Abstract
Simple Summary
In about half of the cases, endometrial stromal sarcomas lack the canonical oncogenic fusions JAZF1-SUZ12 or YWHAE-NUTM2, which are mutually exclusive. The aim of this study was to explore by RNA sequencing a retrospective series of uterine sarcomas diagnosed as endometrial stromal sarcomas but negative for JAZF1 and/or YWHAE rearrangement in FISH, in order to provide a better description of their molecular landscape, improve the classification of endometrial stromal sarcomas and provide guidance for disease management.
Abstract
A series of 42 patient tumors diagnosed as endometrial stromal sarcoma (ESS) based on the morphology but negative for JAZF1 and/or YWHAE rearrangement in FISH was analyzed by RNA-sequencing. A chromosomal rearrangement was identified in 31 (74%) of the cases and a missense mutation in known oncogenes/tumor suppressor genes in 11 (26%). Cluster analyses on the expression profiles from this series together with a control cohort composed of five samples of low grade ESS harboring a JAZF1-SUZ12 fusion, one high grade ESS harboring a BCOR-ITD, two uterine tumors resembling ovarian sex cord tumors, two samples each of uterine leiomyoma and leiomyosarcomas and a series of BCOR-rearranged family of tumor (n = 8) indicated that tumors could be gather in three distinct subgroups: one mainly composed of BCOR-rearranged samples that contained seven ESS samples, one mainly composed of JAZF1-fused ESS (n = 15) and the last composed of various molecular subtypes (n = 19). These three subgroups display different gene signatures, different in silico cell cycle scores and very different clinical presentations, natural history and survival (log-rank test, p = 0.004). While YWHAE-NUTM2 fusion genes may be present in both high and low grade ESS, the high-grade presents with additional BCOR or BCORL1 gene mutations. RNAseq brings clinically relevant molecular classification, enabling the reclassification of diseases and the guidance of therapeutic strategy.
Keywords: endometrial stromal sarcomas, RNA-sequencing, gene fusions, prognostic, uterine, sarcoma, expression profile
1. Introduction
Endometrial stromal sarcoma (ESS) is a rare disease accounting for less than 1% of all uterine tumors [1]. It represents the second most common type of uterine mesenchymal neoplasm, after leiomyosarcoma (LMS). ESS gathers a heterogeneous group of tumors with distinct clinical, histological and genetic characteristics. According to the 2014 WHO (World Health Organization) Classification [2], ESS is divided into three categories: low-grade ESS (LG-ESS), high-grade ESS (HG-ESS) and undifferentiated uterine sarcoma (UUS).
In addition to morphological and immunohistochemical data, the integration of molecular methods enables a more precise diagnosis and nosological classification. It may be important for guiding clinical management in entities with different natural histories. The current state of the art indicates that ESS is characterized by different types of recurrent genetic events, most commonly chromosomal translocations that frequently involve chromatin remodeling genes. The two most frequent gene fusions are JAZF1-SUZ12 [3], known to be associated with LG-ESS and favorable prognosis, and YWHAE-NUTM2 [4], known to be related to HG-ESS and high risk of rapid relapse and death. Adjuvant therapy is not recommended for JAZF1-fused LG-ESS, and in the case of advanced disease, aromatase-inhibitors (AIs) are the standard treatment [5]. Conversely, YWHAE-fused HG-ESS are considered as high risk tumors for which adjuvant chemotherapy and radiotherapy is often recommended, and in the case of advanced disease, they are considered to be insensitive to hormonal therapies, and cytotoxic chemotherapy is considered appropriate [6,7].
Grading and molecular classification are therefore of importance to classify ESS and decide on a treatment. In current routine practice, JAZF1-SUZ12 and YWHAE-NUTM2 fusion genes are screened by fluorescence in situ hybridization (FISH), which has limitations: (i) the most commonly used FISH test being a break-apart probe allows detection of only one of the rearranged genes and may induce false negatives; (ii) about half of ESS does not harbor a fusion of JAZF1 or YWHAE. Involvement of the PHF1 gene in chromosomal rearrangements targeting band 6p21 has been found in LG-ESS with different partners from JAZF1, EPC1, MEAF6 and BRD8 [8,9,10], and some other recurrent rearrangements have been reported involving BCOR [11,12,13], NCOA [14,15] or MBTD1 [16]. Recently, the expression of BCOR using IHC (Immunohistochemistry) was proposed as a criterion to classify HG-ESS carrying a BCOR rearrangement or a YWHAE-NUTM2 fusion [17].
In order to improve ESS classification and provide guidance for disease management, we characterized the molecular alteration of LG- and HG-ESS lacking canonical translocations, investigating a retrospective series of “FISH-negative” ESS using RNA-sequencing. We hypothesized that not only RNA sequencing would help in a better risk stratification of ESS but would also provide a better description of their molecular landscape by gathering their genetic abnormalities and specific expression profiles.
2. Results
2.1. Clinical and Pathological Features
This translational study was conducted on a retrospective series of 42 ESS negative for JAZF1 rearrangement (n = 22), YWHAE rearrangement (n = 11) or both (n = 9) (Figure S1). Patients and tumors characteristics are presented in Table 1 and Table S1.
Table 1.
Clinical and pathological characteristics of the investigational series of endometrial stromal sarcoma (ESS).
| Characteristics | N = 42 |
|---|---|
| Median Age (years); range | 53; 26–82 |
| Median size of the primary tumor (mm) | 90 |
| Pathological diagnosis (review) | |
| Low grade ESS | 26 |
| (Sex cord elements) | (4) |
| High grade ESS | 7 |
| Undifferentiated uterine sarcoma | 8 |
| No possibility of gradation * | 1 |
| FISH results | |
| JAZF1 negative (YWHAE not performed) | 22 |
| YWHAE negative (JAZF1 not performed) | 11 |
| Both JAZF1 and YWHAE negative | 9 |
| Stage at diagnosis | |
| Localized | 30 |
| Metastatic | 12 |
* One case initially diagnosed as “ESS with indefinite grade” because of unusual features for a low grade sarcoma (tumor necrosis, epithelioid cells, high cyclin D1 expression).
