Abstract
The powerful analgesic effects of opioid drugs have captivated the interest of physicians and scientists for millennia, and the ability of opioid drugs to produce serious undesired effects has been recognized for a similar period of time (Kieffer and Evans, 2009). Many of these develop progressively with prolonged or repeated drug use and then persist, motivating particular interest in understanding how opioid drugs initiate adaptive or maladaptive modifications in neural function or regulation. Exciting advances have been made over the past several years in elucidating drug-induced changes at molecular, cellular, and physiologic scales of analysis. The present review will highlight some recent cellular studies that we believe bridge across scales and will focus on optical imaging approaches that put opioid drug action “under the microscope.”
SIGNIFICANCE STATEMENT
Opioid receptors are major pharmacological targets, but their signaling at the cellular level results from a complex interplay between pharmacology, regulation, subcellular localization, and membrane trafficking. This minireview discusses recent advances in understanding the cellular biology of opioid receptors, emphasizing particular topics discussed at the 50th anniversary of the International Narcotics Research Conference. Our goal is to highlight distinct signaling and regulatory properties emerging from the cellular biology of opioid receptors and discuss potential relevance to therapeutics.
Cellular Basis of Opioid Receptor Regulation
Early interest in a cellular basis for opioid adaptations was motivated by the ability of prolonged morphine exposure to produce complex regulatory effects on signaling in neuroblastoma cells (Sharma et al., 1975) and to reduce receptor reserve in tissue explants (Chavkin and Goldstein, 1982). This was followed by the identification of agonist-induced downregulation of opioid receptors associated with endocytic delivery to lysosomes (Law et al., 1984). A more rapid and nondestructive process of receptor internalization was later detected, first pharmacologically and then using cellular imaging (Von Zastrow et al., 1993; von Zastrow et al., 1994). This led to the delineation of clathrin- and dynamin-dependent endocytosis of opioid receptors promoted by agonist-induced receptor phosphorylation followed by engagement of a class of cytoplasmic adaptor and scaffolding proteins called β-arrestins. Regulated endocytosis by this conserved mechanism has now been demonstrated for all three opioid receptor types: μ (MOP-R), δ (DOP-R), and κ (KOP-R) (Keith et al., 1996; Zhang et al., 1996, 1998; Whistler and von Zastrow, 1998; Li et al., 1999).
Once internalized, receptors can recycle nondestructively to the plasma membrane or traffic to lysosomes for proteolytic downregulation. The decision of which route receptors take is made by receptor-specific molecular sorting operations that occur in endosomes (Tsao and von Zastrow, 2000; Whistler et al., 2002). Internalized MOP-R and KOP-R typically recycle efficiently and downregulate slowly, whereas DOP-R, although also capable of nondestructive recycling, traffics preferentially to lysosomes and downregulates more rapidly (Keith et al., 1996; Li et al., 1999; Tsao and von Zastrow, 2000; Tanowitz and von Zastrow, 2003). Such molecular sorting is highly selective and directed by discrete structural determinants in the receptor. For example, a short sequence present in the cytoplasmic tail of MOP-R is necessary for efficient recycling of this opioid receptor, and when fused to the cytoplasmic tail of DOP-R, it is sufficient to redirect receptors from lysosomal to recycling pathways. Further, an MOP-R splice variant that naturally lacks this sequence, generated by alternative processing of the receptor transcript, recycles less efficiently and downregulates more rapidly during prolonged agonist exposure (Tanowitz and von Zastrow, 2003; Tanowitz et al., 2008). Additional sorting occurs later during transit to lysosomes through translocation of receptors from the endosome limiting membrane to small membrane vesicles that are formed within the endosome lumen. This process is promoted by ubiquitination of receptors on lysine residues in the first cytoplasmic loop (Hislop et al., 2011).
Such observations delineated a basic framework for the cellular regulation of opioid receptors (Fig. 1) that largely comports with a conserved paradigm of receptor desensitization established through studies of a variety of GPCRs (DeWire et al., 2007). Although much of the original work elaborating this regulatory framework relied on recombinant receptor expression and the use of simplified model systems, key elements of it are now validated in physiologic context. In particular, agonist-induced endocytosis of MOP-R and DOP-R has been directly demonstrated in native neurons (Sternini et al., 1996; Scherrer et al., 2006), and endocytic delivery of DOP-R to lysosomes has been convincingly associated with long-term downregulation of functional antinociception in vivo (Pradhan et al., 2009). Further, the same phosphorylation sites defined initially by phosphoproteomic mapping of heterologously expressed MOP-R (Lau et al., 2011) have been shown to be essential for functional desensitization of postsynaptic signaling and agonist-induced internalization of MOP-R in acute brain slices, as well as for antinociceptive tolerance assessed behaviorally in vivo after repeated administration of morphine (Arttamangkul et al., 2018; Kliewer et al., 2019).
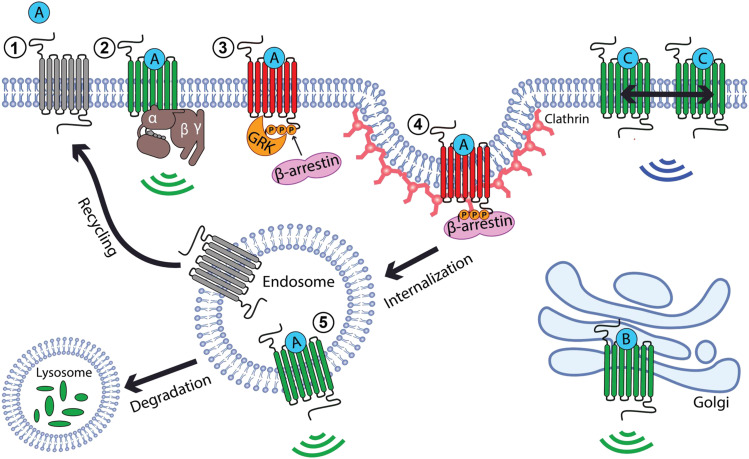
Fig. 1.
Agonist-induced signaling and trafficking of opioid receptors. Inactive opioid receptors (gray) become activated (green) after binding to an agonist (A, steps 1 to 2). This enables signaling via G proteins (green ripples, step 2) and triggers phosphorylation of the receptor tail (P) by GRKs, followed by receptor engagement of β-arrestins (step 3) and endocytosis via clathrin-coated pits (step 4). These events inactivate G protein signaling (red receptor) and assure signal termination from the plasma membrane by endocytic removal of receptors. After receptors arrive in early endosomes, they have the capacity to signal again by engaging G proteins in the endosome membrane (green ripples, step 5). Receptors also engage molecular sorting mechanisms in the endosome limiting membrane (not shown), which determine whether internalized receptors are delivered to lysosomes for proteolytic downregulation or are nondestructively recycled to restore surface receptor responsiveness. Many nonpeptide agonist drugs (drug B) are sufficiently membrane-permeant to activate a discrete pool of opioid receptors at the Golgi apparatus and activate receptors from this location (green ripples). Some agonists (drug C) induce receptor reorganization in the plasma membrane to change surface signaling (blue ripples).
