Abstract
Phytopharmaceuticals have always reported vital roles in the field of medicine hence the need to investigate safe and efficient drugs for treating metabolic disorders is very significant. Roots of Selinum vaginatum have therapeutic benefits and are widely used by the people of the Rohtang region for treating diabetes and its associated complications. The present study focusses on the isolation of the bioactive from the S. vaginatum roots for estimating acute toxicity studies, anti-diabetic and diabetic neuropathy protective action along with the mechanism of action in STZ induced Wistar rats. The Selinum vaginatum roots were collected from the Rohtang region, Himalayas. Chlorogenic acid was isolated and underwent identification by UV, HPLC, 1H NMR, C13 NMR, Mass, and FTIR spectroscopy methods. Chlorogenic acid was dosed at 10 and 20 mg/kg to observe the effects on experimentally induced diabetes and with time generated diabetic neuropathic complications. Biomarkers TNF-α, superoxide dismutase, nitrosative stress, lipid peroxide profile, and membrane-bound inorganic phosphate were analyzed. Histopathological evaluation of the liver and sciatic nerve was performed for all groups. Parameters like blood glucose levels, body weight, food intake, Thermal Hyperalgesia, Writhing, Cold Hyperalgesia Responses, Mechanical hyperalgesia, Grip Strength, Spontaneous Locomotor (Exploratory) Test, Neuromuscular Coordination tests, and lipid profile analysis showcased the anti-diabetic and diabetic neuropathy protective action of the drug. Inflammation, degradation, and necrosis were found to be reduced in the liver and sciatic nerve cells of treated groups. All the biomarkers used to analyze the oxidative pathway were significantly replenished indicates that chlorogenic acid produces these effects through this pathway.
Keywords: Natural product chemistry, Pharmaceutical science, Molecular biology, Toxicology, Evidence based medicine, Environmental toxicology, Physiology, Selinum vaginatum, Sciatic nerve, Chlorogenic acid, Acute toxicity, Diabetic neuropathy, Diabetes mellitus, Agricultural science, Environmental science, Biological sciences, Veterinary medicine, Health sciences
Natural product chemistry; Pharmaceutical science; Molecular biology; Toxicology; Evidence based medicine; Environmental toxicology; Physiology; Selinum vaginatum; Sciatic nerve; Chlorogenic acid; Acute toxicity; Diabetic neuropathy; Diabetes mellitus; Agricultural science; Environmental science; Biological sciences; Veterinary medicine; Health sciences.
1. Introduction
Diabetes mellitus is a metabolic disorder that produces chronic effects hence is seen as hyperglycemia in patients with complications like neuropathy, nephropathy, retinopathy, etc. It is one of the prominent causes of death in the world and is affecting around 6% of the complete population of the world with majorly hitting the low and middle-income countries (Adeghate et al., 2006). The rapidly increasing incidences worldwide are responsible for the impact on health, life expectancy and quality of life of people as well as on the healthcare systems (Saraswat et al., 2018). As per the International Diabetes Federation's (IDF) by 2030 approximately 80% of the complete diabetic population will be from the lower- and middle-income countries. The improper output of hepatic glucose, as well as a decrease in the uptake of glucose by the skeletal muscles with the lesser synthesis of glycogen, leads to the condition of hyperglycemia (Rang et al., 2012). Extreme weight loss, polyphagia, hypotension, blurred vision, polyuria, wasting, and tachycardia are the signs of hyperglycemia (American Diabetes Association, 2011, 2018; Genuth et al., 2003; Mohan, 2018).
The neuropathy, associated with DM (Diabetes Mellitus) is a condition that is progressive and silent as it shows any symptoms or pathological severity during its progress. Often, they are categorized as symmetrical neuropathy and asymmetrical neuropathy (Dyck and Thomas, 1999). When the patients are undergoing neurological examinations in the clinics they are often diagnosed with diabetes or by impaired glucose tolerance which is mostly undetected previously (Singleton et al., 2001). Diabetic sensorimotor polyneuropathy is a type of neuropathy that often leads to pathophysiological complications like foot ulceration (McNeely et al., 1995; Reiber et al., 1999).
Selinum vaginatum is a dwarf to medium-sized plant which belongs to the family Apiaceae. This grows in humus-rich lands of Himalayas, S Africa, and Andean mountain ranges at a height of 6000–14000 feet. The Selinum genus has around thirty-five perennial species. The rhizomes resemble Nardostachys jatamansi in their appearance but have different anatomies when studied closely (Mehra and Jolly, 1963; Seshadri and Sood, 1967). The root powder of Paeonia emodi Wallich is mixed with powder of Selinum vaginatum and given as half teaspoon up to six months for treating epilepsy, and hysteria conditions (Tiwari and Rana, 2015).
S. vaginatum has been used for the treatment of hysteria, epilepsy, seizures, nervine sedative, skin disorders, and many other neurological issues, traditionally (Sharma and Samant, 2014). The plant is also used traditionally in treating swelling muscles, and skin diseases (Nand and Naithani, 2018).
It possesses active compounds responsible for its medicinal properties but the active compounds are yet to be figured out which are responsible for the therapeutic actions. Phenols, esters, etc. are powerful antioxidants that have reportedly exhibited many therapeutic actions like anti-carcinogenic, anti-inflammatory, anti-diabetic, antiviral, and vasodilator actions, etc (Mattila and Hellström, 2007; Rice-Evans et al., 1996).
The plant is very popularly used amongst many tribes across India and in the regions falling around the Himalayan belt for treating diseases. Selinum vaginatum is popular amongst Gujjar tribe, Jaad Bhotiya Community Bhotia tribe, Lahaul valley, tribals from Parbati valley, district Chamba of Western Himalaya, Bhotia tribe of Mana village, Nanda Devi Biosphere reserve, local inhabitants of Banaun, tribal inhabitants from Garhwal, Chamoli, Uttarakhand, South India and many other regions (Arya et al., 2018; Balodi et al., 2018; Chauhan, 2014; Gairola et al., 2014; Garbyal et al., 2005; Kumar et al., 2019; Malik et al., 2015; Nand and Naithani, 2018; Radha and Puri, 2019; Radha et al., 2019; Rana et al., 2010, 2013, 2019; Rana and Samant, 2011; Semwal et al., 2010; Sharma et al., 2004, 2011, 2013; Singh et al., 2009; Thakur et al., 2017). However, the effect of Selinum vaginatum in diabetic complications has not been investigated. The aim of the present study was to isolate chlorogenic acid from Selinum vaginatum roots and to evaluate the effect of treatment against STZ induced diabetes and diabetic neuropathy in Wistar rats by assessing various behavioral, and biochemical parameters and histopathological changes.
2. Material and methods
Plant material was authenticated by botanist under reference number GGDCK/Bot./02/2017. The reagents were obtained from the laboratory facility in PSIT, Kanpur. UV, HPLC, and FTIR were performed in PSIT, Kanpur laboratory whereas the C13 NMR, 1H NMR was performed by the Indian Institute of Technology, Kanpur laboratory facility, and Mass spectroscopy from SAIF, CDRI, Lucknow. Animals were obtained from the animal house facility in PSIT. Animals were caged and fed with a regular diet as per the experimental protocol. The instruments used for blood glucose analysis, locomotor activity analysis, etc. were obtained from the PSIT laboratory facility. The blood samples were collected by pricking the tail vein and then FBG – fasting blood glucose was measured by using Accu-chek®. Lipid profiles were analyzed using semi-auto analyzer from Remi Industries Ltd (Mumbai, India).
2.1. Plant material
The plant was collected from Rohtang valley in Himachal Pradesh, India. They were collected in September 2018 from the local market. The roots were then air dried and powdered. They were further extracted with three solvents out of which the ethanolic fraction showed the presence of phenol. Then the ethanol fraction was used for further analysis by using column chromatography.
2.2. Chemicals
Streptozotocin was purchased in the institute by Sigma-Aldrich, St. Louis, USA whereas Glibenclamide was bought from Sanofi India Ltd. All the chemicals used were of analytical grade. This was used for inducing type 2 diabetes (Gundala et al., 2018).
2.3. Animal housing
Adult Wistar rats of weight (180–220 g) of either sex were taken from the animal house in Department Pharmacy at Pranveer Singh Institute of Technology, Kanpur (Reference number 1273/PO/Re/S/09/CPCSEA). The rats were kept in polyacrylic cages which were large and spacious. The temperature in the room was ambient of around 12-h light/12-h dark cycle. The rats were fed with free access to water and a standard pellet diet from Hindustan Lever Ltd. The study was approved by the Institute Animal Ethics Committee and the experimentation was carried out as per the CPCSEA guidelines from the Ministry of Environment, Forests and Climate Change, Government of India (CPCSEA, 2018).
2.4. Packing of column for column chromatography
The residue was adsorbed on neutral alumina to remove the impurities if any. Then it was packed in a glass column where the ratio of the slurry was neutral alumina in petroleum ether (1:2). The column was packed and its dimension was 3.5 × 50 cm and the rate of elution was kept at 2 ml/min thus 50 ml/fraction was isolated. The obtained fractions were eluted with different solvents and then were added together to observe for any residue (Zhang et al., 2018).
2.5. TLC
Fractions 8–12 were added together and evaporated to obtain a yellowish-brown residue which was then dissolved in Ethyl Acetate. This was run on a TLC plate Run on TLC where Ethanol was used as a developing phase & Silica Gel G-as Stationary Phase. 3% methanolic H2SO4 was used as the spraying agent. A dark green spot was observed and the Rf value was reported as 0.45 (Zhang et al., 2018).
2.6. Isolation of phyto-constituent
Roots of the plant were sun-dried and shade dried for 15 days. It was coarsely powdered. The powder went through hot percolation in ethanol. It was stored at room temperature for 7 days and then filtered. The pale blue residue of 1.5 g was removed and the brown filtrate of the ethanolic layer was separated. This filtrate was concentrated to obtain 30 g of residue. It was then undergone through column chromatography with a slurry of neutral alumina in petroleum ether (1:2) in a Column of 3.5 × 50 cm×cm with the rate of elution as 2 ml/min and 50 ml/fraction. A total of 21 fractions were isolated and they were grouped as 1–3 from Acetone, 4–7 from Benzene, 8–12 as ethyl acetate, 13–17 from methanol, and 18–21 from distilled water as a solvent. All fraction groups were given as doses for testing their ability to reduce the blood glucose in the test group induced with diabetes by using streptozotocin. Here the 8–12 fraction from ethyl acetate showed the drop in glucose levels hence this fraction was selected for isolation of the active constituent. Thus, all ethyl acetate fractions were mixed and run on TLC with Ethanol as developing phase & Silica Gel G-as Stationary Phase by using 3% methanolic H2SO4 as a spraying agent. A dark green spot with Rf value = 0.45 was obtained. The melting point was found to be 196–200 °C. The constituent was then recrystallized by distilled water and stored at freezing temperatures hence 102 mg crystals obtained were obtained. They underwent UV, HPLC, C13 and 1H NMR, Mass, FTIR analysis hence the isolated compound was identified as chlorogenic acid as shown in Figures 1, 2, 3, 4, 5, 6 and 7. The dose of 10 and 20 mg/kg of chlorogenic acid was selected as it has shown efficacy in previous studies (Valcheva-Kuzmanova et al., 2015).
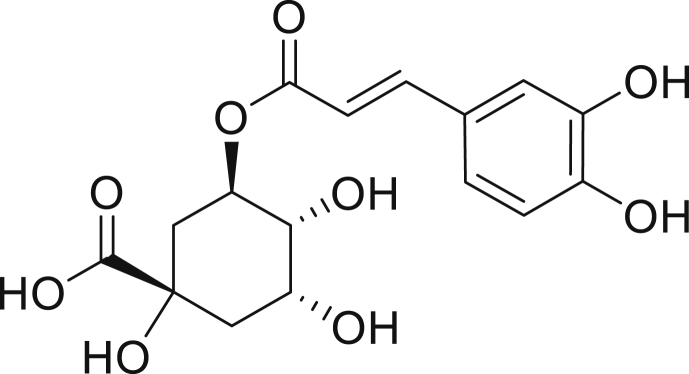
Figure 1.
Structure of chlorogenic acid.