Using IHC, 62% (n = 26) demonstrated a nuclear positivity of ER (Estrogen receptors) and/or PR (Progesterone receptors) and 76% (n = 32) were positive for CD10. Overall, 26 (62%) were considered as LG-ESS using simple morphological classification including 4 (10%) with sex cord-like elements, 7 (16%) as HG-ESS and 8 (19%) as undifferentiated uterine sarcomas (UUS); one (2%) could not be graded. Median age at diagnosis was 53 years (26 to 82 years). Thirty-nine (93%) were located in the uterus (two primary ESS of the ovary and one of the peritoneum). Twelve patients (29%) were metastatic at diagnosis (FIGO stage IV), four LG, three HG and five UUS. The median overall survival (mOS) of the whole cohort was 11.3 years. For FIGO stage IV disease, treatments received by patients (and duration) are detailed in Table S2.
2.2. Molecular Classification Using RNA-Sequencing
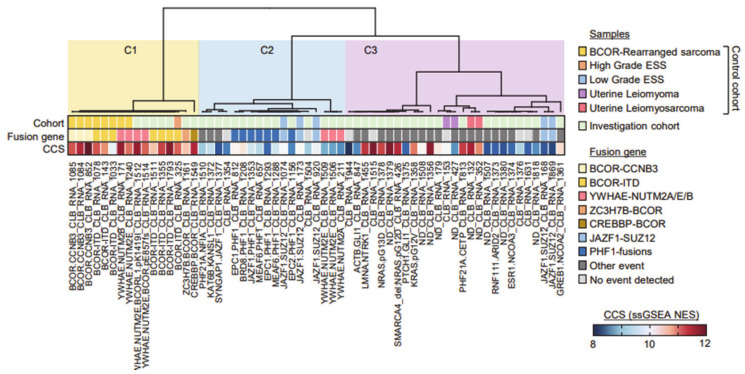
All but one RNA-sequencing successfully passed the quality controls (98%, n = 41). A chromosomal rearrangement was identified in 31 (74%) samples. The detected gene alterations are listed in Table S3. Briefly, an YWHAE-NUTM2E gene fusion was identified in 5 cases (12%), 10 samples (24%) carried a fusion involving a gene of the PHD family (PHF1, PHF21A or KAT6B), BCOR-rearrangements were observed in 5 (12%) samples, including 3 BCOR internal tandem duplications (BCOR-ITD), one ZC3H7B-BCOR fusion and a novel fusion, CREBBP-BCOR (Figure S2), and two (5%) samples carried a fusion involving genes of the NCOA family. In a single sample each, a SYNGAP1-JAZF1 and a new gene fusion involving a chromatin remodeling gene (RNF111-ARID2) were identified. In nine remaining samples, fusion genes could be identified but were either numerous, unrelated to the chromatin remodeling process and of unknown function or described in other neoplasia than ESS. Among the latter, two cases (5%) harbored a fusion implicating GLI1 relating them to the previously described “malignant epithelioid neoplasm with GLI1 gene rearrangements”, and one case (2%) harbored a LMNA-NTRK1 fusion corresponding to the previously described NTRK-fused uterine sarcomas [18]. Finally, in eight samples (19%) we could not detect any in-frame fusion gene. Of note, five (11%) cases harbored complex genomic profiles with numerous gene fusions and/or had mutations involving either TP53, KRAS, NRAS or BRAF, consistent with a diagnosis of UUS, and one case presented a deletion of SMARCA4 (together with a NRAS mutation), consistent with a SMARCA4-deficient undifferentiated uterine sarcoma. Of note, any time a rearrangement involving PHF1, YWHAE or JAZF1 was revealed by the RNAseq, a FISH (PHF1 or YWHAE or JAZF1) was performed in addition. The expression profiles of this series was matched to a control cohort of 20 recently diagnosed cases, including 5 samples of LG-ESS harboring a JAZF1-SUZ12 fusion, 1 HG-ESS harboring a BCOR-ITD, 2 uterine tumors resembling ovarian sex cord tumors (UTROSCT) and 2 samples each of uterine leiomyoma and LMS. Because of the similarity of their genetic alterations, we also included eight samples of soft-tissues and bone sarcomas carrying a BCOR-rearrangement (two pediatric undifferentiated round cell soft tissue sarcomas (BCOR-ITD), two pediatric undifferentiated round cell bone sarcomas (BCOR-CCNB3) and one pediatric brain tumor (BCOR-ITD)] or an YWHAE-NUTM2 fusion (two pediatric undifferentiated round cell bone sarcomas). Unsupervised consensus hierarchical clustering divided the samples into three subgroups (Figure 1 and Figure S3).
Figure 1.
Consensus hierarchical unsupervised clustering of expression profiles extracted from FFPE tumor samples. Consensus for k = 3 cluster is shown. Sample, tumor types and identified fusion genes are indicated. Single sample GSEA (Gene Set Enrichment Analysis) G2/M cell cycle score (CCS) for each samples is represented in a blue–red normalized scale.
Cluster C1 contained all the BCOR-rearranged sarcomas, and four samples carried a YWHAE-NUTM2 fusion (two ESS and two from the control cohort). Cluster C2 was composed of most JAZF1-fused and all PHF1-fused ESSs from both the control and investigation cohorts, together with three YWHAE-NUTM2 positive ESS samples. Cluster C3 grouped various molecular subtypes, including the NCOA-fused ESS, close to the cases of uterine LMS from the control cohort, and contained all the samples consistent with UUS.