A main takeaway from the cellular regulatory framework summarized above is that receptor trafficking and signaling operations are intricately interconnected. A critical group of enzymes that regulate opioid receptors (and many other GPCRs) are the so-called GPCR kinases, or GRKs (Komolov and Benovic, 2018). Phosphorylation by isoforms of protein kinase C has also been shown to produce significant effects on MOP-R trafficking and signaling and contributes to differentiating regulatory effects of morphine relative to opioid peptide agonists on this receptor (Bailey et al., 2009; Bowman et al., 2015; Civciristov et al., 2019; Kunselman et al., 2019). GRK-mediated phosphorylation is a general mechanism for inhibiting the ability of GPCRs to engage G proteins and for promoting binding to β-arrestins (Shenoy and Lefkowitz, 2011; Williams et al., 2013; Kang et al., 2014). Accordingly, phosphorylation promotes two processes that appear to act redundantly to assure that opioid signaling from the plasma membrane is terminated: functional uncoupling of receptors from G proteins followed by physical removal of receptors from the cell surface (Fig. 1).
Toward an Expanded Understanding of the Signaling-Trafficking Relationship
According to the conserved regulatory framework summarized above, endocytosis of opioid receptors is inextricably associated with receptor inactivation, effectively operating as a redundant mechanism to assure that surface-delimited signaling is terminated after receptor phosphorylation and engagement of β-arrestin in the plasma membrane. However, it is increasingly clear that opioid receptors can produce additional signaling effects that are promoted, rather than inhibited, by receptor engagement with β-arrestin. The potential of β-arrestins to act as regulated scaffold proteins promoting the activation of kinase cascades by various GPCRs has been well described (DeWire et al., 2007), and studies of KOP-R provide strong support for the physiologic relevance of such signaling to opioid receptors (Bruchas and Chavkin, 2010). Recent studies of MOP-R and DOP-R suggest, in addition, that opioid receptors use β-arrestin–promoted endocytosis to enable G protein signaling from internal membrane compartments. Moreover, studies of MOP-R suggest a discrete regulatory scheme that enables opioid receptors to maintain sensitive signaling via G proteins at the plasma membrane. Key observations supporting each of these emerging views are summarized below.
Evidence for endosomal activation of opioid receptor signaling via G proteins emerged from the development of conformational biosensors, which detect and localize activated MOP-R and DOP-R in living cells. Single-domain antibodies (nanobodies), generated initially as tools to stabilize active-conformation receptors for structural studies (Huang et al., 2015; Manglik et al., 2017), were later adapted to detect conformational activation of receptors in living cells when expressed as fluorescent fusion proteins in the cytoplasm (Stoeber et al., 2018). Imaging live cells using total internal reflection fluorescence microscopy, a method useful for examining events close to the cell surface, demonstrated agonist-induced recruitment of biosensor to the plasma membrane, as expected. Imaging by confocal microscopy, enabling intracellular compartments to be resolved in optical sections, also revealed biosensor recruitment to endosome membranes after receptor internalization. Experiments contrasting the effects of membrane-permeant and membrane-impermeant antagonists provided functional evidence supporting the ability of DOP-R activation in internal membrane compartments to contribute to a relatively sustained component of opioid receptor–mediated inhibition of cellular adenylyl cyclase activity. These observations add support to the overall hypothesis that phosphorylation and β-arrestin–promoted endocytosis of opioid receptors is not associated exclusively with signal termination. Rather, the conserved phosphorylation-endocytosis machinery appears to enable a second “wave” of G protein–mediated signaling that is initiated from the endosome limiting membrane (Fig. 1).
Evidence suggesting a distinct effect of opioid receptor endocytosis on G protein signaling from the plasma membrane emerged through studies using a combination of fluorescence imaging methods to investigate the relationship between MOP-R trafficking and signaling in neurons and specifically focusing on this relationship in axons. MOP-R mediates both postsynaptic and presynaptic neuromodulatory effects through G proteins (Williams et al., 2013). At the postsynaptic plasma membrane, MOP-R stimulates a hyperpolarizing current through G protein–dependent activation of G protein–coupled inwardly rectifying potassium (GIRK) channels (Williams et al., 1982). This response normally desensitizes over several minutes in the continuous presence of agonist and then recovers after agonist washout (Dang and Williams, 2004). Desensitization of the postsynaptic GIRK response occurs even when internalization of MOP-R is prevented (Arttamangkul et al., 2006), supporting the hypothesis that endocytosis acts redundantly in surface-delimited signal termination, and receptor recycling after endocytosis parallels recovery of MOP-R signaling from the desensitized state (Arttamangkul et al., 2008; Yu et al., 2010; Quillinan et al., 2011). Further, mutation of phosphorylation sites in the MOP-R cytoplasmic tail that are required for agonist-induced internalization of receptors in the somatodendritic compartment blocks functional desensitization of postsynaptic GIRK signaling measured in brain slice preparations (Alvarez et al., 2002; Just et al., 2013; Yousuf et al., 2015; Arttamangkul et al., 2018). MOP-R is also known to inhibit postsynaptic calcium transients, but interestingly, this response does not rapidly desensitize. This difference appears to occur as a consequence of classic receptor reserve because reducing the overall number of functional receptors by preexposing tissue slices to the irreversible opioid antagonist β-chlornaltrexamine unmasks rapid desensitization of this postsynaptic response as well (Fox and Hentges, 2017). Accordingly, MOP-R signaling, which occurs in the somatodendritic plasma membrane of neurons, conforms to the classic cellular regulatory paradigm, with receptor phosphorylation followed by internalization producing a net loss of functional signaling responsiveness.
However, there is now evidence for a different relationship between MOP-R signaling and trafficking in axons. MOP-R inhibits transmitter release at presynaptic terminals by local G protein–dependent inhibition of voltage-gated calcium channels and components of the vesicular release machinery (Wilding et al., 1995; Bourinet et al., 1996; Zurawski et al., 2019). This response is more highly sensitive to opioid peptides than postsynaptic GIRK signaling mediated by MOP-R, and it remains highly sensitive even under conditions of prolonged agonist exposure that strongly desensitize the postsynaptic GIRK response (Fyfe et al., 2010; Pennock et al., 2012; Lowe and Bailey, 2015). Further, in contrast to what is observed for postsynaptic calcium transients, reducing the overall number of functional opioid receptors by irreversible antagonist preexposure fails to unmask desensitization of presynaptic inhibition mediated by MOP-R (Pennock et al., 2012). Accordingly, MOP-R regulation in the presynaptic compartment appears to differ fundamentally from that occurring in the postsynaptic compartment based on its higher overall ligand sensitivity and resistance to desensitization, even when overall receptor reserve is reduced.
