Key Points
Question
Is prehospital plasma administration associated with improved survival among severely injured patients with traumatic brain injury (TBI)?
Findings
In this secondary analysis of a predefined subgroup of 166 severely injured patients with trauma from a cluster randomized clinical trial, prehospital plasma administration was associated with lower mortality in patients with TBI.
Meaning
Future studies are needed to confirm these clinical benefits, but prehospital plasma administered soon after injury may improve survival for patients with TBI.
This secondary analysis of a cluster randomized clinical trial assesses the association between prehospital plasma administration and survival in patients with traumatic brain injury.
Abstract
Importance
Prehospital plasma administration improves survival in injured patients at risk for hemorrhagic shock and transported by air ambulance. Traumatic brain injury (TBI) is a leading cause of death following trauma, but few early interventions improve outcomes.
Objective
To assess the association between prehospital plasma and survival in patients with TBI.
Design, Setting, and Participants
The Prehospital Air Medical Plasma (PAMPer) trial was a pragmatic, multicenter, phase 3, cluster randomized clinical trial involving injured patients who were at risk for hemorrhagic shock during air medical transport to a trauma center. The trial was conducted at 6 US sites with 9 level-I trauma centers (comprising 27 helicopter emergency services bases). The original trial analyzed 501 patients, including 230 patients who were randomized to receive plasma and 271 randomized to standard care resuscitation. This secondary analysis of a predefined subgroup included patients with TBI. Data analysis was performed from October 2019 to February 2020.
Interventions
Patients were randomized to receive standard care fluid resuscitation or 2 units of thawed plasma.
Main Outcomes and Measures
The primary outcome was mortality at 30 days. Patients with TBI were prespecified as a subgroup for secondary analysis and for measurement of markers of brain injury. The 30-day survival benefit of prehospital plasma in subgroups with and without TBI as diagnosed by computed tomography was characterized using Kaplan-Meier survival analysis and Cox proportional hazard regression.
Results
In total, 166 patients had TBI (median [interquartile range] age, 43.00 [25.00-59.75] years; 125 men [75.3%]). When compared with the 92 patients who received standard care, the 74 patients with TBI who received prehospital plasma had improved 30-day survival even after adjustment for multiple confounders and assessment of the degree of brain injury with clinical variables and biomarkers (hazard ratio [HR], 0.55; 95% CI, 0.33-0.94; P = .03). Receipt of prehospital plasma was associated with improved survival among patients with TBI with a prehospital Glasgow Coma Scale score of less than 8 (HR, 0.56; 95% CI, 0.35-0.91) and those with polytrauma (HR, 0.50; 95% CI, 0.28-0.89). Patients with TBI transported from the scene of injury had improved survival following prehospital plasma administration (HR, 0.45; 95% CI, 0.26-0.80; P = .005), whereas patients who were transferred from an outside hospital showed no difference in survival for the plasma intervention (HR, 1.00; 95% CI, 0.33-3.00; P = .99).
Conclusions and Relevance
These findings are exploratory, but they suggest that receipt of prehospital plasma is associated with improved survival in patients with computed tomography–positive TBI. The prehospital setting may be a critical period to intervene in the care of patients with TBI. Future studies are needed to confirm the clinical benefits of early plasma resuscitation following TBI and concomitant polytrauma.
Trial Registration
ClinicalTrials.gov Identifier: NCT01818427
Introduction
Traumatic injury is a leading cause of preventable death,1,2 which results primarily from hemorrhage and traumatic brain injury (TBI).3 Worldwide, it is estimated that there are 50 million cases of TBI each year, generating a global annual economic burden of approximately US $400 billion.4 Despite efforts to address this global health problem,5,6 there are limited therapies to lessen the morbidity and mortality associated with TBI.4 Increasingly, prehospital interventions following traumatic injury result in improved outcomes and survival,7,8,9 and this phase of care may be especially relevant to the treatment of TBI.4,10,11
The Prehospital Air Medical Plasma (PAMPer) trial8 demonstrated that prehospital plasma resuscitation improved 30-day survival by 10% in severely injured trauma patients at risk for hemorrhagic shock and transported by air ambulance. Several underlying mechanisms to explain this survival benefit are hypothesized, including volume expansion to restore perfusion, an alteration in the inflammatory response,12,13 a reduction in endothelial injury,14,15 and the prevention or mitigation of coagulopathy.10 Retrospective studies and animal models16,17 suggest that plasma administered soon after injury may improve neurological function after TBI. Prehospital interventions that mitigate secondary injury from hypotension, hypoxia, and hypocarbia have also been shown to improve TBI outcomes.11,18,19,20 Preliminary, unadjusted subgroup analysis in the PAMPer trial suggested a survival benefit in patients with severe head injury, as defined by Abbreviated Injury Severity (AIS) scores for the head.8 However, to our knowledge, the association of early plasma administration with survival has not been fully characterized in the subgroup of patients with TBI as confirmed by computed tomography (CT) while adjusting for potential confounding factors.
Identifying patients who benefit from early interventions enables the targeted allocation of resources to the most appropriate patients.21,22,23 Whether the beneficial effect of prehospital plasma differs across the spectrum of TBI is unknown. In this analysis, we sought to characterize the survival benefit associated with prehospital plasma among patients with TBI using data derived from a recently completed prehospital plasma clinical trial.
Methods
We performed a post hoc secondary analysis of a prespecified subgroup from the PAMPer trial. The PAMPer trial was a pragmatic, multicenter, phase 3, cluster randomized clinical superiority trial designed to test the effect of administering plasma to severely injured trauma patients on air ambulances before arrival to definitive trauma care. We randomized patients to receive either standard care fluid resuscitation (crystalloid or crystalloid and packed red blood cells) or 2 units of thawed plasma followed by standard care fluid resuscitation. This study was approved under an Emergency Exception From Informed Consent protocol from the Human Research Protection Office of the US Army Medical Research and Material Command and by the appropriate institutional review boards as previously described.8 The full trial protocol is available in Supplement 1. Additional information regarding study inclusion and exclusion criteria and model analysis methods can be found in the eAppendix in Supplement 2. This study follows the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
TBI was defined in the study protocol as a prespecified subgroup for a post hoc, secondary analysis. For the primary analysis, TBI was defined by AIS score for the head greater than 2.8 CT imaging is more accurate than head AIS score for determining the presence of TBI. In this study, we assessed imaging results and defined TBI as brain injury documented by CT scan. TBI was defined as any finding consistent with TBI as defined by a radiologist at initial head CT. We analyzed glial fibrillary acidic protein (GFAP) and ubiquitin C-terminal hydrolase (UCH-L1) to assess biological markers of TBI across trial groups as described elsewhere.24,25
Statistical Analysis
We first evaluated the association of prehospital plasma with 30-day mortality across groups with and without TBI (binned as a dichotomous variable) using a generalized estimating equations model in the geepack software package version 1.2-1 (R Project for Statistical Computing).26 The plasma and TBI interaction was assessed for statistical significance, accounting for trial cluster effects and multiple confounders. We then performed Kaplan-Meier survival analysis comparing prehospital plasma vs standard care resuscitation across groups according to the presence of TBI. Log-rank P values were calculated and survival curves were built using the Survminer software package version 0.4.3 (R Project for Statistical Computing).27
To verify these unadjusted findings, we performed an analysis of survival with the use of a Cox proportional hazard with shared frailty model to evaluate the treatment with adjustment for possible confounding factors and site clustering on survival.28 We generated the model for the primary outcome and assessed prehospital differences across the groups when randomization for prehospital plasma occurred. We assessed whether our fitted Cox regression model adequately described the data using the Survival software package version 2.41-3 (R Project for Statistical Computing).29 We evaluated variance inflation factors with the rms software package version 5.1-3.1 (R Project for Statistical Computing)30 to ensure that the variance of our regression coefficients was not due to multicollinearity. This model met the proportional hazards assumption on the basis of Schoenfeld residuals.
