Bats host a large numbers of viruses, many of which are zoonotic. In the United States, the big brown bat (Eptesicus fuscus) is widely distributed and lives in small colonies that roost in cavities, often in human dwellings, leading to frequent human interaction. Viral metagenomic sequencing of samples from an E. fuscus bat submitted for rabies testing identified the first exogenous bat Deltaretrovirus. The E. fuscus deltaretrovirus (EfDRV) genome consists of the typical deltaretrovial 5′-gag-pro-pol-env-3′ genes along with genes encoding two putative transcriptional transactivator proteins distantly related to the Tax protein of human T-cell lymphotrophic virus and nuclear antigen 3B of Epstein-Barr virus. Searches of the E. fuscus genome sequence failed to identify endogenous EfDRV. RT-PCR targeting the EfDRV pol gene identified 4/60 (6.7%) bats with positive results. Together, these results suggest that EfDRV is exogenous. As all members of Deltaretrovirus are associated with T- and B-cell malignancies or neurologic disease, further studies on possible zoonosis are warranted.
KEYWORDS: bat, emerging virus, public health, retrovirus, zoonosis
ABSTRACT
Bats are the reservoir for a large number of zoonotic viruses, including members of Coronaviridae (severe acute respiratory syndrome coronavirus [SARS-CoV] and SARS-CoV-2), Paramyxoviridae (Hendra and Nipah viruses), Rhabdoviridae (rabies virus), and Filoviridae (Ebola virus) as exemplars. Many retroviruses, such as human immunodeficiency virus, are similarly zoonotic; however, only infectious exogenous gammaretroviruses have recently been identified in bats. Here, viral metagenomic sequencing of samples from bats submitted for rabies virus testing, largely due to human exposure, identified a novel, highly divergent exogenous Deltaretrovirus from a big brown bat (Eptesicus fuscus) in South Dakota. The virus sequence, corresponding to Eptesicus fuscus deltaretrovirus (EfDRV), comprised a nearly complete coding region comprised of canonical 5′-gag-pro-pol-env-3′ genes with 37% to 51% identity to human T-lymphotropic virus (HTLV), an infectious retrovirus that causes T-cell lymphoma. A putative tax gene with 27% identity to HTLV was located downstream of the pol gene along with a gene harbored in an alternative reading frame which possessed a conserved domain for an Epstein-Barr virus nuclear antigen involved in gene transactivation, suggesting a regulatory function similar to that of the deltaretrovirus rex gene. A TaqMan reverse transcriptase PCR (RT-PCR) targeting the pol gene identified 4/60 (6.7%) bats as positive for EfDRV, which, combined with a search of the E. fuscus genome that failed to identify sequences with homology to EfDRV, suggests that EfDRV is an infectious exogenous virus. As all known members of Deltaretrovirus can cause malignancies and E. fuscus is widely distributed in the Americas, often with a colonial roosting behavior in human dwellings, further studies are needed to investigate potential zoonosis.
IMPORTANCE Bats host a large numbers of viruses, many of which are zoonotic. In the United States, the big brown bat (Eptesicus fuscus) is widely distributed and lives in small colonies that roost in cavities, often in human dwellings, leading to frequent human interaction. Viral metagenomic sequencing of samples from an E. fuscus bat submitted for rabies testing identified the first exogenous bat Deltaretrovirus. The E. fuscus deltaretrovirus (EfDRV) genome consists of the typical deltaretrovial 5′-gag-pro-pol-env-3′ genes along with genes encoding two putative transcriptional transactivator proteins distantly related to the Tax protein of human T-cell lymphotrophic virus and nuclear antigen 3B of Epstein-Barr virus. Searches of the E. fuscus genome sequence failed to identify endogenous EfDRV. RT-PCR targeting the EfDRV pol gene identified 4/60 (6.7%) bats with positive results. Together, these results suggest that EfDRV is exogenous. As all members of Deltaretrovirus are associated with T- and B-cell malignancies or neurologic disease, further studies on possible zoonosis are warranted.
