Abstract
Background
Infertility is a global health issue and hysterosalpingography (HSG) is a valuable radiological tool in infertility workup and remains a main modality for investigating female infertility. However, the HSG findings of infertility are not the same worldwide.
This study aimed at evaluating the incidence of HSG findings in patients investigated for primary and secondary infertility, correlating these findings with their clinical data that reflect the infertility causes and comparing the findings with previous international studies.
Methods
A prospective descriptive study of 75 female patients referred, as cases of infertility, for HSG examination in Elrebat Hospital and Khartoum Advanced Diagnostic Center. HSG was performed in the first half of the cycle. The procedure and its complications, were explained to the patients and informed consents were obtained. Patients with active pelvic infection and active uterine or vaginal bleeding were excluded from the study. Using aseptic technique and with proper patient’s positioning, iodinated contrast was introduced into the cervix under fluoroscopic monitoring, to demonstrate the uterine cavity, fallopian tubes and free spillage into the peritoneal cavity. Personal data, clinical data and HSG findings were analyzed using SPSS version 23.
Results
The commonest age group seen was 26–36 years. Close incidences of primary and secondary infertility were detected. 52.7% had abnormal findings in HSG. Tubal pathology (42.7%) being the most common abnormality, followed by uterine and combined abnormalities. There was strong association between past medical history suggesting pelvic inflammatory disease (PID) or past history suggesting tubal blockage secondary to abdominopelvic surgery and tubal abnormalities.
Conclusion
HSG examinations revealed that the most common abnormality was tubal blockage possibly complicating PID and abdominopelvic surgeries. This reflects the HSG diagnostic and therapeutic role in the assessment of female infertility and the further needs for more preventive measures targeting the reduction of tubal pathologies in developing countries.
Keywords: Abdominopelvic surgery, Hysterosalpingography, Infertility, Pelvic inflammatory disease, Tubal blockage
1. Introduction
Infertility is the failure to establish a clinical pregnancy after 12 months of regular, unprotected sexual intercourse or due to an impairment of a person’s capacity to reproduce either as an individual or with the partner (Zegers-Hochschild et al., 2017). Infertility affects approximately 15% of reproductive-aged couples with recent prevalence between 9 and 18% of the general population (Pundir and ELToukhy, 2010, Aghajanova et al., 2017). It is classified into primary, in which the couples have never conceived at any stage, or secondary when previous pregnancy, although not necessarily a live birth, has occurred (Subedi et al., 2016).
Due to the rising prevalence of male infertility and the delay in child bearing in females, infertility rates are increasing (Thable, et al., 2020).
Infertility causes have been categorized into male infertility causes, due to poor semen parameters and female infertility causes when it is due to occluded fallopian tubes, uterine abnormalities or anovulation (Panti and Sununu, 2014).
Tubal pathologies constitute 35–40% of infertility causes. Tubal function can be impaired by infection or surgical damage. Blockage can involve the proximal, mid or distal part. Peritubal adhesions due to inflammation, infection, tuberculosis, previous surgeries, endometriosis and ectopic pregnancy are peritoneal factors resulting in tubal subfertility (Jain and Jain, 2019).
Abnormalities of the uterine cavity can be a cause of subfertility in about 10% of females. These include endometrial fibroids and polyps, intrauterine adhesions or synechiae and congenital Müllerian duct abnormalities (Schankath et al., 2012).
Anovulation could be due to functional hypothalamic amenorrhea, premature ovarian failure or polycystic ovary syndrome. Regular menstrual cycles history suggests ovulation and a luteal phase progesterone level above 30 nmol/L confirms it. Morphology of the ovaries and antral follicle count can be assessed by transabdominal and transvaginal ultrasound (Thurston et al., 2019).
Female fertility decreases with female age (Thable, et al., 2020). In a recent study, 52.05% of female infertility causes were due to anovulation (Elhussein et al., 2019).
Causes of male infertility include azoospermia, teratospermia, oligozoospermia and asthenospermia, assessed by semen analyses (Elhussein et al., 2019).
Assessment of the female genital tract anatomy is an essential component of infertility investigations where imaging plays an essential role in the diagnostic evaluation of infertility (Choussein and Vlahos, 2012). HSG is radiographic test used to evaluate the patency of the fallopian tubes, morphology of the uterus and cervix. It is a simple and an inexpensive modality when compared to other methods (Jain and Jain, 2019).