We explored the association between prehospital plasma and both TBI severity and concomitant polytrauma by comparison of hazard ratios (HRs) from a fitted Cox proportional hazard model. We analyzed survival for patients with TBI transported from the scene and those transferred from an outside hospital as proxies for early vs late prehospital plasma fluid administration.
We analyzed data using R statistical software version 3.4.1 (R Project for Statistical Computing).31 The analysis code is publicly available on GitHub.32 Categorical variables are presented as frequencies and percentages and were tested using the Pearson χ2 test. Continuous variables are expressed as medians and interquartile ranges (IQRs) and were tested using Mann-Whitney U or Kruskal-Wallis test, as appropriate; all statistical tests were 2-sided. Statistical significance was determined at the P < .05 level. Data analysis was performed from October 2019 to February 2020.
Results
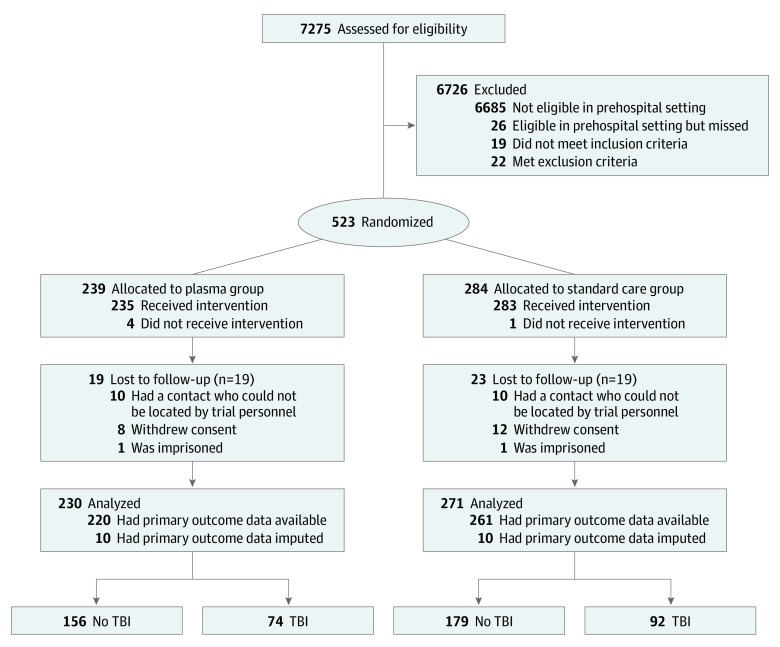
Of the 501 PAMPer patients, 166 patients (median [IQR] age, 43.00 [25.00-59.75] years) sustained a TBI as indicated by the presence of brain injury on CT imaging. Patients with TBI were mostly male (125 men [75.3%]), all of them had blunt trauma injuries (100%), and they had a median (IQR) Injury Severity Score of 29 (22-38) (Figure 1 and Table 1).
Figure 1. Patient Selection Flowchart.
TBI indicates traumatic brain injury.
Table 1. Comparison of Patient and Injury Characteristics and Outcomes Across Patients With and Without TBI.
| Variable | Patients, No. (%) | P valuea | |
|---|---|---|---|
| No TBI (n = 335) | TBI (n = 166) | ||
| Demographic characteristics | |||
| Age, median (IQR), y | 46.00 (31.00-59.50) | 43.00 (25.00-59.75) | .46 |
| Male | 239 (71.3) | 125 (75.3) | .41 |
| Race | |||
| White | 287 (85.7) | 148 (89.2) | .10 |
| Black | 35 (10.4) | 8 (4.8) | |
| Asian | 1 (0.3) | 0 | |
| Other | 3 (0.9) | 5 (3.0) | |
| Unknown | 9 (2.7) | 5 (3.0) | |
| Medications receiving | |||
| Vitamin K antagonist | 12 (4.4) | 2 (1.8) | .33 |
| Antiplatelets | 27 (9.7) | 11 (9.6) | >.99 |
| Injury characteristics | |||
| Injury Severity Score, median (IQR) | 17 (9-25) | 29 (22-38) | <.001 |
| Abbreviated Injury Severity score, median (IQR) | |||
| Head | 0 (0-2) | 4 (3-5) | <.001 |
| Chest | 2 (0-3) | 3 (2-3) | .01 |
| Abdomen | 2 (0-3) | 2 (0-2) | .09 |
| Extremity | 2 (0-3) | 2 (0-3) | .47 |
| Glasgow Coma Scale score | |||
| Prehospital | |||
| <8 | 95 (29.3) | 123 (75.5) | <.001 |
| 8-12 | 34 (10.5) | 13 (8.0) | |
| 13-15 | 195 (60.2) | 27 (16.6) | |
| Emergency department | |||
| <8 | 144 (43.8) | 132 (81.0) | <.001 |
| 8-12 | 5 (1.5) | 8 (4.9) | |
| 13-15 | 180 (54.7) | 23 (14.1) | |
| Prehospital systolic blood pressure <70 mm Hg | 164 (49.1) | 82 (49.4) | >.99 |
| Spinal cord injury | 21 (6.3) | 17 (10.3) | .16 |
| Blunt injury | 247 (73.7) | 166 (100.0) | <.001 |
| Blunt mechanism | |||
| Fall | 21 (8.5) | 14 (8.4) | .32 |
| Motor vehicle crash | |||
| Occupant | 137 (55.5) | 89 (53.6) | |
| Motorcyclist | 40 (16.2) | 35 (21.1) | |
| Pedestrian or cyclist | 13 (5.2) | 15 (9.0) | |
| Unknown | 2 (0.8) | 1 (0.6) | |
| Struck by or against | 16 (6.5) | 3 (1.8) | |
| Other | 18 (7.3) | 9 (5.4) | |
| Prehospital | |||
| Intubation | 131 (39.1) | 125 (75.3) | <.001 |
| Cardiopulmonary resuscitation | 19 (5.7) | 12 (7.2) | .63 |
| Plasma intervention | 156 (46.6) | 74 (44.6) | .75 |
| Crystalloid fluid, median (IQR), mL | 600.00 (0.00-1440.00) | 812.50 (0.00-1475.00) | .35 |
| Packed red blood cells, median (IQR), units | 0.00 (0.00-1.50) | 0.00 (0.00-1.00) | .93 |
| Received packed red blood cells | 115 (34.3) | 59 (35.5) | .87 |
| Transport time, median (IQR), min | 41.00 (32.25-52.00) | 42.50 (35.00-53.00) | .19 |
| Transferred from facility | 77 (23.1) | 34 (20.6) | .60 |
