Abstract
Vitamin D3 is the precursor of 1α,25-dihydroxyvitamin D3 (1,25(OH)2D3), a pleiotropic hormone that is a major regulator of the human genome. 1,25(OH)2D3 modulates the phenotype and physiology of many cell types by controlling the expression of hundreds of genes in a tissue- and cell-specific fashion. Vitamin D deficiency is common among cancer patients and numerous studies have reported that 1,25(OH)2D3 promotes the differentiation of a wide panel of cultured carcinoma cells, frequently associated with a reduction in cell proliferation and survival. A major mechanism of this action is inhibition of the epithelial–mesenchymal transition, which in turn is largely based on antagonism of the Wnt/β-catenin, TGF-β and EGF signaling pathways. In addition, 1,25(OH)2D3 controls the gene expression profile and phenotype of cancer-associated fibroblasts (CAFs), which are important players in the tumorigenic process. Moreover, recent data suggest a regulatory role of 1,25(OH)2D3 in the biology of normal and cancer stem cells (CSCs). Here, we revise the current knowledge of the molecular and genetic basis of the regulation by 1,25(OH)2D3 of the differentiation and stemness of human carcinoma cells, CAFs and CSCs. These effects support a homeostatic non-cytotoxic anticancer action of 1,25(OH)2D3 based on reprogramming of the phenotype of several cell types.
Keywords: vitamin D, cell differentiation, stemness, cancer, carcinoma cells, cancer-associated fibroblasts, cancer stem cells, organoids, epithelial-mesenchymal transition, Wnt/β-catenin
1. Introduction
Vitamin D3, or cholecalciferol, is a secosteroid molecule synthesized in the human skin by the action of solar UV-B light on 7-dehydrocholesterol. A second, limited source (around 10%) of vitamin D3 in the human organism is the diet, particularly fatty fish such as salmon, herring and sardines. Cholecalciferol is biologically inert. It enters the bloodstream and is hydroxylated at position C-25 in the liver by cytochrome CYP2R1 to render 25-hydroxyvitamin D3 (25(OH)D3, calcidiol or calcifediol). 25(OH)D3 is then subjected to another hydroxylation at position C-1 by cytochrome CYP27B1, mainly by kidney tubular cells and also by several types of epithelial and immune cells, to form 1α,25-dihydroxyvitamin D3 (1,25(OH)2D3, calcitriol) [1,2,3]. Both 25(OH)D3 and 1,25(OH)2D3 bind to the vitamin D receptor (VDR) protein, one of the 48 members of the ligand-activated transcription factor superfamily of nuclear hormone receptors. Although some controversy exists, 1,25(OH)2D3 appears to bind VDR with higher affinity and efficacy in terms of gene regulation than 25(OH)D3. Ligand binding promotes the formation of heterodimers between VDR and RXR, the receptor for 9-cis-retinoic acid, and the binding of these VDR-RXR heterodimers to DNA [1,2,3].
Chromatin immunoprecipitation sequencing assays have revealed the presence of around ten thousand VDR DNA binding sites in the human genome [4]. They are distributed throughout the whole genome. A subset is located close to the transcription initiation site of the genes that are directly regulated by 1,25(OH)2D3, but in many cases they are located upstream of the transcription initiation site or in introns, in the coding sequence, or downstream target genes. In line with the high number of VDR DNA binding sites, 1,25(OH)2D3 regulates the expression of hundreds of genes that vary between tissues, cell types and context [5,6]. Accordingly, 1,25(OH)2D3 displays a whole set of functions in the organism. The long-term knowledge of the association of vitamin D deficiency with rickets in children and osteomalacia in adults led to the consideration of calcium and phosphate metabolism and bone biology as the main roles of vitamin D in humans. However, during evolution, VDR was probably involved first in energy metabolism and defense against infections, and 1,25(OH)2D3 is today considered an important multifaceted regulator of the immune system [7].
1,25(OH)2D3 was initially linked to cancer in 1981, when the groups of D. Feldman and T. Suda reported its effect of inhibiting the proliferation of cultured human melanoma cells and inducing the differentiation of mouse myeloid leukemic cells, respectively [8,9]. During the last four decades, many studies have confirmed that 1,25(OH)2D3 attenuates the proliferation rate of many types of cancer cells, usually in association with a differentiation-inducing effect. In recent years, it has been shown that 1,25(OH)2D3 regulates the gene expression profile and phenotype of stromal fibroblasts present in the tumor microenvironment. Moreover, several studies have indicated that 1,25(OH)2D3 modulates cell stemness in a few cancer systems. Here, we review this set of actions, positing 1,25(OH)2D3 as a crucial regulator of homeostasis in the organism and as a candidate for non-cytotoxic anticancer therapies based on the regulation of cancer cell differentiation and stemness.
2. Effects of 1,25(OH)2D3 on Cancer Cell Differentiation
2.1. Carcinomas: The Epithelial–Mesenchymal Transition
Carcinomas are the most frequent (around 90%) type of solid cancer. They arise from epithelial cells that, in the early steps of the tumorigenic process, lose control of their proliferation and two features of their differentiated phenotype: (i) apical–basal polarity, which is the differential distribution of proteins and lipids at distinct cell surface areas that in this way are functionally diverse, and (ii) adhesiveness, which is the ability to bind strongly to neighboring epithelial cells and to the extracellular matrix (ECM) by means of a series of specialized cell adhesion structures. The loss of epithelial differentiation results from the acquisition of a cellular program called epithelial–mesenchymal transition (EMT). EMT involves drastic changes in the pattern of gene expression, triggered by a group of transcription factors (EMT-TFs: mainly SNAIL1, SNAIL2, ZEB1, ZEB2 and TWIST1) that cause repression of the epithelial phenotype and the induction of a mesenchymal state [10,11]. Thus, diverse epithelial cell surface proteins responsible for adhesiveness—including components of adherens junctions such as E-cadherin (considered a hallmark of the adhesive differentiated epithelial phenotype), tight junctions such as claudins and occludin, and desmosomes, as well as cytoskeletal components (cytokeratins), some ECM-binding integrins and polarity regulators (such as the Crumbs, Par and Scribble complexes)—are replaced by others typical of motile fibroblastic cells. These include N-cadherin, a distinct panel of integrins, vimentin and ECM-degrading metalloproteases (MMP). As a result, epithelial cells remodel their cytoskeletons, lose cell–cell and cell–ECM adhesion, change to a front–back polarity and acquire a fibroblastic-like phenotype with motility and basal membrane invasion capacities [11,12]. EMT provides tumor cells with other features of malignancy, such as stemness and diminished apoptosis, which causes resistance to cytotoxic chemo- and radiotherapies and to immunotherapy [11].
Thus, the appearance of a carcinoma involves an initial increased proliferation capacity and also the loss of the differentiated phenotype by epithelial cells that is mostly associated with the EMT program. The increased proliferation capacity gives rise to a mass of initially benign tumor cells growing at their original tissue location, whereas the loss of the differentiated phenotype gives these cells the ability to migrate (the first requirement for cancer dissemination and metastasis) and a higher survival capacity. The EMT process is activated by a variety of agents and signals that induce or activate the EMT-TFs such as transforming growth factor (TGF)-β, Wnt, Notch and ligands of several receptors with tyrosine kinase activity and cytokine receptors. These signals usually act cooperatively and in many cases are produced by stromal cells of the tumor microenvironment [11]. EMT is a reversible and usually a partial phenomenon. It does not affect all cells in a tumor and does not completely abrogate cell polarity and adhesiveness; thus, it generates intratumoral phenotypic heterogeneity. Later during tumorigenesis, EMT is reversed by the opposite process of mesenchymal–epithelial transition (MET). By restoring cell aggregation and binding to ECM, MET probably facilitates the survival of tumor cells during circulation in the bloodstream and the initial colonization of metastatic niches [10,11].
2.2. Effects of 1,25(OH)2D3 on the Differentiation of Carcinoma Cells
Many studies on the effects of 1,25(OH)2D3 on carcinoma cells have been performed in colon and breast cancer due to their high incidence and mortality and due to abundant reports pointing to these two neoplasias as those that are most commonly associated with vitamin D deficiency [13]. As in most cancer cell types, 1,25(OH)2D3 reduces proliferation at the cell cycle G0–G1 transition via the inhibition of retinoblastoma protein phosphorylation by cyclin/cyclin-dependent kinase (CDK) complexes, in part through the induction of CDK inhibitors and the repression of the c-MYC gene [1,2,3]. Usually, the inhibition of proliferation is accompanied by a reduction in cell survival due to sensitization to apoptotic stimuli, and both effects are linked to the induction of cell differentiation.
2.2.1. Colon Cancer
1,25(OH)2D3 Induces Epithelial Differentiation and Inhibits EMT
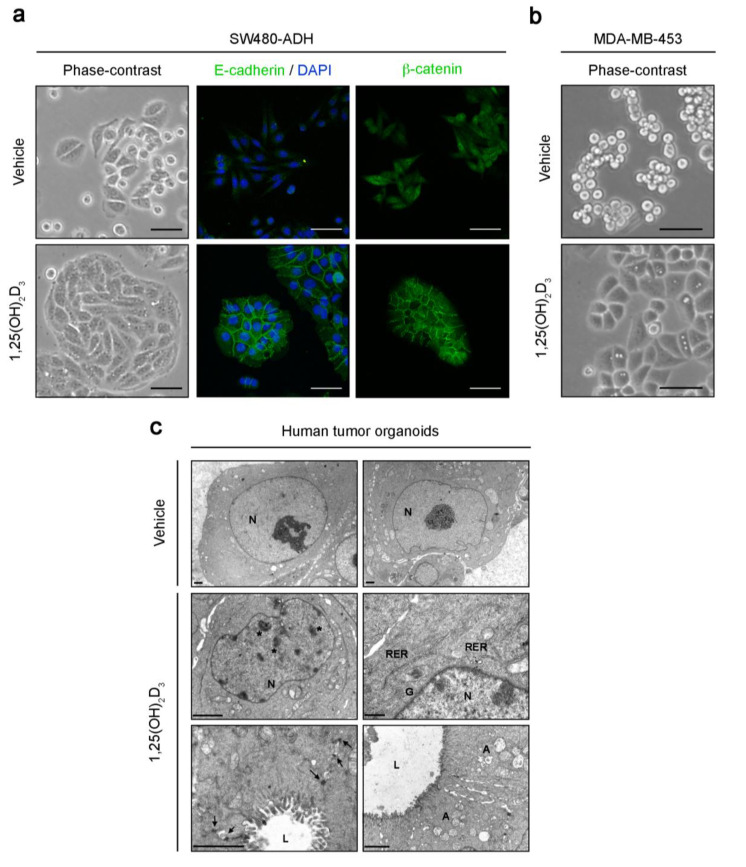
In line with its physiological role in the intestine, promoting the absorption of calcium and phosphate, the intestinal epithelium barrier function and xenobiotic metabolism, 1,25(OH)2D3 induces the differentiation of normal colon epithelial cells through the upregulation of many epithelial enzymes and markers and through maintaining the morphology typical of the epithelial differentiated phenotype [14,15]. Concordantly, in colon carcinoma cells 1,25(OH)2D3 induces a change in morphology that increases cell–cell adhesion and cell flattening (Figure 1a), which is paralleled by a decrease in proliferation. This effect is variably profound and directly related to the level of expression of VDR [16,17]. Immunofluorescence and global gene expression analyses showed that 1,25(OH)2D3 upregulates an array of intercellular adhesion molecules, including E-cadherin (Figure 1a), occludin, claudin-2 and -12, and zonula occludens/tight junction protein-1 and -2 [16,18]. 1,25(OH)2D3 induces and/or redistributes several cytokeratins, F-actin, vinculin, plectin, filamin A and paxillin, which modulate the actin cytoskeleton and the intermediate filament network, changing stress fibers and the ECM binding structures (focal adhesion contacts and hemidesmosomes) [16,17]. Thus, by controlling a large set of genes and proteins, 1,25(OH)2D3 increases cell–cell and cell–ECM adhesion (Figure 2). 1,25(OH)2D3 also induces expression of the calcium sensing receptor (CASR) that regulates calcium homeostasis and the differentiation of colon normal epithelium and carcinoma cells [19,20,21,22,23]. Curiously, 1,25(OH)2D3 has distinct effects on inhibitors of differentiation (ID)-1 and -2, two members of the ID family of proteins that control the differentiation, proliferation, migration and invasion of multiple cell types. Thus, in SW480-ADH human colon carcinoma cells, 1,25(OH)2D3 induces ID-1 but decreases ID-2 expression [24].