Despite this distinction, live-cell imaging of tagged MOP-R in neurons revealed agonist-induced phosphorylation and phosphorylation-dependent endocytosis in axons, with these events mediated by similar machinery as that mediating rapid desensitization of postsynaptic MOP-R signaling (Jullié et al., 2020). A clue to how these conserved cellular regulatory events are compatible with a lack of functional desensitization at the presynapse became apparent from experiments using various imaging methods to examine in detail the localization and dynamics of MOP-R in axons. To visualize sites of endocytic uptake of MOP-R in axons of living neurons, a pH-sensitive GFP variant (SEP) was fused to the extracellular domain of MOP-R. Taking advantage of rapid quenching of surface-accessible SEP fluorescence by perfusion of an acidic buffer, newly formed endocytic vesicles containing SEP-labeled MOP-R were visualized in live image series as abruptly appearing acid-resistant fluorescent punctae (Merrifield et al., 2005). In contrast to clathrin-mediated endocytosis at the somatodendritic plasma membrane that occurs throughout the membrane surface at densely distributed sites (Rosendale et al., 2017), endocytosis of MOP-R in axons was found to be restricted to sparsely distributed sites, which are localized almost exclusively within terminals.
One might expect such local endocytosis to cause even stronger desensitization at the presynapse than at the postsynaptic plasma membrane. However, when individual MOP-R proteins were imaged using single-molecule localization microscopy, receptors were found to be uniformly distributed and not detectably accumulated at presynaptic terminals. Moreover, analysis of single-receptor trajectories revealed that MOP-R is freely mobile and rapidly diffuses on the axon surface. Confocal fluorescence microscopy and wide field imaging of axons under oblique illumination (a method that is useful for examining organelle dynamics in axons), revealed that MOP-R–containing endosomes accumulate in terminals but also move bidirectionally on the axon shaft. These endosomes support local surface recycling of MOP-R because membrane fusion events that insert receptors into the axon plasma membrane, imaged as local bursts of increased fluorescence intensity (due to dequenching of SEP upon receptor exposure at the axon surface), appear adjacent to individual endosomes both in terminals and in the axon shaft domain. Together these observations suggest that local recycling enables axons to maintain a diffusive surface pool of MOP-R even after prolonged agonist exposure and that receptors present on the axon shaft but outside of terminals have the potential to support local signaling at terminals through lateral diffusion and collisional coupling (Fig. 2).
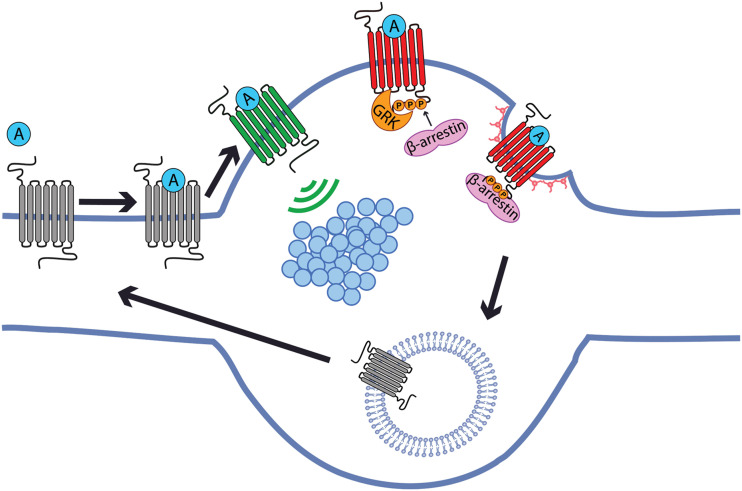
Fig. 2.
Cellular basis for sensitive presynaptic neuromodulation by opioid receptors. Opioid receptors inhibit synaptic vesicle (blue circles) exocytosis by locally regulating effectors that are restricted in terminals and positioned in, or adjacent to, individual presynaptic active zones. Opioid receptors are not immobilized at terminals, however, and instead are laterally mobile throughout the axon surface and collisionally couple to effectors at the presynapse. After ligand-induced activation (A), presynaptic opioid receptors undergo phosphorylation (P) and endocytosis directly at terminals. receptors are then are locally recycled and reinserted to the axon surface both within and outside of synapses to replenish the diffusible surface pool. Lateral diffusion of receptors is sufficiently fast for terminals to “sample” agonist-receptor complexes formed outside of synapses. The net effect of these events is to maintain a mobile surface receptor pool that is capable of mediating sensitive signaling at the presynapse by leveraging lateral diffusion and the allosteric nature of opioid receptor signaling by heterotrimeric G proteins.
Indeed, the measured diffusion rate of MOP-R on the axon surface is sufficient to enable agonist-receptor complexes formed outside of synapses to diffuse into an adjacent active zone before the agonist dissociates. This suggests that resistance to rapid desensitization, a distinguishing characteristic of MOP-R signaling at the presynapse, arises from a different type of receptor reserve. In the classic model, resistance to desensitization is achieved by an overall excess of receptors. In axons, the extrasynaptic receptor fraction is protected because the conserved phosphorylation-endocytosis machinery is restricted to terminals. Presynaptic inhibition by opioids thus appears to resist desensitization by leveraging a discrete principle of “lateral” receptor reserve, based on ligand-receptor complexes signaling after diffusing into the synapse and being protected from inactivation when outside of it. Lateral receptor reserve, unlike classic receptor reserve, enables the presynapse to remain opioid-responsive and resist desensitization with low receptor number. It also carries other new physiologic implications, which remain to be investigated. These include: 1) enhancing reliability of presynaptic neuromodulation by increasing the rate at which individual terminals can sample independent agonist-receptor binding events, 2) achieving high absolute sensitivity to opioid ligands by using the axon as an extended “antenna” to increase the extracellular volume that an individual presynaptic terminal can effectively sample, and 3) enabling the same active zone to be regulated by multiple GPCR types without steric inhibition, despite the typically small size of terminals and limited number of relevant effectors immobilized at individual active zones (Jullié et al., 2020).
