Abstract
Background
The authors investigated the durability of vaccine efficacy (VE) against human papillomavirus (HPV)16 or 18 infections and antibody response among nonrandomly assigned women who received a single dose of the bivalent HPV vaccine compared with women who received multiple doses and unvaccinated women.
Methods
HPV infections were compared between HPV16 or 18-vaccinated women aged 18 to 25 years who received one (N = 112), two (N = 62), or three (N = 1365) doses, and age- and geography-matched unvaccinated women (N = 1783) in the long-term follow-up of the Costa Rica HPV Vaccine Trial. Cervical HPV infections were measured at two study visits, approximately 9 and 11 years after initial HPV vaccination, using National Cancer Institute next-generation sequencing TypeSeq1 assay. VE and 95% confidence intervals (CIs) were estimated. HPV16 or 18 antibody levels were measured in all one- and two-dose women, and a subset of three-dose women, using a virus-like particle-based enzyme-linked immunosorbent assay (n = 448).
Results
Median follow-up for the HPV-vaccinated group was 11.3 years (interquartile range = 10.9–11.7 years) and did not vary by dose group. VE against prevalent HPV16 or 18 infection was 80.2% (95% CI = 70.7% to 87.0%) among three-dose, 83.8% (95% CI = 19.5% to 99.2%) among two-dose, and 82.1% (95% CI = 40.2% to 97.0%) among single-dose women. HPV16 or 18 antibody levels did not qualitatively decline between years four and 11 regardless of the number of doses given, although one-dose titers continue to be statistically significantly lower compared with two- and three-dose titers.
Conclusion
More than a decade after HPV vaccination, single-dose VE against HPV16 or 18 infection remained high and HPV16 or 18 antibodies remained stable. A single dose of bivalent HPV vaccine may induce sufficiently durable protection that obviates the need for more doses.
Cervical cancer is a leading cause of cancer and cancer death in many countries, particularly in those with a low human development index (1). Human papillomavirus (HPV) vaccines could prevent most cervical cancers, yet uptake is insufficient to make much of an impact on global cancer rates (2). Over the next 65 years, current vaccination strategies are projected to avert only 3% of the nearly 20 million new cases and 10 million deaths from cervical cancer globally (3).
Compared with the recommended two- and three-dose regimens, single-dose HPV vaccination could reduce costs and logistical barriers, which consequently could increase vaccine implementation and uptake. We demonstrated in post hoc analyses that a single dose of the bivalent HPV vaccine protected against cervical HPV16 or 18 infection 7 years after initial vaccination compared with age-matched unvaccinated women and elicited a stable systemic antibody response, albeit at lower levels than those induced by three doses, thus necessitating evaluation of virologic endpoints (4).
Modeling efforts suggest durability of the protection is a key component for long-term reduction in HPV prevalence and ultimately cervical cancer (5). Here, we update our nonrandomized analysis of dose-specific HPV vaccine efficacy (VE) against prevalent cervical HPV infection a median of 11 years after vaccination for women who received one, two, and three doses of the bivalent HPV vaccine, compared with unvaccinated women, in the Costa Rica HPV Vaccine Trial (CVT) (6,7).
Methods
Study Participants and Procedures
Participants were enrolled in the publicly funded, community-based, randomized phase III CVT (Clinicaltrials.gov NCT00128661) (7). Between 2004 and 2005, 7466 women aged 18 to 25 years consented and were randomly assigned to receive either the AS04-HPV-16/18 vaccine (Cervarix, GlaxoSmithKline Biologicals, Rixensart, Belgium) or a control hepatitis A vaccine (Havrix, GlaxoSmithKline Biologicals) in a 1:1 ratio at zero, 1, and 6 months and followed up for 4 years. At enrollment and follow-up visits, all participants provided serum, and women who reported intercourse received a pelvic exam, at which time cervical cells were collected for cytology and HPV DNA testing. At the end of the 4-year trial, participants were offered the vaccine they had not received at enrollment and most in the original HPV vaccine arm were invited to participate in a long-term follow-up study (6). During this unblinded follow-up, participants in the original HPV-vaccine arm were followed biennially, and each clinic visit included a pelvic exam with collection of cervical and blood samples. A new unvaccinated control group (UCG, n = 2836) was recruited to replace the control arm, with similar characteristics to trial participants. The 11-year study visit, our final assessment of virologic endpoints given the expected lower rates of HPV acquisition in women in their 30s, finished in August 2017. Protocols were approved by Institutional Review Boards at the US NCI and in Costa Rica; all participants signed informed consent.
Approximately 20% of women received fewer than three doses of their assigned vaccine (8). Reasons for missing vaccine doses were independent of trial arm and mostly due to pregnancy and colposcopic referral (8).
