the same gel, or from different gels. (G) Electrodensity of the mast cells granules after saline and acid perfusion. (H) Similar ultrastructure of the duodenal epithelium after saline and acid perfusion showing no changes in cell morphology or integrity of the epithelium (×3000). Data are mean ± SEM; ***P < 0.001. MBP, eosinophilic major basic protein.
Part 2: The mast cell stabilizer DSCG does not affect the acid-induced activation of the duodenogastric reflex and the altered duodenal epithelial integrity
Symptoms and duodenal pH
To investigate whether mast cell activation plays a role in activation of the duodenogastric reflex and decreased mucosal integrity resulting from duodenal acid perfusion, we performed a similar acid perfusion study following a 2-week treatment with the mast cell stabilizer DSCG or placebo in a randomized cross-over fashion. For this part of the study, another group of 10 healthy volunteers (3 men, 7 women; age 23.7 ± 1.2 years) was included. There was no difference in dyspeptic symptom score during acid perfusion after treatment with placebo or DSCG (all P > 0.05; results not shown). The mean pH during acid perfusion was comparable in both conditions (7.70 ± 0.12 vs. 3.96 ± 0.41 after placebo; 7.45 ± 0.09 vs. 3.89 ± 0.43 after DSCG; between groups P = 0.91).
Intragastric pressure
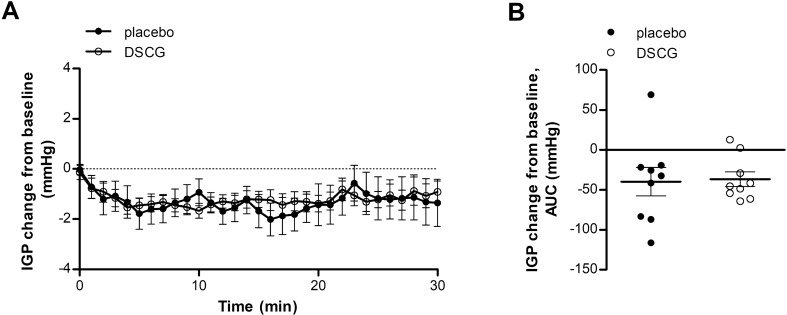
Activation of the duodenogastric reflex after acid perfusion was not modified by DSCG treatment compared with placebo treatment, as both groups showed a similar drop in IGP (AUC: − 39.8 ± 17.8 vs. − 36.7 ± 9.1 mmHg, P = 0.86) (Fig. 5A,B).
Figure 5.
Acid-induced IGP drop is not influenced by mast cell stabilization. IGP during acid perfusion was evaluated after treatment with placebo (black dots) and DSCG (white dots) using a high-resolution manometry catheter. (A) Time curve of IGP during acid perfusion after treatment with placebo and DSCG. (B) AUC of IGP during acid perfusion after placebo and DSCG treatment. n = 9 for placebo and n = 10 for DSCG. Data are mean ± SEM. AUC, area under the curve; DSCG, disodiumcromoglycate; IGP, intragastric pressure.
Low-grade inflammation
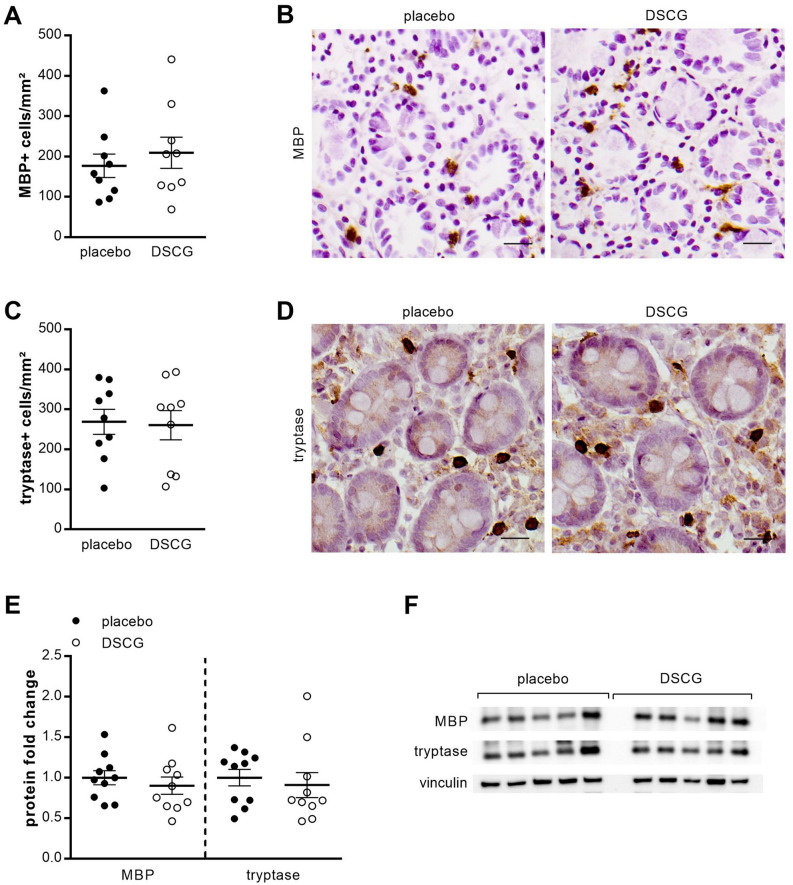
Eosinophil (176.9 ± 28.9 vs. 209.2 ± 38.7 MBP + cells mm−2, P = 0.53) (Fig. 6A,B) and mast cell (268.8 ± 31.5 vs. 260.5 ± 36.4 tryptase + cells mm−2, P = 0.78) (Fig. 6C,D) counts were comparable between the placebo group and the DSCG group. No difference in mRNA expression of the eosinophil marker MBP (1.26-fold ± 0.15 vs. 1.09 ± 0.14, P = 0.67) and the mast cell marker tryptase (1.21-fold ± 0.12 vs. 1.06 ± 0.13, P = 0.49) was found after DSCG treatment compared with placebo treatment. Furthermore, the protein expression of MBP (0.90-fold, P = 0.29) and tryptase (0.91-fold, P = 0.15) was similar in both groups (Fig. 6E,F).