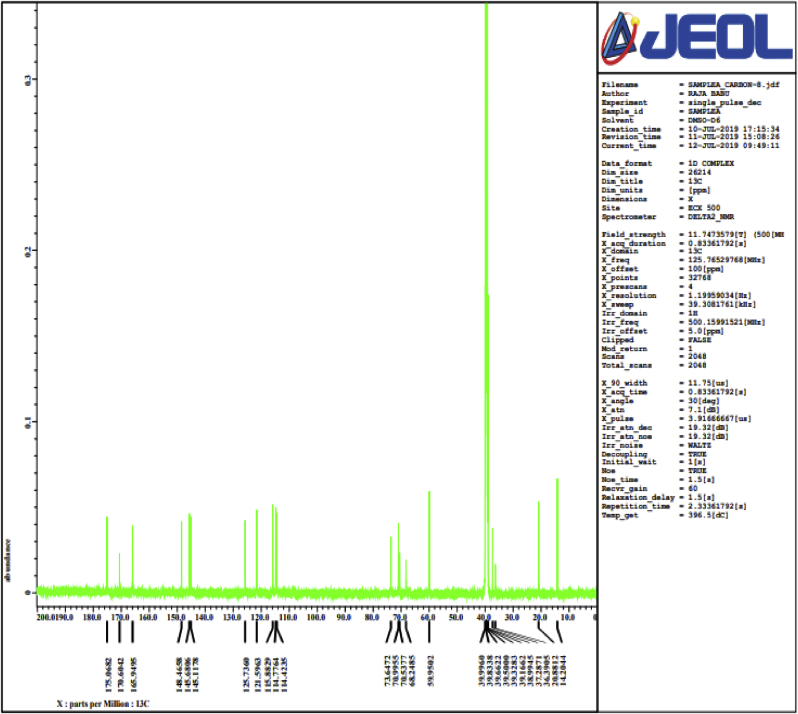
Figure 2.
C 13 NMR spectra of isolated compound (chlorogenic acid) obtained from roots of Selinum vaginatum.
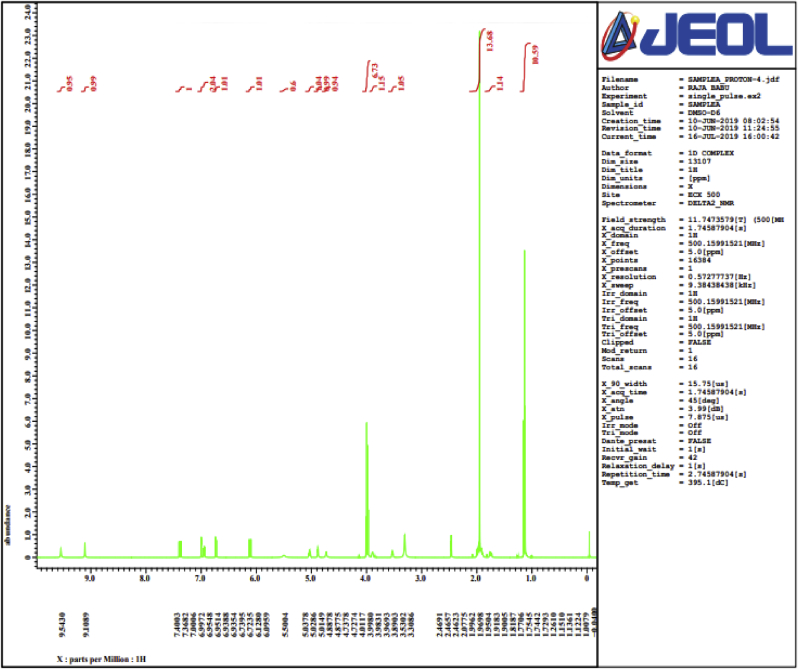
Figure 3.
1 H NMR spectra of isolated compound (chlorogenic acid) obtained from roots of Selinum vaginatum.
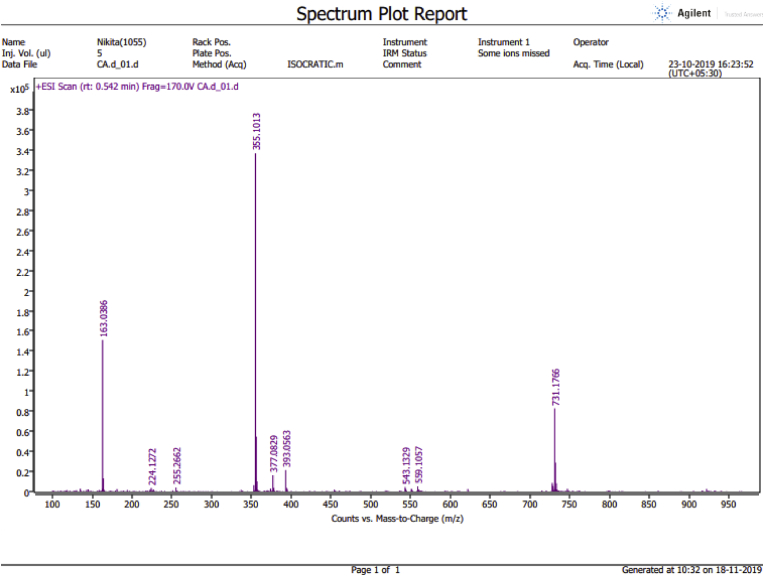
Figure 4.
HRMS of isolated compound (chlorogenic acid) obtained from roots of Selinum vaginatum.
Figure 5.
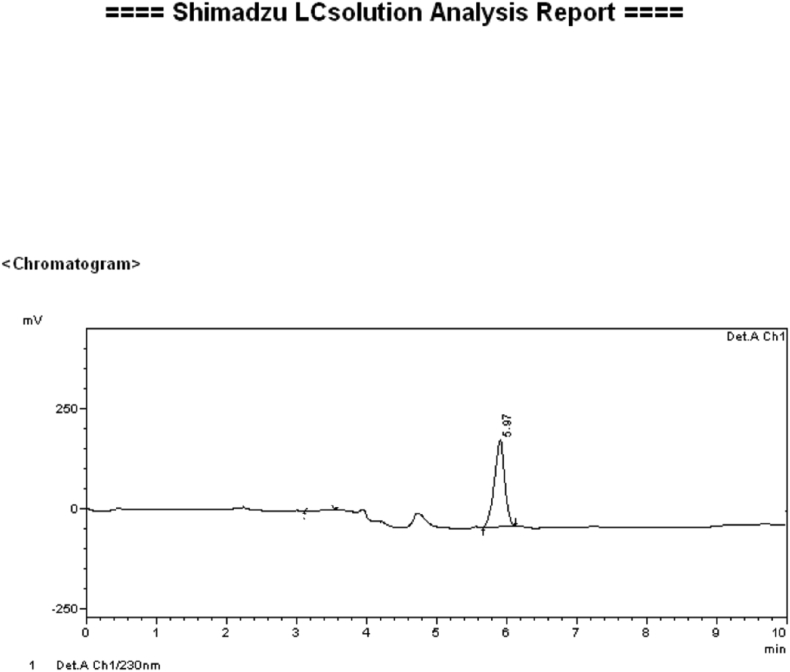
HPLC obtained of the isolated compound (chlorogenic acid) from roots of Selinum vaginatum.
Figure 6.
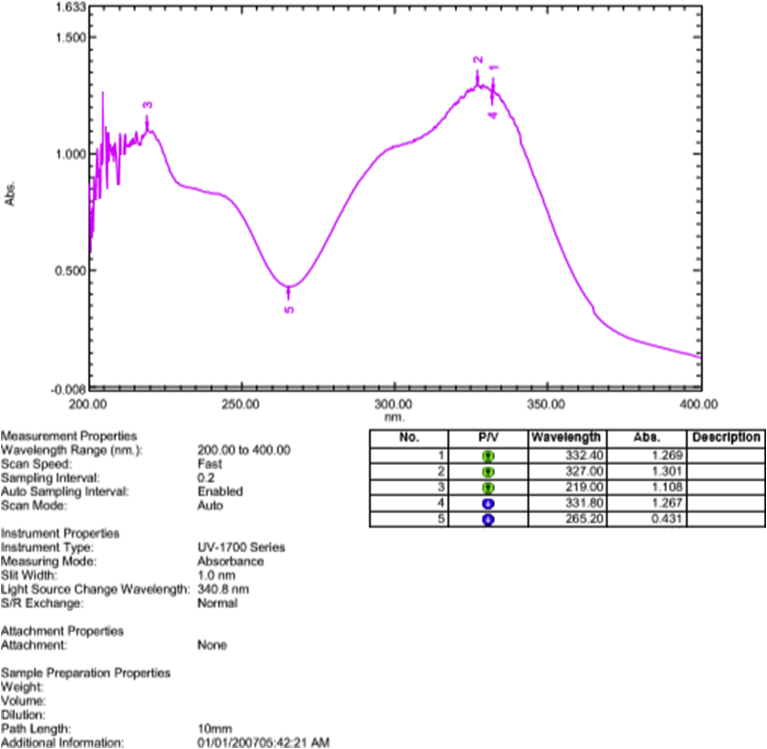
The graph above is showing the UV spectra of isolated compound (chlorogenic acid) from roots of Selinum vaginatum.
Figure 7.
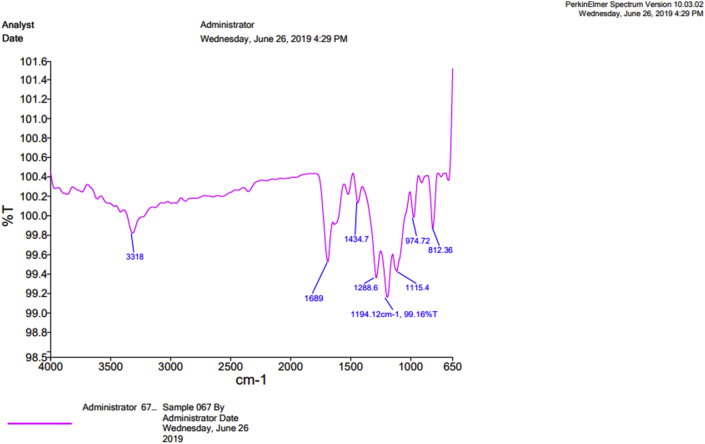
FTIR spectra of isolated compound (chlorogenic acid) obtained from roots of Selinum vaginatum.
2.7. Spectroscopic studies
The solid residue collected after the isolation and evaporation of extract was taken for the spectroscopic analysis. UV, HPLC and FTIR spectroscopy was performed in the laboratory facility in PSIT, Kanpur. Mass Spectroscopy was performed in CDRI, Lucknow where ethanol was used as a solvent. 1H NMR and C 13 NMR were performed at the Indian Institute of Technology, Kanpur laboratory facility where the solvent used was DMSO-D6. All the spectroscopic analysis confirmed the presence of chlorogenic acid which has a molecular formula of C16H18O9. The interpretations made us perform the Mass spectroscopy through which it was confirmed that the compound is chlorogenic acid. The spectroscopies are linked.
2.8. Acute toxicity studies
The acute toxicity studies of the isolated chlorogenic acid were performed as per the Organization for Economic Co-operation and Development guidelines 423 (OECD, 2001). Post overnight fasting of the treated Wistar rats they were orally administered with the isolated chlorogenic acid where increased dose levels of 5, 50, 300, 2000 mg/kg were given respectively from the stock solution of the isolated crystals from fraction. Then the animals were observed for behaviors like (restlessness, alertness, irritability, and fearfulness) as well for the neurological examinations (touch response, spontaneous activity, pain response, and gait) as well as for the autonomic reflexes like (urination and defecation) for 24 h. Post 24 h duration the animals also observed for 14 days for mortality (Parasuraman, 2011). The animals were kept under supervision up to 14 days for any sign of toxicity or mortality then animals were sacrificed and organs were also isolated to see for any visible changes morphologically (Mohamed et al., 2011; OECD, 2001). The organs were tested for any visible signs of organ toxicity and results are shown in Figure 8.
Figure 8.
Observation of visible changes on Organs post-Acute toxicity studies. A: Kidney; B: Spleen; C: Pancreas; D: Heart.
2.9. Induction of diabetes
The induction of diabetes was carried out by administering 45 mg/kg of Streptozotocin (Sigma Aldrich, St. Louis, USA) through intraperitoneal route (i.p.) to overnight fasted rats (Koneri et al., 2014). The development of increased blood glucose levels was confirmed post 72 h after the injection of STZ. Post induction of diabetes the groups were divided into four groups and treated as per the protocol. Fasting Blood Glucose was measured with the help of a glucometer (Accu-chek®, Roche) at days 7, 14, 21, and 28 (Koneri et al., 2014).
