Supplemental Digital Content is available in the text.
Keywords: defibrillators, incidence, infections, mortality, population
Background:
In the WRAP-IT trial (Worldwide Randomized Antibiotic Envelope Infection Prevention), adjunctive use of an absorbable antibacterial envelope resulted in a 40% reduction of major cardiac implantable electronic device infection without increased risk of complication in 6983 patients undergoing cardiac implantable electronic device revision, replacement, upgrade, or initial cardiac resynchronization therapy defibrillator implant. There is limited information on the cost-effectiveness of this strategy. As a prespecified objective, we evaluated antibacterial envelope cost-effectiveness compared with standard-of-care infection prevention strategies in the US healthcare system.
Methods:
A decision tree model was used to compare costs and outcomes of antibacterial envelope (TYRX) use adjunctive to standard-of-care infection prevention versus standard-of-care alone over a lifelong time horizon. The analysis was performed from an integrated payer-provider network perspective. Infection rates, antibacterial envelope effectiveness, infection treatment costs and patterns, infection-related mortality, and utility estimates were obtained from the WRAP-IT trial. Life expectancy and long-term costs associated with device replacement, follow-up, and healthcare utilization were sourced from the literature. Costs and quality-adjusted life years were discounted at 3%. An upper willingness-to-pay threshold of $150 000 per quality-adjusted life year was used to determine cost-effectiveness, in alignment with the American College of Cardiology/American Heart Association practice guidelines and as supported by the World Health Organization and contemporary literature.
Results:
The base case incremental cost-effectiveness ratio of the antibacterial envelope compared with standard-of-care was $112 603/quality-adjusted life year. The incremental cost-effectiveness ratio remained lower than the willingness-to-pay threshold in 74% of iterations in the probabilistic sensitivity analysis and was most sensitive to the following model inputs: infection-related mortality, life expectancy, and infection cost.
Conclusions:
The absorbable antibacterial envelope was associated with a cost-effectiveness ratio below contemporary benchmarks in the WRAP-IT patient population, suggesting that the envelope provides value for the US healthcare system by reducing the incidence of cardiac implantable electronic device infection.
Registration:
URL: https://www.clinicaltrials.gov. Unique identifier: NCT02277990.
WHAT IS KNOWN?
Infection is one of the most serious complications of cardiac implantable electronic device (CIED) therapy, often requiring prolonged hospitalization and complete device extraction until the infection is resolved.
Impacts of CIED infection include increased risk of mortality, reduced quality of life, disruptions in CIED therapy, and significant costs to payers, hospitals, and patients.
In the WRAP-IT trial (Worldwide Randomized Antibiotic Envelope Infection Prevention), adjunctive use of an absorbable antibacterial envelope (TYRX) resulted in a 40% reduction of major CIED infection without increased risk of complication in 6983 patients undergoing CIED revision, replacement, upgrade, or initial cardiac resynchronization therapy defibrillator implant.
WHAT THE STUDY ADDS?
This analysis is the first to leverage randomized controlled trial data to quantify the lifetime impact of using the absorbable antibacterial envelope adjunctive to standard-of-care infection prevention, on healthcare resource use, costs, and patient-centric outcomes in the US healthcare system.
The absorbable antibacterial envelope was associated with a cost-effectiveness ratio below contemporary benchmarks in the WRAP-IT patient population, from the perspective of an integrated payer-provider network.
Infection is one of the most serious complications of cardiac implantable electronic device (CIED) therapy, often times requiring prolonged hospitalization and complete device extraction.1–3 In a recent report based on the global WRAP-IT trial (Worldwide Randomized Antibiotic Envelope Infection Prevention), CIED infections were associated with a greater than 3-fold increase in all-cause mortality, a reduction in quality of life for 6 months, and a disruption in CIED therapy in 36% of patients.4 Costs in the United States were estimated at $55K for the hospital, and assuming Medicare fee-for-service or Medicare Advantage: $26K for the payer and $2.1K for the patient, or $57K to the payer and $1.5K to the patient, respectively. Given the significant clinical and financial burden on patients and the healthcare system, infection prevention is of prime importance.
The WRAP-IT trial was designed to evaluate the safety and efficacy of an antibacterial envelope (TYRX absorbable antibacterial envelope, Medtronic, MN), and reported a 40% reduction in major CIED infection with antibacterial envelope use, with no increase in procedure- or system-related complications in patients undergoing CIED revision, replacement, upgrade, or initial cardiac resynchronization therapy defibrillator (CRT-D) implant.5,6 The use of the antibacterial envelope was recently recommended in the 2019 European Heart Rhythm Association International Consensus document, which was endorsed by a number of professional societies/associations comprised of experts in cardiology.7 This analysis evaluates the cost-effectiveness of the antibiotic envelope in the entire WRAP-IT trial cohort and in defined clinical subsets within the US healthcare system.
Methods
We adapted a published decision tree model8 to compare costs and outcomes of antibacterial envelope use (TYRX absorbable antibacterial envelope, Medtronic, MN) adjunctive to standard-of-care infection prevention strategies versus standard-of-care alone over a lifelong time horizon. The WRAP-IT trial protocol was approved by the ethics committee at each participating institution and associated national and local regulatory agencies. All patients provided written informed consent. Due to the proprietary nature of the data collected for this trial, data will not be made publicly available.
All analyses were performed from the perspective of an integrated payer-provider entity (eg, provider-owned health plan), which provides the most comprehensive overview of financial impact to the hospital and healthcare system. The analysis was performed over the full patient lifetime and used a willingness-to-pay (WTP) threshold of $150K per quality-adjusted life year (QALY) in alignment with American College of Cardiology/American Heart Association practice guidelines9 on cost/value methodology and supported by the World Health Organization10 and contemporary literature.11,12
Model Structure
The decision tree model (Figure 1) was developed in Microsoft Excel (Microsoft Corporation, Redmond, WA) to assess the cost-effectiveness of the antibacterial envelope versus standard-of-care infection prevention alone. Each event following the initial procedure was characterized by a node that occurred with a given probability, and each branch represents a mutually exclusive pathway. Total costs and payoffs were calculated by summing the product of the pathway probabilities and the corresponding costs and outcomes. The decision tree models events over the 12 months; however, the overall model was extended to a lifetime perspective by assigning lumped cost/benefit estimates at each 12-month end point. The structure of the model remains the same for all analyses, only model inputs change.
