Abstract
The living state is low entropy, highly complex organization, yet it is part of the energy cycle of the environment. Due to the recurring presence of the resting state, stimulus and its response form a thermodynamic cycle of perception that can be modeled by the Carnot engine. The endothermic reversed Carnot engine relies on energy from the environment to increase entropy (i.e., the synaptic complexity of the resting state). High entropy relies on mental energy, which represents intrinsic motivation and focuses on the future. It increases freedom of action. The Carnot engine can model exothermic, negative emotional states, which direct the focus on the past. The organism dumps entropy and energy to its environment, in the form of aggravation, anxiety, criticism, and physical violence. The loss of mental energy curtails freedom of action, forming apathy, depression, mental diseases, and immune problems. Our improving intuition about the brain’s intelligent computations will allow the development of new treatments for mental disease and novel find applications in robotics and artificial intelligence.
Keywords: Mental energy, Carnot engine, Consciousness, Depression, Emotions, Mental disease
Mental energy or intrinsic motivation Cognitive and physical effort is associated with different cost functions (Kool and Botvinick 2018). Mental energy entails metacognitive monitoring, related to intrinsic motivation, which predicts enhanced performance, learning, and creativity, and it plays a vital role in personality development and wellness across the lifespan (Ryan et al. 2016). It is a long-term ability based on mental fluidity that allows trust, belief, and confidence. Intrinsic motivation allows a consistent exertion of mental effort toward achievement by increasing future freedom of action.
Mental effort Mental effort is how hard a person tries to perform on some task. Prolonged periods of demanding cognitive activity or disease lead to fatigue that negatively affect performance. Mental fatigue has a more significant detrimental effect on physical performance than physical exhaustion (Coutinho et al. 2018), which can be remedied by rest or the recovery of health.
Mental fatigue Mental fatigue is a psychobiological state caused by prolonged exertion that can damage cognitive and exercise performance (Meeusen et al. 2020).
…economists instinctively assume thinking as a costly activity…Mental effort is like physical effort-people dislike both, and will do more of both if you pay them more.—Camerer and Hogarth (1999)
Introduction
The connection between the mind and the brain has remained an intriguing puzzle throughout human history. Idealism, represented by Kant, Hegel, and many Eastern beliefs, asserts the primacy of consciousness. Materialism is the dominant metaphysical framework in science. Neuropsychology has a general expectation that brain mechanisms are sufficient to explain all psychologically described phenomena (Schwartz et al. 2005). Dennett, Churchland, and other materialists, who consider the mind just an illusion produced by brain circuits, favor the idea. The two contrasting views have found a compromise in dualism. Descartes, echoed by Popper, Eccles, Chalmers, and others in western thought, posited the separation of physical bodies and the world of mental states. The following argument contributes to recent findings in neuroscience and psychology to the discussion.
The hierarchical organization of information processing gives rise to the time dependence and subjectivity of motivation. If motivation were a vector, its length would represent the amplitude or intensity of pursuit, and the angle of the vector would represent its focus on a specific goal (Simpson and Balsam 2015). An uncanny ability to automatically (independent of consciousness) reorient itself against disturbances imposed on it by the environment forms the basis of the mind’s homeostatic regulation. Applying homeostatic principles to simulated networks improves their correspondence significantly to empirical fMRI data (Rocha et al. 2018). Self-determination theory (SDT) considers competence, which is protected by a restorative response, a basic psychological need for well-being (Fang et al. 2018). Active Inference Theory applies the free energy principle to action (Friston 2012, 2018; Allen and Friston 2016). Any adaptive change in the brain will minimize free-energy (prediction error, cost). The following discussion incorporates the above theories by showing how psychology and emotions arise from the self-regulation of the cortical brain (Deli 2015).
The stability of the inner world of consciousness (Smitha et al. 2017) forms the resting-state networks (RSNs). In the absence of stimuli, mind-wandering, autobiographical memory, future thinking, and introspection (Grieder et al. 2018) integrate motor and sensory information into an abstract model of the world (Musall et al. 2019). The constancy of the self depends on the stability provided by the resting state, which is maintained independently of conscious awareness. Its extensive geodesic distance from primary sensory and motor regions permits abstract representational hierarchies of dense information integration, connectivity, and routing hubs (van den Heuvel et al. 2012; Margulies et al. 2016). The integration of the organism’s past and present orchestrate future behavior based on the brain’s internal model (Fingelkurts and Fingelkurts 2014).
High signal complexity (entropy) of the default mode network (DMN) appears to be crucial for cognitive function and the maintenance of mental health (Grieder et al. 2018). Resting neuroimaging signals show that fluid access to variable neural states predicts complex behavioral performance and intellectual capacity. An almost equal likelihood occurrence of ripples of activations (representing microstates) represents high resting entropy (Fingelkurts and Fingelkurts 2014). Although resting thoughts are fleeting, the high degrees of freedom grants the ability to produce almost any thought.
Boltzmann explained entropy as the number of microstates of the system. On the other hand, information entropy (Shannon’s entropy) is the average rate at which a stochastic source of data produces information. Measuring and evaluating the entropy of the neuronal system might open a new way for diagnosis and therapy in psychology and psychiatry, and cognitive sciences (Belavkin and Ritter 2003; Gabora 2016; Hirsh et al. 2012; Sayood 2018; Smith et al. 2018).
Traditional approaches to entropy estimate the degree of regularity of a time series on a single time scale (Li et al. 2018). Complexity, such as the dynamics of activities, can be defined as the amount of nonlinear information that a time series conveys over time (Omidvarnia et al. 2018). The simultaneous coexistence of subcritical and supercritical dynamics in different neural regions stretches the parameter range forming self-organized criticality (Hesse and Gross 2014). Entropy in several crucial brain areas correlates with intelligence in both verbal and performance measures (Saxe et al. 2018). The relation was most strongly observed in the prefrontal cortex, inferior temporal lobes, and cerebellum. The multiscale entropy (MSE) approach makes use of a method termed “coarse-graining,” which provides an entropy profile across multiple time scales and differentiates meaningful complexity from uncorrelated randomness (Li et al. 2018). The multiscale entropy (MSE) and functional connectivity (FC) show high associations at lower temporal frequencies (Wang et al. 2018).
Due to the increasing entropy in the environment, a constant flow of information bombards our sensory system (Schrödinger 1945). The cognitive or computational resource of the neural tissue is limited at any one time (Inzlicht et al. 2018), while neuronal signalling and metabolism are tightly coupled at the local level. The stabilized internal representations stay closer to their attractor fixed-points. Perceptual binding and feedback/feedforward waves improve the perception of external inputs by reducing neural noise (Buzsaki et al. 2013). The energy need of mental effort (focus) inspires utilizing physical principles for the analysis of cognitive processes (Deli et al. 2018; Fry 2017; Street 2016). Recent efforts in consciousness science have studied the thermodynamic consequences of signal processing. Although the energetic cost of emotions in signal processing has been established, their specific role in the brain’s energy cycle awaits resolution. We examined the thermodynamic consequences of basic emotions posited by the fermionic mind hypothesis (FMH) (Deli 2020).
Discussion of mental energy
The energy need of muscle action is straightforward, but the mental effort is independent of physiological variables traditionally associated with endurance performance (heart rate, blood lactate, oxygen uptake, cardiac output, and maximal aerobic capacity). Nevertheless, cognitive or computational effort, such as thinking, focus, and even meditation, is taxing (Kool and Botvinick 2018; Martin et al. 2018), and conscious control drains mental resources in proportion to task difficulty (Warm et al. 2008; Van Cutsem et al. 2017; McMorris et al. 2018). However, mental energy permits one to engage in and enjoy effortful cognitive activities for their own sake (Inzlicht et al. 2018). Mental energy (or g factor) is autonomous self-regulation (Ryan and Deci 2008) toward the pursuit of internal goals.
Affective neuroscience has revealed the functional integration of emotions and cognition. Emotions are part of the brain’s energy architecture (Barbey et al. 2014; Touroutoglou et al. 2015). As such, conflict elicits many of the hallmark features of emotion, including valence judgments, physiological arousal, and subjective emotional experiences (Inzlicht et al. 2018; Saunders et al. 2017). Cognitive control can be understood as an emotional process (Inzlicht et al. 2015); therefore, emotional intelligence (the ability to identify and manage emotions) is closely related to mental energy. For example, in long-distance runners, emotional intelligence was found to influence performance to a more considerable extent than rigorous training (Rubaltelli et al. 2018).
The energy need of attention means that decision-making, empathy (Cameron et al. 2017), focus and vigilance (Zohar et al. 2003; Buzsaki et al. 2013; Manohar et al. 2018) depletes vitality, resulting in fatigue and negative emotions (Inzlicht et al. 2015). Several neurotransmitter systems (dopamine and adenosine) in the prefrontal cortex and the anterior cingulate cortex can compromise endurance performance (Loy et al. 2018; Meeusen et al. 2020),
Lack of mental energy means real, formidable challenges to achievement and should not be confused with fatigue. While fatigue can compromise performance temporarily, mental energy is a consistent ability (Schwartz et al. 2005). Although small lifestyle changes may improve feelings of fatigue, enhancing mental energy requires long-term development (Boolani et al. 2019) and comprehensive interventions. Intellect is flexible (Heintzelman et al. 2013). In studies, mental energy could be improved by elevated dopamine and norepinephrine transmission and binding (Loy et al. 2018), whereas fatigue was related to serotonin and inflammatory cytokines and reduced histamine binding.
What is the neurological background of differences in performance? The prolonged performance of a demanding cognitive task increases cerebral adenosine accumulation, which may lead to a higher perception of effort experienced during subsequent endurance performance (Pageaux et al. 2014). Highly anxious individuals must exert considerable cognitive effort to perform at the same level as less anxious people (Inzlicht et al. 2015; Saunders et al. 2017). Distress encompasses negative moods, and a lack of confidence and worry reflects negative self-referent cognitions. Insecurity causes avoidance of mental exertion whenever possible (Inzlicht et al. 2015). There is ample evidence that immune signalling is intimately tied to the neural processes governing social behaviors (Kopec et al. 2019). Because immune challenges impair social behavior by altering neuro-immune signaling in brain regions important for reward/motivation, the dopaminergic reward circuitry is engaged during social behaviors and impacts immune function. Motivation, such as the expectation of reward or goal-enhancing events, mitigates fatigue, and can push beyond the usual performance limitations (Manohar et al. 2018; Zohar et al. 2003).