Insight into the Molecular and Cellular Specificity of Opioid Drug Effects
In addition to its impact on physiologic desensitization, the principle of receptor reserve (in its classic meaning) is important for understanding opioid drug action, in particular agonist efficacy and partial agonism. Opioid receptors have been prototypical receptors for experimentally illustrating the impact of receptor reserve on the efficacy of drugs (Borgland et al., 2003; McPherson et al., 2010). Reduction of receptor reserve using irreversible antagonists, such as β-funaltrexamine or chlornaltrexamine, has been used extensively to identify partial agonists that show full maximal responses in highly amplified or very efficiently coupled signaling pathways. Morphine, for example, shows lower intrinsic efficacy at MOP-R than enkephalins or fentanyl yet can produce maximal responses in systems with high receptor reserve (Borgland et al., 2003). According to classic receptor theory, partial agonism is explained by the degree to which the agonist stabilizes a single activated GPCR state. However, recent biophysical evidence indicates that GPCRs dynamically fluctuate between multiple conformational states (Weis and Kobilka, 2018), and conformational heterogeneity has been explicitly demonstrated for MOP-R (Sounier et al., 2015). The significance of multiple receptor states to physiologic signaling by opioid peptides or the effects of drugs remains unclear and defines an exciting area of current investigation.
Interest in partial agonism has experienced a recent renaissance, partially motivated by such emerging biophysical evidence and preceded by various functional and cellular observations, which together suggest that agonists have the potential to differ in more than one “dimension” of intrinsic efficacy at a given GPCR (Galandrin et al., 2007; Urban et al., 2007; Kenakin, 2017). A current elaboration of this concept is the proposed pharmacological paradigm of biased agonism, determined by agonists differentially promoting receptor coupling to G protein relative to β-arrestin–mediated pathways. This paradigm gained particular traction through studies of β-arrestin knockout mice, in which morphine administration resulted in enhanced antinociception but diminished respiratory depression and constipation (Bohn et al., 1999; Raehal et al., 2005). Based on this, G protein–biased agonists—namely, agonists that selectively promote opioid receptors to engage G proteins over β-arrestins—were proposed to provide a new avenue for safer opioid analgesics. This hypothesis motivated the development of compounds such as TRV130 (oliceridine) (DeWire et al., 2013), PZM21 (Manglik et al., 2016), and SR-17018 (Schmid et al., 2017) as putative G protein–biased opioid receptor agonists with improved therapeutic profiles. The physiologic validity of the bias hypothesis, in its present elaboration, is still being evaluated (Hill et al., 2018). In particular, the presence of reduced respiratory depression in β-arrestin knockout mice was recently challenged (Kliewer et al., 2020). Further, the degree to which physiologically relevant differences between opioid drugs truly result from G protein bias, or reflect low agonist efficacy overall (typically lower than morphine), remains a matter of active investigation (Conibear and Kelly, 2019). Moreover, there is accumulating evidence for additional factors impacting bias profile, particularly kinetic effects on trafficking and signaling (Thompson et al., 2016; Weinberg et al., 2017). In the future, we anticipate that structural, biophysical, and computational approaches will provide increasingly precise understanding of the underpinnings of agonist efficacy, bias, and allosteric modulation, leading to drugs with improved therapeutic window (Filizola, 2019; Hu et al., 2019, 2020; Zarzycka et al., 2019).
Another facet of bias has recently emerged thanks, in part, to the new applications of genetically encoded biosensors and advanced imaging approaches that allow unprecedented spatiotemporal resolution of receptor localization and signaling events. As mentioned above, these sensors have facilitated the detection of receptor signals in distinct parts of the cell, from diffusion across the plasma membrane (Gondin et al., 2019; Metz et al., 2019; Tobin et al., 2019) to different subcellular compartments, including endosomes and Golgi membranes (Fig. 1) (Stoeber et al., 2018). The molecular environment surrounding receptors is already well known to contribute to cell and tissue type–specific differences in signaling and drug effects. Location bias adds another dimension of selectivity based on differences in the molecular environment around receptors at a subcellular level (Lobingier et al., 2017), suggesting interesting new directions for improving drug efficacy and selectivity. This concept is relevant not only to trafficking of receptors between membrane compartments but also to the lateral redistribution of opioid receptors between plasma membrane domains. For example, morphine and an opioid peptide full agonist were shown to produce different spatiotemporal signaling profiles by changing the selectivity with which MOP-R engages distinct protein networks, mediated by a phosphorylation-dependent change in the surface distribution of receptors (Fig. 1) (Civciristov et al., 2019).
New Approaches to Investigate Opioid Receptor Biology In Vivo
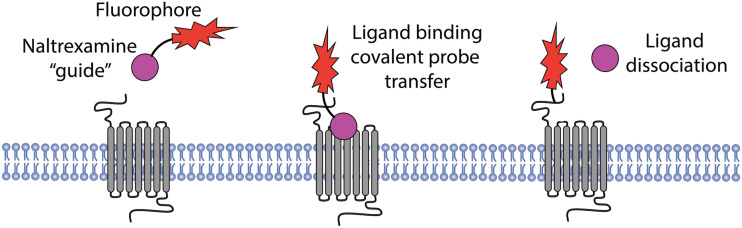
Ultimately, for these new directions to be exploited, it is key to better understand the physiologic context in which opioid receptors operate. Cells expressing opioid receptors (and GPCRs in general) are the nodes at which physiology, cell biology, and biophysical concepts need to converge. Traditional approaches such as electrophysiology, immunohistochemistry, and behavioral pharmacology, together with more recently developed genetic strategies to ablate or modify expression of opioid receptors in different parts of the nervous system, have paved the way for incisive studies of native tissue preparations and are beginning to illustrate the complexity of opioid drug action in vivo (Corder et al., 2018). Transgenic expression of mutant MOP-R in knockout animals is also revealing additional complexity of opioid receptor regulation by phosphorylation in vivo (Arttamangkul et al., 2019a). In particular, knockin mice expressing mutant or tagged receptors are unveiling physiologic regulation of opioid receptors in their native environment and delineating functional consequences in vivo (Scherrer et al., 2006; Wang et al., 2018; Ehrlich et al., 2019; Kliewer et al., 2019). Further, engineered opioid receptors that are activated by light allow precise spatiotemporal control of opioid signaling in defined neural circuits and relevance to behavior (Siuda et al., 2015). Moreover, new chemical tools are being developed to enable the detection of endogenous receptors in vivo without genetic modification. For example, an elegant chemical method was described recently that exploits the selectivity of drug-receptor interactions as a pharmacological “guide” to covalently label endogenous receptors without impairing receptor activity (Fig. 3). Using this method, opioid receptors were imaged live in native brain slices using two-photon fluorescence microscopy, without the need for genetically encoded tags or indirect (e.g., antibody) labeling methods (Arttamangkul et al., 2019b).
Fig. 3.
A nonperturbing labeling strategy for opioid receptors using ligand-guided chemistry. Naltrexamine-acylimidazole-Alexa594 binds to the orthosteric binding site of opioid receptors, with the pharmacophore “guiding” covalent coupling to residues located outside of the ligand binding pocket. The coupling reaction releases the pharmacophore (upon washout), leaving the native receptor fluorescently labeled and functional to undergo subsequent activation by an orthosteric agonist after labeling.