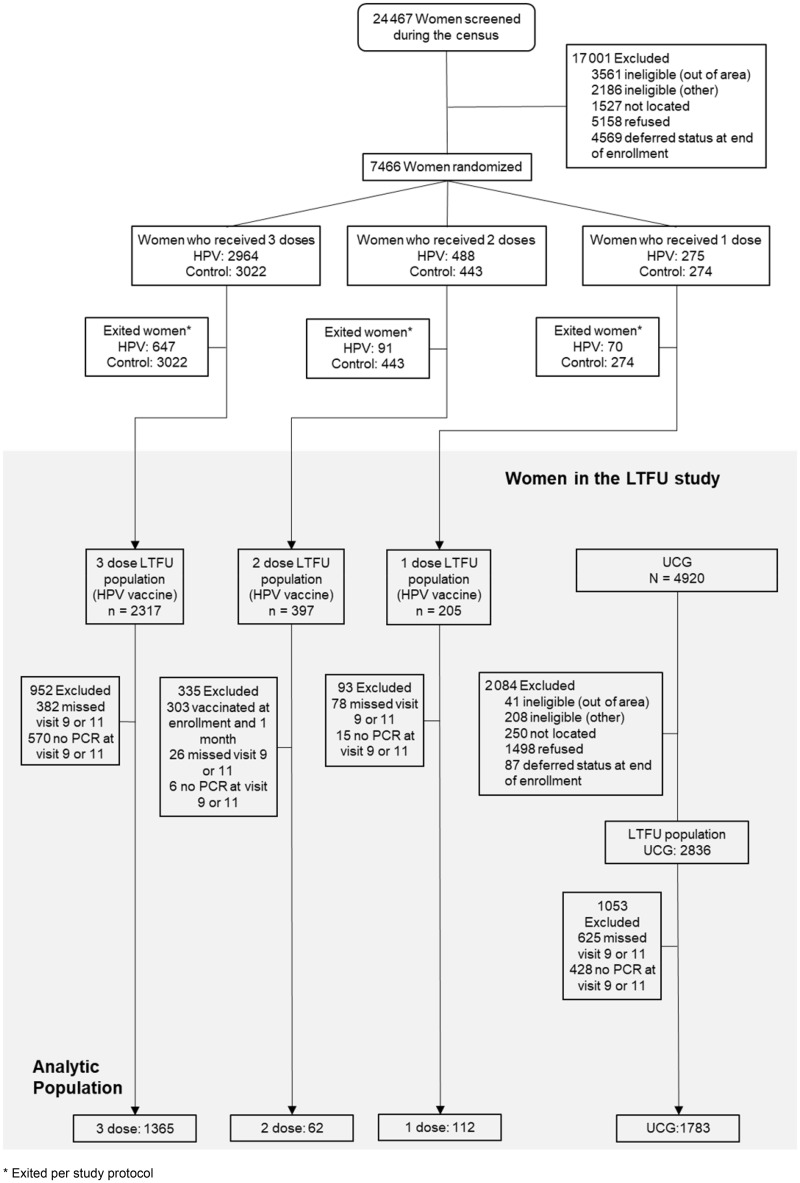
This evaluation focused on HPV-vaccinated women by dose group and the UCG, restricted to women who attended and provided samples at both the 9- and 11-year study visits, to avoid the possibility that discrepancies in the proportion of women who had two visits between the one-, two-, and three-dose groups could bias the conclusions. For virologic endpoints, we analyzed: 112 of 126 women who initially received a single HPV vaccine dose; 62 of 67 women who received two doses at enrollment and 6 months later; 1365 of 1887 women who received three doses; and 1783 of 2189 UCG. For groups 3 and 4, random sampling was used to initially select 50% of each group for testing, and subsequent laboratory testing resulted in additional results being available for inclusion in this analysis (Figure 1).
Figure 1.
Consolidated standards of reporting trials (CONSORT diagram of women in the nonrandomly assigned extension of the Costa Rica HPV vaccine trial (CVT). This CONSORT diagram shows the women’s progress through the randomly assigned phase of the CVT. The CONSORT then details the nonrandomly assigned extension of CVT, the long-term follow-up study (LTFU), where a new unvaccinated control group (UCG) was recruited to replace the control arm, with similar characteristics to trial participants.
For serologic endpoints, all available serum from the one- and two-dose women who had serum available at both the 9- and 11-year timepoints (n = 205 and 93) was tested. A random subset of the three-dose group was selected, restricted to women or timepoints included in previous rounds of testing who had sufficient serum (n = 150) (4). The benchmark for natural immunity was inferred from previous testing (4).
Laboratory Methods
HPV DNA
HPV DNA detection and genotyping from cervical specimens were performed at the National Cancer Institute (NCI) Cancer Genomics Research Laboratory using the TypeSeq assay, which detects 51 HPV genotypes based on next-generation sequencing (9,10). A binary result of positive or negative was reported for the human positive control and for each of the 51 HPV types. See the Supplementary Methods (available online) for further details.
HPV Binding Antibody Measurement
HPV16 and HPV18 serum antibody levels were measured by a virus like particle (VLP)-based enzyme-linked immunosorbent assay (ELISA) at the NCI HPV Immunology Laboratory (11–13). The prespecified seropositivity cutoffs for HPV16 and HPV18 were 8 EU (ELISA units)/mL and 7 EU/mL, respectively. Higher cutoffs (HPV16 = 19 EU/mL and HPV18 = 18 EU/mL) (14) were evaluated in a sensitivity analysis. Serology data in the results section of the manuscript are presented using EU/mL; we additionally convert the serology output in years 9 and 11 to International Units in Supplementary Table 1 (available online). Laboratory-blinded replicates (n = 66) were included: the inter-plate coefficient of variation was 2.6% for HPV16 and 3.0% for HPV18. See the Supplementary Methods (available online) for further details.
HPV Neutralizing Antibody Measurement
Secreted alkaline phosphatase-based pseudovirion neutralization assay (SEAP-NA) was used to measure anti-HPV16 and anti-HPV18 neutralizing antibody titers. See the Supplementary Methods (available online) for further details.
Statistical Analysis
The primary analysis compared the percentage of women with a prevalent HPV infection at the 9- and/or 11-year study visit among the analytic groups (one, two, and three HPV vaccine doses and UCG). A prevalent infection was defined as a type-specific HPV infection at either or both the 9- and 11-year study visits and was counted as a single outcome. The secondary analysis compared the percentage of women with an incident HPV infection at the 11-year study visit (ie, no HPV infection at the 9-year study visit) by dose group; this endpoint was defined as an infection (type-specific) being present in year 11 that was not present in year nine (in some cases there may have been intervening clinical management visits; these were ignored); prevalent infections require no prior HPV-infection status information.
For each endpoint, the percentage of women with the endpoint and the VE is reported for each of the dose groups. P values comparing the endpoints in the one- and two-dose groups with the endpoint in the three-dose group were computed using Fisher’s test.
VE was estimated as 1 – PV/PU, where Pv and PU are, respectively, the prevalence or incidence of infection in the vaccinated and unvaccinated women. Confidence intervals (CIs) for the VE were based on the exact test, conditioning on the total number of events, and using the mid-p adjustment. VE is reported as a composite endpoint for the vaccine types HPV16 or 18 and individually HPV16 and HPV18. Prevalence of carcinogenic HPV types unrelated to HPV vaccination (ie, HPV35, 39, 51, 52, 56, 58, 59) and noncarcinogenic HPV types were evaluated to assess balance in HPV exposure by dose group.