HSG can detect tubal abnormalities including tubal blockage, hydrosalpinx, polyps, salpingitis isthmica nodosa [SIN] and peritubal adhesions as well as, uterine abnormalities including leiomyomas, polyps, adenomyosis, synechiae, surgical changes and congenital anomalies (Simpson et al., 2006). Although, it provides an image of the uterine cavity, it does not show the external contour of the uterus, which is important for differentiating the different types of uterine anomalies (Kiridi et al., 2015). On the other hand, magnetic resonance imaging [MRI] is used to evaluate the myometrium and congenital Müllerian duct anomalies, but has a limited role in assessment of tubal abnormalities. Laparoscopy and hysteroscopy are radiation free procedures used for evaluation of the uterus, cervix and fallopian tubes but are invasive and lack the clear tubal definition that HSG offers (Onwuchekwa and Oriji, 2017). Hysterosalpingo-contrast sonography (HyCoSy) is a valuable method for assessment of uterine wall and cavity, ovaries, peritoneum and fallopian tubes patency, with the advantage of being radiation free (Saanida and Beenamol, 2013). Although, being relatively safe, it can be complicated by pelvic cramps, fever, nausea and vagal symptoms (Yang et al., 2013). The use of three dimensional HyCoSy together with B- mode HyCoSy, in the assessment of the walls and patency of fallopian tubes, is very accurate (Chen et al., 2019). Moreover, HyCoSy has similar accuracy to magnetic resonance-hysterosalpingography, for assessment of tubal patency (Chen, et al., 2020).
The main contraindications for HSG are pregnancy, active pelvic infection, recent tubal or uterine surgery and active vaginal or uterine bleeding (Chalazonitis et al., 2009).
Although, HSG is associated with some disadvantages such as patient discomfort and exposure to radiation, it remains the gold-standard modality used for evaluating female infertility not only in developing countries including Sudan, but also in the United States and Europe (Kaproth-Joslin and Dogra, 2013, Tros et al., 2019, Bukar et al., 2011). However, the HSG pattern of infertility in Sudanese patients remains not fully understood.
Hence, this study aimed at evaluating the incidence of HSG findings in patients investigated for primary and secondary infertility and correlating these findings with the patients’ age, type of infertility and with tubal and peritoneal causes of infertility provided from the patients’ PMH, as well as correlating the HSG findings with personal and clinical data reflecting ovarian and male factors of infertility.
2. Materials and methods
2.1. Study design and participant eligibility
This was a prospective descriptive study during a period of 15 months. A total of 75 Sudanese patients of childbearing age, referred by gynecologists, as cases of infertility for HSG examination in Elrebat Hospital and Khartoum Advanced Diagnostic Center, in Sudan, agreed to participate in the study.
Patients included in the study had a thorough clinical history with special emphasis on the age, type of infertility, menstrual cycle regularity, previous history of pelvic inflammatory disease [PID], tubal blockage or dysfunction, as well as the partner’s seminal investigations
Patients with active PID, lower genital tract infection, active uterine or vaginal bleeding or severe allergy to iodine-based contrast material were excluded as these are contraindications for the study.
2.2. Hysterosalpingography procedure
Trained radiologists, radiographers and nursing team performed the examinations and the reporting was conducted by experienced radiologists. The procedure, and its complications, were explained to the patients after their arrival to the x- ray department and informed consents were obtained from the patients.
HSG procedure was performed as previously described (Onwuchekwa and Oriji, 2017). The patients were scheduled for HSG on 7th to 12th day of the menstrual cycle and before having any sexual intercourse to ensure a thin endometrium that facilitates image interpretation and ensures that there is no pregnancy which is an absolute contraindication for HSG examination. Pregnancy test was requested for some patients, who had irregular menstrual cycles with possibility of pregnancy.
As patients may experience discomfort during hysterosalpingography, they were advised to take a nonsteroidal anti-inflammatory drug, such as ibuprofen 400 mg, half an hour before the procedure, as a preparation.
Each patient was placed in the lithotomy position at the foot end of the table and using aseptic technique, a vaginal speculum was inserted after applying local anesthetic. Then, a uterine sound was passed for uterine size and direction assessment. A syringe filled with 15 ml of 60% urografin was fixed to the end of the cannula and air being removed from the cannula. With a gentle traction on a vulsellum forceps placed on the anterior cervical lip, the cannula was then inserted and fixed to the cervix and the patient moved up the table. Patient position was adjusted and contrast injected slowly under fluoroscopic monitoring. Films were taken with the patient in the supine position after injection of about 5 ml of contrast to demonstrate the cavity of the uterus and after injection of another 5 ml to demonstrate free spillage into the peritoneal cavity. Normal saline and life -saving drugs were available beside the patient. Apart from mild procedural pain, no HSG complication occurred in this study. Patients were informed that vaginal bleeding may occur for one to two days following the procedure. Antibiotics were needed and prescribed for some patients.
2.3. Statistical analysis
A well-constructed questionnaire was used. A descriptive statistical data analysis including personal information, clinical history and radiological findings was performed with the SPSS (Statistical Package for Social Sciences, version 23, SPSS Inc, Chicago, Illinois, USA). The clinical and procedural categorical data of the patients were expressed as frequencies and percentages. Chi-square test was performed to analyze and identify any associations between qualitative variables. P < 0.05 was considered statistically significant.
