Abstract
For decades the broad role of opioids in addiction, neuropsychiatric disorders, and pain states has been somewhat well established. However, in recent years, with the rise of technological advances, not only is the existing dogma being challenged, but we are identifying new disease areas in which opioids play a critical role. This review highlights four new areas of exploration in the opioid field. The most recent addition to the opioid family, the nociceptin receptor system, shows promise as the missing link in understanding the neurocircuitry of motivation. It is well known that activation of the kappa opioid receptor system modulates negative affect and dysphoria, but recent studies now implicate the kappa opioid system in the modulation of negative affect associated with pain. Opioids are critical in pain management; however, the often-forgotten delta opioid receptor system has been identified as a novel therapeutic target for headache disorders and migraine. Lastly, changes to the gut microbiome have been shown to directly contribute to many of the symptoms of chronic opioid use and opioid related behaviors. This review summarizes the findings from each of these areas with an emphasis on identifying new therapeutic targets.
SIGNIFICANCE STATEMENT
The focus of this minireview is to highlight new disease areas or new aspects of disease in which opioids have been implicated; this includes pain, motivation, migraine, and the microbiome. In some cases, this has resulted in the pursuit of a novel therapeutic target and resultant clinical trial. We believe this is very timely and will be a refreshing take on reading about opioids and disease.
Introduction
Traditionally, reviews of the opioid system in behavior have focused on the role of the mu, delta, and kappa opioid receptors in addiction, pain, and more recently stress. In celebration of the International Narcotics Research Conference’s 50th anniversary we have chosen to honor the pioneering research on the diverse functions of the opioid system in disease by highlighting the most recent and more novel research actively ongoing. This includes the nociceptin system, which has gained traction in recent years as a key player in modulation of motivated states. It is well known that the kappa opioid system drives negative affective states; however, recent findings show that negative affect is a critical component in pain processing. The delta opioid system, which has had a turbulent journey through behavioral research, shows promise in the regulation of headache disorders and migraine. Finally, the most widely studied opioid receptor, the mu opioid receptor, greatly impacts gut microbiota composition and contributes to symptoms of chronic opioid use disorder. The fact that the behaviors highlighted in this review are somewhat independent and diverse and involve opioid receptors expressed both peripherally and centrally adds strength to the ever-growing field of opioid receptor function in behavior and disease.
Nociceptin Neurocircuitry and Motivation
Motivation- and reward-related neurocircuitry serves a critical role in regulating the internal states and responses to environmental conditions that enable an organism to adapt and survive. Particularly, dysfunction within these motivational processes can lead to reward-related behaviors that manifest into severe behavioral phenotypes including mood, substance use, and eating disorders (Volkow et al., 2011; Russo and Nestler, 2013). Additionally, environmental stressors can have an enormous impact on the output of this neurocircuitry, resulting in adaptive changes that can have long-term effects on reward-seeking behaviors (Koob and Volkow, 2016). Efforts to understand the anatomic specificity of reward motivation and its regulation by neuropeptides have provided some converging evidence that supports the nociceptin system as a candidate to modulate stress responsivity and reward-seeking behaviors (Toll et al., 2016). The endogenous neuropeptide, nociceptin (N/OFQ), and its receptor, nociceptin opioid peptide receptor (NOP) are widely distributed throughout the brain (Darland et al., 1998). Not only has this system been implicated in modulating normal appetitive behaviors, it is also implicated in a variety of psychiatric illnesses related to depression, substance abuse, binge eating, and anxiety (Mollereau et al., 1994; Mollereau and Mouledous, 2000; Norton et al., 2002; Zheng et al., 2002; Ozawa et al., 2015; Toll et al., 2016; Der-Avakian et al., 2017). Importantly, many of the underlying negative affective states integral to these illnesses may be modulated by a wide and diverse network of N/OFQ-NOP neurocircuitry. It is well known that N/OFQ and NOP mRNA expression dramatically overlap with multiple key feeding-, reward-, and stress-related brain regions. Specifically, anatomic studies highlight this neuromodulator system’s network of peptide and receptor interactions spanning multiple stress- and reward-related brain nuclei including the nucleus accumbens (NAc), striatum, bed nucleus of stria terminalis, the central nucleus of the amygdala, hippocampus, ventral tegmental area (VTA), and several hypothalamic areas (Anton et al., 1996; Darland et al., 1998; Mollereau and Mouledous, 2000). This system has been suggested to have a natural role in regulating an animal’s food-seeking behavior and as a result has modulatory influence in reward-seeking behaviors, generally. Additionally, its presence within the hypothalamic pituitary axis circuitry places it as a prominent candidate to influence stress responsivity and affective state (Devine et al., 2001). Yet how, where, and what specific neurocircuitry coordinates this intersection of stress, reward, and motivation remain unresolved and an important avenue for research. As such, recent investigations have pursued NOP agonists and antagonists as alternative therapeutics for mood disorders, such as depression and anxiety, as well as binge-eating and substance use disorders.
N/OFQ-NOP in Motivation and Major Depressive Disorder.
One in six Americans suffer from major depressive disorder (MDD), and an estimated one-third of these patients treated with current therapies are resistant to treatment (Rush et al., 2006; Greenberg et al., 2015). Mood disorders, including MDD and bipolar disorder, have been linked to reward-processing deficiencies that contribute to the functional impairments that define these disorders (American Psychiatric Association, 2013; Whitton et al., 2015). These reward-processing and motivational deficits are generally classified as anhedonia, a loss of interest or pleasure, and avolition, a lack of motivation to perform tasks. Recent evidence proposes that different psychiatric disorders may present subtle differences in particular reward processes, such as consummatory pleasure, motivation, and reward learning (Treadway and Zald, 2011; Barch et al., 2016). Additionally, studies have found that patients with MDD and anhedonia have poorer prognoses (Vrieze et al., 2014) and have a higher prevalence of treatment failure (McMakin et al., 2012). This prevalence of anhedonia and avolition suggests that aberrant reward processing is a core feature underlying depression pathophysiology. Moreover, stressful conditions can precipitate these conditions or the expression of related symptoms in healthy individuals (Berenbaum and Connelly, 1993; Kendler et al., 1999; Charney and Manji, 2004), as well as deficits in brain reward system function in laboratory animals (Der-Avakian et al., 2014; Donahue et al., 2014). Although pharmacotherapies are widely prescribed, significant limitations such as intolerable side effects, delayed antidepressant action, and low efficacy after treatment remain as consistent barriers to successful treatment (Berton and Nestler, 2006). As such, there is an urgent need for a better understanding of the pharmacological, behavioral, and neuroanatomical aspects of neuropeptidergic systems that could present more effective therapeutic approaches in the development of successful antidepressants (Werner and Coveñas, 2010).
Early preclinical studies unveiled the N/OFQ-NOP receptor system as a potential candidate to modulate mood-related disorders as NOP antagonist administration was demonstrated to have antidepressant-like effects in rodent models of depression. Specifically, NOP receptor antagonists reduced immobility time in the forced swimming test in mice, a measure considered indicative of antidepressant-like behavior (Redrobe et al., 2002). Additionally, converging evidence from genetic knockout studies demonstrated that NOP knockout mice and rats display reduced immobility time in forced swimming and tail suspension tests compared with wild-type controls (Gavioli et al., 2003, 2004). Furthermore, investigators at Eli Lilly developed the potent and selective NOP receptor antagonist, LY294009448, now named BTRX-246040 (Toledo et al., 2014), and found antidepressant-like behavioral effects in the forced swimming test in mice, which was absent in NOP knockout animals (Witkin et al., 2016). Other recent studies have corroborated these previous findings by demonstrating NOP antagonists effect to reduce depressive-like behaviors induced by lipopolysaccharide administration (Medeiros et al., 2015); repeated, uncontrollable foot-shock (Holanda et al., 2016); and unpredictable chronic mild stress (Vitale et al., 2009), which all can be reversed by classic antidepressants. Additionally, Der-Avakian et al. (2017) found that repeated social defeat stress induced reward-learning deficits in rats that resulted in increased N/OFQ mRNA expression in the nucleus accumbens shell as well as increased NOP mRNA in the VTA. This study also found that repeated social defeat stress reduced Fos mRNA expression in the VTA, indicating a reduction in neuronal activity.
Given the previously described data, it is critical to better understand how changes in reward neurocircuitry could manifest as depressive-like symptoms in humans. Early clinical investigation presented evidence of higher plasma levels of N/OFQ across different patient populations with depression with a reduction of these peptide levels after treatment with antidepressants (Gu et al., 2003; Zhang et al., 2009). Although these data suggest that elevated N/OFQ levels are associated with depression states, how these affective states are specifically driven by N/OFQ action is not completely understood. Complimentary studies examining different means of N/OFQ detection in real time are warranted. Given these findings, recent clinical investigation of the NOP antagonist BTRX-246040 as a novel oral treatment of MDD has demonstrated possible clinical potential (Toledo et al., 2014; Post et al., 2016). This double-blind, parallel-group, fixed-dose, placebo-controlled, 8-week proof-of-concept study randomized 136 patients to receive BTRX-246040 (N = 70) or placebo (N = 66) at 11 different sites in the United States. Patients who met criteria for MDD without psychotic features (as defined by the Diagnostic and Statistical Manual of Mental Disorders 4th Edition Test Revision) were evaluated using the GRID-Hamilton-17 Depression Rating Scale, 17 items (Hamilton, 1959; Williams et al., 2008). Once daily oral dosing (40 mg) of BTRX-246040 in these patients was evaluated for 8 weeks and with efficacy of treatment based on a change from baseline to 8 weeks when compared with placebo treatment. The least squares mean differences from placebo was −1.5 (95% confidence interval, −4.7, 1.7), and the probability that BTRX-246040 was better than placebo was 82.9%. Although this did not meet the predefined, proof-of-concept criterion, when analyses included the poststudy 9–10 week follow-up data, this least squares mean change was −2.9, and the probability that treatment with BTRX-246040 had a greater reduction in GRID-Hamilton-17 Depression Rating Scale total score than placebo was 97.4%. These findings established the first human data that provided evidence of NOP receptor antagonism as a potential strategy for the treatment of MDD. BTRX-246040 is currently under investigation in a double-blind, placebo-controlled phase 2a study for MDD treatment.
These previous studies have laid the foundational work that presents the N/OFQ-NOP receptor system as a promising target for the treatment of MDD. However, how this activity is modulated pharmacologically and what specific neurocircuitry facilitates depressive-like behaviors has not yet been fully elucidated. In particular, understanding differences in particular reward processes, such as affect, motivation, and reward learning, could provide clinically relevant information to guide treatment. One major component of MDD is avolition, or the lack of motivation to perform tasks. Motivation involved in reward-seeking behavior is thought to be mediated via dynamic activity and neuroplastic alterations of VTA dopamine neurons, and this activity is coordinated through a plethora of inputs, including nociceptin neurocircuitry. Notably, NOP is broadly expressed on VTA neurons positive for tyrosine hydroxylase, the rate-limiting enzyme for dopamine, and electrophysiological studies have reported that slice application of N/OFQ can inhibit dopamine neuron activity (Norton et al., 2002; Zheng et al., 2002). Furthermore, converging behavioral pharmacology evidence has demonstrated that systemic activation of the NOP receptor can reduce reward-related behaviors, including food consumption or preference for drugs of abuse (Ciccocioppo et al., 2000; Kotlińska et al., 2002; Zhao et al., 2003; Zaveri, 2011; Witkin et al., 2014; Kallupi et al., 2017). In particular, disruptions in activity of the mesolimbic dopaminergic system, specifically within the VTA, have been implicated in regulating reward consumption, effort, and motivational drive (Stauffer et al., 2016; Morales and Margolis, 2017). Recently, there has been important progress in identifying and characterizing neurocircuits that impact motivational states through regulation of VTA dopamine activity (Juarez and Han, 2016); however, how neuromodulators and specific endogenous neuropeptides engage and regulate motivation through the VTA and other limbic areas remains mostly unexplored. Recent evidence has suggested that there is remarkable heterogeneity of neuronal subtypes and anatomic localization within the VTA, as well as transmitter and neuropeptide systems that engage dopaminergic outputs (Jhou et al., 2009; Tan et al., 2012; van Zessen et al., 2012; Morales and Margolis, 2017). Given this framework, new research has sought to understand the mechanisms by which the N/OFQ-NOP receptor system is interconnected to modulate reward-seeking behavior through circuit-based investigation on endogenous VTA regulation and how mesolimbic NOP activation impacts motivation and depressive-like states.
