Abstract
Vibrios can degrade chitin surfaces to soluble N-acetyl glucosamine oligosaccharides (GlcNAcn) that can be utilized as a carbon source and also induce a state of natural genetic competence. In this study, we characterized chitin-dependent growth and natural competence in Vibrio parahaemolyticus and its regulation. We found that growth on chitin was regulated through chitin sensors ChiS (sensor histidine kinase) and TfoS (transmembrane transcriptional regulator) by predominantly controlling the expression of chitinase VPA0055 (ChiA2) in a TfoX-dependent manner. The reduced growth of ΔchiA2, ΔchiS and ΔtfoS mutants highlighted the critical role played by ChiA2 in chitin breakdown. This growth defect of ΔchiA2 mutant could be recovered when chitin oligosaccharides GlcNAc2 or GlcNAc6 were supplied instead of chitin. The ΔtfoS mutant was also able to grow on GlcNAc2 but the ΔchiS mutant could not, which indicates that GlcNAc2 catabolic operon is dependent on ChiS and independent of TfoS. However, the ΔtfoS mutant was unable to utilize GlcNAc6 because the periplasmic enzymes required for the breakdown of GlcNAc6 were found to be downregulated at the mRNA level. We also showed that natural competence can be induced only by GlcNAc6, not GlcNAc2, because the expression of competence genes was significantly higher in the presence of GlcNAc6 compared to GlcNAc2. Moreover, this might be an indication that GlcNAc2 and GlcNAc6 were detected by different receptors. Therefore, we speculate that GlcNAc2-dependent activation of ChiS and GlcNAc6-dependent activation of TfoS might be crucial for the induction of natural competence in V. parahaemolyticus through the upregulation of the master competence regulator TfoX.
Keywords: chitin, chitinase, GlcNAc6, natural competence, ChiA2, ChiS, TfoS
1. Introduction
Vibrio parahaemolyticus is responsible for food-borne gastroenteritis globally since isolation of the first pandemic O3:K6 strains in 1996 [1]. This Gram-negative, halophilic bacterium is widely disseminated in estuarine, marine and coastal surroundings either in a free-swimming state or affixed to abiotic and biotic surfaces including zooplankton, fish and shellfish [2,3]. The most abundant biomolecule in this habitat is the insoluble polysaccharide known as chitin, composed of β→1,4 linked N-acetyl glucosamine (GlcNAc) residues. Through the action of secreted chitinase, Vibrios can degrade the chitin surfaces into soluble GlcNAcn oligosaccharides and utilize them as a source of carbon and nitrogen [4]. Thus, Vibrio species in the aquatic environment are the key players in the recycling of chitin [5]. According to the Vibrio cholerae chitin utilization pathway, it is known that the chitinase enzymes degrade chitin into GlcNAc2-6 oligosaccharides which then enter either through porin or chitoporin, depending on their sizes. Then, further enzymatic degradation takes place in the periplasm to primarily release GlcNAc2 from higher oligosaccharides along with some GlcNAc residues [6,7]. The binding of GlcNAc2 with CBP (chitin oligosaccharide binding protein) activates ChiS (sensor histidine kinase) which is the regulator of genes required for degradation and utilization of chitin, such as chitinases for chitin breakdown, chitoporin for transport of GlcNAcn residues into the periplasm and GlcNAc2 catabolic operon to metabolize GlcNAc2 in cytoplasm [4,8].
In addition to its role as a nutrient source, chitin can also activate a cascade of gene expression to induce natural competence in Vibrios. Natural competence is the ability to uptake extracellular DNA (eDNA) from the environment and this eDNA might get integrated by homologous recombination to provide novel traits. Chitin-induced natural competence and uptake of eDNA was first reported in V. cholerae in 2005 [9]. Thereafter, it was observed in several species of the Vibrionaceae family such as Vibrio vulnificus, Vibrio fischeri and V. parahaemolyticus [10,11,12]. This process of horizontal gene transfer is known as natural transformation and is one of the reasons behind the high levels of genomic diversity among Vibrionaceae [9,10,11,12]. In V. cholerae, the competence for genetic transformation is triggered by chitin-induced transcription factor TfoX, which regulates the genes required for DNA uptake [9]. The exposure of chitin oligosaccharides induces the transcription of Hfq-dependent small RNA (sRNA), tfoR, which is critical for the translation of TfoX [13]. Recently, two independent studies characterized a novel chitin-sensing regulator, TfoS, that is responsible for transcriptional activation of tfoR [14,15]. Yamamoto et al. reported that TfoS does not possess the signature domain of a two-component system (TCS), but the activity of TfoS is dependent on ChiS.
Due to the conserved nature of chitin utilization and natural transformation among Vibrios, these two processes were primarily studied in V. cholerae. However, a recent report showed the existence of variability in the regulation of natural transformation among Vibrio species [16]. So, we used V. parahaemolyticus in our study to determine the role of chitinases, ChiS and TfoS in terms of chitin utilization and uptake of eDNA. We also studied the effect of GlcNAc2 and GlcNAc6 on the natural competence of V. parahaemolyticus which will highlight a new aspect of regulation related to this conserved phenomenon.
2. Materials and Methods
2.1. Bacterial Strains, Plasmids Used and Growth Conditions
V. parahaemolyticus strain RIMD2210633 (Wild-type; WT), an O3:K6 serotype [1] was obtained from the Laboratory for Culture Collection, Research Institute for Microbial Diseases, Osaka University. This strain was used for the construction of deletion mutants and for functional studies. The WT strain was grown aerobically (250 rpm) at 30 °C for 16 h in 5 mL marine Luria–Bertani (MLB) broth (LB broth containing 3% NaCl). For this study, we had generated a spontaneous streptomycin-resistant (SmR) mutant of the WT strain as described previously [17] and designated as VPS. The frequency at which we isolated SmR mutant was 1 × 10−10. The genomic DNA (gDNA) isolated from VPS was used in chitin-induced natural transformation assays.
