Abstract
Background
Physiotherapy is a commonly prescribed intervention for people with Parkinson’s disease (PD). Conventional types of physiotherapy have been studied extensively, while novel modalities are being developed and evaluated.
Objective
To evaluate the effectiveness of conventional and more recent physiotherapy interventions for people with PD. The meta-analysis performed as part of the 2014 European Physiotherapy Guideline for PD was used as the starting point and updated with the latest evidence.
Methods
We performed a systematic search in PubMed, CINAHL, Embase, and Web of Science. Randomized controlled trials comparing any physiotherapy intervention with no intervention or sham treatment were included. Trials were classified into 12 categories: conventional physiotherapy, resistance training, treadmill training, strategy training, dance, martial arts, aerobic exercises, hydrotherapy, balance and gait training, dual tasking, exergaming, and Nordic walking. Outcomes included motor symptoms, balance, gait, and quality of life, and are presented as standardized mean differences. The GRADE (Grading of Recommendations, Assessment, Development and Evaluation) approach was used to systematically appraise methodological quality.
Results
A total of 191 trials with 7998 participants were included. Conventional physiotherapy significantly improved motor symptoms, gait, and quality of life. Resistance training improved gait. Treadmill training improved gait. Strategy training improved balance and gait. Dance, Nordic walking, balance and gait training, and martial arts improved motor symptoms, balance, and gait. Exergaming improved balance and quality of life. Hydrotherapy improved balance. Finally, dual task training did not significantly improve any of the outcomes studied.
Conclusions
This meta-analysis provides a comprehensive overview of the evidence for the effectiveness of different physiotherapy interventions in the management of PD, allowing clinicians and patients to make an evidence-based decision for specific treatment modalities. Further work is needed to directly compare the relative efficacy of the various treatments.
Keywords: Parkinson’s disease, physiotherapy, meta-analysis, review, guideline, European Physiotherapy Guideline
Introduction
Parkinson’s disease (PD) is a complex and progressive disorder characterized by various motor and non-motor symptoms.1 Cardinal motor features include rest tremor, rigidity, bradykinesia, postural instability and an altered walking pattern, including freezing of gait.2,3 Moreover, PD patients have an increased risk of falls, even in the early disease stages, which may have widespread consequences, such as fractures, hospitalization, and death.4,5 As the disease progresses, these symptoms result in progressive difficulties in daily living, greater dependence and social isolation, with a significant impact on the quality of life of patients and their families.6 Complementary to pharmacotherapy and neurosurgical treatments, physiotherapy aims to improve multiple PD-related impairments, including problems related to physical capacity, physical (in)activity, gait, posture, transfers, balance, and falls.7,8
Physiotherapy consists of many different treatment modalities, and novel physiotherapy interventions are continuously being developed. Examples include martial arts9 and dance.10 During the past decades, numerous studies have evaluated the effectiveness of the various physiotherapy modalities,11 including comparisons across different interventions.12 However, the methodologies applied in these studies were highly variable and many different outcomes were used. This inconsistency has been a great limitation in interpreting the available evidence and in providing clear and concise treatment recommendations to people with PD.
Here, we aim to evaluate the effectiveness of multiple physiotherapy modalities, namely conventional physiotherapy, resistance training, treadmill training, strategy training, dance, martial arts, aerobic exercises, hydrotherapy, balance and gait training, dual tasking, exergaming, and Nordic walking by means of a meta-analysis. Although many systematic reviews and meta-analyses previously discussed the effectiveness of various physiotherapy modalities in PD, we here provide a comprehensive, updated overview, including randomized controlled trials (RCTs) . This is the most complete meta-analysis about physiotherapy in PD to date, evaluating all present, commonly used physiotherapy modalities. In addition, since 2012 (when the most comprehensive meta-analysis so far was published by Tomlinson et al13), many new studies have been published, creating a much larger body of evidence. For the present study, the meta-analysis as described in the European Physiotherapy Guideline for Parkinson’s Disease was used as a starting point (published in 2014),14 and updated with the latest scientific evidence until June 2020. Our comprehensive review evaluates the effect of various physiotherapy interventions on motor symptoms, balance, gait and quality of life. Additionally, areas that have been studied less extensively and that require further research are identified.
Methods
Search Strategy
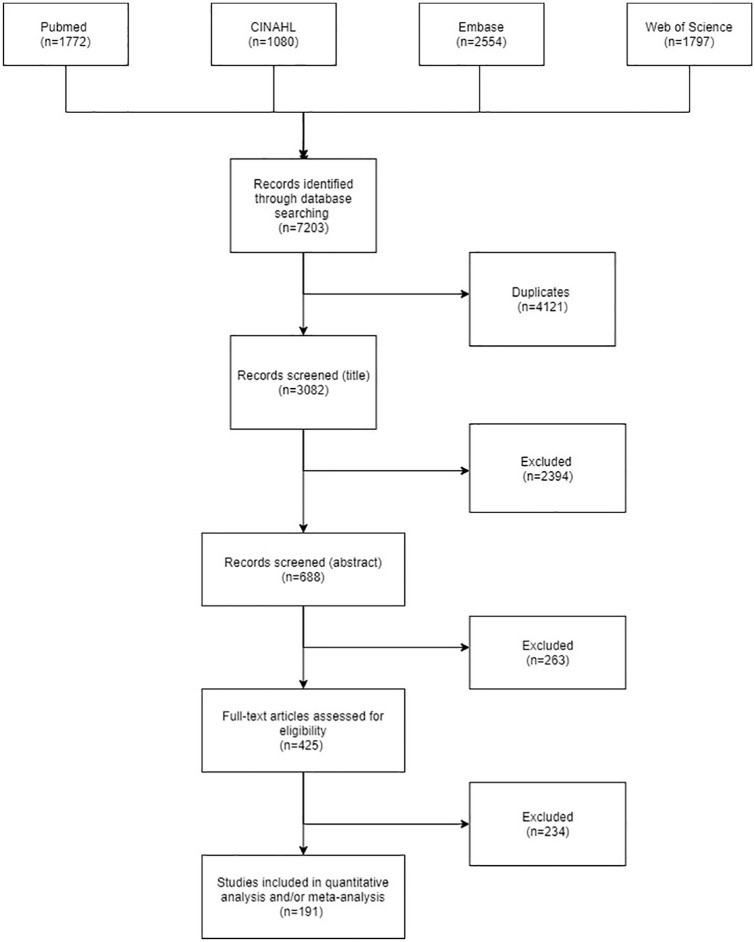
A systematic literature search including articles published until June 2020 in the PubMed (Medline), CINAHL, Embase, and Web of Science databases was conducted. The search strategy was identical to the one used in the European Guideline.14 The search criteria were kept broad and trials were included when they evaluated (a) any type of physiotherapy intervention, (b) people with PD, and (c) any outcomes related to motor function or quality of life. Search terms included “Parkinson’s disease,” “Parkinson disease,” “physiotherapy,” “randomized controlled trial,” “controlled clinical trial,” “systematic review,” and “guideline.” A flowchart describing the literature selection is presented in Figure 1. Full-text articles, published abstracts, and conference proceedings were included. In addition, reference lists were hand searched to identify further relevant articles.
