Abstract
Human milk contains high amounts of complex oligosaccharides, which can be utilized especially by Bifidobacterium species in the infant gut as a carbon and energy source. N-acetyl-d-glucosamine is a building block of these oligosaccharides, and molecular details on the release and utilization of this monosaccharide are not fully understood. In this work we have studied some of the enzymatic properties of three N-acetyl-β-D-hexosaminidases encoded by the genome of the intestinal isolate Bifidobacterium longum subsp. infantis ATCC 15697 and the gene expression of the corresponding genes during bacterial growth on human milk oligosaccharides. These enzymes belong to the glycosyl hydrolase family 20, with several homologs in bifidobacteria. Their optimum pH was 5.0 and optimum temperature was 37 °C. The three enzymes were active on the GlcNAcβ1-3 linkage found in lacto-N-tetraose, the most abundant human milk oligosaccharide. Blon_0459 and Blon_0732, but not Blon_2355, cleaved branched GlcNAcβ1-6 linkages found in lacto-N-hexaose, another oligosaccharide abundant in breast milk. Bifidobacterium infantis N-acetyl-β-D-hexosaminidases were induced during early growth in vitro on human milk oligosaccharides, and also during growth on lacto-N-tetraose or lacto-N-neotetraose. The up-regulation of enzymes that convert this monosaccharide into UDP-N-acetylglucosamine by human milk oligosaccharides suggested that this activated sugar is used in peptidoglycan biosynthesis. These results emphasize the complexity of human milk oligosaccharide consumption by this infant intestinal isolate, and provide new clues into this process.
Keywords: Bifidobacterium longum subsp. infantis, Human milk oligosaccharides, N-acetylglucosamine, β-Hexosaminidase, Glycosyl hydrolase, Prebiotics
1. Introduction
Human milk represents the first food introduced to breast-fed infants. It meets all the nutritional requirements of the newborn, and its composition has been refined through evolution to ensure the wellbeing of the infant [1]. An increasing number of positive health outcomes has been linked to breast milk intake in early infancy [2,3].
Breastfeeding has a profound impact on the establishment of the intestinal microbiota. Human milk oligosaccharides (HMO) are present in high concentrations in breast milk [4], and certain bacterial populations in the infant gut microbiota are able to use HMO as a carbon source, especially those belonging to the Bifidobacterium genus [5,6]. Evidence has also assigned HMO a protective role against pathogen colonization [7].
HMO are composed by five monosaccharides: glucose, galactose, fucose, N-acetylneuraminic acid (or sialic acid,) and N-acetylglucosamine (GlcNAc). Several combinations of glycosidic bonds add structural complexity to HMO [4,9], which are not degradable by intestinal enzymes. Newer analytical techniques have recently expanded the number of known HMO species [10,11], revealing a constellation of structures that are not represented in other mammals [12].
GlcNAc forms part of the building blocks found in HMO, such as lacto-N-biose (Galβ1-3GlcNAc) and N-acetyllactosamine (Galβ1-4GlcNAc), as well as different core structures found in glycoproteins and glycolipids abundant in intestinal lumen secretions [13]. GlcNAc is also a structural component of bacterial peptidoglycan, for which metabolic pathways have been well described, based on the de novo synthesis of UDP-GlcNAc [14,15].
Bifidobacterium longum subsp. infantis (Bifidobacterium infantis) is among the few species consistently found to colonize the gastrointestinal tract in breast-fed infants [16,17]. The ability of this bacterium to vigorously utilize HMO as the sole carbon source in vitro [18] has been explained by a unique genetic divergence from other bifidobacteria to metabolize these substrates [19]. Some of these adaptations include the replacement of certain gene clusters for other genes associated to the utilization of fucose and sialic acid [20]; an abundance of ABC transporters and associated Family 1 Solute Binding Proteins (F1SBPs) with affinity for different HMO structures [21]; and finally a higher number of predicted glycosyl hydrolases that target linkages present in HMO. These studies suggest that consumption of HMO by B. infantis includes the import of intact oligosaccharides [21,22] followed by degradation of HMO via the action of intracellular α1-2/3/4 fucosidases [23], α2-3/6-sialidases [24], and β1-3/4 galactosidases [25]. N-acetyl-β-D-hexosaminidases are another set of enzymes thought to play an important role in the consumption of HMO by B. infantis. In this work we examined some of the properties of the three N-acetyl-β-D-hexosaminidases present in B. infantis, as well as the different patterns of gene expression on GlcNAc metabolic pathways induced by different oligosaccharides including HMO.
2. Materials and methods
2.1. Bacteria and media
For routine experiments, B. longum subsp. infantis ATCC 15697 was grown on de Mann–Rogosa–Sharp broth, supplemented with 0.05% w/v l-cysteine (Sigma–Aldrich, St. Louis, MO). Cells were anaerobically grown at 37 °C in a vinyl chamber (Coy Laboratory Products, Grass Lake, MI) containing 5% carbon dioxide, 5% hydrogen, and 90% nitrogen. Competent Escherichia coli BL21 Star and Top10 cells were from Invitrogen (Carlsbad, CA).