OBSERVATION
Bats are a large, ancient, and incredibly diverse order of mammals that inhabit all continents and ecosystems other than Antarctica (1). Importantly, bats are the reservoir for a large number of viruses, many of which are zoonotic (2). Recent significant human epidemics resulting from zoonotic transmission from bats include those caused by severe acute respiratory syndrome coronavirus, henipaviruses (Hedra and Nipah viruses), Menangle virus, and Ebola virus and, likely most recently, the global pandemic caused by severe acute respiratory syndrome coronavirus 2 (3). Additionally, bats are a common reservoir for rabies virus.
The family Retroviridae consists of a large, diverse array of single-stranded RNA (ssRNA) viruses that cause a number of clinically and economically significant diseases (4). A distinguishing feature of the retrovirus life cycle is the formation of a linear double-stranded DNA (dsDNA) genome that can integrate into the host genome to form a provirus. This integration into the host genome can disrupt key cell cycle pathways, leading to malignant transformations and disease such as cancer, immunodeficiencies, autoimmune disorders, or inflammatory diseases. Retroviruses can spread horizontally as infectious agents referred to as exogenous or vertically once integrated into the genome as a provirus. Remnant or “fossil” retroviruses are common in vertebrate genomes and comprise significant amounts of the host genome (5, 6).
Compared to other Retroviridae genera, Deltaretrovirus consists of only a small number of viruses with limited host range (4). There are three recognized species of primate T-lymphotrophic virus (TLV) that infect humans (HTLV) and simians (STLV). While the persistent infection is often asymptomatic, 4% to 5% of HTLV-1 infections progress to leukemia or lymphoma (7). HTLV-1 can also cause HTLV-1-associated myelopathy/tropical spastic paraparesis (8).
The remaining Deltaretrovirus species, bovine leukemia virus (BLV), causes losses to the U.S. dairy industry in excess of 200 million dollars annually due to decreased cow longevity, milk production, and sale price due to carcass condemnation resulting from lymphoma (9). Additionally, BLV has an immunosuppressive effect on the bovine immune system (10). BLV has been eradicated from many countries, so it also represents a trade barrier. More troubling are multiple studies that have associated the detection of BLV in human breast tissue with breast cancer (11).
Compared to domestic animals, very little viral surveillance is performed in wildlife. Bats are a reservoir of rabies virus and represent the most commonly detected rabies carriers in the United States. Additionally, bats are the reservoir of numerous zoonotic diseases worldwide and so are of particular concern when exposed to humans (1, 12). Here, we utilized big brown bats (Eptesicus fuscus) submitted for rabies detection that tested negative for rabies virus to screen for potential novel and zoonotic viruses using viral metagenomic sequencing.
All of the bats (n = 60) were submitted to the South Dakota State University Animal Disease Research and Diagnostic Laboratory between March and July 2020. The lungs and heart were dissected from 20 bats and homogenized in a minimal volume of phosphate-buffered saline before centrifugation was performed at 20,000 × g for 5 min to pellet debris. Next, 80 μl of sample was transferred to a microcentrifuge tube and treated with a cocktail of nucleases to digest unprotected nucleic acids. Nucleic acid isolation, reverse transcription, second strand synthesis, and amplification were performed as previously described (13). Sequencing libraries were constructed for each bat individually using a Nextera XT library preparation kit followed by sequencing on a MiSeq instrument using paired 151-bp reads. Approximately 200,000 to 500,000 reads were generated per sample. Sequenced reads were trimmed of adapter sequences using onboard software before being exported to CLC Genomics and assembled de novo. Contig sequences were analyzed by BLASTX using the BLAST2Go plugin incorporated into the software package.
One sample contained three contigs (691 to 1,259 bp) most similar to HTLV. Metagenomic sequencing was performed on this sample multiple times. Approximately 1.1 million combined sequencing reads were used to assemble a contig 6,597 bp in length that was verified by Sanger sequencing. The sample was collected from a bat found dead in a private residence in South Dakota.