Figure 1.
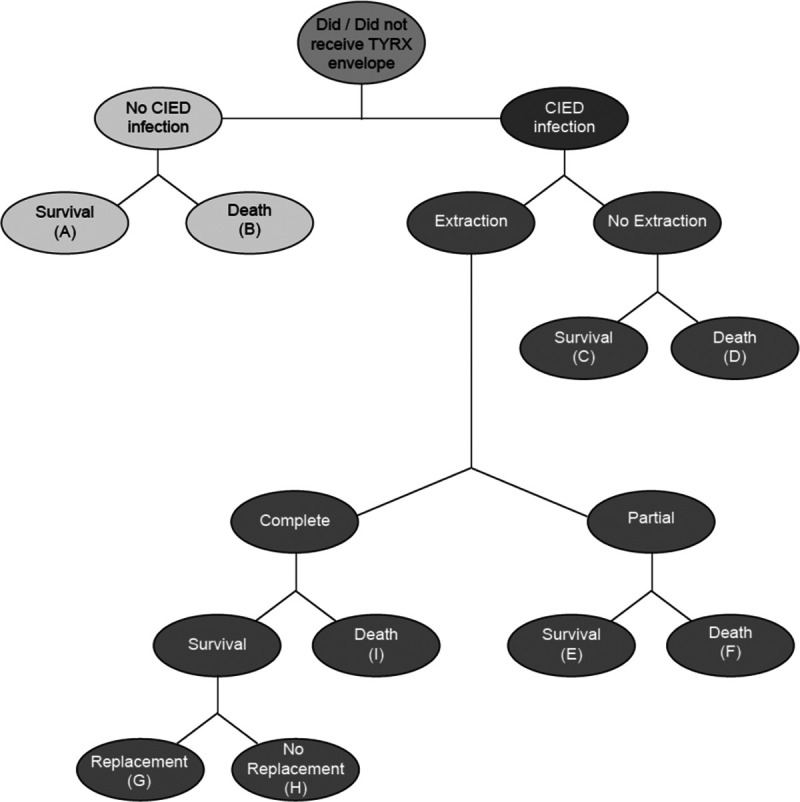
Decision tree analysis. The decision tree model was developed in Microsoft Excel (Microsoft Corporation, Redmond, WA). Each event following the initial procedure was characterized by a node that occurred with a given probability, and each branch represents a mutually exclusive pathway. The total costs and payoffs associated with either treatment option was calculated by multiplying the pathway probabilities by the corresponding costs and outcomes and summing the expected costs and payoffs. The decision tree time horizon is 12 mo, and the model is extended to a lifetime perspective by assigning lumped cost/benefit estimates at end points A–H. CIED indicates cardiac implantable electronic device.
Model Inputs
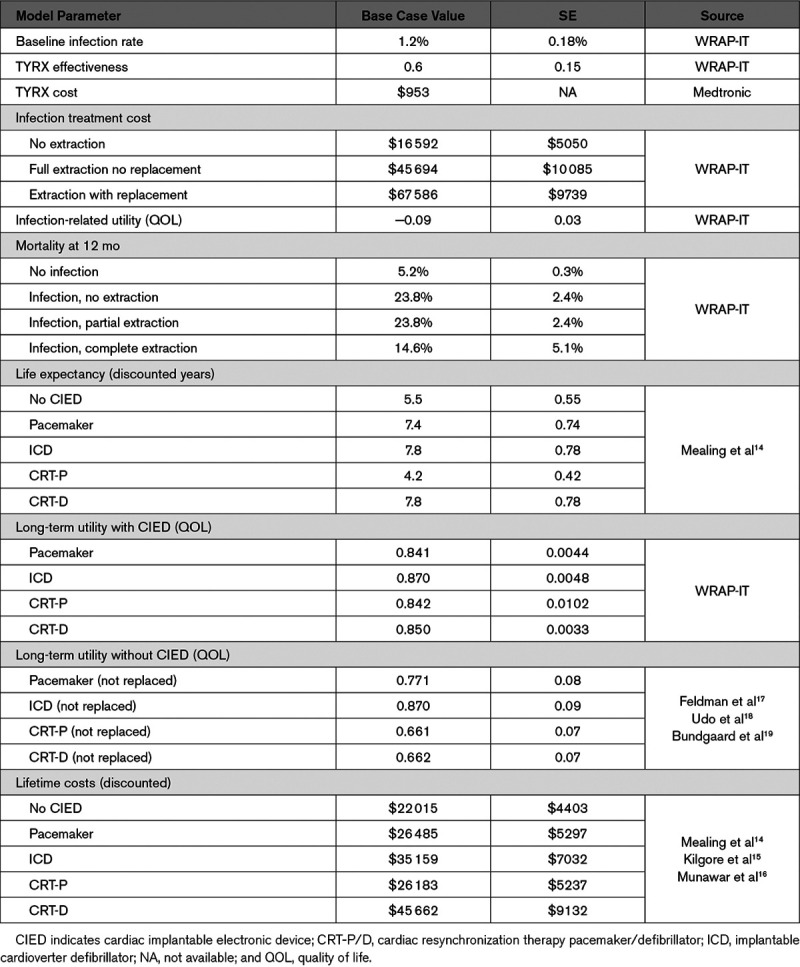
Clinical inputs to the model including the standard-of-care infection rate, antibacterial envelope effectiveness, infection treatment costs and patterns, mortality at 12 months, and quality of life were based on WRAP-IT trial results.4,6 Long-term life expectancy and utilities (single cardinal values between 0 and 1 reflecting the health-related quality of life of an individual at a point in time)13 were derived from prior cost-effectiveness modeling.14 Hospital costs were used as a surrogate for integrated payer-provider entity costs. Expected lifetime costs for device follow-up, replacement, and healthcare utilization were derived from prior estimates.14–16 Model input values for the base case (the primary analysis) and SEs are included in Table 1, and more detail behind the model inputs are included in the Data Supplement (Tables I through IV in the Data Supplement).
Table 1.