Figure 1.
Effects of 1,25(OH)2D3 on the phenotype of human colon and breast carcinoma cells and colon tumor organoids. (a) Phase-contrast and immunofluorescence confocal microscopy images of SW480-ADH human colon carcinoma cells treated with 100 nM 1,25(OH)2D3 or vehicle for 72 h. Scale bars, 50 μm. (b) Phase-contrast microscopy images of MDA-MB-453 human breast carcinoma cells treated with 100 nM 1,25(OH)2D3 or vehicle for 72 h. Scale bars, 50 μm. (c) Electron microscopy images of human colon tumor organoids treated with 100 nM 1,25(OH)2D3 or vehicle for 96 h. Scale bars, 2 μm. A, autophagic vacuoles; G, Golgi complexes; L, lumen; N, nucleus; RER, rough endoplasmic reticulum; arrows, desmosomes; asterisks, heterochromatin aggregates.
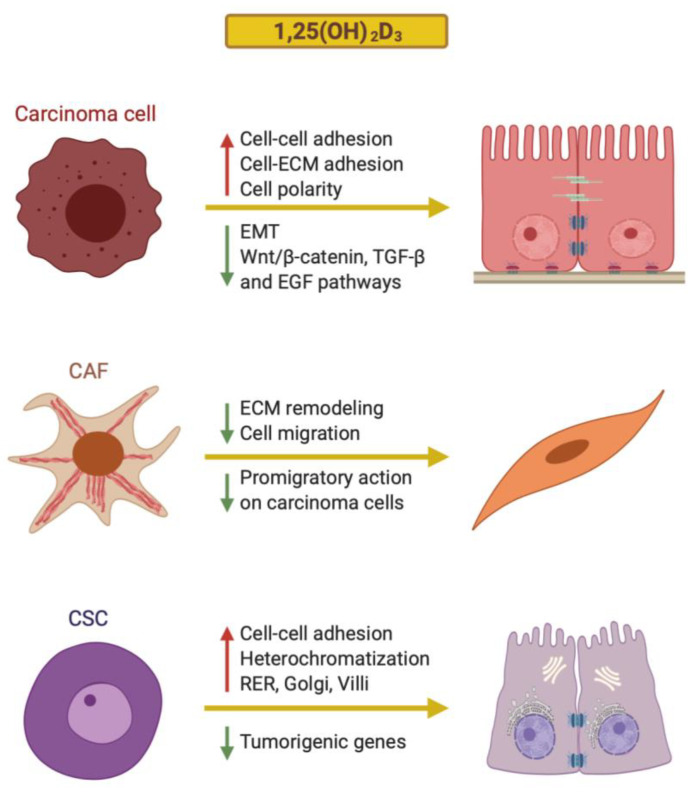
Figure 2.
Schematic representation of the mechanisms by which 1,25(OH)2D3 regulates the differentiation of human colon carcinoma cells, cancer-associated fibroblasts (CAFs) and cancer stem cells (CSCs).
An in-depth study revealed that the induction of the E-cadherin protein by 1,25(OH)2D3 depends on the activation of an extranuclear signaling pathway involving the entry of Ca2+ from the external medium into the cytosol and the cascade activation of the RhoA small GTPase and the kinases ROCK, p38MAPK and MSK1. The activation of this pathway potentiates transcription of the CDH1/E-cadherin gene promoted by ligand-activated VDR, leading to the accumulation of the E-cadherin protein at the adherens junctions, along with the enhancement of cell–cell adhesion [17]. In a separate study, the kinase PIP4K2B has been reported to be necessary for E-cadherin induction by 1,25(OH)2D3 in colon carcinoma cells [25].
TGF-β is a strong inhibitor of epithelial cell proliferation and a major inducer of EMT-TFs in many cell systems. 1,25(OH)2D3 inhibits the induction of EMT by TGF-β in normal epithelial cells, which prevents the downregulation of E-cadherin [26]. Notably, TGF-β signaling is inactivated by mutation of TGFBR2/TGF-β receptor type II in around 30% of colon cancers or, less frequently, by that of the signal transducers SMAD2 or SMAD4, which limits the EMT-promoting role of TGF-β in this neoplasia. However, Chen et al. reported that 1,25(OH)2D3 attenuates the induction of EMT by TGF-β in colon carcinoma cells and inhibits SNAIL1 and SNAIL2 expression, the E-cadherin/N-cadherin switch, and the secretion of MMP2 and MMP9 [27]. Likewise, 1,25(OH)2D3 reduces the induction of EMT by interleukin-1β via repression of the long noncoding RNA lncTCF7 [28].
Antagonism of the Wnt/β -Catenin Signaling Pathway by 1,25(OH)2D3
The Wnt/β-catenin signaling pathway is abnormally activated by mutations in APC, or less frequently in CTNNB1/β-catenin or other genes, in nearly all primary human colon tumors and their metastases [29,30]. In addition, autocrine or paracrine stimulation by Wnt factors and diminished expression of Wnt inhibitors, such as members of the dickkopf (DKK) and secreted frizzled-related protein families, may potentiate this pathway [31]. As a result of these alterations, β-catenin protein accumulates within the cell nucleus of colon epithelial cells and acts as a transcriptional regulator, forming complexes with members of the T-cell factor (TCF) family and activating a specific gene expression program that largely coincides with that of normal intestinal stem cells [32]. This leads to epithelial dedifferentiation, induction of EMT and acquisition of stemness. Thus, abnormal Wnt/β-catenin pathway activation is responsible for the initiation and probably also for the progression of colon cancer via the increase in colon epithelial cell survival and proliferation and the loss of the differentiated phenotype [31,32,33].
First in colon carcinoma cells and later in other types of cancer cells, 1,25(OH)2D3 has been shown to antagonize the activation of the Wnt/β-catenin pathway at several levels [34]. Ligand-activated nuclear VDR binds to the β-catenin protein, which hampers the formation of transcriptionally competent β-catenin-TCF complexes and thus blocks the expression of their target genes [16,35]. In addition, 1,25(OH)2D3 induces DKK-1 gene expression, which encodes an extracellular inhibitor of Wnt signaling that acts on Wnt receptor complexes at the cell surface [36]. Another mechanism of Wnt/β-catenin pathway inactivation by 1,25(OH)2D3 derives from increased accumulation of the E-cadherin protein, which, due to its high affinity, sequesters the newly synthesized cytosolic β-catenin protein at the subcortical/surface adherens junctions [16] (Figure 1a). However, this mechanism is non-essential, as 1,25(OH)2D3 inhibits the Wnt/β-catenin pathway even in cells that lack E-cadherin expression [16].
Other mechanisms of Wnt/β-catenin pathway deactivation by 1,25(OH)2D3 have been proposed: the induction of TCF4 in colon and breast carcinoma cells [37], and a paracrine action decreasing the production and release by stromal macrophages of interleukin-1β, a cytokine that inhibits the phosphorylation-mediated degradation of β-catenin protein and so increases β-catenin-TCF transcriptional activity in colon carcinoma cells [38]. In addition, in the non-malignant LT97 colon adenoma cell line, 1,25(OH)2D3 reduces the level of nuclear β-catenin and increases cellular differentiation [39].
The relevance of the vitamin D system for Wnt/β-catenin activity in colon cancer in vivo was first studied in animals. Larriba et al. [40] and Zheng et al. [41] reported that the absence of Vdr (Vdr-deficient mice) increases colonic tumor burden and the amount of nuclear β-catenin protein in colon cancer cells in the ApcMin/+ colon cancer mouse model. These findings support a role of VDR/1,25(OH)2D3 in repressing the Wnt/β-catenin pathway and the growth of intestinal tumors. More importantly, in a randomized, double-blinded, placebo-controlled clinical trial, Bostick et al. showed that supplementation with vitamin D3 increases the expression of E-cadherin and CASR, as well as other differentiation markers and potentially protective genes, in the healthy colon mucosa of patients with colorectal adenomas [42,43]. These authors also found an increase in the APC/β-catenin ratio in the colonic mucosa of the vitamin D3-supplemented group, which is compatible with an inhibitory effect of vitamin D3 on the Wnt/β-catenin pathway [44].
Other 1,25(OH)2D3 Target Genes Are Involved in Colon Cancer Cell Differentiation
Work by our group has led to the identification of several mediators of the prodifferentiation action of 1,25(OH)2D3 in colon carcinoma cells. These mediators include cystatin D, miR-22, KDM6B and Sprouty-2 (SPRY2).
Cystatin D is a multifunctional protein that acts as a cysteine protease inhibitor in the cytosol and extracellular region and as a transcriptional regulator within the cell nucleus [45]. The transcription of the CST5 gene, encoding cystatin D, is strongly induced by 1,25(OH)2D3 in colon carcinoma cells via direct binding of VDR to its promoter region [46]. Remarkably, cystatin D overexpression induces the expression of E-cadherin, occludin and other adhesion proteins, and represses that of several genes encoding EMT-TFs (SNAI1, SNAI2, ZEB1, ZEB2). Moreover, cystatin D antagonizes the Wnt/β-catenin pathway and inhibits colon carcinoma cell proliferation and migration [46].
In a microarray-based study, we identified several microRNAs (miRs) regulated by 1,25(OH)2D3 in SW480-ADH human colon carcinoma cells. One of these targets is miR-22, which is induced by 1,25(OH)2D3 and contributes to the effects of 1,25(OH)2D3 on gene expression and cell proliferation and migration [47]. Subsequently, other groups have reported that miR-22 inhibits EMT, invasiveness and tumor growth in colon cancer and a few other cancer systems [48,49].
KDM6B is an enzyme that demethylates di- and tri-methyl-lysine 27 on histone H3 (H3K27me2/3), an epigenetic mark that usually correlates with gene repression. Thus, KDM6B is expected to enable the activation of genes, although this histone demethylase appears to have additional transcriptional effects unrelated to histone demethylation. We found that 1,25(OH)2D3 upregulates the KDM6B gene by activating its promoter, and that KDM6B knockdown reduces the induction of E-cadherin and cell differentiation and the inhibition of β-catenin transcriptional activity promoted by 1,25(OH)2D3 in colon carcinoma cells [50]. KDM6B knockdown also upregulates SNAIL1 and ZEB1, the latter possibly through the decrease of miR-200b and miR-200c, and it downregulates the adhesion proteins E-cadherin, claudin-1 and -7 [50,51].