| Hospital | |||
| International normalized ratio, median (IQR) | 1.20 (1.10-1.40) | 1.30 (1.10-1.60) | .001 |
| Thromboelastography values, median (IQR) | |||
| α Angle, ° | 70.30 (61.90-74.90) | 67.80 (59.75-73.47) | .11 |
| Activated clotting time, s | 113.00 (97.00-128.00) | 121.00 (100.00-136.00) | .31 |
| Kappa, min | 1.75 (1.20-2.79) | 2.08 (1.50-3.10) | .08 |
| Maximum amplitude, mm | 58.00 (49.00-64.15) | 55.90 (48.85-61.15) | .11 |
| Lysis at 30 min, % | 1.40 (0.00-20.00) | 2.30 (0.00-34.38) | .19 |
| Transfusion in 24 h, median (IQR), units | 4.00 (1.00-13.00) | 4.00 (2.00-13.00) | .27 |
| Packed red blood cells in 24 h, median (IQR), units | 3.00 (1.00-7.50) | 3.50 (1.00-7.75) | .61 |
| Plasma in 24 h, median (IQR), units | 0.00 (0.00-3.00) | 0.00 (0.00-4.00) | .20 |
| Platelets in 24 h, median (IQR), units | 0.00 (0.00-1.00) | 0.00 (0.00-1.00) | .12 |
| Crystalloid fluid in 24 h, median (IQR), mL | 4300.00 (2297.50-6500.00) | 4725.00 (3103.00-6753.25) | .20 |
| Vasopressors in 24 h | 139 (41.5) | 103 (62.0) | <.001 |
| Outcome | |||
| 30-d mortality | 65 (19.4) | 77 (46.4) | <.001 |
| 24-hour mortality | 47 (14.0) | 45 (27.1) | .001 |
| Multiple organ failure | 192 (57.3) | 109 (65.7) | .09 |
| Intensive care unit length of stay, median (IQR), d | 3 (1-10) | 7 (2-14) | <.001 |
| Hospital length of stay, median (IQR), d | 8 (3-21) | 10 (2-20) | .97 |
| Ventilator duration, median (IQR), d | 2 (1-6) | 4 (1-10) | <.001 |
Abbreviations: IQR, interquartile range; TBI, traumatic brain injury.
To calculate P values for between-group comparisons, we performed the Pearson χ2 test for categorical variables, the Mann-Whitney U test for nonparametric, continuous variables with 2 or fewer groups, and the Kruskal-Wallis test for nonparametric, continuous variables with more than 2 groups.
A comparison of patients with and without TBI revealed no significant differences in patient demographic characteristics. However, patients with TBI were generally more severely injured, had higher incidence of prehospital intubation, were more likely to receive in-hospital vasopressors, and had higher intensive care unit length of stay, more days on mechanical ventilation, and higher 24-hour and 30-day mortality rates (Table 1). Approximately one-half of the TBI cohort (86 patients [51.8%]) sustained a subdural hematoma or hemorrhage (eTable 1 in Supplement 2), and of the patients with TBI who did not survive to 30 days, approximately one-half (42 patients [54.5%]) had a cause of death attributed to TBI (eTable 2 in Supplement 2).
Patients with TBI were balanced across the 2 trial groups, with 74 in the plasma group and 92 in the standard care group. Among patients with TBI, demographic and injury characteristics did not differ across groups of the trial. There were no significant differences in fluid administration during the duration of prehospital transport other than the plasma intervention. However, in-hospital transfusion requirements did differ across trial groups. Patients with TBI who were treated with prehospital plasma received less crystalloid fluid, vasopressors, and packed red blood cells in the first 24 hours. In addition, patients with TBI who received prehospital plasma had lower international normalized ratios (median [IQR], 1.20 [1.10-1.40] vs 1.40 [1.20-1.80]). Patients with TBI who received prehospital plasma had lower unadjusted 24-hour mortality (16.2% vs 35.9%) and 30-day mortality (35.1% vs 55.4%). These patients also had higher incidence of multiple organ failure and intensive care unit and hospital length of stay (Table 2).
Table 2. Comparison of Patient and Injury Characteristics and Outcomes Among Patients with Traumatic Brain Injury Across Groups of the Prehospital Air Medical Plasma Trial.