2.2. RNA extraction and quantitative PCR
B. infantis cells were grown on chemically defined Zhang-Mills-Block 1 media (ZMB-1 [26],), supplemented with 2% w/v of carbon sources such as lactose or glucose (Sigma); purified HMO [27]; and lacto-N-tetraose (LNT) or lacto-N-neotetraose (LNnT; V-labs, Covington LA). Optical density at 600 nm was continuously assayed using a PowerWave microplate spectrophotometer (BioTek Instruments, Inc., Winoosky, VT), under anaerobic conditions. Each experiment was done at least in duplicate. Cells at exponential phase growing on the different substrates were centrifuged at 12000 × g for 2 min, and resuspended in 1 ml of RNA later (Ambion, Austin, TX), stored overnight at 4 °C and then at −80 °C until use. Cells growing on HMO were recovered at different OD600 values representing early exponential phase, mid-exponential phase I, mid-exponential phase II, late exponential phase and stationary phase (Figure S1). B. infantis cells growing on LNT or LNnT were also recovered at early and mid-exponential phase. RNA extraction and cDNA conversion were performed as previously described [21]. RNA quality was checked in a Bioanalyzer 2100 using the RNA 6000 Nano Kit (Agilent Technologies, Foster City, CA). Relative quantification for each gene listed in Table S1 was obtained as previously described [21], using the Fast Sybr Green Master Mix (Applied Biosystems, Foster City, CA), and reaction conditions as recommended by the manufacturer. Primers for qPCR were designed using the Primer3 software (Table S1;http://frodo.wi.mit.edu).
2.3. Bioinformatic analyses
The Integrated Microbial Genomes [28] database was used to examine the genetic landscapes of genes predicted to participate in GlcNAc metabolism in the genome of B. infantis ATCC 15697 and to infer metabolic pathways found in the genome of B. infantis ATCC 15697.
2.4. Molecular cloning and protein purification
β-Hexosaminidase genes (Blon_0459, Blon_0732 and Blon_2355) were PCR-amplified from chromosomal DNA of B. infantis ATCC 15697 using the primers in Table S1. PCR reactions contained 0.5 μM of each primer, 1 ng DNA, 0.2 mM dNTPs (Fermentas, Glen Burnie, MD), and 2 U of Phusion DNA Polymerase (Finnzymes, Vantaa, Finland) in a 150 μl final volume. PCR was performed in a PTC200 Thermo Cycler (MJ Research, Ramsey, MN), using the following program: initial denaturation at 98 °C for 30 s, 35 cycles of denaturation at 98 ° C 10 s, annealing at 58 °C for 30 s, extension at 72 °C 2 min, and a final extension at 72 °C for 7 min. PCR products were gel purified (Qiaquick Gel Extraction Kit, Qiagen, Valencia CA) and cloned into pET101 using the Champion pET101 Directional TOPO Expression Kit (Invitrogen, Carlsbad, CA). Inserts were sequenced using primers T7prom and T7term (Invitrogen). BL21 Star clones containing the recombinant genes were grown in 100 ml LB broth with 50 μg/ml carbenicillin at 37 °C in a shaker at 250 rpm (Innova-4000, New Brunswick Scientific, Edison, NJ) until cultures reached an OD600 of 0.6, and induced for 6 h with 0.5 mM IPTG at 28 °C (for Blon_0459 and Blon_0732), or with 1 mM IPTG at 37 ° C (for Blon_2355). Bacterial cells were pelleted at 2000 × g in an Eppendorf 5804 centrifuge (Eppendorf, Hauppauge, NY) for 20 min at 4° and stored at −80 °C. Cell pellets were resuspended in Bug-buster Protein Extraction Reagent (EMD Chemicals, Gibbstown, NJ), using 5 ml of the detergent for every 50 ml of culture. To obtain complete lysis, lysozyme (Sigma Aldrich; 50 μl of 50 mg/ml stock), and 200 U DNase I (Roche Applied Sciences, IN) were added. The suspension was vortexed and incubated for 10 min at room temperature, and centrifuged for 20 min at 12000 × g at 4 °C. The supernatant was applied to Bio-Scale Mini Profinity IMAC cartridges, connected to an EP-1 Econo-pump (Bio-Rad, Hercules, CA), and proteins were purified as recommended by the manufacturer. Purity and molecular weight of recombinant proteins were evaluated using 10% SDS-PAGE gels (Bio-Rad). Buffer was exchanged for PBS using Amicon Ultra-15 Centrifugal Filter Units, with a cut-off of 10 kDa (Millipore, Billerica, CA).