SPRY2 was found to be a gene that is strongly repressed by 1,25(OH)2D3 in a microarray analysis performed in SW480-ADH cells [18]. Its encoded protein is a modulator of tyrosine kinase receptor signaling, with receptor- and cell type-dependent inhibitory or enhancing effects. Thus, SPRY2 inhibits fibroblast growth factor signaling but potentiates the activation of RAS-ERK by epidermal growth factor (EGF). In human colon carcinoma cells, SPRY2 promotes EMT through the upregulation of ZEB1 and the downregulation of epithelial splicing regulator ESRP1. Consequently, SPRY2 represses CDH1/E-cadherin and genes encoding the tight junction proteins claudin-7 and occludin, as well as the important regulators of the polarized epithelial phenotype LLGL2, PATJ and ST14 [52,53]. SPRY2 expression is induced by β-catenin in cooperation with the transcription factor FOXO3a. Accordingly, it correlates with nuclear β-catenin and FOXO3a colocalization in human colon carcinomas and is indicative of poor prognosis [54]. In summary, our data indicate that repression of SPRY2 makes an important contribution to the prodifferentiation action of 1,25(OH)2D3.
Crosstalk between 1,25(OH)2D3 and EMT
The above data clearly indicate that 1,25(OH)2D3 promotes epithelial differentiation and inhibits EMT in colon carcinoma cells. Conversely, it has been shown that SNAIL1 and SNAIL2 repress VDR expression and abolish 1,25(OH)2D3 responsiveness [55,56,57]. Thus, 1,25(OH)2D3 and EMT are reciprocally downregulated and the balance between 1,25(OH)2D3 and EMT-inducing signals determines cell phenotype. Supporting this, VDR RNA expression correlates directly with differentiation and inversely with SNAI1 and SNAI2 RNA expression in human colon tumors [55,57,58,59,60]. In addition to the mechanisms described in previous sections, the mutual antagonism between 1,25(OH)2D3 and EMT is probably also a consequence of the multilevel cross-inhibition of 1,25(OH)2D3 and the EMT-inducing pathways Wnt/β-catenin, TGF-β and EGF [34,61,62] (Figure 2). For example, by repressing SPRY2 and inducing E-cadherin, which downregulates EGFR [63], 1,25(OH)2D3 may inhibit EGF signaling. In addition, cross-inhibition of VDR and EGFR has been described in colon carcinoma cells [64,65,66]. Similar functional crosstalk applies to 1,25(OH)2D3 and insulin-like growth factor (IGF)-I [61].
2.2.2. Breast Cancer
Breast cancer is a highly heterogeneous disease. Two key studies have respectively proposed five or ten molecular subtypes on the basis of a global gene expression study [67] or a more comprehensive genomic and transcriptomic analysis [68]. Analyzing the expression of estrogen receptor (ER), progesterone receptor (PR), and epidermal growth factor receptor 2 (ERBB2, NEU or HER2) is still the basis for breast cancer stratification and an important determinant of breast carcinoma cell phenotype. Cancers that lack or express very low levels of ER, PR and HER2 proteins (triple negative breast cancers or TNBC) have the poorest prognosis and, concordantly, ER- PR- HER2- cells are highly dedifferentiated (anaplastic). Interestingly, TNBC patients have very low 25(OH)D blood levels and some in vitro and clinical studies suggest the possibility of a protective effect of 1,25(OH)2D3 against TNBC [69].
1,25(OH)2D3 contributes to the normal development and function of the mammary gland [70]. Accordingly, VDR is expressed in normal breast tissue and in many, but not all, breast carcinoma cell lines and tumors [71,72,73,74]. Several excellent reviews have summarized the effects of 1,25(OH)2D3 on breast cancer cells, animal models and patients [70,75,76,77]. The study of 1,25(OH)2D3 effects in a panel of human breast carcinoma cell lines revealed that it induces a cell-specific change in morphology (Figure 1b) and gene expression. In some cases, 1,25(OH)2D3 alters the cytoarchitecture of actin filaments and microtubules and induces cytoplasmic extensions (filopodia and lamellipodia). It also increases adhesion to substrate by promoting the accumulation of focal adhesion kinase, paxillin and αv- and β5-integrins in focal adhesion plaques. Additionally, in several cell lines 1,25(OH)2D3 upregulates E-cadherin and represses the myoepithelial proteins P-cadherin, smooth muscle α-actin and α6- and β4-integrins [72,78]. Other studies have described the inhibition by 1,25(OH)2D3 of breast carcinoma cell migration, invasion and metastatic capacities via a reduction in the expression and/or the activity of N-cadherin, the ECM components tenascin C and periostin, several metalloproteases (MMP-1, MMP-9) and serine proteases (plasminogen activator), as well as the induction of their inhibitors [79,80,81,82,83,84]. In addition, upregulation of the actin cytoskeleton adaptor protein PDLIM2 is crucial for the pro-adhesive, antimigratory and anti-invasive actions of 1,25(OH)2D3 in breast cancer cells [85]. In contrast to its induction in colon carcinoma cells [24], 1,25(OH)2D3 suppresses the expression of ID-1 in human breast carcinoma cells and xenografted tumors, which conceivably contributes to promoting differentiation [86].
2.2.3. Other Solid Cancers
As in colon and breast cancers, 1,25(OH)2D3 induces cell differentiation, sensitizes to apoptosis and inhibits proliferation, migration and invasion in a series of other solid neoplasias. Again, its effects on differentiation are largely due to inhibition of the EMT and antagonism of the Wnt/β-catenin, EGF and TGF-β pathways.
The antagonism of the Wnt/β-catenin pathway by 1,25(OH)2D3 has been described in several solid cancers as a consequence of a variety of mechanisms: (1) by promoting the lysosomal degradation of LRP6, a member of the Wnt surface receptor complex, in pancreatic adenocarcinoma cells [87]; (2) via ligand-activated VDR-β-catenin interaction in renal carcinoma cells [88] and mouse skin tumorigenesis [89,90]; and (3) via ligand-activated VDR-β-catenin interaction and DKK-1 induction in Kaposi’s sarcoma cells [91]. The inhibition of the TGF-β pathway by 1,25(OH)2D3 and other VDR agonists and the subsequent repression of EMT-TFs and upregulation of epithelial markers have been reported in anaplastic thyroid cancer cells [92], ovarian cancer cells [93], lung adenocarcinoma cells [94] and renal carcinoma cells [88]. Likewise, VDR agonists repress EGF signaling in squamous cell carcinoma cells [95], epidermoid cells [96] and psoriatic keratinocytes [97].
Altogether, these effects show that 1,25(OH)2D3 is a strong, multifaceted promotor of human carcinoma cell differentiation. As discussed by Gocek and Studzinski [75], the prodifferentiation effect of 1,25(OH)2D3 on carcinoma cells does not result in the restoration of a complete normal epithelial phenotype, but it probably has an antitumor action due to the associated diminution of carcinoma cell proliferation; survival; and migratory, invasive and metastatic capacities.
2.3. Effects of 1,25(OH)2D3 on the Differentiation of Hematological Cancer Cells
As in solid cancers, vitamin D deficiency is common among patients with hematological malignancies, and 1,25(OH)2D3 and other VDR agonists inhibit the proliferation of leukemia, lymphoma and myeloma cells, as well as favoring their apoptosis upon cytotoxic treatments. A widely reported effect of 1,25(OH)2D3 in these cells is the inhibition of STAT-1 and STAT-3 signaling and the subsequent repression of a large number of cytokines [75,98]. However, the induction of differentiation seems to be a less important protective mechanism of 1,25(OH)2D3 in hematological malignancies than in solid cancers. Although VDR is expressed by all immune cell types, 1,25(OH)2D3 induces differentiation of myeloid leukemia cells almost exclusively [99,100]. Most results have been obtained in the acute myeloid leukemia (AML) cell lines HL60, THP-1 and U937. Thus, 1,25(OH)2D3 increases the expression of markers of the monocyte-macrophage phenotype, such as CD14 and some proteins involved in phagocytosis and adherence to the substratum, including CD11b [75,98]. A number of genes and proteins have been proposed as mediators of this prodifferentiation action of 1,25(OH)2D3, such as CEBPB and CDKN1A, which respectively encode the CCAAT enhancer binding protein β transcription factor and the p21CIP1 cyclin-dependent kinase inhibitor [101,102,103]. Other studies have proposed ERK, JNK, PI3K and PKC-α and -β as cytosolic mediators of these 1,25(OH)2D3 effects [104,105,106]. Notably, Muto et al. reported that 1,25(OH)2D3 induces differentiation of the retinoic acid-resistant acute promyelocytic leukemia UF-1 cell line, associated with the expression of the p21CIP1 and p27KIP1 cell cycle inhibitors [107]. Prodifferentiation effects of VDR agonists have also been reported in follicular non-Hodgkin’s lymphoma SU-DHL4 cells, with increased expression of mature B-cell markers [108].
3. Effects of 1,25(OH)2D3 on the Differentiation of Tumor Stromal Fibroblasts
Although the crucial importance of the tumor microenvironment during all stages of carcinogenesis is today widely accepted, studies on the action of 1,25(OH)2D3 on tumor stromal cells are scarce. Cancer-associated fibroblasts (CAFs) are the most abundant cell type in the tumor microenvironment. Recent data indicate that CAFs are a heterogeneous population of cells generated from diverse origins in response to signals secreted by tumor cells or by other cells of the tumor stroma. Thus, they can originate from the phenotypic change (activation) of resident fibroblasts, from the recruitment and activation of bone marrow-derived fibrocytes and mesenchymal stem cells, or from the transdifferentiation of other cell types (epithelial, endothelial or smooth muscle cells, adipocytes or pericytes) [109,110]. Usually, but not always, CAFs have tumor promoter effects that favor the malignancy of cancer cells by altering the ECM and secreting protumorigenic and drug-resistance factors [109,111].
Several strategies for CAF-directed anticancer therapy are possible [110]. Since CAF elimination unexpectedly rendered an acceleration of pancreatic cancer [112,113], the option of their deactivation or reprogramming to a less protumorigenic phenotype has become attractive. In this context, the VDR agonist calcipotriol has been reported to inhibit pancreatic stellate cell activation and differentiation into myofibroblasts, and thus to reduce inflammation and fibrosis in a pancreatitis mouse model and to enhance the efficacy of anticancer therapy in a pancreatic cancer model [114]. Likewise, calcipotriol reduces liver inflammation and fibrosis through the inhibition of hepatic stellate cell activation [115,116]. Accordingly, other protective effects of 1,25(OH)2D3 against fibrosis have been described and summarized elsewhere [62].
A global gene expression study, performed with human CAFs isolated from tumor biopsies of five breast cancer patients, identified 123 genes regulated by 1,25(OH)2D3 [117]. This gene signature reflects an antiproliferative and anti-inflammatory effect of 1,25(OH)2D3, as it includes the downregulation of the growth factor NRG1 and other genes with proliferation promotion effects, as well as the upregulation of DUSP1 (a phosphatase that inactivates MAPKs) and NFKBIA (an inhibitor of NFĸB). In paired normal fibroblasts (NFs), 1,25(OH)2D3 modulates the expression of 126 genes (55% of them are also regulated by 1,25(OH)2D3 in CAFs), including among the upregulated genes a few involved in antiproliferative, apoptosis and differentiation processes [117]. However, this study lacked functional analyses.