Detailed Model Inputs
Analysis
Total lifetime costs and QALYs were simulated to calculate the incremental cost-effectiveness ratio (ICER) for the base case. We conducted 1-way sensitivity analyses and a probabilistic sensitivity analysis to assess the impact of model inputs and parameter uncertainty. Cost-effectiveness for subgroups defined based on known CIED infection risk factors (number of prior procedures, age, depressed renal function, immunocompromised, and procedure type)20 were also calculated by adjusting the standard-of-care infection rate of each subgroup with all other model inputs unchanged. Lifetime costs and QALYs were discounted at a discount rate of 3% for all analyses. All currency reflects 2017 US dollars.
Results
Base Case Analysis
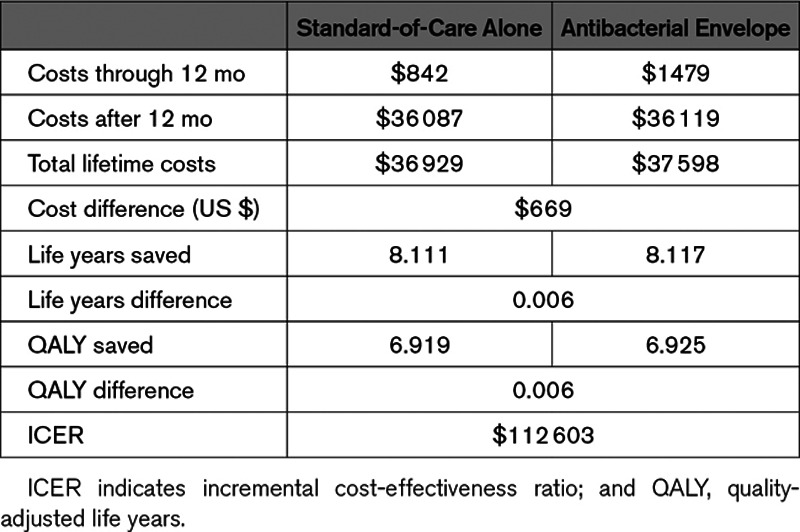
Table 2 shows the results of the base case analysis. The antibacterial envelope resulted in 8.117 life years (6.925 QALYs) saved at a cost of $37 598, while standard-of-care alone resulted in 8.111 life years (6.919 QALYs) saved at a cost of $36 929. The ICER of the antibacterial envelope compared with standard-of-care alone was $112 603/QALY; the antibacterial envelope is cost-effective at a WTP of $150K.
Table 2.
Base Case Analysis Results
Sensitivity Analyses
Results of the 1-way sensitivity analyses show that the ICER was most sensitive to infection-related mortality, life expectancy, and infection cost (Figure 2). Additional parameter values were considered during the 1-way sensitivity analysis (Table V in the Data Supplement); however, the model was not overly sensitive to these values (Table VI in the Data Supplement). Figure 3 shows the results of the probabilistic sensitivity analysis, where each dot corresponds to the resulting cost per QALY of a model iteration, and the continuous line shows the WTP threshold of $150K per QALY. The probabilistic sensitivity analysis found that the ICER remained lower than the WTP threshold in the majority (74%) of iterations.
Figure 2.
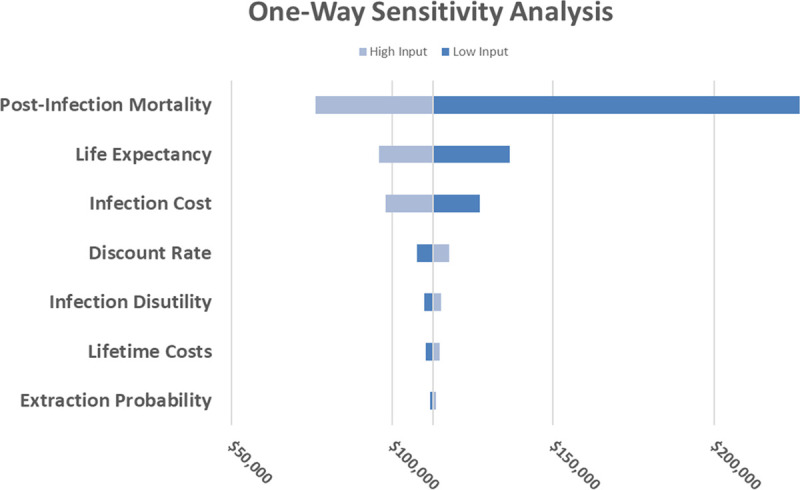
One-way sensitivity analysis. Tornado chart of the range of incremental cost-effectiveness ratio (ICER) across high and low parameter input values (input values are available in Table V in the Data Supplement). Each input was varied with all others held constant. ICER values remained below the $150K willingness-to-pay (WTP) benchmark for the majority of input variations.
Figure 3.
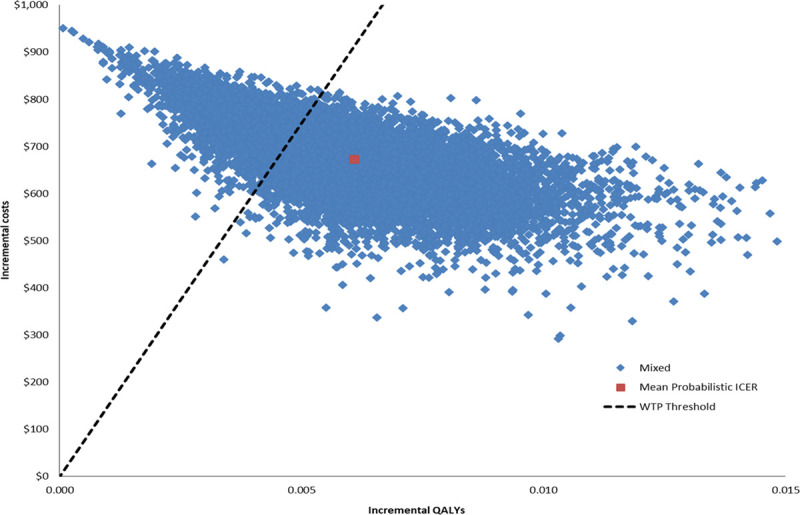
Probablistic sensitivity analysis. The scatterplot depicts the range of incremental cost-effectiveness ratio (ICER) given probabilistic variation in model inputs. Blue dots represent individual ICER data points, whereas the red dot represents the mean ICER from the probabilistic data points. The dashed line represents the willingness-to-pay (WTP) benchmark. The ICER remained lower than the WTP in the majority (74%) of iterations. QALY indicates quality-adjusted life year.
