Abstract
Background
Lower protein intake in older adults is associated with loss of muscle mass and strength. The present study aimed to provide a pooled estimate of the overall prevalence of protein intake below recommended (according to different cut‐off values) among community‐dwelling older adults, both within the general older population and within specific subgroups.
Methods
As part of the PRevention Of Malnutrition In Senior Subjects in the EU (PROMISS) project, a meta‐analysis was performed using data from four cohorts (from the Netherlands, UK, Canada, and USA) and four national surveys [from the Netherlands, Finland (two), and Italy]. Within those studies, data on protein and energy intake of community‐dwelling men and women aged ≥55 years were obtained by either a food frequency questionnaire, 24 h recalls administered on 2 or 3 days, or food diaries administered on 3 days. Protein intake below recommended was based on the recommended dietary allowance of 0.8 g/kg body weight (BW)/d, by using adjusted BW (aBW) instead of actual BW. Cut‐off values of 1.0 and 1.2 were applied in additional analyses. Prevalences were also examined for subgroups according to sex, age, body mass index (BMI), education level, appetite, living status, and recent weight loss.
Results
The study sample comprised 8107 older persons. Mean ± standard deviation protein intake ranged from 64.3 ± 22.3 (UK) to 80.6 ± 23.7 g/d [the Netherlands (cohort)] or from 0.94 ± 0.38 (USA) to 1.17z ± 0.30 g/kg aBW/d (Italy) when related to BW. The overall pooled prevalence of protein intake below recommended was 21.5% (95% confidence interval: 14.0–30.1), 46.7% (38.3–55.3), and 70.8% (65.1–76.3) using the 0.8, 1.0, and 1.2 cut‐off value, respectively. A higher prevalence was observed among women, individuals with higher BMI, and individuals with poor appetite. The prevalence differed only marginally by age, education level, living status, and recent weight loss.
Conclusions
In community‐dwelling older adults, the prevalence of protein intake below the current recommendation of 0.8 g/kg aBW/d is substantial (14–30%) and increases to 65–76% according to a cut‐off value of 1.2 g/kg aBW/d. To what extent the protein intakes are below the requirements of these older people warrants further investigation.
Keywords: Protein, Diet, Old age, Prevalence, Recommendations, Multi‐cohort
Introduction
A substantial proportion of community‐dwelling older adults has a protein intake below the current recommended dietary allowance (RDA) of 0.8 g/kg body weight (BW)/d, 1 , 2 the intake level assumed to be sufficient to prevent protein deficiencies. A growing body of epidemiological and short‐term metabolic studies indicate, however, that a protein intake above the RDA further benefits muscle mass, strength, and function among older adults, 3 with better muscle health in old age being associated with less disability, 3 , 4 , 5 , 6 higher quality of life, 3 , 7 and lower mortality risk. 8 , 9 For these reasons, experts suggest that the recommended protein intake should be increased to 1.0–1.2 g/kg BW/d. 10 , 11 Applying those higher cut‐off values for optimal protein intake will yield a higher proportion of older adults with a protein intake below recommended, but it is unknown to what degree.
Reported prevalence rates of protein intake <0.8 g/kg aBW/d vary between 10% and 43%. 12 , 13 , 14 , 15 , 16 Besides differences in country‐specific dietary habits and dietary assessment methods that may contribute to the observed variation in prevalence, this variation may also be related to personal characteristics. To illustrate, older women 12 , 15 , 17 and older persons with poor self‐rated health 18 are more likely to report a lower protein intake. Furthermore, the prevalence increases with higher age, 12 , 17 , 19 higher body mass index (BMI), 15 , 20 , 21 and lower education level. 18 Insight into such differences may contribute to the identification of target groups for dietary interventions.
The aim of the present study is to provide a pooled estimate of the prevalence of protein intake below recommended, according to different cut‐off values, among community‐dwelling older adults across different countries. Furthermore, estimates for subgroups according to sex, age, education level, BMI, living status, appetite, and recent weight loss will be provided to explain variations in prevalence.
Methods
This study is part of the PRevention Of Malnutrition in Senior Subjects in the EU (PROMISS) project, which aims to understand the context of malnutrition among community‐dwelling older adults and to develop preventive strategies for it. Cohorts and national surveys of the PROMISS consortium that have data on dietary intake and anthropometry from older adults aged ≥55 years were eligible for the present study. Data from four cohorts, that is, the Health, Aging and Body Composition Study (Health ABC) from the USA, the Longitudinal Aging Study Amsterdam (LASA) from the Netherlands, the Newcastle 85+ Study (Newcastle 85+) from the UK, and the Quebec Longitudinal Study on Nutrition and Aging (NuAge) from Canada, and four national surveys, that is, the Dutch National Food Consumption Survey‐Older Adults (DNFCS) from the Netherlands, the National FINDIET Studies of 2007 and 2012 (FINDIET 2007, FINDIET 2012) from Finland, and the Third Italian National Food Consumption Survey (INRAN‐SCAI) from Italy, were used (Table 1). Ethical approval for the respective studies was obtained from the ethics committees of the local institutions. The studies were conducted in accordance with the ethical principles laid down in the 1964 Declaration of Helsinki and its later amendments.
TABLE 1.
Details of the studies and their community‐dwelling older adults included in the PRevention Of Malnutrition In Senior Subjects in the EU project meta‐analysis on protein intake below recommended
| Study | Country | n total | Women | Age, years | BMI, kg/m2 | Method of dietary assessment | Year of dietary assessment |
|---|---|---|---|---|---|---|---|
| % | Mean ± SD | Mean ± SD | |||||
| Health ABC | USA | 2660 | 51.6 | 74.7 ± 2.9 | 27.2 ± 4.8 | FFQ (108 items) | 1998–1999 |
| LASA | the Netherlands | 1345 | 52.4 | 69.4 ± 8.4 | 27.0 ± 4.3 | FFQ (76 items on 238 food products) | 2014–2015 |
| Newcastle 85+ | UK | 719 | 60.0 | 85.5 ± 0.4 | 24.5 ± 4.4 | 24 h recall (2 days) | 2006–2007 |
| NuAge | Canada | 1286 | 53.5 | 78.4 ± 4.2 | 27.7 ± 4.6 | 24 h recall (3 days) | 2007–2008 |
| DNFCS | the Netherlands | 709 | 48.9 | 76.9 ± 5.1 | 27.4 ± 3.8 | 24 h recall (2 days) | 2010–2012 |
| FINDIET 2007 | Finland | 463 | 50.5 | 68.9 ± 2.8 | 28.3 ± 4.6 | 24 h recall (2 days) | 2007 |
| FINDIET 2012 | Finland | 410 | 49.5 | 68.8 ± 2.8 | 28.2 ± 4.4 | 24 h recall (2 days) | 2012 |
| INRAN‐SCAI | Italy | 515 | 61.2 | 74.6 ± 7.3 | 25.7 ± 4.1 | Food diaries (3 days) | 2005–2006 |
Studies included the Health, Aging and Body Composition Study (Health ABC), Longitudinal Aging Study Amsterdam (LASA), the Newcastle 85+ Study (Newcastle 85+), Quebec Longitudinal Study on Nutrition and Aging (NuAge), Dutch National Food Consumption Survey‐Older adults (DNFCS), National FINDIET Survey 2007 and 2012 (FINDIET 2007 and 2012), and the Third Italian National Food Consumption Survey (INRAN‐SCAI).
