Abstract
The effect of biochar alone or co-applied with triple superphosphate on rhizosphere soil characteristics, nodule formation, phytoconstituents and antioxidant property of cowpea (Vigna uguiculata) is yet to be adequately examined in sub Saharan Africa. A field experiment was conducted where cowpea (Vigna unguiculata) was grown in a tropical sandy loam soil amended with biochar at 1.5 t ha−1 and 2.5 t ha−1 solely or together with inorganic phosphate fertilizer (Triple superphosphate), applied at a rate of 60 kg P ha −1. At 50% flowering, changes in selected rhizosphere soil properties (pH, total nitrogen, available phosphorus, soil organic carbon, cation exchange capacity), nodule count, phytochemicals (phenols, flavonoids, alkaloids, tannins, saponins) and antioxidant property of cowpea roots and leaves were determined by standard laboratory procedures. Differences between means of the measured parameters were established using ANOVA, and relationships among the parameters were explored using Pearson correlation (p < 0.05). Addition of biochar solely or in combination with TSP significantly (p < 0.05) increased soil pH, total nitrogen, available phosphorus, soil organic carbon, cation exchange capacity and root nodule count. Flavonoids, phenols, alkaloids, saponin, tannin contents and antioxidant activity in the roots and leaves were significantly (p < 0.05) higher in the amended soils compared with the unamended soil. Similarly, soil flavonoids, phenols, alkaloids and antioxidant activity were significantly higher in amended soils compared with control. Significant, positive inter and intra correlation with varying strength was found between soil properties, nodule number and phytoconstituents. This is an indication that biochar can be co-applied with triple superphosphate to sustain soil fertility, improve nodulation and enhance concentrations of phytoconstituents in soil, cowpea roots and leaves.
Keywords: Biochar, Phytochemicals, Triple superphosphate, Nodule count, Antioxidant activity, Agricultural soil science, Agronomy, Climatology, Ecology, Soil science, Environmental management, Microbiology, Biotechnology, Transcriptomics, Biochemistry
Biochar, Phytochemicals, Triple superphosphate, Nodule count, Antioxidant activity, Agricultural soil science, Agronomy, Climatology, Ecology, Soil science, Environmental management, Microbiology, Biotechnology, Transcriptomics, Biochemistry.
1. Introduction
Large tracts of arable lands in sub-Saharan Africa (SSA) are characterized by poor fertility, particularly with low nitrogen and phosphorus content (Lal, 2019). These soils often show high aluminum toxicity and low percentage base saturation (Sade et al., 2016). Biochar application has received a heightened research attention as a novel approach to restoring soil fertility especially in acidic and low nutrient SSA soils (Mensah and Frimpong, 2018; Raboin et al., 2016; Rondon et al., 2007). Biochar is a stable C by-product obtained from pyrolysis or thermochemical decomposition of organic feedstock in a high temperature and no or limited oxygen environment (Lehmann and Joseph, 2015). Biochar improves soil quality by increasing soil pH (Chan et al., 2008), cation exchange capacity (Lehmann, 2007; Uzoma et al., 2011), soil nutrient retention capacity (Gao et al., 2016), enhancing microbial biomass and activity (Lehmann et al., 2011; Phares et al., 2017) and improving soil moisture retention capacity (Duku et al., 2011).
Studies have shown that biochar produced from crop residues such as corn cob and rice husk, often have low nitrogen (Deenik and Cooney, 2016; Liu et al., 2019) and phosphorus (Deenik and Cooney, 2016) contents. Then again, when animal residue is charred, P bioavailability in the charred biomass is reduced (Wu et al., 2012). Dai et al. (2016) explained that the reduction in P after charring animal residue is due to bonding of P with multivalent metal cations in biochar which affects P bioavailability. It is therefore imperative to supplement the N and P in biochar amended soils with inorganic or organic inputs. Previous studies involving biochar co-applied with compost (Naeem et al., 2018), poultry manure (Phares et al., 2017) or inorganic fertilizer (NPK) (Naeem et al., 2018; Yu et al., 2017) respectively have shown significant additive effects of the combined amendments on soil physicochemical and biological properties and crop yields.
Here, we examined the potential of combined biochar and inorganic phosphorus application to sustainably overcome phosphorus deficiency in acidic Ghanaian soils. We hypothesize that the liming effect of the added biochar will address the potential phosphorus retention and low phosphate availability to crops in acidic soils amended with inorganic phosphorus fertilizer. Our hypothesis is underpinned by the fact that available phosphorus is limited in acidic tropical soils due to precipitation by mainly Al3+ and Fe3+ ions. Thus, biochar addition offers the potential to raise soil's pH beyond the threshold where P fixation by Al3+ and Fe3+ ions is likely to occur, resulting in higher phosphorus availability for plant uptake. The liming effect of the applied biochar can also increase the CEC of acid soils through a reduction of soluble Al3+ and Fe3+, which could also increase the availability of P and other nutrients. It is also postulated in our study that the availability of phosphorus is necessary for nodule formation, nitrogen fixation and growth of cowpea.
Cowpea is an important legume in most SSA countries as the grains, roots and leaves serve as major source of protein for people and as sources of feed for animals (Quaye et al., 2009; Timko and Singh, 2008). Cowpea is reported to be rich in phytochemicals, such as phenols, flavonoids and alkaloids (Alidu et al., 2020; Sivakumar et al., 2018; Sombié et al., 2018). The phytochemicals have medicinal uses and as important secondary metabolites, they moderate, soil-plant-microorganism interactions including alellopathy, defense against cowpea diseases and pest, nodulation, nutrient fixation and N uptake (Aniszewski, 2007; Bhattacharya et al., 2010; Hu et al., 2005). For instance, flavonoids together with phenols regulates how rhizobia colonize legume roots, and enhances rhizobia survival in the rhizosphere (Cooper, 2004). Allahdadi and Farzane (2018) reported that high soil N application decreased total phenolics, flavonoids and antioxidant activity in artichoke leaves while Gomaa et al. (2015) observed an increase in flavonoid content of Trifolium alexandrinum following the application of Sonchus oleraceus residue. In contrast, Ibrahim et al. (2013) found that addition of chicken dung enhanced the production of secondary metabolites such as total phenolics, flavonoids, saponin and improved antioxidant activity.
So far, there is paucity of information regarding the effect of biochar and inorganic phosphorus fertilizer on rhizosphere soil properties, nodule formation and phytoconstituents contents of cowpea grown on tropical sandy loam soils and under field condition. Therefore, this study was undertaken to examine the effect of biochar applied solely or in combination with inorganic P fertilizer on; (i) Soil pH, total N, SOC and available P contents, and CEC as well as cowpea root nodule counts and (ii) Flavonoids, phenols, alkaloids, saponin, tannin contents and antioxidant activity and in the rhizosphere soil, roots and leaves of cowpea grown on a tropical sandy loam soil.
2. Materials and methods
2.1. Site description, experimental layout and planting
A micro plot field experiment was carried out between December, 2019 and February, 2020, at the Research and Teaching Farm of the School of Agriculture, University of Cape Coast. The experimental site is located in the coastal savannah agro - ecological zone with coordinates (5.131782, -1.294073). The area experiences a bimodal rainfall i.e. a major rainy season and a minor rainy season, followed by a dry season. The soils of the area belong to a local classification called Benya series belonging to Haplic Acrisol (FAO, I. Working Group WRB, 2015), which is part of the Edina catena. It developed from Sekondian rocks; mainly sandstones, shales, and conglomerates (Asamoa, 1973).
Biochar was produced from pyrolysis of dry rice husk feedstock at approximately 400 °C for 35 min, using a locally manufactured top-lit updraft kiln (TLUD). The properties of the soil and biochar used in the study are summarized in Tables 1 and 2. The design used for the experiment was randomized complete block design with four replications. The size of a plot per treatment was 1.5 m × 2.5 m.
