Abstract
PURPOSE:
Early detection and management of symptoms in patients with cancer improves outcomes. However, the optimal approach to symptom monitoring and management is unknown. InSight Care is a mobile health intervention that captures symptom data and facilitates patient-provider communication to mitigate symptom escalation.
PATIENTS AND METHODS:
Patients initiating antineoplastic treatment at a Memorial Sloan Kettering regional location were eligible. Technology supporting the program included the following: a predictive model that identified patient risk for a potentially preventable acute care visit; a secure patient portal enabling communication, televisits, and daily delivery of patient symptom assessments; alerts for concerning symptoms; and a symptom-trending application. The main outcomes of the pilot were feasibility and acceptability evaluated through enrollment and response rates and symptom alerts, and perceived value evaluated on the basis of qualitative patient and provider interviews.
RESULTS:
The pilot program enrolled 100 high-risk patients with solid tumors and lymphoma (29% of new treatment starts v goal of 25%). Over 6 months of follow-up, the daily symptom assessment response rate was 56% (the goal was 50%), and 93% of patients generated a severe symptom alert. Patients and providers perceived value in the program, and archetypes were developed for program improvement. Enrolled patients were less likely to use acute care than were other high-risk patients.
CONCLUSION:
InSight Care was feasible and holds the potential to improve patient care and decrease facility-based care. Future work should focus on optimizing the cadence of patient assessments, the workforce supporting remote symptom management, and the return of symptom data to patients and clinical teams.
INTRODUCTION
Complications of therapy have rapidly increased emergency department (ED) visits by patients with cancer, growing 5.5-fold faster than overall ED visits from 2006 to 2015.1 At the same time, potentially preventable admissions for patients with metastatic cancer grew by 34% in the United States.2 The Department of Health and Human Services identified the care of patients receiving antineoplastic treatment as an area of health care needing improvement and cited the root causes of care gaps as (1) the delayed onset of chemotherapy symptoms that patients must manage at home, (2) patients assuming little can be done and not seeking assistance until their symptoms worsen, and (3) limited access to and communication with providers who can tailor care to individual patient needs.4
Innovations in care delivery have sought to close these care gaps. Proactive symptom reporting increased quality of life, reduced ED visits, and improved overall survival.5,6 Providing high-risk patients with enhanced access and care coordination decreases ED visits, hospitalizations, and intensive care unit admissions.7,8 Connected health efforts to intensively monitor high-risk patients have been promoted as another solution.9 There is an opportunity for new models of care delivery that provide proactive, coordinated, and participatory care to patients receiving antineoplastic treatment through digital management and engagement.
We describe a technology-enabled program called InSight Care,10 which identifies patients initiating antineoplastic therapy at high risk of a potentially preventable acute care visit (PPACV), monitors the symptoms of enrolled patients daily, and intervenes as necessary. We assess enrollment characteristics, response rates, symptom alerts, and patient and provider perceptions. In an exploratory analysis, we also compare acute care use for InSight Care–enrolled patients with high-risk patients not enrolled in the program.
PATIENTS AND METHODS
This study received a waiver of informed consent from the Memorial Sloan Kettering Cancer Center (MSK) Institutional Review Board.
Program Description, Participants, Enrollment
We conducted a single-arm pilot study in the medical oncology clinics at MSK Westchester between October 15, 2018, and July 10, 2019. Eligible patients (1) were 18 years of age or older; (2) had a diagnosis of a solid tumor or lymphoma; (3) were starting intravenous antineoplastic therapy, including a cytotoxic, immunologic, or biologic agent, that was initial therapy or had been at least 6 months since their last treatment; (4) had access to a smartphone, tablet, or computer; (5) were enrolled in the patient portal; and (6) were identified by our risk stratification model or clinical criteria as at high risk of a PPACV. The risk stratification model, described elsewhere,11 used data from the electronic medical record (EMR) to prospectively estimate the risk of a PPACV within the next 6 months for patients starting intravenous therapy. The top quartile of patients identified by this model were categorized as “high risk” and were offered enrollment in the program. Patients in the other 3 quartiles could be enrolled if they possessed 1 or more of the following clinical criteria for high risk identified by their clinical team: (1) comorbidities that increased the risk of a treatment complication; (2) provider-identified barriers to care; (3) non-MSK ED visits or hospitalizations; (4) inability to aliment sufficiently; (5) high tumor burden or site of metastasis concerning for symptom elicitation; (6) high psychosocial distress or multiple symptomatic complaints; (7) dose reduction with initial antineoplastic treatment; or (8) combined modality therapy. These criteria were developed on the basis of discussions with clinicians about relevant risk factors and were features missed by the predictive model because representative EMR data were lacking at treatment start. Patients were excluded if they were in a therapeutic clinical trial or if they could not speak English.