The isogenic deletion mutants were constructed by double-crossover allelic exchange using the R6K-ori suicide vector pXAC623 [18] and was maintained in Escherichia coli β2155 λ pir, a diaminopimelic acid (DAP) auxotrophic mutant [19]. For TA cloning, we used pGEMT easy vector (Promega, Madison, WI, USA) and was maintained in E. coli JM109. E. coli strains JM109 and β2155 were routinely cultured in LB broth at 37 °C. However, 0.5 mM DAP (Wako, Osaka, Japan) was added for the growth of E. coli β2155. The medium was also supplemented with appropriate antibiotics whenever necessary.
2.2. Construction of Isogenic Deletion Mutants of V. parahaemolyticus RIMD2210633
All the in-frame deletion mutants (Table 1) were created using splicing by overlap extension (SOE) PCR and allelic exchange [20]. Primers were designed using the V. parahaemolyticus RIMD2210633 genome sequence [21] as the template. For each gene deletion, approximately 500–600 bps flanking sequence of the genes (vp2478: chiS; vpa1177: chiA; vpa0055: chiA2 and vp0854: tfoS) were amplified using two sets of primers. These upstream and downstream flanking PCR products were then fused by PCR to get an in-frame truncated version of the respective gene. This fusion product was amplified and cloned into pGEM-T Easy vector. This plasmid was then digested with a pair of restriction enzymes and the insert was then ligated into the suicide vector pXAC623. The resulting plasmid was transformed into E. coli β2155 λ pir (donor) and then mobilized into WT strain (recipient) by filter mating. In brief, the donor and the recipient strains were grown until the OD600 reached 0.4–0.5. Then, the donor and recipient strains were mixed in a 1:1 ratio and spotted upon 0.22 μm filter membrane (Millipore, Burlington, MA, USA) placed on LB plates and kept at 30 °C for overnight. The transconjugants were selected by the absence of DAP and presence of chloramphenicol in the selection plates. These colonies were streaked on MLB plates with 10% sucrose without chloramphenicol and incubated at 30 °C for overnight to select for colonies with desired gene deletion. Double-crossover deletion mutants were screened by PCR based assay using specific primers.
Table 1.
List of strains and plasmids used in this study.
| Strains or Plasmids | Description | Source |
|---|---|---|
| Strains | ||
| V. parahaemolyticus RIMD22106333 | Clinical isolate of serotype O3:K6; wild-type (WT) strain | [1] |
| VPS | Spontaneous streptomycin-resistant (SmR) mutant | |
| ΔchiS | Deletion in vp2478 of WT, sensor kinase | This study |
| ΔchiA | Deletion in vpa1177 of WT, Chitinase A | This study |
| ΔchiA2 | Deletion in vpa0055 of WT, chitinase | This study |
| ΔchiAΔchiA2 | Double mutant | This study |
| ΔtfoS | Deletion in vp0854 of WT, AraC family transcriptional regulator | This study |
| Escherichia coli | ||
| β2155 | thrB1004 pro thi strA hsdS Δ(lacZ)ΔM15 [F′ Δ(lacZ)M15 lacIq traD36 proA+proB+] ΔdapA::erm(Emr), pir::RP4; kan(Kmr) from SM10 | [19] |
| JM109 | recA1 endA1 gyrA96 thi hsdR17 (rK−,mK+) relA1 supE44 ∆(lac-proAB) [F′, traD36, proAB, lacIqZ∆M15] | Promega |
| Plasmids | ||
| pGEM-T easy | TA-cloning vector; AmpR | Promega |
| pXAC623 | Suicide vector derived from pKTN701 containing the sacBgene of Bacillus subtilis; CmR | [18] |
AmpR, ampicillin-resistant; CmR, chloramphenicol-resistant; SmR, streptomycin-resistant.
2.3. Growth Curve Analysis
From overnight grown cultures, fresh MLB broth was inoculated in 1:100 dilution and grown until log phase was reached. Then the log phase cultures were harvested by centrifugation and washed with defined artificial sea water, DASW [9]. 30 mL of DASW supplemented with 1% shrimp shell chitin flakes (Sigma-Aldrich, St. Louis, MO, USA) was inoculated with approximately 104 cfu mL−1. The cultures were incubated at 30 °C under shaking of 100 rpm for 72 h. In case of chitin oligosaccharides, 5 mM GlcNAc2 and 1.25 mM GlcNAc6 supplemented DASW was used and growth was checked for 48 h at 30 °C.
2.4. Chitinase Plate Assay
Colloidal chitin was prepared from chitin flakes derived from shrimp shells as previously described [22]. Colloidal chitin plates were made by mixing 2% w/v colloidal chitin in DASW. Strains were grown in MLB broth at 30 °C until OD600 reached 0.4. Then, the cell suspension was washed twice with DASW and diluted in DASW so that the OD600 became ~0.4. 10 μL of each bacterial suspension was spotted on the chitin agar plate. The plate was incubated at 30 °C for 5 days and the zone of chitin clearing was recorded.
2.5. Natural Transformation Assays in the Presence of Chitin Flakes and Purified Chitin Oligosaccharides
Natural transformation assays were performed as previously described [23] with some modifications. WT and isogenic mutant strains were inoculated in MLB broth for overnight growth. The overnight grown culture was diluted with fresh MLB broth in a 1:100 ratio and grown until the OD600 reached 0.3–0.4. The bacterial pellet was then washed twice with DASW and diluted in DASW + 0.2% lactate so that the OD600 became ~0.2. This 4 mL of bacterial suspension was added to the conical flask having 200 mg sterilized chitin flakes and incubated statically at 30 °C for overnight. Next day, planktonic phase was removed and fresh 4 mL DASW added along with 50 μg of gDNA (conc. 12.5 μg mL−1) isolated from streptomycin resistant strain VPS and incubated at 30 °C for 8 h under static condition. In negative control only TE buffer was added. After 8 h incubation period, the conical flask was vigorously vortexed to release the attached bacteria. The appropriate dilutions were then plated onto MLB plates with or without streptomycin (200 μg mL−1). The transformation efficiency was calculated as the number of colonies in antibiotic plates divided by the number of colonies on plates without antibiotic.
To determine the frequency of transformation in liquid culture, cells were grown to late-log phase in DASW supplemented with different chitin oligosaccharides (Carbosynth Limited, Berkshire, UK) in the form of GlcNAc2 (5 mM) and GlcNAc6 (1.25 mM). Approximately, 12.5 μg mL−1 genomic DNA was added and incubated at 30 °C for 8 h under static condition. In negative control only TE buffer was added. The appropriate dilutions were then plated onto MLB plates with or without streptomycin (200 μg mL−1).
