Key Points
Question
What is the association between human papillomavirus (HPV) and survival outcomes in patients with nonoropharyngeal squamous cell carcinoma (non-OPSCC)?
Findings
This systematic review and meta-analysis of 22 observational and 2 randomized clinical trials including 24 854 patients with HPV-positive non-OPSCC showed that, in oral cavity locations, overall survival (OS) was not significantly associated with HPV positivity; however, HPV-positive tumors showed worse disease-free survival. Laryngeal and hypopharyngeal HPV-positive tumors were associated with improved OS whereas in nasopharyngeal locations HPV did not appear to affect OS or disease-specific survival.
Meaning
The findings of this meta-analysis may be useful for future clinical studies of laryngeal and hypopharyngeal tumors and whether HPV status should be incorporated in prognostication of patients with these cancers.
Abstract
Importance
Although the survival impact of human papillomavirus (HPV) in oropharyngeal squamous cell carcinoma (OPSCC) is well known, there has been conflicting and scarce evidence on the role of HPV in non-OPSCC.
Objective
To undertake a Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA)–compliant systematic review and meta-analysis of all published studies on the association between HPV status and survival outcomes in patients with non-OPSCC, analyzing each site separately.
Data Sources
PubMed, CINAHL, and Embase were searched from 1946 to December 16, 2019, for English-language articles.
Study Selection
Analysis comprised randomized clinical trials or observational studies that each included at least 10 patients with non-OPSCC in which the presence of HPV was analyzed, survival outcomes were reported, and a clinical follow-up of 1 year or more was performed. Studies excluded were those in which data on OPSCC and non-OPSCC were not distinguished between both cohorts and studies on patients with distant metastatic tumors at diagnosis. Final analysis included outcomes that were analyzed in at least 3 studies.
Data Extraction and Synthesis
Two reviewers independently abstracted the data. Risk of bias was estimated with the Newcastle-Ottawa Scale. Meta-analysis was performed using the random-effects model.
Main Outcomes and Measures
The primary end point was overall survival (OS); secondary end points were disease-specific survival (DSS) and disease-free survival (DFS).
Results
Of the 3947 articles screened, a total of 22 observational and 2 randomized clinical trials were included in the analysis, representing 24 854 patients. In oral cavity locations, OS was not significantly associated with HPV positivity (hazard ratio [HR], 1.16; 95% CI, 0.83-1.61; I2 = 71%); however, HPV-positive tumors showed worse DFS (HR, 1.81; 95% CI, 1.12-2.91; I2 = 47%). Laryngeal and hypopharyngeal HPV-positive tumors were associated with improved OS (HR, 0.71; 95% CI, 0.54-0.92; I2 = 38% and HR, 0.60; 95% CI, 0.47-0.76; I2 = 0%), respectively, whereas, in nasopharyngeal locations HPV was not associated with OS (HR, 0.82; 95% CI, 0.49-1.38; I2 = 46%) or DSS (HR, 0.55; 95% CI, 0.22-1.42; I2 = 65%).
Conclusions and Relevance
In this meta-analysis of 24 studies, HPV was associated with improved OS in laryngeal and hypopharyngeal locations but not in the oral cavity and the nasopharynx. This information may be useful for future clinical studies of laryngeal and hypopharyngeal tumors and whether HPV status should be incorporated in prognostication of patients with these cancers.
This meta-analysis examines the association between human papillomavirus and survival in patients with oropharyngeal squamous cell carcinoma.
Introduction
There has been a substantial treatment shift in oropharyngeal squamous cell carcinomas (OPSCC) after the assessment of human papillomavirus (HPV) in this location.1 The impact of HPV as a risk stratification biomarker in OPSCC is well known, and guidelines recommend that all patients with OPSCC should be tested for the presence of the virus.2,3,4 Currently, research efforts are being directed toward an improvement both in survival and quality-of-life outcomes of patients with HPV-associated OPSCC.5 Treatment paths and prognosis differ greatly between HPV-negative and HPV-positive tumors, being more favorable for the latter cohort.
Despite being present in 7% to 25% of non-OPSCC tumors,6,7,8,9 the role of HPV in these tumors is not clearly understood.10 Literature on the subject is controversial, and few studies have analyzed the role of HPV in these locations.11 Even fewer studies have looked at the implications of HPV at each nonoropharyngeal site.12 To our knowledge, there has been no synthesis of the available literature to determine whether HPV has prognostic significance in non-OPSCC as it does in the oropharynx. Our objective was to undertake a systematic review and meta-analysis, analyzing all of the non-OPSCC sites separately, to examine whether there is a survival advantage of HPV-positive tumors in non-OPSCC sites.
Methods
Methods of the analysis and inclusion criteria were defined in advance, documented, and registered with PROSPERO (CRD42018100092) in a prespecified protocol. This study followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline.
Inclusion criteria were randomized clinical trials or observational studies including at least 10 patients with HPV non-OPSCC, the presence of HPV was analyzed, and survival outcomes were reported. Exclusion criteria were studies of OPSCC, studies that included OPSCC and non-OPSCC with indistinguishable data between the cohorts, studies with distant metastasis at presentation, and those that did not provide hazard ratios (HRs) or CIs.
Study Selection
A systematic search was conducted using Ovid MEDLINE In-Process & Other Non-Indexed Citations, MEDLINE, Embase, Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, ClinicalTrials.gov, CINAHL, and Scopus. The article search was performed from each database’s earliest records up to and including December 16, 2019, by an experienced librarian with the input of 2 of us (A.S. and S.D.M.). Only studies published in the English language were included, and bibliographies of selected articles were reviewed for additional relevant studies. Gray literature and conference abstracts were also tracked (eMethods in the Supplement).
Two of us (A.S. and M.H.K.) independently selected studies on the basis of the inclusion and exclusion criteria. Disparities in selection were resolved by the lead investigator (S.D.M.). Data extraction was compiled in a spreadsheet and the following information was obtained from each study: (1) study characteristics (year, location, number of patients), (2) tumor sites, (3) HPV assessment method (p16 immunohistochemistry [IHC] or HPV DNA in situ hybridization and DNA by polymerase chain reaction [PCR] or real-time PCR), (4) prevalence of HPV-positive tumors, and (5) outcomes (overall survival [OS], disease-free survival [DFS], and disease-specific survival [DSS]).
The Newcastle-Ottawa Scale13 was used to assess the quality of the studies. Each reviewer generated a score, and this value was reviewed. For studies that had high risk of bias (score <6), reviewers would deliberate on whether such studies should be excluded.
Statistical Analysis
Effect estimates presented as HRs and associated 95% CIs for HPV status of different anatomic sites and outcome of interest were extracted. Analyses included studies with estimates from univariate or multivariate models. Meta-analysis was performed using ReviewManager, version 5.3 (The Nordic Cochrane Centre, The Cochrane Collaboration, 2014). Random-effects models were adopted for all analyses. For each model, outcomes for HPV non-OPSCC were included within the meta-analysis only if relevant data from a minimum of 3 studies were provided.