Figure 6.
Low-grade inflammation. Duodenal biopsy samples after placebo (black dots) and DSCG treatment (white dots), were stained for eosinophils using eosinophilic MBP (A,B) and for mast cells using tryptase (C,D). Representative images of MBP (B) and tryptase (D) immunohistochemistry in mucosal biopsy specimens obtained after placebo (left) and DSCG treatment (right). Scale bar: 20 µm. (E) Protein expression of MBP and tryptase was measured by western blot after intraduodenal acid perfusion with placebo (black dots) and DSCG (white dots) pretreatment. (n = 10 for both groups). (F) Representative western blot of five saline perfused and five acid perfused subjects. Bands were cropped from different parts of the same gel, or from different gels. Data are mean ± SEM. MBP, eosinophilic major basic protein.
Duodenal mucosal barrier function
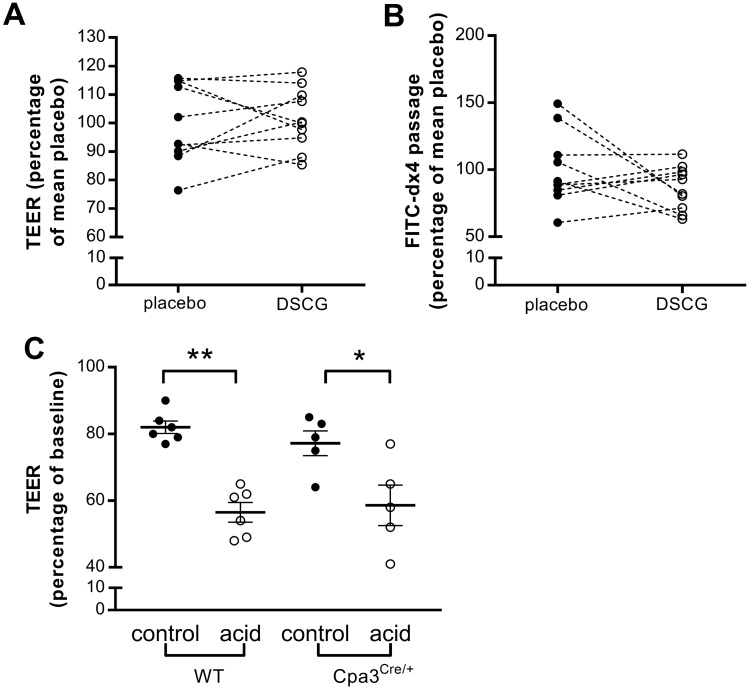
There was no difference in TEER (100.0 ± 4.4% vs. 101.5 ± 3.4%, P = 0.70) (Fig. 7A) and passage of FITC-dx4 (100.0 ± 8.5% vs. 86.5 ± 5.2%, P = 0.21) (Fig. 7B) between the placebo and the DSCG group after duodenal acid perfusion, suggesting that mast cell stabilization with DSCG is not sufficient to reverse the acid-induced barrier dysfunction.
Figure 7.
Acid-induced impairment of the duodenal mucosal barrier is not mediated by mast cells. Mucosal barrier function after acid perfusion following treatment with placebo (black dots) and DSCG (white dots) was evaluated in Ussing chambers by measuring TEER (A) and passage of FITC-dx4 (B). n = 10 for both groups. (C) Acid-induced reduction in TEER in wild type and in deficient mast cell Cpa3Cre/+ mice. Results are expressed relative to the mean of the control group. DSCG, disodiumcromoglycate; FITC-dx4, fluorescently labeled dextran of 4 kDa; TEER, transepithelial electrical resistance; WT, wild type.
To further confirm the absence of a role of mast cells in the acid-induced alterations in the epithelial barrier function, we exposed to acid the duodenum of mice deficient in mast cells (Cpa3Cre/+) and their wild type littermates ex vivo. In both the wild type and the Cpa3Cre/+ mice, acid exposure resulted in a decreased TEER (81.0% (78.5–85.5) vs. 57.5% (48.8–62.8); 79.0% (69.5–84.0) vs. 58.0% (46.5–71.0); both p < 0.05, Fig. 7C) compared with control buffer. However, there was no difference in TEER after acid exposure between wild type mice and mast cell deficient mice. All together, these results suggest that the impaired barrier function induced by acid is not mediated by mast cells.
Discussion
In this study, we have demonstrated that 30 min of acid perfusion of the duodenum in healthy volunteers resulted in activation of a duodenogastric reflex causing a relaxation of the proximal stomach. Duodenal acidification also impaired the mucosal integrity, shown by a decreased TEER and an increased passage of a paracellular probe that is associated with a reduced expression of the tight junction protein CLDN3. Duodenal acid perfusion additionally may lead to activation of mast cells, demonstrated by an increased expression of tryptase. Moreover, this study suggests that activation of the duodenogastric reflex and impaired mucosal integrity resulting from duodenal acidification occurs independently from mast cell activation, as pre-treatment with the mast cell stabilizer DSCG did not influence changes in IGP and barrier function in healthy controls, and a similarly impaired barrier function was found in mast cell deficient and wild type mice.