2.6. Total RNA Isolation and Real-Time PCR
WT and isogenic mutant strains were inoculated in MLB broth for overnight growth. Overnight grown culture was added to fresh MLB broth in 1:100 dilution until the OD600 reached 0.3–0.4. The bacterial pellet was then washed twice and diluted in DASW + 0.2% lactate so that the OD600 became ~0.2. This 5 mL of bacterial suspension was added to the conical flask having 100 mg sterilized chitin flakes and incubated statically at 30 °C for 24 h. Then, the conical flask was vigorously vortexed to release the attached bacteria and the complete bacterial suspension was pellet down. Next, this bacterial pellet was dissolved in 500 μL PBS and 1 mL RNAprotect solution and incubated for 5 min at room temperature. The RNA extraction was done according to manufacturer protocol by using RNeasy kits (QIAGEN, Hilden, Germany). The enzymatic lysis of V. parahaemolyticus was performed with 200 μL of lysozyme (5 mg mL−1) for 15 min at room temperature. In-column DNase treatment was performed for 15 min at room temperature.
For the mRNA expression analysis in the presence of purified chitin oligosaccharides, we used 2.5 mM GlcNAc2 and 0.625 mM GlcNAc6. Overnight grown culture was added to fresh MLB broth in 1:100 dilution until the OD600 reached 0.3. The bacterial pellet was then washed twice and diluted in DASW + 0.2% lactate + GlcNAcn so that the OD600 became ~0.2. This bacterial suspension was incubated at 30 °C for 5 h and then processed for RNA isolation. The primers were designed by Primer Quest tool of Integrated DNA Technologies (IDT, Coralville, IA, USA) such that the amplicon size should be approximately 80–165 bp. We used iTaq Universal SYBR Green One-Step kit for real time RT-PCR (Bio-Rad, Hercules, CA, USA) with 50 ng of RNA for each reaction in Mini Opticon Real-time PCR system. The relative expression of the target transcripts was calculated according to Livak method [24] using recA as an internal control. We also used pvsA as an alternate house-keeping gene for the mRNA expression analysis of competence related gene such as tfoX, pilA, comEA and the data were given as supplementary information.
2.7. Statistical Analysis
The data were analyzed by Student’s t-test. A probability level (p) value of ≤0.05 was considered statistically significant. Three independent experiments were done, and the data represents mean ± SE of these independent events.
3. Results
3.1. ChiS-Dependent Chitinase VPA0055 Is the Major Chitinase Required for Growth in the Presence of Chitin
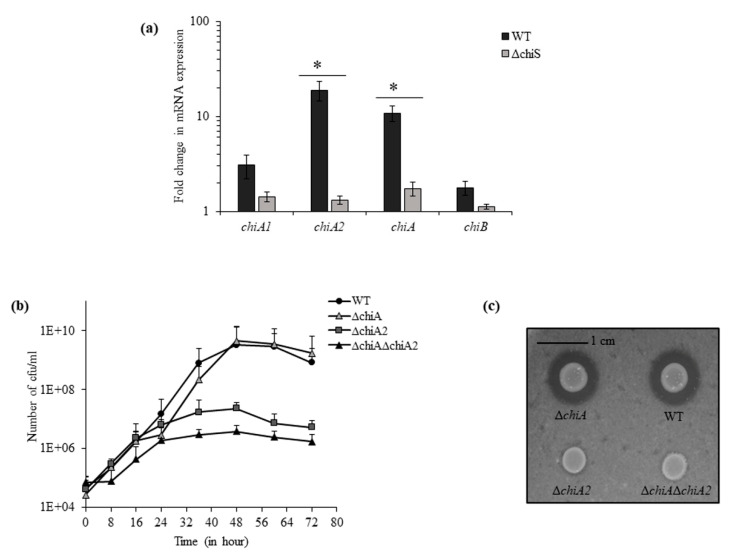
The genes of chitin utilization pathway are known to be controlled by a sensor histidine kinase, ChiS in Vibrios [8]. In the presence of chitin flakes, the real-time RT-PCR showed 6-fold downregulation in the expression of chitinase gene vpa1177 (chitinase A or chiA) and 14-fold downregulation in chitinase gene vpa0055 (chiA2) in ΔchiS mutant as compared to WT. However, the expression of other two chitinase genes vp0619 (chiB) and vp2338 (chiA1) did not show any significant change between ΔchiS mutant and WT (Figure 1a).
Figure 1.
VPA0055 or ChiA2 is the major ChiS-dependent chitinase required for the growth of V. parahaemolyticus in the presence of chitin. (a) The relative expression of four chitinase genes at mRNA level was checked for the ΔchiS mutant compared to the WT strain. recA was used as an internal control. Each bar indicates the mean ± SE of three independent experiments. Asterisks represents p < 0.05, where a fold change in mRNA expression of the ΔchiS mutant was significantly affected. (b) Growth curve of the indicated isogenic mutants and WT in DASW with chitin as sole carbon source. Each point represents the mean ± SE of three independent experiments. (c) Chitinase plate assay. Wild type and indicated isogenic mutants of V. parahaemolyticus were assayed for chitinase activity using DASW plate containing 2% colloidal chitin.
Next, we used isogenic mutants ΔchiA, ΔchiA2 and ΔchiAΔchiA2 for growth curve analysis compared to the WT strain in the presence of 1% chitin flakes. After 48 h, the maximum growth was observed and the viable count of WT, ΔchiA, ΔchiA2 and ΔchiAΔchiA2 was 3.25 × 109, 4.6 × 109, 2.3 × 107 and 3.8 × 106 cfu mL−1, respectively (Figure 1b,c). So, the mutants ΔchiA2 and ΔchiAΔchiA2 showed 140-fold and 850-fold reduced growth compared to the WT strain but the ΔchiA mutant showed WT-like growth. This suggests that in the absence of ChiA2, the activity of other three chitinase could support only minimal level of growth in chitin and thus it can be concluded that ChiA2 played the major role in the breakdown of chitin.