The role of interaction in mental change
The brain’s rich club organization ensures minimal energy conformation during state transitions (van den Heuvel et al. 2012; van den Heuvel and Sporns 2011). Active inference minimizes an organism’s exposure to uncertainty or surprise (Pepperell 2018a, b). We propose that such minimal energy conformation is analog to the principle of least action in physics. The stationary action has a temporal equivalent in the predictive intelligent processing that calculates its response based on experience. As material systems observe the principle of least action when moving in space, intelligent systems optimize their action repertoire between the past and the future (Friston et al. 2017).
Energy metabolism is necessary for proper cell function and viability, but it is also crucial in higher brain functions such as memory processing and behavior. Investigating the brain’s energy relationships can uncover the physical underpinning of consciousness (Pepperell 2018a, b). The brain maintains homeostasis because incompatibility with expectations triggers emotional reactions (Selye 1974). Repeated activation of the same neuronal connections requires less energy, resulting in less and less emotional involvement, forming automatic activation patterns.
Sensory interaction, the basis of mental homeostasis
The neural tissue generates spontaneous oscillations. In unconscious states, such as anesthesia, electric activities are limited to the anatomical repertoire (Uhrig et al. 2018). The energy-requirement of consciousness states (Buzsaki et al. 2013; Manohar et al. 2018; Kyong et al. 2015; Pepperell 2018a, b; Street 2016; Inzlicht et al. 2018) drive oscillations beyond the connection map. Specific neural activation patterns (i.e., dorsal anterior cingulate cortex) as well as accompanying autonomic indices (i.e., skin conductance response) hold predictive information on individual performance (Köhler et al. 2018). Because the cost of cognition is also often evaluated as the amount of information needed to update earlier beliefs, the effort of tasks is highly variable and subjective (Koechlin 2007).
Evoked potential in the human brain is a complex play of sharply changing potentials (such as N100 and P300), see Fig. 1 for a greatly simplified representation (Sur and Sinha 2009). Electromagnetic gradients form a highly fluid, wave-like activation pattern on the cortical surface (reviewed by Muller et al. 2018). Fast oscillations from sensory areas flow toward the frontal, associative regions, whereas direction flow reverses via slow oscillations (Fig. 1b), which recover the DMN. The sensory transmission toward the sensory cortex by fast oscillations and response by slow oscillations was confirmed in humans (Buzsaki et al. 2013) but should be typical in all mammals.
Fig. 1.
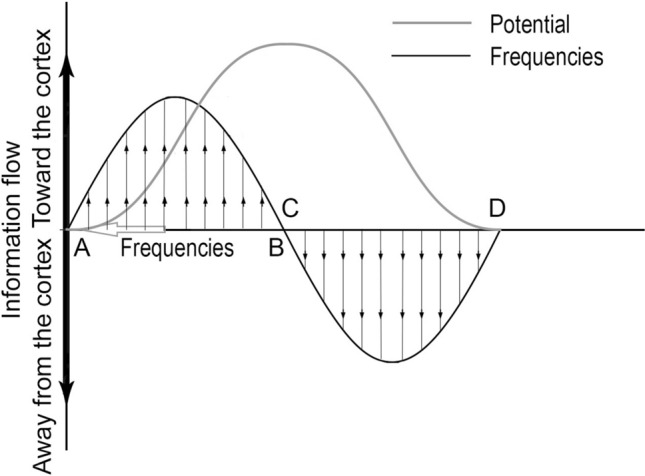
Changes in energy balance due to the stimulus. The brain frequencies change from high, on the left (A), to low, toward the right (D), determining the direction of information flow in the brain (shown by thin arrows). The resting state of the brain is energy neutral before stimulus (A) and after a response (D). The first part of the cycle (AB) is driven by the stimulus, whereas the evoked potential restores the resting state via self-regulation (CD). The enhanced brain frequencies represent high temperature and robust energy need; the low frequencies expand by forming high amplitude, which correlates to a cooling down Adapted from Deli et al. 2018)
The electromagnetic flows of sensory perception occurring between the limbic system and the cortex form polarity effects (Deli et al. 2018). In rats, high-frequency (40–100 Hz) stimulation of central thalamus relay neurons in vivo caused widespread forebrain activation, but low brain frequency stimulation generated a jerking strain, or convolution (Liu et al. 2015) indicating the strict reliance on incoming stimuli (information).
Thermodynamic considerations
According to Landauer’s principle, it takes energy to erase one bit of information (Landauer 1961). Let us consider the brain as a computing object. Evoked activities reflect an enhanced ‘temperature,’ which makes it possible to calculate the thermodynamic cost of neural computation (Fry 2017; Deli et al. 2018; Street 2016). Because goal-directed activities reflect the size of their originating impetus, the characteristic path-length, and the correlation coefficient is proportional to the effort (Kyong et al. 2015) and can approach zero in a randomly organized network (Wu et al. 2012).
Cerebral metabolism depends on a constant supply of both glucose and oxygen. The extensive oxygen use supports steady-state energy metabolism, via neurovascular coupling mechanisms. The subtle Spatio-temporal regulation of the neurovascular coupling might be mediated by neuronal signalling mechanisms (i.g., glial pathways), in addition to sensing energy consumption (Pasley and Freeman 2008). Despite the refined regulation, neurovascular uncoupling and ATP depletion may play a physiological role (Fig. 2, Trevisiol et al. 2017).
Fig. 2.
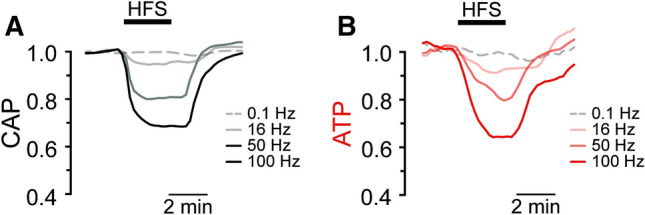
Compound action potentials (CAP) during high-frequency stimulation (HFS). a The CAP area decreases over time during high-frequency stimulation (HFS). The decay amplitude deviates from the absence of HFS, indicated by the dashed line (0.1 Hz, used for normalization to 1.0), and increases progressively with the increase in stimulation frequency (16 Hz, 50 Hz, 100 Hz). b Axonal ATP levels also decrease with increasing stimulation frequency, reaching a new steady-state level, which depends on the stimulation frequency (Reproduced from Figure [4] (Trevisiol et al. 2017), eLife, published under the Creative Commons Attribution 4.0 International Public License (CC BY 4.0; https://creativecommons.org/licenses/by/4.0/)
Incompatibility with expectations triggers emotions that instigate response that restores the resting state but modifies synaptic organization (i.e., mental energy); state transitions depend on the weighted energy contributions of regions (Betzel et al. 2016; Pop-Jordanova and Pop-Jordanov 2005), see Fig. 1. The evolution of the energy need and synaptic weight of connections leads to memory and learning. The energy need of oscillations (and the resulting mental change) is frequency-dependent. The brain, a voracious consumer of information, is intertwined with the energy/information cycle of the environment.
Sensory activation compresses the spatial signal according to an Eigenvalue. Like the musical notes representing a complex musical piece, harmonic brain modes, defined as connectome harmonics, yield the frequency-specific building blocks of cortical activity (Fingelkurts and Fingelkurts 2014; Atasoy et al. 2016). The energy difference between harmonic modes means that it takes energy to switch between emotions. Thus, emotions represent the brain’s operating temperature and its intellectual limit. The feelings of autonomy (volition) and competence (mastery) cultivate intrinsic motivation (Deci et al. 1999).
An intelligent response to a stimulus increases future degrees of freedom (Wissner-Gross and Freer 2013), but stress and anxiety reduce it (Rowe and Fitness 2018). An intelligent response to the stimulus corresponds to the mind being an abstract mirror of the environment and adopting the laws of physics.
The resting-state functions as a heat bath. The amount of thermal energy transferred in the Carnot cycle:
| 1 |
The efficiency of the Carnot cycle is the amount of work output ()
| 2 |
where is the activity (temperature) of the evoked state, and is the activity (temperature) of the resting state. Energy is lost, and complexity decreases.
The efficiency of the reversed Carnot cycle () is the heat removed/the amount of work input
| 3 |
where is the temperature (activities) of the low frequency of the evoked state of positive emotions, is the temperature of the resting state. Energy accumulates as the available connection states (complexity).
The role of emotions
A phylogenetic consequence of emotions “allows” mammals and birds to be warm-blooded, or care for their offspring, form the mysterious inner world of consciousness, display impressive learning ability, and develop a nuanced social life. An intelligent response to the incessant stimulus develops the mind into an abstract mirror of the environment. Emotions trigger a response because they represent the fundamental forces of motivation. Emotions have typical brain activity profiles, but based on their energetic profile, only positive and negative emotions exist (active inference framework), which formulate the processing polarities of the brain (Kao et al. 2015). Negative emotions might trigger exothermic processes, which reconstruct the past (Carnot cycle), whereas positive emotions are endothermic processes (reverse Carnot cycle) that control the future and boost mental energy (Deli et al. 2018; Fry 2017), see Fig. 3.
Fig. 3.