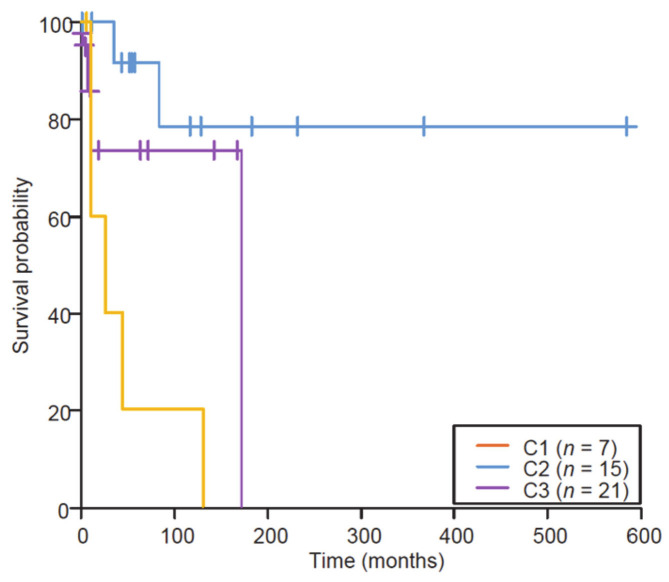
Evaluation of a cell cycle score (CCS), based on single sample GeneSet Enrichment Analyses (ssGSEA) on the hallmark G2/M MsigDB geneset, showed that C1 samples had a high CCS, C2 samples had a rather low CCS and the C3 cluster was mixed. These clustering data were significantly correlated to survival (Figure 2; log-rank test, p = 0.004).
Figure 2.
Kaplan–Meier survival analysis of the retrospective series of primary diagnosed ESS stratified by the clusters (log-rank, p = 0.004; Wilcoxon); x-axis, time in months. The three subgroups display different gene signatures and different in silico cell cycle scores that significantly correlated with different overall survivals.
Indeed, ESSs from the C1 group (high grade; HG) showed a more aggressive clinical behavior (mOS reached at 18 months), while ESSs from the C2 group (low grade; LG) were much more clinically indolent (mOS not reached), and C3 (intermediate grade; IG) showed clinically intermediate behaviors (mOS not reached). Amongst 12 cases that had synchronous metastasis, 3 clustered in the C1/HG group (43%, n = 3/7), and 8 clustered in the C3/IG group (38%, n = 8/21), but only one clustered in the C2/LG group (7%, n = 1/15), C2 tumors being less frequently metastatic at diagnosis (Fisher’s exact test, p = 0.03).
Finally, the proportion of ER and/or PR positive cases according to the cluster analysis was 2/7 (29%) in C1/HG, 14/14 (100%) in C2/LG and 12/21 (57%) in C3/IG (Fisher’s exact test, p = 0.0009).
Overall, as shown in Figure S4, the alteration detection coupled to expression profile clustering enabled reclassification of 12 cases (29%). Seven cases diagnosed as LG-ESS were reclassified either as HG-ESS with BCOR rearrangement (n = 2), malignant epithelioid neoplasm with GLI1 rearrangement (n = 2), NCOA fusion-positive uterine tumors (n = 2) or uterine leiomyoma (n = 1). Two cases diagnosed as HG-ESS were reclassified as UUS, and three cases diagnosed as UUS were reclassified either as HG-ESS (n = 1), NTRK fusion-positive uterine sarcomas (n = 1) or SMARCA4-deficient uterine sarcoma (n = 1). Finally, one case diagnosed as ESS without the possibility of gradation was classified as LG-ESS.
2.3. Differentially Expressed Genes and Pathways
Supervised analyses highlighted genes specifically expressed in the defined clusters (Figure S5A–E and Table S4). We identified a significant difference regarding genes involved in the cell cycle/proliferation pathways, morphogenesis/development, translation and spliceosome (upregulated in the C1 as compared to the C2 and C3) and genes involved in immunity (downregulated in the C1 as compared to the C2 and C3). Comparing C2 and C3, we also identified significant differences regarding genes involved in the cell cycle, inflammation and immunity (down-regulated in C2 as compared to C3) and regarding genes involved in neuron/synapse and translation (up-regulated in C2 as compared to C3).
We noticed that the WT1 gene was often absent or with low expression levels in the C1 tumors as compared as the C2/3 tumors (Figure S4E), and the level of expression of ESR1 was also significantly higher in the C2/3 than in the C1.
2.4. YWAHE-NUTM2B Fusion Are Not Uncommon in LG-ESS
Interestingly, the five cases harboring the YWAHE-NUTM2B fusion were divided by the clustering: two cases clustered as expected in the C1 group (HG), whereas three cases clustered in the C2 group (LG), with pure low-grade morphological features (Figure S6). To rule out the fusion gene being expressed by a small number of high-grade cells within low-grade cells, we first looked at the normalized number of reads covering the fusion point without finding differences between the HG and LG samples (around one supporting read per million of mapped reads in both groups). Second, we found at least 80% of the cells having the YWHAE rearrangement in FISH analyses. However, the analysis of the two HG showed two nucleotide variation events identified: one mutation in BCOR (CLB_RNA_1514: NM_017745: exon4:c.2570_2571del:p.E857fs; AAR = 0.28, validated by Sanger sequencing) (Figure S7), and one in BCORL1 (CLB_RNA_1512: NM_021946: exon7:c.A4256T:p.K1419I; AAR = 0.67).
The analysis of the differentially expressed genes/pathways between the two groups’ YWHAE-NUTM2B-postive cases (Figure S8 and Table S5) identified upregulated genes involved in cell cycle/proliferation pathways and mitochondria in the HG cases, while genes involved in immune response, inflammation, angiogenesis and the extracellular matrix were found downregulated in the HG cases. All LG YWHAE-NUTM2B-postive cases expressed high levels of ESR1, while it was not expressed in the HG cases (Figure S8C), a result that was further confirmed by IHC.
We then looked specifically to the medical records of those five patients. All three patients in the C2 group were alive without disease (21, 12 and 7 years after the diagnosis). Conversely both patients of the C1 group died of the disease 10 months after the diagnosis for the patient with initial stage 4 and 12 years after diagnosis (for the second patient who had a late relapse at 10 years).
2.5. BCOR Rearrangements Are Associated with a Poor Outcome
Unlike YWHAE-NUTM2 positive cases, all the BCOR-rearranged tumors clustered in the C1 group. Among the five cases of our series, we had clinical data for three patients who had all died of the disease, 10 months, 2 years and 3 years, respectively, after the diagnosis.