3.2. ChiA2 Is Essential for Chitin Induced Natural Transformation
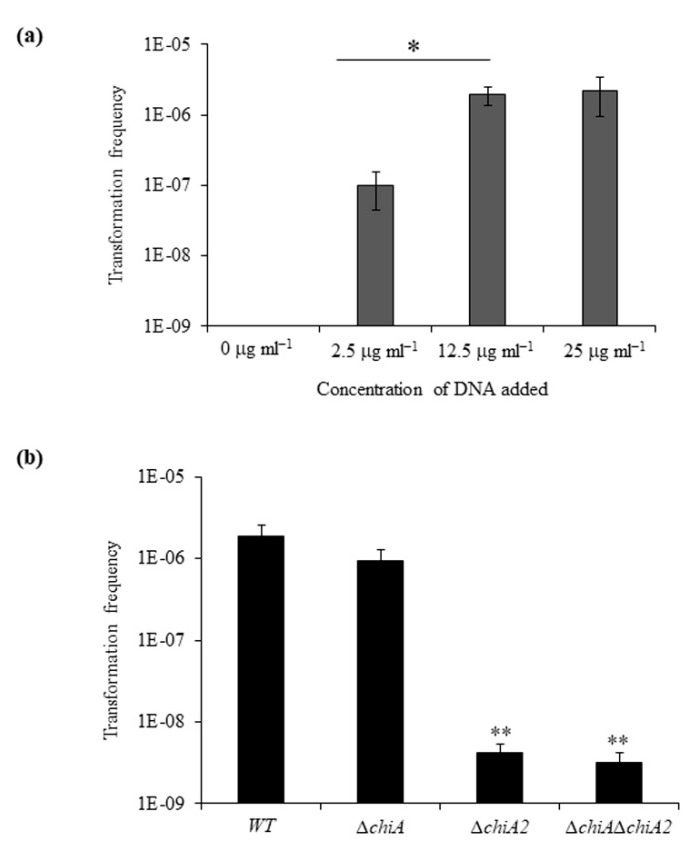
We tested the transformation frequency of WT strain in chitin flakes by adding increasing amounts of donor genomic DNA (gDNA) ranging from 0 to 25 µg mL−1 (Figure 2a). We observed increasing transformation frequencies of 1 × 10−7, 1.9 × 10−6 and 2.2 × 10−6 with gDNA concentration of 2.5 µg mL−1, 12.5 µg mL−1 and 25 µg mL−1, respectively. The frequency of spontaneous SmR-resistant mutants in a sample without DNA was below the limit of detection i.e., between 8 × 10−10 to 2 × 10−10. The transformation frequency difference between the addition of 2.5 µg mL−1 and 12.5 µg mL−1 was statistically significant.
Figure 2.
ChiA2 is essential for chitin-induced natural transformation (a) WT strain incubated with increasing concentration of extracellular DNA for natural transformation. (b) WT and isogenic mutant strains ΔchiA, ΔchiA2 and ΔchiAΔchiA2 were naturally transformed on shrimp chitin flakes with 12.5 μg/mL extracellular DNA. Average of at least three independent experiments were represented. * Statistically significant difference between lowest and other concentrations of donor gDNA (p < 0.05); ** Statistically significant difference between the transformation frequency of WT and mutant strains (p < 0.05).
Next, we compared the transformation frequency of WT, ΔchiA, ΔchiA2 and ΔchiAΔchiA2. The transformation efficiency of WT, ΔchiA, ΔchiA2 and ΔchiAΔchiA2 was 1.89 × 10−6, 9.4 × 10−7, 4.2 × 10−9 and 3.2 × 10−9, respectively (Figure 2b). So, the mutants ΔchiA, ΔchiA2 and ΔchiAΔchiA2 showed a 2-fold, 450-fold and 590-fold reduction in transformation frequency compared to the WT strain. Among the two ChiS regulated chitinases, the absence of ChiA2 drastically reduced the transformation frequency in V. parahaemolyticus. The transformation frequency difference between the WT and ΔchiA mutant was found to be statistically non-significant. The reason behind the high transformation frequencies of the ΔchiA mutant was the ability to utilize chitin efficiently. Therefore, like previous reports, this study also confirms that the ability to degrade chitin into soluble GlcNAcn is directly linked to the DNA uptake efficiency [4,25].
3.3. Role of Transmembrane Regulators ChiS and TfoS in Natural Competence
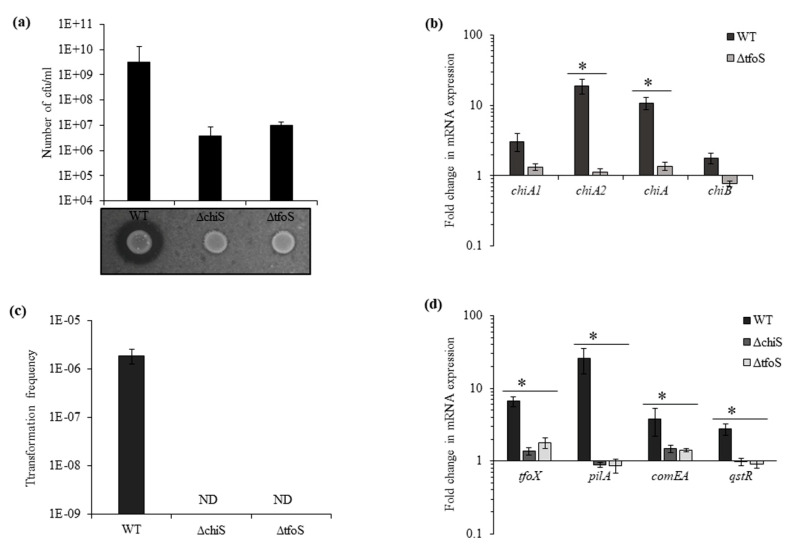
In addition to ChiS, TfoS was also depicted as a membrane-bound transcriptional regulator that links chitin sensing and induction of natural competence by activating TfoX [14,15] in V. cholerae. To determine the role of the homologous gene in V. parahaemolyticus in chitin utilization and natural transformation, we used isogenic mutants ΔchiS and ΔtfoS. The mutants were found to grow poorly in chitin as the sole carbon source, and the viable count for ΔchiS was 3.6 × 106 cfu mL−1 and for ΔtfoS 9.7 × 106 cfu mL−1 after 48 h (Figure 3a). The real-time RT-PCR indicates downregulation in the expression of chitinase genes in ΔtfoS mutant, chiA2 showed 16-fold and chiA showed 8-fold downregulation (Figure 3b). This downregulation of chitinase genes in ΔtfoS mutant was similar to the ΔchiS mutant (Figure 1a).