2.5. Determination of kinetic parameters
Relative enzymatic activity was determined using 2 mg/ml of 4-nitrophenyl-N-acetyl-β-d-glucosaminide (GlcNAc-pnp; Sigma) and 10 μg of each recombinant enzyme. For optimum pH determinations, McIlvaine buffers [29], with pH values from 4.0 to 8.0 were prepared, and 100 μl reactions contained 80 μl of each buffer, 10 μl of substrate, and 10 μg of enzyme. Reactions were performed in triplicate in 96 microwell plates and incubated for 10 min at 37 °C. After stopping each reaction by the addition of an equal volume of 1 M Na2CO3 the absorbance at 405 nm was read (Synergy2 microplate reader, Biotek, Winoosky, VT). Optimum temperatures were determined at each enzyme optimum pH, and reactions were carried out at 4 °C, 20 °C, 30 °C, 37 °C, 45 °C, 55 °C and 65 °C. Relative activity was determined from A405 reads. Kinetic constants were obtained using substrate concentrations in the range of 0.1 and 4 mM of GlcNAc-pnp and 100 μg of each enzyme in 50 mM of Na2HPO4 under optimum conditions and times within the initial rate of the reaction. Amounts of p-nitrophenol produced were estimated from a standard curve and A405 values. Non-linear regression was used to determine Km and Vmax, using the tool Solver in Microsoft Excel. Enzymatic specificity using 4-nitrophenyl- conjugated substrates was also determined using 10 μg of enzyme, under optimal conditions for 10 min, with 10 μl of each of these substrates at 2 mg/ml in a 100 μl volume reaction (purchased from Sigma unless mentioned): 4-nitrophenyl-β-gal-actopyranoside (Acros Organics, Pittsburgh, PA), 4-nitrophenyl β-d-glucopyranoside, 4-nitrophenyl-α-d-fucopyranoside, 4-nitrophenyl N-acetyl-α-d-glucosaminide and 4-nitrophenyl N-acetyl-β-d-galactosaminide.
2.6. Thin layer chromatography
Chitobiose (GlcNAcβ1-4GlcNAc; V-labs) at a concentration of 1 mg/ml was coincubated with 10 mg of each β-hexosaminidase in 50 mM Na2HPO4 at pH 5.0. Reactions were carried out for 60 min at 37 °C, and products were inactivated at 95 °C for 5 min. LNT and lacto-N-hexaose (LNH) at 1 mg/ml (0.5 μg final) were also coincubated with these enzymes, but with and without the addition of 10 units of β1-3 or β1-4 galactosidase, or both (New Englands Biolabs), for 1 h at 37 °C and pH 5.5. Reactions were heat inactivated at 95 °C for 5 min and spotted in TLC glass Silica-gel plates (Sigma). A mixture of ethyl acetate, acetic acid and water in a 2:1:1 ratio was used as solvent. After drying, plates were sprayed with 0.5% α-napthol and 5% H2SO4 in ethanol. Plates were dried and revealed at 150 °C for 10 min.
3. Results
3.1. Distribution of β-hexosaminidase genes in B. infantis
A search on the genome of B. infantis ATCC 15697 indicated three genes encoding N-acetyl-β-D-hexosaminidases, each containing the pfam motif 00728 and belonging to the glycosyl hydrolase family 20 (GH20). Sequence identity was on average 50% among them, and the encoded enzymes are putatively located intracellularly since they lack of signal peptides. While Blon_0459 and Blon_0732 are located separately in the genome with no evident functional association to other neighboring genes, Blon_2355 is part of the HMO cluster I, a unique locus found in B. infantis species that contains several F1SBPs, ABC permeases and glycosyl hydrolases related to the import and degradation of HMO [19,30]. Interestingly, the protein sequences of B. infantis N-acetyl-β-D-hexosaminidases do not significantly resemble those of Bifidobacterium bifidum, another infant intestinal isolate with known ability to consume HMO [31].
3.2. Enzymatic properties and substrate specificity of B. infantis β-hexosaminidases
In order to understand the possible role of these enzymes on HMO degradation, we cloned, expressed and purified these proteins from E. coli using an N-terminal his-tag. A partial enzymatic characterization using 4-nitrophenyl N-acetyl-β-d-glucosaminide (GlcNAc-pnp) indicated that these three enzymes had a pH optimum of 5.0 and reached maximum activity at 37 °C(Table 1). While Km and kcat values for GlcNAc-pnp were higher for Blon_0732, Blon_2355 showed the higher enzymatic efficiency compared to the other N-acetyl-β-D-hexosaminidases as determined by the kcat/Km ratio (Table 1). Interestingly, none of these enzymes showed appreciable β-N-acetyl-d-galactosaminidase activity, and no detectable activity was observed against Glc-β-pnp, Gal-β-pnp, GlcNAc-α-pnp, GalNAc-α-pnp or Fuc-α-pnp substrates (data not shown).
Table 1.