More recently, a study designed to characterize the effects of 1,25(OH)2D3 on colon cancer stromal fibroblasts rendered some interesting data [118]. First, the analysis of tumor biopsies from 658 patients showed that high VDR expression in CAFs is associated with better patient overall and progression-free survival, independently of the level of VDR expression in carcinoma cells. Second, a global gene expression analysis of seven primary cultures of CAFs and NFs established from colon cancer patient biopsies revealed that 1,25(OH)2D3 imposes in CAFs a 48-gene signature that correlates with longer patient survival in several colon cancer cohorts. Around one thousand genes are regulated by 1,25(OH)2D3 in CAFs and NFs, with a 21% overlap. The identified 1,25(OH)2D3 target genes are involved in cell adhesion, differentiation and migration; tissue remodeling; blood vessel development and inflammatory response. These genes encode mainly for ECM components and cytokines. Third, 1,25(OH)2D3 inhibits two protumoral properties in CAFs and in NFs: the paracrine promigratory action on carcinoma cells and the capacity to contract collagen gels, which is considered a hallmark of fibroblastic activation [118] (Figure 2). These results show that 1,25(OH)2D3 promotes profound reprogramming of the CAF gene expression profile, which leads to inhibition of their protumoral phenotype and contributes to protection against colon cancer. In addition, this study reveals that 1,25(OH)2D3 attenuates the malignant phenotype of colon carcinoma cells not only via a direct effect on these cells, but also indirectly through the de-activation of CAFs. Concordantly, the analysis of a large cohort of colon cancer patients indicated that the expression of VDR in CAFs and carcinoma cells has an additive protective effect, extending the overall survival of patients [118]. These findings are clinically relevant, as they indicate that colon cancer patients may benefit from an adequate vitamin D status or from treatment with VDR agonists, provided their CAFs express VDR, even though their carcinoma cells may be VDR-deficient, for instance due to SNAIL1 and/or SNAIL2 upregulation.
Remarkably, the regulation by 1,25(OH)2D3 of signaling molecules (cytokines, growth factors) secreted by CAFs suggests that it may also affect the biology of carcinoma cells and other cell types in the tumor microenvironment, such as immune and endothelial cells, in a paracrine manner. In line with these data, Kong et al. [119] reported that 1,25(OH)2D3 decreases the amount of miR-10a-5p found in the exosomes secreted by human pancreatic CAFs, which attenuates the promigratory and pro-invasive effects that these CAFs exert on pancreatic carcinoma cells. Notably, the possible interplay between 1,25(OH)2D3 and Wnt3A in colon fibroblasts has also been reported. Both agents are strong regulators of the gene expression profile and phenotype of these cells. However, in contrast to the antagonism reported in carcinoma cells, they have an additive and partially overlapping effect [120,121].
These results show that 1,25(OH)2D3 action extends to tumor stromal fibroblasts and that 1,25(OH)2D3 is an important regulator of CAF differentiation. Together with other findings suggesting anti-inflammatory and anti-angiogenic effects of 1,25(OH)2D3 at the level of immune and endothelial cells [122], they widen the prodifferentiation action of 1,25(OH)2D3 to cells of the tumor microenvironment, which may have important consequences on cancer development.
4. Effects of 1,25(OH)2D3 on Cancer Stem Cells
The concept of cancer stem cells (CSCs) states that tumors are initiated, progress and probably become resistant to therapies due to the accumulation of genetic and epigenetic alterations in tissue stem cells that become CSCs. This has led to investigations focused on identifying markers of undifferentiated stem cells in each tissue that could be used to isolate and specifically target CSCs with appropriate drugs or antibodies. However, although some relatively selective markers have been identified in a few cancer types, the idea of culturing or targeting tumor-specific CSCs has clashed with the finding of stem cell plasticity. This term refers to the dedifferentiation of cells at intermediate or even terminal differentiation stages, in order to restore the stem cell compartment following a lethal injury in normal tissues, or as a consequence of the acquisition of genetic alterations, and/or in response to signals from the tumor microenvironment in cancer [123,124,125]. Thus, stemness is today considered a usually transient cellular state that is lost in the process of differentiation. Differentiated cells can re-acquire stemness properties when the stem cell reservoir needs to be regenerated. Consequently, no stable CSCs seem to exist within tumors that can be isolated and studied as optimal targets for anticancer therapies [126].
Several studies have reported that VDR agonists inhibit the formation of floating spheroids called mammospheres (a feature attributed to CSCs) in breast cancer stem-like cells obtained from established cell lines. Interestingly, this effect could be overcome by β-catenin overexpression, which suggests that the inhibition of the Wnt/β-catenin pathway mediates this action of VDR agonists [127]. Likewise, in human triple negative and basal-like breast cancer cells, the 1,25(OH)2D3 analog BXL0124 reduces mammosphere-forming efficiency and downregulates the expression of stemness markers (OCT4, CD44) and Notch pathway genes (NOTCH1, JAG1/2) [128,129,130]. In addition, 1,25(OH)2D3 depleted the cancer stem-like cell subpopulation present in a human ovarian cancer cell line, thus reducing cell capacity to form spheres and initiate tumor formation. Additionally in this case, the main mechanism responsible for these effects of 1,25(OH)2D3 is antagonism of the Wnt/β-catenin pathway [131]. 1,25(OH)2D3 reduced sphere formation and the RNA expression of several stem cell markers (Cd44, Nanog, Oct4, Sox2, Klf4 and Abcg2) in a stem-like subpopulation of mouse malignant ovarian epithelial cells [132]. Similarly, 1,25(OH)2D3 downregulates NANOG and OCT4 in embryonal carcinoma and seminoma cells [133].
The above studies share the weakness of analyzing 1,25(OH)2D3 effects on cell populations which are enriched in stem cell characteristics but which are isolated from immortalized cell lines that have been growing in culture for a long time. Organoids clearly resemble the in vivo situation more closely, as they are three-dimensional structures generated by primary normal or cancer stem cells isolated from patients on culturing. Our group has recently described the effects of 1,25(OH)2D3 on human colon normal and tumor organoids, generated from biopsies of healthy and tumor tissue obtained from colon cancer patients [134]. Remarkably, 1,25(OH)2D3 induces cell differentiation in colon tumor organoids by changing their typical blastic cell appearance to a more epithelial differentiated phenotype that includes cell–cell adhesion structures, heterochromatin, villi, abundant rough endoplasmic reticulum, Golgi complexes and autophagic vacuoles [134] (Figure 1c). Global transcriptomic RNA-seq analysis revealed that 1,25(OH)2D3 promotes an enrichment in the colon differentiation signature EPHB2low vs. EPHB2high in colon tumor organoids, as well as the repression of genes involved in proliferation and tumorigenesis (Figure 2). However, unexpectedly, 1,25(OH)2D3 does not substantially modulate the expression of stemness or Wnt/β-catenin target genes. Contrarily, in colon normal organoids, 1,25(OH)2D3 upregulates several key stemness genes (LGR5, SMOC2, LRIG1, MSI1, PTK7 and MEX3A) and, concordantly, it does not affect the undifferentiated cell phenotype [134]. Importantly, recent RNA-seq analyses showed that 1,25(OH)2D3 has very similar effects on the global pattern of gene expression in colon and rectum normal organoids [135]. Likewise, the transcriptomic profiles induced by 1,25(OH)2D3 in colon and rectal tumor organoids are highly comparable [135]. As occurs in colon tissue [134], 1,25(OH)2D3 upregulates LGR5, LRIG1, SMOC2 and MSI1 stemness genes and downregulates the differentiation genes MUC2 and TFF2 in normal rectum organoids, but not in rectal tumor organoids, which indicates a homeostatic action of 1,25(OH)2D3 on the normal stem cell population in both intestinal areas (colon and rectum) [135]. Accordingly, studies by Augenlicht’s group have revealed that feeding mice with a low vitamin D3 and calcium diet or specific-inactivation of Vdr in Lgr5+ intestinal stem cells compromises stem cell properties and function, and thus alters the maturation of Lgr5+ progeny and intestinal homeostasis [136,137,138]. Interestingly, in human colon carcinoma cell lines, the miR-372/373 cluster, which is upregulated by the Wnt/β-catenin pathway and enhances stemness, has been found to downregulate VDR RNA and a panel of differentiation genes [139]. Together, these results indicate that 1,25(OH)2D3 exerts a homeostatic action in colon normal stem cells and a prodifferentiation effect on colon CSCs.
Recently, reduced VDR expression has been found to be associated with impaired myeloid progenitor differentiation and a poor prognostic factor in AML. The observed VDR repression is mainly due to gene promoter methylation, blocking differentiation and promoting self-renewal and proliferation in myeloid precursor cells. Accordingly, VDR agonists inhibit cell stemness in normal bone marrow and AML [140].
5. Conclusions
The active vitamin D metabolite 1,25(OH)2D3 and other synthetic VDR agonists are differentiation agents that enforce an epithelial state in carcinoma cells largely through the induction of key epithelial proteins and the inhibition of EMT. Work done predominantly in colon and breast cancer shows that the latter is mainly a consequence of the antagonism that these molecules exert on Wnt/β-catenin, TGF-β and EGF signaling pathways. Additionally, 1,25(OH)2D3 profoundly changes the gene expression profile of tumor stromal fibroblasts. It attenuates their activated phenotype and decreases their protumoral effects. It also exerts prodifferentiation actions on CSCs. Collectively, these data indicate that 1,25(OH)2D3, or VDR agonists in general, are candidates for cancer differentiation strategies.
Acknowledgments
We thank Miguel Lafarga for his continuous support and help with microscopy analyses, and Lucille Banham for her valuable assistance in the preparation of the English manuscript.
Funding
The work in the authors’ laboratory is funded by the Agencia Estatal de Investigación (PID2019-104867RB-I00/AEI/10.13039/501100011033), the Agencia Estatal de Investigación-Fondo Europeo de Desarrollo Regional (SAF2016-76377-R, MINECO/AEI/FEDER, EU), the Ministerio de Economía y Competitividad (SAF2017-90604-REDT/NuRCaMeIn), and the Instituto de Salud Carlos III-Fondo Europeo de Desarrollo Regional (CIBERONC; CB16/12/00273).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the writing of the manuscript or in the decision to publish it.