Anti-HPV16 and anti-HPV18 antibody seropositivity are presented for each assay by the three HPV vaccine dose groups using both standard and updated cutoffs. Geometric mean (GM) in ELISA units per milliliter of the serum antibody levels at the 9- and 11-year visits and the ratio of those GMs are reported. We estimate 95% confidence intervals by assuming the log-level or log-ratio is normally distributed and exponentiating the confidence interval for the mean of the log-transformed measure. All P values are two-sided and considered statistically significant at P less than .05. See the Supplementary Methods (available online) for further details.
Results
Participant Characteristics
Median follow-up for the HPV-vaccinated group was 11.3 years (interquartile range [IQR] = 10.9–11.7 years) and did not vary by dose group (all P ≥ .18). From the 4-year study visit (when the UCG was enrolled), the UCG had slightly less follow-up time (median 6.3 years; IQR = 5.9–6.7 years) compared with the HPV-vaccinated groups (one dose: 6.6 years, two doses: 6.6 years, three doses: 6.6 years). At the final visit, the vaccinated group and the UCG were similar by age, sex partners, and smoking (all P ≥ .24) and differed in pregnancies, OC use, and number of study visits (Table 1).
Table 1.
Characteristics at the 11-year study visit among the HPV-vaccinated women, by vaccine dose group, and HPV-unvaccinated women in the CVT
| Characteristic* | Vaccine doses, No. |
||||
|---|---|---|---|---|---|
| 3 | 2 (0 and 6)† | 1 | Unvaccinated | ||
| No. (%) | No. (%) | No. (%) | No. (%) | P ‡ | |
| Age, y | |||||
| 26–32 | 683 (50.0) | 32 (51.6) | 50 (44.6) | 937 (52.6) | .26 |
| 33–38 | 682 (50.0) | 30 (48.4) | 62 (55.4) | 846 (47.4) | |
| Sex partners, no. | |||||
| 0–1 | 224 (16.4) | 12 (19.4) | 17 (15.2) | 322 (18.1) | .60 |
| 2 | 202 (14.8) | 7 (11.3) | 19 (17.0) | 288 (16.2) | |
| 3+ | 939 (68.8) | 43 (69.4) | 76 (67.9) | 1173 (65.8) | |
| Pregnancies, no. | |||||
| 0 | 162 (11.9) | 7 (11.3) | 5 (4.5) | 129 (7.2) | <.001 |
| 1 | 363 (26.6) | 18 (29.0) | 25 (22.3) | 351 (19.7) | |
| 2+ | 840 (61.5) | 37 (59.7) | 82 (73.2) | 1303 (73.1) | |
| Oral contraceptive use | |||||
| Unknown | 0 | 0 | 0 | 2 | <.001 |
| No | 88 (6.4) | 2 (3.2) | 5 (4.5) | 203 (11.4) | |
| Yes | 1277 (93.6) | 60 (96.8) | 107 (95.5) | 1578 (88.6) | |
| Smoking status | |||||
| Unknown | 1 | 0 | 0 | 2 | |
| Never | 1091 (80.0) | 49 (79.0) | 98 (87.5) | 1446 (81.2) | .24 |
| Former | 209 (15.3) | 12 (19.4) | 12 (10.7) | 245 (13.8) | |
| Current | 64 (4.7) | 1 (1.6) | 2 (1.8) | 90 (5.1) | |
| Follow-up visits during unblinded, long-term follow-up phase of study, no. | |||||
| 0–3 | 974 (71.4) | 48 (77.4) | 82 (73.2) | 1044 (58.6) | <.001 |
| 4 | 277 (20.3) | 8 (12.9) | 21 (18.8) | 494 (27.7) | |
| 5+ | 114 (8.4) | 6 (9.7) | 9 (8.0) | 245 (13.7) | |
To accommodate unvaccinated women enrolled later into long-term follow-up, demographic characteristics were compared at the 11-year study visit, using standard contingency table methods. CVT = Costa Rica HPV Vaccine Trial; HPV = human papillomavirus.
The two doses were administered at time 0 and 6 months, per the vaccine label.
Chi-square two-sided P value.
Virologic Endpoints
VE against prevalent HPV16 or 18 infections more than a decade after initial vaccination was high regardless of dose group (Table 2). VE was 80.2% (95% CI = 70.7% to 87.0%) for three doses, 83.8% (95% CI = 19.5% to 99.2%) for two doses, and 82.1% (95% CI = 40.2% to 97.0%) for a single dose. No statistically significant differences in VE or infection rates were present across dose groups. The background prevalence of nonvaccine carcinogenic HPV genotypes (HPV35, 39, 51, 52, 56, 58, 59) and noncarcinogenic HPV types were similarly high in the vaccinated arms (by dose group) and the UCG, suggesting the groups were at similar risk for HPV infection (Table 2).
Table 2.