To assess the degree of heterogeneity, the I2 statistic was also calculated, and a value exceeding 50% implied substantial heterogeneity.14 Forest plots were constructed for all associations of HPV status of the different nonoropharyngeal sites and outcomes. Funnel plots were also obtained to assess the existence of publication bias.
Results
Study Selection
There were 3947 records initially identified. After 430 duplicates were removed, a total of 3517 records remained for title and abstract analysis, and 73 studies were reviewed in full text. Twenty-four articles (22 observational studies and 2 randomized clinical trials) were included in the final analysis (eFigure 1 in the Supplement).
There were some discrepancies among the pooled studies in relation to the diagnostic method used to identify HPV-positive tumors. Whenever possible, we performed analysis grouping studies in p16 IHC and HPV DNA to avoid biases. We also found reports from different institutions using the same databases in coincident time periods and analyzing the same anatomic sites and outcomes.11,12,15 To prevent overlapping cohorts, we selected the studies that combined the largest data set of patients.
The 24 studies10,12,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37 included in the final analysis comprised 24 854 patients. Categorization of the studies by tumor site showed that there were 1474 patients with nasopharyngeal tumors, 11 458 with oral cavity tumors, 9793 with laryngeal tumors, and 2129 with hypopharyngeal tumors (Table). Overall survival in studies of the oral cavity (n = 13), nasopharynx (n = 5), larynx (n = 9), and hypopharynx (n = 3); DFS in studies of the oral cavity (n = 5); and DSS in studies of the nasopharynx (n = 3) were included in the final models.
Table. Characteristics of the Included Studies.
| Source | Duration | Design | Location | No. of patients (tumor site) | HPV diagnostic method (HPV type) | HPV prevalence, % | Outcome | Median follow-up, mo | |
|---|---|---|---|---|---|---|---|---|---|
| Positive | Negative | ||||||||
| Chuang et al,16 2012 | 2001-2010 | OSI | Taiwan | 178 (Oral cavity) | p16 IHC | 52 | 48 | OS, RFS | 6 |
| Chung et al,10 2014 | Multiple trials | RCT | US | 80 (Oral cavity), 181 (larynx), 61 (hypopharynx) | p16 IHC | 26.3 | 73.8 | OS, PFS | 60 |
| Fakhry et al,17 2017 | 1995-2012 | OSI | US | 243 (Larynx), 125 (nasopharynx) | HPV DNA, ISH | 5 (Larynx), 10 (nasopharynx) | 95 (Larynx), 90 (nasopharynx) | OS | 42 |
| Hernandez et al,18 2014 | 1993-2004 | PBO | US | 148 (Larynx) | HPV DNA, PCR (NS) | 20.9 | 79.1 | OS | NS |
| Jiang et al,19 2016 | 2000-2014 | OSI | US | 86 (Nasopharynx) | p16 IHC | 15 (p16+/EBV+) | 85 | OS | 36 |
| Kang et al,20 2016 | 1993-2016 | OSI | South Korea | 46 (Nasopharynx) | p16 IHC | 67.4 | 32.6 | DSS | NS |
| Lai et al,21 2017 | 2000-2014 | OSI | Australia | 95 (Oral cavity) | p16 IHC, HPV DNA, ISH | 61 | 39 | OS, DFS | NS |
| Lam et al,22 2018 | 2005-2010 | OSI | China | 85 (Larynx) | p16 IHC | 12.9 | 87.1 | OS | NS |
| Lassen et al,23 2014 | Multiple trials | RCT | Denmark | 321 (Larynx), 137 (hypopharynx) | p16 IHC | 13.7 (Larynx), 15.3 (hypopharynx) | 86.3 (Larynx), 84.7 (hypopharynx) | OS | 60 |
| Lee et al,24 2012 | 2004-2006 | OSI | Taiwan | 333 (Oral cavity) | HPV DNA, PCR (16) | 21.3 | 78.7 | OS, DFS | 58 |
| Lee et al,25 2015 | 2004-2011 | OSI | Taiwan | 1002 (Oral cavity) | HPV DNA, PCR (16) | 19 | 81 | OS | 69 |
| Li et al,12 2018 | 2010-2014 | PBO | US | 9080 (Oral cavity), 7725 (larynx), 1931 (hypopharynx), 1006 (nasopharynx) | HPV DNA (NS) | 13.5 (Oral cavity), 12.2 (larynx), 16.9 (hypopharynx), 32.5 (nasopharynx) | 86.5 (Oral cavity), 87.8 (larynx), 83.1 (hypopharynx), 67.5 (nasopharynx) | OS | 60 |
| Loeschke et al,26 2016 | 2000-2004 | OSI | Germany | 85 (Oral cavity) | p16 IHC | 9.5 | 90.5 | OS, DFS | NS |
| Ni et al,27 2019 | 2013-2014 | OSI | China | 147 (Oral cavity) | p16 IHC | 14.3 | 85.7 | OS | NS |
| Ramshankar et al,28 2014 | 1995-2007 | OSI | India | 167 (Oral cavity) | p16, HPV DNA, PCR (16) | 15.4 | 84.6 | OS, DFS | 74 |
| Rodríguez-Santamarta et al,29 2016 | 1996-2007 | OSI | Spain | 125 (Oral cavity) | p16 IHC | 11 | 89 | OS | NS |
| Ruuskanen et al,30 2019 | 1990-2009 | PBO | Finland | 150 (Nasopharynx) | HPV DNA, ISH | 14 (HPV+/EBV+) | 86 | OS, DSS | NS |
| Saghravanian et al,31 2016 | 2001-2013 | OSI | Iran | 114 (Oral cavity) | HPV DNA, PCR (6, 11) | 13 | 87 | OS, DFS | 35.5 |
| Stanley et al,32 2019 | NS | OSI | US | 55 (Larynx) | p16 IHC | 33 | 67 | Mortality (OS) | 30 |
| Stenmark et al,33 2014 | 1985-2011 | OSI | US | 61 (Nasopharynx) | p16 IHC | 30 (HPV+/EBV−) | 70 | OS, DSS | 84 |
| Tong et al,34 2018 | NS | OSI | China | 211 (Larynx) | HPV PCR and ISH (11, 13,16, 18, 31, 33, 35, 39, 45, 51, 52, 58, 59, 68, 70) | 54 | 46 | OS | NS |
| Wotman et al,35 2019 | 2013-2015 | PBO | US | 517 (Nasopharynx) | HPV status (NS) | 34.8 | 65.2 | CSS (DSS) | NS |
| Young et al,36 2015 | 2002-2012 | OSI | Australia | 307 (Larynx) | p16 IHC | 6.5 | 93.5 | OS | 41 |
| Zhao et al,37 2009 | 1991-2001 | OSI | China | 52 (Oral cavity) | HPV DNA, PCR (6, 11, 16, 18) | 40.4 | 59.6 | OS | 50.7 |
Abbreviations: CSS, cancer-specific survival; DSS, disease-specific survival; EBV, Epstein-Barr virus; EBV+, EBV positive; EBV−, EBV negative; HPV, human papillomavirus; IHC, immunohistochemistry; ISH, in situ hybridization; NS, not specified; OS, overall survival; OSI, observational single institutional; PBO, population-based observational; PCR, polymerase chain reaction; RCT, randomized clinical trial.