| Variable | Patients, No. (%) | P valuea | |
|---|---|---|---|
| Standard care (n = 92) | Plasma (n = 74) | ||
| Demographic characteristics | |||
| Age, median (IQR), y | 44.00 (25.00-59.25) | 42.50 (25.25-60.25) | .80 |
| Male | 75 (81.5) | 50 (67.6) | .06 |
| Race | |||
| White | 80 (87.0) | 68 (91.9) | .67 |
| Black | 5 (5.4) | 3 (4.1) | |
| Asian | 0 | 0 | |
| Other | 3 (3.3) | 2 (2.7) | |
| Unknown | 4 (4.3) | 1 (1.4) | |
| Medications receiving | |||
| Vitamin K antagonist | 1 (1.8) | 1 (1.7) | >.99 |
| Antiplatelets | 3 (5.3) | 8 (13.8) | .22 |
| Injury characteristics | |||
| Injury Severity Score, median (IQR) | 29 (22-37) | 29 (20-41) | .92 |
| Abbreviated Injury Severity score, median (IQR) | |||
| Head | 4 (3-5) | 3 (3-4) | .33 |
| Chest | 3 (2-3) | 3 (2-30) | .23 |
| Abdomen | 0 (0-2) | 2 (0-2) | .26 |
| Extremity | 2 (0-3) | 2 (0-3) | .41 |
| Glasgow Coma Scale score | |||
| Prehospital | |||
| <8 | 71 (79.8) | 52 (70.3) | .37 |
| 8-12 | 6 (6.7) | 7 (9.5) | |
| 13-15 | 12 (13.5) | 15 (20.3) | |
| Emergency department | |||
| <8 | 77 (84.6) | 55 (76.4) | .17 |
| 8-12 | 2 (2.2) | 6 (8.3) | |
| 13-15 | 12 (13.2) | 11 (15.3) | |
| Prehospital systolic blood pressure <70 mm Hg | 49 (53.3) | 33 (44.6) | .34 |
| Spinal cord injury | 11 (12.0) | 6 (8.2) | .60 |
| Blunt injury | 92 (100.0) | 74 (100.0) | >.99 |
| Blunt mechanism | |||
| Fall | 9 (9.8) | 5 (6.8) | .86 |
| Motor vehicle crash | |||
| Occupant | 51 (55.4) | 38 (51.4) | |
| Motorcyclist | 20 (21.7) | 15 (20.3) | |
| Pedestrian or cyclist | 6 (6.5) | 9 (12.5) | |
| Unknown | 0 | 1 (1.4) | |
| Struck by or against | 2 (2.2) | 1 (1.4) | |
| Other | 4 (4.3) | 5 (7.8) | |
| Prehospital | |||
| Intubation | 72 (78.3) | 53 (71.6) | .42 |
| Cardiopulmonary resuscitation | 9 (9.8) | 3 (4.1) | .27 |
| Crystalloid fluid, median (IQR), mL | 1000.00 (0.00-1500.00) | 700.00 (0.00-1287.50) | .20 |
| Packed red blood cells, median (IQR), units | 0.00 (0.00-2.00) | 0.00 (0.00-1.00) | .13 |
| Received packed red blood cells | 38 (41.3) | 21 (28.4) | .12 |
| Transport time, median (IQR), min | 41.00 (33.75-50.25) | 45.00 (36.00-54.25) | .08 |
| Transferred from facility | 12 (13.2) | 22 (29.7) | .02 |
| Hospital | |||
| International normalized ratio, median (IQR) | 1.40 (1.20-1.80) | 1.20 (1.10-1.40) | .001 |
| Thromboelastography values, median (IQR) | |||
| α Angle, ° | 66.10 (56.97-71.97) | 69.70 (62.48-75.03) | .10 |
| Activated clotting time, s | 121.00 (97.00-136.00) | 121.00 (105.00-132.00) | .97 |
| Kappa, min | 2.15 (1.56-3.50) | 1.80 (1.25-2.60) | .10 |
| Maximum amplitude, mm | 55.70 (47.23-59.83) | 56.00 (52.25-63.30) | .14 |
| Lysis at 30 min, % | 2.15 (0.00-40.00) | 2.75 (0.00-30.00) | .63 |
| Transfusion in 24 h, median (IQR), units | 5.00 (2.00-16.00) | 3.00 (0.00-10.00) | .03 |
| Packed red blood cells in 24 h, median (IQR), units | 4.00 (2.00-8.00) | 3.00 (0.00-6.00) | .03 |
| Plasma in 24 h, median (IQR), units | 0.00 (0.00-4.00) | 0.00 (0.00-3.00) | .34 |
| Platelets in 24 h, median (IQR), units | 0.00 (0.00-1.00) | 0.00 (0.00-1.00) | .47 |
| Crystalloid fluid in 24 h, median (IQR), mL | 4999.00 (3850.00-7000.00) | 4312.50 (2280.75-5867.50) | .05 |
| Vasopressors in 24 h | 67 (72.8) | 36 (48.6) | .002 |
| Outcome | |||
| 30-d mortality | 51 (55.4) | 26 (35.1) | .01 |
| 24-hour mortality | 33 (35.9) | 12 (16.2) | .008 |
| Multiple organ failure | 52 (56.5) | 57 (77.0) | .009 |
| Intensive care unit length of stay, median (IQR), d | 6 (1-12) | 8 (3-15) | .04 |
| Hospital length of stay, median (IQR), d | 8 (1-19) | 13 (6-20) | .05 |
| Ventilator duration, median (IQR), d | 4 (1-9) | 5 (2-11) | .12 |
Abbreviation: IQR, interquartile range.
To calculate P values for between-group comparisons, we performed the Pearson χ2 test for categorical variables, the Mann-Whitney U test for nonparametric, continuous variables with 2 or fewer groups, and the Kruskal-Wallis test for nonparametric, continuous variables with more than 2 groups.
We next evaluated 30-day mortality across patients with and without TBI using a generalized estimating equations model to account for trial cluster effects. The plasma and TBI association was statistically significant even after accounting for multiple confounders, including age, head AIS score, sex, Injury Severity Score, prehospital resuscitation fluid requirements, mechanism of injury, severe prehospital shock (systolic blood pressure <70 mm Hg), prehospital Glasgow Coma Scale (GCS) score, and transport time (eTable 3 and eTable 4 in Supplement 2).
We then performed Kaplan-Meier survival analysis comparing prehospital plasma patients vs standard care across subgroups with and without TBI (Figure 2). In the cohort of patients without TBI, there was no difference in 30-day survival across groups of the trial (HR, 0.80; 95% CI, 0.49-1.30; P = .38). In the cohort of patients with TBI, patients who received prehospital plasma had improved 30-day survival (HR, 0.52; 95% CI, 0.33-0.84; P = .006).
Figure 2. Unadjusted Kaplan-Meier Survival Analysis for 30-Day Survival Comparing Prehospital Plasma and Standard Care Patients With and Without Traumatic Brain Injury.
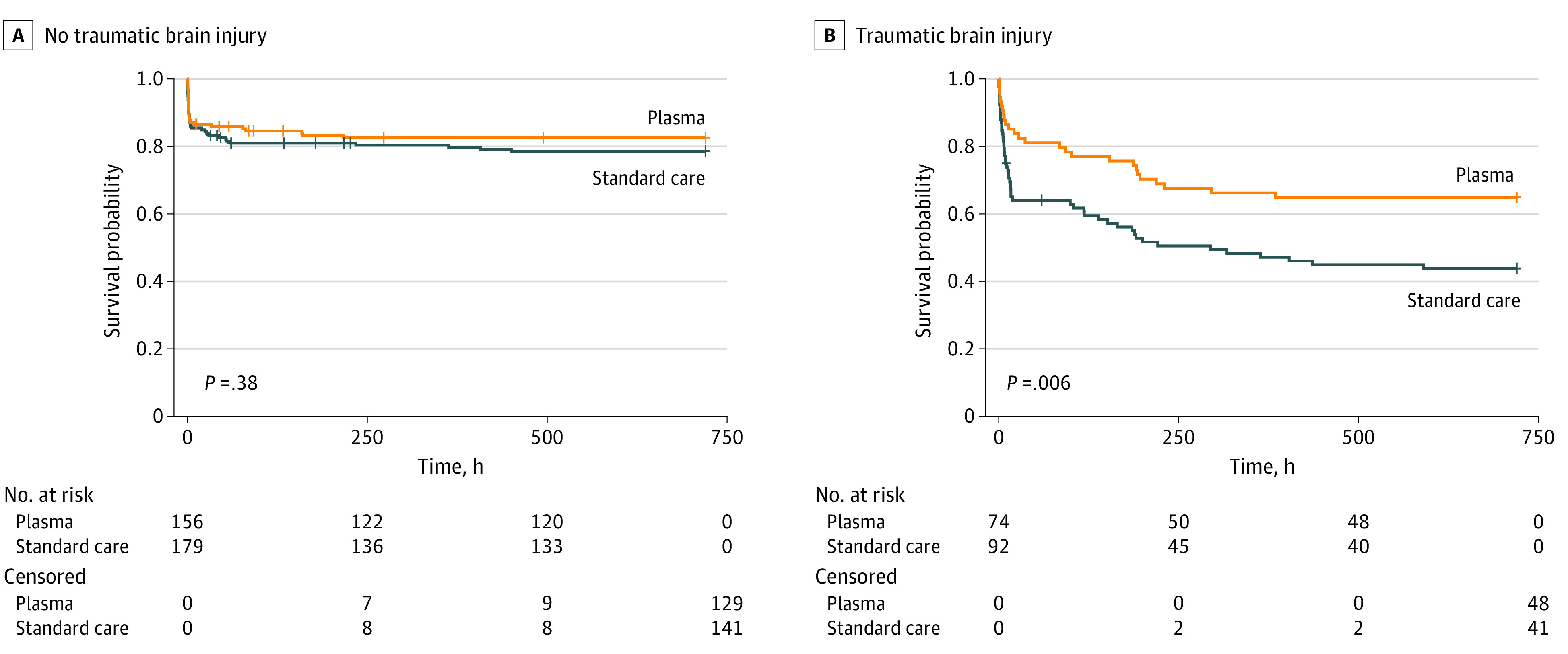
P values were calculated with log-rank testing.