Figure 1.
Flowchart describing the search strategy.
Inclusion Criteria
RCTs that studied a physiotherapy intervention compared to no intervention, sham therapy (eg, stretching exercise or usual care with no specific exercise component) or active therapy in people with PD were included, regardless of disease stage and severity. There was no start date for trials to be included; the oldest included trial is from 1994 and the most recent one from 2020. Trials that included subjects with atypical or secondary parkinsonism were not considered. Based on the categories used for the development of the European Guideline, we included conventional physiotherapy, treadmill training, strategy training, dance, martial arts, Nordic walking, whole body vibration, and massage. These categories were identified by representatives of 20 European professional associations and people with PD.14 In addition, trials investigating one of the following categories were included: resistance training, aerobic exercises, hydrotherapy, balance and gait training, dual tasking, and exergaming. To be included, trials had to report a precise description of the applied exercise intervention in terms of training regime, frequency, intensity, duration, and progression. Trials also had to be written in English. Trials were excluded if exercise interventions (experimental or control) were not adequate for meta-analytic comparisons, for example, when mean (differences) and/or standard deviations were not described or if the intervention was not considered a physiotherapy intervention (eg, experimental trials with only 1 day of treatment). Also, multidisciplinary interventions were excluded because the effectiveness of physiotherapy alone cannot be derived from these trials.
Conventional physiotherapy was defined as all active (exercise) interventions traditionally used by physiotherapists to manage people with PD, such as traditional physiotherapy techniques or multifaceted interventions combining different physiotherapy techniques.8,15 Interventions using transcranial direct current stimulation were also included in this category. Hydrotherapy and trials using dual task training were treated as separate categories. The strategy training category included strategies for complex motor sequences and cueing interventions that use temporal or spatial external stimuli associated with the initiation and ongoing facilitation of motor activity, particularly gait. In the dance category, various types of dance were included, such as tango, American ballroom, and waltz/foxtrot. Tai Chi and Qigong were included in the martial arts category. The treadmill training category included trials using treadmill training as a stand-alone intervention, just as the Nordic walking category included trials that used Nordic walking as a stand-alone intervention. Finally, we considered exercise or training interventions with a therapeutic goal as a physiotherapy intervention.
Outcome Measures
Based on the European Guideline, where representatives of 20 European professional associations and patients graded these as being critical,14 the following outcomes were selected:
Motor symptoms: the (Movement Disorders Society–sponsored revision of the) – Unified Parkinson’s Disease Rating Scale part III ((MDS)-UPDRS-III);
Balance outcomes: the Timed Up and Go test (TUG), Berg Balance Scale (BBS), Mini Balance Evaluation Systems Test (Mini-BESTest), Functional Reach, Falls Efficacy Scale International (FES-I) and the Activities-specific Balance Confidence Scale (ABC);
Gait outcomes: the 6-Minute Walk Test (6MWT), 10-Meter Walk Test (10MWT), gait speed, stride length, step length, cadence and the (New)-Freezing of Gait Questionnaire ((N)FOG-Q);
Quality of life: the Parkinson’s Disease Questionnaire-39 (PDQ-39), Parkinson’s Disease Quality of Life Questionnaire (PDQL) and the EuroQol Five Dimensions Questionnaire (EQ-5D).
Data Extraction
For articles published until the end of 2012, SK and the Guideline Development Group performed the data extraction and subsequent analysis. For this present update, one reviewer (DR) screened all identified trials on title and abstract and any doubt was discussed with another independent reviewer (NdV). Final inclusion was based on consensus between the two reviewers. Data extraction was performed using a standardized data collection form. We extracted the main characteristics of each included study, including information about the sample (initial sample size, age, gender), intervention (experimental and control), and physiotherapy outcomes (assessment and follow-up). Adverse events were not taken into account. Only data assessed immediately after the intervention period were used; data from longer follow-up periods were not taken in to account due to the large variability in intervals. Moreover, only few trials reported data on longer-term follow-up (eg, 6 months or longer). For trials with more than 2 intervention arms, the 2 arms most likely to show the largest effect were included (eg, intervention group and no intervention control group, instead of sham therapy group). We contacted authors via email (sending 1 reminder) if descriptive information or data for statistical analysis were missing, such as mean (differences) and/or standard deviations regarding the included outcome measures.
Methodological Quality
For trials published until the end of 2012, SK and the Guideline Development Group assessed the methodological quality using the Grading of Recommendations Assessment Development and Evaluation (GRADE) quality assessment tool.16 For this present update, one reviewer (DR) assessed the methodological quality using GRADE. A second reviewer (JD) assessed a sample of the included trials to check for consensus, independent from the first reviewer. This sample was selected randomly and included one-third of all included trials. The reviewers agreed on the final quality grading of all these trials. GRADE is endorsed by many major organizations, such as the Cochrane Collaboration, the World Health Organization, and the British Medical Journal. It is a valid measure of the methodological quality of clinical trials. Each study was rated based on the comprehensiveness of the reported information, which led to an overall methodological quality rating of high, moderate, low, or very low. RCTs started at the high level. Downgrading was based on incorrect or unclear description of the randomization procedures, (single) masked, concealment of allocation, eligibility criteria, differences between treatment groups at baseline and failure to use an intention to treat analysis. Finally, the GRADE quality rating was summarized per outcome measure in each category, also taking into account heterogeneity between trials, imprecision and publication bias (Appendix, Table 3).
Data Synthesis
We used the Cochrane Collaboration’s software Review Manager (RevMan version 5.3, Copenhagen) to perform the meta-analysis (available from the website for free: http://tech.cochrane.org/revman/download). All outcome variables used for analysis were continuous data and were entered in the database as mean values with standard deviations of postintervention outcome scores. The extracted continuous data from different scales were converted to standardized mean differences (SMD) with 95% confidence intervals (CIs) and adjusted for sample size. We calculated SMDs in order to combine data of outcomes with different units. For scales indicating improvement by decrease (eg, Unified Parkinson’s Disease Rating Scale, Timed Up and Go test), mean values were adjusted by multiplication with −1.17 A significant effect size of less than 0.2 was considered a small effect, a size of 0.2 to 0.5 a moderate effect, and an effect size of more than 0.8 a large effect.18 The meta-analysis results were expressed as pooled effects, with corresponding 95% CIs and P values.