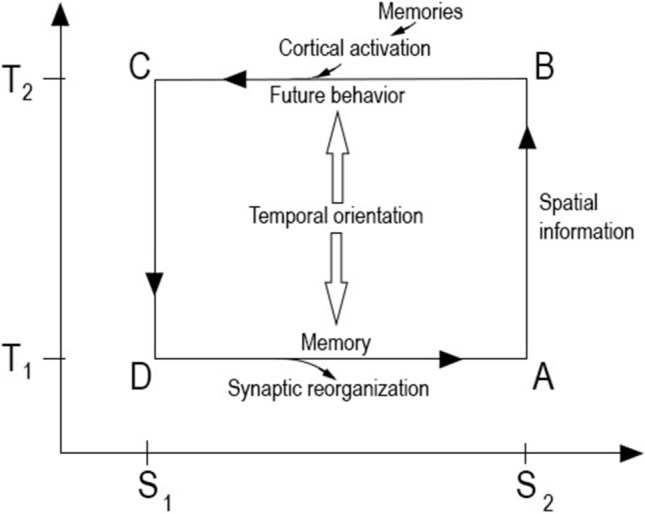
The reversed Carnot cycle illustrated on a temperature-entropy plane. The cycle operates via self-regulation between the high frequency, evoked state (T2), and the resting state (T1); the horizontal axis represents entropy. Stimulus increases the frequencies during the compression phase (AB). The energy state of synaptic potentials permits the electric flow to spread through the cortex (BC). The resulting potential reverses the direction of the electric flow (CD); these slower oscillations expand to formulate a response. Synaptic reorganization accumulates complexity as memory. Synaptic changes prepare the neural system to better respond to stimuli in the future (DA). The electrical activities reformulate the high entropy, resting state (DA). In deep learning, computation depends on the depth and number of connections of the network, and the phases of information processing are very similar to the cycle of neural computation
Potentials and electric flows between the cortex and the limbic brain form the evoked cycle that formulates automatic and involuntary energy-information exchange with the environment and inspires the particle-like stability of the mind. Because evoked states build on the resting potential, they can be analyzed with the tools of thermodynamics (Deli et al. 2018; Fry 2017). Emotions have a typical brain activity profile but represent only positive or negative states based on their energy use. The central tenet here is that by considering mental energy as an analog to potential energy in physics, the Carnot cycle can model the brain’s operation.
Physical processes can be dissipative, which reconstructs the past, and intelligent, those that anticipate the future (Cox 1979). The first kind, exothermic process dumps entropy, and energy into its environment, whereas the latter, endothermic actions absorb entropy while requiring energy to operate. The thermodynamic computation by a cortical neuron provides an example of the second possibility. Fry analyzed the thermodynamics of action potentials generated by one neuron (Fry 2017). The quasi-hierarchical nature of the brain allows us to generalize his conclusions for the energy-information exchange of stimulus. Extending the thermodynamic considerations onto the whole brain delineates the role of energy in cognition (Deli et al. 2018).
We examine the Carnot cycles. The thermodynamic considerations of positive and negative emotional states as they relate to mental effort and mental energy can answer the crucial questions in psychology and aid AI research.
Positive emotions: the reversed Carnot cycle
The ability to form an intelligent response to a stimulus depends on the flexibility and complexity of the resting brain. In diverse cortical areas, spike frequency depends on both the environmental inputs and internal conditions (Mildner and Tamir 2019; Piscopo et al. 2018). In other words, the history and current state of the system are just as crucial in determining the quality of neuronal activation as the stimulus itself. Consequently, the state of the brain, i.e., the observer, determines the information value of the stimulus.
High mental plasticity inspires exploration for its own sake, as a goal in itself (DeYoung 2013). The willingness to work hard for rewards, even in cases of the low probability of payout, is the function of mental energy (Di Domenico and Ryan 2017; Treadway et al. 2012). Mental energy is synaptic flexibility that reflects higher degrees of freedom, manifested as trust and confidence (Ryan et al. 2016). Self–confidence encourages a can-do attitude that builds upon the robust and satisfying mental state. Mental energy deflects negativity and conflict, inspiring creativity, success, and even longevity (Inzlicht et al. 2018; Kaczmarek et al. 2017; Stellar et al. 2015; Diener and Chan 2011; Aknin et al. 2012; Steptoe and Wardle 2005; Ryan and Deci 2017).
Intrinsic motivation has been attributed mostly to the dopaminergic systems (Gottlieb and Oudeyer 2018; Grolnick and Ryan 1987; Kobayashi and Hsu 2019; Treadway et al. 2012; Salamone and Correa 2012). It inspires natural curiosity and interest, to seek out challenges, and to develop and advance the self (Di Domenico and Ryan 2017; Panksepp 1998). Intrinsic motivation is an energetic, powerful state that is associated with better physical and mental health (Boehm and Lyubomirsky 2008; Csikszentmihalyi and Hunter 2003; Diener and Chan 2011; Koivumaa-Honkanen et al. 2004).
Recent neuroimaging data suggest that anterior midcingulate cortex (aMCC) connectivity plays a crucial role in volition and can indicate grit, persistence, and better academic performance (Touroutoglou et al. 2019). Higher levels of energy productions correlate positively with better performance, even in animals (Biro and Stamps 2008; Careau and Garland 2012). High performers in intelligence tasks showed lower brain activations, indicating higher neural efficiency (Haier et al. 1988; Poldrack 2015) accompanied by slower oscillations (Bethell et al. 2012; Seo et al. 2008), which in turn expand time perception (Neupert and Allaire 2012; Remmers and Zander 2018a, b).
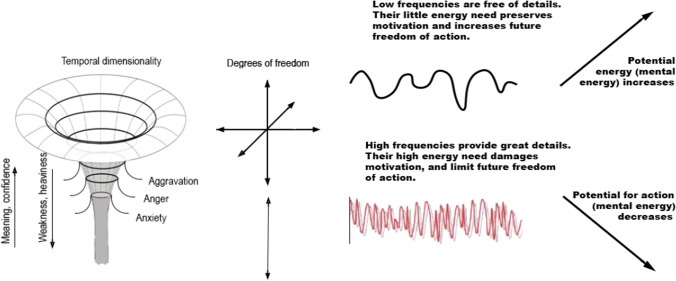
The resulting negative temporal curvatures correlate with increasing temporal dimensionality (Tozzi et al. 2017, Tozzi and Peters 2017; see also Table 1), as well as neurotransmitter action, such as dopamine (Soares et al. 2016; Fredrickson and Joiner 2002; Rudd et al. 2012; Peters et al. 2017). For example, being in the present moment or experiencing awe, trust, and belief correlate with the sense of temporal excess (Csikszentmihalyi and Hunter 2003; Neupert and Allaire 2012; Rudd et al. 2012). The subjective sense about the wealth of time provides a calm disposition, which inspires better decision-making (Mitchell et al. 2018). Higher degrees of freedom also enable intelligent responses.
Table 1.
The physiological consequences of different brain states
| Reversed Carnot cycle | Carnot cycle | |
|---|---|---|
| High entropy resting state | Low entropy resting state | |
| Mental state | Positive emotions | Negative emotions |
| Frequencies | Slow oscillations expand—information poor | High frequencies contract—detail-oriented, accumulate information |
| Temporal dimensionality | Positive temporal curvature—lower dimensionality | Negative temporal curvature—higher dimensionality |
| Subjective sense of time | Time perception expand (the luxury of time inspires confidence) | Time perception expands (details overwhelm with stress) |
| Future degrees of freedom | Degrees of freedom enhances | Degrees of freedom reduces |
| Thermodynamic consequences | An endothermic cycle absorbs energy and entropy from the environment | An exothermic cycle dumps energy and entropy onto the environment |
| Consequences for the organism | High mental energy (intellect) | Degradation of mental energy → mental and immune problems |
The thermodynamic and psychological consequences of basic emotions
The enthusiasm and energy that characterize awe and other positive emotions reflect an information-free state. Because low frequencies lack details (Figs. 3 and 4) and engage in more broad cortical areas, they inspire associative representations (Kounios and Beeman 2009; Machado and Cantilino 2016). The slower oscillations can access to a high number of microstates, to produce almost any thought. The temporal variability of the resting-state connectivity, i.e., high entropy, correlates with fluid intelligence (Yang et al. 2019). These endothermic processes control the future by enhancing intellect (Fry 2017; Wissner-Gross and Freer 2013).
Fig. 4.
The relationship between emotions and mental energy (future freedom of action). a Mental energy permits positive emotions and meaning that expand the future freedom of action (top). The process, such as acceptance, learning, and meditation, requires energy. The higher energy needs of aggravation, anger, and anxiety constitute stress, which urges decisions that decrease the future freedom of action (Adopted from, Deli 2020). b Low frequencies leave the brain energy resources intact, allowing a sense of energy and enthusiasm. (top) High-frequency oscillations confuse with overwhelming details; their high energy need triggers an immune reaction, sows uncertainty, and impatience that corrupt mental freedom
Similarly, low-frequency brain stimulation boosts creativity (Lustenberger et al. 2015), which increases synaptic complexity and future freedom of action. Likewise, low-frequency optogenetic stimulation of the anterior cingulate areas decreased anxiety in mice (Kiefer and Pulvermüller 2012). Mindfulness meditation training, for example, is an energy-requiring slowing down that can reverse negative attitudes (Fig. 4, for explanation). Boosting mental energy this way may improve synaptic complexity and mental flexibility.
In summary, the energy frugal slow oscillations correlate with a flexible and dynamic synaptic map of an energetic mental state, and this can be modeled by the reversed Carnot cycle. Landauer’s principle shows that creativity, acceptance, and flow must be information-free conditions (Fig. 4, top). Endothermic processes control the future (enhance mental energy), and exothermic processes reconstruct the past by degrading mental energy (Deli et al. 2018; Fry 2017), see Fig. 3. Positive emotional states not only feel powerful but represent more energetic conditions, the so-called mental energy. The energy from the environment fuels the endothermic process, underlining the observation that mental energy is a social characteristic and evolves closely intertwined with the surrounding. The endothermic cycle is the source of intellect and should be the objective of all education.
In Eastern beliefs, equanimity is a state of acceptance (i.e., absorbing energy). High intellect is an energetic state that can produce a full range of emotions and permits better control of the future (Deli et al. 2018). Learning and various spiritual practices, such as meditation, acceptance, and gratitude, which boost creativity, happiness, and wisdom, can reformulate the mind.