After analysis of 30-day survival with the use of an adjusted Cox proportional hazard model, the association between survival and prehospital plasma in the TBI subgroup remained statistically significant (HR, 0.55; 95% CI, 0.33-0.94; P = .03), representing a 45% lower risk of mortality (eTable 5 in Supplement 2). When patients without TBI were similarly analyzed, no significant association with survival was found for the plasma variable (HR, 0.67; 95% CI, 0.39-1.14; P = .14).
We assayed blood samples from patients for key markers of TBI. GFAP and UCH-L1 levels were both associated with TBI at 0 and 24 hours from admission (eFigure 1 in Supplement 2), indicating their utility as diagnostic markers of TBI. However, there was no difference in GFAP or UCH-L1 levels across groups of the trial (eFigure 2 in Supplement 2), suggesting no underlying differences in brain injury across trial groups.
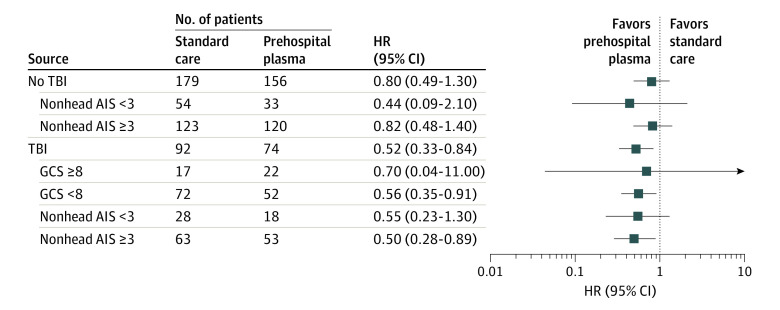
In an exploratory subgroup analysis of patients with TBI, we assessed whether initial neurological deficits or concomitant injury burden were associated with the survival benefit of plasma. We found that prehospital plasma was associated with the greatest survival benefit among patients with TBI with GCS score less than 8 (HR, 0.56; 95% CI, 0.35-0.91), for a 44% reduction in the risk of mortality, and among patients with TBI and polytrauma (HR 0.50, 95% CI 0.28-0.89), for a 50% reduction in the risk of mortality (Figure 3).
Figure 3. Hazard Ratios (HRs) for Each Subgroup Derived From a Cox Proportional Hazard Model.
HRs are reported for prehospital plasma compared with standard care. HRs less than or equal to 1 indicate a decreased risk of death; HRs greater than 1 indicate an increased risk of death. Traumatic brain injury (TBI) with mild to moderate traumatic injury in other body regions is defined by the presence of TBI and Abbreviated Injury Severity (AIS) score less than 3 in nonhead AIS categories (abdomen, chest, and extremity). Polytrauma with TBI is defined by the presence of TBI and AIS score of 3 or higher in the abdomen, chest, or extremity categories. GCS indicates Glasgow Coma Scale.
We also assessed the importance of transport origin as a proxy for the time between plasma administration and time of injury in the prehospital environment. In the cohort of patients transferred from an outside hospital, there was no difference in 30-day survival across groups of the trial (HR, 1.00; 95% CI, 0.33-3.00; P = .99) (eFigure 3 in Supplement 2). In the cohort of patients who were transported from the scene of injury, patients who received prehospital plasma had improved 30-day survival (HR, 0.45; 95% CI, 0.26-0.80; P = .005), suggesting that minimizing the time between injury and plasma administration is important.
Discussion
Damage-control resuscitation, including prioritization of the early administration of blood products, improves survival.8,9,33,34 Prehospital resuscitation with blood products may prevent downstream complications of traumatic injury8,35 and may have implications for mitigating the secondary injury following TBI.10 The results of this analysis demonstrate a strong survival benefit associated with prehospital plasma in patients with TBI. This was more robust after adjustment and demonstrated a 45% lower risk of mortality for patients who received prehospital plasma. This secondary analysis suggests that patients with TBI who are administered prehospital plasma have greater survival than the overall PAMPer cohort. A persistent and guiding tenet of TBI treatment is the prevention of hypotension and secondary brain injury.11,18,19,20,36 Although the underlying mechanisms for the survival benefit associated with plasma remain unknown, these findings suggest that early resuscitation with blood products including plasma may benefit patients with TBI and risk of hemorrhagic shock.