Figure 3.
Transmembrane regulators ChiS and TfoS are essential for growth and natural transformation in the presence of chitin. (a) Growth of the indicated isogenic mutants and WT in DASW with chitin as sole carbon source after 48 h and chitinase activity on colloidal chitin plate. (b) The relative expression of four chitinase genes at mRNA level was checked for the ΔtfoS mutant compared to the WT strain. recA was used as an internal control. Each bar indicates the mean ±SE of three independent experiments. Asterisks represent p < 0.05. (c) WT and isogenic mutant strains ΔchiS and ΔtfoS were naturally transformed in the presence of shrimp chitin flakes. ND = not detected. (d) The relative expression of competence genes tfoX, pilA, comEA and qstR at mRNA level was checked for WT, ΔchiS and ΔtfoS mutant. Each bar indicates the mean ±SE of three independent experiments. Asterisks represents p < 0.05, where fold change in mRNA expression between wild type and mutant was significantly affected.
In the presence of chitin, the natural transformation was undetectable in the ΔchiS and ΔtfoS mutants (Figure 3c). Along with downregulation in the expression of chitinases, ΔchiS and ΔtfoS mutants also showed a reduction in the mRNA expression of competence-related genes such as tfoX, pilA, comEA and qstR (Figure 3d and Figure S1). Therefore, it can be concluded that in the presence of chitin, the lack of chitinase activity in the ΔchiS and ΔtfoS mutant prevents the release of chitin oligosaccharides. Due to which, there was no upregulation in the expression of master competence regulator tfoX and other competence genes to induce the natural competence state in V. parahaemolyticus. Neither ChiS nor TfoS could independently upregulate the expression of tfoX.
3.4. ChiS and TfoS in Chitin Oligosaccharide Sensing
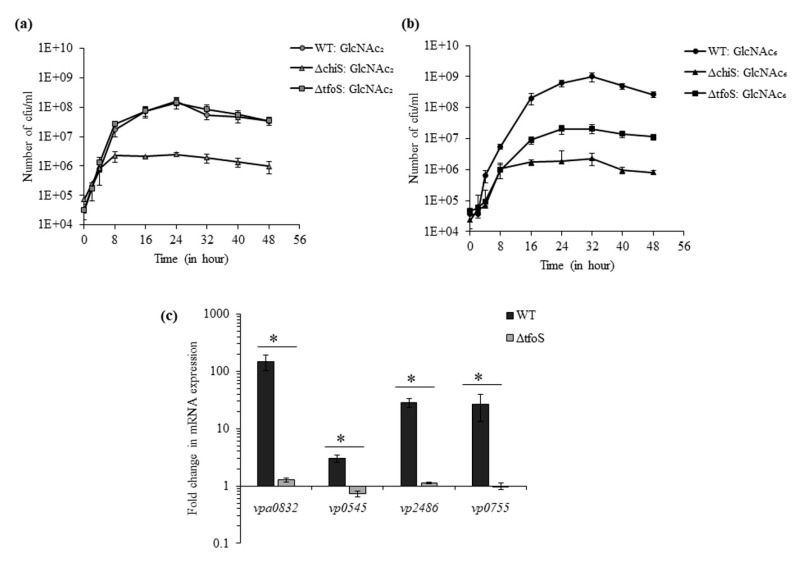
We used the smallest chitin oligosaccharide, GlcNAc2, and the largest chitin oligosaccharide, GlcNAc6, for the growth analysis of WT, ΔtfoS and ΔchiS. In the presence of GlcNAc2, WT and ΔtfoS showed similar growth whereas ΔchiS mutant could not grow (Figure 4a). Therefore, it can be interpreted that the absence of tfoS did not inhibit GlcNAc2-induced ChiS-dependent activation of GlcNAc2 catabolic operon.
Figure 4.
Role of transmembrane regulators ChiS and TfoS in chitin oligosaccharide sensing. (a) Growth curve of WT and isogenic mutant strains ΔchiS and ΔtfoS in 5 mM GlcNAc2 supplemented DASW for 48 h at 30 °C. (b) Growth curve of WT and isogenic mutant strains ΔchiS and ΔtfoS in 1.25mM GlcNAc6 supplemented DASW for 48 h at 30 °C. (c) The relative mRNA expression of the enzymes (vpa0832, vp0545, vp2486 and vp0755) involved in the breakdown of GlcNAc6 was checked for WT and ΔtfoS mutant. Each bar indicates the mean ± SE of three independent experiments. * represents a p-value < 0.05.
However, in the presence of GlcNAc6, both ΔchiS and ΔtfoS mutant showed reduction in growth compared to WT (Figure 4b). After 32 h, the viable count of WT, ΔchiS, ΔtfoS was 9.9 × 108, 2.06 × 107 and 2.3 × 106 cfu mL−1, respectively (Figure 4c). So, the mutant ΔtfoS could grow just 10-fold more compared to the ΔchiS mutant. The ΔchiS mutant could not metabolize chitin oligosaccharides because GlcNAc2 catabolic operon was ChiS-dependent. As we already mentioned that GlcNAc2 catabolic operon was TfoS-independent, the reason behind reduced growth of the ΔtfoS mutant in GlcNAc6 might be related to the downstream processing of GlcNAC6 inside periplasm. The breakdown of GlcNAc6 inside the periplasm might depend on activities of chitodextrinase (vpa0832:cdx); N,N′-diacetylchitobiase (vp0755: chb) and two β-N-acetyl hexosaminidase (vp2486: bNha and vp0545: ha). Therefore, we compared the mRNA expression of these four enzymes in the presence of GlcNAc6 between the WT and ΔtfoS mutant. The ΔtfoS mutant showed 116.6-, 4.1-, 25.4- and 26.2-fold downregulation in vpa0832, vp0545, vp2486 and vp0755, respectively (Figure 4c). So, the ΔtfoS mutant could not metabolize GlcNAC6 due to the downregulation of these four periplasmic enzymes.