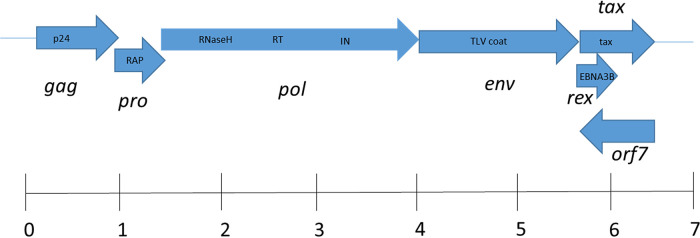
Open reading frame (ORF) and BLASTP analysis identified viral proteins Gag-Pro-Pol-Env with 37% to 51% identity to proteins encoded by HTLV and STLV (Fig. 1). Translation of the overlapping Gag, Pro, and Pol proteins presumably occurs due to ribosomal −1 frameshifts, as a presumptive slippage sequence, 6(A)-9 nt (nucleotides)-5(G)-10 nt-5(C), was identified at the 3′ end of gag in the pro overlap region that is very similar to the conserved slippage sequence 6(A)-8 nt-6(G)-11 nt-6(C). Similarly, a ribosomal slippage sequence matching HTLV-1 and HTLV-2 (TTTAAAC) was identified near the predicted 3′ end of pro in the region that overlaps pol. A putative Tax homolog was identified immediately downstream of env based on 25% identity with HTLV. An ORF-overlapping env encoded a predicted 238-amino-acid (a.a.) protein which failed to show significant homology to known proteins by BLASTP; however, it did possess a conserved domain for an Epstein-Barr virus nuclear antigen involved in gene transactivation, suggesting a regulatory role in viral replication similar to that identified for the deltaretrovirus rex gene (14). A final ORF encoding a 316-a.a. protein was identified on the complementary strand that overlaps the putative tax and rex ORFs. This predicted protein showed no similarity to known proteins by BLASTP analysis. Similarly to other deltaretroviruses, the nucleotide composition strongly favored cytosine (32.4%) compared to adenine, guanine, and thymine (21.0% to 24.4%).
FIG 1.
Genomic structure of Eptesicus fuscus deltaretrovirus. Open reading frames (ORF) corresponding to gag, pro, pol, env, and tax were identified by BLASTP based on homology with primate T-cell lymphotropic virus. The ORF harboring the putative rex gene contains an Epstein-Barr virus nuclear antigen 3B (EBNA3B) conserved domain. Conserved retroviral domains identified by BLASTP are shown in boxes within each ORF. ORF7 had no similarity to known proteins or conserved domains. Abbreviations: retroviral aspartyl protease (RAP), reverse transcriptase (RT), integrase (IN). The scale bar indicates size in kilobases.
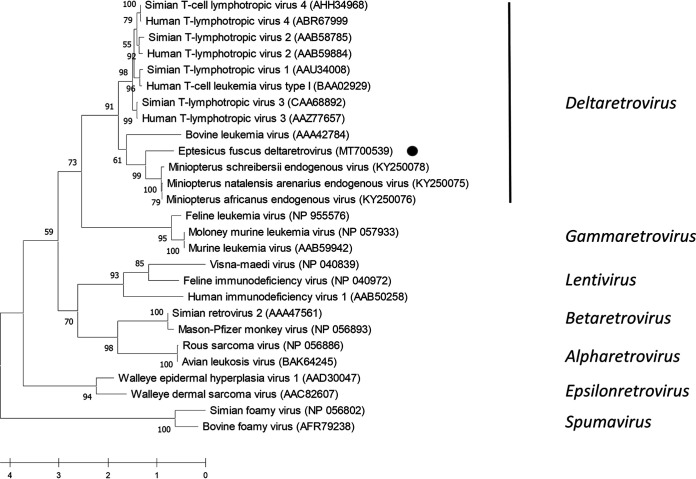
Phylogenetic analysis was performed on partial Gag amino acid sequences using representatives from all Retroviridae genera. Also included in this analysis were Gag sequences from an endogenous Deltaretrovirus identified in Miniopteridae genomes (15). Sequences were aligned in MegaX by the use of ClustalW followed by phylogenetic reconstruction using the best-fitting Whelan and Goldman frequency model (16). Tree topology was evaluated with 1,000 bootstrap replicates. EfDRV formed a sister group to endogenous Miniopteridae deltaretroviruses in a clade with BLV in the ancestral position (Fig. 2). A sister clade contained primate T-lymphotrophic viruses. Similar analyses performed with the Pol and Env amino acid sequences placed EfDRV in the same taxonomic position (data not shown). Previous work found that deltaretrovirus endogenization in Miniopteridae occurred 20 to 45 million years ago, suggesting that EfDRV has been circulating in bats for at least that amount of time (15). More recently, endogenous deltaretrovirus sequences were identified in cetaceans, insectivores, and carnivores (17).