At their first treatment visit, patients received an orientation to the InSight Care program (approximately 30 minutes), which included completing a baseline patient-reported outcome (PRO) symptom assessment and viewing an informational video.12 The purpose of the orientation was to provide a program overview, introduce the technology interface, and emphasize the role of the symptom assessments in their care.
Symptom Monitoring
A dedicated team of oncology registered nurses (RNs) and nurse practitioners (NPs), the InSight Care team, monitored and managed the symptoms reported. These clinicians were based at MSK Westchester and acted as an extension of the primary oncology team, assisting with patient management exclusively through the digital platform without ambulatory clinic visits. If symptoms required evaluation, they referred patients to the appropriate health care setting. These clinicians were recruited from MSK oncology practice nursing and the Supportive Care Service and received additional training in the technology platform used in this program. Two RNs and 1 NP actively monitored and managed patient symptoms from 7 am to 7:30 pm. After-hours calls were taken as home calls by an InSight NP. Care was documented for all clinical teams to access in the AllScripts EMR in a specifically designed InSight Care templated note that allowed for the incorporation of patient-reported outcomes (PROs) and portal secure messages.
Technology Support
riskExplorer and enrollment decision support.
The MSK Web application known as riskExplorer was integrated into the InSight Care digital platform to display the top 10 features contributing to each patient’s estimated risk of a PPACV within 6 months of therapy initiation.
Messaging and televisit platform.
The Portal Secure Messaging via the MSK patient portal facilitated asynchronous, bidirectional communication between the patient and the InSight Care team and sent patients prompts to complete daily symptom assessments. Interaction also occurred by phone or televisit when appropriate (Appendix Fig A1, online only).
Symptom assessments and symptom alerts.
Starting the day of treatment initiation, enrolled patients received daily electronic symptom assessments through the MSK patient portal. Survey questions were based on the Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events and the ongoing Electronic Patient Reporting of Symptoms During Cancer Treatment (PRO-TECT) trial.13,14 Assessments focused on symptoms driving PPACVs for MSK patients receiving antineoplastic treatment and included pain, activity, anorexia/dehydration, nausea, vomiting, constipation, diarrhea, dyspnea, anxiety, and depression.15
The survey was designed for a 1-time daily interaction that could range from less than a minute to several minutes, depending on the patient’s symptoms. Purposeful design elements of the survey included (1) completion via mobile device or desktop computer; (2) branching logic for reported symptoms to ask additional questions about extent, severity, and interference with daily activities; (3) cross-question consistency (eg, patients with constipation were not asked about diarrhea); (4) an avatar to localize pain symptoms; (5) prior day responses prepopulated so only changes needed to be entered; and (6) a free text box for additional symptom information.
Logic was developed to generate alerts, red for severe symptoms and yellow for mild/moderate symptoms, which were sent via portal messaging to the InSight Care team; red alerts required an immediate response often within minutes of submission. Red alerts also generated the following response to the patient: “You have a symptom that your care team will help manage. During evenings, call your doctor’s office and say you are part of InSight Care. In an emergency, call 911 or go to your local ER.”
Symptom tracker.
We developed an internal Web-based application that assisted the InSight Care team in trending symptom data. This application indicated the patient’s last antineoplastic therapy and included tabs to other EMR data such as pathology and radiology to provide clinical context for symptoms (Appendix Fig A1).
Feasibility
The program was designed to care for the quartile of patients most likely to require a PPACV on the basis of the predictive model and clinician-identified risk. Feasibility was defined as the enrollment of ≥ 25% of patients starting antineoplastic therapy. Model risk scores and clinical risk criteria for patients not in the top risk quartile were recorded. Response rate was evaluated as the proportion of daily assessments completed, with a goal of > 50% survey completion in the 6 months after treatment start. We also tracked the type of symptoms reported and the number of red and yellow alerts generated.
Acceptability and Perceived Value
Acceptability and perceived value were assessed through qualitative interviews and observations by the MSK Design Innovation Group, composed of designers skilled in ethnographic-style research; affinity mapping; and identifying social, experiential, and systems insight to guide care delivery innovation. Ten patients enrolled in InSight Care; 7 follow-up clinic visits and 3 clinic days and 1 nonclinic day were observed. Eleven patients, 10 participating medical oncologists, and 7 oncology practice nurses were interviewed. These assessments were used to develop insights about the challenges faced and whether the program met patient and primary oncology team needs. Archetypes of patients’ perceptions of the program were created to further the development of patient-centric value propositions.