Negative emotional states: the Carnot cycle
Mental energy ensures emotional stability and permits a focus on long-term goals. Its lack degrades perseverance. The resulting impulsiveness thwarts people’s feelings of autonomy (volition), competence (mastery) (Deci et al. 1999), and intrinsic motivation. We will examine the negative mind in comparison to positive emotions.
Energetic consideration (Erosion of mental energy)
Negative emotional states have more significant energy requirements than positive emotional or neutral mental states (Joffily and Coricelli 2013; Saarimäki et al. 2015, 2016; Saarimäki 2017). The preponderance of negative emotions parallels high-frequency limbic activity and decreased prefrontal functioning (Seo et al. 2008). The detailed and deterministic oscillations waste energy, narrow focus, and reduce temporal dimensionality. Enhanced brain frequencies may trigger long-term potentiation (Bliss and Lømo 1973), which increases the likelihood of activating the same synaptic path. Stress and anxiety reduce the degrees of freedom (Rowe and Fitness 2018), blinding people for the possibilities open to them, see Table 1 and Fig. 4, bottom (Lupien et al. 2007).
High-frequency brain waves produce a constricted feeling and increase time perception (Fredrickson and Joiner 2002; Yamada and Kawabe 2011; Peters et al. 2017) even for unconscious stimuli (Oei et al. 2012). Rumination, regret, and remorse waste mental resources and degrade the ability to carry out intentions (Joffily and Coricelli 2013). The preoccupation with the past is an indication of compromised mental energy and information accumulation. Due to the higher energy need of all negative emotions, highly anxious individuals must exert considerably more cognitive effort (Joffily and Coricelli 2013; Saarimäki et al. 2015, 2016; Saarimäki 2017), which exhausts motivation (Trevisiol et al. 2017), see Fig. 2. In anxious individuals (Moran et al. 2015), decreased theta band synchrony typically produces task-irrelevant signals and inferior post-error behavior (Inzlicht et al. 2015). Insecure and guilty people perceive themselves as heavier and their chores more burdensome (Day and Bobocel 2013).
Intolerance of uncertainty (e.g., generalized anxiety disorder), impulsivity enhances the propensity to develop problems with alcohol, drugs, gambling, overspending, and overeating (Hamilton et al. 2015; London 2016). In humans, recurring automatic thought patterns of revenge thinking, regret, or remorse represent insecurity that increases the likelihood of contradiction, critical tendency, aggravation, or complete withdrawal. Over the long-term, the adverse effects curtail freedom and induce vulnerability to immune problems and mental diseases (Gehring et al. 2018; Stanghellini et al. 2016). Immune challenges further impair social behavior by altering neuro-immune signaling in brain regions important for reward/motivation.
Anxiety and depression represent energy-poor states (Wise et al. 2017). In recent rodent’s experiments, repeated stress exposure, which reduced plasticity by corrupting connectivity within the medial PFC, drove depressive behavior (Li et al. 2018; Yang et al. 2018). The stagnation of vital processes and an incapacitating slackening the flow of time characterize depression (Stanghellini et al. 2016). The reduced motivation in depression can be addressed by dopamine and norepinephrine (Loy et al. 2018), for example, catechol-O-methyltransferase (COMT) inhibitor tolcapone increases dopamine tone in the frontal cortex (Mitchell et al. 2018) indicating the lack of sufficient mental energy.
Finally, the degradation of mental energy and performance might be the first symptom of mental diseases. Neurodegeneration in areas critical for memory, such as the medial temporal lobes, the entorhinal cortex, and the hippocampus, might be the precursor for various forms of dementia. For example, the compromised integrity of medial frontal regions (Apostolova et al. 2007) might lead to apathy in Alzheimer’s disease (AD, Drago et al. 2010; Kumfor et al. 2018; Nobis and Husain 2018). Other studies found a relationship between the lack of mental energy and apathy (Patzelt et al. 2019) in AD (Perri et al. 2018) and other conditions. The “impairment of intrinsic motivation is one of the characteristics of schizophrenia” (Takeda et al. 2018), Parkinson’s disease (Muhammed et al. 2016), neurodegenerative diseases such as frontotemporal dementia (Bertoux et al. 2015; Mitchell et al. 2018) and other clinically-related traits (e.g., anhedonia and apathy) (Patzelt et al. 2019).
Resting entropy
A high signal complexity, particularly in the DMN, is essential for cognitive functionality (Grieder et al. 2018; Sokunbi et al. 2013). All negative emotional states degrade the entropy of the DMN (Low et al. 2018; Saxe et al. 2018). DMN disruptions and reduced resting-state entropy have been reported in schizophrenia spectrum disorder (Smith et al. 2018), depression (Wise et al. 2017), autism (Padmanabhan et al. 2017; Hogeveen et al. 2018), and AD (Jones et al. 2011; Gray and Thompson 2004; Wang et al. 2017). Altered entropy in AD indicates a disturbance of both local information processing and information transfer between distal areas. Specifically, the entropy decrease correlates with the progression of AD (Grieder et al. 2018).
As mentioned earlier, the reduction of entropy in negative emotions is an exothermic condition, which damages emotional regulation and intellect. Mental damage might occur as a consequence of the unsustainable accumulation of information. In other words, the extended effort to process information overwhelms the hormonal system and deteriorates into immune and mental problems.
Antipsychotic medications that successfully ameliorate the negative symptoms of schizophrenia (delusions, hallucinations, and others) have been available for some time; however, they leave patients with residual symptoms, of which lack of motivation is the primary driver of poor outcome and low quality of life (Kiang et al. 2003). Problems with motivation and particularly the corruption of mental energy might lead to dramatic differences in health outcomes, intellectual, and social performance (D’Acquisto 2017; Kopec et al. 2019; Sizemore et al. 2018).
In major depression, the less variable resting-state functional connectivity leads to repetitive thought patterns (Charney 2016; Kaiser et al. 2016; Murrough et al. 2016), indicating a lack of freedom. It is suspected that antidepressants ameliorate the deficits in synaptic plasticity (Abdallah et al. 2015). Tolcapone, a catechol-O-methyltransferase (COMT) inhibitor, may reduce impulsivity (Mitchell et al. 2018). Although stroke lesions are restricted to one hemisphere, entropy reduction extends to nodes from the contralesional hemisphere (Bastos et al. 2014), indicating the unity of the mind and the global nature if entropy in underpinning mental changes.
Time perception
Stress distorts time perception, which causes “the non-specific response of the body to any demand upon it” (Selye 1974) and a loss of control. The impaired time perception (Mitchell et al. 2018) shows a close relationship with emotion regulation and correlates with some clinical conditions, involving problems in decision making. Conflict inherently enhances the neural activity and triggers a negative mindset, a state more prone to activation; worried people fail to produce the connectivity for theta phase synchrony (Cavanagh and Frank 2014) between medial and lateral sites (Moran et al. 2015). The lack of freedom leads to a compromised response such as aggravation, and the fight or flight response (Verma et al. 2011). For example, prisons enhance impulsiveness, which reducing emotional coloring and trust, thereby inspires criminal personalities (Meijers et al. 2018) and addictions (Hamilton et al. 2015; London 2016) (Fig. 4, bottom).
The connection of slower oscillations with positive emotions and enhanced brain frequencies with negative mental states has been corroborated in numerous studies (Bethell et al. 2012; Seo et al. 2008). The bottom line is that low frequencies form negative, whereas negative emotions form positive temporal curvatures with increasing and decreasing temporal dimensionality modifications, respectively (Tozzi et al. 2017). Importantly, in both instances, time perception expands (Neupert and Allaire 2012; Remmers and Zander 2018a, b). Tolcapone, catechol-O-methyltransferase (COMT) inhibitor, may reduce impulsive decision-making and lessen the distorted time perception via dopamine effects (Mitchell et al. 2018).
Emotional flatness
As discussed above, emotional problems are often the primary symptoms in mental disease and immune disruptions. Because the intellect is related to the production of an expansive range of emotions, mental decline correlates with the greying of emotions (Meijers et al. 2018). AD patients evaluate the pleasant images as less pleasant, the negative scenes as less disturbing, and prosody recognition is also impaired (Amlerova et al. 2017). An emotional flatness, deficits in emotional memory, and an inability to overcome prepotent response tendencies are often seen in patients with frontotemporal dementia (FTD) and AD (Chen et al. 2017). Lower brain signal complexity is also associated with a higher degree of cognitive decline (Grieder et al. 2018; Li et al. 2018) and AD-related pathology.
Even without structural atrophy and with typical performance on cognitive tests, amyloid-positive AD patients have higher global connectivity within the right anterior insula and superior temporal sulcus (STS). Entropic changes due to hyper-connectivity might represent pre-symptomatic indicators of the disease, causing mental and emotional rigidity (lower degrees of freedom) (Fig. 4, bottom). Indeed, increased reactivity and negativity, even at a single time-point, increase the risk of late-onset AD (Fredericks et al. 2018). The temporal anti-correlation between task-positive (i.e., functional networks during task execution) and the task-negative RSNs (i.e., DMN), typically found in healthy subjects, is attenuated in progressed stages of AD Grieder et al. 2018).
Hyper-connectivity degrades cognitive flexibility. Hyper-connectivity of the superior temporal sulcus (STS), a brain region that plays crucial roles in social processing, is associated with lower interpersonal warmth and a trend toward increasing emotional reactivity (Fredericks et al. 2018). Recent results have indicated that reduced temporal dimensionality and structural connectomics might be behind problems of emotional regulation (Sizemore et al. 2018; Tozzi and Peters 2017). The above energetic considerations lend further support to the possible dimensionality changes.
The emerging rule is that the loss of mental energy precedes emotional flatness; corrupted cognitive functioning (Sapey-Triomphe et al. 2015; Bourgin et al. 2018) is concerned with the past, which engenders insecurity. The process must be modeled by the Carnot cycle, where mental degradation is connected to lower resting entropy. Negative states are exothermic processes that dump energy onto the environment via aggravation, rumination, and critical tendency. Over the long-term, the loss of complexity, i.e., functional and synaptic changes, corrupts intellect and leads to anatomically detectable problems. Recognizing the role of thermodynamic changes in stress and anxiety might inspire novel treatments for mental diseases.