In studies of the oral cavity, OS was not significantly associated with HPV positivity (HR, 1.16; 95% CI, 0.83-1.61, I2 = 71%) (Figure 1A). In subgroup analysis by diagnostic method, no significant differences were found for p16 IHC (HR, 1.11; 95% CI, 0.60-2.06; I2 = 63%) or HPV DNA (HR, 1.17; 95% CI, 0.73-1.88; I2 = 77%) subgroups. Heterogeneity scores were substantial in these models. When the only population-based study was removed from the analysis,12 the test for overall effect was nonsignificant, but the I2 score was reduced to 50% (HR, 1.25; 95% CI, 0.91-1.73). Removing the studies with unadjusted HRs also did not substantially reduce the heterogeneity or change the overall effect (HR, 1.20; 95% CI, 0.77-1.88; I2 78%). The HPV-positive tumors showed worse DFS (HR, 1.81; 95% CI, 1.12-2.91; I2 = 47%) (Figure 1B).
Figure 1. Overall and Disease-Free Survival in Studies of the Oral Cavity.
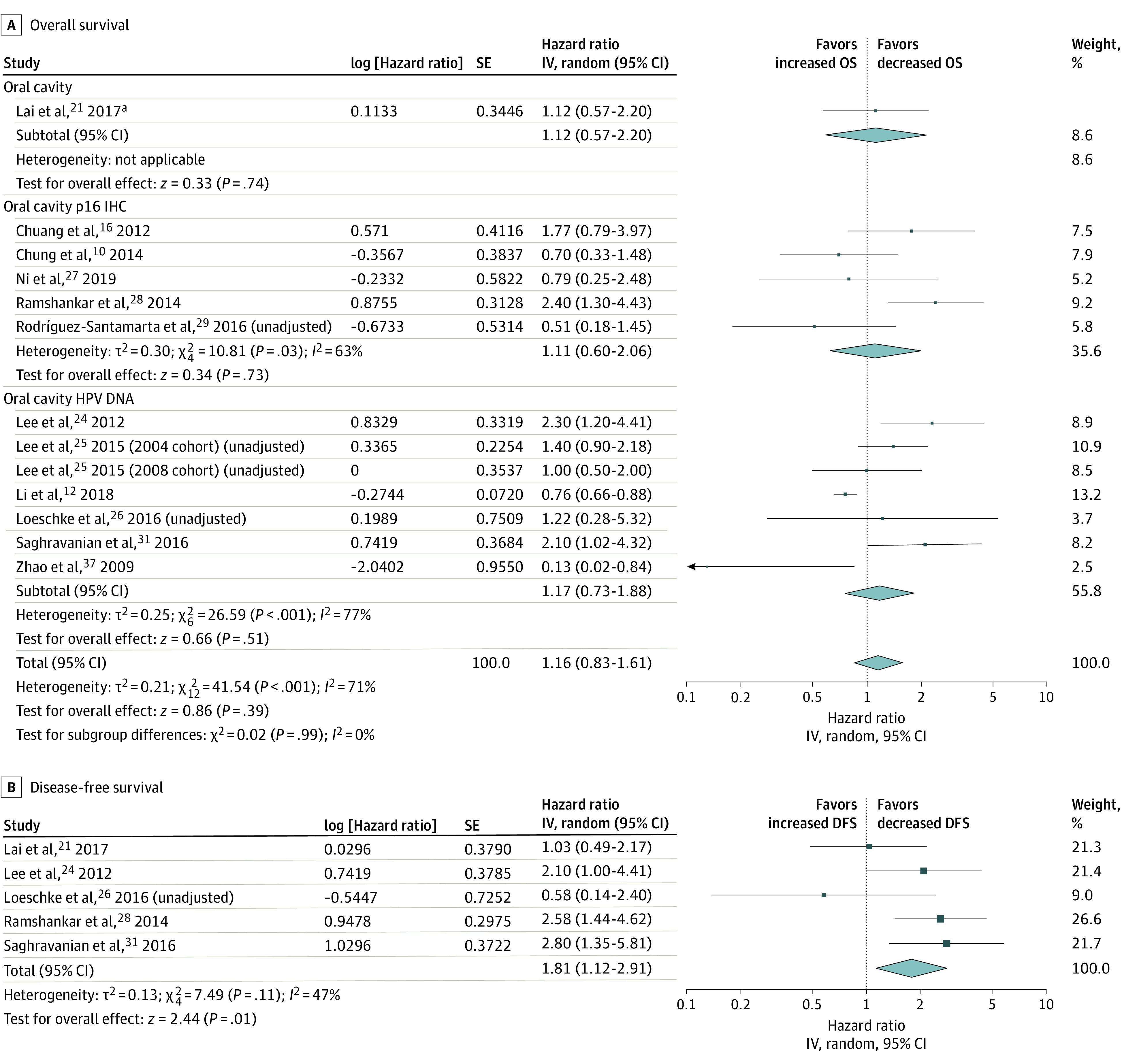
IV indicates instrumental variable.
aTested both p16 IHC and HPV DNA.
In studies of the nasopharynx, HPV positivity did not affect OS (HR, 0.82; 95% CI, 0.49-1.38; I2 = 46%). Tumors found to have HPV DNA also failed to show any difference in survival (HR, 0.69; 95% CI, 0.37-1.30; I2 = 59%) (Figure 2). In addition, DSS was not influenced by HPV (HR, 0.55; 95% CI, 0.22-1.42; I2 = 65%).
Figure 2. Overall and Disease-Specific Survival in Studies of the Nasopharynx.