Although previous studies have paired pharmacological and behavioral techniques to interrogate the N/OFQ-NOP receptor system, relatively new preclinical tools such as optogenetics and chemogenetics have given investigators the opportunity to examine this system with cell-specific precision. Our recent work (Parker et al., 2019) used optogenetics, chemogenetics, and fiber photometry (alongside genetic knock studies) to interrogate the role NOP and nociceptin neurocircuitry might play in the VTA, specifically in the modulation of dopamine cell activity and its relation to reward motivation. Although several studies have demonstrated a role for NOP activation to inhibit dopamine activity, few studies have evaluated the behavioral consequence of NOP stimulation in VTA dopamine neurons. Here, we sought to reveal the nociceptinergic neurocircuitry that had functional connectivity with the VTA. In this study, we uncovered a unique subpopulation of prepronociceptin (Pnoc) neurons located within the paranigral nucleus of the VTA (pnVTA) that are engaged during reward-seeking behavior. These studies used a progressive ratio operant conditioning task in which the effort required to receive a sucrose reward exponentially increases after each subsequent reward presentation. This task is designed to directly measure the effort an animal is willing to expend to receive a reward. Eventually, the animal will reach a point at which it will no longer seek the reward, called the “breakpoint” (Richardson and Roberts, 1996). Our data demonstrated that chemogenetic and optogenetic stimulation of these pnVTAPnoc neurons suppress the motivation to seek sucrose in the progressive ratio test, resulting in lower breakpoint and fewer rewards presented, whereas inhibition or ablation of these neurons increased animals’ performance. Using fiber photometry, we also discovered that these pnVTA neurons are especially engaged during low-yield reward-seeking behavior and most active during the final nose poke immediately preceding the animal’s breakpoint. Additionally, this study found that pnVTAPnoc neuron stimulation was aversive, both in real time and conditioned place preference tests. Furthermore, we demonstrated that global NOP knockout, conditional NOP knockout within the VTA, and specific deletion of NOP from VTA dopamine cells dramatically enhanced reward-seeking behaviors in a manner similar to pnVTAPnoc neuron ablation and inhibition. We also demonstrated that the conditional rescue and stimulation of NOP in VTA dopamine neurons greatly diminishes motivation to seek sucrose rewards.
Collectively, these data demonstrate a uniquely specific role of intra-VTA nociceptin neuropeptide release and NOP activation that acts to constrain dopamine neuron activity during reward-seeking behavior. In our case, an absent VTA nociceptin or NOP system allowed excessive reward-seeking under conditions in which a normally functioning NOP system would typically engage and promote appropriate operant responding for sucrose through dopamine neuron inhibition. Although this investigation provides insights into the coordination of motivated behavior, these data likely represent only a small portion of the neurocircuitry involved. Taken together, our study supports the conclusion that VTA NOP expression is critical for maintaining natural reward-seeking behavior and that functional changes in expression could manifest into altered reward seeking. It is possible that aberrant changes in this nociceptinergic neurocircuitry activity could manifest as the depressive-like symptomology present in individuals with MDD. Avolition represents only a portion of MDD symptomology, yet these behavioral differences could also reveal insights into the comorbidity of MDD and motivational dysregulation disorders such as substance use disorder. Other considerations include the role sex and gender have in the prevalence in MDD in patient populations. Although our data did not reveal any sex differences in the role of pnVTA nociceptinergic circuitry in reward motivation in our study, there is considerable evidence supporting a robust difference in MDD, with almost twice as many women suffering from MDD than men (Hyde et al., 2008; Salk et al., 2017; Hyde and Mezulis, 2020). Importantly, there is limited investigation into whether nociceptin neurocircuitry may be differentially affected among these distinct MDD populations, and new research should explicitly interrogate these avenues to provide better insights into appropriate therapeutic strategies for the treatment of MDD.
Conclusions.
It is well known that depression and other mood disorders are complicated disease states, driven by a confluence of physiologic and environmental factors that often require several therapeutic approaches. Currently, preclinical studies are seeking to better understand the pharmacological and neuroanatomical means by which the nociceptin/NOP system and NOP ligands affect emotional states and subsequent motivated behaviors. The identification of successful small molecule candidates like BTRX-246040 offer therapeutic potential, yet our understanding of how this system is engaged during these treatments is still not clear. However, given the preliminary success of these ligands preclinically and clinically, researchers need only to resolutely identify any side effects of chronic treatment and provide insight into the therapeutic advantages of NOP ligands in ameliorating depressive-like states in patients.
The Kappa Opioid System in the Regulation of the Negative Affective Component of Pain.
Negative affect refers to the experience of a negative emotional (affective) state. This is often described as an aversive/dysphoria-like or depressive-like state. Negative affect is manifested in a number of neuropsychiatric diseases such as addiction and pain. Here we will focus on negative affect in pain processing and mechanisms involved.
It has been well established that activation of kappa opioid receptors (KORs) induces dysphoria- and aversive-like states in rodent models (Mucha and Herz, 1985; Shippenberg et al., 1993; Knoll and Carlezon, 2010). In fact, it was a study in humans in which activation of KOR was first described to elicit “dysphoric and psychotomimetic effects” (Pfeiffer et al., 1986). The mechanisms underlying negative affective states are not entirely clear, but we do know that the NAc region seems to be highly and consistently implicated. Interestingly, clinical studies show reduced NAc activity and alteration in reward evaluation, decision making, and motivation in patients with pain (Oluigbo et al., 2012; Baliki and Apkarian, 2015). The NAc is part of the mesolimbic pathway, which is the reward-mediating pathway in the mammalian brain, composed of dopaminergic neurons projecting from the VTA of the midbrain to the NAc in the forebrain. Dopamine from these VTA dopaminergic neurons is released in the NAc in response to reinforcers, such as food, social interaction, or drugs of abuse.
KORs are expressed in the NAc on presynaptic terminals of dopaminergic neurons (Werling et al., 1988; Ebner et al., 2010; Al-Hasani and Bruchas, 2011), and activation of KORs decreases dopamine release (Spanagel et al., 1992; Margolis et al., 2003), which is known to drive aversive and negative emotional states (Wadenberg, 2003; Cahill et al., 2014; Wise and Koob, 2014). This dopaminergic dysfunction is thought to contribute to development of chronic pain (Cahill et al., 2014; Taylor et al., 2014, 2016; Yalcin and Barrot, 2014; Cahill and Taylor, 2017; Liu et al., 2019). This is somewhat validated by many who report reduced motivation for goal-directed behaviors during pain and has become a well known feature of pain-induced negative affect (Narita et al., 2005; Leitl et al., 2014; Schwartz et al., 2014; Hipólito et al., 2015; Liu et al., 2019; Massaly et al., 2019). Furthermore, intra-VTA opioid reward and evoked dopamine release within the NAc are reduced in chronic pain states (Taylor et al., 2015). It has also been shown that pain states alter reward processing; for example, animals exposed to chronic pain developed a preference for the morphine-paired side in a conditioned place preference (CPP) model when the dose of morphine was increased; known reinforcing doses of morphine do not induce a place preference under painful conditions (Wu et al., 2014). It was not until the last few years that laboratories began studying the kappa opioid receptor system in the context of negative affect and pain processing.
A recent study found that the morphine CPP described above is blocked upon administration of KOR antagonist JDTic, implicating a crucial role for KORs in the modulation of the aversive/negative affective component of pain in a chronic peripheral nerve injury (PNI) model (Liu et al., 2019). The same group also showed that blockade of KOR restores the blunted CPP after intra-VTA activation of μ opioid receptors (MORs) in neuropathic pain states. This suggests that KORs contribute to the loss of MOR reward-related behaviors in neuropathic pain (Liu et al., 2019). This was further supported by measuring evoked dopamine release, demonstrating that KOR modulation of dopamine release contributes to the tonic aversive component of pain. In addition, it has been shown that KORs are also necessary for the decrease in motivated behaviors characteristic in pain states. Blocking KORs, specifically in the contralateral and ventral NAc shell (vNAcSh) in rats and mice, respectively, prevents inflammatory pain–induced decrease in motivation, as measured in an operant task (Massaly et al., 2019). Furthermore, a decrease in sucrose self-administration is observed after local administration of the KOR agonist U50488 into the NAc shell. Together, these studies confirm both the necessity and sufficiency of KOR in pain-induced negative affect.
Further studies to better understand the mechanisms by which KOR modulates pain-induced negative affect show that both KOR mRNA in male but not female mice, and dynorphin mRNA in both male and female mice, are increased in the NAc after PNI (Liu et al., 2019). An increase in GTPδS binding was also observed in the NAc after PNI in males but not females (Liu et al., 2019) and in an inflammatory pain model (Massaly et al., 2019). These findings suggest that there may be an increase in KOR receptor expression and function in chronic pain states, which appears to also be sex dependent.
It is well known that a large proportion of medium spiny neurons in the NAcSh contain dynorphin and locally control presynaptic neurotransmitter release (Nestler and Carlezon, 2006; Al-Hasani et al., 2015); however, until recently little was known about their role in modulation of pain-induced negative affect. Interestingly, photo-stimulation of dynorphin-containing neurons in the vNAcSh decreases motivation in a sucrose self-administration paradigm, but this is not potentiated in an inflammatory pain model (Massaly et al., 2019), suggesting that pain does not potentiate dynorphin-mediated aversion but, based on findings summarized above, KORs are required to modulate negative affect.
Though it appears that dynorphin is not directly involved in modulation of affect, as shown in behavioral paradigms, evidence suggests that dynorphin-containing neurons may in fact be involved in the described KOR compensatory changes (Liu et al., 2019; Massaly et al., 2019). In a rat complete Freud’s adjuvant (CFA) model, expression of dynorphin A is increased, as measured by immunohistochemistry, as well as an observed enhancement in excitability in dynorphin-containing neurons, as measured in whole cell patch clamp recordings, together suggesting an increase in dynorphin tone during inflammatory pain (Massaly et al., 2019). Further investigation shows that both frequency and amplitude of spontaneous inhibitory postsynaptic current is decreased in dynorphin-containing neurons, suggesting that disinhibition of these neurons during inflammatory pain increases excitability in the vNAcSh (Massaly et al., 2019). When dynorphin-containing neurons are silenced using inhibitory designer receptors exclusively activated by designer drugs, CFA-treated rats no longer show the characteristic decrease in motivation associated with pain, implicating a critical role for dynorphin, as well as KOR, in the NAc in processing of pain-induced negative affect. This is further confirmed by positron emission tomography imaging studies, showing a decrease in radioligand binding at KORs in the NAc after CFA, suggesting an elevation in dynorphin.