Conclusions
Traditionally, neuroscience, which studies the brain, and psychology, which studies the mind, sharply differed in their methodologies and objectives. Synthesizing their insights into the cohesive framework of consciousness is the intriguing challenge of our time. This review has attempted to connect the energy metabolism of the neural system with psychology and intellect based on the FMH.
The brain’s recurrent energy-information exchange via stimulus places it within the thermodynamic cycle of the environment and allows the examination of the brain based on information and energy. Intelligent systems are sensitive to changes in the environment. Meaningful perception is an abstract representation of the physical world.
Incoming information induces electric flows resulting in minute potential differences that modify the global (brain-wide) synaptic map. Thus, synaptic organization and complexity, which represent an energy potential of memory and learning, formulate the basis of future behavior. As material systems observe the principle of least action when moving in space, intelligent systems optimize their action repertoire between the past and the future. The temporal equivalent of the stationary action in physics is a minimum energy configuration toward the future.
We have shown that basic physical and information-theoretic principles can describe intelligent computation. Establishing the thermodynamic basis of intellect can inspire educational and social reforms. High intellect is an energetic state that can produce a full range of emotions. Mental energy is so intertwined with the immune and hormonal functions that erosion of mental energy causes mental problems and increases the vulnerability to diseases. Verifying the thermodynamic underpinning of mental changes could revolutionize psychological and social sciences.
The above findings corroborate the recent findings by respected laboratories on consciousness; intellect correlates with increasing resting entropy, and compromises of mental energy compromise intellectual performance, lead to mental diseases and immune problems. Emotions can be considered the fundamental forces of the mind, which inspire interaction and mental change. Mindfulness, learning, meditation, gratitude, and other mental practices might improve well-being by increasing mental freedom of action. The highest productivity of the brain results from turning challenges into positive emotional states and reversing mental degradation via learning and various spiritual practices.
The neural system performs computations with thermodynamic efficiency in orders of magnitude higher than current supercomputers. Our improving intuition about intelligent computations will allow the development of novel techniques and applications in the rapidly changing field of thermodynamics, AI, and robotics.
Acknowledgement
Supported by National Brain Research Program of Hungary (NAP2, 2017-1.2.1-NKP-2017-00002) to ZK.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abdallah CG, Jackowski A, Sato JR, et al. Prefrontal cortical GABA abnormalities are associated with reduced hippocampal volume in major depressive disorder. Eur Neuropsychopharmacol. 2015;25:1082–1090. doi: 10.1016/j.euroneuro.2015.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aknin LB, Hamlin JK, Dunn EW. Giving leads to happiness in young children. PLoS ONE. 2012;7(6):e39211. doi: 10.1371/journal.pone.0039211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen M, Friston KJ (2016) From cognitivism to autopoiesis: towards a computational framework for the embodied mind. Synthese 1–24 [DOI] [PMC free article] [PubMed]
- Amlerova J, et al. Recognition of emotions from voice in mild cognitive impairment and Alzheimer’s disease dementia. Alzheimers Dement. 2017;13(7):1148. [Google Scholar]
- Apostolova LG, Akopyan GG, Partiali N, Steiner CA, Dutton RA, Hayashi KM, Dinov I, Toga AW, Cummings JL, Thompson PM. Structural correlates of apathy in Alzheimer’s disease. Dement Geriatr Cogn Disord. 2007;24:91–97. doi: 10.1159/000103914. [DOI] [PubMed] [Google Scholar]
- Atasoy S, Donnelly I, Pearson J. Human brain networks function in connectome specific harmonic waves. Nat Commun. 2016;7:10340. doi: 10.1038/ncomms10340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbey AK, Colom R, Grafman J. Distributed neural system for emotional intelligence revealed by lesion mapping. Soc Cogn Affect Neurosci. 2014;9(3):265–272. doi: 10.1093/scan/nss124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastos AM, Briggs F, Alitto HJ, Mangun GR, Usrey WM. Simultaneous recordings from the primary visual cortex and lateral geniculate nucleus reveal rhythmic interactions and a cortical source for gamma-band oscillations. J Neurosci. 2014;34(22):7639–7644. doi: 10.1523/JNEUROSCI.4216-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belavkin RV, Ritter FE (2003) The use of entropy for analysis and control of cognitive models. In: F. Detje, D. Doerner, H. Schaub (eds) Proceedings of the fifth international conference on cognitive modeling. Bamberg, pp 21–26
- Bertoux M, de Souza LC, Zamith P, Dubois B, Bourgeois-Gironde S. Discounting of future rewards in behavioural variant frontotemporal dementia and Alzheimer’s disease. Neuropsychology. 2015;29(6):933–939. doi: 10.1037/neu0000197. [DOI] [PubMed] [Google Scholar]
- Bethell EJ, Holmes A, MacLarnon A, Semple S. Evidence that emotion mediates social attention in Rhesus Macaques. PLoS ONE. 2012;7(8):e44387. doi: 10.1371/journal.pone.0044387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betzel RF, Gu S, Medaglia JD, Pasqualetti F, Bassett DS. Optimally controlling the human connectome: the role of network topology. Sci Rep. 2016;6:30770. doi: 10.1038/srep30770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biro PA, Stamps JA. Are animal personality traits linked to life-history productivity? Trends in Ecol Evol. 2008;23:361–368. doi: 10.1016/j.tree.2008.04.003. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Lømo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973;232(2):331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm JK, Lyubomirsky S. Does happiness promote career success? J Career Assess. 2008;16:101–116. [Google Scholar]
- Boolani A, O’Connor PJ, Reid J, et al. Predictors of feelings of energy differ from predictors of fatigue. Fatigue Biomed Health Behav. 2019;7(1):12–28. [Google Scholar]
- Bourgin J, Guyader N, Chauvin A, Juphard A, Sauvée M, Moreaud O, Silvert L, Hot P. Early emotional attention is impacted in Alzheimer’s disease: an eye-tracking study. J Alzheimers Dis. 2018;63(4):1445–1458. doi: 10.3233/JAD-180170. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Logothetis N, Singer W. Scaling brain size, keeping timing: evolutionary preservation of brain rhythms. Neuron. 2013;80(4):751–764. doi: 10.1016/j.neuron.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camerer CF, Hogarth RM. The effects of financial incentives in experiments: a review and capital-labor production framework. J. Risk Uncertain. 1999;19:7–42. [Google Scholar]
- Cameron D, Hutcherson C, Ferguson A, Scheffer JA, Hadjiandreou E, Inzlicht M (2017) Empathy is hard work: people choose to avoid empathy because of its cognitive costs. J Exp Psychol General 2887903 [DOI] [PubMed]
- Careau V, Garland T., Jr Performance, personality, and energetics: correlation, causation, and mechanism. Physiol Biochem Zool. 2012;85:543–571. doi: 10.1086/666970. [DOI] [PubMed] [Google Scholar]
- Cavanagh JF, Frank MJ. Frontal theta as a mechanism for cognitive control. Trends Cogn Sci. 2014;18(8):414–421. doi: 10.1016/j.tics.2014.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charney DS. Reduced global functional connectivity of the medial prefrontal cortex in major depressive disorder. Hum Brain Mapp. 2016;37(9):3214–3223. doi: 10.1002/hbm.23235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen KH, Lwi SJ, Hua AY, Haase CM, Miller BL, Levenson RW. Increased subjective experience of non-target emotions in patients with frontotemporal dementia and Alzheimer’s disease. Curr Opin Behav Sci. 2017;15:77–84. doi: 10.1016/j.cobeha.2017.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutinho D, Goncalves B, Wong DP, Travassos B, Coutts AJ, Sampaio J. Exploring the effects of mental and muscular fatigue in soccer players’ performance. Hum Mov Sci. 2018;58:287–296. doi: 10.1016/j.humov.2018.03.004. [DOI] [PubMed] [Google Scholar]
- Cox RT (1979) Of inference and inquiry, an essay in inductive logic. In: Proceedings of the maximum entropy formalism—first maximized entropy workshop, Boston, MA, USA, pp 119–168
- Csikszentmihalyi M, Hunter J. Happiness in everyday life: the uses of. J Happiness Stud. 2003;4:185–199. [Google Scholar]
- D’Acquisto F. Affective immunology: where emotions and the immune response converge. Dialogues Clin Neurosci. 2017;19(1):9–19. doi: 10.31887/DCNS.2017.19.1/fdacquisto. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day MV, Bobocel DR. The weight of a guilty conscience: subjective body weight as an embodiment of guilt. PLoS ONE. 2013;8(7):e69546. doi: 10.1371/journal.pone.0069546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deci EL, Koestner R, Ryan RM. A meta-analytic review of experiments examining the effects of extrinsic rewards on intrinsic motivation. Psychol Bull. 1999;125:627–668. doi: 10.1037/0033-2909.125.6.627. [DOI] [PubMed] [Google Scholar]
- Deli E (2015) The science of consciousness: how a new understanding of time and space infers the evolution of the mind. Self-Published Hungary/USA
- Deli E. Can the fermionic mind hypothesis (fmh) explain consciousness the physics of selfhood. Act Nerv Super. 2020 doi: 10.1007/s41470-020-00070-4. [DOI] [Google Scholar]