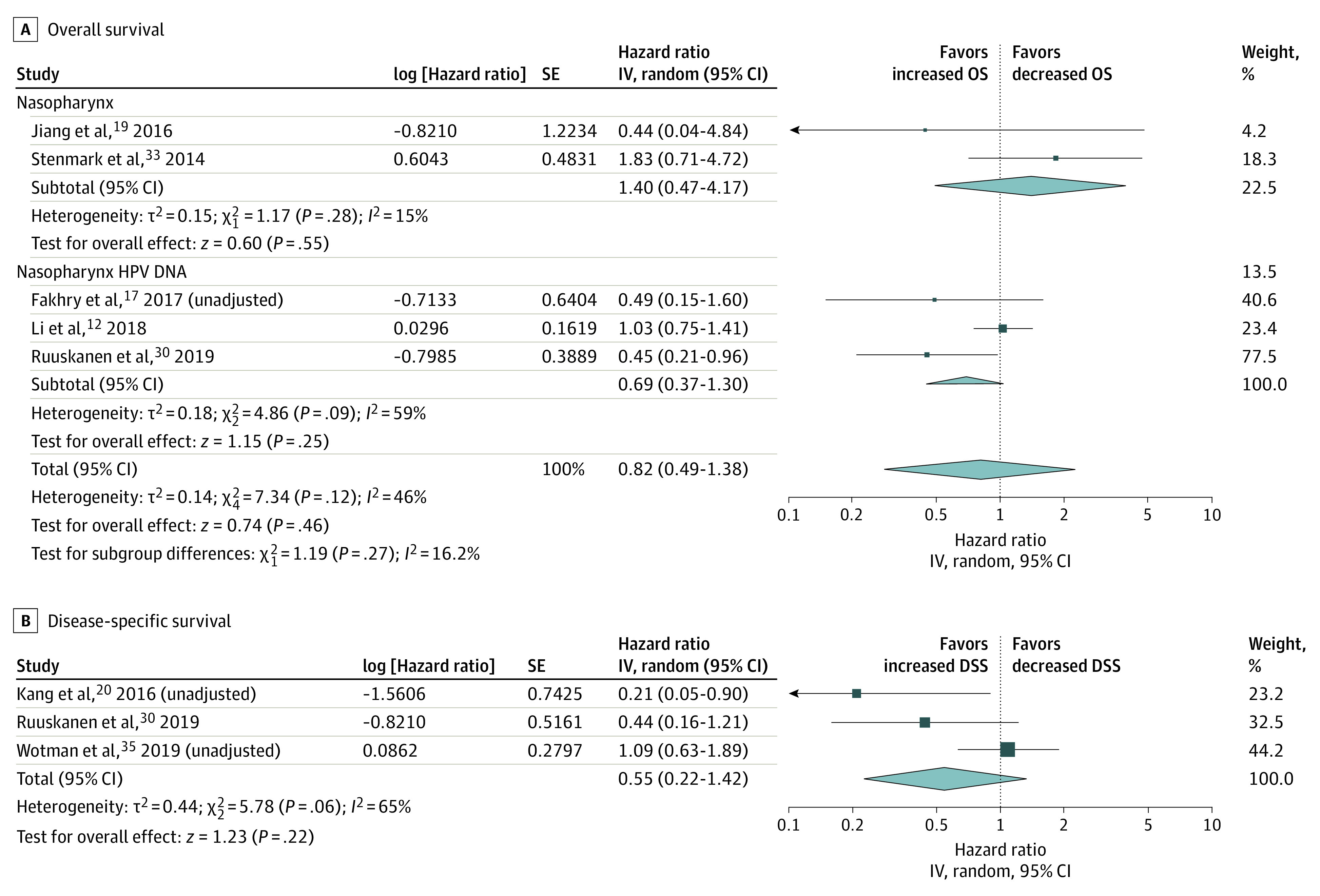
IV indicates instrumental variable.
In studies of the larynx, patients with HPV-positive tumors had improved OS (HR, 0.71; 95% CI, 0.54-0.92; I2 = 38%) (Figure 3). Subgroup analysis by p16 also showed a survival improvement in this site (HR, 0.61; 95% CI, 0.39-0.93; I2 = 29%), but not when grouped by HPV-DNA (HR, 0.79; 95% CI, 0.52-1.20; I2 = 56%) . In the latter analysis, when 1 of the 2 population-based studies18 was not included, the I2 value was 48%, but the test for overall effect was not significant (HR, 0.69; 95% CI, 0.45-1.07). We excluded this study in an attempt to reduce the heterogeneity of the HPV-DNA laryngeal cancers model. The HPV-positive hypopharyngeal tumors had improved OS (HR, 0.60; 95% CI, 0.47-0.76; I2 = 0%) (Figure 4).
Figure 3. Overall Survival (OS) in Studies of the Larynx.
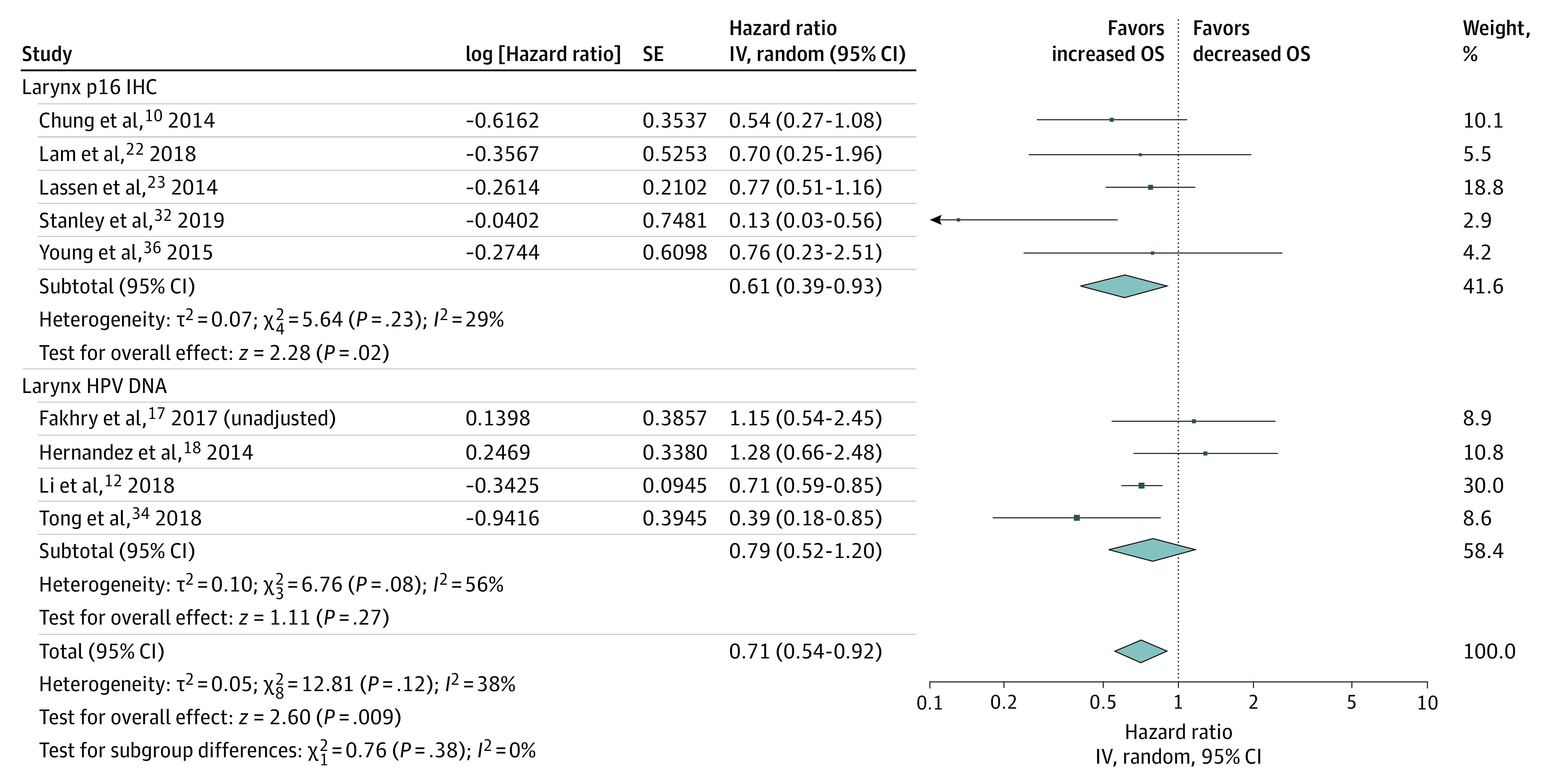
IV indicates instrumental variable.