Subgroup Analyses
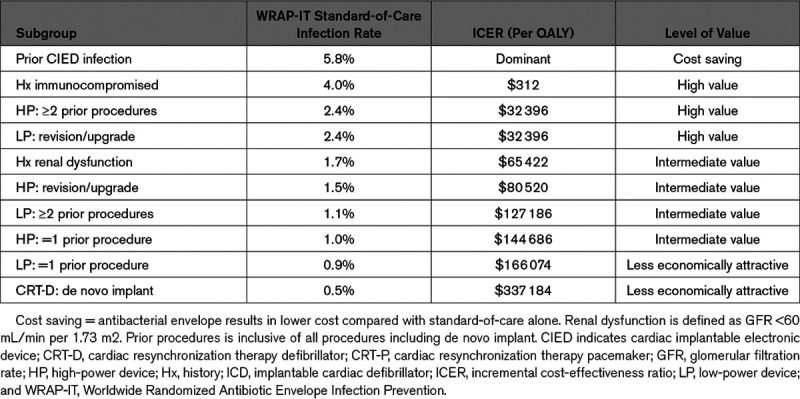
The following cut points for levels of cost-effectiveness are calculated from the model by varying the standard-of-care infection rate (while holding all other model inputs constant): the therapy is cost-effective (ICER below the $150K WTP threshold) when the standard-of-care infection rate is ≥1.0%, is highly cost-effective (ICER below $50K) when the standard-of-care infection rate is ≥2.0%, and is cost saving when the standard-of-care infection rate is ≥4.0%. The therapy is less economically attractive at standard-of-care infection rates below 1%. See Table 3 for the cost-effectiveness of subgroups based on known CIED infection risk factors.
Table 3.
Cost-Effectiveness of Subgroups
Discussion
This analysis is the first to leverage randomized controlled trial data to quantify the lifetime impact of using the absorbable antibacterial envelope adjunctive to standard-of-care infection prevention, on healthcare resource use, costs, and patient-centric outcomes in the US healthcare system. We found that when used in patients with an increased risk of CIED infection, the antibacterial envelope has an ICER of $112 603/QALY compared with standard-of-care alone, which is below the WTP level of $150K/QALY. This finding is robust to the sensitivity analyses, showing ICERs lower than the WTP with most reasonable variations in model inputs.
An estimated 1.5 million patients worldwide receive a CIED each year.21 Infections are one of the most feared complications leading to substantial morbidity and 3-fold risk of mortality.4 In spite of best practice, 1% to 4% of CIED procedures are associated with infection within 12 months,6,22–25 and risk accumulates over the lifetime of the patient.26 The impact of a CIED infection to the healthcare system is substantial, with each event costing $48 000 to $55 000 to treat.1,25,27–29 To date, only the TYRX absorbable antibacterial envelope has been shown to significantly reduce the risk of CIED infection with the addition of preoperative antibiotics.6 In addition to the prior finding that the antibacterial envelope is clinically effective, this analysis suggests that the envelope is also cost-effective in the US healthcare system.
When considering the most appropriate perspective for a cost-effectiveness analysis in the US healthcare system, competing incentives between stakeholders (third-party payers, hospitals, and clinicians) serve to distort economic analyses from traditional perspectives. For example, from a strict third-party payer perspective there is no reimbursement for the antibacterial envelope, so the use of the antibacterial envelope would result in no cost. From a strict hospital perspective, reimbursement from the payer for the treatment of an infection limits the impact of true costs. Further complicating matters, prior reports have shown significant discrepancies between hospital costs and reimbursement.4 Such discrepancies are plausible given the mechanism of reimbursement under Medicare Severity Diagnosis Related Groups. Patients with a CIED infection typically are treated by a hospital admission involving removal of the infected device, a prolonged hospital stay (2–4 weeks) on intravenous antibiotics and temporary device therapy to clear the infection, then a replacement CIED implant. Because of the Medicare Severity Diagnosis Related Groups payment mechanism, the hospitals receive only one payment for the entire inpatient stay. This payment is usually driven by the most expensive procedure performed during the inpatient stay, which is usually the implant of a new CIED. This means that while the hospital has spent significant resources stabilizing the patient, removing the infected system, and having the patient occupy a bed for several weeks, the hospital is not paid for these costly activities—instead the hospital is paid the same as if the patient had arrived for a de novo CIED implant. To fairly and comprehensively account for all costs and benefits experienced by the health care ecosystem, this analysis was performed from the perspective of an integrated payer-provider entity, such as a provider-owned health plan.
We think the integrated entity described above represents the concept of value in healthcare as described by the Porter Model for value in health.30 It collects all costs and outcomes of the full cycle of care into a single perspective, minimizing conflicting goals; and the ICER is the inverse of the Porter Model definition of value (patient-centric health outcomes achieved per dollar spent). Although not fully aligned with payers and providers that are not part of an integrated system, our findings are aimed to inform the decision-making process at the time of a CIED procedure by accounting for the patient, provider, and payer perspectives.
The tacit WTP for the US healthcare system has been $50K per QALY. While the origin of this threshold is unclear, it is sometimes attributed to cost-effectiveness analyses of hemodialysis that has its origin approximately 50 years ago.31 Assuming acceptance of this threshold as a fair benchmark for value at the time, it is reasonable to consider updating it over time at the standard rate of inflation.32 A simple inflation adjustment of $50K from 1980 would yield a WTP in 2017 dollars of $169K per QALY.33 More recent estimates of the cost-effectiveness of standard, hospital-based hemodialysis for end-stage renal disease are consistent with this rough gauge and range from $125K to $150K per QALY.