Results
Study Characteristics
We found 425 potentially eligible trials and these were screened for inclusion. A total of 191 full-text papers fulfilled the inclusion criteria and were included in the analysis (references in Supplementary Material 15). The remaining 234 studies were excluded for various reasons, mainly due to missing data for statistical analysis. The type of exercise interventions varied considerably. In general, the interventions included either a specific type of exercise (eg, Tai Chi, tango dance, or aerobic training) or a combination of various exercise components (eg, strength, flexibility, and balance training). Intervention characteristics also varied extensively; however, on average interventions lasted between 4 and 12 weeks, sporadically up to 6 months or even 2 years. Training sessions were organized between 1 and 7 times a week, but on average 3 to 4 times. Sessions lasted an average of 67 ± 52 minutes. The study characteristics of all included article are described in Supplementary through 12 in the Supplementary Table 1. There were insufficient data to perform a meta-analysis on whole body vibration, massage, boxing, yoga, and fall prevention programs. These interventions are briefly discussed in the section “Other Physiotherapy Techniques”. Among the eligible outcome measures, the (MDS)-UPDRS-III, TUG, and gait speed were most frequently reported. Table 1 shows the results of the meta-analysis regarding the selected types of interventions and main outcome measures. Supplementary File 3 shows the forest plots accompanying Table 1.
Table 1.
Results of the Outcome Measures Related to the Type of Intervention.a
| Motor symptoms | Balance outcomes |
Gait outcomes |
Quality-of-life outcomes |
||||||
|---|---|---|---|---|---|---|---|---|---|
| (MDS-) UPDRS | TUG | BBS | 6MWT | 10MWT | Gait speed | Stride length | Cadence | PDQ-39 | |
| Conventional PT (n = 45) | 0.48 [0.35, 0.60]**
n = 26 |
0.11 [−0.07, 0.29] n = 14 |
0.03 [−0.25, 0.31] n = 3 |
0.13 [−0.01, 0.28] n = 6 |
0.30 [0.01, 0.59] n = 56** |
0.24 [0.03, 0.45] n = 11 |
0.28 [−0.11, 0.67] n = 4 |
0.52 [0.11, 0.92] n = 4** |
0.11 [0.01, 0.22] n = 17* |
| Resistance training (n = 17) | 0.20 [−0.02, 0.42] n = 7 |
0.19 [−0.05, 0.43] n = 6 |
0.31 [−0.47, 1.09] n = 1 |
0.67 [0.09, 1.24] n = 2* |
−0.07[−0.30, 0.16] n = 6 |
−0.63 [−1.20, −0.06] n = 2 |
0.23 [0.01, 0.43] n = 7 |
||
| Treadmill training (n = 32) | 0.10 [−0.09, 0.29] n = 16 |
0.07 [−0.19, 0.34] n = 8 |
0.21 [−0.13, 0.55] n = 5 |
0.29 [0.04, 0.55]*
n = 9 |
0.47 [0.08, 0.85] n = 4* |
0.52 [0.34, 0.69] n = 23** |
0.20 [− 0.04, 0.44] n = 12 |
0.12 [−0.15, 0.39] n = 9 |
−0.06 [−0.49, 0.36] n = 4 |
| Strategy training (n = 14) | 0.43 [−0.32, 1.18] n = 1 |
0.53 [0.23, 0.82] n = 6** |
0.12 [−0.43, 0.68] n=1 |
−0.02 [−0.90, 0.85] n = 1 |
−0.04 [−0.60, 0.51] n = 1 |
0.45 [0.13, 0.76] n = 6** |
0.52 [0.11, 0.93] n = 4 |
0.47 [−0.01, 0.95] n = 3 |
0.17 [−0.34, 0.67] n = 2 |
| Dance (n = 11) | 0.72 [0.44, 1.01] n = 8** |
0.49 [0.19, 0.80] n = 8** |
0.59 [0.27, 0.91] n = 7** |
0.51 [0.10, 0.91] n = 4* |
0.32 [−0.02, 0.66] n = 6 |
0.11 [− 0.36, 0.58] n = 3 |
1.33 [0.31, 2.34] n = 1* |
−0.13 [−0.63, 0.38] n = 3 |
|
| Martial arts (n = 11) | 0.26 [0.08, 0.43] n = 10* |
0.56 [0.36, 0.77] n = 7** |
0.24 [−0.02, 0.49] n = 4 |
0.20 [−0.15, 0.55] n = 3 |
0.29 [0.07, 0.52] n = 6* |
0.32 [0.07, 0.56] n = 3* |
−0.09 [−0.66, 0.47] n = 2 |
||
| Nordic walking (n = 3) | 0.74 [0.24, 1.24] n = 3** |
0.55 [0.06, 1.04] n = 3* |
0.99 [0.48, 1.50] n = 3** |
0.94 [0.28, 1.60] n = 2* |
0.37 [−0.15, 0.90] n = 2 |
0.46 [−0.18, 1.11] n = 1 |
|||
| Aerobic exercises (n = 5) | 0.92 [0.61, 1.22] n = 4** |
0.80 [0.44, 1.15] n = 1** |
[−0.81, 0.83] n = 1 |
1.02 [0.69, 1.34] n = 3** |
0.20 [−0.11, 0.52] n = 2 |
||||
| Balance and gait training (n = 28) | 0.34 [0.11, 0.56] n = 12* |
0.36 [0.15, 0.58] n = 11** |
0.57 [0.35, 0.79] n = 12** |
−0.12 [−0.48, 0.23] n = 4 |
0.15 [−0.56, 0.85] n = 2 |
0.28 [0.12, 0.44] n = 17** |
0.36 [0.12, 0.59] n = 10* |
0.24 [−0.01, 0.48] n = 8 |
0.28 [−0.04, 0.60] n = 6 |
| Hydrotherapy (n = 8) | −0.11 [−0.41, 0.19] n = 5 |
0.50 [0.25, 0.75] n = 8** |
0.31 [0.04, 0.59] n = 7 |
0.39 [−0.01, 0.79] n = 3 |
|||||
| Dual task (n = 3) | −0.18 [−0.79, 0.42] n = 2 |
−0.36 [−1.39, 0.66] n = 1 |
−0.25 [−0.85, 0.34] n = 2 |
−0.08 [−0.46, 0.30] n = 1 |
−0.29 [−0.65, 0.07] n = 2 |
−0.04 [−0.78, 0.71] n = 1 |
|||
| Exergaming (n = 9) | 0.58 [0.29, 0.87] n = 7** |
0.47 [0.17, 0.77] n = 6* |
0.23 [−0.20, 0.67] n = 3 |
−1.66 [−2.84, −0.48] n = 1* |
0.30 [−0.02, 0.62] n = 4 |
0.77 [−0.07, 1.60] n = 1 |
0.45 [0.13, 0.77] n = 4* |
||
Abbreviations: n, number of included studies; PT, physiotherapy; (MDS-)UPDRS-III, Movement Disorder Society–sponsored revision of the) Unified Parkinson’s Disease Rating Scale part III; TUG, Timed Up and Go; BBS, Berg Balance Scale; 6MWT, 6-Minute Walking Test; 10MWT, 10-Minute Walking Test; PDQ-39, Parkinson’s Disease Questionnaire–39; PDQL, Parkinson’s Disease Quality of Life Questionnaire.
Data are presented as standardized mean difference [95% CI]. Positive effect estimates mean positive results on each outcome measure. Red cells indicate small significant effect (≤0.2), orange cells indicate moderate significant effect (0.2-0.5), light green cells indicate moderately large significant effect (0.5-0.8), and dark green cells indicate large significant effect (≥0.8).
Statistically significant effect, P ≤ .05.