BMI, body mass index; FFQ, food frequency questionnaire; SD, standard deviation
For each cohort, the most recent measurement cycle at which dietary intake was assessed was used for the present study (Table 1). Time of dietary assessment ranged from 1998–1999 (Health ABC) to 2014–2015 (LASA). The (time‐dependent) factors used to define subgroups (i.e. BMI, living status, appetite, and recent weight loss) were obtained from the same measurement cycle or—if not available—from the closest cycle (Supporting Information, Table S1).
For each cohort and survey, a researcher was appointed to be responsible for harmonization of the covariates and provision of the cohort‐specific prevalences, based on a predefined protocol for this study (Appendix S2).
Participants
We excluded participants (Figure 1) who were institutionalized (n = 51), from whom sex was unknown (n = 2), who had very low cognitive status (Mini‐Mental State Examination score < 18 or dementia), and who had no help from a proxy during the dietary assessment, as this may bias reported dietary intake (n = 155), with missing dietary intake data or a high number of missing items in the available data (n = 427), with very high reported energy intakes, that is, >3500 kcal/d for women or >4000 kcal/d for men, as these intakes are implausible 22 (n = 107), or for whom BMI was unknown (n = 114). The total sample of all studies comprised 8107 participants aged ≥55 years.
FIGURE 1.
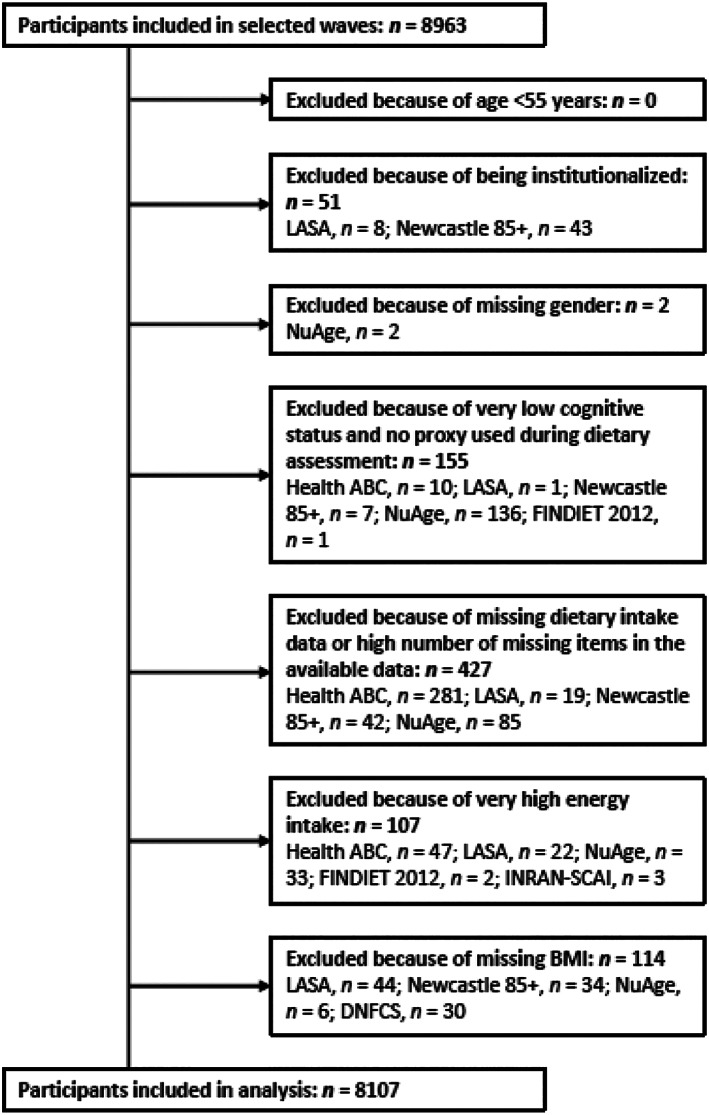
Flow chart representing the participants included in the analysis. Studies included the Health, Aging and Body Composition Study (Health ABC), Longitudinal Aging Study Amsterdam (LASA), the Newcastle 85+ Study (Newcastle 85+), Quebec Longitudinal Study on Nutrition and Aging (NuAge), Dutch National Food Consumption Survey‐Older adults (DNFCS), National FINDIET Survey 2007 and 2012 (FINDIET 2007 and 2012), and the Third Italian National Food Consumption Survey (INRAN‐SCAI).
Dietary intake
Dietary intake was assessed by a food frequency questionnaire (FFQ) in Health ABC and LASA. In Health ABC, a 108‐item interviewer‐administered FFQ 23 that reflected the preceding 12 months was used. In LASA, a self‐administered FFQ, 24 available as paper and online version, was used. This FFQ consisted of 76 items about 238 food products and reflected the preceding 4 weeks. In the other cohorts and in three surveys, multiple 24 h recalls were used. In Newcastle 85+, two recalls were conducted by trained research nurses on non‐consecutive weekdays at least 1 week apart, 25 with portion sizes estimated by a photographic food atlas. In NuAge, three non‐consecutive recalls were conducted by trained registered dietitians on two randomly chosen weekdays and one weekend day, one face to face and two by telephone, 26 with portion sizes estimated by portion size models. In DNFCS, two recalls were conducted during home visits by trained dieticians on two week or weekend days within a period of 2 to 6 weeks. 27 Participants filled in a food diary on the day to be recalled, which served as a memory aid and as a check for household measures. In both FINDIET surveys, two recalls were conducted face to face by trained nutritionists on consecutive days, 28 with portion sizes estimated by a picture book of food portions. In INRAN‐SCAI, dietary intake was measured by three food diaries, filled in by the participant on consecutive days and checked by trained field workers, 29 with portion sizes estimated by household measures, guidance notes, and a photographs atlas. In all studies, intakes of energy and protein were calculated by using country‐specific food composition databases properly adapted to the survey data to comply with the surveyed food matching needs. Within studies that assessed dietary intake by 24 h recalls or food diaries, individual intakes of protein and energy were averaged over the 2 or 3 days.
Protein intake was expressed per kilogramme of adjusted BW (aBW), as suggested by Berner and colleagues. 17 The main reason for this concerns the different protein requirements of fat mass and lean mass. In overweight people, most excess BW is fat mass, which contributes little to protein turnover. In underweight people, the availability of protein is often insufficient to maintain muscle mass. Protein intake expressed relative to actual BW will likely give an overestimation of the true requirements, and as such, by expressing protein intake relative to actual BW, the prevalence of protein intake below recommended will probably be overestimated. By using aBW, we attempted to control (at least in part) for the excess BW or insufficient protein availability. aBW was calculate as the nearest BW that would place a participant with an undesirable BW in the healthy BMI range of 18.5 to 24.9 kg/m2 for adults aged <71 years and of 22.0 to 27.0 kg/m2 for adults aged ≥71 years. 17 Protein intake was dichotomized according to three cut‐off values: <0.8, <1.0, and <1.2 g/kg aBW/d.