2.6. Immune Landscape
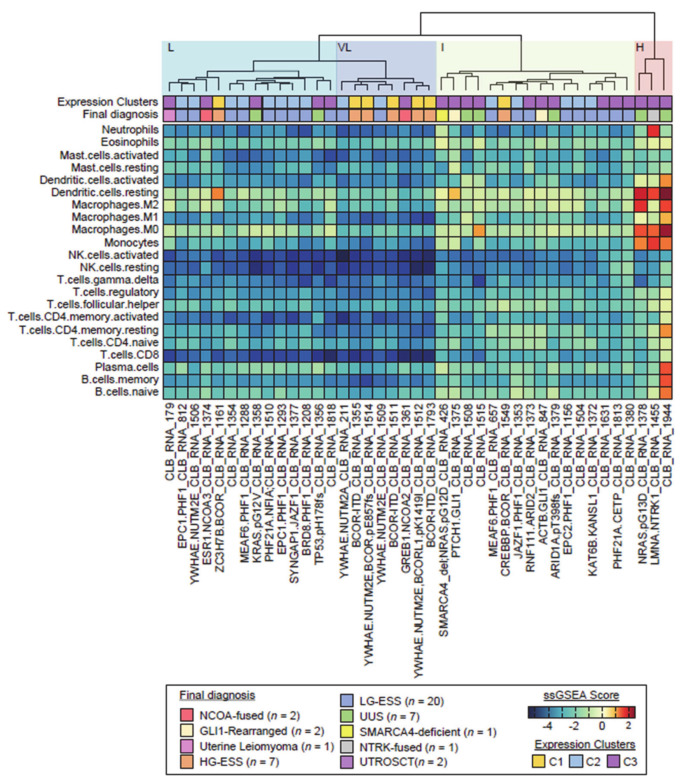
The 41 ESS samples were classified according to immune infiltrate: four main different groups were identified by cluster analysis (Figure 3).
Figure 3.
Hierarchical unsupervised clustering of the investigation cohort (n = 43) upon immune infiltrate population profiles. Cluster analyses generated one group with high (H) immune infiltrate and one group with low immune infiltrate subdivided in 3 slightly different subtypes: very low (VL), low (L) and intermediate (I). Clusters from Figure 1 and final diagnosis are also reported.
Importantly, a small subset of tumors (composed of a case of UUS that harbored a NRAS G13D mutation, a case of uterine sarcoma with LMNA-NTRK1 fusion and a case of UUS without relevant genomic alteration) stood out consistently from the other tumors. Those three tumors were highly infiltrated, mostly by macrophage immune cell types (subgroup H; high immune infiltrate). The other 38 tumors had limited or no immune cell infiltrates. Nevertheless, three slightly different subgroups could be distinguished among those tumors: VL (very low immune infiltrate), L (low immune infiltrate) and I (intermediate). The VL subgroup (n = 8; including four YWHAE-NUTM2 fusion, three BCOR-ITD and the GREB1-NCOA2 positive tumors) was characterized by a very low immune infiltrate for all cell types, while the L subgroup (n = 14; including most PHF1-fused tumors) harbored higher densities of macrophages, and the I subgroup I (n = 16; including 3 UUS and the two GLI1-rearranged neoplasm) was characterized by higher densities of CD4+ T cells and macrophages.
3. Discussion
In the present work, we analyzed ESS lacking the canonical fusion genes and described a novel classification of these rare sarcomas, which provides guidance to predict their prognosis and to guide treatments. ESS includes indeed a variety of different disease types with different genomic alterations and natural histories. RNA-sequencing identified cytogenetically negative ESS and enabled us to distinguish three biologically homogeneous risk groups by combining fusion gene discovery and expression profiling. The HG group is composed of ESS harboring a BCOR rearrangement, while the LG group is composed of ESS that harbors a fusion of a PRC2 zinc finger protein (e.g., JAZF1, PHF1). In addition, a third group considered as the IG group, close to the LMS of the control cohort, was composed of various molecular subtypes including NTRK fusion, SMARCA4 loss, NCOA fusion and other genetic abnormalities such as NRAS/KRAS mutations. Importantly, we showed that YWHAE-NUTM2 tumors are split in the HG and LG group.
Currently, the natural history prognosis and standard treatment of ESS depend on the histological grade of the tumor. According to the current WHO classification [19], YWHAE-fused ESS represents a clinically aggressive subtype of ESS classified as HG-ESS, distinct from the usual LG-ESS with JAZF1 rearrangement and from UUS with no identifiable molecular aberration. In detail, standard local treatment of LG-ESS is “en bloc” total hysterectomy (including bilateral salpingo-oophorectomy), and in cases of advanced disease, AIs are usually preferred. In the case of HG-ESS and UUS, previous clinical studies have shown that adjuvant radiotherapy and adjuvant chemotherapy might improve overall survival [20], and in cases of advanced disease, conventional systemic chemotherapy is recommended [7]. Considering the molecular classification using RNA sequencing, the LG group has a very good prognosis, and adjuvant therapy should not be considered while in case of advanced disease, AIs should remain the standard. Conversely, the HG group has a very poor prognosis, and as they grouped together with the BCOR–CCNB3 positive samples (so called “Ewing-like” sarcomas) from the control cohort, describing a very-well defined cluster, the therapeutic strategy should be discussed, both in the adjuvant and the metastatic setting, with potentially a more intensive multiagent chemotherapy regimen. The IG group shows an intermediate behavior, the role of AIs in advanced disease is much more uncertain than in the LG group, and RNAseq is crucial as it could guide the treatment (e.g., NTRK-fused uterine sarcomas). Finally, RNAseq is essential for the YWHAE-NUTM2 positive ESS in order to split the tumors in HG/LG and guide the therapeutic strategy.