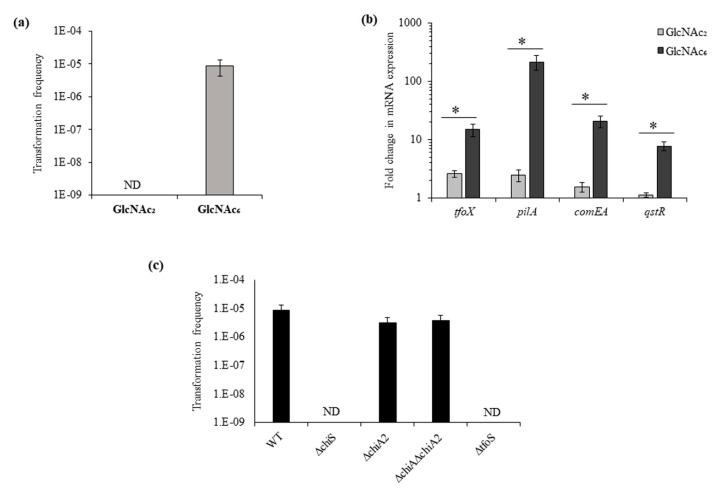
3.5. ChiS and TfoS Are Indispensable for the GlcNAc6 Induced Competence State
We found that GlcNAc6 could induce natural competence in the WT strain with a transformation frequency of 7.7 ± 3.6 × 10−6 (Figure 5a). It was reported that the periplasmic chitodextrinase could cleave GlcNAc6 into smaller molecules [6]. Therefore, we used GlcNAc2 as an inducer of natural transformation, but we could not detect transformants (below the limit of detection). So, we studied the mRNA expression of competence genes tfoX, pilA, comEA and qstR in the presence of GlcNAc2 and GlcNAc6. The GlcNAc6 induced upregulation as compared to GlcNAc2 was 5.7-, 87.5-, 13.6- and 6.9-fold for tfoX, pilA, comEA and qstR, respectively (Figure 5b and Figure S2). This showed that GlcNAc6 could upregulate the expression of these genes more significantly than GlcNAc2 (Figure 5b).
Figure 5.
GlcNAc6 induced competence state in V. parahaemolyticus require ChiS as well as TfoS. (a) Induction of natural competence in WT in the presence of chitin oligosaccharides: 5 mM GlcNAc2 and 1.25 mM GlcNAc6 supplemented DASW. (b) The relative mRNA expression of competence genes tfoX, pilA, comEA and qstR was compared in the presence of GlcNAc2 and GlcNAc6 in the wild type strain. Each bar indicates the mean ±SE of three independent experiments. * represents a p-value < 0.05 (c) GlcNAc6 used for natural transformation in WT and indicated mutants. Each bar represents the mean ± SE of three independent experiments.
Then, we checked natural competence in the presence of GlcNAc6 for mutants which showed no transformation in the presence of chitin. In the growth medium of the ΔchiA2 and ΔchiAΔchiA2 mutant when supplemented with purified GlcNAc6 instead of chitin, the mutant became competent and could uptake eDNA (Figure 5c). This result suggests that the reason behind the lack of competence in these two mutants was their inability to release GlcNAc6 moieties from chitin. Therefore, it can be concluded that ChiA2 plays a vital role in degradation of chitin to release GlcNAc6 residues which then induce a competence state. However, the ΔchiS and ΔtfoS mutants were unable to undergo transformation even in the presence of purified GlcNAc6 (Figure 5c). This indicates the inter-dependence of ChiS and TfoS because the absence of either lead to complete inhibition ofs GlcNAc6 induced competence in V. parahaemolyticus.
4. Discussion
In this study, we found that chitinase ChiA2 plays an essential role during growth on chitin as a sole carbon source and chitin-induced natural transformation. In the ΔchiA2 mutant, the activity of three other chitinase (ChiA, ChiA1 and ChiB) could support only minimal level of growth (2.3 × 107 cfu mL−1) which was just six-fold more when compared to the growth of ΔchiS mutant (3.8 × 106 cfu mL−1). This suggests that chitinase VPA0055 (ChiA2) played the major role in breakdown of chitin in V. parahaemolyticus. This ChiS regulated chitinase, VPA0055 is 76.5% identical to VCA0027 (ChiA2) of V. cholerae and it was among the two major extracellular chitinases [4,25]. In addition to the major role played by ChiA2 in chitin-dependent growth, the activity of ChiA2 is critical for natural transformation in V. parahaemolyticus. Interestingly, the transformation frequency was drastically reduced in the ΔchiA2 mutant, but not like ΔchiS or ΔtfoS mutant, where it was reduced beyond the detection limit. Similar observation was reported in V. cholerae, where absence of ChiA2 still support a low level of chitin-induced transformation due to other chitinases [25].
In V. cholerae, ChiS and TfoS were depicted as transmembrane regulators that link chitin sensing and the induction of natural competence by activating TfoX [13,14,15]. It was shown in V. cholerae that ΔchiS and ΔtfoS mutants were phenotypically different because ΔchiS could not utilize chitin oligomers, while a ΔtfoS mutant could [15]. However, in this study we found that the ΔtfoS mutant of V. parahaemolyticus showed a different growth phenotype depending on the size of the chitin oligosaccharide. In the presence of GlcNAc2, the growth phenotype of WT and ΔtfoS mutant was similar which suggests that, like V. cholerae, GlcNAc2 catabolic operon in V. parahaemolyticus is dependent on ChiS and independent of TfoS [14]. However, the inability of ΔtfoS mutant to utilize GlcNAc6 was found to be due to downregulation of chitodextrinase (vpa0832), N,N′-diacetylchitobiase (vp0755) and β-N-acetyl hexosaminidase (vp0545 and vp2486). Therefore, ΔtfoS mutant could not utilize GlcNAc6 completely even in the presence of ChiS.
