Abstract
Background
The relationship between triglyceride (TG) level and the mortality risk of all-cause and cardiovascular disease is not entirely consistent among adults.
Methods
The present analysis included adult participants from National Health and Nutrition Examination Surveys (NHANES) between the periods 1999–2014. The levels of TG were categorized into < 150, 150–199, 200–250 and ≥ 250 mg/dL respectively. Multivariate Cox regression analysis, stratified analysis and generalized additive model were conducted to reveal the correlation between TG and mortality risk. Results were presented in hazard ratio (HRs) and 95% confidence intervals (CIs).
Results
There were 18,781 (9130 males, mean age was 45.64 years) participants being included in the analysis. The average follow-up period was 8.25 years, where 1992 (10.61%) cases of all-cause and 421 (2.24%) cardiovascular death have occurred. In the multivariate Cox model, every 1 mg/dL raise in TG has significantly associated with all-cause mortality (HR: 1.08, 95% CI: 1.02, 1.15) but not cardiovascular mortality (HR: 1.10, 95% CI: 0.97, 1.24). When using TG < 150 mg/dL as reference, TG ≥ 250 mg/dL associated with death from all-cause (HR = 1.34, 95% CI: 1.12, 1.60; P = 0.0016 but not cardiovascular death (HR = 1.26, 95% CI: 0.85, 1.88; P = 0.2517). According to smoothing spline plots, the risk of all-cause was the lowest when TG was approximately 135 mg/dL.
Conclusion
TG might have a dose-independent association with all-cause mortality among adults in United States.
Keywords: Triglyceride, All-cause mortality, Cardiovascular mortality, Adult population, Nonlinear, Dose-independent
Introduction
Multiple epidemiological and clinical studies have reported the linkage between elevated triglyceride (TG) concentrations and cardiovascular diseases (CVD) [1–5]. The Bezafibrate Infarction Prevention Registry study revealed that elevated TG associated with higher mortality risk in patients with coronary heart disease [6]. More recently, a meta-analysis of 61 cohorts demonstrated relationship between TG levels and CVD in a dose-response manner [7]. Another meta-regression analysis of 49 randomized trials has found that lowering TG could reduce the risk of major vascular events, which was independent from the levels of circulating low density lipoprotein cholesterol (LDL-C) [8]. However, the relation between TG and mortality was often attenuated after being adjusting for cardiovascular risk factors. Currently, whether triglyceride concentration was independently related to all-cause or cause-specific mortality has been controversial. Davis, et al. [9] suggested that the levels of TG had no independent association with mortality due to coronary disease. Khawaja et al. [10] demonstrated that lower TG (TG < 200 mg/dL) at admission was associated with a higher risk of mortality in participants with myocardial infarction. Considering the discrepancies in findings, the prospective relationship between serum TG levels and mortality was explored among adults in United States (US).
Methods
Subjects enrollment
The data source was from National Health and Nutrition Examination Surveys (NHANES) between the periods 1999–2014. NHANES was a nationwide study conducted by Centers for Disease Control and Prevention (CDC) in United States. Serum TG was determined among NHANES participants aged ≥18 years old. After excluding participants younger than 18, people with missing serum TG levels or with cancer at baseline, 18,781 participants were included (Fig. 1). The Institutional Review Board of the CDC has approved the protocol of survey (Protocol 98–12, 2005–06 and 2011–17). Participants provided informed consent in written form before the start of the study.
Fig. 1.
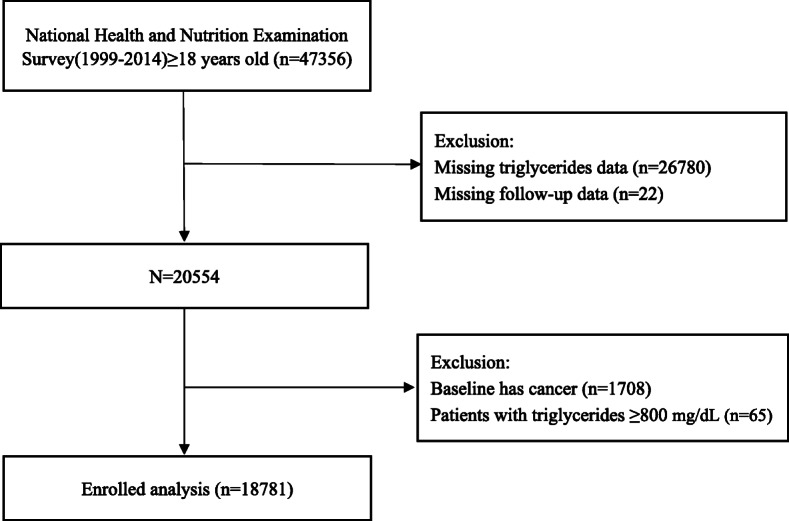
Research flow chart
Assessment of exposure
Standardized procedures and methods were used for collecting serum samples. Serum samples were collected from venous vessels in the morning. Serum circulating TG and total cholesterol (TC) levels were measured enzymatically, while direct immunoassay or precipitation was used to determine the level of high-density lipoprotein cholesterol (HDL-C) [11]. Hitachi 704 Analyzer (Boehringer Mannheim Diagnostics, Indianapolis, IN) was used to measure serum TC, HDL-C and TG [12]. If TG was ≤400 mg/dL, Friedewald formula was adapted to calculate the value of LDL-C [13].
Assessment of covariates
At baseline, socio-demographics and lifestyle factors (including age, race/ethnicity, gender, smoking status, diet habits and education levels), data on physical examination (height, weight, blood pressure, estimated glomerular filtration rate (eGFR)), disease history and medication history were acquired. Hypertension was defined by self-reported history, systolic/diastolic blood pressure (SBP/DBP) ≥ 140/90 mmHg, or taking drugs to reduce blood pressure [14]. Diabetes was defined by self-reported history, taking hypoglycemic medications, fasting serum glucose level ≥ 7.0 mmol/L or hemoglobin A1C ≥ 6.5% [15].