The wide variety of symptoms observed in patients with FD suggests the involvement of multiple pathophysiological mechanisms3. Although earlier studies reported abnormalities in gastric sensorimotor function in the pathophysiology of FD, later studies have also shown functional and structural alterations of the duodenum3. It has been demonstrated that FD patients display an increased duodenal acid exposure during the daytime and postprandially10,11, even though they are reported to have normal gastric acid secretion17. Excessive duodenal acid exposure might be important in the origin of dyspeptic symptoms, as a subset of patients with FD benefits from anti-secretory therapy with proton-pump inhibitors18. The mechanism underlying increased duodenal acid exposure in FD is suggested to be at least in part attributable to delayed duodenal acid clearance as FD patients display decreased duodenal motor activity in response to acid perfusion19–21.
Esophageal acid perfusion in healthy humans provoked decreased esophageal mucosal barrier function22,23. Other studies have demonstrated that acid perfusion of the duodenum in rats increased duodenal permeability24,25. We now translated these findings to humans, showing that acid perfusion of the duodenum during 30 min in healthy volunteers results in reduced TEER and increased passage to a paracellular probe, indicative of impaired duodenal barrier function. In addition, we detected a decreased expression of the tight junction protein CLDN3 in duodenal biopsy samples after acid perfusion. Claudin-3 is a sealing or barrier-forming claudin of the tight junctional protein complex. Transfecting MDCK II cell lines with human claudin-3 increased TEER and decreased permeability to a 4 kDa dextran26. Based on these limited data, the reduced claudin-3 expression may explain the observed barrier defect in our study, although it cannot be excluded that other, not measured, components of the junctional complex are involved as well. A decreased expression of CLDN3 had already been shown in a rat model with chronic acid-induced esophagitis27,28 and in an airway epithelium cell line exposed to acid29. These results suggest that CLDN3 expression could be a specific indicator for excessive acid exposure. Although the patients with FD of our previous study5 did not present with a decreased expression of CLDN3, this does not necessarily imply that excessive duodenal acid exposure is not a potential pathophysiological mechanism in this disorder. FD is a heterogeneous disorder, so it is conceivable that different pathways resulting in impaired intestinal barrier function are activated in different subgroups, underlying the varied expression profiles of the tight junction proteins. Not all FD patients present an increased spontaneous duodenal acid exposure and it is possible that other factors (e.g. stress) contribute to reduced intestinal integrity through alterations in the expression of other tight junction proteins. It is also possible that with a chronic exposure to duodenal acid—unlike the acute exposure that has been used in our study—the expression of other tight junction proteins is affected. Furthermore, a reduced expression of CLDN3 can also be a feature of decreased epithelial integrity in an earlier phase and may trigger a cascade of events that lead to other molecular abnormalities. For example, reduced CLDN3 expression can lead to increased permeability, resulting in more pronounced activation of immune cells, which in turn alters the expression of other cell-to-cell adhesion proteins including tight junctions.
Besides an altered duodenal barrier function, we also reported that duodenal acid perfusion leads to an increased expression of tryptase in the mucosa, which may be indicative of mast cell activation. This finding may confirm earlier studies in the opossum, where it has been shown that intraluminal esophageal acid perfusion is associated with mast cell activation30–33. We did not detect an increased number of mast cells or eosinophils after acid perfusion, unlike what we previously described in patients with FD4,5. This could be explained by the short time span between the acid perfusion and obtaining biopsy samples to observe an actual increased infiltration of immune cells in the lamina propria. Within this time frame, we aimed to mimic duodenal conditions of FD patients11 that enabled us to identify degranulation of mast cell, potentially related to an acid luminal content. Unfortunately, the experimental setting did not allow us to demonstrate significant degranulation, presumably due to the acute versus chronic exposure as happens in FD patients and the absence of additional immune and/or non-immune stimuli driving mast cell activation and degranulation34. Nevertheless, additional research should focus on chronic exposure to clarify the role of mast cells and other mast cell mediators besides tryptase such as histamine, within the duodenal acid environment in FD.
Because of the bidirectional communication between mast cells and neurons in the gastrointestinal tract35,36, which might mediate a possible effect of mast cell activation on gastric motility—we evaluated whether mast cell stabilization could prevent the decrease in IGP during duodenal acidification. Our results showed a similar drop in IGP during acid perfusion after treatment with the mast cell blocker compared with placebo, suggesting that mast cells are not involved in activation of the duodenogastric reflex during duodenal acidification. It is also currently unknown whether increased duodenal permeability occurs because of direct contact with acid or if it involves an indirect mechanism, perhaps triggered by mast cell activation. Impaired intestinal integrity and inflammation have already been shown to be closely related, but a cause-consequence relationship between these alterations has not been established and is the subject of ongoing discussion. Mast cell activation has been shown to play a key role in impaired epithelial barrier function37–40. Moreover, a previous study of our group observed increased small intestinal permeability after acute psychological stress in healthy volunteers, which was prevented by the mast cell stabilizer DSCG41. These results suggest that mast cell activation can be a pivotal element in the disruption of intestinal barrier function. Nonetheless, the opposite explanation—i.e. that impaired barrier function results in mast cell activation—remains possible as studies in animal models have demonstrated attenuation of inflammation after prevention of elevated intestinal permeability42,43.