Socio‐demographic and anthropometric characteristics
In order to make the variables for the subgroup analyses comparable across the different cohorts and surveys, we harmonized these variables as much as possible with regard to methods of assessment and categorization, according to a predefined protocol (Appendix S2). Education level was based on the self‐reported highest level of education attained (Health ABC, LASA, DNFCS, FINDIET 2007 and 2012, INRAN‐SCAI) or years of full time education (Newcastle 85+, NuAge) and categorized into low (finished lower vocational education or lower; or ≤9 years of education), medium [finished general intermediate or attended (but not finished) higher vocational education, or 10 to 14 years of education], and high (finished higher vocational education or university, or ≥15 years of education). BMI, calculated as BW (in kilogramme) divided by height (in metre) squared, was based on measured BW and measured height in all studies except Newcastle 85+ (height was calculated from measured demi‐span) and INRAN‐SCAI (self‐reported weight and height were used). Living status was assessed by the number of household members in all studies except DNFCS (asked for whom the participant lives together with) and INRAN‐SCAI (unavailable). If the data were unavailable (INRAN‐SCAI) or missing, marital status was used. Living status was divided into living alone (0 household members or widowed, divorced, separated, and never married) and living with another (≥1 household member or married). Appetite was assessed by the level of appetite over the past month (Health ABC, NuAge) or in general (LASA) on a 5 (Health ABC, LASA) or 10 point scale (NuAge), for example, by the question ‘Over the last four weeks, what was your appetite level on a scale from 0 (no appetite) to 10 (very good appetite)?’ (NuAge). In DNFCS, the participant was asked for loss of appetite in the past week (yes/no). Appetite was categorized into good (very good, good, average, or score ≥7, or no) and poor (poor, very poor, moderate, or score ≤6, or yes). No data on appetite were available for Newcastle 85+, FINDIET 2007 and 2012, and INRAN‐SCAI. Recent weight loss was determined using self‐reported recent weight loss of ≥4 kg over the past 6 months (Health ABC, LASA, NuAge, DNFCS) or—if not available—over the past year (FINDIET 2007 and 2012). No data on self‐reported weight loss were available for Newcastle 85+ and INRAN‐SCAI.
Statistical analysis
The cohort‐specific prevalence was calculated locally by each researcher and the survey‐specific prevalence by J. M. A. B., according to the predefined protocol (Appendix S2). Analyses were performed by SPSS Statistics version 24 (IBM Corp., Armonk, NY, USA), SAS version 9.4 (SAS Institute Inc., Cary, NC, USA), and R version 3.2.2. 30
Meta‐analyses using a random effects model were performed with the meta package version 4.3‐2 31 to estimate the pooled prevalence with 95% confidence interval (CI) of protein intake below recommended. To correct for the error that arises when confidence limits fall outside the range of 0.1 to 0.9 and for the error resulting from study variance that points towards zero when prevalence rates are very small or very large, we applied the Freeman–Tukey double arcsine transformation before pooling. 32 Otherwise, logit transformation was applied. The pooled prevalence of protein intake <0.8, <1.0, and <1.2 g/kg aBW/d was estimated within the total study sample and within subgroups according to seven predefined characteristics: sex, age, education level, BMI, living status, appetite, and recent weight loss. We considered a difference in prevalence between subgroups of ≥5% relevant. Heterogeneity between studies was shown by I 2 statistics, which reflect the percentage of the total variation in prevalence resulting from between‐study variation rather than within‐study variation or chance. I 2 values >75% are considered to reflect high heterogeneity. 33 Forest plots were created, graphically showing the prevalence of each study and the overall pooled prevalence. Because the current protein recommendations are based on actual BW, we estimated the prevalence of protein intake below recommended based on protein intake in g/kg actual BW/d as a sensitivity analysis.
Results
The number of participants included per study ranged from 410 (FINDIET 2012) to 2660 (Health ABC) (Table 1). Between 48.9% (DNFCS) and 61.2% (INRAN‐SCAI) of the participants were women. On average, Finnish participants were youngest (2007, 68.9 ± 2.8 years; 2012, 68.8 ± 2.8 years) and UK participants were oldest (85.5 ± 0.4 years). Mean BMI was lowest among UK participants (24.5 ± 4.4 kg/m2) and highest among Finnish participants (2007, 28.3 ± 4.6 kg/m2; 2012, 28.2 ± 4.4 kg/m2).
Mean ± standard deviation protein intake ranged from 64.3 ± 22.3 (Newcastle 85+) to 80.6 ± 23.7 g/d (LASA) or from 0.94 ± 0.38 (Health ABC) to 1.17 ± 0.30 g/kg aBW/d (INRAN‐SCAI) (Table 2) when related to BW. Mean energy intake ranged from 1627 ± 534 (FINDIET 2007) to 2082 ± 580 kcal/d (LASA).
TABLE 2.
Energy and protein intake among the community‐dwelling older adults included in the PRevention Of Malnutrition In Senior Subjects in the EU project meta‐analysis on protein intake below recommended
| Name study | n total | Energy intake (kcal/d) | Protein intake (g/d) | Protein intake (g/kg BW/d) | Protein intake < 0.8 g/kg BW/d | Protein intake (g/kg aBW/d) a | Protein intake < 0.8 g/kg aBW/d | ||
|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | n | % | Mean ± SD | n | % | ||
| Health ABC | 2660 | 1827 ± 655 | 65.5 ± 25.9 | 0.90 ± 0.38 | 1197 | 45.0 | 0.94 ± 0.38 | 1044 | 39.2 |
| LASA | 1345 | 2082 ± 580 | 80.6 ± 23.7 | 1.04 ± 0.32 | 290 | 21.6 | 1.12 ± 0.32 | 193 | 14.3 |
| Newcastle 85+ | 719 | 1683 ± 505 | 64.3 ± 22.3 | 1.03 ± 0.37 | 209 | 29.1 | 1.01 ± 0.33 | 200 | 27.8 |
| NuAge | 1286 | 1839 ± 499 | 72.9 ± 21.7 | 1.04 ± 0.34 | 332 | 25.8 | 1.10 ± 0.32 | 213 | 16.5 |
| DNFCS | 709 | 1981 ± 466 | 76.6 ± 19.5 | 1.01 ± 0.28 | 163 | 23.0 | 1.06 ± 0.26 | 108 | 15.2 |
| FINDIET 2007 | 463 | 1627 ± 534 | 69.2 ± 24.4 | 0.91 ± 0.33 | 193 | 41.7 | 1.01 ± 0.33 | 125 | 27.0 |
| FINDIET 2012 | 410 | 1670 ± 551 | 70.7 ± 24.4 | 0.91 ± 0.33 | 159 | 38.8 | 1.02 ± 0.33 | 109 | 26.6 |
| INRAN‐SCAI | 515 | 2002 ± 539 | 77.6 ± 21.1 | 1.13 ± 0.31 | 68 | 13.2 | 1.17 ± 0.30 | 53 | 10.3 |
Studies included the Health, Aging and Body Composition Study (Health ABC), Longitudinal Aging Study Amsterdam (LASA), the Newcastle 85+ Study (Newcastle 85+), Quebec Longitudinal Study on Nutrition and Aging (NuAge), Dutch National Food Consumption Survey‐Older adults (DNFCS), National FINDIET Survey 2007 and 2012 (FINDIET 2007 and 2012), and the Third Italian National Food Consumption Survey (INRAN‐SCAI).
aBW, adjusted body weight; BMI, body mass index; SD, standard deviation
Adjusted body weight is the nearest body weight that would place the participant with an undesirable body weight in the healthy BMI range of 18.5–25.0 kg/m2 (age <71 years) and of 22.0–27.0 kg/m2 (age ≥ 71 years).