FIG 2.
Phylogenetic analysis of the partial Gag protein (374 amino acids). Sequences were aligned by the use of ClustalW, and the phylogenetic tree was inferred by maximum-likelihood analysis using the Whelan and Goldman +F model implemented in MegaX (16). The robustness of the tree topology was evaluated with 1,000 bootstrap replicates. The scale bar indicates number of amino acid substitutions per site. Positions with less than 80% coverage were eliminated from the analysis. Eptesicus fuscus deltaretrovirus (EfDRV) is indicated by a bullet symbol (•).
A BLASTN search of the Eptesicus fuscus genome failed to find any significant matches (expectation values ≥ 0.03) to the EfDRV genome. Next, a TaqMan PCR was designed targeting the EfDRV pol gene using the following primers and probe: forward primer, 5′-CTATTTCCTGGCTACTGACACC; reverse primer, 5′-GGTAAGGTAGTGATGGTGCG; probe, 5′-6-carboxyfluorescein (FAM)-CCTCGTGATTTGGCTCCTTGGGT. Quantitative reverse transcriptase PCR (QRT-PCR) was performed on the 20 bat samples subjected to metagenomic sequencing and additionally on 40 viscera homogenates prepared from bats submitted for rabies virus testing. Four of the 60 (6.7%) bats were positive for EfDRV (threshold cycle [CT] values, 26.5 to 33.5), including the sample where EfDRV was identified by metagenomic sequencing. Amplification and sequencing of an 847-bp product spanning parts of the pro and pol genes using primers 621F (5′-CTCCTGCGAGCTTGTGCTAATG) and 1624R (5′-GGGAGAACTTCCCAAGCGTAAC) found 97% to 100% identity to the EfDRV genome. Treatment of sample nucleic acids with DNase did not affect Eptesicus fuscus deltaretrovirus (EfDRV) detection by QRT-PCR, while treatment with RNase rendered EfDRV undetectable. Together, these results suggest that EfDRV is an exogenous infectious virus.
Recently, multiple exogenous gammaretroviruses related to koala retrovirus were identified in bats from Asia and Australia (18). Prior to this report, only endogenous retroviruses originating from multiple genera had been identified in bat genomes (15, 19, 20). The infection of koalas with koala retrovirus occurs both vertically and horizontally (21, 22). Koala retrovirus has been endogenized in Northern Australia koalas, while animals in the south are infected horizontally. Infection with koala retrovirus has been associated with the development of lymphoma and immunosuppression linked to Chlamydia infection (21, 23–25). Interestingly, EfDRV shows a well-supported evolutionary relationship to Miniopterus endogenous retrovirus.
EfDRV is the first described bat Deltaretrovirus and represents only the second report of an exogenous bat retrovirus. The identification of EfDRV from a bat with human exposure raises questions of possible zoonosis, considering that all members of this genus cause disease. Further research and surveillance are needed to determine if EfDRV poses a risk to human or animal health.
Data availability.
The sequence described in this work was submitted to GenBank under accession no. MT700539. Sequence reads were archived as BioProject PRJNA659740.