The results presented here show that molecular analysis is therefore important to assess the prognosis and guide the clinical management. Indeed, 50% of ESSs do not harbor a JAZF1 and YWHAE rearrangement by FISH, which represents an insufficient examination for the characterization of these cancers. Given the heterogeneity of these cancers and the frequent absence of canonical mutations, RNAseq represents an efficient diagnostic tool in routine to identify accurately the different subtypes of ESS with completely opposite natural history and prognosis. Importantly, RNAseq also enabled the reclassification of a quarter of the ESS, such as a tumor initially classified as LG-ESS and finally corresponding to a ZC3H7B-BCOR HG-ESS (CLB_RNA_1161) and a tumor initially classified as HG-ESS and finally harboring a LMNA-NTRK fusion (CLB_RNA_1455). The first patient presented with a stage IV FIGO disease at diagnosis. She had a 90 mm uterine tumor, microscopically composed of ovoid cells with a myxoid background, low mitotic count, no tumor necrosis and no vascular invasion, strongly positive for CD10 and Cyclin D1, weakly positive for hormone receptors. She received an AI as a first line treatment. Unfortunately, she progressed rapidly and died in less than six months. With the benefit of hindsight, those ZC3H7B-BCOR ESSs are indeed known to be associated with abundant myxoid stroma and with aggressive clinical behavior. The second patient had a rapidly progressive disease after an anthracycline-based chemotherapy regimen and did not have time to enter an NTRK inhibitor trial or routine treatment. Similarly, UUS reclassified as SMARCA4-deficient uterine sarcoma (CLB_RNA_426), also called “malignant rhabdoid tumor of the uterus”, is a candidate to anti-PD1 in a clinical trial given the high response rates to immunotherapy in this entity (ClinicalTrials.gov Identifier: NCT03012620). In addition, a case initially diagnosed as LG-ESS was reclassified as leiomyoma (CLB_RNA_179). Microscopically, the tumor cells were strongly positive for Desmin, Cyclin D1 and WT1, moderately positive for ER/PR, weakly positive for SMA (smooth muscle actin) and negative for CD 10, and the expert pathologist concluded it was “ESS with smooth muscle cells differentiation”. It is well known that, by histopathological examination and IHC, the distinction between ESS with smooth muscle cell differentiation and highly cellular leiomyomas is challenging [21,22]. At any rate, RNAseq and clustering data helped us correct this diagnosis.
While Sumathi et al. [21] previously showed that diffuse WT-1 positivity is characteristic of endometrial stromal neoplasms and may be of value in diagnosis, we noticed that the WT1 gene is often absent or with low expression levels in HG tumors as compared to the others (Figure S4E), suggesting that WT-1 might be a prognosis factor in ESS. Of note, the level of expression of ESR1 was also significantly higher in the C2/3 than in the C1 cluster (HG), which is consistent with the known prognosis of ER in sarcomas [23]. Importantly, the YWHAE-NUTM2 fusion gene does not seem to be specific to HG-ESS. Aisagbonhi et al. [24] previously described a case of ESS with a typical morphology of low-grade (without high-grade areas) and a YWHAE rearrangement. Here, we demonstrate that (i) LG-ESS with YWHAE-NUTM2 fusions are not uncommon; (ii) they differ from their HG counterparts not only by the expression of genes involved in cell cycle/proliferation pathways and absence of mutation of the BCOR pathway, but also by those involved in the immune response or angiogenesis and (iii) they may be identified by ER positivity in IHC. Investigation of a larger cohort may confirm these conclusions. Expression data also identified some potentially new therapeutic targets, such as NTRK3 and the histone–lysine N-methyltransferase enzyme, EZH2, which has a higher expression in C1 cluster samples. Inhibition of HDAC (Histone deacetylases) therefore provides a very interesting approach to treatment for this subtype of sarcoma. Preclinical studies have previously shown that ESS may be sensitive to HDAC inhibition, and clinical data of the phase II trial of panobinostat in advanced sarcoma are consistent [25], even if only three ESSs were enrolled in the study. Moreover, a recent paper suggested that ESS with BCOR-rearrangement harbors MDM2 amplifications [26]. Among the four HG-ESS-BCOR of our series, only one (CLB_RNA_1549) carries a MDM2-CDK4 amplification (Figure S9).
Finally, while cases of the HG group show very low immune infiltrate for all tumor microenvironment cell types, some of the LG and IG group display moderate immune infiltrates with especially a significant presence of activated T cells (CD3+ CD8+) and regulatory T cells that might be of interest for immunotherapy approaches [27]. Of note, a trial of Pembrolizumab in rare subtypes of sarcomas is ongoing but included only few ESS (ClinicalTrials.gov Identifier: NCT03012620).
By showing the molecular landscape of fusions genes in ESS, RNAseq analysis also helps in understanding the biology and the mechanisms of tumorigenesis. The common altered genes identified in ESS are involved in chromatin remodeling, as SUZ12 belongs to the PRC2 complex while PHF1 promotes the recruitment of PRC2 to the chromatin. Polycomb Repressive Complexes (PRC) 1 and 2 are responsible for silencing the chromatin via histone methylation and ubiquitination. The JAZF1-SUZ12 fusion disrupts the PRC2 complex, preventing its binding, and thus impairs the chromatin repression of oncogenes such as HOXA9 [28]. BCOR is part of the non-canonical PRC1.1 complex that binds to BCL6 and acts as a corepressor. The precise mechanisms underlying the different BCOR-rearrangements are still unknown, but they possibly disrupt PRC1.1 complex formation [29]. The SYNGAP1-JAZF1 fusion may be linked to JAZF1-PHF1 fusion, as both partners are adjacent genes and that a PHF1-SYNGAP1 is a known readthrough. The CREBBP-BCOR fusion may be functionally related to the CREBBP-BCORL1 recently described in two cases of ossifying fibromyxoid tumors [16], a tumor type that shares fusion genes similar to those of ESS [30]. In three cases of “JAZF1-negative” ESS, we identified other genetic abnormalities involving genes related to chromatin remodeling. In one case (CLB_RNA_1818), we detected a single mutation in the Tet methyl cytosine dioxygenase 2 (TET2) gene. TET2 increases the activity of HDAC2 [31], and mutations of this gene have previously been described in other neoplasms such as AML (acute myeloid leukemia) with an unfavorable prognostic value [32]. In one case (CLB_RNA_1372), we detected a KAT6B-KANSL1 fusion that was previously described in two cases of leiomyomas [33,34]. KAT6B encodes a lysine acetyltransferase involved in histone tail modifications. A RNF111-ARID2 fusion was identified in one patient (CLB_RNA_1373). ARID2 is a subunit of the PBAF chromatin-remodeling complex, and mutations of this gene have been described in various neoplasms (hepatocellular carcinoma, melanoma). Altogether and amongst all tumors that we finally classified as ESS (see Table S3), only five samples remained without having any detectable alteration of a chromatin remodeling gene.