N-acetyl-β-D-hexosaminidases optimum reaction conditions and apparent kinetics.
| Optimum pH | Optimum temperature | Km (mM) | Kcat(s−1) | kcat/Km (s−1 M−1) | |
|---|---|---|---|---|---|
| Blon_0459 | 5.0 | 37 °C | 0.33 ± 0.04 | 6.92 ± 1.34 | 2.14 × 105 ± 6.49 × 103 |
| Blon_0732 | 5.0 | 37 °C | 1.64 ± 0.85 | 104.16 ± 46.2 | 6.5 × 104 ± 5.43 × 102 |
| Blon_2355 | 5.0 | 37 °C | 0.48 ± 0.02 | 74.73 ± 44.1 | 1.56 × 106 ± 3.73 × 104 |
All three enzymes degraded chitobiose (GlcNAcβ1-4GlcNAc; Figure S2), as determined using TLC. LNT and LNH are abundant isomers found in human milk, and they were used to study some of the linkage preferences of these enzymes after galactose removal by β1-3 or β1-4 specific galactosidases (Fig. 1). Incubation of LNT with a β1-3 galactosidase generated galactose and lacto-N-triose. Coincubation of this β-galactosidase and Blon_0459, Blon_0732 or Blon_2355 generated a spot with the same migration as lactose, indicating that these three enzymes are able to cleave the GlcNAcβ1-3Gal linkage (Fig. 1, lanes 9–12).
Fig. 1.
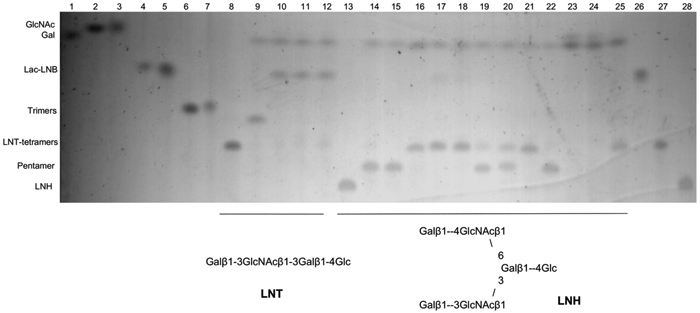
Thin layer chromatography of co-incubations of B. infantis N-acetyl-β-D-hexosaminidases with LNT or LNH after treatment with β-galactosidases. Structures are illustrated below the figure. Lanes 1–8 and 26–28: standards (as indicated in the figure); lane 9: LNT with specific β1-3 galactosidase; lanes 10–12: LNT with a β1-3 galactosidase and Blon_0459, Blon_0732 or Blon_2355. Lane 13: LNH; lanes 14–16: LNH with either a β1-3, a β1-4 or both specific galactosidases; lanes 17–19: LNH with a β1-3 galactosidase and Blon_0459, Blon_0732 and Blon_2355, respectively; lanes 20–22: LNH with a β1-4 galactosidase and either Blon_0459, Blon_0732 and Blon_2355; lanes 23–25: LNH with both β1-3 and β1-4 galactosidase, as well as either Blon_0459, Blon_0732 and Blon_2355.
Unlike LNT, LNH is a branched oligosaccharide (Fig. 1, lane 13). Using a β1-3 galactosidase, LNH was reduced to a pentamer (Fig. 1, lane 14), exposing a GlcNAcβ1-3Gal linkage that was successfully cleaved by Blon_0459 and Blon_0732, and to a lesser extent by Blon_2355 (Fig. 1, lanes 17–19). Using a β1-4 galactosidase, the pentasaccharide generated (Fig. 1, lane 15) exposed now a GlcNAcβ1-6Gal linkage, which was cleaved by Blon_0732, partially by Blon_0459, but not at all by Blon_2355 (Fig. 1, lanes 20–22). Finally, coincubation of LNH with the two specific β-galactosidases generated a tetramer (Fig. 1, lane 16), which after addition of Blon_0459 or Blon_0732 was reduced to galactose, GlcNAc and glucose (Fig. 1, lanes 23–25). This later catalytic activity was not observed for Blon_2355.
3.3. Gene expression of N-acetyl-β-D-hexosaminidases in B. infantis under growth on different carbohydrates
The levels of gene expression of the three encoded N-acetyl-β-D-hexosaminidases in B. infantis were first evaluated at different time points during growth on HMO as the sole carbon source (Figure S1). These levels were compared to those of B. infantis cells growing exponentially on lactose. We observed that all three genes were induced over two-fold at early exponential phase (Fig. 2A), suggesting a role for these proteins in HMO metabolism given their substrate specificities. Interestingly, a gradual decrease in their expression was observed at mid and late exponential phase, as well as during stationary phase where these genes were apparently repressed.
Fig. 2.