Our study shows that pretreatment with the mast cell stabilizer DSCG does not block acid-induced epithelial barrier dysfunction, suggesting that impaired duodenal integrity after acid perfusion is a primary consequence of acid perfusion, and not a mast cell-dependent mechanism. Moreover, the lack of effect of DSCG on the drop of IGP and altered duodenal barrier function induced by acid, together with the ultrastructural studies assessing degranulation could suggest that acid do not directly activate mast cells as probably occurs in the esophagus32. Nevertheless, the increased amount of tryptase in the mucosa after acid perfusion suggests the opposite. Our and other studies show that the assessment of the activation of mast cells in physiological and pathophysiological conditions is complex. Secretion of mediators can occur without evidence of degranulation, and even mediators stored within the same granule can be selectively released in a discriminatory pattern44. Interestingly, Gottwald et al. found that electrical vagal stimulation increases histamine levels in intestinal tissues without degranulation of mast cells45. Furthermore, IL-1 stimulates secretion of IL-6 from mast cells without release of tryptase46. These and other data suggest the possibility of activation/modulation of mast cells without degranulation. Whether the specific synthesis and release of certain mediators without decrease in granular content can be blocked by DSCG is unknown. Further experiments are needed to confirm that duodenal acid perfusion activates mast cells in healthy subjects. Nevertheless, irrespective of the outcome of these studies, mast cell activation does not seem to be involved in the acid-induced barrier defect based on our human and mice studies.
Limitations of the study include the fact that the study was performed in HV and not in patients and that, the acid perfusion was set at a short perfusion time to limit the amount of acid infused. Moreover, our data cannot demonstrate that DSCG treatment sufficiently stabilized the mast cells are since tryptase expression levels were similar between the active and the placebo arm. Nevertheless, we previously used the same dosing and treatment duration in another study where DSCG counteracted the effect of stress on small intestinal permeability during psychological stress41. However, we cannot exclude a potential effect of more potent mast cell stabilizers such as ketotifen or blockers of mast cell products such as histamine, e.g. the histamine receptor 1-blocker ebastine.
In conclusion, we demonstrated that duodenal acid perfusion in HV decreases IGP, disrupts epithelial integrity and promotes tryptase production in mucosal mast cells. An increased duodenal acid exposure could therefore underlie gastric dysfunction, altered duodenal permeability and low-grade inflammation observed in FD and can thus be considered a potential pathophysiological mechanism contributing to dyspeptic symptom generation. This study additionally suggests that mast cell activation is not implicated in activation of the duodenogastric reflex and increased permeability resulting from duodenal acidification. Our data support further evaluation of duodenal acid as a therapeutic target in FD but oppose the idea of using DSCG as a possible treatment in acid-induced duodenal barrier dysfunction and gastric dysmotility.
Material and methods
Study subjects
Healthy volunteers were recruited from a mailing list after exclusion of gastrointestinal symptoms or a history of gastrointestinal disease and were included in a double-blind, randomized, cross-over study. Exclusion criteria were regular use of medication besides oral contraceptives, type 1 or 2 diabetes or first-degree family members with type 1 diabetes, celiac disease or inflammatory bowel disease. Non-steroidal anti-inflammatory drugs were not allowed in the month before and alcohol in the last 3 days before the study procedures. Written informed consent was obtained prior to inclusion in the study and the human ethical committee of the University of Leuven approved the protocol. All methods were performed in accordance with our institution guidelines and regulations. The study in healthy volunteers was registered on https://www.clinicaltrials.gov as NCT02664051 (registered in 26/01/2016).
Ex vivo study in mice
Cpa3Cre/+ gene-targeted mice have been described previously47. Mice were kept at the KU Leuven animal facility under SPF conditions. All experimental procedures were approved by the Animal Ethics Committee of the Medical Faculty of the KU Leuven (Leuven, Belgium). All methods were performed in accordance with our institution guidelines and regulations.
Study design
Part 1: randomized cross-over acid or saline perfusion study
For the first part of this study, an assembly including a pH electrode with an antimony pH sensor and a thin infusion tube (2 mm diameter) was introduced transnasally and positioned in the second portion of the duodenum after an overnight fast. The pH electrode was calibrated using commercial buffer solutions at pH 7.0 and pH 4.0 before insertion. Duodenal pH was continuously monitored during the study period and recorded using an ambulatory data-logger (MicroDigitrapper; Synectics Medical, Stockholm, Sweden). Subsequently, a high-resolution manometry (HRM) catheter (36 channels spaced 1 cm apart; Manoscan 360, Sierra Scientific Instruments, Los Angeles, California, USA) was inserted through the nose and positioned in the gastric fundus to measure intragastric pressure (IGP) as a read-out of gastric relaxation or contraction. This method has been developed in our lab as a minimally invasive alternative to the barostat48. After a stabilization period of 20 min, one investigator (TV) started the infusion of 0.1 N HCl (acid) or saline in the duodenum at a rate of 5 mL min−1 during 30 min, in a randomized, cross-over manner. The solutions were prepared in the absence of the participant and the perfusion bags looked identical. The order of perfusion was based on an automatically generated random sequence. Perfusions were done with at least 2 weeks in between as it is known that the intestinal epithelium takes 4–7 days to renew49. The participants and a second investigator (HV), who was taking care of the ex vivo experiments and analyses, were blinded to the nature (acid or saline) of the infusion. During the perfusion, occurrence of symptoms (fullness, bloating, belching, nausea, satiation, epigastric burning and epigastric pain) was scored using a 100-mm visual analogue scale (VAS) before and every 5 min during the perfusion. Thirty minutes after perfusion, endoscopic duodenal biopsies were obtained (see below).
Part 2: randomized cross-over acid perfusion study with mast cell stabilization or placebo
For the second part of this study, a second group of participants was treated with oral placebo (190 mg mannitol) or disodiumcromoglycate (DSCG; Nalcrom, Italchimici SpA, Rome, Italy), a mast cell stabilizer, 200 mg qid for 2 weeks41. Between both treatments, there was a washout period of at least 2 weeks. The order of the treatment was based on an automatically generated random sequence. Capsules and packaging of placebo and DSCG looked identical. Participants and the investigator performing the ex vivo experiments and analyses were blinded to the nature of the treatment. After treatment, the study design was as described in part 1 except that acid perfusions were performed at both study visits.