Acute Care Usage
Staffed 24/7, the MSK Urgent Care Center (UCC) is the central point of entry for unplanned hospital admissions. Visit rates to the UCC by enrolled patients were compared with rates among nonparticipating high-risk patients.
RESULTS
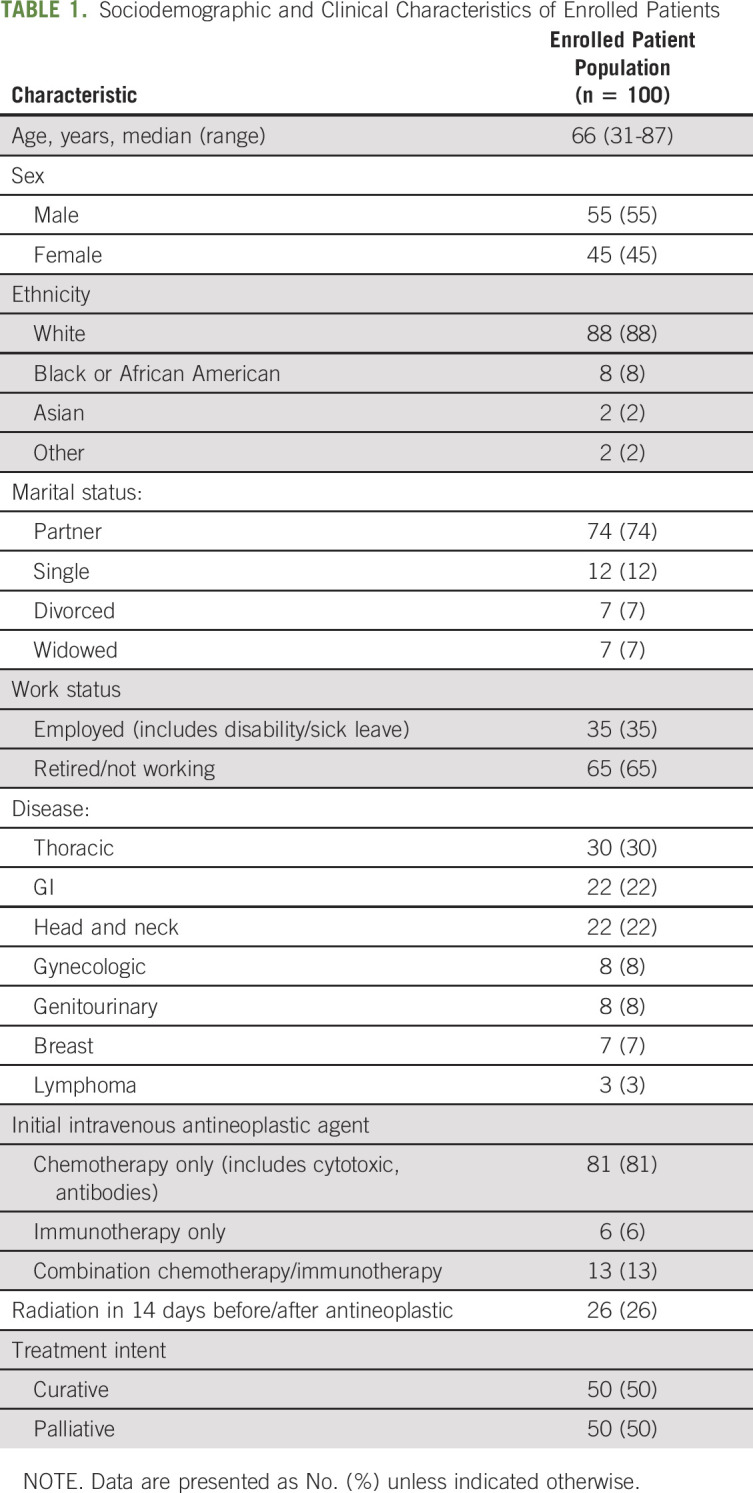
From October 15, 2018, to July 10, 2019, there were 342 patients with eligible intravenous antineoplastic therapy starts at MSK Westchester; 100 (29%) were enrolled. The average age of the enrolled patients was 66 years (range, 31-87 years), and 45% were female. Thirty percent of the patients had a thoracic malignancy, followed by GI (22%), head and neck (22%), and other malignancies (26%; Table 1). Most patients entered the program through clinician identification of high-risk criteria (74%), with multimodality therapy (39%), high tumor burden or site of metastasis concerning for symptom elicitation (39%), and high psychosocial distress or multiple symptomatic complaints (30%) the most common clinical criteria. Reasons patients were not enrolled in InSight Care included logistic reasons (n = 17; did not complete program onboarding before their initial treatment), could not access technology (n = 7), declined to participate (n = 5), did not speak English (n = 3), and other reasons (n = 1). Providers could select multiple reasons per patient (n = 28 model-identified high-risk patients did not enroll). Six months after enrollment, 59 enrolled patients exited the program and 41 remained. Patients exited for the following reasons: 24 completed treatment, 21 died or transitioned to hospice, 11 chose to stop participating, and 3 transitioned care to another team or institution. The average enrollment duration of the exited patients was 90 days.