Table 1.
Initial physical and chemical properties of experimental soil (n = 3).
| Soil properties | Mean | Standard deviation |
|---|---|---|
| pH | 5.43 | 0.02 |
| Total nitrogen (%) | 0.08 | 0.01 |
| Available phosphorus (mg kg-1) | 8.5 | 0.34 |
| Soil organic carbon (%) | 0.73 | 0.05 |
| Exchangeable acidity (cmol kg-1) | 0.92 | 0.06 |
| CEC (cmol kg-1) | 4.47 | 0.28 |
| Sand∗ | 68% | |
| Silt∗ | 10% | |
| Clay∗ | 22% |
Soil parameter had only two replicates.
Table 2.
Properties of rice husk biochar used for the experiment (n = 3).
| Property | Mean | Standard deviation |
|---|---|---|
| pH | 7.9 | 0.35 |
| Total organic carbon (%) | 71.2 | 3.40 |
| Total nitrogen (%) | 0.48 | 0.06 |
| C:N ratio | 149.5 | 20.44 |
| Available phosphorus (g kg-1) | 0.51 | 0.04 |
| Total phosphorus (%) | 0.17 | 0.03 |
| Total potassium (%) | 1.2 | 0.04 |
| CEC (cmol kg−1) | 1.4 | 0.03 |
| Ash (%) | 15 | 1.53 |
| EC (dS m-1) | 0.6 | 0.08 |
Biochar was applied solely or in combination with triple superphosphate (TSP). Table 3 indicates rates and treatment combinations of biochar and triple superphosphate (TSP) used for the experiment. Potassium (K) was supplied to all plots from muriate of potash (MOP).
Table 3.
Rates and treatment combinations of biochar and triple superphosphate (TSP) used for the experiment (number of replications = 4).
| Treatment | BC (t ha−1) | TSP (kg ha −1) |
|---|---|---|
| CTRL | 0 | 0 |
| BC1.5 | 1.5 | 0 |
| BC2.5 | 2.5 | 0 |
| BC1.5 + P | 1.5 | 60 |
| BC2.5 + P | 2.5 | 60 |
CTRL: control, BC1.5 = biochar at 1.5 t ha−1, BC2.5 = biochar at 2.5 t ha−1, P = phosphorus fertilizer TSP: triple superphosphate.
2.2. Planting and agronomic practices
The Department of Molecular Biology and Biotechnology of the University of Cape Coast provided the cowpea seeds used in the study. Biochar was incorporated uniformly into the soil to a depth of 0.1 m, 5 days before the cowpea seeds were sowed to ensure that the biochar had equilibrated in the soil and the spike in heat at the beginning of biochar mineralization has subsided. Three cowpea seeds were sown per hill at a spacing of 0.3 m within row × 0.5 m between row. The cowpea seedlings were thinned to two plants per hill, 8 days after emergence with each plot having 50 plant stands. Triple superphosphate (60 kg P ha−1) and MOP (45 kg K ha−1) was applied on the 14th day after planting (DAP), using side dressing where they were applied to the base of the crop. These fertilizers were dissolved in tap water to ensure the minimum electrical conductance that will not adversely affect physiological functions of the plants by dissolving 3.8 g of each fertilizer in 50 mL of tap water per plant.
The seedlings were watered every week to 70% field capacity by using time domain reflectometry (TDR) method to check loss in soil moisture. Field capacity was estimated as described by Walker (1989). Weeding was done on three occasions during the growing period. Neem extract was applied to control pest and disease as described by Badii et al. (2008) and Ganiyu et al. (2017). The experimented was conducted for 35 days after emergence.
2.3. Data collection
2.3.1. Sample collection, preparation and nodule count
Six plants and their rhizosphere soil were respectively collected when the cowpea plants had attained 50% flowering. Plants within the central rows of each treatment plot previously tagged were carefully uprooted using a spade with a ball of earth to a depth of approximately 40 cm. Prior to roots excavation, plots were moistened to allow for ease of excavation to reduce retention of nodules in the soil. Soils attached to the cowpea roots were carefully shaken into a bowl and the roots were carefully washed under running water to enable removal of the nodules attached to the roots. The nodules were counted with the aid of a magnifying glass and placed into labeled ziplock bags. The cowpea roots and leaves were separated and dried to about 10% moisture content. The dry root and aboveground biomass samples were then ground and stored in clean and labeled ziplock bags for laboratory analysis 2 days afterwards. The rhizosphere soil collected under the roots of the uprooted cowpea plants were sieved through 2 mm mesh; stored in ziplock bags and sent to the laboratory for further investigation.
2.3.2. Effective nodule count
The number of effective nodules was estimated by observing the internal colour of the nodule (Kawaka et al., 2018; Thrall et al., 2011). The nodules were cut into two halves to observe the colour of the interior. Nodules which showed pink-reddish colouration were designated effective and assumed to be able to fix nitrogen while the nodules that showed green or no coloration were described as ineffective nodules.
2.4. Laboratory analyses
2.4.1. Analysis of experimental soil physicochemical properties
The pH of experimental soil was measured using the glass electrode of a Suntex SP-701 pH meter in 1:2.5 soil: water (w/v) suspension. The soil organic carbon (SOC) content was determined by the modified wet oxidation method (Nelson and Sommers, 1996). Total nitrogen in soil was determined using the micro Kjeldahl method as described by Stewarte et al. (1974). Available phosphorus (AvP) content in the soil was analyzed following the Bray-1 acid method (Maghanga et al., 2015). The ammonium acetate (at pH 7) extraction method was used to determine the cation exchange capacity (CEC) of soil (Dohrmann, 2006) and acid (KCl) titration was used to determine exchangeable acidity of soil (Anderson and Ingram, 1993). The hydrometer method (Ashworth et al., 2001) was used to analyze the soil particle size distribution and the textural class of the soil was determined with the USDA textural triangle.
2.4.2. Analysis of biochar physicochemical properties
The pH of biochar was determined using the glass electrode of a Suntex SP-701 pH meter in 1:5 water: biochar (w/v) suspension. Electrical conductivity (EC) of biochar was determined using conductivity meter (LF91, Germany). Loss on ignition method was used for biochar carbon and ash determination by respectively heating biochar at 550 °C (Mikos-Szymańska et al., 2019) and 900 °C (Ascough et al., 2018) for 4 h. Total nitrogen in biochar was determined using the micro Kjeldahl method as described by Stewarte et al. (1974). Available phosphorus (AvP) content in biochar was analyzed by Bray-1 phophomolybdate blue method (Li et al., 2018) and total phosphorus using acid digestion and colorimetric P measurement as described by Song and Guo (2012). The ammonium acetate (at pH 7) method was used to determine the cation exchange capacity (CEC) of biochar (Munera-Echeverri et al., 2018).
2.4.3. Phytochemical and antioxidant analysis of leaves, root and soil
2.4.3.1. Sample extraction, phytochemical screening
Leaves and roots were prepared by cold maceration procedure (Ncube et al., 2008) whiles rhizosphere soil was prepared by oven-drying method (Iannucci et al., 2013). Methanol was used as a solvent (Iannucci et al., 2013; Kong et al., 2006; Ncube et al., 2008) to obtain extracts for the determination of phytochemicals in the cowpea roots, above ground biomass and soil, respectively. The extracts were screened to detect phenols, flavonoids, alkaloids, saponins and tannins following standard protocols described by Adegoke et al. (2010).