Statistically significant effect, P ≤ .001.
Methodological Quality
The GRADE assessment showed that the methodological quality of the included trials varied considerably, with several quality indicators not fully discussed in many publications. Only 132 (66.7%) trials reported the randomization method used. Information on concealment of treatment allocation was poorly reported (57, 28.9%). Masked assessors were reported in 146 (73.8%) trials, and masking of patients was not possible in most trials due to the intervention characteristics. Finally, 45 (22.8%) trials described whether they used an intention to treat analysis, 185 (93.4%) trials reported on the eligibility criteria and in 169 (85.3%) trials the intervention and control group were similar at baseline. Table 3 in the Appendix shows the summary of findings table according to GRADE with detailed evidence per outcome measure. In summary, the evidence for conventional physiotherapy, resistance training, and balance and gait training, was considered to be moderate to high; for treadmill training and martial arts was moderate; for strategy training, dance, dual-task training, Nordic walking, aerobic exercise, hydrotherapy, and exergaming was low to moderate.
Conventional Physiotherapy
A total of 45 trials (n = 2608) investigated the effect of conventional physiotherapy compared with no exercise or sham treatment. Table 1 shows that pooled effect estimates indicate beneficial effects of conventional physiotherapy on motor symptoms, gait, and quality of life outcomes in people with PD. There was a moderate effect on the (MDS)-UPDRS (n = 26; SMD 0.48, 95% CI 0.35 to 0.60, P < .001), 10MWT (n = 5; SMD 0.30, 95% CI 0.01 to 0.59, P < .04), and cadence (n = 4; SMD 0.52, 95% CI 0.11 to 0.92, P = .01). In addition to the outcomes presented in Table 1, a meta-analysis was performed on 6 other outcomes, including the FES-I (n = 3; SMD 0.38, 95% CI 0.14 to 0.61, P = .002), ABC Scale (n = 4; SMD −0.15, 95% CI −0.45 to 0.15, P = .3), Mini-BESTest (n = 5; SMD 0.23, 95% CI −0.04 to 0.49, P = .10), Functional Reach (n = 3; SMD 0.26, 95% CI 0.00 to 0.51, P = .05), (N)FOG-Q (n = 6; SMD 0.24, 95% CI 0.04 to 0.43, P = .02), and the EQ-5D (n = 4; SMD 0.21, 95% CI −0.01 to 0.43, P = .06). These outcomes confirm the positive effect of conventional physiotherapy in reducing fear of falling and freezing of gait.
Treadmill Training
A total of 32 trials (n = 823) directly compared treadmill training with no exercise or sham treatment. Pooled data revealed that treadmill training improved gait outcomes. There was a moderate effect on the 10MWT and a moderately large effect on gait speed in favor of treadmill training. No other significant effects were found (Table 1).
Strategy Training (Including Cueing)
A total of 14 trials (n = 364) reported data on the efficacy of strategy training interventions compared with no exercise or sham treatment (Table 1). The pooled meta-analysis results showed that strategy training significantly improved balance and gait outcomes compared with the control group. There was a moderate to large effect on gait speed and the TUG. Strategy training did not have a significant effect on freezing of gait as measured with the (N)FOG-Q (n = 2; SMD 0.55, 95% CI −0.11 to 1.21, P = .10).
Dance
We found a total of 11 trials (n = 339) investigating the effect of dancing compared with no exercise or sham treatment. Table 1 shows a beneficial effect of dance interventions on motor symptoms, balance, and gait. Dancing had a moderately large effect on the (MDS)-UPDRS and BBS, as well as a moderate effect on the TUG. Dance did not improve freezing of gait as measured with the (N)FOG-Q (n = 6; SMD −0.08, 95% CI −0.40 to 0.23, P = .61). The positive effect of dance on balance was further confirmed with the Mini-BESTest (n = 3; SMD 0.81, 95% CI 0.39 to 1.23, P < .001).
Martial Arts
Pooled data from 11 trials (n = 580) directly comparing martial arts interventions (Tai Chi and Qigong) with no exercise or sham treatment revealed a beneficial effect on motor symptoms, balance, and gait parameters. Martial arts had a moderately large effect on the TUG and a moderate effect on the (MDS)-UPDRS, gait speed, and stride length (Table 1). Moreover, martial arts also had a moderately large effect on functional reach (n = 2; SMD 0.64, 95% CI 0.31 to 0.97, P < .001).
Nordic Walking
Nordic walking interventions significantly improve motor symptoms, balance, and gait outcomes. After analyzing pooled data of 3 trials (n = 73) comparing Nordic walking interventions with no exercise or sham treatment, Nordic walking had a moderately large effect on motor symptoms and a large effect on the BBS and the 6MWT (Table 1).
Resistance Training
A total of 17 trials (n = 663) reporting the outcomes of interest in the present meta-analysis were included. Those trials investigated the effect of resistance training in comparison with no exercise or sham treatment. Pooled data revealed a moderately large effect on the 6MWT in favor of resistance training. No other significant effects were found (Table 1).
Aerobic Exercises
Five trials, including a total of 231 participants, compared aerobic exercises to standard care or no exercise. Pooled data indicated that aerobic exercises interventions significantly improves motor symptoms, balance, and gait outcome (Table 1).
Balance and Gait Training
We found a significant effect of balance and gait training on improving motor symptoms, balance, and gait outcomes. A total of 28 trials (n = 1069) directly compared balance and gait training with no exercise, sham, or active treatment. Those trials revealed a moderate effect on MDS-UPDRS, gait speed, and stride length (Table 1). A moderately large effect was found on BBS, further confirmed by a large significant effect on Mini-BEST test (n = 6; SMD 0.90, 95% CI 0.69 to 1.12, P < .001). A moderately significant effect was also found for step length (n = 4; SMD 0.46, 95% CI 0.19 to 0.73, P = .001) and ABC scale (n = 5; SMD 0.49, 95% CI 0.23 to 0.74, P < .001).
Hydrotherapy
A total of 8 trials (n = 230) investigated hydrotherapy interventions in comparison with standard physiotherapy or no exercise. Pooled data indicated a moderately large effect of hydrotherapy on the TUG (Table 1). Moreover, a moderately large effect was found for fear of falling—FES-I (n = 2; SMD 0.72, 95% CI 0.19 to 1.26, P < .05).
Dual Tasking
A total of 3 trials (n = 167) investigated the effect of solely dual tasking training interventions in comparison to no or sham intervention. Pooled data indicated dual task training did not significantly improve any of the outcomes described in this meta-analysis (Table 1).
Exergaming
Table 1 shows that pooled data of 9 trials (n = 303) indicate a moderately large effect on the TUG, BBS, and PDQ-39 of exergaming in comparison with sham or no intervention. The effect of exergaming on quality of life was further confirmed by a large effect on the EQ5D (n = 2; SMD 1.41, 95% CI 0.94 to 1.87, P < .001).