The above considerations are consistent with those discussed in the 2014 American College of Cardiology/American Heart Association practice guidelines,9 United States-based healthcare value assessment organization statements,11 and perspectives published in peer-reviewed journals,12 which generally recommend consideration of $150K per QALY as a reasonable WTP. Given this understanding, we think it is reasonable to use a contemporary benchmark of $150K per QALY, and to further characterize subgroups that have an ICER below $50K per QALY as high value, those with an ICER between $50K and $150K as intermediate value, and those with an ICER above $150K as less economically attractive.
The ICER for the antibacterial envelope reported here lies within the range of cost-effectiveness results seen for other cardiovascular therapies. Based on a 4-year time horizon, CRT-D therapy had an ICER of $58K as compared to ICD therapy alone in patients with standard CRT-D indications.34 The CardioMems device was found to have an ICER of $71K in New York Heart Association Class III patients.35 The cost-effectiveness of sacubitril versus valsartan in patients with systolic heart failure ranged from $45K to $144K in separate estimates.36,37 Transcatheter aortic valve replacement had ICER estimates that ranged from $39 964 to $116 500 when compared with medical management; however, it was considered a disruptive therapy for a population with no other treatment options.38 Transcatheter aortic valve replacement ICER estimates also ranged from $32 000 to $252 400 when compared with surgical valve replacement in patients with high risk of surgical mortality. At a base case ICER of $112 603and the range of ICERs reported for higher risk subgroups, the antibacterial envelope therapy is commensurate with these other therapies.
To date, there has been little evidence directly addressing the economic implications of using an antibacterial envelope. A report on cost-effectiveness in the United Kingdom healthcare system8 used a pooled estimate of effectiveness from trials published before WRAP-IT. This report found the antibacterial envelope to be cost-saving for patients with an ICD or CRT-D device and cost-effective when baseline probabilities of infection exceeded 1.38% to 1.95% depending on clinical subset. Given the paucity of such evidence, there have been attempts to use surrogate measures of efficiency, such as number needed to treat. The number needed to treat that can be calculated directly from the WRAP-IT study is ≈200; however, the number needed to treat cannot be interpreted in isolation from a full consideration of the benefits, risks, and costs of the therapy, which we take up in the current report. Out of context, the number needed to treat may appear to be a large value; however, the WRAP-IT trial established that there was benefit without increased risk of complications, and this report suggests that the costs are commensurate with the benefits of the envelope.
Different patients present for CIED therapy distinctly, for bradycardia, ventricular arrhythmias, and systolic dysfunction. In addition, the preprocedural comorbidities and the intensity of the CIED procedure distinguish these patients clinically and has an impact on the estimates of cost-effectiveness (Table 3). For example, envelope use in patients who have experienced a prior CIED infection could reasonably be considered high value, while patients with pacemakers and one or fewer prior procedures and no other risk factors could be considered less economically attractive. Recent evidence from the PADIT trial (Prevention of Arrhythmia Device Infection Trial)20,39 provides a tool to estimate infection risk based on patient-specific factors, which may further inform use of the antibacterial envelope. While the primary finding of this analysis is that the antibacterial envelope is cost-effective in the WRAP-IT trial population, the sub-analyses accounting for patient-specific factors provides some insight into how to assess the use of the antibacterial envelope in these clinical subsets of patients.
It is important to keep this economic analysis in perspective as helpful in broad, policy-level decision-making, without applying it as a sole decision criterion in the context of an individual patient. In particular, there may be patient-level heterogeneity that was not modeled in this analysis. For example, a patient receiving a pacemaker without other risk factors may not appear to warrant envelope use from an economic perspective alone. However, if that patient also had a prosthetic valve, the consequence of a CIED infection may lead to consequences related to the valve as well. Other examples include a patient with a simple generator change with older leads leading to multiple postinfection procedures including lead extractions, a patient with no venous access on the opposite side leading to the need for either a trans-iliac or epicardial system, or a CRT patient where the ability to access the same coronary venous branch after extraction and replacement would be compromised.40 There is no substitute for clinical judgment in cases that are complicated by factors that are hard to measure, inclusive of potential consequences of infection should it occur.
Our analysis has several limitations that should be acknowledged. Some model inputs were derived from sources outside of the WRAP-IT trial (Table 1); however, data were selected from WRAP-IT whenever available, and most inputs did represent an in-trial analysis. Costs and benefits were modeled beyond the directly observed results from the WRAP-IT trial; however, the results were robust to the sensitivity analyses. The model did not explicitly account for age-related impacts to utility or healthcare costs; however, the impact of these factors is attenuated by the compound discounting of costs and outcomes over time. The model was particularly sensitive to postinfection mortality with the ICER above the WTP range toward the limit of the sensitivity analysis; however, mortality risk after infection has been demonstrated in other analyses.41,42 The utilities for patients with and without CIED therapy were modeled independently and may have led to an overestimation of variability of the probabilistic sensitivity analysis, but this is a conservative assumption. Patients from the WRAP-IT trial were not all from the United States, but the clinical application and outcomes of CIED therapy are relatively standardized around the world, and costs were estimated from the US subset of patients. Subgroup analyses were not powered in the original trial and represent hypothesis level evidence only. Infection risk factors used in the subgroup analyses represent clinical factors known before the procedure as these are reasonably well established in prior literature, other less well-understood risk factors, such as those related to the procedure, could also impact baseline infection risk resulting in directional changes in cost-effectiveness. Patients on chronic oral immunosuppressive agents or on hemodialysis were excluded from the WRAP-IT trial; however, history of immunosuppressive therapy and history of renal dysfunction (glomerular filtration rate <60 mL/min per 1.73 m2) were used as surrogates for the subgroup analysis. The results of this analysis are applicable only to the patient populations that were defined by the WRAP-IT trial inclusion criteria and may not be generalizable to the full CIED procedure population.
Conclusions
The absorbable antibacterial envelope was associated with a cost-effectiveness ratio below contemporary benchmarks in the WRAP-IT patient population, suggesting that the envelope provides value for the US healthcare system by reducing the incidence of CIED infection.
Acknowledgments
We thank Joanne Krueger, Sarah Willey, and Kayce Valerius of Medtronic, Inc for their management of the WRAP-IT trial (Worldwide Randomized Antibiotic Envelope Infection Prevention) and all the trial sites and investigators that contributed to this data set.