Improvements in TBI care have primarily focused on the in-hospital setting.37 Our results suggest that the benefit of interventions may be even more robust if delivered in the prehospital setting, as close to the time of injury as feasible. Early plasma resuscitation may act by attenuating endothelial damage and inflammation,13,14,15,38,39,40 systems affected by the complex molecular derangements following TBI. Evidence from 2 retrospective studies41,42 suggests a survival benefit associated with early in-hospital plasma administration among patients with TBI. The postulated benefits of plasma in patients with burn injuries include a restoration of intravascular volume and reduction of burn injury–associated endotheliopathy.43
The association between prehospital plasma and survival may depend on the severity of TBI and the presence of concomitant polytrauma. Preliminary data from an exploratory subgroup analysis suggest that patients with impaired neurological function (GCS score <8) who received prehospital plasma had an associated 44% reduction in the risk of mortality (HR, 0.56; 95% CI, 0.35-0.91). Our subgroup analysis also reveals that receipt of prehospital plasma among patients with TBI with polytrauma (concomitant injury burden) and the inherently higher risk of hemorrhage was associated with a 50% reduction in the risk of mortality (HR, 0.50; 95% CI, 0.28-0.89). However, these results are exploratory and should be assessed in a larger, more appropriately powered trial. These preliminary findings are nonetheless consistent with the hypothesis that prehospital plasma improves survival by minimizing the detrimental effects of shock and endothelial injury, as well as reducing the effects of secondary brain injury.44 During both prehospital and emergency department resuscitation, injured patients often receive large volumes of crystalloid fluid. Although this may improve perfusion, it may worsen inflammation, coagulopathy, endotheliopathy, and tissue oxygenation. Another benefit of early plasma administration may be a reduced reliance on crystalloid resuscitative fluid. Our exploratory analyses suggest that patients transported directly from the scene derived a greater benefit from prehospital plasma than those transferred from other hospitals. Origin of transport (from the scene or referral emergency department) may represent a surrogate for the interval between injury and plasma intervention. Although other confounding variables may contribute to these benefits of plasma, the greatest benefit was associated with plasma administration early after injury.
Mortality from both hemorrhage and TBI occurs early after injury but follows a different time course for each cause. Hemorrhage causes most early (<6 hours of hospital admission) deaths, after which the proportion of deaths due to TBI dramatically increases.45 The survival curves for the plasma and standard care groups of patients with TBI separate prior to 6 hours following hospital admission, indicating that plasma appears to have beneficial effects early in the pathophysiology of polytrauma with TBI.45 Although there was no apparent difference in thrombelastography parameters across groups, patients with TBI did have reduced international normalized ratio values, consistent with the overall study findings.8 Future studies are needed to determine the mechanisms behind this survival benefit. Nonetheless, the finding that improved survival is associated with prehospital plasma is a critical first step in optimizing resuscitation for hypotensive patients with TBI with severe injuries.
Circulating markers of brain injury (GFAP and UCH-L1) measured at hospital admission and 24 hours after admission corroborated the CT findings and are an objective means for TBI diagnostics, as previously reported.24 TBI marker concentrations also suggested no underlying brain injury differences across groups of the trial. This is the first analysis, to our knowledge, to use these biomarkers in this capacity.
In this analysis, patients without TBI did not have a plasma-associated survival benefit. Although it is possible that the benefit of plasma is only for patients with TBI, study limitations or differences between these groups may also contribute to this result. Patients with TBI had a greater incidence of polytrauma, greater injury severity, and overall greater mortality compared with patients without TBI. However, in our analysis, the plasma variable was significant even after accounting for differences in patient and injury characteristics.
Overall, prehospital plasma was associated with improved 30-day survival among patients with TBI. We observed a greater incidence of multiple organ failure and longer intensive care unit and hospital length of stay in the plasma group, suggesting that patients who have a survival benefit are also more likely to develop organ dysfunction or have a longer recovery time. This could result from adverse events associated with prehospital plasma administration. However, these findings may represent a survival bias. We interpret these results to mean that plasma reduced mortality for a group of unexpected survivors, patients who would have died had they not received this intervention.46 These patients may also require more aggressive postinjury care. Similarly, the observed association between prehospital packed red blood cells and mortality may be due to the fluid intervention. Alternatively, we hypothesize that more severely injured patients are more likely to receive prehospital packed red blood cells, consistent with the primary analysis.8
Limitations
Several limitations are inherent to a secondary analysis of a prospective, randomized clinical trial and should be considered in the interpretation of our results. First, the study was not blinded, and CT was used to diagnose TBI, factors that may result in unanticipated bias. Misclassification or misdiagnosis of TBI are potential limitations of this study. The limitations of sampling and variability in prehospital and in-hospital factors (treatment before the arrival of emergency medical services, prehospital times, and physician-level differences) also introduce bias. In addition, this study was not randomized across groups with and without TBI, and patients who received prehospital plasma also received less crystalloid fluid and blood component products. To address these issues, we adjusted for possible confounding variables across groups including patient and injury characteristics. We did not adjust for multiple comparisons. We are unable to determine whether procedures (eg, medication administration) or non-TBI factors (eg, hypotension or alcohol use) influenced variables in our analysis such as GCS score. However, we use the earliest (initial, prehospital) GCS scores as a proxy for brain injury severity. Our analysis may be underpowered to detect differences in small subgroups (eg, TBI with mild traumatic injury to other body regions) or to suggest that plasma has no beneficial effects for patients without TBI. Despite being smaller, the TBI subgroup had a greater survival benefit associated with prehospital plasma. The Cox proportional hazard model is adjusted for baseline covariates; however, there is a risk of residual imbalance and unmeasured confounding. There may be unknown differences across groups with and without TBI that are not accounted for in the current analysis. However, our biomarker results suggest that these findings do not result from major head injury differences. Although these markers did not differ across groups of the trial, this does not exclude potential differences in other unmeasured variables. In the primary trial, the time of injury was unable to be defined; thus, there may be variable time of randomization to time of injury. We quantified the time of randomization as a proxy for early or late prehospital plasma administration by transport origin and assume that there was a longer time interval if transferred from an outside hospital. During prehospital transport from the scene of an accident, without the benefit of CT imaging, we are unable to determine whether a patient has a brain injury. Although patients with TBI who received plasma have a higher frequency of multiple organ failure and longer length of stay, this may be due to survival bias. The application of these results may be limited by the fact that AIS score, Injury Severity Score, and head CT imaging are not available in the prehospital setting. Furthermore, this study was not designed to assess long-term functional neurological outcomes, although this may be an important area of future investigation.
Conclusions
Early administration of prehospital plasma to patients with TBI is associated with improved survival, particularly among those with polytrauma and risk of hemorrhagic shock. Future studies are needed to confirm the clinical benefits of early plasma resuscitation. Our results are exploratory, but the prehospital setting may be a critical time to intervene in the care of patients with TBI.