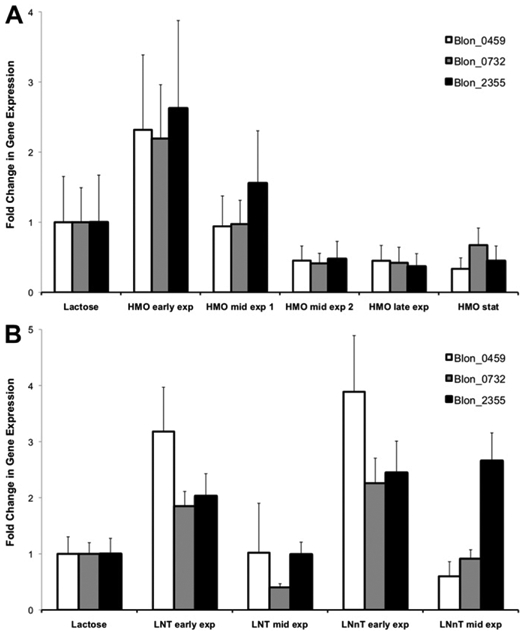
Relative quantification of the gene expression levels of β-hexosaminidase genes at different time points during growth on HMO (A), LNT or LNnT (B). Expression is relative to their levels during growth on lactose. Error bars represent two biological replicates.
A similar trend was observed for B. infantis cells growing on LNT or LNnT (Fig. 2B). The three encoded N-acetyl-β-D-hexosamini-dases were up-regulated during early growth on these substrates, but their expression drastically decreased during mid-exponential growth. Only Blon_2355 was still considerably expressed at mid-exponential growth on LNnT, but not on LNT.
3.4. Gene expression of GlcNAc-related metabolic pathways
In previous studies aspects of HMO metabolism in B. infantis have been studied using proteomics during mid-exponential phase [19]. B. infantis possesses several gene clusters that potentially participate in the metabolism of GlcNAc and other monosaccharides besides the HMO cluster I (Figure S3). Changes in gene expression of these genes were also studied by qPCR at exponential phase. Several genes in the LNB/GNB cluster (genes Blon_2171 to Blon_2177 in B. infantis) were shown to be up-regulated to a different extent during growth on LNT or LNnT at mid-exponential phase (Fig. 3A). These genes include Blon_2173, encoding an N-acetylhexosamine-1-kinase, Blon_2174, a lacto-N-biose phosphorylase, and Blon_2172, galactose-1-phosphate uridyltransferase. The expression levels for Blon_2171 were not significantly altered but this gene showed a constitutive expression [19]. Their enzymatic products constitute a Leloir-like pathway that generates Glc-1-P and UDP-GlcNAc using LNB or GNB as substrates [32,33].
Fig. 3.
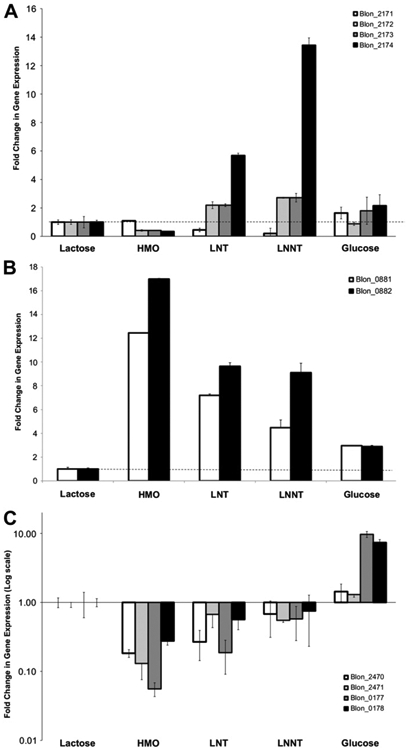
Fold change in gene expression for genes predicted to participate in the metabolism of GlcNAc in B. infantis after exponential growth on the substrates listed in the x-axis. Results are normalized to levels on lactose (dashed line), and error bars represent three biological replicates. A: Genes in the LNB/GNB pathway; B: GlcNAc-6-P deacetylase and GlcN-6-P isomerase; C: (results are presented in log scale) a PTS system putative for GlcNAc import and metabolism.
Blon_0882, a gene encoding a GlcNAc-6-phosphate deacetylase (nagA), and Blon_0881, which gene product is a glucosamine-6-phosphate isomerase (nagB), were induced during exponential growth on HMO, LNT and LNnT (Fig. 3B). These genes are located next to an ABC importer that was previously shown to bind specific mono- and disaccharides containing GlcNAc [21].
Finally another set of genes in the genome of this bacterium represents a phosphotransferase (PTS) system with predicted affinity for GlcNAc. These genes, Blon_2470 (IIA subunit) and Blon_2471 (IIBC subunit), as well as other components in the PTS system, were repressed between 5 and 10-fold during logarithmic growth using HMO, LNT or LNnT as the sole carbon source (Fig. 3C). Only Blon_0177 and Blon_0178, encoding a phosphocarrier protein and a phosphoenolpyruvate protein phosphotransferase, respectively, were induced by glucose, probably associated to another PTS system that imports glucose in B. infantis (Blon_2183).