2.4.3.2. Quantitative determination of phytoconstituents
Phenols content in sample extracts were determined following a modified Folin-Ciocalteu reagent assay protocol described by Adusei et al. (2019) and results reported as gallic acid equivalent (GAE) (mg g−1) of dry extract. The aluminum chloride colorimetric procedure was followed to determine the flavonoid content of the extract using quercetin as a standard (Chantiratikul et al., 2009). The total flavonoid content was expressed as quercetin equivalent (QE) (mg g−1) of dry extract. To determine percent alkaloid content hydrochloric acid dilution coupled with complete precipitation method was used (Adegoke et al., 2010) and the alkaloid content (%) was estimated from Eq. (1);
| (1) |
Saponin content was determined using a protocol based on vanillin-sulphuric acid colorimetric reaction (Adusei et al., 2019) and the result expressed as diosgenin equivalent (DE) (mg g−1) of dry extract. Tannins content in each extract was estimated following protein precipitation binding protocol, using tannic acid as a standard. Results obtained for tannin content was reported as tannic acid equivalent (TAE) (mg g−1) of dry extract. Phosphomolybdenum assay method was used to determine antioxidant activity using ascorbic acid as a reference standard and the results expressed as ascorbic acid equivalent (AAE) (mg g−1) of dry weight extract (Akinmoladun et al., 2007).
2.5. Data and statistical analysis
All the data generated in the study were analyzed using the Genstat statistical software (GenStat 14.1, VSN International, Oxford, UK). The data was generally presented as mean ±1 standard deviations. A one-way analysis of variance (ANOVA) was used to compare means of the various parameters studied. Significant differences between means were established at 5% probability unless otherwise stated. A mean ranking was done using Fisher's Least Significant Difference (LSD) comparison assuming equal means. Pearson correlation was used to establish relationship between parameters measured.
3. Results
3.1. Effect of biochar and triple superphosphate fertilizer on rhizosphere soil properties
The effects of biochar and triple superphosphate fertilizer on rhizosphere soil properties are presented in Table 4.
Table 4.
Rhizosphere soil properties following application of biochar and inorganic phosphorus fertilizer (TSP) (n = 3).
| Treatment | pH | Total N (%) | AvP (mg kg−1) | SOC (%) | CEC (cmolc kg−1) |
|---|---|---|---|---|---|
| Control | 5.43 ± 0.03a | 0.09 ± 0.02a | 7.34 ± 0.09a | 0.72 ± 0.00a | 4.43 ± 0.10a |
| BC1.5 | 5.83 ± 0.03b | 0.22 ± 0.04b | 11.25 ± 0.43b | 1.12 ± 0.03b | 5.05 ± 0.07b |
| BC2.5 | 6.10 ± 0.01c | 0.30 ± 0.02c | 13.92 ± 0.67c | 1.34 ± 0.02c | 6.38 ± 0.05c |
| BC1.5 + P | 5.86 ± 0.01b | 0.36 ± 0.03d | 23.52 ± 0.79d | 1.16 ± 0.04b | 5.08 ± 0.10b |
| BC2.5 + P | 6.11 ± 0.01c | 0.43 ± 0.03e | 26.78 ± 0.65e | 1.36 ± 0.02c | 6.41 ± 0.04c |
| %CV | 0.4 | 9.5 | 3.5 | 2.3 | 1.4 |
BC1.5 = biochar at 1.5 t ha−1, BC2.5 = biochar at 2.5 t ha−1, P = phosphorus fertilizer; CV: coefficient of variation, AvP = available phosphorus, SOC = soil organic carbon, CEC = cation exchange capacity. Each value is presented as mean ±1 standard deviation. Means in the same column and lettered with same alphabet superscripts are not significantly different at p < 0.05 using Fisher's protected LSD test.
3.1.1. Soil pH
All the treatments significantly (p < 0.05) increased soil pH compared with the control (Table 4), with the highest value (6.11) recorded in the combined biochar at 2.5 t ha−1 and TSP treatment, whiles the lowest value (5.43) recorded for the control plot. Soil pH increased with increasing biochar rates, such that when biochar rate was increased from 1.5 t ha−1 to 2.5 t ha−1, pH increased from 5.83 to 6.10. However, when biochar at respective rates (1.5 t ha−1 and 2.5 t ha−1) were co-applied with TSP fertilizer, no significant increases were recorded compared with biochar alone. The rise in soil pH followed the order; control < BC1.5 < BC1.5 + P < BC2.5 < BC2.5 + P.
3.1.2. Total nitrogen
The amendments applied to soil resulted in significant (p < 0.05) increases in total soil nitrogen content compared with the control (Table 4). The lowest total soil nitrogen content (0.09%) was observed in the control plot and the highest (0.43%) was observed when 2.5 t ha−1 biochar and 60 kg P TSP were applied together. In addition, increasing biochar rates from 1.5 to 2.5 t ha−1 increased total N by 36% compared with the former. It was also observed that when biochar at the respective rates was co-applied with TSP, total N increased significantly (p < 0.05).
3.1.3. Available phosphorus
The incorporation of biochar solely or in combination with TSP significantly (p < 0.05) increased available soil phosphorus in all the treatments. The soil analysis results showed that soil available P contents ranged from 7.34 mg kg−1 to 26.78 mg kg−1 respectively for the control field and the plots that received 2.5 t ha−1 biochar in combination with TSP.
3.1.4. Soil organic carbon
The addition of biochar and triple superphosphate significantly (p < 0.05) increased SOC (Table 4). Notably, when biochar rate was increased from 1.5 t ha−1 to 2.5 t ha−1, SOC increased by 19.6%. No significant change in soil SOC occurred when biochar at rates of 1.5 and 2.5 t ha−1 were co-applied with TSP compared to the treatments without TSP.
3.1.5. Cation exchange capacity
CEC increased significantly in all amended plots above that observed in the control treatment. Although increasing the rate of biochar applied solely resulted in an increase in CEC (Table 4), the combined addition of TSP and biochar did not yield any further increases in CEC.
3.1.6. Nodule number and effective nodule number
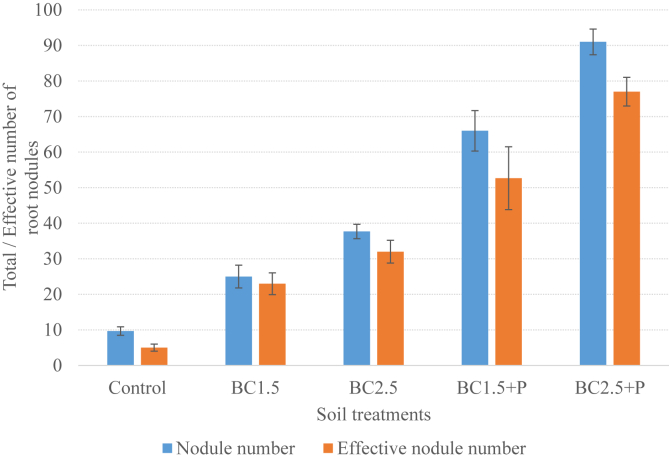
The total and effective numbers of root nodules found in the study are summarized in Figure 1.
Figure 1.
Nodule number and effective nodule number in response to biochar and triple superphosphate fertilizer. BC1.5 = biochar at 1.5 t ha−1, BC2.5 = biochar at 2.5 t ha−1. Data show mean ± standard error of the mean, n = 3.
Application of biochar and TSP greatly increased both the absolute and effective number of root nodules compared with the unamended soil (Figure 1). The number of effective root nodules number followed the increasing order; control < BC1.5 < BC2.5 < BC1.5 + P < BC2.5 + P.
3.2. Effect of biochar and triple superphosphate fertilizer on phytoconstituents, antioxidant activity of cowpea roots, leaves and rhizosphere soil
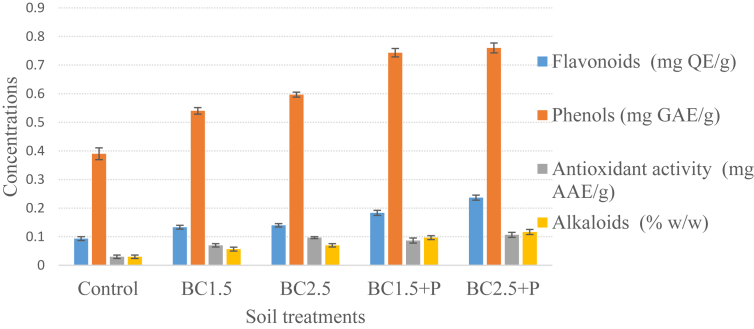
Table 5, summarizes the content of phenols, flavonoids, alkaloids, tannins, saponins and antioxidant activity of root and leaves. The results of the treatments effect on total phenols, total flavonoids, alkaloids and antioxidant activity of rhizosphere soil are shown in Figure 2.