In total, 2045 of the 8107 participants (25.2%) had a protein intake <0.8 g/kg aBW/d (Table 3). The meta‐analysis of the eight studies yielded an overall pooled prevalence of 21.5% (95% CI: 14.0–30.1, I 2 : 98.7%) (Figure 2). Based on those eight studies, more women than men had a protein intake <0.8 g/kg aBW/d [23.6% (16.4–31.5) vs. 18.8% (10.9–28.4), respectively; Figure 3A]. Furthermore, the pooled prevalence increased with increasing BMI, up to BMI category ≥27 to <30, which was comparable with category ≥30 kg/m2 (Figure 3B). Based on the four studies with data on appetite, the prevalence was higher among participants with poor compared with good appetite [27.9% (16.0–44.0) vs. 18.6% (9.0–34.4), respectively; Figure 3C]. No substantial differences in prevalence were observed between subgroups defined by age, education level, living status, and recent weight loss. In all analyses, even within subgroups, observed heterogeneity was high (I 2 ≥ 80.6%).
TABLE 3.
Number of studies and their community‐dwelling older adults included in the meta‐analyses and pooled prevalences of protein intake below 0.8 g/kg aBW/d
| Characteristics | n studies | Pooled prevalence (95% CI) | Heterogeneity | |||
|---|---|---|---|---|---|---|
| n cases/total | I 2 (%) | P value | ||||
| Total sample | 8 | 2045/8107 | 21.5 | (14.0–30.1) | 98.7 | <0.01 |
| Sex | ||||||
| Men | 8 | 925/3812 | 18.8 | (10.9–28.4) | 97.8 | <0.01 |
| Women | 8 | 1120/4295 | 23.6 | (16.4–31.5) | 96.9 | <0.01 |
| Age, years | ||||||
| ≥55 to <65 | 1 | 58/478 | ‐ | ‐ | ‐ | |
| ≥65 to <75 | 7 | 978/3682 | 20.2 | (11.8–30.2) | 97.9 | <0.01 |
| ≥75 to <85 | 5 | 769/2952 | 18.5 | (10.6–30.1) | 97.6 | <0.01 |
| ≥85 | 5 | 240/995 | 17.4 | (10.7–25.4) | 80.6 | <0.01 |
| Education level | ||||||
| Low | 8 | 692/2999 | 21.2 | (13.8–29.7) | 96.6 | <0.01 |
| Medium | 8 | 756/3017 | 23.1 | (15.3–32.0) | 96.3 | <0.01 |
| High | 8 | 586/2043 | 19.4 | (11.2–29.2) | 94.6 | <0.01 |
| BMI, kg/m2 | ||||||
| BMI <22 a | 8 | 153/915 | 12.9 | (8.0–20.2) | 83.1 | <0.01 |
| BMI ≥22 to <27 | 8 | 767/3450 | 18.5 | (11.3–27.1) | 97.1 | <0.01 |
| BMI ≥27 to <30 | 8 | 583/1959 | 26.1 | (16.6–36.8) | 96.0 | <0.01 |
| BMI ≥30 | 8 | 542/1783 | 26.5 | (18.4–35.6) | 93.5 | <0.01 |
| Living status | ||||||
| Living alone | 8 | 693/2692 | 23.1 | (15.8–31.4) | 95.6 | <0.01 |
| Living with another | 8 | 1335/5317 | 20.7 | (12.9–29.8) | 98.2 | <0.01 |
| Appetite | ||||||
| Poor a | 4 | 356/1037 | 27.9 | (16.0–44.0) | 93.9 | <0.01 |
| Good a | 4 | 1182/4876 | 18.6 | (9.0–34.4) | 99.1 | <0.01 |
| Recent weight loss b | ||||||
| Yes a | 6 | 288/1629 | 24.6 | (14.5–38.5) | 93.1 | <0.01 |
| No | 6 | 1452/5032 | 23.2 | (13.8–34.2) | 98.4 | <0.01 |
aBW, adjusted body weight; BMI, body mass index; CI, confidence interval
Estimations based on logit transformation instead of Freeman–Tukey double arcsine transformation.
Recent weight loss of ≥4 kg in the past 6 months.
FIGURE 2.
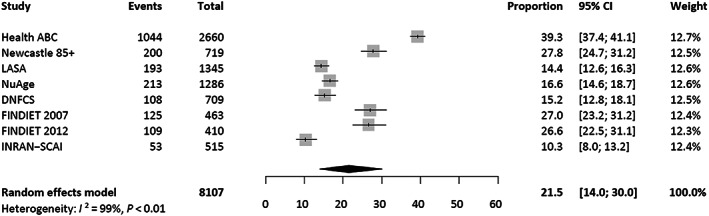
Forest plot representing both the study‐specific prevalences and the overall pooled prevalence of protein intake below the recommended dietary allowance of 0.8 g/kg aBW/d among community‐dwelling older adults. The pooled proportion with 95% confidence interval was obtained from a meta‐analysis using a random‐effects model. Studies included the Health, Aging and Body Composition Study (Health ABC), Longitudinal Aging Study Amsterdam (LASA), the Newcastle 85+ Study (Newcastle 85+), Quebec Longitudinal Study on Nutrition and Aging (NuAge), Dutch National Food Consumption Survey‐Older adults (DNFCS), National FINDIET Survey 2007 and 2012 (FINDIET 2007 and 2012), and the Third Italian National Food Consumption Survey (INRAN‐SCAI).
FIGURE 3.