4. Discussion
Certain infant-borne Bifidobacterium species are known to efficiently consume HMO, one of the most abundant components of human milk [20,22]. B. infantis is characterized by the preferential import of intact HMO with different degrees of polymerization [18]. The lack of lacto-n-biosidase activity in the strain ATCC 15697 suggests that glycosyl hydrolases act sequentially on different HMO isomers. An α-sialidase encoded by the HMO cluster I released sialic acid from HMO [24]. Recently α-fucosidases with activity on different fucosylated HMO [23] and β-galactosidases acting on β1-3/4 linkages of HMO have been characterized [25].
In this work we have studied the properties and expression of three N-acetyl-β-D-hexosaminidases encoded in the genome of B. infantis ATCC 15697. Since GlcNAc in HMO is not located in terminal positions, the activity of N-acetyl-β-D-hexosaminidases requires previous cleavage by the glycosyl hydrolases aforementioned. B. infantis N-acetyl-β-D-hexosaminidases share only 26–28% identity with BbhI, a β-hexosaminidase found in B. bifidum JCM 1254 and active on LNnT and partially on LNH [31]. Their low identities suggest that this enzymatic activity in these microorganisms is convergent and in line with their remarkably different HMO consumption strategies [34]. Interestingly, β-hexosaminidases in B. infantis showed different substrates affinities. Blon_2355 activity was apparently limited to linear oligosaccharides such as LNT, while Blon_0732 and to a lesser extent Blon_0459 cleaved both linear GlcNAcβ1-3Gal in LNT and branched GlcNAcβ1-6Gal as found in LNH. The higher enzymatic efficiency of Blon_2355, given by its kcat/km ratio (Table 1) might also indicate that this enzyme is highly specialized in the release of GlcNAc from linear oligosaccharides.
Considering their enzymatic activities and induction during growth on HMO, the results of this study suggest that Blon_0459, Blon_0732 and Blon_2355 participate in the release of GlcNAc from HMO. Interestingly, their up-regulation was restricted to the early exponential phase, and conversely their expression apparently was down-regulated during mid and late logarithmic growth as well as stationary phase. This result was unexpected, since other important genes associated to HMO consumption and degradation are readily induced during mid and late exponential phase [21,23]. The tight regulation of the expression of these genes might still allow B. infantis N-acetyl-β-D-hexosaminidases to be active during subsequent phases of growth. Bacterial transcriptional responses to HMO are therefore complex and dynamic, and this is likely to be related to the heterogeneous nature of HMO, based on chemically diverse isomers found at different concentrations.
Genomic analysis indicated three different genetic clusters potentially involved in the metabolism of GlcNAc by B. infantis (Figure S3). Genes present in the LNB/GNB cluster were shown to be up-regulated during B. infantis growth on LNT and LNnT (Fig. 3A; [32]). It is possible that an N-acetylhexosamine-1-kinase in this cluster (Blon_2173; NahK) participates in GlcNAc metabolism. This enzyme generates GlcNAc-1-P from GlcNAc (Figure S4A; [35]), but downstream pathways metabolizing GlcNAc to glycolysis require GlcNAc-6-P [36,37]. The genome of B. infantis does not contain any evident function that can interconvert these substrates, except genes that participate in the de novo biosynthesis of UDP-GlcNAc (Figure S4B), catalyzing the inverse reaction. Another gene in the LNB/GNB cluster induced by HMO, LNT and LNnT is Blon_2172, an encoded galactose-1-phosphate uridyltransferase that converts GlcNAc-1-P into UDP-GlcNAc [33]. It is possible that under these conditions, the UDP-GlcNAc formed is used directly in peptidoglycan synthesis, linking HMO consumption and GlcNAc release with cell wall biogenesis. The idea of GlcNAc being used as a peptidoglycan building block is also supported by the down-regulation of a PTS system specific for GlcNAc import (Fig. 3C), which generates GlcNAc-6-P from GlcNAc.
Growth on HMO and some of their isomers also led to the up-regulation of GlcNAc-6-P deacetylase (Blon_0882; nagB) and Blon_0881 (glucosamine-6-P isomerase). Considering that metabolism of sialic acid includes the formation of GlcNAc-6-P (Figure S4C), it is possible that the induction of these genes leads to utilization of sialic acid in glycolysis. Other genes in this pathway were also shown to be up-regulated during growth on HMO compared to glucose and lactose (Figure S5). Therefore, these results suggest that under these conditions sialic acid present in HMO represents a carbon and energy source for B. infantis, while GlcNAc might be used as a building block in peptidoglycan synthesis. Further mutational and biochemical experiments are required to support this hypothesis.
In summary, this study presents evidence for the molecular mechanisms found in the infant intestinal isolate B. infantis, associated to the release of GlcNAc from complex HMO. The activity of N-acetyl-β-D-hexosaminidases is complementary to previously characterized α-sialidases, α-fucosidases and β-galactosidases active on HMO. B. infantis N-acetyl-β-D-hexosaminidases cleaved linear or branched HMO, and they are up-regulated exclusively during early growth on HMO, LNT or LNnT. This work also suggests that GlcNAc could be directly used as a substrate for peptidoglycan synthesis, while other monosaccharides found in HMO could be used in glycolysis. These results further contribute to unraveling the adaptations that this microorganism has evolved to respond and utilize human milk components such as HMO.