Assessment of outcome
The outcomes of this study were death from all-cause and CVD. Mortality status was ascertained from NHANES until death or 31st December 2015, whichever occurred first. Codes in the 10th edition of the International Classification of Diseases including I00-I09, I20-I51, I11 and I13 were used to derive the cause of CVD death [16].
Statistical analyses
Descriptive statistics was presented according to the levels of TG (< 150, 150–200, 200–249, ≥ 250 mg/dL) at baseline. Differences by TG levels were explored by one-way analysis of variance, Kruscal Whallis H test and chi-square tests. Multivariate Cox regression models with hazard ratios (HRs) and 95% confidence intervals (CIs) were built to assess the death from all-cause and CVD. Model I was crude model and no confounders were included. In Model II, age, sex and body mass index (BMI) were adjusted. In Model III, race, level of education, smoking status, SBP, energy intake, C-reactive protein, eGFR, TC, HDL-C, disease history and the use of medication were additionally adjusted. Differences in survival rates by TG levels were analyzed by Kaplan-Meier curves. Restricted cubic spline analysis was used to reveal how serum TG might relate to all-cause and CVD mortality. A generalized additive model was used to assess any nonlinear relationship. If a nonlinear relationship was detected, Cox proportional hazards models were built on both sides of the inflection point. Results from Cox regression were stratified by age at baseline (< 65 or ≥ 65 years), sex (man or woman), ethnicity (White or non-White), BMI categories (< 25 or ≥ 25 kg/m2), history of diabetes or hypertension, history of CVD and the use of lipid-lowering drugs (all categorized into yes or no). Statistical analyses were performed by R software with the version of 3.3.2 (Vienna, Austria) and P < 0.05 was regarded as statistically significant.
Results
Baseline characteristics
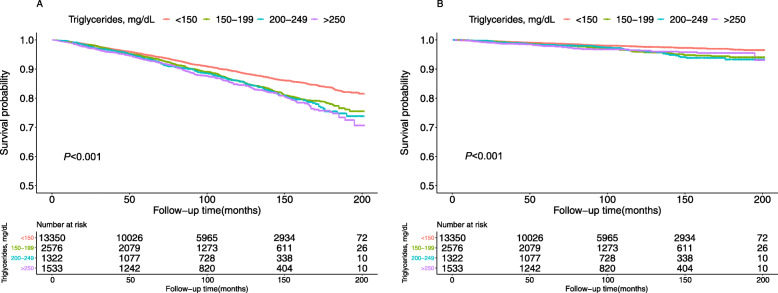
The present study included 18,781 participants (9130 males, mean age was 45.64 years). During the average follow-up period of 8.25 years, 1992 (10.61%) all-cause and 421 (2.24%) cardiovascular death occurred. Table 1 has summarized the characteristics of participants at baseline. There were significant subgroup differences for all baseline variables according to TG levels. The differences in survival rate of all-cause (Fig. 2a) and cardiovascular (Fig. 2b) mortality according to the levels of TG were demonstrated in Fig. 2.
Table 1.
Demographic and clinical characteristics according to triglyceride levels
| Characteristic | Triglycerides, mg/dL | P-value | ||||
|---|---|---|---|---|---|---|
| Total | < 150 | 150–199 | 200–250 | ≥ 250 | ||
| Number | 18,781 | 13,350 | 2576 | 1322 | 1533 | |
| Age, years | 45.64 ± 18.94 | 44.30 ± 19.15 | 49.09 ± 18.45 | 49.63 ± 18.23 | 48.12 ± 16.92 | < 0.001 |
| Gender, n (%) | < 0.001 | |||||
| Male | 9130 (48.61) | 6246 (46.79) | 1292 (50.16) | 726 (54.92) | 866 (56.49) | |
| Female | 9651 (51.39) | 7104 (53.21) | 1284 (49.84) | 596 (45.08) | 667 (43.51) | |
| Race, n (%) | < 0.001 | |||||
| Non-white | 10,628 (56.59) | 7806 (58.47) | 1367 (53.07) | 675 (51.06) | 780 (50.88) | |
| White | 8153 (43.41) | 5544 (41.53) | 1209 (46.93) | 647 (48.94) | 753 (49.12) | |
| Smoking, n (%) | < 0.001 | |||||
| No | 9463 (54.54) | 6953 (57.31) | 1201 (48.86) | 619 (48.59) | 690 (46.43) | |
| Yes | 7887 (45.46) | 5179 (42.69) | 1257 (51.14) | 655 (51.41) | 796 (53.57) | |
| Education level, n (%) | < 0.001 | |||||
| Less than high school | 5010 (29.15) | 3226 (26.90) | 829 (33.93) | 437 (34.46) | 518 (34.91) | |
| High school or above | 12,177 (70.85) | 8766 (73.10) | 1614 (66.07) | 831 (65.54) | 966 (65.09) | |