2.4. Ethical statements
The study protocol was planned in accordance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. All procedures performed in this study were in accordance with the ethical standards of the institutional ethics committee. The anonymity of participants was maintained.
3. Results
3.1. Demographic and clinical characteristics of the participants
The mean age of the participants was 31.71 ± 6.30 (SD) years with a median of 32. The patients aged 15–45 years. 14 (18.7%) of them aged 15–25 years. The majority 43 (57.3%) were within the range of 26–36 years. While, 18 (24%) their ages vary from 37 to 45 years. There were 37 (49.3%) cases of primary infertility and 38 (50.7%) cases with secondary infertility.
Primary infertility affected 14 (100%) of women aged 15–25 years, 19 (44.2%) of 26–36 years age group and 4 (22.2%) of those aged 37–45 years. Secondary infertility didn’t affect the first age group. But, affected 24 (55.8%) of the second and (77.8%) of the third group, respectively (Fig. 1). The mean age of women with primary infertility was 28.57 ± 6.26 (SD) years, while that for those with secondary infertility was 34.76 ± 4.67 (SD) years.
Fig. 1.
Distribution of age groups of patients referred for HSG according to type of infertility.
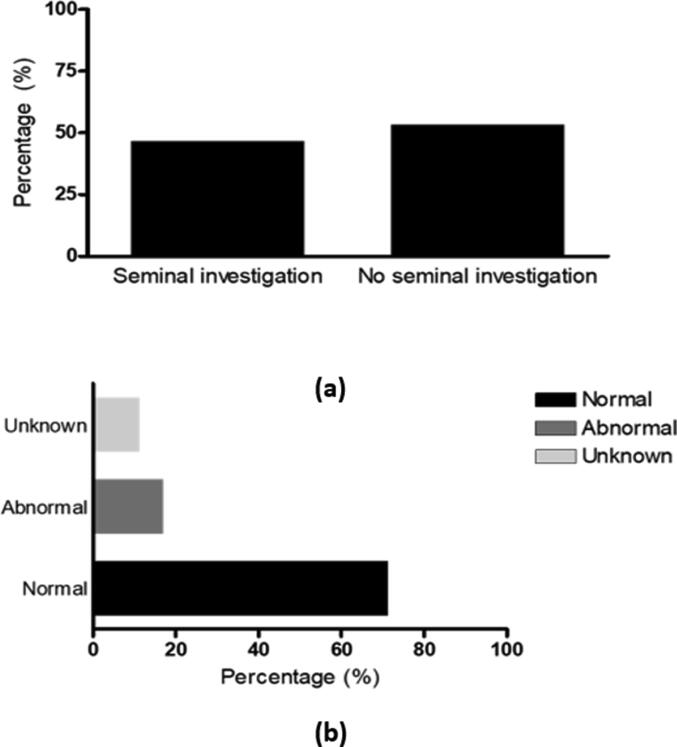
Of 75 male partners, 35 (46.7%) had undergone seminal investigations. Of these, 25 (71.4%) had normal results, 6 (17.1%) had abnormal results and 4 (11.4%) with unknown results (Fig. 2).
Fig. 2.
Seminal investigation (a) The percentage of male partners who undergone seminal investigation. (b) Male partners seminal investigation results.
Most of participants 51 (68%) had regular menstrual cycles, while 24 (32%) had irregular cycles (Fig. 3).
Fig. 3.
Regularity of the menstrual cycle among the patients.
The presence of factors suggesting PID among patients showed that 12 (16%) had past medical history [PMH] of diagnosed gonococcal or chlamydial infection. Also, 7 (9.3%) patients had PMH of intrauterine contraceptive device [IUCD]-provoked infection. Only 2 (2.7%) patients had history of post abortion infection or puerperal infection. Whereas, 35 (46.7%) patients had PMH of PID symptoms (table 1).
Table 1.
Distribution of patients according to presence of factors suggesting PID.
| Yes | No | |
|---|---|---|
| Frequency N (%) | Frequency N (%) | |
| PMH of Diagnosed Gonococcal or chlamydial infection | 12 (16%) | 63 (84%) |
| PMH of IUCD - provoked infection | 7 (9.3%) | 68 (90.7%) |
| PMH of post abortion infection | 2 (2.7%) | 73 (97.3%) |
| PMH of puerperal infection | 2 (2.7%) | 73 (97.3%) |
| PMH of PID symptoms | 35 (46.7%) | 40 (53.3%) |
Concerning the presence of factors suggesting tubal blockage among the participants, (table 2) illustrates the results. 13 (17.3%) patients had PMH of abdominal or pelvic surgery. 9 (12.0%) had previous HSG showing blocked tubes. Only 2 (2.7%) with history of ectopic pregnancy. While no history of tuberculosis was reported.
Table 2.