Supplementary Material
Acknowledgements
Daniel Garrido was funded in part through a Fulbright-Conicyt Chile scholarship, and Santiago Ruiz-Moyano was supported by the Ministry of Education and Science of Spain and University of Extremadura, Spain. This work was supported by grants from the University of California Discovery Grant Program, the California Dairy Research Foundation and National Institutes of Health Awards R01HD065122, and R01HD061923. The authors would like to thank Dr. David Sela for his important help on the elaboration of this work.
Abbreviations:
- HMO
human milk oligosaccharides
- LNT
lacto-N-tetraose
- LNnT
lacto-N-neotetraose
- LNB
lacto-N-biose
- GlcNAc
N-acetylglucosamine
- GNB
galacto-N-biose
- LNH
lacto-N-hexaose
Footnotes
Appendix A. Supplementary material
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.anaerobe.2012.04.012.
References
- [1].German JB, Freeman SL, Lebrilla CB, Mills DA. Human milk oligosaccharides: evolution, structures and bioselectivity as substrates for intestinal bacteria. Nestle Nutr Workshop Ser Pediatr Program 2008;62:205–18. discussion 18–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Le Huerou-Luron I, Blat S, Boudry G. Breast- v. formula-feeding: impacts on the digestive tract and immediate and long-term health effects. Nutr Res Rev 2010;23:23–36. [DOI] [PubMed] [Google Scholar]
- [3].Adlerberth I, Wold AE. Establishment of the gut microbiota in Western infants. Acta Paediatr 2009;98:229–38. [DOI] [PubMed] [Google Scholar]
- [4].Kunz C, Rudloff S, Baier W, Klein N, Strobel S. Oligosaccharides in human milk: structural, functional, and metabolic aspects. Annu Rev Nutr 2000;20:699–722. [DOI] [PubMed] [Google Scholar]
- [5].Marcobal A, Barboza M, Froehlich JW, Block DE, German JB, Lebrilla CB, et al. Consumption of human milk oligosaccharides by gut-related microbes. J Agric Food Chem 2010;58:5334–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kiyohara M, Tachizawa A, Nishimoto M, Kitaoka M, Ashida H, Yamamoto K. Prebiotic effect of lacto-N-biose I on bifidobacterial growth. Biosci Biotechnol Biochem 2009;73:1175–9. [DOI] [PubMed] [Google Scholar]
- [7].Newburg DS, Ruiz-Palacios GM, Morrow AL. Human milk glycans protect infants against enteric pathogens. Annu Rev Nutr 2005;25:37–58. [DOI] [PubMed] [Google Scholar]
- [9].Ninonuevo MR, Park Y, Yin H, Zhang J, Ward RE, Clowers BH, et al. A strategy for annotating the human milk glycome. J Agric Food Chem 2006;54:7471–80. [DOI] [PubMed] [Google Scholar]
- [10].Wu S, Tao N, German JB, Grimm R, Lebrilla CB. Development of an annotated library of neutral human milk oligosaccharides. J Proteome Res 2010;9: 4138–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Wu S, Grimm R, German JB, Lebrilla CB. Annotation and structural analysis of sialylated human milk oligosaccharides. J Proteome Res 2011;10:856–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Tao N, Wu S, Kim J, An HJ, Hinde K, Power ML, et al. Evolutionary glycomics: characterization of milk oligosaccharides in primates. J Proteome Res 2011;10:1548–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Varki A Essentials of glycobiology. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 2009. [PubMed] [Google Scholar]
- [14].Foley S, Stolarczyk E, Mouni F, Brassart C, Vidal O, Aissi E, et al. Characterisation of glutamine fructose-6-phosphate amidotransferase (EC 2.6.1.16) and N-acetylglucosamine metabolism in Bifidobacterium. Arch Microbiol 2008; 189:157–67. [DOI] [PubMed] [Google Scholar]
- [15].Ghosh S, Rao KH, Sengupta M, Bhattacharya SK, Datta A. Two gene clusters coordinate for a functional N-acetylglucosamine catabolic pathway in Vibrio cholerae. Mol Microbiol 2011;80:1549–60. [DOI] [PubMed] [Google Scholar]
- [16].Favier CF, de Vos WM, Akkermans AD. Development of bacterial and bifidobacterial communities in feces of newborn babies. Anaerobe 2003;9:219–29. [DOI] [PubMed] [Google Scholar]
- [17].Roger LC, McCartney AL. Longitudinal investigation of the faecal microbiota of healthy full-term infants using fluorescence in situ hybridization and denaturing gradient gel electrophoresis. Microbiology 2010;156:3317–28. [DOI] [PubMed] [Google Scholar]
- [18].LoCascio RG, Ninonuevo MR, Freeman SL, Sela DA, Grimm R, Lebrilla CB, et al. Glycoprofiling of bifidobacterial consumption of human milk oligosaccharides demonstrates strain specific, preferential consumption of small chain glycans secreted in early human lactation. J Agric Food Chem 2007;55:8914–9. [DOI] [PubMed] [Google Scholar]