Duodenal biopsies
Biopsy specimens were taken with a standard biopsy forceps in the second part of the duodenum by an experienced endoscopist (JT) during an esophagogastroduodenoscopy. Three biopsies were put in ice-cold oxygenated Krebs–Ringer bicarbonate buffer for Ussing chamber experiments to assess epithelial barrier function. Two biopsies were placed in RNAlater solution (Qiagen, Hilden, Germany) for RNA isolation and real-time reverse transcriptase polymerase chain reaction (RT-PCR), to assess gene expression. Two biopsies were snap frozen in liquid nitrogen for further protein extraction and identification by western blot. One biopsy was fixed in formalin and embedded in paraffin for immunofluorescence and immunohistochemistry for specific histological analysis. Another biopsy was fixed with 2.5% (v/v) glutaraldehyde (Sigma-Aldrich, St. Louis, Missouri, USA) and 2% (v/v) paraformaldehyde (Sigma-Aldrich) in phosphate buffer at pH 7.4 for ultrastructural evaluation by transmission electron microscopy (TEM).
Experimental methods
Ussing chamber experiments
Duodenal biopsies were mounted in modified 3 mL Ussing chambers (Mussler Scientific Instruments, Aachen, Germany) as described previously5. TEER was recorded every 30 min during 2 h. Passage through the biopsy was evaluated with the paracellular probe fluorescently labelled dextran (FITC-dx4; MW = 4000 Da, 1 mg mL−1; Sigma-Aldrich, St. Louis, USA). FITC-dx4 was added to the mucosal compartment and serosal samples were collected every 30 min during 2 h, of which the fluorescence level was measured using a fluorescence reader (FLUOstar Omega; BMG Labtech, Ortenberg, Germany). The average values of time points 60, 90 and 120 min was taken. Results of TEER and FITC-dx4 passage were presented as values relative to the mean of the control group.
RNA isolation, c-DNA synthesis and quantitative real time PCR
Real-time reverse transcription polymerase chain reaction (RT-PCR) was performed as we previously described5 and it is described in detail in the Supplementary Methods. Primer sequences are specified in Supplementary Table S2.
Western blot
Western blot was performed as we previously described5. Equal amounts of protein per sample were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to a polyvinylidene difluoride membrane. Blots were incubated overnight with primary antibodies: rabbit anti-CLDN1 (1:500; Abcam, Cambridge, UK), rabbit anti-CLDN2 (1:500; Abcam), rabbit anti-CLDN3 (1:500; Abcam), mouse anti-CLDN4 (1:1000; Invitrogen, Carlsbad, USA), rabbit anti-OCLN (1:1000; Invitrogen), rabbit anti-DSC2 (1:500; Abcam), mouse anti-DSG2 (1:1000; Abcam), mouse anti-tryptase (1:500; Dako, Glostrup, Denmark) or mouse anti-MBP (1:500; AbD Serotec, Kidlington, UK). All membranes were stained with mouse anti-vinculin (1:5000; Sigma-Aldrich), as a protein loading control. Peroxidase-conjugated goat anti-rabbit IgG or goat anti-mouse IgG (both 1:5000; Thermo Scientific) were used as secondary antibodies. Bands were quantified by densitometry using ImageJ software (National Institutes of Health; https://rsb.info.nih.gov.ij/). Fold change was determined relative to the average of the group perfused with the saline solution (part 1) or the placebo oral treatment (part 2).
Immunofluorescence
Immunofluorescence was performed as described before5. Deparaffinization and rehydration were performed following standard procedures using xylene and graded solutions on 5 µm sections. Tissues were then blocked with Protein Blocking Solution (Dako) and incubated during 60 min at room temperature in mouse anti-ZO-1 (1:50; Invitrogen), rabbit anti-β-catenin (1:250; Abcam) or mouse anti-E-cadherin (1:50; Abcam). Alexa Fluor 488 goat anti-mouse IgG and Alexa Fluor 594 donkey anti-rabbit IgG (both 1:1000; Invitrogen) were used as secondary antibodies. Ten representative non-overlapping confocal images were obtained with a LSM510 Meta Laser Scanning microscope at 630× magnification (Zeiss, Oberkochen, Germany). ImageJ software was used to quantify protein in a blinded manner, measuring the average and the area of fluorescence intensity at the apical pole.
Immunohistochemistry
Immunohistochemistry was performed as we previously described4,5. After deparaffinization, sections were blocked with REAL Peroxidase Blocking (Dako) and Protein Blocking Solution (Dako). Eosinophils and mast cells were stained by incubating sections at room temperature for 60 min in mouse anti-MBP (1:20) or 30 min in mouse anti-mast cell tryptase (1:200) or anti-cKit (1:250), respectively. Sections were incubated with secondary horse anti-mouse biotinylated antibody (1:200; Vector Laboratories, Burlingame, California, USA) and diaminobenzidine was used as the chromogen, followed by counterstaining with Harris’s haematoxylin. Pictures of at least seven representative non-overlapping high-power fields (HPFs) at 400× magnification were taken on an optical microscope (BX41 Olympus; Olympus, Aartselaar, Belgium) in a blinded manner. The area of the lamina propria was measured using ImageJ software and positive cells were counted. Results are expressed as positive cells per mm2.
Transmission electron microscopy
Transmission electron microscopy technique is described in detail in the Supplementary Methods.
Ex vivo acid exposure in mice
Duodenal tissue from mice deficient in mast cells (Cpa3Cre/+) and their wild type littermates was mounted in Ussing chambers as described above for duodenal biopsies. After a stabilization period of 30 min, one tissue of each mouse was exposed to HCl (pH 1.3) during 30 min, while one tissue was used as a control (krebs buffer). TEER was recorded just before acid exposure and 30 min after acid exposure.