Trial Protocol
eAppendix. Supplemental Methods
eTable 1. Traumatic Brain Injury (TBI) Subtypes
eTable 2. Causes of Death for Patients With CT-Positive Traumatic Brain Injury (TBI)
eTable 3. Generalized Estimating Equations (GEE) Model Coefficients Without Adjustment for Confounders
eTable 4. Generalized Estimating Equations (GEE) Model Coefficients
eTable 5. Cox Proportional Hazard Model for 30-Day Mortality Among Patients With TBI
eFigure 1. TBI Biomarker Concentration in Patients With and Without TBI at 0, 24, and 72 Hours Post Admission
eFigure 2. TBI Biomarker Concentration Compared Across the Trial Arms at 0, 24, and 72 Hours Post Admission
eFigure 3. Unadjusted Kaplan Meier 30-Day Survival Analysis Comparing Plasma and Standard Care Arms Among Patients With TBI and Grouped by Transport Origin
eReferences
Data Sharing Protocol
References
- 1.Rhee P, Joseph B, Pandit V, et al. . Increasing trauma deaths in the United States. Ann Surg. 2014;260(1):13-21. doi: 10.1097/SLA.0000000000000600 [DOI] [PubMed] [Google Scholar]
- 2.Eastridge BJ, Hardin M, Cantrell J, et al. . Died of wounds on the battlefield: causation and implications for improving combat casualty care. J Trauma. 2011;71(1)(suppl):S4-S8. doi: 10.1097/TA.0b013e318221147b [DOI] [PubMed] [Google Scholar]
- 3.Galvagno SM Jr, Fox EE, Appana SN, et al. ; PROPPR Study Group . Outcomes after concomitant traumatic brain injury and hemorrhagic shock: a secondary analysis from the Pragmatic, Randomized Optimal Platelets and Plasma Ratios trial. J Trauma Acute Care Surg. 2017;83(4):668-674. doi: 10.1097/TA.0000000000001584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maas AIR, Menon DK, Adelson PD, et al. ; InTBIR Participants and Investigators . Traumatic brain injury: integrated approaches to improve prevention, clinical care, and research. Lancet Neurol. 2017;16(12):987-1048. doi: 10.1016/S1474-4422(17)30371-X [DOI] [PubMed] [Google Scholar]
- 5.Stocchetti N, Carbonara M, Citerio G, et al. . Severe traumatic brain injury: targeted management in the intensive care unit. Lancet Neurol. 2017;16(6):452-464. doi: 10.1016/S1474-4422(17)30118-7 [DOI] [PubMed] [Google Scholar]
- 6.Johnson WD, Griswold DP. Traumatic brain injury: a global challenge. Lancet Neurol. 2017;16(12):949-950. doi: 10.1016/S1474-4422(17)30362-9 [DOI] [PubMed] [Google Scholar]
- 7.Shackelford SA, Del Junco DJ, Powell-Dunford N, et al. . Association of prehospital blood product transfusion during medical evacuation of combat casualties in Afghanistan with acute and 30-day survival. JAMA. 2017;318(16):1581-1591. doi: 10.1001/jama.2017.15097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sperry JL, Guyette FX, Brown JB, et al. ; PAMPer Study Group . Prehospital plasma during air medical transport in trauma patients at risk for hemorrhagic shock. N Engl J Med. 2018;379(4):315-326. doi: 10.1056/NEJMoa1802345 [DOI] [PubMed] [Google Scholar]
- 9.Pusateri AE, Moore EE, Moore HB, et al. . Association of prehospital plasma transfusion with survival in trauma patients with hemorrhagic shock when transport times are longer than 20 minutes: a post hoc analysis of the PAMPer and COMBAT clinical trials. JAMA Surg. 2019;155(2):e195085. doi: 10.100/jamasurg.2019.5085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maegele M, Schöchl H, Menovsky T, et al. . Coagulopathy and haemorrhagic progression in traumatic brain injury: advances in mechanisms, diagnosis, and management. Lancet Neurol. 2017;16(8):630-647. doi: 10.1016/S1474-4422(17)30197-7 [DOI] [PubMed] [Google Scholar]
- 11.Spaite DW, Bobrow BJ, Keim SM, et al. . Association of statewide implementation of the prehospital traumatic brain injury treatment guidelines with patient survival following traumatic brain injury: the Excellence in Prehospital Injury Care (EPIC) Study. JAMA Surg. 2019;154(7):e191152. doi: 10.1001/jamasurg.2019.1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang R, Holcomb JB, Johansson PI, Pati S, Schreiber MA, Wade CE. Plasma resuscitation improved survival in a cecal ligation and puncture rat model of sepsis. Shock. 2018;49(1):53-61. doi: 10.1097/SHK.0000000000000918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pati S, Peng Z, Wataha K, Miyazawa B, Potter DR, Kozar RA. Lyophilized plasma attenuates vascular permeability, inflammation and lung injury in hemorrhagic shock. PLoS One. 2018;13(2):e0192363. doi: 10.1371/journal.pone.0192363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diebel LN, Martin JV, Liberati DM. Microfluidics: a high-throughput system for the assessment of the endotheliopathy of trauma and the effect of timing of plasma administration on ameliorating shock-associated endothelial dysfunction. J Trauma Acute Care Surg. 2018;84(4):575-582. doi: 10.1097/TA.0000000000001791 [DOI] [PubMed] [Google Scholar]
- 15.Barelli S, Alberio L. The role of plasma transfusion in massive bleeding: protecting the endothelial glycocalyx? Front Med (Lausanne). 2018;5:91. doi: 10.3389/fmed.2018.00091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Halaweish I, Bambakidis T, He W, et al. . Early resuscitation with fresh frozen plasma for traumatic brain injury combined with hemorrhagic shock improves neurologic recovery. J Am Coll Surg. 2015;220(5):809-819. doi: 10.1016/j.jamcollsurg.2015.01.057 [DOI] [PubMed] [Google Scholar]
- 17.Hernandez MC, Thiels CA, Aho JM, et al. . Prehospital plasma resuscitation associated with improved neurologic outcomes after traumatic brain injury. J Trauma Acute Care Surg. 2017;83(3):398-405. doi: 10.1097/TA.0000000000001581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spaite DW, Hu C, Bobrow BJ, et al. . Association of out-of-hospital hypotension depth and duration with traumatic brain injury mortality. Ann Emerg Med. 2017;70(4):522–530. doi: 10.1016/j.annemergmed.2017.03.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spaite DW, Hu C, Bobrow BJ, et al. . Mortality and prehospital blood pressure in patients with major traumatic brain injury: implications for the hypotension threshold. JAMA Surg. 2017;152(4):360-368. doi: 10.1001/jamasurg.2016.4686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spaite DW, Hu C, Bobrow BJ, et al. . The effect of combined out-of-hospital hypotension and hypoxia on mortality in major traumatic brain injury. Ann Emerg Med. 2017;69(1):62-72. doi: 10.1016/j.annemergmed.2016.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reitz KM, Moore HB, Guyette FX, et al. . Prehospital plasma in injured patients is associated with survival principally in blunt injury: results from two randomized prehospital plasma trials. J Trauma Acute Care Surg. 2020;88(1):33-41. doi: 10.1097/TA.0000000000002485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anto VP, Guyette FX, Brown J, et al. . Severity of hemorrhage and the survival benefit associated with plasma: results from a randomized prehospital plasma trial. J Trauma Acute Care Surg. 2020;88(1):141-147. doi: 10.1097/TA.0000000000002530 [DOI] [PubMed] [Google Scholar]