Table 5.
Phytochemicals, antioxidant activity of cowpea roots and leaves following application of biochar and inorganic phosphorus fertilizer (TSP).
| Treatment | Sample | Phenols (mg GAE g−1) | Flavonoids (mg QE g−1) | Antioxidant activity (mg AAE g−1) | Alkaloids (% w/w) | Tannin (mg TAE g−1) | Saponin (mg DE g−1) |
|---|---|---|---|---|---|---|---|
| Control | Leaves | 1.39 ± .03c | 0.37 ± 0.02de | 0.07 ± 0.01b | 0.65 ± 0.04c | 0.11 ± .02b | 1.02 ± .02de |
| Roots | 0.89 ± .04a | 0.13 ± 0.02a | 0.05 ± 0.01a | 0.31 ± 0.02a | 0.07 ± 0.01a | 0.64 ± .04a | |
| BC1.5 | Leaves | 2.32 ± 0.03g | 0.74 ± 0.03f | 0.11 ± 0.01de | 0.90 ± 0.04d | 0.15 ± .02cd | 1.10 ± .02f |
| Roots | 1.10 ± 0.02b | 0.20 ± 0.02b | 0.08 ± 0.01bc | 0.46 ± 0.02b | 0.10 ± .01b | 0.83 ± .02b | |
| BC2.5 | Leaves | 4.16 ± 0.04h | 0.78 ± 0.03g | 0.14 ± 0.01f | 1.05 ± 0.04e | 0.17 ± .02d | 1.14 ± .01f |
| Roots | 1.81 ± 0.02d | 0.27 ± 0.02c | 0.09 ± 0.02cd | 0.50 ± 0.01b | 0.10 ± 0.02b | 0.91 ± 0.04c | |
| BC1.5 + P | Leaves | 4.29 ± 0.04i | 1.04 ± 0.02h | 0.16 ± 0.01g | 1.31 ± 0.05f | 0.23 ± 0.01e | 1.29 ± 0.10g |
| Roots | 1.92 ± 0.04e | 0.34 ± 0.02d | 0.12 ± 0.02e | 0.58 ± 0.02c | 0.1433 ± 0.01c | 1.013 ± 0.02d | |
| BC2.5 + P | Leaves | 5.02 ± 0.09j | 1.11 ± 0.03i | 0.19 ± 0.01h | 1.86 ± 0.12g | 0.28 ± 0.02f | 1.35 ± 0.03ef |
| Roots | 2.02 ± 0.02f | 0.39 ± 0.02e | 0.12 ± 0.01e | 0.61 ± 0.03c | 0.15 ± 0.01cd | 1.08 ± 0.02g | |
| % CV | 1.6 | 3.7 | 9.4 | 5.8 | 10 | 3.6 |
BC1.5 = biochar at 1.5 t ha−1, BC2.5 = biochar at 2.5 t ha−1, GAE = Gallic acid equivalent, QE = Quercetin equivalent, DE = Diosgenin equivalent, w = Weight. AA = Antioxidant activity, TAE = Tannic acid equivalent. Each value is presented as mean ± standard deviation. Means in the same column lettered with same alphabets superscript are not significantly different at p < 0.05 using Fisher's protected LSD test.
Figure 2.
Phytoconstituents and antioxidant activity of rhizosphere soil in response to biochar and TSP application. BC1.5 = biochar at 1.5 t ha−1, BC2.5 = biochar at 2.5 t ha−1. Data show mean ± standard error of the mean, n = 3.
The content of phenols, flavonoids, alkaloids, tannins, saponins and antioxidant activity of root and leaves respectively differed significantly (p < 0.05) with treatments (Table 5). Similarly, phenols, flavonoids, alkaloids and antioxidant activity were significantly (p < 0.05) higher in amended soil compared with unamended soil.
The values of phytochemicals in soil, roots and leaves followed an increasing order of; control < BC1.5 < BC2.5 < BC1.5 + P < BC2.5 + P. Additionally, phytoconstituents and antioxidant activity, were in the increasing order of; rhizosphere soil < roots < leaves, across all treatments. Total phenol content (TPC) in the leaves ranged between 1.39 in the control to 5.02 mg GAE g−1 in the 2.5 t ha−1 biochar plus TSP, respectively. A similar trend was shown for TPC distribution in roots, which ranged from 0.89 to 2.02 mg GAE g−1.
The highest leaf flavonoid content (1.11 mg QE g−1) was found in the 2.5 t ha−1 biochar plus TSP plots and the least (0.37 mg QE g−1) from the control treatment. Consistent with the leaf flavonoid contents, the roots showed similar increases in flavonoid content measured in the differently amended soils with the lowest (0.13 mg QE g−1) being found in the leaves from the control treatment and highest (0.39 mg QE g−1) from plot treated with 2.5 t ha−1 biochar co-applied with TSP.
The leaves from the control treatment recorded the lowest (0.65 %) alkaloids content while the highest alkaloid content (1.86 %) was measured in the leaves from the combined biochar and TSP treatment. The alkaloid contents of the roots from the control plot were the lowest (0.31 %) whereas the highest (0.61 %) was found in the combined 2.5 t ha−1 biochar and phosphorus amended plots. (The range of leaf saponin contents was 1.02–1.35 mg DE g−1 whiles that of the roots were 0.64–1.08 mg DE g−1. Tannin content was the lowest of all the phytoconstituents and showed a range of 0.11–0.28 mg TAE g−1 in the leaves and 0.07–0.15 mg TAE g−1 in the roots.
Varying antioxidant capacity was observed as a result of applying biochar and inorganic phosphorus fertilizer. The leaves showed higher antioxidant property than the roots across all treatments. The increase of the antioxidant property was in order; control < BC1.5 < BC2.5 < BC1.5 + P < BC2.5 + P. The range of antioxidant activity was 0.07–0.19 mg AAE g−1 for leaves and 0.05–0.12 mg AAE g−1 for roots.
The outcome of the study indicated a significant rise in phenols, flavonoids, alkaloids and antioxidant activity of rhizosphere soil following the application of biochar alone or co-applied with inorganic P fertilizer. The content of this metabolites in soil ranged between 0.39 to 0.76 mg GAE g−1 for phenols, 0.09–0.24 mg QE g−1 for flavonoids and 0.03–0.12 % for alkaloids. Antioxidant activity in the rhizosphere soil ranged between 0.03 to 0.10 mg AAE g−1.
4. Discussion
4.1. Effect of biochar and triple superphosphate fertilizer on rhizosphere soil properties
Addition of biochar and TSP increased soil pH, total nitrogen, available phosphorus, soil organic carbon and cation exchange capacity in the present study. The increases in pH positively correlated strongly with increases in CEC (r = 0.93, p < 0.001) (Table 6). Biochar is reported to contain high amount of carbonates and oxides of basic cations (Fidel et al., 2017) which through liming reaction replaces H+ and Al3+ on the soil colloidal complex, consequently raising the soil's pH (Brady and Weil, 2016). The increase in soil pH following sole biochar addition and with increasing rates of biochar application is consistent with previous findings (Steiner et al., 2007; Zhang et al., 2012). Moreover, when TSP was co–applied with biochar, a slight increase in soil pH was observed compared with the corresponding sole biochar additions. This is a clear indication for including biochar and TSP in sustainable soil fertility management policies, since it would optimize soil pH for P availability. The soil pH range (5.4–6.1) observed in the biochar and TSP amended soils fall within the optimum range for soil microbial activity, nutrient availability (N, P, S, Ca, Mg) and growth of crops (McCauley et al., 2009; Neina, 2019).