The induction of natural transformation with GlcNAc6 not by GlcNAc2 establishes GlcNAc6 as the key molecule required for this phenomenon. In addition, this might also suggest the presence of different sensors for the detection of GlcNAc2 and GlcNAc6. Because in the absence of tfoS, GlcNAc2 was able to activate ChiS-dependent GlcNAc2 catabolic operon. This observation proves ChiS as the sensor for the detection of GlcNAc2. The periplasmic sensor domain of TfoS is structurally related to hybrid two-component system (HTCS) proteins which can couple nutrient sensing to metabolic regulation [14,26]. It has been shown that the deletion of periplasmic domain of TfoS could abolish the natural transformation phenomenon in V. cholerae [15]. As we could detect natural transformation only in the presence of GlcNAc6, this might suggest a possible interaction between TfoS and GlcNAc6 either directly with the periplasmic loop or indirectly with the help of some unknown factor. So, TfoS might act as the sensor for GlcNAc6. However, none of them act independently because GlcNAc6 could not induce natural competence either in the ΔchiS or ΔtfoS mutant. Therefore, we speculate GlcNAc2 induced activation of ChiS and GlcNAc6 induced stimulation of TfoS; these two independent events might mutually control the transcriptional activation of tfoR and thus, the translation of master competence regulator TfoX.
Altogether, chitin-induced natural transformation is a common trait observed among Vibrio species, yet there are differences in the regulation of this phenomenon. A regulatory variation was observed by Simpson et al., where they mentioned quorum sensing is expendable for the natural transformation in V. campbellii DS40M4 and V. parahaemolyticus RIMD2201633 [16] but is required in V. cholerae to activate the competence regulator QstR. In this study, we found another variation where natural competence can only be induced by largest chitin oligosaccharide GlcNAc6 in V. parahaemolyticus whereas in V. cholerae, even smallest chitin oligosaccharide GlcNAc2 could induce the state of competence [14,15]. The significance of GlcNAc6-dependent natural transformation lies in the fact that GlcNAc6 makes this process more specific for Vibrios because relatively few other microbes can take up long chitin oligosaccharides compared to mono- and di-saccharides [27] and that ultimately leads to the acquisition of new features. As a consequence, novel strains of Vibrios could emerge with heightened ecological fitness and pathogenicity [28]. Moreover, in a nutrient-poor marine environment, the ability to uptake GlcNAc6 might provide them a competitive advantage over other microbial species. In future, we would like to know whether GlcNAc6-dependent competence is a strain specific phenomenon or a general trait for V. parahaemolyticus and more detailed genetic analysis will be done to elaborate GlcNAc6 induced competence in V. parahaemolyticus.
Acknowledgments
The authors are grateful to Teruo Kuroda at Graduate School of Biomedical and Health Sciences, Hiroshima University for providing us the suicide vector, pXAC623 and Escherichia coli β2155 λ pir.
Abbreviations
| MDPI | Multidisciplinary Digital Publishing Institute |
| DOAJ | Directory of open access journals |
| TLA | Three letter acronym |
| LD | linear dichroism |
| TfoS | ChiS-dependent tfoR regulator |
| DASW | Defined artificial sea water |
| GlcNAc | N-acetyl glucosamine |
| GlcNAc2 | Diacetylchitobiose |
| GlcNAc6 | Hexaacetylchitohexaose |
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-2607/8/9/1303/s1, Figure S1: The relative expression of competence genes tfoX, pilA, comEA and qstR at mRNA level was checked for WT, ΔchiS and ΔtfoS mutant in the presence of chitin; Figure S2: The relative mRNA expression of competence genes tfoX, pilA, comEA and qstR was compared in the presence of GlcNAc2 and GlcNAc6 in wild type strain.
Author Contributions
For research articles with several authors, a short paragraph specifying their individual contributions must be provided. The following statements should be used “conceptualization, A.D.; methodology, A.D.; formal analysis, A.D.; writing—original draft preparation, A.D.; writing—review and editing, T.M. and S.-i.M.; supervision, T.M. and S.-i.M.; funding acquisition, S.-i.M.”, please turn to the CRediT taxonomy for the term explanation. Authorship must be limited to those who have contributed substantially to the work reported. All authors have read and agreed to the published version of the manuscript.
Funding
This investigation is supported by the Japan Initiative for Global Research Network on Infectious Diseases (J-GRID; JP19fm0108002) from Ministry of Education, Culture, Sport, Science and Technology in Japan (MEXT) and Japan Agency for Medical Research and Development (AMED). The funders had no role in study design, data collection and analysis, or preparation of the manuscript.
Conflicts of Interest
The authors declare that there are no conflict of interest.
References
- 1.Wong H.C., Liu S.H., Wang T.K., Lee C.L., Chiou C.S., Liu D.P., Nishibuchi M., Lee B.K. Characteristics of Vibrio parahaemolyticus O3:K6 from Asia. Appl. Environ. Microbiol. 2000;66:3981–3986. doi: 10.1128/AEM.66.9.3981-3986.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gode-Potratz C.J., Kustusch R.J., Breheny P.J., Weiss D.S., McCarter L.L. Surface sensing in Vibrio parahaemolyticus triggers a programme of gene expression that promotes colonization and virulence. Mol. Microbiol. 2011;79:240–263. doi: 10.1111/j.1365-2958.2010.07445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ceccarelli D., Hasan N.A., Huq A., Colwell R.R. Distribution and dynamics of epidemic and pandemic Vibrio parahaemolyticus virulence factors. Front. Cell Infect. Microbiol. 2013;3:97. doi: 10.3389/fcimb.2013.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meibom K.L., Li X.B., Neilson A.T., Wu C.Y., Roseman S., Schoolnik G.K. The Vibrio cholerae chitin utilization program. Proc. Natl. Acad. Sci. USA. 2004;101:2524–2529. doi: 10.1073/pnas.0308707101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thompson F.L., Iida T., Swings J. Biodiversity of vibrios. Microbiol. Mol. Biol. Rev. 2004;68:403–431. doi: 10.1128/MMBR.68.3.403-431.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keyhani N.O., Roseman S. The chitin catabolic cascade in the marine bacterium Vibrio furnissii molecular cloning, isolation, and characterization of a periplasmic chitodextrinase. J. Biol. Chem. 1996;271:33414–33424. doi: 10.1074/jbc.271.52.33414. [DOI] [PubMed] [Google Scholar]