TABLE 1.
Sociodemographic and Clinical Characteristics of Enrolled Patients
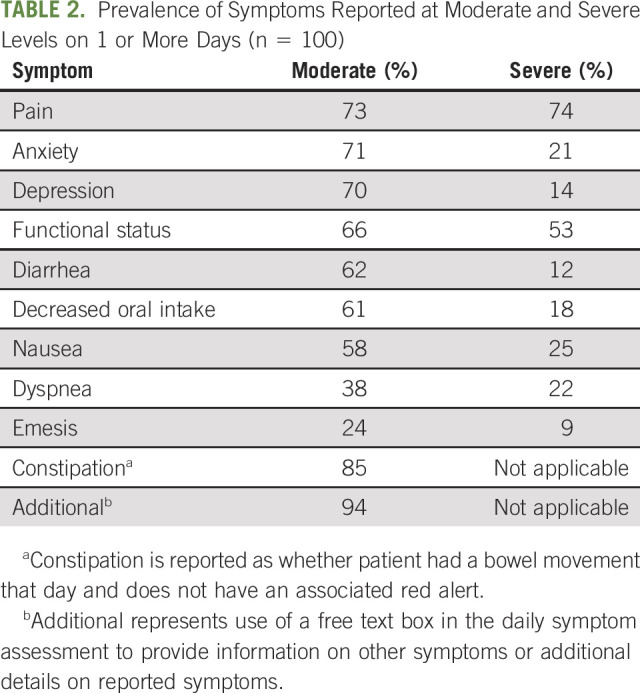
Response rate (completion of daily assessments) in the first 30 days of the program was 67%, and it decreased gradually with time in the program. Response rate in days 31 to 90 of the program was 60% and in days 91 to 180 of the program, 56%. Ninety-three percent of patients generated at least 1 red alert, with 74% of patients generating a red alert for pain, 53% for activity, and 25% for nausea (Table 2). The most frequent moderate/mild symptoms were constipation (85%), pain (73%), and activity (66%). Ninety-four percent of patients made use of the free text box, mainly to add details about their symptoms. The In Sight Care team and enrolled patients shared 5,010 symptom-related portal messages during the 6-month follow-up period.
TABLE 2.
Prevalence of Symptoms Reported at Moderate and Severe Levels on 1 or More Days (n = 100)
Patient interviews suggest that they valued the following:
Speedy responses: Patients appreciated rapid responses after submitting a survey reporting issues. “One time I included this issue in my survey, they reached out in 5 minutes. Nothing but good.” – Patient
The feeling of having a safety net: Patients were reassured by having 24/7 access to clinicians. “The best part is I don’t feel alone in this. They [InSight Care Team] have been a lifesaver for me.” – Patient
Convenience: Patients appreciated the ability to address issues without an in-person visit. “That was good. I didn’t have to get dressed and go to another doctor’s appointment.”– Patient
We created archetypes of the patients on the basis of these interviews (Fig 1), showing (1) motivators for completing the survey; (2) value drivers; (3) barriers to participation; (4) impact of program reinforcement from the primary oncologist; and (5) met and unmet patient needs. The archetypes helped us identify improvement areas, which include additional patient education, reinforcement by primary oncologists, and assessment usability.
Fig 1.