In summary, it is known that KOR-mediated aversion and negative affect occurs in the NAc by altered dopamine transmission from the VTA, but the studies described here are the first to directly show that KOR also mediates negative affect in pain states and to explore the mechanisms. Evidence suggests that compensatory changes occur in the KOR system during pains states, as shown by increased function and expression. It seems likely that dynorphin-expressing medium spiny neurons modulate these KOR effects in turn with other neuropeptide systems yet to be explored. It also worth noting that there is evidence to suggest that stress together with pain may dysregulate KOR signaling (Massaly et al., 2016). It has been shown that stress causes activation of both KOR and p38 mitogen-activated protein kinase, coexpressed in GABAergic neurons, in the nucleus accumbens, cortex, and hippocampus (Bruchas et al., 2007). Studies from this same group went on to show that p38α mitogen-activated protein kinase in serotonergic neurons plays a critical role in the modulation of affective behavioral responses (Bruchas et al., 2011).
KORs are attractive targets as they are antinociceptive without the unwanted side effects that are commonly associated with activation of MOR (respiratory depression and constipation) (Porreca and Burks, 1983; Porreca et al., 1984; Unterwald et al., 1987; Shippenberg et al., 1988; Di Chiara, 1998; Field et al., 1999; Kivell and Prisinzano, 2010). Antinociception is achieved by activation of central or peripheral KOR (Porreca et al., 1987; Millan et al., 1988; Stein et al., 1989, 1990; Stein, 1991; Horan and Porreca, 1993; Vanderah et al., 2008); however, as described above, activation of central KOR drives a negative affective state: hence the push toward the development of peripherally restricted drugs (Aldrich and Vigil‐Cruz, 2003; Beck et al., 2019). Most recently, a KOR agonist, JT09, shows promise as it is peripherally restricted, matches morphine’s therapeutic properties, and is orally active (Beck et al., 2019). It is not yet known how targeting KORs peripherally might, if at all, alter central pain processing and negative affect. An important consideration is that the effects of KOR depend on the pain model. For example, KOR is not involved in the aversion induced in acute pain states (Leitl et al., 2014a,b; Bagdas et al., 2016) but is involved in modulation of negative affect in chronic pain states (Liu et al., 2019; Massaly et al., 2019). One final, important consideration is that the effects of KOR modulation in pain are sex dependent. This has been reported both in preclinical animal models and in human imaging studies. Positron emission tomography studies in humans show that KOR binding is greater in men than in women in many brain regions, including the anterior cingulate cortex, which has been associated with pain affect (Vijay et al., 2016). These findings hold promise for the continued development of KOR targets in the treatment of pain, but the mechanisms are clearly complex and dependent on a number of factors that need to be considered throughout the drug development process.
Opioids and Headache Disorders.
Headache disorders are highly disabling conditions that are widespread throughout the world. According to the most recent Global Burden of Disease Study, headache was ranked as third most prevalent, and a common type of headache, migraine, was ranked as sixth most prevalent. The greatest burden for headache is carried by women in their reproductive years, and globally in this population, migraine produced 20.3 million years lost due to disability in 2016 alone (GBD 2016 Headache Collaborators, 2018). There is still a limited understanding of the mechanisms underlying headache, and as with other pain conditions, opioid receptors appear to play an important role in the regulation of headache, with all four opioid receptors being implicated in migraine pathophysiology.
Clinically available opioids are often prescribed for migraine (Bigal and Lipton, 2009), and these analgesics act primarily through MOR (Borsodi et al., 2019). Chronic opioid use can result in a paradoxical increase in pain, known as opioid-induced hyperalgesia (OIH) (Hayhurst and Durieux, 2016). This phenomenon is clearly observed in patients with headache and is classified as mediation overuse headache (Headache Classification Committee of the International Headache Society, 2018). In this case opioids initially provide relief but in the long term can result in increased frequency and severity of headache symptoms (Bigal and Lipton, 2009; Buse et al., 2012). Medication overuse headache not only is difficult to treat but can result in opioid dependence and abuse, which has contributed to the opioid epidemic (Reid et al., 2002; Colás et al., 2004; Loder, 2006; Diener et al., 2016).
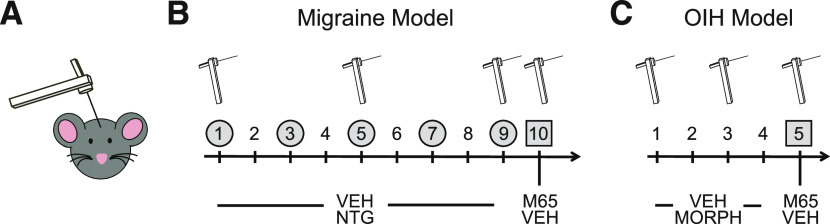
Epidemiologically, evidence clearly shows that the use of opioids can facilitate the transition from episodic to chronic migraine (Bigal and Lipton, 2009) and increase associated disability (Buse et al., 2012). The mechanisms underlying this facilitation were recently examined preclinically in a massive exploratory peptidomic study (Anapindi et al., 2019). This investigation determined whether there were shared mechanisms between OIH and chronic migraine. Both conditions are characterized by dysregulation in neuropeptides, and it was hypothesized that there may be overlapping alterations that could bridge these two conditions. Mice underwent a classic OIH paradigm in which they received escalating doses of morphine over 4 days. Another group of mice were treated according to a chronic migraine model (Pradhan et al., 2014a), in which they received chronic intermittent injection of nitroglycerin (NTG), a known human migraine trigger (Fig. 2). NTG is used as a human experimental model of migraine (Ashina et al., 2017), and its use in rodents has been shown to produce migraine-associated effects such as allodynia, altered meningeal blood flow, and photophobia (Demartini et al., 2019). Both models resulted in severe cephalic allodynia, and multiple brain and peripheral regions were collected for label-free, nonbiased liquid chromatography–mass spectrometry. Although there were significant changes in neuropeptide expression and levels in either paradigm alone, there were very few overlapping peptides that were affected in both OIH and chronic migraine. Of these shared peptides, pituitary adenylate cyclase-activating polypeptide (PACAP), vasoactive intestinal peptide, and secretogranin consistently showed significant changes in chronic migraine and OIH. As a confirmation of these findings, the role of PACAP was reexamined in both OIH and chronic migraine models. PACAP can bind to three different receptors (VPAC1, VPAC2, and PAC1), but PAC1 is most commonly associated with migraine-related effects. The PAC1 antagonist, M65, was found to inhibit cephalic allodynia induced by both OIH and chronic NTG treatment, thus confirming the role of this peptide in regulating both disorders (Anapindi et al., 2019). Previous studies have identified PACAP as a target for migraine, but it has not been considered as treatment of OIH, and this work indicates that it may be a mechanistic link through which opioids facilitate migraine chronicity.
Fig. 2.
Mouse models of chronic migraine–associated pain and opioid-induced hyperalgesia. (A) Mechanical thresholds are determined by von Frey hair stimulation of the periorbital region. (B) To model chronic migraine–associated pain, mice are injected every other day with vehicle or nitroglycerin (10 mg/kg i.p.) and tested on days 1, 5, and 9. To test the effect of PAC1 inhibition, mice were treated with M65 (0.1 mg/kg i.p.) or vehicle on day 10. (C) For OIH, mice were injected twice daily with morphine or vehicle. On days 1–3 they received 20 mg/kg, and on day 4 they received 40 mg/kg s.c. morphine/injection; they are tested on days 1 and 3 of treatment. The effect of M65 was determined on day 5, when cephalic OIH had been established.
Fig. 1.
N/OFQ-NOP receptor fields of research. Promising new research areas discussed within this review are highlighted.
In contrast to the promigraine effects of MOR activation, delta opioid receptor (DOR) agonists appear to mitigate migraine-related symptoms, and DOR has been identified as a novel therapeutic target for headache disorders (Charles and Pradhan, 2016). Although DOR agonists are not highly effective in models of acute pain, they have shown efficacy in a number of models of chronic pain, including inflammatory and neuropathic pain (Vicente-Sanchez et al., 2016). Increased evidence indicates that DORs are functionally upregulated during chronic pain states (Cahill et al., 2007; Pradhan et al., 2013; Gendron et al., 2016; Vicente-Sanchez et al., 2016) and may serve as a protective mechanism in the face of increased pain processing, including in multiple headache models. An initial report showed that three different selective DOR agonists, SNC80, ARM390, and JNJ20788560, inhibited migraine-associated mechanical allodynia induced by NTG (Pradhan et al., 2014b). Another group confirmed that SNC80 could block the effects of NTG, in this case using thermal hyperalgesia as an endpoint (Dripps et al., 2018). It is important to note that agonists that promote both high (SNC80) and low (ARM390) internalization of DOR were active in this migraine model, indicating that this is not a ligand-biased effect (Vicente-Sanchez and Pradhan, 2018). These studies indicate that DOR agonists may be effective as acute migraine therapies.
DOR has also shown promise in other types of headache disorders. Chronic migraine is defined as >15 headache days per month over a 3-month period (Headache Classification Committee of the International Headache Society, 2018) and affects ∼1%–3% of the population. These patients are often refractory to treatment and suffer from overwhelming disability. SNC80 was shown to inhibit established cephalic allodynia in an NTG model of chronic migraine–associated pain (Moye et al., 2019b). Acute injection of this DOR agonist was also effective in a related model of chronic migraine induced by mild traumatic brain injury, otherwise known as posttraumatic migraine (Moye and Pradhan, 2017; Moye et al., 2019b). Importantly, long-term treatment with SNC80 also prevented the development of chronic migraine in this same model of posttraumatic headache (Moye et al., 2019a). These data suggest that DOR may be effective for both episodic headache and could also protect from the induction of headache chronicity. In addition, SNC80 was also shown to effectively block cephalic and peripheral allodynia in the model of OIH described above (Moye et al., 2019b). These findings particularly stress that MOR and DORs distinctly regulate head pain processing, as chronic treatment with the mu agonist, morphine, induced pain that was still blocked by DOR activation. Furthermore, medication overuse headache can also be induced by triptans, a class of drugs specifically developed for the treatment of migraine, and SNC80 also inhibited allodynia induced by chronic sumatriptan treatment (Moye et al., 2019b). These studies suggest that DOR may be an effective target not just for migraine but for headache disorders more broadly. Importantly, in contrast to morphine and sumatriptan, chronic DOR agonist treatment produced a limited form of medication overuse headache/opioid-induced hyperalgesia (Moye et al., 2019b), further supporting the development of DOR as a target for headache.
DOR activation may also modulate headache symptoms beyond pain. In a conditioning paradigm, SNC80 prevented the development of a conditioned place aversion to NTG (Pradhan et al., 2014b). This study would suggest that DOR may also regulate negative affective state induced by migraine. Especially considering that DOR agonists have antidepressant and anxiolytic properties, they may be ideal candidates for addressing the high comorbidity between headache and emotional disorders (Silberstein et al., 2007). In addition, DOR activation was also found to beneficially modulate migraine aura–related symptoms. Cortical spreading depression (CSD) is thought to be the physiologic correlate of migraine aura, the visual disturbances experienced by ∼30% of patients with migraine (Charles and Baca, 2013). CSDs are slowly propagated waves of depolarization followed by inhibition of brain activity. They can be induced experimentally by dripping potassium chloride onto the dura and measuring changes in optical reflectance and corresponding direct current shifts in local potentials (Brennan et al., 2007). Migraine preventives have been shown to decrease CSD events (Ayata et al., 2006; Bogdanov et al., 2011), and is used as a screening tool for migraine prophylactics. SNC80 significantly decreased the number of CSD events evoked by potassium chloride (Pradhan et al., 2014b), showing that DORs can regulate the cortical excitability associated with migraine, which again suggests that DOR agonists could also prove to be migraine preventives.