HPV VE using prevalent and incident HPV infections as the endpoint among women tested at both years nine and 11
| HPV endpoint | Unvaccinated (n = 1783) |
3 Doses (n = 1365) |
2 Doses (0 and 6)* (n = 62) |
1 Dose (n = 112) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. HPV + | % HPV+ (95% CI) | No. HPV + | % HPV+ (95% CI) | VE, % (95% CI) | No. HPV + | % HPV+ (95% CI) | VE, % (95% CI) | P † | No. HPV + | % HPV+ (95% CI) | VE, % (95% CI) | P † | |
| Prevalent HPV infections at year 9 or 11 | |||||||||||||
| 16 or 18 | 178 | 10.0 (8.7 to 11.4) | 27 | 2.0(1.3 to 2.8) | 80.2(70.7 to 87.0) | 1 | 1.6(0.1 to 7.7) | 83.8(19.5 to 99.2) | 1.00 | 2 | 1.8(0.3 to 5.8) | 82.1(40.2 to 97.0) | 1.00 |
| 16 | 111 | 6.2(5.2 to 7.4) | 22 | 1.6(1.0 to 2.4) | 74.1(59.7 to 84.0) | 1 | 1.6(0.1 to 7.7) | 74.1(−30.0 to 98.7) | 1.00 | 2 | 1.8(0.3 to 5.8) | 71.3(3.3 to 95.2) | .70 |
| 18 | 74 | 4.2(3.3 to 5.2) | 6 | 0.4(0.2 to 0.9) | 89.4(77.0 to 95.8) | 0 | 0.0 §(0.0 to 4.7) | 100.0(−18.8 to 100) | 1.00 | 0 | 0.0 §(0.0 to 2.6) | 100.0(34.2 to 100) | 1.00 |
| Carcinogenic HPV unrelated to vaccine‡ | 426 | 23.9(22.0 to 25.9) | 339 | 24.8(22.6 to 27.2) | −3.9(−19.9 to 9.9) | 19 | 30.6(20.2 to 42.9) | −28.3(−98.8 to 21.2) | .30 | 25 | 22.3(15.3 to 30.7) | 6.6(−37.6 to 38.8) | .65 |
| Noncarcinogenic | 898 | 50.4(48.0 to 52.7) | 722 | 52.9(50.2 to 55.5) | −5.0(−15.8 to 4.8) | 37 | 59.7(47.2 to 71.3) | −18.5(−62.8 to 15.9) | .30 | 68 | 60.7(51.4 to 69.4) | −20.5(−53.3 to 6.4) | .12 |
| Incident HPV infections at year 11 | |||||||||||||
| 16 or 18 | 69 | 3.9(3.1 to 4.9) | 8 | 0.6(0.3 to 1.1) | 84.9(69.8 to 93.2) | 1 | 1.6(0.1 to 7.7) | 58.4(−110.9 to 97.9) | .33 | 2 | 1.8(0.3 to 5.8) | 53.9(−57.1 to 92.4) | .17 |
| 16 | 37 | 2.2(1.6 to 2.9) | 8 | 0.6(0.3 to 1.1) | 72.6(43.2 to 88.1) | 1 | 1.6(0.1 to 7.7) | 25.5(−286.6 to 96.4) | .33 | 2 | 1.8(0.3 to 5.8) | 17.5(−189.0 to 86.6) | .17 |
| 18 | 32 | 1.8(1.3 to 2.6) | 0 | 0.0 §(0.0 to 0.2) | 100.0(87.4 to 100) | 0 | 0.0 §(0.0 to 4.7) | 100.0(−175.6 to 100) | N/A | 0 | 0.0 §(0.0 to 2.6) | 100.0(−52.6 to 100) | N/A |
| Carcinogenic HPV unrelated to vaccine‡ | 198 | 11.1(9.7 to 12.6) | 142 | 10.4(8.9 to 12.1) | 6.3(−16.1 to 24.6) | 6 | 9.7(4.0 to 19.0) | 12.9(−84.1 to 65.1) | 1.00 | 16 | 14.3(8.7 to 21.7) | −28.6(−109.1 to 25.2) | .20 |
| Noncarcinogenic | 514 | 28.8(26.8 to 31.0) | 425 | 31.1(28.7 to 33.6) | −8.0(−22.8 to 5.0) | 14 | 22.6(13.5 to 34.2) | 21.7(−29.4 to 55.7) | .16 | 44 | 39.3(30.6 to 48.6) | −36.3(−83.7 to 0.9) | .09 |
The two doses were administered at time 0 and 6 months, per the vaccine label. CI = confidence interval; HPV = human papillomavirus; N/A = not applicable; VE = vaccine efficacy.
P value comparing rate in specified dose group to rate in three-dose group.
Includes HPV35, 39, 51, 52, 56, 58, and 59.
Cell with zero observations.
No statistically significant differences were observed in point estimates for VE against incident HPV16 or 18 infection at the study visit that occurred, on average, 11 years after initial vaccination; 95% confidence intervals were wide, especially for the one- and two-dose groups, because few incident infections were observed. VEs were qualitatively different between dose groups: 84.9% (95% CI = 69.8% to 93.2%) for the three-dose group, 58.4% (95% CI = −110.9% to 97.9%) for the two-dose group, and 53.9% (95% CI = −57.1% to 92.4%) for the one-dose group. Further, likely due to small sample sizes for the one- and two-dose groups, the estimated VEs were not statistically different from 0 (Table 2).
Immunologic Endpoints
Using our standard threshold for positivity based on the ELISA assay, 100% of HPV-vaccinated women remained seropositive at years nine and 11 regardless of number of doses received. In the sensitivity analysis using higher cutoffs, seropositivity at the 9-year study visit was 97.7% (95% CI = 93.8% to 99.4%) for HPV16 and 96.1% (95% CI = 91.6% to 98.6%) for HPV18 among one-dose women, 100% (95% CI = 96.2% to 100%) for HPV16 and 18 for two-dose women, and 100% (95% CI = 97.8% to 100%) for HPV16 and 18 for three-dose women. Seropositivity at the 11-year visit was 96.7% (95% CI = 93.3% to 98.7%) for HPV16 and 92.9% (95% CI = 88.5% to 96.0%) for HPV18 for one-dose women, 98.7% (95% CI = 93.8% to 99.9%) for HPV16 and 100% (95% CI = 96.2% to 100%) for HPV18 for two-dose women, and 100% (95% CI = 97.9% to 100%) for HPV16 and 18 for three-dose women (data not shown). Using the higher cutoff, 0.0% and 4.2% of one-dose women went from HPV16 and HPV18 seropositive to seronegative between years nine and 11, respectively.
Based on ELISA, antibody concentration levels for HPV16 and HPV18 remained relatively constant between years nine and 11 for all dose groups, although GMs did show a slight decrease for three- and two-dose women along with a slight but nonstatistically significant increase for one-dose women (Table 3). Among women in the three-, two-, and one-dose groups, the changes in the GMs of the HPV16 antibody concentration levels were −1.6% (95% CI = −8.6%% to 5.5%), −17.9% (95% CI = −28.5% to −7.4%), and +0.3% (95% CI = −13.4% to 13.9%), respectively. In the same groups, the change in the GMs of the HPV18 antibody concentration levels, respectively, were −4.0% (95% CI = −13.6% to 5.6%), −2.1% (95% CI = −22.5% to 18.4%), and −1.3% (95% CI = −14.0% to 11.5%). Using SEAP-NA, results were similar: percent change from year nine to year 11 was only statistically significant for HPV16 in the two-dose group and ratios of the GMTs that excluded 1.0 were HPV16 by ELISA for the two-dose group and HPV16 by SEAP-NA for the two- and three-dose groups.