Data analysis
Data analysis was performed as we previously described11,48. The severity VAS scores of each symptom during perfusion were averaged and corrected for the score before the perfusion. For IGP measurements, an interpolated thermal compensation was done on the recording to correct for thermal drift during the measurement. The original data were exported from the recording software (Manoview Analysis, Sierra Scientific Instrument, Los Angeles, USA) to Microsoft Excel. To avoid influences on IGP from movement, coughing, swallowing or sneezing, a moving median was calculated per channel over a 30 s frame. Per channel, a baseline value was calculated from the moving median data as the average pressure in the last 5 min of the stabilization period. Data were presented per minute as the difference of the minimum moving median value in that minute and the baseline value of the five selected channels below the lower esophageal sphincter. The area under the curve (AUC) at each minute was calculated and averaged over the 30 min perfusion period.
Statistical analysis
Differences between groups were analyzed using paired Student’s t-tests or Wilcoxon signed rank tests when appropriate and data are presented as mean ± SEM or median (IQR) respectively. Differences between more than two groups were analyzed using Kruskal–Wallis, followed by post-hoc testing (Dunns correction for multiple testing). All results were analyzed using SAS 9.2 (SAS Institute, Cary, USA) and values were considered statistically significant when P < 0.05. Bonferroni correction for multiple testing was performed.
Supplementary information
Acknowledgements
We thank the study nurses of the Gastrointestinal Motility Unit, Leuven University Hospitals. We are grateful to Alejandro Sánchez-Chardi and Francisca Cardoso from Servei de Microscopia, Universitat Autònoma de Barcelona (Spain) for excellent technical support in sample preparation for electron microscopy analysis. We thank Dr. Erwin Dreesen for his help with the statistical analysis. Confocal imaging was performed in the Cell Imaging Core (KU Leuven, Belgium). Nalcrom was kindly provided free of charge by Italchimici SpA, Rome, Italy.
Author contributions
H.V., R.F., T.V.: performed the research, designed the research study, analyzed the data, wrote the paper. M.V., D.B., J.To.: performed the research. S.C., A.A.: performed the research, analyzed the data. L.W.: wrote the paper. H.-R.R., G.M., G.B.: gave crucial critical revision of the manuscript and supplied the Cpa3Cre/+ mice. G.D.H.: performed the research (anti-cKit staining). J.T.: designed the research study, gave crucial critical revision of the manuscript. All reviewed the paper for important intellectual content.
Funding
Funding was provided by a Methusalem grant from Leuven University to JT. HV is a postdoctoral fellow and LW a predoctoral fellow supported by the Research Foundation Flanders (Fonds Wetenschappelijk Onderzoek Vlaanderen, FWO). TV is a senior clinical investigator of the Research Foundation Flanders (FWO Vlaanderen). Funding was also provided by the Fondo de Investigación Sanitaria and CIBERehd, Instituto Carlos III, Subdirección General de Investigación Sanitaria, Ministerio de Ciencia e Innovación (PI16/00583 & CPII16/00031 to MV and EHD16PI02 to MV and RF). CIBERehd is funded by the Instituto de Salud Carlos III.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors jointly supervised this work: Ricard Farré and Tim Vanuytsel.
Supplementary information
is available for this paper at 10.1038/s41598-020-74491-1.
References
- 1.Drossman DA, Hasler WL. Rome IV-functional GI disorders: Disorders of gut–brain interaction. Gastroenterology. 2016;150:1257–1261. doi: 10.1053/j.gastro.2016.03.035. [DOI] [PubMed] [Google Scholar]
- 2.Stanghellini V, et al. Rome IV—gastroduodenal disorders. Gastroenterology. 2016;150:1380–1392. doi: 10.1053/j.gastro.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 3.Vanheel H, Farre R. Changes in gastrointestinal tract function and structure in functional dyspepsia. Nat. Rev. Gastroenterol. Hepatol. 2013;10:142–149. doi: 10.1038/nrgastro.2012.255. [DOI] [PubMed] [Google Scholar]
- 4.Vanheel H, et al. Activation of eosinophils and mast cells in functional dyspepsia: An ultrastructural evaluation. Sci. Rep. 2018;8:5383. doi: 10.1038/s41598-018-23620-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vanheel H, et al. Impaired duodenal mucosal integrity and low-grade inflammation in functional dyspepsia. Gut. 2014;63:262–271. doi: 10.1136/gutjnl-2012-303857. [DOI] [PubMed] [Google Scholar]
- 6.Vanuytsel T, et al. Role of duodenal mucosal nerve endings in the acid-induced duodenogastric sensorimotor reflex: Effect of benzocaine in healthy humans. Neurogastroenterol. Motil. 2013;25:e353–e361. doi: 10.1111/nmo.12126. [DOI] [PubMed] [Google Scholar]
- 7.Ji Y, et al. Activation of rat intestinal mucosal mast cells by fat absorption. Am. J. Physiol. Gastrointest. Liver Physiol. 2012;302:G1292–G1300. doi: 10.1152/ajpgi.00011.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Juanola C, Giralt M, Jimenez M, Mourelle M, Vergara P. Mucosal mast cells are involved in CCK disruption of MMC in the rat intestine. Am. J. Physiol. 1998;275:G63–G67. doi: 10.1152/ajpgi.1998.275.1.G63. [DOI] [PubMed] [Google Scholar]