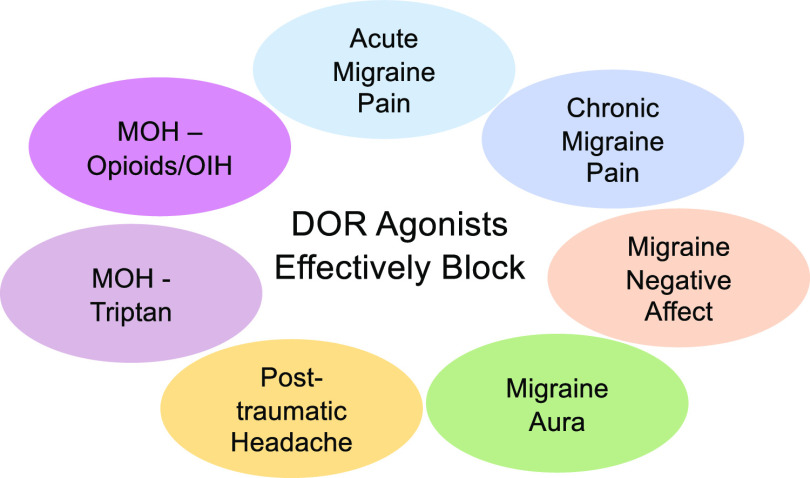
DOR is highly expressed in brain regions that are involved in head pain processing, further supporting its role in headache pathophysiology. In dorsal root ganglia, DOR are found on a small subset of neurons that express the promigraine peptide calcitonin gene related peptide (CGRP) (Bardoni et al., 2014). There is also evidence from rodent and human postmortem tissue studies showing the expression of DOR in trigeminal ganglia—first order neurons innervating the head (Mansour et al., 1988; Mennicken et al., 2003; Pradhan et al., 2011; Rice et al., 2017), as well as the dura (Rice et al., 2017). Furthermore, DOR is also expressed in central regions modulating migraine, including the trigeminal nucleus caudalis and cortex (Mansour et al., 1988; Peckys and Landwehrmeyer, 1999; Mennicken et al., 2003), as well as in regions more broadly regulating pain perception (Pradhan and Clarke, 2005; Gendron et al., 2016). The expression of DOR in limbic regions such as the hippocampus, striatum, and amygdala highlight its role in emotional regulation (Lutz and Kieffer, 2013), which could be beneficial in light of the high comorbidity between headache disorders and anxiety and depression. Considering the finding that PACAP facilitates migraine and OIH, whereas DOR agonists are inhibitory in these models, future studies will focus on the relationship between the PACAPergic system and DOR. There is also high anatomic coexpression between PACAP/PAC1 and DOR in many central nervous system regions (Anapindi et al., 2019), thus further supporting an interplay between these two systems. Unlike MOR activation, DOR agonists have low abuse liability and do not appear to cause physical dependence (Negus et al., 1998; Brandt et al., 2001; Stevenson et al., 2005; Do Carmo et al., 2009). In addition, DOR agonists cause less respiratory depression and fewer effects on gastrointestinal transit (May et al., 1989; Gallantine and Meert, 2005; Poole et al., 2011) relative to MOR agonists. One caveat to the development of DOR agonists is that some of them are proconvulsant. However, this appears to be due to ligand-directed signaling, and it may be possible to distinguish this adverse behavior from pain-relieving effects through drug development (Pradhan et al., 2012). Figure 3 summarizes all of the headache models in which DOR agonists have been shown to be effective.
Fig. 3.
DOR agonists have been shown to be effective in models of acute and chronic migraine–associate pain (nitroglycerin), negative affect (conditioned place aversion), and aura (cortical spreading depression), as well as in models of posttraumatic headache and medication overuse headache (MOH) related to sumatriptan or morphine (OIH).
DOR agonists are actively being developed for migraine treatment. A clinical trial (phase 1) was recently performed by Trevena Inc. to test the DOR agonist, TRV250, for acute migraine. This agonist was specifically developed with reduced seizure liability, without compromising headache-relieving effects. The data have only been disclosed in a press release from the company and indicate that in healthy volunteers TRV250 has safety, tolerability, and a pharmacokinetic profile that would support moving to phase 2 trials for efficacy in migraine. Along with these clinical studies, studies are ongoing to determine the molecular mechanisms through which DOR regulates headache.
KOR has also recently emerged as a therapeutic target for headache disorders, and in this case an antagonist strategy is being proposed. Upregulation of the endogenous KOR ligand, dynorphin, has been identified as a marker of stress (Bruchas et al., 2010; Carlezon and Krystal, 2016), and this may be one way in which stress mechanistically results in migraine. Stress is commonly identified as a migraine trigger (Maleki et al., 2012), and in support of this idea, KOR antagonists were recently shown to be effective in a stress-induced headache model (Xie et al., 2017). Treatment of rats with chronic sumatriptan to induce medication overuse headache concurrently resulted in hypersensitivity to a bright light stress cue. Subsequent exposure to this cue produced an increase in plasma CGRP, a promigraine peptide, as well as induction of cephalic and peripheral allodynia. Systemic injection of either a long- (norbinaltorphimine) and short- (CYM51317) acting KOR antagonist blocked both the augmented CGRP and allodynia. Furthermore, chronic treatment with sumatriptan increased dynorphin and phosphorylated KOR in the amygdala, and intraamygdala injection of norbinaltorphimine was also pain relieving. These results open the possibility of targeting KOR antagonists for the treatment of migraine, especially in patients with increased sensitivity to stress.
Finally, NOP receptor has also been implicated in headache pathophysiology. Nociceptin is highly expressed in human trigeminal ganglia (Hou et al., 2003), with up to 70% of neurons being nociceptin positive. In addition, in an electrophysiological model of migraine, nociceptin significantly inhibited neurogenic dural vasodilation. Nociceptin levels have also been examined in a population of patients with migraine. Interictally, the levels of the endogenous NOP ligand, N/OFQ, were significantly lower in the plasma of patients with migraine compared with healthy controls, and N/OFQ levels further dropped during a migraine attack (Ertsey et al., 2005). Together, these studies promote further investigation into the role of NOP and its receptor in headache disorders.
Opioid-Related Behaviors and the Gut Microbiome.
The effects of opioids—particularly mu opioid agonists—in the gut is well known. The slowing of gut motility is a desirable effect of over-the-counter opioid agonists, such as loperamide, for the treatment of diarrhea. On the other hand, severe constipation and abdominal cramping is a common and often dose-limiting side effect of chronic opioid use. More recently, however, opioids have been shown to affect the gastrointestinal environment beyond their influence on gut motility.
The gut microbiome refers to the collection of microorganisms that reside in the gut and is increasingly acknowledged to contribute to brain development and behavior (Mayer et al., 2014) It is now well established that mu opioid agonists significantly change the composition of gut bacteria (Acharya et al., 2017; Xu et al., 2017; Wang et al., 2018). In fact, the influence of mu opioid agonists on gut bacteria composition is incredibly labile, with changes to the gut microbiome detected after only 1 day of morphine treatment (Wang et al., 2018). The gut microbiome also fluctuates significantly after intermittent morphine exposure as the gut cycles through phases of drug onset and withdrawal (Lee et al., 2018). Importantly, we now know that changes to the gut microbiome directly contribute to many of the symptoms of chronic opioid use. For example, depleting the gut microbiome with oral antibiotics inhibits the development of morphine tolerance (Kang et al., 2017), although this relationship seems to depend on the type and length of antibiotics used (Lee et al., 2018). Moreover, fecal microbiota transfer from morphine withdrawn to drug-naïve mice was sufficient to induce symptoms of withdrawal including hyperalgesia, negative affect, and inflammation (Lee et al., 2018). Although more research is needed, these early studies suggest the gut microbiome is an important contributor to the full expression of behaviors associated with chronic opioid use.
A key question is, what is the mechanism by which gut bacteria influence opioid-related behaviors? Compiled analysis of the multitude of studies describing the gut microbiota after opioid exposure reveal some common themes. Although opioid treatment does not alter overall species richness (how many different types of bacteria), it does alter the abundance of specific key bacterial taxa. For example, chronic opioid treatment leads to a relative reduction in Gram-negative bacteria in the Bacteroidetes phyla and a relative increase in Gram-positive bacteria in the Firmicutes phyla (Feng et al., 2006; Babrowski et al., 2012; Banerjee et al., 2016; Wang and Roy, 2017; Lee et al., 2018; Wang et al., 2018). The reduction in the Bacteroidetes/Firmicutes ratio has been correlated with inflammation in several diseases, including obesity and irritable bowel syndrome (Power et al., 2014), and may contribute to the inflammation associated with chronic opioid use (Cahill and Taylor, 2017).
Gut bacteria perform many functions within the gut, including fiber fermentation, carbohydrate digestion, bile acid formation, and drug metabolism. Interestingly, a role for gut bacteria in morphine metabolism has been described. Certain gut bacteria have beta-glucuronidase activity, an enzyme responsible for hydrolyzing morphine metabolites (morphine-3-glucuronide and morphine-6-glucuronide) back into morphine (Walsh and Levine, 1975; Koster et al., 1985). Therefore, transformation of morphine-3-glucuronide and morphine-6-glucuronide into morphine by gut bacteria may influence the pharmacokinetic profile of morphine by reversing drug metabolism and prolonging morphine effects. Reductions in bacteria with beta-glucuronidase activity has been proposed to contribute to opioid tolerance (Wang et al., 2018), although further research is necessary to further confirm these ideas.
Another important area of opioid-induced dysbiosis is bile acid formation. Chronic opioid treatment leads to reductions in both primary and secondary bile acid transformation (Banerjee et al., 2016; Wang et al., 2018; Sindberg et al., 2019). Bile acids are not only important for nutrient absorption and metabolism but also inhibit the expansion and translocation of certain pathogenic bacteria (Yoon et al., 2017). To this point, reduction of bile acid after chronic morphine treatment is associated with the expansion of pathogenic bacteria Enterococcus faecalis (Banerjee et al., 2016). Treatment of drug-naïve mice with E. faecalis was sufficient to increase morphine tolerance, suggesting that it is a key bacterial species driving opioid dependence (Wang et al., 2018).
Treatment with chronic opioids also leads to a significant loss in gut barrier integrity and bacterial migration (Koster et al., 1985; Banerjee et al., 2016). Both Gram-negative and Gram-positive bacteria have been shown to colonize the liver, spleen, and lymph nodes after chronic opioid treatment (Hilburger et al., 1997; Meng et al., 2015). This process has been shown to be dependent on toll-like receptor 2, given that toll-like receptor 2 knockout mice do not show morphine-induced gut epithelial barrier dysfunction (Meng et al., 2013). The loss of gut barrier integrity after opioid treatment can exacerbate viral infection and sepsis, which has serious clinical implications given the propensity to use opioids in clinical emergency settings (Meng et al., 2015; Banerjee et al., 2016; Shakhsheer et al., 2016; Zhang et al., 2018).
Interventions that restore the gut microbiome are an obvious target to improve opioid efficacy and minimize the effects of withdrawal. Fecal microbiota transfer has provided initial proof of principle that manipulating the gut microbiome can influence opioid-dependent behaviors (Lee et al., 2018). Moreover, dietary supplementation with omega 3 polyunsaturated fatty acids restored the gut microbiome and reduced inflammation and opioid-seeking behaviors in a contingent opioid dependence model (Hakimian et al., 2019). Although these initial studies are promising, much more research is needed to fully explore the therapeutic potential of targeting the gut microbiome in mitigating the effects of chronic opioids in clinical populations. In particular, studies examining how the gut microbiome contributes to opioid function in both males and females are urgently needed. To date, the vast majority of preclinical research cited above was performed in males only. Studies examining these effects in both males and females is required to fully capture the relationship of the gut microbiome and opioids as it relates to human clinical populations.