References
- 1.Fleet J.C., DeSmet M., Johnson R., Li Y. Vitamin D and cancer: A review of molecular mechanisms. Biochem. J. 2012;441:61–76. doi: 10.1042/BJ20110744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feldman D., Krishnan A.V., Swami S., Giovannucci E., Feldman B.J. The role of vitamin D in reducing cancer risk and progression. Nat. Rev. Cancer. 2014;14:342–357. doi: 10.1038/nrc3691. [DOI] [PubMed] [Google Scholar]
- 3.Christakos S., Dhawan P., Verstuyf A., Verlinden L., Carmeliet G. Vitamin D: Metabolism, Molecular Mechanism of Action, and Pleiotropic Effects. Physiol. Rev. 2016;96:365–408. doi: 10.1152/physrev.00014.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tuoresmaki P., Vaisanen S., Neme A., Heikkinen S., Carlberg C. Patterns of genome-wide VDR locations. PLoS ONE. 2014;9:e96105. doi: 10.1371/journal.pone.0096105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campbell M.J. Vitamin D and the RNA transcriptome: More than mRNA regulation. Front. Physiol. 2014;5:181. doi: 10.3389/fphys.2014.00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carlberg C., Munoz A. An update on vitamin D signaling and cancer. Semin. Cancer Biol. 2020 doi: 10.1016/j.semcancer.2020.05.018. in press. [DOI] [PubMed] [Google Scholar]
- 7.Hanel A., Carlberg C. Vitamin D and evolution: Pharmacologic implications. Biochem. Pharmacol. 2020;173:113595. doi: 10.1016/j.bcp.2019.07.024. [DOI] [PubMed] [Google Scholar]
- 8.Abe E., Miyaura C., Sakagami H., Takeda M., Konno K., Yamazaki T., Yoshiki S., Suda T. Differentiation of mouse myeloid leukemia cells induced by 1 alpha,25-dihydroxyvitamin D3. Proc. Natl. Acad. Sci. USA. 1981;78:4990–4994. doi: 10.1073/pnas.78.8.4990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colston K., Colston M.J., Feldman D. 1,25-dihydroxyvitamin D3 and malignant melanoma: The presence of receptors and inhibition of cell growth in culture. Endocrinology. 1981;108:1083–1086. doi: 10.1210/endo-108-3-1083. [DOI] [PubMed] [Google Scholar]
- 10.Nieto M.A. Context-specific roles of EMT programmes in cancer cell dissemination. Nat. Cell Biol. 2017;19:416–418. doi: 10.1038/ncb3520. [DOI] [PubMed] [Google Scholar]
- 11.Dongre A., Weinberg R.A. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat. Rev. Mol. Cell Biol. 2019;20:69–84. doi: 10.1038/s41580-018-0080-4. [DOI] [PubMed] [Google Scholar]
- 12.Yang J., Antin P., Berx G., Blanpain C., Brabletz T., Bronner M., Campbell K., Cano A., Casanova J., Christofori G., et al. Guidelines and definitions for research on epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2020;21:341–352. doi: 10.1038/s41580-020-0237-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grant W.B. Review of Recent Advances in Understanding the Role of Vitamin D in Reducing Cancer Risk: Breast, Colorectal, Prostate, and Overall Cancer. Anticancer Res. 2020;40:491–499. doi: 10.21873/anticanres.13977. [DOI] [PubMed] [Google Scholar]
- 14.Barbachano A., Fernandez-Barral A., Ferrer-Mayorga G., Costales-Carrera A., Larriba M.J., Munoz A. The endocrine vitamin D system in the gut. Mol. Cell. Endocrinol. 2017;453:79–87. doi: 10.1016/j.mce.2016.11.028. [DOI] [PubMed] [Google Scholar]
- 15.Christakos S., Li S., De La Cruz J., Shroyer N.F., Criss Z.K., Verzi M.P., Fleet J.C. Vitamin D and the intestine: Review and update. J. Steroid Biochem. Mol. Biol. 2020;196:105501. doi: 10.1016/j.jsbmb.2019.105501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palmer H.G., Gonzalez-Sancho J.M., Espada J., Berciano M.T., Puig I., Baulida J., Quintanilla M., Cano A., de Herreros A.G., Lafarga M., et al. Vitamin D(3) promotes the differentiation of colon carcinoma cells by the induction of E-cadherin and the inhibition of beta-catenin signaling. J. Cell Biol. 2001;154:369–387. doi: 10.1083/jcb.200102028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ordonez-Moran P., Larriba M.J., Palmer H.G., Valero R.A., Barbachano A., Dunach M., de Herreros A.G., Villalobos C., Berciano M.T., Lafarga M., et al. RhoA—ROCK and p38MAPK-MSK1 mediate vitamin D effects on gene expression, phenotype, and Wnt pathway in colon cancer cells. J. Cell Biol. 2008;183:697–710. doi: 10.1083/jcb.200803020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palmer H.G., Sanchez-Carbayo M., Ordonez-Moran P., Larriba M.J., Cordon-Cardo C., Munoz A. Genetic signatures of differentiation induced by 1alpha,25-dihydroxyvitamin D3 in human colon cancer cells. Cancer Res. 2003;63:7799–7806. [PubMed] [Google Scholar]
- 19.Fetahu I.S., Hummel D.M., Manhardt T., Aggarwal A., Baumgartner-Parzer S., Kallay E. Regulation of the calcium-sensing receptor expression by 1,25-dihydroxyvitamin D3, interleukin-6, and tumor necrosis factor alpha in colon cancer cells. J. Steroid Biochem. Mol. Biol. 2014;144:228–231. doi: 10.1016/j.jsbmb.2013.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aggarwal A., Hobaus J., Tennakoon S., Prinz-Wohlgenannt M., Graca J., Price S.A., Heffeter P., Berger W., Baumgartner-Parzer S., Kallay E. Active vitamin D potentiates the anti-neoplastic effects of calcium in the colon: A cross talk through the calcium-sensing receptor. J. Steroid Biochem. Mol. Biol. 2016;155:231–238. doi: 10.1016/j.jsbmb.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 21.Aggarwal A., Kallay E. Cross Talk between the Calcium-Sensing Receptor and the Vitamin D System in Prevention of Cancer. Front. Physiol. 2016;7:451. doi: 10.3389/fphys.2016.00451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tennakoon S., Aggarwal A., Kallay E. The calcium-sensing receptor and the hallmarks of cancer. Biochim. Biophys. Acta (BBA)-Bioenerg. 2016;1863:1398–1407. doi: 10.1016/j.bbamcr.2015.11.017. [DOI] [PubMed] [Google Scholar]
- 23.Yang W., Liu L., Masugi Y., Qian Z.R., Nishihara R., Keum N., Wu K., Smith-Warner S., Ma Y., Nowak J.A., et al. Calcium intake and risk of colorectal cancer according to expression status of calcium-sensing receptor (CASR) Gut. 2018;67:1475–1483. doi: 10.1136/gutjnl-2017-314163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fernandez-Garcia N.I., Palmer H.G., Garcia M., Gonzalez-Martin A., del Rio M., Barettino D., Volpert O., Munoz A., Jimenez B. 1alpha,25-Dihydroxyvitamin D3 regulates the expression of Id1 and Id2 genes and the angiogenic phenotype of human colon carcinoma cells. Oncogene. 2005;24:6533–6544. doi: 10.1038/sj.onc.1208801. [DOI] [PubMed] [Google Scholar]
- 25.Kouchi Z., Fujiwara Y., Yamaguchi H., Nakamura Y., Fukami K. Phosphatidylinositol 5-phosphate 4-kinase type II beta is required for vitamin D receptor-dependent E-cadherin expression in SW480 cells. Biochem. Biophys. Res. Commun. 2011;408:523–529. doi: 10.1016/j.bbrc.2011.04.045. [DOI] [PubMed] [Google Scholar]
- 26.Ricca C., Aillon A., Viano M., Bergandi L., Aldieri E., Silvagno F. Vitamin D inhibits the epithelial-mesenchymal transition by a negative feedback regulation of TGF-beta activity. J. Steroid Biochem. Mol. Biol. 2019;187:97–105. doi: 10.1016/j.jsbmb.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 27.Chen S., Zhu J., Zuo S., Ma J., Zhang J., Chen G., Wang X., Pan Y., Liu Y., Wang P. 1,25(OH)2D3 attenuates TGF-beta1/beta2-induced increased migration and invasion via inhibiting epithelial-mesenchymal transition in colon cancer cells. Biochem. Biophys. Res. Commun. 2015;468:130–135. doi: 10.1016/j.bbrc.2015.10.146. [DOI] [PubMed] [Google Scholar]
- 28.Li T., Zhu J., Zuo S., Chen S., Ma J., Ma Y., Guo S., Wang P., Liu Y. 1,25(OH)2D3 Attenuates IL-1beta-Induced Epithelial-to-Mesenchymal Transition Through Inhibiting the Expression of lncTCF7. Oncol. Res. 2019;27:739–750. doi: 10.3727/096504018X15360541345000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cancer Genome Atlas Network Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487:330–337. doi: 10.1038/nature11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yaeger R., Chatila W.K., Lipsyc M.D., Hechtman J.F., Cercek A., Sanchez-Vega F., Jayakumaran G., Middha S., Zehir A., Donoghue M.T.A., et al. Clinical Sequencing Defines the Genomic Landscape of Metastatic Colorectal Cancer. Cancer Cell. 2018;33:125–136.e123. doi: 10.1016/j.ccell.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nusse R., Clevers H. Wnt/beta-Catenin Signaling, Disease, and Emerging Therapeutic Modalities. Cell. 2017;169:985–999. doi: 10.1016/j.cell.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 32.Merlos-Suarez A., Barriga F.M., Jung P., Iglesias M., Cespedes M.V., Rossell D., Sevillano M., Hernando-Momblona X., da Silva-Diz V., Munoz P., et al. The intestinal stem cell signature identifies colorectal cancer stem cells and predicts disease relapse. Cell Stem Cell. 2011;8:511–524. doi: 10.1016/j.stem.2011.02.020. [DOI] [PubMed] [Google Scholar]
- 33.Krausova M., Korinek V. Wnt signaling in adult intestinal stem cells and cancer. Cell Signal. 2014;26:570–579. doi: 10.1016/j.cellsig.2013.11.032. [DOI] [PubMed] [Google Scholar]
- 34.Larriba M.J., Gonzalez-Sancho J.M., Barbachano A., Niell N., Ferrer-Mayorga G., Munoz A. Vitamin D Is a Multilevel Repressor of Wnt/b-Catenin Signaling in Cancer Cells. Cancers. 2013;5:1242–1260. doi: 10.3390/cancers5041242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shah S., Islam M.N., Dakshanamurthy S., Rizvi I., Rao M., Herrell R., Zinser G., Valrance M., Aranda A., Moras D., et al. The molecular basis of vitamin D receptor and beta-catenin crossregulation. Mol. Cell. 2006;21:799–809. doi: 10.1016/j.molcel.2006.01.037. [DOI] [PubMed] [Google Scholar]
- 36.Aguilera O., Pena C., Garcia J.M., Larriba M.J., Ordonez-Moran P., Navarro D., Barbachano A., Lopez de Silanes I., Ballestar E., Fraga M.F., et al. The Wnt antagonist DICKKOPF-1 gene is induced by 1alpha,25-dihydroxyvitamin D3 associated to the differentiation of human colon cancer cells. Carcinogenesis. 2007;28:1877–1884. doi: 10.1093/carcin/bgm094. [DOI] [PubMed] [Google Scholar]