- 9.Fried M, Feinle C. The role of fat and cholecystokinin in functional dyspepsia. Gut. 2002;51(Suppl 1):i54–i57. doi: 10.1136/gut.51.suppl_1.i54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bratten J, Jones MP. Prolonged recording of duodenal acid exposure in patients with functional dyspepsia and controls using a radiotelemetry pH monitoring system. J. Clin. Gastroenterol. 2009;43:527–533. doi: 10.1097/MCG.0b013e31818e37ab. [DOI] [PubMed] [Google Scholar]
- 11.Lee KJ, et al. A pilot study on duodenal acid exposure and its relationship to symptoms in functional dyspepsia with prominent nausea. Am. J. Gastroenterol. 2004;99:1765–1773. doi: 10.1111/j.1572-0241.2004.30822.x. [DOI] [PubMed] [Google Scholar]
- 12.Hunt JN, Knox MT. The slowing of gastric emptying by four strong acids and three weak acids. J. Physiol. 1972;222:187–208. doi: 10.1113/jphysiol.1972.sp009793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee KJ, Kim JH, Cho SW. Dyspeptic symptoms associated with hypersensitivity to gastric distension induced by duodenal acidification. J. Gastroenterol. Hepatol. 2006;21:515–520. doi: 10.1111/j.1440-1746.2005.03976.x. [DOI] [PubMed] [Google Scholar]
- 14.Lee KJ, Vos R, Janssens J, Tack J. Influence of duodenal acidification on the sensorimotor function of the proximal stomach in humans. Am. J. Physiol. Gastrointest. Liver Physiol. 2004;286:G278–G284. doi: 10.1152/ajpgi.00086.2003. [DOI] [PubMed] [Google Scholar]
- 15.Simren M, Vos R, Janssens J, Tack J. Acid infusion enhances duodenal mechanosensitivity in healthy subjects. Am. J. Physiol. Gastrointest. Liver Physiol. 2003;285:G309–G315. doi: 10.1152/ajpgi.00242.2002. [DOI] [PubMed] [Google Scholar]
- 16.Vanuytsel T, Karamanolis G, Van Oudenhove L, Vos R, Tack J. Influence of ondansetron on gastric sensorimotor responses to short duodenal acid infusion in healthy volunteers. Neurogastroenterol. Motil. 2011;23:226–232.e115. doi: 10.1111/j.1365-2982.2010.01631.x. [DOI] [PubMed] [Google Scholar]
- 17.Collen MJ, Loebenberg MJ. Basal gastric acid secretion in nonulcer dyspepsia with or without duodenitis. Dig. Dis. Sci. 1989;34:246–250. doi: 10.1007/BF01536059. [DOI] [PubMed] [Google Scholar]
- 18.Pinto-Sanchez MI, Yuan Y, Hassan A, Bercik P, Moayyedi P. Proton pump inhibitors for functional dyspepsia. Cochrane Database Syst. Rev. 2017;11:CD011194. doi: 10.1002/14651858.CD011194.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Samsom M, Verhagen MA, vanBerge Henegouwen GP, Smout AJ. Abnormal clearance of exogenous acid and increased acid sensitivity of the proximal duodenum in dyspeptic patients. Gastroenterology. 1999;116:515–520. doi: 10.1016/S0016-5085(99)70171-X. [DOI] [PubMed] [Google Scholar]
- 20.Schwartz MP, Samsom M, Smout AJ. Chemospecific alterations in duodenal perception and motor response in functional dyspepsia. Am. J. Gastroenterol. 2001;96:2596–2602. doi: 10.1111/j.1572-0241.2001.04103.x. [DOI] [PubMed] [Google Scholar]
- 21.Schwartz MP, Samsom M, Van Berge Henegouwen GP, Smout AJ. Effect of inhibition of gastric acid secretion on antropyloroduodenal motor activity and duodenal acid hypersensitivity in functional dyspepsia. Aliment Pharmacol. Ther. 2001;15:1921–1928. doi: 10.1046/j.1365-2036.2001.01123.x. [DOI] [PubMed] [Google Scholar]
- 22.Farre R, et al. Acid and weakly acidic solutions impair mucosal integrity of distal exposed and proximal non-exposed human oesophagus. Gut. 2010;59:164–169. doi: 10.1136/gut.2009.194191. [DOI] [PubMed] [Google Scholar]
- 23.Farre R, et al. Evaluation of oesophageal mucosa integrity by the intraluminal impedance technique. Gut. 2011;60:885–892. doi: 10.1136/gut.2010.233049. [DOI] [PubMed] [Google Scholar]
- 24.Hallgren A, Wilander E, Nylander O. Acid-induced increase in duodenal mucosal permeability is augmented by nitric oxide inhibition and vasopressin. Acta Physiol. Scand. 1997;160:363–370. doi: 10.1046/j.1365-201X.1997.00171.x. [DOI] [PubMed] [Google Scholar]
- 25.Nylander O, Holm L, Wilander E, Hallgren A. Exposure of the duodenum to high concentrations of hydrochloric acid. Effects on mucosal permeability, alkaline secretion, and blood flow. Scand. J. Gastroenterol. 1994;29:437–444. doi: 10.3109/00365529409096835. [DOI] [PubMed] [Google Scholar]
- 26.Milatz S, et al. Claudin-3 acts as a sealing component of the tight junction for ions of either charge and uncharged solutes. Biochim. Biophys. Acta. 2010;1798:2048–2057. doi: 10.1016/j.bbamem.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 27.Asaoka D, et al. Altered localization and expression of tight-junction proteins in a rat model with chronic acid reflux esophagitis. J. Gastroenterol. 2005;40:781–790. doi: 10.1007/s00535-005-1628-6. [DOI] [PubMed] [Google Scholar]
- 28.Miwa H, et al. Experimental oesophagitis in the rat is associated with decreased voluntary movement. Neurogastroenterol. Motil. 2009;21:296–303. doi: 10.1111/j.1365-2982.2008.01221.x. [DOI] [PubMed] [Google Scholar]