Distribution of patients according to presence of factors suggesting tubal blockage.
| Yes | No | |
|---|---|---|
| Frequency N (%) | Frequency N (%) | |
| PMH of Ectopic pregnancy | 2 (2.7%) | 73 (97.3%) |
| PMH abdominal or pelvic surgery | 13 (17.3%) | 62 (82.7%) |
| History of tuberculosis | 0% | 75 (100%) |
| Previous HSG showing blocked tubes | 9 (12%) | 66 (88%) |
3.2. Hysterosalpingography findings
Abnormal findings at HSG were found in 39 (52.7%) patients. Abnormalities involving fallopian tubes were seen in 32 (42.7%) patients. This was in the form of bilateral tubal blockage in 11 (14.9%) patients, unilateral tubal blockage in 17 (23%) patients and hydrosalpinx in 8 (10.8%) patients. The blockage was noted at the proximal end of the tube and at the distal end in 12 (16.2%) and 17 (23%) patients, respectively. Uterine abnormalities were found in 18 (24%) patients. 12 (16%) patients in the form of well-defined filling defects in the uterus. Irregular uterine outlines or irregular filling defects were noted in only 3 (4%) patients, possibly due to intrauterine adhesions. While, congenital abnormalities of uterine shape were seen in 5 (6.8%) patients. Also, 5 (6.8%) patients had other findings such as SIN and loculated spill which indicates peritubal adhesions. Only 9 (12%) patients had combined tubal and uterine abnormalities (Fig. 4).
Fig. 4.
HSG findings (a) The incidence of normal and abnormal hysterosalpingography (HSG) findings among the patients. (b) The incidence of tubal, uterine and combined abnormalities among the patients. (c) Pattern of fallopian tube abnormalities and uterine abnormalities.
3.3. HSG findings association with personal and clinical data
HSG findings associations with patients’ age groups, type of infertility and PMH suggesting PID or tubal blockage were studied. The associations between variables reflecting ovarian and male factors of infertility and HSG findings were studied as well.
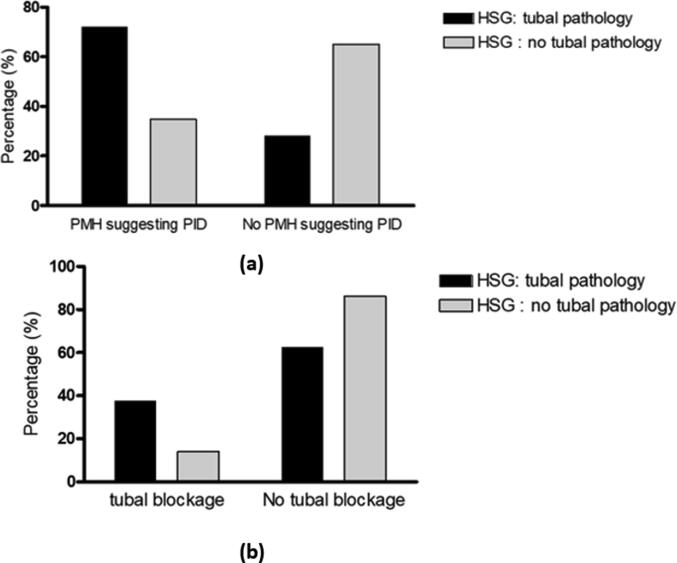
As illustrated in Fig. 5, HSG tubal abnormalities were found in 23 (71.9%) patients with PMH suggesting PID and 12 (37.5%) patients with past history suggesting tubal blockage. While, normal HSG tubal findings were seen in 15 (34.9%) and 6 (14.0%) patients who had PMH suggestive of PID and tubal blockage, respectively. This reflects highly significant association between HSG tubal pathology and PMH suggesting PID or tubal blockage, (P = 0.002, P = 0.018, respectively).
Fig. 5.
HSG pathology correlations (a): Correlation between HSG tubal pathology and factors suggesting PID. P-value = 0.002. (b): Correlation between HSG tubal pathology and factors suggesting tubal blockage. P-value = 0.018. * PMH: past medical history. PID: pelvic inflammatory disease.
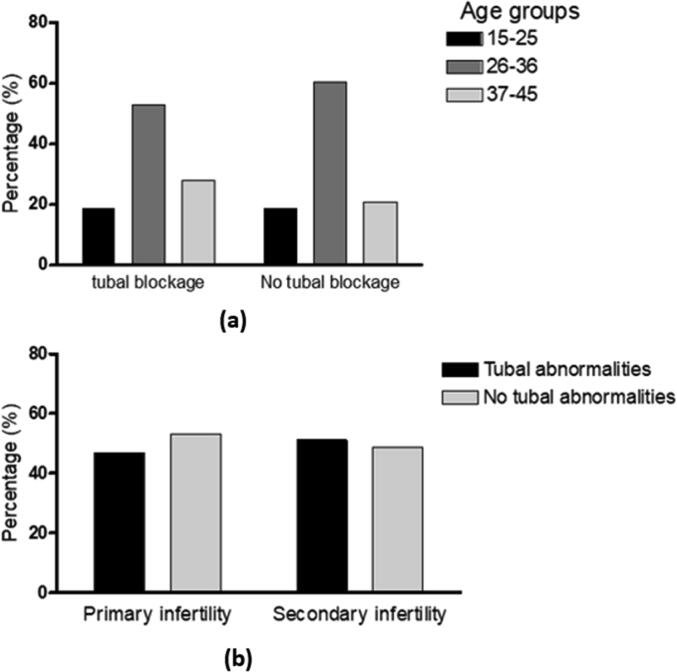
No significant associations were found between the negative or positive HSG tubal patency findings and the patients’ age or type of infertility (P = 0.75, P = 0.71, respectively), (Fig. 6).