Table 6.
Pearson correlation matrix of nodule number, selected phytochemicals and rhizosphere soil properties of cowpea.
| pH | TN (%) | SOC | AvP | Nodule count | Flavonoids in soil | |
|---|---|---|---|---|---|---|
| pH | 0.70∗∗ | |||||
| TN (%) | 0.84∗∗∗ | 0.92∗∗∗ | ||||
| SOC (g kg−1) | 0.99∗∗∗ | 0.86∗∗∗ | 0.72∗∗ | |||
| AvP (mg kg−1) | 0.66∗∗ | 0.92∗∗∗ | 0.68∗∗ | 0.98∗∗∗ | ||
| Flavonoid (soil) | 0.71∗∗ | 0.93∗∗∗ | 0.73∗∗ | 0.95∗∗∗ | 0.97∗∗∗ | |
| Flavonoids (roots) | 0.81∗∗∗ | 0.97∗∗∗ | 0.82∗∗∗ | 0.96∗∗∗ | 0.95∗∗∗ | 0.93∗∗∗ |
| Phenols (soil) | 0.70∗∗ | 0.93∗∗∗ | 0.71∗∗ | 0.98∗∗∗ | 0.98∗∗∗ | 0.96∗∗∗ |
| Phenols (root) | 0.83∗∗∗ | 0.94∗∗∗ | 0.84∗∗∗ | 0.89∗∗∗ | 0.88∗∗∗ | 0.83∗∗∗ |
| AAS | 0.11ns | 0.32ns | 0.09ns | 0.32ns | 0.19ns | 0.23ns |
| Alkaloids soil | 0.75 | 0.91∗∗∗ | 0.75∗∗ | 0.94∗∗∗ | 0.93∗∗∗ | 0.94∗∗∗ |
| CEC | 0.93∗∗∗ | 0.74∗∗ | 0.89∗∗∗ | 0.55∗ | 0.62∗ | 0.63∗ |
| Saponins (leaves) | 0.66∗∗ | 0.91∗∗∗ | 0.7∗∗ | 0.96∗∗∗ | ||
| Saponins (root) | 0.84∗∗∗ | 0.97∗∗∗ | 0.86∗∗∗ | 0.94∗∗∗ | ||
| Taninns (leaves) | 0.7∗∗ | 0.93∗∗∗ | 0.72∗∗ | 0.96∗∗∗ | ||
| Tannins (root) | 0.68∗∗ | 0.91∗∗∗ | 0.73∗∗ | 0.94∗∗∗ | ||
| Antioxidant activity (leaves) | 0.81∗∗∗ | 0.98∗∗∗ | 0.83∗∗∗ | 0.95∗∗∗ | ||
| Antioxidant activity (roots) | 0.75∗∗ | 0.90∗∗ | 0.79∗∗∗ | 0.87∗∗∗ | ||
| Alkaloids (leaves) | 0.75∗∗ | 0.87∗∗∗ | 0.74∗∗ | 0.87∗∗∗ | ||
| Alkaloids (root) | 0.81∗∗∗ | 0.94∗∗∗ | 0.82∗∗∗ | 0.94∗∗∗ |
AAS = antioxidant activity in soil, TN = total nitrogen, AvP = available phosphorus, SOC = soil organic carbon, CEC = cation exchange capacity, significant at; ∗ for 0.05, ∗∗ for 0.01 and ∗∗∗ for 0.001 probability level, ns = non significant.
The observed increase in total nitrogen in our study could be associated with fresh addition of nitrogen from biochar applied, as well as symbiotic nitrogen fixation resulting from increase in nodule numbers (r = 0.92, p < 0.001) in the present study. The direct influence of biochar towards favourable soil conditions to favour availability of nitrogen can be one of the mechanisms to explain the increased total nitrogen. This is further shown in the positive and significant correlation between soil total N, available P and flavonoid contents of the roots (r = 0.92, p < 0.001) and (r = 0.97, p < 0.001) (Table 6). Cross and Sohi (2011) explained that mineralization of biochar occur in soil and leads to the release of its labile compounds such as nitrogen; usually at rapid rate initially and continues slowly after sometime. Although it has been shown that plant based biochar contain small amount of mineral nitrogen, they largely contribute to the total nitrogen pool (Cui et al., 2017). Cowpea nodules have been documented to be involved in symbiotic nitrogen fixation (Ouma et al., 2016).
The increase in soil available phosphorus content could be associated with the positive correlation with soil pH (r = 0.61, p < 0.01) and the contribution of labile phosphorus from biochar as well as triple superphosphate application. This suggests that combined application of biochar and TSP could potentially have a synergistic effect on soil available P content. The result of our study could be very instrumental in developing strategies for improving phosphorus solubility and availability in low pH tropical soils. In tropical soils, key factors such as low initial phosphorus content of the soil and the dominance of soluble acidic cations (Al3+, Fe3+), reduce the solubility and availability of phosphorus (Brady and Weil, 2016). Particularly, labile phosphorus is precipitated by these ions in most acidic tropical soil. Our study indicates that this challenge can be tackled by increasing soil pH through biochar application and supplementing the native soil P with inorganic phosphorus fertilizer. Biochar possess acid neutralizing effect as it adsorbs cations especially, Al3+ onto its negatively charged surfaces, and in the process reduce exchangeable acidity (Al3+, H+) of the soil (Novak et al., 2009). In addition, it causes the precipitation of Al and Fe as Fe(OH)3 and Al(OH)3, which increases phosphorus availability in soil (Gerke, 1994). The reduction in exchangeable acidity content by the added biochar coupled with the release of fresh available phosphorus from the TSP might have reflected in the elevated available P concentration in the combined biochar and TSP treatments.
The increase in SOC content following biochar addition is probably due to additional C added by the biochar. Biochar is reported to contain a large amount of recalcitrant C that is not easily decomposed though not entirely inert. It undergoes mineralisation and release labile fractions of C into soil. The observed increase in SOC could also be related to positive priming effect which stimulates the mineralization of native soil organic carbon. Singh and Cowie (2014) espoused that biochar supports the proliferation and metabolic activities of microbes to mineralize native carbon.
Significant increases in cation exchange capacity in our study is an encouraging outcome which reinforces the need to apply biochar to tropical acid soil. The increase could be due to the high surface area, porous characteristics and increased surface negative charges on the added biochar resulting from ionization of functional groups, chiefly carboxylic and phenolic groups (Liang et al., 2006). Elevated cation exchange capacity positively correlated with pH increases (r = 0.93, p < 0.001) found in the amended soils. In agreement with Jien and Wang (2013) and Novak et al. (2019), this implies that biochar application can improve soil CEC and enhance nutrient retention capacity of tropical soil.