Conclusion
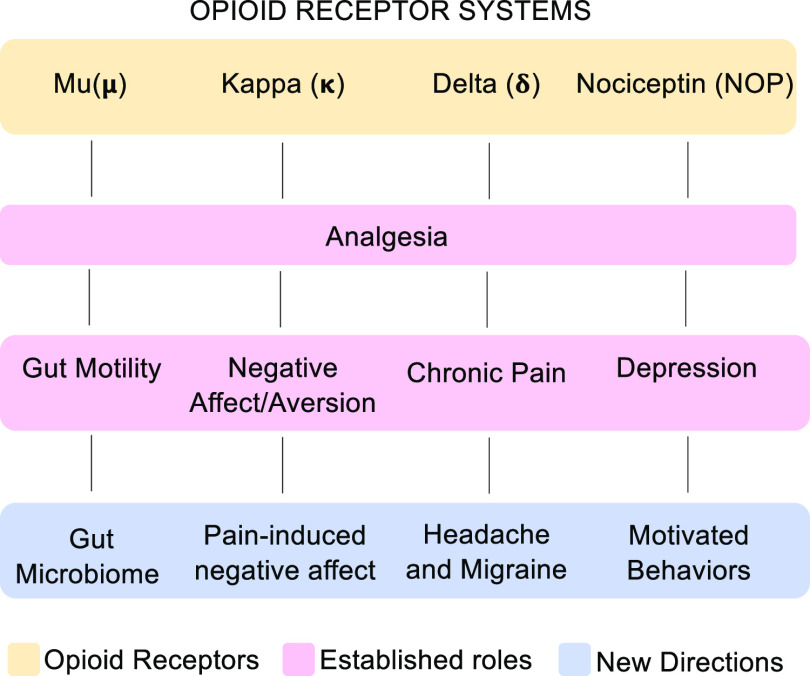
In this review we have highlighted four distinct emerging fields of opioid research that have exciting translational potential (Fig. 4). The fact that these areas of research are somewhat unrelated and do not all overlap implies that perhaps selective drug targeting to specific opioid receptors may be easier. Here we show that the opioid system can be involved in peripheral and/or central regulation in the modulation of certain disease states, which is very interesting, especially in light of the development of many restricted opioid compounds and the fact that much of this research has already led to opioid compounds entering clinical trials. Together these findings highlight the dynamic nature and importance of opioids in a number of diseases and remind us that there is still so much to learn. The ongoing development of new therapeutic targets for opioids is very encouraging, and the field will likely see significant developments over the next decade.
Fig. 4.
Summary of topics reviewed. All four opioid receptors modulate analgesia, yet they each have unique established roles, which has led to research into more specialized functions. Mu opioid receptor system has long been known to alter gut motility (prevents diarrhea vs. constipation), but more recently its role in gut microbiome composition has had implications in chronic opioid use disorder; the established primary role for the kappa opioid system is regulation of negative affect with a more recently discovered role in pain-induced negative affect; the delta opioid receptor system has been identified as a promising target for treating headache disorders and migraine, a significant advancement from its more generalized role in chronic pain. Finally, most research thus far has focused on the role of the nociceptin system in depression; here we review recent advances studying its role in motivation as a mechanism to refine treatment approaches.
Abbreviations
- CFA
complete Freud’s adjuvant
- CGRP
calcitonin gene related peptide
- CPP
conditioned place preference
- CSD
cortical spreading depression
- DOR
delta opioid receptor
- KOR
kappa opioid receptor
- MDD
major depressive disorder
- MOR
mu opioid receptor
- N/OFQ
nociceptin
- NAc
nucleus accumbens
- N/OFQ
nociceptin
- NOP
nociceptin opioid peptide receptor
- NTG
nitroglycerin
- OIH
opioid-induced hyperalgesia
- PACAP
pituitary adenylate cyclase-activating polypeptide
- PNI
peripheral nerve injury
- Pnoc
prepronociceptin
- pnVTA
paranigral nucleus of the VTA
- vNAcSh
ventral NAc shell
- VTA
ventral tegmental area
Authorship Contributions
Wrote or contributed to the writing of the manuscript: Parker, Sugiarto, Taylor, Pradhan, Al-Hasani.
Footnotes
References
- Acharya C, Betrapally NS, Gillevet PM, Sterling RK, Akbarali H, White MB, Ganapathy D, Fagan A, Sikaroodi M, Bajaj JS. (2017) Chronic opioid use is associated with altered gut microbiota and predicts readmissions in patients with cirrhosis. Aliment Pharmacol Ther 45:319–331. [DOI] [PubMed] [Google Scholar]
- Aldrich JV, Vigil‐Cruz SC. (2003) Narcotic analgesics, Burger’s Medicinal Chemistry and Drug Discovery pp 331–482, Wiley, New York. [Google Scholar]
- Al-Hasani R, Bruchas MR. (2011) Molecular mechanisms of opioid receptor-dependent signaling and behavior. Anesthesiology 115:1363–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Hasani R, McCall JG, Shin G, Gomez AM, Schmitz GP, Bernardi JM, Pyo C-O, Park SI, Marcinkiewcz CM, Crowley NA, et al. (2015) Distinct subpopulations of nucleus accumbens dynorphin neurons drive aversion and reward. Neuron 87:1063–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association (2013) Diagnostic and Statistical Manual of Mental Disorders: DSM-5, 5th ed, American Psychiatric Association, Washington, D.C.. [Google Scholar]
- Anapindi KDB, Yang N, Romanova EV, Rubakhin SS, Tipton A, Dripps I, Sheets Z, Sweedler JV, Pradhan AA. (2019) PACAP and other neuropeptide targets link chronic migraine and opioid-induced hyperalgesia in mouse models. Mol Cell Proteomics 18:2447–2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton B, Fein J, To T, Li X, Silberstein L, Evans CJ. (1996) Immunohistochemical localization of ORL-1 in the central nervous system of the rat. J Comp Neurol 368:229–251. [DOI] [PubMed] [Google Scholar]
- Ayata C, Jin H, Kudo C, Dalkara T, Moskowitz MA. (2006) Suppression of cortical spreading depression in migraine prophylaxis. Ann Neurol 59:652–661. [DOI] [PubMed] [Google Scholar]
- Babrowski T, Holbrook C, Moss J, Gottlieb L, Valuckaite V, Zaborin A, Poroyko V, Liu DC, Zaborina O, Alverdy JC. (2012) Pseudomonas aeruginosa virulence expression is directly activated by morphine and is capable of causing lethal gut-derived sepsis in mice during chronic morphine administration. Ann Surg 255:386–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagdas D, Muldoon PP, AlSharari S, Carroll FI, Negus SS, Damaj MI. (2016) Expression and pharmacological modulation of visceral pain-induced conditioned place aversion in mice. Neuropharmacology 102:236–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliki MN, Apkarian AV. (2015) Nociception, pain, negative moods, and behavior selection. Neuron 87:474–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee S, Sindberg G, Wang F, Meng J, Sharma U, Zhang L, Dauer P, Chen C, Dalluge J, Johnson T, et al. (2016) Opioid-induced gut microbial disruption and bile dysregulation leads to gut barrier compromise and sustained systemic inflammation. Mucosal Immunol 9:1418–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM, Pagliaccio D, Luking K. (2016) Mechanisms underlying motivational deficits in psychopathology: similarities and differences in depression and schizophrenia. Curr Top Behav Neurosci 27:411–449. [DOI] [PubMed] [Google Scholar]
- Bardoni R, Tawfik VL, Wang D, François A, Solorzano C, Shuster SA, Choudhury P, Betelli C, Cassidy C, Smith K, et al. (2014) Delta opioid receptors presynaptically regulate cutaneous mechanosensory neuron input to the spinal cord dorsal horn. Neuron 81:1443. [DOI] [PubMed] [Google Scholar]
- Beck TC, Reichel CM, Helke KL, Bhadsavle SS, Dix TA. (2019) Non-addictive orally-active kappa opioid agonists for the treatment of peripheral pain in rats. Eur J Pharmacol 856:172396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berenbaum H, Connelly J. (1993) The effect of stress on hedonic capacity. J Abnorm Psychol 102:474–481. [DOI] [PubMed] [Google Scholar]
- Berton O, Nestler EJ. (2006) New approaches to antidepressant drug discovery: beyond monoamines. Nat Rev Neurosci 7:137–151. [DOI] [PubMed] [Google Scholar]
- Bigal ME, Lipton RB. (2009) Excessive opioid use and the development of chronic migraine. Pain 142:179–182. [DOI] [PubMed] [Google Scholar]
- Bogdanov VB, Multon S, Chauvel V, Bogdanova OV, Prodanov D, Makarchuk MY, Schoenen J. (2011) Migraine preventive drugs differentially affect cortical spreading depression in rat. Neurobiol Dis 41:430–435. [DOI] [PubMed] [Google Scholar]
- Borsodi A, Bruchas M, Caló G, Chavkin C, Christie MJ, Civelli O, Connor M, Cox BM, Devi LA, Evans C, et al. (2019) Opioid receptors (version 2019.4) in the IUPHAR/BPS Guide to Pharmacology Database. IUPHAR/BPS Guide to Pharmacology CITE 2019.