Fig. 6.
Tubal findings correlations. (a): Correlation between tubal findings and the patients’ age groups. P-value = 0.753. (b): Correlation between tubal findings and type of infertility. P-value = 0.713.
Similarly, (Fig. 7) shows that no significant associations were found between negative or positive HSG uterine findings and patients’ age groups or type of infertility (P = 0.25 for both).
Fig. 7.
Uterine abnormalities correlations (a): Correlation of uterine abnormalities with the patients’ age groups. P = 0.251. (b) Correlation between uterine abnormalities and type of infertility. P = 0.252.
Fig. 8 illustrates the association of HSG findings with variables that reflect ovarian and male factors of infertility. No significant association was found between normal and abnormal HSG findings and menstrual cycle being regular or irregular (P = 0.86) (Fig. 8a). Similarly, there was no significant association between normal and abnormal HSG findings and the results of seminal analysis (P = 0.06), (Fig. 8b).
Fig. 8.
The correlation of normal HSG findings with variables that reflect ovarian and male factors of infertility. (a) The correlation of normal HSG findings with the menstrual cycle. P = 0.861. (b) The correlation between normal HSG findings and the results of seminal analysis. P = 0.06.
Samples of HSG images of females presenting with infertility are shown in Fig. 9.
Fig. 9.
Sample of HSG images. Image (a): HSG showing normal filling of the uterine cavity and fallopian tubes with free bilateral intraperitoneal spill of contrast. Image (b): HSG showing normal uterus with bilateral proximal tubal blockage. Image (c): HSG showing normal left fallopian tube with free peritoneal spill. Right fallopian tube is not outlined and no peritoneal spill is seen on the right, indicating proximal right tubal blockage. Image (d): HSG showing arcuate uterus. The distal right fallopian tube is prominent with no intraperitoneal spillage of contrast denoting distal blockage. Normal left fallopian tube showing free contrast spillage. Image(e): HSG showing right hydrosalpinx. The right fallopian tube is dilated and filled with contrast with absence of free spillage. Normal uterine cavity and left fallopian tube. Image (f): HSG showing divided uterus, suggestive of a septate or bicornuate uterus. Image (g): HSG during the early filling stage of the uterus, showing a round well-defined filling defect in the uterus consistent with submucous leiomyoma. Image (h1): HSG, spot radiograph obtained with the uterus fully distended with contrast material and portions of the fallopian tubes are opacified. (h2): HSG, spot radiograph showing free intraperitoneal contrast material spillage at the site of the right fallopian tube and loculated spill at the site of the left fallopian tube indicating peri tubal pelvic adhesions.
4. Discussion
Infertility affects one of every seven couples (Thurston et al., 2019). Thus it affects a significant proportion of population, with a significant overall burden. Further research is required for implementation and integration of infertility diagnosis and treatment in developing countries, where infertility burden is greatest (Fathalla, 2019).
Moreover, causes of infertility vary with the characteristics of local population. Therefore, it is essential to study infertility causes locally and manage accordingly (Deshpande and Gupta, 2019).
Despite its existence for more than hundred years, hysterosalpingography remains one of the first line imaging tools in infertility work up (Omidiji et al., 2019). Being available, cheap, less invasive and easily interpreted, it is still widely used, despite the presence of other complementary methods (Onwuchekwa and Oriji, 2017).
In consonance with a previous study (Ibekwe et al., 2010), high prevalence of infertility was found in woman aged 26–36 years. It seems reasonable, because it is the peak of female reproductive stage. The incidence of infertility whether primary or secondary, differs in various regions of the world. In our study, the incidence of these two types were nearly equal. This is different from what has been found previously by (Bukar et al., 2011) in Nigeria, (Aziz et al., 2015) in Pakistan and (Al-Turki et al., 2016) in Saudi Arabia, where secondary infertility was more common. On the other hand, primary infertility was more common than secondary infertility in a study conducted in India (Deshpande and Gupta, 2019). Interestingly, a significant association between the type of infertility and the age of the patients was found. Primary infertility affects patients who are younger than 25 years. While, secondary infertility remains the most prevalent among patients above 37 years, as the advanced age of couples and long duration of marriage reduce their ability to reproduce and have a new child. This is comparable with results from a recent study (Benksim et al., 2018).