- 37.Beildeck M.E., Islam M., Shah S., Welsh J., Byers S.W. Control of TCF-4 expression by VDR and vitamin D in the mouse mammary gland and colorectal cancer cell lines. PLoS ONE. 2009;4:e7872. doi: 10.1371/journal.pone.0007872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaler P., Augenlicht L., Klampfer L. Macrophage-derived IL-1beta stimulates Wnt signaling and growth of colon cancer cells: A crosstalk interrupted by vitamin D3. Oncogene. 2009;28:3892–3902. doi: 10.1038/onc.2009.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Groschel C., Aggarwal A., Tennakoon S., Hobaus J., Prinz-Wohlgenannt M., Marian B., Heffeter P., Berger W., Kallay E. Effect of 1,25-dihydroxyvitamin D3 on the Wnt pathway in non-malignant colonic cells. J. Steroid Biochem. Mol. Biol. 2016;155:224–230. doi: 10.1016/j.jsbmb.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 40.Larriba M.J., Ordonez-Moran P., Chicote I., Martin-Fernandez G., Puig I., Munoz A., Palmer H.G. Vitamin D receptor deficiency enhances Wnt/beta-catenin signaling and tumor burden in colon cancer. PLoS ONE. 2011;6:e23524. doi: 10.1371/journal.pone.0023524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zheng W., Wong K.E., Zhang Z., Dougherty U., Mustafi R., Kong J., Deb D.K., Zheng H., Bissonnette M., Li Y.C. Inactivation of the vitamin D receptor in APC(min/+) mice reveals a critical role for the vitamin D receptor in intestinal tumor growth. Int. J. Cancer. 2012;130:10–19. doi: 10.1002/ijc.25992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bostick R.M. Effects of supplemental vitamin D and calcium on normal colon tissue and circulating biomarkers of risk for colorectal neoplasms. J. Steroid Biochem. Mol. Biol. 2015;148:86–95. doi: 10.1016/j.jsbmb.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fedirko V., Bostick R.M., Flanders W.D., Long Q., Sidelnikov E., Shaukat A., Daniel C.R., Rutherford R.E., Woodard J.J. Effects of vitamin d and calcium on proliferation and differentiation in normal colon mucosa: A randomized clinical trial. Cancer Epidemiol. Biomark. Prev. 2009;18:2933–2941. doi: 10.1158/1055-9965.EPI-09-0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ahearn T.U., Shaukat A., Flanders W.D., Rutherford R.E., Bostick R.M. A randomized clinical trial of the effects of supplemental calcium and vitamin D3 on the APC/beta-catenin pathway in the normal mucosa of colorectal adenoma patients. Cancer Prev. Res. 2012;5:1247–1256. doi: 10.1158/1940-6207.CAPR-12-0292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ferrer-Mayorga G., Alvarez-Diaz S., Valle N., De Las Rivas J., Mendes M., Barderas R., Canals F., Tapia O., Casal J.I., Lafarga M., et al. Cystatin D locates in the nucleus at sites of active transcription and modulates gene and protein expression. J. Biol. Chem. 2015;290:26533–26548. doi: 10.1074/jbc.M115.660175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alvarez-Diaz S., Valle N., Garcia J.M., Pena C., Freije J.M., Quesada V., Astudillo A., Bonilla F., Lopez-Otin C., Munoz A. Cystatin D is a candidate tumor suppressor gene induced by vitamin D in human colon cancer cells. J. Clin. Investig. 2009;119:2343–2358. doi: 10.1172/JCI37205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alvarez-Diaz S., Valle N., Ferrer-Mayorga G., Lombardia L., Herrera M., Dominguez O., Segura M.F., Bonilla F., Hernando E., Munoz A. MicroRNA-22 is induced by vitamin D and contributes to its antiproliferative, antimigratory and gene regulatory effects in colon cancer cells. Hum. Mol. Genet. 2012;21:2157–2165. doi: 10.1093/hmg/dds031. [DOI] [PubMed] [Google Scholar]
- 48.Liu Y., Chen X., Cheng R., Yang F., Yu M., Wang C., Cui S., Hong Y., Liang H., Liu M., et al. The Jun/miR-22/HuR regulatory axis contributes to tumourigenesis in colorectal cancer. Mol. Cancer. 2018;17:11. doi: 10.1186/s12943-017-0751-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu M., Li J., Wang X., Meng S., Shen J., Wang S., Xu X., Xie B., Liu B., Xie L. MiR-22 suppresses epithelial-mesenchymal transition in bladder cancer by inhibiting Snail and MAPK1/Slug/vimentin feedback loop. Cell Death Dis. 2018;9:209. doi: 10.1038/s41419-017-0206-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pereira F., Barbachano A., Silva J., Bonilla F., Campbell M.J., Munoz A., Larriba M.J. KDM6B/JMJD3 histone demethylase is induced by vitamin D and modulates its effects in colon cancer cells. Hum. Mol. Genet. 2011;20:4655–4665. doi: 10.1093/hmg/ddr399. [DOI] [PubMed] [Google Scholar]
- 51.Pereira F., Barbachano A., Singh P.K., Campbell M.J., Munoz A., Larriba M.J. Vitamin D has wide regulatory effects on histone demethylase genes. Cell Cycle. 2012;11:1081–1089. doi: 10.4161/cc.11.6.19508. [DOI] [PubMed] [Google Scholar]
- 52.Barbachano A., Ordonez-Moran P., Garcia J.M., Sanchez A., Pereira F., Larriba M.J., Martinez N., Hernandez J., Landolfi S., Bonilla F., et al. SPROUTY-2 and E-cadherin regulate reciprocally and dictate colon cancer cell tumourigenicity. Oncogene. 2010;29:4800–4813. doi: 10.1038/onc.2010.225. [DOI] [PubMed] [Google Scholar]
- 53.Barbachano A., Fernandez-Barral A., Pereira F., Segura M.F., Ordonez-Moran P., Pau E.C.D.S., Gonzalez-Sancho J.M., Hanniford D., Martinez N., Costales-Carrera A., et al. SPROUTY-2 represses the epithelial phenotype of colon carcinoma cells via upregulation of ZEB1 mediated by ETS1 and miR-200/miR-150. Oncogene. 2016;35:2991–3003. doi: 10.1038/onc.2015.366. [DOI] [PubMed] [Google Scholar]
- 54.Ordonez-Moran P., Irmisch A., Barbachano A., Chicote I., Tenbaum S., Landolfi S., Tabernero J., Huelsken J., Munoz A., Palmer H.G. SPROUTY2 is a beta-catenin and FOXO3a target gene indicative of poor prognosis in colon cancer. Oncogene. 2014;33:1975–1985. doi: 10.1038/onc.2013.140. [DOI] [PubMed] [Google Scholar]
- 55.Palmer H.G., Larriba M.J., Garcia J.M., Ordonez-Moran P., Pena C., Peiro S., Puig I., Rodriguez R., de la Fuente R., Bernad A., et al. The transcription factor SNAIL represses vitamin D receptor expression and responsiveness in human colon cancer. Nat. Med. 2004;10:917–919. doi: 10.1038/nm1095. [DOI] [PubMed] [Google Scholar]
- 56.Larriba M.J., Valle N., Palmer H.G., Ordonez-Moran P., Alvarez-Diaz S., Becker K.F., Gamallo C., de Herreros A.G., Gonzalez-Sancho J.M., Munoz A. The inhibition of Wnt/beta-catenin signalling by 1alpha,25-dihydroxyvitamin D3 is abrogated by Snail1 in human colon cancer cells. Endocr. Relat. Cancer. 2007;14:141–151. doi: 10.1677/ERC-06-0028. [DOI] [PubMed] [Google Scholar]
- 57.Larriba M.J., Martin-Villar E., Garcia J.M., Pereira F., Pena C., de Herreros A.G., Bonilla F., Munoz A. Snail2 cooperates with Snail1 in the repression of vitamin D receptor in colon cancer. Carcinogenesis. 2009;30:1459–1468. doi: 10.1093/carcin/bgp140. [DOI] [PubMed] [Google Scholar]
- 58.Pena C., Garcia J.M., Silva J., Garcia V., Rodriguez R., Alonso I., Millan I., Salas C., de Herreros A.G., Munoz A., et al. E-cadherin and vitamin D receptor regulation by SNAIL and ZEB1 in colon cancer: Clinicopathological correlations. Hum. Mol. Genet. 2005;14:3361–3370. doi: 10.1093/hmg/ddi366. [DOI] [PubMed] [Google Scholar]
- 59.Pena C., Garcia J.M., Garcia V., Silva J., Dominguez G., Rodriguez R., Maximiano C., de Herreros A.G., Munoz A., Bonilla F. The expression levels of the transcriptional regulators p300 and CtBP modulate the correlations between SNAIL, ZEB1, E-cadherin and vitamin D receptor in human colon carcinomas. Int. J. Cancer. 2006;119:2098–2104. doi: 10.1002/ijc.22083. [DOI] [PubMed] [Google Scholar]
- 60.Pena C., Garcia J.M., Larriba M.J., Barderas R., Gomez I., Herrera M., Garcia V., Silva J., Dominguez G., Rodriguez R., et al. SNAI1 expression in colon cancer related with CDH1 and VDR downregulation in normal adjacent tissue. Oncogene. 2009;28:4375–4385. doi: 10.1038/onc.2009.285. [DOI] [PubMed] [Google Scholar]
- 61.Larriba M.J., Gonzalez-Sancho J.M., Bonilla F., Munoz A. Interaction of vitamin D with membrane-based signaling pathways. Front. Physiol. 2014;5:60. doi: 10.3389/fphys.2014.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Larriba M.J., de Herreros A.G., Munoz A. Vitamin D and the Epithelial to Mesenchymal Transition. Stem. Cells Int. 2016;2016:6213872. doi: 10.1155/2016/6213872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Andl C.D., Rustgi A.K. No one-way street: Cross-talk between e-cadherin and receptor tyrosine kinase (RTK) signaling: A mechanism to regulate RTK activity. Cancer Biol. Ther. 2005;4:28–31. doi: 10.4161/cbt.4.1.1431. [DOI] [PubMed] [Google Scholar]
- 64.Tong W.M., Kallay E., Hofer H., Hulla W., Manhardt T., Peterlik M., Cross H.S. Growth regulation of human colon cancer cells by epidermal growth factor and 1,25-dihydroxyvitamin D3 is mediated by mutual modulation of receptor expression. Eur. J. Cancer. 1998;34:2119–2125. doi: 10.1016/S0959-8049(98)00267-6. [DOI] [PubMed] [Google Scholar]
- 65.Tong W.M., Hofer H., Ellinger A., Peterlik M., Cross H.S. Mechanism of antimitogenic action of vitamin D in human colon carcinoma cells: Relevance for suppression of epidermal growth factor-stimulated cell growth. Oncol. Res. 1999;11:77–84. [PubMed] [Google Scholar]
- 66.Dougherty U., Mustafi R., Sadiq F., Almoghrabi A., Mustafi D., Kreisheh M., Sundaramurthy S., Liu W., Konda V.J., Pekow J., et al. The renin-angiotensin system mediates EGF receptor-vitamin d receptor cross-talk in colitis-associated colon cancer. Clin. Cancer Res. 2014;20:5848–5859. doi: 10.1158/1078-0432.CCR-14-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Perou C.M., Sorlie T., Eisen M.B., van de Rijn M., Jeffrey S.S., Rees C.A., Pollack J.R., Ross D.T., Johnsen H., Akslen L.A., et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 68.Curtis C., Shah S.P., Chin S.F., Turashvili G., Rueda O.M., Dunning M.J., Speed D., Lynch A.G., Samarajiwa S., Yuan Y., et al. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature. 2012;486:346–352. doi: 10.1038/nature10983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Blasiak J., Pawlowska E., Chojnacki J., Szczepanska J., Fila M., Chojnacki C. Vitamin D in Triple—Negative and BRCA1-Deficient Breast Cancer-Implications for Pathogenesis and Therapy. Int. J. Mol. Sci. 2020;21:3670. doi: 10.3390/ijms21103670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Welsh J. Function of the vitamin D endocrine system in mammary gland and breast cancer. Mol. Cell. Endocrinol. 2017;453:88–95. doi: 10.1016/j.mce.2017.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Berger U., Wilson P., McClelland R.A., Colston K., Haussler M.R., Pike J.W., Coombes R.C. Immunocytochemical detection of 1,25-dihydroxyvitamin D receptors in normal human tissues. J. Clin. Endocrinol. Metab. 1988;67:607–613. doi: 10.1210/jcem-67-3-607. [DOI] [PubMed] [Google Scholar]
- 72.Pendas-Franco N., Gonzalez-Sancho J.M., Suarez Y., Aguilera O., Steinmeyer A., Gamallo C., Berciano M.T., Lafarga M., Munoz A. Vitamin D regulates the phenotype of human breast cancer cells. Differentiation. 2007;75:193–207. doi: 10.1111/j.1432-0436.2006.00131.x. [DOI] [PubMed] [Google Scholar]