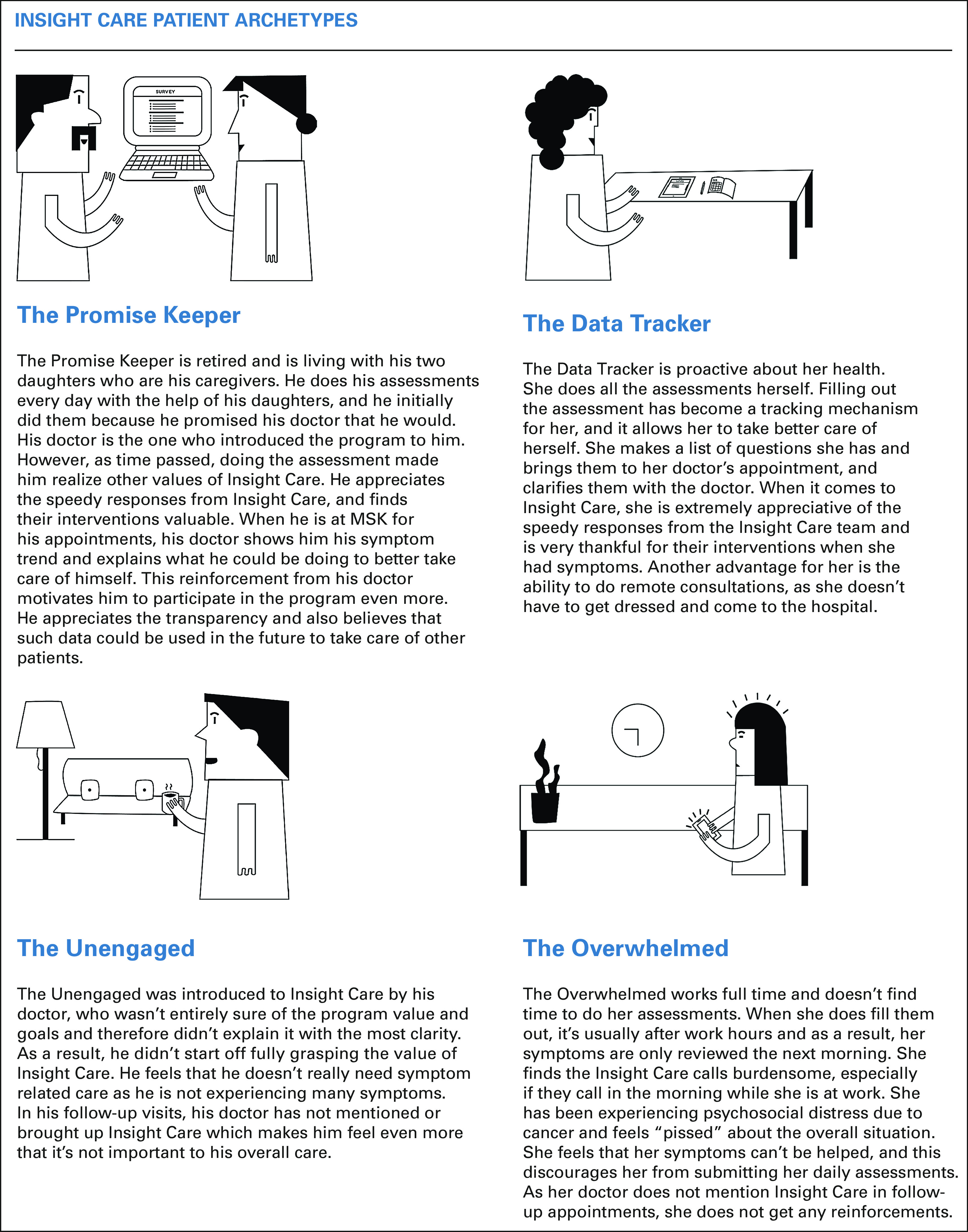
InSight Care patient archetypes.
Providers with a high proportion of their patients enrolled in InSight Care saw tangible benefits of the program and expressed that InSight Care acts like an extension of the nurse and provides continuity in care. Office practice nurses felt like InSight Care reduced some of their workload, especially since the program focused on high-risk patients. In contrast, providers with fewer patients enrolled in the program indicated that they received too many updates on only a few patients in their clinic and were concerned about fragmentation of care with a centralized team. Nurses felt a lack of clarity in roles and responsibilities between themselves and the InSight Care nurses.
Presentation rates to the MSK UCC within 6 months of enrollment were 22% (22 of 100) in the InSight Care cohort and 39% (11 of 28) for model-identified high-risk patients who did not enroll. Although this pilot was not designed as an effectiveness study, it does provide an early signal that the intervention may reduce acute care visits.
DISCUSSION
The InSight Care pilot was developed to extend provider interactions with patients receiving antineoplastic therapy wherever and whenever they were needed. Our results show that a dedicated clinical team can monitor symptoms using a digital platform and can manage care. The pilot was feasible, acceptable, and perceived to be of value by high-risk patients and primary oncology teams with a high proportion of enrolled patients.