Other Physiotherapy Techniques
Due to lack of data, it was not possible to perform a meta-analysis on whole body vibration, massage, boxing, yoga, and fall prevention programs. We briefly summarize the most recent findings, while recommending future trials to investigate the effects in more detail. Sharififar et al19 published a systematic review in 2014 about whole body vibration for people with PD. Six trials were included. The authors concluded that whole body vibration demonstrated to have limited, but potentially beneficial effects on balance and mobility.
Furthermore, in 2014 Donoyama et al20 evaluated the effectiveness of Anma (Japanese) massage therapy including 21 people with PD. After a single session and also after 7 weekly sessions, visual analogue scale scores were significantly lower for muscle stiffness, movement difficulties, pain, and fatigue compared with the control group; and gait speed and stride length had significantly improved. Puymbroeck and colleagues21 performed an RCT in which 27 people with PD were allocated to a yoga intervention group and a conventional physiotherapy control group. Both groups underwent an 8-week intervention. Results showed that yoga group improved motor functioning (MDS-UPDRS), and gait (FOG and Functional Gait Assessment).
The efficacy of boxing as an intervention has been investigated by Combs and colleagues.22 The authors randomized a convenience sample of 31 people with PD to either a boxing intervention group or a conventional physiotherapy control group. Their results showed improvements on gait velocity and endurance that were only found in the boxing intervention group.
Finally, Ashburn and colleagues23 performed a multicenter randomized trial involving 474 persons with PD. The intervention group received an individually tailored, progressive, home-based, fall strategy program during 12 supervised sessions with additionally 30 minutes of unsupervised training daily. The control group received standard care and educative material about PD. After 6 months, the number of reported falls in the intervention group was not significantly lower than in the control group.
Discussion
Here, we have summarized the evidence on physiotherapy modalities in people with PD, as an update to the European Physiotherapy Guideline for Parkinson’s disease that was published in 2014. Implementation of the recommendations of the European Guideline into daily clinical practice is essential to enhance the expertise of therapists, to improve outcomes for patients, and to reduce the risk of adverse events.24,25 In that regard, it is necessary to continuously update the European Guideline as new evidence emerges, and this is what we offer here.
We found that physiotherapy leads to significant improvements in all preselected outcomes. However, the different intervention types seem to have variable treatment effects. For example, conventional physiotherapy, dance, martial arts, Nordic walking, and balance and gait training improved PD motor symptoms ((MDS)-UPDRS-III), in contrast to treadmill training and strategy training. Balance outcomes did not improve after treadmill training but did improve with other interventions such as strategy training and dance (as measured using the TUG or BBS). All treatment modalities, with the exception of dual tasking training and exergaming, improved gait, with the evidence being most convincing for treadmill training and strategy training, since these are the only interventions showing moderately large to large effects (improving gait speed and step length). Finally, quality of life only improved with conventional physiotherapy, and exergaming but in general there was a lack of studies that evaluated changes in quality of life. Importantly, however, all these comparisons are made indirectly, and they should be interpreted with caution.
In terms of effect sizes, most of the observed differences between the intervention and control groups were of moderate size. Large effect sizes were noted for BBS and 6MWT (following Nordic a walking intervention), the Mini-BEST test (following balance and gait training) and the EQ5D (following exergaming). However, note that the 6MWT in only 2 Nordic walking trials. Additionally, even though large effects sizes signal that the intervention is successful and therefore should be implemented, actual implementation of a physiotherapy intervention in clinical practice comes with challenges that may impact the size of the effect. For example, the motivation to participate in a clinical trial is often high for both patients and therapist, this may lead to exceptionally high-quality treatments and good adherence, which is difficult to accomplish in clinical practice. Therefore, caution is warranted in generalizing these results to the general PD population and future trials remain necessary to confirm these positive findings. Because some of the included interventions were compared with a control group that may be considered as active control, one may argue that the effect sizes could have been larger with the exclusion of these studies. However, this is only a small proportion of all included papers. Furthermore, our aim was to provide a complete overview and comparison with active control interventions were therefore included.
Limitations
A first major strength of the present analysis is the fact that different commonly used types of physiotherapy were included, while other trials mostly focused on specific areas of physiotherapy, such as strength training26 or treadmill training.27 We provide a comprehensive overview of the effectiveness of physiotherapy for people with PD, thus enabling an easy comparison of all available interventions. A second strength is that the outcome measures used were previously graded as being critical by 20 European professional associations and patients in the European Guideline.14 Finally, the rigorous GRADE standards were used to systematically appraise the methodological quality of all included trials.
It can be difficult to perform a meta-analysis on a heterogeneous subject such as physiotherapy. First, included trials varied enormously in the number of exercise sessions per week, exercise intensity, and duration of the intervention. Also, many different interventions were used for the control groups, ranging from usual care to stretching exercises or doing low-intensity exercises at home. All these trials were included since the exercise interventions of these active control groups were expected to have no significant impact on outcome measures. Second, there was great heterogeneity across trials regarding the outcomes used, even to assess the same variable. For example, “number of falls” was measured over different time frames, while near-falls were variably included. Furthermore, “muscle strength” was measured using highly variable methods, which made it difficult to compare trial results. Therefore, trials using these outcomes were excluded from the meta-analysis. Third, the methodological quality and reporting was highly variable, and often inadequate. Lack of information does not necessarily indicate poor quality of the trial, but the level of possible bias was simply difficult to assess. Finally, most trials had relatively small sample sizes, and many evaluated short-term interventions (<12 weeks). Follow-up durations varied as well, at best spanning 6 months to several years, but mostly considerably shorter. Therefore, it was not possible to perform a meta-analysis on long-term effects of physiotherapy interventions. However, a recent review did evaluate the long-term effects of exercise and physiotherapy interventions for people with PD.28 The authors suggest a potential long-term effect on motor symptoms and physical functioning. Balance training had the longest carry-over effect (up to 12 months), followed by gait and Tai Chi training (up to 6 months). It appears that a training period longer than 12 weeks is needed to achieve clinically meaningful improvement in motor function (MDS-UPDRS-III).
The present meta-analysis found that physiotherapy is effective in improving specific outcomes. Therapists and patients can therefore choose from a variety of treatment strategies, based on the specific symptoms they seek to improve and based on the patients’ personal preferences regarding what exercise modality they relate to. This will facilitate a more patient-centered approach, where patients and therapists have an evidence-based choice between interventions. Being able to adjust the treatment according to patient preferences will be important to enhance motivation and increase long-term adherence to therapy. However, different strategies were only rarely compared back to back, hence any specific choice should be made cautiously until direct comparisons of the various treatment modalities become available.
Future studies should focus on improved methodological quality, including larger sample sizes, longer follow-up and use of relevant, reliable, and sensitive outcomes. Recently, the International Consortium for Health Outcomes Measurement published a consensus set of outcomes for PD and this may be a valuable tool for future trials.29 Moreover, this meta-analysis highlights the variety of physiotherapy interventions used to treat PD. Specific trials are needed to directly compare different interventions with standardized characteristics regarding training regime, frequency, intensity, duration, and progression. Such work is needed to underpin the most appropriate intervention, including for specific PD subgroups. Elaborating on this, young-onset PD patients are typically underrepresented in research and future work should also focus on the effects of different physiotherapy interventions in younger cohorts. Future studies should also assess the economic benefit and address the risk of adverse events (eg, possible increase in number of falls when patients are stimulated to move more) and determine adequate dosing. Finally, more work is needed to study the appropriate implementation strategies in the community to improve long-term adherence.