ACKNOWLEDGMENTS
This work was supported in part by the USDA and by South Dakota Agricultural Experiment Station Animal Health and Hatch funds.
REFERENCES
- 1.Calisher CH, Childs JA, Field HE, Holmes KV, Schountz T. 2006. Bats: important reservoir hosts of emerging viruses. Clin Microbiol Rev 19:531–545. doi: 10.1128/CMR.00017-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Letko M, Seifert SN, Olival KJ, Plowright RK, Munster VJ. 2020. Bat-borne virus diversity, spillover and emergence. Nat Rev Microbiol 18:461–411. doi: 10.1038/s41579-020-0394-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lau SKP, Luk HKH, Wong ACP, Li KSM, Zhu L, He Z, Fung J, Chan TTY, Fung KSC, Woo PCY. 2020. Possible bat origin of severe acute respiratory syndrome coronavirus 2. Emerg Infect Dis 26:1542–1547. doi: 10.3201/eid2607.200092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stoye JP, Blomberg J, Coffin JM, Fan H, Hahn B, Neil J, Quackenbush S, Rethwilm A, Tristem M. 2011. Family: Retroviridae. International Committee on Taxonomy of Viruses, ninth report. https://talk.ictvonline.org/ictv-reports/ictv_9th_report/reverse-transcribing-dna-and-rna-viruses-2011/w/rt_viruses/161/retroviridae.
- 5.Herniou E, Martin J, Miller K, Cook J, Wilkinson M, Tristem M. 1998. Retroviral diversity and distribution in vertebrates. J Virol 72:5955–5966. doi: 10.1128/JVI.72.7.5955-5966.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Griffiths DJ. 2001. Endogenous retroviruses in the human genome sequence. Genome Biol 2:reviews1017. doi: 10.1186/gb-2001-2-6-reviews1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Phillips AA, Harewood JC. 2018. Adult T cell leukemia-lymphoma: state of the art. Curr Hematol Malig Rep 13:300–307. doi: 10.1007/s11899-018-0458-6. [DOI] [PubMed] [Google Scholar]
- 8.Nagasaka M, Yamagishi M, Yagishita N, Araya N, Kobayashi S, Makiyama J, Kubokawa M, Yamauchi J, Hasegawa D, Coler-Reilly ALG, Tsutsumi S, Uemura Y, Arai A, Takata A, Inoue E, Hasegawa Y, Watanabe T, Suzuki Y, Uchimaru K, Sato T, Yamano Y. 2020. Mortality and risk of progression to adult T cell leukemia/lymphoma in HTLV-1 associated myelopathy/tropical spastic paraparesis. Proc Natl Acad Sci U S A 117:11685–11691. doi: 10.1073/pnas.1920346117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ott SL, Johnson R, Wells SJ. 2003. Association between bovine-leukosis virus seroprevalence and herd-level productivity on U.S. dairy farms. Prev Vet Med 61:249–262. doi: 10.1016/j.prevetmed.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 10.Frie MC, Coussens PM. 2015. Bovine leukemia virus: a major silent threat to proper immune responses in cattle. Vet Immunol Immunopathol 163:103–114. doi: 10.1016/j.vetimm.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 11.Baltzell KA, Shen HM, Krishnamurthy S, Sison JD, Nuovo GJ, Buehring GC. 2018. Bovine leukemia virus linked to breast cancer, but no coinfection with human papillomavirus: case-control study of women in Texas. Cancer 124:1342–1349. doi: 10.1002/cncr.31169. [DOI] [PubMed] [Google Scholar]
- 12.Ma X, Monroe BP, Cleaton JM, Orciari LA, Gigante CM, Kirby JD, Chipman RB, Fehlner-Gardiner C, Gutiérrez Cedillo V, Petersen BW, Olson V, Wallace RM. 2020. Rabies surveillance in the United States during 2018. J Am Vet Med Assoc 256:195–208. doi: 10.2460/javma.256.2.195. [DOI] [PubMed] [Google Scholar]