4.2. Effect of biochar and triple superphosphate on nodule formation
Nodule number positively correlated with pH, available phosphorus, total nitrogen, soil organic carbon, cation exchange capacity, flavonoids, phenols, and alkaloids in the present study. Nodules contribute to fixing of nitrogen into the soil through a process called symbiotic nitrogen fixation (SNF). The process, SNF is regulated by soil conditions (Morón et al., 2005) which influence the survival, infection and activity of the rhizobia. In our study, as pH increased from 5.8 to 6.11, nodule number also increased from 10 to 91. This is congruent with the findings of Morón et al. (2005) who reported high number of nodules at pH 5.5 and above, but decreased at pH 7. Appunu and Dhar (2006) argued that presence of toxic metal ions (Al3+, Cu2+ and Mn2+) in acid soil negatively affect the growth, abundance and persistence of non acid-tolerant rhizobia leading to reduced root infection and nodulation (Mendoza-Soto et al. 2015). In consistent, the activity of these metals were reduced in the present study due to pH increase. The elevated concentration of available phosphorus correlated positively with increasing nodule number, which is an indication that nodule initiation and development was possibly influenced by the availability of phosphorus in this study. This confirm previous reports that phosphorus is involved in nodulation through crop growth stimulation, initiation of nodule formation as well as enhancement of rhizobium-legume interaction (Karikari et al., 2015; Kyei-Boahen et al., 2017; Nkaa et al., 2014). The improvement of other soil properties such as cation exchange capacity, soil organic carbon and total nitrogen is evident of an increased soil fertility, which would have enhanced nodule formation. Rhizosphere flavonoids and phenols, both in the root and soil, showed positive correlation with nodule formation. The release of flavonoid into the soil has been shown to be affected by external soil nutrient input as shown for dissolved organic carbon (Del Valle et al., 2020) and phosphorus (Juszczuk et al., 2004) in related previous studies. In the present study, biochar for instance optimized pH, increased available phosphorus, soil organic carbon, which could have stimulated the release of these metabolites. The elevation of these molecules in the rhizosphere potentially enhanced the formation of nodules and subsequently nitrogen fixation in this study. Additionally, the present study demonstrated that biochar did not suppress the release of flavonoids and its function as communication molecule for rhizobia infection of roots as seen in the increased effective nodule numbers.
4.3. Effect of biochar and triple superphosphate fertilizer on phytoconstituents, antioxidant activity of cowpea roots, leaves and rhizosphere soil
Biochar and triple superphosphate were observed to increase the phytoconstituents and antioxidant properties of rhizosphere soil, leaves and roots of cowpea. Research finding on biochar and triple superphosphate on phytoconstituents of cowpea is rudimentary for comparison. However, the improvement of these phytoconstituents and antioxidant capacity could be attributed to the improvement in soil fertility (Ibrahim et al., 2013; Jansen et al., 2012; Taha et al., 2009; Zhu et al., 2009). The present study showed positive correlations among many soil properties, phytoconstituents and antioxidant activity of roots and leaves. Specific interaction of biochar with the phytoconstituents needs attention to elucidate the fate and behavior of these molecules; particularly in soil amended with biochar.
Increase in phytochemicals, particularly in the leaves and roots reinforce the need to explore medicinal potential of the roots and leaves of cowpea apart from its been consumed as food and or animal feed. In soil, phenols and flavonoids act as signaling molecule in the establishment of plant-microbe association particularly arbuscular mycorrhizal and legume-rhizobia symbioses, provide adsorption sites for complexation of ions implicated in phosphorus precipitation, consequently increasing phosphorus availability in soil (Bhattacharya et al., 2010; Hu et al., 2005). Similarly, alkaloid aids in nitrogen fixation and functions as biofertilizers as well as carbon source for growing plant and soil microbes (Aniszewski, 2007).
5. Conclusion
Tropical soils’ have low pH and largely deficient in phosphorus and nitrogen so every effort to raise the pH and improve the levels of these nutrients is needed. In this study, we demonstrated that combined biochar and TSP application increased soil pH, improved soil fertility, nodulation and nitrogen fixation. Additionally, co-application of biochar and TSP is a pragmatic strategy to improve leaf and root total phenolic, total flavonoids, alkaloids, saponin, tannins concentrations and antioxidant capacity.
Declarations
Author contribution statement
Christian Adler Phares: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools; Wrote the paper.
Kofi Atiah: Conceived and designed the experiments; Performed the experiments; Contributed reagents, materials, analysis tools; Wrote the paper.
Kwame Agyei Frimpong,Samira Aggor-Woananu,Andrews Danquah: Performed the experiments; Contributed reagents, materials, analysis tools; Wrote the paper.
Aaron T. Asare: Conceived the experiments; Contributed reagents, materials, analysis tools.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Adegoke A.A., Iberi P.A., Akinpelu D.A., Aiyegoro O.A., Mboto C.I. Studies on phytochemical screening and antimicrobial potentials of Phyllanthus amarus against multiple antibiotic resistant bacteria. Int. J. Appl. Res. Nat. Prod. 2010;3(3):6–12. [Google Scholar]
- Adusei S., Otchere J.K., Oteng P., Mensah R.Q., Tei-Mensah E. Phytochemical analysis, antioxidant and metal chelating capacity of Tetrapleura tetraptera. Heliyon. 2019;5(11) doi: 10.1016/j.heliyon.2019.e02762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akinmoladun A.C., Ibukun E.O., Afor E., Obuotor E.M., Farombi E.O. Phytochemical constituent and antioxidant activity of extract from the leaves of Ocimum gratissimum. Sci. Res. Essays. 2007;2(5):163–166. [Google Scholar]
- Allahdadi M., Farzane P. Influence of different levels of nitrogen fertilizer on some phytochemical characteristics of artichoke (Cynara scolymus L.) leaves. J. Med. Plants Stud. 2018;6(1):109–115. [Google Scholar]
- Alidu M.S., Asante I.K., Mensah H.K. Evaluation of nutritional and phytochemical variability of cowpea Recombinant Inbred Lines under contrasting soil moisture conditions in the Guinea and Sudan Savanna Agro-ecologies. Heliyon. 2020;6(2) doi: 10.1016/j.heliyon.2020.e03406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J.M., Ingram J.S. Tropical soils biology and fertility. In: Anderson J.M., Ingram J.S., editors. A Handbook of Methods. second ed. CAB International; Wallingford: 1993. p. 221. [Google Scholar]
- Aniszewski T. Elsevier; 2007. Alkaloids-Secrets of Life: Alkaloid Chemistry, Biological Significance, Applications and Ecological Role. [Google Scholar]
- Appunu C., Dhar B. Symbiotic effectiveness of acid-tolerant Bradyrhizobium strains with soybean in low pH soil. Afr. J. Biotechnol. 2006;5(10) [Google Scholar]
- Asamoa G.K. Soil Research Institute (C.S.I.R.); 1973. Soils of Proposed Farm Sites of the University of Cape Coast. Technical (Report No. 88), Kwadaso, Kumasi. [Google Scholar]
- Ascough P.L., Bird M.I., Meredith W., Snape C., Large D., Tilston E. Dynamics of charcoal alteration in a tropical biome: a biochar-based study. Front. Earth Sci. 2018;6:61. [Google Scholar]