Supplemental Material
Supplemental material, NNR_Supplementary_Material_10_Radder_Revised_Version_2 for Physiotherapy in Parkinson’s Disease: A Meta-Analysis of Present Treatment Modalities by Danique L. M. Radder, Ana Lígia Silva de Lima, Josefa Domingos, Samyra H. J. Keus, Marlies van Nimwegen, Bastiaan R. Bloem and Nienke M. de Vries in Neurorehabilitation and Neural Repair
Supplemental material, NNR_Supplementary_Material_11_Radder_Revised_Version_2 for Physiotherapy in Parkinson’s Disease: A Meta-Analysis of Present Treatment Modalities by Danique L. M. Radder, Ana Lígia Silva de Lima, Josefa Domingos, Samyra H. J. Keus, Marlies van Nimwegen, Bastiaan R. Bloem and Nienke M. de Vries in Neurorehabilitation and Neural Repair
Supplemental material, NNR_Supplementary_Material_12_Radder_Revised_Version_2 for Physiotherapy in Parkinson’s Disease: A Meta-Analysis of Present Treatment Modalities by Danique L. M. Radder, Ana Lígia Silva de Lima, Josefa Domingos, Samyra H. J. Keus, Marlies van Nimwegen, Bastiaan R. Bloem and Nienke M. de Vries in Neurorehabilitation and Neural Repair
Supplemental material, NNR_Supplementary_Material_13_Radder for Physiotherapy in Parkinson’s Disease: A Meta-Analysis of Present Treatment Modalities by Danique L. M. Radder, Ana Lígia Silva de Lima, Josefa Domingos, Samyra H. J. Keus, Marlies van Nimwegen, Bastiaan R. Bloem and Nienke M. de Vries in Neurorehabilitation and Neural Repair
Supplemental material, NNR_Supplementary_Material_14_Radder for Physiotherapy in Parkinson’s Disease: A Meta-Analysis of Present Treatment Modalities by Danique L. M. Radder, Ana Lígia Silva de Lima, Josefa Domingos, Samyra H. J. Keus, Marlies van Nimwegen, Bastiaan R. Bloem and Nienke M. de Vries in Neurorehabilitation and Neural Repair
Supplemental material, NNR_Supplementary_Material_15_Radder_revised for Physiotherapy in Parkinson’s Disease: A Meta-Analysis of Present Treatment Modalities by Danique L. M. Radder, Ana Lígia Silva de Lima, Josefa Domingos, Samyra H. J. Keus, Marlies van Nimwegen, Bastiaan R. Bloem and Nienke M. de Vries in Neurorehabilitation and Neural Repair
Supplemental material, NNR_Supplementary_Material_1_Radder_Revised_Version_2 for Physiotherapy in Parkinson’s Disease: A Meta-Analysis of Present Treatment Modalities by Danique L. M. Radder, Ana Lígia Silva de Lima, Josefa Domingos, Samyra H. J. Keus, Marlies van Nimwegen, Bastiaan R. Bloem and Nienke M. de Vries in Neurorehabilitation and Neural Repair
Supplemental material, NNR_Supplementary_Material_2_Radder_Revised_Version_2 for Physiotherapy in Parkinson’s Disease: A Meta-Analysis of Present Treatment Modalities by Danique L. M. Radder, Ana Lígia Silva de Lima, Josefa Domingos, Samyra H. J. Keus, Marlies van Nimwegen, Bastiaan R. Bloem and Nienke M. de Vries in Neurorehabilitation and Neural Repair
Supplemental material, NNR_Supplementary_Material_3_Radder_Revised_Version_2 for Physiotherapy in Parkinson’s Disease: A Meta-Analysis of Present Treatment Modalities by Danique L. M. Radder, Ana Lígia Silva de Lima, Josefa Domingos, Samyra H. J. Keus, Marlies van Nimwegen, Bastiaan R. Bloem and Nienke M. de Vries in Neurorehabilitation and Neural Repair
Supplemental material, NNR_Supplementary_Material_4_Radder_Revised_Version_2 for Physiotherapy in Parkinson’s Disease: A Meta-Analysis of Present Treatment Modalities by Danique L. M. Radder, Ana Lígia Silva de Lima, Josefa Domingos, Samyra H. J. Keus, Marlies van Nimwegen, Bastiaan R. Bloem and Nienke M. de Vries in Neurorehabilitation and Neural Repair
Supplemental material, NNR_Supplementary_Material_5_Radder_Revised_Version_2 for Physiotherapy in Parkinson’s Disease: A Meta-Analysis of Present Treatment Modalities by Danique L. M. Radder, Ana Lígia Silva de Lima, Josefa Domingos, Samyra H. J. Keus, Marlies van Nimwegen, Bastiaan R. Bloem and Nienke M. de Vries in Neurorehabilitation and Neural Repair
Supplemental material, NNR_Supplementary_Material_6_Radder_Revised_Version_2 for Physiotherapy in Parkinson’s Disease: A Meta-Analysis of Present Treatment Modalities by Danique L. M. Radder, Ana Lígia Silva de Lima, Josefa Domingos, Samyra H. J. Keus, Marlies van Nimwegen, Bastiaan R. Bloem and Nienke M. de Vries in Neurorehabilitation and Neural Repair
Supplemental material, NNR_Supplementary_Material_7_Radder_Revised_Version_2 for Physiotherapy in Parkinson’s Disease: A Meta-Analysis of Present Treatment Modalities by Danique L. M. Radder, Ana Lígia Silva de Lima, Josefa Domingos, Samyra H. J. Keus, Marlies van Nimwegen, Bastiaan R. Bloem and Nienke M. de Vries in Neurorehabilitation and Neural Repair
Supplemental material, NNR_Supplementary_Material_8_Radder_Revised_Version_2 for Physiotherapy in Parkinson’s Disease: A Meta-Analysis of Present Treatment Modalities by Danique L. M. Radder, Ana Lígia Silva de Lima, Josefa Domingos, Samyra H. J. Keus, Marlies van Nimwegen, Bastiaan R. Bloem and Nienke M. de Vries in Neurorehabilitation and Neural Repair
Supplemental material, NNR_Supplementary_Material_9_Radder_Revised_Version_2 for Physiotherapy in Parkinson’s Disease: A Meta-Analysis of Present Treatment Modalities by Danique L. M. Radder, Ana Lígia Silva de Lima, Josefa Domingos, Samyra H. J. Keus, Marlies van Nimwegen, Bastiaan R. Bloem and Nienke M. de Vries in Neurorehabilitation and Neural Repair
Acknowledgments
We thank all the people who performed the trials that contributed to this meta-analysis and all the patients who took part in these trials. We thank all the people that were part of the Guideline Development Group of the European Physiotherapy Guideline for Parkinson’s Disease (15), who provided the base for this meta-analysis: Marten Munneke, Mariella Graziano, Jaana Paltamaa, Elisa Pelosin, Susanne Brühlmann, Bhanu Ramaswamy, Jan Prins, Chris Struiksma, Lynn Rochester, and Alice Nieuwboer.