Conclusion and Outlook
Many of the imaging methods highlighted above have focused on studies of MOP-R. Given the distinct and important effects of DOP-R and KOP-R, we anticipate that future studies using these methods will provide additional insights into the cellular basis of opioid receptor function and drug action. We also anticipate that advances in the study of other GPCRs will find application to the study of opioid receptors. For example, single-molecule imaging has been used to detect “hot spots” on the plasma membrane at which GPCRs interact with cognate G proteins (Sungkaworn et al., 2017). Such imaging approaches can be used to examine GPCR interactions with other proteins as well, including receptor-receptor interactions (Calebiro and Grimes, 2020). We note that interesting progress has already been made on this front for opioid receptors, with single-molecule imaging of receptors bound to a high-affinity fluorescent ligand revealing short-lived MOP-R homodimers in the plasma membrane (Gentzsch et al., 2019).
We also discussed above how structural studies enabled the development of conformational biosensors to detect opioid receptor activation in living cells. In particular, cryoelectron microscopy has emerged as a powerful approach for resolving structural details of GPCRs in complex with other proteins. A pioneering example was the determination of a structure of agonist-activated MOP-R in complex with Gi (Koehl et al., 2018). Recently, cryoelectron microscopy was used successfully for structural determination of agonist-activated GPCRs in association with β-arrestin, a physiologically important complex that has eluded conventional X-ray crystallography (Zhou et al., 2016; Yin et al., 2019; Huang et al., 2020; Staus et al., 2020). It has also enabled structural determination of a GPCR bound to both β-arrestin and G protein simultaneously, a complex which has been proposed to form on the endosome membrane and transduce a sustained form of signaling by certain GPCRs after they undergo ligand-induced internalization (Vilardaga et al., 2012; Nguyen et al., 2019). Such structural and biophysical advances will undoubtedly fuel new progress at the cellular level. For example, cryoelectron microscopy revealed a subtle difference between the conformation of activated MOP-R when in complex with Gi relative to when in complex with a nanobody (Huang et al., 2015; Koehl et al., 2018). Inspired by this, a recent cell-based study compared recruitment by opioid receptors of engineered protein probes derived from corresponding G protein or nanobody folds. Considerable selectivity of protein probe recruitment to MOP-R and KOP-R was observed in living cells, depending on receptor activation by partial, biased, or full agonists (Stoeber et al., 2020).
In sum, the past few years have seen major advances in illuminating opioid pharmacology under the microscope. The field is now poised to resolve with unprecedented clarity fundamental receptor signaling and regulatory processes across molecular, cellular, and physiologic scales. Such approaches have already yielded new insight into the actions of existing drugs, are motivating new therapeutic hypotheses that are presently under investigation, and may enable future development of improved opioid therapies. In addition, because opioid receptors are widely considered prototypes for the large GPCR family, we anticipate that such efforts will continue to drive GPCR-directed therapeutic innovation more broadly.
Acknowledgments
We thank many colleagues for their valuable contributions to the field and critical discussion and regret that we were able to highlight only a limited subset of relevant studies in the present brief review.
Abbreviations
- DOP-R
δ-opioid receptor
- GIRK
G protein–coupled inwardly rectifying potassium channel
- GPCR
G protein–coupled receptor
- GRK
GPCR kinase
- KOP-R
κ-opioid receptor
- MOP-R
μ-opioid receptor
- SEP
superecliptic pHluorin
Authorship Contributions
Wrote or contributed to the writing of the manuscript: Jullié, Gondin, von Zastrow, Canals.
Footnotes
Work in the authors’ laboratories was supported by research grants from the US National Institutes of Health/National Institute on Drug Abuse (R01DA010711 and R01DA012864 to M.v.Z.) and the UCSF Program for Breakthrough Biomedical Research (D.J.).
References
- Alvarez VA, Arttamangkul S, Dang V, Salem A, Whistler JL, Von Zastrow M, Grandy DK, Williams JT. (2002) μ-Opioid receptors: ligand-dependent activation of potassium conductance, desensitization, and internalization. J Neurosci 22:5769–5776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arttamangkul S, Heinz DA, Bunzow JR, Song X, Williams JT. (2018) Cellular tolerance at the µ-opioid receptor is phosphorylation dependent. eLife 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arttamangkul S, Leff ER, Koita O, Birdsong WT, Williams JT. (2019a) Separation of acute desensitization and long-term tolerance of µ-opioid receptors is determined by the degree of C-terminal phosphorylation. Mol Pharmacol 96:505–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arttamangkul S, Plazek A, Platt EJ, Jin H, Murray TF, Birdsong WT, Rice KC, Farrens DL, Williams JT. (2019b) Visualizing endogenous opioid receptors in living neurons using ligand-directed chemistry. eLife 8:e49319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arttamangkul S, Quillinan N, Low MJ, von Zastrow M, Pintar J, Williams JT. (2008) Differential activation and trafficking of micro-opioid receptors in brain slices. Mol Pharmacol 74:972–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arttamangkul S, Torrecilla M, Kobayashi K, Okano H, Williams JT. (2006) Separation of mu-opioid receptor desensitization and internalization: endogenous receptors in primary neuronal cultures. J Neurosci 26:4118–4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey CP, Oldfield S, Llorente J, Caunt CJ, Teschemacher AG, Roberts L, McArdle CA, Smith FL, Dewey WL, Kelly E, et al. (2009) Involvement of PKC alpha and G-protein-coupled receptor kinase 2 in agonist-selective desensitization of mu-opioid receptors in mature brain neurons. Br J Pharmacol 158:157–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn LM, Lefkowitz RJ, Gainetdinov RR, Peppel K, Caron MG, Lin FT. (1999) Enhanced morphine analgesia in mice lacking beta-arrestin 2. Science 286:2495–2498. [DOI] [PubMed] [Google Scholar]
- Borgland SL, Connor M, Osborne PB, Furness JB, Christie MJ. (2003) Opioid agonists have different efficacy profiles for G protein activation, rapid desensitization, and endocytosis of mu-opioid receptors. J Biol Chem 278:18776–18784. [DOI] [PubMed] [Google Scholar]