2.10. Treatment schedule and experimental protocol
A total of thirty animals were taken for the study and six animals were in the normal control group and rest all were induced with diabetes. Control Group (Group 1) animals (n = 6) were receiving distilled water and maintained non-diabetic. Three days post-injection of Streptozotocin the rats were divided into four groups (n = 6). The Group II Diabetic control group consisted of the animals received vehicle (1 ml/kg, p. o.). Group III received glibenclamide (0.2 mg/kg) whereas Group IV and Group V received isolated chlorogenic acid (10 mg/kg) and (20 mg/kg) respectively for twelve weeks. This dose of chlorogenic acid was selected as it has been previously reported to show activity at this concentration (Valcheva-Kuzmanova et al., 2015). The body weight, fasting blood glucose (from trail region) were recorded from the study. The mean average of food (on the basis of pellets consumed) and water intake per day was calculated for all the groups (Williams and Saffran, 1996).
2.11. Development of diabetic neuropathy
The treatment was given in continuation from week 0 to week 12 to evaluate the effects of diabetic neuropathy. The development of diabetic neuropathy was properly accessed in all groups by the help of Thermal Hyperalgesia –Eddy's hot plate method, Number of Writhing Responses in 10 min Cold Hyperalgesia (Acetone drop test), Mechanical Hyperalgesia (Pinprick test), Grip Strength test, Actophotometer and Rotarod tests on 2nd, 4th, 8th and 12th week in all groups respectively.
2.12. Fasting blood glucose
The rats were kept on fasting in all groups’ post 28 days of experimentation and the blood glucose levels were recorded by collecting blood from the tail region. The body weight, food, and water consumption were also recorded and analyzed at the end of experimentation (Koneri et al., 2014).
2.13. Body weight, food intake and water intake
The body weight will be measured at the initial day of selection of animals, induction of experimental diabetes, at seven days interval in the duration of therapy at and the end of the experiment. The food intake will be calculated on the basis of the number of pellets consumed per day. In the experiment, calibrated water bottles will be used for the estimation of the water intake (Williams and Saffran, 1996).
2.14. Lipid profile
The lipid profile was conducted by performing the lipid profile tests after collecting blood by retro-orbital methods on the 12th week of experimentation. Serum was separated from plasma by the help of centrifugation on Remi Industries Ltd (Mumbai, India) for 10 min at a speed of 15000 rpm. The serum was used for performing analysis like LDL, HDL, total cholesterol, and Triglyceride analysis. The manufacturers of the kit were (Span Diagnostics Ltd. India).
2.15. Thermal Hyperalgesia: Eddy's hot plate method
In this test, the animals from each group were kept individually on hot plate analgesiometer (Columbus Instruments, USA) where the temperature was adjusted to 55 ± 1 °C. As the signs of licking of paw or jumping, were seen for the first time it was recorded and hence the index of pain threshold was calculated. The cut-off time for the experiment was 10 s to avoid any kind of damage to the paws of animals. This study was assessed on week 2, 4th, 8th, and 12th (Kuhad and Chopra, 2009).
2.16. Writhing responses
Assessment of neuropathic pain was also assessed by the help of the Acetic acid writhing method in animals of all groups. The animals were treated with 1% v/v acetic acid prepared in distilled water. 60 minutes post administration of doses the rats were injected through i. p. route with 1% acetic acid in the volume of around 0.1 ml/10g body weight. Then episodes were recorded for 30 min and the stretching movements like the elongation of the body, stretching of back, and extension of limbs was counted (Zhang et al., 2018).
2.17. Cold hyperalgesia: acetone drop test
The responses of rats for cold sensitivity were recorded by performing the acetone drop test method. Rats from all groups were separately kept on a mesh cage to make them adjustable to the environment. Then a fresh drop of 50μL acetone was applied onto the paw gently (on the mid plantar surface). A cold stimulation was generated post 2–5 s of the application of acetone and the responses observed were shaking, licking of paw, rubbing or mild paw withdraws. Such reactions were recorded as the nociceptive responses while the no responses were termed as anti-nociceptive effects. These tests were performed on week 2, week 4, week 8, and week 12 of the experiment. The tests were performed on both paws at a time interval of 5 min and the mean responses were recorded (Bennett and Xie, 1988).
2.18. Mechanical hyperalgesia: pinprick test
The hind paw of the rat was touched with a bent gauge needle at 90° and it was made sure that there was no piercing with the needle just a pick was made. The withdrawal responses as a reflex were observed and recorded in all groups. The time duration of paw withdrawal was recorded (in a sec) where the cut off time was 20 s. The results were recorded in all groups for weeks 2, 4, 8, and 12 (Kanaan et al., 1996).
2.19. Grip strength
Grip strength test was performed for the determination of the neuromuscular strength. Animals were measured for their grip strength by hanging them on their forelimbs with the help of a fine metal wire which was held tight at the end of poles. Time duration taken for holding the wire before falling on the surface was recorded which a determination of their muscle strength. An animal with the weekend or damaged muscle will fall soon. The time duration of hanging was recorded (Ali et al., 2004).
2.20. Spontaneous Locomotor (exploratory) test
The spontaneous motor exploratory test was performed to record and interpret animal behavior with the help of actophotometer. Here the animals were kept in a closed actophotometer (30 × 30 × 30 cm) with photocells on the outer wall. The digital counter recorded the interruptions in the beam (Anjaneyulu and Chopra, 2004; Kar et al., 2003).
2.21. Neuromuscular coordination test (motor coordination)
Rotarod was used as an apparatus to record the neuromuscular coordination in rats of all groups on weeks 2, 4, 8, and 12. The rats were kept on the rotarod apparatus at a rotation speed of 25 rpm. The fall-off time for each group was recorded during the first 5 min of the run (Carter et al., 1999).
2.22. Nerve fiber analysis (histopathology)
The sciatic nerve was isolated from the thigh region of rat in all groups on the last day of the 24th week of the experimentation. The nerve was isolated when the rat was sacrificed on the last day and the sciatic vein was isolated by pulling the hind limb from the left hand and the base of the tail was pulled by the right hand. Then an incision was made around the thigh region near to hind limb to expose and cut out the thick whitish cord-like structure (the sciatic nerve) (Nasiry et al., 2017). The isolated sciatic nerve was fixed in 4% formaldehyde solution (Sigma Aldrich) buffered, pH 6.9. Sections were frozen for 24 h and then the histopathology studies were carried outpost 24 h. The sections were obtained using a fine blade post fixing them in resin. The samples were stained with Eosin solution. The process of isolation of the sciatic nerve section is shown in Figure 9. Isolation of Liver Tissue: Histopathology: The animals were sacrificed at the end of the 12th week and the liver was isolated from animals of each group. The organ was stored in 4% formalin for 24 h. Post the storage tissue was isolated and stained with eosin solution (Yaman et al., 2017).
Figure 9.
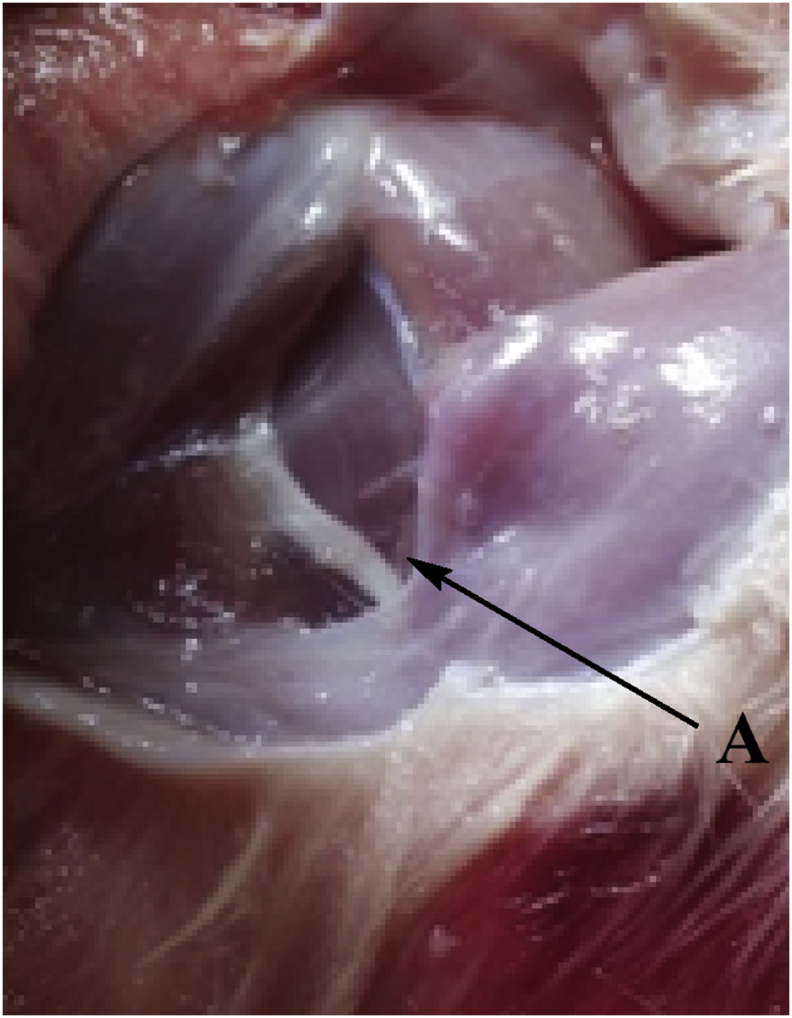
Isolation of Sciatic Nerve from the thigh region (near lower limb) A: Sciatic Nerve (post isolation of nerve from each group the nerve was frozen in formalin solution and sections of the nerve were stained and observed under microscope).
2.23. Biochemical estimations
2.23.1. Preparation of sciatic nerve homogenate
When the animals were sacrificed at the end of the 12-week study the sciatic nerves were isolated from them. Then a tissue homogenate was prepared using buffer 0.1 M Tris–HCl of pH 7.4 and supernatant of homogenate was used to estimate SOD (superoxide dismutase), NO content (nitric oxide), lipid peroxidation (MDA content-LPO), Na+K+ATPase (membrane-bound inorganic phosphate) and TNF-α (Bonting, 1970; Miranda et al., 2001; Misra and Fridovich, 1972; Slater and Sawyer, 1971).
2.23.2. Estimation of TNF-α
TNF-α quantification was performed by the help of Thermo Scientific, USA Rat TNF-α immunoassay kit for measuring 4.5 h solid-phase ELISA of TNF-α levels in rats. The assay involved the use of a monoclonal antibody for pre-coating the microplate. 50 μL of buffer was added to each well. Then sciatic nerve homogenate samples from normal, control, standard, and treated groups of 50 μL were incubated for 1 h at R.T. The presence of TNF-α will show binding to immobilized antibodies. Post washing the unbound substances biotinylated antibody reagent was poured in each well and incubated 1 h at R.T. Then washing was done and streptavidin-HPR reagent 100 μL was added in every well followed by adding an antibody-enzyme reagent to remove any unbound antibody. A substrate solution of 100 μL of TMB was added which turns a blue color of the product to yellow. The color intensity was checked at 550 nm and sample values were noted.
2.24. Statistical analysis
All the experiments were performed wherein every group n = 6. The quantitative data has been tabulated as Mean ± S.E.M. and were tested with one-way ANOVA followed by post-hoc test Tukey-Kramer multiple comparison test.
3. Results
3.1. Fasting blood glucose
The results were recorded as shown in Table 1. When the tests were performed on day 28 of the experimentation the treated group 2 has shown a reduction of blood glucose and was recorded as 125.11 and 119.10 mg/dl in Group IV and V respectively. The results are substantial when compared with the results of Group II (Diabetic control) from week 12.
Table 1.
Effect of chlorogenic acid on fasting blood sugar level during the course of anti-diabetic study model (Post induction of Diabetes).
| S. No. | Treatment Type |
Fasting Blood glucose level in mg/dl |
|||
|---|---|---|---|---|---|
| Group Type | Day 7 | Day 14 | Day 21 | Day 28 | |
| 1 | Normal Control | 96.1 ± 1.23 | 95.29 ± 0.93 | 95.21 ± 2.17 | 96.13 ± 1.24 |
| 2 | Diabetic Control | 161.52 ± 1.36a | 261.29 ± 2.36a | 282.12 ± 0.28a | 302.41 ± 1.12a |
| 3 | Standard | 157.14 ± 1.23∗∗∗ | 220.25 ± 2.43∗∗∗ | 192.51 ± 1.93∗∗∗ | 178.31 ± 1.45∗∗∗ |
| 4 | Treated 1 | 156.49 ± 2.35∗∗∗ | 199.50 ± 1.89∗∗∗ | 146.21 ± 1.23∗∗∗ | 125.11 ± 1.21∗∗∗ |
| 5 | Treated 2 | 148.21 ± 1.32∗∗∗ | 192.1 ± 1.32∗∗∗ | 137.12 ± 1.33∗∗∗ | 119.10 ± 3.21∗∗∗ |
Values are expressed as Mean ± SEM; n = 6. One-way ANOVA; followed by Tukey-Kramer multiple comparisons test: aP< 0.001 in comparison with normal control and ∗∗∗P < 0.001 in comparison with the diabetic control.