- Brandt MR, Furness MS, Rice KC, Fischer BD, Negus SS. (2001) Studies of tolerance and dependence with the delta-opioid agonist SNC80 in rhesus monkeys responding under a schedule of food presentation. J Pharmacol Exp Ther 299:629–637. [PubMed] [Google Scholar]
- Brennan KC, Beltrán-Parrazal L, López-Valdés HE, Theriot J, Toga AW, Charles AC. (2007) Distinct vascular conduction with cortical spreading depression. J Neurophysiol 97:4143–4151. [DOI] [PubMed] [Google Scholar]
- Bruchas MR, Land BB, Aita M, Xu M, Barot SK, Li S, Chavkin C. (2007) Stress-induced p38 mitogen-activated protein kinase activation mediates kappa-opioid-dependent dysphoria. J Neurosci 27:11614–11623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchas MR, Land BB, Chavkin C. (2010) The dynorphin/kappa opioid system as a modulator of stress-induced and pro-addictive behaviors. Brain Res 1314:44–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchas MR, Schindler AG, Shankar H, Messinger DI, Miyatake M, Land BB, Lemos JC, Hagan CE, Neumaier JF, Quintana A, et al. (2011) Selective p38α MAPK deletion in serotonergic neurons produces stress resilience in models of depression and addiction. Neuron 71:498–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buse DC, Pearlman SH, Reed ML, Serrano D, Ng-Mak DS, Lipton RB. (2012) Opioid use and dependence among persons with migraine: results of the AMPP study. Headache 52:18–36. [DOI] [PubMed] [Google Scholar]
- Cahill CM, Holdridge SV, Morinville A. (2007) Trafficking of delta-opioid receptors and other G-protein-coupled receptors: implications for pain and analgesia. Trends Pharmacol Sci 28:23–31. [DOI] [PubMed] [Google Scholar]
- Cahill CM, Taylor AM. (2017) Neuroinflammation-a co-occurring phenomenon linking chronic pain and opioid dependence. Curr Opin Behav Sci 13:171–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill CM, Taylor AMW, Cook C, Ong E, Morón JA, Evans CJ. (2014) Does the kappa opioid receptor system contribute to pain aversion? Front Pharmacol 5:253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon WA, Jr., Krystal AD. (2016) Kappa-opioid antagonists for psychiatric disorders: from bench to clinical trials. Depress Anxiety 33:895–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles A, Pradhan AA. (2016) Delta-opioid receptors as targets for migraine therapy. Curr Opin Neurol 29:314–319. [DOI] [PubMed] [Google Scholar]
- Charles AC, Baca SM. (2013) Cortical spreading depression and migraine. Nat Rev Neurol 9:637–644. [DOI] [PubMed] [Google Scholar]
- Charney DS, Manji HK. (2004) Life stress, genes, and depression: multiple pathways lead to increased risk and new opportunities for intervention. Sci STKE 2004:re5. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Angeletti S, Panocka I, Massi M. (2000) Nociceptin/orphanin FQ and drugs of abuse. Peptides 21:1071–1080. [DOI] [PubMed] [Google Scholar]
- Colás R, Muñoz P, Temprano R, Gómez C, Pascual J. (2004) Chronic daily headache with analgesic overuse: epidemiology and impact on quality of life. Neurology 62:1338–1342. [DOI] [PubMed] [Google Scholar]
- Darland T, Heinricher MM, Grandy DK. (1998) Orphanin FQ/nociceptin: a role in pain and analgesia, but so much more. Trends Neurosci 21:215–221. [DOI] [PubMed] [Google Scholar]
- Der-Avakian A, D’Souza MS, Potter DN, Chartoff EH, Carlezon WA, Jr., Pizzagalli DA, Markou A. (2017) Social defeat disrupts reward learning and potentiates striatal nociceptin/orphanin FQ mRNA in rats. Psychopharmacology (Berl) 234:1603–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Der-Avakian A, Mazei-Robison MS, Kesby JP, Nestler EJ, Markou A. (2014) Enduring deficits in brain reward function after chronic social defeat in rats: susceptibility, resilience, and antidepressant response. Biol Psychiatry 76:542–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devine DP, Watson SJ, Akil H. (2001) Nociceptin/orphanin FQ regulates neuroendocrine function of the limbic-hypothalamic-pituitary-adrenal axis. Neuroscience 102:541–553. [DOI] [PubMed] [Google Scholar]
- Di Chiara G. (1998) A motivational learning hypothesis of the role of mesolimbic dopamine in compulsive drug use. J Psychopharmacol 12:54–67. [DOI] [PubMed] [Google Scholar]
- Diener H-C, Holle D, Solbach K, Gaul C. (2016) Medication-overuse headache: risk factors, pathophysiology and management. Nat Rev Neurol 12:575–583. [DOI] [PubMed] [Google Scholar]
- Do Carmo GP, Folk JE, Rice KC, Chartoff E, Carlezon WA, Jr., Negus SS. (2009) The selective non-peptidic delta opioid agonist SNC80 does not facilitate intracranial self-stimulation in rats. Eur J Pharmacol 604:58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue RJ, Muschamp JW, Russo SJ, Nestler EJ, Carlezon WA., Jr. (2014) Effects of striatal ΔFosB overexpression and ketamine on social defeat stress-induced anhedonia in mice. Biol Psychiatry 76:550–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dripps IJ, Boyer BT, Neubig RR, Rice KC, Traynor JR, Jutkiewicz EM. (2018) Role of signalling molecules in behaviours mediated by the δ opioid receptor agonist SNC80. Br J Pharmacol 175:891–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner SR, Roitman MF, Potter DN, Rachlin AB, Chartoff EH. (2010) Depressive-like effects of the kappa opioid receptor agonist salvinorin A are associated with decreased phasic dopamine release in the nucleus accumbens. Psychopharmacology (Berl) 210:241–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ertsey C, Hantos M, Bozsik G, Tekes K. (2005) Plasma nociceptin levels are reduced in migraine without aura. Cephalalgia 25:261–266. [DOI] [PubMed] [Google Scholar]
- Feng P, Truant AL, Meissler JJ, Jr., Gaughan JP, Adler MW, Eisenstein TK. (2006) Morphine withdrawal lowers host defense to enteric bacteria: spontaneous sepsis and increased sensitivity to oral Salmonella enterica serovar Typhimurium infection. Infect Immun 74:5221–5226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field MJ, Carnell AJ, Gonzalez MI, McCleary S, Oles RJ, Smith R, Hughes J, Singh L. (1999) Enadoline, a selective kappa-opioid receptor agonist shows potent antihyperalgesic and antiallodynic actions in a rat model of surgical pain. Pain 80:383–389. [DOI] [PubMed] [Google Scholar]
- Gallantine EL, Meert TF. (2005) A comparison of the antinociceptive and adverse effects of the mu-opioid agonist morphine and the delta-opioid agonist SNC80. Basic Clin Pharmacol Toxicol 97:39–51. [DOI] [PubMed] [Google Scholar]
- Gavioli EC, Marzola G, Guerrini R, Bertorelli R, Zucchini S, De Lima TCM, Rae GA, Salvadori S, Regoli D, Calo G. (2003) Blockade of nociceptin/orphanin FQ-NOP receptor signalling produces antidepressant-like effects: pharmacological and genetic evidences from the mouse forced swimming test. Eur J Neurosci 17:1987–1990. [DOI] [PubMed] [Google Scholar]
- Gavioli EC, Vaughan CW, Marzola G, Guerrini R, Mitchell VA, Zucchini S, De Lima TCM, Rae GA, Salvadori S, Regoli D, et al. (2004) Antidepressant-like effects of the nociceptin/orphanin FQ receptor antagonist UFP-101: new evidence from rats and mice. Naunyn Schmiedebergs Arch Pharmacol 369:547–553. [DOI] [PubMed] [Google Scholar]
- GBD 2016 Headache Collaborators (2018) Global, regional, and national burden of migraine and tension-type headache, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 17:954–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendron L, Cahill CM, von Zastrow M, Schiller PW, Pineyro G. (2016) Molecular pharmacology of δ-opioid receptors. Pharmacol Rev 68:631–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg PE, Fournier A-A, Sisitsky T, Pike CT, Kessler RC. (2015) The economic burden of adults with major depressive disorder in the United States (2005 and 2010). J Clin Psychiatry 76:155–162. [DOI] [PubMed] [Google Scholar]
- Gu H, Hu D, Hong XR, Mao J, Cui Y, Hui N, Sha JY. (2003) [Changes and significance of orphanin and serotonin in patients with postpartum depression]. Zhonghua Fu Chan Ke Za Zhi 38:727–728. [PubMed] [Google Scholar]
- Hamilton M. (1959) The assessment of anxiety states by rating. Br J Med Psychol 32:50–55. [DOI] [PubMed] [Google Scholar]
- Hayhurst CJ, Durieux ME. (2016) Differential opioid tolerance and opioid-induced hyperalgesia: a clinical reality. Anesthesiology 124:483–488. [DOI] [PubMed] [Google Scholar]
- Headache Classification Committee of the International Headache Society (2018) The International Classification of Headache Disorders, 3rd edition, Cephalalgia 38, pp 1–211. [DOI] [PubMed] [Google Scholar]
- Hilburger ME, Adler MW, Truant AL, Meissler JJ, Jr., Satishchandran V, Rogers TJ, Eisenstein TK. (1997) Morphine induces sepsis in mice. J Infect Dis 176:183–188. [DOI] [PubMed] [Google Scholar]
- Hipólito L, Wilson-Poe A, Campos-Jurado Y, Zhong E, Gonzalez-Romero J, Virag L, Whittington R, Comer SD, Carlton SM, Walker BM, et al. (2015) Inflammatory pain promotes increased opioid self-administration: role of dysregulated ventral tegmental area μ opioid receptors. J Neurosci 35:12217–12231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holanda VAD, Medeiros IU, Asth L, Guerrini R, Calo’ G, Gavioli EC. (2016) Antidepressant activity of nociceptin/orphanin FQ receptor antagonists in the mouse learned helplessness. Psychopharmacology (Berl) 233:2525–2532. [DOI] [PubMed] [Google Scholar]
- Horan PJ, Porreca F. (1993) Lack of cross-tolerance between U69,593 and bremazocine suggests kappa-opioid receptor multiplicity in mice. Eur J Pharmacol 239:93–98. [DOI] [PubMed] [Google Scholar]
- Hou M, Uddman R, Tajti J, Edvinsson L. (2003) Nociceptin immunoreactivity and receptor mRNA in the human trigeminal ganglion. Brain Res 964:179–186. [DOI] [PubMed] [Google Scholar]
- Hyde JS, Mezulis AH. (2020) Gender differences in depression: biological, affective, cognitive, and sociocultural factors. Harv Rev Psychiatry 28:4–13. [DOI] [PubMed] [Google Scholar]
- Hyde JS, Mezulis AH, Abramson LY. (2008) The ABCs of depression: integrating affective, biological, and cognitive models to explain the emergence of the gender difference in depression. Psychol Rev 115:291–313. [DOI] [PubMed] [Google Scholar]
- Jhou TC, Geisler S, Marinelli M, Degarmo BA, Zahm DS. (2009) The mesopontine rostromedial tegmental nucleus: a structure targeted by the lateral habenula that projects to the ventral tegmental area of Tsai and substantia nigra compacta. J Comp Neurol 513:566–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juarez B, Han M-H. (2016) Diversity of dopaminergic neural circuits in response to drug exposure. Neuropsychopharmacology 41:2424–2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallupi M, Scuppa G, de Guglielmo G, Calò G, Weiss F, Statnick MA, Rorick-Kehn LM, Ciccocioppo R. (2017) Genetic deletion of the nociceptin/orphanin FQ receptor in the rat confers resilience to the development of drug addiction. Neuropsychopharmacology 42:695–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang M, Mischel RA, Bhave S, Komla E, Cho A, Huang C, Dewey WL, Akbarali HI. (2017) The effect of gut microbiome on tolerance to morphine mediated antinociception in mice. Sci Rep 7:42658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Karkowski LM, Prescott CA. (1999) Causal relationship between stressful life events and the onset of major depression. Am J Psychiatry 156:837–841. [DOI] [PubMed] [Google Scholar]
- Kivell B, Prisinzano TE. (2010) Kappa opioids and the modulation of pain. Psychopharmacology (Berl) 210:109–119. [DOI] [PubMed] [Google Scholar]
- Knoll AT, Carlezon WA., Jr. (2010) Dynorphin, stress, and depression. Brain Res 1314:56–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. (2016) Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry 3:760–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster AS, Frankhuijzen-Sierevogel AC, Noordhoek J. (1985) Distribution of glucuronidation capacity (1-naphthol and morphine) along the rat intestine. Biochem Pharmacol 34:3527–3532. [DOI] [PubMed] [Google Scholar]