Figure 4. Overall Survival (OS) in Studies of the Hypopharynx.
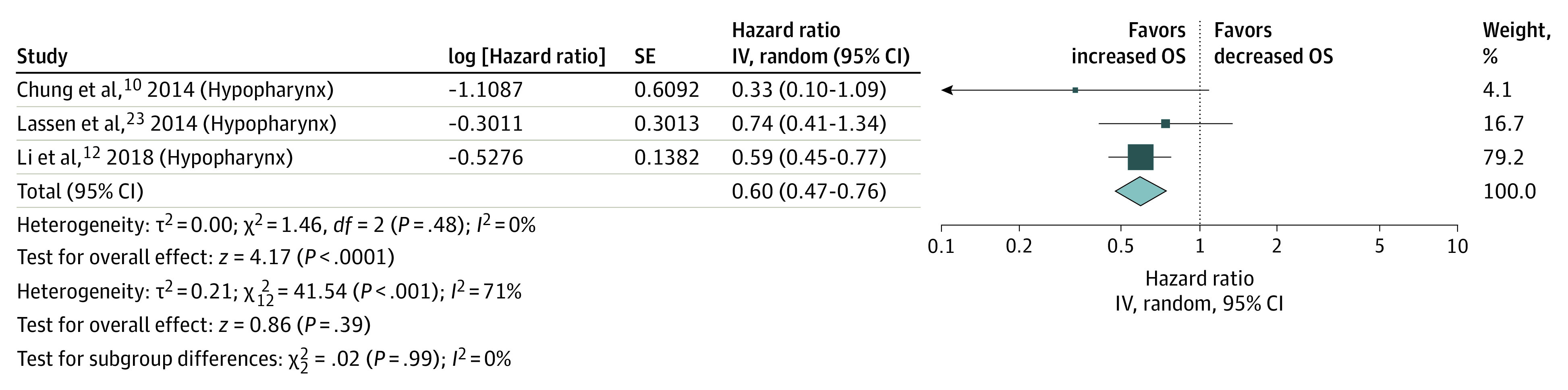
IV indicates instrumental variable.
All of the studies had quality values greater than 6 on the Newcastle-Ottawa Scale. The lowest scores were obtained in the comparability aspect owing to the different methods of determining HPV associations of the tumors (eTable in the Supplement). A funnel-shaped distribution was shown on visual inspection, indicating low risk of publication bias (eFigure 2 in the Supplement).
Discussion
To our knowledge, this is the largest and most comprehensive meta-analysis analyzing survival outcomes of HPV in non-OPSCCs separated by sites. Our analysis noted that, in laryngeal and hypopharyngeal locations, patients with HPV-positive tumors appeared to experience improved OS. In the oral cavity and in the nasopharynx, HPV status was not associated with OS. Disease-free survival was able to be calculated only for oral cavity tumors, and we found that patients with HPV-positive tumors had poorer DFS. Disease-specific survival could be measured only in nasopharyngeal tumors, where the presence of HPV did not appear to be a significant predictor of survival.
Our results suggest that HPV has different prognostic significance among the anatomic sites within the upper aerodigestive tract, highlighting the importance of comparing each site separately. Past studies have looked at HPV as a prognostic marker grouping non-OPSCCs.38 Although these studies contributed to understanding this disease, we believe that conclusions from these reports should be interpreted carefully.10 Each region possesses unique anatomic and functional characteristics; therefore, it is reasonable to think that they react diversely toward the same pathologic stimuli. In addition, treatments differ substantially in locations such as the oral cavity or larynx, with surgical and nonsurgical predominance, respectively,23 making comparisons even more problematic.
Overall survival was not influenced by HPV status in the oral cavity in the overall comparison, even after subgrouping the studies based on their diagnostic methods. We found more compelling differences in DFS, where HPV-positive tumors had decreased DFS. Most of the non-OPSCC studies pooled by our search analyzed oral cavity cancers, and more conflicting data in terms of survival can be found at that site. Contrary to our report, studies that used the US National Cancer Database11,12 found an association with HPV and improved OS. We surmise that our discrepancies with these large studies could potentially be attributed to ethnic differences in the samples, as we included Asian countries in the pooled studies,24,25,28,31,37 where there are different risk factors for oral cavity cancers. Primary site locations of oral cavity cancers differ geographically, and as the oral cavity comprises many different subsites, it is also possible that HPV plays a more meaningful role in one oral cavity subsite over another.39 Further limitations in the National Cancer Database, including variable reporting for risk factors, such as alcohol and smoking, type of HPV testing used, selection bias for HPV testing, and misclassification of the cancer site, may have contributed to conflicting results.
We were unable to find any difference in OS and DSS of HPV in nasopharyngeal tumors. Comparison of nasopharyngeal tumor data is inherently difficult due to the Epstein-Barr virus (EBV) contribution to carcinogenesis and prognostication in this location, adding another variable to the analysis.19,35 It is therefore particularly important to perform a granular analysis with all the possible combinations of HPV and EBV status. For example, Stenmark et al33 reported that HPV positivity worsened OS but in an EBV-negative cohort, and Fakhry et al17 and Li et al12 did not report the incidence of EBV in their nasopharyngeal cohort. A recent meta-analysis of nasopharyngeal cancers40 found that HPV positivity was marginally associated with improved OS but only in a subgroup analysis excluding smaller studies. The association between HPV and EBV is unclear, and future analyses should report results with all the EBV and HPV combinations.
Perhaps the most important finding of our study was the association of HPV with laryngeal and hypopharyngeal tumor outcomes. Most series report a low prevalence of HPV-positive laryngeal cancers (approximately 25%) and an even lower prevalence (approximately 6%) in the hypopharynx.9,41,42 Survival outcomes of the pooled laryngeal and hypopharyngeal studies were more aligned. In contrast to our findings, large, single-institutional studies22,23,36 did not find meaningful differences, and this discrepancy is where the value of meta-analysis is clear: pooling data from different studies allowed us to find a possible difference in studies that separately could not find a prognostic impact. Two studies17,18 reported that HPV-positive laryngeal tumors had worse OS, albeit the comparisons did not reach statistical significance in their analysis. Using the Surveillance, Epidemiology, and End Results database, Hernandez et al18 were able to adjust their findings only to glottic locations and localized vs advanced stages, but there was no information about other risk factors, such as smoking or alcohol use. When we excluded this study from the final random model effect, the I2 score became nonsignificant in the HPV-positive laryngeal tumors diagnosed by HPV DNA. In concordance with other studies, Fahkry et al17 also did not find differences in HPV DNA–positive laryngeal tumors, but we were unable to identify the possible reasons why HPV was associated with worse survival. Fewer studies analyzed prognosis in the hypopharynx, but we noted an OS improvement.