As a limitation of our study, it is important to mention that our study cohort included only five cases of YWHAE-NUTM2 positive ESS. In addition, without paired normal samples, we do not know whether the variations identified by RNAseq are somatic or even driver mutations. The few variations we reported here are not described in normal tissues and most are present in the COSMIC database [35]. Even so, we cannot definitely rule out private or germline variations, and it will require germline DNA to ascertain this point. Finally, our study did not include any validation cohort, and future studies of larger cohorts of uterine stromal sarcomas are needed to confirm and to expand upon our results and are currently ongoing in our center.
4. Materials and Methods
All patients signed informed consent to participate to research according to the French laws. Of note, an Institutional Review Board (The Centre Léon Bérard Clinical Trial Review Committee, Ethical code: N° CMT-2016-013) reviewed and agreed with the study protocol and the informed consent form on the 24th March 2016. The histological diagnosis and grading was established by an expert gynecologic pathologist according to the World Health Organization (WHO) Classification of Tumors [16]. Detailed methods are presented in the Supplementary Materials.
5. Conclusions
To our knowledge, our study represents the largest series of RNA-sequenced uterine stromal sarcomas. Altogether, ESSs represent a heterogeneous group of tumors, in terms of clinical behavior, pathology, genetics and immune infiltrate. FISH negative uterine stromal sarcomas present new and recurrent molecular variants of ESS, some of which are previously unreported. RNAseq also allowed the reclassification of a quarter of the tumors and helped in the identification of ESS with opposite natural history and prognosis, which is crucial for treatment decision. The functional similarities between the classical JAZF1-SUZ12 and the alternative transcripts suggest they activate an analogous oncogenic pathway. We also show that while BCOR-rearrangements seem always related to HG and poor prognosis, the detection of YWHAE-NUTM2B fusion is not sufficient to consider tumors as HG. Finally, RNA-seq is a powerful tool to enable nosological classification, identify target genes, predict natural history and guide treatment for patients with uterine sarcomas.
Supplementary Materials
The following are available online at https://www.mdpi.com/2072-6694/12/9/2604/s1, Figure S1: CONSORT diagram of investigational and control cohorts, Figure S2: Schematic of the previously unreported fusion gene CREBBP-BCOR, Figure S3: Consensus hierarchical unsupervised clustering of the investigation cohort only, Figure S4: Tumor reclassifications, Figure S5: Supervised analyses between clusters from Figure 1, Figure S6: Photomicrograph shows sheets or fascicles of ovoid to spindle cells with low mitotic count and no tumor necrosis, Figure S7: Schematic of the BCOR frameshift mutation in the CLB_RNA_1514 sample (HG YWHAE-NUTM2B), Figure S8: Supervised analyses between clusters 1 and 2 of the YWHAE-NUTM2-positive ESS samples, Figure S9: CGH array analysis of the CLB_RNA_1661 sample (HG ZC3H7B-BCOR), Table S1: Patients and tumors characteristics, Table S2: Systemic treatments received by patients in case of metastatic disease and duration of response (Progression free survival in months), Table S3: Details of the RNAseq fusion and mutation detection, Table S4: Differentially expressed genes between clusters, Table S5: Differentially expressed genes between high grade (C1 cluster) and low grade (C2 cluster) YWHAE-NUTM2-positive ESS.
Author Contributions
Conceptualization, J.-Y.B.; Data curation, T.F., D.P., P.M., M.K. and A.M.; Methodology, F.T.; Supervision, I.T., J.-Y.B. and F.T.; Validation, F.T.; Visualization, M.C., M.-P.S. and A.B.; Writing—original draft, M.B.; Writing—review and editing, I.R.-C., A.D., H.V., J.-Y.B. and F.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by NetSarc/RREPS/RESOS (INCA and DGOS) and LYRICAN (INCA-DGOS-INSERM 12563), Association DAM’s, Ensemble contre Le GIST, Eurosarc (FP7-278742), Fondation ARC, Infosarcome, InterSARC (INCA), LabEx DEvweCAN (ANR-10-LABX-0061), Ligue de l’Ain contre le Cancer Ligue contre le Cancer, EURACAN (EC 739521), Institut National du Cancer (grant INCa-PLBIO16-208).
Conflicts of Interest
The authors have declared that no conflict of interest exists.