- Kotlińska J, Wichmann J, Legowska A, Rolka K, Silberring J. (2002) Orphanin FQ/nociceptin but not Ro 65-6570 inhibits the expression of cocaine-induced conditioned place preference. Behav Pharmacol 13:229–235. [DOI] [PubMed] [Google Scholar]
- Lee K, Vuong HE, Nusbaum DJ, Hsiao EY, Evans CJ, Taylor AMW. (2018) The gut microbiota mediates reward and sensory responses associated with regimen-selective morphine dependence. Neuropsychopharmacology 43:2606–2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitl MD, Onvani S, Bowers MS, Cheng K, Rice KC, Carlezon WA, Jr., Banks ML, Negus SS. (2014a) Pain-related depression of the mesolimbic dopamine system in rats: expression, blockade by analgesics, and role of endogenous κ-opioids. Neuropsychopharmacology 39:614–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitl MD, Potter DN, Cheng K, Rice KC, Carlezon WA, Jr., Negus SS. (2014b) Sustained pain-related depression of behavior: effects of intraplantar formalin and complete freund’s adjuvant on intracranial self-stimulation (ICSS) and endogenous kappa opioid biomarkers in rats. Mol Pain 10:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SS, Pickens S, Burma NE, Ibarra-Lecue I, Yang H, Xue L, Cook C, Hakimian JK, Severino AL, Lueptow L, et al. (2019) Kappa opioid receptors drive a tonic aversive component of chronic pain. J Neurosci 39:4162–4178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loder E. (2006) Post-marketing experience with an opioid nasal spray for migraine: lessons for the future. Cephalalgia 26:89–97. [DOI] [PubMed] [Google Scholar]
- Lutz P-E, Kieffer BL. (2013) Opioid receptors: distinct roles in mood disorders. Trends Neurosci 36:195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maleki N, Becerra L, Borsook D. (2012) Migraine: maladaptive brain responses to stress. Headache 52 (Suppl 2):102–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour A, Khachaturian H, Lewis ME, Akil H, Watson SJ. (1988) Anatomy of CNS opioid receptors. Trends Neurosci 11:308–314. [DOI] [PubMed] [Google Scholar]
- Margolis EB, Hjelmstad GO, Bonci A, Fields HL. (2003) Kappa-opioid agonists directly inhibit midbrain dopaminergic neurons. J Neurosci 23:9981–9986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massaly N, Copits BA, Wilson-Poe AR, Hipólito L, Markovic T, Yoon HJ, Liu S, Walicki MC, Bhatti DL, Sirohi S, et al. (2019) Pain-induced negative affect is mediated via recruitment of the nucleus accumbens kappa opioid system. Neuron 102:564–573.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massaly N, Morón JA, Al-Hasani R. (2016) A trigger for opioid misuse: chronic pain and stress dysregulate the mesolimbic pathway and kappa opioid system. Front Neurosci 10:480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May CN, Dashwood MR, Whitehead CJ, Mathias CJ. (1989) Differential cardiovascular and respiratory responses to central administration of selective opioid agonists in conscious rabbits: correlation with receptor distribution. Br J Pharmacol 98:903–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer EA, Knight R, Mazmanian SK, Cryan JF, Tillisch K. (2014) Gut microbes and the brain: paradigm shift in neuroscience. J Neurosci 34:15490–15496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMakin DL, Olino TM, Porta G, Dietz LJ, Emslie G, Clarke G, Wagner KD, Asarnow JR, Ryan ND, Birmaher B, et al. (2012) Anhedonia predicts poorer recovery among youth with selective serotonin reuptake inhibitor treatment-resistant depression. J Am Acad Child Adolesc Psychiatry 51:404–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medeiros IU, Ruzza C, Asth L, Guerrini R, Romão PRT, Gavioli EC, Calo G. (2015) Blockade of nociceptin/orphanin FQ receptor signaling reverses LPS-induced depressive-like behavior in mice. Peptides 72:95–103. [DOI] [PubMed] [Google Scholar]
- Meng J, Banerjee S, Li D, Sindberg GM, Wang F, Ma J, Roy S. (2015) Opioid exacerbation of gram-positive sepsis, induced by gut microbial modulation, is rescued by IL-17a neutralization. Sci Rep 5:10918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng J, Yu H, Ma J, Wang J, Banerjee S, Charboneau R, Barke RA, Roy S. (2013) Morphine induces bacterial translocation in mice by compromising intestinal barrier function in a TLR-dependent manner. PLoS One 8:e54040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennicken F, Zhang J, Hoffert C, Ahmad S, Beaudet A, O’Donnell D. (2003) Phylogenetic changes in the expression of delta opioid receptors in spinal cord and dorsal root ganglia. J Comp Neurol 465:349–360. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Członkowski A, Morris B, Stein C, Arendt R, Huber A, Höllt V, Herz A. (1988) Inflammation of the hind limb as a model of unilateral, localized pain: influence on multiple opioid systems in the spinal cord of the rat. Pain 35:299–312. [DOI] [PubMed] [Google Scholar]
- Mollereau C, Mouledous L. (2000) Tissue distribution of the opioid receptor-like (ORL1) receptor. Peptides 21:907–917. [DOI] [PubMed] [Google Scholar]
- Mollereau C, Parmentier M, Mailleux P, Butour JL, Moisand C, Chalon P, Caput D, Vassart G, Meunier JC. (1994) ORL1, a novel member of the opioid receptor family. Cloning, functional expression and localization. FEBS Lett 341:33–38. [DOI] [PubMed] [Google Scholar]
- Morales M, Margolis EB. (2017) Ventral tegmental area: cellular heterogeneity, connectivity and behaviour. Nat Rev Neurosci 18:73–85. [DOI] [PubMed] [Google Scholar]
- Moye LS, Novack ML, Tipton AF, Krishnan H, Pandey SC, Pradhan AA. (2019a) The development of a mouse model of mTBI-induced post-traumatic migraine, and identification of the delta opioid receptor as a novel therapeutic target. Cephalalgia 39:77–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moye LS, Pradhan AA. (2017) From blast to bench: a translational mini-review of posttraumatic headache. J Neurosci Res 95:1347–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moye LS, Tipton AF, Dripps I, Sheets Z, Crombie A, Violin JD, Pradhan AA. (2019b) Delta opioid receptor agonists are effective for multiple types of headache disorders. Neuropharmacology 148:77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucha RF, Herz A. (1985) Motivational properties of kappa and mu opioid receptor agonists studied with place and taste preference conditioning. Psychopharmacology (Berl) 86:274–280. [DOI] [PubMed] [Google Scholar]
- Narita M, Kishimoto Y, Ise Y, Yajima Y, Misawa K, Suzuki T. (2005) Direct evidence for the involvement of the mesolimbic kappa-opioid system in the morphine-induced rewarding effect under an inflammatory pain-like state. Neuropsychopharmacology 30:111–118. [DOI] [PubMed] [Google Scholar]
- Negus SS, Gatch MB, Mello NK, Zhang X, Rice K. (1998) Behavioral effects of the delta-selective opioid agonist SNC80 and related compounds in rhesus monkeys. J Pharmacol Exp Ther 286:362–375. [PubMed] [Google Scholar]
- Nestler EJ, Carlezon WA., Jr. (2006) The mesolimbic dopamine reward circuit in depression. Biol Psychiatry 59:1151–1159. [DOI] [PubMed] [Google Scholar]
- Norton CS, Neal CR, Kumar S, Akil H, Watson SJ. (2002) Nociceptin/orphanin FQ and opioid receptor-like receptor mRNA expression in dopamine systems. J Comp Neurol 444:358–368. [DOI] [PubMed] [Google Scholar]
- Oluigbo CO, Salma A, Rezai AR. (2012) Targeting the affective and cognitive aspects of chronic neuropathic pain using basal forebrain neuromodulation: rationale, review and proposal. J Clin Neurosci 19:1216–1221. [DOI] [PubMed] [Google Scholar]
- Ozawa A, Brunori G, Mercatelli D, Wu J, Cippitelli A, Zou B, Xie XS, Williams M, Zaveri NT, Low S, et al. (2015) Knock-in mice with NOP-eGFP receptors identify receptor cellular and regional localization. J Neurosci 35:11682–11693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker KE, Pedersen CE, Gomez AM, Spangler SM, Walicki MC, Feng SY, Stewart SL, Otis JM, Al-Hasani R, McCall JG, et al. (2019) A paranigral VTA nociceptin circuit that constrains motivation for reward. Cell 178:653–671.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peckys D, Landwehrmeyer GB. (1999) Expression of mu, kappa, and delta opioid receptor messenger RNA in the human CNS: a 33P in situ hybridization study. Neuroscience 88:1093–1135. [DOI] [PubMed] [Google Scholar]
- Pfeiffer A, Brantl V, Herz A, Emrich HM. (1986) Psychotomimesis mediated by kappa opiate receptors. Science 233:774–776. [DOI] [PubMed] [Google Scholar]
- Poole DP, Pelayo J-C, Scherrer G, Evans CJ, Kieffer BL, Bunnett NW. (2011) Localization and regulation of fluorescently labeled delta opioid receptor, expressed in enteric neurons of mice. Gastroenterology 141:982–991.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porreca F, Burks TF. (1983) The spinal cord as a site of opioid effects on gastrointestinal transit in the mouse. J Pharmacol Exp Ther 227:22–27. [PubMed] [Google Scholar]
- Porreca F, Mosberg HI, Hurst R, Hruby VJ, Burks TF. (1984) Roles of mu, delta and kappa opioid receptors in spinal and supraspinal mediation of gastrointestinal transit effects and hot-plate analgesia in the mouse. J Pharmacol Exp Ther 230:341–348. [PubMed] [Google Scholar]
- Porreca F, Mosberg HI, Omnaas JR, Burks TF, Cowan A. (1987) Supraspinal and spinal potency of selective opioid agonists in the mouse writhing test. J Pharmacol Exp Ther 240:890–894. [PubMed] [Google Scholar]
- Post A, Smart TS, Krikke-Workel J, Dawson GR, Harmer CJ, Browning M, Jackson K, Kakar R, Mohs R, Statnick M, et al. (2016) A selective nociceptin receptor antagonist to treat depression: evidence from preclinical and clinical studies. Neuropsychopharmacology 41:1803–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power SE, O’Toole PW, Stanton C, Ross RP, Fitzgerald GF. (2014) Intestinal microbiota, diet and health. Br J Nutr 111:387–402. [DOI] [PubMed] [Google Scholar]
- Pradhan A, Smith M, McGuire B, Evans C, Walwyn W. (2013) Chronic inflammatory injury results in increased coupling of delta opioid receptors to voltage-gated Ca2+ channels. Mol Pain 9:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan AA, Befort K, Nozaki C, Gavériaux-Ruff C, Kieffer BL. (2011) The delta opioid receptor: an evolving target for the treatment of brain disorders. Trends Pharmacol Sci 32:581–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan AA, Smith ML, Kieffer BL, Evans CJ. (2012) Ligand-directed signalling within the opioid receptor family. Br J Pharmacol 167:960–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan AA, Smith ML, McGuire B, Tarash I, Evans CJ, Charles A. (2014a) Characterization of a novel model of chronic migraine. Pain 155:269–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan AA, Smith ML, Zyuzin J, Charles A. (2014b) δ-Opioid receptor agonists inhibit migraine-related hyperalgesia, aversive state and cortical spreading depression in mice. Br J Pharmacol 171:2375–2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan AAA, Clarke PBS. (2005) Comparison between delta-opioid receptor functional response and autoradiographic labeling in rat brain and spinal cord. J Comp Neurol 481:416–426. [DOI] [PubMed] [Google Scholar]
- Redrobe JP, Calo’ G, Regoli D, Quirion R. (2002) Nociceptin receptor antagonists display antidepressant-like properties in the mouse forced swimming test. Naunyn Schmiedebergs Arch Pharmacol 365:164–167. [DOI] [PubMed] [Google Scholar]
- Reid MC, Engles-Horton LL, Weber MB, Kerns RD, Rogers EL, O’Connor PG. (2002) Use of opioid medications for chronic noncancer pain syndromes in primary care. J Gen Intern Med 17:173–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice FL, Xie JY, Albrecht PJ, Acker E, Bourgeois J, Navratilova E, Dodick DW, Porreca F. (2017) Anatomy and immunochemical characterization of the non-arterial peptidergic diffuse dural innervation of the rat and Rhesus monkey: implications for functional regulation and treatment in migraine. Cephalalgia 37:1350–1372. [DOI] [PubMed] [Google Scholar]
- Richardson NR, Roberts DC. (1996) Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods 66:1–11. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, Niederehe G, Thase ME, Lavori PW, Lebowitz BD, et al. (2006) Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry 163:1905–1917. [DOI] [PubMed] [Google Scholar]