3.2. Body weight, food intake and water intake
Group V and group IV rats gained weight respectively when compared from week 2 to week 12. The gain of weight is opposite to the symptom of weight loss is diabetes hence improvement in health is seen in treated groups where average weight was recorded as 201.11 and 211.12 g while the diabetic group has recorded as the mean weight of 120.73 g. The results are significant when compared with the results of Group II (Diabetic control) from week 12. The results are plotted in Tables 2 and 3.
Table 2.
Effect of chlorogenic acid on the body weight post induction of diabetes in wistar albino rats.
| S. No. | Treatment Type |
Body Weight (g) |
|||
|---|---|---|---|---|---|
| Group Type | 2nd week | 4th week | 8th week | 12th week | |
| 1 | Normal Control | 150.5 ± 3.23 | 170.31 ± 2.01 | 201.31 ± 2.24 | 252.77 ± 1.31 |
| 2 | Diabetic Control | 161.60 ± 1.990a | 153.70 ± 2.12a | 145.52 ± 0.62a | 120.73 ± 1.31a |
| 3 | Standard | 159.97 ± 2.456 | 160.53 ± 1.59 | 162.40 ± 1.81∗∗∗ | 168.4 ± 2.00∗∗∗ |
| 4 | Treated 1 | 159.11 ± 0.91 | 168.2 ± 0.33∗∗∗ | 178.14 ± 2.32∗∗∗ | 201.11 ± 2.45∗∗∗ |
| 5 | Treated 2 | 165.77 ± 0.57 | 169.35 ± 3.30∗∗∗ | 180.28 ± 2.78∗∗∗ | 211.12 ± 2.13∗∗∗ |
Values are expressed as Mean ± SEM; n = 6. One-way ANOVA; Tukey-Kramer multiple comparisons test: aP< 0.001 in comparison with normal control; and ∗∗∗P < 0.001 in comparison with the diabetic control.
Table 3.
Effect of chlorogenic acid on the food and water consumption post treatment in wistar albino rats.
| S. No. | Treatment Type |
Food & Water Consumption |
|
|---|---|---|---|
| Group Type | Water intake (ml/day) | Food intake (g/day) | |
| 1 | Normal Control | 12.75 ± 1.41 | 19.13 ± 0.58 |
| 2 | Diabetic Control | 37.34 ± 8.93a | 29.19 ± 1.24a |
| 3 | Standard | 24.25 ± 3.74∗∗∗ | 20.22 ± 3.34∗∗∗ |
| 4 | Treated 1 | 20.23 ± 2.37∗∗∗ | 22.30 ± 2.15∗∗∗ |
| 5 | Treated 2 | 18.15 ± 8.42∗∗∗ | 21.23 ± 1.34∗∗∗ |
Values are expressed as Mean ± SEM; n = 6. One-way ANOVA; Tukey-Kramer multiple comparisons test: aP< 0.001 in comparison with normal control; and ∗∗∗P < 0.001 in comparison with the diabetic control.
3.3. Lipid profile
The lipid levels of Group IV and Group V are showing better responses when compared to the diabetic control group. Total cholesterol levels were reported as 79.08 and 77.13, triglyceride levels were reported as 64.25 and 67.31, LDL levels were reported as 39.21, and 36.31, HDL was reported as 28.47 and 26.23 and VLDL were reported as 15.23 and 15.56 mg/dl in Group IV and V respectively. The results are significant when compared with the results of Group II (Diabetic control) from week 12. The results are presented in Table 4.
Table 4.
Effect of chlorogenic acid on lipid profile of groups in wistar albino rats.
| S. No. | Treatment Type |
Lipid profile (mg/dl) post treatment |
||||
|---|---|---|---|---|---|---|
| Group Type | TC | Triglyceride | LDL | HDL | VLDL | |
| 1 | Normal Control | 75.12 ± 1.23 | 67.21 ± 1.16 | 34.28 ± 2.24 | 30.15 ± 1.72 | 14.19 ± 1.59 |
| 2 | Diabetic Control | 145.13 ± 1.43a | 159.15 ± 1.39a | 95.41 ± 1.23a | 14.21 ± 1.71a | 40.13 ± 1.45a |
| 3 | Standard | 78.27 ± 0.42∗∗∗ | 69.15 ± 2.33∗∗∗ | 38.83 ± 2.14∗∗∗ | 29.1 ± 2.15∗∗∗ | 14.16 ± 0.23∗∗∗ |
| 4 | Treated 1 | 79.08 ± 2.31∗∗∗ | 64.25 ± 0.83∗∗∗ | 39.21 ± 1.64∗∗∗ | 28.47 ± 0.34∗∗∗ | 15.23 ± 0.25∗∗∗ |
| 5 | Treated 2 | 77.13 ± 1.21∗∗∗ | 67.31 ± 1.91∗∗∗ | 36.31 ± 0.35 ∗∗∗ | 26.23 ± 0.53∗∗∗ | 15.56 ± 0.23∗∗∗ |
Values are expressed as Mean ± SEM; n = 6. One-way ANOVA; Tukey-Kramer multiple comparisons test: aP< 0.001 in comparison with normal control; and ∗∗∗P < 0.001 in comparison with the diabetic control.
3.4. Thermal Hyperalgesia: Eddy's hot plate method
The results are mentioned in Table 5. Treatment Group IV has shown a mean reaction latency of 4.88 s and 4.53 s in Group IV and V in response on week 12. The results are significant when compared with the results of Group II (Diabetic control) from week 12.
Table 5.
Effect of chlorogenic acid on Thermal Hyperalgesia: Eddy's hot plate test to assess diabetic neuropathy in Wistar albino rats.
| S. No. | Treatment Type |
Thermal Hyperalgesia –Eddy's hot plate method Response Time (s) |
|||
|---|---|---|---|---|---|
| Group Type | Reaction Latency (2nd week) | Reaction Latency (4th weeks) | Reaction Latency (8th weeks) | Reaction Latency (12th weeks) | |
| 1 | Normal Control | 4.15 ± 0.31 | 4.23 ± 1.28 | 4.43 ± 2.18 | 4.11 ± 1.81 |
| 2 | Diabetic Control | 6.91 ± 2.31a | 7.24 ± 0.22a | 8.94 ± 0.21a | 9.95 ± 0.19a |
| 3 | Standard | 6.12 ± 0.12 | 5.60 ± 2.32∗∗∗ | 5.01 ± 0.12∗∗∗ | 5.14 ± 0.21∗∗∗ |
| 4 | Treated 1 | 6.03 ± 0.48 | 5.11 ± 0.12∗∗∗ | 4.91 ± 1.31∗∗∗ | 4.88 ± 2.61∗∗∗ |
| 5 | Treated 2 | 6.01 ± 0.14 | 5.01 ± 0.20∗∗∗ | 4.21 ± 2.91∗∗∗ | 4.53 ± 0.48∗∗∗ |
Values are expressed as Mean ± SEM; n = 6. One-way ANOVA; followed by Tukey-Kramer multiple comparisons test: aP< 0.001 in comparison with normal control; ∗∗∗P < 0.001 in comparison with the diabetic control.
3.5. Writhing responses
Group IV has shown an average of 16.52 and Group V has shown an average of 14.81writhing responses in 10 min. When results were compared with the responses from the Diabetes control group (Group II) of experimentation on the 12th week the results were quite significant. The results are mentioned in Table 6.
Table 6.
Effect of chlorogenic acid on the writhing responses to assess the neuropathic pain in Wistar albino rats.
| S. No. | Treatment Type |
Number of Writhing Responses |
|||
|---|---|---|---|---|---|
| Group Type | (2nd week) | (4th week) | (8th week) | (12th week) | |
| 1 | Normal Control | 15.41 ± 2.31 | 12.21 ± 2.02 | 10.13 ± 1.70 | 8.4 ± 1.21 |
| 2 | Diabetic Control | 25.11 ± 0.22a | 26.86 ± 1.58a | 27.39 ± 0.52a | 29.13 ± 0.34a |
| 3 | Standard | 21.14 ± 1.78 | 24.13 ± 1.18∗∗∗ | 23.44 ± 0.91∗∗∗ | 18.54 ± 0.45∗∗∗ |
| 4 | Treated 1 | 21.19 ± 0.36 | 22.64 ± 1.31∗∗∗ | 22.41 ± 2.91∗∗∗ | 16.52 ± 0.71∗∗∗ |
| 5 | Treated 2 | 20.17 ± 2.91 | 21.28 ± 2.91∗∗∗ | 20.17 ± 2.91∗∗∗ | 14.81 ± 1.31∗∗∗ |
Values are expressed as Mean ± SEM; n = 6. One-way ANOVA; Tukey-Kramer multiple comparisons test: aP< 0.001 in comparison with normal control; and ∗∗∗P < 0.001 in comparison with the diabetic control.
3.6. Cold hyperalgesia: acetone drop test
The results were documented in Table 7. Group IV has recorded average readings of 4.91and, Group V has shown a response of 4.53 in cold hyperalgesia responses when compared with the results from week 12 the results were quite significant. Prolonged responses were seen in Group II (Diabetic group) where the diabetes-induced group has shown potential nerve damage due to recorded delayed responses in hyperalgesia.
Table 7.
Effect of chlorogenic acid on Cold hyperalgesia: Acetone drop test in wistar albino rats.
| S. No. | Treatment Type |
Cold Hyperalgesia (Acetone drop test) |
|||
|---|---|---|---|---|---|
| Group Type | Allodynia Score in sec (2nd week) | Allodynia Score in sec (4th week) | Allodynia Score in sec (8th week) | Allodynia Score in sec (12th week) | |
| 1 | Normal Control | 4.91 ± 0.19 | 4.13 ± 1.34 | 4.19 ± 2.03 | 4.62 ± 1.42 |
| 2 | Diabetic Control | 7.77 ± 3.23a | 8.11 ± 1.23a | 8.75 ± 1.28a | 9.12 ± 0.71a |
| 3 | Standard | 5.28 ± 1.15 | 4.98 ± 0.25∗∗∗ | 4.37 ± 0.25∗∗∗ | 6.98 ± 2.93∗∗∗ |
| 4 | Treated 1 | 5.97 ± 2.02 | 5.11 ± 1.23∗∗∗ | 5.01 ± 3.15∗∗∗ | 4.91 ± 2.51∗∗∗ |
| 5 | Treated 2 | 4.93 ± 1.65 | 4.84 ± 0.26∗∗∗ | 4.76 ± 0.35∗∗∗ | 4.53 ± 3.61∗∗∗ |
Values are expressed as Mean ± SEM; n = 6. One-way ANOVA; followed by Tukey-Kramer multiple comparisons test: aP< 0.001 in comparison with normal control; ∗∗∗P < 0.001 in comparison with the diabetic control.
3.7. Mechanical hyperalgesia: pinprick test
The results are compared in Table 8. The responses of Group IV and Group V have improved to 4.16 and 3.11 respectively on comparison from week 12 data. When results were compared with the responses from the Diabetes control group (Group II) of experimentation on 12th week the results were quite significant.
Table 8.
Effect of chlorogenic acid on Mechanical hyperalgesia: Pin prick test in wistar albino rats.
| Treatment Type |
Mechanical Hyperalgesia (Pin prick test) |
||||
|---|---|---|---|---|---|
| S. No. | Group Type | Response latency in sec (2nd week) | Response latency in sec (4th week) | Response latency in sec (8th week) | Response late Response latency in sec (12th week) |
| 1 | Normal Control | 2.13 ± 0.82 | 2.21 ± 0.16 | 1.99 ± 0.66 | 2.31 ± 0.31 |
| 2 | Diabetic Control | 6.16 ± 1.36a | 6.96 ± 1.04a | 7.71 ± 0.48a | 8.78 ± 1.04a |
| 3 | Standard | 6.86 ± 1.19 | 7.98 ± 1.02∗∗∗ | 8.92 ± 0.28∗∗∗ | 4.99 ± 0.37∗∗∗ |
| 4 | Treated 1 | 6.03 ± 0.64 | 7.79 ± 0.21∗∗∗ | 8.41 ± 1.39∗∗∗ | 4.16 ± 1.32∗∗∗ |
| 5 | Treated 2 | 6.44 ± 1.06 | 7.94 ± 1.23∗∗∗ | 4.05 ± 0.12∗∗∗ | 3.11 ± 0.62∗∗∗ |
Values are expressed as Mean ± SEM; n = 6. One-way ANOVA; Tukey-Kramer multiple comparisons test: aP< 0.001 in comparison with normal control; and ∗∗∗P < 0.001 in comparison with the diabetic control.