- Bourinet E, Soong TW, Stea A, Snutch TP. (1996) Determinants of the G protein-dependent opioid modulation of neuronal calcium channels. Proc Natl Acad Sci USA 93:1486–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman SL, Soohoo AL, Shiwarski DJ, Schulz S, Pradhan AA, Puthenveedu MA. (2015) Cell-autonomous regulation of Mu-opioid receptor recycling by substance P. Cell Rep 10:1925–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchas MR, Chavkin C. (2010) Kinase cascades and ligand-directed signaling at the kappa opioid receptor. Psychopharmacology (Berl) 210:137–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calebiro D, Grimes J. (2020) G protein-coupled receptor pharmacology at the single-molecule level. Annu Rev Pharmacol Toxicol 60:73–87. [DOI] [PubMed] [Google Scholar]
- Chavkin C, Goldstein A. (1982) Reduction in opiate receptor reserve in morphine tolerant guinea pig ilea. Life Sci 31:1687–1690. [DOI] [PubMed] [Google Scholar]
- Civciristov S, Huang C, Liu B, Marquez EA, Gondin AB, Schittenhelm RB, Ellisdon AM, Canals M, Halls ML. (2019) Ligand-dependent spatiotemporal signaling profiles of the μ-opioid receptor are controlled by distinct protein-interaction networks. J Biol Chem 294:16198–16213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conibear AE, Kelly E. (2019) A biased view of µ-opioid receptors? Mol Pharmacol 96:542–549 DOI: 10.1124/mol.119.115956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corder G, Castro DC, Bruchas MR, Scherrer G. (2018) Endogenous and exogenous opioids in pain. Annu Rev Neurosci 41:453–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang VC, Williams JT. (2004) Chronic morphine treatment reduces recovery from opioid desensitization. J Neurosci 24:7699–7706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWire SM, Ahn S, Lefkowitz RJ, Shenoy SK. (2007) Beta-arrestins and cell signaling. Annu Rev Physiol 69:483–510. [DOI] [PubMed] [Google Scholar]
- DeWire SM, Yamashita DS, Rominger DH, Liu G, Cowan CL, Graczyk TM, Chen X-T, Pitis PM, Gotchev D, Yuan C, et al. (2013) A G protein-biased ligand at the μ-opioid receptor is potently analgesic with reduced gastrointestinal and respiratory dysfunction compared with morphine. J Pharmacol Exp Ther 344:708–717. [DOI] [PubMed] [Google Scholar]
- Ehrlich AT, Semache M, Gross F, Da Fonte DF, Runtz L, Colley C, Mezni A, Le Gouill C, Lukasheva V, Hogue M, et al. (2019) Biased signaling of the mu opioid receptor revealed in native neurons. iScience 14:47–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filizola M. (2019) Insights from molecular dynamics simulations to exploit new trends for the development of improved opioid drugs. Neurosci Lett 700:50–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox PD, Hentges ST. (2017) Differential desensitization observed at multiple effectors of somatic μ-opioid receptors underlies sustained agonist-mediated inhibition of proopiomelanocortin neuron activity. J Neurosci 37:8667–8677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyfe LW, Cleary DR, Macey TA, Morgan MM, Ingram SL. (2010) Tolerance to the antinociceptive effect of morphine in the absence of short-term presynaptic desensitization in rat periaqueductal gray neurons. J Pharmacol Exp Ther 335:674–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galandrin S, Oligny-Longpré G, Bouvier M. (2007) The evasive nature of drug efficacy: implications for drug discovery. Trends Pharmacol Sci 28:423–430. [DOI] [PubMed] [Google Scholar]
- Gentzsch C, Seier K, Drakopoulos A, Jobin M-L, Lanoiselée Y, Koszegi Z, Maurel D, Sounier R, Hübner H, Gmeiner P, et al. (2019) Selective and wash-resistant fluorescent dihydrocodeinone derivatives allow single-molecule imaging of μ-ppioid receptor dimerization. Angew Chem Int Ed Engl DOI: 10.1002/anie.201912683 [published ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gondin AB, Halls ML, Canals M, Briddon SJ. (2019) GRK mediates μ-opioid receptor plasma membrane reorganization. Front Mol Neurosci 12:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill R, Disney A, Conibear A, Sutcliffe K, Dewey W, Husbands S, Bailey C, Kelly E, Henderson G. (2018) The novel μ-opioid receptor agonist PZM21 depresses respiration and induces tolerance to antinociception. Br J Pharmacol 175:2653–2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hislop JN, Henry AG, von Zastrow M. (2011) Ubiquitination in the first cytoplasmic loop of μ-opioid receptors reveals a hierarchical mechanism of lysosomal down-regulation. J Biol Chem 286:40193–40204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Provasi D, Ramsey S, Filizola M. (2020) Mechanism of μ-opioid receptor-magnesium interaction and positive allosteric modulation. Biophys J 118:909–921 DOI: 10.1016/j.bpj.2019.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Wang Y, Hunkele A, Provasi D, Pasternak GW, Filizola M. (2019) Kinetic and thermodynamic insights into sodium ion translocation through the μ-opioid receptor from molecular dynamics and machine learning analysis. PLOS Comput Biol 15:e1006689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Manglik A, Venkatakrishnan AJ, Laeremans T, Feinberg EN, Sanborn AL, Kato HE, Livingston KE, Thorsen TS, Kling RC, et al. (2015) Structural insights into µ-opioid receptor activation. Nature 524:315–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Masureel M, Qu Q, Janetzko J, Inoue A, Kato HE, Robertson MJ, Nguyen KC, Glenn JS, Skiniotis G, et al. (2020) Structure of the neurotensin receptor 1 in complex with β-arrestin 1. Nature 579:303–308 DOI: 10.1038/s41586-020-1953-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jullié D, Stoeber M, Sibarita J-B, Zieger HL, Bartol TM, Arttamangkul S, Sejnowski TJ, Hosy E, von Zastrow M. (2020) A discrete presynaptic vesicle cycle for neuromodulator receptors. Neuron 105:663–677.e8 DOI: 10.1016/j.neuron.2019.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just S, Illing S, Trester-Zedlitz M, Lau EK, Kotowski SJ, Miess E, Mann A, Doll C, Trinidad JC, Burlingame AL, et al. (2013) Differentiation of opioid drug effects by hierarchical multi-site phosphorylation. Mol Pharmacol 83:633–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang DS, Tian X, Benovic JL. (2014) Role of β-arrestins and arrestin domain-containing proteins in G protein–coupled receptor trafficking. Curr Opin Cell Biol 27:63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith DE, Murray SR, Zaki PA, Chu PC, Lissin DV, Kang L, Evans CJ, von Zastrow M. (1996) Morphine activates opioid receptors without causing their rapid internalization. J Biol Chem 271:19021–19024. [DOI] [PubMed] [Google Scholar]
- Kenakin T. (2017) Signaling bias in drug discovery. Expert Opin Drug Discov 12:321–333. [DOI] [PubMed] [Google Scholar]