Table 3.
Distributions of ELISA serum antibody concentration levels and SEAP neutralization titers for HPV16 and HPV18 at years nine and 11
| Assay | Metric | Three doses |
Two doses (0/6 mo) |
One dose |
|||
|---|---|---|---|---|---|---|---|
| GM (95% CI) | IQR | GM (95% CI) | IQR | GM (95% CI) | IQR | ||
| HPV 16 ELISA antibody concentration | |||||||
| ELISA antibody concentration, EU/mL | Year 9 | 699 (606 to 807) | 394–1265 | 414 (328 to 524) | 197–813 | 172 (141 to 209) | 83–319 |
| Year 11 | 664 (570 to 772) | 383–1309 | 340 (267 to 434) | 170–684 | 176 (145 to 214) | 84–370 | |
| Change, % (95%CI) | –1.6 (–8.6 to 5.5) | –17.9 (–28.5 to –7.4) | 0.3 (–13.4 to 13.9) | ||||
| Ratio of GM | 0.95 (0.89–1.01) | 0.82 (0.76–0.89) | 1.03 (0.94–1.13) | ||||
| HPV18 ELISA antibody concentration | |||||||
| ELISA antibody concentration, EU/mL | Year 9 | 292 (249 to 342) | 163–532 | 210 (171 to 259) | 119–426 | 102 (83 to 125) | 48–223 |
| Year 11 | 275 (234 to 323) | 160–518 | 194 (156 to 241) | 117–384 | 109 (89 to 133) | 51–220 | |
| Change, % (95%CI) | –4.0 (–13.6 to 5.6) | –2.1 (–22.5 to 18.4) | –1.3 (–14.0 to 11.5) | ||||
| Ratio of GM | 0.94 (0.88–1.01) | 0.92 (0.83–1.02) | 1.07 (0.99–1.16) | ||||
| HPV 16 SEAP-NA neutralization titer | |||||||
| SEAP-NA antibody titer | Year 9 | 1776 (1389 to 2270) | 1014–3114 | 771 (612 to 970) | 382–1484 | 285 (229 to 355) | 120–654 |
| Year 11 | 1565 (1227 to 1997) | 924–2916 | 626 (491 to 798) | 323–1405 | 285 (233 to 349) | 133–608 | |
| Change, % (95%CI) | –10.5 (–21.7 to 0.7) | –17.2 (–28.1 to –6.4) | –11.5 (–29.7 to 6.7) | ||||
| Ratio of GM | 0.88 (0.81–0.96) | 0.81 (0.75–0.88) | 1.00 (0.90–1.11) | ||||
| HPV18 SEAP-NA neutralization titer | |||||||
| SEAP-NA antibody titer | Year 9 | 856 (645 to 1136) | 425–1615 | 531 (415 to 678) | 295–1092 | 216 (170 to 273) | 93–504 |
| Year 11 | 792 (588 to 1065) | 371–1573 | 489 (371 to 644) | 217–1080 | 233 (184 to 296) | 96–551 | |
| Change, % (95%CI) | –2.6 (–15.9 to 10.6) | 14.6 (–33.2 to 62.4) | 6.8 (–7.4 to 21.0) | ||||
| Ratio of GM | 0.92 (0.81–1.06) | 0.92 (0.80–1.06) | 1.08 (0.98–1.19) | ||||
CI = confidence interval; ELISA = enzyme-linked immunosorbent assay; GM = geometric mean; IQR = interquartile range; SEAP-NA = secreted alkaline phosphatase-based pseudovirion neutralization assay.
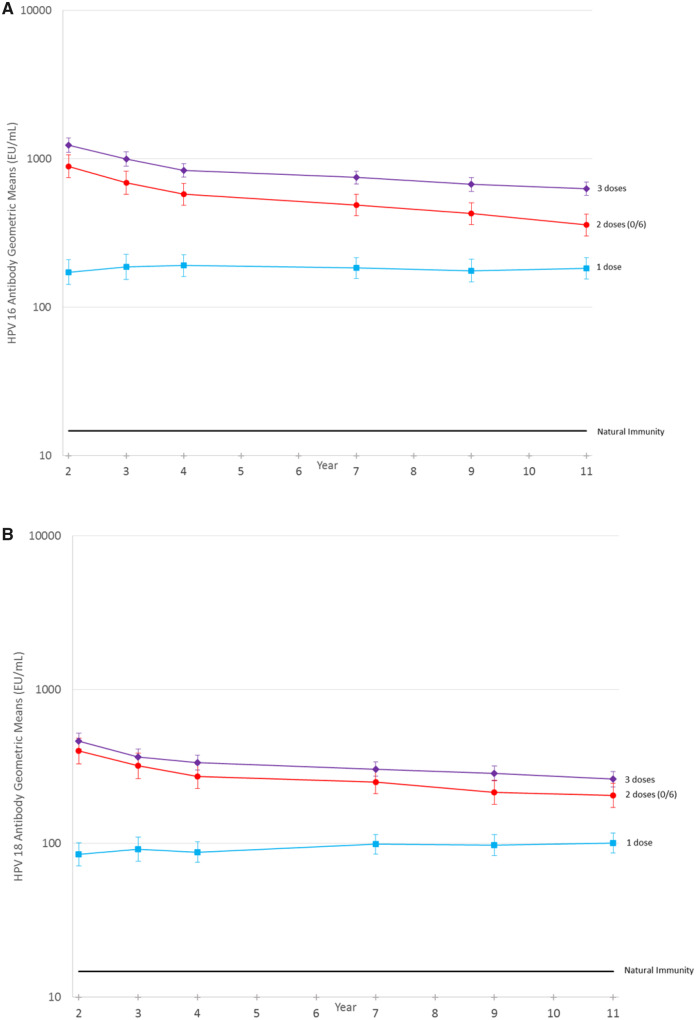
We evaluate trends in GMs across the six study visits (ie, years two, three, four, seven, nine, and 11) in Figure 2 and Supplementary Figures 1–3 (available online). As expected, there were small decreases in antibody levels between years four and 11 in the two- and three-dose women. In one-dose women, there was no change (P = .72) in HPV16 antibody levels over time and actually a small, statistically significant increase in the HPV18 antibody levels over time, with an average per-year increase in the log-level of 0.017 (95% CI = 0.005 to 0.029) driven by the modest shift between years one to four and years seven to 11 (Figure 2). Adjustment for testing batch did not qualitatively modify results. This effect disappears when limiting analyses to women with measurements at all years. In this group, the average per-year increase in the log-level of the HPV18 titer was 0.015 (95% CI = −0.002 to 0.032) (Supplementary Figure 3, available online). HPV seropositivity based on the higher threshold and comparing years nine and 11, greater than 90% of HPV-vaccinated women were HPV16 and 18 seropositive at both timepoints, and 2.5% of one-dose HPV-vaccinated women were HPV16 and 18 seronegative at both timepoints (Supplementary Table 2, available online).