Footnotes
Supplementary material for this article is available on the Neurorehabilitation & Neural Repair website at https://journals.sagepub.com/home/nnr.
Author Contributions: For studies published until the 31st of December 2012, SK and the Guideline Development Group designed and implemented the searches, selected the studies and undertook data extraction and assessment of risk of bias. For this present update, DR, NdV, and AL designed and implemented the searches. DR, NdV, and AL selected the studies. DR, JD, and AL undertook data extraction and assessment of risk of bias. DR, AL performed the data analysis. All authors were involved in interpretation of the review. BB is the study guarantor.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Professor Bas Bloem was supported by a research grant of the Parkinson’s Foundation. Danique Radder, Nienke de Vries, and Ana Lígia Silva de Lima were supported by a research grant of ZonMw (The Netherlands Organisation for Health Research and Development). The European Physiotherapy Guideline for Parkinson’s disease was mainly financed by ParkinsonNet and the Royal Dutch Society for Physical Therapy (KNGF), the Netherlands.
ORCID iD: Danique L. M. Radder  https://orcid.org/0000-0001-7363-6681
https://orcid.org/0000-0001-7363-6681
References
- 1. Jankovic J. Parkinson’s disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry. 2008;79:368-376. [DOI] [PubMed] [Google Scholar]
- 2. Bartels AL, Leenders KL. Parkinson’s disease: the syndrome, the pathogenesis and pathophysiology. Cortex. 2009;45:915-921. [DOI] [PubMed] [Google Scholar]
- 3. Moustafa AA, Chakravarthy S, Phillips JR, et al. Motor symptoms in Parkinson’s disease: a unified framework. Neurosci Biobehav Rev. 2016;68:727-740. [DOI] [PubMed] [Google Scholar]
- 4. Voss TS, Elm JJ, Wielinski CL, et al. Fall frequency and risk assessment in early Parkinson’s disease. Parkinsonism Relat Disord. 2012;18:837-841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Allen NE, Schwarzel AK, Canning CG. Recurrent falls in Parkinson’s disease: a systematic review. Parkinson’s Dis. 2013;2013:906274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schrag A, Jahanshahi M, Quinn N. How does Parkinson’s disease affect quality of life? A comparison with quality of life in the general population. Mov Disord. 2000;15:1112-1118. [DOI] [PubMed] [Google Scholar]
- 7. van der Kolk NM, King LA. Effects of exercise on mobility in people with Parkinson’s disease. Mov Disord. 2013;28:1587-1596. [DOI] [PubMed] [Google Scholar]
- 8. Keus SH, Bloem BR, Hendriks EJM, et al. Evidence-based analysis of physical therapy in Parkinson’s disease with recommendations for practice and research. Mov Disord. 2007;22:451-460. [DOI] [PubMed] [Google Scholar]
- 9. Yang Y, Li XY, Gong L, Zhu YL, Hao YL. Tai Chi for improvement of motor function, balance and gait in Parkinson’s disease: a systematic review and meta-analysis. PLoS One. 2014;9:102942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shanahan J, Morris ME, Ni Bhriain O, Saunders J, Clifford AM. Dance for people with Parkinson disease: what is the evidence telling us? Arch Phys Med Rehabil. 2015;96:141-153. [DOI] [PubMed] [Google Scholar]
- 11. Rocha PA, McClelland J, Morris ME. Complementary physical therapies for movement disorders in Parkinson’s disease: a systematic review. Eur J Phys Rehabil Med. 2015;51:693-704. [PubMed] [Google Scholar]
- 12. Tomlinson CL, Herd CP, Clarke CE, et al. Physiotherapy for Parkinson’s disease: a comparison of techniques. Cochrane Database Syst Rev. 2014;2014(6):CD002815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tomlinson CL, Patel S, Meek C, et al. Physiotherapy intervention in Parkinson’s disease: systematic review and meta-analysis. BMJ. 2012;345:e5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Keus S, Munneke M, Graziano M, et al. European Physiotherapy Guideline for Parkinson’s Disease. KNGF/ParkinsonNet; 2014. Accessed August 8, 2020 https://www.parkinsonnet.nl/app/uploads/sites/3/2019/11/eu_guideline_parkinson_guideline_for_pt_s1.pdf [Google Scholar]
- 15. Morris ME. Movement disorders in people with Parkinson disease: a model for physical therapy. Phys Ther. 2000;80:578-597. [PubMed] [Google Scholar]
- 16. Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction—GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64:383-394. [DOI] [PubMed] [Google Scholar]
- 17. Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1. 0. Accessed August 8, 2020 https://handbook-5-1.cochrane.org/
- 18. Cohen J. Statistical Power Analysis for the Behavioral Sciences. Lawrence Erlbaum; 1988:18-74. [Google Scholar]
- 19. Sharififar S, Coronado RA, Romero S, Azari H, Thigpen M. The effects of whole body vibration on mobility and balance in Parkinson disease: a systematic review. Iran J Med Sci. 2014;39:318-326. [PMC free article] [PubMed] [Google Scholar]
- 20. Donoyama N, Suoh S, Ohkoshi N. Effectiveness of Anma massage therapy in alleviating physical symptoms in outpatients with Parkinson’s disease: a before-after study. Complement Ther Clin Pract. 2014;20:251-261. [DOI] [PubMed] [Google Scholar]
- 21. Van Puymbroeck M, Walter AA, Hawkins BL, et al. Functional improvements in Parkinson’s disease following a randomized trial of yoga. Evid Based Complement Alternat Med. 2018;2018:8516351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Combs SA, Diehl MD, Chrzastowski C, et al. Community-based group exercise for persons with Parkinson disease: a randomized controlled trial. NeuroRehabilitation. 2013;32:117-124. [DOI] [PubMed] [Google Scholar]
- 23. Ashburn A, Pickering R, Rochester L, et al. The PDSAFE falls prevention programme for people with Parkinson’s: a multicentre randomised controlled trial. Mov Disord. 2018;33:S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Aminoff MJ, Christine CW, Friedman JH, et al. Management of the hospitalized patient with Parkinson’s disease: current state of the field and need for guidelines. Parkinsonism Relat Disord. 2011;17:139-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Weerkamp NJ, Tissingh G, Poels PJE, et al. Parkinson disease in long term care facilities: a review of the literature. J Am Med Dir Assoc. 2014;15:90-94. [DOI] [PubMed] [Google Scholar]