- Kieffer BL, Evans CJ. (2009) Opioid receptors: from binding sites to visible molecules in vivo. Neuropharmacology 56 (Suppl 1):205–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliewer A, Gillis A, Hill R, Schmidel F, Bailey C, Kelly E, Henderson G, Christie MJ, Schulz S. (2020) Morphine-induced respiratory depression is independent of β-arrestin2 signalling. Br J Pharmacol DOI: 10.1111/bph.15004 [published ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliewer A, Schmiedel F, Sianati S, Bailey A, Bateman JT, Levitt ES, Williams JT, Christie MJ, Schulz S. (2019) Phosphorylation-deficient G-protein-biased μ-opioid receptors improve analgesia and diminish tolerance but worsen opioid side effects. Nat Commun 10:367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehl A, Hu H, Maeda S, Zhang Y, Qu Q, Paggi JM, Latorraca NR, Hilger D, Dawson R, Matile H, et al. (2018) Structure of the µ-opioid receptor-Gi protein complex. Nature 558:547–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komolov KE, Benovic JL. (2018) G protein–coupled receptor kinases: past, present and future. Cell Signal 41:17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunselman JM, Zajac AS, Weinberg ZY, Puthenveedu MA. (2019) Homologous regulation of mu opioid receptor recycling by G βγ, protein kinase C, and receptor phosphorylation. Mol Pharmacol 96:702–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau EK, Trester-Zedlitz M, Trinidad JC, Kotowski SJ, Krutchinsky AN, Burlingame AL, von Zastrow M. (2011) Quantitative encoding of the effect of a partial agonist on individual opioid receptors by multisite phosphorylation and threshold detection. Sci Signal 4:ra52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law PY, Hom DS, Loh HH. (1984) Down-regulation of opiate receptor in neuroblastoma x glioma NG108-15 hybrid cells. Chloroquine promotes accumulation of tritiated enkephalin in the lysosomes. J Biol Chem 259:4096–4104. [PubMed] [Google Scholar]
- Li JG, Luo LY, Krupnick JG, Benovic JL, Liu-Chen LY. (1999) U50,488H-induced internalization of the human kappa opioid receptor involves a beta-arrestin- and dynamin-dependent mechanism. Kappa receptor internalization is not required for mitogen-activated protein kinase activation. J Biol Chem 274:12087–12094. [DOI] [PubMed] [Google Scholar]
- Lobingier BT, Hüttenhain R, Eichel K, Miller KB, Ting AY, von Zastrow M, Krogan NJ. (2017) An approach to spatiotemporally resolve protein interaction networks in living cells. Cell 169:350–360.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe JD, Bailey CP. (2015) Functional selectivity and time-dependence of μ-opioid receptor desensitization at nerve terminals in the mouse ventral tegmental area. Br J Pharmacol 172:469–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manglik A, Kobilka BK, Steyaert J. (2017) Nanobodies to study G protein-coupled receptor structure and function. Annu Rev Pharmacol Toxicol 57:19–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manglik A, Lin H, Aryal DK, McCorvy JD, Dengler D, Corder G, Levit A, Kling RC, Bernat V, Hübner H, et al. (2016) Structure-based discovery of opioid analgesics with reduced side effects. Nature 537:185–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson J, Rivero G, Baptist M, Llorente J, Al-Sabah S, Krasel C, Dewey WL, Bailey CP, Rosethorne EM, Charlton SJ, et al. (2010) μ-opioid receptors: correlation of agonist efficacy for signalling with ability to activate internalization. Mol Pharmacol 78:756–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrifield CJ, Perrais D, Zenisek D. (2005) Coupling between clathrin-coated-pit invagination, cortactin recruitment, and membrane scission observed in live cells. Cell 121:593–606. [DOI] [PubMed] [Google Scholar]
- Metz MJ, Pennock RL, Krapf D, Hentges ST. (2019) Temporal dependence of shifts in mu opioid receptor mobility at the cell surface after agonist binding observed by single-particle tracking. Sci Rep 9:7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen AH, Thomsen ARB, Cahill TJ, III, Huang R, Huang L-Y, Marcink T, Clarke OB, Heissel S, Masoudi A, Ben-Hail D, et al. (2019) Structure of an endosomal signaling GPCR-G protein-β-arrestin megacomplex. Nat Struct Mol Biol 26:1123–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennock RL, Dicken MS, Hentges ST. (2012) Multiple inhibitory G-protein-coupled receptors resist acute desensitization in the presynaptic but not postsynaptic compartments of neurons. J Neurosci 32:10192–10200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan AAA, Becker JAJ, Scherrer G, Tryoen-Toth P, Filliol D, Matifas A, Massotte D, Gavériaux-Ruff C, Kieffer BL. (2009) In vivo delta opioid receptor internalization controls behavioral effects of agonists. PLoS One 4:e5425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quillinan N, Lau EK, Virk M, von Zastrow M, Williams JT. (2011) Recovery from mu-opioid receptor desensitization after chronic treatment with morphine and methadone. J Neurosci 31:4434–4443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raehal KM, Walker JKL, Bohn LM. (2005) Morphine side effects in beta-arrestin 2 knockout mice. J Pharmacol Exp Ther 314:1195–1201. [DOI] [PubMed] [Google Scholar]
- Rosendale M, Jullié D, Choquet D, Perrais D. (2017) Spatial and temporal regulation of receptor endocytosis in neuronal dendrites revealed by imaging of single vesicle formation. Cell Rep 18:1840–1847. [DOI] [PubMed] [Google Scholar]
- Scherrer G, Tryoen-Tóth P, Filliol D, Matifas A, Laustriat D, Cao YQ, Basbaum AI, Dierich A, Vonesh J-L, Gavériaux-Ruff C, et al. (2006) Knockin mice expressing fluorescent delta-opioid receptors uncover G protein–coupled receptor dynamics in vivo. Proc Natl Acad Sci USA 103:9691–9696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid CL, Kennedy NM, Ross NC, Lovell KM, Yue Z, Morgenweck J, Cameron MD, Bannister TD, Bohn LM. (2017) Bias factor and therapeutic window correlate to predict safer opioid analgesics. Cell 171:1165–1175.