- Deli E, Peters J, Tozzi A. The thermodynamic analysis of neural computation. J Neurosci Clin Res. 2018;3:1. [Google Scholar]
- DeYoung CG. The neuromodulator of exploration: a unifying theory of the role of dopamine in personality. Front Hum Neurosci. 2013;7:762. doi: 10.3389/fnhum.2013.00762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diener Ed, Chan MY. Happy people live longer: subjective well-being contributes to health and longevity. Appl Psychol: Health Well-Being. 2011;3(1):1–43. [Google Scholar]
- Di Domenico SI, Ryan RM. The emerging neuroscience of intrinsic motivation: a new frontier in self-determination research. Front Hum Neurosci. 2017;11:145. doi: 10.3389/fnhum.2017.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drago V, Foster PS, Chanei L, Rembisz J, Meador K, Finney G. Emotional indifference in Alzheimer’s disease. J Neuropsychiatry Clin Neurosci. 2010;22:236–242. doi: 10.1176/jnp.2010.22.2.236. [DOI] [PubMed] [Google Scholar]
- Fang H, He B, Fu H, Zhang H, Mo Z, Meng L. A surprising source of self-motivation: prior competence frustration strengthens one’s motivation to win in another competence-supportive activity. Front Hum Neurosci. 2018;12:314. doi: 10.3389/fnhum.2018.00314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fingelkurts AA, Fingelkurts AA. Present moment, past, and future: mental kaleidoscope. Front Psychol. 2014;5:395. doi: 10.3389/fpsyg.2014.00395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredericks CA, Sturm VE, Brown JA, Hua AY, Bilgel M, et al. Early affective changes and increased connectivity in preclinical Alzheimer’s disease. Alzheimers Dement (Amst) 2018;10:471–479. doi: 10.1016/j.dadm.2018.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrickson BL, Joiner T. Positive emotions trigger upward spirals toward emotional well-being. Am Psychol Soc. 2002;13(2):172–175. doi: 10.1111/1467-9280.00431. [DOI] [PubMed] [Google Scholar]
- Friston KJ. A free energy principle for biological systems. Entropy. 2012;14:2100–2121. doi: 10.3390/e14112100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ. Active inference and cognitive consistency. Psychol Inq. 2018;29(2):67–73. doi: 10.1080/1047840x.2018.1480693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K, FitzGerald T, Rigoli F, Schwartenbeck P, Pezzulo G. Active inference: a process theory. Neural Comput. 2017;29(1):1–49. doi: 10.1162/NECO_a_00912. [DOI] [PubMed] [Google Scholar]
- Fry R. Physical intelligence and thermodynamic computing. Entropy. 2017;19:107. [Google Scholar]
- Gabora L (2016) A possible role for entropy in creative cognition. In: Proceedings of the 3rd international electronic conference on entropy and its applications, 1–10 November; Sciforum Electronic Conference Series 3, E001, 10.3390/ecea-3-e001
- Gehring WJ, Coles MGH, Meyer DE, Donchin E. The error-related negativity: an event-related brain potential accompanying errors. Psychophysiology. 2018;27:S34. [Google Scholar]
- Gottlieb J, Oudeyer PY. Towards a neuroscience of active sampling and curiosity. Nat Rev Neurosci. 2018;19(12):758–770. doi: 10.1038/s41583-018-0078-0. [DOI] [PubMed] [Google Scholar]
- Gray JR, Thompson PM. Neurobiology of intelligence: health implications? Discov Med. 2004;4:157–162. [PubMed] [Google Scholar]
- Grieder M, Wang D, Dierks T, Wahlund LO, Jann K. Default mode network complexity and cognitive decline in mild Alzheimer’s disease. Front Neurosci. 2018;12:770. doi: 10.3389/fnins.2018.00770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grolnick WS, Ryan RM. Autonomy in children’s learning: an experimental and individual difference investigation. J Pers Soc Psychol. 1987;52:890–898. doi: 10.1037//0022-3514.52.5.890. [DOI] [PubMed] [Google Scholar]
- Haier RJ, Siegel BV, Nuechterlein KH, Hazlett E, Wu JC, Paek J, Browning HL, Buchsbaum MS. Cortical glucose metabolic rate correlates of abstract reasoning and attention studied with positron emission tomography. Intelligence. 1988;12(2):199–217. [Google Scholar]
- Hamilton KR, Mitchell MR, Wing VC, Balodis IM, Bickel WK, Fillmore M, Moeller FG. Choice impulsivity: definitions, measurement issues, and clinical implications. Pers Disord. 2015;6(2):182–198. doi: 10.1037/per0000099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heintzelman SJ, Trent J, King LA. Encounters with objective coherence and the experience of meaning in life. Psychol Sci. 2013;24:991–998. doi: 10.1177/0956797612465878. [DOI] [PubMed] [Google Scholar]
- Hesse J, Gross T. Self-organized criticality as a fundamental property of neural systems. Front Syst Neurosci. 2014;23(8):166. doi: 10.3389/fnsys.2014.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsh JB, Mar RA, Peterson JB. Psychological entropy: a framework for understanding uncertainty-related anxiety. Psychol Rev. 2012;119:304–320. doi: 10.1037/a0026767. [DOI] [PubMed] [Google Scholar]
- Hogeveen J, Krug MK, Elliott MV, Solomon M. Insula-retrosplenial cortex overconnectivity increases internalizing via reduced insight in autism. Biol Psychiatry. 2018;84:287–294. doi: 10.1016/j.biopsych.2018.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inzlicht M, Bartholow BD, Hirsh JB. Emotional foundations of cognitive control. Trends Cogn Sci. 2015;19(3):126–132. doi: 10.1016/j.tics.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inzlicht M, Shenhav A, Olivola CY. The effort paradox: effort is both costly and valued. Trends Cogn Sci. 2018;22(4):337–349. doi: 10.1016/j.tics.2018.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joffily M, Coricelli G. Emotional valence and the free-energy principle. PLoS Comput Biol. 2013;9(6):e1003094. doi: 10.1371/journal.pcbi.1003094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DT, Machulda MM, Vemuri P, Mcdade EM, Zeng G, Senjem ML, et al. Age-related changes in the default mode network are more advanced in Alzheimer disease. Neurology. 2011;77:1524–1531. doi: 10.1212/WNL.0b013e318233b33d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczmarek LD, Behnke M, Kashdan TB, Kusiak A, Marzec K, Mistrzak M, Włodarczyk M (2017) Smile intensity in social networking profile photographs is related to greater scientific achievements. J Posit Psychol 1–5
- Kaiser RH, Whitfield S, Gabrieli DG, et al. Dynamic resting-state functional connectivity in major depression. Neuropsychopharmacol. 2016;41(7):1822–1830. doi: 10.1038/npp.2015.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao F-C, Wang SR, Chang YJ. Brainwaves analysis of positive and negative emotions. ISAA. 2015;12:1263–1266. [Google Scholar]
- Kiang M, Christensen BK, Remington G, Kapur S. Apathy in schizophrenia: clinical correlates and association with functional outcome. Schizophr Res. 2003;63:79–88. doi: 10.1016/s0920-9964(02)00433-4. [DOI] [PubMed] [Google Scholar]
- Kiefer M, Pulvermüller F. Conceptual representations in mind and brain: theoretical developments, current evidence and future directions. Cortex. 2012;48:805–825. doi: 10.1016/j.cortex.2011.04.006. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Hsu M. Common neural code for reward and information value. Proc Natl Acad Sci. 2019 doi: 10.1073/pnas.1820145116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koechlin EC. Summerfield an information theoretical approach to prefrontal executive function. Trends Cogn Sci. 2007;11:229–235. doi: 10.1016/j.tics.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Köhler S, Schumann A, Cruz FD, Wagner G, Bär K-J. Towards response success prediction: an integrative approach using high-resolution fMRI and autonomic indices. Neuropsychologia. 2018;119:182–190. doi: 10.1016/j.neuropsychologia.2018.08.003. [DOI] [PubMed] [Google Scholar]
- Koivumaa-Honkanen H, Koskenvuo M, Honkanen RJ, Viinamäki H, Heikkilä K, Kaprio J. Life dissatisfaction and subsequent work disability in an 11-year follow-up. Psychol Med. 2004;34:221–228. doi: 10.1017/s0033291703001089. [DOI] [PubMed] [Google Scholar]
- Kool W, Botvinick M. Mental labour. Nat Hum Behav. 2018 doi: 10.1038/s41562-018-0401-9. [DOI] [PubMed] [Google Scholar]
- Kopec AM, et al. Neuro-immune mechanisms regulating social behavior: dopamine as mediator? Trends Neurosci. 2019;42(5):337–348. doi: 10.1016/j.tins.2019.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kounios J, Beeman M. The aha! moment: the cognitive neuroscience of insight. Curr Dir Psychol Sci. 2009;18(4):210–216. [Google Scholar]
- Kumfor F, Zhen A, Hodges JR, Piguet O, Irish M. Apathy in Alzheimer’s disease and frontotemporal dementia: distinct clinical profiles and neural correlates. Cortex. 2018;103:350–359. doi: 10.1016/j.cortex.2018.03.019. [DOI] [PubMed] [Google Scholar]
- Kyong J, Chung CK, Kim JS. The entropic brain: effortful speech reflected in organisation of the network graph. J Cogn Sci. 2015;16:61–72. [Google Scholar]
- Landauer R. Irreversibility and heat generation in the computing process. IBM J Res Dev. 1961;5:183–191. [Google Scholar]
- Li X, Zhu Z, Zhao W, Sun Y, Wen D, Xie Y, et al. Decreased resting-state brain signal complexity in patients with mild cognitive impairment and Alzheimer’s disease: a multiscale entropy analysis. Biomed Opt Express. 2018;9:1916–1929. doi: 10.1364/BOE.9.001916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, LeeHJ WeitzAJ, Fang Z, Lin P, Choy M, et al. Frequency selective control of cortical and subcortical networks by central thalamus. Elife. 2015;4:e09215. doi: 10.7554/elife.09215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London ED. Impulsivity, stimulant abuse, and dopamine receptor signaling. Adv Pharmacol. 2016;76:67–84. doi: 10.1016/bs.apha.2016.01.002. [DOI] [PubMed] [Google Scholar]