- 13.Hause BM, Collin EA, Anderson J, Hesse RA, Anderson G. 2015. Bovine rhinitis viruses are common in U.S. cattle with bovine respiratory disease. PLoS One 10:e0121998. doi: 10.1371/journal.pone.0121998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ambinder RF, Mullen MA, Chang YN, Hayward GS, Hayward SD. 1991. Functional domains of Epstein-Barr virus nuclear antigen EBNA-1. J Virol 65:1466–1478. doi: 10.1128/JVI.65.3.1466-1478.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farkašová H, Hron T, Pačes J, Hulva P, Benda P, Gifford RJ, Elleder D. 2017. Discovery of an endogenous Deltaretrovirus in the genome of long-fingered bats (Chiroptera: Miniopteridae). Proc Natl Acad Sci U S A 114:3145–3150. doi: 10.1073/pnas.1621224114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whelan S, Goldman N. 2001. A general empirical model of protein evolution derived from multiple protein families using a maximum-likelihood approach. Mol Biol Evol 18:691–699. doi: 10.1093/oxfordjournals.molbev.a003851. [DOI] [PubMed] [Google Scholar]
- 17.Hron T, Elleder D, Gifford RJ. 2019. Deltaretroviruses have circulated since at least the Paleogene and infected a broad range of mammalian species. Retrovirology 16:33. doi: 10.1186/s12977-019-0495-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hayward JA, Tachedjian M, Kohl C, Johnson A, Dearnley M, Jesaveluk B, Langer C, Solymosi PD, Hille G, Nitsche A, Sánchez CA, Werner A, Kontos D, Crameri G, Marsh GA, Baker ML, Poumbourios P, Drummer HE, Holmes EC, Wang L-F, Smith I, Tachedjian G. 2020. Infectious KoRV-related retroviruses circulating in Australian bats. Proc Natl Acad Sci U S A 117:9529–9536. doi: 10.1073/pnas.1915400117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hayward JA, Tachedjian M, Cui J, Field H, Holmes EC, Wang LF, Tachedjian G. 2013. Identification of diverse full length endogenous betaretroviruses in megabats and microbats. Retrovirology 10:35. doi: 10.1186/1742-4690-10-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cui J, Tachedjian G, Tachedjian M, Holmes EC, Zhang S, Wang LF. 2012. Identification of diverse groups of endogenous gammaretroviruses in mega- and microbats. J Gen Virol 93:2037–2045. doi: 10.1099/vir.0.043760-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tarlinton R, Meers J, Hanger J, Young P. 2005. Real-time reverse transcriptase PCR for the endogenous koala retrovirus reveals an association between plasma viral load and neoplastic disease in koalas. J Gen Virol 86:783–787. doi: 10.1099/vir.0.80547-0. [DOI] [PubMed] [Google Scholar]
- 22.Tarlinton RE, Meers J, Young PR. 2006. Retroviral invasion of the koala genome. Nature 442:79–81. doi: 10.1038/nature04841. [DOI] [PubMed] [Google Scholar]
- 23.Canfield PJ, Sabine JM, Love DN. 1988. Virus particles associated with leukemia in a koala. Aust Vet J 65:327–328. doi: 10.1111/j.1751-0813.1988.tb14518.x. [DOI] [PubMed] [Google Scholar]
- 24.Quigley BL, Ong VA, Hanger J, Timms P. 2017. Molecular dynamics and mode of transmission of koala retrovirus as it invades and spreads through a wild Queensland koala population. J Virol 92:e01871-17. doi: 10.1128/JVI.01871-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Waugh CA, Hanger J, Loader J, King A, Hobbs M, Johnson R, Timms P. 2017. Infection with koala retrovirus subgroup B (KoRV-B), but not KoRV-A, is associated with chlamydial disease in free-ranging koalas (Phascolarctos cinereus). Sci Rep 7:134. doi: 10.1038/s41598-017-00137-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The sequence described in this work was submitted to GenBank under accession no. MT700539. Sequence reads were archived as BioProject PRJNA659740.