- 7.Keyhani N.O., Roseman S. The chitin catabolic cascade in the marine bacterium Vibrio furnissii molecular cloning, isolation, and characterization of a periplasmic beta-N-acetyl glucosaminidase. J. Biol. Chem. 1996;271:33425–33432. doi: 10.1074/jbc.271.52.33425. [DOI] [PubMed] [Google Scholar]
- 8.Li X., Roseman S. The chitinolytic cascade in Vibrios is regulated by chitin oligosaccharides and a two-component chitin catabolic sensor/kinase. Proc. Natl. Acad. Sci. USA. 2004;101:627–631. doi: 10.1073/pnas.0307645100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meibom K.L., Blokesch M., Dolganov N.A., Wu C.Y., Schoolnik G.K. Chitin induces natural competence in Vibrio cholerae. Science. 2005;310:1824–1827. doi: 10.1126/science.1120096. [DOI] [PubMed] [Google Scholar]
- 10.Gulig P.A., Tucker M.S., Thiaville P.C., Joseph J.L., Brown R.N. USER friendly cloning coupled with chitin-based natural transformation enables rapid mutagenesis of Vibrio vulnificus. Appl. Environ. Microbiol. 2009;75:4936–4949. doi: 10.1128/AEM.02564-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pollack-Berti A., Wollenberg M.S., Ruby E.G. Natural transformation of Vibrio fischeri requires tfoX and tfoY. Environ. Microbiol. 2010;12:2302–2311. doi: 10.1111/j.1462-2920.2010.02250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen Y., Dai J., Morris J.G., Jr., Johnson J.A. Genetic analysis of the capsule polysaccharide (K antigen) and exopolysaccharide genes in pandemic Vibrio parahaemolyticus O3:K6. BMC Microbiol. 2010;10:274. doi: 10.1186/1471-2180-10-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamamoto S., Izumiya H., Mitobe J., Morita M., Arakawa E., Ohnishi M., Watanabe H. Identification of a chitin-inducd small RNA that regulates translation of tfoX gene, encoding a positive regulator of natural competence in Vibrio cholerae. J. Bacteriol. 2011;193:1953–1965. doi: 10.1128/JB.01340-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamamoto S., Mitobe J., Ishikawa T., Wai S.N., Ohnishi M., Watanabe H., Izumiya H. Regulation of natural competence by the orphan two-component system sensor kinase ChiS involves a non-canonical transmembrane regulator in Vibrio cholerae. Mol. Microbiol. 2014;91:326–347. doi: 10.1111/mmi.12462. [DOI] [PubMed] [Google Scholar]
- 15.Dalia A.B., Lazinski D.W., Camilli A. Identification of a membrane-bound transcriptional regulator that links chitin and natural competence in Vibrio cholerae. mBio. 2014;5:e01028:1–e01028:13. doi: 10.1128/mBio.01028-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simpson C.A., Podicheti R., Rusch D.B., Dalia A.B., van Kessel J.C. Diversity in natural transformation frequencies and regulation across Vibrio species. mBio. 2019;10:e02788:1–e02788:19. doi: 10.1128/mBio.02788-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Whitaker W.B., Parent M.A., Naughton L.M., Richards G.P., Blumerman S.L., Boyd E.F. Modulation of responses of Vibrio parahaemolyticus O3:K6 to pH and temperature stresses by growth at different salt concentrations. Appl. Environ. Microbiol. 2010;76:4720–4729. doi: 10.1128/AEM.00474-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuroda T., Mizushima T., Tsuchiya T. Physiological roles of three Na+/H+ antiporters in the halophilic bacterium Vibrio parahaemolyticus. Microbiol. Immunol. 2005;49:711–719. doi: 10.1111/j.1348-0421.2005.tb03662.x. [DOI] [PubMed] [Google Scholar]
- 19.Demarre G., Guérout A.M., Matsumoto-Mashimo C., Rowe-Magnus D.A., Marlière P., Mazel D. A new family of mobilizable suicide plasmids based on broad host range R388 plasmid (IncW) and RP4 plasmid (IncPα) conjugative machineries and their cognate Escherichia coli host strains. Res. Microbiol. 2005;156:245–255. doi: 10.1016/j.resmic.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 20.Skorupski K., Taylor R.K. Positive selection of vectors for allelic exchange. Gene. 1996;169:47–52. doi: 10.1016/0378-1119(95)00793-8. [DOI] [PubMed] [Google Scholar]
- 21.Makino K., Oshima K., Kurokawa K., Yokoyama K., Uda T., Tagomori K., Iijima Y., Najima M., Nakano M., Yamashita A., et al. Genome sequence of Vibrio parahaemolyticus: A pathogenic mechanism distinct from that of V. cholerae. Lancet. 2003;361:743–749. doi: 10.1016/S0140-6736(03)12659-1. [DOI] [PubMed] [Google Scholar]
- 22.Hsu S.C., Lockwood J.L. Powdered chitin agar as a selective medium for enumeration of actinomycetes in water and soil. Appl. Microbiol. 1975;29:422–426. doi: 10.1128/AEM.29.3.422-426.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marvig R.L., Blokesch M. Natural transformation of Vibrio cholerae as a tool—Optimizing the procedure. BMC Microbiol. 2010;10:155. doi: 10.1186/1471-2180-10-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 25.Hayes C.A., Dalia T.N., Dalia A.B. Systematic genetic dissection of chitin degradation and uptake in Vibrio cholerae. Environ. Microbiol. 2017;19:4154–4163. doi: 10.1111/1462-2920.13866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sonnenburg E.D., Sonnenburg J.L., Manchester J.K., Hansen E.E., Chiang H.C., Gordon J.I. A hybrid two-component system protein of a prominent human gut symbiont couples glycan sensing in vivo to carbohydrate metabolism. Proc. Natl. Acad. Sci. USA. 2006;103:8834–8839. doi: 10.1073/pnas.0603249103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suginta W., Chumjan W., Mahendran K.R., Janning P., Schulte A., Winterhalter M. Molecular uptake of chitooligosaccharides through chitoporin from the marine bacterium Vibrio harveyi. PLoS ONE. 2013;8:e55126. doi: 10.1371/journal.pone.0055126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mooi F.R., Bik E.M. The evolution of epidemic Vibrio cholerae strains. Trends Microbiol. 1997;5:161–165. doi: 10.1016/S0966-842X(96)10086-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.