There were different HPV diagnostic methods in the included studies. Expression of HPV E6/E7 RNA indicates active viral oncogene transcription, and reverse transcriptase real-time PCR is considered the standard to determine HPV infection.43,44 Because reverse transcriptase real-time PCR requires additional steps for preparation of the sample and larger amount of tumor cells, the most widespread diagnostic methods are HPV DNA using PCR or in situ hybridization and p16 IHC. However, in regions where HPV infection is less common than in the oropharynx, concordance in sensitivity of p16 IHC compared with high-risk HPV E6/E7 real-time RNA expression is 79%, specificity is 93%, positive predictive value is 41%, and negative predictive value is 99%, indicating that p16 IHC is a poor surrogate biomarker of HPV infection in non-OPSCC.8,10 To mitigate this risk, we performed subgroup analysis, analyzing HPV DNA and p16 IHC groups separately, acknowledging that there are different diagnostic techniques within HPV DNA testing groups. We believe it is paramount to account for this difference in HPV diagnostic methods when analyzing our data. Although p16 IHC was used in 50% of the studies that fulfilled the inclusion criteria, it might not be the most adequate method to reflect active HPV infection. In an ideal setting, outcomes should be reported based on the standard diagnostic technique. The evidence comparing p16 and HPV DNA outside the oropharynx is scarce, but results showed that survival in the oral cavity, larynx, and hypopharynx was significantly higher for p16-positive with HPV DNA-positive findings, whereas p16-positive, HPV DNA-negative and p16-negative with HPV DNA-positive findings had similar outcomes. p16-Negative with HPV-DNA negative findings had the worse survival of all combinations.45 One further aspect to be considered in the diagnostic method was that, in cases where HPV DNA PCR was used, some studies selected different subtypes of HPV in their analysis (Table).
We found significant heterogeneity among studies in some models. In the oral cavity, heterogeneity was still present after subgroup analysis by diagnostic method was performed. After extracting the only population-based study12 from the model, the I2 level decreased to 50%, even though the P value for heterogeneity remained significant. The subgroup analysis of OS in HPV DNA laryngeal tumors also had a high I2 score. In this case, by removing only 1 of the 2 population-based studies, the heterogeneity was not significant. Population-based studies can sometimes contribute to heterogeneity in the random-effect models. One of the reasons can be the lack of granularity in their analysis. When heterogeneity is significant, it is worthwhile to explore whether I2 scores are decreased in the calculations by removing them and report the result of the removal. Nevertheless, it is unwise to remove population-based studies from the meta-analysis as this may introduce bias. We could not find any other clear variables to perform subgroup analysis to explore heterogeneity. In the remaining analyses with I2 scores higher than 50%, we were unable to perform subgroup analysis or meta-regression because the models had too few studies. Another aspect to consider is the heterogeneity in reporting survival data in meta-analyses. There are situations in which studies report different survival outcomes that might not be directly combinable as, for example, locoregional control and locoregional recurrence. On occasions, this issue can be addressed by inverting the corresponding effect estimates and 95% CIs, but it is not always possible. Moreover, OS is usually reported as a 5-year outcome, but that can also vary. We scrutinized studies and pooled data only when the same outcomes were reported among studies, but there were cases where median follow-up or survival years were not reported (Table).26,30
There is no validated explanation to the clinical difference and prognostic impact of HPV between OPSCC and non-OPSCC. Microtumoral environment and immunologic response differences have been reported as plausible reasons.46 Oropharyngeal squamous cell carcinomas are in close proximity with lymphoid tissues and it is hypothesized that there is an ongoing interaction between the immune system and viral antigens that can contribute to an enhanced radiosensitivity.47 Some reports that found HPV significance in hypopharyngeal and nasopharyngeal locations have surmised that there can be tumor extension from the inferior pole of the oropharynx extending to the hypopharynx and superior pole of the oropharynx extending to the nasopharynx.48 Our finding that HPV-positive laryngeal tumors have improved OS does not account for tumor extension or misclassification because laryngeal tumors are far from the oropharynx. All of these uncertainties suggest that preclinical research in this area would be beneficial.
We noted that some of the studies presented an unusually high prevalence of HPV-associated tumors.20,27,32,34 In those reports several HPV subtypes were included, but the threshold to measure HPV positiveness as not specified. In the oral cavity, it can be surmised that the high prevalence of HPV-associated tumors was attributed to higher T categories and to the extension of those tumors to the oropharynx. Nevertheless, those studies clearly stated that all the tumors were from oral cavity locations, and most of the tumors were T1 and T2 categories,16,21,29 which makes the oropharyngeal extension hypothesis less likely. Overall, it is important that the same criteria are used when reporting the association with HPV, even though there is no standardized reporting principle for tumors outside the oropharynx.
Strengths and Limitations
Among the strengths of our study is that our protocol was specified a priori, defining in advance the criteria of our analysis and following PRISMA guidelines. In addition, the literature search was performed by an experienced librarian. We perused all the available literature in an effort to exclude studies with overlapping cohorts and obtained results from studies with large patient populations.
The limitations of this study are represented by the retrospective, single-cohort nature of the included articles with inherent biases. Our meta-analysis was predominantly of observational studies, with variability among the rigor of site classification, risk factors for survival, and type of HPV testing method. Our study was also limited by statistical and clinical heterogeneity. Statistical heterogeneity was high for oral cavity and nasopharynx sites. Despite performing sensitivity analysis, we were not able to account for the high level of heterogeneity. There was clinical heterogeneity between the studies, including geographic differences, testing prevalence and methods for HPV status, subtypes of HPV reported, and prognostic factors. In our meta-analysis, we pooled studies that reported unadjusted and adjusted survival outcomes. Given that there are several prognostic factors for survival in head and neck cancer, lack of adjustment for these known risk factors may introduce bias in the pooled results. We attempted to account for this possible bias using our sensitivity analysis. The site classification of tumors can also have its flaws in each study as, in many cases, identification of the originating site for tumors involving contiguous anatomic sites can be challenging, especially in tumors of higher T categories, with the risk of misclassifying sites.