3.8. Grip strength
The results are mentioned in Table 9. Grip strength of Group IV and Group V improved to 25.11 and 29.13 at the end of the experiment. When results were compared with the responses from the Diabetes control group (Group II) of experimentation on 12th week the results were quite significant.
Table 9.
Effect of chlorogenic acid on Grip Strength to record the Response latency in wistar albino rats.
| S. No. | Treatment Type |
Grip Strength for each animal in a group ±SEM, n = 6. |
|||
|---|---|---|---|---|---|
| Group Type | Response latency in sec (2th week) | Response latency in sec (4th week) | Response latency in sec (8th week) | Response latency in sec (12th week) | |
| 1 | Normal Control | 31.02 ± 1.35 | 29.81 ± 0.51 | 30.01 ± 2.31 | 30.11 ± 0.58 |
| 2 | Diabetic Control | 12.13 ± 0.21a | 9.96 ± 1.35a | 7.13 ± 0.52a | 4.36 ± 1.13a |
| 3 | Standard | 19.26 ± 1.92∗∗∗ | 20.19 ± 0.87∗∗∗ | 22.23 ± 0.73∗∗∗ | 23.05 ± 2.36∗∗∗ |
| 4 | Treated 1 | 20.71 ± 1.36∗∗∗ | 21.44 ± 2.13∗∗∗ | 23.71 ± 1.36∗∗∗ | 25.11 ± 1.49∗∗∗ |
| 5 | Treated 2 | 23.13 ± 0.21∗∗∗ | 25.09 ± 0.18∗∗∗ | 28.32 ± 1.72∗∗∗ | 29.13 ± 0.91∗∗∗ |
Values are expressed as Mean ± SEM; n = 6. One-way ANOVA; followed by Tukey-Kramer multiple comparisons test: aP< 0.001 in comparison with normal control; ∗∗∗P < 0.001 in comparison with the diabetic control.
3.9. Spontaneous Locomotor (exploratory) test: actophotometer
The results are shown in Table 10. The locomotor activity of Group improved to 98.13 and 110.34 in Group IV and Group V after receiving doses for 12 weeks. When results were compared with the responses from the Diabetes control group (Group II) of experimentation on 12th week the results were quite significant.
Table 10.
Effect of chlorogenic acid on Spontaneous Locomotor (Exploratory) Test: Actophotometer in wistar albino rats.
| S. No. | Treatment Type |
Actophotometer: Counts per 5 min |
|||
|---|---|---|---|---|---|
| Group Type | Counts (2nd week) | Counts (4th week) | Counts (8th week) | Counts (12th week) | |
| 1 | Normal Control | 122.50 ± 8.744 | 121.83 ± 7.452 | 122.83 ± 8.565 | 121.33 ± 9.501 |
| 2 | Diabetic Control | 30.33 ± 7.236a | 26.98 ± 5.324a | 20.17 ± 6.882a | 14.33 ± 6.784a |
| 3 | Standard | 40.17 ± 6.492∗∗∗ | 61.67 ± 6.25∗∗∗ | 77.26 ± 5.262∗∗∗ | 89.17 ± 5.168∗∗∗ |
| 4 | Treated 1 | 42.67 ± 6.02∗∗∗ | 62.22 ± 6.25∗∗∗ | 81.17 ± 7.22∗∗∗ | 98.13 ± 5.28∗∗∗ |
| 5 | Treated 2 | 45.67 ± 6.22∗∗∗ | 69.24 ± 8.37∗∗∗ | 89.63 ± 5.57∗∗∗ | 110.34 ± 3.61∗∗∗ |
Values are expressed as Mean ± SEM; n = 6. One-way ANOVA; followed by Tukey-Kramer multiple comparisons test: aP< 0.001 in comparison with normal control; ∗∗∗P < 0.001 in comparison with the diabetic control.
3.10. Neuromuscular coordination test (motor coordination): rota rod test
The results are shown in Table 11. Neuromuscular coordination recorded an improvement of 84.27 and 112.23 in Group IV and Group V animals. When results were compared with the responses from the Diabetes control group (group II) of experimentation on 12th week the results were quite significant. The results of Treated Group IV and V, when expressed in Mean ± SEM when analyzed by One-way ANOVA followed by Tukey-Kramer multiple comparisons test, have shown P < 0.001 significance when compared to diabetic control groups (group II). Hence, emphasizing the healing and protective effect of chlorogenic acid.
Table 11.
Effect of chlorogenic acid on Neuromuscular Coordination test (Motor coordination): Rota Rod Test in wistar albino rats.
| S. No. | Treatment Type |
Rota Rod Test |
|||
|---|---|---|---|---|---|
| Group Type | Counts (2nd week) | Counts (4th weeks) | Counts (8th weeks) | Counts (12th weeks) | |
| 1 | Normal Control | 125.41 ± 1.11 | 129.2 ± 1.12 | 128.21 ± 1.02 | 128.13 ± 6.04 |
| 2 | Diabetic Control | 44.31 ± 2.11a | 22.15 ± 5.61a | 18.34 ± 5.61a | 9.12 ± 6.13a |
| 3 | Standard | 52.31 ± 6.14 | 84.13 ± 1.13∗∗∗ | 99.62 ± 5.33∗∗∗ | 102.14 ± 1.34∗∗∗ |
| 4 | Treated 1 | 56.50 ± 6.11∗∗∗ | 68.31 ± 6.92∗∗∗ | 78.23 ± 6.32∗∗∗ | 84.27 ± 2.13∗∗∗ |
| 5 | Treated 2 | 54.12 ± 2.11 | 70.12 ± 6.12∗∗∗ | 86.02 ± 8.31∗∗∗ | 112.23 ± 4.22∗∗∗ |
Values are expressed as Mean ± SEM; n = 6. One-way ANOVA; Tukey-Kramer multiple comparisons test: aP< 0.001 in comparison with normal control; ∗∗∗P < 0.001 in comparison with the diabetic control.
3.11. Nerve fiber analysis: histopathology
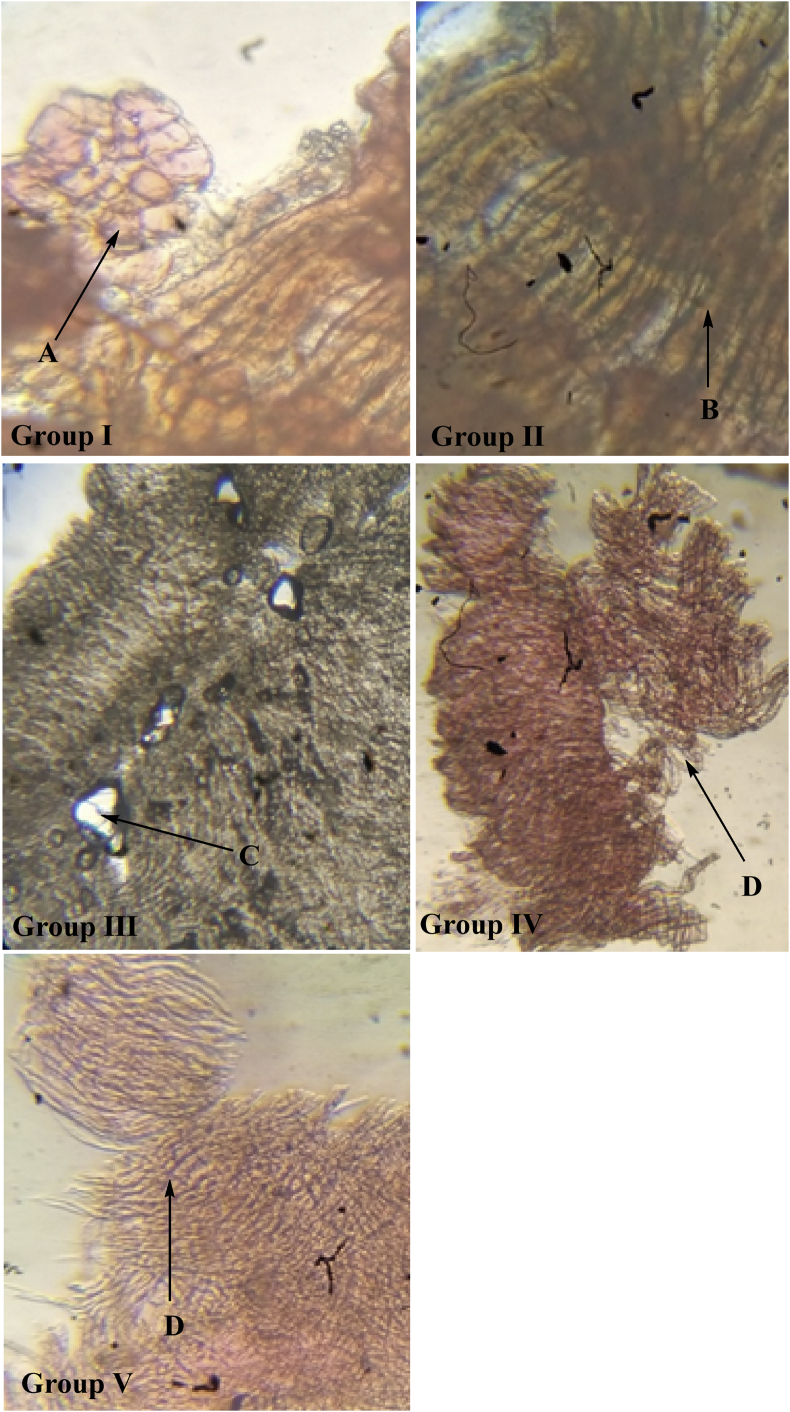
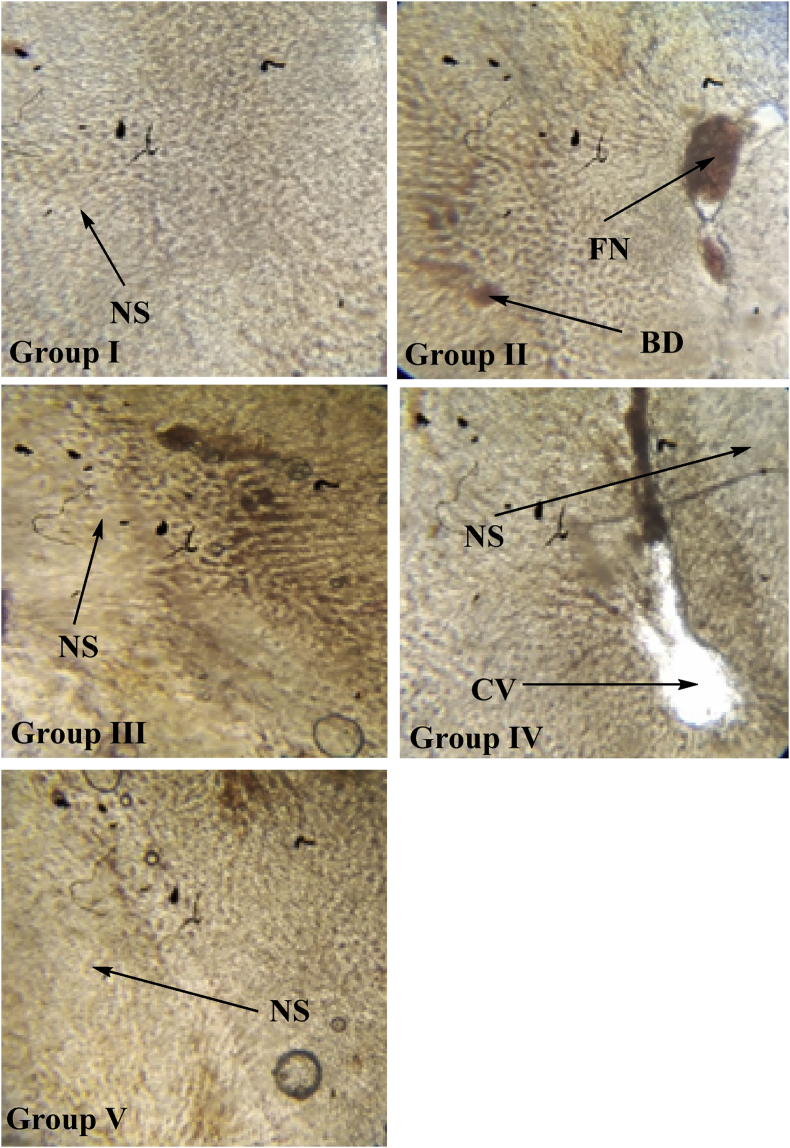
The effects of isolated chlorogenic acid obtained from Selinum vaginatum were recorded by dosing the animals for 12 weeks and studying their histopathological sections. The Longitudinal vasa nervorum analysis of the sciatic nerves in the 12th week shows Normal nerve tissue without any sign of inflammation or degenerative changes in the control group (non-diabetic). Lipid degenerated axons showing focal peripheral axonal loss were seen in the case of the diabetic group (untreated) which is a characteristic feature of diabetes. Mild focal axonal loss in the peripheral region of the standard group was noted and minimum axonal degeneration changes in treated groups (healing effect) were seen. These images show healing action in changes in treated groups IV and V. No fatty necrosis or balloon degradation was observed in liver cells of treated rats hence showing the anti-diabetic and healing action of isolated chlorogenic acid. The sections captured are shown in Figures 10 and 11.