Analysis of the factors suggesting PMH of PID among the participants revealed that, almost half of them had PID symptoms, with only (16%) having PMH of proven gonococcal or chlamydial infection. This finding needs to be interpreted with caution. In line with previous evidence (Abdella et al., 2015), this may be due to the small numbers of patients who were adequately investigated for sexually transmitted diseases [STDs]. This revealed that the prevalence of STDs, such as genital Chlamydial infection in many developing countries lacks the reliable estimates due to limitations in diagnostic and treatment programs. Also, (9.3%) of patients had PMD of IUCD-provoked infection and as anticipated only minority (2.7%) had PMH of post abortion or puerperal infection, as many women deliver or being managed for miscarriages in hospitals with good medical facilities and proper sanitary conditions.
Analysis of PMH suggesting blockage of the fallopian tubes among the patients showed that, (32%) of patients had PMH suggesting tubal blockage, with PMH of abdominal or pelvic surgery (17.3%) and previous HSG with blocked tubes (12%) being the most common, only few (2.7%) with PMH of ectopic pregnancy and no patient with a history of tuberculosis.
Discussing the history that reflects ovarian and male factors of infertility, the majority of referred women had regular menstrual cycle reflecting the small contribution of the ovarian factor of infertility in this population. Although, the semen findings were normal in most of the investigated males, it is highly questionable whether the male partner of an infertile couple can be considered healthy, as more than half of the male partners were not investigated for infertility.
In Sudan, local research showed that some societies still do not acknowledge men’s contribution to infertility. Women should be the ones to seek management and bear the social stigma (Khalifa and Ahmed, 2012).
In this study, more than half of the infertile females had HSG abnormalities, and this is comparable to the previously reported findings in Nigeria (Danfulani et al., 2014). Tubal abnormalities had been the most common in agreement with other studies (Okafor et al., 2010) and (Al-Turki et al., 2016). But the incidence in the latter was much higher (81.2%). Tubal abnormalities were followed by uterine abnormalities, and then with combined tubal and uterine pathologies. This is in contrast to a study done by (Onwuchekwa and Oriji, 2017) where uterine abnormalities were more common than tubal abnormalities.
Available evidence (Kitilla, 2010), supports higher incidence of distal tubal blockage as compared to proximal blockage.
In our sample, unilateral tubal blockage was more common than bilateral blockage, similar to (Aziz et al., 2015). Hydrosalpinx was detected in 10.8% approximately similar to (Bhattarai and Pokhrel, 2017). Other findings such as SIN and loculated spill were found in fewer patients close to the findings in (Onwuchekwa and Oriji, 2017).
Well-defined filling defects in the uterus, possibly due to leiomyomas or endometrial polyps, were the most common uterine abnormality, in agreement with (Onwuchekwa and Oriji, 2017), followed by congenital uterine malformations, and then irregular uterine filling defects.
The significant association detected between HSG tubal pathology findings and PMH suggesting PID indicates that PID is common in Sudan and being a common cause of infertility. Furthermore, our study revealed a significant association between PMH suggesting tubal blockage, mainly history of previous abdominopelvic surgery and HSG tubal pathology findings. This corroborated previous evidences (Famurewa et al., 2013).
In contrast with earlier findings (Bhattarai and Pokhrel, 2017), no significant associations between HSG tubal or uterine findings and the type of infertility was found. In that study, uterine abnormalities were common in cases with primary infertility. While tubal abnormalities were common in women with secondary infertility, being explained by the possibility of poor health care after delivery or abortion and the increased risk of PID and STDs.
Abdominal pain and minimal postprocedural vaginal spotting were the main complications experienced by patients who had participated in this study. Other HSG drawbacks include exposure to ionizing radiation, possibility of infection, allergic reaction and rarely intravasation of contrast media. (Bhattarai and Pokhrel, 2017). The rate of complications is higher when oil soluble contrast media is used (Burks and Hansen, 2020).
History about menstrual cycle regularity and results of seminal analysis, being normal or abnormal, were considered the indicators for ovarian and male factors of infertility. These are considered limitations of this study. A broader study that includes ovarian and male partners’ investigations is needed for more accurate correlation of these factors with HSG findings.
5. Conclusion
Hysterosalpingogram, an important tool in infertility assessment, has a major role in outlining pathologies of female reproductive pathway. However, complementary tests might remain necessary to confirm some infertility patterns.
HSG findings of infertility vary in different regions. In our study, tubal blockage was the most prevalent HSG abnormality, associated mainly with PID. Thus, preventive measures and proper management of pelvic infections are vital to decrease the incidence of infertility caused by tubal pathologies. Moreover, as both males and females contribute to couple infertility, female partners should not be considered as the root of infertility. Encouraging male partners to undergo infertility investigations remains necessary to improve the outcome through responding favorably to diagnosis and infertility treatment.