- [19].Sela DA, Chapman J, Adeuya A, Kim JH, Chen F, Whitehead TR, et al. The genome sequence of Bifidobacterium longum subsp. infantis reveals adaptations for milk utilization within the infant microbiome. Proc Natl Acad Sci U S A 2008;105:18964–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Sela DA, Mills DA. Nursing our microbiota: molecular linkages between bifidobacteria and milk oligosaccharides. Trends Microbiol 2010;18:298–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Garrido D, Kim JH, German JB, Raybould HE, Mills DA. Oligosaccharide binding proteins from Bifidobacterium longum subsp. infantis reveal a preference for host glycans. PLoS One 2011;6:e17315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Asakuma S, Hatakeyama E, Urashima T, Yoshida E, Katayama T, Yamamoto K, et al. Physiology of consumption of human milk oligosaccharides by infant gut-associated bifidobacteria. J Biol Chem 2011;286:34583–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Sela DA, Garrido D, Lerno L, Wu S, Tan K, Eom HJ, et al. Bifidobacterium longum subsp. infantis ATCC 15697 alpha-fucosidases are active on fucosylated human milk oligosaccharides. Appl Environ Microbiol 2012;78:795–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Sela DA, Li Y, Lerno L, Wu S, Marcobal AM, German JB, et al. An infant-associated bacterial commensal utilizes breast milk sialyloligosaccharides. J Biol Chem 2011;286:11909–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Yoshida E, Sakurama H, Kiyohara M, Nakajima M, Kitaoka M, Ashida H, et al. Bifidobacterium longum subsp. infantis uses two different beta-galactosidases for selectively degrading type-1 and type-2 human milk oligosaccharides. Glycobiology 2012;22:361–8. [DOI] [PubMed] [Google Scholar]
- [26].Zhang G, Mills DA, Block DE. Development of chemically defined media supporting high-cell-density growth of lactococci, enterococci, and streptococci. Appl Environ Microbiol 2009;75:1080–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Ward RE, Ninonuevo M, Mills DA, Lebrilla CB, German JB. In vitro fermentation of breast milk oligosaccharides by Bifidobacterium infantis and Lactobacillus gasseri. Appl Environ Microbiol 2006;72:4497–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Markowitz VM, Korzeniewski F, Palaniappan K, Szeto E, Werner G, Padki A, et al. The integrated microbial genomes (IMG) system. Nucleic Acids Res 2006;34:D344–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].McIlvaine TC. A buffer solution for colorimetric comparison. J Biol Chem 1921;49:183–6. [Google Scholar]
- [30].LoCascio RG, Desai P, Sela DA, Weimer B, Mills DA. Broad conservation of milk utilization genes in Bifidobacterium longum subsp. infantis as revealed by comparative genomic hybridization. Appl Environ Microbiol 2010;76:7373–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Miwa M, Horimoto T, Kiyohara M, Katayama T, Kitaoka M, Ashida H, et al. Cooperation of beta-galactosidase and beta-N-acetylhexosaminidase from bifidobacteria in assimilation of human milk oligosaccharides with type 2 structure. Glycobiology 2010;20:1402–9. [DOI] [PubMed] [Google Scholar]
- [32].Kitaoka M, Tian J, Nishimoto M. Novel putative galactose operon involving lacto-N-biose phosphorylase in Bifidobacterium longum. Appl Environ Microbiol 2005;71:3158–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Nishimoto M, Kitaoka M. Identification of N-acetylhexosamine 1-kinase in the complete lacto-N-biose I/galacto-N-biose metabolic pathway in Bifidobacterium longum. Appl Environ Microbiol 2007;73:6444–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Garrido D, Barile D, Mills DA. A molecular basis for bifidobacterial enrichment in the infant gastrointestinal tract. Adv Nutr 2012;3:415S–21S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Cai L, Guan W, Kitaoka M, Shen J, Xia C, Chen W, et al. A chemoenzymatic route to N-acetylglucosamine-1-phosphate analogues: substrate specificity investigations of N-acetylhexosamine 1-kinase. Chem Commun (Camb);2009:2944–6. [DOI] [PubMed] [Google Scholar]
- [36].Vogler AP, Lengeler JW. Analysis of the nag regulon from Escherichia coli K12 and Klebsiella pneumoniae and of its regulation. Mol Gen Genet 1989;219:97–105. [DOI] [PubMed] [Google Scholar]
- [37].Vincent F, Davies GJ, Brannigan JA. Structure and kinetics of a monomeric glucosamine 6-phosphate deaminase: missing link of the NagB superfamily? J Biol Chem 2005;280:19649–55. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