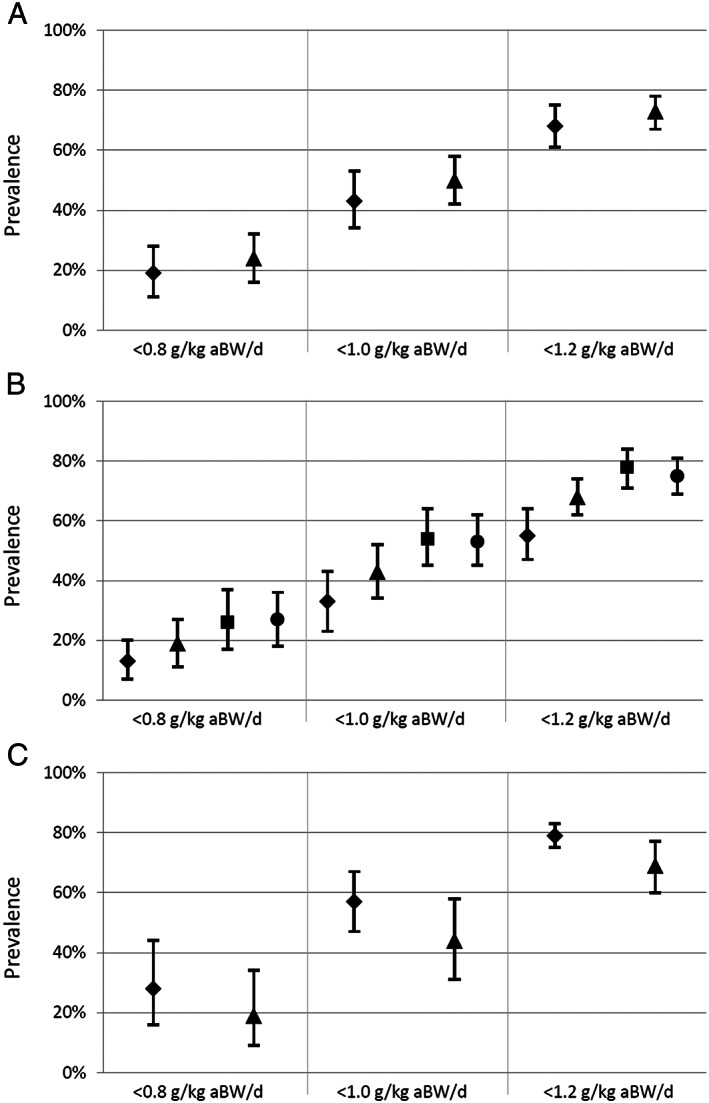
Pooled prevalence of protein intake below recommended among community‐dwelling older adults according to the cut‐off values of <0.8, <1.0, and <1.2 g/kg aBW/d, presented for subgroups according to sex (A; ♦ men, ▲ women), body mass index (B; ♦ <22, ▲ 22–27, ■ 27–30, ● ≥30 kg/m2), and appetite (C; ♦ poor, ▲ good). Pooled proportions with 95% confidence intervals were obtained from meta‐analyses using a random‐effects model. Studies included the Health, Aging and Body Composition Study (Health ABC), Longitudinal Aging Study Amsterdam (LASA), the Newcastle 85+ Study (Newcastle 85+), Quebec Longitudinal Study on Nutrition and Aging (NuAge), Dutch National Food Consumption Survey‐Older adults (DNFCS), National FINDIET Survey 2007 and 2012 (FINDIET 2007 and 2012), and the Third Italian National Food Consumption Survey (INRAN‐SCAI).
The overall pooled prevalence of protein intake <1.0 and <1.2 g/kg aBW/d was 46.7% (38.3–55.3) and 70.8% (65.1–76.3), respectively (Tables S3 and S4). The differences in prevalence between subgroups (e.g. between those with good and poor appetite) for these cut‐offs were comparable with the 0.8 cut‐off value, with one exception. Whereas we observed no substantial differences in prevalence based on the 0.8 cut‐off value between subgroups defined by education level, we did observe a difference based on the cut‐off values of 1.0 and 1.2: the prevalence was higher among medium‐educated than low‐educated or high‐educated individuals.
Sensitivity analysis
The overall pooled prevalence of protein intake <0.8, <1.0, and <1.2 g/kg actual BW/d was 29.1% (21.2–37.8), 54.3% (46.7–61.8), and 75.7% (70.7–80.4), respectively (Table S5).
Discussion
This is the first multi‐country study to provide pooled estimates of the prevalence of protein intake below recommended among community‐dwelling older adults. Based on 8107 adults aged ≥55 years from eight studies, we found the prevalence of protein intake below the current RDA of <0.8 g/kg aBW/d to be substantial (21.5%). Applying the cut‐off values of 1.0 and 1.2 suggested by experts 10 , 11 yielded a prevalence of 46.7% and 70.8%, respectively. Women, older adults with a higher BMI, and those with poor appetite more often had a protein intake below recommended.
Variation in prevalence
Our study showed large variation in the prevalence of protein intake below recommended between the studies, reflected by the high heterogeneity. This was not entirely unexpected, because the eight populations differed with regard to, for example, age, BMI, and dietary habits. Some factors, including sex, 12 , 15 , 17 age, 15 , 17 education level, 18 and BMI, 15 , 16 , 21 are known to be associated with protein intake. We therefore expected these characteristics to partly account for the heterogeneity, but this was not the case. Although prevalences differed between subgroups, I 2 statistics only marginally declined within each group. Methodological differences, such as eligibility criteria and dietary assessment methods, also existed between the studies. However, heterogeneity was not explained by race (Caucasian vs. African–American) or method of dietary assessment (24 h recalls vs. FFQ) (data not shown). In sum, the variation in prevalence is most likely a result of factors other than those we assessed and may include health status 34 and country‐specific dietary habits. 35 , 36
We identified subgroups for which protein intake was more often below recommended, consistently for all three cut‐off values: women, individuals with higher BMI, and individuals with poor appetite. The finding for sex (difference based on 0.8 cut‐off value: 4.8%) is in line with findings among US older adults participating in NHANES 17 and Chinese older adults 37 but differed from those among US older adults participating in the Framingham Osteoporosis Study. 38 Generally, women consume lower amounts of energy than men and have therefore lower protein intakes, although in the Framingham Osteoporosis Study, 38 mean absolute protein intakes were similar for men and women. Apart from a real difference in protein intake, this relatively small difference may also be due to underreporting as women tend to underreport energy intake—and possibly protein intake as well—to a larger extent than men. 39 , 40 The lower protein intake (g/kg aBW) observed in women compared with men may partly contribute to the poorer muscle strength and function generally observed among women. 41 It may therefore be a potential explanation for the well‐established male–female health‐survival paradox; women live longer but have more physical disabilities than men. 42 Women have, however, relatively less muscle mass and consequently may require less protein per kilogramme of BW compared with men. This may suggest that the cut‐off value for adequate protein intake differs for sex. Whether these hypotheses hold should be further explored. Our finding for BMI is in line with previous studies showing that people with higher BMI reported lower protein intakes, 19 , 37 , 43 even though our study used adjusted instead of actual BW to express protein intake. This may again be explained by underreporting as overweight people tend to underreport their protein intake to a greater extent than normal‐weight people. 44 , 45 Our finding of lower protein intakes among those reporting poor appetite confirmed the well‐established phenomenon called anorexia of ageing, that is, the accelerated loss of appetite with advancing age, 46 which increases the risk of reduced food intake. Being aware of the anorexia of ageing, 47 we also expected to find lower protein intakes with higher age, but we observed no differences in prevalence between age subgroups. Other studies both confirm 37 , 43 and contradict 12 , 17 , 19 our findings. Sex may partly explain those differences, 17 , 19 but other factors associated with both (chronological) age and protein intake, such as health status, 19 , 43 may have also caused variation.