Conclusions
This study provides physicians and researchers an up-to-date methodologic summary of the outcome evidence for HPV in non-OPSCC. These data can be used in continued research on tumor sites, such as the larynx and hypopharynx, where there seems to be an association with survival in HPV-positive tumors. Subsequent research in these areas should aim to implement homogeneous diagnostic methods for HPV detection.
eMethods. Search Strategy
eFigure 1. Study Inclusion Flow Diagram
eFigure 2. Funnel Plots of Each Subsite
eTable. Newcastle-Ottawa Scale for Assessing the Quality of Nonrandomized Studies
References
- 1.Ang KK, Harris J, Wheeler R, et al. . Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363(1):24-35. doi: 10.1056/NEJMoa0912217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller MC, Goldenberg D; Education Committee of the American Head and Neck Society (AHNS) . AHNS Series: do you know your guidelines? principles of surgery for head and neck cancer: a review of the National Comprehensive Cancer Network guidelines. Head Neck. 2017;39(4):791-796. doi: 10.1002/hed.24654 [DOI] [PubMed] [Google Scholar]
- 3.Quon H, Vapiwala N, Forastiere A, et al. . Radiation therapy for oropharyngeal squamous cell carcinoma: American Society of Clinical Oncology endorsement of the American Society for Radiation Oncology evidence-based clinical practice guideline. J Clin Oncol. 2017;35(36):4078-4090. doi: 10.1200/JCO.2017.73.8633 [DOI] [PubMed] [Google Scholar]
- 4.Lewis CM, Nurgalieva Z, Sturgis EM, Lai SY, Weber RS. Improving patient outcomes through multidisciplinary treatment planning conference. Head Neck. 2016;38(suppl 1):E1820-E1825. doi: 10.1002/hed.24325 [DOI] [PubMed] [Google Scholar]
- 5.Nichols AC, Theurer J, Prisman E, et al. . Radiotherapy versus transoral robotic surgery and neck dissection for oropharyngeal squamous cell carcinoma (ORATOR): an open-label, phase 2, randomised trial. Lancet Oncol. 2019;20(10):1349-1359. doi: 10.1016/S1470-2045(19)30410-3 [DOI] [PubMed] [Google Scholar]
- 6.Chaturvedi AK, Anderson WF, Lortet-Tieulent J, et al. . Worldwide trends in incidence rates for oral cavity and oropharyngeal cancers. J Clin Oncol. 2013;31(36):4550-4559. doi: 10.1200/JCO.2013.50.3870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaturvedi AK, Engels EA, Pfeiffer RM, et al. . Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29(32):4294-4301. doi: 10.1200/JCO.2011.36.4596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lingen MW, Xiao W, Schmitt A, et al. . Low etiologic fraction for high-risk human papillomavirus in oral cavity squamous cell carcinomas. Oral Oncol. 2013;49(1):1-8. doi: 10.1016/j.oraloncology.2012.07.002 [DOI] [PubMed] [Google Scholar]
- 9.Kreimer AR, Clifford GM, Boyle P, Franceschi S. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review. Cancer Epidemiol Biomarkers Prev. 2005;14(2):467-475. doi: 10.1158/1055-9965.EPI-04-0551 [DOI] [PubMed] [Google Scholar]
- 10.Chung CH, Zhang Q, Kong CS, et al. . p16 Protein expression and human papillomavirus status as prognostic biomarkers of nonoropharyngeal head and neck squamous cell carcinoma. J Clin Oncol. 2014;32(35):3930-3938. doi: 10.1200/JCO.2013.54.5228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ko HC, Harari PM, Sacotte RM, et al. . Prognostic implications of human papillomavirus status for patients with non-oropharyngeal head and neck squamous cell carcinomas. J Cancer Res Clin Oncol. 2017;143(11):2341-2350. doi: 10.1007/s00432-017-2481-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li H, Torabi SJ, Yarbrough WG, Mehra S, Osborn HA, Judson B. Association of human papillomavirus status at head and neck carcinoma subsites with overall survival. JAMA Otolaryngol Head Neck Surg. 2018;144(6):519-525. doi: 10.1001/jamaoto.2018.0395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wells GA, Shea B, O’Connell D PJ, Welch V, Losos M TP. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-analyses. The Ottawa Hospital Research Institute; 2014. [Google Scholar]
- 14.Higgins JPT, Thomas J, Chandler J, et al. , eds. Cochrane Handbook for Systematic Reviews of Interventions version 6.0. Updated July 2019. Accessed September 14, 2020. http://www.training.cochrane.org/handbook
- 15.Verma V, Simone CB II, Lin C. Human papillomavirus and nasopharyngeal cancer. Head Neck. 2018;40(4):696-706. doi: 10.1002/hed.24978 [DOI] [PubMed] [Google Scholar]
- 16.Chuang CY, Sung WW, Wang L, et al. . Differential impact of IL-10 expression on survival and relapse between HPV16-positive and -negative oral squamous cell carcinomas. PLoS One. 2012;7(10):e47541. doi: 10.1371/journal.pone.0047541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fakhry C, Westra WH, Wang SJ, et al. . The prognostic role of sex, race, and human papillomavirus in oropharyngeal and nonoropharyngeal head and neck squamous cell cancer. Cancer. 2017;123(9):1566-1575. doi: 10.1002/cncr.30353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hernandez BY, Goodman MT, Lynch CF, et al. ; HPV Typing of Cancer Workgroup . Human papillomavirus prevalence in invasive laryngeal cancer in the United States. PLoS One. 2014;9(12):e115931. doi: 10.1371/journal.pone.0115931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang W, Chamberlain PD, Garden AS, et al. . Prognostic value of p16 expression in Epstein-Barr virus–positive nasopharyngeal carcinomas. Head Neck. 2016;38(S1)(suppl 1):E1459-E1466. doi: 10.1002/hed.24258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kang H, Kwon M, Park JJ, et al. . Clinical implications of human papilloma virus and other biologic markers in nasopharyngeal cancer. Oral Oncol. 2016;55:e7-e10. doi: 10.1016/j.oraloncology.2016.02.002 [DOI] [PubMed] [Google Scholar]
- 21.Lai K, Killingsworth M, Matthews S, et al. . Differences in survival outcome between oropharyngeal and oral cavity squamous cell carcinoma in relation to HPV status. J Oral Pathol Med. 2017;46(8):574-582. doi: 10.1111/jop.12535 [DOI] [PubMed] [Google Scholar]
- 22.Lam EWH, Chan MMH, Wai CKC, et al. . The role of human papillomavirus in laryngeal cancer in Southern China. J Med Virol. 2018;90(6):1150-1159. doi: 10.1002/jmv.25058 [DOI] [PubMed] [Google Scholar]
- 23.Lassen P, Primdahl H, Johansen J, et al. ; Danish Head and Neck Cancer Group (DAHANCA) . Impact of HPV-associated p16-expression on radiotherapy outcome in advanced oropharynx and non-oropharynx cancer. Radiother Oncol. 2014;113(3):310-316. doi: 10.1016/j.radonc.2014.11.032 [DOI] [PubMed] [Google Scholar]