Figure 2.
Human papillomavirus (HPV)16 (graph A) and HPV18 (graph B) antibody levels over time by number of doses received. A linear mixed model using all titer results including replicate testing was used to calculate the Geometric mean titers (GMTs) and 95% confidence intervals (CIs) for single-dose women using continuous time, adjusted by testing batch and using random effects to account for the correlation within a patient and within replicate testing of a visit. For HPV16, the average per-year change in the log titer level for the 221 women with one dose is −0.002 (95% CI = −0.015 to 0.011, P = .72). For HPV18, the average per-year change in the log titer level for the 221 women with one dose is 0.017 (95% CI = 0.005 to 0.029, P = .005).
Over the course of the study, the NCI Immunology Laboratory conducted three major batches of serologic testing by ELISA [4-year (15), 7-year (4), and 11-year analyses]. HPV16 and 18 antibody results were compared across batches; the reproducibility was excellent (Supplementary Table 3 and Supplementary Figure 4, available online). Correlations between ELISA and SEAP-NA from the final batch of testing, collapsing across years nine and 11, were also very high (Supplementary Table 4, available online).
Extensive analyses were conducted to confirm antibody stability over time by dose (Supplementary Figures 5–9, available online). Supplementary Figure 6 (available online) shows the HPV antibody levels in most women do not qualitatively change over time and only a minority of one-dose women experienced a qualitative increase, suggesting that sexual exposure to HPV16 or 18 does not boost antibodies in the majority of women and thus does not explain the observed persistence in antibody levels.
Discussion
We present our final assessment of HPV infection, more than a decade after the initial bivalent HPV vaccination, in this post hoc evaluation of HPV vaccine protection for women who received one, two, and three doses compared with UCG. High VE against HPV16 or 18 infections was similar for all dose groups, suggesting that the protection afforded by even a single dose may be sufficient to produce a lifelong impact on cancer prevention. Although these women were not randomly assigned to dose group and differences were observed for OC use and pregnancy, the similarity in HPV prevalence for nonvaccine carcinogenic and noncarcinogenic types indicates their risk for HPV exposure was similar, allaying concerns that bias or differential HPV exposure could explain these findings.
VE was assessed among all women with available test results in years nine and 11 using an endpoint of prevalent HPV infection to maximize power (instead of incident persistent HPV infection). VE analyses in such cohorts and using such endpoints result in attenuated VE estimates. Nonetheless, VE among women who received a single HPV vaccine dose was 82% and was not statistically different from the observed VE for women who received three doses, the standard of care in this age group. It is important to note that the lower bound of the 95% confidence interval of the VE estimate for the one-dose group was 40.2%, meaning that a substantially lower VE cannot be ruled out; tighter confidence intervals were not possible given the small sample size of the single-dose group. Notably, all HPV-vaccinated women, regardless of number of doses received, remained HPV16 and 18 seropositive using our traditional cutoff more than a decade after initial vaccination, and the average drop in antibody levels between years nine and 11 was modest. Even with the more stringent cutoff, very high seropositivity was observed. Surpassing the 10-year benchmark is important given modeling efforts showing that a single-dose routine vaccination program requires durable protection to avert a substantial number of cervical cancers (16).
Other antivirion antibody responses induced by viral infection or live viral vaccines, which present the same type of high-density repetitive display of surface epitopes as HPV VLPs, have been shown to persist at essentially constant levels for many decades (17). Yet the sustained immunological responses observed among single-dose HPV-vaccinated participants were unexpected because it has not been observed with other subunit vaccines. For example, anti-Hepatitis B (HB) positivity in young adults (median age of 25 years) after single-dose HB vaccination was 4% and dropped to 0% after 2 years (18), with antibody levels below the threshold considered necessary for protection against HBV infection (19). However, the subviral particles that comprise the HBV vaccine may not be sufficiently “virus-like” to efficiently induce durable antibody responses. Antibody responses to a single dose of the HPV vaccine more closely resemble those to a live virus infection, which can persist indefinitely at a relatively steady-state level (20). In keeping with the idea first proposed by Bachman and Zinkernagel, we speculate that the rigid and densely ordered display of antigens on the VLP surface, as with the display of antigens on many authentic virions, is specifically recognized as foreign by the humoral immune system, with the cross-linking of the B-cell receptors on cognate naïve B cells by the repetitive antigen inducing exceptionally strong activation and survival signals and leading to the generation of long-lived plasma cells that continuously secrete antibodies in the absence of further antigen stimulation (17,21,22). Of note, while we document protection with a single vaccine dose against homologous HPV types in this work, complementary findings against heterologous types show strong efficacy with one, two, and three doses (23).