This program has 2 key areas of innovation. The first is the focus on medical oncology patients at high risk of a PPACV, identified using a risk stratification model and clinical criteria. The second is our leveraging of a digital platform to enable staff to collect and visualize clinical and patient-reported information and then to connect with patients with minimal barriers.
This pilot has raised additional research questions about operationalizing PROs and remote monitoring strategies:
Patient identification: Previous studies have shown that identifying high-risk patients and providing them with intensive attention reduces their acute care use and can improve survival.5-8 PPACVs frequently arise during treatment initiation, so recognition of high-risk patients must occur early.16 Predictive analytic models can focus resources on those most in need; however, few have been implemented in clinical practice.11 We found that risk stratification models must work in collaboration with the clinician. Most patients were enrolled in the program on the basis of clinical risk criteria and not their model-predicted high-risk status. Future work should focus on understanding the individual contributions, and/or synergy, of the risk model and clinical judgment in identifying patients who benefit from programs such as InSight Care.
PRO cadence: There is a balance between the patient burden of completing assessments, including the psychological stress of daily reminders of their disease and the value of frequent check-ins to identify symptoms before they intensify. We saw that the response rate declined with time but remained more than 50% (every other day completion) at 6 months of enrollment. In interviews, patients did not view the daily assessment as a burden and valued it as an ability to connect with the team. However, the optimal delivery of symptom assessments, including whether this should be customized to each patient, requires additional evaluation and becomes increasingly important , because the Centers for Medicare and Medicaid Innovation recently announced that electronic PROs would be mandated for practices participating in the Oncology Care First Model.17
Workforce: Multiple models have been implemented for monitoring and managing symptoms reported through PROs. Mooney et al19 found that symptom outcomes improved when assessments were received by a dedicated advanced practice provider. Basch et al6 found that returning PRO assessments to the primary oncology team improved outcomes. In our pilot, we found that the optimal staffing model must provide knowledgeable, disease-specific, real-time responses and care coordination to patients, but how best to leverage advanced practice providers, oncology nurses, and other clinic staff is unclear, and variations in this care delivery have been proposed.19a
Return of information: The mechanisms by which monitoring PROs extend life and reduce acute care use are unknown. Our symptom tracking tools were developed for the dedicated InSight Care team. Effectively communicating symptom data to the primary clinical team will require summarization and integration into their workflow. Earlier detection of symptoms by the primary team could result in modification of treatment or earlier scans to identify disease progression. It also may be valuable to provide symptom data to patients. The ability of patients to view PROs may improve patient activation, self-realization, and coping by providing more transparency regarding their symptoms. There might also be an opportunity to share symptom reports of other patients to help patients understand symptom norms.19b Optimally integrating PROs into the patient’s treatment is an area of active exploration for future iterations of InSight Care.
This was a pilot feasibility and acceptability study without a control arm conducted at a single site. We did not attempt to establish efficacy. A future study is planned with a larger patient population and a matched control group to accurately measure the effect of InSight Care on acute care use. The cost-benefit of this intervention in its current form is also unclear. From a staffing perspective, we plan to refine this operating model over time as we learn more about the volume and nature of symptom data being generated and the corresponding interventions. The cost-effectiveness analysis would need to include the staffing costs for the InSight Care team, as well as savings from prevented acute care and potential redeployment of nursing from symptom calls to more clinic-based encounters.
This pilot has raised significant research questions about the optimal implementation of predictive analytics and remote monitoring of high-risk patients through a digital platform. This becomes increasingly important in a post-COVID-19 environment; with initial data suggesting that patients with cancer are twice as likely to become infected and are at high risk of severe clinical events, there is a greater need to minimize their facility-based encounters and to provide more care through remote methods.20,21 As more care is shifted to virtual encounters,22 creating a digital platform that allows patients with cancer to feel seen, have their symptom needs met, and connect to their oncology teams is crucial, all while ensuring that the implementation optimizes clinical workflows.
ACKNOWLEDGMENT
We are grateful to Clare Wilhelm, PhD, for his thorough review and assistance with our manuscript.
Appendix
Fig A1.