| Body mass index, kg/m2 | 28.46 ± 6.68 | 27.75 ± 6.78 | 29.94 ± 6.26 | 30.29 ± 6.08 | 30.58 ± 5.78 | < 0.001 |
| Systolic blood pressure, mmHg | 122.53 ± 19.03 | 121.15 ± 18.72 | 124.98 ± 19.31 | 126.52 ± 19.49 | 126.96 ± 19.23 | < 0.001 |
| Diastolic blood pressure, mmHg | 68.88 ± 13.25 | 68.22 ± 12.88 | 70.26 ± 13.41 | 70.28 ± 14.15 | 71.12 ± 14.73 | < 0.001 |
| Energy, kcal | 2149.28 ± 1020.81 | 2146.82 ± 1032.72 | 2112.49 ± 991.50 | 2144.78 ± 957.29 | 2237.06 ± 1015.15 | 0.003 |
| eGFR, mg/min/1.73m2 | 92.49 ± 29.80 | 93.07 ± 28.43 | 90.16 ± 31.21 | 91.43 ± 34.66 | 92.23 ± 34.06 | < 0.001 |
| C-reactive protein, mg/L | 0.45 ± 0.86 | 0.42 ± 0.87 | 0.51 ± 0.97 | 0.51 ± 0.66 | 0.53 ± 0.72 | < 0.001 |
| Triglycerides | ||||||
| mg/dL | 131.38 ± 87.24 | 89.44 ± 30.20 | 171.66 ± 14.46 | 222.04 ± 14.32 | 350.73 ± 107.34 | < 0.001 |
| mmol/L | 1.48 ± 0.98 | 1.01 ± 0.34 | 1.94 ± 0.16 | 2.51 ± 0.16 | 3.96 ± 1.21 | < 0.001 |
| Low density lipoprotein cholesterol | ||||||
| mg/dL | 114.90 ± 35.92 | 111.57 ± 33.69 | 125.44 ± 37.33 | 125.36 ± 40.70 | 118.22 ± 44.07 | < 0.001 |
| mmol/L | 2.97 ± 0.93 | 2.89 ± 0.87 | 3.24 ± 0.97 | 3.24 ± 1.05 | 3.06 ± 1.14 | < 0.001 |
| Total cholesterol | ||||||
| mg/dL | 194.24 ± 42.18 | 185.88 ± 38.09 | 207.80 ± 40.78 | 215.37 ± 44.77 | 226.00 ± 48.30 | < 0.001 |
| mmol/L | 5.02 ± 1.09 | 4.81 ± 0.99 | 5.37 ± 1.05 | 5.57 ± 1.16 | 5.84 ± 1.25 | < 0.001 |
| High density lipoprotein cholesterol | ||||||
| mg/dL | 53.33 ± 15.65 | 56.43 ± 15.46 | 48.01 ± 13.17 | 45.95 ± 13.05 | 41.73 ± 12.96 | < 0.001 |
| mmol/L | 1.38 ± 0.40 | 1.46 ± 0.40 | 1.24 ± 0.34 | 1.19 ± 0.34 | 1.08 ± 0.34 | < 0.001 |
| Diabetes, n (%) | < 0.001 | |||||
| No | 15,898 (84.68) | 11,739 (87.95) | 2066 (80.26) | 1006 (76.15) | 1087 (70.91) | |
| Yes | 2877 (15.32) | 1608 (12.05) | 508 (19.74) | 315 (23.85) | 446 (29.09) | |
| Hypertension, n (%) | < 0.001 | |||||
| No | 11,387 (60.70) | 8587 (64.38) | 1384 (53.83) | 678 (51.32) | 738 (48.20) | |
| Yes | 7374 (39.30) | 4751 (35.62) | 1187 (46.17) | 643 (48.68) | 793 (51.80) | |
| Cardiovascular disease, n (%) | < 0.001 | |||||
| No | 15,712 (91.26) | 11,076 (92.22) | 2197 (89.64) | 1118 (88.03) | 1321 (88.96) | |
| Yes | 1504 (8.74) | 934 (7.78) | 254 (10.36) | 152 (11.97) | 164 (11.04) | |
| Antihypertensive drugs, n (%) | < 0.001 | |||||
| No | 14,358 (76.45) | 10,557 (79.08) | 1835 (71.23) | 913 (69.06) | 1053 (68.69) | |
| Yes | 4423 (23.55) | 2793 (20.92) | 741 (28.77) | 409 (30.94) | 480 (31.31) | |
| Hypoglycemic agents, n (%) | < 0.001 | |||||
| No | 17,309 (92.16) | 12,516 (93.75) | 2328 (90.37) | 1167 (88.28) | 1298 (84.67) | |
| Yes | 1472 (7.84) | 834 (6.25) | 248 (9.63) | 155 (11.72) | 235 (15.33) | |
| Lipid-lowering drugs, n (%) | < 0.001 | |||||
| No | 16,560 (88.17) | 11,956 (89.56) | 2193 (85.13) | 1113 (84.19) | 1298 (84.67) | |
| Yes | 2221 (11.83) | 1394 (10.44) | 383 (14.87) | 209 (15.81) | 235 (15.33) | |
| Antiplatelet drugs, n (%) | < 0.001 | |||||
| No | 18,460 (98.29) | 13,153 (98.52) | 2508 (97.36) | 1292 (97.73) | 1507 (98.30) | |
| Yes | 321 (1.71) | 197 (1.48) | 68 (2.64) | 30 (2.27) | 26 (1.70) | |
| Cardiovascular mortality, n (%) | < 0.001 | |||||
| No | 18,360 (97.76) | 13,108 (98.19) | 2493 (96.78) | 1275 (96.44) | 1484 (96.80) | |
| Yes | 421 (2.24) | 242 (1.81) | 83 (3.22) | 47 (3.56) | 49 (3.20) | |
| All-cause mortality, n (%) | < 0.001 | |||||
| No | 16,789 (89.39) | 12,114 (90.74) | 2243 (87.07) | 1131 (85.55) | 1301 (84.87) | |
| Yes | 1992 (10.61) | 1236 (9.26) | 333 (12.93) | 191 (14.45) | 232 (15.13) | |
Note: Subgroup differences were tested by one-way analysis of variance for continuous variables and by chi-square for categorical variables
Values are mean ± standardized differences or number (%)
n number; eGFR estimated glomerular filtration rate
Fig. 2.