- 73.Lopes N., Sousa B., Martins D., Gomes M., Vieira D., Veronese L.A., Milanezi F., Paredes J., Costa J.L., Schmitt F. Alterations in Vitamin D signalling and metabolic pathways in breast cancer progression: A study of VDR, CYP27B1 and CYP24A1 expression in benign and malignant breast lesions. BMC Cancer. 2010;10:483. doi: 10.1186/1471-2407-10-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Al-Azhri J., Zhang Y., Bshara W., Zirpoli G., McCann S.E., Khoury T., Morrison C.D., Edge S.B., Ambrosone C.B., Yao S. Tumor Expression of Vitamin D Receptor and Breast Cancer Histopathological Characteristics and Prognosis. Clin. Cancer Res. 2017;23:97–103. doi: 10.1158/1078-0432.CCR-16-0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gocek E., Studzinski G.P. Vitamin D and differentiation in cancer. Crit. Rev. Clin. Lab. Sci. 2009;46:190–209. doi: 10.1080/10408360902982128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Narvaez C.J., Matthews D., LaPorta E., Simmons K.M., Beaudin S., Welsh J. The impact of vitamin D in breast cancer: Genomics, pathways, metabolism. Front. Physiol. 2014;5:213. doi: 10.3389/fphys.2014.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Welsh J. Vitamin D and breast cancer: Past and present. J. Steroid Biochem. Mol. Biol. 2018;177:15–20. doi: 10.1016/j.jsbmb.2017.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lopes N., Carvalho J., Duraes C., Sousa B., Gomes M., Costa J.L., Oliveira C., Paredes J., Schmitt F. 1Alpha,25-dihydroxyvitamin D3 induces de novo E-cadherin expression in triple-negative breast cancer cells by CDH1-promoter demethylation. Anticancer Res. 2012;32:249–257. [PubMed] [Google Scholar]
- 79.Gonzalez-Sancho J.M., Alvarez-Dolado M., Munoz A. 1,25-Dihydroxyvitamin D3 inhibits tenascin-C expression in mammary epithelial cells. FEBS Lett. 1998;426:225–228. doi: 10.1016/S0014-5793(98)00348-2. [DOI] [PubMed] [Google Scholar]
- 80.Koli K., Keski-Oja J. 1alpha,25-dihydroxyvitamin D3 and its analogues down-regulate cell invasion-associated proteases in cultured malignant cells. Cell Growth Differ. 2000;11:221–229. [PubMed] [Google Scholar]
- 81.Sundaram S., Beckman M.J., Bajwa A., Wei J., Smith K.M., Posner G.H., Gewirtz D.A. QW-1624F2-2, a synthetic analogue of 1,25-dihydroxyvitamin D3, enhances the response to other deltanoids and suppresses the invasiveness of human metastatic breast tumor cells. Mol. Cancer Ther. 2006;5:2806–2814. doi: 10.1158/1535-7163.MCT-06-0092. [DOI] [PubMed] [Google Scholar]
- 82.LaPorta E., Welsh J. Modeling vitamin D actions in triple negative/basal-like breast cancer. J. Steroid Biochem. Mol. Biol. 2014;144:65–73. doi: 10.1016/j.jsbmb.2013.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wilmanski T., Barnard A., Parikh M.R., Kirshner J., Buhman K., Burgess J., Teegarden D. 1alpha,25-Dihydroxyvitamin D Inhibits the Metastatic Capability of MCF10CA1a and MDA-MB-231 Cells in an In Vitro Model of Breast to Bone Metastasis. Nutr. Cancer. 2016;68:1202–1209. doi: 10.1080/01635581.2016.1213868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hansen C.M., Frandsen T.L., Brunner N., Binderup L. 1 alpha,25-Dihydroxyvitamin D3 inhibits the invasive potential of human breast cancer cells in vitro. Clin. Exp. Metastasis. 1994;12:195–202. doi: 10.1007/BF01753887. [DOI] [PubMed] [Google Scholar]
- 85.Vanoirbeek E., Eelen G., Verlinden L., Carmeliet G., Mathieu C., Bouillon R., O’Connor R., Xiao G., Verstuyf A. PDLIM2 expression is driven by vitamin D and is involved in the pro-adhesion, and anti-migration and -invasion activity of vitamin D. Oncogene. 2014;33:1904–1911. doi: 10.1038/onc.2013.123. [DOI] [PubMed] [Google Scholar]
- 86.Williams J.D., Aggarwal A., Swami S., Krishnan A.V., Ji L., Albertelli M.A., Feldman B.J. Tumor Autonomous Effects of Vitamin D Deficiency Promote Breast Cancer Metastasis. Endocrinology. 2016;157:1341–1347. doi: 10.1210/en.2015-2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Arensman M.D., Nguyen P., Kershaw K.M., Lay A.R., Ostertag-Hill C.A., Sherman M.H., Downes M., Liddle C., Evans R.M., Dawson D.W. Calcipotriol Targets LRP6 to Inhibit Wnt Signaling in Pancreatic Cancer. Mol. Cancer Res. 2015;13:1509–1519. doi: 10.1158/1541-7786.MCR-15-0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Xu S., Zhang Z.H., Fu L., Song J., Xie D.D., Yu D.X., Xu D.X., Sun G.P. Calcitriol inhibits migration and invasion of renal cell carcinoma cells by suppressing Smad2/3-, STAT3- and beta-catenin-mediated epithelial-mesenchymal transition. Cancer Sci. 2020;111:59–71. doi: 10.1111/cas.14237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Palmer H.G., Anjos-Afonso F., Carmeliet G., Takeda H., Watt F.M. The vitamin D receptor is a Wnt effector that controls hair follicle differentiation and specifies tumor type in adult epidermis. PLoS ONE. 2008;3:e1483. doi: 10.1371/journal.pone.0001483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jiang Y.J., Teichert A.E., Fong F., Oda Y., Bikle D.D. 1alpha,25(OH)2-dihydroxyvitamin D3/VDR protects the skin from UVB-induced tumor formation by interacting with the beta-catenin pathway. J. Steroid Biochem. Mol. Biol. 2013;136:229–232. doi: 10.1016/j.jsbmb.2012.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tapia C., Suares A., De Genaro P., Gonzalez-Pardo V. In vitro studies revealed a downregulation of Wnt/beta-catenin cascade by active vitamin D and TX 527 analog in a Kaposi’s sarcoma cellular model. Toxicol. In Vitro. 2020;63:104748. doi: 10.1016/j.tiv.2019.104748. [DOI] [PubMed] [Google Scholar]
- 92.Chiang K.C., Kuo S.F., Chen C.H., Ng S., Lin S.F., Yeh C.N., Chen L.W., Takano M., Chen T.C., Juang H.H., et al. MART-10, the vitamin D analog, is a potent drug to inhibit anaplastic thyroid cancer cell metastatic potential. Cancer Lett. 2015;369:76–85. doi: 10.1016/j.canlet.2015.07.024. [DOI] [PubMed] [Google Scholar]
- 93.Hou Y.F., Gao S.H., Wang P., Zhang H.M., Liu L.Z., Ye M.X., Zhou G.M., Zhang Z.L., Li B.Y. 1alpha,25(OH)(2)D(3) Suppresses the Migration of Ovarian Cancer SKOV-3 Cells through the Inhibition of Epithelial-Mesenchymal Transition. Int. J. Mol. Sci. 2016;17:1285. doi: 10.3390/ijms17081285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jiang F., Yang Y., Xue L., Li B., Zhang Z. 1alpha,25-dihydroxyvitamin D3 Attenuates TGF-beta-Induced Pro-Fibrotic Effects in Human Lung Epithelial Cells through Inhibition of Epithelial-Mesenchymal Transition. Nutrients. 2017;9:980. doi: 10.3390/nu9090980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lee E., Jeon S.H., Yi J.Y., Jin Y.J., Son Y.S. Calcipotriol inhibits autocrine phosphorylation of EGF receptor in a calcium-dependent manner, a possible mechanism for its inhibition of cell proliferation and stimulation of cell differentiation. Biochem. Biophys. Res. Commun. 2001;284:419–425. doi: 10.1006/bbrc.2001.4943. [DOI] [PubMed] [Google Scholar]
- 96.Cordero J.B., Cozzolino M., Lu Y., Vidal M., Slatopolsky E., Stahl P.D., Barbieri M.A., Dusso A. 1,25-Dihydroxyvitamin D down-regulates cell membrane growth- and nuclear growth-promoting signals by the epidermal growth factor receptor. J. Biol. Chem. 2002;277:38965–38971. doi: 10.1074/jbc.M203736200. [DOI] [PubMed] [Google Scholar]
- 97.Boisseau-Garsaud A.M., Donatien P., Margerin C., Taieb A. EGF receptor expression and growth of psoriatic and normal human keratinocytes are modulated by 1.25 (OH)2-vitamin D3 ex vivo. Arch. Dermatol. Res. 1996;288:453–457. doi: 10.1007/BF02505234. [DOI] [PubMed] [Google Scholar]
- 98.Kulling P.M., Olson K.C., Olson T.L., Feith D.J., Loughran T.P., Jr. Vitamin D in hematological disorders and malignancies. Eur. J. Haematol. 2017;98:187–197. doi: 10.1111/ejh.12818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tanaka H., Abe E., Miyaura C., Shiina Y., Suda T. 1 alpha,25-dihydroxyvitamin D3 induces differentiation of human promyelocytic leukemia cells (HL-60) into monocyte-macrophages, but not into granulocytes. Biochem. Biophys. Res. Commun. 1983;117:86–92. doi: 10.1016/0006-291X(83)91544-9. [DOI] [PubMed] [Google Scholar]
- 100.Koeffler H.P., Amatruda T., Ikekawa N., Kobayashi Y., DeLuca H.F. Induction of macrophage differentiation of human normal and leukemic myeloid stem cells by 1,25-dihydroxyvitamin D3 and its fluorinated analogues. Cancer Res. 1984;44:5624–5628. [PubMed] [Google Scholar]
- 101.Liu M., Lee M.H., Cohen M., Bommakanti M., Freedman L.P. Transcriptional activation of the Cdk inhibitor p21 by vitamin D3 leads to the induced differentiation of the myelomonocytic cell line U937. Genes Dev. 1996;10:142–153. doi: 10.1101/gad.10.2.142. [DOI] [PubMed] [Google Scholar]
- 102.Ji Y., Studzinski G.P. Retinoblastoma protein and CCAAT/enhancer-binding protein beta are required for 1,25-dihydroxyvitamin D3-induced monocytic differentiation of HL60 cells. Cancer Res. 2004;64:370–377. doi: 10.1158/0008-5472.CAN-03-3029. [DOI] [PubMed] [Google Scholar]
- 103.Marchwicka A., Marcinkowska E. Regulation of Expression of CEBP Genes by Variably Expressed Vitamin D Receptor and Retinoic Acid Receptor alpha in Human Acute Myeloid Leukemia Cell Lines. Int. J. Mol. Sci. 2018;19:1918. doi: 10.3390/ijms19071918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hmama Z., Nandan D., Sly L., Knutson K.L., Herrera-Velit P., Reiner N.E. 1alpha,25-dihydroxyvitamin D(3)-induced myeloid cell differentiation is regulated by a vitamin D receptor-phosphatidylinositol 3-kinase signaling complex. J. Exp. Med. 1999;190:1583–1594. doi: 10.1084/jem.190.11.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Studzinski G.P., Wang X., Ji Y., Wang Q., Zhang Y., Kutner A., Harrison J.S. The rationale for deltanoids in therapy for myeloid leukemia: Role of KSR-MAPK-C/EBP pathway. J. Steroid Biochem. Mol. Biol. 2005;97:47–55. doi: 10.1016/j.jsbmb.2005.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cao H., Xu Y., de Necochea-Campion R., Baylink D.J., Payne K.J., Tang X., Ratanatharathorn C., Ji Y., Mirshahidi S., Chen C.S. Application of vitamin D and vitamin D analogs in acute myelogenous leukemia. Exp. Hematol. 2017;50:1–12. doi: 10.1016/j.exphem.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 107.Muto A., Kizaki M., Yamato K., Kawai Y., Kamata-Matsushita M., Ueno H., Ohguchi M., Nishihara T., Koeffler H.P., Ikeda Y. 1,25-Dihydroxyvitamin D3 induces differentiation of a retinoic acid-resistant acute promyelocytic leukemia cell line (UF-1) associated with expression of p21(WAF1/CIP1) and p27(KIP1) Blood. 1999;93:2225–2233. doi: 10.1182/blood.V93.7.2225. [DOI] [PubMed] [Google Scholar]