Patient-facing interface for receiving notifications and communication with InSight Care team. (A) Patients receive a message notification on the Patient Portal to complete their survey. Bidirectional, self-directed communication is provided through telephone, televisits, and the Patient Portal. Design elements include the following: the notifications patients receive to complete their daily symptom survey, a tab allowing patients direct communication with the InSight Care team on the Patient Portal’s home page, and general communication drop-down that routes the patient to the InSight Care team. (B) Patient-facing portal home page. (C) Portal secure messages drop-down menu. (D–E) Clinician-facing interfaces to support clinical work. (D) Symptom data on the symptom tracker. The InSight Care clinicians can trend symptom data as shown, which allows the team to notice gradual changes in symptoms over time. (E) Portal secure message (PSM) alerts for a fictional patient. Both the InSight Care team and the Primary Oncology Teams receive PSMs with the patient’s survey responses once complete, as well as any other communications about symptoms made by the patient to the InSight Care team.
PRIOR PRESENTATION
Presented in part at the June 2019 and June 2020 Annual Meetings of the American Society of Clinical Oncology, Chicago, IL.
SUPPORT
Supported in part through National Institutes of Health National Cancer Institute Grant No. P30 CA008748. The funder had no role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
AUTHOR CONTRIBUTIONS
Conception and design: Bobby Daly, Gilad Kuperman, Abigail Baldwin Medsker, Alice S. Ro, Javiera Arenas, Hrudaya Veena Yanamandala, Raj Kottamasu, Jessie Holland, Chasity B. Walters, Aaron Begue,
Melissa Zablocki, Amandeep K. Dhami, Yeneat O. Chiu, Isaac Wagner, Stephen R. Veach, Brett A. Simon
Financial support: Aaron Begue
Administrative support: Chasity B. Walters, Aaron Begue, Isaac Wagner
Provision of study material or patients: Bobby Daly, Chasity B. Walters, Aaron Begue
Collection and assembly of data: Bobby Daly, Gilad Kuperman, Alice S. Ro, Jessie Holland, Aaron Begue, Nicholas Silva, Stefania Sokolowski, Isaac Wagner, Stephen R. Veach, Rachel N. Grisham
Data analysis and interpretation: Bobby Daly, Gilad Kuperman, Alice S. Ro, Jessie Holland, Kim Chow, Aaron Begue, Nicholas Silva, Stefania Sokolowski, Isaac Wagner, Brett A. Simon
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
InSight Care Pilot Program: Redefining Seeing a Patient
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/op/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Bobby Daly
Leadership: Quadrant Holdings
Stock and Other Ownership Interests: Quadrant Holdings (I), Lilly (I), Pfizer (I), Cigna (I), Baxter (I), Zoetis (I), CVS Health, Walgreens Boots Alliance, IBM
Other Relationship: AstraZeneca
Open Payments Link: https://openpaymentsdata.cms.gov/physician/2785286
Raj Kottamasu
Stock and Other Ownership Interests: Amgen, Bristol Myers Squibb, Gilead Sciences, Anthem, BMY, AbbVie
Isaac Wagner
Consulting or Advisory Role: Nan Fung Life Sciences
Rachel N. Grisham
Consulting or Advisory Role: Mateon Therapeutics, Clovis Oncology, Regeneron
Research Funding: Context Therapeutics (Inst)
Travel, Accommodations, Expenses: EMD Serono
Other Relationship: Prime Oncology, MCM Education, OncLive
Diane L. Reidy-Lagunes
Honoraria: Novartis
Consulting or Advisory Role: Ipsen, Novartis, Lexicon, AAA
Research Funding: Novartis, Ipsen
Brett A. Simon
Patents, Royalties, Other Intellectual Property: Patent application US2015/0290418, patent application US13/118109
No other potential conflicts of interest were reported.
REFERENCES
- 1. Jairam V, Lee V, Park HS, et al. Treatment-related complications of systemic therapy and radiotherapy. JAMA Oncol. 2019;5:1028–1035. doi: 10.1001/jamaoncol.2019.0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Daly B, Abougergi MS. National trends in admissions for potentially preventable conditions among patients with metastatic solid tumors, 2004-2014. J Clin Oncol. 2018;36:26. [Google Scholar]
- 3. Reference deleted.