Strengths and limitations
This study has several strengths. First, we applied advanced statistical techniques to obtain a pooled estimate of protein intake below recommended levels. Second, we included data from a large number of older adults representing multiple countries, age strata, and levels of education. Third, we used adjusted instead of actual BW, so our findings are less driven by excess body fat among overweight people or insufficient protein availability among underweight people. Some limitations have to be mentioned as well. First, the limited number of studies did not allow us to test differences in prevalence between subgroups (e.g. women vs. men). Second, only cohorts and surveys from the PROMISS consortium were selected for inclusion, which may have led to selection bias. Whether this may have resulted in an overestimation or underestimation of the observed prevalence is difficult to predict. Third, the different methods used by the studies to assess dietary intake and other variables may have influenced the results. However, we harmonized all covariates according to a predefined protocol in order to increase comparability across studies. Moreover, neither the method of dietary assessment nor the subgroups were found to be a major source of heterogeneity. Food databases were not harmonized, meaning that in each national food composition database, protein intake was calculated based on slightly different (food‐specific) nitrogen–protein conversion factors, but we expect this to have only a minor influence on the heterogeneity. Fourth, certain limitations are inherent to dietary assessment. An FFQ is designed to measure usual food intake, but it captures only regularly consumed foods and is therefore not infinite. This most often leads to an underestimation of usual (energy and protein) intake 44 and consequently to an overestimation of the prevalence of protein intake below recommended. For example, the prevalence in the Health ABC cohort was substantially higher than in the other studies. The number of items in the respective FFQ (n = 108) may have been too low to catch all protein‐containing foods in sufficient detail. Furthermore, FFQs require good memory—which may be hampered in old age—and are prone to recall bias and thus may be less accurate. 48 A 24 h recall measures short‐term intake but is prone to day‐to‐day variation. 48 Administering two or three recalls per person gives a better estimate of the usual intake, 49 , 50 but it seems insufficient to eliminate all day‐to‐day variation. 51 Consequently, the observed prevalence is most likely overestimated. 52 , 53 To improve the estimation of protein intake, studies including data on biomarkers of energy and protein intake, for example 24 h urine nitrogen, are recommended. 19 , 44 , 54 , 55
Implications for clinical practice
For correct interpretation, it is important to understand the meaning of the various terms to indicate dietary reference intakes. The estimated average requirement, which is based on nitrogen‐balance studies, is the average daily amount of a nutrient that meets the requirement of half of the healthy individuals in a particular population. 56 The RDA, which is commonly used for dietary guidelines, is the average daily amount of a nutrient that is sufficient to meet the requirement of 97.5% of the individuals. It is thus important to realize that the estimated 21.5% is the proportion of older adults that does not meet the RDA of 0.8 g/kg aBW/d, which does not necessarily mean that their protein intake is inadequate (compared with their needs). Furthermore, from the available data, protein inadequacy on an individual level cannot be assessed.
Before proceeding to increase the RDA to 1.0 or 1.2 g/kg BW/d, evidence on the clinical benefits of higher protein intake should be convincing. Although a growing number of studies show that protein intake above 0.8 g/kg BW/d is associated with improved muscle health, evidence is not univocal. 57 Furthermore, concern is expressed about the potential negative effects of higher protein intake, particularly on renal function. Although current evidence indicates no adverse effect of higher protein intake on renal function in healthy individuals, 58 , 59 potential (other) negative health effects of higher protein intake should also be considered. Last, the practical feasibility of increasing the recommendations for protein intake should be well considered as many parties in society will be involved.
Conclusions
Despite its variation, the prevalence of protein intake below the current recommendation of 0.8 g/kg aBW/d in old age is substantial (14–30%). Over two‐thirds of community‐dwelling older adults have a protein intake below 1.2 g/kg aBW/d. A few subgroups of older adults that had a higher prevalence of protein intake below recommended were distinguished: women, individuals with higher BMI, and individuals with poor appetite. These findings may be of importance for the development of nutritional guidelines, prevention strategies, and health care policy. It remains for future research to obtain more precise estimates of the proportion of older adults whose protein intake is inadequate, based on a large number of cohorts with data on usual intake, and to examine whether sex differences in protein intake and requirement may contribute to the male–female health‐survival paradox.
Funding
European Horizon 2020 PROMISS Project ‘PRevention Of Malnutrition In Senior Subjects in the EU’, (grant agreement no. 678732). The content only reflects the author's view and the commission is not responsible for any use that may be made of the information it contains.
Author contributions
L. M. H., M. V., J. M. A. B., C. J., N. M., N. P., and H. A. H. W. designed the research; L. M. H., J. M. A. B., M. W. H., N. M., and N. P. performed statistical analyses; L. M. H., J. M. A. B., C. J., N. M., H. A. H. W., and M. V. wrote the paper; all authors were involved in the interpretation of the data and provided substantial feedback on draft manuscripts; L. M. H. had primary responsibility for the final content; all authors read and approved the final manuscript.
Ethical authorship
All authors certify that they comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle. 60
Conflicts of interest
None declared.
Supporting information
Table S1. Overview of measurement cycles of dietary intake and socio‐demographic and anthropometric factors, presented per study
Table S2.1. Harmonization approach per variable for the four cohorts included
Table S2.2. Harmonization approach per variable for the four surveys included
Table S3. Number of studies and their community‐dwelling older adults included in the meta‐analyses and pooled prevalence rates of protein intake below 1.0 g/kg aBW/d
Table S4. Number of studies and community‐dwelling older adults included in the meta‐analyses and pooled prevalence rates of protein intake below 1.2 g/kg aBW/d
Table S5. Number of studies and community‐dwelling older adults included in the meta‐analyses and pooled prevalence rates of protein intake below 0.8, 1.0, and 1.2 g/kg actual BW/d
Hengeveld L. M., Boer J. M. A., Gaudreau P., Heymans M. W., Jagger C., Mendonça N., Ocké M. C., Presse N., Sette S., Simonsick E. M., Tapanainen H., Turrini A., Virtanen S. M., Wijnhoven H. A. H., and Visser M. (2020) Prevalence of protein intake below recommended in community‐dwelling older adults: a meta‐analysis across cohorts from the PROMISS consortium, Journal of Cachexia, Sarcopenia and Muscle, 11, 1212–1222, doi: 10.1002/jcsm.12580
References
- 1. (EFSA) EFSA, Dietary reference values for nutrients: Summary report. 2017.