- 108.Hickish T., Cunningham D., Colston K., Millar B.C., Sandle J., Mackay A.G., Soukop M., Sloane J. The effect of 1,25-dihydroxyvitamin D3 on lymphoma cell lines and expression of vitamin D receptor in lymphoma. Br. J. Cancer. 1993;68:668–672. doi: 10.1038/bjc.1993.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ohlund D., Elyada E., Tuveson D. Fibroblast heterogeneity in the cancer wound. J. Exp. Med. 2014;211:1503–1523. doi: 10.1084/jem.20140692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chen X., Song E. Turning foes to friends: Targeting cancer-associated fibroblasts. Nat. Rev. Drug Discov. 2019;18:99–115. doi: 10.1038/s41573-018-0004-1. [DOI] [PubMed] [Google Scholar]
- 111.Augsten M. Cancer-associated fibroblasts as another polarized cell type of the tumor microenvironment. Front. Oncol. 2014;4:62. doi: 10.3389/fonc.2014.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ozdemir B.C., Pentcheva-Hoang T., Carstens J.L., Zheng X., Wu C.C., Simpson T.R., Laklai H., Sugimoto H., Kahlert C., Novitskiy S.V., et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell. 2014;25:719–734. doi: 10.1016/j.ccr.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rhim A.D., Oberstein P.E., Thomas D.H., Mirek E.T., Palermo C.F., Sastra S.A., Dekleva E.N., Saunders T., Becerra C.P., Tattersall I.W., et al. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell. 2014;25:735–747. doi: 10.1016/j.ccr.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sherman M.H., Yu R.T., Engle D.D., Ding N., Atkins A.R., Tiriac H., Collisson E.A., Connor F., Van Dyke T., Kozlov S., et al. Vitamin D receptor-mediated stromal reprogramming suppresses pancreatitis and enhances pancreatic cancer therapy. Cell. 2014;159:80–93. doi: 10.1016/j.cell.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ding N., Yu R.T., Subramaniam N., Sherman M.H., Wilson C., Rao R., Leblanc M., Coulter S., He M., Scott C., et al. A vitamin D receptor/SMAD genomic circuit gates hepatic fibrotic response. Cell. 2013;153:601–613. doi: 10.1016/j.cell.2013.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Duran A., Hernandez E.D., Reina-Campos M., Castilla E.A., Subramaniam S., Raghunandan S., Roberts L.R., Kisseleva T., Karin M., Diaz-Meco M.T., et al. p62/SQSTM1 by Binding to Vitamin D Receptor Inhibits Hepatic Stellate Cell Activity, Fibrosis, and Liver Cancer. Cancer Cell. 2016;30:595–609. doi: 10.1016/j.ccell.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Campos L.T., Brentani H., Roela R.A., Katayama M.L., Lima L., Rolim C.F., Milani C., Folgueira M.A., Brentani M.M. Differences in transcriptional effects of 1alpha,25 dihydroxyvitamin D3 on fibroblasts associated to breast carcinomas and from paired normal breast tissues. J. Steroid Biochem. Mol. Biol. 2013;133:12–24. doi: 10.1016/j.jsbmb.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 118.Ferrer-Mayorga G., Gomez-Lopez G., Barbachano A., Fernandez-Barral A., Pena C., Pisano D.G., Cantero R., Rojo F., Munoz A., Larriba M.J. Vitamin D receptor expression and associated gene signature in tumour stromal fibroblasts predict clinical outcome in colorectal cancer. Gut. 2017;66:1449–1462. doi: 10.1136/gutjnl-2015-310977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kong F., Li L., Wang G., Deng X., Li Z., Kong X. VDR signaling inhibits cancer-associated-fibroblasts’ release of exosomal miR-10a-5p and limits their supportive effects on pancreatic cancer cells. Gut. 2019;68:950–951. doi: 10.1136/gutjnl-2018-316627. [DOI] [PubMed] [Google Scholar]
- 120.Niell N., Larriba M.J., Ferrer-Mayorga G., Sanchez-Perez I., Cantero R., Real F.X., Del Peso L., Munoz A., Gonzalez-Sancho J.M. The human PKP2/plakophilin-2 gene is induced by Wnt/beta-catenin in normal and colon cancer-associated fibroblasts. Int. J. Cancer. 2018;142:792–804. doi: 10.1002/ijc.31104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ferrer-Mayorga G., Niell N., Cantero R., Gonzalez-Sancho J.M., Del Peso L., Munoz A., Larriba M.J. Vitamin D and Wnt3A have additive and partially overlapping modulatory effects on gene expression and phenotype in human colon fibroblasts. Sci. Rep. 2019;9:8085. doi: 10.1038/s41598-019-44574-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wu X., Hu W., Lu L., Zhao Y., Zhou Y., Xiao Z., Zhang L., Zhang H., Li X., Li W., et al. Repurposing vitamin D for treatment of human malignancies via targeting tumor microenvironment. Acta Pharm. Sin. B. 2019;9:203–219. doi: 10.1016/j.apsb.2018.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Schwitalla S., Fingerle A.A., Cammareri P., Nebelsiek T., Goktuna S.I., Ziegler P.K., Canli O., Heijmans J., Huels D.J., Moreaux G., et al. Intestinal tumorigenesis initiated by dedifferentiation and acquisition of stem-cell-like properties. Cell. 2013;152:25–38. doi: 10.1016/j.cell.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 124.Plaks V., Kong N., Werb Z. The cancer stem cell niche: How essential is the niche in regulating stemness of tumor cells? Cell Stem Cell. 2015;16:225–238. doi: 10.1016/j.stem.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Murata K., Jadhav U., Madha S., van Es J., Dean J., Cavazza A., Wucherpfennig K., Michor F., Clevers H., Shivdasani R.A. Ascl2-Dependent Cell Dedifferentiation Drives Regeneration of Ablated Intestinal Stem Cells. Cell Stem Cell. 2020;26:377–390.e376. doi: 10.1016/j.stem.2019.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chaffer C.L., Brueckmann I., Scheel C., Kaestli A.J., Wiggins P.A., Rodrigues L.O., Brooks M., Reinhardt F., Su Y., Polyak K., et al. Normal and neoplastic nonstem cells can spontaneously convert to a stem-like state. Proc. Natl. Acad. Sci. USA. 2011;108:7950–7955. doi: 10.1073/pnas.1102454108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Jeong Y., Swami S., Krishnan A.V., Williams J.D., Martin S., Horst R.L., Albertelli M.A., Feldman B.J., Feldman D., Diehn M. Inhibition of Mouse Breast Tumor-Initiating Cells by Calcitriol and Dietary Vitamin D. Mol. Cancer Ther. 2015;14:1951–1961. doi: 10.1158/1535-7163.MCT-15-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.So J.Y., Wahler J., Das Gupta S., Salerno D.M., Maehr H., Uskokovic M., Suh N. HES1-mediated inhibition of Notch1 signaling by a Gemini vitamin D analog leads to decreased CD44(+)/CD24(-/low) tumor-initiating subpopulation in basal-like breast cancer. J. Steroid Biochem. Mol. Biol. 2015;148:111–121. doi: 10.1016/j.jsbmb.2014.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wahler J., So J.Y., Cheng L.C., Maehr H., Uskokovic M., Suh N. Vitamin D compounds reduce mammosphere formation and decrease expression of putative stem cell markers in breast cancer. J. Steroid Biochem. Mol. Biol. 2015;148:148–155. doi: 10.1016/j.jsbmb.2014.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Shan N.L., Wahler J., Lee H.J., Bak M.J., Gupta S.D., Maehr H., Suh N. Vitamin D compounds inhibit cancer stem-like cells and induce differentiation in triple negative breast cancer. J. Steroid Biochem. Mol. Biol. 2017;173:122–129. doi: 10.1016/j.jsbmb.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Srivastava A.K., Rizvi A., Cui T., Han C., Banerjee A., Naseem I., Zheng Y., Wani A.A., Wang Q.E. Depleting ovarian cancer stem cells with calcitriol. Oncotarget. 2018;9:14481–14491. doi: 10.18632/oncotarget.24520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ji M., Liu L., Hou Y., Li B. 1alpha,25Dihydroxyvitamin D3 restrains stem celllike properties of ovarian cancer cells by enhancing vitamin D receptor and suppressing CD44. Oncol. Rep. 2019;41:3393–3403. doi: 10.3892/or.2019.7116. [DOI] [PubMed] [Google Scholar]
- 133.Jensen M.B., Jorgensen A., Nielsen J.E., Steinmeyer A., Leffers H., Juul A., De Meyts E.R. Vitamin D metabolism and effects on pluripotency genes and cell differentiation in testicular germ cell tumors in vitro and in vivo. Neoplasia. 2012;14:952–963. doi: 10.1593/neo.121164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Fernandez-Barral A., Costales-Carrera A., Buira S.P., Jung P., Ferrer-Mayorga G., Larriba M.J., Bustamante-Madrid P., Dominguez O., Real F.X., Guerra-Pastrian L., et al. Vitamin D differentially regulates colon stem cells in patient-derived normal and tumor organoids. FEBS J. 2020;287:53–72. doi: 10.1111/febs.14998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Costales-Carrera A., Fernandez-Barral A., Bustamante-Madrid P., Dominguez O., Guerra-Pastrian L., Cantero R., del Peso L., Burgos A., Barbachano A., Muñoz A. Comparative study of organoids from patient-derived normal and tumor colon and rectal tissue. Cancers. 2020;12:2302. doi: 10.3390/cancers12082302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Peregrina K., Houston M., Daroqui C., Dhima E., Sellers R.S., Augenlicht L.H. Vitamin D is a determinant of mouse intestinal Lgr5 stem cell functions. Carcinogenesis. 2015;36:25–31. doi: 10.1093/carcin/bgu221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Augenlicht L.H. Environmental Impact on Intestinal Stem Cell Functions in Mucosal Homeostasis and Tumorigenesis. J. Cell. Biochem. 2017;118:943–952. doi: 10.1002/jcb.25719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Li W., Zimmerman S.E., Peregrina K., Houston M., Mayoral J., Zhang J., Maqbool S., Zhang Z., Cai Y., Ye K., et al. The nutritional environment determines which and how intestinal stem cells contribute to homeostasis and tumorigenesis. Carcinogenesis. 2019;40:937–946. doi: 10.1093/carcin/bgz106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wang L.Q., Yu P., Li B., Guo Y.H., Liang Z.R., Zheng L.L., Yang J.H., Xu H., Liu S., Zheng L.S., et al. miR-372 and miR-373 enhance the stemness of colorectal cancer cells by repressing differentiation signaling pathways. Mol. Oncol. 2018;12:1949–1964. doi: 10.1002/1878-0261.12376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Paubelle E., Zylbersztejn F., Maciel T.T., Carvalho C., Mupo A., Cheok M., Lieben L., Sujobert P., Decroocq J., Yokoyama A., et al. Vitamin D Receptor Controls Cell Stemness in Acute Myeloid Leukemia and in Normal Bone Marrow. Cell Rep. 2020;30:739–754.e734. doi: 10.1016/j.celrep.2019.12.055. [DOI] [PubMed] [Google Scholar]