- 29.Xu R, et al. The degradation of airway tight junction protein under acidic conditions is probably mediated by transient receptor potential vanilloid 1 receptor. Biosci. Rep. 2013;33:e00078. doi: 10.1042/BSR20130087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barclay RL, Dinda PK, Morris GP, Paterson WG. Morphological evidence of mast cell degranulation in an animal model of acid-induced esophageal mucosal injury. Dig. Dis. Sci. 1995;40:1651–1658. doi: 10.1007/BF02212685. [DOI] [PubMed] [Google Scholar]
- 31.Feldman MJ, Morris GP, Dinda PK, Paterson WG. Mast cells mediate acid-induced augmentation of opossum esophageal blood flow via histamine and nitric oxide. Gastroenterology. 1996;110:121–128. doi: 10.1053/gast.1996.v110.pm8536848. [DOI] [PubMed] [Google Scholar]
- 32.Paterson WG. Role of mast cell-derived mediators in acid-induced shortening of the esophagus. Am. J. Physiol. 1998;274:G385–G388. doi: 10.1152/ajpgi.1998.274.2.G385. [DOI] [PubMed] [Google Scholar]
- 33.Paterson WG, et al. Intraluminal acid induces oesophageal shortening via capsaicin-sensitive neurokinin neurons. Gut. 2007;56:1347–1352. doi: 10.1136/gut.2006.115881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wouters MM, Vicario M, Santos J. The role of mast cells in functional GI disorders. Gut. 2016;65:155–168. doi: 10.1136/gutjnl-2015-309151. [DOI] [PubMed] [Google Scholar]
- 35.Barbara G, Stanghellini V, De Giorgio R, Corinaldesi R. Functional gastrointestinal disorders and mast cells: Implications for therapy. Neurogastroenterol. Motil. 2006;18:6–17. doi: 10.1111/j.1365-2982.2005.00685.x. [DOI] [PubMed] [Google Scholar]
- 36.Van Nassauw L, Adriaensen D, Timmermans JP. The bidirectional communication between neurons and mast cells within the gastrointestinal tract. Auton Neurosci. 2007;133:91–103. doi: 10.1016/j.autneu.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 37.Jacob C, et al. Mast cell tryptase controls paracellular permeability of the intestine. Role of protease-activated receptor 2 and beta-arrestins. J. Biol. Chem. 2005;280:31936–31948. doi: 10.1074/jbc.M506338200. [DOI] [PubMed] [Google Scholar]
- 38.Lee JW, et al. Subjects with diarrhea-predominant IBS have increased rectal permeability responsive to tryptase. Dig. Dis. Sci. 2010;55:2922–2928. doi: 10.1007/s10620-009-1094-8. [DOI] [PubMed] [Google Scholar]
- 39.Wallon C, et al. Corticotropin-releasing hormone (CRH) regulates macromolecular permeability via mast cells in normal human colonic biopsies in vitro. Gut. 2008;57:50–58. doi: 10.1136/gut.2006.117549. [DOI] [PubMed] [Google Scholar]
- 40.Zheng PY, et al. Psychological stress induces eosinophils to produce corticotrophin releasing hormone in the intestine. Gut. 2009;58:1473–1479. doi: 10.1136/gut.2009.181701. [DOI] [PubMed] [Google Scholar]
- 41.Vanuytsel T, et al. Psychological stress and corticotropin-releasing hormone increase intestinal permeability in humans by a mast cell-dependent mechanism. Gut. 2014;63:1293–1299. doi: 10.1136/gutjnl-2013-305690. [DOI] [PubMed] [Google Scholar]
- 42.Arrieta MC, Madsen K, Doyle J, Meddings J. Reducing small intestinal permeability attenuates colitis in the IL10 gene-deficient mouse. Gut. 2009;58:41–48. doi: 10.1136/gut.2008.150888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clayburgh DR, Musch MW, Leitges M, Fu YX, Turner JR. Coordinated epithelial NHE3 inhibition and barrier dysfunction are required for TNF-mediated diarrhea in vivo. J. Clin. Investig. 2006;116:2682–2694. doi: 10.1172/JCI29218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kraeuter Kops S, Theoharides TC, Cronin CT, Kashgarian MG, Askenase PW. Ultrastructural characteristics of rat peritoneal mast cells undergoing differential release of serotonin without histamine and without degranulation. Cell Tissue Res. 1990;262:415–424. doi: 10.1007/BF00305238. [DOI] [PubMed] [Google Scholar]
- 45.Gottwald TP, Hewlett BR, Lhotak S, Stead RH. Electrical stimulation of the vagus nerve modulates the histamine content of mast cells in the rat jejunal mucosa. NeuroReport. 1995;7:313–317. doi: 10.1097/00001756-199512290-00075. [DOI] [PubMed] [Google Scholar]
- 46.Kandere-Grzybowska K, et al. IL-1 induces vesicular secretion of IL-6 without degranulation from human mast cells. J. Immunol. 2003;171:4830–4836. doi: 10.4049/jimmunol.171.9.4830. [DOI] [PubMed] [Google Scholar]
- 47.Feyerabend TB, et al. Cre-mediated cell ablation contests mast cell contribution in models of antibody- and T cell-mediated autoimmunity. Immunity. 2011;35:832–844. doi: 10.1016/j.immuni.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 48.Janssen P, et al. Intragastric pressure during food intake: A physiological and minimally invasive method to assess gastric accommodation. Neurogastroenterol. Motil. 2011;23(316–22):e153–e154. doi: 10.1111/j.1365-2982.2011.01676.x. [DOI] [PubMed] [Google Scholar]
- 49.Jeon MK, Klaus C, Kaemmerer E, Gassler N. Intestinal barrier: Molecular pathways and modifiers. World J. Gastrointest. Pathophysiol. 2013;4:94–99. doi: 10.4291/wjgp.v4.i4.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.