CRediT authorship contribution statement
Hind Toufig: Conceptualization, Data curation, Formal analysis, Investigation. Methodology, Software, Visualization, Writing-Original draft, Writing-review and editing. Tarek Benameur: Data curation, Formal analysis, Software, Visualization, Writing-Original draft, Writing-review and editing. Mohammed-Elfatih Twfieg: Formal analysis, Visualization, Writing-Original draft, Writing-review and editing. Hiba Omer: Formal analysis, Software, Visualization, Writing-Original draft, Writing- review and editing. Tamara El-Musharaf: Formal analysis, Visualization, Writing-review and editing.
Declaration of Competing Interest
All authors declare that there are no conflicts of interest and no personal financial interests. The research did not receive any specific grant funding agencies in the public or commercial sectors.
Acknowledgements
We are grateful to Mr. Mamoun Elkheir, Mr. Khalid Bo Hassan, in KFU and Dr. Abdulkhaliq Kardesh for their great support in completing this work.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abdella R.M.A. Screening for Chlamydia trachomatis in Egyptian women with unexplained infertility, comparing real-time PCR techniques to standard serology tests: case control study. BMC Womens Health. 2015;15(45) doi: 10.1186/s12905-015-0202-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aghajanova L., Hoffman J., Mok-Lin E., Herndon C.N. Obstetrics and gynecology residency and fertility needs. Reprod. Sci. 2017;24(3):428–434. doi: 10.1177/1933719116657193. [DOI] [PubMed] [Google Scholar]
- Al-Turki H.A. Uterine and tubal abnormalities in infertile Saudi Arabian women: a teaching hospital experience. Saudi J. Med. Med. Sci. 2016;2(4):89–92. doi: 10.4103/1658-631X.178293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aziz M.U., Anwar S., Mahmood S. Hysterosalpingographic evaluation of primary and secondary infertility. Pak. J. Med. Sci. 2015;31(5):1188–1191. doi: 10.12669/pjms.315.7545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benksim A. Difference between primary and secondary infertility in Morocco: frequencies and associated factors. Int. J. Fertil. Steril. 2018;12(2):142–146. doi: 10.22074/ijfs.2018.5188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattarai M., Pokhrel S. Hysterosalpingographic evaluation of uterus and fallopian tubes of infertile women. J. Nobel Medical College. 2017;6(10):63–71. [Google Scholar]
- Bukar M., Takai U., Mustapha Z., Tahir A. Hysterosalpingographic findings in infertile women: A seven year review. Nigerian J. Clin. Pract. 2011;14(2):168–170. doi: 10.4103/1119-3077.84008. [DOI] [PubMed] [Google Scholar]
- Burks H.R., Hansen K.R. Oil or water-based contrast for hysterosalpingography? Fertil. Steril. 2020;114(1):75–76. doi: 10.1016/j.fertnstert.2020.04.034. [DOI] [PubMed] [Google Scholar]
- Chalazonitis A. Hysterosalpingography: Technique and Applications. Curr. Probl. Diagn. Radiol. 2009;38(5):199–205. doi: 10.1067/j.cpradiol.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Chen L.-S. Hysterosalpingo-contrast-sonography vs. magnetic resonance-hysterosalpingography for diagnosing fallopian tubal patency: A systematic review and meta-analysis. Eur. J. Radiol. 2020;125 doi: 10.1016/j.ejrad.2020.108891. [DOI] [PubMed] [Google Scholar]
- Chen, S., Du, X., Chen, Q., Chen, S., 2019. Combined real-time three-dimensional hysterosalpingo-contrast sonography with B mode hysterosalpingo-contrast sonography in the evaluation of fallopian tube patency in patients undergoing infertility investigations. [DOI] [PMC free article] [PubMed]
- Choussein S., Vlahos N.F. Female fertility assessment. Curr. Obstetrics Gynecol. Reports. 2012;1(4):174–181. [Google Scholar]
- Danfulani M. Tubal abnormalities on hysterosalpingography in primary and secondary infertility in Sokoto, Northwestern-Nigeria. Asian J. Med. Sci. 2014;6(2):47–50. [Google Scholar]
- Deshpande P.S., Gupta A.S. Causes and prevalence of factors causing infertility in a public health facility. J. Hum. Reprod. Sci. 2019;12(4):278–293. doi: 10.4103/jhrs.JHRS_140_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elhussein O.G. Epidemiology of infertility and characteristics of infertile couples requesting assisted reproduction in a low-resource setting in Africa, Sudan. Fertil. Res. Pract. 2019;5:7. doi: 10.1186/s40738-019-0060-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Famurewa O., Adeyemi A., Ibitoye O., Ogunsemoyin O. Association between history of abdominopelvic surgery and tubal pathology. Afr. Health Sci. 2013;13(2):441–446. doi: 10.4314/ahs.v13i2.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fathalla, M., 2019. Infertility is a global public health issue. Sexual and reproductive health. [Online] Available at: https://www.who.int/reproductivehealth/topics/infertility/perspective/en/.