Kaplan-Meier survival curves for all-cause (a) and cardiovascular (b) mortality
The relationship between triglyceride and mortality
As revealed in Table 2, for every 1 mmol/L increment in TG, TG (Model 3 HR = 1.08, 95%CI: 1.02, 1.15; P = 0.0085) was significantly associated with all-cause mortality, but not for cardiovascular mortality (Model 3 HR = 1.10, 95%CI: 0.97, 1.24; P = 0.1482). When using TG < 150 mg/dL as referent, the HRs for all-cause death were 0.97 (0.84, 1.12), 1.06 (0.89, 1.27) and 1.34 (1.12, 1.60) for TG levels at 150–200, 200–249 and ≥ 250 mg/dL in Model 3, respectively. (P for trend = 0.0070). Similarly, the HRs for death due to CVD were 1.01 (0.75, 1.37), 1.19 (0.82, 1.71) and 1.26 (0.85, 1.88) (P for trend = 0.2030), respectively.
Table 2.
Multivariate Cox regression analysis of triglyceride levels with mortality in different models
| Model I HR (95%CI), P-value |
Model II HR (95%CI), P-value |
Model III HR (95%CI), P-value |
|
|---|---|---|---|
| All-cause mortality | |||
| Triglycerides (per mmol/L increment) | 1.17 (1.13, 1.21) < 0.0001 | 1.07 (1.02, 1.12) 0.0021 | 1.08 (1.02, 1.15) 0.0085 |
| Triglycerides groups, mg/dL | |||
| < 150 | 1.0 | 1.0 | 1.0 |
| 150–200 | 1.31 (1.16, 1.48) < 0.0001 | 1.00 (0.89, 1.13) 0.9414 | 0.97 (0.84, 1.12) 0.7249 |
| 200–249 | 1.39 (1.19, 1.62) < 0.0001 | 1.03 (0.88, 1.20) 0.7255 | 1.06 (0.89, 1.27) 0.5172 |
| ≥ 250 | 1.48 (1.28, 1.70) < 0.0001 | 1.32 (1.14, 1.51) 0.0001 | 1.34 (1.12, 1.60) 0.0016 |
| P for trend | < 0.001 | 0.002 | 0.007 |
| Cardiovascular mortality | |||
| Triglycerides (per mmol/L increment) | 1.22 (1.13, 1.31) < 0.0001 | 1.16 (1.06, 1.27) 0.0009 | 1.10 (0.97, 1.24) 0.1482 |
| Triglycerides groups, mg/dL | |||
| < 150 | 1.0 | 1.0 | 1.0 |
| 150–200 | 1.67 (1.30, 2.14) < 0.0001 | 1.28 (1.00, 1.65) 0.0504 | 1.01 (0.75, 1.37) 0.9349 |
| 200–249 | 1.77 (1.29, 2.41) 0.0004 | 1.31 (0.96, 1.79) 0.0909 | 1.19 (0.82, 1.71) 0.3544 |
| ≥ 250 | 1.61 (1.18, 2.18) 0.0025 | 1.53 (1.12, 2.08) 0.0068 | 1.26 (0.85, 1.88) 0.2517 |
| P for trend | < 0.001 | 0.002 | 0.203 |
Notes: Multivariate Cox regression was performed to examine the association between triglyceride levels and mortality
Data are shown in HRs and 95%CI
HR hazard ratios; CI confidence intervals
Model I adjust for none
Model II adjust for age, gender and BMI
Model III adjust for age, gender, race, education level, smoking, body mass index, systolic blood pressure, estimated glomerular filtration rate, energy, C-reactive protein, total cholesterol, high density lipoprotein cholesterol, hypertension, diabetes, and medicine using (antihypertensive drugs, hypoglycemic agents, lipid-lowering drugs, and antiplatelet drugs)
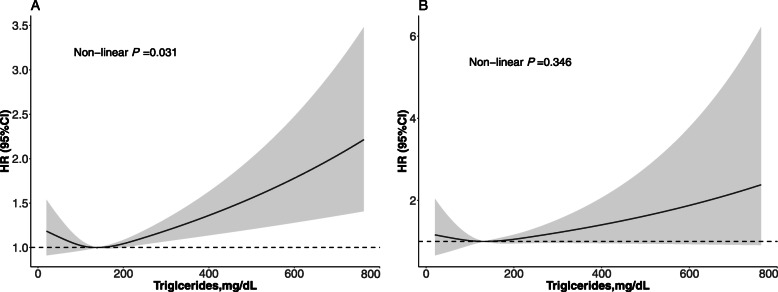
As demonstrated in Table 3, the cut-off values of TG to predict death due to all-cause and cardiovascular diseases were 1.52 mmol/L (135 mg/dL) and 1.10 mmol/L (97 mg/dL), respectively. Below the cut-off value, the HRs for of all-cause and CVD death were 0.87 (95%CI: 0.71, 1.06) and 0.59 (95%CI: 0.25, 1.39) for every 1 mmol/L elevation in the level of serum TG. Above the cut-off value of TG levels, the HRs for of all-cause and CVD death were 1.12 (95%CI: 1.05, 1.19) and 1.12 (95%CI: 0.99, 1.27) for every 1 mmol/L elevation in the level of serum TG, respectively. The risk of death due to all-cause was the lowest when TG was approximately 135 mg/dL. Results from smoothing spline plots suggested that TG was linked with all-cause death in dose-independent manner (Fig. 3a), but it was close to a linear relationship with cardiovascular mortality (Fig. 3b).
Table 3.