Figure 10.
Longitudinal vasa nervorum analysis in sciatic nerves of wistar albino rats in all groups on 12th week. Representative the images from the sciatic nerve longitudinal section of the control group, diabetic group, standard group, treated 1 and treated 2 groups from 6th week of treatment age. The samples were stained with eosin. Group II shows swelling in the neurons. Effects of isolated chlorogenic acid obtained from Selinum vaginatum on the eosin histopathological sections of sciatic nerves in diabetic rats. Following figures indicating: A: Normal nerve tissue which is not showing any sign of inflammation or degenerative changes in the control group (non-diabetic). B: Lipid degenerated axons showing focal peripheral axonal loss in case of diabetic group (untreated); C: mild focal axonal loss in the peripheral region in standard group; D: minimum axonal degeneration changes in treated group 1 and treated group 2.
Figure 11.
Histopathological analysis of liver tissue obtained from wistar albino rats in all groups on 6th week. Representative the images from the liver tissues obtained from animals of control group, diabetic group, standard group, treated 1 and treated 2 groups from 6th week of age. The samples were stained with eosin. Fatty necrosis was observed in group II post 12 weeks of induced diabetic neuropathy. CV- Central Vein, NS- Narrow sinusoids; FN-Fatty necrosis; BD-Ballooning degradation.
3.12. Effect on superoxide dismutase
A significant decrease in superoxide dismutase was recorded in the sciatic nerve of diabetic control group rats (5.41 ± 1.34 U/mg of protein) when compared to the normal group (27.13 ± 1.75 U/mg of protein). Whereas the treated group rats have shown an increase in SOD levels -21.35 ± 1.34 U/mg of protein and 25.28 ± 1.42 U/mg of protein in Group IV and V respectively (Table 12). This has shown significant and dose-dependent results in rats being treated with chlorogenic acid.
Table 12.
Effect of chlorogenic acid on endogenous biomarkers in rats.
| S. No. | Treatment Type |
Endogenous Biomarkers |
||||
|---|---|---|---|---|---|---|
| Group Type | SOD (U/mg of protein) | NO (μg/mL) | LPO (nM/mg of protein) | Na+K+ATPase (μmol/mg of protein) | TNF-α (pg/mL) | |
| 1 | Normal Control | 27.13 ± 1.75 | 108.17 ± 2.62 | 2.59 ± 1.29 | 10.13 ± 1.46 | 53.48 ± 2.14 |
| 2 | Diabetic Control | 5.41 ± 1.34a | 306.12 ± 3.13a | 9.13 ± 1.26a | 2.77 ± 1.25a | 161.12 ± 2.32a |
| 3 | Standard | 16.14 ± 1.32∗ | 227.34 ± 2.35∗ | 6.26 ± 1.11∗ | 6.38 ± 1.25∗ | 120.21 ± 3.14∗ |
| 4 | Treated 1 | 21.35 ± 1.34∗ | 160.10 ± 4.49∗ | 4.21 ± 1.31∗ | 7.13 ± 4.19∗ | 91.18 ± 1.39∗ |
| 5 | Treated 2 | 25.28 ± 1.42∗ | 131.23 ± 5.11∗ | 2.91 ± 1.25∗ | 9.41 ± 2.51∗ | 69.43 ± 1.72∗ |
Values are expressed as Mean ± SEM; n = 6. One-way ANOVA; Tukey-Kramer multiple comparisons test: aP< 0.05 in comparison with normal control; ∗P < 0.05 in comparison with the diabetic control.
3.13. Effect in the nitrosative stress
The levels of neural nitrite in the sciatic nerve of the diabetic control group animals were increased significantly to 306.12 ± 3.13 μg/mL, P < 0.05 after 12 weeks of STZ injection when compared to the normal group animals where value recorded was 108.17 ± 2.62 μg/mL, P < 0.05. Whereas in treated groups IV and V the values were recorded as 160.10 ± 4.49 and 131.23 ± 5.11 that are significantly lower when compared to the diabetic control group (Table 12).
3.14. Effect in lipid peroxide profile
LPO (Neutral Lipid Peroxidase) levels after 12 weeks of treatment were noted in all groups. Diabetic control groups have shown a significant increase in the LPO levels (9.13 ± 1.26 nM/mg of protein, P < 0.05) when compared to the normal group. Levels of treated groups were decreased significantly and recorded as 4.21 ± 1.31 and 2.91 ± 1.25 nM/mg of protein, P < 0.05 in group V and V respectively when compared to the diabetic group (Table 12).
3.15. Effect in membrane-bound inorganic phosphate
After 12 weeks of study Na+K+ATPase level (membrane-bound inorganic phosphate) was found to be 2.77 ± 1.25 μmol/mg of protein P < 0.05 in the diabetes group which is significantly lower when compared to the normal group. In treated groups, the values were significantly increased in comparison to the diabetic groups. Group IV and V have reported values of 7.13 ± 4.19 and 9.41 ± 2.51 μmol/mg of protein, P < 0.05 (Table 12).
3.16. Effect in the TNF-α
Wistar rats treated with STZ in diabetic groups have shown a significant increase in TNF- α levels in the sciatic nerves 161.12 ± 2.32 pg/mL when compared to the control group 53.48 ± 2.14 pg/mL. Group IV and V have shown a significant reduction in values (91.18 ± 1.39 and 69.43 ± 1.72 pg/mL) when compared to the diabetic group (Table 12).
4. Discussion
The results from this study suggest that chlorogenic acid has an ameliorative effect on the experimentally induced Diabetes and Diabetic Neuropathy in Wistar rats. Hence it was hypothesized this alleviation was due to the isolated chlorogenic acid from Selinum vagiantum on the experimentally induced hyperglycemia and diabetic neuropathy. Diabetic Neuropathy is amongst the commonly observed complication of diabetes, recorded in ~50% of the patients suffering from diabetes. This complication is surfaced majorly due to induced free radical oxidative stress observed in the pathogenesis of diabetic neuropathy (van Dam, 2002). As the rhizomatous roots of S. vaginatum have been used for the treatment of neurological disorders like hysteria, insomnia, convulsions, epilepsy and, diabetes etc. It has been reported by researchers that it has lipid peroxidation and antioxidant functions (Pandey et al., 2013; Rana et al., 2010). Thus, this plant has proven to have medicinal values.
In the current study, the rats suffering from diabetes exhibited a prominent delay in the tail, paw withdraw latency in comparison to the control rats. This signifies that the rats were having thermal and mechanical hyperalgesia which delayed the response time and became worse with increasing weeks of study. The thermal algesia responses, writhing responses, pinprick improved over the 12 weeks’ time in treated groups, most prominently in Group V hence emphasizing the neuroprotective action of chlorogenic acid. The grip strength, locomotor responses recorded by actophotometer, rotarod responses were also improved in treated groups as compared to the diabetic control groups. As when the animals suffer from diabetes with time they start developing lethargic responses, inflammation in nerves which induces pain in limbs hence the locomotor responses are delayed and the animals tend to lose activeness with the progression of diabetes and the development of diabetic neuropathy (AlSharari et al., 2014; Kamboj et al., 2010).
In this study, a significant reduction was recorded in the glucose levels for four weeks when compared with the control group. This result has proved the anti-diabetic action of Selinum vaginatum. The experiment results suggest that an administered isolated chlorogenic acid from Selinum vaginatum has shown an ameliorative effect against streptozotocin-induced Diabetes and Diabetic Neuropathy in Wistar rats (Vinagre et al., 2010).
As a reduction in the bodyweight is a characteristic feature of identification of diabetes, hence we monitored the bodyweight of animals to ensure the action of isolated chlorogenic acid. Bodyweight in diabetic control group animals was found to be decreased which is a characteristic feature of diabetes, whereas the bodyweight of animals improved in treated groups. This marks the healing property of the treated groups (Kumar et al., 2019).
Variations in lipid profiles is also a notable feature of prolonged diabetes. Diabetes cases report high levels of TC, alterations in LDL, high TG, and low levels of HDL. In this experimentation Lipid profiles of the treated groups improved by the end of experimentation which showed the ameliorating effect and anti-diabetic action of the isolated chlorogenic acid (Howard et al., 2000).
Oxidative stress can be closely correlated to SOD and MDA which are endogenous enzymes. Endogenous enzymes and Superoxide are responsible for endothelial damages. The superoxide anions lead to an increase in the protein kinase C and aldose reductase activity which is correlated to the pain perceptions. SOD functions as antioxidant protection from superoxide anions by transforming them into H2O2. MDA depicts the elevated stress conditions and is majorly responsible for the disruption of lipid membranes by rearranging the bonds in unsaturated FA (fatty Acids) of the membrane caused tissue damage. An increase in lipid peroxidation provides the index of oxidative stress whose levels are reduced in the drug treatment. NO-an intracellular messenger (Nitric oxide) has a vital role in various pathological processes. It reacts with ROS (reactive oxygen species) which acts as an anti-oxidant. This oxidation is non-specific and affects cell molecules (Cotter and Cameron, 2003; Kamei et al., 2001).
An increase in the NO levels is caused by reaction with superoxide anions hence forming of peroxynitrite which leads to DNA damage, fast nitrosylation, lipid peroxidation, and cell death. This ultimately causes aggregation and elevated pain. Fe (III) tetrakis-2-(N-triethylene glycol monomethyl ether) pyridyl porphyrin is the peroxynitrite decomposition catalyst which interacts with sensory neuropathy in diabetic groups. The results have been in accordance with previous studies performed (Coppey et al., 2002; Drel et al., 2007; Levy and Zochodne, 2004).
The Uniform distribution of enzyme Na+K+ATPase is correlated to the generation as well as transmission of bioelectricity. Conditions of chronic hyperglycemia lead to a reduction in Na+K+ATPase enzyme activity which causes inactivation of phosphate. It also causes activation of the polyol pathways. Hence the enzyme Na+K+ATPase is inhibited and their restoration is preferred. In the current study chlorogenic acid has restored the levels of Na+K+ATPase thus showing neuroprotective action (Chiu, 2005; Drel et al., 2007; Hirata and Okada, 1990).
In microvascular and neural tissues, the acceleration of TNF-α production is a characteristic feature of Diabetic neuropathy which leads to nerve damage and micro-vascular permeability microangiopathy. Usage of agents that suppress the cytokine elevation for treatment of diabetic neuropathy complications is advised. Hence, the dose-dependency in the reduction of TNF-α by isolated chlorogenic acid shows its role in the inhibition of the elevated cytokines (Lee et al., 2009; Satoh et al., 2003; Tiwari et al., 2011).
This study also demonstrated the strong association between developments of Diabetic Neuropathy in diabetic rats with prominent damage observed in diabetic rats over a period of 12 weeks. An increase in levels of inflammation is a prominent factor for the development of hyperglycemia and neuropathy in diabetes (Brownlee, 2005; Navarro-Gonzalez and Mora-Fernandez, 2008). With a persistent condition of diabetes, the inflammatory biomarkers are overexpressed and are the cause behind cell death and dysfunction. The liver tissues and the isolated sciatic nerve has shown inflammation and infiltration in the case of the diabetic control group animals while the treated groups show healthy tissues (Djordjevic et al., 2015; Li et al., 2013; Nasiry et al., 2017; Thorup et al., 2000).
Chlorogenic acid was observed to improve locomotor changes, lipid profile, and response latencies and induce attenuation in the histopathological alterations in the liver and sciatic nerves of rats. Hence it is hypothesized that the improvement was the result of the administered isolated chlorogenic acid of concentrations 10 mg/kg and 20 mg/kg respectively to Wistar rats with STZ-induced hyperglycemia and observe the reduced inflammation injury in sciatic nerves as a sign of reduced neuropathy–the prolonged side-effect of diabetes (Nasiry et al., 2017). Such infiltrations observed in diabetic control group animals in their liver and sciatic nerve tissues are a sign of hyperglycemia and diabetic neuropathy (Thorup et al., 2000). Using medicinal plants as a source to cure various disease conditions is a commonly followed practice in many parts of the world. A majority of the population still relies on using traditional medicines, ayurvedic remedies (extracts, ghritas, churnas), herb combinations, and herbal home remedies to cure disease (Chandra et al., 2012, 2015a, 2015b; Nikita et al., 2020; Pal et al., 2011; Yadav et al., 2012).