- Low I, Kuo PC, Tsai CL, Liu YH, Lin MW, Chao HT, et al. Interactions of BDNF Val66Met polymorphism and menstrual pain on brain complexity. Front Neurosci. 2018;12:826. doi: 10.3389/fnins.2018.00826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loy BD, Cameron MH, O’Connor PJ. Perceived fatigue and energy are independent unipolar states: supporting evidence. Med Hypotheses. 2018;113:46–51. doi: 10.1016/j.mehy.2018.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, Maheu F, Tu M, Fiocco A, Schramek TE. The effects of stress and stress hormones on human cognition. Implications for the field of brain and cognition. Brain Cogn. 2007;65(3):209–237. doi: 10.1016/j.bandc.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Lustenberger C, et al. Functional role of frontal alpha oscillations in creativity. Cortex. 2015;67:74–82. doi: 10.1016/j.cortex.2015.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado L, Cantilino A. A systematic review of the neural correlates of positive emotions. Rev Bras Psiquiatr. 2016;39:172–179. doi: 10.1590/1516-4446-2016-1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manohar SG, Muhammed K, Fallon SJ, et al. Motivation dynamically increases noise resistance by internal feedback during movement. Neuropsychologia. 2018;123:19–29. doi: 10.1016/j.neuropsychologia.2018.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulies DS, Ghosh SS, Goulas A, Falkiewicz M, Huntenburg JM, Langs G, et al. Situating the default-mode network along a principal gradient of macroscale cortical organization. PNAS. 2016;113(44):12574–12579. doi: 10.1073/pnas.1608282113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin K, Meeusen R, Thompson KG, et al. Mental fatigue impairs endurance performance: a physiological explanation. Sports Med. 2018;48:2041. doi: 10.1007/s40279-018-0946-9. [DOI] [PubMed] [Google Scholar]
- McMorris T, Barwood M, Hale B, Dicks M, Corbett J. Cognitive fatigue effects on physical performance. J Phys Behav. 2018;188:103–107. doi: 10.1016/j.physbeh.2018.01.029. [DOI] [PubMed] [Google Scholar]
- Meeusen R, Van Cutsem J, Roelands B. Endurance exercise-induced and mental fatigue and the brain. Exp Psychol. 2020 doi: 10.1113/EP088186. [DOI] [PubMed] [Google Scholar]
- Meijers J, Harte JM, Meynen G, Cuijpers P, Scherder EJ. Reduced self-control after three months of imprisonment; a pilot study. Front Psychol. 2018;9:69. doi: 10.3389/fpsyg.2018.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mildner JN, Tamir DI. Spontaneous thought as an unconstrained memory process. Trends Neurosci. 2019;42(11):763–777. doi: 10.1016/j.tins.2019.09.001. [DOI] [PubMed] [Google Scholar]
- Mitchell JM, Weinstein D, Vega T, Kayser AS. Dopamine, time perception, and future time perspective. Psychopharmacology. 2018;235(10):2783–2793. doi: 10.1007/s00213-018-4971-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran TP, Bernat EM, Aviyente S, Schroder HS, Moser JS. Sending mixed signals: worry is associated with enhanced initial error processing but reduced call for subsequent cognitive control. Soc Cogn Affect Neurosci. 2015;10(11):1548–1556. doi: 10.1093/scan/nsv046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhammed K, Manohar S, Ben Yehuda M, Chong TT-J, Tofaris G, Lennox G, et al. Reward sensitivity deficits modulated by dopamine are associated with apathy in Parkinson’s disease. Brain. 2016;139(10):2706–2721. doi: 10.1093/brain/aww188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller L, Chavane F, Reynolds J, Sejnowski TJ. Cortical travelling waves: mechanisms and computational principles. Nat Rev Neurosci. 2018;19:255–268. doi: 10.1038/nrn.2018.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrough JW, Abdallah CG, Anticevic A, Collins KA, Geha P, Averill LA, Schwartz J, DeWilde KE, et al. Reduced global functional connectivity of the medial prefrontal cortex in major depressive disorder. Hum Brain Mapp. 2016;37(9):3214–3223. doi: 10.1002/hbm.23235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musall S, Kaufman MT, Juavinett AL, Gluf S, Churchland AK. Single-trial neural dynamics are dominated by richly varied movements. Nat Neurosci. 2019;22:1677–1686. doi: 10.1038/s41593-019-0502-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neupert SD, Allaire JC. I think I can, I think I can: examining the within-person coupling of control beliefs and cognition in older adults. Psychol Aging. 2012;2(2):145–152. doi: 10.1037/a0026447. [DOI] [PubMed] [Google Scholar]
- Nobis L, Husain M. Apathy in Alzheimer’s disease. Curr Opin Behav Sci. 2018;22:7–13. doi: 10.1016/j.cobeha.2017.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oei NYL, Rombouts SARB, Soeter RP, van Gerven JM, Both S. Dopamine modulates reward system activity during subconscious processing of sexual stimuli. Neuropsychopharmacology. 2012;37:1729–1737. doi: 10.1038/npp.2012.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omidvarnia A, Mesbah M, Pedersen M, Jackson G (2018) Range entropy: a bridge between signal complexity and self-similarity. arXiv:1809.06500 [DOI] [PMC free article] [PubMed]
- Padmanabhan A, Lynch CJ, Schaer M, Menon V. The default mode network in autism. Biol Psychiatry Cogn Neurosci Neuroimaging. 2017;2:476–486. doi: 10.1016/j.bpsc.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pageaux B, et al. Response inhibition impairs subsequent self-paced endurance performance. Eur J Appl Physiol. 2014;114(5):1095–1105. doi: 10.1007/s00421-014-2838-5. [DOI] [PubMed] [Google Scholar]
- Panksepp J. Affective neuroscience: the foundations of human and animal emotions. New York: Oxford University Press; 1998. [Google Scholar]
- Pasley BN, Freeman RD. Neurovascular coupling. Scholarpedia. 2008;3(3):5340. [Google Scholar]
- Patzelt EH, Kool W, Millner AJ, Gershman SJ. The transdiagnostic structure of mental effort avoidance. Sci Rep. 2019;9(1):1689. doi: 10.1038/s41598-018-37802-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepperell R. Consciousness as a physical process caused by the organization of energy in the brain. Front Psychol. 2018;1(9):2091. doi: 10.3389/fpsyg.2018.02091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepperell R. Consciousness as a physical process caused by the organization of energy in the brain. Front Psychol. 2018 doi: 10.3389/fpsyg.2018.02091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perri R, Carlesimo GA, Costa A. The contribution of neuropsychological and neuroimaging research to the definition of the neurocognitive correlates of apathy. Neuropsychologia. 2018;118(Pt B):1–3. doi: 10.1016/j.neuropsychologia.2018.08.013. [DOI] [PubMed] [Google Scholar]
- Peters A, McEwen BS, Friston K. Uncertainty and stress: why it causes diseases and how it is mastered by the brain. Prog Neurobiol. 2017;156:164–188. doi: 10.1016/j.pneurobio.2017.05.004. [DOI] [PubMed] [Google Scholar]
- Piscopo D, Weible A, Rothbart MK, Posner MI, Niell CM. Changes in white matter in mice resulting from low frequency brain stimulation. Proc Natl Acad Sci USA. 2018;115(27):6639–6646. doi: 10.1073/pnas.1802160115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack RA. Is “efficiency” a useful concept in cognitive neuroscience? Dev Cogn Neurosci. 2015;11:12–17. doi: 10.1016/j.dcn.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pop-Jordanova N, Pop-Jordanov N. Spectrum-weighted EEG frequency (“brain-rate”) as a quantitative indicator of mental arousal. Prilozi. 2005;26(2):35–42. [PubMed] [Google Scholar]
- Remmers C, Zander T. Why you don’t see the forest for the trees when you are anxious: anxiety impairs intuitive decision making. Clin Psycholog Sci. 2018;6:48–62. [Google Scholar]
- Remmers C, Zander T. Why you don’t see the forest for the trees when you are anxious: anxiety impairs intuitive decision making. Clin Psychol Sci. 2018;6:48–62. [Google Scholar]
- Rocha RP, et al. Homeostatic plasticity and emergence of functional networks in a whole-brain model at criticality. Sci Rep. 2018;8:1–5. doi: 10.1038/s41598-018-33923-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe AD, Fitness J. Understanding the role of negative emotions in adult learning and achievement: a social functional perspective. Behav Sci (Basel) 2018;8(2):27. doi: 10.3390/bs8020027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubaltelli E, Agnoli S, Leo I. Emotional intelligence impact on half marathon finish times. Pers Individ Diff. 2018;128:107–112. [Google Scholar]
- Rudd M, Aaker J, Vohs K. Awe expands people’s perception of time, alters decision making, and enhances well-being. Psychol Sci. 2012;23(10):1130–1136. doi: 10.1177/0956797612438731. [DOI] [PubMed] [Google Scholar]
- Ryan RM, Deci EL. From ego depletion to vitality: theory 242 WEINSTEIN AND RYAN and findings concerning the facilitation of energy available to the self. Soc Pers Psychol Compass. 2008;2:702–717. [Google Scholar]
- Ryan RM, Deci EL. Self-determination theory: basic psychological needs in motivation development and wellness. New York: Guilford Press; 2017. [Google Scholar]
- Ryan RM, Deci EL, Vansteenkiste M (2016) Autonomy and autonomy disturbances in self-development and psychopathology: research on motivation, attachment, and clinical process. In: D. Cicchetti (eds) Developmental psychopathology, vol. 1, 3rd edn. Theory and Method, pp 385–438
- Saarimäki H et al (2017) Distributed affective space represents multiple emotion categories across the brain. 10.1101/123521 [DOI] [PMC free article] [PubMed]
- Saarimäki H, Gostopoulos A, Jääskeläinen IP, Lampinen J, Vuilleumier P, Sams M, Nummenmaa L (2015) Discrete neural signatures of basic emotions. Cereb Cortex 1–11 [DOI] [PubMed]