- Ibekwe P., Udensi M., Imo A. Hysterosalpingographic findings in patients with infertility in South Eastern Nigeria. Nigerian J. Med. 2010;19(2) doi: 10.4314/njm.v19i2.56510. [DOI] [PubMed] [Google Scholar]
- Jain V., Jain D.S. A study on hysterosalpingography findings in patients with infertility. Int. J. Clin. Obstetrics Gynaecol. 2019;2(4):35–38. [Google Scholar]
- Kaproth-Joslin K., Dogra V. Imaging of female infertility: a pictorial guide to the hysterosalpingography, ultrasonography, and magnetic resonance imaging findings of the congenital and acquired cause of female infertility. Radiol. Clin. North Am. 2013;51(6):967–981. doi: 10.1016/j.rcl.2013.07.002. [DOI] [PubMed] [Google Scholar]
- Khalifa D.M.S., Ahmed M.A. Reviewing Infertility care in Sudan; socio-cultural, policy and ethical barriers. FVV ObGyn. 2012:53–58. [Google Scholar]
- Kiridi E., Ibrahim I., Lawani L. Hysterosalpingography: still relevant in the evaluation of infertility in the Niger Delta. Int. J. Med. Biomed. Res. 2015;4(1):50–54. [Google Scholar]
- Kitilla, T., 2010. Hysterosalpingography in the evaluation of infertility: a five years review. (FGAE, 2001 -5). Ethiop. Med. J. 48(4), pp. 267–276. [PubMed]
- Okafor C.O., Okafor C.I., Okpala O.C., Umeh E. The pattern of hysterosalpingographic findings in women being investigated for infertility in Nnewi, Nigeria. Niger J. Clin. Pract. 2010;13(3):264–267. [PubMed] [Google Scholar]
- Omidiji O.A. Hysterosalpingographic findings in infertility – what has changed over the years? African Health Sci. 2019;19(2) doi: 10.4314/ahs.v19i2.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onwuchekwa C.R., Oriji V.K. Hysterosalpingographic (HSG) pattern of infertility in women of reproductive age. J. Human Reproductive Sci. 2017;10(3):178–184. doi: 10.4103/jhrs.JHRS_121_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panti A.A., Sununu Y.T. The profile of infertilityin a teaching Hospital in North West Nigeria. Sahel Med. J. 2014;17(1):7. [Google Scholar]
- Pundir J., ELToukhy T. Uterine cavity assessment prior to IVF. Women's Health. 2010;6(6):841–848. doi: 10.2217/whe.10.61. [DOI] [PubMed] [Google Scholar]
- Saanida M., Beenamol S. A comparative study between sonohysterosalpingography and hysterosalpingography in the evaluation of infertility. J. Evolut. Med. Dental Sci. 2013;2(7):702–708. [Google Scholar]
- Schankath A. Hysterosalpingography in the workup of female infertility: indications, technique and diagnostic findings. Insights Imaging. 2012;3(5):475–483. doi: 10.1007/s13244-012-0183-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson W.L., Beitia L.G., Mester J. Hysterosalpingography: a reemerging study. Radiographics. 2006;26(2):419–431. doi: 10.1148/rg.262055109. [DOI] [PubMed] [Google Scholar]
- Subedi S., Lamichhane S., Chhetry M. Study of infertile couples attending a teaching hospital in Eastern Nepal. J. Nepal Med. Assoc. 2016;55(203):22–25. [PubMed] [Google Scholar]
- Thable A., Duff E., Dika C. Infertility management in primary care. Nurse Practitioner. 2020;45(5):48–54. doi: 10.1097/01.NPR.0000660356.18430.0a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurston L., Abbara A., Dhillo W. Investigation and managment of subfertility. J. Clin. Pathol. 2019;9(72):579–587. doi: 10.1136/jclinpath-2018-205579. [DOI] [PubMed] [Google Scholar]
- Tros R. The capacity of transvaginal hydrolaparoscopy versus hysterosalpingography to diagnose tubal pathology in the work-up of subfertile women, a randomised clinical trial. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019;236:127–132. doi: 10.1016/j.ejogrb.2019.02.035. [DOI] [PubMed] [Google Scholar]
- Yang T. Sonohysterography: Principles, technique and role in diagnosis of endometrial pathology. World J. Radiol. 2013;5(3):81–87. doi: 10.4329/wjr.v5.i3.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zegers-Hochschild F. The international glossary on infertility and fertility care, 2017. Hum. Reprod. 2017;32(9):1786–1801. doi: 10.1093/humrep/dex234. [DOI] [PMC free article] [PubMed] [Google Scholar]