- 4. https://www.gpo.gov/fdsys/pkg/FR-2016-08-22/pdf/2016-18476.pdf Department of Health and Human Services Centers for Medicare & Medicaid Services: Medicare program: Hospital inpatient prospective payment systems for acute care hospitals and the long-term care hospital prospective payment system and policy changes and fiscal year 2017 rates; quality reporting requirements for specific providers; graduate medical education; hospital notification procedures applicable to beneficiaries receiving observation services; technical changes relating to costs to organizations and Medicare cost reports; finalization of interim final rules with comment period on LTCH PPS payments for severe wounds, modifications of limitations on redesignation by the Medicare Geographic Classification Review Board, and extensions of payments to MDHs and low volume hospitals. [PubMed]
- 5. Basch E, Deal AM, Dueck AC, et al. Overall survival results of a trial assessing patient-reported outcomes for symptom monitoring during routine cancer treatment. JAMA. 2017;318:197–198. doi: 10.1001/jama.2017.7156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Basch E, Deal AM, Kris MG, et al. Symptom monitoring with patient-reported outcomes during routine cancer treatment: A randomized controlled trial. J Clin Oncol. 2016;34:557–565. doi: 10.1200/JCO.2015.63.0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rocque GB, Partridge EE, Pisu M, et al. The Patient Care Connect Program: Transforming health care through lay navigation. J Oncol Pract. 2016;12:e633–e642. doi: 10.1200/JOP.2015.008896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rocque GB, Pisu M, Jackson BE, et al. Resource use and Medicare costs during lay navigation for geriatric patients with cancer. JAMA Oncol. 2017;3:817–825. doi: 10.1001/jamaoncol.2016.6307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Handley NR, Schuchter LM, Bekelman JE. Best practices for reducing unplanned acute care for patients with cancer. J Oncol Pract. 2018;14:306–313. doi: 10.1200/JOP.17.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Daly B, Baldwin-Medsker A, Perchick W. Using remote monitoring to reduce hospital visits for cancer patients. Harv Bus Rev. 2019 https://hbr.org/2019/11/using-remote-monitoring-to-reduce-hospital-visits-for-cancer-patients [Google Scholar]
- 11. Daly B, Gorenshteyn D, Nicholas KJ, et al. Building a clinically relevant risk model: Predicting risk of a potentially preventable acute care visit for patients starting antineoplastic therapy. JCO Clin Cancer Inform. 2020;4:275–289. doi: 10.1200/CCI.19.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Memorial Sloan Kettering Cancer Center: Introduction to InSight Care at Memorial Sloan Kettering Cancer Center, 2018. https://www.youtube.com/watch?v=FPYPif3XBJQ.
- 13. ClinicalTrials.gov: Electronic Patient Reporting of Symptoms During Cancer Treatment (PRO-TECT). https://clinicaltrials.gov/ct2/show/NCT03249090.
- 14. Basch E, Reeve, Mitchell SA, et al. Development of the National Cancer Institute's Patient-Reported Outcomes Version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE) J Natl Cancer Inst. 2014;106:dju244. doi: 10.1093/jnci/dju244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Daly B, Nicholas K, Gorenshteyn D, et al. Misery loves company: Presenting symptom clusters to urgent care by patients receiving antineoplastic therapy. J Oncol Pract. 2018;14:e484–e495. doi: 10.1200/JOP.18.00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Whitney RL, Bell JF, Tancredi DJ, et al. Unplanned hospitalization among individuals with cancer in the year after diagnosis. J Oncol Pract. 2019;15:e20–e29. doi: 10.1200/JOP.18.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. The Centers for Medicare and Medicaid Innovation: Oncology Care First Model: Informal request for information. https://innovation.cms.gov/files/x/ocf-informalrfi.pdf.
- 18. Reference deleted.
- 19. Mooney KH, Beck SL, Wong B, et al. Automated home monitoring and management of patient-reported symptoms during chemotherapy: Results of the symptom care at home RCT. Cancer Med. 2017;6:537–546. doi: 10.1002/cam4.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19a. Daly B, Baldwin Medsker A: Patient-reported outcomes 2.0. 2019. https://jcoopblog.org/blog/2019/6/11/patient-reported-outcomes-20.
- 19b. Stabile C, Temple LK, Ancker JS, et al. Ambulatory cancer care electronic symptom self-reporting (ACCESS) for surgical patients: A randomised controlled trial protocol. BMJ Open 2019. 2019;9:e030863. doi: 10.1136/bmjopen-2019-030863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liang W, Guan W, Chen R, et al. Cancer patients in SARS-CoV-2 infection: A nationwide analysis in China. Lancet Oncol. 2020;21:335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yu J, Ouyang W, Chua MLK, et al. SARS-CoV-2 transmission in patients with cancer at a tertiary care hospital in Wuhan, China. JAMA Oncol. 2020 doi: 10.1001/jamaoncol.2020.0980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. doi: 10.1200/CCI.20.00033. Patt D: Using clinical informatics to navigate a crisis: How technology and policy change can influence cancer care delivery. JCO Clinical Cancer Inform 4:318-320, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]