The current study has shown the behavioral, histopathological, and biochemical effects of chlorogenic acid. The standard drug has restored the levels of glucose but is not very effective in restoring the biomarkers hence not as effective as chlorogenic acid in treating diabetic neuropathy. However, the chlorogenic acid concentrations have attenuated the diabetic condition's and also restored the neuropathic pain by modulating the inflammatory cytokine release, oxidative stress, tumor necrosis factor-α (TNF-α) which are important factors in mediation of neuropathic pain, apoptotic conditions, and restoration of membrane-bound inorganic phosphate enzyme activity. Chlorogenic acid acted significantly in inhibiting diabetic neuropathy pain by involving mechanisms leading to inhibition of the elevated cytokines, restoration of levels of Na+K+ATPase, decrease in the NO levels, and reduction of TNF-α. Thus, showcasing the anti-oxidant and healing action of chlorogenic acid isolated from the herb.
5. Conclusion
This study confirmed the neuroprotective action and anti-diabetic activity of the isolated chlorogenic acid from the Selinum vaginatum roots in Wistar rats through an antioxidant mechanism of action and also, the traditional claim of the tribes.
Declarations
Author contribution statement
N. Saraswat: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
N. Sachan and P. Chandra: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
I am highly thankful to the Institute of Pharmacy at PSIT for the laboratory facilities. I am also thankful to the Indian Institute of Technology, Kanpur and Central Drug Research Institute, Lucknow for the laboratory facilities.
References
- Adeghate E., Schattner P., Dunn E. An update on the etiology and epidemiology of diabetes mellitus. Ann. N. Y. Acad. Sci. 2006;1084:1–29. doi: 10.1196/annals.1372.029. [DOI] [PubMed] [Google Scholar]
- Ali A., Ahmad F.J., Pillai K.K., Vohora D. Evidence of the antiepileptic potential of amiloride with neuropharmacological benefits in rodent models of epilepsy and behavior. Epilepsy & behavior. E&B. 2004;5:322–328. doi: 10.1016/j.yebeh.2004.01.005. [DOI] [PubMed] [Google Scholar]
- AlSharari S.D., Al-Rejaie S.S., Abuohashish H.M., Aleisa A.M., Parmar M.Y., Ahmed M.M. Ameliorative potential of morin in streptozotocin-induced neuropathic pain in rats. Trop. J. Pharmaceut. Res. 2014;13:1429–1436. [Google Scholar]
- American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2011;1(34 Suppl):S62–S69. doi: 10.2337/dc11-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Diabetes Association 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2018. Diabetes Care. 2018;41:S13–S27. doi: 10.2337/dc18-S002. [DOI] [PubMed] [Google Scholar]
- Anjaneyulu M., Chopra K. Quercetin attenuates thermal hyperalgesia and cold allodynia in STZ-induced diabetic rats. Indian J. Exp. Biol. 2004;42:766–769. [PubMed] [Google Scholar]
- Arya D., Goel S., Joshi G.C., Sharma O.R., S.K S. Medico-ethno botanical practices among Bhotia tribe of Kumaon himalaya: a case study from Bageshwar district, Uttarkhand, India. J Drug Res Ayurvedic Sci. 2018;3:43–47. [Google Scholar]
- Balodi K.N., Purohit M.V., Shridhar V., Arunachalam K. Ethno-medicinal uses of various plants species among the Jaad Bhotiya community of Uttarakhand, western himalaya. Ethno Med. 2018;18:189–197. [Google Scholar]
- Bennett G.J., Xie Y.K. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- Bonting S.L.J.M.I.T. 1970. Sodium-potassium activated adenosine triphosphatase and cation transport. [Google Scholar]
- Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54:1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- Carter R.J., Lione L.A., Humby T., Mangiarini L., Mahal A., Bates G.P., Dunnett S.B., Morton A.J. Characterization of progressive motor deficits in mice transgenic for the human Huntington's disease mutation. J. Neurosci : Offic.J. Soc Neuroscience. 1999;19:3248–3257. doi: 10.1523/JNEUROSCI.19-08-03248.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra P., Sachan N., Kishore K., Ghosh A.K. Acute, sub-chronic oral toxicity studies and evaluation of antiulcer activity of Sooktyn in experimental animals. "J. Adv. Pharm. Technol. Research"" (JAPTR)". 2012;3:117–123. doi: 10.4103/2231-4040.97290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra P., Sachan N., Pal D. Protective effect of Dalbergia sissoo Roxb. ex DC. (family: fabaceae) leaves against experimentally induced diarrhoea and peristalsis in mice. Toxicol. Ind. Health. 2015;31:1229–1235. doi: 10.1177/0748233713491815. [DOI] [PubMed] [Google Scholar]
- Chandra P., Yadav E., Mani M., Ghosh A.K., Sachan N. Protective effect of Lygodium flexuosum (family: lgodiaceae) against excision, incision and dead space wounds models in experimental rats. Toxicol. Ind. Health. 2015;31:274–280. doi: 10.1177/0748233712471704. [DOI] [PubMed] [Google Scholar]
- Chauhan A. Ethno-medicine of Bhotia tribe in Mana village of Uttarakhand. Int. J. Sociol. Anthropol. 2014;6:296–304. [Google Scholar]
- Chiu S. Peripheral Neuropathy. Elsevier; 2005. Channel function in mammalian axons and support cells; pp. 95–112. [Google Scholar]
- Coppey L.J., Gellett J.S., Davidson E.P., Dunlap J.A., Yorek M.A. Effect of treating streptozotocin-induced diabetic rats with sorbinil, myo-inositol or aminoguanidine on endoneurial blood flow, motor nerve conduction velocity and vascular function of epineurial arterioles of the sciatic nerve. Int. J. Exp. Diabetes Res. 2002;3:21–36. doi: 10.1080/15604280212525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter M.A., Cameron N.E. Effect of the NAD(P)H oxidase inhibitor, apocynin, on peripheral nerve perfusion and function in diabetic rats. Life Sci. 2003;73:1813–1824. doi: 10.1016/s0024-3205(03)00508-3. [DOI] [PubMed] [Google Scholar]
- Cpcsea . Government of India; 2018. Compendium of CPCSEA 2018. Committee for the purpose of control and supervision of experiments on animals. Ministery of environment, Forest and Climate Change; pp. 1–213. [Google Scholar]
- Djordjevic A., Bursac B., Velickovic N., Vasiljevic A., Matic G. The impact of different fructose loads on insulin sensitivity, inflammation, and PSA-NCAM-mediated plasticity in the hippocampus of fructose-fed male rats. Nutr. Neurosci. 2015;18:66–75. doi: 10.1179/1476830513Y.0000000098. [DOI] [PubMed] [Google Scholar]
- Drel V.R., Pacher P., Vareniuk I., Pavlov I.A., Ilnytska O., Lyzogubov V.V., Bell S.R., Groves J.T., Obrosova I.G. Evaluation of the peroxynitrite decomposition catalyst Fe(III) tetra-mesitylporphyrin octasulfonate on peripheral neuropathy in a mouse model of type 1 diabetes. Int. J. Mol. Med. 2007;20:783–792. [PMC free article] [PubMed] [Google Scholar]
- Dyck P.J., Thomas P.K. W.B. Saunders; Philadelphia: 1999. Diabetic Neuropathy. [Google Scholar]
- Gairola S., Sharma J., Bedi Y.S. A cross-cultural analysis of Jammu, Kashmir and Ladakh (India) medicinal plant use. J. Ethnopharmacol. 2014;155:925–986. doi: 10.1016/j.jep.2014.06.029. [DOI] [PubMed] [Google Scholar]
- Garbyal S., Aggarwal K., Babu C. Traditionally used medicinal plants in Dharchula Himalayas of Pithoragarh district, Uttaranchal. Indian J. Tradit Knowledge. 2005;4:199–207. [Google Scholar]
- Genuth S., Alberti K.G., Bennett P., Buse J., Defronzo R., Kahn R., Kitzmiller J., Knowler W.C., Lebovitz H., Lernmark A., Nathan D., Palmer J., Rizza R., Saudek C., Shaw J., Steffes M., Stern M., Tuomilehto J., Zimmet P. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26:3160–3167. doi: 10.2337/diacare.26.11.3160. [DOI] [PubMed] [Google Scholar]
- Gundala N.K., Naidu V.G., Das U.N.J.B. Vol. 496. 2018. Amelioration of streptozotocin-induced type 2 diabetes mellitus in Wistar rats by arachidonic acid; pp. 105–113. communications, b.r. [DOI] [PubMed] [Google Scholar]
- Hirata Y., Okada K. Relation of Na+, K(+)-ATPase to delayed motor nerve conduction velocity: effect of aldose reductase inhibitor, ADN-138, on Na+, K(+)-ATPase activity. Metabolism. 1990;39:563–567. doi: 10.1016/0026-0495(90)90019-9. [DOI] [PubMed] [Google Scholar]
- Howard B.V., Robbins D.C., Sievers M.L., Lee E.T., Rhoades D., Devereux R.B., Cowan L.D., Gray R.S., Welty T.K., Go O.T., Howard W.J. LDL cholesterol as a strong predictor of coronary heart disease in diabetic individuals with insulin resistance and low LDL: the Strong Heart Study. Arterioscler. Thromb. Vasc. Biol. 2000;20:830–835. doi: 10.1161/01.atv.20.3.830. [DOI] [PubMed] [Google Scholar]
- Kamboj S.S., Vasishta R.K., Sandhir R. N-acetylcysteine inhibits hyperglycemia-induced oxidative stress and apoptosis markers in diabetic neuropathy. J. Neurochem. 2010;112:77–91. doi: 10.1111/j.1471-4159.2009.06435.x. [DOI] [PubMed] [Google Scholar]
- Kamei J., Mizoguchi H., Narita M., Tseng L.F. Therapeutic potential of PKC inhibitors in painful diabetic neuropathy. Expet Opin. Invest. Drugs. 2001;10:1653–1664. doi: 10.1517/13543784.10.9.1653. [DOI] [PubMed] [Google Scholar]
- Kanaan S.A., Saade N.E., Haddad J.J., Abdelnoor A.M., Atweh S.F., Jabbur S.J., Safieh-Garabedian B. Endotoxin-induced local inflammation and hyperalgesia in rats and mice: a new model for inflammatory pain. Pain. 1996;66:373–379. doi: 10.1016/0304-3959(96)03068-0. [DOI] [PubMed] [Google Scholar]
- Kar A., Choudhary B.K., Bandyopadhyay N.G. Comparative evaluation of hypoglycaemic activity of some Indian medicinal plants in alloxan diabetic rats. J. Ethnopharmacol. 2003;84:105–108. doi: 10.1016/s0378-8741(02)00144-7. [DOI] [PubMed] [Google Scholar]
- Koneri R.B., Samaddar S., Simi S.M., Rao S.T. Neuroprotective effect of a triterpenoid saponin isolated from Momordica cymbalaria Fenzl in diabetic peripheral neuropathy. Indian J. Pharmacol. 2014;46:76–81. doi: 10.4103/0253-7613.125179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhad A., Chopra K. Tocotrienol attenuates oxidative-nitrosative stress and inflammatory cascade in experimental model of diabetic neuropathy. Neuropharmacology. 2009;57:456–462. doi: 10.1016/j.neuropharm.2009.06.013. [DOI] [PubMed] [Google Scholar]
- Kumar J.U., Santhosh, Chaitanya M.J., Krishna, Semotiuk A.J., Krishna V. Indigenous knowledge on medicinal plants used by ethnic communities of South India. Ethnobotany Res. Applications 18, J. 2019 [Google Scholar]
- Lee E.J., Kim D.I., Kim W.J., Moon S.K. Naringin inhibits matrix metalloproteinase-9 expression and AKT phosphorylation in tumor necrosis factor-alpha-induced vascular smooth muscle cells. Mol. Nutr. Food Res. 2009;53:1582–1591. doi: 10.1002/mnfr.200800210. [DOI] [PubMed] [Google Scholar]
- Levy D., Zochodne D.W. NO pain: potential roles of nitric oxide in neuropathic pain. Pain Practice : Off. J World Institute of Pain. 2004;4:11–18. doi: 10.1111/j.1533-2500.2004.04002.x. [DOI] [PubMed] [Google Scholar]