- Russo SJ, Nestler EJ. (2013) The brain reward circuitry in mood disorders. Nat Rev Neurosci 14:609–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salk RH, Hyde JS, Abramson LY. (2017) Gender differences in depression in representative national samples: meta-analyses of diagnoses and symptoms. Psychol Bull 143:783–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz N, Temkin P, Jurado S, Lim BK, Heifets BD, Polepalli JS, Malenka RC. (2014) Chronic pain. Decreased motivation during chronic pain requires long-term depression in the nucleus accumbens. Science 345:535–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakhsheer BA, Versten LA, Luo JN, Defazio JR, Klabbers R, Christley S, Zaborin A, Guyton KL, Krezalek M, Smith DP, et al. (2016) Morphine promotes colonization of anastomotic tissues with collagenase - producing Enterococcus faecalis and causes leak. J Gastrointest Surg 20:1744–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shippenberg TS, Bals-Kubik R, Herz A. (1993) Examination of the neurochemical substrates mediating the motivational effects of opioids: role of the mesolimbic dopamine system and D-1 vs. D-2 dopamine receptors. J Pharmacol Exp Ther 265:53–59. [PubMed] [Google Scholar]
- Shippenberg TS, Stein C, Huber A, Millan MJ, Herz A. (1988) Motivational effects of opioids in an animal model of prolonged inflammatory pain: alteration in the effects of kappa- but not of mu-receptor agonists. Pain 35:179–186. [DOI] [PubMed] [Google Scholar]
- Silberstein SD, Dodick D, Freitag F, Pearlman SH, Hahn SR, Scher AI, Lipton RB. (2007) Pharmacological approaches to managing migraine and associated comorbidities--clinical considerations for monotherapy versus polytherapy. Headache 47:585–599. [DOI] [PubMed] [Google Scholar]
- Sindberg GM, Callen SE, Banerjee S, Meng J, Hale VL, Hegde R, Cheney PD, Villinger F, Roy S, Buch S. (2019) Morphine potentiates dysbiotic microbial and metabolic shifts in acute SIV infection. J Neuroimmune Pharmacol 14:200–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanagel R, Herz A, Shippenberg TS. (1992) Opposing tonically active endogenous opioid systems modulate the mesolimbic dopaminergic pathway. Proc Natl Acad Sci USA 89:2046–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauffer WR, Lak A, Kobayashi S, Schultz W. (2016) Components and characteristics of the dopamine reward utility signal. J Comp Neurol 524:1699–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein C. (1991) Peripheral analgesic actions of opioids. J Pain Symptom Manage 6:119–124. [DOI] [PubMed] [Google Scholar]
- Stein C, Gramsch C, Herz A. (1990) Intrinsic mechanisms of antinociception in inflammation: local opioid receptors and beta-endorphin. J Neurosci 10:1292–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein C, Millan MJ, Shippenberg TS, Peter K, Herz A. (1989) Peripheral opioid receptors mediating antinociception in inflammation. Evidence for involvement of mu, delta and kappa receptors. J Pharmacol Exp Ther 248:1269–1275. [PubMed] [Google Scholar]
- Stevenson GW, Folk JE, Rice KC, Negus SS. (2005) Interactions between delta and mu opioid agonists in assays of schedule-controlled responding, thermal nociception, drug self-administration, and drug versus food choice in rhesus monkeys: studies with SNC80 [(+)-4-[(alphaR)-alpha-((2S,5R)-4-allyl-2,5-dimethyl-1-piperazinyl)-3-methoxybenzyl]-N,N-diethylbenzamide] and heroin. J Pharmacol Exp Ther 314:221–231. [DOI] [PubMed] [Google Scholar]
- Tan KR, Yvon C, Turiault M, Mirzabekov JJ, Doehner J, Labouèbe G, Deisseroth K, Tye KM, Lüscher C. (2012) GABA neurons of the VTA drive conditioned place aversion. Neuron 73:1173–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AMW, Becker S, Schweinhardt P, Cahill C. (2016) Mesolimbic dopamine signaling in acute and chronic pain: implications for motivation, analgesia, and addiction. Pain 157:1194–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AMW, Castonguay A, Taylor AJ, Murphy NP, Ghogha A, Cook C, Xue L, Olmstead MC, De Koninck Y, Evans CJ, et al. (2015) Microglia disrupt mesolimbic reward circuitry in chronic pain. J Neurosci 35:8442–8450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AMW, Murphy NP, Evans CJ, Cahill CM. (2014) Correlation between ventral striatal catecholamine content and nociceptive thresholds in neuropathic mice. J Pain 15:878–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo MA, Pedregal C, Lafuente C, Diaz N, Martinez-Grau MA, Jiménez A, Benito A, Torrado A, Mateos C, Joshi EM, et al. (2014) Discovery of a novel series of orally active nociceptin/orphanin FQ (NOP) receptor antagonists based on a dihydrospiro(piperidine-4,7′-thieno[2,3-c]pyran) scaffold. J Med Chem 57:3418–3429. [DOI] [PubMed] [Google Scholar]
- Toll L, Bruchas MR, Calo’ G, Cox BM, Zaveri NT. (2016) Nociceptin/orphanin FQ receptor structure, signaling, ligands, functions, and interactions with opioid systems. Pharmacol Rev 68:419–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Zald DH. (2011) Reconsidering anhedonia in depression: lessons from translational neuroscience. Neurosci Biobehav Rev 35:537–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unterwald E, Sasson S, Kornetsky C. (1987) Evaluation of the supraspinal analgesic activity and abuse liability of ethylketocyclazocine. Eur J Pharmacol 133:275–281. [DOI] [PubMed] [Google Scholar]
- Vanderah TW, Largent-Milnes T, Lai J, Porreca F, Houghten RA, Menzaghi F, Wisniewski K, Stalewski J, Sueiras-Diaz J, Galyean R, et al. (2008) Novel D-amino acid tetrapeptides produce potent antinociception by selectively acting at peripheral kappa-opioid receptors. Eur J Pharmacol 583:62–72. [DOI] [PubMed] [Google Scholar]
- van Zessen R, Phillips JL, Budygin EA, Stuber GD. (2012) Activation of VTA GABA neurons disrupts reward consumption. Neuron 73:1184–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicente-Sanchez A, Pradhan AA. (2018) Ligand-directed signaling at the delta opioid receptor. Handb Exp Pharmacol 247:73–85. [DOI] [PubMed] [Google Scholar]
- Vicente-Sanchez A, Segura L, Pradhan AA. (2016) The delta opioid receptor tool box. Neuroscience 338:145–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijay A, Wang S, Worhunsky P, Zheng M-Q, Nabulsi N, Ropchan J, Krishnan-Sarin S, Huang Y, Morris ED. (2016) PET imaging reveals sex differences in kappa opioid receptor availability in humans, in vivo. Am J Nucl Med Mol Imaging 6:205–214. [PMC free article] [PubMed] [Google Scholar]
- Vitale G, Ruggieri V, Filaferro M, Frigeri C, Alboni S, Tascedda F, Brunello N, Guerrini R, Cifani C, Massi M. (2009) Chronic treatment with the selective NOP receptor antagonist [Nphe 1, Arg 14, Lys 15]N/OFQ-NH 2 (UFP-101) reverses the behavioural and biochemical effects of unpredictable chronic mild stress in rats. Psychopharmacology (Berl) 207:173–189. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang G-J, Fowler JS, Tomasi D, Telang F. (2011) Addiction: beyond dopamine reward circuitry. Proc Natl Acad Sci USA 108:15037–15042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrieze E, Demyttenaere K, Bruffaerts R, Hermans D, Pizzagalli DA, Sienaert P, Hompes T, de Boer P, Schmidt M, Claes S. (2014) Dimensions in major depressive disorder and their relevance for treatment outcome. J Affect Disord 155:35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadenberg M-LG. (2003) A review of the properties of spiradoline: a potent and selective kappa-opioid receptor agonist. CNS Drug Rev 9:187–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh CT, Levine RR. (1975) Studies of the enterohepatic circulation of morphine in the rat. J Pharmacol Exp Ther 195:303–310. [PubMed] [Google Scholar]
- Wang F, Meng J, Zhang L, Johnson T, Chen C, Roy S. (2018) Morphine induces changes in the gut microbiome and metabolome in a morphine dependence model. Sci Rep 8:3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Roy S. (2017) Gut homeostasis, microbial dysbiosis, and opioids. Toxicol Pathol 45:150–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werling LL, Frattali A, Portoghese PS, Takemori AE, Cox BM. (1988) Kappa receptor regulation of dopamine release from striatum and cortex of rats and Guinea pigs. J Pharmacol Exp Ther 246:282–286. [PubMed] [Google Scholar]
- Werner F-M, Coveñas R. (2010) Classical neurotransmitters and neuropeptides involved in major depression: a review. Int J Neurosci 120:455–470. [DOI] [PubMed] [Google Scholar]
- Whitton AE, Treadway MT, Pizzagalli DA. (2015) Reward processing dysfunction in major depression, bipolar disorder and schizophrenia. Curr Opin Psychiatry 28:7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JBW, Kobak KA, Bech P, Engelhardt N, Evans K, Lipsitz J, Olin J, Pearson J, Kalali A. (2008) The GRID-HAMD: standardization of the Hamilton Depression Rating Scale. Int Clin Psychopharmacol 23:120–129. [DOI] [PubMed] [Google Scholar]
- Wise RA, Koob GF. (2014) The development and maintenance of drug addiction. Neuropsychopharmacology 39:254–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin JM, Rorick-Kehn LM, Benvenga MJ, Adams BL, Gleason SD, Knitowski KM, Li X, Chaney S, Falcone JF, Smith JW, et al. (2016) Preclinical findings predicting efficacy and side-effect profile of LY2940094, an antagonist of nociceptin receptors. Pharmacol Res Perspect 4:e00275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin JM, Statnick MA, Rorick-Kehn LM, Pintar JE, Ansonoff M, Chen Y, Tucker RC, Ciccocioppo R. (2014) The biology of Nociceptin/Orphanin FQ (N/OFQ) related to obesity, stress, anxiety, mood, and drug dependence. Pharmacol Ther 141:283–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Na X, Zang Y, Cui Y, Xin W, Pang R, Zhou L, Wei X, Li Y, Liu X. (2014) Upregulation of tumor necrosis factor-alpha in nucleus accumbens attenuates morphine-induced rewarding in a neuropathic pain model. Biochem Biophys Res Commun 449:502–507. [DOI] [PubMed] [Google Scholar]
- Xie JY, De Felice M, Kopruszinski CM, Eyde N, LaVigne J, Remeniuk B, Hernandez P, Yue X, Goshima N, Ossipov M, et al. (2017) Kappa opioid receptor antagonists: a possible new class of therapeutics for migraine prevention. Cephalalgia 37:780–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Xie Z, Wang H, Shen Z, Guo Y, Gao Y, Chen X, Wu Q, Li X, Wang K. (2017) Bacterial diversity of intestinal microbiota in patients with substance use disorders revealed by 16S rRNA gene deep sequencing. Sci Rep 7:3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalcin I, Barrot M. (2014) The anxiodepressive comorbidity in chronic pain. Curr Opin Anaesthesiol 27:520–527. [DOI] [PubMed] [Google Scholar]
- Yoon S, Yu J, McDowell A, Kim SH, You HJ, Ko G. (2017) Bile salt hydrolase-mediated inhibitory effect of Bacteroides ovatus on growth of Clostridium difficile. J Microbiol 55:892–899. [DOI] [PubMed] [Google Scholar]
- Zaveri NT. (2011) The nociceptin/orphanin FQ receptor (NOP) as a target for drug abuse medications. Curr Top Med Chem 11:1151–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang LL, Zheng H, Ma C, He ZG, Zheng C. (2009) The plasma orphanin FQ in patients with depression before and after treatment. Chin J Psychiatry 42:138–142. [Google Scholar]
- Zhang R, Meng J, Lian Q, Chen X, Bauman B, Chu H, Segura B, Roy S. (2018) Prescription opioids are associated with higher mortality in patients diagnosed with sepsis: a retrospective cohort study using electronic health records. PLoS One 13:e0190362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao R-J, Woo R-S, Jeong M-S, Shin B-S, Kim D-G, Kim K-W. (2003) Orphanin FQ/nociceptin blocks methamphetamine place preference in rats. Neuroreport 14:2383–2385. [DOI] [PubMed] [Google Scholar]
- Zheng F, Grandy DK, Johnson SW. (2002) Actions of orphanin FQ/nociceptin on rat ventral tegmental area neurons in vitro. Br J Pharmacol 136:1065–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]