Sources of Funding
The WRAP-IT trial (Worldwide Randomized Antibiotic Envelope Infection Prevention) was supported by Medtronic, Inc.
Disclosures
Dr Wilkoff receives honoraria/consultant fees from Abbott, Medtronic, and Philips. Dr Boriani receives honoraria/consultant fees from Boston, Biotronik, and Medtronic. Dr Mittal receives honoraria/consultant fees from Abbott, Boston Scientific, and Medtronic. J.E. Poole receives honoraria/consultant fees from Boston Scientific, EBR Solutions, Kestra, and Medtronic. Dr Kennergren receives honoraria/consultant fees from Biotronik, Boston Scientific, Medtronic, and Philips. Dr Corey receives honoraria/consultant fees from Arsanis, Basilea, Bayer, Contrafect, Medtronic, Melinta, Motif, Paratek, Pfizer, Quintiles, Tetraphase, The Medicines Company, Theravance, Bio2 Medical, Cempra, Meiji Seika Pharm Co, Novella, Regeneron, and SC Pharma. Drs Schloss and Pickett receive honoraria/consultant fees from Boston Scientific and Medtronic. Dr Evonich receives honoraria/consultant fees from Abbott, Allergan, Astra Zeneca, Biosense Webster, Boehringer Ingelheim, Boston Scientific, Coherex Medical, CorMatrix Cardiovascular, CVRx, EISAI, EKOS, Eli Lily, E.R. Squibb & Sons, Hamilton Health Sciences, Janssen Pharmaceuticals, Novartis, Pfizer, Preventice Services, Roxwood Medical, Sanofi-Aventis, SentreHEART, Siemens Medical Solutions, Terumo Medical, and Zoll Services. Dr Silver receives honoraria/consultant fees from Medtronic. Dr Tarakji receives honoraria/consultant fees from AliveCor and Medtronic. Dr Krahn receives honoraria/consultant fees from Medtronic. Dr Gallastegui, Dr Roark, Dr Sorrentino, Dr Sholevar, E.M. Cronin, Dr Berman, Dr Riggio, Dr Khan, Dr Collier, and Dr Eldadah receive institutional funding from Medtronic. R. Holbrook, Dr Lande, Dr Lexcen, and Dr Seshadri are employed by Medtronic.
Supplementary Material
Nonstandard Abbreviations and Acronyms
- CIED
- cardiac implantable electronic device
- CRT-D
- cardiac resynchronization therapy defibrillator
- ICD
- implantable cardioverter-defibrillator
- ICER
- incremental cost-effectiveness ratio
- PADIT
- Prevention of Arrhythmia Device Infection Trial
- QALY
- quality-adjusted life year
- WRAP-IT
- Worldwide Randomized Antibiotic Envelope Infection Prevention Trial
- WTP
- willingness-to-pay
For Sources of Funding and Disclosures, see page 1081.
The Data Supplement is available at https://www.ahajournals.org/doi/suppl/10.1161/CIRCEP.120.008503.
References
- 1.Sohail MR, Henrikson CA, Braid-Forbes MJ, Forbes KF, Lerner DJ. Mortality and cost associated with cardiovascular implantable electronic device infections. Arch Intern Med. 2011; 171:1821–1828. doi: 10.1001/archinternmed.2011.441 [DOI] [PubMed] [Google Scholar]
- 2.Ahmed FZ, Fullwood C, Zaman M, Qamruddin A, Cunnington C, Mamas MA, Sandoe J, Motwani M, Zaidi A. Cardiac implantable electronic device (CIED) infections are expensive and associated with prolonged hospitalisation: UK retrospective observational study. PLoS One. 2019; 14:e0206611 doi: 10.1371/journal.pone.0206611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Essebag V, Verma A, Healey JS, Krahn AD, Kalfon E, Coutu B, Ayala-Paredes F, Tang AS, Sapp J, Sturmer M, et al. ; BRUISE CONTROL Investigators. Clinically significant pocket hematoma increases long-term risk of device infection: BRUISE CONTROL INFECTION study. J Am Coll Cardiol. 2016; 67:1300–1308. doi: 10.1016/j.jacc.2016.01.009 [DOI] [PubMed] [Google Scholar]
- 4.Wilkoff BL, Boriani G, Mittal S, Poole JE, Kennergren C, Corey GR, Love JC, Augostini R, Faerestrand S, Wiggins SS, et al. ; WRAP-IT Investigators. Impact of cardiac implantable electronic device infection: a clinical and economic analysis of the WRAP-IT trial. Circ Arrhythm Electrophysiol. 2020; 13:e008280 doi: 10.1161/CIRCEP.119.008280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tarakji KG, Mittal S, Kennergren C, Corey R, Poole J, Stromberg K, Lexcen DR, Wilkoff BL. Worldwide randomized antibiotic envelope infection prevention trial (WRAP-IT). Am Heart J. 2016; 180:12–21. doi: 10.1016/j.ahj.2016.06.010 [DOI] [PubMed] [Google Scholar]
- 6.Tarakji KG, Mittal S, Kennergren C, Corey R, Poole JE, Schloss E, Gallastegui J, Pickett RA, Evonich R, Philippon F, et al. ; WRAP-IT Investigators. Antibacterial envelope to prevent cardiac implantable device infection. N Engl J Med. 2019; 380:1895–1905. doi: 10.1056/NEJMoa1901111 [DOI] [PubMed] [Google Scholar]
- 7.Blomström-Lundqvist C, Traykov V, Erba PA, Burri H, Nielsen JC, Bongiorni MG, Poole J, Boriani G, Costa R, Deharo JC, et al. ; ESC Scientific Document Group. European Heart Rhythm Association (EHRA) international consensus document on how to prevent, diagnose, and treat cardiac implantable electronic device infections-endorsed by the Heart Rhythm Society (HRS), the Asia Pacific Heart Rhythm Society (APHRS), the Latin American Heart Rhythm Society (LAHRS), International Society for Cardiovascular Infectious Diseases (ISCVID) and the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur J Cardiothorac Surg. 2020; 57:e1–e31. doi: 10.1093/ejcts/ezz296 [DOI] [PubMed] [Google Scholar]