- Li J., Wei G.H., Huang H., Lan Y.P., Liu B., Liu H., Zhang W., Zuo Y.X. Nerve injury-related autoimmunity activation leads to chronic inflammation and chronic neuropathic pain. Anesthesiology. 2013;118:416–429. doi: 10.1097/ALN.0b013e31827d4b82. [DOI] [PubMed] [Google Scholar]
- Malik Z.A., Bhat J.A., Ballabha R., Bussmann R.W., Bhatt A.B. Ethnomedicinal plants traditionally used in health care practices by inhabitants of Western Himalaya. J. Ethnopharmacol. 2015;172:133–144. doi: 10.1016/j.jep.2015.06.002. [DOI] [PubMed] [Google Scholar]
- Mattila P., Hellström J. Phenolic acids in potatoes, vegetables, and some of their products. J. Food Compos. Anal. 2007;20:152–160. [Google Scholar]
- McNeely M.J., Boyko E.J., Ahroni J.H., Stensel V.L., Reiber G.E., Smith D.G., Pecoraro R.F. The independent contributions of diabetic neuropathy and vasculopathy in foot ulceration. How great are the risks? Diabetes Care. 1995;18:216–219. doi: 10.2337/diacare.18.2.216. [DOI] [PubMed] [Google Scholar]
- Mehra P.N., Jolly S.S. The identity and pharmacognosy of the adulterant of Nardostachzys jatamansi DC. Planta Med. 1963;11:8–15. [Google Scholar]
- Miranda K.M., Espey M.G., Wink D.A. A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide : Biol. Chem. 2001;5:62–71. doi: 10.1006/niox.2000.0319. [DOI] [PubMed] [Google Scholar]
- Misra H.P., Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972;247:3170–3175. [PubMed] [Google Scholar]
- Mohamed E.A., Lim C.P., Ebrika O.S., Asmawi M.Z., Sadikun A., Yam M.F. Toxicity evaluation of a standardised 50% ethanol extract of Orthosiphon stamineus. J. Ethnopharmacol. 2011;133:358–363. doi: 10.1016/j.jep.2010.10.008. [DOI] [PubMed] [Google Scholar]
- Mohan H. eighth ed. Jaypee Brothers Medical Publishers; 2018. Textbook of pathology. [Google Scholar]
- Nand K., Naithani S. Ethnobotanical uses of wild medicinal plants by the local community in the Asi Ganga Sub-basin, Western Himalaya. J Complement Med Res. 2018;9:34. [Google Scholar]
- Nasiry D., Khalatbary A.R., Ahmadvand H., Talebpour Amiri F., Akbari E. Protective effects of methanolic extract of Juglans regia L. leaf on streptozotocin-induced diabetic peripheral neuropathy in rats. BMC Compl. Alternative Med. 2017;17:476. doi: 10.1186/s12906-017-1983-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro-Gonzalez J.F., Mora-Fernandez C. The role of inflammatory cytokines in diabetic nephropathy. J. Am Soc Nephrol : JASN (J. Am. Soc. Nephrol.) 2008;19:433–442. doi: 10.1681/ASN.2007091048. [DOI] [PubMed] [Google Scholar]
- Nikita S., Neetu S., Phool C. A review on ethnobotanical, phytochemical, pharmacological and traditional aspects of indigenous Indian herb Trachyspermum ammi (L) Current Traditional Medicine. 2020;6:172–187. [Google Scholar]
- Oecd . France; Paris: 2001. OECD Guideline 423: Acute Oral Toxicity - Acute Toxic Class Method. OECD guideline for the testing of chemicals. Organization for Economic Cooperation and Development; pp. 1–14. [Google Scholar]
- Pal D., Mishra P., Sachan N., Ghosh A.K. Biological activities and medicinal properties of Cajanus cajan (L) Millsp. "J. Adv. Pharm. Technol. Research"" (JAPTR)". 2011;2:207–214. doi: 10.4103/2231-4040.90874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey M.M., Katara A., Pandey G., Rastogi S., Rawat A.K. An important Indian traditional drug of ayurveda jatamansi and its substitute bhootkeshi: chemical profiling and antioxidant activity. Evid Based Complement Alternat Med. 2013;2013:142517. doi: 10.1155/2013/142517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parasuraman S. Toxicological screening. J. Pharmacol. Pharmacother. 2011;2:74–79. doi: 10.4103/0976-500X.81895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radha, Puri S. Study of wild medicinal plants used by tribal migratory shepherds in hills of shimla district, Himachal Pradesh. Plant Archives. 2019;19:785–790. [Google Scholar]
- Radha Puri S., Pundir A. Survey of wild medicinal plants used by migratory shepherds in summer hill of district Shimla in Himachal Pradesh. Bio Bulletin. 2019;5:18–24. [Google Scholar]
- Rana C.S., Sharma A., Kumar N., Dangwal L.R., Tiwari J.K. Ethnopharmacology of some important medicinal plants of Nanda Devi national park (NDNP) Uttarakhand, India. Nat. Sci. 2010;8:9–14. [Google Scholar]
- Rana C.S., Tiwari J.K., Dangwal L.R., Gairola S. Faith herbal healer knowledge document of Nanda Devi Biosphere reserve, Uttarakhand, India. Indian J. Traditional Knowledge. 2013;12:308–314. [Google Scholar]
- Rana D., Bhatt A., Lal B. Ethnobotanical knowledge among the semi-pastoral Gujjar tribe in the high altitude (Adhwari's) of Churah subdivision, district Chamba, Western Himalaya. J. Ethnobiol. Ethnomed. 2019;15:10. doi: 10.1186/s13002-019-0286-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rana M.S., Samant S.S. Diversity, indigenous uses and conservation status of medicinal plants in manali wildlife sanctuary, north western himalaya. Indian J. Tradit Knowledge. 2011;10:439–459. [Google Scholar]
- Rang H.P., Dale M.M., Ritter J.M., Flower R.J., Henderson G. seventh ed. Churchill Livingstone; London: 2012. Rang and Dale’s Pharmacology. [Google Scholar]
- Reiber G.E., Vileikyte L., Boyko E.J., del Aguila M., Smith D.G., Lavery L.A., Boulton A.J. Causal pathways for incident lower-extremity ulcers in patients with diabetes from two settings. Diabetes Care. 1999;22:157–162. doi: 10.2337/diacare.22.1.157. [DOI] [PubMed] [Google Scholar]
- Rice-Evans C.A., Miller N.J., Paganga G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radical Biol. Med. 1996;20:933–956. doi: 10.1016/0891-5849(95)02227-9. [DOI] [PubMed] [Google Scholar]
- Saraswat N., Chandra P., Sachan N. A deep insight on diabetic neuropathy: the silent complication of diabetes, with inputs on its causes, diagnosis, pathways, and treatments. Asian J. Pharmaceut. Clin. Res. 2018;11:112–119. [Google Scholar]
- Satoh J., Yagihashi S., Toyota T. The possible role of tumor necrosis factor-alpha in diabetic polyneuropathy. Exp. Diabesity Res. 2003;4:65–71. doi: 10.1155/EDR.2003.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semwal D., Saradhi P.P., Kala C., Sajwan B. Medicinal plants used by local Vaidyas in Ukhimath block, Uttarakhand. Indian J. Tradit Knowledge. 2010;9:480–485. [Google Scholar]
- Seshadri T.R., Sood M.S. Chemical comparison of the roots of Selinum vaginatum and Nardostachys jatamansi. Phytochemistry. 1967;6:445–446. [Google Scholar]
- Sharma J., Gairola S., Gaur R.D., Painuli R.M., Siddiqi T.O. Ethnomedicinal plants used for treating epilepsy by indigenous communities of sub-Himalayan region of Uttarakhand, India. J. Ethnopharmacol. 2013;150:353–370. doi: 10.1016/j.jep.2013.08.052. [DOI] [PubMed] [Google Scholar]
- Sharma P., Samant S.S. Diversity, distribution and indigenous uses of medicinal plants in Parbati valley of kullu district in Himachal Pradesh, northwestern himalaya. Asian J Adv Basic Sci. 2014;2:77–98. [Google Scholar]
- Sharma P.K., Chauhan N.S., Lal B. Observations on the traditional phytotherapy among the inhabitants of Parvati valley in western Himalaya, India. J. Ethnopharmacol. 2004;92:167–176. doi: 10.1016/j.jep.2003.12.018. [DOI] [PubMed] [Google Scholar]
- Sharma P.K., Thakur S.K., Manuja S., Rana R.K., Kumar P., Sharma S., Chand J., Singh A., Katoch K.K. Observations on traditional phytotherapy among the inhabitants of Lahaul valley through amchi system of medicine—a cold desert area of Himachal Pradesh in north western Himalayas, India. Chin. Med. 2011;2:11. [Google Scholar]
- Singh A., Lal M., Samant S.S. Diversity, indigenous uses and conservation prioritization of medicinal plants in Lahaul valley, proposed Cold Desert Biosphere Reserve, India. Int. J. Biodivers. Sci. Manag. 2009;5:132–154. [Google Scholar]
- Singleton J.R., Smith A.G., Bromberg M.B. Increased prevalence of impaired glucose tolerance in patients with painful sensory neuropathy. Diabetes Care. 2001;24:1448–1453. doi: 10.2337/diacare.24.8.1448. [DOI] [PubMed] [Google Scholar]
- Slater T.F., Sawyer B.C. The stimulatory effects of carbon tetrachloride and other halogenoalkanes on peroxidative reactions in rat liver fractions in vitro. General features of the systems used. Biochem. J. 1971;123:805–814. doi: 10.1042/bj1230805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur D., Sharma A., Uniyal S.K. Why they eat, what they eat: patterns of wild edible plants consumption in a tribal area of Western Himalaya. J. Ethnobiol. Ethnomed. 2017;13:70. doi: 10.1186/s13002-017-0198-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorup C., Ollerstam A., Persson A.E., Torffvit O. Increased tubuloglomerular feedback reactivity is associated with increased NO production in the streptozotocin-diabetic rat. J. Diabetes Complicat. 2000;14:46–52. doi: 10.1016/s1056-8727(00)00056-8. [DOI] [PubMed] [Google Scholar]
- Tiwari R., Rana C. Phytomedicine for the diabetes : a traditional approach. Ann. Phytomed. 2015;4:108–110. [Google Scholar]
- Tiwari V., Kuhad A., Chopra K. Emblica officinalis corrects functional, biochemical and molecular deficits in experimental diabetic neuropathy by targeting the oxido-nitrosative stress mediated inflammatory cascade. Phytother Res. 2011;25:1527–1536. doi: 10.1002/ptr.3440. [DOI] [PubMed] [Google Scholar]
- Valcheva-Kuzmanova S., Georgieva A., Belcheva I., Tashev R. Anxiolytic-like effect of chlorogenic acid administered subchronically to rats. Comptes rendus de l'Académie bulgare des sciences : sciences mathématiques et naturelles. 2015;68:1463–1470. [Google Scholar]
- van Dam P.S. Oxidative stress and diabetic neuropathy: pathophysiological mechanisms and treatment perspectives. Diabetes/Metabolism Research Reviews. 2002;18:176–184. doi: 10.1002/dmrr.287. [DOI] [PubMed] [Google Scholar]
- Vinagre A.S., Rönnau Â.D.S.R.O., Pereira S.F., Silveira L.U.D., Wiilland E.d.F., Suyenaga E.S. Anti-diabetic effects of Campomanesia xanthocarpa (Berg) leaf decoction. Braz. J. Pharm. Sci. 2010;46:169–177. [Google Scholar]
- Williams F., Saffran M. Insulin in the drinking water of rats. Drug Deliv. 1996;3:81–85. [Google Scholar]
- Yadav E., Mani M., Chandra P., Sachan N., Ghosh A.K. A review on therapeutic potential of Lygodium flexuosum Linn. Pharm. Rev. 2012;6:107–114. doi: 10.4103/0973-7847.99944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaman T., Uyar A., Celik I., Alkan E.E., Keles O.F., Yener Z. Histopathological and immunohistochemical study of antidiabetic effects of Heracleum persicum extract İn experimentally diabetic rats. Indian J. Pharmaceutical Educ. Res. 2017;51:s450–s457. [Google Scholar]
- Zhang Q.W., Lin L.G., Ye W.C. Techniques for extraction and isolation of natural products: a comprehensive review. Chin. Med. 2018;13:20. doi: 10.1186/s13020-018-0177-x. [DOI] [PMC free article] [PubMed] [Google Scholar]