The main limitations of our study are the small sample sizes of the one- and two-dose groups, which are fixed and thus have limited power to detect small differences in HPV attack rates by dose. Because women were not randomly assigned to dose group, we have done extensive work to rule out bias (22,24), including documenting similar antibody responses by dose group 1 month after the initial dose, and balance in the attack rate of non-16 or 18 HPV types by dose in years four, seven, nine, and 11. Yet these data do not afford the same reassurance against selection bias and level of evidence as a randomized trial comparing single with multiple doses. To overcome this, we implemented the Scientific Evaluation of One or Two Doses of the Bivalent or Nonavalent Prophylactic HPV Vaccines (the ESCUDDO study), a randomized clinical trial to definitively determine the protection afforded by single-dose regimens of the HPV vaccines (ClinicalTrials.gov identifier: NCT03180034). ESCUDDO is a four-arm randomized, noninferiority efficacy trial in Costa Rica that aims to evaluate whether, in adolescent girls, one dose or two doses of the bivalent or nonavalent vaccines can confer strong, durable protection against persistent HPV infections (sample size approximately 20 000). Analyses will be conducted to estimate VE vs no vaccination using a concurrent population survey of comparable, initially unvaccinated females in the same region; results are anticipated in 2025.
From the global perspective, women who are at the greatest lifetime risk of cervical cancer are not being vaccinated (3). Our nonrandomized data suggest that a single dose of the HPV vaccine protects against HPV infection a decade after vaccination, with documented stabilization of antibody responses. Given the small number of women who received a single dose, we cannot be certain that one dose will be as protective against HPV infection as two or three doses. But the very strong differences between the one-dose and the unvaccinated groups are unlikely to be explained by biases or chance findings, providing compelling evidence that a single dose of the HPV vaccine is superior to not vaccinating.
Funding
The CVT is a long-standing collaboration between investigators in Costa Rica and the National Cancer Institute (NCI). The trial is sponsored and funded by the NCI (contract N01-CP-11005), with funding support from the National Institutes of Health Office of Research on Women’s Health. GlaxoSmithKline Biologicals (GSK) provided vaccine and support for aspects of the trial associated with regulatory submission needs of the company under a Clinical Trials Agreement (FDA BB-IND 7920) during the 4-year, randomized blinded phase of our study. The long-term follow-up was funded by the NCI with support from the National Institutes of Health Office of Research on Women’s Health.
Notes
The NCI and Costa Rica investigators are responsible for the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation of the manuscript. JTS and DRL report that they are named inventors on US Government-owned HPV vaccine patents that are licensed to GSK and Merck and for which the NCI receives licensing fees. They are entitled to limited royalties as specified by federal law. The other authors declare that they have no conflicts of interest. Where authors are identified as personnel of the International Agency for Research on Cancer, World Health Organization, the authors alone are responsible for the views expressed in this article, and they do not necessarily represent the decisions, policy, or views of the International Agency for Research on Cancer, World Health Organization.
Investigators in the Costa Rica HPV Vaccine Trial (CVT) Group: Proyecto Epidemiológico Guanacaste, Fundación INCIENSA, San José, Costa Rica: Bernal Cortés (specimen and repository manager), Paula González (long-term follow-up study [LTFU]: co-principal investigator), Rolando Herrero (CVT: co-principal investigator), Silvia E. Jiménez (trial coordinator), Carolina Porras (co-investigator), Ana Cecilia Rodríguez (co-investigator).
United States National Cancer Institute, Bethesda, MD, USA: Allan Hildesheim (co-principal investigator and NCI co-project officer), Aimée R. Kreimer (LTFU: co-principal investigator and NCI co-project officer), Douglas R. Lowy (HPV virologist), Mark Schiffman (CVT: medical monitor and co-project officer), John T. Schiller (HPV virologist), Mark Sherman (CVT: QC pathologist), Sholom Wacholder (statistician).
Leidos Biomedical Research, Inc., Frederick National Laboratory for Cancer Research, Frederick, MD, USA (HPV Immunology Laboratory): Ligia A. Pinto, Troy J. Kemp.
Georgetown University, Washington, DC, USA: Mary K. Sidawy (CVT: histopathologist).
DDL Diagnostic Laboratory, the Netherlands (HPV DNA testing): Wim Quint, Leen-Jan van Doorn, Linda Struijk.
University of California, San Francisco, CA, USA: Joel M. Palefsky (expert on anal HPV infection and disease diagnosis and management), Teresa M. Darragh (pathologist and clinical management).
University of Virginia, Charlottesville, VA, USA: Mark H. Stoler (QC pathologist).
We extend a special thanks to the women of Guanacaste and Puntarenas, Costa Rica, who gave of themselves in participating in this effort. In Costa Rica, we acknowledge the tremendous effort and dedication of the staff involved in this project; we would like to specifically acknowledge the meaningful contributions by Carlos Avila, Loretto Carvajal, Rebeca Ocampo, Cristian Montero, Diego Guillen, Jorge Morales, and Mario Alfaro. In the United States, we extend our appreciation to the team from Information Management Services (IMS) responsible for the development and maintenance of the data system used in the trial and who serve as the data management center for this effort, especially Jean Cyr, Julie Buckland, John Schussler, and Brian Befano. We thank Dr Diane Solomon (CVT: medical monitor and QC pathologist) for her invaluable contributions during the randomized blinded phase of the trial and the design of the LTFU and Nora Macklin (CVT) and Kate Torres (LTFU) for the expertise in coordinating the study. We thank the members of the Data and Safety Monitoring Board charged with protecting the safety and interest of participants during the randomly assigned, blinded phase of our study (Steve Self, Chair, Adriana Benavides, Luis Diego Calzada, Ruth Karron, Ritu Nayar, and Nancy Roach) and members of the external Scientific HPV Working Group who have contributed to the success of our efforts over the years (Joanna Cain and Elizabeth Fontham, Co-Chairs, Diane Davey, Gypsyamber D’Souza, Anne Gershon, Elizabeth Holly, Silvia Lara, Henriette Raventós, Wasima Rida, Richard Roden, Maria del Rocío Sáenz Madrigal, and Margaret Stanley).