The results of two-piecewise linear regression model between triglyceride and mortality
| All-cause mortality HR (95% CI) P-value |
Cardiovascular disease mortality HR (95% CI) P-value |
|
|---|---|---|
| Cutoff value, mmol/L | 1.52 mmol/L (135 mg/dL) | 1.10 mmol/L (97 mg/dL) |
| < Cut-off value | 0.87 (0.71, 1.06) 0.1656 | 0.59 (0.25, 1.39) 0.2247 |
| ≥ Cut-off value | 1.12 (1.05, 1.19) 0.0006 | 1.12 (0.99, 1.27) 0.0747 |
| P for log likelihood ratio test | 0.026 | 0.159 |
Notes: Multivariate linear regression was performed to examine the association between triglyceride levels and mortality
Data are shown in HRs and 95%CI
HR hazard ratios; CI confidence intervals
Effect: age, gender, race, education level, smoking, body mass index, systolic blood pressure, estimated glomerular filtration rate, energy, C-reactive protein, total cholesterol, high density lipoprotein cholesterol, hypertension, diabetes, and medicine using (antihypertensive drugs, hypoglycemic agents, lipid-lowering drugs, and antiplatelet drugs)
Fig. 3.
Adjusted spline curves analyze for the association of triglyceride with all-cause (a) and cardiovascular mortality. Age, gender, race, education level, smoking, body mass index, systolic blood pressure, estimated glomerular filtration rate, energy, C-reactive protein, total cholesterol, high density lipoprotein cholesterol, hypertension, diabetes, and medicine using (antihypertensive drugs, hypoglycemic agents, lipid-lowering drugs, and antiplatelet drugs)were all adjusted
Subgroup analysis
Table 4 has summarized the results of subgroup analysis. When TG ≥ 135 mg/dL, TG independently associated with all-cause death in subjects aged ≥65 years (HR: 1.09, 95%CI: 0.99, 1.19), males (HR: 1.14, 95%CI: 1.05, 1.23), people with BMI ≥ 25 kg/m2 (HR: 1.11, 95%CI: 1.03, 1.19),White population (HR: 1.16, 95%CI: 1.07, 1.27), people without taking lipid-lowering drugs (HR: 1.12, 95%CI: 1.05, 1.20), people without diabetes (HR: 1.11, 95%CI: 1.03, 1.19), people with hypertension (HR: 1.14, 95%CI: 1.06, 1.22), people with diabetes (HR: 1.11, 95%CI: 1.01, 1.22), and people with CVD (HR: 1.11, 95%CI: 1.03, 1.19). However, when TG was ≥97 mg/dL, TG associated with cardiovascular mortality in subjects with BMI < 25 kg/m2 (HR: 1.44, 95%CI: 1.09, 1.90), males (HR: 1.21, 95%CI: 1.05, 1.40), people with hypertension (HR: 1.25, 95%CI: 1.07, 1.46) and people with CVD (HR: 1.26, 95%CI: 1.04, 1.52).
Table 4.
Subgroup analysis of triglycerides levels with mortality
| Number | All-cause mortality HR (95% CI) P-value |
P for log likelihood ratio test | Cardiovascular disease mortality HR (95% CI) P-value |
P for log likelihood ratio test | |||
|---|---|---|---|---|---|---|---|
| Cutoff value, mmol/L | < 1.52 | ≥ 1.52 | < 1.10 | ≥ 1.10 | |||
| Age, years | |||||||
| ≥ 65 | 2502 | 0.78 (0.60, 1.01) 0.0558 | 1.09 (0.99, 1.19) 0.0654 | 0.024 | 0.54 (0.19, 1.51) 0.2400 | 1.00 (0.84, 1.19) 0.9968 | 0.267 |
| < 65 | 9135 | 1.21 (0.87, 1.68) 0.2640 | 1.05 (0.95, 1.15) 0.3327 | 0.436 | 1.49 (0.30, 7.35) 0.6273 | 1.15 (0.96, 1.38) 0.1206 | 0.757 |
| Gender | |||||||
| Male | 5659 | 0.91 (0.69, 1.19) 0.4874 | 1.14 (1.05, 1.23) 0.0016 | 0.136 | 0.85 (0.29, 2.50) 0.7651 | 1.21 (1.05, 1.40) 0.0096 | 0.527 |
| Female | 5978 | 0.89 (0.65, 1.22) 0.4596 | 1.08 (0.97, 1.20) 0.1491 | 0.277 | 0.44 (0.10, 2.00) 0.2873 | 0.92 (0.72, 1.18) 0.5239 | 0.367 |
| Body mass index, kg/m2 | |||||||
| ≥ 25 | 8102 | 0.90 (0.70, 1.16) 0.4312 | 1.11 (1.03, 1.19) 0.0036 | 0.143 | 0.70 (0.22, 2.19) 0.5437 | 1.09 (0.94, 1.26) 0.2783 | 0.471 |
| < 25 | 3535 | 0.78 (0.54, 1.12) 0.1821 | 1.23 (1.03, 1.47) 0.0244 | 0.050 | 0.43 (0.11, 1.67) 0.2241 | 1.44 (1.09, 1.90) 0.0095 | 0.105 |
| Race | |||||||
| Non-white | 6114 | 1.00 (0.74, 1.35) 0.9952 | 1.05 (0.96, 1.16) 0.2861 | 0.757 | 0.34 (0.10, 1.11) 0.0738 | 1.17 (0.98, 1.39) 0.0916 | 0.054 |
| White | 5523 | 0.79 (0.60, 1.05) 0.1088 | 1.16 (1.07, 1.27) 0.0007 | 0.016 | 1.08 (0.30, 3.96) 0.9055 | 1.07 (0.89, 1.27) 0.4852 | 0.982 |
| Lipid-lowering drugs | |||||||
| No | 10,251 | 0.91 (0.73, 1.15) 0.4476 | 1.12 (1.05, 1.20) 0.0015 | 0.114 | 0.53 (0.20, 1.42) 0.2078 | 1.14 (0.98, 1.32) 0.0813 | 0.147 |
| Yes | 1386 | 0.75 (0.48, 1.17) 0.2035 | 1.11 (0.96, 1.29) 0.1696 | 0.126 | 1.05 (0.16, 6.85) 0.9565 | 1.07 (0.83, 1.37) 0.6183 | 0.990 |
| Hypertension | |||||||
| No | 6779 | 0.87 (0.59, 1.29) 0.4962 | 1.07 (0.94, 1.23) 0.2792 | 0.350 | 2.16 (0.22, 21.49) 0.5106 | 0.95 (0.68, 1.34) 0.7752 | 0.483 |
| Yes | 4858 | 0.81 (0.64, 1.03) 0.0903 | 1.14 (1.06, 1.22) 0.0006 | 0.013 | 0.46 (0.18, 1.17) 0.1035 | 1.16 (1.01, 1.33) 0.0358 | 0.066 |
| Diabetes | |||||||
| No | 9867 | 0.90 (0.71, 1.14) 0.3882 | 1.14 (1.04, 1.25) 0.0065 | 0.101 | 0.85 (0.30, 2.43) 0.7648 | 0.91 (0.72, 1.14) 0.4063 | 0.911 |
| Yes | 1770 | 0.82 (0.55, 1.22) 0.3276 | 1.11 (1.01, 1.22) 0.0287 | 0.162 | 0.43 (0.09, 2.17) 0.3091 | 1.25 (1.07, 1.46) 0.0054 | 0.223 |
| CVD | |||||||
| No | 10,646 | 0.93 (0.74, 1.18) 0.5569 | 1.10 (1.02, 1.18) 0.0142 | 0.217 | 0.83 (0.28, 2.43) 0.7333 | 1.01 (0.85, 1.20) 0.8977 | 0.727 |