- 24.Lee LA, Huang CG, Liao CT, et al. . Human papillomavirus-16 infection in advanced oral cavity cancer patients is related to an increased risk of distant metastases and poor survival. PLoS One. 2012;7(7):e40767. doi: 10.1371/journal.pone.0040767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee LA, Huang CG, Tsao KC, et al. . Human papillomavirus infections are common and predict mortality in a retrospective cohort study of Taiwanese patients with oral cavity cancer. Medicine (Baltimore). 2015;94(47):e2069. doi: 10.1097/MD.0000000000002069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Loeschke S, Ohlmann AK, Bräsen JH, Holst R, Warnke PH. Prognostic value of HMGA2, P16, and HPV in oral squamous cell carcinomas. J Craniomaxillofac Surg. 2016;44(9):1422-1429. doi: 10.1016/j.jcms.2016.06.009 [DOI] [PubMed] [Google Scholar]
- 27.Ni Y, Zhang X, Wan Y, et al. . Relationship between p16 expression and prognosis in different anatomic subsites of OSCC. Cancer Biomark. 2019;26(3):375-383. doi: 10.3233/CBM-192402 [DOI] [PubMed] [Google Scholar]
- 28.Ramshankar V, Soundara VT, Shyamsundar V, Ramani P, Krishnamurthy A. Risk stratification of early stage oral tongue cancers based on HPV status and p16 immunoexpression. Asian Pac J Cancer Prev. 2014;15(19):8351-8359. doi: 10.7314/APJCP.2014.15.19.8351 [DOI] [PubMed] [Google Scholar]
- 29.Rodríguez-Santamarta T, Rodrigo JP, García-Pedrero JM, et al. . Prevalence of human papillomavirus in oral squamous cell carcinomas in northern Spain. Eur Arch Otorhinolaryngol. 2016;273(12):4549-4559. doi: 10.1007/s00405-016-4152-9 [DOI] [PubMed] [Google Scholar]
- 30.Ruuskanen M, Irjala H, Minn H, et al. . Epstein-Barr virus and human papillomaviruses as favorable prognostic factors in nasopharyngeal carcinoma: a nationwide study in Finland. Head Neck. 2019;41(2):349-357. doi: 10.1002/hed.25450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saghravanian N, Zamanzadeh M, Meshkat Z, Afzal Aghaee M, Salek R. Evaluation of the prevalence rate and the prognostic effect of human papilloma virus infection in a group of patients with oral cavity squamous cell carcinoma. Iran J Cancer Prev. 2016;9(3):e3998. doi: 10.17795/ijcp-3998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stanley AJ, Srivastava A, Contreras J, et al. . p16 as a prognostic biomarker in squamous cell carcinoma of the supraglottic larynx. Int J Radiat Oncol Biol Phys. 2019;105(1)(suppl):E367-E368. doi: 10.1016/j.ijrobp.2019.06.1710 [DOI] [Google Scholar]
- 33.Stenmark MH, McHugh JB, Schipper M, et al. . Nonendemic HPV-positive nasopharyngeal carcinoma: association with poor prognosis. Int J Radiat Oncol Biol Phys. 2014;88(3):580-588. doi: 10.1016/j.ijrobp.2013.11.246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tong F, Geng J, Yan B, et al. . Prevalence and prognostic significance of HPV in laryngeal squamous cell carcinoma in northeast China. Cell Physiol Biochem. 2018;49(1):206-216. doi: 10.1159/000492858 [DOI] [PubMed] [Google Scholar]
- 35.Wotman M, Oh EJ, Ahn S, Kraus D, Costantino P, Tham T. HPV status in patients with nasopharyngeal carcinoma in the United States: a SEER database study. Am J Otolaryngol. 2019;40(5):705-710. doi: 10.1016/j.amjoto.2019.06.007 [DOI] [PubMed] [Google Scholar]
- 36.Young RJ, Urban D, Angel C, et al. . Frequency and prognostic significance of p16(INK4A) protein overexpression and transcriptionally active human papillomavirus infection in laryngeal squamous cell carcinoma. Br J Cancer. 2015;112(6):1098-1104. doi: 10.1038/bjc.2015.59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao D, Xu QG, Chen XM, Fan MW. Human papillomavirus as an independent predictor in oral squamous cell cancer. Int J Oral Sci. 2009;1(3):119-125. doi: 10.4248/IJOS.09015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.D’Souza G, Westra WH, Wang SJ, et al. . Differences in the prevalence of human papillomavirus (HPV) in head and neck squamous cell cancers by sex, race, anatomic tumor site, and HPV detection method. JAMA Oncol. 2017;3(2):169-177. doi: 10.1001/jamaoncol.2016.3067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shield KD, Ferlay J, Jemal A, et al. . The global incidence of lip, oral cavity, and pharyngeal cancers by subsite in 2012. CA Cancer J Clin. 2017;67(1):51-64. doi: 10.3322/caac.21384 [DOI] [PubMed] [Google Scholar]
- 40.Tham T, Teegala S, Bardash Y, Herman SW, Costantino P. Is human papillomavirus and p16 expression associated with survival outcomes in nasopharyngeal cancer?: a systematic review and meta-analysis. Am J Otolaryngol. 2018;39(6):764-770. doi: 10.1016/j.amjoto.2018.07.005 [DOI] [PubMed] [Google Scholar]
- 41.Isayeva T, Li Y, Maswahu D, Brandwein-Gensler M. Human papillomavirus in non-oropharyngeal head and neck cancers: a systematic literature review. Head Neck Pathol. 2012;6(S1)(suppl 1):S104-S120. doi: 10.1007/s12105-012-0368-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wendt M, Romanitan M, Näsman A, et al. . Presence of human papillomaviruses and p16 expression in hypopharyngeal cancer. Head Neck. 2014;36(1):107-112. doi: 10.1002/hed.23394 [DOI] [PubMed] [Google Scholar]
- 43.Shi W, Kato H, Perez-Ordonez B, et al. . Comparative prognostic value of HPV16 E6 mRNA compared with in situ hybridization for human oropharyngeal squamous carcinoma. J Clin Oncol. 2009;27(36):6213-6221. doi: 10.1200/JCO.2009.23.1670 [DOI] [PubMed] [Google Scholar]
- 44.Jordan RC, Lingen MW, Perez-Ordonez B, et al. . Validation of methods for oropharyngeal cancer HPV status determination in US cooperative group trials. Am J Surg Pathol. 2012;36(7):945-954. doi: 10.1097/PAS.0b013e318253a2d1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stephen JK, Divine G, Chen KM, Chitale D, Havard S, Worsham MJ. Significance of p16 in site-specific HPV positive and HPV negative head and neck squamous cell carcinoma. Cancer Clin Oncol. 2012;2(1):51-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chakravarthy A, Henderson S, Thirdborough SM, et al. . Human papillomavirus drives tumor development throughout the head and neck: improved prognosis is associated with an immune response largely restricted to the oropharynx. J Clin Oncol. 2016;34(34):4132-4141. doi: 10.1200/JCO.2016.68.2955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rieckmann T, Tribius S, Grob TJ, et al. . HNSCC cell lines positive for HPV and p16 possess higher cellular radiosensitivity due to an impaired DSB repair capacity. Radiother Oncol. 2013;107(2):242-246. doi: 10.1016/j.radonc.2013.03.013 [DOI] [PubMed] [Google Scholar]
- 48.Wilson DD, Crandley EF, Sim A, et al. . Prognostic significance of p16 and its relationship with human papillomavirus in pharyngeal squamous cell carcinomas. JAMA Otolaryngol Head Neck Surg. 2014;140(7):647-653. doi: 10.1001/jamaoto.2014.821 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Search Strategy
eFigure 1. Study Inclusion Flow Diagram
eFigure 2. Funnel Plots of Each Subsite
eTable. Newcastle-Ottawa Scale for Assessing the Quality of Nonrandomized Studies