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma SK, Klee WA, Nirenberg M. (1975) Dual regulation of adenylate cyclase accounts for narcotic dependence and tolerance. Proc Natl Acad Sci USA 72:3092–3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoy SK, Lefkowitz RJ. (2011) β-Arrestin-mediated receptor trafficking and signal transduction. Trends Pharmacol Sci 32:521–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siuda ER, Copits BA, Schmidt MJ, Baird MA, Al-Hasani R, Planer WJ, Funderburk SC, McCall JG, Gereau RW, IV, Bruchas MR. (2015) Spatiotemporal control of opioid signaling and behavior. Neuron 86:923–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sounier R, Mas C, Steyaert J, Laeremans T, Manglik A, Huang W, Kobilka BK, Déméné H, Granier S. (2015) Propagation of conformational changes during μ-opioid receptor activation. Nature 524:375–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staus DP, Hu H, Robertson MJ, Kleinhenz ALW, Wingler LM, Capel WD, Latorraca NR, Lefkowitz RJ, Skiniotis G. (2020) Structure of the M2 muscarinic receptor-β-arrestin complex in a lipid nanodisc. Nature 579:297–302 DOI: 10.1038/s41586-020-1954-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternini C, Spann M, Anton B, Keith DE, Jr, Bunnett NW, von Zastrow M, Evans C, Brecha NC. (1996) Agonist-selective endocytosis of mu opioid receptor by neurons in vivo. Proc Natl Acad Sci USA 93:9241–9246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeber M, Jullié D, Li J, Chakraborty S, Majumdar S, Lambert NA, Manglik A, von Zastrow M. (2020) Agonist-selective recruitment of engineered protein probes and of GRK2 by opioid receptors in living cells. eLife 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeber M, Jullié D, Lobingier BT, Laeremans T, Steyaert J, Schiller PW, Manglik A, von Zastrow M. (2018) A genetically encoded biosensor reveals location bias of opioid drug action. Neuron 98:963–976.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sungkaworn T, Jobin M-L, Burnecki K, Weron A, Lohse MJ, Calebiro D. (2017) Single-molecule imaging reveals receptor-G protein interactions at cell surface hot spots. Nature 550:543–547. [DOI] [PubMed] [Google Scholar]
- Tanowitz M, Hislop JN, von Zastrow M. (2008) Alternative splicing determines the post-endocytic sorting fate of G-protein-coupled receptors. J Biol Chem 283:35614–35621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanowitz M, von Zastrow M. (2003) A novel endocytic recycling signal that distinguishes the membrane trafficking of naturally occurring opioid receptors. J Biol Chem 278:45978–45986. [DOI] [PubMed] [Google Scholar]
- Thompson GL, Lane JR, Coudrat T, Sexton PM, Christopoulos A, Canals M. (2016) Systematic analysis of factors influencing observations of biased agonism at the mu-opioid receptor. Biochem Pharmacol 113:70–87. [DOI] [PubMed] [Google Scholar]
- Tobin SJ, Wakefield DL, Terenius L, Vukojević V, Jovanović-Talisman T. (2019) Ethanol and naltrexone have distinct effects on the lateral nano-organization of mu and kappa opioid receptors in the plasma membrane. ACS Chem Neurosci 10:667–676. [DOI] [PubMed] [Google Scholar]
- Tsao PI, von Zastrow M. (2000) Type-specific sorting of G protein–coupled receptors after endocytosis. J Biol Chem 275:11130–11140. [DOI] [PubMed] [Google Scholar]
- Urban JD, Clarke WP, von Zastrow M, Nichols DE, Kobilka B, Weinstein H, Javitch JA, Roth BL, Christopoulos A, Sexton PM, et al. (2007) Functional selectivity and classical concepts of quantitative pharmacology. J Pharmacol Exp Ther 320:1–13. [DOI] [PubMed] [Google Scholar]
- Vilardaga J-P, Gardella TJ, Wehbi VL, Feinstein TN. (2012) Non-canonical signaling of the PTH receptor. Trends Pharmacol Sci 33:423–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Zastrow M, Keith DE, Jr, Evans CJ. (1993) Agonist-induced state of the delta-opioid receptor that discriminates between opioid peptides and opiate alkaloids. Mol Pharmacol 44:166–172. [PubMed] [Google Scholar]
- von Zastrow M, Keith D, Zaki P, Evans C. (1994) Intracellular trafficking of epitope-tagged opioid receptors: different effects of morphine and enkephalin. Regul Pept 54:315–316. [Google Scholar]
- Wang D, Tawfik VL, Corder G, Low SA, François A, Basbaum AI, Scherrer G. (2018) Functional divergence of delta and mu opioid receptor organization in CNS pain circuits. Neuron 98:90–108.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg ZY, Zajac AS, Phan T, Shiwarski DJ, Puthenveedu MA. (2017) Sequence-specific regulation of endocytic lifetimes modulates arrestin-mediated signaling at the µ opioid receptor. Mol Pharmacol 91:416–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis WI, Kobilka BK. (2018) The molecular basis of G protein-coupled receptor activation. Annu Rev Biochem 87:897–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whistler JL, Enquist J, Marley A, Fong J, Gladher F, Tsuruda P, Murray SR, Von Zastrow M. (2002) Modulation of postendocytic sorting of G protein–coupled receptors. Science 297:615–620. [DOI] [PubMed] [Google Scholar]
- Whistler JL, von Zastrow M. (1998) Morphine-activated opioid receptors elude desensitization by beta-arrestin. Proc Natl Acad Sci USA 95:9914–9919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilding TJ, Womack MD, McCleskey EW. (1995) Fast, local signal transduction between the mu opioid receptor and Ca2+ channels. J Neurosci 15:4124–4132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JT, Egan TM, North RA. (1982) Enkephalin opens potassium channels on mammalian central neurones. Nature 299:74–77. [DOI] [PubMed] [Google Scholar]
- Williams JT, Ingram SL, Henderson G, Chavkin C, von Zastrow M, Schulz S, Koch T, Evans CJ, Christie MJ. (2013) Regulation of μ-opioid receptors: desensitization, phosphorylation, internalization, and tolerance. Pharmacol Rev 65:223–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin W, Li Z, Jin M, Yin Y-L, de Waal PW, Pal K, Yin Y, Gao X, He Y, Gao J, et al. (2019) A complex structure of arrestin-2 bound to a G protein–coupled receptor. Cell Res 29:971–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousuf A, Miess E, Sianati S, Du Y-P, Schulz S, Christie MJ. (2015) Role of phosphorylation sites in desensitization of µ-opioid receptor. Mol Pharmacol 88:825–835. [DOI] [PubMed] [Google Scholar]
- Yu YJ, Dhavan R, Chevalier MW, Yudowski GA, von Zastrow M. (2010) Rapid delivery of internalized signaling receptors to the somatodendritic surface by sequence-specific local insertion. J Neurosci 30:11703–11714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarzycka B, Zaidi SA, Roth BL, Katritch V. (2019) Harnessing ion-binding sites for GPCR pharmacology. Pharmacol Rev 71:571–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Ferguson SS, Barak LS, Bodduluri SR, Laporte SA, Law PY, Caron MG. (1998) Role for G protein–coupled receptor kinase in agonist-specific regulation of mu-opioid receptor responsiveness. Proc Natl Acad Sci USA 95:7157–7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Yu Y, Mackin S, Weight FF, Uhl GR, Wang JB. (1996) Differential mu opiate receptor phosphorylation and desensitization induced by agonists and phorbol esters. J Biol Chem 271:11449–11454. [DOI] [PubMed] [Google Scholar]
- Zhou XE, Gao X, Barty A, Kang Y, He Y, Liu W, Ishchenko A, White TA, Yefanov O, Han GW, et al. (2016) X-ray laser diffraction for structure determination of the rhodopsin-arrestin complex. Sci Data 3:160021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurawski Z, Yim YY, Alford S, Hamm HE. (2019) The expanding roles and mechanisms of G protein-mediated presynaptic inhibition. J Biol Chem 294:1661–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]