References
- 1.Puliyath G., Nair M.K. Endometrial stromal sarcoma: A review of the literature. Indian J. Med. Paediatr. Oncol. 2012;33:1–6. doi: 10.4103/0971-5851.96960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jo V.Y., Fletcher C.D.M. WHO classification of soft tissue tumours: An update based on the 2013 (4th) edition. Pathology. 2014;46:95–104. doi: 10.1097/PAT.0000000000000050. [DOI] [PubMed] [Google Scholar]
- 3.Koontz J.I., Soreng A.L., Nucci M., Kuo F.C., Pauwels P., Van Den Berghe H., Dal Cin P., Fletcher J.A., Sklar J. Frequent fusion of the JAZF1 and JJAZ1 genes in endometrial stromal tumors. Proc. Natl. Acad. Sci. USA. 2001;98:6348–6353. doi: 10.1073/pnas.101132598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee C.-H., Ou W.-B., Mariño-Enriquez A., Zhu M., Mayeda M., Wang Y., Guo X., Brunner A.L., Amant F., French C.A., et al. 14-3-3 fusion oncogenes in high-grade endometrial stromal sarcoma. Proc. Natl. Acad. Sci. USA. 2012;109:929–934. doi: 10.1073/pnas.1115528109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amant F., Floquet A., Friedlander M., Kristensen G., Mahner S., Nam E.J., Powell M.A., Ray-Coquard I., Siddiqui N., Sykes P., et al. Gynecologic Cancer InterGroup (GCIG) consensus review for endometrial stromal sarcoma. Int. J. Gynecol. Cancer. 2014;24(Suppl. 3):S67–S72. doi: 10.1097/IGC.0000000000000205. [DOI] [PubMed] [Google Scholar]
- 6.Meurer M., Floquet A., Ray-Coquard I., Bertucci F., Auriche M., Cordoba A., Piperno-Neumann S., Salas S., Delannes M., Chevalier T., et al. Localized high grade endometrial stromal sarcoma and localized undifferentiated uterine sarcoma: A retrospective series of the French Sarcoma Group. Int. J. Gynecol. Cancer. 2019;29:691–698. doi: 10.1136/ijgc-2018-000064. [DOI] [PubMed] [Google Scholar]
- 7.Casali P.G., Abecassis N., Aro H.T., Bauer S., Biagini R., Bielack S., Bonvalot S., Boukovinas I., Bovee J.V.M.G., Brodowicz T., et al. Soft tissue and visceral sarcomas: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018;29(Suppl. 4):iv51–iv67. doi: 10.1093/annonc/mdy096. [DOI] [PubMed] [Google Scholar]
- 8.Micci F., Panagopoulos I., Bjerkehagen B., Heim S. Consistent rearrangement of chromosomal band 6p21 with generation of fusion genes JAZF1/PHF1 and EPC1/PHF1 in endometrial stromal sarcoma. Cancer Res. 2006;66:107–112. doi: 10.1158/0008-5472.CAN-05-2485. [DOI] [PubMed] [Google Scholar]
- 9.Micci F., Brunetti M., Dal Cin P., Nucci M.R., Gorunova L., Heim S., Panagopoulos I. Fusion of the genes BRD8 and PHF1 in endometrial stromal sarcoma. Genes Chromosomes Cancer. 2017;56:841–845. doi: 10.1002/gcc.22485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Micci F., Gorunova L., Gatius S., Matias-Guiu X., Davidson B., Heim S., Panagopoulos I. MEAF6/PHF1 is a recurrent gene fusion in endometrial stromal sarcoma. Cancer Lett. 2014;347:75–78. doi: 10.1016/j.canlet.2014.01.030. [DOI] [PubMed] [Google Scholar]
- 11.Lewis N., Soslow R.A., Delair D.F., Park K.J., Murali R., Hollmann T.J., Davidson B., Micci F., Panagopoulos I., Hoang L.N., et al. ZC3H7B-BCOR high-grade endometrial stromal sarcomas: A report of 17 cases of a newly defined entity. Mod. Pathol. 2018;31:674–684. doi: 10.1038/modpathol.2017.162. [DOI] [PubMed] [Google Scholar]
- 12.Mariño-Enriquez A., Lauria A., Przybyl J., Ng T.L., Kowalewska M., Debiec-Rychter M., Ganesan R., Sumathi V., George S., McCluggage W.G., et al. BCOR Internal Tandem Duplication in High-grade Uterine Sarcomas. Am. J. Surg. Pathol. 2018;42:335–341. doi: 10.1097/PAS.0000000000000993. [DOI] [PubMed] [Google Scholar]
- 13.Allen A.J., Ali S.M., Gowen K., Elvin J.A., Pejovic T. A recurrent endometrial stromal sarcoma harbors the novel fusion JAZF1-BCORL1. Gynecol. Oncol. Rep. 2017;20:51–53. doi: 10.1016/j.gore.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dickson B.C., Childs T.J., Colgan T.J., Sung Y.-S., Swanson D., Zhang L., Antonescu C.R. Uterine Tumor Resembling Ovarian Sex Cord Tumor: A Distinct Entity Characterized by Recurrent NCOA2/3 Gene Fusions. Am. J. Surg. Pathol. 2019;43:178–186. doi: 10.1097/PAS.0000000000001153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brunetti M., Panagopoulos I., Gorunova L., Davidson B., Heim S., Micci F. RNA-sequencing identifies novel GREB1-NCOA2 fusion gene in a uterine sarcoma with the chromosomal translocation t(2;8)(p25;q13) Genes Chromosomes Cancer. 2018;57:176–181. doi: 10.1002/gcc.22518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dewaele B., Przybyl J., Quattrone A., Finalet Ferreiro J., Vanspauwen V., Geerdens E., Gianfelici V., Kalender Z., Wozniak A., Moerman P., et al. Identification of a novel, recurrent MBTD1-CXorf67 fusion in low-grade endometrial stromal sarcoma. Int. J. Cancer. 2014;134:1112–1122. doi: 10.1002/ijc.28440. [DOI] [PubMed] [Google Scholar]
- 17.Chiang S., Lee C.-H., Stewart C.J.R., Oliva E., Hoang L.N., Ali R.H., Hensley M.L., Arias-Stella J.A., Frosina D., Jungbluth A.A., et al. BCOR is a robust diagnostic immunohistochemical marker of genetically diverse high-grade endometrial stromal sarcoma, including tumors exhibiting variant morphology. Mod. Pathol. 2017;30:1251–1261. doi: 10.1038/modpathol.2017.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chiang S., Cotzia P., Hyman D.M., Drilon A., Tap W.D., Zhang L., Hechtman J.F., Frosina D., Jungbluth A.A., Murali R., et al. NTRK Fusions Define a Novel Uterine Sarcoma Subtype With Features of Fibrosarcoma. Am. J. Surg. Pathol. 2018;42:791–798. doi: 10.1097/PAS.0000000000001055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Conklin C.M.J., Longacre T.A. Endometrial stromal tumors: The new WHO classification. Adv. Anat. Pathol. 2014;21:383–393. doi: 10.1097/PAP.0000000000000046. [DOI] [PubMed] [Google Scholar]