| Yes | 991 | 0.64 (0.42, 0.98) 0.0405 | 1.18 (1.04, 1.34) 0.0112 | 0.012 | 0.35 (0.07, 1.64) 0.1826 | 1.26 (1.04, 1.52) 0.0192 | 0.123 |
Notes: Multivariate Cox regression was performed to examine the association between triglyceride levels and mortality
Data are shown in HRs and 95%CI
HR hazard ratios; CI confidence intervals; CAD coronary heart disease
When analyzing a subgroup variable, age, gender, race, education level, smoking, body mass index, systolic blood pressure, estimated glomerular filtration rate, C-reactive protein, energy, total cholesterol, high density lipoprotein cholesterol, hypertension, diabetes, medicine use (antihypertensive drugs, hypoglycemic agents, lipid-lowering drugs, and antiplatelet drugs) were all adjusted except the variable itself
Discussion
In this study, results showed that elevated TG had independent association with all-cause mortality. When compared to people with TG < 150 mg/dL, TG ≥ 250 mg/dL increased the risk of all-cause mortality increased by 34%.
How serum TG related to all-cause mortality in the present study agreed with some previous studies [2, 6, 7]. In this study, all-cause mortality risk was increased by 8% per 1 mmol/L TG increment. Meanwhile, the association between TG and CVD death did not reach significance level. A meta-analysis demonstrated that the risk of CVD death elevated by 13% (P < 0.001) per 1 mmol/L TG increment, suggesting TG associated with CVD mortality and was independent from multiple cardiovascular risk factors [7]. In addition, adjusted spline curves analyze showed when TG was greater than 135 mg/dL, the risk of all-cause mortality was elevated, suggesting a nonlinear relationship between TG and mortality. This finding was similar to previous studies [7, 17]. When TG < 135 mg/dL, TG was inversely associated with all-cause mortality. However, there was a study found that TG ranged 100 to 149 mg/dL might also increase the risk for mortality [18]. The differences in the study population may be the main reasons for the discrepancy in findings.
In the present study, the stratified analysis indicated no significant relationship between TG and cardiovascular mortality in all subgroups. However, the relationship was differed by age, gender, race, BMI, comorbidities, and the use of lipid-lowering drugs. Female participants have a lower risk of death than males, and could be explained by the role of estrogen. Previous research suggested that TG levels were significantly influenced by the endogenous hormonal environment [19–21]. It was also found that the relationship between TG and death was stronger among Whites, probably due to the difference in eating habits and genetic variations between ethnic groups.
The specific mechanism by which TG increased the risk of death has not been fully elucidated. First, animal and human experiments indicated that excessive TG levels were often accompanied by higher inflammation or oxidative stress [22, 23]. Second, circulating TG could passed the blood-brain and induce insulin receptor resistance [24]. Third, genetic variation of lipoprotein lipase, apolipoprotein C3 and lipase maturation factor-1 may play an important role [25]. Apolipoprotein C-III could associate with hypertriglyceridemia and CVD [26]. Although there were still many uncertainties in blood lipid metabolism, proprotein convertase subtilisin/kexin type 9 (PCSK9) has demonstrated an important role in lipid metabolism [27–29]. Moreover, high intrahepatic or circulating PCSK9 levels could increase TG storage and secretion, thus leading to a higher risk of CVD. These observations suggested the use of PCSK9 inhibitors to prevent CVD [30].
Study strength and limitations
The strength of this study was to link with national data, which helped us to elucidate prospective relationship between TG and mortality. However, this study has some limitations. First, some covariates were self-reported. Second, there were other confounders not being adjusted, such as exercise and cardiovascular risk scores. Third, data on serum lipid was only collected once at baseline, and it was unclear how the changes in TG over time might influence the association with mortality. In addition, sample size was reduced due to incomplete data collection of serum lipids in NHANES. Finally, the study findings were mainly applicable to the American population and cannot be extrapolated to other countries.
Conclusion
Elevated TG was independently associated with all-cause mortality, but no significant relationship with cardiovascular death. The results might also suggest non-linear correlation between TG and all-cause death. More attention should be paid to the association of TG with CVD-related mortality, and the management and monitoring of TG should be strengthened. In addition, the relationship between TG and cause-specific deaths is still unclear. More basic and clinical researches are still needed to clarify this relationship.