- 2. WHO, Protein and amino acid requirements in human nutrition, in WHO Technical Report Series, Organization WH, Editor . 2007, WHO Press: Geneva, Switzerland: p 1‐265. [PubMed] [Google Scholar]
- 3. Wolfe RR. The role of dietary protein in optimizing muscle mass, function and health outcomes in older individuals. Br J Nutr 2012;108:S88–S93. [DOI] [PubMed] [Google Scholar]
- 4. Deer RR, Volpi E. Protein intake and muscle function in older adults. Curr Opin Clin Nutr Metab Care 2015;18:248–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Paddon‐Jones D, Campbell WW, Jacques PF, Kritchevsky SB, Moore LL, Rodriguez NR, et al. Protein and healthy aging. Am J Clin Nutr 2015;101:1339S–1345S. [DOI] [PubMed] [Google Scholar]
- 6. Mendonca N, Granic A, Hill TR, Siervo M, Mathers JC, Kingston A, et al. Protein intake and disability trajectories in very old adults: the Newcastle 85+ study. J Am Geriatr Soc 2019;67:50–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mijnarends DM, Meijers JM, Halfens RJ, ter Borg S, Luiking YC, Verlaan S, et al. Validity and reliability of tools to measure muscle mass, strength, and physical performance in community‐dwelling older people: a systematic review. J Am Med Dir Assoc 2013;14:170–178. [DOI] [PubMed] [Google Scholar]
- 8. Hardy SE, Kang Y, Studenski SA, Degenholtz HB. Ability to walk 1/4 mile predicts subsequent disability, mortality, and health care costs. J Gen Intern Med 2011;26:130–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ruiz JR, Sui X, Lobelo F, Morrow JR, Jackson AW, Sjostrom M, et al. Association between muscular strength and mortality in men: prospective cohort study. BMJ 2008;337:a439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bauer J, Biolo G, Cederholm T, Cesari M, Cruz‐Jentoft AJ, Morley JE, et al. Evidence‐based recommendations for optimal dietary protein intake in older people: a position paper from the PROT‐AGE Study Group. J Am Med Dir Assoc 2013;14:542–559. [DOI] [PubMed] [Google Scholar]
- 11. Deutz NE, Bauer JM, Barazzoni R, Biolo G, Boirie Y, Bosy‐Westphal A, et al. Protein intake and exercise for optimal muscle function with aging: recommendations from the ESPEN Expert Group. Clin Nutr 2014;33:929–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fulgoni VL. Current protein intake in America: analysis of the National Health and Nutrition Examination Survey, 2003‐2004. Am J Clin Nutr 2008;87:1554S–1557S. [DOI] [PubMed] [Google Scholar]
- 13. Houston DK, Tooze JA, Garcia K, Visser M, Rubin S, Harris TB, et al. Protein intake and mobility limitation in community‐dwelling older adults: the Health ABC study. J Am Geriatr Soc 2017;65:1705–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Isanejad M, Mursu J, Sirola J, Kroger H, Rikkonen T, Tuppurainen M, et al. Dietary protein intake is associated with better physical function and muscle strength among elderly women. Br J Nutr 2016;115:1281–1291. [DOI] [PubMed] [Google Scholar]
- 15. Mendonca N, Granic A, Mathers JC, Hill TR, Siervo M, Adamson AJ, et al. Prevalence and determinants of low protein intake in very old adults: insights from the Newcastle 85+ Study. Eur J Nutr 2018;57:2713–2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hengeveld LM, Pelgrom ADA, Visser M, Boer JMA, Haveman‐Nies A, Wijnhoven HAH. Comparison of protein intake per eating occasion, food sources of protein and general characteristics between community‐dwelling older adults with a low and high protein intake. Clin Nutr ESPEN 2019;29:165–174. [DOI] [PubMed] [Google Scholar]
- 17. Berner LA, Becker G, Wise M, Doi J. Characterization of dietary protein among older adults in the United States: amount, animal sources, and meal patterns. J Acad Nutr Diet 2013;113:809–815. [DOI] [PubMed] [Google Scholar]
- 18. Granic A, Mendonca N, Sayer AA, Hill TR, Davies K, Adamson A, et al. Low protein intake, muscle strength and physical performance in the very old: the Newcastle 85+ study. Clin Nutr 2018;37:2260–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Beasley JM, LaCroix AZ, Neuhouser ML, Huang Y, Tinker L, Woods N, et al. Protein intake and incident frailty in the Women's Health Initiative observational study. J Am Geriatr Soc 2010;58:1063–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hengeveld LM, Wijnhoven HAH, Olthof MR, Brouwer IA, Harris TB, Kritchevsky SB, et al. Prospective associations of poor diet quality with long‐term incidence of protein‐energy malnutrition in community‐dwelling older adults: the Health, Aging, and Body Composition (Health ABC) study. Am J Clin Nutr 2018;107:155–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lemieux FC, Filion ME, Barbat‐Artigas S, Karelis AD, Aubertin‐Leheudre M. Relationship between different protein intake recommendations with muscle mass and muscle strength. Climacteric 2014;17:294–300. [DOI] [PubMed] [Google Scholar]
- 22. Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr 1997;65:1220S–1228S, discussion 1229S–1231S. [DOI] [PubMed] [Google Scholar]
- 23. Block G, Hartman AM, Dresser CM, Carroll MD, Gannon J, Gardner L. A data‐based approach to diet questionnaire design and testing. Am J Epidemiol 1986;124:453–469. [DOI] [PubMed] [Google Scholar]
- 24. Elstgeest LEM, Visser M. Nutrition and Food‐related Behaviour study 2014–2015 (side study). 2017; Available from: https://www.lasa-vu.nl/themes/physical/nutrition.html
- 25. Mendonca N, Hill TR, Granic A, Davies K, Collerton J, Mathers JC, et al. Macronutrient intake and food sources in the very old: analysis of the Newcastle 85+ study. Br J Nutr 2016;115:2170–2180. [DOI] [PubMed] [Google Scholar]
- 26. Farsijani S, Payette H, Morais JA, Shatenstein B, Gaudreau P, Chevalier S. Even mealtime distribution of protein intake is associated with greater muscle strength, but not with 3‐y physical function decline, in free‐living older adults: the Quebec longitudinal study on Nutrition as a Determinant of Successful Aging (NuAge study). Am J Clin Nutr 2017;106:113–124. [DOI] [PubMed] [Google Scholar]
- 27. Ocké MC, Buurma‐Rethans EJM, de Boer EJ, Wilson‐van den Hooven C, Etemad‐Ghameslou Z, Drijvers JJMM, et al. Diet of community‐dwelling older adults. Dutch National Food Consumption Survey Older Adults 2010–2012. Bilthoven: National Institute for Public Health and the Environment; 2013. [Google Scholar]
- 28. Pietinen P, Paturi M, Reinivuo H, Tapanainen H, Valsta LM. FINDIET 2007 Survey: energy and nutrient intakes. Public Health Nutr 2010;13:920–924. [DOI] [PubMed] [Google Scholar]
- 29. Leclercq C, Arcella D, Piccinelli R, Sette S, Le Donne C, Turrini A, et al. The Italian National Food Consumption Survey INRAN‐SCAI 2005‐06: main results in terms of food consumption. Public Health Nutr 2009;12:2504–2532. [DOI] [PubMed] [Google Scholar]
- 30. R Core Team . A language and environment for statistical computing R Foundation for Statistical Computing. Vienna, Austria; 2018. Available online at https://www.R-project.org/. [Google Scholar]
- 31. Schwarzer G, meta: an R package for meta‐analysis, in R News. 2007. p. 40–45.