- 8.Kay G, Eby EL, Brown B, Lyon J, Eggington S, Kumar G, Fenwick E, Sohail MR, Wright DJ. Cost-effectiveness of TYRX absorbable antibacterial envelope for prevention of cardiovascular implantable electronic device infection. J Med Econ. 2018; 21:294–300. doi: 10.1080/13696998.2017.1409227 [DOI] [PubMed] [Google Scholar]
- 9.Anderson JL, Heidenreich PA, Barnett PG, Creager MA, Fonarow GC, Gibbons RJ, Halperin JL, Hlatky MA, Jacobs AK, Mark DB, et al. ; ACC/AHA Task Force on Performance Measures; ACC/AHA Task Force on Practice Guidelines. ACC/AHA statement on cost/value methodology in clinical practice guidelines and performance measures: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures and Task Force on Practice Guidelines. Circulation. 2014; 129:2329–2345. doi: 10.1161/CIR.0000000000000042 [DOI] [PubMed] [Google Scholar]
- 10.Hutubessy R, Chisholm D, Edejer TT. Generalized cost-effectiveness analysis for national-level priority-setting in the health sector. Cost Eff Resour Alloc. 2003; 1:8 doi: 10.1186/1478-7547-1-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Institute for Clinical and Economic Review. Overview of the ICER Value Assessment Framework and Update for 2017–2019. Accessed August 15, 2019. https://icer-review.org/wp-content/uploads/2018/03/ICER-value-assessment-framework-update-FINAL-062217.pdf
- 12.Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness–the curious resilience of the $50,000-per-QALY threshold. N Engl J Med. 2014; 371:796–797. doi: 10.1056/NEJMp1405158 [DOI] [PubMed] [Google Scholar]
- 13.Torrance GW. Utility approach to measuring health-related quality of life. J Chronic Dis. 1987; 40:593–603. doi: 10.1016/0021-9681(87)90019-1 [DOI] [PubMed] [Google Scholar]
- 14.Mealing S, Woods B, Hawkins N, Cowie MR, Plummer CJ, Abraham WT, Beshai JF, Klein H, Sculpher M. Cost-effectiveness of implantable cardiac devices in patients with systolic heart failure. Heart. 2016; 102:1742–1749. doi: 10.1136/heartjnl-2015-308883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kilgore M, Patel HK, Kielhorn A, Maya JF, Sharma P. Economic burden of hospitalizations of Medicare beneficiaries with heart failure. Risk Manag Healthc Policy. 2017; 10:63–70. doi: 10.2147/RMHP.S130341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Munawar DA, Mahajan R, Linz D, Wong GR, Khokhar KB, Thiyagarajah A, Kadhim K, Emami M, Mishima R, Elliott AD, et al. Predicted longevity of contemporary cardiac implantable electronic devices: a call for industry-wide “standardized” reporting. Heart Rhythm. 2018; 15:1756–1763. doi: 10.1016/j.hrthm.2018.07.029 [DOI] [PubMed] [Google Scholar]
- 17.Feldman AM, de Lissovoy G, Bristow MR, Saxon LA, De Marco T, Kass DA, Boehmer J, Singh S, Whellan DJ, Carson P, et al. Cost effectiveness of cardiac resynchronization therapy in the comparison of medical therapy, pacing, and defibrillation in heart failure (COMPANION) trial. J Am Coll Cardiol. 2005; 46:2311–2321. doi: 10.1016/j.jacc.2005.08.033 [DOI] [PubMed] [Google Scholar]
- 18.Udo EO, van Hemel NM, Zuithoff NP, Nijboer H, Taks W, Doevendans PA, Moons KG. Long term quality-of-life in patients with bradycardia pacemaker implantation. Int J Cardiol. 2013; 168:2159–2163. doi: 10.1016/j.ijcard.2013.01.253 [DOI] [PubMed] [Google Scholar]
- 19.Bundgaard JS, Thune JJ, Nielsen JC, Videbæk R, Haarbo J, Bruun NE, Videbæk L, Aagaard D, Korup E, Jensen G, et al. The impact of implantable cardioverter-defibrillator implantation on health-related quality of life in the DANISH trial. Europace. 2019; 21:900–908. doi: 10.1093/europace/euz018 [DOI] [PubMed] [Google Scholar]
- 20.Birnie DH, Wang J, Alings M, Philippon F, Parkash R, Manlucu J, Angaran P, Rinne C, Coutu B, Low RA, et al. Risk factors for infections involving cardiac implanted electronic devices. J Am Coll Cardiol. 2019; 74:2845–2854. doi: 10.1016/j.jacc.2019.09.060 [DOI] [PubMed] [Google Scholar]
- 21.Mond HG, Proclemer A. The 11th world survey of cardiac pacing and implantable cardioverter-defibrillators: calendar year 2009–a World Society of Arrhythmia’s project. Pacing Clin Electrophysiol. 2011; 34:1013–1027. doi: 10.1111/j.1540-8159.2011.03150.x [DOI] [PubMed] [Google Scholar]
- 22.Tarakji KG, Ellis CR, Defaye P, Kennergren C. Cardiac implantable electronic device infection in patients at risk. Arrhythm Electrophysiol Rev. 2016; 5:65–71. doi: 10.15420/aer.2015.27.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greenspon AJ, Patel JD, Lau E, Ochoa JA, Frisch DR, Ho RT, Pavri BB, Kurtz SM. 16-year trends in the infection burden for pacemakers and implantable cardioverter-defibrillators in the United States 1993 to 2008. J Am Coll Cardiol. 2011; 58:1001–1006. doi: 10.1016/j.jacc.2011.04.033 [DOI] [PubMed] [Google Scholar]
- 24.Prutkin JM, Reynolds MR, Bao H, Curtis JP, Al-Khatib SM, Aggarwal S, Uslan DZ. Rates of and factors associated with infection in 200 909 Medicare implantable cardioverter-defibrillator implants: results from the National Cardiovascular Data Registry. Circulation. 2014; 130:1037–1043. doi: 10.1161/CIRCULATIONAHA.114.009081 [DOI] [PubMed] [Google Scholar]