- 26. Ramazzina I, Bernazzoli B, Costantino C. Systematic review on strength training in Parkinson’s disease: an unsolved question. Clin Interv Aging. 2017;12:619-628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mehrholz J, Kugler J, Storch A, Pohl M, Hirsch K, Elsner B. Treadmill training for patients with Parkinson’s disease. An abridged version of a Cochrane Review. Eur J Phys Rehabil Med. 2016;52:704-713. [PubMed] [Google Scholar]
- 28. Mak MK, Wong-Yu IS, Shen X, Chung CL. Long-term effects of exercise and physical therapy in people with Parkinson disease. Nat Rev Neurol. 2017;13:689-703. [DOI] [PubMed] [Google Scholar]
- 29. De Roos P, Bloem BR, Kelley TA, et al. A consensus set of outcomes for Parkinson’s disease from the International Consortium for Health Outcomes Measurement. J Parkinsons Dis. 2017;7:533-543. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, NNR_Supplementary_Material_10_Radder_Revised_Version_2 for Physiotherapy in Parkinson’s Disease: A Meta-Analysis of Present Treatment Modalities by Danique L. M. Radder, Ana Lígia Silva de Lima, Josefa Domingos, Samyra H. J. Keus, Marlies van Nimwegen, Bastiaan R. Bloem and Nienke M. de Vries in Neurorehabilitation and Neural Repair
Supplemental material, NNR_Supplementary_Material_11_Radder_Revised_Version_2 for Physiotherapy in Parkinson’s Disease: A Meta-Analysis of Present Treatment Modalities by Danique L. M. Radder, Ana Lígia Silva de Lima, Josefa Domingos, Samyra H. J. Keus, Marlies van Nimwegen, Bastiaan R. Bloem and Nienke M. de Vries in Neurorehabilitation and Neural Repair
Supplemental material, NNR_Supplementary_Material_12_Radder_Revised_Version_2 for Physiotherapy in Parkinson’s Disease: A Meta-Analysis of Present Treatment Modalities by Danique L. M. Radder, Ana Lígia Silva de Lima, Josefa Domingos, Samyra H. J. Keus, Marlies van Nimwegen, Bastiaan R. Bloem and Nienke M. de Vries in Neurorehabilitation and Neural Repair
Supplemental material, NNR_Supplementary_Material_13_Radder for Physiotherapy in Parkinson’s Disease: A Meta-Analysis of Present Treatment Modalities by Danique L. M. Radder, Ana Lígia Silva de Lima, Josefa Domingos, Samyra H. J. Keus, Marlies van Nimwegen, Bastiaan R. Bloem and Nienke M. de Vries in Neurorehabilitation and Neural Repair
Supplemental material, NNR_Supplementary_Material_14_Radder for Physiotherapy in Parkinson’s Disease: A Meta-Analysis of Present Treatment Modalities by Danique L. M. Radder, Ana Lígia Silva de Lima, Josefa Domingos, Samyra H. J. Keus, Marlies van Nimwegen, Bastiaan R. Bloem and Nienke M. de Vries in Neurorehabilitation and Neural Repair
Supplemental material, NNR_Supplementary_Material_15_Radder_revised for Physiotherapy in Parkinson’s Disease: A Meta-Analysis of Present Treatment Modalities by Danique L. M. Radder, Ana Lígia Silva de Lima, Josefa Domingos, Samyra H. J. Keus, Marlies van Nimwegen, Bastiaan R. Bloem and Nienke M. de Vries in Neurorehabilitation and Neural Repair
Supplemental material, NNR_Supplementary_Material_1_Radder_Revised_Version_2 for Physiotherapy in Parkinson’s Disease: A Meta-Analysis of Present Treatment Modalities by Danique L. M. Radder, Ana Lígia Silva de Lima, Josefa Domingos, Samyra H. J. Keus, Marlies van Nimwegen, Bastiaan R. Bloem and Nienke M. de Vries in Neurorehabilitation and Neural Repair
Supplemental material, NNR_Supplementary_Material_2_Radder_Revised_Version_2 for Physiotherapy in Parkinson’s Disease: A Meta-Analysis of Present Treatment Modalities by Danique L. M. Radder, Ana Lígia Silva de Lima, Josefa Domingos, Samyra H. J. Keus, Marlies van Nimwegen, Bastiaan R. Bloem and Nienke M. de Vries in Neurorehabilitation and Neural Repair
Supplemental material, NNR_Supplementary_Material_3_Radder_Revised_Version_2 for Physiotherapy in Parkinson’s Disease: A Meta-Analysis of Present Treatment Modalities by Danique L. M. Radder, Ana Lígia Silva de Lima, Josefa Domingos, Samyra H. J. Keus, Marlies van Nimwegen, Bastiaan R. Bloem and Nienke M. de Vries in Neurorehabilitation and Neural Repair
Supplemental material, NNR_Supplementary_Material_4_Radder_Revised_Version_2 for Physiotherapy in Parkinson’s Disease: A Meta-Analysis of Present Treatment Modalities by Danique L. M. Radder, Ana Lígia Silva de Lima, Josefa Domingos, Samyra H. J. Keus, Marlies van Nimwegen, Bastiaan R. Bloem and Nienke M. de Vries in Neurorehabilitation and Neural Repair
Supplemental material, NNR_Supplementary_Material_5_Radder_Revised_Version_2 for Physiotherapy in Parkinson’s Disease: A Meta-Analysis of Present Treatment Modalities by Danique L. M. Radder, Ana Lígia Silva de Lima, Josefa Domingos, Samyra H. J. Keus, Marlies van Nimwegen, Bastiaan R. Bloem and Nienke M. de Vries in Neurorehabilitation and Neural Repair
Supplemental material, NNR_Supplementary_Material_6_Radder_Revised_Version_2 for Physiotherapy in Parkinson’s Disease: A Meta-Analysis of Present Treatment Modalities by Danique L. M. Radder, Ana Lígia Silva de Lima, Josefa Domingos, Samyra H. J. Keus, Marlies van Nimwegen, Bastiaan R. Bloem and Nienke M. de Vries in Neurorehabilitation and Neural Repair
Supplemental material, NNR_Supplementary_Material_7_Radder_Revised_Version_2 for Physiotherapy in Parkinson’s Disease: A Meta-Analysis of Present Treatment Modalities by Danique L. M. Radder, Ana Lígia Silva de Lima, Josefa Domingos, Samyra H. J. Keus, Marlies van Nimwegen, Bastiaan R. Bloem and Nienke M. de Vries in Neurorehabilitation and Neural Repair
Supplemental material, NNR_Supplementary_Material_8_Radder_Revised_Version_2 for Physiotherapy in Parkinson’s Disease: A Meta-Analysis of Present Treatment Modalities by Danique L. M. Radder, Ana Lígia Silva de Lima, Josefa Domingos, Samyra H. J. Keus, Marlies van Nimwegen, Bastiaan R. Bloem and Nienke M. de Vries in Neurorehabilitation and Neural Repair
Supplemental material, NNR_Supplementary_Material_9_Radder_Revised_Version_2 for Physiotherapy in Parkinson’s Disease: A Meta-Analysis of Present Treatment Modalities by Danique L. M. Radder, Ana Lígia Silva de Lima, Josefa Domingos, Samyra H. J. Keus, Marlies van Nimwegen, Bastiaan R. Bloem and Nienke M. de Vries in Neurorehabilitation and Neural Repair