- 20.Pautier P., Nam E.J., Provencher D.M., Hamilton A.L., Mangili G., Siddiqui N.A., Westermann A.M., Reed N.S., Harter P. Ray-Coquard; Gynecologic Cancer InterGroup (GCIG) consensus review for high-grade undifferentiated sarcomas of the uterus. Int. J. Gynecol. Cancer. 2014;24(Suppl. 3):S73–S77. doi: 10.1097/IGC.0000000000000281. [DOI] [PubMed] [Google Scholar]
- 21.Sumathi V.P., Al-Hussaini M., Connolly L.E., Fullerton L., McCluggage W.G. Endometrial stromal neoplasms are immunoreactive with WT-1 antibody. Int. J. Gynecol. Pathol. 2004;23:241–247. doi: 10.1097/01.pgp.0000130051.04396.13. [DOI] [PubMed] [Google Scholar]
- 22.Pujani M., Jairajpuri Z.S., Rana S., Jetley S., Hassan M.J., Jain R. Cellular leiomyoma versus endometrial stromal tumor: A pathologists’ dilemma. J. Midlife Health. 2015;6:31–34. doi: 10.4103/0976-7800.153619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Valkov A., Sorbye S., Kilvaer T.K., Donnem T., Smeland E., Bremnes R.M., Busund L.-T. Estrogen receptor and progesterone receptor are prognostic factors in soft tissue sarcomas. Int. J. Oncol. 2011;38:1031–1040. doi: 10.3892/ijo.2011.920. [DOI] [PubMed] [Google Scholar]
- 24.Aisagbonhi O., Harrison B., Zhao L., Osgood R., Chebib I., Oliva E. YWHAE Rearrangement in a Purely Conventional Low-grade Endometrial Stromal Sarcoma that Transformed Over Time to High-grade Sarcoma: Importance of Molecular Testing. Int. J. Gynecol. Pathol. 2018;37:441–447. doi: 10.1097/PGP.0000000000000451. [DOI] [PubMed] [Google Scholar]
- 25.Cassier P.A., Lefranc A., Amela E.Y., Chevreau C., Bui B.N., Lecesne A., Ray-Coquard I., Chabaud S., Penel N., Berge Y., et al. A phase II trial of panobinostat in patients with advanced pretreated soft tissue sarcoma. A study from the French Sarcoma Group. Br. J. Cancer. 2013;109:909–914. doi: 10.1038/bjc.2013.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kommoss F.K., Chang K.T., Stichel D., Banito A., Jones D.T., Heilig C.E., Fröhling S., Sahm F., Stenzinger A., Hartmann W., et al. Endometrial stromal sarcomas with BCOR-rearrangement harbor MDM2 amplifications. [(accessed on 28 May 2020)];J. Pathol. Clin. Res. 2020 6:178–184. doi: 10.1002/cjp2.165. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/cjp2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keung E.Z., Burgess M., Salazar R., Parra Cuentas E., Rodrigues-Canales J., Bolejack V., Van Tine B.A., Schuetze S.M., Attia S., Riedel R.F., et al. Correlative Analyses of the SARC028 Trial Reveal an Association Between Sarcoma-Associated Immune Infiltrate and Response to Pembrolizumab. Clin. Cancer Res. 2020;26:1258–1266. doi: 10.1158/1078-0432.CCR-19-1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma X., Wang J., Wang J., Ma C.X., Gao X., Patriub V., Sklar J.L. The JAZF1-SUZ12 fusion protein disrupts PRC2 complexes and impairs chromatin repression during human endometrial stromal tumorogenesis. Oncotarget. 2017;8:4062–4078. doi: 10.18632/oncotarget.13270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Z., Gearhart M.D., Lee Y.-W., Kumar I., Ramazanov B., Zhang Y., Hernandez C., Lu A.Y., Neuenkirchen N., Deng J., et al. A Non-canonical BCOR-PRC1.1 Complex Represses Differentiation Programs in Human ESCs. Cell Stem Cell. 2018;22:235–251. doi: 10.1016/j.stem.2017.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Antonescu C.R., Sung Y.-S., Chen C.-L., Zhang L., Chen H.-W., Singer S., Agaram N.P., Sboner A., Fletcher C.D. Novel ZC3H7B-BCOR, MEAF6-PHF1, and EPC1-PHF1 fusions in ossifying fibromyxoid tumors—molecular characterization shows genetic overlap with endometrial stromal sarcoma. Genes Chromosomes Cancer. 2014;53:183–193. doi: 10.1002/gcc.22132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Q., Zhao K., Shen Q., Han Y., Gu Y., Li X., Zhao D., Liu Y., Wang C., Zhang X., et al. Tet2 is required to resolve inflammation by recruiting Hdac2 to specifically repress IL-6. Nature. 2015;525:389–393. doi: 10.1038/nature15252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gaidzik V.I., Paschka P., Späth D., Habdank M., Köhne C.-H., Germing U., von Lilienfeld-Toal M., Held G., Horst H.-A., Haase D., et al. TET2 mutations in acute myeloid leukemia (AML): Results from a comprehensive genetic and clinical analysis of the AML study group. J. Clin. Oncol. 2012;30:1350–1357. doi: 10.1200/JCO.2011.39.2886. [DOI] [PubMed] [Google Scholar]
- 33.Ainsworth A.J., Dashti N.K., Mounajjed T., Fritchie K.J., Davila J., Mopuri R., Jackson R.A., Halling K.C., Bakkum-Gamez J.N., Schoolmeester J.K. Leiomyoma with KAT6B-KANSL1 fusion: Case report of a rapidly enlarging uterine mass in a postmenopausal woman. Diagn. Pathol. 2019;14:32. doi: 10.1186/s13000-019-0809-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Panagopoulos I., Gorunova L., Bjerkehagen B., Heim S. Novel KAT6B-KANSL1 fusion gene identified by RNA sequencing in retroperitoneal leiomyoma with t(10;17)(q22;q21) PLoS ONE. 2015;10:e0117010. doi: 10.1371/journal.pone.0117010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tate J.G., Bamford S., Jubb H.C., Sondka Z., Beare D.M., Bindal N., Boutselakis H., Cole C.G., Creatore C., Dawson E., et al. COSMIC: The Catalogue of Somatic Mutations in Cancer. Nucleic Acids Res. 2019;47:D941–D947. doi: 10.1093/nar/gky1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.