- 25.Eby EL, Bengtson LGS, Johnson MP, Burton ML, Hinnenthal J. Economic impact of cardiac implantable electronic device infections: cost analysis at one year in a large United States health insurer. J Med Econ. 2020; 23:698–705. doi: 10.1080/13696998.2020.1751649 [DOI] [PubMed] [Google Scholar]
- 26.Dai M, Cai C, Vaibhav V, Sohail MR, Hayes DL, Hodge DO, Tian Y, Asirvatham R, Cochuyt JJ, Huang C, et al. Trends of cardiovascular implantable electronic device infection in 3 decades: a population-based study. JACC Clin Electrophysiol. 2019; 5:1071–1080. doi: 10.1016/j.jacep.2019.06.016 [DOI] [PubMed] [Google Scholar]
- 27.Greenspon AJ, Eby EL, Petrilla AA, Sohail MR. Treatment patterns, costs, and mortality among Medicare beneficiaries with CIED infection. Pacing Clin Electrophysiol. 2018; 41:495–503. doi: 10.1111/pace.13300 [DOI] [PubMed] [Google Scholar]
- 28.Sohail MR, Eby EL, Ryan MP, Gunnarsson C, Wright LA, Greenspon AJ. Incidence, treatment intensity, and incremental annual expenditures for patients experiencing a cardiac implantable electronic device infection: evidence from a large US payer database 1-year post implantation. Circ Arrhythm Electrophysiol. 2016; 9:e003929 doi: 10.1161/CIRCEP.116.003929 [DOI] [PubMed] [Google Scholar]
- 29.Shariff N, Eby E, Adelstein E, Jain S, Shalaby A, Saba S, Wang NC, Schwartzman D. Health and economic outcomes associated with use of an antimicrobial envelope as a standard of care for cardiac implantable electronic device implantation. J Cardiovasc Electrophysiol. 2015; 26:783–789. doi: 10.1111/jce.12684 [DOI] [PubMed] [Google Scholar]
- 30.Porter ME. What is value in health care? N Engl J Med. 2010; 363:2477–2481. doi: 10.1056/NEJMp1011024 [DOI] [PubMed] [Google Scholar]
- 31.Grosse SD. Assessing cost-effectiveness in healthcare: history of the $50,000 per QALY threshold. Expert Rev Pharmacoecon Outcomes Res. 2008; 8:165–178. doi: 10.1586/14737167.8.2.165 [DOI] [PubMed] [Google Scholar]
- 32.Ubel PA, Hirth RA, Chernew ME, Fendrick AM. What is the price of life and why doesn’t it increase at the rate of inflation? Arch Intern Med. 2003; 163:1637–1641. doi: 10.1001/archinte.163.14.1637 [DOI] [PubMed] [Google Scholar]
- 33.$50,000 in 1979 → 2017 | Inflation Calculator. Official Inflation Data, Alioth Finance; Accessed August 16, 2019. https://www.officialdata.org/1979-dollars-in-2017?amount=50000 [Google Scholar]
- 34.Noyes K, Veazie P, Hall WJ, Zhao H, Buttaccio A, Thevenet-Morrison K, Moss AJ. Cost-effectiveness of cardiac resynchronization therapy in the MADIT-CRT trial. J Cardiovasc Electrophysiol. 2013; 24:66–74. doi: 10.1111/j.1540-8167.2012.02413.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sandhu AT, Goldhaber-Fiebert JD, Owens DK, Turakhia MP, Kaiser DW, Heidenreich PA. Cost-effectiveness of implantable pulmonary artery pressure monitoring in chronic heart failure. JACC Heart Fail. 2016; 4:368–375. doi: 10.1016/j.jchf.2015.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gaziano TA, Fonarow GC, Claggett B, Chan WW, Deschaseaux-Voinet C, Turner SJ, Rouleau JL, Zile MR, McMurray JJ, Solomon SD. Cost-effectiveness analysis of sacubitril/valsartan vs enalapril in patients with heart failure and reduced ejection fraction. JAMA Cardiol. 2016; 1:666–672. doi: 10.1001/jamacardio.2016.1747 [DOI] [PubMed] [Google Scholar]
- 37.Zueger PM, Kumar VM, Harrington RL, Rigoni GC, Atwood A, DiDomenico RJ, Touchette DR. Cost-effectiveness analysis of sacubitril/valsartan for the treatment of heart failure with reduced ejection fraction in the United States. Pharmacotherapy. 2018; 38:520–530. doi: 10.1002/phar.2108 [DOI] [PubMed] [Google Scholar]
- 38.Gialama F, Prezerakos P, Apostolopoulos V, Maniadakis N. Systematic review of the cost-effectiveness of transcatheter interventions for valvular heart disease. Eur Heart J Qual Care Clin Outcomes. 2018; 4:81–90. doi: 10.1093/ehjqcco/qcx049 [DOI] [PubMed] [Google Scholar]
- 39.Krahn AD, Longtin Y, Philippon F, Birnie DH, Manlucu J, Angaran P, Rinne C, Coutu B, Low RA, Essebag V, et al. Prevention of arrhythmia device infection trial: the PADIT trial. J Am Coll Cardiol. 2018; 72:3098–3109. doi: 10.1016/j.jacc.2018.09.068 [DOI] [PubMed] [Google Scholar]
- 40.Tarakji KG. Cardiovascular implantable electronic device infection: procedure versus lifetime risk. JACC Clin Electrophysiol. 2019; 5:1081–1083. doi: 10.1016/j.jacep.2019.05.026 [DOI] [PubMed] [Google Scholar]
- 41.Tarakji KG, Wazni OM, Harb S, Hsu A, Saliba W, Wilkoff BL. Risk factors for 1-year mortality among patients with cardiac implantable electronic device infection undergoing transvenous lead extraction: the impact of the infection type and the presence of vegetation on survival. Europace. 2014; 16:1490–1495. doi: 10.1093/europace/euu147 [DOI] [PubMed] [Google Scholar]
- 42.Rizwan Sohail M, Henrikson CA, Jo Braid-Forbes M, Forbes KF, Lerner DJ. Increased long-term mortality in patients with cardiovascular implantable electronic device infections. Pacing Clin Electrophysiol. 2015; 38:231–239. doi: 10.1111/pace.12518 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