Acknowledgements
None.
Abbreviations
- CDC
Centers for Disease Control and Prevention
- eGFR
Estimated glomerular filtration rate
- CVD
Cardiovascular diseases
- NHANES
National Health and Nutrition Examination Surveys
- HR
Hazard ratio
- CI
Confidence interval
- TG
Triglyceride
- LDL-C
Low density lipoprotein cholesterol
- HDL-C
High density lipoprotein cholesterol
- TC
Total cholesterol
- SBP
Systolic blood pressure
- DBP
Diastolic blood pressure
- BMI
Body mass index
- HbA1C
Hemoglobin A1C
Authors’ contributions
Yu-qing HUANG, Ying-qing FENG and Bin ZHANG contributed to study design. Yu-qing HUANG, Xiao-cong LIU and Kenneth Lo contributed to data analysis and manuscript drafting. Yu-qing HUANG, Xiao-cong LIU, Kenneth Lo contributed to data downloading. All authors contributed to manuscript revising and have approved the final article.
Funding
This work was supported by the Science and Technology Plan Program of Guangzhou (No. 201803040012), the Key Area R&D Program of Guangdong Province (No. 2019B020227005) and the National Key Research and Development Program of China (No.2017YFC1307603).
Availability of data and materials
Data are from the NHANES Study. Data are available in a public, open access repository.
Ethics approval and consent to participate
The survey protocol was approved by the Institutional Review Board of the Centers for Disease Control and Prevention (Protocol 98–12, 2005–06 and 2011–17). All patients received informed consent before the start of the study.
Consent for publication
All authors approved and agreed to publish the final version of the manuscript.
Competing interests
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yu-qing Huang and Xiao-cong Liu contributed equally to this work.
Contributor Information
Ying-qing Feng, Email: 651792209@qq.com.
Bin Zhang, Email: 3418989350@qq.com.
References
- 1.Sarwar N, Danesh J, Eiriksdottir G, Sigurdsson G, Wareham N, Bingham S, et al. Triglycerides and the risk of coronary heart disease: 10,158 incident cases among 262,525 participants in 29 Western prospective studies. Circulation. 2007;115(4):450–458. doi: 10.1161/CIRCULATIONAHA.106.637793. [DOI] [PubMed] [Google Scholar]
- 2.Kasai T, Miyauchi K, Yanagisawa N, Kajimoto K, Kubota N, Ogita M, et al. Mortality risk of triglyceride levels in patients with coronary artery disease. Heart. 2013;99(1):22–29. doi: 10.1136/heartjnl-2012-302689. [DOI] [PubMed] [Google Scholar]
- 3.Toth PP, Philip S, Hull M, Granowitz C. Elevated triglycerides (>/=150 mg/dL) and high triglycerides (200-499 mg/dL) are significant predictors of new heart failure diagnosis: a real-world analysis of high-risk statin-treated patients. Vasc Health Risk Manag. 2019;15:533–538. doi: 10.2147/VHRM.S221289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Toth PP, Philip S, Hull M, Granowitz C. Association of Elevated Triglycerides with Increased Cardiovascular Risk and Direct Costs in statin-treated patients. Mayo Clin Proc. 2019;94(9):1670–1680. doi: 10.1016/j.mayocp.2019.03.028. [DOI] [PubMed] [Google Scholar]
- 5.Patel A, Barzi F, Jamrozik K, Lam TH, Ueshima H, Whitlock G, et al. Serum triglycerides as a risk factor for cardiovascular diseases in the Asia-Pacific region. Circulation. 2004;110(17):2678–2686. doi: 10.1161/01.CIR.0000145542.24347.18. [DOI] [PubMed] [Google Scholar]
- 6.Haim M, Benderly M, Brunner D, Behar S, Graff E, Reicher-Reiss H, et al. Elevated serum triglyceride levels and long-term mortality in patients with coronary heart disease: the Bezafibrate infarction prevention (BIP) registry. Circulation. 1999;100(5):475–482. doi: 10.1161/01.CIR.100.5.475. [DOI] [PubMed] [Google Scholar]
- 7.Liu J, Zeng FF, Liu ZM, Zhang CX, Ling WH, Chen YM. Effects of blood triglycerides on cardiovascular and all-cause mortality: a systematic review and meta-analysis of 61 prospective studies. Lipids Health Dis. 2013;12:159. doi: 10.1186/1476-511X-12-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marston NA, Giugliano RP, Im K, Silverman MG, O'Donoghue ML, Wiviott SD, et al. Association between triglyceride lowering and reduction of cardiovascular risk across multiple lipid-lowering therapeutic classes: a systematic review and meta-regression analysis of randomized controlled trials. Circulation. 2019;140(16):1308–1317. doi: 10.1161/CIRCULATIONAHA.119.041998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Criqui MH, Heiss G, Cohn R, Cowan LD, Suchindran CM, Bangdiwala S, et al. Plasma triglyceride level and mortality from coronary heart disease. N Engl J Med. 1993;328(17):1220–1225. doi: 10.1056/NEJM199304293281702. [DOI] [PubMed] [Google Scholar]
- 10.Khawaja OA, Hatahet H, Cavalcante J, Khanal S, Al-Mallah MH. Low admission triglyceride and mortality in acute coronary syndrome patients. Cardiol J. 2011;18(3):297–303. [PubMed] [Google Scholar]
- 11.Bucholz EM, Rodday AM, Kolor K, Khoury MJ, de Ferranti SD. Prevalence and predictors of cholesterol screening, awareness, and statin treatment among US adults with familial hypercholesterolemia or other forms of severe dyslipidemia (1999-2014) Circulation. 2018;137(21):2218–2230. doi: 10.1161/CIRCULATIONAHA.117.032321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doran B, Guo Y, Xu J, Weintraub H, Mora S, Maron DJ, et al. Prognostic value of fasting versus nonfasting low-density lipoprotein cholesterol levels on long-term mortality: insight from the National Health and nutrition examination survey III (NHANES-III) Circulation. 2014;130(7):546–553. doi: 10.1161/CIRCULATIONAHA.114.010001. [DOI] [PubMed] [Google Scholar]