Preliminary findings for this analysis were presented at the International Papillomavirus Conference in Sydney, Australia, in October 2018.
Supplementary Material
References
- 1. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018; 68(6):394–424. [DOI] [PubMed] [Google Scholar]
- 2. Bruni L, Diaz M, Barrionuevo-Rosas L, et al. Global estimates of human papillomavirus vaccination coverage by region and income level: a pooled analysis. Lancet Global Health. 2016;4(7):E453–E463. [DOI] [PubMed] [Google Scholar]
- 3. Bruni L. Global vaccine uptake and projected cervical cancer disease reductions. HPV World. 2017;1(19):6–9. [Google Scholar]
- 4. Safaeian M, Sampson JN, Pan YJ, et al. Durability of protection afforded by fewer doses of the HPV16/18 vaccine: the CVT trial. J Natl Cancer Inst. 2018;110(2):205–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brisson M, Benard E, Drolet M, et al. Population-level impact, herd immunity, and elimination after human papillomavirus vaccination: a systematic review and meta-analysis of predictions from transmission-dynamic models. Lancet Public Health. 2016;1(1):e8–e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gonzalez P, Hildesheim A, Herrero R, et al. Rationale and design of a long term follow-up study of women who did and did not receive HPV 16/18 vaccination in Guanacaste, Costa Rica. Vaccine. 2015;33(18):2141–2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Herrero R, Hildesheim A, Rodriguez AC, et al. Rationale and design of a community-based double-blind randomized clinical trial of an HPV 16 and 18 vaccine in Guanacaste, Costa Rica. Vaccine. 2008;26(37):4795–4808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kreimer AR, Rodriguez AC, Hildesheim A, et al. ; for the CVT Vaccine Group. Proof-of-principle evaluation of the efficacy of fewer than three doses of a bivalent HPV16/18 vaccine. J Natl Cancer Inst. 2011;103(19):1444–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wagner S, Roberson D, Boland J, et al. ; The CVT Group. Evaluation of TypeSeq, a novel high-throughput, low-cost, next-generation sequencing based assay for detection of 51 HPV genotypes. J Infect Dis. 2019;220(10):1609–1619. 10.1093/infdis/jiz324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wagner S, Roberson D, Boland J, et al. Development of the TypeSeq assay for detection of 51 human papillomavirus genotypes by next-generation sequencing. J Clin Microbiol. 2019;57(5):e01794–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Safaeian M, Ghosh A, Porras C, et al. Direct comparison of HPV16 serological assays used to define HPV-naive women in HPV vaccine trials. Cancer Epidemiol Biomarkers Prev. 2012;21(9):1547–1554. [DOI] [PubMed] [Google Scholar]
- 12. Kemp TJ, Garcia-Pineres A, Falk RT, et al. Evaluation of systemic and mucosal anti-HPV16 and anti-HPV18 antibody responses from vaccinated women. Vaccine. 2008;26(29–30):3608–3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dessy FJ, Giannini SL, Bougelet CA, et al. Correlation between direct ELISA, single epitope-based inhibition ELISA and pseudovirion-based neutralization assay for measuring anti-HPV-16 and anti-HPV-18 antibody response after vaccination with the AS04-adjuvanted HPV-16/18 cervical cancer vaccine. Hum Vaccin. 2008;4(6):425–434. [DOI] [PubMed] [Google Scholar]
- 14. Romanowski B, Schwarz TF, Ferguson L, et al. Sustained immunogenicity of the HPV-16/18 AS04-adjuvanted vaccine administered as a two-dose schedule in adolescent girls: five-year clinical data and modeling predictions from a randomized study. Hum Vaccin Immunother. 2016;12(1):20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Safaeian M, Porras C, Pan Y, et al. for the CVT Group. Durable antibody responses following one dose of the bivalent human papillomavirus L1 virus-like particle vaccine in the Costa Rica Vaccine Trial. Cancer Prev Res (Phila). 2013;6(11):1242–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Burger EA, Campos NG, Sy S, et al. Health and economic benefits of single-dose HPV vaccination in a Gavi-eligible country. Vaccine. 2018;36(32):4823–4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Amanna IJ, Slifka MK.. Mechanisms that determine plasma cell lifespan and the duration of humoral immunity. Immunol Rev. 2010;236(1):125–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yoshioka N, Deguchi M, Hagiya H, et al. Durability of immunity by hepatitis B vaccine in Japanese health care workers depends on primary response titers and durations. PLoS One. 2017;12(11):e0187661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. West DJ, Calandra GB.. Vaccine induced immunologic memory for hepatitis B surface antigen: implications for policy on booster vaccination. Vaccine. 1996;14(11):1019–1027. [DOI] [PubMed] [Google Scholar]
- 20. Amanna IJ, Carlson NE, Slifka MK.. Duration of humoral immunity to common viral and vaccine antigens. N Engl J Med. 2007;357(19):1903–1915. [DOI] [PubMed] [Google Scholar]
- 21. Bachmann MF, Jennings GT.. Vaccine delivery: a matter of size, geometry, kinetics and molecular patterns. Nat Rev Immunol. 2010;10(11):787–796. [DOI] [PubMed] [Google Scholar]
- 22. Schiller J, Lowy D.. Explanations for the high potency of HPV prophylactic vaccines. Vaccine. 2018;36(32):4768–4773. 10.1016/j.vaccine.2017.12.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tsang SH, Sampson JN, Schussler J, Porras C, Wagner S,Boland J, Cortes B, Lowy DR, Schiller JT, Schiffman M, Kemp TJ, Rodriguez AC,Quint W, Gail MH, Pinto LA, Gonzalez P, Hildesheim A, Kreimer AR, Herrero R. Durability of Cross-Protection by Different Schedules of the Bivalent HPVVaccine: the CVT Trial. J Natl Cancer Inst. 2020 Feb 24. doi:10.1093/jnci/djaa010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kreimer AR, Herrero R, Sampson JN, et al. Evidence for single-dose protection by the bivalent HPV vaccine—review of the Costa Rica HPV vaccine trial and future research studies. Vaccine. 2018;36(32):4774–4782. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.