- Ashworth J., Keyes D., Kirk R., Lessard R. Standard procedure in the hydrometer method for particle size analysis. Commun. Soil Sci. Plant Anal. 2001;32(5-6):633–642. [Google Scholar]
- Badii B.K., Asante S.K., Ayertey J.N. Field evaluation of neem seed extract for the control of major pests of cowpea in northern Ghana. Ghana J. Agric. Sci. 2008;41(2) [Google Scholar]
- Bhattacharya A., Sood P., Citovsky V. The roles of plant phenolics in defence and communication during Agrobacterium and Rhizobium infection. Mol. Plant Pathol. 2010;11(5):705–719. doi: 10.1111/j.1364-3703.2010.00625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady N.C., Weil R.R. fifteenth ed. Pearson; Columbus: 2016. The Nature and Properties of Soils. [Google Scholar]
- Chan K.Y., Van Zwieten L., Meszaros I., Downie A., Joseph S. Agronomic values of green waste biochar as a soil amendment. Soil Res. 2008;45(8):629–634. [Google Scholar]
- Chantiratikul P., Meechai P., Nakbanpotec W. Antioxidant activities and phenolic contents of extracts from Salvinia molesta and Eichornia crassipes. Res. J. Biol. Sci. 2009;4(10):1113–1117. [Google Scholar]
- Cooper J.E. vol. 41. Academic Press; 2004. Multiple responses of rhizobia to flavonoids during legume root infection; pp. 1–62. (Advances in Botanical Research). [Google Scholar]
- Cross A., Sohi S.P. The priming potential of biochar products in relation to labile carbon contents and soil organic matter status. Soil Biol. Biochem. 2011;43(10):2127–2134. [Google Scholar]
- Cui Y.F., Jun M., Wang Q.X., Zhang W.M., Cheng X.Y., Chen W.F. Effects of straw and biochar addition on soil nitrogen, carbon, and super rice yield in cold waterlogged paddy soils of North China. J. Integr. Agric. 2017;16(5):1064–1074. [Google Scholar]
- Dai L., Li H., Tan F., Zhu N., He M., Hu G. Biochar: a potential route for recycling of phosphorus in agricultural residues. Gcb Bioenergy. 2016;8(5):852–858. [Google Scholar]
- Deenik J.L., Cooney M.J. The potential benefits and limitations of corn cob and sewage sludge biochars in an infertile oxisol. Sustainability. 2016;8(2):131. [Google Scholar]
- Del Valle I., Webster T.M., Cheng H.Y., Thies J.E., Kessler A., Miller M.K. Soil organic matter attenuates the efficacy of flavonoid-based plant-microbe communication. Sci. Adv. 2020;6(5) doi: 10.1126/sciadv.aax8254. eaax8254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohrmann R. Problems in CEC determination of calcareous clayey sediments using the ammonium acetate method. J. Plant Nutr. Soil Sci. 2006;169(3):330–334. [Google Scholar]
- Duku M.H., Gu S., Hagan E.B. Biochar production potential in Ghana - a review. Renew. Sustain. Energy Rev. 2011;15(8):3539–3551. [Google Scholar]
- FAO, I. Working Group WRB World reference base for soil resources 2014, update 2015. International soil classification system for naming soils and creating legends for soil maps. World Soil Resources Rep. 2015;106 [Google Scholar]
- Fidel R.B., Laird D.A., Thompson M.L., Lawrinenko M. Characterization and quantification of biochar alkalinity. Chemosphere. 2017;167:367–373. doi: 10.1016/j.chemosphere.2016.09.151. [DOI] [PubMed] [Google Scholar]
- Ganiyu S.A., Popoola A.R., Owolade O.F., Fatona K.A. Control of common bacterial blight disease of cowpea (Vigna unguiculata [L.] Walp) with certain plant extracts in Abeokuta, Nigeria. J. Crop Improv. 2017;31(3):280–288. [Google Scholar]
- Gao S., Hoffman-Krull K., Bidwell A.L., DeLuca T.H. Locally produced wood biochar increases nutrient retention and availability in agricultural soils of the San Juan Islands, USA. Agric. Ecosyst. Environ. 2016;233:43–54. [Google Scholar]
- Gerke J. Kinetics of soil phosphate desorption as affected by citric acid. Z. für Pflanzenernährung Bodenkunde. 1994;157(1):17–22. [Google Scholar]
- Gomaa N.H., Hassan M.O., Fahmy G.M., González L., Hammouda O., Atteya A.M. Flavonoid profiling and nodulation of some legumes in response to the allelopathic stress of Sonchus oleraceus L. Acta Bot. Bras. 2015;29(4):553–560. [Google Scholar]
- Hu H., Tang C., Rengel Z. Influence of phenolic acids on phosphorus mobilization in acidic and calcareous soils. Plant Soil. 2005;268(1):173–180. [Google Scholar]
- Iannucci A., Fragasso M., Platani C., Papa R. Plant growth and phenolic compounds in the rhizosphere soil of wild oat (Avena fatua L.) Front. Plant Sci. 2013;4:509. doi: 10.3389/fpls.2013.00509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim M.H., Jaafar H.Z., Karimi E., Ghasemzadeh A. Impact of organic and inorganic fertilizers application on the phytochemical and antioxidant activity of Kacip Fatimah (Labisia pumila Benth) Molecules. 2013;18(9):10973–10988. doi: 10.3390/molecules180910973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen G., Jürgens H.U., Schliephake E., Ordon F. Effect of the soil pH on the alkaloid content of lupinus angustifolius. Int. J. Agron. 2012;2012 [Google Scholar]
- Jien S.H., Wang C.S. Effects of biochar on soil properties and erosion potential in a highly weathered soil. Catena. 2013;110:225–233. [Google Scholar]
- Juszczuk I.M., Wiktorowska A., Malusá E., Rychter A.M. Changes in the concentration of phenolic compounds and exudation induced by phosphate deficiency in bean plants (Phaseolus vulgaris L.) Plant Soil. 2004;267(1-2):41–49. [Google Scholar]
- Karikari B., Arkorful E., Addy S. Growth, nodulation and yield response of cowpea to phosphorus fertilizer application in Ghana. J. Agron. 2015;14(4):234–240. [Google Scholar]
- Kawaka F., Makonde H., Dida M., Opala P., Ombori O., Maingi J., Muoma J. Genetic diversity of symbiotic bacteria nodulating common bean (Phaseolus vulgaris) in western Kenya. PloS One. 2018;13(11) doi: 10.1371/journal.pone.0207403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyei-Boahen S., Savala C.E., Chikoye D., Abaidoo R. Growth and yield responses of cowpea to inoculation and phosphorus fertilization in different environments. Front. Plant Sci. 2017;8:646. doi: 10.3389/fpls.2017.00646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong C.H., Li H.B., Hu F., Xu X.H., Wang P. Allelochemicals released by rice roots and residues in soil. Plant Soil. 2006;288(1-2):47–56. [Google Scholar]
- Lal R. Tropical soils: distribution, properties and management. Trop. Resour.: Ecol. Dev. 2019;3(1-4):39–52. [Google Scholar]
- Lehmann J. Bio-energy in the black. Front. Ecol. Environ. 2007;5(7):381–387. [Google Scholar]
- Lehmann J., Rillig M.C., Thies J., Masiello C.A., Hockaday W.C., Crowley D. Biochar effects on soil biota–a review. Soil Biol. Biochem. 2011;43(9):1812–1836. [Google Scholar]
- Lehmann J., Joseph S., editors. Biochar for Environmental Management: Science, Technology and Implementation. Routledge; 2015. [Google Scholar]
- Li W., Feng X., Song W., Guo M. Transformation of phosphorus in speciation and bioavailability during converting poultry litter to biochar. Front. Sustain. Food Syst. 2018;2:20. [Google Scholar]
- Liang B., Lehmann J., Solomon D., Kinyangi J., Grossman J., Skjemstad J.O., Thies J. Black carbon increases cation exchange capacity in soils. Soil Sci. 2006:1719–1730. [Google Scholar]
- Liu X., Liao J., Song H., Yang Y., Guan C., Zhang Z. A biochar-based Route for environmentally friendly controlled Release of nitrogen: urea-loaded Biochar and bentonite composite. Sci. Rep. 2019;9(1):1–12. doi: 10.1038/s41598-019-46065-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maghanga K.J., Kituyi L.J., Segor K.F., Kisinyo P. 2015. Comparison of Soil Phosphorous Extraction by Olsen and Double Acid Methods in Acid Soils of Western Kenya. [Google Scholar]