- 13.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. doi: 10.1093/clinchem/18.6.499. [DOI] [PubMed] [Google Scholar]
- 14.James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the eighth joint National Committee (JNC 8) JAMA. 2014;311(5):507–520. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 15.Association AD Classification and diagnosis of diabetes: standards of medical Care in Diabetes-2018. Diabetes Care. 2018;41(Suppl 1):S13–S27. doi: 10.2337/dc18-S002. [DOI] [PubMed] [Google Scholar]
- 16.Huang YQ, Liu L, Huang JY, Lo K, Chen CL, Yu YL, et al. Prehypertension and risk for all-cause and cardiovascular mortality by diabetes status: results from the national health and nutrition examination surveys. Ann Transl Med. 2020;8(6):323. doi: 10.21037/atm.2020.02.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pikhart H, Hubacek JA, Peasey A, Kubinova R, Bobak M. Association between fasting plasma triglycerides, all-cause and cardiovascular mortality in Czech population. Results from the HAPIEE study. Physiol Res. 2015;64(Suppl 3):S355–S361. doi: 10.33549/physiolres.933179. [DOI] [PubMed] [Google Scholar]
- 18.Klempfner R, Erez A, Sagit BZ, Goldenberg I, Fisman E, Kopel E, et al. Elevated triglyceride level is independently associated with increased all-cause mortality in patients with established coronary heart disease: twenty-two-year follow-up of the Bezafibrate infarction prevention study and registry. Circ Cardiovasc Qual Outcomes. 2016;9(2):100–108. doi: 10.1161/CIRCOUTCOMES.115.002104. [DOI] [PubMed] [Google Scholar]
- 19.Jaworski K, Sarkadi-Nagy E, Duncan RE, Ahmadian M, Sul HS. Regulation of triglyceride metabolism. IV. Hormonal regulation of lipolysis in adipose tissue. Am J Physiol Gastrointest Liver Physiol. 2007;293(1):G1–G4. doi: 10.1152/ajpgi.00554.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barnett JB, Woods MN, Lamon-Fava S, Schaefer EJ, McNamara JR, Spiegelman D, et al. Plasma lipid and lipoprotein levels during the follicular and luteal phases of the menstrual cycle. J Clin Endocrinol Metab. 2004;89(2):776–782. doi: 10.1210/jc.2003-030506. [DOI] [PubMed] [Google Scholar]
- 21.Vashishta S, Gahlot S, Goyal R. Effect of menstrual cycle phases on plasma lipid and lipoprotein levels in regularly menstruating women. J Clin Diagn Res. 2017;11(5):C5–C7. doi: 10.7860/JCDR/2017/26031.9799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Al-Aubaidy HA, Jelinek HF. Oxidative stress and triglycerides as predictors of subclinical atherosclerosis in prediabetes. Redox Rep. 2014;19(2):87–91. doi: 10.1179/1351000213Y.0000000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brito A, Lima GM, Farias LM, Rodrigues L, Carvalho V, Pereira C, et al. Lycopene-Rich Extract from Red Guava (Psidium guajava L.) Decreases Plasma Triglycerides and Improves Oxidative Stress Biomarkers on Experimentally-Induced Dyslipidemia in Hamsters. Nutrients. 2019;11(2):393. doi: 10.3390/nu11020393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Banks WA, Farr SA, Salameh TS, Niehoff ML, Rhea EM, Morley JE, et al. Triglycerides cross the blood-brain barrier and induce central leptin and insulin receptor resistance. Int J Obes. 2018;42(3):391–397. doi: 10.1038/ijo.2017.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tada H, Kawashiri MA. Genetic variations, triglycerides, and atherosclerotic disease. J Atheroscler Thromb. 2019;26(2):128–131. doi: 10.5551/jat.ED102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luo M, Peng D. The emerging role of apolipoprotein C-III: beyond effects on triglyceride metabolism. Lipids Health Dis. 2016;15(1):184. doi: 10.1186/s12944-016-0352-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cesaro A, Bianconi V, Gragnano F, Moscarella E, Fimiani F, Monda E, et al. Beyond cholesterol metabolism: the pleiotropic effects of proprotein convertase subtilisin/kexin type 9 (PCSK9). Genetics, mutations, expression, and perspective for long-term inhibition. Biofactors. 2020;46(3):367–380. doi: 10.1002/biof.1619. [DOI] [PubMed] [Google Scholar]
- 28.Lagace TA. PCSK9 and LDLR degradation: regulatory mechanisms in circulation and in cells. Curr Opin Lipidol. 2014;25(5):387–393. doi: 10.1097/MOL.0000000000000114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Demers A, Samami S, Lauzier B, Des Rosiers C, Ngo SE, Ong H, et al. PCSK9 induces CD36 degradation and affects long-chain fatty acid uptake and triglyceride metabolism in adipocytes and in mouse liver. Arterioscler Thromb Vasc Biol. 2015;35(12):2517–2525. doi: 10.1161/ATVBAHA.115.306032. [DOI] [PubMed] [Google Scholar]
- 30.Theocharidou E, Papademetriou M, Reklou A, Sachinidis A, Boutari C, Giouleme O. The role of PCSK9 in the pathogenesis of non-alcoholic fatty liver disease and the effect of PCSK9 inhibitors. Curr Pharm Des. 2018;24(31):3654–3657. doi: 10.2174/1381612824666181010123127. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are from the NHANES Study. Data are available in a public, open access repository.