- 32. Barendregt JJ, Doi SA, Lee YY, Norman RE, Vos T. Meta‐analysis of prevalence. J Epidemiol Community Health 2013;67:974–978. [DOI] [PubMed] [Google Scholar]
- 33. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tieland M, Borgonjen‐Van den Berg KJ, van Loon LJ, de Groot LC. Dietary protein intake in community‐dwelling, frail, and institutionalized elderly people: scope for improvement. Eur J Nutr 2012;51:173–179. [DOI] [PubMed] [Google Scholar]
- 35. Balder HF, Virtanen M, Brants HA, Krogh V, Dixon LB, Tan F, et al. Common and country‐specific dietary patterns in four European cohort studies. J Nutr 2003;133:4246–4251. [DOI] [PubMed] [Google Scholar]
- 36. Huseinovic E, Winkvist A, Slimani N, Park MK, Freisling H, Boeing H, et al. Meal patterns across ten European countries ‐ results from the European Prospective Investigation into Cancer and Nutrition (EPIC) calibration study. Public Health Nutr 2016;19:2769–2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chan R, Leung J, Woo J, Kwok T. Associations of dietary protein intake on subsequent decline in muscle mass and physical functions over four years in ambulant older Chinese people. J Nutr Health Aging 2014;18:171–177. [DOI] [PubMed] [Google Scholar]
- 38. Hannan MT, Tucker KL, Dawson‐Hughes B, Cupples LA, Felson DT, Kiel DP. Effect of dietary protein on bone loss in elderly men and women: the Framingham Osteoporosis study. J Bone Miner Res 2000;15:2504–2512. [DOI] [PubMed] [Google Scholar]
- 39. Briefel RR, Sempos CT, McDowell MA, Chien S, Alaimo K. Dietary methods research in the third National Health and Nutrition Examination Survey: underreporting of energy intake. Am J Clin Nutr 1997;65:1203S–1209S. [DOI] [PubMed] [Google Scholar]
- 40. Johnson RK, Goran MI, Poehlman ET. Correlates of over‐ and underreporting of energy intake in healthy older men and women. Am J Clin Nutr 1994;59:1286–1290. [DOI] [PubMed] [Google Scholar]
- 41. Olsen KM, Dahl SA. Health differences between European countries. Soc Sci Med 2007;64:1665–1678. [DOI] [PubMed] [Google Scholar]
- 42. Oksuzyan A, Juel K, Vaupel JW, Christensen K. Men: good health and high mortality. Sex differences in health and aging. Aging Clin Exp Res 2008;20:91–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gregorio L, Brindisi J, Kleppinger A, Sullivan R, Mangano KM, Bihuniak JD, et al. Adequate dietary protein is associated with better physical performance among post‐menopausal women 60‐90 years. J Nutr Health Aging 2014;18:155–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Freedman LS, Commins JM, Moler JE, Arab L, Baer DJ, Kipnis V, et al. Pooled results from 5 validation studies of dietary self‐report instruments using recovery biomarkers for energy and protein intake. Am J Epidemiol 2014;180:172–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Freisling H, van Bakel MM, Biessy C, May AM, Byrnes G, Norat T, et al. Dietary reporting errors on 24 h recalls and dietary questionnaires are associated with BMI across six European countries as evaluated with recovery biomarkers for protein and potassium intake. Br J Nutr 2012;107:910–920. [DOI] [PubMed] [Google Scholar]
- 46. Chen CC, Schilling LS, Lyder CH. A concept analysis of malnutrition in the elderly. J Adv Nurs 2001;36:131–142. [DOI] [PubMed] [Google Scholar]
- 47. Landi F, Calvani R, Tosato M, Martone AM, Ortolani E, Savera G, et al. Anorexia of aging: risk factors, consequences, and potential treatments. Nutrients 2016;8:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Shim JS, Oh K, Kim HC. Dietary assessment methods in epidemiologic studies. Epidemiol Health 2014;36:e2014009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hoffmann K, Boeing H, Dufour A, Volatier JL, Telman J, Virtanen M, et al. Estimating the distribution of usual dietary intake by short‐term measurements. Eur J Clin Nutr 2002;56:S53–S62. [DOI] [PubMed] [Google Scholar]
- 50. Ma Y, Olendzki BC, Pagoto SL, Hurley TG, Magner RP, Ockene IS, et al. Number of 24‐hour diet recalls needed to estimate energy intake. Ann Epidemiol 2009;19:553–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Dodd KW, Guenther PM, Freedman LS, Subar AF, Kipnis V, Midthune D, et al. Statistical methods for estimating usual intake of nutrients and foods: a review of the theory. J Am Diet Assoc 2006;106:1640–1650. [DOI] [PubMed] [Google Scholar]
- 52. Freedman LS, Midthune D, Carroll RJ, Krebs‐Smith S, Subar AF, Troiano RP, et al. Adjustments to improve the estimation of usual dietary intake distributions in the population. J Nutr 2004;134:1836–1843. [DOI] [PubMed] [Google Scholar]
- 53. Murphy SP, Poos MI. Dietary reference intakes: summary of applications in dietary assessment. Public Health Nutr 2002;5:843–849. [DOI] [PubMed] [Google Scholar]
- 54. Beasley JM, Wertheim BC, LaCroix AZ, Prentice RL, Neuhouser ML, Tinker LF, et al. Biomarker‐calibrated protein intake and physical function in the Women's Health Initiative. J Am Geriatr Soc 2013;61:1863–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Holen T, Norheim F, Gundersen TE, Mitry P, Linseisen J, Iversen PO, et al. Biomarkers for nutrient intake with focus on alternative sampling techniques. Genes Nutr 2016;11:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. in DRI dietary reference intakes: applications in dietary assessment. 2000: Washington (DC). [PubMed] [Google Scholar]
- 57. Volpi E, Campbell WW, Dwyer JT, Johnson MA, Jensen GL, Morley JE, et al. Is the optimal level of protein intake for older adults greater than the recommended dietary allowance? J Gerontol A Biol Sci Med Sci 2013;68:677–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bilancio G, Cavallo P, Ciacci C, Cirillo M. Dietary protein, kidney function and mortality: review of the evidence from epidemiological studies. Nutrients 2019;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Devries MC, Sithamparapillai A, Brimble KS, Banfield L, Morton RW, Phillips SM. Changes in kidney function do not differ between healthy adults consuming higher‐ compared with lower‐ or normal‐protein diets: a systematic review and meta‐analysis. J Nutr 2018;148:1760–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2019. J Cachexia Sarcopenia Muscle 2019;10:1143–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Overview of measurement cycles of dietary intake and socio‐demographic and anthropometric factors, presented per study
Table S2.1. Harmonization approach per variable for the four cohorts included
Table S2.2. Harmonization approach per variable for the four surveys included
Table S3. Number of studies and their community‐dwelling older adults included in the meta‐analyses and pooled prevalence rates of protein intake below 1.0 g/kg aBW/d
Table S4. Number of studies and community‐dwelling older adults included in the meta‐analyses and pooled prevalence rates of protein intake below 1.2 g/kg aBW/d
Table S5. Number of studies and community‐dwelling older adults included in the meta‐analyses and pooled prevalence rates of protein intake below 0.8, 1.0, and 1.2 g/kg actual BW/d