- 23.Guyette FX, Sperry JL, Peitzman AB, et al. . Prehospital blood product and crystalloid resuscitation in the severely injured patient: a secondary analysis of the prehospital air medical plasma trial. Ann Surg. Published online April 13, 2019. doi: 10.1097/SLA.0000000000003324 [DOI] [PubMed] [Google Scholar]
- 24.Bazarian JJ, Biberthaler P, Welch RD, et al. . Serum GFAP and UCH-L1 for prediction of absence of intracranial injuries on head CT (ALERT-TBI): a multicentre observational study. Lancet Neurol. 2018;17(9):782-789. doi: 10.1016/S1474-4422(18)30231-X [DOI] [PubMed] [Google Scholar]
- 25.Yue JK, Yuh EL, Korley FK, et al. ; TRACK-TBI Investigators . Association between plasma GFAP concentrations and MRI abnormalities in patients with CT-negative traumatic brain injury in the TRACK-TBI cohort: a prospective multicentre study. Lancet Neurol. 2019;18(10):953-961. doi: 10.1016/S1474-4422(19)30282-0 [DOI] [PubMed] [Google Scholar]
- 26.Halekoh U, Højsgaard S, Yan J. The R package geepack for generalized estimating equations. J Stat Softw. 2006;15(2):1–11. doi: 10.18637/jss.v015.i02 [DOI] [Google Scholar]
- 27.Kassambara A, Kosinski M Survminer: drawing survival curves using ggplot2. R package version 043. Published 2018. Accessed August 25, 2020. https://rpkgs.datanovia.com/survminer/index.html
- 28.Austin PC. A tutorial on multilevel survival analysis: methods, models and applications. Int Stat Rev. 2017;85(2):185-203. doi: 10.1111/insr.12214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Therneau TM. A package for survival analysis in R. Published 2015. Accessed August 25, 2020. https://cran.r-project.org/web/packages/survival/vignettes/survival.pdf
- 30.Harrell FE. rms: Regression modeling strategies. R package version 51-31. Published 2019. Accessed August 25, 2020. https://cran.r-project.org/web/packages/rms/index.html
- 31.R Core Team R: a language and environment for statistical computing. Published 2017. Accessed August 25, 2020. https://www.r-project.org/
- 32.Gruen DS. PAMPer TBI. Published 2019. Accessed August 25, 2020. https://github.com/dgru/pamper-tbi
- 33.Holcomb JB, Tilley BC, Baraniuk S, et al. . Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA. 2015;313(5):471-482. doi: 10.1001/jama.2015.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Etchill EW, Myers SP, McDaniel LM, et al. . Should all massively transfused patients be treated equally? an analysis of massive transfusion ratios in the nontrauma setting. Crit Care Med. 2017;45(8):1311-1316. doi: 10.1097/CCM.0000000000002498 [DOI] [PubMed] [Google Scholar]
- 35.Brown JB, Guyette FX, Neal MD, et al. . Taking the blood bank to the field: the design and rationale of the Prehospital Air Medical Plasma (PAMPer) Trial. Prehosp Emerg Care. 2015;19(3):343-350. doi: 10.3109/10903127.2014.995851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bratton SL, Chestnut RM, Ghajar J, et al. . Guidelines for the management of severe traumatic brain injury. IX. cerebral perfusion thresholds. J Neurotrauma. 2007;24(1)(suppl):S59-S64. doi: 10.1089/neu.2007.9987 [DOI] [PubMed] [Google Scholar]
- 37.The CRASH-3 Trial Collaborators Effects of tranexamic acid on death, disability, vascular occlusive events and other morbidities in patients with acute traumatic brain injury (CRASH-3): a randomised, placebo-controlled trial. Lancet. 2019;394(10210):1713-1723. doi: 10.1016/S0140-6736(19)32233-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kozar RA, Peng Z, Zhang R, et al. . Plasma restoration of endothelial glycocalyx in a rodent model of hemorrhagic shock. Anesth Analg. 2011;112(6):1289-1295. doi: 10.1213/ANE.0b013e318210385c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gonzalez Rodriguez E, Cardenas JC, Cox CS, et al. . Traumatic brain injury is associated with increased syndecan-1 shedding in severely injured patients. Scand J Trauma Resusc Emerg Med. 2018;26(1):102. doi: 10.1186/s13049-018-0565-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peng Z, Pati S, Potter D, et al. . Fresh frozen plasma lessens pulmonary endothelial inflammation and hyperpermeability after hemorrhagic shock and is associated with loss of syndecan 1. Shock. 2013;40(3):195-202. doi: 10.1097/SHK.0b013e31829f91fc [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jokar TO, Khalil M, Rhee P, et al. . Ratio-based resuscitation in trauma patients with traumatic brain injury: is there a similar effect? Am Surg. 2016;82(3):271-277. [PubMed] [Google Scholar]
- 42.Chang R, Folkerson LE, Sloan D, et al. . Early plasma transfusion is associated with improved survival after isolated traumatic brain injury in patients with multifocal intracranial hemorrhage. Surgery. 2017;161(2):538-545. doi: 10.1016/j.surg.2016.08.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gurney JM, Kozar RA, Cancio LC. Plasma for burn shock resuscitation: is it time to go back to the future? Transfusion. 2019;59(S2):1578-1586. doi: 10.1111/trf.15243 [DOI] [PubMed] [Google Scholar]
- 44.Gruen DS, Brown JB, Guyette FX, et al. . Prehospital plasma is associated with distinct biomarker expression following injury. JCI Insight. 2020;5(8):e135350. doi: 10.1172/jci.insight.135350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fox EE, Holcomb JB, Wade CE, Bulger EM, Tilley BC; PROPPR Study Group . Earlier endpoints are required for hemorrhagic shock trials among severely injured patients. Shock. 2017;47(5):567-573. doi: 10.1097/SHK.0000000000000788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gruen DS, Guyette FX, Brown JB, et al. . Characterization of unexpected survivors following a prehospital plasma randomized trial. J Trauma Acute Care Surg. Published online May 27, 2020. doi: 10.1097/TA.0000000000002816 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eAppendix. Supplemental Methods
eTable 1. Traumatic Brain Injury (TBI) Subtypes
eTable 2. Causes of Death for Patients With CT-Positive Traumatic Brain Injury (TBI)
eTable 3. Generalized Estimating Equations (GEE) Model Coefficients Without Adjustment for Confounders
eTable 4. Generalized Estimating Equations (GEE) Model Coefficients
eTable 5. Cox Proportional Hazard Model for 30-Day Mortality Among Patients With TBI
eFigure 1. TBI Biomarker Concentration in Patients With and Without TBI at 0, 24, and 72 Hours Post Admission
eFigure 2. TBI Biomarker Concentration Compared Across the Trial Arms at 0, 24, and 72 Hours Post Admission
eFigure 3. Unadjusted Kaplan Meier 30-Day Survival Analysis Comparing Plasma and Standard Care Arms Among Patients With TBI and Grouped by Transport Origin
eReferences
Data Sharing Protocol