- Saarimäki H, et al. Discrete neural signatures of basic emotions. Cereb Cortex. 2016;26(6):2563–2573. doi: 10.1093/cercor/bhv086. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M. The mysterious motivational functions of mesolimbic dopamine. Neuron. 2012;76:470–485. doi: 10.1016/j.neuron.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapey-Triomphe L-A, Heckemann RA, Boublay N, Dorey J-M, Hénaff M-A, Rouch I, et al. Neuroanatomical correlates of recognizing face expressions in mild stages of Alzheimer’s disease. PLoS ONE. 2015;10(12):e0143586. doi: 10.1371/journal.pone.0143586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders B, Lin H, Milyavskaya M, Inzlicht M. The emotive nature of conflict monitoring in the medial prefrontal cortex. Int J Psychophysiol. 2017;119:31–40. doi: 10.1016/j.ijpsycho.2017.01.004. [DOI] [PubMed] [Google Scholar]
- Saxe G, Calderone D, Morales L. Brain entropy and human intelligence: a resting-state fMRI study. PLoS ONE. 2018;13:e0191582. doi: 10.1371/journal.pone.0191582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayood K. Information theory and cognition: a review. Entropy. 2018;20:706. doi: 10.3390/e20090706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrödinger E. What is life? the physical aspect of the living cell. Cambridge: Cambridge University Press; 1945. [Google Scholar]
- Schwartz JM, Stapp HP, Beauregard M. Quantum physics in neuroscience and psychology: a neurophysical model of mind-brain interaction. Philos Trans. R Soc Lond B Biol Sci. 2005;360:1309–1327. doi: 10.1098/rstb.2004.1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selye H. Stress without distress. New York: New American Library; 1974. [Google Scholar]
- Seo D, et al. Role of serotonin and dopamine system interactions in the neurobiology of impuive aggression and its comorbidity with other clinical disorders. Aggress Violent Behav. 2008;13(5):383–395. doi: 10.1016/j.avb.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson EH, Balsam PD. The behavioral neuroscience of motivation: an overview of concepts, measures, and translational applications. Curr Top Behav Neurosci. 2015;27:1–12. doi: 10.1007/7854_2015_402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sizemore AE, Giusti C, Kahn A, et al. Cliques and cavities in the human connectome. J Comput Neurosci. 2018;44:115. doi: 10.1007/s10827-017-0672-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RX, Jann K, Dapretto M, Wang DJJ. Imbalance of functional connectivity and temporal entropy in resting-state networks in autism spectrum disorder: a machine learning approach. Front Neurosci. 2018;12:869. doi: 10.3389/fnins.2018.00869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smitha KA, Akhil Raja K, Arun KM, Rajesh PG, Thomas B, Kapilamoorthy TR, Kesavadas C. Resting state fMRI: a review on methods in resting state connectivity analysis and resting state networks. Neuroradiol J. 2017;4:305–317. doi: 10.1177/1971400917697342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares S, Atallah BV, Paton JJ. Midbrain dopamine neurons control judgment of time. Science. 2016;354:1273–1277. doi: 10.1126/science.aah5234. [DOI] [PubMed] [Google Scholar]
- Sokunbi MO, Fung W, Sawlani V, Choppin S, Linden DE, Thome J. Resting state fMRI entropy probes complexity of brain activity in adults with ADHD. Psychiatry Res. 2013;214:341–348. doi: 10.1016/j.pscychresns.2013.10.001. [DOI] [PubMed] [Google Scholar]
- Stanghellini G, Ballerini M, Presenza S, Mancini M, Northoff G, Cutting J. Abnormal time experiences in major depression. An empirical qualitative study. Psychopathology. 2016 doi: 10.1159/000452892. [DOI] [PubMed] [Google Scholar]
- Stellar JE, et al. Positive affect and markers of inflammation: discrete positive emotions predict lower levels of inflammatory cytokines. Emotion. 2015;2:129–133. doi: 10.1037/emo0000033. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Wardle J. Positive affect and biological function in everyday life. Neurobiol Aging. 2005;26(Suppl. 1):108–112. doi: 10.1016/j.neurobiolaging.2005.08.016. [DOI] [PubMed] [Google Scholar]
- Street S. Neurobiology as information physics. Front Syst Neurosci. 2016;10:90. doi: 10.3389/fnsys.2016.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sur S, Sinha VK. Event-related potential: an overview. Ind Psychiatry J. 2009;18(1):70–73. doi: 10.4103/0972-6748.57865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K, Sumiyoshi T, Matsumoto M, Murayama K, Ikezawa S, Matsumoto K, Nakagome K. Neural correlates for intrinsic motivational deficits of schizophrenia; implications for therapeutics of cognitive impairment. Front Psychiatry. 2018;9:178. doi: 10.3389/fpsyt.2018.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipples J. Increased frustration predicts the experience of time slowing-down: evidence from an experience sampling study. T&TP. 2018;6(2):220–230. [Google Scholar]
- Tomasi D, Wang GJ, Volkow ND. Energetic cost of brain functional connectivity. PNAS. 2013;110:13642–13647. doi: 10.1073/pnas.1303346110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touroutoglou A, Lindquist KA, Dickerson BC, Barrett LF. Intrinsic connectivity in the human brain does not reveal networks for “basic” emotions. Soc Cogn Affect Neurosci. 2015 doi: 10.1093/scan/nsv013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touroutoglou A, Andreano JM, Adebayo M, Barrett LF. Motivation in the service of allostasis: the role of the anterior cingulate cortex. Adv Motiv Sci. 2019;6:1–25. doi: 10.1016/bs.adms.2018.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tozzi A, Peters JF. From abstract topology to real thermodynamic brain activity. Cogn neurodyn. 2017;11(3):283–292. doi: 10.1007/s11571-017-9431-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tozzi A, Peters JF, Fingelkurts AA, Fingelkurts AA, Marijuán PC. Topodynamics of metastable brains. Phys Life Rev. 2017 doi: 10.1016/j.plrev.2017.03.001. [DOI] [PubMed] [Google Scholar]
- Treadway MT, Buckholtz JW, Cowan RL, Woodward ND, Li R, Ansari MS, et al. Dopaminergic mechanisms of individual differences in human effort-based decision-making. J Neurosci. 2012;32(18):6170–6176. doi: 10.1523/JNEUROSCI.6459-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevisiol A, et al. Monitoring ATP dynamics in electrically active white matter tracts. eLife. 2017;6:e24241. doi: 10.7554/eLife.24241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhrig L, Sitt JD, Jacob A, et al. Resting-state dynamics as a cortical signature of anesthesia in monkeys. Anesthesiology. 2018;129(5):942–958. doi: 10.1097/ALN.0000000000002336. [DOI] [PubMed] [Google Scholar]
- Van Cutsem J, Marcora S, De Pauw K, et al. The effects of mental fatigue on physical performance: a systematic review. Sports Med. 2017;47(8):1569–1588. doi: 10.1007/s40279-016-0672-0. [DOI] [PubMed] [Google Scholar]
- van den Heuvel MP, Sporns O. Rich-club organization of the human connectome. J Neurosci. 2011;31:15775–15786. doi: 10.1523/JNEUROSCI.3539-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP, Kahn RS, Goni J, Sporns O. High-cost, high-capacity backbone for global brain communication. Proc Natl Acad Sci USA. 2012;109:11372–11377. doi: 10.1073/pnas.1203593109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veltkamp M, Aarts H, Custers R. Unravelling the motivational yarn: a framework for understanding the instigation of implicitly motivated behaviour resulting from deprivation and positive affect. Eur Rev Soc Psychol. 2009;20:345–381. [Google Scholar]
- Verma R, Balhara YP, Gupta CS. Gender differences in stress response: role of developmental and biological determinants. Ind Psychiatry J. 2011;20(1):4–10. doi: 10.4103/0972-6748.98407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Niu Y, Miao L, Cao R, Yan P, Guo H, et al. Decreased complexity in Alzheimer’s disease: resting-state fMRI evidence of brain entropy mapping. Front Aging Neurosci. 2017;9:378. doi: 10.3389/fnagi.2017.00378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang DJ, Jann K, Fan C, Qiao Y, Zang Y-F, Lu H, et al. Neurophysiological basis of multiscale entropy of brain complexity and its relationship with functional connectivity. Front Neurosci. 2018;12:352. doi: 10.3389/fnins.2018.00352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warm JS, Parasuraman R, Matthews G. Vigilance requires hard mental work and is stressful. Hum Factors. 2008;50(3):433–441. doi: 10.1518/001872008X312152. [DOI] [PubMed] [Google Scholar]
- Wise T, Marwood L, Perkins AM, Herane-Vives A, Joules R, Lythgoe DJ, et al. Instability of default mode network connectivity in major depression: a two-sample confirmation study. Transl Psychiatry. 2017;7(4):e1105. doi: 10.1038/tp.2017.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wissner-Gross AD, Freer CE. Causal entropic forces. Phys Rev Lett. 2013;110:168702. doi: 10.1103/PhysRevLett.110.168702. [DOI] [PubMed] [Google Scholar]
- Wu J, Zhang J, Liu C, Liu D, Ding X, Zhou C. Graph theoretical analysis of EEG functional connectivity during music perception. Brain Res. 2012;1483:71–81. doi: 10.1016/j.brainres.2012.09.014. [DOI] [PubMed] [Google Scholar]
- Yamada Y, Kawabe T. Emotion colors time perception unconsciously. Conscious Cogn. 2011;20(4):1–7. doi: 10.1016/j.concog.2011.06.016. [DOI] [PubMed] [Google Scholar]
- Yang X, Li L, Yang Q, Wang Y, Wang X, Zou Q, et al. Spatial complexity of brain signal is altered in patients with generalized anxiety disorder. J Affect Disord. 2018;246:387–393. doi: 10.1016/j.jad.2018.12.107. [DOI] [PubMed] [Google Scholar]
- Yang S, Zhao Z, Cui H, et al. Temporal variability of cortical gyral-sulcal resting state functional activity correlates with fluid intelligence. Front Neural Circuits. 2019;13(36):1–12. doi: 10.3389/fncir.2019.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zohar D, Tzischinski O, Epstein R. Effects of energy availability on immediate and delayed emotional reactions to work events. J Appl Psychol. 2003;88:1082–1093. doi: 10.1037/0021-9010.88.6.1082. [DOI] [PubMed] [Google Scholar]