- McCauley A., Jones C., Jacobsen J. Soil pH and organic matter. Nutr. Manage. Mod. 2009;8(2):1–12. [Google Scholar]
- Mendoza-Soto A.B., Naya L., Leija A., Hernández G. Responses of symbiotic nitrogen-fixing common bean to aluminum toxicity and delineation of nodule responsive microRNAs. Front. Plant Sci. 2015;6:587. doi: 10.3389/fpls.2015.00587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mensah A.K., Frimpong K.A. Biochar and/or compost APPLICATIONS improve soil properties, growth, and yield of maize grown in acidic rainforest and coastal savannah Soils in Ghana. Int. J. Agron. 2018;2018 [Google Scholar]
- Mikos-Szymańska M., Schab S., Rusek P., Borowik K., Bogusz P., Wyzińska M. Preliminary Study of a Method for obtaining Brown Coal and biochar based granular compound fertilizer. Waste Biomass Valoriz. 2019;10(12):3673–3685. [Google Scholar]
- Morón B., Soria-Díaz M.E., Ault J., Verroios G., Noreen S., Rodríguez-Navarro D.N. Low pH changes the profile of nodulation factors produced by Rhizobium tropici CIAT899. Chem. Biol. 2005;12(9):1029–1040. doi: 10.1016/j.chembiol.2005.06.014. [DOI] [PubMed] [Google Scholar]
- Munera-Echeverri J.L., Martinsen V., Strand L.T., Zivanovic V., Cornelissen G., Mulder J. Cation exchange capacity of biochar: An urgent method modification. Sci. Total Environ. 2018;642:190–197. doi: 10.1016/j.scitotenv.2018.06.017. [DOI] [PubMed] [Google Scholar]
- Naeem M.A., Khalid M., Aon M., Abbas G., Amjad M., Murtaza B. Combined application of biochar with compost and fertilizer improves soil properties and grain yield of maize. J. Plant Nutr. 2018;41(1):112–122. [Google Scholar]
- Ncube N.S., Afolayan A.J., Okoh A.I. Assessment techniques of antimicrobial properties of natural compounds of plant origin: current methods and future trends. Afr. J. Biotechnol. 2008;7(12) [Google Scholar]
- Neina D. The role of soil pH in plant nutrition and soil remediation. Appl. Environ. Soil Sci. 2019;2019 [Google Scholar]
- Nelson D.W., Sommers L.E. Total carbon, organic Carbon and organic matter. pp. 961 – 1010 in: methods of soil analysis part 3 – chemical methods. In: Sparks D.L., Page A.L., Helmke P.A., Loeppert R.H., editors. Soil Seince Society of America, Americal Society of Agronomy, Madison, WI. 1996. [Google Scholar]
- Nkaa F., Nwokeocha O.W., Ihuoma O. Effect of phosphorus fertilizer on growth and yield of cowpea (Vigna unguiculata) J. Pharm. Biol. Sci. (IOSR-JPBS) 2014;9(5):74–82. [Google Scholar]
- Novak J.M., Busscher W.J., Laird D.L., Ahmedna M., Watts D.W., Niandou M.A. Impact of biochar amendment on fertility of a southeastern coastal plain soil. Soil Sci. 2009;174(2):105–112. [Google Scholar]
- Ouma E.W., Asango A.M., Maingi J., Njeru E.M. Elucidating the potential of native rhizobial isolates to improve biological nitrogen fixation and growth of common bean and soybean in smallholder farming systems of Kenya. Int. J. Agron. 2016;1:1–7. [Google Scholar]
- Phares C.A., Osei B.A., Tagoe S. Effects of Biochar and poultry Manure on the Composition of phosphorus solubilizing Fungi and soil available phosphorus Concentration in an oxisol. J. Agric. Ecol. Res. Int. 2017:1–15. [Google Scholar]
- Quaye W., Adofo K., Madode Y., Abizari A.R. Exploratory and multidisciplinary survey of the cowpea network in the Tolon-Kumbungu district of Ghana: a food sovereignty perspective. Afr. J. Agric. Res. 2009;4(4):311–320. [Google Scholar]
- Raboin L.M., Razafimahafaly A.H.D., Rabenjarisoa M.B., Rabary B., Dusserre J., Becquer T. Improving the fertility of tropical acid soils: liming versus biochar application? A long term comparison in the highlands of Madagascar. Field Crop. Res. 2016;199:99–108. [Google Scholar]
- Rondon M.A., Lehmann J., Ramírez J., Hurtado M. Biological nitrogen fixation by common beans (Phaseolus vulgaris L.) increases with bio-char additions. Biol. Fertil. Soils. 2007;43(6):699–708. [Google Scholar]
- Sade H., Meriga B., Surapu V., Gadi J., Sunita M.S.L., Suravajhala P., Kishor P.K. Toxicity and tolerance of aluminum in plants: tailoring plants to suit to acid soils. Biometals. 2016;29(2):187–210. doi: 10.1007/s10534-016-9910-z. [DOI] [PubMed] [Google Scholar]
- Singh B.P., Cowie A.L. Long-term influence of biochar on native organic carbon mineralisation in a low-carbon clayey soil. Sci. Rep. 2014;4:3687. doi: 10.1038/srep03687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivakumar D., Chen L., Sultanbawa Y. A comprehensive review on beneficial dietary phytochemicals in common traditional Southern African leafy vegetables. Food Sci. Nutr. 2018;6(4):714–727. doi: 10.1002/fsn3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sombié P., Compaoré M., Coulibaly A.Y., Ouédraogo J.T., Tignégré J., Kiendrébéogo M. Antioxidant and phytochemical studies of 31 cowpeas (Vigna unguiculata (L. Walp.)) genotypes from Burkina Faso. Foods. 2018;7(9):143. doi: 10.3390/foods7090143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W., Guo M. Quality variations of poultry litter biochar generated at different pyrolysis temperatures. J. Anal. Appl. Pyrol. 2012;94:138–145. [Google Scholar]
- Steiner C., Teixeira W.G., Lehmann J., Nehls T., de Macêdo J.L.V., Blum W.E., Zech W. Long term effects of manure, charcoal and mineral fertilization on crop production and fertility on a highly weathered Central Amazonian upland soil. Plant Soil. 2007;291(1-2):275–290. [Google Scholar]
- Stewarte E.A., Grimshaw H.M., Parkinson J.A., Quarmby C. Blackwell Scientific Publication; Oxford: 1974. Chemical Analysis of Ecological Materials. [Google Scholar]
- Taha H.S., El Bahr M.K., Seif E.N.M. 2009. In Vitro Studies on Egyption Catharanthus Roseus (L.). Ii. Effect of Biotic and Abiotic Stress on Indole Alkaloids Production. [Google Scholar]
- Thrall P.H., Laine A.L., Broadhurst L.M., Bagnall D.J., Brockwell J. Symbiotic effectiveness of rhizobial mutualists varies in interactions with native Australian legume genera. PloS One. 2011;6(8) doi: 10.1371/journal.pone.0023545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timko M.P., Singh B.B. Genomics of Tropical Crop Plants. Springer; New York, NY: 2008. Cowpea, a multifunctional legume; pp. 227–258. [Google Scholar]
- Uzoma K.C., Inoue M., Andry H., Fujimaki H., Zahoor A., Nishihara E. Effect of cow manure biochar on maize productivity under sandy soil condition. Soil Use Manag. 2011;27:205–212. [Google Scholar]
- Walker W.R. FAO; Rome): 1989. Guidelines for Designing and Evaluating Surface Irrigation System. FAO Irrigation and Drainage Paper No. 45. [Google Scholar]
- Wu W., Yang M., Feng Q., McGrouther K., Wang H., Lu H., Chen Y. Chemical characterization of rice straw-derived biochar for soil amendment. Biomass Bioenergy. 2012;47:268–276. [Google Scholar]
- Yu L., Lu X., He Y., Brookes P.C., Liao H., Xu J. Combined biochar and nitrogen fertilizer reduces soil acidity and promotes nutrient use efficiency by soybean crop. J. Soils Sediments. 2017;17(3):599–610. [Google Scholar]
- Zhang A., Bian R., Pan G., Cui L., Hussain Q., Li L. Effects of biochar amendment on soil quality, crop yield and greenhouse gas emission in a Chinese rice paddy: a field study of 2 consecutive rice growing cycles. Field Crop. Res. 2012;127:153–160. [Google Scholar]
- Zhu Z., Liang Z., Han R., Wang X. Impact of fertilization on drought response in the medicinal herb Bupleurum chinense DC.: growth and saikosaponin production. Ind. Crop. Prod. 2009;29(2-3):629–633. [Google Scholar]